Abstract
In recent years, the management of male factor infertility has undergone important changes with the introduction of novel concepts, advanced testing, and therapeutic interventions. This review highlights some of these changes and discusses their impact to routine clinical practice. First, we discuss the recent changes in the World Health Organization (WHO) laboratory methods and reference values for the examination of human semen. Second, we examine the role of sperm chromatin integrity tests in light of increasing evidence of the detrimental effect of sperm DNA fragmentation on reproductive outcomes. Third, we summarize the main findings of varicocele-related infertility and the outcomes of microsurgical varicocele repair to different case scenarios. Lastly, we critically discuss the current management of men with nonobstructive azoospermia seeking fertility and the new opportunities that emerged to help these men achieve biological fatherhood.
Keywords: Andrology, Male infertility, Microsurgery, Nonobstructive azoospermia, Semen analysis, Sperm DNA fragmentation, Varicocele
Introduction
Infertility affects nearly 10 to 15 % of men in their prime reproductive age [1]. Despite its multifactorial nature, male factor infertility is yet not fully understood and approximately half of the cases are deemed unexplained or idiopathic [2]. Investigation of the conditions that compromise male fertility is usually undertaken by history, physical examination, and semen analysis. During this investigation, special attention should be given to issues that have major implication for diagnosis and management, including semen analysis, varicocele, and azoospermia [1].
In approximately 15 % of the cases, results of the conventional semen analysis do not reveal obvious abnormalities [3]. However, it has been shown that spermatozoa of infertile men have lower DNA integrity than fertile men [4–6]. This is important because genetic information passed on to the next generation depends on sperm DNA integrity [7] . Since several etiological factors have been implicated in the impairment of sperm DNA content, assessment of sperm DNA fragmentation (SDF) may offer an opportunity to better understand and treat such sperm dysfunctions [7, 8].
Varicocele is considered the leading cause of male infertility as it can impair spermatogenesis through several distinct pathophysiological mechanisms [9]. Current evidence supports oxidative stress as the key element in the pathophysiology of varicocele-related infertility [10]. From the laboratory perspective, measurement of markers of oxidative stress, including SDF, can provide valuable information about the extent of oxidative stress and might guide therapeutic interventions [11]. From the clinical perspective, repair of varicocele has been advocated for alleviating oxidative stress-associated infertility, which may improve both natural fertility and assisted reproductive technology (ART) outcomes [12, 13].
Among infertile men, 5 to 10 % present with nonobstructive azoospermia (NOA), a condition characterized by a lack of sperm in ejaculates due to spermatogenic failure [14]. Despite being invariably infertile, these men do not necessarily have an unattainable potential to initiate a pregnancy [15]. However, proper counseling and management of men with NOA represent a challenge for andrologists, urologists, and reproductive medicine specialists alike. Notwithstanding, advances in molecular biology testing, hormone therapy, and microsurgical sperm retrieval, as well as state-of-the-art in vitro fertilization (IVF) techniques, have offered revived hope for such men to achieve biological fatherhood (reviewed by Esteves [14]).
This review highlights the aforementioned issues and discusses their impact to routine clinical practice. Our aim is to provide a useful guide for professionals involved in the health care of infertile males.
World Health Organization (WHO) laboratory methods for the examination of human semen: implications for practice
In 2010, the WHO introduced important changes to the methods for conducting and reporting results of routine semen analysis. The alterations specifically included assessments of (i) volume by weight rather than graduated pipette; (ii) motility by two categories, namely progressive and nonprogressive, in contrast with four categories in previous WHO manual editions; and (iii) morphology by strict criteria (Tygerberg criteria) as opposed to the WHO criteria in the previous manuals [16, 17]. The reason for changing the volume measurements relied on the observations that aspirating semen with a serological pipette underestimates the volume by approximately 0.5 mL (range 0.3–0.8 mL) compared with weighing [18, 19]. In another study, the use of serological pipettes decreased the “true” volume estimation by approximately 17 % compared with weight, thus influencing the total sperm count [20]. Along the same lines, changes in the methods for assessing sperm motility were aimed to decrease interobserver subjectivity, whereas the adoption of the strict criteria for sperm morphology analysis is in line with the low percentage of spermatozoa classified as normal in the cervical mucus [21, 22].
Furthermore, the WHO introduced the first semen criteria based on a population-based study of recent fathers with time-to-pregnancy (TTP) of 1 year or less [23]. Data from nearly 1900 men was obtained to generate percentile distributions for semen volume, and sperm count, motility, vitality, and morphology. The fifth centile was established as the lower cutoff limits for normality, making the reference values markedly lower than those previously considered to be consistent with normal male fertility as reported in the previous manual (Table 1). Interestingly, recent studies evaluating the impact of these changes noted that approximately 15 % of patients would have their semen analysis results reclassified as within normal ranges had the 2010 WHO reference values been adopted [24]. On the other hand, it seems unquestionable that WHO manuals will remain widely used for laboratory examination of human semen as they have been since 1980. It has been therefore suggested that caution should be exercised to interpret a semen analysis in view of the new changes. Issues related to the new format of data generation and semen analysis methods might explain the lower cutoff limits [17, 22].
Table 1.
Cutoff reference values for semen characteristics as published in the 1999 and 2010 World Health Organization (WHO) manuals for laboratory examination of human semen. The reference limits in the 2010 WHO manual were set as the values within the fifth centile distribution of semen parameters of approximately 2000 men whose partners had a time-to-pregnancy of 12 months or less. The corresponding 50th and 95th centiles are also provided (adapted from Cooper et al [23] and Esteves et al. [22])
| Semen parameters | WHO 1999a | WHO 2010b | ||
|---|---|---|---|---|
| 5th centile | 50th centile | 95th centile | ||
| Volume (mL) | ≥2 | 1.5 | 3.7 | 6.8 |
| Sperm count (106/mL) | ≥20 | 15 | 73.0 | 213.0 |
| Total sperm count (106) | ≥40 | 39 | 255.0 | 802.0 |
| Total motility (% motile) | ≥50 | 40 | 61 | 78 |
| Progressive motility | ≥25 %c | 32 %d | 55 | 72 |
| Vitality (% alive) | ≥75 | 58 | 15 | 44 |
| Morphology (% normal forms) | (14)e | 4f | 79 | 91 |
aReference values based on the clinical experience of investigators who have studied populations of healthy fertile men of unknown time-to-pregnancy
bReference limits obtained from the 5th centile values
cGrade a = rapid progressive motility (>25 μm/s)
dProgressive motility irrespective of grades
eValue not defined, but strict criterion is suggested
fStrict (Tygerberg) criterion
To illustrate the importance of semen analysis in the management of male factor infertility, the most influential male infertility guidelines, including those issued by the American Urological Association (AUA), American Society for Reproductive Medicine (ASRM), and European Association of Urology (EAU), still rely to a large extent upon the concept of abnormal semen analysis to recommend interventions [25]. However, different interpretations may be expected with regard to the degree of abnormality depending on the manual version adopted, with obvious implications for counseling, diagnosis, and management of men seeking fertility evaluation.
In a recent meta-analysis, we examined the impact of the WHO criteria for semen analysis in infertile men with clinical varicocele [26]. This is important because current varicocele guidelines recommend treatment only when the palpable varicocele is accompanied by abnormal semen parameters [27]. For example, affected men deemed eligible for treatment might be considered ineligible depending on which WHO criteria is applied. But our results not only indicated that varicocele was unequivocally associated with reduced sperm count (mean difference [MD] −44.48 × 106 mL−1; 95 % CI −61.45, −27.51 × 106 mL−1; P < 0.001), motility (MD −26.67 %; 95 % CI −34.27, −19.08; P < 0.001), and morphology (MD −19.68 %; 95 % CI −29.28, −10.07; P < 0.001) but also that the magnitude of size effect was not significantly influenced by the WHO laboratory manual edition used for semen analysis [26]. This means that the application of the new WHO criteria is unlikely to have a significant impact on the indication for varicocele repair.
In another systematic review and meta-analysis comprising 5865 men, we examined the impact of the WHO criteria when evaluating the semen quality of smokers and nonsmokers [28]. We found that cigarette smoking was unequivocally associated with reduced sperm count and motility and that deterioration of semen quality was associated with moderate and heavy smoking. Furthermore, the latest WHO laboratory methods for the examination of human semen had only minimal impact on the magnitude of effect size with regard to the aforementioned semen parameters, thus reassuring the observed negative effect of smoking on semen quality irrespective of the WHO manual used for semen assessment [28]. Notwithstanding, adoption of 2010 WHO manual impacted the evaluation of sperm morphology and resulted in a loss of perception of the negative effects of smoking on this important parameter.
In conclusion, although it seems logical to adopt the latest (fifth edition) WHO laboratory methods for the evaluation of human semen, a careful examination of its characteristics and limitations is advisable. Importantly, clinicians should not expect an analysis of the widely ranging parameters of the whole ejaculate to give robust discriminatory information of the male fertility potential.
Sperm DNA fragmentation testing and clinical implications for male factor infertility
Despite being the parameters most frequently utilized in clinical settings, sperm count, motility, and morphology may not be the best predictors of a given man fertility potential as they do not necessarily associate with the presence of immature sperm chromatin or fragmented DNA [3, 17, 29]. Perhaps the major problem is that impaired sperm chromatin is found not only in men with abnormal semen parameters [30] but also in those exhibiting semen analysis within normal ranges [31]. SDF may originate from the testis and excurrent duct system. Abortive apoptosis and defective protamination theories are proposed to explain the generation of DNA fragmentation within the testis. However, oxidative stress affecting sperm during transit through the epididymis and after ejaculation is the major cause of SDF outside the testis [32]. Several etiological factors have been implicated in the impairment of sperm DNA integrity, including cigarette smoking, radiation, chemotherapy, leukocytospermia, varicocele, cancer, obesity, and advanced paternal age [10, 29, 30, 32].
Reduced fertility rates have been reported in cases of high SDF [33]. Impaired embryo development [34], compromised integrity of the embryonic genome [35], and increased rates of miscarriage [36, 37] have also been associated with high sperm DNA damage. Collectively, increasing evidence suggests a negative impact of high SDF to natural conception and pregnancy outcomes in IVF and intracytoplasmic sperm injection (ICSI) cycles (reviewed by Esteves et al. [8]). In this context, the evaluation of SDF in addition to conventional semen analysis would be ideal as it evaluates an independent attribute of sperm quality [29].
Several tests have been introduced to measure the sperm DNA damage [reviewed by Esteves et al. [8] and Majzoub et al. [11]). The most commonly used are the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), sperm chromatin dispersion test (SCD), and the sperm chromatin structure assay (SCSA). These tests measure different expressions of sperm DNA damage; hence, results are not necessarily interchangeable [8]. SCSA requires expensive equipment such as the flow cytometer; therefore, samples are usually sent to a central laboratory. Unlike SCSA, TUNEL and SCD are advantageous because they require less costly equipment and can be performed in-house [38]. In a previous study, we compared SDF rates measured by SCD and TUNEL assays in men with unexplained infertility [38]. The SCD method is a two specific step process, namely, (i) controlled DNA denaturation and (ii) controlled protamine depletion, which measures the susceptibility of DNA to denaturation [39]. In contrast, TUNEL measures “true” single- and double-stranded DNA fragmentation by incorporating modified nucleotides into the site of damage [40]. We found that SCD was more sensitive, easier, and quicker to perform than TUNEL, but results obtained by these methods were not comparable (r = 0.29) [38]. Notwithstanding, it has been recently reported that the TUNEL assay can be performed with a modified protocol using bench top flow cytometer that allows accurate and quick measurement of DNA damage in a large number of samples [40]. At present, however, consensus has not been reached as to which test should be considered the gold standard for SDF measurement. Possible solutions would involve standardization, validation, interpretation of results, and establishment of reference ranges among the several methods available.
Clinical utility
We have advocated the routine use of SDF testing in the clinical evaluation of male factor infertility [8, 11, 29, 32, 38]. In our settings, the improved SCD test is our preferred approach and cutoff levels of 30 % are used to discriminate specimens with normal and abnormal results [41]. Importantly, we ask our patients to abstain from ejaculation for a fixed period 1–2 days before semen collection due to the influence of longer abstinence periods on SDF results [42]. Test results may provide diagnostic and prognostic information complementary to, but distinct from routine semen analysis, and are useful to guide management and monitoring intervention outcomes [8, 29]. Despite recognizing that our position on SDF as an integral part of semen analysis is debatable, the Practice Committee of the ASRM has recently conceded that determining the values of SDF may be clinically informative for intrauterine insemination (IUI) or IVF and ICSI outcomes [43]. The importance of sperm DNA fragmentation testing in various case scenarios is discussed below.
Varicocele
Among several male infertility factors, varicocele—a common medical condition found in about 15 % of the general male population and 40 % of infertile counterparts—has been shown to compromise sperm DNA quality [11, 30]. The underlying mechanism is the increased oxidative stress which has been implicated as a central element in the pathophysiology of varicocele [10, 44, 45]. Reactive oxygen species (ROS) can cause damage to both nuclear and mitochondrial DNA, which may lead to base modification, strand breaks, and chromatin cross-links [32]. Moreover, increased oxidative stress can trigger an apoptosis-like process affecting sperm maturation and nuclear protamination [10]. In a multicenter study involving 593 infertile men attending infertility clinics, we observed that SDF rates were increased in all etiology categories and were higher than those of fertile controls [30]. Interestingly, both men with varicocele (35.7 ± 18.3 %) and those with leukocytospermia (41.7 ± 17.6 %) exhibited the highest SDF rates. Notably, a subpopulation of sperm exhibiting massive DNA damage (termed “degraded sperm”) was more prevalent in men with clinical varicocele (P < 0.001). A sperm DNA degradation index (DDSi) of 0.33 or greater identified men with clinical varicocele with 94 % accuracy. The DDSi represented the proportion of sperm with degraded DNA in the whole population with fragmented DNA. Our data shows that determining the proportion of degraded sperm, as a function of the whole population of spermatozoa exhibiting fragmented DNA, may be useful to identify subjects with varicocele [30].
Repair of clinical varicocele has been shown to reduce SDF. This decrease is usually observed 3 months after varicocelectomy [46, 47]. A meta-analysis involving 177 patients summarized the evidence regarding the effects of varicocelectomy on SDF rates [48]. The authors found a 3.4 % reduction (95 % CI 4.1 to 2.7; P < 0.00001) in SDF rates after varicocele repair. Due to the low magnitude of effect size and heterogeneity in the methods for assessing SDF, a final response to the clinical significance of varicocele treatment on SDF will require further well-designed studies taking into count the SDF method, varicocele grade, and fertility outcomes. Notwithstanding, we have examined the impact of repairing clinical varicoceles in a group of infertile men with severe male factor infertility undergoing ICSI [50]. Eighty patients were subjected to subinguinal microsurgical varicocelectomy prior to ICSI and 162 had ICSI with untreated varicocele. In this study, the chance of achieving clinical pregnancy (OR = 1.82; 95 % CI 1.06–3.15) and live birth (OR = 1.87, 95 % CI 1.08–3.25) was significantly increased while the chance of miscarriage was decreased (OR = 0.433, 95 % CI 0.22–0.84) had the varicocele been treated. Our results were recently corroborated by a recent meta-analysis involving 870 patients which showed that the clinical pregnancy rates (OR = 1.59; 95 % CI 1.19–2.12) and live birth rates (OR = 2.17; 95 % CI 1.55–3.06) were increased in the varicocelectomy group compared to the group subjected to ICSI without previous varicocelectomy [13]. It is hypothesized that improvements in sperm function, including sperm chromatin integrity, would be a plausible explanation for the observed beneficial effect of prior varicocelectomy on ICSI outcomes.
Assisted reproductive technology
The literature is rich in studies examining the relationship between SDF and pregnancy rates after IUI, IVF, and ICSI. It has been shown that SDF rates higher than 30 % by SCSA are associated with decreased delivery rates after IUI (OR = 9.9; 95 % CI 2.37 to 41.51) [50]. Similarly, IUI with greater than 12 % TUNEL-positive sperm is unlikely to result in a live birth [51]. As far as IVF and ICSI are concerned, a recent meta-analysis evaluating nine IVF and five ICSI studies showed that the odds of clinical pregnancy in IVF were higher (OR = 1.742, 95 % CI 1.38 to 2.19) when SDF was below 27 %, but results did not differ when ICSI was used (OR = 0.895, 95 % CI 0.629 to 1.273) [52]. The association between SDF and live birth rates was also examined in a contemporary study pooling six observational studies and 998 couples. Couples whose male partners had lower SDF achieved higher live birth rates after both IVF (relative risk 1.27; 95 % CI 1.05 to 1.52) and ICSI (relative risk 1.11; 95 % CI 1.00, 1.23), but the magnitude of effect size was more pronounced for IVF, thus suggesting that ICSI is preferable as an ART modality in cases involving high SDF [53].
Several strategies have been attempted to overcome SDF in couples subjected to ART. In addition to varicocele repair, oral antioxidant intake, short ejaculatory abstinence, and sperm selection techniques have been used. Oral antioxidants were shown to reduce SDF by approximately 14 % (95 % CI 13.8 to 17.7) [54], but the ideal regimen and duration are still to be determined. SDF also varies as a function of ejaculatory abstinence. Daily ejaculations over a 2-week period were shown to decrease SDF by 27 % (P = 0.03) [55]. In a recent study involving healthy normozoospermic men, we assessed SDF using the flow cytometry TUNEL assay after varying ejaculatory abstinence periods in the same men [42]. Short abstinence (1 day) yielded the least amount of SDF (mean 9.9 %; 95 % CI 6.6–13.2 ) compared to the WHO recommended period of 2 to 7 days (mean 12.8 %, 95 % CI 9.3–16.3; P = 0.02) and longer periods of >7 days (mean 17.8 %, 95 % CI 13.3–22.3 %; P = 0.007). This may be due to epididymal stasis that is kept at a minimum by short abstinence, thus decreasing the exposure to oxidative stress. Regarding the use of sperm selection techniques to deselect sperm with DNA damage for ICSI, a variable reduction in the proportion of SDF in the processed specimen is noted after sperm selection using density centrifugation (22 to 44 % relative reduction) [56], hyaluronic acid binding [57], sperm magnetic sorting [58], and ultra-high magnification sperm selection [59]. However, the clinical validity of the aforementioned methods to bypass the detrimental effect of SDF on ART outcomes is still unclear.
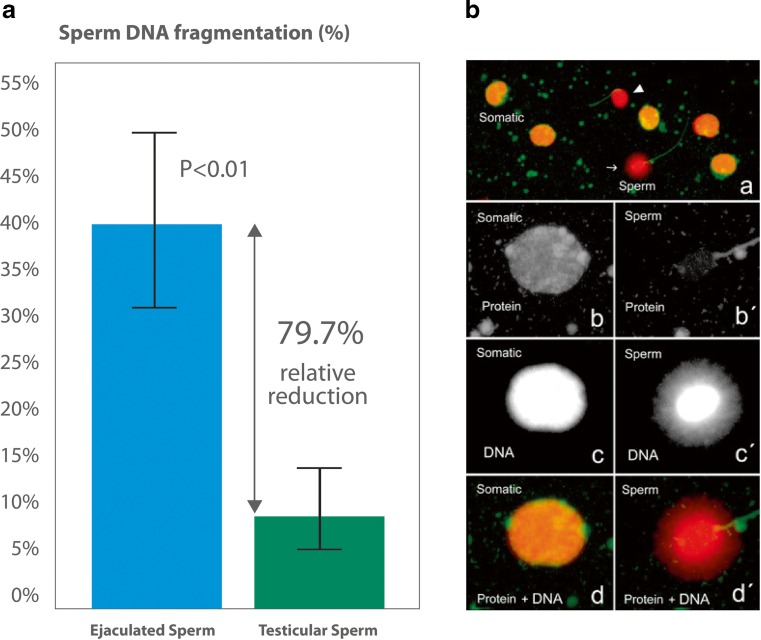
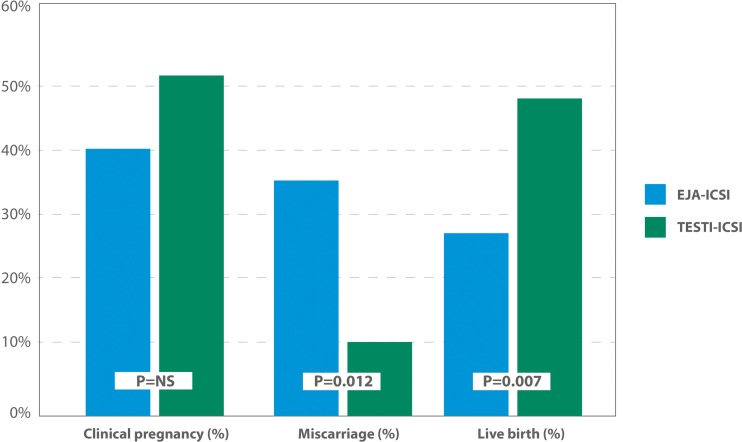
Another attempted strategy has been the use of testicular sperm for ICSI due to the tendency of testicular sperm to have better DNA quality than ejaculated counterparts. In one study involving 18 patients, SDF assessed by TUNEL was lower in the testicle (4.8 ± 3.6 %) than in the ejaculate (23.6 ± 5.1 %; P < 0.001), and pregnancy rates by ICSI favored testicular sperm (44.4 vs. 6 %; P < 0.05) [60]. Using the same method, Moskovtsev et al. studied 12 men and found that the SDF was 3-fold higher in the ejaculate than in the testis (39.7 ± 14.8 % vs. 13.3 ± 7.3 %; P < 0.001) [61]. To shed light onto this matter, we recently conducted a prospective comparative study involving 172 couples undergoing ICSI in which the male partners had idiopathic oligozoospermia and high SDF despite taking oral antioxidant for at least 3 months [41]. Sperm injections were carried out with ejaculated sperm (EJA-ICSI; n = 91) or testicular sperm (TESTI-ICSI; n = 81) retrieved by either testicular sperm extraction (TESE) or testicular sperm aspiration (TESA). SDF levels were reassessed on the day of oocyte retrieval using a variant of the SCD test that combines a dual fluorescent cocktail probe to discriminate somatic cells from spermatozoa (Fig. 1). We found that SDF was 5-fold lower in testicular sperm compared with ejaculated sperm (40.7 ± 9.9 vs. 8.3 ± 5.3 %; P < 0.001). Moreover, the use of testicular sperm for ICSI was associated with significantly better outcomes (Fig. 2). For the TESTI-ICSI group versus the EJA-ICSI group, respectively, the live birth rates were 46.7 and 26.4 % (P = 0.007), with a relative risk of 1.76 (95 % CI 1.15 to 2.70) favoring testicular sperm. And miscarriage rates were 3-fold lower in the TESTI-ICSI group (10.0 % versus 34.3 %; P = 0.012), with a relative risk of 0.29 (95 % CI: 0.10 - 0.82) favoring testicular sperm. Importantly, clinical pregnancy rates were not statistically different between the two groups (51.9 vs. 40.2 %) therefore indicating that this parameter may not be a valid outcome measure in SDF studies evaluating pregnancy rates [41]. Specifically, the risk of miscarriage after ART in couples whose male partners have high SDF has been recently highlighted in recent meta-analyses [36, 37, 62]. The odds for pregnancy loss were 2.2× (95 % CI 1.54 to 3.03) and 2.5× (95 % CI 1.52 to 4.04) higher in couples whose male partners had high SDF than counterparts with normal SDF [36, 37].
Fig. 1.
a Comparison of sperm DNA fragmentation rates in ejaculated and testicular sperm of 81 infertile men undergoing ICSI. Use of testicular sperm for ICSI resulted in an absolute reduction of 32.6 % (relative reduction of 79.7 %) in SDF. b Sperm chromatin dispersion (SCD) test for assessing SDF in testicular sperm. A variant of the Halosperm test (Halotech DNA, Spain) that combines a dual fluorescent cocktail probe to discriminate somatic cells from spermatozoa was used. Spermatozoa and somatic cells exhibit differences in the wavelength emission associated with each fluorochrome (green for proteins and red for DNA). Spermatozoa exhibit only red fluorescence on the sperm head owing to protamine removal, while nonsperm cells fluoresce yellow as a result of the combined emission of both fluorochromes (a). Spermatozoa exhibiting red fluorescence with a green flagellum and no halo of chromatin dispersion represented those with fragmented DNA (arrow cap). In contrast, spermatozoa exhibiting red fluorescence with a green flagellum and haloes of chromatin dispersion represented those with nonfragmented DNA (arrow). A somatic cell with its typical high protein and DNA contents and a spermatozoon with its characteristic low protein remnant and high DNA content are seen in b and c, respectively, using a single channel fluorescence emission. After merging the information provided by protein and DNA selective staining, somatic cells and spermatozoa can be easily distinguished (d and d′). In addition, the sperm tail fluoresces in green, and this feature also helps to distinguish spermatozoa from other cell elements (a and d′). Adapted with permission from Esteves et al. [41]
Fig. 2.
Clinical pregnancy, miscarriage, and live birth rates after sperm injections using either ejaculated sperm (EJA-ICSI; n = 91) or testicular sperm retrieved by TESE or TESA (TESTI-ICSI; n = 81) cohorts. Adapted with permission from Esteves et al. [41]
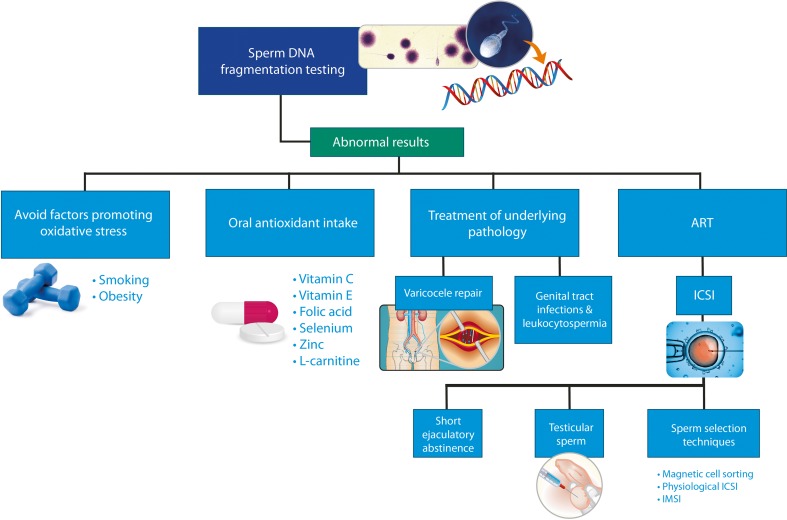
Collectively, emerging evidence indicates that SDF tests provide valuable information to both diagnosis and management of male factor infertility. Testing may be recommended at initial workup to all men but those with azoospermia and severe oligozoospermia in whom the method may not be feasible. Abnormal testing results identify men in whom SDF is a contributory factor to infertility. SDF tests can be used to monitor results of interventions and to select the best ART strategy, as depicted in Fig. 3.
Fig. 3.
Possible treatment alternatives to overcome high sperm DNA fragmentation. The figure highlights the role of SDF testing to better manage couples facing infertility. Possible treatment strategies to overcome high SDF are indicated. ART assisted reproductive technology, ICSI intracytoplasmic sperm injection, IMSI ultra-high magnification sperm injection
Novel concepts in the management of infertile men with nonobstructive azoospermia
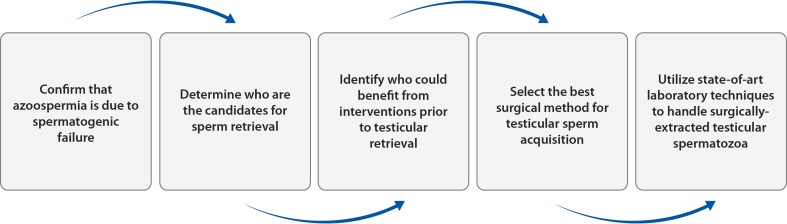
Nonobstructive azoospermia is a serious type of male factor infertility, affecting approximately 1 % of all men and 10 to 15 % of infertile males [14, 63]. Despite being untreatable and resulting from a spectrum of testicular disorders, approximately 50 % of men with NOA have sparse foci of sperm production within their dysfunctional testes, which can be extracted and used for ICSI [64]. From a clinical perspective, we propose that the management of infertile men with NOA should take into account the (i) differential diagnosis of azoospermia, (ii) selection of the eligible patients for sperm retrieval using molecular biology diagnosis, (iii) identification of the affected men that might benefit of interventions prior to sperm retrieval, (iv) application of the best method to surgically retrieve testicular spermatozoa, and (v) use of state-of-the-art IVF techniques (Fig. 4; Table 2) [14]. A coordinated multidisciplinary effort involving infertility specialists, including urologists/andrologists, reproductive endocrinologists, geneticists, and embryologists, will offer the best possible chance of biological offspring for men with NOA as discussed below.
Fig. 4.
Step-by-step approach in the clinical management of men with nonobstructive azoospermia seeking fertility (adapted with permission from Esteves [14])
Table 2.
Clinical management steps, interventions, and suggested actions in the management of infertile men with nonobstructive azoospermia
| Clinical management step | Interventions | Action taken | Interpretation |
|---|---|---|---|
| Azoospermia differential diagnosis | Medical history, physical examination, endocrine profile (FSH and TT levels at a minimum; LH, prolactin, thyroid hormones, and estradiol are added as needed), and examination of pelleted semen in multiple occasions. Testicular biopsy may be considered in the few cases in which the differential diagnosis cannot be determined. | Confirmation that azoospermia is due to NOA and identification of patients with the presence of few spermatozoa in the ejaculate. | A differential diagnosis between OA, hypogonadotropic hypogonadism, and NOA should be performed as treatment strategy and outcome vary according to the type of azoospermia. |
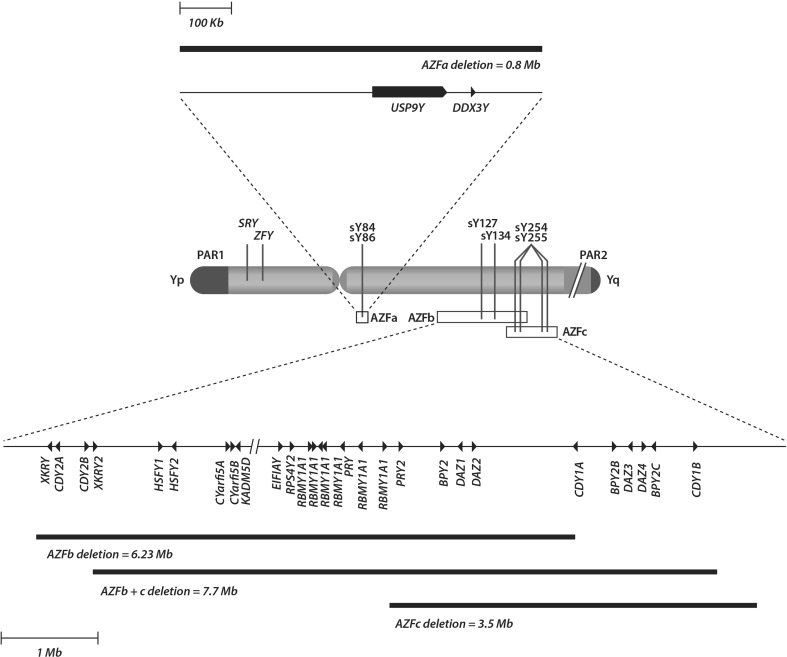
| Determination of the candidates for sperm retrieval | Y chromosome microdeletion screening using multiplex (PCR) blood test. The basic set of PCR primers recommended by the EAA/EMQN to be used in multiplex PCR reactions for the diagnosis of Yq microdeletion includes sY14 (SRY), ZFX/ZFY, sY84 and sY86 (AZFa), sY127 and sY134 (AZFb), and sY254 and sY255 (AZFc). | Deselect men with microdeletions involving subregions AZFa, AZFb, and AZFb + c | Approximately 10 % of NOA men harbor microdeletions within the AZF region. The SR success in the affected subjects with YCMD involving the subregions AZFa, AZFb, and AZFb + c are virtually nonexistent and such patients should be counseled accordingly. The SR success in those men with AZFc deletions range from 50 to 70 %, and genetic counseling should be offered to these men because testicular spermatozoa used for ICSI will invariably transmit the deletion from father to son. |
| Identification of patients who may benefit from interventions prior to sperm retrieval | Determination of TT and estradiol serum levels | Medical treatment with exogenous gonadotropins, aromatase inhibitors, and clomiphene citrate should be considered, especially for men with hypogonadism (TT< 300 ng/dL) or T/E ratio <10. | Patients should be advised that evidence regarding a positive effect of medical treatment is currently equivocal and that such interventions are considered empirical. |
| Physical examination to identify the presence of clinical varicocele and analysis of testicular biopsy results (if available) | Microsurgical repair of clinical varicocele | Microsurgical varicocele repair prior to SR is associated with higher SR success than SR in the presence of an untreated varicocele. But patients with testicular histopathology showing Sertoli cell-only are unlikely to benefit from varicocele repair. The quality of evidence regarding the beneficial effect of varicocele repair in NOA is overall moderate, and therefore, patients should be counseled accordingly. | |
| Selection of the most effective and efficient surgical method for testicular sperm acquisition | Analysis of testicular biopsy results (if available) and whether sperm have been obtained in previous retrievals and by which method | Micro-TESE is the preferred approach to harvest sperm in NOA. Conventional TESE may be considered in cases of previous success with TESE, particularly when testicular histopathology shows hypospermatogenesis. | Overall, micro-TESE is associated with 1.5-fold higher SR success rate than conventional TESE. The minimal tissue extraction facilitates laboratory sperm processing and reduces testicular damage. |
| Application of state-of-the-art laboratory techniques to handle surgically extracted testicular spermatozoa | Extraction of a minimum volume of tissue by micro-TESE facilitates tissue processing and search for sperm. Testicular tissue preparation techniques include mechanical and enzymatic mincing and erythrocyte lysis. | Sterile techniques, stable pH and temperature, and laboratory air quality conditions useful to optimize micromanipulation efficiency and safety assurance | Spermatozoa collected from men with NOA should be handled with great care because they are often compromised in quality and are more fragile than ejaculated counterparts. The reproductive potential of such gametes used for ICSI is differentially affected by NOA. |
| Excess sperm not used for ICSI should be cryopreserved for future attempts. |
ICSI intracytoplasmic sperm injection, micro-TESE microdissection testicular sperm extraction, NOA nonobstructive azoospermia, OA obstructive azoospermia, PCR polymerase chain reaction, SR sperm retrieval, T/E testosterone to estradiol ratio (T and E units of measurement: ng/dL and pg/mL, respectively), TESE testicular sperm extraction, TT total testosterone
Differential diagnosis
Information obtained from the medical history, physical examination, and hormone analysis provides >90 % accuracy to determine the type of azoospermia (obstructive vs. nonobstructive) [65]. Etiology conditions associated with NOA include congenital and genetic abnormalities, endocrine disorders, exposure to gonadotoxins, postinfectious, varicocele, trauma, and idiopathic [63]. Unlike NOA, obstructive azoospermia (OA) is attributed to a mechanical blockage along the reproductive tract and is associated with a favorable prognosis since spermatogenesis is intact [66]. Although a rare entity, it is equally important is to identify those men with azoospermia due to hypogonadotropic hypogonadism (HH). HH is an endocrine disorder characterized by failure of sperm production due to the lack of appropriate stimulation by gonadotropins (reviewed by Fraietta et al. [67]).
Men with NOA usually have normal epididymides and palpable vasa. Small-sized testes (<15 mL) are otherwise encountered since about 85 % of testicular parenchyma is involved in spermatogenesis [1, 14]. However, testicular volume is often normal in men with spermatogenic maturation arrest, a condition characterized by a lack of mature spermatozoa despite the presence of normal numbers of germ cells [68]. The serum levels of follicle-stimulating hormone (FSH) are usually elevated while low testosterone levels (<300 ng/dL) are found in approximately 50 % of the affected men [69]. Abnormal testosterone levels reflect Leydig cell insufficiency, which is usually accompanied by elevated luteinizing hormone (LH) levels [69].
Ejaculates of men with NOA usually have normal volume and pH, which indicates patent ejaculatory ducts and functional seminal vesicles [1]. It is important that such specimens are examined after high-speed (>1000g) centrifugation to exclude the presence of rare spermatozoa [70]. The finding of any spermatozoa may offer the chance of ART to be performed with ejaculated sperm, thus avoiding sperm retrieval (SR) methods. We perform centrifugation at 3000g for 15 min, which is followed by a careful examination of the pellet semen [14]. Equally important is the examination of multiple specimens and in several occasions as azoospermia may be transient due to conditions such as fever and use of medication [71].
The gold-standard test for the confirmation of NOA is testicular histopathology analysis [65]. Hypospermatogenesis, germ cell maturation arrest, germ cell aplasia (Sertoli cell-only syndrome, SCO), tubular sclerosis, or a combination of those, are the common phenotypes. Histopathology results may also predict sperm retrieval outcome. Evaluating 356 men with NOA, we found that patients with SCO had lower SR rates (19.5 %) than those with maturation arrest (40.3 %; P = 0.007) [72]. Both aforementioned groups had lower SR rates than the group with hypospermatogenesis (SR = 100.0 %; P < 0.001). However, testicular biopsies carried out with the sole purpose of histopathology evaluation could remove the rare foci of sperm production and thus jeopardize the chances of future retrieval attempts [72]. Hence, we only perform diagnostic testicular biopsies when the type of azoospermia is equivocal. In such cases, a specimen is taken for wet examination in addition to conventional histopathology analysis. We routinely cryopreserve testicular spermatozoa when mature spermatozoa are found on wet specimens [73].
Prognostic factors for sperm retrieval success
The uncertainty of sperm acquisition in NOA makes prognostic factors very desirable. However, etiology of NOA, testicular volume, endocrine profile, and testicular histopathology reflect only the global spermatogenic function. Overall, these factors are not useful to predict SR success [72, 74–76].
On the other hand, it has been estimated that 10 % of men with NOA harbor microdeletions within the long arm of the Y chromosome, which clusters the genes involved in spermatogenesis regulation [77–79]. The application of molecular technology has allowed the recognition of three AZF subregions, namely AZFa, AZFb, and AZFc, each one including a major candidate gene [79] (Fig. 5). Deletions differentially affecting these AZF subregions cause a distinct disruption of germ cell development. AZFa deletions affecting the entire AZFa are invariably associated with complete absence of spermatogenesis. AZFb and AZFbc deletions are similar to AZFa deletions, thus meaning that SR success is virtually nil (reviewed by Krausz et al. [79]). In contrast, AZFc deletions are often associated with residual spermatogenesis and the SR success varies from 50 to 70 % [77–80]. Notwithstanding, the male offspring originated from fathers with AZFc microdeletions will inherit the Y chromosome microdeletion and as a result infertility [81]. However, there is a potential risk for the 45,X0 karyotype and to the mosaic 45,X0/46,XY in the offspring, which may lead to spontaneous abortion and genital ambiguity [82–84]. Genetic counseling is, therefore, essential to give information about the risks of conceiving a son with infertility and other genetic abnormalities. To sum up, NOA candidates for SR and ICSI should be screened for Y chromosome microdeletions since diagnosing a deletion has prognostic value and influence the therapeutic options. Sperm retrieval is not recommended to men with complete deletions involving the AZFa and/or AZFb regions. Lastly, genetic counseling should be offered to the patients with AZFc deletions because such deletions will be invariably transmit from father to son.
Fig. 5.
Human Y chromosome map depicting the AZF subregions and gene content. The AZFa region maps from approximately 12.9 to 13.7 Mb of the chromosome and contains two single copy genes, USP9Y and DDX3Y. AZFb spans from approximately 18 to 24.7 Mb of the chromosome and AZFc from approximately 23 to 26.7 Mb. Both regions contain multiple genes as depicted in the bottom of the figure. The location of the basic set of sequence-tagged sites primers to be investigated in azoospermic men with spermatogenic failure, according to the European Association of Andrology and the European Molecular Genetics Quality Network 2013 guidelines, is identified by solid vertical lines (adapted with permission from Esteves [14])
The role of interventions prior to sperm retrieval
Medical therapy
Although it is generally believed that therapy is ineffective in NOA due to the already high levels of serum gonadotropins, the effect of interventions has been revisited in light of recent findings suggesting that enhancing intratesticular testosterone (ITT) production might stimulate residual spermatogenesis [85, 86]. Drugs that have been utilized include clomiphene citrate, gonadotropins (human chorionic gonadotropin and FSH), and aromatase inhibitors (reviewed by Esteves [14] and Kumar [87]).
Among these, our preference has been hCG that specifically binds to LH receptors at the Leydig cell level and boost ITT production [14]. In one study involving 20 men with NOA treated with hCG, ITT levels were significantly higher after treatment (pre 273.6 ± 134.4; post 1348.1 ± 505.4 ng/mL; P < 0.0001) [88]. Concomitantly, endogenous FSH levels are suppressed in half of the individuals subjected to hCG treatment through a negative feedback mechanism of elevated serum testosterone [89]. Such an effect seems beneficial to upregulate the expression of FSH receptors that may increase Sertoli cell function [89].
In a study involving 442 NOA men subjected to medical therapy (clomiphene citrate and hCG) prior to SR, the authors aimed at achieving 600 to 800 ng/dL posttreatment serum testosterone [90]. In this study, significantly higher retrieval rates were obtained in the group of patients achieving the desired hormonal level postmedical therapy (57 vs. 33.6 %). On the other hand, others have found no beneficial effect of aromatase inhibitors, clomiphene citrate, and hCG with regard to sperm retrieval success [91]. Notwithstanding, it has been hypothesized that hCG may be beneficial in NOA as it stimulates spermiogenesis and spermatogonia DNA synthesis, provided residual areas of complete or incomplete spermatogenesis exist [88]. These effects might result in the formation of well-differentiated seminiferous tubules that could be detected during sperm retrieval [89].
Varicocele repair
Varicocele is found in approximately 5 % of men with NOA [44, 92]. Whereas it is debatable whether varicocele is merely coincidental or contributory to spermatogenesis disruption, surgical repair of clinical varicoceles has been carried out in an attempt to improve sperm production in such men [92, 93].
In a recent systematic review aimed at evaluating the benefit of repairing clinical varicocele in infertile men with NOA, 18 studies accounting for 468 patients were included [94]. Among these, 16 noncontrolled studies including a total of 344 men reported postoperative semen analysis results after varicocele repair. In 43.9 % of these patients (range 20.8–55.0 %), spermatozoa were found in postoperative ejaculates. The postoperative sperm count and motility were 1.82 million/mL (95 % CI 0.98–2.77) and 22.9 % (95 % CI 12.5–33.2 %), respectively. Whereas varicocele grade did not appear to have influenced the results, the appearance of sperm in postoperative ejaculates was associated with testicular histopathology findings (9.7 % in SCO, 35.3 % in maturation arrest, and 56.2 % in hypospermatogenesis). Overall, the odds for sperm in the ejaculate were 2.35 (95 % CI 1.04–5.29; P = 0.04) and 12.0 (95 % CI 4.34–33.17; P < 0.001) when patients with hypospermatogenesis (HS) were compared to those with maturation arrest (MA) and SCO, respectively, and 5.09 (95 % CI 1.83–14.10; P = 0.001) favoring MA over SCO. The reported pregnancy rate (natural conception and ART) in this subgroup of men was 26.1 % [94].
In this aforementioned study, three controlled studies specifically evaluated sperm retrieval outcomes in men with varicocele and NOA. Sperm retrieval success was significantly higher in the group of men who had the varicocele treated than those with untreated varicocele (OR 2.65; 95 % CI 1.69–4.14; P < 0.0001). Despite achieving higher pregnancy rates by ICSI with the use of testicular sperm, results favoring the varicocele repair group were only marginally significant (OR for clinical pregnancy 2.07, 95 % CI 0.92–4.65; P = 0.08; OR for live birth 2.19, 95 % CI 0.99–4.83; P = 0.05) [94]. Overall, our findings indicate that repair of palpable varicoceles increases sperm retrieval success in NOA. Added to this, approximately 44 % of the treated men will have enough sperm in the ejaculate to avoid sperm retrieval. But given the low/moderate quality of the evidence, it is advisable that doctors discuss with their patients the risks and benefits of the intervention.
Sperm retrieval method in NOA
Sperm retrieval in NOA is aimed at offering the highest possible chance of harvesting sperm, which can be used fresh for ICSI or cryopreserved. Retrieval methods should also minimize testicular damage thus preserving androgen production and the possibility of repeat SR [95].
Two recent systematic reviews have examined SR success as a function of the surgical retrieval method. In one report involving seven comparative studies and 1062 patients, microdissection testicular sperm extraction (micro-TESE) yielded higher SR success rates (42.9 to 63 %) than conventional TESE (16.7 to 45 %) [96]. In another study, 15 studies with a total of 1890 patients were included. Micro-TESE was compared with conventional TESE and percutaneous TESA. Micro-TESE was 1.5 times more likely (95 % CI 1.4–1.6) to result in SR success than TESE. Furthermore, TESE was 2.0 times more likely (95 % CI 1.8–2.2) to result in SR success than TESA [97].
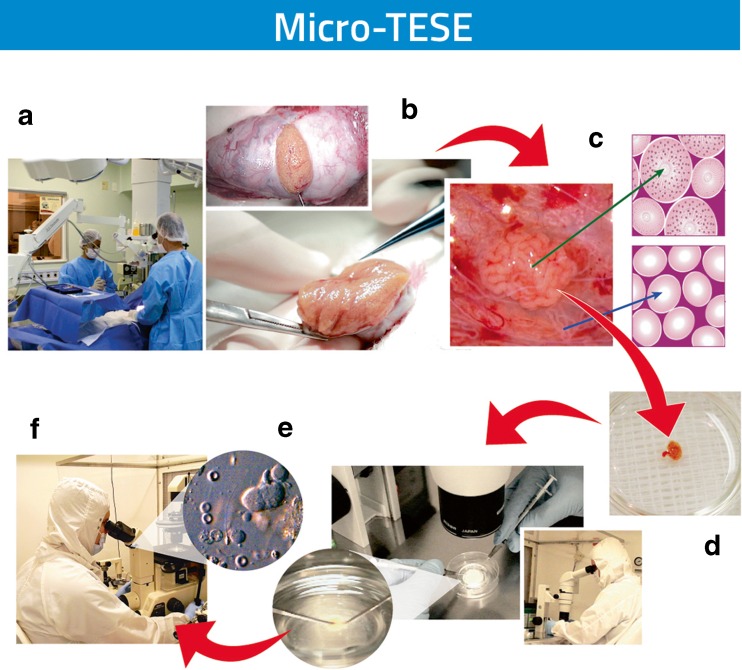
Our experience with micro-TESE has also been reassuring [98]. The method rescues approximately one third of the cases that failed in previous non-microsurgical retrieval attempts and is particularly useful for men with NOA presenting the worst-case scenarios [99, 100]. The lower tissue extraction lessens testicular damage and facilitates laboratory processing and sperm search thus increasing efficiency [74, 98, 101, 102] (Fig. 6). In a study involving 356 patients with NOA subjected to micro-TESE, SR success according to histopathology characteristics of hypospermatogenesis, maturation arrest, and SCO was 100, 40.3, and 19.5 %, respectively [72].
Fig. 6.
Microdissection testicular sperm extraction (micro-TESE). The flowchart illustrates the consecutive steps from the microsurgical procedure to the laboratory processing of testicular specimens. The rationale of micro-TESE is to identify focal areas of sperm production within the testes, based on the size and appearance of the seminiferous tubules, with the aid of the operating microscope (A). A large incision is made in an avascular area of the tunica albuginea and the testicular parenchyma is widely exposed (B). The parenchyma is then dissected at ×16 to ×25 magnification to enable the search and isolation of seminiferous tubules exhibiting larger diameter in comparison with nonenlarged or collapsed counterparts (C). These enlarged tubules are more likely to contain germ cells and eventually normal sperm production. Microsurgical-guided biopsies are performed by carefully removing such tubules, which are sent to the laboratory for examination (D). The minimal tissue extracted facilitates laboratory processing and sperm search thus increasing the process efficiency (E). The initial laboratory step involves mechanical mincing of the seminiferous tubules and examination of specimens for sperm identification (F). The use of optical magnification also reduces the chances of vascular injury by proper identification of testicular blood supply, thus reducing the chances of hematoma formation and testicular devascularization
Role of IVF laboratory
After sperm retrieval, the testicular tissue is immediately transferred to the IVF laboratory for processing. Special care is needed during handling because such spermatozoa are more fragile than ejaculated counterparts [102]. Furthermore, sperm DNA fragmentation and aneuploidy rates are higher in testicular sperm obtained from men with NOA than ejaculated sperm from infertile men with other etiology categories [103, 104]. As a result, lower fertilization, embryo development as well as pregnancy rates have been achieved when the gametes retrieved from men with NOA are used for ICSI [100, 105, 106].
The extraction of low amounts of tissue by micro-TESE is advantageous as it makes it easier and quicker to handle the specimens [101]. On the other hand, processing large testicular tissue fragments as routinely extracted by conventional TESE is extremely labor-intensive and may miss the rare spermatozoa in the sea of cells and noncellular elements. Testicular tissue preparation techniques include mechanical mincing and enzymatic digestion with collagenase. These methods ensure tubular wall breakdown and cellular content loss [102, 107, 108]. After the proper disintegration of the seminiferous tubules, specimens are processed to eliminate surplus tissue elements and red blood cells [102, 109]. Lastly, Petri dishes are prepared with a series of microdroplets of sperm culture medium covered with mineral oil to which aliquots of processed testicular tissue are loaded. Meticulous examination of the specimens allows identification and isolation of testicular sperm [102]. This final step is carried out in the ICSI workstation. Throughout these processes, temperature and pH of working solutions should be kept constant, and state-of-the-art laboratory practices, including sterile techniques and control of air quality conditions, are important to ensure efficiency and safety [102, 110]. In our center, sperm retrieval and micromanipulation of testicular sperm, which includes tissue processing, sperm injection, embryo culture, embryo transfer, and cryopreservation, are carried out in controlled environments [111–113].
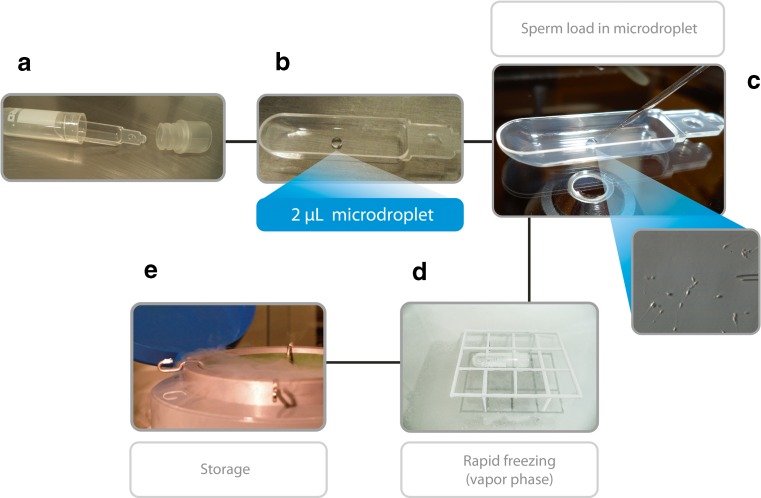
Sperm retrieval can be either coordinated with oocyte collection to allow ICSI to be carried out with fresh sperm or performed to intentionally cryopreserve testicular sperm for future use. In the former, cryopreservation of surplus testicular sperm is highly recommended, as NOA patients often require more than one ICSI attempt until a pregnancy is established but repeat retrievals are not always possible. At present, we cryopreserve testicular sperm using the Cell Sleeper method [114]. Cell Sleepers are nonbiological closed devices in which a few spermatozoa can be frozen in microdroplets (Fig. 7). The method involves rapid freezing and has shown to be reliable for freezing surgically retrieved testicular sperm [114, 115]. In a recent case series including 12 ICSI cycles, six of which involved sperm retrieval by micro-TESE in NOA patients, spermatozoa frozen in Cell Sleepers were used for sperm injections. Overall, the postthaw sperm recovery rate and motility were 94 and 55.8 %, respectively. In this study, the 2PN fertilization rate was 65.9 % whereas the clinical pregnancy rate was 60 % [115].
Fig. 7.
The Cell Sleeper method for low count sperm freezing. The Cell Sleeper (Nipro, Japan) consists of an outer vial, an inner tray, and screw cap (A). The inner tray is removed from the vial, placed in the lid of a large culture dish, and a 2-μL droplet of cryopreservation solution is pipetted into the tray, in a central position (B). Spermatozoa are aspirated and ejected into the droplet on the tray with the aid of a microinjection pipette (C). Immediately thereafter, the tray is returned to the vial and the vial is closed with the screw cap. The vial is placed in a horizontal position 4–5 cm above the surface of liquid nitrogen (D). After 2 min, the vial is submerged in LN2 and secured into a cryopreservation cane for storage (E)
Motility stimulants (e.g., pentoxifylline) and other methods to select viable sperm (e.g., hypoosmotic swelling test and sperm tail flexibility test) are utilized when only immotile sperm (fresh or cryo-thawed) are available for microinjection (reviewed by Esteves and Varghese [102]).
ART outcomes
Overall, ICSI outcomes using testicular sperm extracted from men with NOA are lower than both ejaculated counterparts and obstructive azoospermia [100, 106, 116]. It appears that such cells have a higher tendency to carry deficiencies related to the centrioles and genetic material, which ultimately affect their capability to activate the oocytes and trigger the development of a viable embryo [103, 104].
In an early series involving 330 patients with different infertility conditions including 53 azoospermic men with NOA, we evaluated ICSI outcomes according to the source of spermatozoa and the type of azoospermia [106]. We noted that the 2PN fertilization rates were significantly lower when testicular sperm of men with NOA were compared to both ejaculated sperm, and testicular/epididymal sperm of men with OA (52.2, 71.1, and 73.6 % in NOA, ejaculated sperm, and OA, respectively; P < 0.05). Embryo development and pregnancy rates are also negatively affected by NOA [106]. In two recent series involving a larger cohort of azoospermic men with NOA, we analyzed the health of offspring according to the source of sperm and the type of azoospermia. In one study, 182 women were subjected to ICSI using sperm from partners with NOA, and their outcomes were compared with 182 and 465 women whose partners had obstructive azoospermia and other forms of nonazoospermia infertility, respectively [100]. Live birth rates after ICSI were significantly lower in the NOA group (21.4 %) compared with the OA (37.5 %) and ejaculated sperm (32.3 %) groups (P = 0.003). A total of 326 deliveries resulted in 427 babies born. Overall, differences were not observed in gestational age, preterm birth, birth weight, and low birth weight. In another series, we compared 365 men with NOA subjected to micro-TESE and ICSI with their own sperm to 40 men with NOA who used donor sperm for ICSI due to failed retrievals [106]. Both groups were compared to a group of 146 men with OA subjected to percutaneous sperm retrieval and ICSI with their own sperm. The overall SR success rate in NOA was 41.4 %, but the results were lower than those of the OA group (100 %; adjusted OR 0.033; 95 % CI 0.007–0.164; P < 0.001). Live birth rates were also lower in the NOA group subjected to ICSI with own testicular sperm (19.9 %) than the group that used donor sperm (37.5 %; adjusted OR 0.377 (95 % CI 0.233–0.609; P < 0.001)) and obstructive azoospermia (34.2 %; adjusted OR 0.403 (95 % CI 0.241–0.676; P = 0.001)). Neither miscarriage rates nor the newborn parameters (gestational age, birth weight, malformation rate, perinatal mortality) of infants conceived significantly differed among the groups. Although the health of resulting offspring after ICSI using sperm of men with NOA has been reassuring, only a few studies to date have evaluated the neonatal profile of such babies [100, 106, 117, 118]. On the contrary, studies on the physical, neurological, and developmental outcomes of children conceived are warranted.
NOA: a glance toward the future
Aspermatogenesis is defined as a severe impairment of spermatogenesis in which germ cells are either lacking or present only in immature forms. This extreme type of male infertility affects approximately 25–45 % of patients with NOA [119]. In such cases, in vitro fertilization with immature germ cells and in vitro culture of these cells have been attempted. However, ICSI with immature germ cells has yielded conflicting results, and despite the reported deliveries of healthy offspring, the method has extremely low efficiency as currently used [120]. Furthermore, concerns related to the potential transmission of genomically imprinted disorders raise doubt about the safety of these procedures [121].
Since ART requires mature germ cells, research efforts are now focused on the differentiation of immature germ cells and production/derivation of sperm from somatic cells. Breakthrough advancements have been accomplished by using stem cells from mouse embryos to create primordial germ cells, which differentiated in spermatozoa after transplantation to mice testis [122]. In humans, the formation of human haploid-like cells was obtained from pluripotent stem cells of somatic origin using the technique of in vitro sperm derivation. Spermatogonial stem cell (SCC) is another venue for both reproductive and regenerative medicine [123]. These cells have unlimited potentials including pluripotency, self-renewal, differentiation, and transdifferentiation [126]. However, many issues have to be clarified, including the dedifferentiation and transdifferentiation mechanisms, optimal induction protocols, and origin of the embryonic stem-like cells. At present, the production of human gametes in the laboratory is yet to be fully translated to the bedside [119].
Conclusions
The following list summarizes the clinical and laboratory concepts in male factor infertility discussed in this review.
Semen analysis remains the cornerstone of infertility evaluation as it provides information on the status of the epididymis, seminiferous tubules, and accessory sex glands. The adoption of the latest (fifth edition) WHO laboratory methods for the evaluation of human semen calls for a careful examination of its characteristics and limitations. Conventional semen analysis as routinely performed does not give robust discriminatory information about the male fertility potential.
Fair evidence indicates that sperm DNA fragmentation tests provide valuable information to both diagnosis and management of male factor infertility. Test results have both diagnostic and prognostic information complementary to, but distinct from conventional sperm parameters, and are useful to guide management and monitoring intervention outcomes. Determining the values of SDF may be clinically informative for IUI or IVF and ICSI outcomes. Strategies to alleviate SDF include varicocele repair, oral antioxidant intake, short ejaculatory abstinence, laboratory sperm selection techniques, and use of testicular sperm for ICSI. At present, a consensus has not been reached as to which test should be considered the gold standard for SDF measurement. Possible solutions would involve standardization, validation, interpretation of results, and establishment of reference ranges among the several methods available.
The importance of varicocele to male factor infertility has been revisited. Oxidative stress is a central element in its pathophysiology and may lead to SDF. Repair of clinical varicocele is effective to improve not only the conventional semen parameters but also sperm chromatin integrity. Furthermore, treatment of clinical varicoceles may improve both natural fertility and ART outcomes.
Nonobstructive azoospermia is the most severe presentation of male infertility. The optimal management of infertile men with NOA involves a series of steps that includes: (i) differential diagnosis of azoospermia, (ii) selection of the eligible patients for sperm retrieval using molecular biology diagnosis, (iii) identification of the affected men that might benefit of interventions prior to sperm retrieval, (iv) application of the best method to surgically retrieve testicular spermatozoa, and (v) use of state-of-the-art IVF techniques.
Acknowledgments
The author is grateful to Dr. Hakan Yarali for the invitation to develop this review as a supplementary material to the keynote lecture “Novel concepts in male factor infertility: clinical and laboratory perspectives”, delivered at the “Excellence in ART: Clinical and Laboratory Perspectives” Meeting, Cappadocia, Turkey April 2016.
Compliance with ethical standards
Conflict of interest
The author declares that he has no conflicts of interest.
Footnotes
Expanded from a Keynote Lecture presented by the author at the “Excellence in ART: Clinical and laboratory Perspectives” Meeting, Cappadocia, Turkey April 2016.
Capsule
Management of male infertility has undergone unprecedented changes in recent years with the introduction of advanced laboratory testing and therapeutic interventions. This review highlights some of these issues and discusses their impact to routine clinical practice, making it a valuable guide to health-care providers.
References
- 1.Esteves SC, Miyaoka R, Agarwal A. An update on the clinical assessment of the infertile male. [corrected] Clinics (Sao Paulo) 2011;66:691–700. doi: 10.1590/S1807-59322011000400026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandes M, Hamilton CJ, De Bruin JP, Nelen WL, Kremer JA. The relative contribution of IVF to the total ongoing pregnancy rate in a subfertile cohort. Hum Reprod. 2010;25:118–26. doi: 10.1093/humrep/dep341. [DOI] [PubMed] [Google Scholar]
- 3.Hamada A, Esteves SC, Nizza M, Agarwal A. Unexplained male infertility: diagnosis and management. Int Braz J Urol. 2012;38:576–94. doi: 10.1590/S1677-55382012000500002. [DOI] [PubMed] [Google Scholar]
- 4.Zini A, Bielecki R, Phang D, Zenzes MT. Correlations between two markers of sperm DNA integrity, DNA denaturation and DNA fragmentation, in fertile and infertile men. Fertil Steril. 2001;75:674–77. doi: 10.1016/S0015-0282(00)01796-9. [DOI] [PubMed] [Google Scholar]
- 5.Ahmad G, Moinard N, Esquerre-Lamare C, Mieusset R, Bujan L. Mild induced testicular and epididymal hyperthermia alters sperm chromatin integrity in men. Fertil Steril. 2012;97:546–53. doi: 10.1016/j.fertnstert.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 6.Brahem S, Mehdi M, Elghezal H, Saad A. The effects of male aging on semen quality, sperm DNA fragmentation and chromosomal abnormalities in an infertile population. J Assist Reprod Genet. 2011;28:425–32. doi: 10.1007/s10815-011-9537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis SE. Should sperm DNA fragmentation testing be included in the male infertility work-up? Reprod Biomed Online. 2015;31:134–7. doi: 10.1016/j.rbmo.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Esteves SC, Sharma RK, Gosalvez J, Agarwal A. A translational medicine appraisal of specialized andrology testing in unexplained male infertility. Int Urol Nephrol. 2014;46:1037–52. doi: 10.1007/s11255-014-0715-0. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal A, Esteves SC. Varicocele and male infertility: current concepts and future perspectives. Asian J Androl. 2016;18:161–2. doi: 10.4103/1008-682X.172819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho CL, Esteves SC, Agarwal A. Novel insights into the pathophysiology of varicocele and its association with reactive oxygen species and sperm DNA fragmentation. Asian J Androl. 2016;18:186–93. doi: 10.4103/1008-682X.170441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majzoub A, Esteves SC, Gosálvez J, Agarwal A. Specialized sperm function tests in varicocele and the future of andrology laboratory. Asian J Androl. 2016;18:205–12. doi: 10.4103/1008-682X.172642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tiseo BC, Esteves SC, Cocuzza MS. Summary evidence on the effects of varicocele treatment to improve natural fertility in subfertile men. Asian J Androl. 2016;18:239–45. doi: 10.4103/1008-682X.172639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esteves SC, Roque M, Agarwal A. Outcome of assisted reproductive technology in men with treated and untreated varicocele: systematic review and meta-analysis. Asian J Androl. 2016;18:254–8. doi: 10.4103/1008-682X.163269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esteves SC. Clinical management of infertile men with nonobstructive azoospermia. Asian J Androl. 2015;17:459–70. doi: 10.4103/1008-682X.148719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esteves SC, Agarwal A. The azoospermic male: current knowledge and future perspectives. Clinics. 2013;68(Suppl 1):1–4. doi: 10.6061/clinics/2013(Sup01)01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization . WHO laboratory manual for the examination and processing of human semen. 5. Geneva: World Health Organization; 2010. p. 271. [Google Scholar]
- 17.Esteves SC. Clinical relevance of routine semen analysis and controversies surrounding the 2010 World Health Organization criteria for semen examination. Int Braz J Urol. 2014;40:443–53. doi: 10.1590/S1677-5538.IBJU.2014.04.02. [DOI] [PubMed] [Google Scholar]
- 18.Brazil C, Swan SH, Drobnis EZ, Liu F, Wang C, Redmon JB, et al. Standardized methods for semen evaluation in a multicenter research study. J Androl. 2004;25:635–44. doi: 10.1002/j.1939-4640.2004.tb02835.x. [DOI] [PubMed] [Google Scholar]
- 19.Iwamoto T, Nozawa S, Yoshiike M, Hoshino T, Baba K, Matsushita T, et al. Semen quality of 324 fertile Japanese men. Hum Reprod. 2006;21:760–5. doi: 10.1093/humrep/dei362. [DOI] [PubMed] [Google Scholar]
- 20.Pompeu C, Feijo C, Esteves S. Comparison between analytical scale and graduated serological pipette for semen volume analysis: a cross sectional study. Hum Reprod. 2015;30(Suppl 1):i331–2. [Google Scholar]
- 21.Van Waart J, Kruger TF, Lombard CJ, Ombelet W. Predictive value of normal sperm morphology in intrauterine insemination (IUI): a structured literature review. Hum Reprod Update. 2001;7:495–500. doi: 10.1093/humupd/7.5.495. [DOI] [PubMed] [Google Scholar]
- 22.Esteves SC, Zini A, Aziz N, Alvarez JG, Sabanegh ES, Jr, Agarwal A. Critical appraisal of World Health Organizations new reference values for human semen characteristics and effect on diagnosis and treatment of subfertile men. Urology. 2012;79:16–22. doi: 10.1016/j.urology.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–45. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 24.Murray KS, James A, McGeady JB, Reed ML, Kuang WW, Nangia AK. The effect of the new 2010 World Health Organization criteria for semen analyses on male infertility. Fertil Steril. 2012;98:1428–31. doi: 10.1016/j.fertnstert.2012.07.1130. [DOI] [PubMed] [Google Scholar]
- 25.Esteves SC, Chan P. A systematic review of recent clinical practice guidelines and best practice statements for the evaluation of the infertile male. Int Urol Nephrol. 2015;47:1441–56. doi: 10.1007/s11255-015-1059-0. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal A, Sharma R, Harlev A, Esteves SC. Effect of varicocele on semen characteristics according to the new 2010 World Health Organization criteria: a systematic review and meta-analysis. Asian J Androl. 2016;18:163–70. doi: 10.4103/1008-682X.172638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shridharani A, Owen RC, Elkelany OO, Kim ED. The significance of clinical practice guidelines on adult varicocele detection and management. Asian J Androl. 2016;18:269–75. doi: 10.4103/1008-682X.172641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma R, Harlev A, Agarwal A, Esteves SC. Cigarette smoking and semen quality: a new meta-analysis examining the effect of the 2010 World Health Organization laboratory methods for the examination of human semen. Eur Urol. 2016. [DOI] [PubMed]
- 29.Agarwal A, Cho CL, Esteves SC. Should we evaluate and treat sperm DNA fragmentation? Curr Opin Obstet Gynecol. 2016;28:164–71. doi: 10.1097/GCO.0000000000000271. [DOI] [PubMed] [Google Scholar]
- 30.Esteves SC, Gosálvez J, López-Fernández C, Núñez-Calonge R, Caballero P, Agarwal A, et al. Diagnostic accuracy of sperm DNA degradation index (DDSi) as a potential noninvasive biomarker to identify men with varicocele-associated infertility. Int Urol Nephrol. 2015;47:1471–7. doi: 10.1007/s11255-015-1053-6. [DOI] [PubMed] [Google Scholar]
- 31.Saleh RA, Agarwal A, Nelson DR, Nada EA, El-Tonsy MH, Alvarez JG, et al. Increased sperm nuclear DNA damage in normozoospermic infertile men: a prospective study. Fertil Steril. 2002;78:313–8. doi: 10.1016/S0015-0282(02)03219-3. [DOI] [PubMed] [Google Scholar]
- 32.Gosalvez J, Lopez-Fernandez C, Fernandez JL, Esteves SC, Johnston SD. Unpacking the mysteries of sperm DNA fragmentation: ten frequently asked questions. J Reprod Biotechnol Fertil. 2015;4:1–16. doi: 10.1177/2058915815594454. [DOI] [Google Scholar]
- 33.Sun JG, Jurisicova A, Casper RF. Detection of deoxyribonucleic acid fragmentation in human sperm: correlation with fertilization in vitro. Biol Reprod. 1997;56:602–7. doi: 10.1095/biolreprod56.3.602. [DOI] [PubMed] [Google Scholar]
- 34.Seli E, Gardner DK, Schoolcraft WB, Moffatt O, Sakkas D. Extent of nuclear DNA damage in ejaculated spermatozoa impacts on blastocyst development after in vitro fertilization. Fertil Steril. 2004;82:378–83. doi: 10.1016/j.fertnstert.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 35.Aitken RJ, Krausz C. Oxidative stress, DNA damage and the Y chromosome. Reproduction. 2001;122:497–506. doi: 10.1530/rep.0.1220497. [DOI] [PubMed] [Google Scholar]
- 36.Zini A, Boman JM, Belzile E, Ciampi A. Sperm DNA damage is associated with an increased risk of pregnancy loss after IVF and ICSI: systematic review and meta-analysis. Hum Reprod. 2008;23:2663–8. doi: 10.1093/humrep/den321. [DOI] [PubMed] [Google Scholar]
- 37.Robinson L, Gallos ID, Conner SJ, Rajkhowa M, Miller D, Lewis S, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod. 2012;27:2908–17. doi: 10.1093/humrep/des261. [DOI] [PubMed] [Google Scholar]
- 38.Feijo CM, Esteves SC. Diagnostic accuracy of sperm chromatin dispersion test to evaluate sperm deoxyribonucleic acid damage in men with unexplained infertility. Fertil Steril. 2014;101:58–63. doi: 10.1016/j.fertnstert.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Gosálvez J, Rodríguez-Predreira M, Mosquera A, López-Fernández C, Esteves SC, Agarwal A, et al. Characterisation of a subpopulation of sperm with massive nuclear damage, as recognised with the sperm chromatin dispersion test. Andrologia. 2014;46:602–9. [DOI] [PubMed]
- 40.Sharma R, Ahmad G, Esteves SC, Agarwal A. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using bench top flow cytometer for evaluation of sperm DNA fragmentation in fertility laboratories: protocol, reference values, and quality control. J Assist Reprod Genet. 2016;33:291–300. doi: 10.1007/s10815-015-0635-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esteves SC, Sánchez-Martín F, Sánchez-Martín P, Schneider DT, Gosálvez J. Comparison of reproductive outcome in oligozoospermic men with high sperm DNA fragmentation undergoing intracytoplasmic sperm injection with ejaculated and testicular sperm. Fertil Steril. 2015;104:1398–405. doi: 10.1016/j.fertnstert.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 42.Agarwal A, Gupta S, Du Plessis S, Sharma R, Esteves SC, Sabanegh E. Abstinence time and its impact on basic and advanced semen parameters. Urology. 2016. [DOI] [PubMed]
- 43.Practice Committee of the American Society for Reproductive Medicine Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril. 2015;103:e18–e25. doi: 10.1016/j.fertnstert.2014.12.103. [DOI] [PubMed] [Google Scholar]
- 44.Agarwal A, Hamada A, Esteves SC. Insight into oxidative stress in varicocele-associated male infertility: part 1. Nat Rev Urol. 2012;9:678–90. doi: 10.1038/nrurol.2012.197. [DOI] [PubMed] [Google Scholar]
- 45.Hamada A, Esteves SC, Agarwal A. Insight into oxidative stress in varicocele-associated male infertility: part 2. Nat Rev Urol. 2013;10:26–37. doi: 10.1038/nrurol.2012.198. [DOI] [PubMed] [Google Scholar]
- 46.Miyaoka R, Esteves SC. A critical appraisal on the role of varicocele in male infertility. Adv Urol. 2012;2012:597495. doi: 10.1155/2012/597495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dada R, Shamsi MB, Venkatesh S, Gupta NP, Kumar R. Attenuation of oxidative stress & DNA damage in varicocelectomy: implications in infertility management. Indian J Med Res. 2010;132:728–30. [PMC free article] [PubMed] [Google Scholar]
- 48.Wang YJ, Zhang RQ, Lin YJ, Zhang RG, Zhang WL. Relationship between varicocele and sperm DNA damage and the effect of varicocele repair: a meta-analysis. Reprod Biomed Online. 2012;25:307–14. doi: 10.1016/j.rbmo.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Esteves SC, Oliveira FV, Bertolla RP. Clinical outcome of intracytoplasmic sperm injection in infertile men with treated and untreated clinical varicocele. J Urol. 2010;184:1442–6. [DOI] [PubMed]
- 50.Bungum M, Humaidan P, Axmon A, Spano M, Bungum L, Erenpreiss J, et al. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod. 2007;22:174–9. doi: 10.1093/humrep/del326. [DOI] [PubMed] [Google Scholar]
- 51.Duran EH, Morshedi M, Taylor S, Oehninger S. Sperm DNA quality predicts intrauterine insemination outcome: a prospective cohort study. Hum Reprod. 2002;17:3122–8. doi: 10.1093/humrep/17.12.3122. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Z, Zhu L, Jiang H, Chen H, Chen Y, Dai Y. Sperm DNA fragmentation index and pregnancy outcome after IVF or ICSI: a meta-analysis. J Assist Reprod Genet. 2015;32:17–26. doi: 10.1007/s10815-014-0374-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Osman A, Alsomait H, Seshadri S, El-Toukhy T, Khalaf Y. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: a systematic review and meta-analysis. Reprod Biomed Online. 2015;30:120–7. doi: 10.1016/j.rbmo.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 54.Showell MG, Mackenzie-Proctor R, Brown J, et al. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2014;12:CD007411. doi: 10.1002/14651858.CD007411.pub3. [DOI] [PubMed] [Google Scholar]
- 55.Sanchez-Martin P, Sanchez-Martin F, Gonzalez-Martinez M, Gosalvez J. Increased pregnancy after reduced male abstinence. Syst Biol Reprod Med. 2013;59:256–60. doi: 10.3109/19396368.2013.790919. [DOI] [PubMed] [Google Scholar]
- 56.Gosálvez J, González-Martínez M, López-Fernández C, Fernández JL, Sánchez-Martín P. Shorter abstinence decreases sperm deoxyribonucleic acid fragmentation in ejaculate. Fertil Steril. 2011;96:1083–6. doi: 10.1016/j.fertnstert.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 57.Parmegiani L, Cognigni GE, Bernardi S, Troilo E, Taraborrelli S, Arnone A, et al. Comparison of two ready-to-use systems designed for sperm-hyaluronic acid binding selection before intracytoplasmic sperm injection: PICSI vs. Sperm Slow: a prospective, randomized trial. Fertil Steril. 2012;98:632–7. doi: 10.1016/j.fertnstert.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 58.Said TM, Grunewald S, Paasch U, Rasch M, Agarwal A, Glander HJ. Advantage of combining magnetic cell separation with sperm preparation techniques. Reprod Biomed Online. 2005;10:740–6. doi: 10.1016/S1472-6483(10)61118-2. [DOI] [PubMed] [Google Scholar]
- 59.Teixeira DM, Barbosa MA, Ferriani RA, Navarro PA, Raine-Fenning N, Nastri CO, et al. Regular (ICSI) versus ultra-high magnification (IMSI) sperm selection for assisted reproduction. Cochrane Database Syst Rev. 2013;7:CD010167. doi: 10.1002/14651858.CD010167.pub2. [DOI] [PubMed] [Google Scholar]
- 60.Greco E, Scarselli F, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, et al. Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoa. Hum Reprod. 2005;20:226–30. doi: 10.1093/humrep/deh590. [DOI] [PubMed] [Google Scholar]
- 61.Moskovtsev SI, Jarvi K, Mullen JB, Cadesky KI, Hannam T, Lo KC. Testicular spermatozoa have statistically significantly lower DNA damage compared with ejaculated spermatozoa in patients with unsuccessful oral antioxidant treatment. Fertil Steril. 2010;93:1142–6. doi: 10.1016/j.fertnstert.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 62.Zhao J, Zhang Q, Wang Y, Li Y. Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: a systematic review and metaanalysis. Fertil Steril. 2014;102:998–1005. doi: 10.1016/j.fertnstert.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 63.Cocuzza M, Alvarenga C, Pagani R. The epidemiology and etiology of azoospermia. Clinics. 2013;68(S1):15–26. doi: 10.6061/clinics/2013(Sup01)03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Esteves SC, Miyaoka R, Orosz JE, Agarwal A. An update on sperm retrieval techniques for azoospermic male. Clinics. 2013;68(S1):99–110. doi: 10.6061/clinics/2013(Sup01)11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schoor RA, Elhanbly S, Niederberger CS, Ross LS. The role of testicular biopsy in the modern management of male infertility. J Urol. 2002;167:197–200. doi: 10.1016/S0022-5347(05)65411-0. [DOI] [PubMed] [Google Scholar]
- 66.Esteves SC, Lee W, Benjamin DJ, Seol B, Verza S, Jr, Agarwal A. Reproductive potential of men with obstructive azoospermia undergoing percutaneous sperm retrieval and intracytoplasmic sperm injection according to the cause of obstruction. J Urol. 2013;189:232–7. doi: 10.1016/j.juro.2012.08.084. [DOI] [PubMed] [Google Scholar]
- 67.Fraietta R, Zylberstejn DS, Esteves SC. Hypogonadotropic hypogonadism revisited. Clinics (Sao Paulo) 2013;68(Suppl 1):81–8. doi: 10.6061/clinics/2013(Sup01)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hung AJ, King P, Schlegel PN. Uniform testicular maturation arrest: a unique subset of men with nonobstructive azoospermia. J Urol. 2007;178:608–12. doi: 10.1016/j.juro.2007.03.125. [DOI] [PubMed] [Google Scholar]
- 69.Bobjer J, Naumovska M, Giwercman YL, Giwercman A. High prevalence of androgen deficiency and abnormal lipid profile in infertile men with non-obstructive azoospermia. Int J Androl. 2012;35:688–94. doi: 10.1111/j.1365-2605.2012.01277.x. [DOI] [PubMed] [Google Scholar]
- 70.Monteiro RAC, Pariz JR, Pieri PC, Hallak J. An easy, reproducible and cost-effective method for andrologists to improve the laboratory diagnosis of non-obstructive azoospermia: a novel microcentrifugation technique. Int Braz J Urol. 2016;42:132–8. doi: 10.1590/S1677-5538.IBJU.2015.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aziz N. The importance of semen analysis in the context of azoospermia. Clinics (Sao Paulo) 2013;68(Suppl 1):35–8. doi: 10.6061/clinics/2013(Sup01)05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Esteves SC, Agarwal A. Re: sperm retrieval rates and intracytoplasmic sperm injection outcomes for men with non-obstructive azoospermia and the health of resulting offspring. Asian J Androl. 2014;16:642. doi: 10.4103/1008-682X.127817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Esteves SC, Miyaoka R, Agarwal A. Sperm retrieval techniques for assisted reproduction. Int Braz J Urol. 2011;37:570–83. doi: 10.1590/S1677-55382011000500002. [DOI] [PubMed] [Google Scholar]
- 74.Esteves SC, Verza S., Jr . PESA/TESA/TESE sperm processing. In: Nagy ZP, Varghese AC, Agarwal A, editors. Practical manual of in vitro fertilization. New York: Springer; 2012. pp. 207–20. [Google Scholar]
- 75.Verza S, Jr, Esteves SC. Microsurgical versus conventional single-biopsy testicular sperm extraction in nonobstructive azoospermia: a prospective controlled study. Fertil Steril. 2011;96(Suppl):S53. doi: 10.1016/j.fertnstert.2011.07.201. [DOI] [Google Scholar]
- 76.Tournaye H, Verheyen G, Nagy P, Ubaldi F, Goossens A, Silber S, et al. Are there any predictive factors for successful testicular sperm recovery in azoospermic patients? Hum Reprod. 1997;12:80–6. doi: 10.1093/humrep/12.1.80. [DOI] [PubMed] [Google Scholar]
- 77.Simoni M, Tuttelmann F, Gromoll J, Nieschlag E. Clinical consequences of microdeletions of the Y chromosome: the extended Munster experience. Reprod Biomed Online. 2008;16:289–303. doi: 10.1016/S1472-6483(10)60588-3. [DOI] [PubMed] [Google Scholar]
- 78.Hamada AJ, Esteves SC, Agarwal A. A comprehensive review of genetics and genetic testing in azoospermia. Clinics (Sao Paulo) 2013;68(Suppl 1):39–60. doi: 10.6061/clinics/2013(Sup01)06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krausz C, Hoefsloot L, Simoni M, Tüttelmann F. EAA/EMQN best practice guidelines for molecular diagnosis of Y-chromosomal microdeletions: state-of-the-art 2013. Andrology. 2014;2:5–19. doi: 10.1111/j.2047-2927.2013.00173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peterlin B, Kunej T, Sinkovec J, Gligorievska N, Zorn B. Screening for Y chromosome microdeletions in 226 Slovenian subfertile men. Hum Reprod. 2002;17:17–24. doi: 10.1093/humrep/17.1.17. [DOI] [PubMed] [Google Scholar]
- 81.Navarro-Costa P, Plancha CE, Gonçalves J. Genetic dissection of the AZF regions of the human Y chromosome: thriller or filler for male (in)fertility? J Biomed Biotechnol. 2010;2010:936569. doi: 10.1155/2010/936569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Patsalis PC, Sismani C, Quintana-Murci L, Taleb-Bekkouche F, Krausz C, McElreavey K. Effects of transmission of Y chromosome AZFc deletions. Lancet. 2000;360:1222–4. doi: 10.1016/S0140-6736(02)11248-7. [DOI] [PubMed] [Google Scholar]
- 83.Siffroi JP, Le Bourhis C, Krausz C, Barbaux S, Quintana-Murci L, Kanafani S, et al. Sex chromosome mosaicism in males carrying Y chromosome long arm deletions. Hum Reprod. 2000;15:2559–62. doi: 10.1093/humrep/15.12.2559. [DOI] [PubMed] [Google Scholar]
- 84.Rajpert-De Meyts E, Ottesen AM, Garn ID, Aksglaede L, Juul A. Deletions of the Y chromosome are associated with sex chromosome aneuploidy but not with Klinefelter syndrome. Acta Paed. 2011;100:900–2. doi: 10.1111/j.1651-2227.2011.02169.x. [DOI] [PubMed] [Google Scholar]
- 85.Coviello AD, Bremner WJ, Matsumoto AM, Herbst KL, Amory JK, Anawalt BD, et al. Intratesticular testosterone concentrations comparable with serum levels are not sufficient to maintain normal sperm production in men receiving a hormonal contraceptive regimen. J Androl. 2004;25:931–938. doi: 10.1002/j.1939-4640.2004.tb03164.x. [DOI] [PubMed] [Google Scholar]
- 86.Kato Y, Shiraishi K, Matsuyama H. Expression of testicular androgen receptor in non-obstructive azoospermia and its change after hormonal therapy. Andrology. 2014;2:734–40. doi: 10.1111/j.2047-2927.2014.00240.x. [DOI] [PubMed] [Google Scholar]
- 87.Kumar R. Medical management of non-obstructive azoospermia. Clinics. 2013;68(S1):75–9. doi: 10.6061/clinics/2013(Sup01)08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shinjo E, Shiraishi K, Matsuyama H. The effect of human chorionic gonadotropin-based hormonal therapy on intratesticular testosterone levels and spermatogonial DNA synthesis in men with non-obstructive azoospermia. Andrology. 2013;1:929–35. doi: 10.1111/j.2047-2927.2013.00141.x. [DOI] [PubMed] [Google Scholar]
- 89.Shiraishi K, Ohmi C, Shimabukuro T, Matsuyama H. Human chorionic gonadotrophin treatment prior to microdissection testicular sperm extraction in non-obstructive azoospermia. Hum Reprod. 2012;27:331–9. doi: 10.1093/humrep/der404. [DOI] [PubMed] [Google Scholar]
- 90.Hussein A, Ozgok Y, Ross L, Rao P, Niederberger C. Optimization of spermatogenesis-regulating hormones in patients with non-obstructive azoospermia and its impact on sperm retrieval: a multicentre study. BJU Int. 2013;111(3, pt. B):E110–4. doi: 10.1111/j.1464-410X.2012.11485.x. [DOI] [PubMed] [Google Scholar]
- 91.Reifsnyder JE, Ramasamy R, Husseini J, Schlegel PN. Role of optimizing testosterone before microdissection testicular sperm extraction in men with nonobstructive azoospermia. J Urol. 2012;188:532–6. doi: 10.1016/j.juro.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 92.Weedin JW, Khera M, Lipshultz LI. Varicocele repair in patients with nonobstructive azoospermia: a meta-analysis. J Urol. 2010;183:2309–15. doi: 10.1016/j.juro.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 93.Esteves SC, Glina S. Recovery of spermatogenesis after microsurgical subinguinal varicocele repair in azoospermic men based on testicular histology. Int Braz J Urol. 2005;31:541–8. doi: 10.1590/S1677-55382005000600005. [DOI] [PubMed] [Google Scholar]
- 94.Esteves SC, Miyaoka R, Roque M, Agarwal A. Outcome of varicocele repair in men with nonobstructive azoospermia: systematic review and meta-analysis. Asian J Androl. 2016;18:246–53. doi: 10.4103/1008-682X.169562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schlegel PN, Su LM. Physiological consequences of testicular sperm extraction. Hum Reprod. 1997;12:1688–92. doi: 10.1093/humrep/12.8.1688. [DOI] [PubMed] [Google Scholar]
- 96.Deruyver Y, Vanderschueren D, Van der Aa F. Outcome of microdissection TESE compared with conventional TESE in non-obstructive azoospermia: a systematic review. Andrologia. 2014;2:20–24. doi: 10.1111/j.2047-2927.2013.00148.x. [DOI] [PubMed] [Google Scholar]
- 97.Bernie AM, Mata DA, Ramasamy R, Schlegel PN. Comparison of microdissection testicular sperm extraction, conventional testicular sperm extraction, and testicular sperm aspiration for nonobstructive azoospermia: a systematic review and meta-analysis. Fertil Steril. 2015;104:1099–103. doi: 10.1016/j.fertnstert.2015.07.1136. [DOI] [PubMed] [Google Scholar]
- 98.Esteves SC. Microdissection testicular sperm extraction (micro-TESE) as a sperm acquisition method for men with nonobstructive azoospermia seeking fertility: operative and laboratory aspects. Int Braz J Urol. 2013;39:440–1. doi: 10.1590/S1677-5538.IBJU.2013.03.21. [DOI] [PubMed] [Google Scholar]
- 99.Ashraf MC, Singh S, Raj D, Ramakrishnan S, Esteves SC. Micro-dissection testicular sperm extraction as an alternative for sperm acquisition in the most difficult cases of azoospermia: technique and preliminary results in India. J Hum Reprod Sci. 2013;6:111–23. doi: 10.4103/0974-1208.117175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Esteves SC, Agarwal A. Reproductive outcomes, including neonatal data, following sperm injection in men with obstructive and nonobstructive azoospermia: case series and systematic review. Clinics (Sao Paulo) 2013;68(Suppl. 1):141–50. doi: 10.6061/clinics/2013(Sup01)16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schlegel PN. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod. 1999;14:131–5. doi: 10.1093/humrep/14.1.131. [DOI] [PubMed] [Google Scholar]
- 102.Esteves SC, Varghese AC. Laboratory handling of epididymal and testicular spermatozoa: what can be done to improve sperm injections outcome. J Hum Reprod Sci. 2012;5:233–43. doi: 10.4103/0974-1208.106333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Meseguer M, Santiso R, Garrido N, Gil-Salom M, Remohí J, Fernandez JL. Sperm DNA fragmentation levels in testicular sperm samples from azoospermic males as assessed by the sperm chromatin dispersion test. Fertil Steril. 2009;92:1638–45. doi: 10.1016/j.fertnstert.2008.08.106. [DOI] [PubMed] [Google Scholar]
- 104.Vozdova M, Heracek J, Sobotka V, Rubes J. Testicular sperm aneuploidy in non-obstructive azoospermic patients. Hum Reprod. 2012;27:2233–9. doi: 10.1093/humrep/des115. [DOI] [PubMed] [Google Scholar]
- 105.Verza S, Jr, Esteves SC. Sperm defect severity rather than sperm source is associated with lower fertilization rates after intracytoplasmic sperm injection. Int Braz J Urol. 2008;34:49–56. doi: 10.1590/S1677-55382008000100008. [DOI] [PubMed] [Google Scholar]
- 106.Esteves SC, Prudencio C, Seol B, Verza S, Knoedler C, Agarwal A. Comparison of sperm retrieval and reproductive outcome in azoospermic men with testicular failure and obstructive azoospermia treated for infertility. Asian J Androl. 2014;16:602–6. doi: 10.4103/1008-682X.126015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aydos K, Demirel LC, Baltaci V, Unlü C. Enzymatic digestion plus mechanical searching improves testicular sperm retrieval in non-obstructive azoospermia cases. Eur J Obstet Gynecol Reprod Biol. 2005;120:80–6. doi: 10.1016/j.ejogrb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 108.Baukloh V. Retrospective multicentre study on mechanic and enzymatic preparation of fresh and cryopreserved testicular biopsies. Hum Reprod. 2002;17:1788–94. doi: 10.1093/humrep/17.7.1788. [DOI] [PubMed] [Google Scholar]
- 109.Ozkavukcu S, Ibis E, Kizil S, Isbacar S, Aydos K. A laboratory modification to testicular sperm preparation technique improves spermatogenic cell yield. Asian J Androl. 2014;16:852–7. doi: 10.4103/1008-682X.132468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Popal W, Nagy ZP. Laboratory processing and intracytoplasmic sperm injection using epididymal and testicular spermatozoa: what can be done to improve outcomes. Clinics. 2013;68(S1):125–30. doi: 10.6061/clinics/2013(Sup01)14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Esteves SC, Bento FC. Implementation of cleanroom technology in reproductive laboratories: the question is not why but how. Reprod Biomed Online. 2016;32(1):9–11. doi: 10.1016/j.rbmo.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 112.Esteves SC, Bento FC. Air quality control in the ART laboratory is a major determinant of IVF success. Asian J Androl. 2015;18:596–9. [DOI] [PMC free article] [PubMed]
- 113.Esteves SC, Bento FC. Implementation of air quality control in reproductive laboratories in full compliance with the Brazilian Cells and Germinative Tissue Directive. Reprod Biomed Online. 2013;26:9–21. doi: 10.1016/j.rbmo.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 114.Endo Y, Fujii Y, Shintani K, Seo M, Motoyama H, Fu H. Simple vitrification for small numbers of human spermatozoa. Reprod Biomed Online. 2012;24:301–7. doi: 10.1016/j.rbmo.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 115.Coetzee K, Ozgur K, Berkkanoglu M, Bulut H, Isikli A. Reliable single sperm cryopreservation in Cell Sleepers for azoospermia management. Andrologia. 2016;48:203–10. doi: 10.1111/and.12434. [DOI] [PubMed] [Google Scholar]
- 116.He X, Cao Y, Zhang Z, Zhao J, Wei Z, Zhao J, et al. Spermatogenesis affects the outcome of ICSI for azoospermic patients rather than sperm retrieval method. Syst Biol Reprod Med. 2010;56:457–64. doi: 10.3109/19396368.2010.513078. [DOI] [PubMed] [Google Scholar]
- 117.Fedder J, Gabrielsen A, Humaidan P, Erb K, Ernst E, Loft A. Malformation rate and sex ratio in 412 children conceived with epididymal or testicular sperm. Hum Reprod. 2007;22:1080–5. doi: 10.1093/humrep/del488. [DOI] [PubMed] [Google Scholar]
- 118.Belva F, De Schrijver F, Tournaye H, Liebaers I, Devroey P, Haentjens P, et al. Neonatal outcome of 724 children born after ICSI using non-ejaculated sperm. Hum Reprod. 2011;26:1752–8. doi: 10.1093/humrep/der121. [DOI] [PubMed] [Google Scholar]
- 119.Aponte PM, Schlatt S, Franca LR. Biotechnological approaches to the treatment of aspermatogenic men. Clinics (Sao Paulo) 2013;68(Suppl. 1):157–67. doi: 10.6061/clinics/2013(Sup01)18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vloeberghs V, Verheyen G, Tournaye H. Intracytoplasmic spermatid injection and in vitro maturation: fact or fiction? Clinics (Sao Paulo) 2013;68(Suppl. 1):151–6. doi: 10.6061/clinics/2013(Sup01)17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kumar M, Kumar K, Jain S, Hassan T, Dada R. Novel insights into the genetic and epigenetic paternal contribution to the human embryo. Clinics (Sao Paulo) 2013;68(Suppl 1):5–14. doi: 10.6061/clinics/2013(Sup01)02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sato T, Katagiri K, Gohbara A, Inoue K, Ogonuki N, Ogura A, et al. In vitro production of functional sperm in cultured neonatal mouse testes. Nature. 2011;471:504–7. doi: 10.1038/nature09850. [DOI] [PubMed] [Google Scholar]
- 123.Lee DR, Kim KS, Yang YH, Oh HS, Lee SH, Chung TG, et al. Isolation of male germ stem cell-like cells from testicular tissue of non-obstructive azoospermic patients and differentiation into haploid male germ cells in vitro. Hum Reprod. 2006;21:471–6. doi: 10.1093/humrep/dei319. [DOI] [PubMed] [Google Scholar]