Abstract
Systemic lupus erythematosus (SLE) is a chronic autoimmune disorder that predominately affects women of reproductive age. Hypertension is an important cardiovascular risk factor that is prevalent in this patient population. Despite the high incidence of hypertension in women with SLE, the pathophysiological mechanisms underlying the development of hypertension remain poorly understood. This review will focus on disease-related factors, including inflammation, autoantibodies, and sex hormones that may contribute to hypertension in patients with SLE. In addition, we will highlight studies performed by our laboratory using the female NZBWF1 (F1 hybrid of New Zealand Black and New Zealand White strains) mouse model, a spontaneous model of SLE that mimics human disease and develops hypertension and renal injury. Specifically, using female NZBWF1 mice, we have demonstrated that multiple factors contribute to the pathogenesis of hypertension, including the inflammatory cytokine, tumor necrosis factor (TNF)-α, oxidative stress, as well as B-cell hyperactivity and autoantibody production.
Keywords: autoantibodies, cardiovascular disease, estrogens, hypertension, inflammation, systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is a chronic multisystem autoimmune disorder that can affect almost all organ systems including the kidneys, skin, joints, and central nervous system. Although the cause of SLE is unknown, genetic and environmental factors as well as sex hormones are likely to be involved in disease pathogenesis. SLE is predominantly a disease of women, since females of reproductive age are affected at a rate of nine females to one male, although the female predilection is less pronounced before menarche and after menopause [Tucker et al. 1995]. It is thought that the complex interaction between genetic, environmental, and hormonal components leads to a breach of immunological tolerance, and that this loss of tolerance results in the production of autoantibodies, most often to self-molecules in the nucleus, cytoplasm and on the cell surface. Antinuclear antibodies are found in ~95% of SLE patients and anti-dsDNA antibodies have been detected in 70% of patients [Reveille, 2004]. The presence of autoantibodies leads to the formation of immune complexes, which can deposit in virtually any tissue in the body. The classification criteria for SLE, as recently revised and validated by the Systemic Lupus International Collaborating Clinics (SLICC) in 2012, are outlined in Table 1. A person is diagnosed with SLE if they have had 4 or more of the 17 criteria outlined, including at least 1 clinical criterion and 1 immunological criterion; alternatively a patient can be diagnosed with biopsy-proven lupus nephritis and positive antinuclear or anti-dsDNA antibodies [Petri et al. 2012].
Table 1.
Systemic Lupus International Collaborating Clinics (SLICC) criteria for the classification of SLE.
| Clinical criteria | Immunological criteria |
|---|---|
| Acute cutaneous lupus (malar rash) | ANA above laboratory reference range |
| Chronic cutaneous lupus (discoid rash) | Anti-dsDNA above laboratory reference range |
| Oral ulcers | Anti-Sm |
| Nonscarring alopecia | Antiphospholipid antibody |
| Synovitis involving two or more joints | Low complement |
| Serositis | Direct Coombs test in the absence of hemolytic anemia |
| Renal | |
| Neurologic | |
| Hemolytic anemia | |
| Leukopenia or lymphopenia | |
| Thrombocytopenia |
ANA, antinuclear antibodies; SLE, systemic lupus erythematosus.
Survival has increased dramatically over the past 50 years for SLE patients, mostly through improved diagnosis and treatment, although 10% of patients still die within 5 years after diagnosis [Smolen, 2002]. Women who survive beyond the first 5 years after diagnosis are most likely to die from cardiovascular disease [Manzi et al. 1997]. The bimodal pattern of mortality in SLE was first described by Urowitz and colleagues in the Toronto lupus cohort: those who die in the first year after diagnosis have active lupus, and those who die later in the course of the disease have inactive lupus and a high incidence of atherosclerosis and myocardial infarction [Urowitz et al. 1976]. This bimodal pattern, as well as the high incidence of death due to cardiovascular disease, has been confirmed in subsequent studies [Mody et al. 1994; Abu-Shakra et al. 1995; Bernatsky et al. 2006]. In addition, women with SLE aged 35–44 were 50 times more likely to have a cardiac event (myocardial infarction or angina pectoris) compared with age-matched controls participating in the Framingham Offspring Study [Manzi et al. 1997]. Hypertension is an important, yet understudied, cardiovascular risk factor that is prevalent in this patient population. This review will discuss both clinical and experimental evidence for the increased prevalence of hypertension and potential underlying mechanisms.
Hypertension in SLE
Hypertension is a major risk factor for the development of cardiovascular disease and is prevalent in patients with SLE [Budman and Steinberg, 1976; Mandell, 1987; Petri, 2000; Selzer et al. 2001; Al-Herz et al. 2003; Sabio et al. 2011; Shaharir et al. 2015]. The incidence of hypertension is especially striking in women younger than 40: in one cohort 40% of women under 40 were hypertensive compared with 11% of the control subjects [Sabio et al. 2011]. While the pathogenesis of hypertension in SLE is not fully understood [Ryan, 2009], a combination of traditional (age, sex, obesity, ethnicity) and disease-related factors (immune system dysfunction, inflammation, renal involvement, drug side effects) may contribute to hypertension in SLE patients [Sabio et al. 2001; Chaiamnuay et al. 2007]. Specifically, renal function, the renin–angiotensin system (RAS), sex hormones, inflammatory cytokines, and autoantibodies will be discussed in this review.
Renal function
In ~50% of SLE patients, the kidneys are affected in the form of immune complex glomerulonephritis, and nearly all patients show evidence of kidney injury on biopsy [Boumpas et al. 1995; Guo et al. 2010]; however, SLE-associated hypertension can occur independently of nephritis [Ward and Studenski, 1992; Petrin et al. 1993]. For example, a recent study by Shaharir and colleagues revealed that 53% of SLE patients in one cohort were hypertensive despite the absence of nephritis [Shaharir et al. 2015]. Because of the importance of the kidney in the long-term control of blood pressure, impaired renal function is certain to contribute to the prevalent hypertension in SLE patients. Impaired renal hemodynamics and tubular function are likely contributors, since the ability of the kidney to excrete sodium and water in response to changes in pressure is critical for effective long-term control of blood pressure. Evidence suggests that both glomerular filtration rate (GFR) and renal plasma flow are impaired in SLE patients [Nakano et al. 1998], and tubular lesions are prevalent [Daniel et al. 2001]; however, whether these changes mechanistically promote SLE-associated hypertension has not been examined extensively. One factor that can promote impaired renal hemodynamics is renal vascular dysfunction. While there are no specific reports on renal vascular endothelial function in SLE, the vascular endothelium is prominently affected in this patient population, as exhibited by the high rates of atherosclerosis in SLE patients [Alves and Ames, 2003; Bijl, 2003; Roman et al. 2003]. In addition, multiple studies have shown vascular endothelial dysfunction in SLE, as measured by brachial artery flow [Lima et al. 2002; Johnson et al. 2004; Piper et al. 2007], and both endothelial-dependent vasodilation and endothelial repair mechanisms are impaired in SLE patients. Therefore, studies designed to evaluate renal vascular function during SLE may yield important insight for understanding the prevalent hypertension.
Renin–angiotensin system
The RAS is critical for blood pressure and volume homeostasis; however, little is known about this system and its relationship to SLE and SLE hypertension. Multiple genetic studies have been conducted to link common angiotensin-converting enzyme (ACE) insertion and deletion polymorphisms and SLE disease; however, no definitive correlations have been made [Kaufman et al. 2001; Lee et al. 2006, 2013]. ACE inhibitors and angiotensin II (ANG II) receptor antagonists are commonly prescribed to SLE patients and have shown to be effective at controlling blood pressure, but mostly in patients with evidence of an activated RAS [Herlitz et al. 1984]. In addition to the RAS having an important role in blood pressure control, ANG II can stimulate the production of endothelin-1 (ET-1), a peptide whose role in blood pressure control results from a balance between vasoconstrictive actions and natriuretic actions in the renal medulla. Plasma ET-1 levels are increased in SLE patients [Julkunen et al. 1991] and serum from SLE patients can induce the production of ET-1 by endothelial cells in vitro [Yoshio et al. 1995].
Inflammatory cytokines
It is widely recognized that the immune dysregulation and low-grade inflammation has a role in the pathophysiology of hypertension [Harrison et al. 2010; Ryan, 2013]. This is supported by the fact that treatment with the immunosuppressive drug mycophenolate mofetil in both animal models and essential hypertensive patients reduces blood pressure [Rodriguez-Iturbe et al. 2002; Herrera et al. 2006; De Miguel et al. 2010; Ferro et al. 2011]. Various inflammatory cytokines, including interleukin (IL)-6, IL-17, IL-18, type I interferons, and tumor necrosis factor (TNF)-α have been implicated in the pathogenesis of SLE [Yap and Lai, 2013]. Similarly, studies report a correlation between cytokines such as IL-6, TNF-α, and C-reactive protein and blood pressure in patients with essential hypertension [Blake et al. 2003; Bautista et al. 2005; Vazquez-Oliva et al. 2005; Sesso et al. 2007]. Inflammatory cytokines interact with important blood pressure regulatory systems, such as the RAS [Brasier et al. 2002; Harrison et al. 2011; Capettini et al. 2012; Pacurari et al. 2014] and the sympathetic nervous system [Pongratz and Straub, 2014]. IL-6, which promotes B-cell hyperreactivity and increased autoantibody production [Cross and Benton, 1999], is elevated in the serum of patients with SLE and generally correlates with disease activity [Ripley et al. 2005]. While the role of IL-6 has not specifically been examined in SLE hypertension, it has been shown to contribute to ANG II-induced hypertension [Zhang et al. 2012]. TNF-α has also been shown to be elevated in the serum of SLE patients and can correlate with disease activity [Weckerle et al. 2012]. Studies report that blockade of TNF-α in SLE patients can decrease urinary protein but increase anti-dsDNA autoantibodies [Aringer et al. 2004]; nevertheless, the role of TNF-α in SLE hypertension remains unclear. The type I interferon IFN-α, which is elevated in the plasma of ~50% of patients with SLE [Baechler et al. 2004], plays a central role in mediating endothelial dysfunction by promoting endothelial progenitor cell (EPC) deletion [Lee et al. 2007]. In addition, there are elevated levels of circulating endothelial cells, which is a marker for vascular injury, in SLE patients [Clancy, 2000]. Whether or not elevated IFN-α impacts renal hemodynamic function as a mechanism to promote hypertension during SLE remains unclear.
Autoantibodies
Patients with essential hypertension or pregnancy-related hypertension have elevated circulating levels of immunoglobulin (Ig)G and IgM [Ebringer and Doyle, 1970; Suryaprabha et al. 1984; Hilme et al. 1989]. In addition, many studies have shown a correlation between the production of pathogenic autoantibodies and hypertension in humans [Gudbrandsson et al. 1981; Wenzel et al. 2008], and specific antibodies to a particular antigen are often increased and may have a role in the pathogenesis [Wallukat et al. 1999; Fu et al. 2000; Jahns et al. 2004]. For example, spontaneously hypertensive rats were previously shown to have autoantibodies in their serum that are cytotoxic to T cells, and transplantation of compatible thymus tissues was sufficient to reduce blood pressure in this model [Ba et al. 1982]. Also, women with preeclampsia reportedly produce activating ANG II type-1 receptor autoantibodies (AT1R-AA) and the antibody titer appears to correlate with disease severity [Siddiqui et al. 2010]. Administration of AT1R-AA isolated from humans to pregnant mice was shown to induce hypertension in those animals [Zhou et al. 2011]. A direct role of autoantibodies in humans was shown in patients with refractory hypertension, in which immunoadsorption of autoantibodies to the α1-adrenergic receptor was sufficient to lower mean arterial pressure [Wenzel et al. 2008]. Autoantibodies to nuclear components are a hallmark characteristic of SLE, and deposition of autoantibody immune complexes in the glomerular and tubular basement membranes are common in lupus nephritis. In addition, there is evidence that autoantibodies can bind directly to intrinsic glomerular antigens [Jang and Stollar, 2003]. Treatment with the B-cell depletion antibody anti-CD20 (Rituximab) has been shown to lower titers of anti-dsDNA antibodies in some studies [Anolik et al. 2004], but no study published to date has reported blood pressure in SLE patients on an anti-CD20 treatment regimen, and the role of specific autoantibodies in the pathogenesis of SLE-associated hypertension remains uncertain.
Sex hormones
Because of the striking female bias in SLE, a role for endogenous sex hormones in disease pathogenesis is likely. Nevertheless, there is conflicting evidence on whether women with SLE have increased estrogens: a meta-analysis of 20 studies found populations of women with SLE have increased, deceased or equivalent levels of 17-β-estradiol when compared with control subjects [McMurray and May, 2003]. While the levels of 17-β-estradiol may be in the normal physiological range, women with SLE do have abnormal estrogen metabolism, as evidenced by the increase in 16α-hydroxylation of estrone [Lahita et al. 1979]. These hydroxylated estrones are more potent and may have a role in increasing inflammatory cell numbers [Cutolo et al. 2004]. Furthermore, women with SLE may have low plasma androgens, including testosterone and dihydrotestosterone [Lahita et al. 1979; Jungers et al. 1982]. Because estrogens have diverse immunomodulatory functions [Lang, 2004; Straub, 2007], it is likely that altered estrogen metabolism in SLE influences multiple aspects of the immune system, including differences in inflammatory cytokine production and both B- and T-cell activation and function. Thus, both abnormal cytokine profiles and autoantibody production could be affected by estrogens and impact blood pressure, although the connection among these factors is not well studied in patients with SLE, particularly as it relates to cardiovascular risk factors such as hypertension.
Mouse models of SLE
In order to better understand the etiology of SLE-associated hypertension, experimental animal models are useful tools. Much has been learned about SLE disease pathogenesis using both spontaneous and induced murine models of SLE. For the purposes of this review, only the most commonly studied spontaneous models will be discussed. For a lengthier discussion of SLE mouse models, the reader is referred to [Theofilopoulos and Dixon, 1985; Perry et al. 2011]. The three most commonly studied spontaneous models are MRL/lpr, BXSB, and NZBWF1 (F1 hybrid of New Zealand Black [NZB] and New Zealand White [NZW] strains). While each of these mouse models varies in their utility, all three develop autoantibodies and immune complex-mediated glomerulonephritis, one of the hallmark characteristics of SLE. MRL/lpr mice have lymphoadenopathy due to an aberrant accumulation of CD4− CD8− T cells that express the B-cell marker B220. In addition, they have high levels of circulating Igs, and begin producing anti-dsDNA and anti-ssDNA antibodies by 12–16 weeks of age [Reilly and Gilkeson, 2002]. The lymphoproliferative phenotype is attributed to a recessive autosomal mutation on chromosome 19, which alters transcription of the Fas receptor [Watson et al. 1992]. MRL/lpr mice develop vasculitis and arthritis as well as severe lupus nephritis, but do not become hypertensive [Rudofsky et al. 1984]. Unlike lupus in humans, male and female MRL/lpr mice are affected at an equal rate. BXSB mice have a translocation of the telomeric end of the X chromosome to the Y chromosome, which results in gene duplications and upregulation of gene expression of many of the duplicated genes. One of the duplicated genes is Toll-like receptor 7 (Tlr7) [Pisitkun et al. 2006; Subramanian et al. 2006], which can bind to exogenous RNA ligands and lead to autoreactive B-cell activation [Leadbetter et al. 2002; Lau et al. 2005]. Because of the nature of the chromosomal translocation, only male BXSB mice develop a SLE-like disease, with symptoms ranging from secondary lymphoid tissue hyperplasia, immune complex mediated glomerulonephritis, monocytosis, and hypergammaglobulinemia. When BXSB mice are crossed with NZW mice, a severe coronary artery disease develops [Hang et al. 1981]. Although these mice are useful in studying coronary artery disease in the context of SLE, they do not have the same gender bias and do not develop hypertension.
The oldest and most extensively studied mouse model of SLE is the NZBWF1. Both NZW and NZB display limited autoimmunity; however the F1 cross of these two strains develops a severe lupus-like phenotype [Theofilopoulos and Dixon, 1985; Perry et al. 2011]. These mice exhibit many of the classic hallmarks of lupus disease, including lymphadenopathy, splenomegaly, elevated serum anti-dsDNA antibodies, and immune complex-mediated glomerulonephritis [Burnett et al. 2004]. NZBWF1 mice typically have a detectable anti-dsDNA autoantibody production by ~17–20 weeks of age [Bassi et al. 2015]. The glomerulonephritis becomes apparent between 5–6 months of age and ultimately leads to kidney failure and death between 10–12 months of age, which is less than half the expected lifespan of a mouse. Unlike the MRL/lpr mice, NZBWF1 mice do not develop arthritis or skin lesions. Importantly, NZBWF1 mice have been shown to develop hypertension [Rudofsky et al. 1984; Ryan et al. 2007]. Like patients with SLE, a strong gender bias exists in NZBWF1 mice and multiple genetic mutations contribute to the development of SLE. Multiple lupus susceptibility loci have been identified that are derived from either the NZB or NZW mice including Sle1, Sle2, and Sle3 [Kono and Theofilopoulos, 2006; Perry et al. 2011]. The Sle1 locus is associated with a selective loss of tolerance to chromatin, Sle2 results in B cells with a lowered cell activation threshold, and Sle3 mediates a dysregulation of CD4+ T cells [Morel et al. 2000]. Contribution of these loci to cardiovascular risk in NZBWF1 mice is still not well understood.
Hypertension in NZBWF1 mice
Because of the strong gender bias and complex genetic component of SLE disease in NZBWF1 mice, this model most closely resembles human SLE, and is especially useful for understanding the pathophysiology of hypertension. Much of the work in our laboratory has made use of female NZBWF1 mice to elucidate the mechanisms of hypertension. NZBWF1 mice were first shown over 30 years ago to have elevated blood pressure using the tail cuff method [Rudofsky et al. 1984]. Our laboratory has shown that NZBWF1 mice have a ~10 mmHg increase in blood pressure, measured in conscious tethered mice via an indwelling carotid artery catheter, when compared to control NZW mice, by 30 weeks of age. This increase in pressure was evident in mice that had no evidence of renal disease as assessed by urinary albumin [Venegas-Pont et al. 2009]. However, by 36 weeks of age, the mice have an even higher mean arterial pressure (Figure 1), and the increase in blood pressure is accompanied by excretion of large amounts of albumin in the urine, indicative of glomerular injury [Ryan and McLemore, 2007]. Histological analyses of the kidneys of NZBWF1 mice also reveal the characteristic ‘wire loop’ glomerular pathology that is observed in human SLE patients with immune complex deposition in the glomerular basement membrane [Carlsoo and Ostberg, 1978; Lemoine et al. 1992]. In addition to immune complex deposition, there are increased numbers of monocytes and macrophages in the renal cortex (Figure 2), indicating that immune cells infiltrate the kidneys and raise the likelihood that they contribute to the pathogenesis of hypertension [Ryan et al. 2006]. Similar to humans, there is a disconnect between nephritis and arterial pressure in murine models of SLE: MRL/lpr, BXSB, and NZBWF1 mice all develop glomerulonephritis; however, only NZBWF1 mice develop hypertension. The hypertension in female NZBWF1 mice is associated with low plasma renin [Rudofsky et al. 1984] and is not sensitive to salt [Mathis et al. 2011]. This suggests that regulation of the RAS is largely intact in this model. Our laboratory has reported on several factors that contribute to hypertension in female NZBWF1 mice, including impaired renal hemodynamic function [Venegas-Pont et al. 2011], vascular endothelial dysfunction [Ryan and McLemore, 2007], altered inflammatory cytokine profiles [Venegas-Pont et al. 2010], oxidative stress [Mathis et al. 2012], adaptive immune system dysfunction [Mathis et al. 2014], and sex hormones [Gilbert et al. 2014; Gilbert and Ryan, 2014].
Figure 1.
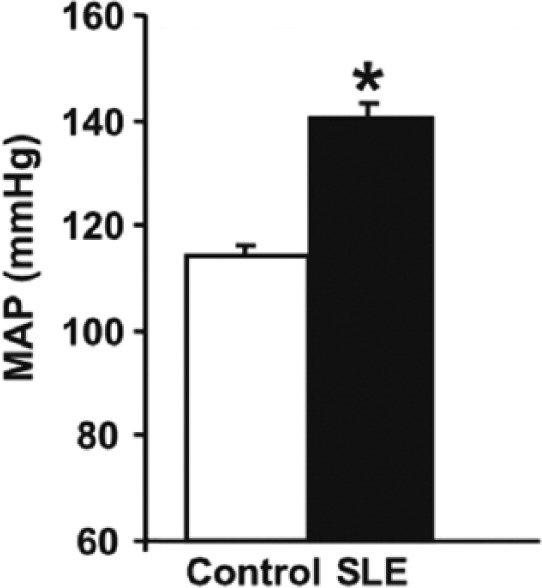
Mean arterial pressure is increased at 36 weeks of age in female NZBWF1 mice with SLE as compared with control NZW mice. Pressure was measured in conscious tethered mice via indwelling carotid artery catheters (*p < 0.05 versus control) [Adapted from Ryan et al. (2006)].
MAP, Mean arterial pressure; NZBWF1, F1 hybrid of NZB and NZW strains; NZW, New Zealand White; SLE, systemic lupus erythematosus.
Figure 2.
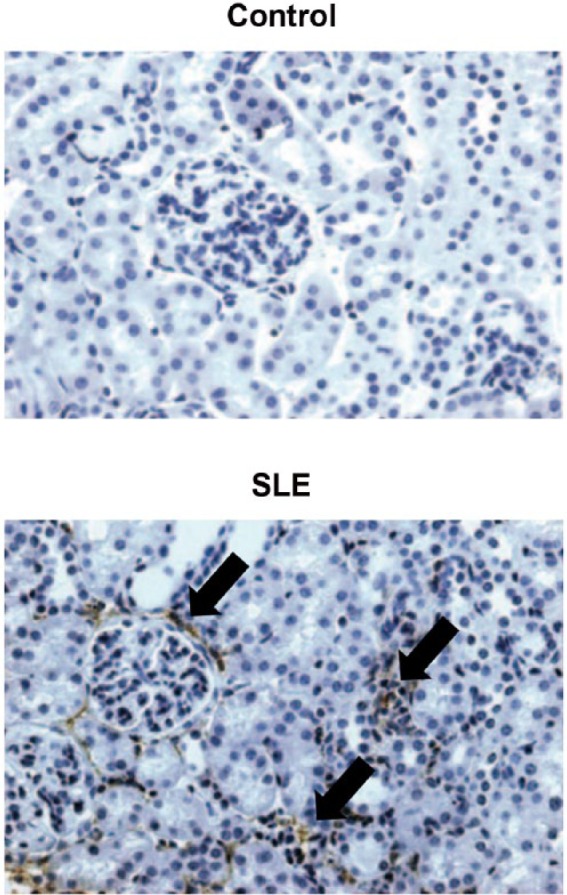
Renal cortices from female NZBWF1 mice have increased monocyte and macrophage infiltration as compared to control NZW mice, as assessed by staining with the monocyte/macrophage antibody F4/80 [Adapted from Ryan et al. (2006)].
NZBWF1, F1 hybrid of NZB and NZW strains; NZW, New Zealand White.
Because the kidneys have a central role in blood pressure regulation through long-term control of sodium and fluid balance, our laboratory has examined renal hemodynamic function as a contributing mechanism for the hypertension. In female NZBWF1 mice, there is a parallel rightward (hypertensive) shift in the pressure natriuresis relationship, demonstrating that at a given salt intake, greater arterial pressure is required to excrete the sodium. Therefore, renal excretory function is impaired in SLE and is an important mechanism for the development of hypertension. In further support of impaired renal hemodynamic function as a potential contributing mechanism for the hypertension, female NZBWF1 mice have an altered renal hemodynamic function as indicated by attenuated renal blood flow [Salvati et al. 1995; Venegas-Pont et al. 2011], increased vascular resistance [Venegas-Pont et al. 2011], and a lower GFR [Kiberd, 1991; Venegas-Pont et al. 2011] when compared to control mice. NZBWF1 mice also have increased blood urea nitrogen and plasma creatinine [Corna et al. 1997; Song et al. 1998]. While it has not been established in NZBWF1 mice whether they have renal vascular dysfunction, there is clear evidence of vascular endothelial dysfunction in these mice. Isolated carotid arteries from NZBWF1 mice displayed impaired relaxation to acetylcholine as compared with control mice at both 20 and 36 weeks of age [Ryan and McLemore, 2007]. NZBWF1 mice exhibit decreased numbers of EPCs in both the spleen and bone marrow at 36 weeks of age, and the EPCs have increased apoptosis and an impaired ability to differentiate into mature endothelial cells in vitro [Thacker et al. 2010]. These data indicate that vascular dysfunction corresponds with hypertension in SLE mice; however, a direct cause and effect relationship between these two has not been established.
While there is limited data on RAS components in mouse models of SLE, treatment of NZBWF1 mice with the ACE inhibitor captopril delays the onset of renal injury and proteinuria [Herlitz et al. 1988; De Albuquerque et al. 2004], and both captopril and enalapril were shown to reduce blood pressure [Herlitz et al. 1988]. However, NZBWF1 mice reportedly have low plasma renin activity [Rudofsky et al. 1984]. In studies by our laboratory, renal hemodynamic responses to an acute infusion of ANG II were examined and NZBWF1 mice were shown to have a greater reduction in RBF after an ANG II infusion, suggesting that the kidneys of NZBWF1 mice have an enhanced sensitivity to ANG II [Venegas-Pont et al. 2011]. This enhanced sensitivity may contribute to the progression of renal disease and hypertension in NZBWF1 mice.
Because of the prominent immune dysregulation in NZBWF1 mice, it is reasonable to conclude that immune factors influence the development of hypertension in these animals. While some studies report lower circulating levels of TNF-α in NZBWF1 mice [Jacob and McDevitt, 1988; Kontoyiannis and Kollias, 2000], our laboratory and others routinely report increased levels of protein expression of renal TNF-α as compared with control mice. In addition, female NZBWF1 mice treated with the TNF-α antagonist etanercept had lower blood pressure and reduced glomerular injury when compared with vehicle-treated animals. Importantly, etanercept decreased monocyte and macrophage infiltration in the kidneys and lowered renal cortical levels of NADPH oxidase [Venegas-Pont et al. 2010]. These data suggest that a TNF-α blockade lowered blood pressure by reducing renal inflammation, which ultimately reduces renal oxidative stress, a recognized contributor to the development of hypertension [Wilcox, 2002; Reckelhoff and Romero, 2003]. The production of both reactive oxygen and nitrogen intermediates is increased in patients with SLE, and multiple studies have shown an increased production of nitric oxide (NO) in autoimmune animal models [Vladutiu, 1995; Oates and Gilkeson, 2006]. Specifically, the increased activity of inducible nitric oxide synthase (NOS2) in murine SLE models correlates with the increased production of pathogenic nitrogen intermediates such as peroxynitrite (ONOO-) [Weinberg et al. 1994; Oates, 2010]. The production of reactive intermediates correlates with glomerular pathology and the onset of nephritis [Oates and Gilkeson, 2006; Oates, 2010]. Our laboratory treated NZBWF1 mice with a combination of tempol and apocynin to directly test whether renal oxidative stress impacted hypertension. This treatment resulted in lowered mean arterial pressures as well as reduced incidence of albuminuria and levels of renal cortical H2O2 and NADPH oxidase expression. Antioxidant therapy did not, however, impact the progression of SLE disease, as measured by anti-dsDNA levels [Mathis et al. 2012]. Recently, our laboratory also treated NZBWF1 mice with anti-CD20 antibody (mouse equivalent of rituximab). As indicated in Figure 3, a 14-week treatment prevented the development of hypertension in NZBWF1 mice. This treatment also lowered the percentage of CD45R+ B cells in the spleen and the quantity of anti-dsDNA antibodies in the plasma. Importantly, this study revealed that a shorter (4-week) treatment with anti-CD20 did not significantly reduce blood pressure, which suggests that once autoantibodies are being produced and the inflammatory process has begun, anti-CD20 therapy may have limited efficacy on hypertension and renal injury [Mathis et al. 2014]. Nevertheless, this study highlights the importance of B cells in the progression of SLE hypertension in NZBWF1 mice.
Figure 3.
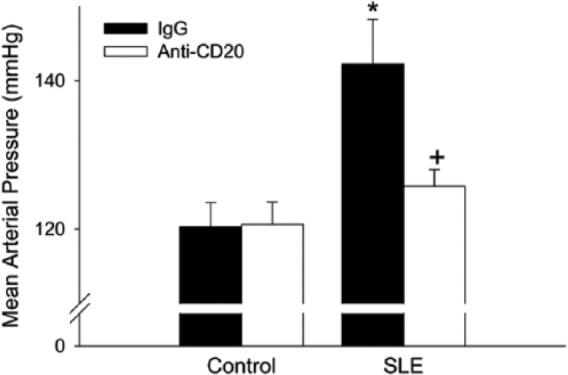
Mean arterial pressure measured in 34-week-old female NZBWF1 (SLE) and NZW (control) mice administered IgG or anti-CD20 for 14 weeks. * p< 0.001 versus control/IgG; +p < 0.01 versus SLE/IgG [Adapted from Mathis et al. (2014)].
IgG, immunoglobulin G; MAP, mean arterial pressure; NZB, New Zealand Black; NZBWF1, F1 hybrid of NZB and NZW strains; NZW, New Zealand White; SLE, systemic lupus erythematosus.
Studies on the NZBWF1 mouse model have also revealed a complex role of estrogens in the pathogenesis of both SLE disease and the associated hypertension. Estrogens have multiple immunoregulatory functions, including modulating cytokines, cytokine receptor production, and effector cell activation [Cunningham and Gilkeson, 2011]. It has been demonstrated that NZBWF1 mice with a pharmacological blockade of estrogen early in life or genetic deletion of estrogen receptor (ER)-α exhibit reduced anti-dsDNA autoantibody levels, kidney damage, and mortality [Wu et al. 2000; Sthoeger et al. 2003; Bynote et al. 2008]. Consistent with these studies, our laboratory recently demonstrated that an early life ovariectomy (OVX) delayed the onset of albuminuria and autoantibody production and also caused an increase in body weight and fat mass. Early life OVX did not, however, alter blood pressure in adult NZBWF1 mice [Gilbert and Ryan, 2014]. Interestingly, the timing of estrogen removal (by OVX) appears to be important. In a recent study by our laboratory, Gilbert and colleagues found that performing OVX on adult (30 weeks) NZBWF1 mice exacerbates the hypertension and albuminuria as compared with sham-operated SLE mice without alterations in the production of anti-dsDNA autoantibodies. When the OVX mice were injected subcutaneously with 17-β-estradiol, the OVX-induced increase in blood pressure was prevented. OVX also caused an increase in renal TNF-α expression [Gilbert et al. 2014]. These data suggest that estrogens have important temporal roles in the pathogenesis of SLE and that while estrogens seem to promote SLE pathogenesis in early life, they may have a cardiovascular protective role in adulthood.
Conclusion
SLE is a chronic autoimmune disorder that predominately affects women of childbearing age. Because of the complex interaction between genes, sex hormones, and the environment, the pathogenesis of hypertension and development of cardiovascular disease is also likely to be multifactorial. Much of the work that has been published to date has focused on local mediators (such as cytokines and reactive oxygen species) that are likely to be downstream of the initial immune system dysregulation. These mediators contribute to local inflammation, which ultimately negatively affects renal function (Figure 4). The role of hyperactive T and B lymphocytes, both central to the development of autoimmune disorders, in the pathogenesis of hypertension remains unclear. Understanding SLE hypertension using NZBWF1 mice will be not only important for reducing cardiovascular risk in patients with SLE, but will also be important for elucidating mechanisms of human essential hypertension.
Figure 4.
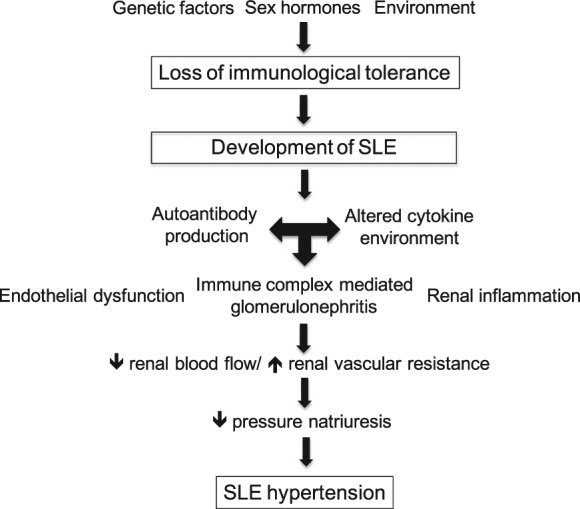
Proposed schematic for factors contributing to the pathogenesis of hypertension during SLE.
SLE, systemic lupus erythematosus.
Footnotes
Funding: This work was partially supported by NIH grants P01HL051971 and P20GM104357.
Conflict of interest statement: The authors declare that there are no conflicts of interest.
Contributor Information
Erin B. Taylor, Department of Physiology and Biophysics, University of Mississippi Medical Center, Jackson, MS, USA
Michael J. Ryan, Department of Physiology and Biophysics, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216, USA.
References
- Abu-Shakra M., Urowitz M., Gladman D., Gough J. (1995) Mortality studies in systemic lupus erythematosus. Results from a single center. I. Causes of death. J Rheumatol 22: 1259–1264. [PubMed] [Google Scholar]
- Al-Herz A., Ensworth S., Shojania K., Esdaile J. (2003) Cardiovascular risk factor screening in systemic lupus erythematosus. J Rheumatol 30: 493–496. [PubMed] [Google Scholar]
- Alves J., Ames P. (2003) Atherosclerosis, oxidative stress and auto-antibodies in systemic lupus erythematosus and primary antiphospholipid syndrome. Immunobiology 207: 23–28. [DOI] [PubMed] [Google Scholar]
- Anolik J., Barnard J., Cappione A., Pugh-Bernard A., Felgar R., Looney R., et al. (2004) Rituximab improves peripheral B cell abnormalities in human systemic lupus erythematosus. Arthritis Rheum 50: 3580–3590. [DOI] [PubMed] [Google Scholar]
- Aringer M., Graninger W., Steiner G., Smolen J. (2004) Safety and efficacy of tumor necrosis factor alpha blockade in systemic lupus erythematosus: an open-label study. Arthritis Rheum 50: 3161–3169. [DOI] [PubMed] [Google Scholar]
- Ba D., Takeichi N., Kodama T., Kobayashi H. (1982) Restoration of T cell depression and suppression of blood pressure in spontaneously hypertensive rats (SHR) by thymus grafts or thymus extracts. J Immunol 128: 1211–1216. [PubMed] [Google Scholar]
- Baechler E., Gregersen P., Behrens T. (2004) The emerging role of interferon in human systemic lupus erythematosus. Curr Opin Immunol 16: 801–807. [DOI] [PubMed] [Google Scholar]
- Bassi N., Luisetto R., Ghirardello A., Gatto M., Valente M., Della Barbera M., et al. (2015) 17-Beta-estradiol affects blys serum levels and the nephritogenic autoantibody network accelerating glomerulonephritis in NZB/WF1 mice. Lupus 24: 382–391. [DOI] [PubMed] [Google Scholar]
- Bautista L., Vera L., Arenas I., Gamarra G. (2005) Independent association between inflammatory markers (C-reactive protein, interleukin-6 and TNF-Alpha) and essential hypertension. J Hum Hypertens 19: 149–154. [DOI] [PubMed] [Google Scholar]
- Bernatsky S., Boivin J., Joseph L., Manzi S., Ginzler E., Gladman D., et al. (2006) Mortality in systemic lupus erythematosus. Arthritis Rheum 54: 2550–2557. [DOI] [PubMed] [Google Scholar]
- Bijl M. (2003) Endothelial activation, endothelial dysfunction and premature atherosclerosis in systemic autoimmune diseases. Neth J Med 61: 273–277. [PubMed] [Google Scholar]
- Blake G., Rifai N., Buring J., Ridker P. (2003) Blood pressure, C-reactive protein and risk of future cardiovascular events. Circulation 108: 2993–2999. [DOI] [PubMed] [Google Scholar]
- Boumpas D., Austin H., Fessler B., Balow J., Klippel J., Lockshin M. (1995) Systemic lupus erythematosus: emerging concepts. Part 1: renal, neuropsychiatric, cardiovascular, pulmonary and hematologic disease. Ann Intern Med 122: 940–950. [DOI] [PubMed] [Google Scholar]
- Brasier A., Recinos A., Eledrisi M. (2002) Vascular inflammation and the renin-angiotensin system. Arterioscler Thromb Vasc Biol 22: 1257–1266. [DOI] [PubMed] [Google Scholar]
- Budman D., Steinberg A. (1976) Hypertension and renal disease in systemic lupus erythematosus. Arch Intern Med 136: 1003–1007. [PubMed] [Google Scholar]
- Burnett R., Ravel G., Descotes J. (2004) Clinical and histopathological progression of lesions in lupus-prone (NZB X NZW) F1 mice. Exp Toxicol Pathol 56: 37–44. [DOI] [PubMed] [Google Scholar]
- Bynote K., Hackenberg J., Korach K., Lubahn D., Lane P., Gould K. (2008) Estrogen receptor-alpha deficiency attenuates autoimmune disease in (NZB X NZW)F1 mice. Genes Immun 9: 137–152. [DOI] [PubMed] [Google Scholar]
- Capettini L., Montecucco F., Mach F., Stergiopulos N., Santos R., Da Silva R. (2012) Role of renin-angiotensin system in inflammation, immunity and aging. Curr Pharm Des 18: 963–970. [DOI] [PubMed] [Google Scholar]
- Carlsoo B., Ostberg Y. (1978) Ultrastructural observations on the parotitis autoimmunica in the NZB/NZW hybrid mice. Acta Otolaryngol 85:298–306. [DOI] [PubMed] [Google Scholar]
- Chaiamnuay S., Bertoli A., Roseman J., Mcgwin G., Apte M., Duran S., et al. (2007) African-American and Hispanic ethnicities, renal involvement and obesity predispose to hypertension in systemic lupus erythematosus: results from Lumina, a multiethnic cohort (Luminaxlv). Ann Rheum Dis 66: 618–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy R. (2000) Circulating endothelial cells and vascular injury in systemic lupus erythematosus. Curr Rheumatol Rep 2: 39–43. [DOI] [PubMed] [Google Scholar]
- Corna D., Morigi M., Facchinetti D., Bertani T., Zoja C., Remuzzi G. (1997) Mycophenolate mofetil limits renal damage and prolongs life in murine lupus autoimmune disease. Kidney Int 51: 1583–1589. [DOI] [PubMed] [Google Scholar]
- Cross J., Benton H. (1999) The roles of interleukin-6 and interleukin-10 in B cell hyperactivity in systemic lupus erythematosus. Inflamm Res 48: 255–261. [DOI] [PubMed] [Google Scholar]
- Cunningham M., Gilkeson G. (2011) Estrogen receptors in immunity and autoimmunity. Clin Rev Allergy Immunol 40: 66–73. [DOI] [PubMed] [Google Scholar]
- Cutolo M., Sulli A., Capellino S., Villaggio B., Montagna P., Seriolo B., et al. (2004) Sex hormones influence on the immune system: basic and clinical aspects in autoimmunity. Lupus 13: 635–638. [DOI] [PubMed] [Google Scholar]
- Daniel L., Sichez H., Giorgi R., Dussol B., Figarella-Branger D., Pellissier J., et al. (2001) Tubular lesions and tubular cell adhesion molecules for the prognosis of lupus nephritis. Kidney Int 60: 2215–2221. [DOI] [PubMed] [Google Scholar]
- De Albuquerque D., Saxena V., Adams D., Boivin G., Brunner H., Witte D., et al. (2004) An ace inhibitor reduces TH2 cytokines and TGF-beta1 and TGF-beta2 isoforms in murine lupus nephritis. Kidney Int 65: 846–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Miguel C., Das S., Lund H., Mattson D. (2010) T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 298:R1136–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebringer A., Doyle A. (1970) Raised serum IgG levels in hypertension. Br Med J 2: 146–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro C., Edwards N., Hutchison C., Cockwell P., Steeds R., Savage C., et al. (2011) Does immunosuppressant medication lower blood pressure and arterial stiffness in patients with chronic kidney disease? An observational study. Hypertens Res 34: 113–119. [DOI] [PubMed] [Google Scholar]
- Fu M., Herlitz H., Schulze W., Wallukat G., Micke P., Eftekhari P., et al. (2000) Autoantibodies against the angiotensin receptor (AT1) in patients with hypertension. J Hypertens 18: 945–953. [DOI] [PubMed] [Google Scholar]
- Gilbert E., Mathis K., Ryan M. (2014) 17 beta-estradiol protects against the progression of hypertension during adulthood in a mouse model of systemic lupus erythematosus. Hypertension 63: 616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert E., Ryan M. (2014) Impact of early life ovariectomy on blood pressure and body composition in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol 307: R990–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudbrandsson T., Hansson L., Herlitz H., Lindholm L., Nilsson L. (1981) Immunological changes in patients with previous malignant essential hypertension. Lancet 1: 406–408. [DOI] [PubMed] [Google Scholar]
- Guo Q., Lu X., Miao L., Wu M., Lu S., Luo P. (2010) Analysis of clinical manifestations and pathology of lupus nephritis: a retrospective review of 82 cases. Clin Rheumatol 29: 1175–1180. [DOI] [PubMed] [Google Scholar]
- Hang L., Izui S., Dixon F. (1981) (NZW X BXSB)F1 hybrid. A model of acute lupus and coronary vascular disease with myocardial infarction. J Exp Med 154: 216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D., Guzik T., Lob H., Madhur M., Marvar P., Thabet S., et al. (2011) Inflammation, immunity and hypertension. Hypertension 57: 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D., Vinh A., Lob H., Madhur M.S. (2010) Role of the adaptive immune system in hypertension. Curr Opin Pharmacol 10: 203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlitz H., Edeno C., Mulec H., Westberg G., Aurell M. (1984) Captopril treatment of hypertension and renal failure in systemic lupus erythematosus. Nephron 38: 253–256. [DOI] [PubMed] [Google Scholar]
- Herlitz H., Svalander C., Tarkowski A., Westberg G. (1988) Effect of captopril on murine systemic lupus erythematosus disease. J Hypertens Suppl 6: S684–686. [DOI] [PubMed] [Google Scholar]
- Herrera J., Ferrebuz A., Macgregor E., Rodriguez-Iturbe B. (2006) Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol 17: S218–225. [DOI] [PubMed] [Google Scholar]
- Hilme E., Herlitz H., Soderstrom T., Hansson L. (1989) Increased secretion of immunoglobulins in malignant hypertension. J Hypertens 7: 91–95. [PubMed] [Google Scholar]
- Jacob C., McDevitt H. (1988) Tumour necrosis factor-alpha in murine autoimmune ‘lupus’ nephritis. Nature 331: 356–358. [DOI] [PubMed] [Google Scholar]
- Jahns R., Boivin V., Hein L., Triebel S., Angermann C., Ertl G., et al. (2004) Direct evidence for a beta 1-adrenergic receptor-directed autoimmune attack as a cause of idiopathic dilated cardiomyopathy. J Clin Invest 113: 1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang Y., Stollar B. (2003) Anti-DNA antibodies: aspects of structure and pathogenicity. Cell Mol Life Sci 60: 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S., Harvey P., Floras J., Iwanochko M., Ibanez D., Gladman D., et al. (2004) Impaired brachial artery endothelium dependent flow mediated dilation in systemic lupus erythematosus: preliminary observations. Lupus 13: 590–593. [DOI] [PubMed] [Google Scholar]
- Julkunen H., Saijonmaa O., Gronhagen-Riska C., Teppo A., Fyhrquist F. (1991) Raised plasma concentrations of endothelin-1 in systemic lupus erythematosus. Ann Rheum Dis 50: 526–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungers P., Nahoul K., Pelissier C., Dougados M., Tron F., Bach J. (1982) Low plasma androgens in women with active or quiescent systemic lupus erythematosus. Arthritis Rheum 25: 454–457. [DOI] [PubMed] [Google Scholar]
- Kaufman K., Kelly J., Gray-McGuire C., Asundi N., Yu H., Reid J., et al. (2001) Linkage analysis of angiotensin-converting enzyme (ACE) insertion/deletion polymorphism and systemic lupus erythematosus. Mol Cell Endocrinol 177:81–85. [DOI] [PubMed] [Google Scholar]
- Kiberd B. (1991) Murine lupus nephritis. A structure-function study. Lab Invest 65: 51–60. [PubMed] [Google Scholar]
- Kono D., Theofilopoulos A. (2006) Genetics of Sle in mice. Springer Semin Immunopathol 28: 83–96. [DOI] [PubMed] [Google Scholar]
- Kontoyiannis D., Kollias G. (2000) Accelerated autoimmunity and lupus nephritis in nzb mice with an engineered heterozygous deficiency in tumor necrosis factor. Eur J Immunol 30: 2038–2047. [DOI] [PubMed] [Google Scholar]
- Lahita R., Bradlow H., Kunkel H., Fishman J. (1979) Alterations of estrogen metabolism in systemic lupus erythematosus. Arthritis Rheum 22: 1195–1198. [DOI] [PubMed] [Google Scholar]
- Lang T. (2004) Estrogen as an immunomodulator. Clin Immunol 113: 224–230. [DOI] [PubMed] [Google Scholar]
- Lau C., Broughton C., Tabor A., Akira S., Flavell R., Mamula M., et al. (2005) RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med 202: 1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadbetter E., Rifkin I., Hohlbaum A., Beaudette B., Shlomchik M., Marshak-Rothstein A. (2002) Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416: 603–607. [DOI] [PubMed] [Google Scholar]
- Lee P., Li Y., Richards H., Chan F., Zhuang H., Narain S., et al. (2007) Type I interferon as a novel risk factor for endothelial progenitor cell depletion and endothelial dysfunction in systemic lupus erythematosus. Arthritis Rheum 56: 3759–3769. [DOI] [PubMed] [Google Scholar]
- Lee Y., Choi S., Ji J., Song G. (2013) Association between the angiotensin-converting enzyme insertion/deletion polymorphism and susceptibility to systemic lupus erythematosus: a meta-analysis. J Renin Angiotensin Aldosterone Syst 14: 248–254. [DOI] [PubMed] [Google Scholar]
- Lee Y., Rho Y., Choi S., Ji J., Song G. (2006) Angiotensin-converting enzyme insertion/deletion polymorphism and systemic lupus erythematosus: a meta-analysis. J Rheumatol 33: 698–702. [PubMed] [Google Scholar]
- Lemoine R., Berney T., Shibata T., Fulpius T., Gyotoku Y., Shimada H., et al. (1992) Induction of “wire-loop” lesions by murine monoclonal IgG3 cryoglobulins. Kidney Int 41: 65–72. [DOI] [PubMed] [Google Scholar]
- Lima D., Sato E., Lima V., Miranda F., Hatta F. (2002) Brachial endothelial function is impaired in patients with systemic lupus erythematosus. J Rheumatol 29: 292–297. [PubMed] [Google Scholar]
- Mandell B. (1987) Cardiovascular involvement in systemic lupus erythematosus. Semin Arthritis Rheum 17: 126–141. [DOI] [PubMed] [Google Scholar]
- Manzi S., Meilahn E., Rairie J., Conte C., Medsger T., Jansen-McWilliams L., et al. (1997) Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham study. Am J Epidemiol 145: 408–415. [DOI] [PubMed] [Google Scholar]
- Mathis K., Venegas-Pont M., Masterson C., Stewart N., Wasson K., Ryan M. (2012) Oxidative stress promotes hypertension and albuminuria during the autoimmune disease systemic lupus erythematosus. Hypertension 59: 673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis K.W., Venegas-Pont M., Masterson C.W., Wasson K.L., Ryan M.J. (2011) Blood Pressure in a Hypertensive Mouse Model of Sle Is Not Salt-Sensitive. Am J Physiol Regul Integr Comp Physiol 301: R1281–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis K., Wallace K., Flynn E., Maric-Bilkan C., Lamarca B., Ryan M. (2014) Preventing autoimmunity protects against the development of hypertension and renal injury. Hypertension 64: 792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray R., May W. (2003) Sex hormones and systemic lupus erythematosus: review and meta-analysis. Arthritis Rheum 48: 2100–2110. [DOI] [PubMed] [Google Scholar]
- Mody G., Parag K., Nathoo B., Pudifin D., Duursma J., Seedat Y. (1994) High mortality with systemic lupus erythematosus in hospitalized African blacks. Br J Rheumatol 33: 1151–1153. [DOI] [PubMed] [Google Scholar]
- Morel L., Croker B., Blenman K., Mohan C., Huang G., Gilkeson G., et al. (2000) Genetic reconstitution of systemic lupus erythematosus immunopathology with polycongenic murine strains. Proc Natl Acad Sci USA 97: 6670–6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M., Ueno M., Hasegawa H., Watanabe T., Kuroda T., Ito S., et al. (1998) Renal haemodynamic characteristics in patients with lupus nephritis. Ann Rheum Dis 57: 226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oates J. (2010) The biology of reactive intermediates in systemic lupus erythematosus. Autoimmunity 43: 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oates J., Gilkeson G. (2006) The biology of nitric oxide and other reactive intermediates in systemic lupus erythematosus. Clin Immunol 121: 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacurari M., Kafoury R., Tchounwou P., Ndebele K. (2014) The renin-angiotensin-aldosterone system in vascular inflammation and remodeling. Int J Inflam 2014: 689360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D., Sang A., Yin Y., Zheng Y., Morel L. (2011) Murine models of systemic lupus erythematosus. J Biomed Biotechnol 2011: 271694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri M. (2000) Detection of coronary artery disease and the role of traditional risk factors in the Hopkins lupus cohort. Lupus 9: 170–175. [DOI] [PubMed] [Google Scholar]
- Petri M., Orbai A., Alarcon G., Gordon C., Merrill J., Fortin P., et al. (2012) Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 64: 2677–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrin J., Rozman B., Dolenc P., Logar D., Bozic B., Vizjak A., et al. (1993) The dissociation of arterial hypertension and lupus glomerulonephritis in systemic lupus erythematosus. Blood Press 2: 108–112. [DOI] [PubMed] [Google Scholar]
- Piper M., Raza K., Nuttall S., Stevens R., Toescu V., Heaton S., et al. (2007) Impaired endothelial function in systemic lupus erythematosus. Lupus 16: 84–88. [DOI] [PubMed] [Google Scholar]
- Pisitkun P., Deane J., Difilippantonio M., Tarasenko T., Satterthwaite A., Bolland S. (2006) Autoreactive B cell responses to RNA-related antigens due to Tlr7 gene duplication. Science 312: 1669–1672. [DOI] [PubMed] [Google Scholar]
- Pongratz G., Straub R. (2014) The sympathetic nervous response in inflammation. Arthritis Res Ther 16: 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reckelhoff J., Romero J. (2003) Role of oxidative stress in angiotensin-induced hypertension. Am J Physiol Regul Integr Comp Physiol 284: R893–912. [DOI] [PubMed] [Google Scholar]
- Reilly C., Gilkeson G. (2002) Use of genetic knockouts to modulate disease expression in a murine model of lupus, MRL/LPR mice. Immunol Res 25: 143–153. [DOI] [PubMed] [Google Scholar]
- Reveille J. (2004) Predictive value of autoantibodies for activity of systemic lupus erythematosus. Lupus 13: 290–297. [DOI] [PubMed] [Google Scholar]
- Ripley B., Goncalves B., Isenberg D., Latchman D., Rahman A. (2005) Raised levels of interleukin 6 in systemic lupus erythematosus correlate with anaemia. Ann Rheum Dis 64: 849–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Iturbe B., Quiroz Y., Nava M., Bonet L., Chavez M., Herrera-Acosta J., et al. (2002) Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am J Physiol Renal Physiol 282: F191–201. [DOI] [PubMed] [Google Scholar]
- Roman M., Shanker B., Davis A., Lockshin M., Sammaritano L., Simantov R., et al. (2003) Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med 349: 2399–2406. [DOI] [PubMed] [Google Scholar]
- Rudofsky U., Dilwith R., Roths J., Lawrence D., Kelley V., Magro A. (1984) Differences in the occurrence of hypertension among (NZB X NZW)F1, MRL-LPR and BXSB mice with lupus nephritis. Am J Pathol 116: 107–114. [PMC free article] [PubMed] [Google Scholar]
- Ryan M. (2009) The pathophysiology of hypertension in systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol 296: R1258–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M. (2013) An update on immune system activation in the pathogenesis of hypertension. Hypertension 62: 226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M., McLemore G. (2007) Hypertension and impaired vascular function in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol 292: R736–742. [DOI] [PubMed] [Google Scholar]
- Ryan M., McLemore G., Hendrix S. (2006) Insulin resistance and obesity in a mouse model of systemic lupus erythematosus. Hypertension 48: 988–993. [DOI] [PubMed] [Google Scholar]
- Sabio J., Mediavilla J., Fernandez-Torres C., Aliaga L., Jimenez-Alonso J. (2001) Risk factors related to hypertension in a Spanish systemic lupus erythematosus cohort. Lupus 10: 451–452. [DOI] [PubMed] [Google Scholar]
- Sabio J., Vargas-Hitos J., Navarrete-Navarrete N., Mediavilla J., Jimenez-Jaimez J., Diaz-Chamorro A., et al. (2011) Prevalence of and factors associated with hypertension in young and old women with systemic lupus erythematosus. J Rheumatol 38: 1026–1032. [DOI] [PubMed] [Google Scholar]
- Salvati P., Lamberti E., Ferrario R., Ferrario R., Scampini G., Pugliese F., et al. (1995) Long-term thromboxane-synthase inhibition prolongs survival in murine lupus nephritis. Kidney Int 47: 1168–1175. [DOI] [PubMed] [Google Scholar]
- Selzer F., Sutton-Tyrrell K., Fitzgerald S., Tracy R., Kuller L., Manzi S. (2001) Vascular stiffness in women with systemic lupus erythematosus. Hypertension 37: 1075–1082. [DOI] [PubMed] [Google Scholar]
- Sesso H., Wang L., Buring J., Ridker P., Gaziano J. (2007) Comparison of interleukin-6 and C-reactive protein for the risk of developing hypertension in women. Hypertension 49: 304–310. [DOI] [PubMed] [Google Scholar]
- Shaharir S., Mustafar R., Mohd R., Mohd Said M., Gafor H. (2015) Persistent hypertension in lupus nephritis and the associated risk factors. Clin Rheumatol 34: 93–97. [DOI] [PubMed] [Google Scholar]
- Siddiqui A., Irani R., Blackwell S., Ramin S., Kellems R., Xia Y. (2010) Angiotensin receptor agonistic autoantibody is highly prevalent in preeclampsia: correlation with disease severity. Hypertension 55: 386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen J. (2002) Therapy of systemic lupus erythematosus: a look into the future. Arthritis Res 4: S25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Kim H., Baek H., Lee E., Chung E., Hong K. (1998) Paclitaxel reduces anti-dsDNA antibody titer and bun, prolonging survival in murine lupus. Int J Immunopharmacol 20: 669–677. [DOI] [PubMed] [Google Scholar]
- Sthoeger Z., Zinger H., Mozes E. (2003) Beneficial effects of the anti-oestrogen tamoxifen on systemic lupus erythematosus of (NZBXNZW)F1 female mice are associated with specific reduction of IgG3 autoantibodies. Ann Rheum Dis 62: 341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub R. (2007) The complex role of estrogens in inflammation. Endocr Rev 28: 521–574. [DOI] [PubMed] [Google Scholar]
- Subramanian S., Tus K., Li Q., Wang A., Tian X., Zhou J., et al. (2006) A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci USA 103: 9970–9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryaprabha P., Padma T., Rao U. (1984) Increased serum IgG levels in essential hypertension. Immunol Lett 8: 143–145. [DOI] [PubMed] [Google Scholar]
- Thacker S., Duquaine D., Park J., Kaplan M. (2010) Lupus-prone new zealand black/new zealand white F1 mice display endothelial dysfunction and abnormal phenotype and function of endothelial progenitor cells. Lupus 19: 288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilopoulos A., Dixon F. (1985) Murine models of systemic lupus erythematosus. Adv Immunol 37: 269–390. [DOI] [PubMed] [Google Scholar]
- Tucker L., Menon S., Schaller J., Isenberg D. (1995) Adult- and childhood-onset systemic lupus erythematosus: a comparison of onset, clinical features, serology and outcome. Br J Rheumatol 34: 866–872. [DOI] [PubMed] [Google Scholar]
- Urowitz M., Bookman A., Koehler B., Gordon D., Smythe H., Ogryzlo M. (1976) The bimodal mortality pattern of systemic lupus erythematosus. Am J Med 60: 221–225. [DOI] [PubMed] [Google Scholar]
- Vazquez-Oliva G., Fernandez-Real J., Zamora A., Vilaseca M., Badimon L. (2005) Lowering of blood pressure leads to decreased circulating interleukin-6 in hypertensive subjects. J Hum Hypertens 19: 457–462. [DOI] [PubMed] [Google Scholar]
- Venegas-Pont M., Manigrasso M., Grifoni S., Lamarca B., Maric C., Racusen L., et al. (2010) Tumor necrosis factor-alpha antagonist etanercept decreases blood pressure and protects the kidney in a mouse model of systemic lupus erythematosus. Hypertension 56: 643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venegas-Pont M., Mathis K., Iliescu R., Ray W., Glover P., Ryan M. (2011) Blood pressure and renal hemodynamic responses to acute ANG II infusion are enhanced in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol 301: R1286–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venegas-Pont M., Sartori-Valinotti J., Maric C., Racusen L., Glover P., McLemore G., et al. (2009) Rosiglitazone decreases blood pressure and renal injury in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol 296: R1282–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladutiu A. (1995) Role of nitric oxide in autoimmunity. Clin Immunol Immunopathol 76: 1–11. [DOI] [PubMed] [Google Scholar]
- Wallukat G., Homuth V., Fischer T., Lindschau C., Horstkamp B., Jupner A., et al. (1999) Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest 103: 945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward M., Studenski S. (1992) Clinical prognostic factors in lupus nephritis. the importance of hypertension and smoking. Arch Intern Med 152: 2082–2088. [PubMed] [Google Scholar]
- Watson M., Rao J., Gilkeson G., Ruiz P., Eicher E., Pisetsky D., et al. (1992) Genetic analysis of MRL-LPR mice: relationship of the Fas apoptosis gene to disease manifestations and renal disease-modifying loci. J Exp Med 176: 1645–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckerle C., Mangale D., Franek B., Kelly J., Kumabe M., James J., et al. (2012) Large-scale analysis of tumor necrosis factor alpha levels in systemic lupus erythematosus. Arthritis Rheum 64: 2947–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J., Granger D., Pisetsky D., Seldin M., Misukonis M., Mason S., et al. (1994) The role of nitric oxide in the pathogenesis of spontaneous murine autoimmune disease: increased nitric oxide production and nitric oxide synthase expression in MRL-LPR/LPR mice and reduction of spontaneous glomerulonephritis and arthritis by orally administered Ng-monomethyl-l-arginine. J Exp Med 179: 651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel K., Haase H., Wallukat G., Derer W., Bartel S., Homuth V., et al. (2008) Potential relevance of alpha(1)-adrenergic receptor autoantibodies in refractory hypertension. PLoS One 3: e3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox C. (2002) Reactive oxygen species: roles in blood pressure and kidney function. Curr Hypertens Rep 4: 160–166. [DOI] [PubMed] [Google Scholar]
- Wu W., Lin B., Su Y., Suen J., Chiang B. (2000) Tamoxifen decreases renal inflammation and alleviates disease severity in autoimmune NZB/W F1 mice. Scand J Immunol 52: 393–400. [DOI] [PubMed] [Google Scholar]
- Yap D., Lai K. (2013) The role of cytokines in the pathogenesis of systemic lupus erythematosus - from bench to bedside. Nephrology (Carlton) 18: 243–255. [DOI] [PubMed] [Google Scholar]
- Yoshio T., Masuyama J., Mimori A., Takeda A., Minota S., Kano S. (1995) Endothelin-1 release from cultured endothelial cells induced by sera from patients with systemic lupus erythematosus. Ann Rheum Dis 54: 361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Wang W., Yu H., Zhang Y., Dai Y., Ning C., et al. (2012) Interleukin 6 underlies angiotensin II-induced hypertension and chronic renal damage. Hypertension 59: 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C., Irani R., Dai Y., Blackwell S., Hicks M., Ramin S., et al. (2011) Autoantibody-mediated IL-6-dependent endothelin-1 elevation underlies pathogenesis in a mouse model of preeclampsia. J Immunol 186: 6024–6034. [DOI] [PMC free article] [PubMed] [Google Scholar]


