Methodology
The following guidelines were based on MEDLINE and PUBMED searches of English language literature, in addition to consensus conference proceedings. Levels of evidence and grades of recommendation were assigned for each investigation and treatment, as per the modified Oxford Centre for Evidence-Based Medicine grading system. Where the literature was inconsistent or scarce, a consensus expert opinion was generated to provide treatment guidelines.
Introduction
Terminology
Much confusion regarding the diagnosis of this clinical syndrome is due to many changes in definition and nomenclature since its first description in 1887 by Skene.1 The condition classically known as interstitial cystitis (IC) was reserved for patients with typical cystoscopic findings, such as glomerulations, or the classic bladder wall Hunner’s ulcer.2
Up until 2002, the National Institute for Diabetes and Digestive and Kidney Diseases (NIDDK) criteria were used to define IC.3 However, it was recognized that the NIDDK criteria were designed to delineate a homogeneous population for research trials and were overly restrictive for use in routine clinical practice.4 Therefore, in 2002, the International Continence Society defined painful bladder syndrome (PBS) as: “The complaint of suprapubic pain, related to bladder filling accompanied by other symptoms, such as increased daytime and nighttime frequency, in the absence of proven urinary infection or other obvious pathology” of the lower urinary tract.5 Subsequent to this definition, some used IC to reflect patients who meet the classic NIDDK criteria and PBS to reflect those with identical symptoms, but who did not undergo formal hydrodistension or did not meet all of the NIDDK criteria.
Due to similarities with IC/PBS and other chronic pain syndromes, the European Society for the Study of IC/BPS (ESSIC) provided a new definition, which was more descriptive of the clinical syndrome and the underlying pathology. This expanded term, bladder pain syndrome (BPS), describes all patients with “chronic pelvic pain, pressure, or discomfort, perceived to be related to the urinary bladder accompanied by at least one other urinary symptom: persistent urgency or urinary frequency.”6 To include all patients with bladder pain, in 2010, the International Consultation of Incontinence accepted this revised definition.7 In 2009, the Society for Urodynamics and Female Urology (SUFU) defined the term IC/BPS as “an unpleasant sensation (pain, pressure, discomfort) perceived to be related to the urinary bladder, associated with lower urinary tract symptoms for more than six weeks duration, in the absence of infection or other identifiable causes.” This is the definition used by the American Urological Association (AUA) in the most recent guidelines on IC/BPS. This is the definition that will be referred to for the purpose of this guideline.
The corresponding French terminology is cystite interstitielle, cystalgie à urine claire, or cystalgie abacterienne.
Epidemiology
There is wide variation in reported incidence and prevalence of IC/BPS depending on the criteria used for diagnosis. Current studies estimate that between 2.7 and 6.5% of American women have symptoms consistent with a diagnosis of IC/BPS.8,9 The broad range in incidence depends on whether highly sensitive or highly specific defining criteria are used — further highlighting the need for a standardized diagnostic algorithm.
This translates into approximately 3.3–7.9 million women over the age of 18 years in the U.S. affected by symptoms of IC/BPS. Of these women, however, only 9.7% report being diagnosed with IC/BPS.10 In addition, this study found that women with the diagnosis of IC/BPS were significantly more likely to be uninsured, less likely to be married, and had more children than controls. Of patients with IC, 94% are White and the median age is 40 years.
Although the disease can affect both sexes, approximately 90% are female. In addition, the condition is dramatically under-reported in men. There is significant overlap of symptoms of IC/BPS to those of chronic prostatitis/chronic pelvic pain syndrome, with 17% of men found to have symptoms of both complexes.11
Diagnosis of IC/BPS
1. History (MANDATORY, all patients, Grade C, Level 4 evidence)
A thorough general medical history is of paramount importance to identify typical diagnostic symptoms of IC/BPS and other potential mimicking causative conditions. Unfortunately, delay of diagnosis is common, with an average time of three to seven years from the time of presentation to the general practitioner to diagnosis by a specialist.12,13
The characteristic presentation of IC/BPS includes a combination of pain, frequency, nocturia, and urgency. The onset of symptoms may be gradual and/or with only a single voiding symptom; however, pelvic pain is the main descriptor of IC/BPS.14 In early or milder IC/BPS, patients may not describe frank pain, but rather describe sensations of “pressure,” “burning,” “sharp,” or “uncomfortable sensation of having to urinate.” Typically this sensation is felt in the supra-pubic area, but it can be referred to areas located in the pelvis, including the urethra, vagina, labia, inguinal area, perineum, and/or lower abdomen or back.
The location of pain, relation to bladder filling/emptying duration, and a description of the type of pain can all be useful. Pain that occurs only during voiding is not consistent with IC/BPS, and vulvar disorders, which cause pain when urine makes contact with the vulva, should instead be considered. Symptoms of IC/BPS are generally worse a few days prior to menses, in contrast to endometriosis, which is worse during menses. Patients may describe “flares,” or periods of worsening symptoms, which may be triggered by stress, intercourse, menses, or diet. Common triggers include coffee, alcohol, citrus fruits, tomatoes, carbonated beverages, and spicy foods.15
The most common presenting symptom, however, is frequency, estimated to be 92% of one population.12,16 Urgency is also prevalent, however, cannot distinguish IC/BPS from overactive bladder (OAB).17 Typically, the difference is that patients with IC/BPS void to relieve pain, whereas OAB patients void for fear of incontinence. A good response to antimuscarinics suggests OAB, however, be cautious that this may confound the diagnosis, as the disorders may coexist.
Despite the absence of urinary infection (UTI) being a prerequisite at the time of diagnosis, up to 50% of patients will have a previous history of UTI. It is important to elicit a comprehensive medical history, including past pelvic surgery or radiation, medications that can cause cystitis (nonsteroidal anti-inflammatory drugs, cyclophosphamide, and ketamine), fibromyalgia, depression, sexual dysfunction, autoimmune diseases, allergies, and other gynecological conditions (vulvodynia, endometriosis, dyspareunia). Not only is the past medical history important for diagnosis, but also because many of these conditions may co-exist, further stressing the importance of multidisciplinary management. Table 1 summarizes relevant diseases that may be confused with IC/BPS.
Table 1.
Summary of differential diagnoses
| Disease | How they can be excluded or diagnosed* |
|---|---|
| Endometriosis | Pain worse during menses (vs. few days prior) |
| Non-infectious cystitis | History of radiation, nonsteroidal anti-inflammatory drugs, cyclophosphamide, and/or ketamine use |
| Vulvar disorders | Pain occurs only during voiding, when urine contacts vulva, and/or painful sexual intercourse |
| Overactive bladder | Good response to anti-muscarinics, patient voids to avoid incontinence (vs. to relieve pain); no significant perceived bladder pain |
| Pudendal nerve entrapment | Worse with sitting, positional dependency suggests a neurogenic or musculoskeletal process |
| Prostate-related pain | Pain during or after ejaculation, pain on prostate palpation |
| Pelvic floor disorders | Trigger point, fascial or muscle pain or tenderness, spasm on palpation |
IC/BPS may co-exist.
2. Physical examination (MANDATORY, all patients, Grade C, Level 4 evidence)
The physical exam should include an abdominal and pelvic exam, with particular focus on looking for masses, bladder distension, hernias, and tenderness. A musculoskeletal and focused neurological exam may also be contributory. Although there is no physical finding specific to patients with IC/BPS, suprapubic tenderness and bladder neck point tenderness, in both men and women, is very often noted. In men, tenderness may be elicited by palpating the perineal area between the scrotum and anus; in women, palpating the anterior vaginal wall along the course of the urethra up to the bladder neck may elicit pain.
Peters et al demonstrated an association between IC/BPS and pelvic floor dysfunction in a study of 70 women, with 87% experiencing levator pain during pelvic examination.18 Palpation of the levator muscles in both sexes, looking for tenderness, spasm/tight bands, and/or trigger points, is important for both diagnosis and treatment recommendations; pelvic floor or rectal spasms may respond well to pelvic floor physiotherapy. Hypo or hypersensitivity of the perineum, in combination with a weak or absent anal reflex, may suggest pudendal nerve entrapment.
A digital rectal examination (DRE) in men is essential, noting prostate characteristics along with discrete point tenderness of the prostate and pelvic floor muscles. Prostatic massage could be considered if pain appears to be more related to the prostate. Although the diagnosis of IC/BPS and chronic prostatitis/chronic pelvic pain syndrome in men may overlap, differentiation between prostate-related and bladder-related pain generation may help advise treatment strategies.
The female pelvic exam should screen for vulvodynia, vaginitis, atrophic changes, prolapse, cervical pathology, and adnexal masses or tenderness. Point tenderness, a mass, and expression of pus on palpation of the urethra are classic signs of a urethral diverticulum.
3. Ultrasound/pelvic imaging (OPTIONAL, select patients, Grade C, Level 4 evidence)
Abdominal or pelvic ultrasonography, or other imaging modalities, may be useful when alternative clinical conditions are questioned, but are expected to be normal if IC/BPS is the only diagnosis. The appropriate abdominal/pelvic imaging should be completed for patients with microscopic or macroscopic hematuria.4
4. Frequency volume chart (RECOMMENDED all patients, Grade C, Level 3 evidence) +/− post-void residual (OPTIONAL, when indicated, Grade C, Level 4 evidence)
A frequency volume chart is advocated to differentiate polyuria from the classic small voided volumes expected with IC/BPS. In a study of 47 adult women with IC/BPS, the average voided volume was less than 100mL.19 On average, IC/BPS patients void a volume of urine ranging from 86–174mL, compared to an average of 289 mL in an asymptomatic woman. The average number of daytime voids ranges from 17–25 compared to six.20,21
A voiding diary also helps to determine the severity of the storage symptoms and can be used for positive reinforcement related to behavioural and pharmacological intervention.
When a history of poor emptying is obtained and/or the bladder is palpable on exam, measurement of a post-void residual is recommended.
5. Laboratory tests: Urinalysis, culture (RECOMMENDED all patients, Grade C, Level 4 evidence), cytology (OPTIONAL, when indicated, Grade C, Level 4 evidence)
A urine dipstick represents the minimum required laboratory test for IC/BPS. Glucose, leukocytes, hematuria, nitrites, and osmolality may be simply screened for. Absence of leukocytes does not rule out IC/BPS. If signs of UTI are identified, a culture and sensitivity is required and possibly testing for Chlamydia trachomatis, Mycoplasma, Ureaplasma, Corynebacterium species, Candida species, and Mycoplasma tuberculosis if sterile pyuria persists.
Urine cytology is indicated if microscopic hematuria is identified or if there are other risk factors for urothelial carcinoma present, such as smoking. Hematuria has been reported in up to 41% of patients with IC/BPS (only 2/60 were gross hematuria) and none were associated with a life-threatening urological condition.22
6. Symptom scores (RECOMMENDED, all patients, Grade C, Level 3 evidence)
Symptom scores for IC/BPS are useful to establish baseline symptom severity and to track response to therapeutic intervention. Five self-administered symptom scores for IC/BPS have been assessed to variable extents, including: the Interstitial Cystitis Symptom Index (ICSI); the Interstitial Cystitis Problem Index (ICPI);23 The Wisconsin Interstitial Cystitis scale (UW-IC scale);24 the Pain, Urgency, Frequency score (PUF score);25 and the Bladder Pain/IC Symptom Score (BPIC-SS).26
The UW-IC scale, although well-validated and comprehensive, has not been adopted in clinical practice. The combined ICSI/ICPI (also known as the O’Leary Sant Symptom and Problem Index) consist of a four-item symptom and problem index focusing on urgency, frequency, nocturia, and pain, over the past month. It met standards for variability, test retest reliability, internal consistency, and construct validity, as well as responsiveness.23,27 Further studies have re-evaluated the instrument in larger series of IC patients.27–29
The PUF score has not been subject to as extensive a validation process, but has additional items related to pelvic pain and dyspareunia.25 Kushner et al examined the ability of the PUF score and the ICSI/ICPI to distinguish IC from other urinary tract pathologies in a group of 220 clinic patients. All three scales did distinguish IC, and the PUF score (13 or greater) did so more efficiently.30
The BPIC-SS was developed to address a perceived need to reliably identify IC/BPS patients with moderate to severe pain, for inclusion in clinical trials. Through interviewing patients in various countries, the most common symptoms described include bladder pain, persistent urge to urinate, and high urinary frequency. After analyzing patients’ responses to questionnaires and testing the questions’ validity, the researchers came up with eight items that had strong sensitivity and specificity. The authors concluded that patients with a BPIC-SS score of 19 or greater should be included in clinical trials.26
Clinicians must keep in mind that none of the surveys have sufficient specificity to serve as a sole diagnostic indicator, but rather can be used as tools to assist with diagnosis. Based on current literature, the use of the ICSI, ICPI (or its updated version, the BPIC-SS) and/or the PUF score to grade severity of symptoms and follow response to therapeutic intervention in patients with IC/BPS is recommended.
7. Cystoscopy (RECOMMENDED, all patients, Grade C, Level 3 evidence)
Cystoscopy performed alone, without hydrodistension, is expected to be normal (except for discomfort and reduced “functional” bladder capacity) in the majority of patients with IC/BPS. Hunner’s ulcers or lesions can be found with or without hydrodistension under anesthetic in approximately 16%.31 Hunner’s lesions are associated with more severe symptoms and reduced urodynamic and anesthetic capacity.32,33 The classic findings of terminal hematuria and glomerulations are reliably identified only after a formal hydrodistension under anesthetic. However, evidence shows that glomerulations are neither sensitive nor specific for IC.34
As such, the purpose of cystoscopy alone should only be viewed as a tool to rule out bladder cancer/carcinoma in situ, to identify Hunner’s lesions that reflect severe disease or even different disease (information that may impact treatment decisions), to determine effect on pelvic pain during bladder filling and emptying, to objectively evaluate “functional” bladder capacity, to facilitate appropriate pelvic examination, and to reassure the patient.
The incidence of bladder cancer presenting with symptoms compatible with IC/BPS is rare. Tissot et al35 found 1% of 600 patients referred with a diagnosis of IC/BPS had bladder cancer. Most (5/6) with cancer were older than 60 and all except two had microscopic hematuria or positive cytology.
Cystoscopy may be considered optional in a young woman with symptoms of IC/BPS and no risk factors for bladder cancer or other pelvic conditions. This may enable non-urologist physicians to initiate treatment earlier in the stage of disease, when it is potentially more effective.36 It is reasonable to recommend cystoscopy to assist in making a diagnosis before initiating therapy, especially if there is any indication on history, physical examination, urinalysis, or cytology suggesting that other diseases need to be ruled out. Identification of Hunner’s lesions and pelvic floor muscle dysfunction (pelvic floor examination is easily added to a cystoscopic examination) will direct treatment strategies.
8. Potassium sensitivity test (NOT RECOMMENDED, Grade C, Level 3 evidence)
A potassium chloride bladder permeability test was based on the assumption that a “dysfunctional epithelium” (glycosaminoglycan [GAG] layer)37 allowed potassium ions to cross the abnormally permeable urothelium, depolarize nerves and muscles, and result in pain. The technique comparing subjective pain or urgency responses to intravesically instilled 0.4 M potassium chloride vs. water was described by Parsons et al,37 but has been modified by other authors, including variants such as the comparative cystometrogram test.38
This test could potentially direct therapy if a positive potassium sensitivity test correlated well with a favourable response to agents that attempt to replenish the GAG layer, as has been suggested in two small series.39,40 However, in a phase 4 dosing study for pentosan polysulfate (PPS), an epithelial-directed therapy, no difference in response to PPS was observed.41,42
The sensitivity and specificity of the potassium sensitivity test (69.5% and 50%) were found by Chambers et al to be poor, adding no additional use over history and cystoscopy.43 Others have found that the potassium sensitivity test did not correlate with either cystoscopic findings or bladder capacity on urodynamics.43 Further confounding the diagnosis in symptomatic patients, 25% with OAB (almost all with radiation and IC) and 50–84% with chronic pelvic pain syndrome (CPPS) test positive.37,44 In asymptomatic men, a 36% false-positive rate was found.45
At this point, the use of the potassium sensitivity test has not been widely validated and the ability of this test to predict efficacy with GAG-replenishing therapies is not reliable. It is a costly and painful test, with patients experiencing pain both during and after the procedure. For these reasons, the potassium sensitivity test is no longer recommended as a standard evaluation for IC/BPS.
9. Intravesical anesthetic bladder challenge (OPTIONAL, select patients, Grade C, Level 3 evidence)
An anesthetic challenge test, such as an alkalized lidocaine test, instills 10–20 mL of an anesthetic mixture (in this case, 200 mg lidocaine mixed with 8.4% sodium bicarbonate) into an empty bladder. This fluid is held for 10–15 minutes and then drained by catheter. This test can easily be performed after cystoscopy and can provide both relief to the patient, as well as provide diagnostic information and guide future therapy. A patient experiencing relief from the instillation would provide more certainty that the pain is originating from the bladder. Resolution of the pain by intravesical local anesthesia can be both diagnostic and therapeutic.46
To differentiate between the pain originating from urinary bladder from that of other pelvic organs, Taneja et al treated 22 women with pelvic pain with 20 mL of 2% intravesical lidocaine solution. Sixty-eight percent experienced a reduction of pain by 50% or greater. All non-responders were subsequently diagnosed with non-bladder pathology causing their pelvic pain.47
With no risk of symptom flare, the anesthetic bladder challenge may be considered when there is uncertainty as to whether the pain is originating from the bladder.
10. Hydrodistension (OPTIONAL, select patients, Grade C, Level 3 evidence)
Hydrodistension (HD) under general anesthetic allows for stratification of patients into those with more classic disease associated with ulcers and glomerulations from those with no obvious mucosal abnormalities.3 The technique of diagnostic HD generally involves gravity filling of the bladder at 70–100 cmH20 for a minimum of two minutes, performed under general or regional anesthetic. Maximum anesthetic capacity is determined whereby the inflow backs up in the drip chamber or leakage occurs per urethra despite compression against the cystoscope. While severely reduced anesthetic bladder capacities (<400 mL) do correlate with pain,33 more than 50% of patients with IC/BPS show capacities more than 800 mL.
The presence of terminal hematuria upon draining the infusion fluid and the appearance of petechial submucosal hemorrhages (glomerulations) has been suggested to be characteristic of IC/BPS and is one of the prerequisite findings in the NIDDK criteria.3 Glomerulation severity has also been graded. A possible relationship between glomerulations and angiogenic growth factors has been found, suggesting that these growth factors may have an important role in the pathogenesis of IC/BPS.48
Despite the initial adoption of the HD findings of glomerulations as a criteria for the diagnosis of IC/BPS by the National Institutes of Health (NIH), approximately eight percent with a diagnosis of IC/BPS do not show glomerulations.19,32 The severity of glomerulations was found to correlate poorly with symptoms and with histological evidence of inflammation.49 In contrast, Lamale et al found a strong correlation with pain and HD findings.50 Their series was small (12 patients), including perhaps more severe patients, as evidenced by a mean anesthetic bladder capacity of 604 mL, but represented an untreated cohort where they postulated there were no confounders of treatment allowing a true correlation to be identified. In another series of 84 patients, cystoscopy with HD provided little useful information above and beyond the history and physical examination findings.19
Additionally, the specificity of glomerulations was brought into question when Waxman et al found characteristic glomerulations in 45% of 20 normal women who consented to undergo HD at the time of tubal ligation.51
As the literature is conflicting regarding its utility, HD for diagnostic purposes may be appropriate in certain situations. These may include: when a patient is unable to tolerate cystoscopy under local anesthetic and is having a general anesthetic; when a patient has failed other treatment options and HD to assess disease severity may contribute information to the diagnosis; and when assessing a patient for clinical trial eligibility.
11. Urodynamics (UDS) (NOT RECOMMENDED in the routine evaluation of IC/BPS, Grade C, Level 3 evidence)
Filling cystometrogram (CMG) has been advocated by some for the diagnosis of IC/BPS.3,52 Certainly there is overlap between the conditions of OAB-dry and symptoms of IC/BPS, and the finding of detrusor overactivity (DO) on filling CMG may lead the clinician to initiate therapy with anti-cholinergic agents.
According to the NIDDK criteria, the finding of a capacity >350 mL, first sensation of having to void >150 mL, or the presence of DO are exclusionary for a diagnosis of classic IC.3 However, it is recognized that approximately 15% of patients diagnosed with IC/BPS will demonstrate DO53 and, thus, the coexistence of urge incontinence or DO should not preclude a diagnosis of IC/BPS. Other findings on UDS from the IC database study were a reduced first sensation to void (mean 81 ± 64 mL) and maximum sensory capacity (mean 198 ± 107 mL). While these UDS parameters do correlate well with frequency, nocturia, and urgency, they have not been well-correlated to global pain, cystoscopic findings at HD (other than the presence of a Hunner’s lesions), or results of therapeutic intervention.
Bladder capacity may be assessed less invasively and more cost effectively by means of a frequency volume chart with self-measurement of voided volumes; this has been shown to correlate with maximum cystometric capacity and first sensation of having to void in patients with IC/BPS.33,53 If a cystoscopy under local anesthetic is planned, a functional bladder capacity and its relation to the patient’s pain can be assessed with patient awake.
Pressure flow studies, with or without electromyography, may be useful in some situations where there are coexistent voiding symptoms with suspicion of bladder outlet obstruction or voiding dysfunction due to high-tone pelvic floor dysfunction.
Overall, UDS studies are not recommended in the standard diagnostic evaluation of a patient suspected of having IC/BPS.
12. Bladder biopsy (NOT RECOMMENDED in the routine evaluation of IC/BPS, Grade C, Level 3 evidence)
There are no specific features found on bladder biopsy to confirm a diagnosis of IC/BPS. Findings related to chronic inflammation are not specific, overlapping with other etiologies, and they correlate poorly to cystoscopic findings observed during hydrodistension.49 Between 30%49 and 43%54 of patients with a clinical diagnosis of IC/BPS may have normal histology.
However, correlations have been found with specific types of pathological findings and symptoms. Mucosal denudation (i.e., Hunner’s lesions) and submucosal hemorrhage was highly associated with pain; mast cell count on tryptase stain, complete loss of urothelium, granulation tissue in the lamina propria, and vascular density were associated with nocturia in a multivariate analysis of patients from the IC database.55 The importance of mast cells has been controversial. Dundore et al found no significant difference in mast cell counts in the lamina propria or detrusor on Giemsa-stained sections between IC/BPS patients compared to controls.56 Because an association between specific pathological features and symptoms may exist, it is reasonable to include a bladder biopsy and pathological classification in future research studies.
When a biopsy is indicated for research or to rule out carcinoma in situ if suspected by a focal lesion or abnormal cytology, this should be performed from the most abnormal appearing area and should follow HD to avoid increased risk of bladder perforation.
Routine bladder biopsies are not recommended for the diagnosis of IC/BPS, but may be considered in research trials or to rule out other specific diagnosis, such as carcinoma in situ, when clinically indicated.
Treatment of IC/BPS
The purpose of this guideline is to aid clinicians in the treatment of patients diagnosed with IC/BPS. The main goals of treatment should be maximizing symptomatic control and quality of life while avoiding adverse events and treatment complications, recognizing that there is no curative treatment for this condition. Goals of therapy must be realistic and mutually agreed upon between the physician and the patient. IC/BPS can progress to include symptoms outside the bladder, and identification and treatment of associated conditions with early referral to other specialists for multidisciplinary management is of paramount importance.
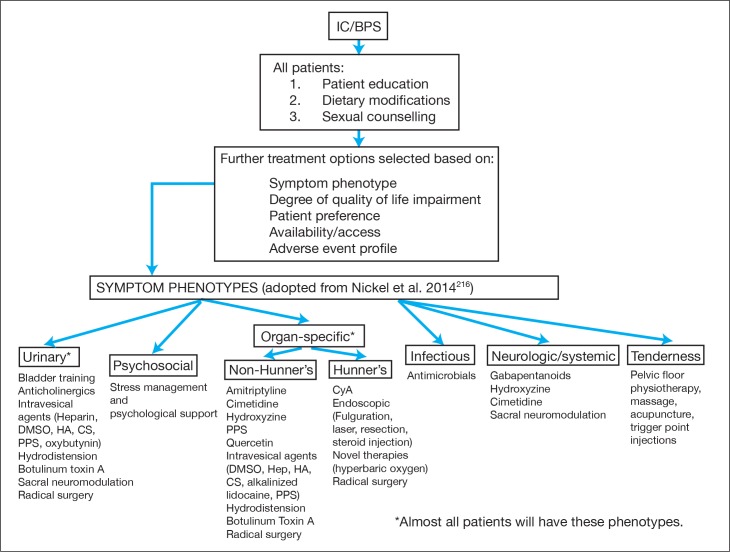
Treatment should be individualized to each patient, with a focus on the specific symptom complex or phenotype of that patient. The application of an algorithmic approach for the treatment of all patients may lead to unsuccessful outcomes. Fig. 1 is provided to aid the clinician with an approach to the treatment of the IC/BPS patient, recognizing that following a single algorithm is not currently appropriate for the treatment of IC/BPS.
Fig. 1.
Proposed management paradigm for the treatment of interstitial cystitis/bladder pain syndrome (IC/BPS); Note: Not intended to be a uniform algorithm, treatment must be individualized; CS: chondroitin sulfate; DMSO: dimethysulfoxide; HA: hyaluronic acid; PPS: pentosan polysulfate.
A. Conservative therapies
1. Patient education (RECOMMENDED in all patients, Grade A) and dietary modifications (RECOMMENDED in all patients, Grade B)
Based on best evidence principles, initial management should focus on conservative strategies. These include patient education, diet and lifestyle changes, and bladder training for all patients. Significant improvement in 45–50% of patients may be expected with only advice and support, as demonstrated in two well-designed, randomized trials.57,58 Bosch et al have developed a practical IC/BPS standard advice checklist to insure all healthcare topics are discussed with the patient.58
Up to 90% of patients have exacerbations of their symptoms after ingesting certain foods or drinks.15,59 Based on survey studies, common food triggers include coffee, tea, citrus fruits, carbonated and alcoholic beverages, bananas, tomatoes, spicy foods, artificial sweeteners, vitamin C, and wheat products.15,59,60 Only one placebo-controlled, randomized, controlled trial (RCT) on the effect of diet in IC/BPS has been published, which failed to report any significant association.61 Dietary modifications, such as a steady intake of water to dilute urine and reduce constipation,62 and an elimination diet trial have been advocated. No standardized protocol exists, but common practice is to instruct patients to avoid all foods on the list for a period varying from one week to three months and then methodically re-introduce one item at a time, with a waiting period of three days to identify potential offenders.60,62
2. Bladder training (RECOMMENDED in motivated patients, Grade B)
Bladder training can be initiated with other lifestyle interventions. The goal is to reduce voiding frequency, potentially increase bladder capacity, and reduce the need to void in response to urgency or pain. Timed voiding or scheduled voiding involves urinating at regular set intervals that disregard the normal urge to void.63 With the urge suppression strategy, patients are instructed to delay urination by gradually increasing the interval from when the urge is felt to when they actually void. Distraction (counting backwards) or relaxation (deep breathing) techniques may be used. The most appropriate protocol is not clear at this point.64 These are quite innocuous, but time-consuming techniques that require a highly motivated patient.65 The effectiveness of such behaviour modification program is supported by prospective data showing symptom improvement for 45–88% of the cohorts.66
3. Stress management techniques and psychological support (RECOMMENDED in patients identified with suffering from stress or psychological dysfunction, Grade B)
Because of its chronic nature, the psychological impact of IC/BPS on the patient’s quality of life should be specifically addressed as an integral part of treatment. A significant number of patients with IC/BPS have reported experiencing depression, anxiety, distress, and various degrees of disability.67 The physician-patient relationship should be emotionally supportive.62 As stress is known to exacerbate symptoms,68 stress-reduction strategies, such as exercising, bathing, reducing working hours, meditation, yoga, and guided imagery62,69 can be beneficial.
Sexual dysfunction should be addressed, as it may worsen IC/BPS symptoms.70 However, treatment of female sexual dysfunction (FSD) is challenging. Management strategies might include counselling, physiotherapy, complementary medications, pharmacologic treatments (hormonal and nonhormonal), or even surgical options.70 Detailed management strategies for FSD are beyond the scope of these guidelines.
Guideline: Based on a large body of literature and the lack of side effects, conservative therapies, including patient education, dietary modifications, bladder retraining, and stress management are recommended as first-line treatment for IC/BPS.
B. Physical therapy techniques
1. Physiotherapy and massage (RECOMMENDED for patients with pelvic floor dysfunction, Grade A)
Many IC/BPS patients have high-tone pelvic floor muscle dysfunction (PFD).71 Those patients who have tenderness on physical exam might benefit from various physical therapy techniques, including: physiotherapy (± biofeedback); myofascial tender points release; or intravaginal Thiele massage. Various techniques have been described that involve skillful, handson maneuvers directed toward relaxation, elongation, stretching, and massaging of tightened muscles. Physical therapists with expertise in pelvic floor muscle relaxation should be involved. Evidence supporting this management option in IC/BPS is more robust, with RCTs and prospective case series reporting moderate or marked improvement of symptoms in 50–62% of patients 72–75 and an additional 21% of patients having complete resolution of symptoms in one study.75
2. Acupuncture (OPTION in motivated patients, Grade B)
Insertion of fine needles into specific points of the body appears to be an effective treatment to alleviate IC/BPS symptoms, according to a systematic review of 23 RCTs.76 However, it was not possible to determine if efficacy was beyond placebo effect due to inconsistencies in protocols across studies. It remains a relatively non-invasive modality that might be used as an adjunct to allopathic medicine.
3. Trigger point injections (OPTION for patients with trigger point pain, Grade D)
Injections of pelvic floor trigger points using a 22 or 25 gauge needle with 1–5 mL of a local anesthetic, with or without glucocorticoid, has also been described, but only anecdotal evidence suggests it may be effective in the treatment of IC/BPS.77
Guideline: Based on Level 1 evidence, pelvic floor physiotherapy can be recommended for patients identified with PFD, while some weak Level 2 evidence suggests that massage techniques, acupuncture, and trigger point injections are options for IC/BPS patients with pelvic floor tenderness.
C. Medical therapies
The only two treatments officially approved by Health Canada for IC/BPS are oral PPS and intravesical dimethysulfoxide (DMSO). All the other treatments discussed are off-label uses. Table 2 provides a summary of the suggested dosages for each treatment option discussed.
Table 2.
IC/BPS treatments
| Medication | Dosage |
|---|---|
| Amitriptyline | 25–75 mg po qhs |
| Cimetidine | 400 mg po bid |
| Hydroxyzine | 10–50 mg po qhs |
| Oral pentosan polysulfate (PPS) | 100 mg po tid |
| Intravesical pentosan polysulfate | 200 mg PPS mixed with 30 mL sterile buffered NS retained for 30–60 minutes |
| Cyclosporine A | 2–3 mg/kg divided bid |
| Gabapentin | 300–2100 mg po divided tid |
| Quercetin | 500 mg po bid |
| Intravesical dimethysulfoxide (DMSO) | 50 mL solution of 50% DMSO (Rimso-50) for 30–60 minutes, once weekly for six weeks; monthly maintenance prn |
| Intravesical heparin | 20 000–40 000 IU of heparin diluted in 10 mL NS for 30–60 minutes, weekly for 4–6 weeks |
| Intravesical hyaluronic acid | 40 mg/50 mL vial (Cystistat®), weekly instillations for 4–12 treatments, then monthly until symptoms resolve |
| Intravesical chondroitin sulfate (CS) | 20 mL vial of 2.0% CS (Uracyst®), retained 30 minutes, weekly for six weeks, then monthly until symptoms resolve |
| Intravesical alkalinized lidocaine | 200 mg lidocaine, alkalinized with a sequential instillation of 8.4% NaHCO3 solution, to a final volume of 10 mL (Urolieve®, PSD597) |
| Intravesical oxybutynin | 10 mg oxybutynin (crushed tablets) diluted in 500 mL NS instilled until first sensation; weekly for six weeks, then monthly for three months |
| Hydrodistension (HD) | Therapeutic HD under spinal or general anesthesia, where the bladder is filled with NS by gravity drainage at a pressure of 80 cm H2O to its capacity and distension is maintained for two to no more than 10 minutes; the bladder is drained at the end and capacity is measured |
| Triamcinolone (steroid) injection for Hunner’s lesions | 1 mL vial of triamcinolone (40 mg/mL) diluted in 9 mL NS (total 10mL), to be injected in aliquots of 1 mL |
| Botulinum toxin A | 100U suburothelial injection ± trigone |
bid: twice daily; IC/BPS: interstitial cystitis/bladder pain syndrome; NaHCO3: sodium bicarbonate; NS: normal saline; po: orally; qhs: every night at bedtime; tid: three times daily.
C1. Oral therapies
1. Amitriptyline (OPTION, Grade B)
Observational studies have shown an improvement in symptoms of IC/BPS with the use of amitriptyline.78,79 Two RCTs have demonstrated a benefit of amitriptyline over placebo.57,80 Van Ophoven et al80 reported a statistically significant improvement in O’Leary-Sant IC symptom and problem index scores from baseline in patients treated with amitriptyline vs. placebo (p=0.05). Overall, 63% vs. 4% of the treatment group vs. placebo group were considered significantly improved at four months followup. Side effects were common, with a reported rate of 92% vs. 21% in the treatment vs. placebo groups, respectively. Most recently, Foster et al57 reported a statistically significant improvement in global response assessment in treatment-naïve patients treated with amitriptyline vs. placebo, but only at a dose of 50 mg or higher (66% vs. 47%, p=0.01). Based on the intention-to-treat analysis, there was no significant difference between groups (55% vs. 45%, p=0.12). Of note, less than 50% of patients tolerated a dose of 50 mg and both groups received standardized education and behavioural modification counselling, which may have contributed to the high response rate in the placebo group. Side effects were common, seen in 88% and 72% of the treatment and placebo groups, respectively.
Guideline: Based on Level 1 and 2 evidence, amitriptyline is an option for the treatment of IC/BPS after conservative therapies have failed.
2. Cimetidine (OPTION, Grade B)
Two very small observational trials81,82 and one placebo-controlled RCT83 have shown an improvement in symptoms of IC/BPS with cimetidine at various dosages. Thilagarajah et al83 randomized 36 patients to cimetidine 400 mg orally twice daily vs. placebo and reported a significant improvement in symptoms in the cimetidine vs. placebo groups, respectively. Suprapubic pain and nocturia were found to be the most improved with cimetidine. No side effects were reported.
Guideline: Based on scarce Level 1, 2, and 3 evidence, cimetidine 400 mg orally twice daily is an option for the treatment of IC/BPS after conservative therapies have failed.
3. Hydroxyzine (OPTION for patients with allergic phenotype, Grade C)
One observational study reported a 40% reduction in symptoms scores and pain compared to baseline.84 One RCT compared placebo vs. hydroxyzine alone vs. PPS alone vs. a combination of hydroxyzine and PPS. There was no significant difference in symptom improvement between the hydroxyzine alone and placebo groups (23% vs. 13%). However, the addition of hydroxyzine to PPS did improve the rate of success compared to PPS alone (40% vs. 28%).85 Side effects were common in all groups and primarily consisted of constitutional symptoms, gastrointestinal symptoms, and pain.
Guideline: There are few studies and conflicting results for the use of hydroxyzine for the treatment of IC/BPS. Observational studies are encouraging and the medication appears safe. Based on Level 3 evidence, hydroxazine may be considered an option (perhaps in patients with an allergy history) after conservative measures have failed.
4. Pentosan polysulfate (PPS) (OPTION, Grade D)
Multiple placebo-controlled RCTs exist comparing PPS to placebo85–89 reporting contradictory results. A meta-analysis including data on 448 patients has summarized the findings of four of these trials.90 The primary outcome of success was defined as a 50% or more improvement in symptoms, including pain, urgency, frequency, and nocturia. The overall success rate of PPS was: pain 37%; urgency 28%; frequency 54%; and nocturia 48%. All were significantly improved over placebo, with the exception of nocturia.
Observational trials have assessed the long-term benefit of PPS with variable results. At a mean followup of 22 months, Alzharani et al91 found that 54.2% of patients treated with PPS reported a >50% improvement in symptoms, whereas Jepsen et al92 reported only 6.2–18.7% of patients maintained a benefit after 18 months of treatment.
Nickel et al93 studied the effect of dose escalation of PPS in a RCT that was not placebo-controlled. They compared 300 mg vs. 600 mg vs. 900 mg of PPS per day in 380 subjects. At 32 weeks, 49.6%, 49.6%, and 45.2% of patients reported a greater than 50% improvement in symptoms, respectively. There was no significant difference between dosages, but importantly, it was found that success rates improved with longer duration of therapy. Common side effects included: diarrhea (25%); headache (18.2%); nausea (15%); pelvic pain (13%); abdominal pain (13%); and alopecia (5%). Twenty-two percent of patients discontinued treatment due to side effects.
Most recently, Nickel et al94 reported the results of 368 patients randomized to placebo vs. PPS 100 mg once daily vs. PPS 100 mg three time daily for 24 weeks. The primary endpoint was defined as a 30% or greater reduction in ICSI total score; 40.7%, 39.8%, and 42.6% of the placebo group, PPS 100 mg once daily, and PPS 100 mg three time daily, respectively, met the primary endpoint with no significant difference between groups.
Guideline: Based on new conflicting Level 1 and 2 evidence, PPS may be offered as an option for the treatment of IC/BPS; however, expected benefits are predicted to be marginal in the majority of patients.
5. Cyclosporine A (CyA) (OPTION as a last resort in patients with inflammation, Grade C)
Multiple observational trials of small sample sizes suggest a positive treatment effect of CyA for IC/BPS.95–98 Patients with Hunner’s lesions seem to derive a better response than those without Hunner’s lesions (68% vs. 30%, respectively).96 A single RCT comparing CyA to PPS showed a significant improvement in IC/BPS symptoms with CyA treatment compared to PPS (59% vs. 13%, p<0.001).99 This trial was not placebo-controlled and side effects occurred in 94% of CyA patients and 56% of PPS patients.
Following the theory that IC/BPS may be caused by an autoimmune/inflammatory reaction, mycophenolate mofetil (MMF) has also been studied in a placebo-controlled RCT. Unfortunately, the study was halted prematurely, but the interim results on 56 patients showed no difference in response between treatment and placebo groups (15% vs. 16%, p=0.67).100
Guideline: Based on Level 3 evidence, CyA may be considered a treatment option for IC/BPS. Close patient monitoring, including blood pressure, Cr and CyA levels are necessary. Due to the potential for serious side effects, CyA should be reserved for severe patients refractory to other treatment options.
6. Gabapentinoids (OPTION in patients with neuropathic pain, Grade C)
Based on success in treating other neuropathic pain conditions, gabapentin has been used for the treatment of IC/BPS. Only two case reports101,102 and three small observational trials exist103–105 The only trial that used gabapentin alone found a 48% improvement in pelvic pain.105
Guideline: Based on scarce Level 3 evidence, gabapentin may be an option in the treatment of IC/BPS refractory to conservative therapies.
7. Quercetin (OPTION, Grade C)
Quercetin has been used to treat male chronic pelvic pain syndrome with success.106 One small observational trial has found a symptomatic improvement in 19/22 patients with IC/BPS after four weeks of Cysta-Q complex (equivalent to quercetin 500 mg orally twice daily).107
Guideline: Based on scarce Level 3 evidence, quercetin may be an option in the treatment of IC/BPS.
C2. Intravesical therapies
Multiple agents have been studied, alone or in combination, for instillation into the bladder for treatment of IC/BPS. Table 3 summarizes various cocktails described in the literature. Treatments may be administered in the clinic setting or at home in some cases. Common side effects include temporary discomfort, hematuria, and UTI. The use of an 8 Fr pediatric feeding tube combined with intraurethral lidocaine may help to improve tolerance to the procedure.108
Table 3.
Intravesical cocktails for IC/BPS
| Ingredients | References |
|---|---|
| 20 mL 0.5% bupivacaine, 20 mL 2% lidocaine jelly, 40 mg triamcinolone, 10–20 000 IU heparin, 80 mg gentamicin | Moldwin217 |
| 8 mL 2% lidocaine, 4 mL 8.4% NaHCO3, 20 000 IU heparin | Welk and Teichman123 |
| 50 mL 0.5% bupivacaine, 50 mL 8.4% NaHCO3 (8.4%), 100 mg hydrocortisone, 10 000 IU heparin, 80 mg gentamicin | Lukban et al.218 |
| 40 mL 0.5% bupivacaine, 10 000 IU heparin, 2 mL dexamethasone, 20 mL NaHCO3 | Mishra219 |
| 50 mL DMSO, 44 mEq (1 amp) NaHCO3, 10 mg triamcinolone, 20 000 IU heparin | Hanno220 |
| 300 mg pentosan polysulfate sodium, 10 mL 2% lidocaine, 10 mL 4.2% NaHCO3; add to this sufficient NaCl 0.9% to reach a total volume of 60 mL | Bade219 |
| 40 000 IU heparin, 8 mL 1% (80 mg) or 2% lidocaine (160 mg), 3 mL 8.4% NaHCO3 suspended in a volume of 15 mL total fluid | Parsons120 |
| 50 mL DMSO, 100 mg hydrocortisone, 10 mL 0.5% bupivacaine, 5 mL NaHCO3 (Optional: add heparin) | Payne219 |
| 5 mL 4% lidocaine followed by 5 mL 8.4% NaHCO3 | Nickel et al.150 |
Adapted from Erickson DR;221 DMSO: dimethysulfoxide; IC/BPS: interstitial cystitis/bladder pain syndrome; NaCl: sodium chloride; NaHCO3: sodium bicarbonate.
1. Dimethylsulfoxide (DMSO) (RECOMMENDED in select patients, Grade B)
DMSO is an organic solvent with anti-inflammatory and analgesic properties. Theoretically, it may cause dissolution of collagen that could potentially cause bladder fibrosis if used on a long-term basis.109 The strongest data supporting its use come from five small RCTs. Perez-Marrero et al compared DMSO to normal saline (NS) and showed a 93% objective improvement and 53% subjective improvement compared to 35% and 18%, respectively, in controls.110 Two RCTs compared DMSO to Bacillus Calmette-Guerin (BCG), one favouring DMSO (30% response rate compared to 10% for BCG, p<0.05)111 and one concluding no benefit with either regimen.112 Finally, two other RCTs compared DMSO to chondroitin sulfate (CS), or to CS plus hyaluronic acid, with significantly better performances of CS groups over DMSO for both objective and subjective outcomes (14–53 % for DMSO against 73% for GAG, p<0.05).113,114 Other observational studies have described use of DMSO in combination with corticoids, bicarbonate, or heparin.52,115–117
Overall, DMSO has a favourable safety profile. Typical side effects include halitosis (garlic-like breath, as it is eliminated through the lungs) and potential flare-up after the first instillation, which usually improves after the second one.118 It is administered as a 50 mL solution of 50% DMSO with a dwell time of 30–60 minutes, once weekly for six weeks. Monthly maintenance doses may be considered.119
Guideline: Based on Level 2 evidence, DMSO is a therapeutic option for IC/BPS.
2. Heparin (RECOMMENDED in select patients, Grade C)
Heparin, as a GAG analogue, may be instilled intravesically with virtually no systemic absorption. It may be used alone by mixing 20 000 to 40 000 IU of heparin diluted in 10 mL NS on a weekly basis for four to six weeks118 and retained for 30–60 minutes. From two prospective, uncontrolled studies, heparin as the sole instillation component rovided symptom improvement for 56–73% of patients at three months with few adverse events reported.120,121 Nowadays, it is most often used within various cocktails (Table 3), mixed with lidocaine, bicarbonate, or other components, making comparisons between studies difficult. Combined with DMSO, it reduced and deferred relapses compared to DMSO alone, for 32% of patients.122 Combined with lidocaine and sodium bicarbonate, three observational studies reported successful outcome for 65–94% of patients at two to four weeks after the last instillation.120,123,124 Lastly, in a multicentre, double-blind, placebo-controlled, crossover trial, Parsons et al showed that a combination of alkalinized lidocaine and heparin provided up to 12 hours of relief from urgency and pain for 42% of patients.125
Guideline: Based on Level 3 evidence, intravesical heparin, alone or in combination, is a therapeutic option for IC/BPS.
3. Hyaluronic acid (HA) (OPTION, Grade C)
HA is thought to help improve or recreate the defective bladder GAG layer in IC/BPS. Several observational studies have reported a wide range of response rates, from 30–87%.39,126–134 Combination therapy with HA and chondroitin sulfate has also shown encouraging results up to three years in small cohorts of patients.135–137
However, the efficacy of intravesical HA has been questioned in three reported, but unpublished RCTs, which found no significant symptom improvement compared to placebo.138–140 Few adverse reactions have been reported except for mild irritative symptoms.
Guideline: Based on published Level 3 evidence, intravesical HA may be considered part of multimodal therapy for IC/BPS. However, it should be kept in mind that three negative trials have been completed without published results.
4. Chondroitin sulfate (CS) (OPTION, Grade D)
In theory, treatment with CS may help replenish the GAG layer of the bladder. A number of small, uncontrolled, single-centre studies have suggested that intravesical CS may ameliorate symptoms in some IC/BPS patients141–143 and a recent prospective, multicentre “real-life” clinical trial further confirmed the potential benefits of this treatment.144 Two underpowered RCTs gathering 163 patients have been recently reported with a tendency for favourable responses with CS compared to placebo (38–39% vs. 23–31%),145,146 but the magnitude of benefit observed could not support its use as a monotherapy of IC/BPS. A higher-power meta-analysis of all patients treated in these studies showed benefit over placebo, but like all intravesical therapies, the magnitude of benefit suggests it should be used only as part of a planned multimodal treatment strategy.147 In a head-to-head small RCT of 15 patients, a low concentration preparation of CS obtained only 17% treatment satisfaction rate compared to 63% with HA.147
Guideline: CS should not be used as monotherapy, but may be considered as part of multimodal therapy for IC/BPS.
5. Lidocaine (RECOMMENDED in select patients, Grade B)
Intravesical lidocaine is better absorbed from the human bladder when alkalinized with sodium bicarbonate.148 Several observational studies have reported therapeutic potential in treatment of acute flares of IC/BPS.120,148,149 In a phase 2 multicentre RCT of 102 patients, Nickel et al reported significant improvement in symptoms compared to placebo after a five-day course of buffered lidocaine three days after last instillation (30% vs. 10%, p=0.012), which was no longer statistically significant at 10 days (24% vs. 12%, p=0.102).150 Electromotive drug administration (EMDA), a modality used to enhance drug absorption by the urothelium through active transport, and the lidocaine-releasing intravesical system (LiRIS), a controlled-release device that prolongs dwell time, have also been recently attempted.151,152
Guideline: Instillation on a daily or weekly basis of alkalinized lidocaine is an option for short-term relief IC/BPS symptoms, primarily bladder pain, based on Level 2 evidence.
6. Resiniferatoxin (RTX) (NOT RECOMMENDED, Grade B)
RTX is a potent analogue of the chili pepper extract capsaicin,153 a neurotoxin that desensitizes C-fiber afferent neurons that transmit pain and, thus, could alleviate pain in IC/BPS.154 In a systematic review, Mourtzoukou et al concluded that results from the six studies currently available (three RCTs, two prospective studies, and one case series) are contradictory regarding the effectiveness of RTX.155 With important tolerability issues, primarily pain following instillation, data are currently insufficient to make a conclusion on its therapeutic efficacy in IC/BPS.
Guideline: Based on conflicting Level 2 evidence and the adverse side effect profile, RTX is not recommended for treatment of IC/BPS.
7. Bacillus Calmette-Guerin (BCG) (NOT RECOMMENDED, Grade B)
BCG instilled into the bladder to stimulate an immunologic response and attenuate symptoms of IC/BPS has been studied. BCG was tested in a large RCT of 265 patients with a response rate of 21% (vs. 12% for placebo, p=0.062).156 Another prospective trial of 30 patients followed for a mean of eight months showed a success rate of 60% compared to 27% for placebo, but did not reach statistical significance (p=0.06).157 In a prospective study of 21 patients with a crossover design, the authors failed to demonstrate any benefit from BCG over DMSO.112
Guideline: Based on Level 2 evidence showing no significant improvement in symptoms and the adverse side effect profile, BCG is not recommended for treatment of IC/BPS
8. Intravesical pentosan polysulfate (PPS) (OPTION, Grade C)
The rationale for intravesical use of PPS, a weak analogue of heparin that may replenish the deficient GAG layer, is that only 1–3% of oral PPS reaches the bladder. It was tested in two small RCTs. Bade et al observed improvement in symptoms for 40% of patients compared to placebo, with rare hematuria reported as the only adverse event.158 Davis et al obtained a 62% response rate at Week 18 for patients treated with both oral and intravesical PPS compared to oral PPS and intravesical NS, with no significant differences in adverse events between treatment groups.159 Finally, in an open-label, uncontrolled study, intravesical PPS encapsulated into liposomes showed efficacy and safety in eight patients over three months.160
Guideline: Based on Level 2 evidence, intravesical PPS, alone or in combination with oral PPS, is a treatment option for IC/BPS.
9. Intravesical oxybutynin (OPTION, Grade C)
In one small RCT, Barbalias et al showed efficacy of intravesical oxybutynin and bladder training compared to bladder training alone (with saline bladder instillations) in improving bladder capacity, frequency, and quality of life scores.161 No adverse events were reported. Intravesical oxybutynin is well-tolerated and has both anticholinergic and anaesthetic properties on the bladder wall.162
Guideline: Based on scarce Level 2 evidence and a favourable side effect profile, intravesical oxybutynin is an option for treatment of IC/BPS.
D. Minimally invasive surgical procedures
1. Hydrodistension (HD) (OPTION, Grade C)
Despite the lack of randomized data to support the use of low-pressure, short-duration HD, it remains one of the most commonly used treatments for IC/BPS.163 Observational studies have shown efficacy rates ranging from 30–54% at one month; 18–56% at two to three months; and 0–37% at five to six months.19,164,165
There is a lack of standardized protocol throughout studies. Complications of HD include flare of symptoms (9%),19 bladder rupture,166 and bladder necrosis.167 Prolonged HD, where the bladder is distended with a balloon from minutes to hours, should be discouraged due to a high complication rate of 20%.153 The long-term complication rate of repeated HD is unknown.
Guideline: Based on Level 3 evidence, short-duration, low-pressure HD is a treatment option for IC/BPS.
2. Treatment of Hunner’s lesions (RECOMMENDED for patients with identified Hunner’s lesions, Grade B)
Several case series have reported various endourologic treatments for Hunner’s lesions. Transurethral resection of Hunner’s lesions with a loop cautery was first described in 1971.168 In the largest series reported by Peeker et al, 90% of 103 patients had symptomatic relief following resection, which lasted more than three years for 40% of patients.169 Fulguration with a Bugbee electrode is another option that led to symptomatic improvement in 76–90% of 150 cases described.170–173 Transurethral coagulation using neodymium:yttrium-aluminum-garnet (Nd:YAG) laser, has been reported in two small observational series with short-term improvements of 78% and 100%.174,175 To prevent bladder or bowel perforation, low bladder filling volume and low-power setting (10–15 W), firing during 1–3 seconds in constant motion until the ulcer is blanched, are recommended.174 Cox et al described direct injection of triamcinolone into the ulcers in 30 patients. They showed a significant improvement for 70%, with a lasting response between 7 and 12 months.176
Complications of these procedures include bladder perforation, hemorrhage, bowel injury,175 and bladder fibrosis.169,172 Lesions tend to recur and retreatment will likely be necessary over time.169,171
Guideline: Based on consistent Level 3 evidence, endoscopic treatment of Hunner’s lesions is recommended for IC/BPS patients with Hunner’s lesions.
3. Botulinum toxin A (BTX-A) (OPTION, Grade C)
Multiple small observational studies have consistently shown a significant improvement in pain, urinary symptoms, and quality of life with intravesical BTX-A.177–181 Two small, double-blind RCTs have been conducted. Manning et al found no significant overall difference between patients randomized to HD plus NS injection vs. HD plus 500 U Dysport®.182 However, Kuo et al reported a 72% vs. 48% success rate in patients randomized to Botox® (100 or 200 U) plus HD vs. HD alone (p=0.032) at three months. 100 U appear to be as effective as 200 U with fewer side effects.183 Unfortunately, it is difficult to comment on the effect of BTX-A alone, as all groups included HD.
The safety and efficacy of repeat BTX-A injections has been shown in observational studies.184–187 The duration (approximately 9–10 months)186 and strength of response seem to be maintained with repeat injections and rate of adverse events low.
Side effects associated with BTX-A include UTI, hematuria, elevated post-void residual, and possible need for temporary clean intermittent catheterization.
Guideline: Based on consistent Level 3 evidence, the use of intravesical BTX-A is an option for the treatment of IC/BPS in patients refractory to other treatments. Repeat injections are safe. Therapy is costly and may not be widely available at all centres. Patients must be counselled on potential side effects, particularly the possibility of urinary retention and need to catheterize.
4. Sacral neuromodulation (SNM) (OPTION, Grade C)
SNM is not yet approved by Health Canada or the FDA for the treatment of IC/BPS, but is indicated for urgency frequency syndrome and urgency urinary incontinence. No RCTs have been completed to assess the effect of SNM on symptoms of IC/BPS. However, multiple observational studies have been reported,188–195 including long-term followup of 86 ± 9.8 months.191 All studies include patients refractory to multiple IC/BPS treatment options.
Based on observational studies, 42–95% of patients experience at least a 50% improvement in overall urinary symptoms, including pain.189,192,193,195 Peters et al192 found a statistically significant decrease in narcotic use postoperatively (from 81.6 to 52 mg/day injectable morphine equivalents, p=0.015) with 4/18 patients stopping narcotic use completely.
Potential side effects of SNM include failure to improve symptoms, painful stimulation, uncomfortable sensations, battery site pain, seroma, infection, mechanical malfunction, and lead migration. There is a surgical revision rate of 27–50%.189,191
Guideline: Based on consistent Level 3 evidence, SNM may be offered as an option for the treatment of IC/BPS to patients who have symptoms refractory to multiple other treatments. Therapy is costly and not widely available at all centres. Patients must be counselled on potential side effects, particularly the need for future surgical revisions.
E. Radical surgery (OPTION for severe refractory patients, Grade C)
Multiple case series exist reporting on the use of invasive surgical techniques for urinary diversion, with or without cystectomy, in severe refractory patients. Supratrigonal cystectomy with augmentation cystoplasty (substitution cystoplasty) has been reported to be beneficial in many series for improving pain, urinary symptoms, and quality of life.196–201 In a recent series, Anderson et al reported 74% of patients were pain-free following urinary diversion and 68% were satisfied. Eight of the 36 patients (22%) who did not have a cystectomy at the original surgery went on to undergo cystectomy for residual symptoms.196 Rossberger et al reported on 47 patients undergoing urinary diversion without cystectomy. A secondary cystectomy was performed in 17% to treat persistent suprapubic pain.200
Based on these retrospective series, patients with identified bladder disease, such as those with Hunner’s lesions199,200 and those with a diminished maximum anesthetic bladder capacity,197,201 were more likely to have improvement in pain and lower urinary tract symptoms postoperatively.
Guideline: Based on Level 3 evidence, major surgery with substitution cystoplasty or urinary diversion ± cystectomy are options for treatment of IC/BPS in patients refractory to all other treatment options with significantly impaired quality of life due to urinary symptoms and pain. Due to the invasiveness of surgery, the benign nature of IC/BPS, and multiple other treatment options available, major surgery should be considered an absolute last resort.
F. Emerging therapies
Novel therapies are emerging for treatment of IC/BPS. Investigational treatments include hyperbaric oxygen, sildenafil, monoclonal antibodies, cannabinoids, and intravesical liposomes.
1. Hyperbaric oxygen (HBO)
HBO has been studied in one case series and two small pilot RCTs. Tanaka et al202 reported successful outcomes in 7/11 patients treated with HBO over 2–4 weeks. Improvements in pain, urgency, frequency, capacity, and symptom scores were maintained for up to 12 months (p<0.05 compared to baseline). Adverse effects included one transient eustachian tube dysfunction and three cases of otitis media. Gallego-Villar et al203 and van Ophoven et al204 both reported small pilot study RCTs. Van Ophoven et al found 3/14 vs. 0/7 responders in the treatment vs. sham groups respectively (p<0.05). Gallego-Villar et al reported success in 10/10 vs. 4/10 patients treated with HBO after DMSO instillations vs. sham treatment after DMSO, respectively. Duration of response was 9.3 vs. 3.1 months in the treatment vs. sham groups (p=0.022).
Guideline: Based on Level 2 and 3 evidence (with small numbers) HBO may be considered in the treatment of IC/BPS for informed patients refractory to other options.
2. Phosphodiesterase-5 (PDE-5) inhibitors
A recent double blind, placebo-controlled RCT compared sildenafil 25 mg orally once daily (n=24) to placebo (n=24) in women with non-ulcerative IC/BPS. They found a significant improvement in symptoms in the treatment vs. placebo arms (P<0.05).205 Side effects were minimal and included flushing and headache.
Guideline: Based on minimal Level 2 evidence, PDE-5 inhibitors may be considered for the treatment of informed patients with IC/BPS.
3. Monoclonal antibodies against TNF-alpha
Adalimumab, an anti-tumour necrosis factor-alpha, has been studied for IC/BPS in a Phase 3 RCT. Adalimumab failed to demonstrate positive proof of concept compared to placebo due to a significant placebo effect.206 Intravenous tanezumab, an anti-nerve growth factor, demonstrated a statistically significant decrease in daily pain score and improvement in global response assessment in a Phase 2 RCT.207 A meta-analysis of all female patients diagnosed with urologic CPPS showed a significant benefit with tanezumab treatment compared to placebo.
Guideline: Monoclonal antibodies are not available or recommended for IC/BPS at this point, but further studies are needed.
4. Cannabinoids
Cannabinoid analgesia is reported for the management of difficult-to-treat pain from chronic illnesses.208,209 Cannabinoids may reduce pain through various interactions with neurotransmitter systems and also have anti-inflammatory and immunomodulatory properties.210 For IC/BPS, only animal studies and case reports are available211–213 but it appears a promising avenue and future studies are needed to assess its efficacy and safety.
Guideline: Use of cannabinoid analgesia is not recommended for IC/BPS at this point, but further studies are needed.
5. Intravesical liposomes
Intravesical liposomes, vesicles of phospholipid bilayers, may serve as a “lotion” for wounded bladder mucosa.214 With an approximately 50% response rate in two observational studies,214,215 it remains investigational, but appears promising for symptomatic flare-ups.215
Guideline: Use of intravesical liposomes is not recommended for IC/BPS at this point, but further studies are needed.
G. Phenotype-directed multimodal therapy (RECOMMENDED for all patients, Grade B)
IC/BPS is a heterogeneous condition challenging to treat for clinicians, with no treatment that is successful for all patients. Patients can be identified with characteristic phenotypic patterns based on proposed mechanisms and symptom complexes and, hopefully in the future, biomarkers. One such categorization of this heterogenous group of patients is the UPOINT phenotypic classification system, which has been described to characterize patients with IC/BPS and guide potential therapies.46,216 Recently, it has been applied as a treatment approach in a prospective, observational trial of 100 patients. At 18.3 months mean followup, a major clinical improvement above baseline was found in 26.9% and a significant improvement in 47.2%; however, it was noted that almost all patients were satisfied with the therapeutic approach.216
A rationale treatment strategy would be to clinically characterize individual patients in regard to symptoms and possible mechanisms and then direct the most appropriate therapy to each of the phenotypic domains.
Conclusion
In conclusion, multiple options exist for the treatment of IC/BPS, ranging from conservative therapies with few side effects to major abdominal surgery. Table 4 outlines the available treatment options based on the data summarized for the purpose of this guideline. An attempt by the consensus panel to identify (when possible) the optimal therapy for each patient has been made. It is the panel’s expert opinion that the traditional and structured tiered monotherapy approach is not the optimal therapeutic strategy. An individualized treatment plan, directed towards that patient’s unique clinical phenotype, based on the recommended diagnostic algorithm, will lead to the best outcomes.216
Table 4.
Summary of guideline for IC/BPS treatment options
| Treatment | Grade | Guideline |
|---|---|---|
| Conservative therapies | ||
| Patient education | A | Recommended in all patients |
| Dietary modifications | B | Recommended in all patients |
| Bladder training | B | Recommended in motivated patients |
| Stress management and psychological support | B | Recommended in patients identified with suffering from stress or psychological dysfunction |
| Physiotherapy and massage | B | Recommended in patients with pelvic floor dysfunction |
| Acupuncture | B | Option in motivated patients |
| Trigger point injections | D | Option for patients with trigger point pain |
| Medical therapies | ||
| Amitriptyline | B | Option |
| Cimetidine | B | Option |
| Hydroxyzine | B | Option for patients with allergic phenotypes |
| Oral pentosan polysulfate | D | Option |
| Cyclosporine A | C | Option as a last resort in patients with inflammation |
| Gabapentinoids | C | Option in patients with neuropathic pain |
| Quercetin | C | Option |
| Intravesical dimethysulfoxide | B | Recommended in selected patients |
| Intravesical heparin | C | Recommended in selected patients |
| Intravesical hyaluronic acid | C | Option |
| Intravesical chondroitin sulfate | D | Option |
| Intravesical alkalinized lidocaine | B | Recommended in selected patients |
| Intravesical resiniferatoxin | B | Not recommended |
| Intravesical Bacillus Calmette-Guerin | B | Not recommended |
| Intravesical pentosan polysulfate | C | Option |
| Intravesical oxybutynin | C | Option |
| Minimally invasive surgical procedures | ||
| Hydrodistension | C | Option |
| Treatment of Hunner’s lesions | B | Recommended for patients with identified Hunner’s lesions |
| Botulinum toxin A | C | Option |
| Sacral neuromodulation | C | Option |
| Radical surgery | C | Option in severe, refractory patients as a last resort |
| Phenotype-directed multimodal therapy | B | Recommended for all patients |
IC/BPS: interstitial cystitis/bladder pain syndrome.
Footnotes
Competing interests: Dr. Cox is has been an Advisory Board member for Asetllas and Ferring; has received grants/honoraria from Astellas and Pfizer; and has participated in clinical trials for Aquinox. Dr. Golda has received grants/honoraria from Astellas and Pfizer. Dr. Nadeau has been an Advisory Board member for Allergan, AMS, Astellas, Ferring, Pfizer, and Red Leaf Medical; a member of the Speakers’ Bureau for Allergan, Astellas, Ferring, Laborie, and Pfizer; and has participated in clinical trials for Astellas and Ipsen. Dr. Nickel has served as a Consultant for Astellas, Auxillium, Eli Lilly, Farr Labs, Ferring, Glaxo-Smith-Kline, Pfizer, Taris Biomedical, Tribute, and Trillium Therapeutics; has been a lecturer for Astellas and Eli Lilly; and has participated in clinical trials for Eli Lilly, Glaxo-Smith-Kline, Johnson & Johnson, Pfizer, and Taris Biomedical. Dr. Carr has been an Advisory Board member for Allergan, Astellas, Gynecare, Janssen, and Pfizer; has been a lecturer for Allergan, Janssen, Pfizer, and Triton. Dr. Corcos has been an Advisory Board member for Allergan, Astellas, and Pfizer; a member of the Speakers’ Bureau for Allergan and Astellas; and has received grants/honoraria from Allergan and Astellas. Dr. Teichman holds investments in Urigen and has participated in clinical trials for Aquinox.
References
- 1.Skene A. Diseases of the bladder and urethra in women. Vol. 167. Wm Wood; New York: 1887. p. 167. [Google Scholar]
- 2.Hunner GL. A rare type of bladder ulcer in women: Report of cases. Boston Med Surg J. 1915;172:660–4. doi: 10.1056/NEJM191505061721802. [DOI] [Google Scholar]
- 3.Gillenwater JY, Wein AJ. Summary of the National Institute of Arthritis, Diabetes, Digestive and Kidney Diseases Workshop on Interstitial Cystitis, National Institutes of Health, Bethesda, Maryland, August 28–29, 1987. J Urol. 1988;140:203–6. doi: 10.1016/s0022-5347(17)41529-1. [DOI] [PubMed] [Google Scholar]
- 4.Hanno PM, Landis JR, Matthews-Cook Y, et al. The diagnosis of interstitial cystitis revisited: Lessons learned from the National Institutes of Health Interstitial Cystitis Database study. J Urol. 1999;161:553–7. doi: 10.1016/S0022-5347(01)61948-7. [DOI] [PubMed] [Google Scholar]
- 5.Abrams P, Cardozo L, Fall M, et al. The standardization of terminology of lower urinary tract function: Report from the Standardization Sub-committee of the International Continence Society. Am J Obstet Gynecol. 2002;187:116–26. doi: 10.1067/mob.2002.125704. [DOI] [PubMed] [Google Scholar]
- 6.van de Merwe JP, Nordling J, Bouchelouche P, et al. Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: An ESSIC proposal. Eur Urol. 2008;53:60–7. doi: 10.1016/j.eururo.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Hanno P, Lin A, Nordling J, et al. Bladder Pain Syndrome Committee of the International Consultation on Incontinence. Neurourol Urodyn. 2010;29:191–8. doi: 10.1002/nau.20847. [DOI] [PubMed] [Google Scholar]
- 8.Berry SH, Bogart LM, Pham C, et al. Development, validation, and testing of an epidemiological case definition of interstitial cystitis/painful bladder syndrome. J Urol. 2010;183:1848–52. doi: 10.1016/j.juro.2009.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konkle KS, Berry SH, Elliott MN, et al. Comparison of an interstitial cystitis/bladder pain syndrome clinical cohort with symptomatic community women from the RAND Interstitial Cystitis Epidemiology study. J Urol. 2012;187:508–12. doi: 10.1016/j.juro.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berry SH, Elliott MN, Suttorp M, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the U.S. J Urol. 2011;186:540–4. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suskind AM, Berry SH, Ewing BA, et al. The prevalence and overlap of interstitial cystitis/bladder pain syndrome and chronic prostatitis/chronic pelvic pain syndrome in men: Results of the RAND Interstitial Cystitis Epidemiology male study. J Urol. 2013;189:141–5. doi: 10.1016/j.juro.2012.08.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito T, Ueda T, Honma Y, et al. Recent trends in patient characteristics and therapeutic choices for interstitial cystitis: Analysis of 282 Japanese patients. Int J Urol. 2007;14:1068–70. doi: 10.1111/j.1442-2042.2007.01863.x. [DOI] [PubMed] [Google Scholar]
- 13.Clemens JQ, Calhoun EA, Litwin MS, et al. A survey of primary care physician practices in the diagnosis and management of women with interstitial cystitis/painful bladder syndrome. Urology. 2010;76:323–8. doi: 10.1016/j.urology.2009.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warren JW, Meyer WA, Greenberg P, et al. Using the International Continence Society’s definition of painful bladder syndrome. Urology. 2006;67:1138–42. doi: 10.1016/j.urology.2006.01.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bassaly R, Downes K, Hart S. Dietary consumption triggers in interstitial cystitis/bladder pain syndrome patients. Female Pelvic Med Reconstr Surg. 2011;17:36–9. doi: 10.1097/SPV.0b013e3182044b5c. [DOI] [PubMed] [Google Scholar]
- 16.Tincello DG, Walker AC. Interstitial cystitis in the UK: Results of a questionnaire survey of members of the Interstitial Cystitis Support Group. Eur J Obstet Gynecol Reprod Biol. 2005;118:91–5. doi: 10.1016/j.ejogrb.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Clemens JQ, Bogart LM, Liu K, et al. Perceptions of urgency in women with interstitial cystitis/bladder pain syndrome or overactive bladder. Neurourol Urodyn. 2011;30:402–5. doi: 10.1002/nau.20974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters KM, Carrico DJ, Kalinowski SE, et al. Prevalence of pelvic floor dysfunction in patients with interstitial cystitis. Urology. 2007;70:16–8. doi: 10.1016/j.urology.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 19.Ottem DP, Teichman JM. What is the value of cystoscopy with hydrodistension for interstitial cystitis? Urology. 2005;66:494–9. doi: 10.1016/j.urology.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Teichman JM, Parsons CL. Contemporary clinical presentation of interstitial cystitis. Urology. 2007;69:41–7. doi: 10.1016/j.urology.2006.08.1111. [DOI] [PubMed] [Google Scholar]
- 21.Koziol JA. Epidemiology of interstitial cystitis. Urol Clin North Am. 1994;21:7–20. [PubMed] [Google Scholar]
- 22.Gomes CM, Sanchez-Ortiz RF, Harris C, et al. Significance of hematuria in patients with interstitial cystitis: Review of radiographic and endoscopic findings. Urology. 2001;57:262–5. doi: 10.1016/S0090-4295(00)00918-3. [DOI] [PubMed] [Google Scholar]
- 23.O’Leary MP, Sant GR, Fowler FJ, Jr, et al. The interstitial cystitis symptom index and problem index. Urology. 1997;49:58–63. doi: 10.1016/S0090-4295(99)80333-1. [DOI] [PubMed] [Google Scholar]
- 24.Keller ML, McCarthy DO, Neider RS. Measurement of symptoms of interstitial cystitis. A pilot study. Urol Clin North Am. 1994;21:67–71. [PubMed] [Google Scholar]
- 25.Parsons CL, Dell J, Stanford EJ, et al. Increased prevalence of interstitial cystitis: Previously unrecognized urologic and gynecologic cases identified using a new symptom questionnaire and intravesical potassium sensitivity. Urology. 2002;60:573–8. doi: 10.1016/S0090-4295(02)01829-0. [DOI] [PubMed] [Google Scholar]
- 26.Humphrey L, Arbuckle R, Moldwin R, et al. The bladder pain/interstitial cystitis symptom score: Development, validation, and identification of a cut score. Eur Urol. 2012;61:271–9. doi: 10.1016/j.eururo.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Propert KJ, Mayer RD, Wang Y, et al. Responsiveness of symptom scales for interstitial cystitis. Urology. 2006;67:55–9. doi: 10.1016/j.urology.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Goin JE, Olaleye D, Peters KM, et al. Psychometric analysis of the University of Wisconsin Interstitial Cystitis Scale: Implications for use in randomized clinical trials. J Urol. 1998;159:1085–90. doi: 10.1016/S0022-5347(01)63840-0. [DOI] [PubMed] [Google Scholar]
- 29.Sirinian E, Azevedo K, Payne CK. Correlation between 2 interstitial cystitis symptom instruments. J Urol. 2005;173:835–40. doi: 10.1097/01.ju.0000152672.83393.61. [DOI] [PubMed] [Google Scholar]
- 30.Kushner L, Moldwin RM. Efficiency of questionnaires used to screen for interstitial cystitis. J Urol. 2006;176:587–92. doi: 10.1016/j.juro.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 31.Peters KM, Killinger KA, Mounayer MH, et al. Are ulcerative and nonulcerative interstitial cystitis/painful bladder syndrome 2 distinct diseases? A study of coexisting conditions. Urology. 2011;78:301–8. doi: 10.1016/j.urology.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 32.Messing E, Pauk D, Schaeffer A, et al. Associations among cystoscopic findings and symptoms and physical examination findings in women enrolled in the Interstitial Cystitis Data Base (ICDB) Study. Urology. 1997;49:81–5. doi: 10.1016/S0090-4295(99)80336-7. [DOI] [PubMed] [Google Scholar]
- 33.Nigro DA, Wein AJ, Foy M, et al. Associations among cystoscopic and urodynamic findings for women enrolled in the Interstitial Cystitis Data Base (ICDB) Study. Urology. 1997;49:86–92. doi: 10.1016/S0090-4295(99)80337-9. [DOI] [PubMed] [Google Scholar]
- 34.Furuya R, Masumori N, Furuya S, et al. Glomerulation observed during transurethral resection of the prostate for patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia is a common finding but no predictor of clinical outcome. Urology. 2007;70:922–6. doi: 10.1016/j.urology.2007.06.1153. [DOI] [PubMed] [Google Scholar]
- 35.Tissot WD, Diokno AC, Peters KM. A referral centre’s experience with transitional cell carcinoma misdiagnosed as interstitial cystitis. J Urol. 2004;172:478–80. doi: 10.1097/01.ju.0000132323.89037.73. [DOI] [PubMed] [Google Scholar]
- 36.Forrest JB, Sebastyanski P, O’Brien-Westbrook M. Observations on the clinical factors affecting the treatment outcomes of interstitial cystitis. Poster presented at: International Pelvic Pain Society (IPPS) Annual Meeting; August 5–7, 2004; Chicago, Ill. [Google Scholar]
- 37.Parsons CL, Greenberger M, Gabal L, et al. The role of urinary potassium in the pathogenesis and diagnosis of interstitial cystitis. J Urol. 1998;159:1862–6. doi: 10.1016/S0022-5347(01)63178-1. [DOI] [PubMed] [Google Scholar]
- 38.Teichman JM, Nielsen-Omeis BJ. Potassium leak test predicts outcome in interstitial cystitis. J Urol. 1999;161:1791–4. doi: 10.1016/S0022-5347(05)68801-5. [DOI] [PubMed] [Google Scholar]
- 39.Gregoire M, Liandier F, Naud A, et al. Does the potassium stimulation test predict cystometric, cystoscopic outcome in interstitial cystitis? J Urol. 2002;168:556–7. doi: 10.1016/S0022-5347(05)64678-2. [DOI] [PubMed] [Google Scholar]
- 40.Daha LK, Riedl CR, Hohlbrugger G, et al. Comparative assessment of maximal bladder capacity, 0.9% NaCl vs. 0.2 M Kcl, for the diagnosis of interstitial cystitis: a prospective controlled study. J Urol. 2003;170:807–9. doi: 10.1097/01.ju.0000081163.46167.82. [DOI] [PubMed] [Google Scholar]
- 41.Gupta SK, Pidcock L, Parr NJ. The potassium sensitivity test: A predictor of treatment response in interstitial cystitis. BJU Int. 2005;96:1063–6. doi: 10.1111/j.1464-410X.2005.05776.x. [DOI] [PubMed] [Google Scholar]
- 42.Parsons CL, Forrest J, Nickel JC, et al. Effect of pentosan polysulfate therapy on intravesical potassium sensitivity. Urology. 2002;59:329–33. doi: 10.1016/S0090-4295(01)01586-2. [DOI] [PubMed] [Google Scholar]
- 43.Chambers GK, Fenster HN, Cripps S, et al. An assessment of the use of intravesical potassium in the diagnosis of interstitial cystitis. J Urol. 1999;162:699–701. doi: 10.1097/00005392-199909010-00018. [DOI] [PubMed] [Google Scholar]
- 44.Hanno P. Is the potassium sensitivity test a valid and useful test for the diagnosis of interstitial cystitis? Against. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:428–9. doi: 10.1007/s00192-005-1306-5. [DOI] [PubMed] [Google Scholar]
- 45.Yilmaz U, Liu YW, Rothman I, et al. Intravesical potassium chloride sensitivity test in men with chronic pelvic pain syndrome. J Urol. 2004;172:548–50. doi: 10.1097/01.ju.0000132411.35214.6c. [DOI] [PubMed] [Google Scholar]
- 46.Nickel JC, Shoskes D, Irvine-Bird K. Clinical phenotyping of women with interstitial cystitis/painful bladder syndrome: A key to classification and potentially improved management. J Urol. 2009;182:155–60. doi: 10.1016/j.juro.2009.02.122. [DOI] [PubMed] [Google Scholar]
- 47.Taneja R. Intravesical lignocaine in the diagnosis of bladder pain syndrome. Int Urogynecol J. 2010;21:321–4. doi: 10.1007/s00192-009-1045-0. [DOI] [PubMed] [Google Scholar]
- 48.Tamaki M, Saito R, Ogawa O, et al. Possible mechanisms inducing glomerulations in interstitial cyst-itis: Relationship between endoscopic findings and expression of angiogenic growth factors. J Urol. 2004;172:945–8. doi: 10.1097/01.ju.0000135009.55905.cb. [DOI] [PubMed] [Google Scholar]
- 49.Denson MA, Griebling TL, Cohen MB, et al. Comparison of cystoscopic and histological findings in patients with suspected interstitial cystitis. J Urol. 2000;164:1908–11. doi: 10.1016/S0022-5347(05)66915-7. [DOI] [PubMed] [Google Scholar]
- 50.Lamale LM, Lutgendorf SK, Hoffman AN, et al. Symptoms and cystoscopic findings in patients with untreated interstitial cystitis. Urology. 2006;67:242–5. doi: 10.1016/j.urology.2005.08.054. [DOI] [PubMed] [Google Scholar]
- 51.Waxman JA, Sulak PJ, Kuehl TJ. Cystoscopic findings consistent with interstitial cystitis in normal women undergoing tubal ligation. J Urol. 1998;160:1663–7. doi: 10.1016/S0022-5347(01)62376-0. [DOI] [PubMed] [Google Scholar]
- 52.Pontari MA, Hanno PM, Wein AJ. Logical and systematic approach to the evaluation and management of patients suspected of having interstitial cystitis. Urology. 1997;49:114–20. doi: 10.1016/S0090-4295(97)00184-2. [DOI] [PubMed] [Google Scholar]
- 53.Kirkemo A, Peabody M, Diokno AC, et al. Associations among urodynamic findings and symptoms in women enrolled in the Interstitial Cystitis Data Base (ICDB) Study. Urology. 1997;49:76–80. doi: 10.1016/S0090-4295(99)80335-5. [DOI] [PubMed] [Google Scholar]
- 54.Mattila J. Vascular immunopathology in interstitial cystitis. Clin Immunol Immunopathol. 1982;23:648–55. doi: 10.1016/0090-1229(82)90327-0. [DOI] [PubMed] [Google Scholar]
- 55.Tomaszewski JE, Landis JR, Russack, et al. Biopsy features are associated with primary symptoms in interstitial cystitis: results from the interstitial cystitis database study. Urology. 2001;57:67–81. doi: 10.1016/S0090-4295(01)01166-9. [DOI] [PubMed] [Google Scholar]
- 56.Dundore PA, Schwartz AM, Semerjian H. Mast cell counts are not useful in the diagnosis of nonulcerative interstitial cystitis. J Urol. 1996;155:885–7. doi: 10.1016/S0022-5347(01)66334-1. [DOI] [PubMed] [Google Scholar]
- 57.Foster HE, Jr, Hanno PM, Nickel JC, et al. Effect of amitriptyline on symptoms in treatment naive patients with interstitial cystitis/painful bladder syndrome. J Urol. 2010;183:1853–8. doi: 10.1016/j.juro.2009.12.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bosch PC. Examination of the significant placebo effect in the treatment of interstitial cystitis/bladder pain syndrome. Urology. 2014;84:321–6. doi: 10.1016/j.urology.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 59.Shorter B, Lesser M, Moldwin RM, et al. Effect of comestibles on symptoms of interstitial cystitis. J Urol. 2007;178:145–52. doi: 10.1016/j.juro.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 60.Friedlander JI, Shorter B, Moldwin RM. Diet and its role in interstitial cystitis/bladder pain syndrome (IC/BPS) and comorbid conditions. BJU Int. 2012;109:1584–91. doi: 10.1111/j.1464-410X.2011.10860.x. [DOI] [PubMed] [Google Scholar]
- 61.Fisher BP, Bavendam TG, Roberts BE. Blinded placebo controlled evaluation on the ingestion of acidic foods and their effect on urinary pH and the symptomatology of interstitial cystitis. J Acad Nutr Diet. 1993;93:A16. doi: 10.1016/0002-8223(93)91040-w. [DOI] [Google Scholar]
- 62.Whitmore KE. Complementary and alternative therapies as treatment approaches for interstitial cystitis. Rev Urol. 2002;4:S28–35. [PMC free article] [PubMed] [Google Scholar]
- 63.Burgio KL, Locher JL, Goode PS. Combined behavioral and drug therapy for urge incontinence in older women. J Am Geriatr Soc. 2000;48:370–4. doi: 10.1111/j.1532-5415.2000.tb04692.x. [DOI] [PubMed] [Google Scholar]
- 64.Smith J. in Incontinence- 4th International Consultation (Health Publications, 2009).
- 65.Arnold J, McLeod N, Thani-Gasalam R, et al. Overactive bladder syndrome - management and treatment options. Aust Fam Physician. 2012;41:878–83. [PubMed] [Google Scholar]
- 66.Chaiken DC, Blaivas JG, Blaivas ST. Behavioural therapy for the treatment of refractory interstitial cystitis. J Urol. 1993;149:1445–8. doi: 10.1016/s0022-5347(17)36411-x. [DOI] [PubMed] [Google Scholar]
- 67.Rabin C, O’Leary A, Neighbors C, et al. Pain and depression experienced by women with interstitial cystitis. Women Health. 2000;31:67–81. doi: 10.1300/J013v31n04_05. [DOI] [PubMed] [Google Scholar]
- 68.Rothrock NE, Lutgendorf SK, Kreder KJ, et al. Stress and symptoms in patients with interstitial cystitis: a life stress model. Urology. 2001;57:422–7. doi: 10.1016/S0090-4295(00)00988-2. [DOI] [PubMed] [Google Scholar]
- 69.Michael YL, Kawachi I, Stampfer MJ, et al. Quality of life among women with interstitial cystitis. J Urol. 2000;164:423–7. doi: 10.1016/S0022-5347(05)67376-4. [DOI] [PubMed] [Google Scholar]
- 70.Liu B, Su M, Zhan H, et al. Adding a sexual dysfunction domain to UPOINT system improves association with symptoms in women with interstitial cystitis and bladder pain syndrome. Urology. 2014;84:1308–13. doi: 10.1016/j.urology.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 71.Bassaly R, Tidwell N, Bertolino S, et al. Myofascial pain and pelvic floor dysfunction in patients with interstitial cystitis. Int Urogynecol J. 2011;22:413–8. doi: 10.1007/s00192-010-1301-3. [DOI] [PubMed] [Google Scholar]
- 72.Fitzgerald MP, Anderson RU, Potts J, et al. Randomized multicenter feasibility trial of myofascial physical therapy for the treatment of urological chronic pelvic pain syndromes. J Urol. 2013;189:S75–85. doi: 10.1016/j.juro.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.FitzGerald MP, Payne CK, Lukacz ES, et al. Randomized multicentre clinical trial of myofascial physical therapy in women with interstitial cystitis/painful bladder syndrome and pelvic floor tenderness. J Urol. 2012;187:2113–8. doi: 10.1016/j.juro.2012.01.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oyama IA, Rejba A, Lukban JC, et al. Modified Thiele massage as therapeutic intervention for female patients with interstitial cystitis and high-tone pelvic floor dysfunction. Urology. 2004;64:862–5. doi: 10.1016/j.urology.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 75.Weiss JM. Pelvic floor myofascial trigger points: manual therapy for interstitial cystitis and the urgency-frequency syndrome. J Urol. 2001;166:2226–31. doi: 10.1016/S0022-5347(05)65539-5. [DOI] [PubMed] [Google Scholar]
- 76.Cummings TM, White AR. Needling therapies in the management of myofascial trigger point pain: a systematic review. Arch Phys Med Rehabil. 2001;82:986–92. doi: 10.1053/apmr.2001.24023. [DOI] [PubMed] [Google Scholar]
- 77.Butrick CW, Sanford D, Hou Q, et al. Chronic pelvic pain syndromes: clinical, urodynamic, and urothelial observations. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:1047–53. doi: 10.1007/s00192-009-0897-7. [DOI] [PubMed] [Google Scholar]
- 78.Hanno PM, Buehler J, Wein AJ. Use of amitriptyline in the treatment of interstitial cystitis. J Urol. 1989;141:846–8. doi: 10.1016/s0022-5347(17)41029-9. [DOI] [PubMed] [Google Scholar]
- 79.van Ophoven A, Hertle L. Long-term results of amitriptyline treatment for interstitial cystitis. J Urol. 2005;174:1837–40. doi: 10.1097/01.ju.0000176741.10094.e0. [DOI] [PubMed] [Google Scholar]
- 80.van Ophoven A, Pokupic S, Heinecke A, et al. A prospective, randomized, placebo controlled, double-blind study of amitriptyline for the treatment of interstitial cystitis. J Urol. 2004;172:533–6. doi: 10.1097/01.ju.0000132388.54703.4d. [DOI] [PubMed] [Google Scholar]
- 81.Dasgupta P, Sharma SD, Womack C, et al. Cimetidine in painful bladder syndrome: A histopathological study. BJU Int. 2001;88:183–6. doi: 10.1046/j.1464-410x.2001.02258.x. [DOI] [PubMed] [Google Scholar]
- 82.Seshadri P, Emerson L, Morales A. Cimetidine in the treatment of interstitial cystitis. Urology. 1994;44:614–6. doi: 10.1016/S0090-4295(94)80074-X. [DOI] [PubMed] [Google Scholar]
- 83.Thilagarajah R, Witherow RO, Walker MM. Oral cimetidine gives effective symptom relief in painful bladder disease: A prospective, randomized, double-blind placebo-controlled trial. BJU Int. 2001;87:207–12. doi: 10.1046/j.1464-410x.2001.02031.x. [DOI] [PubMed] [Google Scholar]
- 84.Theoharides TC, Sant GR. Hydroxyzine therapy for interstitial cystitis. Urology. 1997;49:108–110. doi: 10.1016/S0090-4295(97)00182-9. [DOI] [PubMed] [Google Scholar]
- 85.Sant GR, Propert KJ, Hanno PM, et al. A pilot clinical trial of oral pentosan polysulfate and oral hydroxyzine in patients with interstitial cystitis. J Urol. 2003;170:810–5. doi: 10.1097/01.ju.0000083020.06212.3d. [DOI] [PubMed] [Google Scholar]
- 86.Holm-Bentzen M, Jacobsen F, Nerstrom B, et al. A prospective double-blind clinically controlled multicenter trial of sodium pentosanpolysulfate in the treatment of interstitial cystitis and related painful bladder disease. J Urol. 1987;138:503–7. doi: 10.1016/s0022-5347(17)43241-1. [DOI] [PubMed] [Google Scholar]
- 87.Mulholland SG, Hanno P, Parsons CL, et al. Pentosan polysulfate sodium for therapy of interstitial cystitis. A double-blind placebo-controlled clinical study. Urology. 1990;35:552–8. doi: 10.1016/0090-4295(90)80116-5. [DOI] [PubMed] [Google Scholar]
- 88.Parsons CL, Benson G, Childs SJ, et al. A quantitatively controlled method to study prospectively interstitial cystitis and demonstrate the efficacy of pentosanpolysulfate. J Urol. 1993;150:845–8. doi: 10.1016/s0022-5347(17)35629-x. [DOI] [PubMed] [Google Scholar]
- 89.Parsons CL, Mulholland SG. Successful therapy of interstitial cystitis with pentosanpolysulfate. J Urol. 1987;138:513–6. doi: 10.1016/s0022-5347(17)43243-5. [DOI] [PubMed] [Google Scholar]
- 90.Hwang P, Auclair B, Beechinor D, et al. Efficacy of pentosan polysulfate in the treatment of interstitial cyst-itis: a meta-analysis. Urology. 1997;50:39–43. doi: 10.1016/S0090-4295(97)00110-6. [DOI] [PubMed] [Google Scholar]
- 91.Al-Zahrani AA, Gajewski JB. Long-term efficacy and tolerability of pentosan polysulphate sodium in the treatment of bladder pain syndrome. Can Urol Assoc J. 2011;5:113–8. doi: 10.5489/cuaj.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jepsen JV, Sall M, Rhodes PR, et al. Long-term experience with pentosanpolysulfate in interstitial cystitis. Urology. 1998;51:381–7. doi: 10.1016/S0090-4295(97)00714-0. [DOI] [PubMed] [Google Scholar]
- 93.Nickel JC, Barkin J, Forrest J, et al. Randomized, double-blind, dose-ranging study of pentosan polysulfate sodium for interstitial cystitis. Urology. 2005;65:654–8. doi: 10.1016/j.urology.2004.10.071. [DOI] [PubMed] [Google Scholar]
- 94.Nickel JC, Herschorn S, Whitmore KE, et al. Pentosan polysulfate sodium for treatment of interstitial cystitis/bladder pain syndrome: Insights from a randomized, double-blind, placebo controlled study. J Urol. 2015;193:857–62. doi: 10.1016/j.juro.2014.09.036. [DOI] [PubMed] [Google Scholar]
- 95.Ehren I, Hallen Grufman K, Vrba M, et al. Nitric oxide as a marker for evaluation of treatment effect of cyclosporine A in patients with bladder pain syndrome/interstitial cystitis type 3C. Scand J Urol. 2013;47:503–8. doi: 10.3109/21681805.2013.788552. [DOI] [PubMed] [Google Scholar]
- 96.Forrest JB, Payne CK, Erickson DR. Cyclosporine A for refractory interstitial cystitis/bladder pain syndrome: Experience of 3 tertiary centres. J Urol. 2012;188:1186–91. doi: 10.1016/j.juro.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 97.Forsell T, Ruutu M, Isoniemi H, et al. Cyclosporine in severe interstitial cystitis. J Urol. 1996;155:1591–3. doi: 10.1016/S0022-5347(01)66137-8. [DOI] [PubMed] [Google Scholar]
- 98.Sairanen J, Forsell T, Ruutu M. Long-term outcome of patients with interstitial cystitis treated with low dose cyclosporine A. J Urol. 2004;171:2138–41. doi: 10.1097/01.ju.0000125139.91203.7a. [DOI] [PubMed] [Google Scholar]
- 99.Sairanen J, Tammela TL, Leppilahti M, et al. Cyclosporine A and pentosan polysulfate sodium for the treatment of interstitial cystitis: a randomized comparative study. J Urol. 2005;174:2235–8. doi: 10.1097/01.ju.0000181808.45786.84. [DOI] [PubMed] [Google Scholar]
- 100.Yang CC, Burks DA, Propert KJ, et al. Early termination of a trial of mycophenolate mofetil for treatment of interstitial cystitis/painful bladder syndrome: Lessons learned. J Urol. 2011;185:901–6. doi: 10.1016/j.juro.2010.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hansen HC. Interstitial cystitis and the potential role of gabapentin. South Med J. 2000;93:238–42. doi: 10.1097/00007611-200093020-00021. [DOI] [PubMed] [Google Scholar]
- 102.Takatani J, Takeshima N, Okuda K, et al. A case of perineal pain related to interstitial cystitis which was supposed to be relieved with gabapentin. J Anesth. 2009;23:474–5. doi: 10.1007/s00540-009-0774-z. [DOI] [PubMed] [Google Scholar]
- 103.Kwon WA, Ahn SH, Oh TH, et al. Effect of low-dose triple therapy using gabapentin, amitriptyline, and a nonsteroidal anti-inflammatory drug for overactive bladder symptoms in patients with bladder pain syndrome. Int Neurourol J. 2013;17:78–82. doi: 10.5213/inj.2013.17.2.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee JW, Han DY, Jeong HJ. Bladder pain syndrome treated with triple therapy with gabapentin, amitriptyline, and a nonsteroidal anti-inflammatory drug. Int Neurourol J. 2010;14:256–60. doi: 10.5213/inj.2010.14.4.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sasaki K, Smith CP, Chuang YC, et al. Oral gabapentin (neurontin) treatment of refractory genitourinary tract pain. Tech Urol. 2001;7:47–9. [PubMed] [Google Scholar]
- 106.Shoskes DA, Nickel JC. Quercetin for chronic prostatitis/chronic pelvic pain syndrome. Urol Clin North Am. 2011;38:279–84. doi: 10.1016/j.ucl.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 107.Katske F, Shoskes DA, Sender M, et al. Treatment of interstitial cystitis with a quercetin supplement. Tech Urol. 2001;7:44–6. [PubMed] [Google Scholar]
- 108.Butrick CW. Interstitial cystitis/bladder pain syndrome: Management of the pain disorder: A urogynecology perspective. Urol Clin North Am. 2012;39:377–87. doi: 10.1016/j.ucl.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 109.Sant GR, LaRock DR. Standard intravesical therapies for interstitial cystitis. Urol Clin North Am. 1994;21:73–83. [PubMed] [Google Scholar]
- 110.Perez-Marrero R, Emerson LE, Feltis JT. A controlled study of dimethyl sulfoxide in interstitial cystitis. J Urol. 1988;140:36–9. doi: 10.1016/s0022-5347(17)41478-9. [DOI] [PubMed] [Google Scholar]
- 111.Sairanen J, Leppilahti M, Tammela TL, et al. Evaluation of health-related quality of life in patients with painful bladder syndrome/interstitial cystitis and the impact of four treatments on it. Scand J Urol Nephrol. 2009;43:212–9. doi: 10.1080/00365590802671031. [DOI] [PubMed] [Google Scholar]
- 112.Peeker R, Haghsheno MA, Holmang S, et al. Intravesical bacillus Calmette-Guerin and dimethyl sulfoxide for treatment of classic and nonulcer interstitial cystitis: a prospective, randomized double-blind study. J Urol. 2000;164:1912–5. doi: 10.1016/S0022-5347(05)66916-9. [DOI] [PubMed] [Google Scholar]
- 113.De Ridder DR, Plancke H, Ost D. A prospective, randomized, controlled, multicentre trial comparing DMSO and chondroitin sulfate 2 for painful bladder syndrome/interstitial cystitis. Neurourol Urodyn. 2013;32:691–2. [Google Scholar]
- 114.Cervigni M, Porru D, Ostardo E, et al. A randomized, open-label, multicentre study of efficacy and safety of intravesical hyaluronic acid and chondroitin sulfate vs. DMSO in women with bladder pain syndrome/interstitial cystits. Neurourol Urodyn. 2014;33:665. doi: 10.1002/nau.23091. [DOI] [PubMed] [Google Scholar]
- 115.Ghoniem GM, McBride D, Sood OP, et al. Clinical experience with multiagent intravesical therapy in interstitial cystitis patients unresponsive to single-agent therapy. World J Urol. 1993;11:178–82. doi: 10.1007/bf00211416. [DOI] [PubMed] [Google Scholar]
- 116.Parkin J, Shea C, Sant GR. Intravesical dimethyl sulfoxide (DMSO) for interstitial cystitis—a practical approach. Urology. 1997;49:105–7. doi: 10.1016/S0090-4295(97)00181-7. [DOI] [PubMed] [Google Scholar]
- 117.Gafni-Kane A, Botros SM, Du H, et al. Measuring the success of combined intravesical dimethyl sulfoxide and triamcinolone for treatment of bladder pain syndrome/interstitial cystitis. Int Urogynecol J. 2013;24:303–11. doi: 10.1007/s00192-012-1832-x. [DOI] [PubMed] [Google Scholar]
- 118.Mouracade P, Saussine C. [Interstitial cystitis in 2008] Prog Urol. 2008;18:418–25. doi: 10.1016/j.purol.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 119.DR Erickson. Bladder pain syndrome: Current terminology, diagnosis, and treatment. AUA Update Series. 2009;28 [Google Scholar]
- 120.Parsons CL. Successful downregulation of bladder sensory nerves with combination of heparin and alkalinized lidocaine in patients with interstitial cystitis. Urology. 2005;65:45–8. doi: 10.1016/j.urology.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 121.Kuo HC. Urodynamic results of intravesical heparin therapy for women with frequency urgency syndrome and interstitial cystitis. J Formos Med Assoc. 2001;100:309–14. [PubMed] [Google Scholar]
- 122.Perez-Marrero R, Emerson LE, Maharajh DO, et al. Prolongation of response to DMSO by heparin maintenance. Urology. 1993;41:64–6. doi: 10.1016/0090-4295(93)90198-J. [DOI] [PubMed] [Google Scholar]
- 123.Welk BK, Teichman JM. Dyspareunia response in patients with interstitial cystitis treated with intravesical lidocaine, bicarbonate, and heparin. Urology. 2008;71:67–70. doi: 10.1016/j.urology.2007.09.067. [DOI] [PubMed] [Google Scholar]
- 124.Nomiya A, Naruse T, Niimi A, et al. On- and post-treatment symptom relief by repeated instillations of heparin and alkalized lidocaine in interstitial cystitis. Int J Urol. 2013;20:1118–22. doi: 10.1111/iju.12120. [DOI] [PubMed] [Google Scholar]
- 125.Parsons CL, Zupkas P, Proctor J, et al. Alkalinized lidocaine and heparin provide immediate relief of pain and urgency in patients with interstitial cystitis. J Sex Med. 2012;9:207–12. doi: 10.1111/j.1743-6109.2011.02542.x. [DOI] [PubMed] [Google Scholar]
- 126.Riedl CR, Engelhardt PF, Daha KL, et al. Hyaluronan treatment of interstitial cystitis/painful bladder syndrome. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:717–21. doi: 10.1007/s00192-007-0515-5. [DOI] [PubMed] [Google Scholar]
- 127.Daha LK, Riedl CR, Lazar D, et al. Do cystometric findings predict the results of intravesical hyaluronic acid in women with interstitial cystitis? Eur Urol. 2005;47:393–7. doi: 10.1016/j.eururo.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 128.Morales A, Emerson L, Nickel JC, et al. Intravesical hyaluronic acid in the treatment of refractory interstitial cystitis. J Urol. 1996;156:45–8. doi: 10.1016/S0022-5347(01)65933-0. [DOI] [PubMed] [Google Scholar]
- 129.Kallestrup EB, Jorgensen SS, Nordling J, et al. Treatment of interstitial cystitis with Cystistat: A hyaluronic acid product. Scand J Urol Nephrol. 2005;39:143–7. doi: 10.1080/00365590410015876-1. [DOI] [PubMed] [Google Scholar]
- 130.Kim A, Lim B, Song M, et al. Pretreatment features to influence effectiveness of intravesical hyaluronic Acid instillation in refractory interstitial cystitis/painful bladder syndrome. Int Neurourol J. 2014;18:163–7. doi: 10.5213/inj.2014.18.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Leppilahti M, Hellstrom P, Tammela TL. Effect of diagnostic hydrodistension and four intravesical hyaluronic acid instillations on bladder ICAM-1 intensity and association of ICAM-1 intensity with clinical response in patients with interstitial cystitis. Urology. 2002;60:46–51. doi: 10.1016/S0090-4295(02)01613-8. [DOI] [PubMed] [Google Scholar]
- 132.Porru D, Campus G, Tudino D, et al. Results of treatment of refractory interstitial cystitis with intravesical hyaluronic acid. Urol Int. 1997;59:26–9. doi: 10.1159/000283012. [DOI] [PubMed] [Google Scholar]
- 133.Favre G, Gonzalez M, Zubieta M, et al. Experience of endovesical hyaluronic acid in the treatment of interstitial cystitis/painful bladder syndrome. International Continence Society – Annual Meeting, Barcelona, Spain, 2013, Non-Moderated Poster 666.
- 134.Sambandan N, Briggs K, Sutherland S, et al. The efficacy and safety of intravesical hyaluronic acid in patients with interstitial cystitis/painful bladder syndrome: practice experience. International Continence Society – Annual Meeting, Barcelona, Spain, 2013, Non-Moderated Poster 791.
- 135.Gulpinar O, Kayis A, Suer E, et al. Clinical comparision of intravesical hyaluronic acid and hyaluronic acid-chondroitin sulphate therapy for patients with bladder pain syndrome/interstitital cystitis. Can Urol Assoc J. 2014;8:E610–4. doi: 10.5489/cuaj.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Porru D, Leva F, Parmigiani A, et al. Impact of intravesical hyaluronic acid and chondroitin sulfate on bladder pain syndrome/interstitial cystitis. Int Urogynecol J. 2012;23:1193–9. doi: 10.1007/s00192-011-1546-5. [DOI] [PubMed] [Google Scholar]
- 137.Cervigni M, Natale F, Nasta L, et al. Intravesical hyaluronic acid and chondroitin sulphate for bladder pain syndrome/interstitial cystitis: long-term treatment results. Int Urogynecol J. 2012;23:1187–92. doi: 10.1007/s00192-012-1742-y. [DOI] [PubMed] [Google Scholar]
- 138.Chintea CL, Belal M. Is there enough evidence for the use of intravesical instillations of glycosaminoglycan analogues in interstitial cystitis? BJU Int. 2013;111:192–3. doi: 10.1111/j.1464-410X.2012.11635.x. [DOI] [PubMed] [Google Scholar]
- 139.Chintea CL, Belal M. Is there enough evidence for the use of intravesical instillations of glycosaminoglycan analogues in interstitial cystitis? BJU Int. 2013;111:192–3. doi: 10.1111/j.1464-410X.2012.11635.x. [DOI] [PubMed] [Google Scholar]
- 140.Chintea CL, Belal M. Is there enough evidence for the use of intravesical instillations of glycosaminoglycan analogues in interstitial cystitis? BJU Int. 2013;111:192–3. doi: 10.1111/j.1464-410X.2012.11635.x. [DOI] [PubMed] [Google Scholar]
- 141.Nordling J, van Ophoven A. Intravesical glycosaminoglycan replenishment with chondroitin sulphate in chronic forms of cystitis. A multinational, multicentre, prospective observational clinical trial. Arzneimittelforschung. 2008;58:328–35. doi: 10.1055/s-0031-1296515. [DOI] [PubMed] [Google Scholar]
- 142.Steinhoff G. The efficacy of chondroitin sulphate in treating interstitial cystitis. Eur Urol Suppl. 2003:14–6. [PubMed] [Google Scholar]
- 143.Sorensen RB. Chondroitin sulphate in the treatment of interstitial cystitis and chronic inflammatory disease of the urinary bladder. Eur Urol. 2003:16–8. [Google Scholar]
- 144.Nickel JC, Egerdie B, Downey J, et al. A real-life multicentre clinical practice study to evaluate the efficacy and safety of intravesical chondroitin sulphate for the treatment of interstitial cystitis. BJU Int. 2009;103:56–60. doi: 10.1111/j.1464-410X.2008.08028.x. [DOI] [PubMed] [Google Scholar]
- 145.Nickel JC, Egerdie RB, Steinhoff G, et al. A multicentre, randomized, double-blind, parallel group pilot evaluation of the efficacy and safety of intravesical sodium chondroitin sulfate versus vehicle control in patients with interstitial cystitis/painful bladder syndrome. Urology. 2010;76:804–9. doi: 10.1016/j.urology.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 146.Nickel JC, Hanno P, Kumar K, et al. Second multicentre, randomized, double-blind, parallel-group evaluation of effectiveness and safety of intravesical sodium chondroitin sulfate compared with inactive vehicle control in subjects with interstitial cystitis/bladder pain syndrome. Urology. 2012;79:1220–4. doi: 10.1016/j.urology.2012.01.059. [DOI] [PubMed] [Google Scholar]
- 147.Thakkinstian A, Nickel JC. Efficacy of intravesical chondroitin sulphate in treatment of interstitial cystitis/bladder pain syndrome (IC/BPS): Individual patient data (IPD) meta-analytical approach. Can Urol Assoc J. 2013;7:195–200. doi: 10.5489/cuaj.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Henry R, Patterson L, Avery N, et al. Absorption of alkalized intravesical lidocaine in normal and inflamed bladders: a simple method for improving bladder anesthesia. J Urol. 2001;165:1900–3. doi: 10.1016/S0022-5347(05)66238-6. [DOI] [PubMed] [Google Scholar]
- 149.Henry RA, Patterson L, Nickel C, et al. Alkalinized intravesical lidocaine to treat interstitial cystitis: absorption kinetics in normal and interstitial cystitis bladders. Urology. 2001;57:119. doi: 10.1016/S0090-4295(01)01069-X. [DOI] [PubMed] [Google Scholar]
- 150.Nickel JC, Moldwin R, Lee S, et al. Intravesical alkalinized lidocaine (PSD597) offers sustained relief from symptoms of interstitial cystitis and painful bladder syndrome. BJU Int. 2009;103:910–8. doi: 10.1111/j.1464-410X.2008.08162.x. [DOI] [PubMed] [Google Scholar]
- 151.Nickel JC, Jain P, Shore N, et al. Continuous intravesical lidocaine treatment for interstitial cystitis/bladder pain syndrome: safety and efficacy of a new drug delivery device. Sci Transl Med. 2012;4:143r. doi: 10.1126/scitranslmed.3003804. a100. [DOI] [PubMed] [Google Scholar]
- 152.Rosamilia A, Dwyer PL, Gibson J. Electromotive drug administration of lidocaine and dexamethasone followed by cystodistension in women with interstitial cystitis. Int Urogynecol J Pelvic Floor Dysfunct. 1997;8:142–5. doi: 10.1007/BF02764846. [DOI] [PubMed] [Google Scholar]
- 153.Fall M, Oberpenning F, Peeker R. Treatment of bladder pain syndrome/interstitial cystitis 2008: Can we make evidence-based decisions? Eur Urol. 2008;54:65–75. doi: 10.1016/j.eururo.2008.03.086. [DOI] [PubMed] [Google Scholar]
- 154.Vij M, Srikrishna S, Cardozo L. Interstitial cystitis: diagnosis and management. Eur J Obstet Gynecol Reprod Biol. 2012;161:1–7. doi: 10.1016/j.ejogrb.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 155.Mourtzoukou EG, Iavazzo C, Falagas ME. Resiniferatoxin in the treatment of interstitial cystitis: A systematic review. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1571–6. doi: 10.1007/s00192-008-0663-2. [DOI] [PubMed] [Google Scholar]
- 156.Mayer R, Propert KJ, Peters KM, et al. A randomized controlled trial of intravesical Bacillus Calmette-Guerin for treatment refractory interstitial cystitis. J Urol. 2005;173:1186–91. doi: 10.1097/01.ju.0000152337.82806.e8. [DOI] [PubMed] [Google Scholar]
- 157.Peters K, Diokno A, Steinert B, et al. The efficacy of intravesical Tice strain bacillus Calmette-Guerin in the treatment of interstitial cystitis: A double-blind, prospective, placebo controlled trial. J Urol. 1997;157:2090–4. doi: 10.1016/S0022-5347(01)64682-2. [DOI] [PubMed] [Google Scholar]
- 158.Bade JJ, Laseur M, Nieuwenburg A, et al. A placebo-controlled study of intravesical pentosanpolysulphate for the treatment of interstitial cystitis. Br J Urol. 1997;79:168–71. doi: 10.1046/j.1464-410X.1997.03384.x. [DOI] [PubMed] [Google Scholar]
- 159.Davis EL, El Khoudary SR, Talbott EO, et al. Safety and efficacy of the use of intravesical and oral pentosan polysulfate sodium for interstitial cystitis: a randomized double-blind clinical trial. J Urol. 2008;179:177–85. doi: 10.1016/j.juro.2007.08.170. [DOI] [PubMed] [Google Scholar]
- 160.Lander EB, See JR. Intravesical instillation of pentosan polysulfate encapsulated in a liposome nanocarrier for interstitial cystitis. Am J Clin Exp Urol. 2014;2:145–8. [PMC free article] [PubMed] [Google Scholar]
- 161.Barbalias GA, Liatsikos EN, Athanasopoulos A, et al. Interstitial cystitis: bladder training with intravesical oxybutynin. J Urol. 2000;163:1818–22. doi: 10.1016/S0022-5347(05)67551-9. [DOI] [PubMed] [Google Scholar]
- 162.De Wachter S, Wyndaele JJ. Intravesical oxybutynin: a local anesthetic effect on bladder C afferents. J Urol. 2003;169:1892–5. doi: 10.1097/01.ju.0000049903.60057.4b. [DOI] [PubMed] [Google Scholar]
- 163.Rovner E, Propert KJ, Brensinger C, et al. Treatments used in women with interstitial cystitis: the interstitial cystitis data base (ICDB) study experience. The Interstitial Cystitis Data Base Study Group. Urology. 2000;56:940–5. doi: 10.1016/S0090-4295(00)00845-1. [DOI] [PubMed] [Google Scholar]
- 164.Aihara K, Hirayama A, Tanaka N, et al. Hydrodistension under local anesthesia for patients with suspected painful bladder syndrome/interstitial cystitis: safety, diagnostic potential and therapeutic efficacy. Int J Urol. 2009;16:947–52. doi: 10.1111/j.1442-2042.2009.02396.x. [DOI] [PubMed] [Google Scholar]
- 165.Cole EE, Scarpero HM, Dmochowski RR. Are patient symptoms predictive of the diagnostic and/or therapeutic value of hydrodistention? Neurourol Urodyn. 2005;24:638–42. doi: 10.1002/nau.20200. [DOI] [PubMed] [Google Scholar]
- 166.Rigaud J, Delavierre D, Sibert L, et al. [Hydrodistension in the therapeutic management of painful bladder syndrome] Prog Urol. 2010;20:1054–9. doi: 10.1016/j.purol.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 167.Zabihi N, Allee T, Maher MG, et al. Bladder necrosis following hydrodistention in patients with interstitial cystitis. J Urol. 2007;177:149–52. doi: 10.1016/j.juro.2006.08.095. [DOI] [PubMed] [Google Scholar]
- 168.Kerr WS., Jr Interstitial cystitis: treatment by transurethral resection. J Urol. 1971;105:664–6. doi: 10.1016/s0022-5347(17)61602-1. [DOI] [PubMed] [Google Scholar]
- 169.Peeker R, Aldenborg F, Fall M. Complete transurethral resection of ulcers in classic interstitial cystitis. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11:290–5. doi: 10.1007/s001920070019. [DOI] [PubMed] [Google Scholar]
- 170.Payne RA, O’Connor RC, Kressin M, et al. Endoscopic ablation of Hunner’s lesions in interstitial cystitis patients. Can Urol Assoc J. 2009;3:473–7. doi: 10.5489/cuaj.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hillelsohn JH, Rais-Bahrami S, Friedlander JI, et al. Fulguration for Hunner ulcers: Long-term clinical outcomes. J Urol. 2012;188:2238–41. doi: 10.1016/j.juro.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 172.Chennamsetty A, Khourdaji I, Goike J, et al. Electrosurgical management of Hunner ulcers in a referral centre’s interstitial cystitis population. Urology. 2015;85:74–8. doi: 10.1016/j.urology.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 173.Oliver J. Triamcinolone injection vs. fulguration for treatment of Hunner’s ulcers-type interstital cystitis: Preliminary results of a prospective randomized trial. International Continence Society.
- 174.Rofeim O, Hom D, Freid RM, et al. Use of the neodymium: YAG laser for interstitial cystitis: A prospective study. J Urol. 2001;166:134–6. doi: 10.1016/S0022-5347(05)66093-4. [DOI] [PubMed] [Google Scholar]
- 175.Malloy TR, Shanberg AM. Laser therapy for interstitial cystitis. Urol Clin North Am. 1994;21:141–4. [PubMed] [Google Scholar]
- 176.Cox M, Klutke JJ, Klutke CG. Assessment of patient outcomes following submucosal injection of triamcinolone for treatment of Hunner’s ulcer subtype interstitial cystitis. Can J Urol. 2009;16:4536–40. [PubMed] [Google Scholar]
- 177.Giannantoni A, Costantini E, Di Stasi SM, et al. Botulinum A toxin intravesical injections in the treatment of painful bladder syndrome: A pilot study. Eur Urol. 2006;49:704–9. doi: 10.1016/j.eururo.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 178.Giannantoni A, Mearini E, Del Zingaro M, et al. Two-year efficacy and safety of botulinum a toxin intravesical injections in patients affected by refractory painful bladder syndrome. Curr Drug Deliv. 2010;7:1–4. doi: 10.2174/156720110790396463. [DOI] [PubMed] [Google Scholar]
- 179.Kuo HC. Preliminary results of suburothelial injection of botulinum a toxin in the treatment of chronic interstitial cystitis. Urol Int. 2005;75:170–4. doi: 10.1159/000087173. [DOI] [PubMed] [Google Scholar]
- 180.Liu HT, Kuo HC. Intravesical botulinum toxin A injections plus hydrodistension can reduce nerve growth factor production and control bladder pain in interstitial cystitis. Urology. 2007;70:463–8. doi: 10.1016/j.urology.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 181.Pinto R, Lopes T, Frias B, et al. Trigonal injection of botulinum toxin A in patients with refractory bladder pain syndrome/interstitial cystitis. Eur Urol. 2010;58:360–5. doi: 10.1016/j.eururo.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 182.Manning J, Dwyer P, Rosamilia A, et al. A multicentre, prospective, randomiszed, double-blind study to measure the treatment effectiveness of abobotulinum A (AboBTXA) among women with refractory inter-stitial cystitis/bladder pain syndrome. Int Urogynecol J. 2014;25:593–9. doi: 10.1007/s00192-013-2267-8. [DOI] [PubMed] [Google Scholar]
- 183.Kuo HC, Chancellor MB. Comparison of intravesical botulinum toxin type A injections plus hydrodistention with hydrodistention alone for the treatment of refractory interstitial cystitis/painful bladder syndrome. BJU Int. 2009;104:657–61. doi: 10.1111/j.1464-410X.2009.08495.x. [DOI] [PubMed] [Google Scholar]
- 184.Giannantoni A, Cagini R, Del Zingaro M, et al. Botulinum A toxin intravesical injections for painful bladder syndrome: impact upon pain, psychological functioning and quality of life. Curr Drug Deliv. 2010;7:442–6. doi: 10.2174/156720110793566317. [DOI] [PubMed] [Google Scholar]
- 185.Kuo HC. Repeated intravesical onabotulinumtoxinA injections are effective in treatment of refractory inter-stitial cystitis/bladder pain syndrome. Int J Clin Pract. 2013;67:427–34. doi: 10.1111/ijcp.12113. [DOI] [PubMed] [Google Scholar]
- 186.Pinto R, Lopes T, Silva J, et al. Persistent therapeutic effect of repeated injections of onabotulinum toxin A in refractory bladder pain syndrome/interstitial cystitis. J Urol. 2013;189:548–53. doi: 10.1016/j.juro.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 187.Shie JH, Liu HT, Wang YS, et al. Immunohistochemical evidence suggests repeated intravesical application of botulinum toxin A injections may improve treatment efficacy of interstitial cystitis/bladder pain syndrome. BJU Int. 2013;111:638–46. doi: 10.1111/j.1464-410X.2012.11466.x. [DOI] [PubMed] [Google Scholar]
- 188.Comiter CV. Sacral neuromodulation for the symptomatic treatment of refractory interstitial cystitis: a prospective study. J Urol. 2003;169:1369–73. doi: 10.1097/01.ju.0000053863.96967.5a. [DOI] [PubMed] [Google Scholar]
- 189.Gajewski JB, Al-Zahrani AA. The long-term efficacy of sacral neuromodulation in the management of intractable cases of bladder pain syndrome: 14 years of experience in one centre. BJU Int. 2011;107:1258–64. doi: 10.1111/j.1464-410X.2010.09697.x. [DOI] [PubMed] [Google Scholar]
- 190.Ghazwani YQ, Elkelini MS, Hassouna MM. Efficacy of sacral neuromodulation in treatment of bladder pain syndrome: Long-term followup. Neurourol Urodyn. 2011;30:1271–5. doi: 10.1002/nau.21037. [DOI] [PubMed] [Google Scholar]
- 191.Marinkovic SP, Gillen LM, Marinkovic CM. Minimum 6-year outcomes for interstitial cystitis treated with sacral neuromodulation. Int Urogynecol J. 2011;22:407–12. doi: 10.1007/s00192-010-1235-9. [DOI] [PubMed] [Google Scholar]
- 192.Peters KM, Konstandt D. Sacral neuromodulation decreases narcotic requirements in refractory interstitial cystitis. BJU Int. 2004;93:777–9. doi: 10.1111/j.1464-410X.2003.04745.x. [DOI] [PubMed] [Google Scholar]
- 193.Powell CR, Kreder KJ. Long-term outcomes of urgency-frequency syndrome due to painful bladder syndrome treated with sacral neuromodulation and analysis of failures. J Urol. 2010;183:173–6. doi: 10.1016/j.juro.2009.08.142. [DOI] [PubMed] [Google Scholar]
- 194.Steinberg AC, Oyama IA, Whitmore KE. Bilateral S3 stimulator in patients with interstitial cystitis. Urology. 2007;69:441–3. doi: 10.1016/j.urology.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 195.Zabihi N, Mourtzinos A, Maher MG, et al. Short-term results of bilateral S2-S4 sacral neuromodulation for the treatment of refractory interstitial cystitis, painful bladder syndrome, and chronic pelvic pain. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:553–7. doi: 10.1007/s00192-007-0466-x. [DOI] [PubMed] [Google Scholar]
- 196.Andersen AV, Granlund P, Schultz A, et al. Long-term experience with surgical treatment of selected patients with bladder pain syndrome/interstitial cystitis. Scand J Urol Nephrol. 2012;46:284–9. doi: 10.3109/00365599.2012.669789. [DOI] [PubMed] [Google Scholar]
- 197.Hughes OD, Kynaston HG, Jenkins BJ, et al. Substitution cystoplasty for intractable interstitial cystitis. Br J Urol. 1995;76:172–4. doi: 10.1111/j.1464-410X.1995.tb07668.x. [DOI] [PubMed] [Google Scholar]
- 198.Linn JF, Hohenfellner M, Roth S, et al. Treatment of interstitial cystitis: comparison of subtrigonal and supratrigonal cystectomy combined with orthotopic bladder substitution. J Urol. 1998;159:774–8. doi: 10.1016/S0022-5347(01)63726-1. [DOI] [PubMed] [Google Scholar]
- 199.Peeker R, Aldenborg F, Fall M. The treatment of interstitial cystitis with supratrigonal cystectomy and ileocystoplasty: Difference in outcome between classic and nonulcer disease. J Urol. 1998;159:1479–82. doi: 10.1097/00005392-199805000-00018. [DOI] [PubMed] [Google Scholar]
- 200.Rossberger J, Fall M, Jonsson O, et al. Long-term results of reconstructive surgery in patients with bladder pain syndrome/interstitial cystitis: Subtyping is imperative. Urology. 2007;70:638–42. doi: 10.1016/j.urology.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 201.Webster GD, Maggio MI. The management of chronic interstitial cystitis by substitution cystoplasty. J Urol. 1989;141:287–91. doi: 10.1016/s0022-5347(17)40743-9. [DOI] [PubMed] [Google Scholar]
- 202.Tanaka T, Nitta Y, Morimoto K, et al. Hyperbaric oxygen therapy for painful bladder syndrome/interstitial cystitis resistant to conventional treatments: long-term results of a case series in Japan. BMC Urol. 2011;11:11. doi: 10.1186/1471-2490-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gallego-Vilar D, Garcia-Fadrique G, Povo-Martin I, et al. Maintenance of the response to dimethyl sulf-oxide treatment using hyperbaric oxygen in interstitial cystitis/painful bladder syndrome: A prospective, randomized, comparative study. Urol Int. 2013;90:411–6. doi: 10.1159/000343697. [DOI] [PubMed] [Google Scholar]
- 204.van Ophoven A, Rossbach G, Pajonk F, et al. Safety and efficacy of hyperbaric oxygen therapy for the treatment of interstitial cystitis: A randomized, sham controlled, double-blind trial. J Urol. 2006;176:1442–6. doi: 10.1016/j.juro.2006.06.065. [DOI] [PubMed] [Google Scholar]
- 205.Chen H, Wang F, Chen W, et al. Efficacy of daily low-dose sildenafil for treating interstitial cystitis: Results of a randomized, double-blind, placebo-controlled trial—treatment of interstitial cystitis/painful bladder syndrome with low-dose sildenafil. Urology. 2014;84:51–6. doi: 10.1016/j.urology.2014.02.050. [DOI] [PubMed] [Google Scholar]
- 206.Bosch PC. A randomized, double-blind, placebo controlled trial of adalimumab for interstitial cystitis/bladder pain syndrome. J Urol. 2014;191:77–82. doi: 10.1016/j.juro.2013.06.038. [DOI] [PubMed] [Google Scholar]
- 207.Evans RJ, Moldwin RM, Cossons N, et al. Proof of concept trial of tanezumab for the treatment of symptoms associated with interstitial cystitis. J Urol. 2011;185:1716–21. doi: 10.1016/j.juro.2010.12.088. [DOI] [PubMed] [Google Scholar]
- 208.Russo EB. Cannabinoids in the management of difficult to treat pain. Ther Clin Risk Manag. 2008;4:245–59. doi: 10.2147/tcrm.s1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tripp DA, Nickel JC, Katz L, et al. A survey of cannabis (marijuana) use and self-reported benefit in men with chronic prostatitis/chronic pelvic pain syndrome. Can Urol Assoc J. 2014;8:E901–5. doi: 10.5489/cuaj.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Di Marzo V, Piscitelli F, Mechoulam R. Cannabinoids and endocannabinoids in metabolic disorders with focus on diabetes. Handb Exp Pharmacol. 2011:75–104. doi: 10.1007/978-3-642-17214-4_4. [DOI] [PubMed] [Google Scholar]
- 211.Krenn H, Daha LK, Oczenski W, et al. A case of cannabinoid rotation in a young woman with chronic cystitis. J Pain Symptom Manage. 2003;25:3–4. doi: 10.1016/S0885-3924(02)00601-2. [DOI] [PubMed] [Google Scholar]
- 212.Tambaro S, Casu MA, Mastinu A, et al. Evaluation of selective cannabinoid CB(1) and CB(2) receptor agonists in a mouse model of lipopolysaccharide-induced interstitial cystitis. Eur J Pharmacol. 2014;729:67–74. doi: 10.1016/j.ejphar.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 213.Mukerji G, Yiangou Y, Agarwal SK, et al. Increased cannabinoid receptor 1-immunoreactive nerve fibers in over-active and painful bladder disorders and their correlation with symptoms. Urology. 2010;75:1514.e1515–20. doi: 10.1016/j.urology.2009.12.051. [DOI] [PubMed] [Google Scholar]
- 214.Chuang YC, Lee WC, Chiang PH. Intravesical liposome versus oral pentosan polysulfate for interstitial cystitis/painful bladder syndrome. J Urol. 2009;182:1393–400. doi: 10.1016/j.juro.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 215.Lee WC, Chuang YC, Chiang PH. Safety and dose flexibility clinical evaluation of intravesical liposome in patients with interstitial cystitis or painful bladder syndrome. Kaohsiung J Med Sci. 2011;27:437–40. doi: 10.1016/j.kjms.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Nickel JC, Irvine-Bird K, Jianbo L, et al. Phenotype-directed management of interstitial cystitis/bladder pain syndrome. Urology. 2014;84:175–9. doi: 10.1016/j.urology.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 217.Aguilar VC. in Primary Care for Women, 2nd ed. (ed Peipert JF Leppert PC) Ch. 80, 540 (Lippincott W&W, 2004). [Google Scholar]
- 218.Lukban JC, Whitmore KE, Sant GR. Current management of interstitial cystitis. Urol Clin North Am. 2002;29:649–60. doi: 10.1016/S0094-0143(02)00055-1. [DOI] [PubMed] [Google Scholar]
- 219.International Painful Bladder Foundation Interstitial Cystitis/Painful Bladder Syndrome: Anesthetic Intravesical Cocktails, http://www.painful-bladder.org/pdf/IPBF.intravesicalcocktails.pdf. Accessed September 2008.
- 220. PM Hanno in Campbell-Walsh Urology, 9th ed. (ed Kavoussi LR Wein AJ, Novick AC et al.) Ch. 10, 330–370 (Saunders Elsevier, 2007). ). [Google Scholar]
- 221.DR Erickson. Bladder Pain Syndrome: Current Terminology, Diagnosis and Treatment. AUA Update Series. 2009;28 Lesson 36. [Google Scholar]