Abstract
Object
There is limited literature available to guide transfusion practices for patients with severe traumatic brain injury (TBI). Recent studies have shown that maintaining a higher hemoglobin threshold after severe TBI offers no clinical benefit. The present study aimed to determine if a higher transfusion threshold was independently associated with an increased risk of progressive hemorrhagic injury (PHI), thereby contributing to higher rates of morbidity and mortality.
Methods
The authors performed a secondary analysis of data obtained from a recently performed randomized clinical trial studying the effects of erythropoietin and blood transfusions on neurological recovery after severe TBI. Assigned hemoglobin thresholds (10 g/dl vs 7 g/dl) were maintained with packed red blood cell transfusions during the acute phase after injury. PHI was defined as the presence of new or enlarging intracranial hematomas on CT as long as 10 days after injury. A severe PHI was defined as an event that required an escalation of medical management or surgical intervention. Clinical and imaging parameters and transfusion thresholds were used in a multivariate Cox regression analysis to identify independent risk factors for PHI.
Results
Among 200 patients enrolled in the trial, PHI was detected in 61 patients (30.5%). The majority of patients with PHI had a new, delayed contusion (n = 29) or an increase in contusion size (n = 15). The mean time interval between injury and identification of PHI was 17.2 ± 15.8 hours. The adjusted risk of severe PHI was 2.3 times higher for patients with a transfusion threshold of 10 g/dl (95% confidence interval 1.1–4.7; p = 0.02). Diffuse brain injury was associated with a lower risk of PHI events, whereas higher initial intracranial pressure increased the risk of PHI (p < 0.001). PHI was associated with a longer median length of stay in the intensive care unit (18.3 vs 14.4 days, respectively; p = 0.04) and poorer Glasgow Outcome Scale scores (42.9% vs 25.5%, respectively; p = 0.02) at 6 months.
Conclusions
A higher transfusion threshold of 10 g/dl after severe TBI increased the risk of severe PHI events. These results indicate the potential adverse effect of using a higher hemoglobin transfusion threshold after severe TBI.
Keywords: severe traumatic brain injury, progressive hemorrhagic injury, hemoglobin transfusion threshold, secondary brain injury
Progression of intracranial injury on CT is reported in 8%–67% of patients with blunt traumatic brain injury (TBI).22 Studies focusing on parenchymal contusions show that progressive hemorrhagic injury occurs in more than 50% of the patients on repeat CT scans.9,14 For the majority of patients with TBI, progression of injury is observed in the first 24–48 hours,1,14 although a few patients can show progression as long as 72 hours after injury.6 These events are associated with increased morbidity and mortality as well as poorer neurological outcomes.20
In patients with severe TBI, a disruption of cerebral autoregulation as well as raised intracranial pressure (ICP) can contribute to cerebral hypoperfusion.7,16 Anemia, which is noted in as many as 50% of patients with TBI, can impair oxygen delivery and further contribute to cerebral ischemia.21 To prevent such secondary injury, an adequate hemoglobin concentration (≥ 10 g/dl) is often targeted in the acute phase after TBI. However, a recent randomized controlled trial (RCT) showed no clinical benefit in maintaining a hemoglobin threshold of 10 g/dl as opposed to 7 g/dl.17 Subsequent analyses showed that delayed mortality rates, after adjusting for injury severity, were higher for patients with a transfusion threshold of 10 g/dl.24 To further investigate these findings, we sought to determine if a higher transfusion threshold was independently associated with an increased risk of progressive hemorrhagic injury (PHI).
Methods
Study Design
The present study represents a secondary analysis of data obtained from an RCT studying the effects of erythropoietin (EPO) and blood transfusions on neurological recovery after severe TBI.17 The study was approved by the US FDA and the Institutional Review Boards at each participating institution. This study was registered with the ClinicalTrials.gov database (http://clinicaltrials.gov), and its registration no. is NCT00313716. Written informed consent was obtained from a legally authorized representative during the first year of the study. After approval in August 2007, the study was conducted under the conditions of the Exception from Informed Consent for Emergency Research (Code of Federal Regulations Title 21 CRF §50.24). Study design and results of the RCT have been reported previously.17 In brief, patients with severe TBI were enrolled prospectively within 6 hours of injury and randomized to administration of EPO or placebo and to hemoglobin transfusion thresholds of 7 g/dl or 10 g/dl using a 2 × 2 factorial design. Inclusion criteria were patients with a closed head injury who were admitted to a Level I trauma center and were not able to follow commands after resuscitation. Exclusion criteria were Glasgow Coma Scale (GCS) score of 3 with fixed and dilated pupils, penetrating trauma, pregnancy, life-threatening systemic injuries, use of antiplatelets or anticoagulants, and severe preexisting disease. Study intervention included the administration of EPO or placebo within 6 hours of injury and maintenance of assigned hemoglobin thresholds with transfusion of leukocyte-reduced packed red blood cells until ICP monitoring and ventilator support were discontinued. All patients were managed in a critical care unit according to a detailed standard protocol that conformed to the Guidelines for the Management of Severe Head Injury (see Supplement 1 from Robertson et al.17). Target ICP was < 20 mm Hg, cerebral perfusion pressure > 60 mm Hg, and mean arterial pressure ≥ 80 mm Hg according to the above protocol. Decompressive surgery was performed at admission for patients if indicated by the presence of midline shift, contusions, or cerebral edema.
Follow-up CT scans after the initial admission CT scan were obtained in 194 patients. Follow-up CT imaging data were not obtained in 2 patients who withdrew from the study, 2 patients who recovered rapidly, and 2 patients who died early after admission. An average of 4.4 CT scans were performed per patient enrolled in the study. Our clinical practice involves obtaining a follow-up CT scan within 24 hours after the admission CT scan. The average time between the first and second CT scans was 15.2 ± 1.4 hours. The longest time between the admission CT scan and follow-up CT scan was 77 hrs.
PHI was defined as the presence of new intracerebral hematomas, coalescence of preexisting contusions into a hematoma, delayed or enlargement of subdural or epidural hematomas, or development of unexpected postsurgical hematomas at the operative site. We did not include patients with blood around the surgical site that was likely to be postsurgical in both size and location. These events were recorded up to 240 hours after injury and were classified as severe if the detection of PHI necessitated initiation of pentobarbital coma for refractory raised ICP or a surgical intervention. In patients with more than 1 PHI event, data collected until the first event was used for analysis. Clinical and imaging parameters, transfusion thresholds, and EPO administration for each patient were included in the analysis to determine risk factors for PHI. Outcome at 6 months after the injury was measured using the Glasgow Outcome Scale (GOS). The GOS score was categorized as favorable (good recovery or moderate disability) or unfavorable (severe disability, vegetative, or dead).
Data Analysis
An intent-to-treat statistical analysis was conducted. Baseline characteristics were compared among patients who did or did not have a PHI event using Wilcoxon rank-sum tests for continuous variables and the Fisher’s exact test for categorical variables.
The primary outcome was PHI within 240 hours after injury. Data were censored at the time of withdrawal from the study or date of death, whichever occurred first. “Purposeful” model selection8 was applied to the set of covariates: age, sex, initial GCS motor score in emergency room (ER), Injury Severity Score, initial ICP, arrival time after injury, prehospital hypotension, prehospital hypoxia, EPO group, transfusion group, Marshall classification, basal cistern morphology on initial CT scan, midline shift on initial CT scan, presence of subarachnoid hemorrhage, surgery on admission, and replacement of bone flap after decompressive surgery. Univariate Cox regression models were fit including each covariate at a time. A multivariate model with the variables found statistically significant at the univariate stage (α = 0.25) and the threshold group was fit. Likelihood ratio tests were performed to assess the contribution of each variable in the multivariate model using an α level of 0.10 and assessing changes in the coefficient associated with the threshold group. A reduced model with those variables that had a statistically significant contribution in the multivariate model was fit and was compared with the multivariate model via a likelihood ratio test. Covariates that were not statistically significant in the univariate stage were given a chance to enter in the reduced model using an α level of 0.10. For 7 patients, initial ICP was missing or occurred after the PHI event, and these observations were dropped from the model.
Initial coagulation parameters, platelet count, and toxicology results were not included in the model selection due to missing values. Initial prothrombin time (PT) and partial thromboplastin time (PTT) were missing in 19% of patients, platelets in 12% of patients, blood alcohol levels in 22% of patients, and toxicology results in 32% of patients. Analyses were conducted using SAS (version 9.3, SAS Institute Inc.) or R (version 3.0.2, R Foundation for Statistical Computing) using a 2-sided α level of 0.05.
Results
Two hundred patients were included in the RCT. Demographic and clinical data of patients with and without PHI are shown in Table 1. PHI was detected in 61 patients (30.5%). The majority of patients had a new, delayed contusion (n = 29) or an increase in contusion size (n = 15, Table 2). The mean time interval between injury and identification of PHI was 17.2 ± 15.8 hours.
TABLE 1.
Univariate analysis with demographic and clinical data for patients with and without PHI
| Variable | PHI Present (n = 61) | PHI Absent (n = 139)* | p Value† |
|---|---|---|---|
| Median age in yrs (25th percentile–75th percentile) | 37.0 (28.0–48.0) | 28.0 (21.0–44.0) | 0.004 |
| Females (%) | 7 (11.5) | 19 (13.7) | 0.67 |
| No. of motor components of GCS (%)‡ | |||
| 1–3 | 23 (37.7) | 48 (34.5) | 0.67 |
| 4–5 | 38 (62.3) | 91 (65.5) | |
| Median Injury Severity Score (25th percentile–75th percentile) | 29.0 (25.0–38.0) | 29.0 (25.0–35.0) | 0.87 |
| Median initial ICP (25th percentile–75th percentile)§ | 18.0 (15.0–26.0) | 11.0 (7.0–19.0) | <0.001 |
| Arrival time after injury in min (25th percentile–75th percentile) | 37.0 (30.0–46.0) | 37.0 (30.0–43.0) | 0.63 |
| Prehospital hypotension (%) | 6 (9.8) | 19 (13.7) | 0.45 |
| Prehospital hypoxia (%) | 11 (18.0) | 28 (20.1) | 0.73 |
| EPO group (%) | |||
| 1 regimen | 16 (26.2) | 22 (15.8) | 0.19 |
| 2 regimen | 16 (26.2) | 48 (34.5) | |
| Placebo | 29 (47.5) | 69 (49.6) | |
| Transfusion group (%) | |||
| 7 g/dl | 24 (39.3) | 75 (54.0) | 0.06 |
| 10 g/dl | 37 (60.7) | 64 (46.0) | |
| Marshall classification (%) | |||
| Diffuse (1 or 2) | 12 (19.7) | 77 (55.4) | <0.001 |
| Diffuse (3 or 4) | 12 (19.7) | 34 (24.5) | |
| Mass lesion | 37 (60.7) | 28 (20.1) | |
| Basal cisterns (%) | |||
| Present | 19 (31.2) | 85 (61.2) | <0.001 |
| Compressed | 31 (50.8) | 48 (34.5) | |
| Absent | 11 (18.0) | 6 (4.3) | |
| Midline shift <5 mm (%) | 32 (52.5) | 118 (84.9) | <0.001 |
| Subarachnoid hemorrhage (%) | 46 (75.4) | 92 (66.2) | 0.19 |
| ER values (%) | |||
| PT normal, ≤15 | 38 (62.3) | 98 (70.5) | 0.49 |
| PTT normal, ≤36.4 | 43 (70.5) | 108 (77.7) | 0.73 |
| Platelets normal, ≥150 | 47 (77.1) | 125 (89.9) | 0.07 |
| Surgery on admission (%) | 43 (70.5) | 33 (23.7) | <0.001 |
| Bone flap off (%) | |||
| Yes | 23 (37.7) | 18 (13.0) | <0.001 |
| No | 20 (32.8) | 15 (10.8) | |
| No surgery | 18 (29.5) | 106 (76.3) | |
| Positive alcohol (%) | 28 (45.9) | 86 (61.9) | 0.10 |
| Toxicity screen positive (%) | 21 (34.4) | 58 (41.7) | 0.46 |
No PHI recorded for 6 patients without follow-up CT.
Wilcoxon rank-sum test for continuous variables, chi-square of Fisher’s exact test for categorical variables. Boldface type indicates statistical significance.
Calculated at enrollment; score range is from 1 (no motor response) to 6 (follows commands).
Initial ICP recorded in 136 patients without PHI, and in 61 patients with PHI. In 4 patients with PHI, initial ICP was recorded after PHI occurred and these values were excluded.
TABLE 2.
Description of all PHI events in 61 patients with severe TBI who sustained PHI within 10 days of initial injury
| Event | Value (%) |
|---|---|
| Severe events | 40 (65.6) |
| New delayed event | 37 |
| Contusion | 29 (78.3) |
| Epidural hematoma | 5 (13.5) |
| Subdural hematoma | 3 (8.1) |
| Increased/recurrent event | 29 |
| Contusion | 15 (51.7) |
| Epidural hematoma | 8 (27.6) |
| Subdural hematoma | 6 (20.7) |
| Surgical intervention for PHI* | 13 (21.3) |
In 1 patient, a PHI was observed at surgery using intraoperative ultrasonography, and this patient required a temporal lobectomy.
Univariate Analysis
Univariate analysis showed that patients with PHI were significantly older (p = 0.004), had a higher initial ICP (p < 0.001), had mass lesions on CT (p < 0.001), and were more likely to undergo surgery at admission (p < 0.001). Patients without PHI were more likely to have open basal cisterns and midline shift < 5 mm (p < 0.001; Table 1). There were no significant differences in the initial coagulation parameters or platelet counts between the two groups.
Multivariate Analysis
All PHI Events
Although the risk of PHI was 55% higher for patients in the 10 g/dl transfusion threshold group compared with those in the 7 g/dl threshold group, the threshold effect was not statistically significant (p = 0.11; Table 3). High initial ICP was a significant independent risk factor for all PHI events (Table 3). The presence of diffuse brain injury (Marshal classification I–IV; p < 0.001) was associated with a decreased risk of all PHI events.
TABLE 3.
Multivariate Cox regression analysis for all PHI events*
| PHI Event | HR | 95% CI | p Value |
|---|---|---|---|
| 10 vs 7 g/dl hemoglobin transfusion threshold |
1.55 | 0.90–2.69 | 0.11 |
| Initial ICP | 1.04 | 1.03–1.06 | <0.001 |
| EPO 1 regimen vs placebo | 1.27 | 0.65–2.49 | 0.49 |
| EPO 2 regimen vs placebo | 0.61 | 0.32–1.15 | 0.13 |
| CT category diffuse (1 or 2) vs mass lesion |
0.19 | 0.10–0.38 | <0.001 |
| CT category diffuse (3 or 4) vs mass lesion |
0.20 | 0.09–0.43 | <0.001 |
Boldface type indicates statistical significance.
Severe PHI
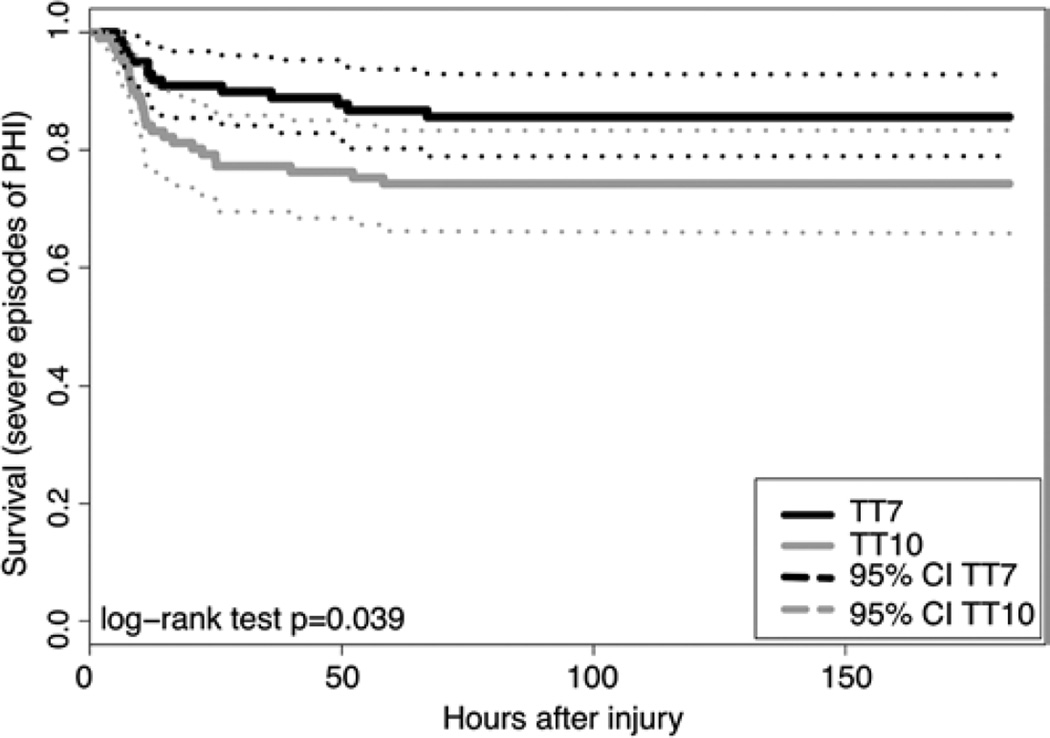
The adjusted risk of severe PHI was 2.3 times higher for patients with a transfusion threshold of 10 g/dl compared with 7 g/dl (p = 0.02; Table 4, Fig. 1). High initial ICP was associated with a higher risk of severe PHI. Surgery at admission showed a trend toward an increased risk of severe PHI, though it did not reach statistical significance (p = 0.07).
TABLE 4.
Multivariate Cox regression analysis for severe PHI events*
| Severe PHI Event | HR | 95% CI | p Value |
|---|---|---|---|
| 10 vs 7 g/dl hemoglobin transfusion threshold |
2.316 | 1.126–4.765 | 0.02 |
| Initial ICP | 1.044 | 1.024–1.064 | <0.001 |
| Midline shift <5 vs ≥5 mm | 3.662 | 1.471–9.118 | 0.01 |
| CT category diffuse (1 or 2) vs mass lesion |
0.186 | 0.040–0.872 | 0.03 |
| CT category diffuse (3 or 4) vs mass lesion |
0.302 | 0.079–1.163 | 0.08 |
| Surgery vs no surgery | 3.171 | 0.894–11.240 | 0.07 |
Boldface type indicates statistical significance.
Fig. 1.
Kaplan-Meier curves showing the impact of hemoglobin transfusion threshold on the incidence of severe episodes of PHI. TT7 = hemoglobin transfusion threshold of 7 g/dl; TT10 = hemoglobin transfusion threshold of 10 g/dl.
Patients with PHI (median 18.3 days, interquartile range 13.1 days) had a significantly longer length of stay in the intensive care unit as compared with those without PHI (median 14.4 days, interquartile range 12.7 days, p = 0.04, Mann-Whitney U-test). The presence of PHI was associated with unfavorable outcomes at 6 months as defined by the GOS score (42.9% vs 25.5%, respectively; p = 0.02, chi-square test)
Although there was a trend, we did not detect an association between severe PHI and unfavorable GOS outcome at 6 months after injury. Seventy-seven percent of patients with severe PHI, 75% of patients with nonsevere PHI, and 58% with no PHI had unfavorable GOS scores (p = 0.06, Fisher’s exact test).
Discussion
In the present study, 90% of PHI events were observed within 48 hours after injury and progression of cerebral contusions was the most common finding. Maintaining a hemoglobin transfusion threshold of 10 g/dl was associated with a higher risk of severe PHI events. PHI was associated with prolonged length of stay in the intensive care unit as well as worse GOS scores at 6 months.
Role of Transfusion Thresholds
A higher transfusion threshold of 10 g/dl was associated with increased risk of severe PHI in this series. In a previous study, we have shown that a transfusion threshold of 10 g/dl can affect certain hemodynamic outcomes.24 Progressive microvascular failure due to mechanical and cellular damage can contribute to PHI after TBI.9 Blood transfusions can introduce less-deformable erythrocytes and affect cerebral microcirculation.12,13 Increased age of transfused blood, which can cause endothelial activation and stimulation of inflammatory cascades,25 can be ineffective in improving cerebral oxygenation after severe TBI.10 Increased frequency of blood transfusions in the 10 g/dl threshold group may have contributed to increased microvascular injury, and thereby led to an increased incidence of severe PHI.
PHI after severe TBI was associated with poorer clinical outcomes at 6 months.19,20 Additionally, these events are associated with higher mortality rates.2,19 We found that PHI increases the length of stay in the intensive care unit and may contribute to further risk of increased morbidity. These results further emphasize the importance of preventing secondary brain injury in this population. Maintaining a lower hemoglobin transfusion threshold, without compromising cerebral perfusion, may reduce the risk of severe PHI and associated morbidity after severe TBI.
Factors Affecting PHI
A number of factors affect the incidence of PHI after severe TBI.1,4,6,14,22 Older patients, male patients,15 and patients with larger initial lesions4 have been shown to have an increased risk of progression of cerebral contusions. Unlike previous retrospective series,4 the present study showed that a higher initial ICP can increase the risk of PHI. These results lend support to the use of ICP monitoring for severe TBI, with the potential for predicting which patients may develop PHI. We believe the association between midline shift < 5 mm and severe PHI (Table 4) is a spurious result because it is contrary to the univariate analysis and was not consistent across different multivariate models.
Diffuse injury with lesions < 25 cm3 (Marshall classification I–IV) was associated with a lower risk of PHI in our series. Servadei et al.19 showed that progression of diffuse injury to a mass lesion can increase the risk of unfavorable clinical outcomes. Large contusions or intracerebral hematomas after severe TBI are more likely to increase in size on repeat imaging,4 as well as lead to neurological deterioration.14 Peri-contusional tissue demonstrates features of hypoperfusion secondary to microvascular injury18 and this ischemic tissue is at risk for secondary injury. These results support repeat CT after severe TBI, particularly for patients with larger hemorrhagic lesions.
We found that surgery at admission, with or without replacement of the bone flap, showed a trend toward an increased risk of PHI. Decompressive craniectomies have been associated with progression of ipsilateral contusions in 6%–58% of patients as well as development of new contralateral hematomas in 6%–28% of patients. Previous studies have shown no significant mortality benefit for patients undergoing decompressive craniectomy versus craniotomy in patients with acute subdural hematomas.5,11,23 The present study, however, was not sufficiently powered to assess the independent effect on PHI when performing a craniotomy versus a craniectomy.
Strengths and Limitations
The present study utilizes data that were prospectively collected for an RCT and adds to the limited literature available to guide transfusion practices for patients with severe TBI.3 Both clinical and radiological data were recorded to perform a multivariate regression analysis to determine the independent effect of transfusion threshold on PHI after severe TBI. The study was limited partly by missing values for coagulation parameters and platelet counts, although complete data were available for more than 80% of the patients. Although a detailed volumetric analysis may have quantified PHI in this study, our results indicate that maintaining a higher hemoglobin transfusion threshold contributed to clinically important PHI events. Despite the limitations of a secondary analysis, our results highlight the potential detrimental effects of targeting a particular hemoglobin value after severe TBI. Indeed, a combination of physiological parameters measuring cerebral and systemic perfusion as well as oxygenation is likely to be more useful in determining when patients with severe TBI require transfusion.
Conclusions
A higher transfusion threshold of 10 g/dl after severe TBI increased the risk of severe PHI events. These results indicate the potential adverse effect of using a higher transfusion threshold after severe TBI.
Acknowledgments
This study was supported by grant no. P01-NS38660 (National Institute of Neurological Disorders and Stroke, NIH).
Abbreviations
- EPO
erythropoietin
- ER
emergency room
- GCS
Glasgow Coma Scale
- GOS
Glasgow Outcome Scale
- ICP
intracranial pressure
- PHI
progressive hemorrhagic injury
- PT
prothrombin time
- PTT
partial thromboplastin time
- RCT
randomized controlled trial
- TBI
traumatic brain injury
Footnotes
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Robertson. Acquisition of data: Gopinath, Yamal, Robertson. Analysis and interpretation of data: all authors. Drafting the article: Vedantam. Critically revising the article: Gopinath, Vedantam, Yamal, Robertson. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Gopinath. Statistical analysis: Vedantam, Yamal, Rubin. Administrative/technical/material support: Gopinath, Robertson. Study supervision: Gopinath, Robertson.
Previous Presentations
Portions of this work were presented as a poster at the 2015 National Neurotrauma Symposium and the abstract was a finalist for the Ethicon AANS/CNS Clinical Neurotrauma Research Award at this meeting.
References
- 1.Alahmadi H, Vachhrajani S, Cusimano MD. The natural history of brain contusion: an analysis of radiological and clinical progression. J Neurosurg. 2010;112:1139–1145. doi: 10.3171/2009.5.JNS081369. [DOI] [PubMed] [Google Scholar]
- 2.Allard CB, Scarpelini S, Rhind SG, Baker AJ, Shek PN, Tien H, et al. Abnormal coagulation tests are associated with progression of traumatic intracranial hemorrhage. J Trauma. 2009;67:959–967. doi: 10.1097/TA.0b013e3181ad5d37. [DOI] [PubMed] [Google Scholar]
- 3.Boutin A, Chassé M, Shemilt M, Lauzier F, Moore L, Zarychanski R, et al. Red blood cell transfusion in patients with traumatic brain injury: a systematic review protocol. Syst Rev. 2014;3:66. doi: 10.1186/2046-4053-3-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang EF, Meeker M, Holland MC. Acute traumatic intraparenchymal hemorrhage: risk factors for progression in the early post-injury period. Neurosurgery. 2006;58:647–656. doi: 10.1227/01.NEU.0000197101.68538.E6. [DOI] [PubMed] [Google Scholar]
- 5.Chen SH, Chen Y, Fang WK, Huang DW, Huang KC, Tseng SH. Comparison of craniotomy and decompressive craniectomy in severely head-injured patients with acute subdural hematoma. J Trauma. 2011;71:1632–1636. doi: 10.1097/TA.0b013e3182367b3c. [DOI] [PubMed] [Google Scholar]
- 6.Gopinath SP, Robertson CS, Contant CF, Narayan RK, Grossman RG, Chance B. Early detection of delayed traumatic intracranial hematomas using near-infrared spectroscopy. J Neurosurg. 1995;83:438–444. doi: 10.3171/jns.1995.83.3.0438. [DOI] [PubMed] [Google Scholar]
- 7.Hlatky R, Contant CF, Diaz-Marchan P, Valadka AB, Robertson CS. Significance of a reduced cerebral blood flow during the first 12 hours after traumatic brain injury. Neurocrit Care. 2004;1:69–83. doi: 10.1385/NCC:1:1:69. [DOI] [PubMed] [Google Scholar]
- 8.Hosmer JDW, Lemeshow S, Sturdivant RX. Applied Logistic Regression. 3. Hoboken: John Wiley & Sons, Inc.; 2013. [Google Scholar]
- 9.Kurland D, Hong C, Aarabi B, Gerzanich V, Simard JM. Hemorrhagic progression of a contusion after traumatic brain injury: a review. J Neurotrauma. 2012;29:19–31. doi: 10.1089/neu.2011.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leal-Noval SR, Muñoz-Gómez M, Arellano-Orden V, Marín-Caballos A, Amaya-Villar R, Marín A, et al. Impact of age of transfused blood on cerebral oxygenation in male patients with severe traumatic brain injury. Crit Care Med. 2008;36:1290–1296. doi: 10.1097/CCM.0b013e3181692dfc. [DOI] [PubMed] [Google Scholar]
- 11.Li LM, Kolias AG, Guilfoyle MR, Timofeev I, Corteen EA, Pickard JD, et al. Outcome following evacuation of acute subdural haematomas: a comparison of craniotomy with decompressive craniectomy. Acta Neurochir (Wien) 2012;154:1555–1561. doi: 10.1007/s00701-012-1428-8. [DOI] [PubMed] [Google Scholar]
- 12.Marik PE, Sibbald WJ. Effect of stored-blood transfusion on oxygen delivery in patients with sepsis. JAMA. 1993;269:3024–3029. [PubMed] [Google Scholar]
- 13.Morisaki H, Sibbald WJ. Tissue oxygen delivery and the microcirculation. Crit Care Clin. 2004;20:213–223. doi: 10.1016/j.ccc.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Narayan RK, Maas AI, Servadei F, Skolnick BE, Tillinger MN, Marshall LF. Progression of traumatic intracerebral hemorrhage: a prospective observational study. J Neurotrauma. 2008;25:629–639. doi: 10.1089/neu.2007.0385. [DOI] [PubMed] [Google Scholar]
- 15.Oertel M, Kelly DF, McArthur D, Boscardin WJ, Glenn TC, Lee JH, et al. Progressive hemorrhage after head trauma: predictors and consequences of the evolving injury. J Neurosurg. 2002;96:109–116. doi: 10.3171/jns.2002.96.1.0109. [DOI] [PubMed] [Google Scholar]
- 16.Robertson CS. Management of cerebral perfusion pressure after traumatic brain injury. Anesthesiology. 2001;95:1513–1517. doi: 10.1097/00000542-200112000-00034. [DOI] [PubMed] [Google Scholar]
- 17.Robertson CS, Hannay HJ, Yamal JM, Gopinath S, Goodman JC, Tilley BC, et al. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: a randomized clinical trial. JAMA. 2014;312:36–47. doi: 10.1001/jama.2014.6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schröder ML, Muizelaar JP, Bullock MR, Salvant JB, Povlishock JT. Focal ischemia due to traumatic contusions documented by stable xenon-CT and ultrastructural studies. J Neurosurg. 1995;82:966–971. doi: 10.3171/jns.1995.82.6.0966. [DOI] [PubMed] [Google Scholar]
- 19.Servadei F, Murray GD, Penny K, Teasdale GM, Dearden M, Iannotti F, et al. The value of the “worst” computed tomographic scan in clinical studies of moderate and severe head injury. Neurosurgery. 2000;46:70–77. doi: 10.1097/00006123-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Stein SC, Spettell C, Young G, Ross SE. Delayed and progressive brain injury in closed-head trauma: radiological demonstration. Neurosurgery. 1993;32:25–31. doi: 10.1227/00006123-199301000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Utter GH, Shahlaie K, Zwienenberg-Lee M, Muizelaar JP. Anemia in the setting of traumatic brain injury: the arguments for and against liberal transfusion. J Neurotrauma. 2011;28:155–165. doi: 10.1089/neu.2010.1451. [DOI] [PubMed] [Google Scholar]
- 22.Wang MC, Linnau KF, Tirschwell DL, Hollingworth W. Utility of repeat head computed tomography after blunt head trauma: a systematic review. J Trauma. 2006;61:226–233. doi: 10.1097/01.ta.0000197385.18452.89. [DOI] [PubMed] [Google Scholar]
- 23.Woertgen C, Rothoerl RD, Schebesch KM, Albert R. Comparison of craniotomy and craniectomy in patients with acute subdural haematoma. J Clin Neurosci. 2006;13:718–721. doi: 10.1016/j.jocn.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 24.Yamal JM, Rubin ML, Benoit JS, Tilley BC, Gopinath S, Hannay HJ, et al. Effect of hemoglobin transfusion threshold on cerebral hemodynamics and oxygenation. J Neurotrauma. 2015;32:1239–1245. doi: 10.1089/neu.2014.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zallen G, Offner PJ, Moore EE, Blackwell J, Ciesla DJ, Gabriel J, et al. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am J Surg. 1999;178:570–572. doi: 10.1016/s0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]