Abstract
Rationale
Several pre-clinical studies suggest that antipsychotic medications cause secondary negative symptoms. However, direct evidence for a relationship among antipsychotic medications, their direct effects on neurotransmitter systems, and negative symptoms in schizophrenia remains controversial.
Objective
The objective of this study was to examine the relationship between antipsychotic-related dopamine D2/3 receptor occupancy and negative symptoms in patients with schizophrenia.
Methods
Forty-one clinically stable outpatients with schizophrenia participated in this prospective dose reduction positron emission tomography (PET) study. Clinical assessments and [11C]-raclopride PET scans were performed before and after participants underwent gradual dose reduction of their antipsychotic medication by up to 40% from the baseline dose.
Results
No significant relationship was found between antipsychotic-related dopamine D2/3 receptor occupancy and negative symptom severity at baseline or follow-up. Similar null findings were found for subdomains of negative symptoms (amotivation and diminished expression). Occupancy was significantly lower following dose reduction; however, negative symptom severity did not change significantly, though a trend toward reduction was noted. Examination of change scores between these two variables revealed no systematic relationship.
Conclusions
Our cross-sectional and longitudinal results failed to find a significant dose-dependent relationship between severity of negative symptoms and antipsychotic-related dopaminergic antagonism in schizophrenia. These findings argue against the notion that antipsychotics necessarily cause secondary negative symptoms. Our results are also in contrast with the behavioural effects of dopaminergic antagonism routinely reported in pre-clinical investigations, suggesting that the role of this variable in the context of chronic treatment and schizophrenia needs to be re-examined.
Keywords: Schizophrenia, Negative symptoms, Motivation, Apathy, Dopamine, Side effects, Extrapyramidal symptoms, Ventral striatum
Introduction
Signs and symptoms such as affective flattening and amotivation, collectively termed negative symptoms (i.e., diminutions in normal behaviour), represent prominent features of schizophrenia that characterize a sizeable number of patients (Kirkpatrick et al. 2006). While these symptoms were described in patients long before the introduction of antipsychotic medications (Bleuler 1950; Kraepelin 1919), it has also been suggested that negative symptoms are in some cases produced or worsened by antipsychotic drugs (Carpenter et al. 1988; Kelley et al. 1999; Schooler 1994). This contention is bolstered by studies showing a worsening of negative symptoms following a single dose of antipsychotic medications in healthy volunteers (Artaloytia et al. 2006; Mas et al. 2013; Saeedi et al. 2006), as well as pre-clinical investigations demonstrating reductions in motivation following administration of these drugs (Salamone and Correa 2012). However, direct evidence for a relationship between antipsychotic medications, their direct effects on neurotransmitter systems, and negative symptoms in schizophrenia remains scarce.
In contrast to the aforementioned studies, clinical studies have shown that negative symptoms generally improve or do not substantially change following treatment with antipsychotic medications (Fusar-Poli et al. 2015; Leucht et al. 2009), and withdrawal of these medications results in worsening of negative symptoms (Breier et al. 1987; Harvey et al. 1996; Miller et al. 1994; Weickert et al. 2003). Several studies have failed to demonstrate a significant association between the severity of negative symptoms and antipsychotic dosage (Fervaha et al. 2015a; Fervaha et al. 2015b; Peralta et al. 2000; Prosser et al. 1987; Tugg et al. 1997) or plasma concentrations (Levinson et al. 1995; Marder et al. 2002; Prosser et al. 1987; Tugg et al. 1997). Action at dopamine D2 receptors (D2R) is the central feature of all antipsychotic drugs – all having antagonistic properties at this neuroreceptor (Kapur and Mamo 2003). Studies examining the association between negative symptoms and antipsychotic D2/3R occupancy are limited. One previous study reported a positive relationship between antipsychotic striatal D2/3R occupancy and negative symptoms (de Haan et al. 2000); however, this finding was not replicated in a subsequent study (de Haan et al. 2003). Another study also failed to observe higher antipsychotic D2/3R occupancy to be linked with greater negative symptom severity (Sakurai et al. 2013), though the former variable was estimated from plasma drug concentrations rather than through molecular neuroimaging techniques. Notably, all the aforementioned investigations quantified negative symptoms utilizing the negative subscale of the Positive and Negative Syndrome Scale (PANSS) (Kay et al. 1987) which includes items tapping into cognitive impairments (e.g., difficulty in abstract thinking). This clearly complicates the interpretation of these past findings, especially as cognition itself has been associated with the antipsychotic dosage (Knowles et al. 2010). Finally, studies to date have not explored the relationship between negative symptoms and antipsychotic occupancy in discrete subregions of the striatum including the ventral striatum, a region more closely associated with motivation than other striatal subregions (Haber and Knutson 2010). Taken together, findings to date are inconclusive regarding the idea of antipsychotic-induced negative symptoms.
In the present study we examined the relationship between antipsychotic D2/3R occupancy measured using carbon 11–labeled ([11C])–raclopride and positron emission tomography (PET) and negative symptoms in schizophrenia. We explored this relationship in a sample of clinically stable outpatients with schizophrenia undergoing a protocolized reduction of their antipsychotic dose, which allowed for the examination of cross-sectional relationships as well as longitudinal inter-relationships between D2/3R occupancy by antipsychotics and negative symptoms. Based on the aforementioned clinical studies, we hypothesized that we would fail to show a large and significant association between negative symptom severity and D2/3R occupancy by antipsychotics, i.e., higher antipsychotic-related D2/3R occupancy would not be positively correlated with levels of negative symptoms. Similarly, we hypothesized that there would be a lack of significant relationship between changes in occupancy and changes in negative symptom severity.
Methods and Materials
Study design and participants
This study represents an extension of a previously reported prospective dose reduction PET study in patients with schizophrenia, for which the details and main results have been published elsewhere (Graff-Guerrero et al. 2015). Briefly, we recruited patients with a DSM-IV diagnosis of schizophrenia or schizoaffective disorder, confirmed with the Structured Clinical Interview for DSM-IV Axis I Disorders (First et al. 2002), aged 50 years or older, who were clinically stable (i.e., no inpatient hospitalization in the past 6 months) and receiving the same dose of risperidone or olanzapine monotherapy for at least 6 months. Patients receiving concomitant psychotropic medications were not excluded. Patients who met criteria for a substance use disorder within the past 6 months, had a urine drug screen positive for substances of abuse, had changed their dose of any psychotropic medication for mental health reasons within the past 6 months, or had an unstable medical condition, were excluded.
The study was approved by the institutional Research Ethics Board, authorized by Health Canada, and registered at ClinicalTrials.gov (NCT00716755). All participants had to be deemed competent to provide consent as per the MacArthur Competence Assessment Tool for Clinical Research (Appelbaum and Grisso 2001) and provided written informed consent before the initiation of any study procedures.
Procedure
Participants were assessed with clinical scales for symptoms and adverse effects at baseline (Graff-Guerrero et al. 2015). A [11C]–raclopride PET scan was performed to estimate antipsychotic D2/3R occupancy. Subsequently, participants underwent a gradual dose reduction of up to 40% of their baseline dose or to the lower limit of the recommended dosage range (i.e., 7.5 mg/d for olanzapine and 1.5 mg/d for risperidone) (Alexopoulos et al. 2004). The dose was reduced weekly by 2.5 mg for olanzapine and 0.5 mg for risperidone. Clinical assessments and a [11C]-raclopride PET scan were performed again at least 2 weeks after the final target dose was attained to ensure steady-state antipsychotic concentration in the brain. The participants were subsequently monitored for at least 3 months with clinical assessments. Clinical deterioration was defined as an increase of at least 20% in the total Brief Psychiatric Rating Scale (BPRS)(Overall and Donald 1962) score from baseline.
On the same day of each of the 2 PET scans (baseline and post-dose reduction), participants’ clinical status was evaluated using the PANSS (Kay et al. 1987), and symptom scores were based on derived factors (Wallwork et al. 2012). Medication-related side effects were evaluated using the Simpson-Angus Scale (SAS) (Simpson and Angus 1970), and the Barnes Rating Scale for Drug-Induced Akathisia (BAS) (Barnes 1989), among other scales (Graff-Guerrero et al. 2015).
Negative symptom assessment
Severity of negative symptoms were evaluated using a consensus-defined factor from the PANSS (Wallwork et al. 2012). Specifically, the following items were summed: Blunted Affect, Emotional Withdrawal, Poor Rapport, Passive/Apathetic Social Withdrawal, Lack of Spontaneity & Flow of Conversation, and Motor Retardation. This negative symptom factor score from the PANSS has been shown to be highly convergent with more detailed measurements of negative symptoms including for example, scores from the Scale for the Assessment of Negative Symptoms (van Erp et al. 2014).
Given the burgeoning evidence for a two-factor structure of negative symptoms in schizophrenia (Messinger et al. 2011), we also calculated two additional scores to evaluate amotivation and expressive deficits separately. Specifically, a social amotivation score was calculated by summing the following items from the PANSS: Emotional Withdrawal, Passive Apathetic Withdrawal, and Active Social Avoidance (Fervaha et al. 2014). This social amotivation score has been shown to have significant convergence with other measures of motivational deficits among patients with schizophrenia (Fervaha et al. 2015b; Luther et al. 2015). A diminished expression score was also calculated by summing the following items from the PANSS: Blunted Affect, Poor Rapport, Lack of Spontaneity & Flow of Conversation, and Motor Retardation (Fervaha et al. 2014). Higher scores on each of these measures reflect greater severity of negative symptom.
Image acquisition and analysis
[11C]-raclopride PET scans were carried out at baseline and again at least 2 weeks after the target antipsychotic dose was reached following dose reduction. The PET scans were performed 14-16 hours after the last dose of the antipsychotic was given. Images were acquired using a high-resolution PET camera system (CPS-HRRT; Siemens Molecular Imaging, USA), which measures radioactivity in 207 brain slices with a thickness of 1.2mm each. The in-plane resolution was ~2.8mm full-width at half-maximum (FWHM). Transmission scans were acquired using a single photon point source to provide attenuation correction (137Cs; T1/2 = 30.2 yr, E = 662 KeV).
After each participant was placed on the scanning bed, a custom-fitted thermoplastic mask (True Scan Imaging) was made and then used during PET scans to decrease movement. A saline solution containing [11C]-raclopride was injected as a bolus through an antecubital vein. The radiosynthesis of [11C]-raclopride and the acquisition of PET images have been described in detail elsewhere (Wilson et al. 2000). The mean (SD) total mass injected, radioactivity dose, and specific activity of [11C]-raclopride for the baseline and follow-up scans were 2.71 (1.96) μg and 2.95 (2.63) μg, 9.69 (0.79) mCi and 9.65 (0.72) mCi, and 1650.89 (701.51 mCi/μmol and 1549.84 (711.59) mCi/μmol, respectively, without any significant differences between the 2 PET scanning sessions (paired-samples t-test; P=0.53, P=0.65, and P=0.38, respectively). Emission data were acquired in list mode for 60 minutes, reconstructed by filtered back projection, and redefined into 28 frames (1–5 of 1-min duration, 6–25 of 2-min duration, and 26–28 of 5-min duration).
Each participant also underwent 3-dimensional, brain volume, T1-weighted magnetic resonance imaging (MRI; inversion time, 650 milliseconds; field of view, 23 cm; 256 × 256; slice thickness, 0.9 mm; flip angle, 8°), performed in a GE Discovery MR750 3.0-T scanner (General Electric Medical Systems) to permit accurate delineation of the brain regions for data analysis. The region of interest (ROI)-based analysis for [11C]-raclopride has been described in detail elsewhere (Graff-Guerrero et al. 2008). Briefly, time activity curves (TACs) from ROIs were obtained from the dynamic PET images in native space with reference to each subjects co-registered MRI image. The co-registration of each subject’s MRI to PET space was done using the normalized mutual information algorithm (Studholme et al. 1997) as implemented in SPM2 (SPM2, Wellcome Department of Cognitive Neurology, London; http://www.fil.ion.ucl.ac.uk/spm). The TACs were analyzed using the Simplified Reference Tissue Method (SRTM) (Lammertsma and Hume 1996), using the cerebellum as the reference region, to derive a quantitative estimate of binding: binding potential relative to the non-displaceable compartment (BPND) as defined by the consensus nomenclature for in vivo imaging of reversibly binding radioligands (Innis et al. 2007). The basis function implementation of the SRTM (Gunn et al. 1997) was applied to the dynamic PET images to generate parametric voxelwise BPND maps using PMOD (v2.7, PMOD Technologies, Zurich, Switzerland). These images were spatially normalized into Montreal Neurological Institute (MNI) brain space by Nearest Neighbour Interpolation with a voxel size fixed in 2 × 2 × 2 mm3 using SPM2. Regional BPND estimates were then derived from ROIs defined in MNI space. The ventral striatum and dorsal striatum (dorsal caudate, hereafter caudate and dorsal putamen, hereafter putamen) were defined according with Mawlawi et al (Mawlawi et al. 2001).
Calculation of occupancy
As we were not able to obtain BPND data from our participants while they were off antipsychotics, to compute D2/3R occupancy we estimated participants’ BPND values using data from antipsychotic-free patients with schizophrenia. Specifically, age- and sex-corrected measures of BPND were estimated for each ROI using a linear regression equation (Nakajima et al. 2015). D2/3R occupancy was calculated using the following formula:
where BPND_Antipsychotic-free is the age- and sex-corrected BPND derived from antipsychotic-free participants with schizophrenia, and BPND_Medicated is the BPND obtained from participants receiving oral risperidone or olanzapine.
Statistical analyses
Initially, a series of paired-samples t-tests were carried out to evaluate whether there were any significant changes on any of the variables of interest following dose reduction (Table 2). We then examined whether negative symptom severity in general was associated with antipsychotic-related D2/3R occupancy at baseline in a dose-dependent fashion using Spearman’s rank correlations. We opted to employ Spearman’s rank correlations rather than Pearson’s product-moment correlations as the former evaluates both linear and non-linear dose-dependent relationships, whereas the latter only evaluates linear relationships. D2/3R occupancy was examined in 3 striatal subregions (caudate, putamen, and ventral striatum) as well as within the whole striatum. After this, we explored whether such a relationship existed for the two discrete subdomains of negative symptoms. Subsequently, and for completeness, an exploratory item-level analysis was carried out. These analyses were repeated using the follow-up values. Finally, we examined whether changes in antipsychotic-related D2/3R occupancy following dose reduction were related to changes in negative symptom severity using Spearman’s rank correlations. For this, difference scores were calculated for both symptom and occupancy values (score at follow-up minus the score at baseline).
Table 2.
Clinical characteristics of participants before and after dose reduction of antipsychotic medication
| Variable | Baseline PET Visit Mean (S.D.) or % |
Follow-up PET Visit Mean (S.D.) or % |
P-valuea |
|---|---|---|---|
| Negative symptom severity | |||
| Negative symptoms total | 15.1 (5.1) | 14.7 (5.1) | 0.06 |
| Social amotivation | 8.0 (2.7) | 7.8 (2.7) | 0.03 |
| Diminished Expression | 9.4 (3.3) | 9.3 (3.2) | 0.17 |
| Other symptoms | |||
| Positive symptoms | 8.1 (2.6) | 8.0 (2.9) | 0.42 |
| Disorganized symptoms | 7.3 (2.4) | 7.2 (2.4) | 0.23 |
| Excitement symptoms | 4.8 (1.3) | 4.7 (1.1) | 0.32 |
| Depression symptoms | 4.4 (1.7) | 4.3 (1.5) | 0.76 |
| Extrapyramidal symptoms | |||
| SAS total | 3.1 (2.5) | 1.8 (1.8) | <0.001 |
| BAS global | 0.7 (1.5) | 0.2 (0.8) | 0.005 |
| Antipsychotic dose (mg/day) | |||
| Olanzapine equivalentsb | 18.4 (8.0) | 12.0 (5.0) | <0.001 |
| Dopamine D2/3 receptor occupancy (%) | |||
| Caudate | 72.9 (8.0) | 65.2 (13.7) | <0.001 |
| Putamen | 69.8 (11.3) | 63.1 (12.9) | <0.001 |
| Ventral striatum | 72.3 (11.3) | 66.7 (13.9) | 0.002 |
| Whole striatum | 71.7 (11.0) | 65.0 (13.0) | <0.001 |
Abbreviations: BAS: Barnes Rating Scale for Drug-Induced Akathisia; PANSS: Positive and Negative Syndrome Scale; SAS: Simpson-Angus Scale.
Based on paired-samples t-tests for individuals with both baseline and follow-up PET data (N=38).
The dose of risperidone was converted to an olanzapine equivalent dosage, calculated using consensus-based antipsychotic dose equivalents based on Gardner et al. (20mg olanzapine = 6mg risperidone) (Gardner et al. 2010). The mean dose of olanzapine was 20.4 mg/day at baseline and 13.2 mg/day at follow-up (change: p<0.001), and the mean dose of risperidone was 4.5 mg/day at baseline and 2.9 mg/day at follow-up (change: p<0.001).
Notably, our study was powered to detect an association with a large effect size (i.e., 80% power to detect a correlation coefficient greater than 0.4 at a two-tailed P-value of 0.05), similar to that reported in one previous study (de Haan et al. 2000).
We also defined prominent negative symptoms as a severity score of 4 (i.e., moderate) or greater on any of the individual negative symptom items comprising the negative symptom factor from the PANSS. Whether antipsychotic-related D2/3 receptor occupancy differed for individuals with and without prominent negative symptoms at baseline was tested using an independent-samples t-test.
Statistical significance was set at P<0.05 (two-tailed) and analyses were carried out using SPSS version 20 (IBM Corporation, Armonk, NY).
Results
Participant characteristics
A total of 41 participants provided both clinical and PET data at baseline, and 38 participants provided follow-up data. Baseline sociodemographic and clinical characteristics of the 41 participants are presented in Table 1. Eighteen participants (43.9%) experienced at least 1 prominent negative symptom.
Table 1.
Baseline demographic and clinical characteristics of 41 participants
| Variable | Mean (S.D.) or % |
|---|---|
| Age (years) | 60.2 (6.7) |
| Sex (% male) | 73.2 |
| Race (% white) | 85.4 |
| Diagnosis (%) | |
| Schizophrenia | 80.5 |
| Schizoaffective disorder | 19.5 |
| Illness duration (years) | 34.3 (10.6) |
| Number of inpatient hospitalizations | 5.9 (5.4) |
| Antipsychotic dose (mg/day) | |
| Olanzapine (N=24) | 20.6 (6.7) |
| Risperidone (N=17) | 4.3 (2.4) |
| Combined (Olanzapine equivalentsa; N=41) | 18.0 (7.9) |
| Participants receiving concomitant medications (N) | |
| Antiparkinsonian | 4 |
| Antidepressant | 15 |
| Mood stabilizer | 7 |
| Benzodiazepine | 13 |
| Any of the above | 24 |
| PANSS total score | 60.2 (13.5) |
| BPRS total score | 41.3 (8.7) |
Abbreviations: BPRS: Brief Psychiatric Rating Scale; PANSS: Positive and Negative Syndrome Scale.
Calculated using consensus-based antipsychotic dose equivalents based on Gardner et al. (20mg olanzapine = 6 mg risperidone) (Gardner et al. 2010).
Negative symptoms before and after antipsychotic dose reduction
Findings from the majority of this sample have been reported previously (Graff-Guerrero et al. 2015). The 3 individuals (7.3%) without follow-up PET data experienced clinical deterioration before reaching the target dose during the dose reduction phase of the study. For the other 38 participants with PET data at both time-points, the mean (SD) time to achieve the dose reduction was 2.2 (1.5) weeks, and the time between the 2 PET scans was 6.5 (2.3) weeks. Clinical and PET data before and after dose reduction are presented in Table 2. Participants’ doses were reduced by a mean (SD) of 34.2% (4.1%). After dose reduction, both participants’ antipsychotic dose and the corresponding D2/3R occupancy were significantly lower. Dose reduction was also associated with lower levels of antipsychotic-related motor side effects. In terms of symptom severity, negative symptoms were slightly, though not statistically significantly, lower following dose reduction; however, in an exploratory analysis using negative symptom subdomain scores, the severity of social amotivation was significantly lower following dose reduction, although the magnitude of change was modest and would not survive correction for multiple comparison testing (Table 2). Notably, though, only 5 of the 39 participants (12.8%) experienced a change in their social amotivation score over time, meaning that for the majority of participants this symptom remained stable following antipsychotic dose reduction. Other symptoms did not significantly change following dose reduction.
Negative symptoms and dopamine D2/3 receptor occupancy
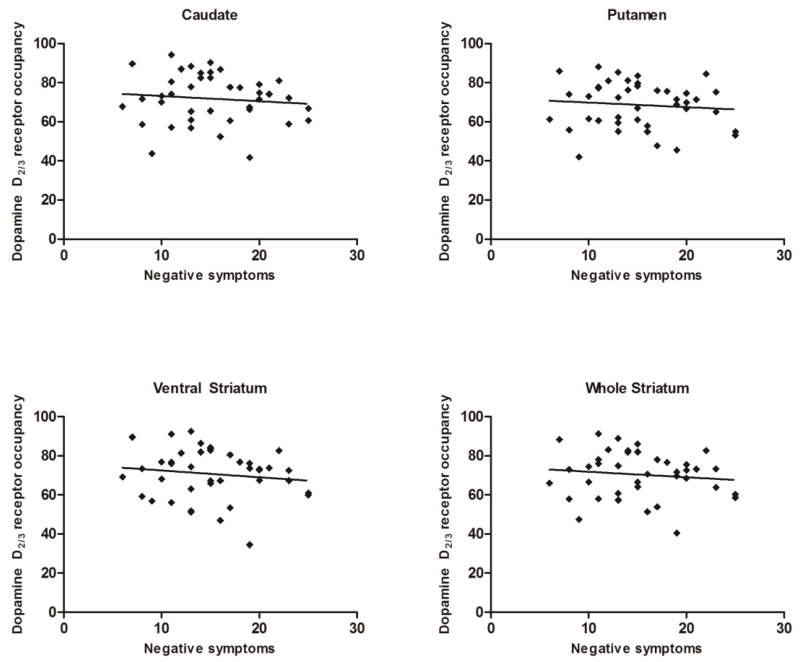
At baseline, negative symptom severity was not associated with antipsychotic-related D2/3R occupancy in any striatal region (Table 3; Figure 1). This finding held true for exploratory analyses with negative symptom subdomain scores and individual item scores. In fact, the magnitude for each bivariate relationship trended toward a negative association, whereby greater negative symptom severity was associated with lower occupancy. A similar pattern of results emerged at follow-up; there was no significant association between any negative symptom measure and occupancy in any striatal region (all P’s>0.05).
Table 3.
Bivariate relationships between negative symptoms and antipsychotic-related dopamine D2/3 receptor occupancy at baseline
| Caudate | Putamen | Ventral striatum |
Whole striatum |
|
|---|---|---|---|---|
| Total score | ||||
| Negative symptoms | −0.10 | −0.14 | −0.13 | −0.10 |
| Subdomains | ||||
| Social amotivation | −0.10 | −0.11 | −0.13 | −0.08 |
| Diminished expression | −0.08 | −0.12 | −0.07 | −0.09 |
| Individual items | ||||
| Blunted affect | −0.03 | −0.03 | −0.04 | −0.01 |
| Emotional withdrawal | −0.19 | −0.17 | −0.16 | −0.16 |
| Poor rapport | −0.03 | −0.13 | −0.13 | −0.09 |
| Passive/apathetic social withdrawal |
−0.10 | −0.13 | −0.10 | −0.09 |
| Lack of spontaneity & flow of conversation |
−0.09 | −0.10 | −0.11 | −0.07 |
| Motor retardation | −0.01 | −0.07 | −0.08 | −0.04 |
Note: All P-values were greater than 0.05.
Figure 1.
Scatterplot of the relationship between negative symptom severity and antipsychotic-related dopamine D2/3 receptor occupancy in various striatal regions.
For completeness, we also re-computed the above tests conducted with the full sample in the two subsamples of participants receiving either olanzapine or risperidone. The occupancy values did not differ between participants receiving olanzapine or risperidone (all P-values>0.05). At baseline, a similar pattern of findings emerged for each drug group as that seen for the full sample; overall negative symptoms were not associated with antipsychotic-related D2/3R occupancy in any striatal region (all P-values>0.05).
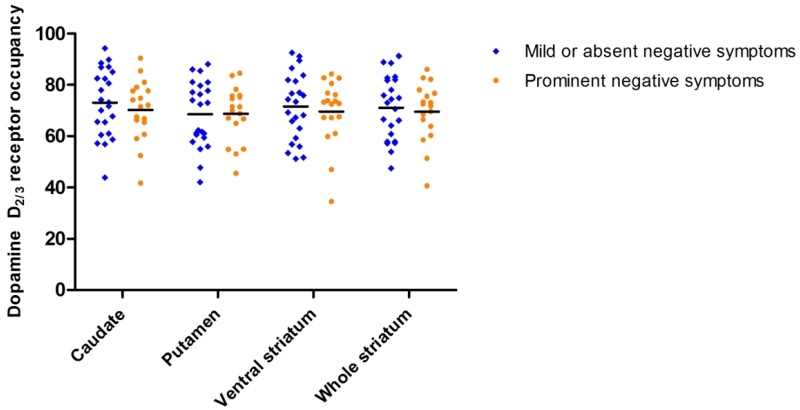
Participants with prominent negative symptoms at baseline did not differ from participants without such symptoms in terms of antipsychotic-related D2/3R occupancy in the caudate (t=0.72, P=0.47), putamen (t=0.04, P=0.97), ventral striatum (t=0.48, P=0.63), or whole striatum (t=0.41, P=0.68; Figure 2). Consistent with the dimensional analysis above, patients with prominent negative symptoms had lower mean occupancy values for all striatal regions, with the exception of the putamen where these values differed less than 0.2%. The validity of the prominent negative symptom classification was underscored by the finding that these individuals had significantly more severe negative symptoms than individuals without prominent negative symptoms (t=8.36, P <0.001) but, importantly, did not differ in the severity of other symptom domains (all P-values>0.05; Supplemental Table 1).
Figure 2.
Dopamine D2/3 receptor occupancy for participants with prominent negative symptoms and those with mild or no negative symptoms. Horizontal line denotes the respective mean value.
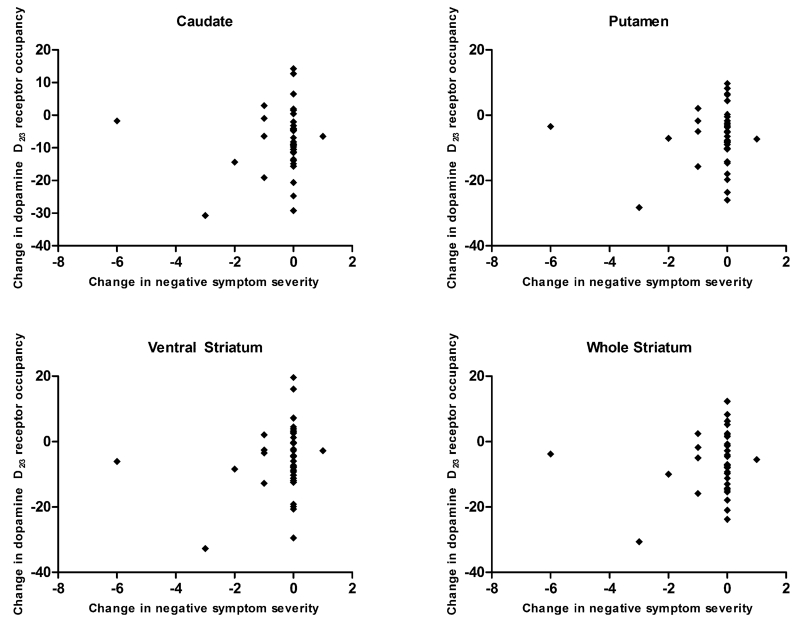
In the longitudinal subsample, changes in negative symptoms were not associated with changes in antipsychotic-related D2/3R occupancy in the caudate (r=0.05, P=0.79), putamen (r=0.02, P=0.90), ventral striatum (r=0.15, P=0.37), or whole striatum (r=0.08, P=0.64; Figure 3). A similar pattern of null findings emerged when examining the relationship between occupancy and negative symptom subdomain scores or individual items (all P-values>0.05; Supplemental Table 2).
Figure 3.
Scatterplot of the relationship between changes in negative symptom severity and changes antipsychotic-related dopamine D2/3 receptor occupancy in various striatal regions following antipsychotic dose reduction.
Discussion
The present study represents an extension of a previously reported dose reduction study (Graff-Guerrero et al. 2015), with analyses focused on negative symptom outcomes. The clinical results of the antipsychotic dose reduction echo those of our previous report in a smaller sample. Specifically, we show that antipsychotic dose can be successfully reduced in the majority of stable patients with schizophrenia who are receiving antipsychotic doses above the recommended minimum. Reduction of antipsychotic dose was associated with improvement in antipsychotic-related motor side effects in the face of stable symptoms (i.e., no substantial changes in symptoms were observed following antipsychotic dose reduction).
The present analysis was carried out to examine the relationship, or lack thereof, between negative symptoms and antipsychotic D2/3R occupancy. To our knowledge, the present study is the largest PET study examining the relationship between antipsychotic occupancy of striatal D2/3R and negative symptoms, and the first to examine the inter-relationship between these variables in the context of a longitudinal dose reduction study, which provides the unique opportunity to explore the impact of changes in dopaminergic antagonism. We failed to find a relationship linking greater occupancy with greater burden of negative symptoms. In fact, the correlation coefficients of this association were in the opposite direction - i.e., greater negative symptoms were associated with lower occupancy, though this relationship was not significant. This bolsters our confidence that the absence of an association was not simply due to a lack of power, though we were adequately powered to detect a moderate to large effect size.
We also explored whether any relationship existed for specific negative symptoms and occupancy and, again, no significant relationship emerged. These lack of associations were seen in the face of a liberal type I error acceptance rate (i.e., α<0.05, without correction for multiple comparison testing). All associations would, of course, be non-significant if a multiple comparison testing correction had been applied. We failed to find any dose-dependent relationship between negative symptoms and occupancy at both baseline and follow-up. Moreover, changes in antipsychotic occupancy level at striatal D2/3R were not related to changes in negative symptom severity. This latter finding is of particular importance as it suggests that after chronic stable treatment with antipsychotics, reduction in occupancy and, by inference, increases in dopaminergic transmission at D2/3R are not sufficient for improvement in negative symptoms. It should be noted that negative symptom severity did trend toward reduction following dose reduction, though the magnitude of this effect was modest. Moreover, the open-label design of the present study cannot rule out a non-specific effect of time (i.e., a placebo effect); we cannot ascribe this nominal reduction in symptom severity to the reduction of antipsychotic dose rather than the benefits of ongoing treatment for example. Therefore, in light of these considerations, this finding should be interpreted with caution.
That no association was found between dopaminergic blockade (i.e., antagonism) at the D2/3R and negative symptoms in general, or motivational deficits in particular, is noteworthy as it stands in contrast to predictions based on pre-clinical investigations and the hypothesized role of dopamine-dependent neural circuitry in motivated behaviour (Salamone and Correa 2012). However, our findings are not inconsistent with these findings and do not rule out dopamine as an important mediator of negative symptoms. Instead, our findings suggest that there does not exist a systematic dose-dependent relationship negative symptoms and occupancy (i.e., antagonism) of striatal D2/3R by antipsychotics. It is certainly possible that basal levels of striatal dopamine are implicated in negative symptom expression and there is, in fact, some evidence for this in schizophrenia (Kegeles et al. 2010). At any rate, our findings reject the notion of dose-dependent antipsychotic-induced negative symptoms. Other (non-iatrogenic) mechanisms need to be explored as causative agents in the expression of these symptoms.
Our study also raises the question of why there was no observed relationship between negative symptoms and dopaminergic blockade. We speculate that several factors might be involved (Fervaha et al. 2015b). Chief among these, we propose that differences in acute dosing paradigms used in pre-clinical and healthy volunteer studies and chronic dosing in patients with schizophrenia account for discrepancies. Some evidence supports that the effects of chronic treatment with antipsychotics are distinct from their acute effects (Amato et al. 2011; Samaha et al. 2007). Future pre-clinical studies are well positioned to resolve this question empirically, focusing on motivated behaviour and examining potential neurobiological mechanisms.
The present study examined variation in antipsychotic-related D2/3R occupancy of olanzapine and risperidone, two second-generation antipsychotic medications. Our study did not include an exhaustive list of other antipsychotic medications including first-generation and other second-generation drugs. While recent clinical data suggest that these two classes of antipsychotics do not substantially differ in terms of their effect on negative symptoms (Darbà et al. 2011; Fusar-Poli et al. 2015), though see (Zhang et al. 2013), there exists evidence that these medication classes have distinct neurofunctional effects (Juckel et al. 2006; Lahti et al. 2009; Schlagenhauf et al. 2008). More specifically, there is some previous work suggesting that it is the first-generation, and not the second-generation, antipsychotics that are associated with reward system dysfunction and (secondary) negative symptoms (Juckel et al. 2006; Schlagenhauf et al. 2008). Thus, it is possible that a relationship between negative symptoms and dopaminergic blockade may be observed in a sample of patients treated with different antipsychotic medications. Data from a previous study that included patients receiving both olanzapine and haloperidol also failed to find a relationship between these two variables (de Haan et al. 2003), arguing against the idea that a relationship necessarily exists when examining patients receiving first-generation antipsychotic medications.
The present study has limitations that should be mentioned. First, it did not include very high doses of antipsychotics, but rather included doses reflective of current practice which were within the range of consensus recommendations (Gardner et al. 2010). Second, baseline medication dosing was not assigned randomly but, instead, determined based on real-world clinical decisions. Third, the present study employed a single-arm open-label study design over a few weeks. Thus the long-term effects of dose reduction or the non-specific effect of time on negative symptoms could not be assessed. However, previous research examining the clinical effects of antipsychotic dose reduction and employing a longer duration of follow-up has also failed to find significant changes in symptom severity (Harris et al. 1997). Fourth, participants included in the study were aged 50 years and above, hence the generalizability of our findings to younger patients or those with first-episode schizophrenia remains an open question. To this end, it should be underscored that our null findings are consistent with other PET studies in younger patients (de Haan et al. 2003), and that previous clinical studies have not found a significant relationship between patient age and negative symptoms (Schultz et al. 1997). Fifth, we focused on antipsychotic occupancy of stiatal D2/3R given their centrality to the mechanism of action of these drugs (Kapur and Mamo 2003), and did not evaluate action at other neuroreceptors (e.g., dopamine D1, serotonin 5-HT2A) or extra-stiatal regions (e.g., prefrontal cortex). Sixth, our assessment of motivational deficits was incomplete. Our measure only evaluated this domain from one perspective (i.e., social amotivation), and we did not measure motivation for non-social goals. It would be of interest to discern whether objective tasks tapping into discrete aspects of negative symptoms are related to antipsychotic occupancy.
It has long been held that antipsychotic medications can induce secondary negative symptoms (Carpenter et al. 1988; Kelley et al. 1999; Schooler 1994). However, direct evidence for this notion is limited. The present study failed to support this, and instead suggests that there is no systematic relationship between antipsychotic occupancy at striatal D2/3R and severity of negative symptoms in schizophrenia. The lack of association was found for both overall and specific negative symptoms, including symptoms reflective of amotivation and diminished expression. Our results therefore suggest that antipsychotic-related dopaminergic antagonism does not necessarily undermine motivation and worsen expressive negative symptoms among stable patients receiving long-term treatment with these medications. It is possible that patients have adapted to the effects of these medications and compensate in some manner. In any case, we failed to find any robust evidence for a link between the effects of these drugs and negative symptoms.
Supplementary Material
Acknowledgements
This work was funded by CIHR grant MOP-97946 (Dr. Graff-Guerrero), US National Institutes of Health grant RO1MH084886 (Dr. Graff-Guerrero), and a Vanier Canada Graduate Scholarship (Dr. Fervaha).
The positron emission tomography center staff at the Centre for Addiction and Mental Health, including Alvina Ng, BS, and Laura Nguyen, BS, provided technical assistance in data collection. The Multimodal Imaging Group Staff at the Centre for Addiction and Mental Health, including Thushanthi Balakumar, Zhe Feng, Kathryn Kalahani-Bargis, and Alex Naber, assisted in participants’ recruitment and data administration.
Dr. Mamo has received research support from Pfizer.
Dr. Mulsant has received research support from Brain Canada, Centre for Addiction and Mental Health (CAMH) Foundation, CIHR, US NIH. He has also received medications for NIH-funded clinical trials from Bristol-Myers Squibb, Eli-Lilly and Company, and Pfizer. He directly own stocks of General Electric (<$5000).
Dr. Nakajima has received fellowship grants from the CIHR, Japan Society for the Promotion of Science, and Nakatomi Foundation and manuscript fees from Dainippon-Sumitomo Pharma and Kyowa Hakko Kirin.
Dr. Gerretsen has received fellowship support from the CAMH Foundation, OMHF, and CIHR.
Dr. Rajji received research support from Brain Canada, Brain and Behavior Research Foundation, Canada Research Chair, Canadian Foundation for Innovation, CIHR, Ontario Ministry of Health and Long-Term Care, Ontario Ministry of Research and Innovation, the US NIH, and the W. Garfield Weston Foundation.
Dr. Remington has received research support from the Schizophrenia Society of Ontario, CIHR, Research Hospital Fund – Canada Foundation for Innovation, Canadian Diabetes Association, Novartis Canada, Medicure Inc., and Neurocrine Bioscience; as a co-investigator he has received research support from the Canadian Psychiatric Research Foundation and Pfizer Inc.; consultant fees from Laboratorios Farmacéuticos ROVI, Synchroneuron, Novartis, and Roche; and speaker’s fees from Novartis.
Dr. Graff-Guerrero has received support from Brain Canada, Canadian Foundation for Innovation, CIHR, Ontario Ministry of Health and Long-Term Care, Ontario Ministry of Research and Innovation, the US National Institute of Health (NIH), Ontario Mental Health Foundation (OMHF), Consejo Nacional de Ciencia y Tecnologia (CONACyT), Instituto de Ciencia y Tecnología del DF (ICyTDF), and Brain & Behavior Research Foundation.
Footnotes
Declaration of Interest
The other authors report no competing interests to disclose.
References
- Alexopoulos GS, Streim J, Carpenter D, Docherty JP. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(Suppl 2):5–99. discussion 100-102; quiz 103-4. [PubMed] [Google Scholar]
- Amato D, Natesan S, Yavich L, Kapur S, Muller CP. Dynamic regulation of dopamine and serotonin responses to salient stimuli during chronic haloperidol treatment. Int J Neuropsychopharmacol. 2011;14:1327–39. doi: 10.1017/S1461145711000010. [DOI] [PubMed] [Google Scholar]
- Appelbaum PS, Grisso T. MacArthur competence assessment tool for clinical research (MacCAT-CR) Professional Resource Press; Sarasota, FL: 2001. [Google Scholar]
- Artaloytia JF, Arango C, Lahti A, Sanz J, Pascual A, Cubero P, Prieto D, Palomo T. Negative signs and symptoms secondary to antipsychotics: a double-blind, randomized trial of a single dose of placebo, haloperidol, and risperidone in healthy volunteers. Am J Psychiatry. 2006;163:488–93. doi: 10.1176/appi.ajp.163.3.488. [DOI] [PubMed] [Google Scholar]
- Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–6. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- Bleuler E. Dementia Praecox or the group of schizophrenias. International Universities Press; New York: 1950. [Google Scholar]
- Breier A, Wolkowitz OM, Doran AR, Roy A, Boronow J, Hommer DW, Pickar D. Neuroleptic responsivity of negative and positive symptoms in schizophrenia. Am J Psychiatry. 1987;144:1549–55. doi: 10.1176/ajp.144.12.1549. [DOI] [PubMed] [Google Scholar]
- Carpenter WT, Jr., Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. 1988;145:578–83. doi: 10.1176/ajp.145.5.578. [DOI] [PubMed] [Google Scholar]
- Darbà J, Minoves A, Rojo E, Jimenez F, Rejas J. Efficacy of second-generation-antipsychotics in the treatment of negative symptoms of schizophrenia: a meta-analysis of randomized clinical trials. Revista de Psiquiatría y Salud Mental (English Edition) 2011;4:126–143. doi: 10.1016/j.rpsm.2011.02.005. [DOI] [PubMed] [Google Scholar]
- de Haan L, Lavalaye J, Linszen D, Dingemans PM, Booij J. Subjective experience and striatal dopamine D(2) receptor occupancy in patients with schizophrenia stabilized by olanzapine or risperidone. Am J Psychiatry. 2000;157:1019–20. doi: 10.1176/appi.ajp.157.6.1019. [DOI] [PubMed] [Google Scholar]
- de Haan L, van Bruggen M, Lavalaye J, Booij J, Dingemans PM, Linszen D. Subjective experience and D2 receptor occupancy in patients with recent-onset schizophrenia treated with low-dose olanzapine or haloperidol: a randomized, double-blind study. Am J Psychiatry. 2003;160:303–9. doi: 10.1176/appi.ajp.160.2.303. [DOI] [PubMed] [Google Scholar]
- Fervaha G, Foussias G, Agid O, Remington G. Motivational and neurocognitive deficits are central to the prediction of longitudinal functional outcome in schizophrenia. Acta Psychiatr Scand. 2014;130:290–9. doi: 10.1111/acps.12289. [DOI] [PubMed] [Google Scholar]
- Fervaha G, Foussias G, Takeuchi H, Agid O, Remington G. Measuring motivation in people with schizophrenia. Schizophr Res. 2015a;169:423–6. doi: 10.1016/j.schres.2015.09.012. [DOI] [PubMed] [Google Scholar]
- Fervaha G, Takeuchi H, Lee J, Foussias G, Fletcher PJ, Agid O, Remington G. Antipsychotics and Amotivation. Neuropsychopharmacology. 2015b;40:1539–48. doi: 10.1038/npp.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York State Psychiatric Institute; New York, NY: 2002. SCID-I/P. [Google Scholar]
- Fusar-Poli P, Papanastasiou E, Stahl D, Rocchetti M, Carpenter W, Shergill S, McGuire P. Treatments of Negative Symptoms in Schizophrenia: Meta-Analysis of 168 Randomized Placebo-Controlled Trials. Schizophr Bull. 2015;41:892–9. doi: 10.1093/schbul/sbu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167:686–93. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- Graff-Guerrero A, Rajji TK, Mulsant BH, Nakajima S, Caravaggio F, Suzuki T, Uchida H, Gerretsen P, Mar W, Pollock BG, Mamo DC. Evaluation of Antipsychotic Dose Reduction in Late-Life Schizophrenia: A Prospective Dopamine D2/3 Receptor Occupancy Study. JAMA Psychiatry. 2015;72:927–34. doi: 10.1001/jamapsychiatry.2015.0891. [DOI] [PubMed] [Google Scholar]
- Graff-Guerrero A, Willeit M, Ginovart N, Mamo D, Mizrahi R, Rusjan P, Vitcu I, Seeman P, Wilson AA, Kapur S. Brain region binding of the D2/3 agonist [11C]-(+)-PHNO and the D2/3 antagonist [11C]raclopride in healthy humans. Hum Brain Mapp. 2008;29:400–10. doi: 10.1002/hbm.20392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage. 1997;6:279–87. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MJ, Heaton RK, Schalz A, Bailey A, Patterson TL. Neuroleptic dose reduction in older psychotic patients. Schizophr Res. 1997;27:241–8. doi: 10.1016/S0920-9964(97)00083-2. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Davidson M, White L, Keefe RS, Hirschowitz J, Mohs RC, Davis KL. Empirical evaluation of the factorial structure of clinical symptoms in schizophrenia: effects of typical neuroleptics on the brief psychiatric rating scale. Biol Psychiatry. 1996;40:755–60. doi: 10.1016/0006-3223(95)00486-6. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–9. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Filonov D, Wustenberg T, Villringer A, Knutson B, Kienast T, Gallinat J, Wrase J, Heinz A. Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology (Berl) 2006;187:222–8. doi: 10.1007/s00213-006-0405-4. [DOI] [PubMed] [Google Scholar]
- Kapur S, Mamo D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1081–90. doi: 10.1016/j.pnpbp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M, Hwang DR, Huang Y, Haber SN, Laruelle M. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67:231–9. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- Kelley ME, van Kammen DP, Allen DN. Empirical validation of primary negative symptoms: independence from effects of medication and psychosis. Am J Psychiatry. 1999;156:406–11. doi: 10.1176/ajp.156.3.406. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Fenton WS, Carpenter WT, Jr., Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32:214–9. doi: 10.1093/schbul/sbj053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles EE, David AS, Reichenberg A. Processing speed deficits in schizophrenia: reexamining the evidence. Am J Psychiatry. 2010;167:828–35. doi: 10.1176/appi.ajp.2010.09070937. [DOI] [PubMed] [Google Scholar]
- Kraepelin E. Dementia Praecox and Paraphrenia. Livingstone; Edinburgh, UK: 1919. [Google Scholar]
- Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Cropsey KL. Modulation of limbic circuitry predicts treatment response to antipsychotic medication: a functional imaging study in schizophrenia. Neuropsychopharmacology. 2009;34:2675–90. doi: 10.1038/npp.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–8. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Leucht S, Arbter D, Engel RR, Kissling W, Davis JM. How effective are second-generation antipsychotic drugs? A meta-analysis of placebo-controlled trials. Mol Psychiatry. 2009;14:429–47. doi: 10.1038/sj.mp.4002136. [DOI] [PubMed] [Google Scholar]
- Levinson DF, Simpson GM, Lo ES, Cooper TB, Singh H, Yadalam K, Stephanos MJ. Fluphenazine plasma levels, dosage, efficacy, and side effects. Am J Psychiatry. 1995;152:765–71. doi: 10.1176/ajp.152.5.765. [DOI] [PubMed] [Google Scholar]
- Luther L, Lysaker PH, Firmin RL, Breier A, Vohs JL. Intrinsic motivation and amotivation in first episode and prolonged psychosis. Schizophr Res. 2015;169:418–22. doi: 10.1016/j.schres.2015.08.040. [DOI] [PubMed] [Google Scholar]
- Marder SR, Aravagiri M, Wirshing WC, Wirshing DA, Lebell M, Mintz J. Fluphenazine plasma level monitoring for patients receiving fluphenazine decanoate. Schizophr Res. 2002;53:25–30. doi: 10.1016/s0920-9964(00)00184-5. [DOI] [PubMed] [Google Scholar]
- Mas S, Gasso P, Fernandez de Bobadilla R, Arnaiz JA, Bernardo M, Lafuente A. Secondary nonmotor negative symptoms in healthy volunteers after single doses of haloperidol and risperidone: a double-blind, crossover, placebo-controlled trial. Hum Psychopharmacol. 2013;28:586–93. doi: 10.1002/hup.2350. [DOI] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, Huang Y, Simpson N, Ngo K, Van Heertum R, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21:1034–57. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- Messinger JW, Tremeau F, Antonius D, Mendelsohn E, Prudent V, Stanford AD, Malaspina D. Avolition and expressive deficits capture negative symptom phenomenology: implications for DSM-5 and schizophrenia research. Clin Psychol Rev. 2011;31:161–8. doi: 10.1016/j.cpr.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DD, Flaum M, Arndt S, Fleming F, Andreasen NC. Effect of antipsychotic withdrawal on negative symptoms in schizophrenia. Neuropsychopharmacology. 1994;11:11–20. doi: 10.1038/npp.1994.31. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Caravaggio F, Mamo DC, Mulsant BH, Chung JK, Plitman E, Iwata Y, Gerretsen P, Uchida H, Suzuki T, Mar W, Wilson AA, Houle S, Graff-Guerrero A. Dopamine D(2)/(3) receptor availability in the striatum of antipsychotic-free older patients with schizophrenia-A [(1)(1)C]-raclopride PET study. Schizophr Res. 2015;164:263–7. doi: 10.1016/j.schres.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JE, Donald RG. The brief psychiatric rating scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- Peralta V, Cuesta MJ, Martinez-Larrea A, Serrano JF. Differentiating primary from secondary negative symptoms in schizophrenia: a study of neuroleptic-naive patients before and after treatment. Am J Psychiatry. 2000;157:1461–6. doi: 10.1176/appi.ajp.157.9.1461. [DOI] [PubMed] [Google Scholar]
- Prosser ES, Csernansky JG, Kaplan J, Thiemann S, Becker TJ, Hollister LE. Depression, parkinsonian symptoms, and negative symptoms in schizophrenics treated with neuroleptics. J Nerv Ment Dis. 1987;175:100–5. doi: 10.1097/00005053-198702000-00006. [DOI] [PubMed] [Google Scholar]
- Saeedi H, Remington G, Christensen BK. Impact of haloperidol, a dopamine D2 antagonist, on cognition and mood. Schizophr Res. 2006;85:222–31. doi: 10.1016/j.schres.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Sakurai H, Bies RR, Stroup ST, Keefe RS, Rajji TK, Suzuki T, Mamo DC, Pollock BG, Watanabe K, Mimura M, Uchida H. Dopamine D2 receptor occupancy and cognition in schizophrenia: analysis of the CATIE data. Schizophr Bull. 2013;39:564–74. doi: 10.1093/schbul/sbr189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76:470–85. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha AN, Seeman P, Stewart J, Rajabi H, Kapur S. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27:2979–86. doi: 10.1523/JNEUROSCI.5416-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlagenhauf F, Juckel G, Koslowski M, Kahnt T, Knutson B, Dembler T, Kienast T, Gallinat J, Wrase J, Heinz A. Reward system activation in schizophrenic patients switched from typical neuroleptics to olanzapine. Psychopharmacology (Berl) 2008;196:673–84. doi: 10.1007/s00213-007-1016-4. [DOI] [PubMed] [Google Scholar]
- Schooler NR. Deficit symptoms in schizophrenia: negative symptoms versus neuroleptic-induced deficits. Acta Psychiatr Scand Suppl. 1994;380:21–6. doi: 10.1111/j.1600-0447.1994.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Schultz SK, Miller DD, Oliver SE, Arndt S, Flaum M, Andreasen NC. The life course of schizophrenia: age and symptom dimensions. Schizophr Res. 1997;23:15–23. doi: 10.1016/S0920-9964(96)00087-4. [DOI] [PubMed] [Google Scholar]
- Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;212:11–9. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- Studholme C, Hill DL, Hawkes DJ. Automated three-dimensional registration of magnetic resonance and positron emission tomography brain images by multiresolution optimization of voxel similarity measures. Med Phys. 1997;24:25–35. doi: 10.1118/1.598130. [DOI] [PubMed] [Google Scholar]
- Tugg LA, Desai D, Prendergast P, Remington G, Reed K, Zipursky RB. Relationship between negative symptoms in chronic schizophrenia and neuroleptic dose, plasma levels and side effects. Schizophr Res. 1997;25:71–8. doi: 10.1016/s0920-9964(97)00009-1. [DOI] [PubMed] [Google Scholar]
- van Erp TG, Preda A, Nguyen D, Faziola L, Turner J, Bustillo J, Belger A, Lim KO, McEwen S, Voyvodic J, Mathalon DH, Ford J, Potkin SG, Fbirn Converting positive and negative symptom scores between PANSS and SAPS/SANS. Schizophr Res. 2014;152:289–94. doi: 10.1016/j.schres.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallwork RS, Fortgang R, Hashimoto R, Weinberger DR, Dickinson D. Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr Res. 2012;137:246–50. doi: 10.1016/j.schres.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert TW, Goldberg TE, Marenco S, Bigelow LB, Egan MF, Weinberger DR. Comparison of cognitive performances during a placebo period and an atypical antipsychotic treatment period in schizophrenia: critical examination of confounds. Neuropsychopharmacology. 2003;28:1491–500. doi: 10.1038/sj.npp.1300216. [DOI] [PubMed] [Google Scholar]
- Wilson AA, Garcia A, Jin L, Houle S. Radiotracer synthesis from [(11)C]-iodomethane: a remarkably simple captive solvent method. Nucl Med Biol. 2000;27:529–32. doi: 10.1016/s0969-8051(00)00132-3. [DOI] [PubMed] [Google Scholar]
- Zhang JP, Gallego JA, Robinson DG, Malhotra AK, Kane JM, Correll CU. Efficacy and safety of individual second-generation vs. first-generation antipsychotics in first-episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2013;16:1205–18. doi: 10.1017/S1461145712001277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.