Abstract
Background
Efavirenz (EFV) has been associated with torsade de pointes despite marginal QT interval lengthening. Since EFV is metabolized by the cytochrome P450 (CYP) 2B6 enzyme, we hypothesized that EFV would lengthen the rate-corrected QT (QTcF) interval in carriers of the CYP2B6*6 decreased functional allele.
Objective
The primary objective of this study was to evaluate EFV-associated QT interval changes with regard to CYP2B6 genotype and to explore mechanisms of QT interval lengthening.
Methods
EFV was administered to healthy volunteers (n=57) as a single 600 mg dose followed by multiple doses to steady-state. Subjects were genotyped for known CYP2B6 alleles and ECGs and EFV plasma concentrations were obtained serially. Whole-cell, voltage-clamp experiments were performed on cells stably expressing hERG and exposed to EFV in the presence and absence of CYP2B6 expression.
Results
EFV demonstrated a gene-dose effect and exceeded the FDA criteria for QTcF interval prolongation in CYP2B6*6/*6 carriers. The largest mean time-matched differences ΔΔQTcF were observed at 6 hrs (14 ms; 95% CI [1; 27]), 12 hrs (18 ms; 95% CI [−4; 40] and 18 hrs (6 ms; 95% CI [−1; 14]) in the CYP2B6*6/*6 genotype. EFV concentrations exceeding 0.4 µg/mL significantly inhibited outward hERG tail currents (P<0.05).
Conclusions
This study demonstrates that homozygous carriers of CYP2B6*6 allele may be at increased risk for EFV-induced QTcF interval prolongation via inhibition of hERG.
Keywords: Efavirenz, QTc interval prolongation, hERG, QTc, torsade de pointes
Introduction
Patients infected with human immunodeficiency virus (HIV) are at an increased risk of cardiovascular disorders including the ventricular tachyarrhythmia, torsade de pointes (TdP).1–3 Both the pathophysiology and treatment of HIV may be associated with rate corrected QT (QTc) interval prolongation to increase the risk for TdP.1–4 Efavirenz (EFV) is non-nucleoside reverse transcriptase inhibitor (NNRTI) that exhibits potent and durable virological suppression. Due to well-documented clinical efficacy and favorable pharmacological properties, EFV 600 mg per day, in combination with 2 nucleoside reverse transcriptase inhibitors (NRTIs), has been extensively used globally as a preferred first line therapy for HIV treatment-naïve patients for over 15 years.5, 6
Efavirenz is generally well-tolerated, but certain patients may be at risk for serious adverse effects. In addition to the well-documented, concentration-dependent central nervous system (CNS) symptoms and hepatic injury,7–10 EFV has been reported to induce QTc interval prolongation and TdP.11 In a thorough QTc (TQT) study, steady-state EFV increased the average time-matched QTc intervals by 4.8 ms.12 This did not meet the mean 5.0 ms threshold defined by the United States Food and Drug Administration (FDA) adopted ICH E14 guidelines.13 The conflicting report of EFV-induced TdP and the thorough QT study suggest that some patients receiving EFV may be at greater risk for QT interval prolongation and TdP.11, 12, 14
Efavirenz is predominately eliminated by the cytochrome P450 (CYP) enzyme, CYP2B6, which is polymorphically expressed.15, 16 The CYP2B6*6 allele is associated with reduced EFV clearance and an increased incidence of EFV-induced CNS toxicity, hepatic injury, and treatment discontinuation.17–19 It follows that pharmacogenetic-based variability due to CYP2B6 polymorphisms may lead to a variability in EFV-induced QTc interval prolongation. However, the CYP2B6 genotype of patients was not assessed in the aforementioned report of EFV induced TdP or in the thorough QTc assessment.11, 12 The primary objective of this study was to evaluate EFV-associated QT interval changes with regard to CYP2B6 genotype and to explore mechanisms of QT interval lengthening. We hypothesized that CYP2B6 genetic variants that are known to slow EFV clearance would be associated with QTc interval lengthening.
Methods
Clinical Trial Study Design
The full inclusion and exclusion criteria and study design have been reported as part of a clinical trial designed to study the effect of steady state EFV on CYP substrates in healthy volunteers.20 Volunteers were included as subjects in the study if they were between 18 and 49 years old, HIV negative, and confirmed to be healthy by physical and medical examination. All enrolled subjects provided written informed consent. The study was approved by the Institutional Review Board (IRB) at Indiana University Purdue University Indianapolis (IUPUI).
The study followed a two-phase sequential design. In the first phase, subjects were administered a single EFV 600 mg dose orally with approximately 240 ml of water during a 24-hour stay in the Indiana Clinical Research Center (ICRC). The second phase of the study was initiated 7 days following the first phase. In the second phase, subjects were given a 17-day supply of EFV and instructed to take 600 mg daily, 2 hours before or after meals and close to bed time for 17 days. Blood samples were collected as outpatients on days 10, 13, 16, 19, and 22 prior to EFV administration. During the single dose phase and on the last day of the second phase, ECGs were collected prior to EFV dosing and every 6 hours after that during the 24-hour inpatient stay in the ICRC. An hour after EFV administration in Phase I and the last day of Phase II, subjects received a single dose of an oral cocktail of cytochrome P450 (CYP) probes as part of the drug interaction study.20 Blood samples were serially collected from one arm through an indwelling venous catheter.
QT Interval Measurements
ECGs were recorded (Marquette Mac 5500, GE Healthcare Bio-Sciences, Pittsburgh, PA) at 0, 6, 12, 18 and 24 hours following EFV administration after the first dose and at steady state achieved after the 17 daily doses. At each measurement point, at least 3 ECGs were recorded 1–3 minutes apart. QT and RR intervals were determined from leads II and V5 manually by an investigator (AA) blinded to time, study phase, and genotype. Inter-individual variability was assessed by an additional investigator (HJ) in a subset of ECGs (>500 complexes) using the kappa statistic. The reliability coefficient (i.e., kappa score) was 92.6%. QT intervals were measured from the earliest QRS deflection to the end of the T wave. The end of the T wave was defined as the intersection of the terminal portion of the T wave and the isoelectric line. In each measured lead, QT and RR intervals were averaged over 5 consecutive beats to obtain a single mean QT interval from each lead from each ECG. To determine the QT interval at each time point, the mean QT interval from 3 ECGs at each time point were averaged for each of the measured leads. QT intervals were measured only from ECGs on which the end of the T wave was clearly discernible.
QT intervals were corrected using the Fridericia correction (QTcF). Time matched difference (TMD) in the QTcF interval between single and multiple EFV dosing at all recording time points (t) were calculated by subtracting QTcF intervals changes from baseline (ΔQTc) following single dose (SD) and at steady state (SS) based on equation 1.
| (Equation 1) |
The TMDs are presented as mean and 95% confidence intervals with significant QTcF interval prolongation defined as a mean TMD greater than 5.0 ms and an upper limit of the 95% confidence interval greater than 10 ms per the ICH E14 guidelines.13
CYP2B6 Genotyping
Genomic DNA was extracted from human blood using standard protocol. DNA was genotyped for the G516T SNP in exon 4 (gene position 15631G>T; NCBI reference sequence: NG_000008.6) that leads to an amino acid change at codon 172 (Q172H) and for the A785G SNP in exon 5 (gene position 18053A>G (NCBI reference sequence: NG_000008.6) that leads to an amino acid substitution (K262R), which together define the common CYP2B6*6 allele. Genotyping for the listed variants was performed by use of the pre-developed TaqMan Assay-Reagents Allelic Discrimination Kits (Applied Biosystems, Foster City, CA) according to the supplier's instructions as previously described.21 The allele specific PCR assays were run using the iQ SYBR Green Supermix using allele specific primers. TaqMan and allele specific PCR assays were run using an iCycler real-time PCR instrument. As a quality control and validation measure for these assays, additional genotyping was performed using a BioTrove Openarray™ chip SNP genotyping platform.
Analytical Methods
Concentrations of efavirenz, 8-hydroxyefavirenz (8-OH EFV), 7-hydroxyefavirenz (7-OH EFV) and 8,14-dihydroxyefavirenz (8,14-diOH EFV) in plasma were quantified using our previously described high performance liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) methods.22, 23
Chemicals
All chemicals for patch-clamp experiments were obtained from Sigma-Aldrich, including EFV (SML0536, Lot# 112M4701V). Stock solutions of 10 mg/ml EFV were prepared in dimethyl sulfoxide (DMSO), filtered, and stored in aliquots at −20 °C. Working solutions were freshly prepared at the time of the experiments by diluting the stock solution with the bath or pipette solution.
CYP2B6*1 cDNA transfection
The assessment of hERG-associated current (IhERG) was performed in human embryonic kidney (HEK) 293 cells, stably expressing hERG.24 Human CYP2B6*1 cDNA in a pCMV4 vector was obtained as a gift from Dr. Ulrich Zanger.25 The hERG-HEK cells were co-transfected with the purified CYP2B6*1 and GFP coding vector in a 5:1 ratio (CYP2B6:GFP) cDNA using Lipofectamine® 2000 reagent (Invitrogen) according to the manufacturer recommendations. Control cells were transfected with the GFP coding vector.
Cellular Electrophysiology
A set of whole-cell, voltage-clamp experiments were performed using a HEKA® EPC 9 amplifier. Data were recorded and analyzed using HEKA® Patchmaster and Fitmaster software. At the time of the recordings, cell culture media was replaced by a bath solution (containing in mM; 137 NaCl, 4 KCl, 1.8 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose, pH adjusted to 7.4 with NaOH) containing different EFV concentrations (0.0, 0.1, 0.2, 0.4, 0.6, 0.8, 1.0, 1.5 and 2.5 µg/mL). Borosilicate glass electrodes (tip resistant 2–5 MΩ) were filled with internal solution (containing in mM; 130 KCl, 1 MgCl2, 5 EGTA, 5 MgATP and 10 HEPES, pH adjusted to 7.2 with KOH). Tail currents were measured at repolarizing test potentials (−100 to +40 mV, 4 seconds each) following a one second depolarizing step to +60 mV from a holding potential of −80 mV. Cell recordings were performed in a minimum 2 plates (3–5 cells/plate) for each EFV concentration and compared to control.
In hERG-HEK cells that were transiently transfected with CYP2B6*1 cDNA, tail current recordings were performed using the following 2-sweep voltage clamp protocol: membrane potential was held at −80 mV, increased to 60 mV (1 second), and currents were measured during two repolarizing steps at −100 and −20 mV (5 seconds each). This protocol was performed twice for each cell at 1 and 2 minutes following cytosolic access and exposure to EFV in the pipette solution in order to evaluate the influence of incubation time on EFV-mediated alterations in hERG channel conductivity. A sigmoidal Emax model was fit to the EFV inhibitory effect on IhERG using Prism (GraphPad Software, Inc., La Jolla, CA).
Cytotoxicity Assay
In order to validate functional expression of CYP2B6 enzyme, transfected cells were incubated at 37°C with different cytotoxic concentrations of EFV. Cytotoxicity was assessed using a Trypan blue assay. Efavirenz concentrations were selected based on a kill curve that was developed to determine the EFV concentration that kills 50% of the hERG-HEK cells (LC50).
Data Analysis
The area under the EFV concentration-time curve from 0 to 24 hours (AUC0–24) was calculated using the linear trapezoidal rule. For multiple comparisons including the temporal changes in TMD and cellular electrophysiological data, the Shapiro-Wilk W test was used to check for normality of the data followed by a one-way ANOVA with Bonferroni correction or Dunnett’s test to determine statistically significant differences among different EFV concentrations or genotype, respectively. The EFV concentration that inhibited 50% of the maximal effect (IC50) was compared between groups using a t-test or ANOVA, as appropriate. The type I error rate for all analyses was set at 5%. Based on recent recommendations to enhance TQT studies, an exposure-response relationship was assessed between EFV concentrations and TMDs.26 A standard Emax model was fit to the TMDs versus EFV concentrations.
Results
Subject Demographics and Efavirenz Plasma Concentrations
Fifty-seven healthy subjects completed both phases of a two-phase sequential design that included a single oral EFV 600 mg dose (Single Dose, Phase I) and 17 days of oral EFV 600 mg administration (Steady-State, Phase II). Subjects with the CYP2B6*1/*1 (n=34) and the CYP2B6*1/*4 (n=3) genotype were collectively grouped based on their phenotype of an EFV concentration-time profile similar to the wild-type metabolizers. Subjects with CYP2B6*1/*6 genotype (n=15) were considered intermediate EFV metabolizers, while subjects homozygous for CYP2B6*6 allele (n=5) represented the slow metabolizers. Demographic information for the study subjects stratified by CYP2B6 genotype are provided in Table 1.
Table 1.
Summary of demographic information
| CYP2B6 Genotype | ||||
|---|---|---|---|---|
| *1/*1 or *1/*4 | *1/*6 | *6/*6 | ||
| n=37 (65%) | n=15 (26%) | n=5 (9%) | ||
| Sex | ||||
| Male | 22 (59%) | 11 (73%) | 2 (40%) | |
| Female | 15 (41%) | 4 (27%) | 3 (60%) | |
| Race | ||||
| White | 28 (76%) | 10 (67%) | 3 (60%) | |
| Black | 8 (22%) | 2 (13%) | 2 (40%) | |
| Asian | 0 (0%) | 2 (13%) | 0 (0%) | |
| Indian | 1 (2%) | 1 (7%) | 0 (0%) | |
| Weight (Kg, mean ± SD) | 73 ± 13 | 79 ± 15 | 65 ± 5 | |
| Age (Years, mean ± SD) | 28 ± 10 | 29 ± 9 | 23 ± 4 | |
| BMI (Kg/m2, mean ± SD) | 24 ± 4 | 26 ± 4 | 21 ± 3 | |
Data presented as number (percent) or mean ± standard deviation
Twenty-four hour exposure to EFV was not different among the CYP2B6 genotypes following the first oral EFV dose (p=0.14). Subjects carrying two CYP2B6*6 alleles displayed significantly higher EFV exposure at steady-state (p<0.05) than carriers of the CYP2B6*1/*6 and CYP2B6*1/*1 with mean ± SD AUC0–24 (µg•hr/mL) of 152.9 ± 56.2, 72.8 ± 14.1, and 58.22 ± 18.1, respectively as displayed in Figure 1.
Figure 1.
Mean ±SD efavirenz AUC0–24 (µg•hr/mL) following a single dose (600 mg) and at steady-state among CYP2B6 genotype groups *1/*1 (n=36), *1/*6 (n=14), and *6/*6 (n=5). *indicates p<0.05
QTcF Interval Changes and CYP2B6 Genotype
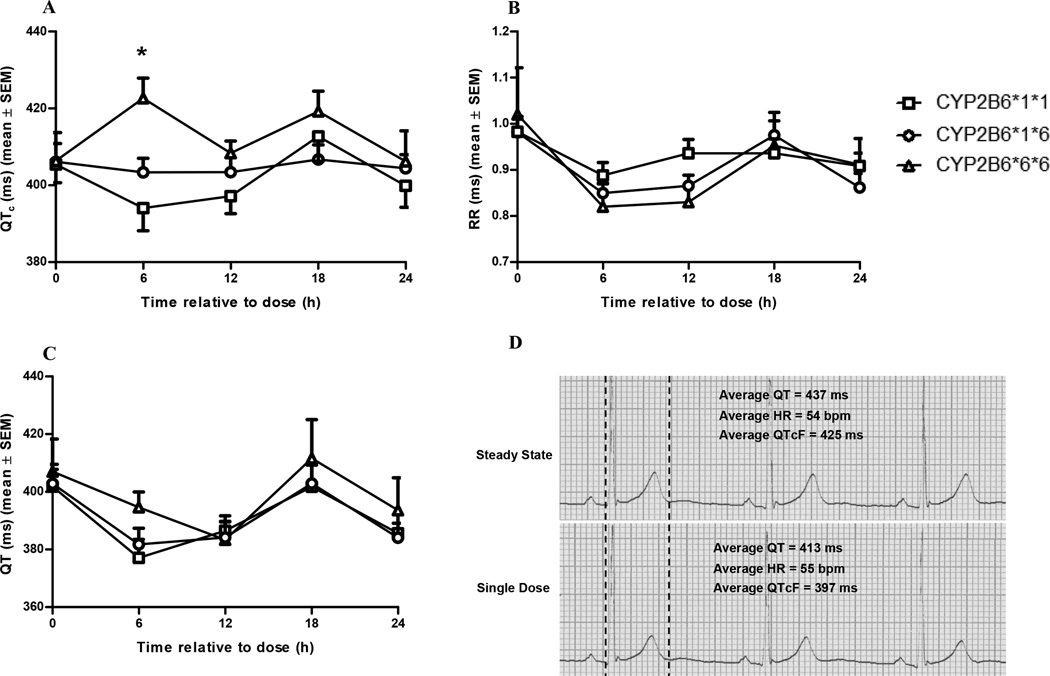
The QTcF, QT, and RR intervals did not change significantly from baseline following a single oral dose of EFV among the CYP2B6 genotypes (Supplemental Figure 1). Following steady-state EFV administration, the QTcF interval was not altered in carriers of the CYP2B6 *1/*1 or *1/*6 alleles (p>0.05 by repeated-measures ANOVA) but was significantly increased 6 hours following EFV administration in the *6/*6 carriers from a mean ± SD baseline of 406 ± 16.4 to 423 ± 11.8 ms (p<0.05 by Dunnett’s test) with an overall p value of 0.02 from the repeated-measures ANOVA (Figure 2A). The RR intervals and the unadjusted QT intervals did not significantly change at steady state (Figures 2B and 2C) with an overall p value > 0.05.
Figure 2.
Mean ± SD (a) QTcF intervals; (b) RR intervals; and (c) uncorrected QT intervals following a 600 mg dose of EFV at steady state (i.e., following 17 doses of EFV 600 mg) among the CYP2B6 genotypes; (d) representative lead II ECG tracing (25 mm/s paper speed) of 3 consecutive beats following a 600 mg dose of EFV at steady state (upper trace) and a single dose (lower trace) in a CYP2B6*6/*6 patient with an average QTcF increase from 397 ms to 425 ms. The mean QTcF interval was significantly increased 6 hours following steady-state EFV administration in the CYP2B6*6/*6 group. The overall p value from the repeated measures ANOVA was equal to 0.02.
*indicates p<0.05 versus time zero
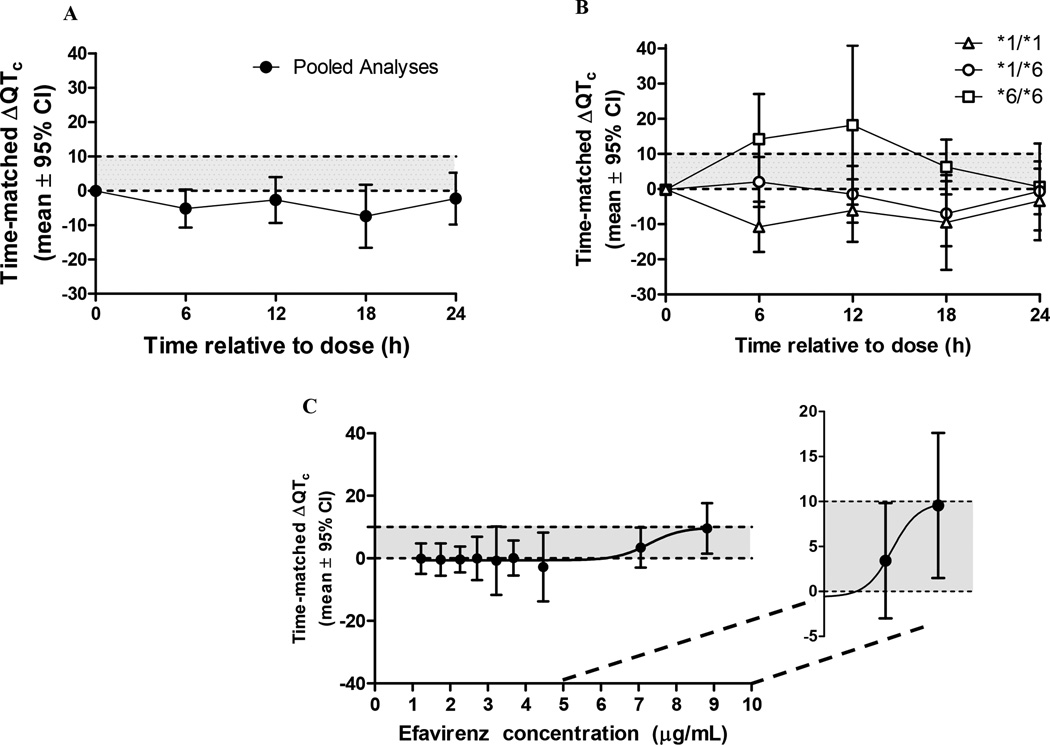
Given the lack of change in the QTcF interval following a single dose and the significant accumulation of EFV given its long half-life (~50 hours), time matched differences (TMD) from baseline in QTcF intervals were corrected between the single and multiple EFV dosing at all recording time points. In the pooled study population, the mean TMD in lead II was decreased for every time measured following EFV administration (Figure 3A). However, a gene-response relationship was observed with the largest TMD in subjects homozygous for the CYP2B6*6 allele (Figure 3B and Table 2). The grouped TMD versus EFV concentration data was best fit to a standard Emax model with an estimated mean ± SEM half maximal inhibitory concentration (IC50) of 7.3 ± 0.5 µg/mL and maximum effect of 10 ± 4.2 ms (Figure 3C).
Figure 3.
Mean ± 95% confidence intervals of QTcF time-matched difference (ΔΔQTc) at steady-state versus single dose for (a) pooled study population and (b) among CYP2B6 genotype groups (*1/*1 (n=36), *1/*6 (n=14), and *6/*6 (n=5)). The shaded area represents the difference between a 0 ms and the upper 95% CI threshold of 10 ms defined by the E14 guidance for thorough QT studies. This threshold is exceeded at 6, 12, and 18 hours post EFV dose among the CYP2B6*6/*6 genotype (C) the mean and 95% CI of TMD were grouped in deciles based on EFV concentrations of the pooled population and fit to a standard Emax model for the exposure-response analysis.
Table 2.
Mean (95% CI) time matched difference in QTcF, (ms)
| Lead II | ||||
|---|---|---|---|---|
| CYP2B6 genotype | ||||
| Pooled Sample | *1/*1 | *1/*6 | *6/*6 | |
| Time (h) | (n = 57) | (n = 37) | (n = 15) | (n=5) |
| 0 | −0.1 (−0.2; 0.0) | 0 (−0.2; 0.1) | −0.2 (−0.4; 0) | −0.2 (−0.4; 0) |
| 6 | −5 (−10; 0) | −11 (−18; −4) | 2 (−5; 9) | 14 (2; 27)† |
| 12 | −3 (−9; 4) | −6 (−15; 3) | −1 (−9; 7) | 18 (−4; 40)† |
| 18 | −7 (−16; 2) | −9 (−23; 4) | −7 (−16; 2) | 6 (−2; 14)† |
| 24 | −2 (−10; 5) | −3 (−15; 8) | −1 (−7; 6) | 1 (−12; 13) |
indicates IEC threshold has been exceeded
The CYP2B6 *6/*6 allele carriers demonstrated the highest EFV concentrations and had a positive relationship between TMD and EFV concentration among CYP2B6*6/*6 allele carriers (β=0.0026, p=0.0497) in the linear model. There was no observed relationship between TMD and EFV concentrations in CYP2B6*1/*6 allele carriers or the CYP2B6*1/*1 allele carriers (β =−0.003, p=0.083). Additionally, there was no observed relationship between TMD and 8-OH, 7-OH, or 8,14-diOH EFV concentrations in CYP2B6*1/*1 allele carriers, CYP2B6*1/*6 allele carriers or CYP2B6*6/*6 allele carriers using linear models (data not shown).
Efavirenz-Associated IHERG Changes
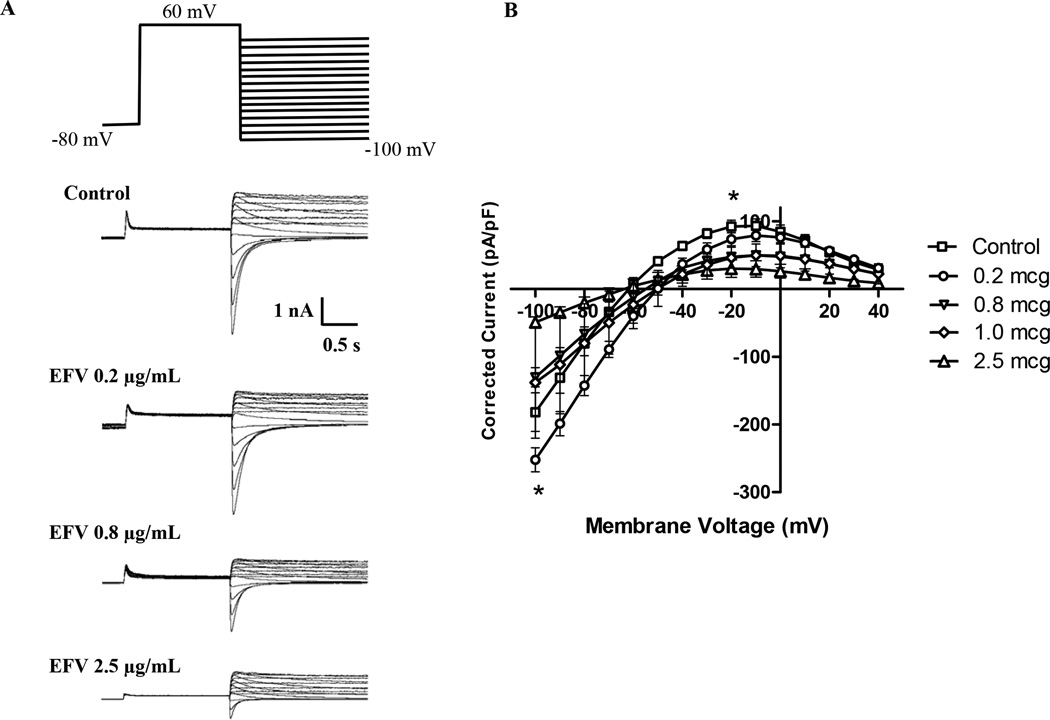
Current-voltage relationships were elicited in HEK293 cells stably expressing hERG using the whole-cell voltage clamp technique following treatment with various EFV concentrations applied to the culture media or directly to the internal (pipette) or external (bath) solutions. Efavirenz 0.2 µg/mL, 0.8 µg/mL and 2.5 µg/mL inhibited both inward and outward IhERG currents in a concentration-dependent manner when applied to the bath solution (Figure 4). EFV also inhibited inward and outward IhERG when applied to the culture media for 24 hours. As expected, the EFV concentration-IhERG and cytotoxicity relationship was shifted when EFV was applied to the culture media since it contains fetal bovine serum (FBS) and EFV is greater than 99.5% protein bound (data not shown).
Figure 4.
hERG current recordings following a fifteen-sweep voltage clamp protocol (a) displaying representative current recordings following exposure to EFV 0.0 (Control), 0.2, 0.8, and 2.5 µg/ml and (b) Mean ± standard error of the mean (SEM) corrected current–voltage relationship of hERG current following EFV concentrations (0.0–2.5 µg/ml) applied to the bath solution. EFV concentrations exceeding 0.4 µg/mL significantly inhibited outward hERG tail currents (overall P<0.0001).
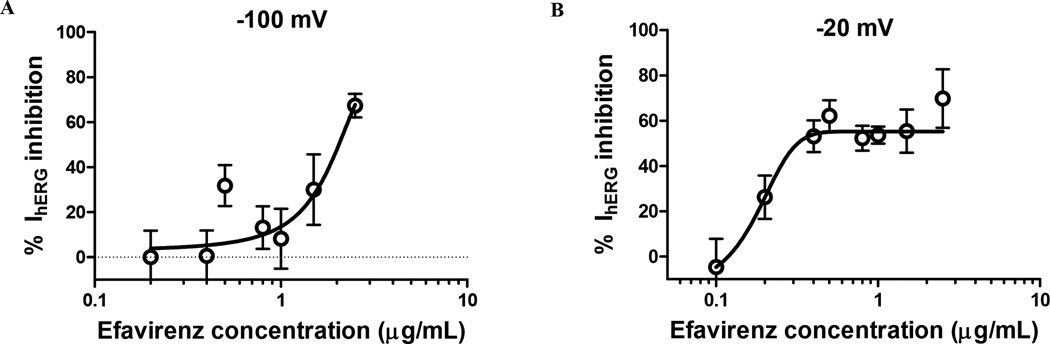
The mean ± standard error of the mean (SEM) maximum observed inhibition of the hERG-related current was 70.7 ± 7.0 percent at 2.5 µg/mL in the bath solution. Sigmoidal concentration-response curves were fit to the EFV-associated inhibition of the inward and outward IhERG currents recorded at −100 mV and −20 mV, respectively (Figure 5). The estimated mean ± SEM maximum inhibition (Emax) was 55.3 ± 3.5%; IC50 was 0.17 ± 0.15 µg/mL; and Hill factor was 7.7 ± 14.4 at −20 mV while at −100 mV the Emax was 92.1 ± 255 %; the IC50 was 1.95 ± 3.23 µg/mL and the Hill factor was 0.79 ± 1.75.
Figure 5.
Concentration-response curve of maximal (a) inward hERG current inhibition at −100 mV and (b) outward hERG current inhibition at −20 mV following exposure to EFV 0.0–2.5 µg/mL in the bath solution. The open circles represent the mean inhibition % while the error bars represent the SEM. The data were fit to the sigmoidal Emax model with the concentration-response curves represented by the best fit line.
CYP2B6 effect on Efavirenz-Associated IhERG Changes
Given the altered relationships between EFV concentrations and TMD among CYP2B6 genotypes, we assessed the potential contributions of EFV metabolites formed by the CYP2B6 isoenzyme to influence IhERG. To assess the potential IhERG effects by EFV metabolites formed by the CYP2B6 pathway, EFV induced IhERG inhibition was assessed in cells transiently transfected with functional CYP2B6*1 and GFP cDNA versus GFP alone. The LC50 for EFV was estimated to be 10.48 ± 0.43 µg/mL at 24 hours and 6.93 ± 0.05 µg/mL at 48 hours in hERG-HEK cells; as displayed by the kill curves in Supplemental Figure 2A. Cells expressing CYP2B6*1 demonstrated significantly less cytotoxicity following 24 and 48 hours incubation with EFV (Supplemental Figures 2B and 2C). This suggests that the cells expressed functional CYP2B6 protein following transient transfection.
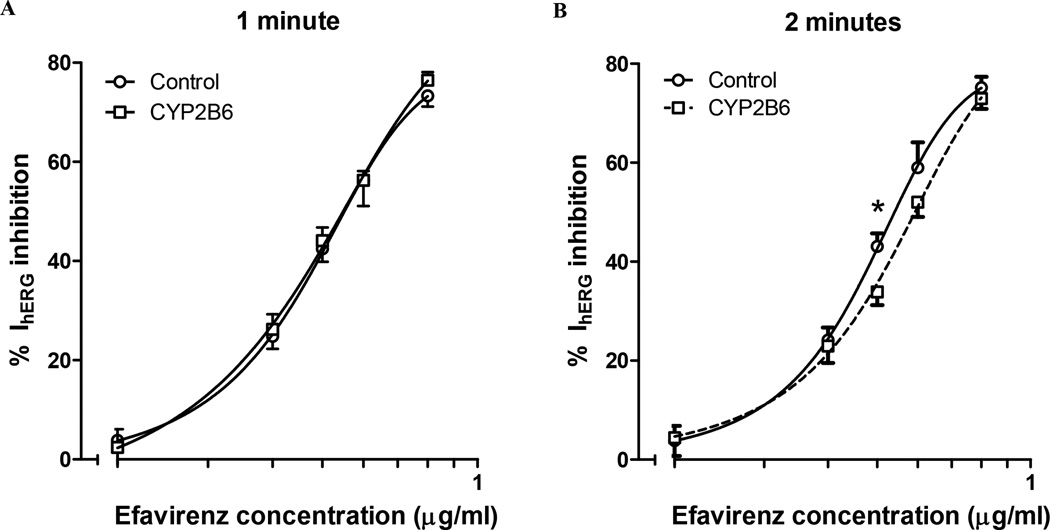
The percent IhERG inhibition at −20 mV was similar in CYP2B6-transfected cells following 1 minute of exposure to that of cells that expressed GFP only (Figure 6A). However, when holding the cells for 2 minutes, there was an observed right-shift in the concentration-effect curve in the same CYP2B6-transfected cells. This resulted in significantly less IhERG inhibition at 0.4 µg/mL in the cells expressing CYP2B6 (Figure 6B). This right shift in the concentration-effect relationship suggest that IhERG inhibition is diminished as parent EFV is metabolized by CYP2B6 in this system.
Figure 6.
Concentration-response curves of outward IhERG inhibition following exposure to EFV 0.0–0.8 µg/mL in the pipette solution. The mean ± SEM percent inhibition of outward current in CYP2B6-GFP (□) and cDNA (○) transfected cells are displayed at (a) 1 minute and (b) 2 minutes following EFV exposure.
*indicates p<0.05
Discussion
In 2005, the FDA adopted the ICH recommendation of clinical TQT studies to investigate the drug-induced, heart rate corrected QT interval prolongation potential.13 The results of this study indicate that EFV prolongs the QTcF interval beyond the E14 threshold in healthy subjects that are carriers of two copies of the CYP 2B6*6 allele while demonstrating a direct concentration-response relationship. The EFV mediated prolongation of the QTcF interval may be due to its ability to inhibit IhERG as demonstrated in stably transfected hERG-HEK cells.
A TQT study has previously been conducted for steady-state EFV using a 600 mg once daily regimen in 60 healthy subjects with an unreported CYP2B6 genotype.12 The TMD in QTcF intervals peaked at 6 hours following EFV administration. The peak TMD was 5.0 ms with an upper limit of the 95% CI just above 8.0 ms, which did not meet the E14 threshold.12 Since there was no available information regarding CYP2B6 genotype in the previous TQT study, the number of CYP2B6*6 carriers cannot be determined. Of note, there were no African American subjects included in the previously reported TQT study with 59 reported as Hispanic or white non-Hispanic and one was reported as an American Indian or Alaskan native.12 The lack of African American subjects may be important since the CYP2B6*6 allele frequency has been reported to range between 8–37% in Caucasian and Hispanic populations and up to 65% in African populations.27 Given the ethnic and race distribution, it is likely that a small portion of patients were carriers of the CYP2B6*6 allele in the previously reported TQT study with even fewer being homozygous for the variant allele.
The propensity for EFV to provoke TdP is not known. A case report indicates EFV was associated with remarkable QTc prolongation (580 ms) and tachyarrhythmia in an African American HIV patient.11 The association of EFV and a prolonged rate corrected QT interval in this case report was based on the absence of other risk factors and the resolution of QTc interval prolongation and tachyarrhythmias following drug discontinuation.11 The CYP2B6 genotype of that patient was not known but the fact that he was African American increases the likelihood that the patient was a CYP2B6*6 carrier.
The current report and several previously reported studies demonstrate a strong association between the CYP2B6*6/*6 genotype and increased EFV exposure through decreased parent drug clearance.15, 16 The mean Cmax was reported to be approximately 5.0 µg/mL in the TQT study and was 4.1, 4.5 and 7.6 µg/mL among CYP2B6*1/*1, *1/*6 and *6/*6 respectively in the current report. A therapeutic window of EFV between 1 to 4 µg/mL has been suggested, with higher concentrations of up to 26 µg/ml being reported in HIV patients with EFV associated CNS toxicity.17
Most drugs that clinically prolong the QTc interval are inhibitors of the rapid component of the delayed rectifier current which is encoded by the hERG gene.28 A concentration-dependent inhibition of IhERG by EFV was observed with an IC50 estimated to be approximately 0.2 µg/mL (i.e. unbound EFV IC50). However, EFV is greater than 99.5% protein bound and the IC50 was estimated to be approximately 2.0 µg/mL in the culture media. The observed concentration-response relationship and the gene-dose effect in the clinical trial coupled with the in vitro demonstration of EFV induced hERG inhibition were important findings to support the observed QTc prolongation in the relatively small number of CYP2B6*6 homozygotes.
Limitations
The clinical study was not designed as a TQT study and did not incorporate a positive control arm. A traditional TQT study would assess TMDs between a placebo phase and a steady-state phase. In this study, the time matched differences in QTcF were assessed between a single dose of EFV and steady-state EFV phase. The long half-life and accumulation potential of EFV limits the likelihood that this design had an impact on the final conclusions. In fact, the concentration-QTcF interval response did not show a positive relationship until EFV concentrations were greater than 4 µg/mL. These concentrations are not achieved following a single dose of EFV. Nonetheless, if using TMD between the single dose and steady state phases were to confound our findings, it would decrease the ability to find a QTc prolonging effect with EFV.
An additional limitation due to the clinical study design was the administration of a CYP substrate cocktail. The influence of this cocktail on the results should be negligible since it was administered as a single dose in both study phases, 1 hour following EFV administration and included drugs that have not been associated with QTc interval prolongation; caffeine, midazolam, omeprazole and tolbutamide.29 Furthermore, omeprazole and midazolam concentrations were decreased in the steady-state EFV phase and tolbutamide and caffeine were unaltered.20 Therefore, the differences in the QTcF interval are not likely due to the drugs in the cocktail.
Conclusions
This study has uncovered the potential risk for QTc interval prolongation associated with EFV in CYP2B6*6/*6 carriers. The mechanism may be due to inhibition of the hERG-related current which was demonstrated through an in vitro system in this study. Prompted by the results of these findings, a TQT study is being performed by the manufacturer that will incorporate CYP2B6 genotype (ClinicalTrials.gov #NCT02164812). The full potential of EFV to prolong the QTc interval cannot be fully verified until the conclusion of that study.
Supplementary Material
Acknowledgments
Supported by R01GM078501 (ZD) from the National Institute of General Medical Sciences and K08HL095655 (BRO) from the National Heart, Lung, and Blood Institute, National Institutes of Health (Bethesda, MD).
Footnotes
JE Tisdale reports honoraria for presentations on the broad topic of drug-induced QT interval prolongation.
Other authors: No disclosures.
References
- 1.Kocheril AG, Bokhari SAJ, Batsford WP, Sinusas AJ. Long QTc and torsades de pointes in human immunodeficiency virus disease. Pacing Clin Electrophysiol. 1997;20:2810–2816. doi: 10.1111/j.1540-8159.1997.tb05439.x. [DOI] [PubMed] [Google Scholar]
- 2.Puri R, Roberts-Thomson KC, Young GD. HIV and Long QT syndrome--cause or coincidence. Int J Cardiol. 2009;133:e9–e10. doi: 10.1016/j.ijcard.2007.08.076. [DOI] [PubMed] [Google Scholar]
- 3.Shimabukuro-Vornhagen A, Rybniker J, Zoghi S, Faetkenheuer G, Michels G, Erdmann E, von Bergwelt-Baildon M, Kochanek M. Acquired Long QT Syndrome and Torsade de Pointes Associated with HIV Infection. Case Report Med. 2010;2010 doi: 10.1155/2010/278427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pugliese A, Gennero L, Vidotto V, Beltramo T, Petrini S, Torre D. A review of cardiovascular complications accompanying AIDS. Cell Biochem Funct. 2004;22:137–141. doi: 10.1002/cbf.1095. [DOI] [PubMed] [Google Scholar]
- 5.Murphy EL, Collier AC, Kalish LA, Assmann SF, Para MF, Flanigan TP, Kumar PN, Mintz L, Wallach FR, Nemo GJ Viral Activation Transfusion Study I. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med. 2001;135:17–26. doi: 10.7326/0003-4819-135-1-200107030-00005. [DOI] [PubMed] [Google Scholar]
- 6.Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, Cahn P, Eron JJ, Gunthard HF, Hammer SM, Reiss P, Richman DD, Rizzardini G, Thomas DL, Jacobsen DM, Volberding PA. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. Jama. 2012;308:387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 7.Fumaz CR, Munoz-Moreno JA, Molto J, Negredo E, Ferrer MJ, Sirera G, Perez-Alvarez N, Gomez G, Burger D, Clotet B. Long-term neuropsychiatric disorders on efavirenz-based approaches: quality of life, psychologic issues, and adherence. J Acquir Immune Defic Syndr. 2005;38:560–565. doi: 10.1097/01.qai.0000147523.41993.47. [DOI] [PubMed] [Google Scholar]
- 8.Ciccarelli N, Fabbiani M, Di Giambenedetto S, Fanti I, Baldonero E, Bracciale L, Tamburrini E, Cauda R, De Luca A, Silveri MC. Efavirenz associated with cognitive disorders in otherwise asymptomatic HIV-infected patients. Neurology. 2011;76:1403–1409. doi: 10.1212/WNL.0b013e31821670fb. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez Martin A, Cabrera Figueroa S, Cruz Guerrero R, Hurtado LP, Hurle AD, Carracedo Alvarez A. Impact of pharmacogenetics on CNS side effects related to efavirenz. Pharmacogenomics. 2013;14:1167–1178. doi: 10.2217/pgs.13.111. [DOI] [PubMed] [Google Scholar]
- 10.Jones M, Nunez M. Liver toxicity of antiretroviral drugs. Semin Liver Dis. 2012;32:167–176. doi: 10.1055/s-0032-1316472. [DOI] [PubMed] [Google Scholar]
- 11.Castillo R, Pedalino RP, El-Sherif N, Turitto G. Efavirenz-associated QT prolongation and Torsade de Pointes arrhythmia. Ann Pharmacother. 2002;36:1006–1008. doi: 10.1345/aph.1A454. [DOI] [PubMed] [Google Scholar]
- 12.Tibotec Pharmaceuticals. ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US); 2000. [cited 2016 Mar 15]. A Study to Assess the Effects of TMC278 and Efavirenz (EFV) on the QT/QTc Interval (Heart Conduction and Heart Rhythm) in Healthy Volunteers. Available from: https://clinicaltrials.gov/ct2/show/NCT00744809 NLM Identifier: NCT000044809. [Google Scholar]
- 13.Harmonisation ICo. The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-antiarrhythmic Drugs. 2005 [PubMed] [Google Scholar]
- 14.Chinello P, Lisena FP, Angeletti C, Boumis E, Papetti F, Petrosillo N. Role of antiretroviral treatment in prolonging QTc interval in HIV-positive patients. J Infect. 2007;54:597–602. doi: 10.1016/j.jinf.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Ward BA, Gorski JC, Jones DR, Hall SD, Flockhart DA, Desta Z. The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J Pharmacol Exp Ther. 2003;306:287–300. doi: 10.1124/jpet.103.049601. [DOI] [PubMed] [Google Scholar]
- 16.Abdelhady AM, Desta Z, Jiang F, Yeo CW, Shin JG, Overholser BR. Population pharmacogenetic-based pharmacokinetic modeling of efavirenz, 7-hydroxy- and 8-hydroxyefavirenz. J Clin Pharmacol. 2014;54:87–96. doi: 10.1002/jcph.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS. 2001;15:71–75. doi: 10.1097/00002030-200101050-00011. [DOI] [PubMed] [Google Scholar]
- 18.Gutierrez F, Navarro A, Padilla S, Anton R, Masia M, Borras J, Martin-Hidalgo A. Prediction of neuropsychiatric adverse events associated with long-term efavirenz therapy, using plasma drug level monitoring. Clin Infect Dis. 2005;41:1648–1653. doi: 10.1086/497835. [DOI] [PubMed] [Google Scholar]
- 19.Gounden V, van Niekerk C, Snyman T, George JA. Presence of the CYP2B6 516G>T polymorphism, increased plasma Efavirenz concentrations and early neuropsychiatric side effects in South African HIV-infected patients. AIDS Res Ther. 2010;7:32. doi: 10.1186/1742-6405-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michaud V, Ogburn E, Thong N, Aregbe AO, Quigg TC, Flockhart DA, Desta Z. Induction of CYP2C19 and CYP3A activity following repeated administration of efavirenz in healthy volunteers. Clin Pharmacol Ther. 2012;91:475–482. doi: 10.1038/clpt.2011.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang T, Klein K, Fischer J, Nussler AK, Neuhaus P, Hofmann U, Eichelbaum M, Schwab M, Zanger UM. Extensive genetic polymorphism in the human CYP2B6 gene with impact on expression and function in human liver. Pharmacogenetics. 2001;11:399–415. doi: 10.1097/00008571-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Cho DY, Ogburn ET, Jones D, Desta Z. Contribution of N-glucuronidation to efavirenz elimination in vivo in the basal and rifampin-induced metabolism of efavirenz. Antimicrob Agents Chemother. 2011;55:1504–1509. doi: 10.1128/AAC.00883-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogburn ET, Jones DR, Masters AR, Xu C, Guo Y, Desta Z. Efavirenz primary and secondary metabolism in vitro and in vivo: identification of novel metabolic pathways and cytochrome P450 2A6 as the principal catalyst of efavirenz 7-hydroxylation. Drug Metab Dispos. 2010;38:1218–1229. doi: 10.1124/dmd.109.031393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Z, Gong Q, Ye B, Fan Z, Makielski JC, Robertson GA, January CT. Properties of HERG channels stably expressed in HEK 293 cells studied at physiological temperature. BIOPHYSICAL JOURNAL. 1998;74:230–241. doi: 10.1016/S0006-3495(98)77782-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lang T, Klein K, Richter T, Zibat A, Kerb R, Eichelbaum M, Schwab M, Zanger UM. Multiple novel nonsynonymous CYP2B6 gene polymorphisms in Caucasians: demonstration of phenotypic null alleles. J Pharmacol Exp Ther. 2004;311:34–43. doi: 10.1124/jpet.104.068973. [DOI] [PubMed] [Google Scholar]
- 26.Darpo B, Benson C, Dota C, Ferber G, Garnett C, Green CL, Jarugula V, Johannesen L, Keirns J, Krudys K, Liu J, Ortemann-Renon C, Riley S, Sarapa N, Smith B, Stoltz RR, Zhou M, Stockbridge N. Results from the IQ-CSRC prospective study support replacement of the thorough QT study by QT assessment in the early clinical phase. Clin Pharmacol Ther. 2015;97:326–335. doi: 10.1002/cpt.60. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Menard V, Benish RL, Jurevic RJ, Guillemette C, Stoneking M, Zimmerman PA, Mehlotra RK. Worldwide variation in human drug-metabolism enzyme genes CYP2B6 and UGT2B7: implications for HIV/AIDS treatment. Pharmacogenomics. 2012;13:555–570. doi: 10.2217/pgs.11.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vandenberg JI, Perry MD, Perrin MJ, Mann SA, Ke Y, Hill AP. hERG K(+) channels: structure, function, and clinical significance. Physiol Rev. 2012;92:1393–1478. doi: 10.1152/physrev.00036.2011. [DOI] [PubMed] [Google Scholar]
- 29.Woosley R, Romero K. QTdrugs List. 1822 Innovation Park Dr., Oro Valley, AZ 85755: AZCERT, Inc.; [accessed 07/06/2015]. www.Crediblemeds.org. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.