Abstract
Purpose
The purpose of this study was to undertake a review of the available evidence comparing the use of a single medium versus sequential media for embryo culture to the blastocyst stage in clinical IVF.
Methods
We searched the Cochrane Central, PubMed, Scopus, ClinicalTrials.gov, Current Controlled Trials and WHO International Clinical Trials Registry Platform to identify randomized controlled trials comparing single versus sequential media for blastocyst culture and ongoing pregnancy rate. Included studies randomized either oocytes/zygotes or women. Eligible oocyte/zygote studies were analyzed to assess the risk difference (RD) and 95 % confidence intervals (CI) between the two media systems; eligible woman-based studies were analyzed to assess the risk ratio (RR) and 95 % CI for clinical pregnancy rate.
Results
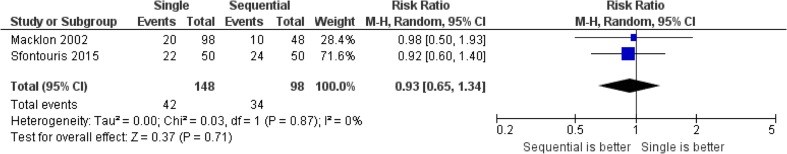
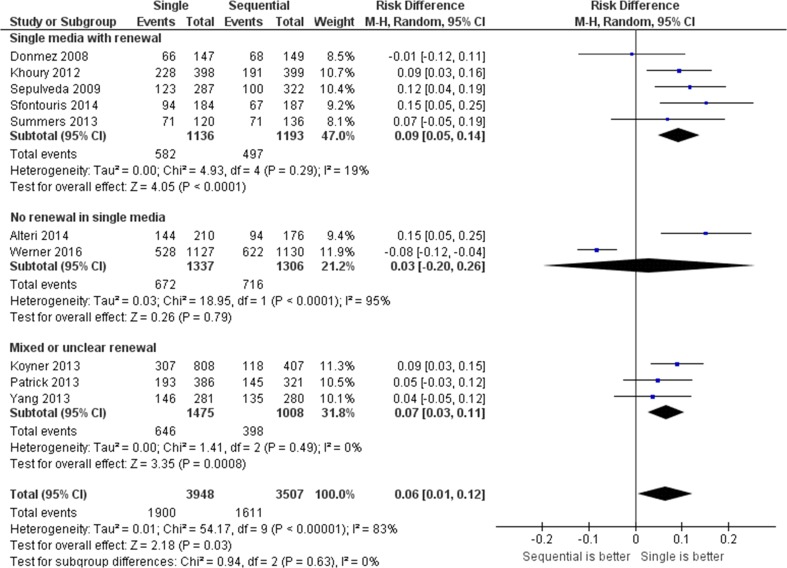
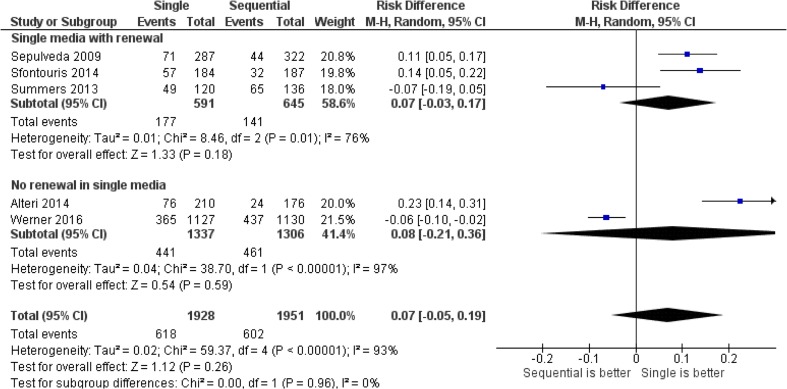
No differences were observed between single and sequential media for either ongoing pregnancy per randomized woman (relative risk (RR) = 0.9, 95 % CI = 0.7 to 1.3, two studies including 246 women, I 2 = 0 %) or clinical pregnancy per randomized woman (RR = 1.0, 95 % CI = 0.7 to 1.4, one study including 100 women); or miscarriage per clinical pregnancy: RR = 1.3, 95 % CI = 0.4 to 4.3, two studies including 246 participants, I 2 = 0 %). Single media use was associated with an increase blastocyst formation per randomized oocyte/zygote (relative distribution (RD) = +0.06, 95 % CI = +0.01 to +0.12, ten studies including 7455 oocytes/zygotes, I 2 = 83 %) but not top/high blastocyst formation (RD = +0.05, 95 % CI = −0.01 to +0.11, five studies including 3879 oocytes/zygotes, I 2 = 93 %). The overall quality of the evidence was very low for all these four outcomes.
Conclusions
Although using a single medium for extended culture has some practical advantages and blastocyst formation rates appear to be higher, there is insufficient evidence to recommend either sequential or single-step media as being superior for the culture of embryos to days 5/6. Future studies comparing these two media systems in well-designed trials should be performed.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-016-0774-5) contains supplementary material, which is available to authorized users.
Keywords: Single, Sequential, Medium, Culture, Extended, Blastocyst
Introduction
The evolution and optimization of culture media that sustain embryo viability and development in vitro have played vital roles in the improvement of the assisted reproductive technologies and the associated increases in pregnancy rates over time. There are now numerous commercial culture media available, which raise the question as to whether any one medium or media system is superior to any other. In addition, increased access to infertility interventions worldwide has resulted in the expansion of IVF laboratories and expertise in embryology, all of which would benefit from global guidance on this issue. Although several studies have been conducted to investigate the comparison of single versus sequential media, few well-designed randomized controlled trials have been performed, and interpretation of study findings is inevitably confounded by IVF laboratory and patient population heterogeneities. Therefore, we performed this systematic review in an attempt to determine whether there is sufficient evidence to prove that one culture medium system for culturing human embryos is superior to another. Unlike a recent Cochrane Review that also evaluated the efficacy of various media for culturing human preimplantation embryos [1], this present review is focused exclusively on comparing single versus sequential media for culturing embryos to the blastocyst stage.
Culture media to support embryo development to the blastocyst stage have been based on two distinct approaches: the “back to nature” sequential approach and the “let the embryo choose” single medium approach [2–4]. Initial attempts of culturing human embryos to the blastocyst stage in a simple single-step media (Earle’s balanced salt solution, T6 medium or MEM) resulted in disappointingly low implantation and pregnancy rates [5–8].
The first reports of a reliable approach for successful extended culture of human embryos depended upon a sequential culture media system, which was designed to meet identified changing metabolic and nutritional requirements of the developing embryo from day 1 through to day 5 or 6 [9–12]. With this sequential media approach, embryos are grown from days 1–3 in a first growth medium and then, at the cleavage stage on day 3, are moved to a second medium (a “blastocyst” medium). The goal with this approach is to expose the embryos to stage-specific media, designed to reflect observed changes in concentrations of pyruvate, lactate, and glucose in the Fallopian tube versus the uterus [11].
Shortly after the development of this first sequential media system [13], there was a renewed interest in the use of a single medium to support development of the embryo during the preimplantation period from days 1 through days 5/6. The first of these single media was potassium simplex optimization (KSOM) medium, originally developed to grow mouse embryos in vitro [14], but which was subsequently proven capable of supporting culture of human embryos [15, 16]. Single culture media aim at allowing developing embryos to choose the nutrients they require, while at the same time minimizing stress from exposure to an abrupt change in their culture environment on day 3. However, single media may be used in a two-step culture, with a medium change on day 3, or in a single-step uninterrupted culture, in which there is no medium replenishment on day 3 [17].
The comparative performance of single and sequential culture media has attracted increased attention, and numerous new generation single media have become available, challenging the necessity of sequential media for extended embryo culture. Available data suggest that both types of media seem to provide adequate support to the developing embryo [3]. However, the clinical efficiency and safety of single versus sequential media are still unclear; the studies that have been performed to date indicate the need for a review of the best available evidence to facilitate a more robust conclusion.
Our objective was to identify, appraise, and summarize the available evidence comparing the efficacy and safety of embryo culture in a single medium with the outcomes of culture in sequential media in women undergoing in vitro fertilization (IVF) treatment with or without the use of intracytoplasmic sperm injection (ICSI). A preliminary analysis was presented during a World Health Organization consultation in order to support its development of global guidance and subsequently updated and revised as requested during consensus.
Materials and methods
Protocol and registration
The protocol for this review was registered at PROSPERO (CRD42015023942).
Eligibility criteria
Only randomized controlled trials (RCTs) comparing sequential versus single media for blastocyst culture were considered eligible, regardless of whether or not the single medium was renewed on day 3 (i.e., whether a fresh, new drop of the same medium was used for culture from day 3 to day 5). We included studies that randomized either oocytes/zygotes or women; however, the studies were analyzed separately as an oocyte/zygote-based review and a woman-based review. As the focus of this review was to compare results of these two media systems on outcomes following extended culture to the blastocyst stage, studies comparing single versus sequential media culture for fertilization/cleavage were not considered eligible.
Information sources
We searched for RCTs in the following electronic databases from their inception: Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, and Scopus. We searched for study protocols and ongoing trials in the following trials registers: ClinicalTrials.gov (www.clinicaltrials.gov); Current Controlled Trials (www.controlled-trials.com/isrctn/); and World Health Organization International Clinical Trials Registry Platform (WHO-ICTRP) (www.who.int/trialsearch/Default.aspx). We searched for conference proceedings in Web of Science (http://apps.webofknowledge.com/). The following terms were used, adjusting for each database as necessary: (sequential OR two-step) AND (continuous OR single OR single-step OR monoculture) AND (culture OR media OR medium) AND (embryo OR blastocyst OR IVF OR “in vitro fertilization” OR ICSI OR “intracytoplasmic sperm injection”). Additionally, we hand-searched the reference list from included trials and similar reviews.
Study selection
The records were screened independently by two of the authors (PAN and IGRV) and full texts were obtained when necessary; disagreements were solved by consulting two other authors (WPM and IAS). Authors corresponded with study investigators to clarify study eligibility when required. There was no limitation regarding language, publication date, or publication status.
Data collection process
We extracted data from included studies using a data extraction form designed and pilot-tested by the authors. We corresponded with study investigators in order to solve any query, as required. Data was extracted independently in a standardized manner by two authors (PAN and IGRV) and checked by another (CON); disagreements were solved by consulting another author (WPM). Where trials had multiple publications, the main trial report was used as the reference and additional details were supplemented from secondary sources.
Data items
Data were sought for the following variables: country, funding sources, conflicts of interest, period of enrollment, inclusion criteria, exclusion criteria, media used, number of women included, number of oocytes included, number of embryos transferred, and age of participants.
For the woman-based review, the primary outcome of effectiveness was live birth per randomized woman. Where live birth was not reported, ongoing pregnancy (clinical pregnancy with reduced chance of miscarriage, usually above 10–16 weeks) was used as a surrogate. The primary outcome for safety was planned to be congenital anomaly per clinical pregnancy; however, no study reported this outcome. As secondary outcomes, we evaluated clinical pregnancy per woman randomized and miscarriage per clinical pregnancy.
For the oocyte-based review, the primary outcome was the number of embryos that developed to the blastocyst stage (D5-6) per oocyte or zygote randomized. For secondary outcomes, we evaluated the proportion of embryos that developed into top/high quality blastocysts (D5-6); both outcomes were calculated per oocyte or embryo randomized. Authors were contacted to obtain missing data; no assumptions were made when data were not obtainable.
Risk of bias in individual studies
Two authors (PAN, IGRV) independently assessed the included studies for the risk of selection bias (random sequence generation and allocation concealment); performance bias (blinding of participants and personnel); detection bias (blinding of outcome assessors); attrition bias (incomplete outcome data); reporting bias (selective outcome reporting); and other potential sources of bias (e.g., difference in the number of embryos transferred, age of participants, co-interventions, early stopping). The risk of bias was checked by a third author (CON) and disagreements were solved by consulting another author (WPM). Blinding was considered to be an important source of detection bias in the oocyte-based review; however, it was not considered to influence the reproductive outcomes in the woman-based review. To judge the risk of bias, we followed the Cochrane Collaboration’s criteria for judging risk of bias [18]: the trials were classified as being at “low,” “high,” or “unclear” risk of bias.
Summary measures
The differences observed between culture media were summarized as risk ratio (RR) for the clinical outcomes and as risk difference (RD) for the laboratory outcomes; the precisions of the estimates were evaluated by the 95 % confidence intervals (CI). We considered the clinical relevance of all comparisons taking into account the precision of the estimates.
Synthesis of results
The results were combined for meta-analysis using Review Manager 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) in a random-effects model because the intervention varied among the studies. Heterogeneity was assessed by the I 2 statistic. The data from primary studies comparing “Single media” versus “Sequential media” were combined. An increase in the risk of a particular outcome associated with Single media, which may be beneficial (e.g., live birth) or detrimental (e.g., miscarriage), was displayed graphically in the meta-analyses to the right of the center line and a decrease in the risk of an outcome to the left of the center line.
Risk of bias across studies
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, the authors attempted to minimize their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data.
Additional analyses
Any observed heterogeneity was taken into account in the interpretation of the estimates. Subgroup analysis was performed by separating the studies with media renewal from those without media renewal in the single media group.
Overall quality of the evidence
We considered the limitations of included studies (e.g., high risk of bias), inconsistency of effect, imprecision, indirectness, and publication bias [19]. Judgments about the quality of evidence were justified, documented, and incorporated into the reporting of results for each outcome. The quality of evidence for each outcome was evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group recommendations: High = We are very confident that the true effect lies close to the observed in this review; Moderate = We are moderately confident in the effect estimate: The true effect is likely to be close to the observed in this review, but there is a possibility that it is substantially different; Low = Our confidence in the effect estimate is limited: The true effect may be substantially different from the observed in this review; Very low = We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the observed in this review.
Results
Study selection
The last electronic search was run on February 20, 2016 and a total of 528 records were retrieved: CENTRAL = 24; PubMed = 133; Scopus = 195; Web of Science = 164; ClinicalTrials = 11; ISRCTN = 1; WHO ICTRP = 0. From the 528 records, we excluded 508 records after reading titles and abstracts: 145 were duplicates and 363 clearly did not meet the eligibility criteria. We further examined 23 studies for eligibility:
A total of three studies were excluded: Two studies used Fertilization and then Cleavage Medium and not a system for Cleavage and then Blastocyst [20, 21], and one study was excluded because a different oxygen tension was used in the different groups [22].
A total of 20 studies were included in this review: Ten studies that randomized oocytes/zygotes [23–32] and two studies that randomized women [33, 34] were included in the forest plots; however, six studies that randomized oocytes/zygotes [35–40] and two studies that randomized women [41, 42] were not included in the forest plots because it was not possible to extract data for the meta-analyses.
Study characteristics
The main characteristics of the included studies are reported in Table 1.
Table 1.
Characteristics of the included studies
| Study | Country | Enrollment | Eligibility criteria | Culture condition (single) | Culture condition (sequential) | Authors’ conclusions |
|---|---|---|---|---|---|---|
| Studies included in the quantitative analysis | ||||||
| Randomization of women | ||||||
| Macklon 2002 | The Netherlands | Sep 1999 to Sep 2000 | Indication for IVF | Rotterdam culture media (Group 1: without change on D3—Rotterdam monoculture system; Group 2: media changed on D3—Rotterdam serial culture system); culture in a closed incubator at 37 °C in 5 % CO2 | Vitrolife G1 until day 3 and Vitrolife G2 until day 5; culture in a closed incubator at 37 °C in 5 % CO2 | No significant differences in blastulation and implantation rates |
| Sfontouris 2015 | Greece | Jan 2014 to Jun 2014 | Age ≤ 40 years; at least 10 retrieved oocytes; fresh cycles with own eggs | Global medium, media was not changed on D3 | Origio (ISM1/BlastAssist), media was changed on D3 | Implantation and pregnancy rates, as well as blastocyst formation and embryo utilization rates were similar |
| Randomization of oocytes/zygotes | ||||||
| Alteri 2014 | Italy | Sep 2014 to Dec 2014 | Women aged ≤39 years | Unclear media, SAGE, media was not changed on D3; culture was in multi-gas incubators (Sanyo) at 37 °C, 5 % 02, 5.5 % CO2 | Unclear media, SAGE, media was changed on D3; culture was in multi-gas incubators (Sanyo) at 37 °C, 5 % 02, 5.5 % CO2 | Single media yielded a significant higher blastocyst formation and top-quality blastocyst rate |
| Donmez 2008 | Turkey | NR | NR | Single-Step Medium (SSM), media was not changed on D3 | Sequential complete P-1 Medium, media was changed on D3 | Single media seems to be more effective on blastomere compaction and blastocyst development |
| Khoury 2012 | USA | NR | NR | Global, media was changed on D3; Global medium was supplemented with Life Global Protein Supplement, overlaid with Global mineral Lite Oil | Quinn’s Advantage (QA) cleavage medium until day 3 and (QA) blastocyst medium until day 6; SAGE media was supplemented with SAGE SPS protein, overlaid with SAGE mineral oil | Single media increased blastocyst formation and number of embryos transferred and resulted in slightly improved clinical pregnancy rate and implantation rate |
| Koyner 2013 | Panama | NR | First IVF treatment | SMS (Global), half renewal and half no renewal media on D3; culture was at 37 °C and 6 % CO2 | Sequential Media System—SQMS (SAGE) media was changed on D3; culture was at 37 °C and 6 % CO2 | The renew of 50 % of media during the embryo culture in SMS increase the embryo cell division and have better potential in achieving good pregnancy and implantation rate |
| Patrick 2013 | North Carolina | NR | NR | Single media: not reported | Sequential media: not reported | Blastocyst formation was similar |
| Sepulveda 2009 | Peru | Mar 2006 to Oct 2006 | Oocytes donors age 19–34 years (not randomized); recipients (randomized) | Global medium, media was change D3; culture was at 5.6 % CO2 | Irvine Early Cleavage Medium (ECM) until day 3 and Multiblast (Irvine Scientific) until day 5–6; culture was at 5.6 % CO2 | A single medium was as good as or better than a sequential media system for human embryo culture from the zygote to blastocyst stage |
| Sfontouris 2014 | Greece | Oct 2013 to Jan 2014 | Age ≤ 40 years; ≥10 retrieved oocytes | Global medium, media was change D3 | Origio (ISM1/BlastAssist), media was changed on D3 | Culture in a single-step medium is associated with higher blastocyst formation rates, better blastocyst quality, and higher blastocyst utilization rates compared to sequential media |
| Summers 2013 | London | NR | Age ≤ 38 years, first or second IVF cycle, ≥6 2PN | Global medium, media was change D3; culture was in Planar BT37 incubators (Planar) humidified at 37 ± 0.5 °C in an atmosphere of 6 % CO2/6 % O2/88 % N2 | Quinn’s Advantage Cleavage medium (SAGE) until day 3 and Quinn’s Advantage Blastocyst medium until day 6; culture was in Planar BT37 incubators (Planar) humidified at 37 ± 0.5 °C in an atmosphere of 6 % CO2/6 % O2/88 % N2 | No differences were noted in the rates of blastocyst development, inner cell mass (ICM), and trophectoderm (TE) scores in the two culture media |
| Werner 2016 | New Jersey | Aug 2013 to Mar 2015 | Age ≤ 42 years, FSH < 12 IU/L, AMH ≥ 1.2 ng/mL, and AFC ≥ 12, not requiring surgical sperm extraction | Continuous Single Culture (Irvine Scientific), media was not change D3; culture was in 5 % O2 | Quinn’s Advantage Cleavage Medium (SAGE) followed by Blast Assist (Origio), media was changed on D3; culture was in 5 % O2 | Blastocyst rate is greatest after culture in the sequential media. However, no difference exists in timing of blastulation, aneuploidy, or sustained implantation rates |
| Yang 2013 | China | NR | Indication for preimplantation genetic screening | Single culture media—CSC, Irvine Scientific, not clear if renewal media on D3 | Vitrolife G1 until day 3 and Vitrolife G2 until days 5 and 6 | The key morphokinetical parameters and percentage of euploid blastocysts are similar |
| Studies not included in the quantitative analysis | ||||||
| Randomization of women | ||||||
| Koscinski 2014 | France | NR | First and second IVF attempts with own eggs, age < 37 years, no indication for PGD | Global medium, media was not changed on D3 | Sequential media Cook, media was changed on D3 | More physiologic embryo development kinetics and higher rates of blastocyst were after culture in the sequential media |
| Scarica 2015 | Italy | Sep 2013 to Sep 2014 | NR | Single-step medium, media was not changed on D3; culture was in a bench\top!incubator (MINC) or in the Embryoscope (Unisense) | Embryo cultured, SAGE, media was changed on D3; culture was in a bench\top!incubator (MINC) or in the Embryoscope (Unisense) | Single medium culture system has an increased blastocyst developmental rate. No significant differences were found in aneuploidy rate and clinical outcomes |
| Randomization of oocytes/zygotes | ||||||
| Ciray 2012 | Turkey | Mar 2011 to Dec 2011 | Women who retrieved 2–6 MII, Age < 40 years, no indication for PGD | Single-Step Media, SSM, media was changed on D3; culture was in time-lapse incubator (Embryoscope TM) at 37 °C, 502, 6 % CO2 | Early Cleavage Media, ECM, until day 3 and MultiBlast Media, MB, until Day 6; culture was in time-lapse incubator (Embryoscope TM) at 37 °C, 5 % 02, 6 % CO2 | The overall clinical and embryological parameters remain similar |
| Hardarson 2015 | Four centers, two in Sweden and two in the USA | Sep 2013 to Feb 2014 | Age ≤ 40 years, ≥ 6 2PN, not requiring surgical sperm extraction | Vitrolife (G-TL), media was not changed on D3; Culture was in time-lapse incubator at 37° C (6–7 % CO2, 5 % O2, and 89 % N2) | Vitrolife G1 until day 3 and Vitrolife G2 until day 5–6; culture was in time-lapse incubator at 37 °C (6–7 % CO2, 5 % O2, and 89 % N2) | Two media systems are equivalent in relation to blastocyst quality and rate |
| Reed 2009 | USA | Jun 2008 to Dec 2008 | ≥2 2PN, no indication for PGD or PGS | Global medium (IVFonline), media was not changed on D3; Culture was at 37 °C and 6 % CO2 | Vitrolife G1 until day 3 and Vitrolife G2 until days 5 and 6; culture was at 37 °C and 6 % CO2 | There is no apparent advantage in using sequential media over a single medium |
| Schneider 2009 | Brazil | NR | NR | Global medium (LifeGlobal), media was change D3 | Vitrolife G1 until day 3 and Vitrolife G2 until day 5 | Single medium is as effective as sequential medium for the development of human embryo to the blastocyst stage |
| Vansteenbrugge 2007 | Belgium | NR | ≥7 retrieved oocytes | Global medium, IVFonline, media was changed on D3; culture was in 6 % CO2 and 5 % O2 at 37 °C | Vitrolife G1 until day 3 and Vitrolife G2 until day 5; culture was in 6 % CO2 and 5 % O2 at 37 °C | The ability of embryos cultured in the single medium to further development to blastocyst and implant is similar to embryos cultured in the sequential media |
| Velez de la Calle 2013 | France | Oct 2012 to Mar 2013 | NR | Continuous single culture—CSC from Irvine, media was not changed on D3; culture was in 6 % CO2 at 37 °C | Ferticult from Fertipro until day 3 and CCM from Vitrolife until day 5; culture was in 6 % CO2 at 37 °C | Single media resulted in more blastocysts, although clinical pregnancy rate was not significantly different |
Risk of bias within studies
From the 20 studies included, only two were deemed to be at low risk of bias in every domain [34, 35]. For nine studies, the information retrieved from the publications and from contacting the authors was insufficient for a complete assessment of the risk of bias; they were therefore deemed to be at unclear risk of bias [23, 25–28, 31, 38–40]. Nine studies were deemed to be at high risk of bias in at least one domain. One was considered at high risk of selection bias because the allocation was not properly concealed [29]; three were at risk of detection bias because the embryologist who assessed the embryo characteristics was not blinded to the allocation [30, 32, 37]; three were at high risk of attrition bias because of loss of participants [33] or because not all embryos were followed up until the blastocyst stage [24, 36]. All the included studies were judged to be at low risk of reporting bias, and two studies were judged to be at high risk of other bias: one study because only preliminary results were published [41] and another study because two types of incubators were used and it is unclear whether the distribution of the embryos in each group was similar or not [42]. The judgments and explanations regarding the risk of bias of the included studies are reported in Supplemental Table 1.
Results of individual studies
The results of the individual studies included in meta-analyses are presented in the forest plots (Figs. 1, 2, and 3 and Supplemental Figures 1 and 2). The main conclusions of all studies are reported in Table 1.
Fig. 1.
Forest plot for ongoing pregnancy per randomized woman
Fig. 2.
Forest plot for blastocyst formation per randomized oocyte/zygote
Fig. 3.
Forest plot for high-/top-quality blastocyst formation per randomized oocyte/zygote
Synthesis of results
No differences were observed between single and sequential media for ongoing pregnancy per randomized woman (relative risk (RR) = 0.9, 95 % CI = 0.7 to 1.3, two studies including 246 women, I 2 = 0 %), clinical pregnancy per randomized woman (RR = 1.0, 95 % CI = 0.7 to 1.4, one study including 100 women), or miscarriage per clinical pregnancy: RR = 1.3, 95 % CI = 0.4 to 4.3, two studies including 246 participants, I 2 = 0 %). Single media use was associated with an increase in blastocyst formation rate per randomized oocyte/zygote (relative distribution (RD) = +0.06, 95 % CI = +0.01 to +0.12, ten studies including 7455 oocytes/zygotes, I 2 = 83 %) but not in top/high blastocyst formation (RD = +0.05, 95 % CI = −0.01 to +0.11, five studies including 3879 oocytes/zygotes, I 2 = 93 %). The pooled results for the outcomes evaluated by this review are presented in Figs. 1, 2, and 3, Supplemental Figures 1 and 2, and Table 2.
Table 2.
Summary of findings for the comparison between single and sequential media for embryo culture to the blastocyst stage
| Clinical outcomes | Absolute riska | RR (95%CI) | N b (studies) | I 2 | Interpretation | Quality of the evidence | |
| Sequential | Single | ||||||
| Ongoing pregnancy | 35 % | 32 % (23–47 %) | 0.9 (0.7; 1.3) | 246 (2) | 0 % | Imprecise for conclusions | Very Lowd,e |
| Clinical pregnancy | 56 % | 54 % (38–77 %) | 1.0 (0.7; 1.4) | 100 (1) | N/A | Imprecise for conclusions | Very Lowd,e |
| Miscarriage | 14 % | 19 % (5–60 %) | 1.3 (0.4; 4.3) | 55 (1) | N/A | Imprecise for conclusions | Very Lowd,e |
| Laboratory outcomes | Absolute riska | RD (95%CI) | N c (studies) | I 2 | Interpretation | Quality of the evidence | |
| Sequential | Single | ||||||
| Blastocyst formation | |||||||
| Single media with renewal | 42 % | 51 % (47–66 %) | 0.09 (0.05; 0.14) | 2329 (5) | 19 % | Single is better | Lowf |
| No renewal in single media | 55 % | 58 % (35–81 %) | 0.03 (−0.20; 0.26) | 2643 (2) | 95 % | Imprecise and inconsistent for conclusions | Very Lowf,g |
| Mixed or unclear renewal | 39 % | 46 % (42–50 %) | 0.07 (0.03; 0.11) | 2483 (3) | 0 % | Single is better | Lowf |
| Overall | 46 % | 52 % (47–58 %) | 0.06 (0.01; 0.12) | 7455 (10) | 83 % | Inconsistent for conclusions | Very Lowf,g |
| Top/high blastocyst | |||||||
| Single media with renewal | 22 % | 29 % (19–39 %) | 0.07 (−0.03; 0.17) | 1236 (3) | 76 % | Imprecise and inconsistent for conclusions | Very Lowf,g |
| No renewal in single media | 35 % | 43 % (14–71 %) | 0.08 (−0.21; 0.36) | 902 (2) | 97 % | Imprecise and inconsistent for conclusions | Very Lowf,g |
| Overall | 31 % | 38 % (26–60 %) | 0.07 (−0.05; 0.19) | 3879 (5) | 93 % | Imprecise and inconsistent for conclusions | Very Lowf,g |
I 2 heterogeneity, RR relative risk, CI confidence interval, RD risk difference
aThe absolute risk in the sequential media was determined as the weighted average risk across studies while the risk in the single media group was determined by assessing the RR/RD and its 95 % CI
bNumber of women randomized
cNumber of oocytes/zygotes randomized
dDowngraded one level because of the limitations of the included studies
eDowngraded up to three levels because of very serious imprecision
fDowngraded up to two levels because of serious limitations of the included studies
gDowngraded up to two levels because of serious inconsistency across studies (high heterogeneity)
Risk of bias across studies
A funnel-plot analysis was performed to assess publication bias for blastocyst formation, the only outcome with at least 10 included studies; this analysis was not suggestive of publication bias (Supplemental Figure 3).
Discussion
In the present meta-analysis, we evaluated the available evidence regarding the comparative efficacy and safety of culturing embryos to the blastocyst stage in a single medium versus in a sequential media system. As health care systems worldwide expand access to fertility care services with development or enhancement of their IVF centers, this analysis can help guide the adaptation of best practice culture technologies into their operating procedures if options for extended culture to the blastocyst stage are feasible. In this review, we show that there is currently insufficient evidence to support the superiority of either of the two media systems for extended embryo culture to blastocyst stage when considering ongoing pregnancy rates. However, increased blastocyst formation rates were associated with use of single media as compared to sequential. Notably, the overall quality of evidence for all outcome measures was considered to be very low and the best interpretation is that we remain very uncertain about which media system is better in this comparison.
The majority of the included studies had methodological limitations such as unclear randomization and concealment, sample sizes that were small or absence of a power calculation. In addition, some studies randomizing oocytes/zygotes had reported outcomes as percentages per participant, thereby preventing data extraction for quantitative analysis. Studies randomizing sibling oocytes or zygotes from a woman’s cohort were considerably more numerous but were characterized by high heterogeneity, with several different culture media being compared. In addition, a major limitation of such sibling oocyte/zygote studies is that they were designed to compare embryo-related outcomes, such as embryo quality and blastocyst formation rates; thus, they have an inherent inability to provide reliable information on important clinical reproductive outcomes, such as implantation, pregnancy, live birth, and miscarriage. These outcomes can only be addressed by prospective RCTs that randomize the women participants.
Strikingly, only two RCTs were eligible for the women-based review: one older, peer-reviewed study from 2002 and one published only in abstract form [33, 34]. Both of these studies reported ongoing pregnancy rates, while only one of them [34] reported early miscarriage; neither of them reported live birth rates. Clearly, RCTs with women as the randomization unit are currently very scarce for this comparison. Nevertheless, the similar reproductive outcomes may suggest that single and sequential media are equally able to support human embryo development in vitro, but the lack of good quality evidence makes solid conclusions regarding the superiority of one system over the other impossible at present.
The studies from the oocyte-based review showed a slight beneficial effect of the single medium in yielding more blastocysts as compared to sequential media (Fig. 2), without any evident effect on blastocyst quality (Fig. 3). Given the observed higher blastocyst formation rate, a putative advantage of a single medium over sequential media requires confirmation by studying cumulative pregnancy rates from all embryos derived from a single cohort of oocytes. Since higher blastocyst formation may equate to more blastocysts being cryopreserved, data from subsequent frozen embryo transfer cycles would greatly enhance our understanding regarding the comparative efficiency of these two media systems on blastocyst viability. However, such studies are currently lacking.
In this context, it is important to differentiate between the formation of blastocysts and the development of viable blastocysts. It has been proposed that the ability of a particular culture medium to give rise to a high percentage of blastocysts does not necessarily mean that such blastocysts are viable or that they have the developmental competence to implant and sustain a pregnancy [13]. Earlier efforts to culture human embryos to the blastocyst stage in less sophisticated media resulted in acceptable blastocyst formation rates but very low pregnancy rates [5–8], indicating that human embryos are capable of developing in diverse culture media, not all of which appear to generate viable embryos with a capacity to implant and result in a pregnancy. The adaptability of human embryos to varying culture conditions has prompted investigation and discussions regarding possible downstream long-term effects of single versus sequential culture on pregnancy outcome and offspring phenotype [43–47].
Indeed, there is some evidence to suggest that the culture environment may affect embryo development, fetal growth, birthweight, and perinatal outcome [47–56], although the majority of these studies have design shortcomings and/or are limited by sample size. Importantly, follow-up of children born following embryo culture in either single or sequential media was not reported in any of the studies included in the present meta-analysis. Of note, the World Health Organization has supported the request to adhere to the IMPRINT CONSORT-based guidelines that recommend RCTs include live birth and neonatal outcome [57, 58].
Whether a single medium should be renewed on day 3 is an important consideration [3, 17]. Previous evidence from animal [59] and human studies [15, 33, 60] suggests that embryo quality and blastocyst development are similar when single media are used in either a continuous (i.e., non-renewed) or interrupted (i.e., renewed) fashion. In the majority of the studies included in the oocyte-based review presented here, the single medium was renewed, while only two studies clearly employed uninterrupted continuous culture. Interestingly, subgroup analysis revealed that the overall significant benefit of a single medium on blastocyst formation was not observed when there was no medium renewal on day 3. However, we cannot exclude the possibility that the beneficial effect would have been maintained if more studies had been available for analysis.
One potential concern with not renewing medium on day 3 is the possible build-up of ammonium caused by breakdown of amino acids, particularly glutamine. However, when used in its stable dipeptide form (either L-alanyl-L-glutamine or glycyl-L-glutamine), ammonium release is minimal. Of note, the two trials included in our review in which the single medium was not renewed [23, 30], did indeed, contain the stable dipeptide form of glutamine. Moreover, a recent study also using a single medium containing the dipeptide form of glutamine showed no difference in blastocyst formation rate when embryos were cultured in renewed versus non-renewed medium [60]. Continuous uninterrupted culture without media renewal confers several potential advantages, including sustained presence in the medium of embryo-secreted autocrine/paracrine factors and the possible reduced stress to the embryo during transfer from one dish to another.
Use of a single medium as compared with a sequential media system has several practical advantages including a reduction in the possibility of unintentional handling errors, a reduction in staff labor and costs related to quality testing, and an overall reduction in the costs of consumables, particularly if the medium is not renewed on day 3 [3, 36, 61]. In lower resource settings or in any setting addressing cost, a single medium could infer distinct economic advantages. Moreover, compatibility with time-lapse systems is undoubtedly a significant benefit and a key reason for the increasing popularity of continuous single medium protocols [20, 35, 40, 60]. Of note, however, current evidence does not suggest a clear benefit of using time-lapse for embryo selection, and these systems generally increase the overall cost of the IVF procedure [62].
In conclusion, there is insufficient evidence to recommend either sequential or single-step media as being superior for the culture of embryos to day 5/6. Very low quality evidence from two RCTs indicates that there is no difference in ongoing pregnancy rates with embryos cultured to the blastocyst stage in a sequential versus a single medium system. Low/very low quality evidence from ten RCTs in which oocytes/zygotes were randomized indicates that there is an increased rate of blastocyst formation following culture in single medium, particularly if the medium is used in a two-step fashion with renewal on day 3. Whether this translates into a higher cumulative pregnancy rate after use of all embryos in a cohort remains to be determined. Nevertheless, using a single medium for extended culture has some practical advantages. Future studies should be performed that compare these two media systems in well-designed trials with outcome indicators that include clinical pregnancy, live birth, and neonatal outcome. Until there is a clear benefit of single or sequential media, each IVF laboratory should identify which system performs best in their own setting.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOC 14.5 kb)
(DOC 14.5 kb)
(DOC 15 kb)
(DOCX 19 kb)
Footnotes
Declaration of Authors’ Roles
Conception and design (CR, LR, WPM, IAS, and NR-F); search strategy (WPM, IAS); data extraction (WPM, PAN, IGRV, CON), analysis, and interpretation (all authors); writing the article (all authors). All authors had full access to all the data in the study and approved the final manuscript.
Capsule
In conclusion, there is insufficient evidence to recommend either sequential or single-step media as being superior for the culture of embryos to days 5/6 blastocyst stage.
References
- 1.Youssef MM, Mantikou E, van Wely M, Van der Veen F, Al-Inany HG, Repping S et al. Culture media for human pre-implantation embryos in assisted reproductive technology cycles. Cochrane Database Syst Rev. 2015;CD007876. [DOI] [PMC free article] [PubMed]
- 2.Summers MC, Biggers JD. Chemically defined media and the culture of mammalian preimplantation embryos: historical perspective and current issues. Hum Reprod Update. 2003;9:557–582. doi: 10.1093/humupd/dmg039. [DOI] [PubMed] [Google Scholar]
- 3.Machtinger R, Racowsky C. Culture systems: single step. In: Smith GD, Swain JE, Pool TB, eds. Embryo Culture. Vol. 912: Humana Press, 2012:199–209. [DOI] [PubMed]
- 4.Quinn P. Culture systems: sequential. Methods Mol Biol. 2012;912:211–230. doi: 10.1007/978-1-61779-971-6_13. [DOI] [PubMed] [Google Scholar]
- 5.Bolton VN, Wren ME, Parsons JH. Pregnancies after in vitro fertilization and transfer of human blastocysts. Fertil Steril. 1991;55:830–832. doi: 10.1016/S0015-0282(16)54257-5. [DOI] [PubMed] [Google Scholar]
- 6.Hardy K, Handyside AH, Winston RM. The human blastocyst: cell number, death and allocation during late preimplantation development in vitro. Development. 1989;107:597–604. doi: 10.1242/dev.107.3.597. [DOI] [PubMed] [Google Scholar]
- 7.Dokras A, Sargent IL, Barlow DH. Human blastocyst grading: an indicator of developmental potential? Hum Reprod. 1993;8:2119–2127. doi: 10.1093/oxfordjournals.humrep.a137993. [DOI] [PubMed] [Google Scholar]
- 8.Noda Y, Goto Y, Umaoka Y, Shiotani M, Nakayama T, Mori T. Culture of human embryos in alpha modification of Eagle’s medium under low oxygen tension and low illumination. Fertil Steril. 1994;62:1022–1027. doi: 10.1016/S0015-0282(16)57068-X. [DOI] [PubMed] [Google Scholar]
- 9.Gardner DK, Lane M, Schoolcraft WB. Culture and transfer of viable blastocysts: a feasible proposition for human IVF. Hum Reprod. 2000;15(Suppl 6):9–23. [PubMed] [Google Scholar]
- 10.Gardner D, Schoolcraft W. In vitro culture of the human blastocyst. In: Jansen R, Mortimer D, editors. Towards reproductive certainty: infertility and genetics beyond 1999. Carnforth, UK: Parthenon Publishing; 1999. pp. 378–388. [Google Scholar]
- 11.Gardner DK, Lane M, Calderon I, Leeton J. Environment of the preimplantation human embryo in vivo: metabolite analysis of oviduct and uterine fluids and metabolism of cumulus cells. Fertil Steril. 1996;65:349–353. doi: 10.1016/S0015-0282(16)58097-2. [DOI] [PubMed] [Google Scholar]
- 12.Gardner DK, Lane M. Culture of viable human blastocysts in defined sequential serum-free media. Hum Reprod. 1998;13(Suppl 3):148–159. doi: 10.1093/humrep/13.suppl_3.148. [DOI] [PubMed] [Google Scholar]
- 13.Gardner D, Lane M. Culture and selection of viable human blastocysts: a feasible proposition of human IVF. Hum Reprod Update. 1997;3:367–382. doi: 10.1093/humupd/3.4.367. [DOI] [PubMed] [Google Scholar]
- 14.Lawitts JA, Biggers JD. Optimization of mouse embryo culture media using simplex methods. J Reprod Fertil. 1991;91:543–556. doi: 10.1530/jrf.0.0910543. [DOI] [PubMed] [Google Scholar]
- 15.Biggers JD, Racowsky C. The development of fertilized human ova to the blastocyst stage in KSOM(AA) medium: is a two-step protocol necessary? Reprod Biomed Online. 2002;5:133–140. doi: 10.1016/S1472-6483(10)61615-X. [DOI] [PubMed] [Google Scholar]
- 16.Wiemer KE, Anderson AR, Kyslinger ML, Weikert ML. Embryonic development and pregnancies following sequential culture in human tubal fluid and a modified simplex optimized medium containing amino acids. Reprod Biomed Online. 2002;5:323–327. doi: 10.1016/S1472-6483(10)61840-8. [DOI] [PubMed] [Google Scholar]
- 17.Biggers J, Summers M. Choosing a culture medium: making informed choices. Fertil Steril. 2008;90:473–483. doi: 10.1016/j.fertnstert.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Higgins J, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011], 2011.
- 19.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 20.Basile N, Morbeck D, Garcia-Velasco J, Bronet F, Meseguer M. Type of culture media does not affect embryo kinetics: a time-lapse analysis of sibling oocytes. Hum Reprod. 2013;28:634–641. doi: 10.1093/humrep/des462. [DOI] [PubMed] [Google Scholar]
- 21.Paternot G, Debrock S, D’Hooghe TM, Spiessens C. Early embryo development in a sequential versus single medium: a randomized study. Reprod Biol Endocrinol. 2010;8:83. doi: 10.1186/1477-7827-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kemeter P, Hajek J, Carli L, Feichtinger W. Current issue: a prospective randomized study comparing two embryo culture systems after IVF/ICSI: sequential media in 5% O2 atmosphere and single medium in 21% O2 atmosphere. J Gynakolo Endokrinol. 2011;21:2–7. [Google Scholar]
- 23.Alteri A, Fabozzi G, Rega E, Starita MF, Giannini P, Piscitelli C, et al. Blastocyst development in single-step versus sequential culture media of the same brand: analysis of 386 sibling oocytes. Hum Reprod. 2014;29:O–105. [Google Scholar]
- 24.Donmez E, Sati L, Tuysuz G, Gokalp-Kaya D, Celik S, Demirel LC. A randomized comparison of sequential and single step culture media systems on sibling oocytes: complete p-1 versus single step medium. Fertil Steril. 2008;90:S431. doi: 10.1016/j.fertnstert.2008.07.1211. [DOI] [Google Scholar]
- 25.Khoury C, Coffler M, Potter D, Frederick J, Battaglia D. Improved blastocyst development using a single step medium versus a sequential medium. Fertil Steril. 2012;97:S4. doi: 10.1016/j.fertnstert.2012.01.008. [DOI] [Google Scholar]
- 26.Koyner P, Castillo-Baso J, Sugasti S, De Armas R, Berbey RD. Embryo development and reproductive outcomes using single medium system (SMS) with 50% of medium renew every 48 hours. Fertil Steril. 2013;100:S26–27. doi: 10.1016/j.fertnstert.2013.07.1743. [DOI] [Google Scholar]
- 27.Patrick JL, Welch L, Teaff N, Whelan J, Crain JMW. Comparative assessment of blastocyst conversion rates in sequential vs continuous culture systems. Fertil Steril. 2013;100:S28. doi: 10.1016/j.fertnstert.2013.07.1748. [DOI] [Google Scholar]
- 28.Sepulveda S, Garcia J, Arriaga E, Diaz J, Noriega-Portella L, Noriega-Hoces L. In vitro development and pregnancy outcomes for human embryos cultured in either a single medium or in a sequential media system. Fertil Steril. 2009;91:1765–1770. doi: 10.1016/j.fertnstert.2008.02.169. [DOI] [PubMed] [Google Scholar]
- 29.Sfontouris IA, Kolibianakis EM, Lainas GT, Zorzovilis IZ, Petsas GK, Lainas TG. Blastocyst development in single-step versus sequential culture media: a prospective randomized study with sibling oocytes. Hum Reprod. 2014;29:i2. [Google Scholar]
- 30.Werner MD, Hong KH, Franasiak JM, Forman EJ, Reda CV, Molinaro TA, et al. Sequential versus Monophasic Media Impact Trial (SuMMIT): a paired randomized controlled trial comparing a sequential media system to a monophasic medium. Fertil Steril. 2016;105:1215–1221. doi: 10.1016/j.fertnstert.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Yang Z, Liu J, Kuang Y, Terigima S, Salem R. Time-lapse and array CGH evaluation of human embryos cultured in single versus sequential media: a randomized pilot study. Hum Reprod. 2013;28:i73. doi: 10.1093/humrep/det179. [DOI] [Google Scholar]
- 32.Summers MC, Bird S, Mirzai FM, Thornhill A, Biggers JD. Human preimplantation embryo development in vitro: a morphological assessment of sibling zygotes cultured in a single medium or in sequential media. Hum Fertil (Camb) 2013;16:278–285. doi: 10.3109/14647273.2013.806823. [DOI] [PubMed] [Google Scholar]
- 33.Macklon NS, Pieters MH, Hassan MA, Jeucken PH, Eijkemans MJ, Fauser BC. A prospective randomized comparison of sequential versus monoculture systems for in-vitro human blastocyst development. Hum Reprod. 2002;17:2700–2705. doi: 10.1093/humrep/17.10.2700. [DOI] [PubMed] [Google Scholar]
- 34.Sfontouris IA, Kolibianakis EM, Lainas GT, Zorzovilis IZ, Petsas GK, Lainas TG. Similar implantation and pregnancy rates using a single medium versus sequential media for embryo culture: a randomized controlled trial. Hum Reprod. 2015;30:i232. doi: 10.1093/humrep/dev136. [DOI] [Google Scholar]
- 35.Hardarson T, Bungum M, Conaghan J, Meintjes M, Chantilis SJ, Molnar L, et al. Noninferiority, randomized, controlled trial comparing embryo development using media developed for sequential or undisturbed culture in a time-lapse setup. Fertil Steril. 2015;104:1452–1459. doi: 10.1016/j.fertnstert.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 36.Reed ML, Hamic A, Thompson DJ, Caperton CL. Continuous uninterrupted single medium culture without medium renewal versus sequential media culture: a sibling embryo study. Fertil Steril. 2009;92:1783–1786. doi: 10.1016/j.fertnstert.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Schneider DT, Verza S, Esteves SC. Single or sequential medium are equally effective for the culture of human embryos to the blastocyst stage: a pilot study. Fertil Steril. 2009;92:S231–232. doi: 10.1016/j.fertnstert.2009.07.1564. [DOI] [Google Scholar]
- 38.Vansteenbrugge A, Vastersaegher C, Fontaine P, Klay C, Rodez D, Pauwels P-C. Comparison of a single medium with sequential media for the development of human zygotes to the blastocyst stage. Fertil Steril. 2007;88:S319. doi: 10.1016/j.fertnstert.2007.07.1071. [DOI] [Google Scholar]
- 39.Velez de la Calle J, Pfeffer J, Taar JP, Prigent Y. Blastocyst outcomes after sequential media culture vs single step media culture in a human IVF program. Fertil Steril. 2013;100:S253. doi: 10.1016/j.fertnstert.2013.07.1192. [DOI] [Google Scholar]
- 40.Ciray HN, Aksoy T, Goktas C, Ozturk B, Bahceci M. Time-lapse evaluation of human embryo development in single versus sequential culture media—a sibling oocyte study. J Assist Reprod Genet. 2012;29:891–900. doi: 10.1007/s10815-012-9818-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koscinski I, Ladureau-Fritsch L, Greze C, Celebi C, Lichtblau I, Teletin M, et al. Comparison of embryo culture with a single medium, global, lifeglobal or with sequential media from Cook laboratories in a prospective study. Hum Reprod. 2014;29:i187. [Google Scholar]
- 42.Scarica C, Ubaldi F, Orlando G, Dovere L, Maggiulli R, Stoppa M, et al. Single step versus sequential culture medium: effects on embryo development, genetic and clinical outcomes. Hum Reprod. 2015;30:i24–25. [Google Scholar]
- 43.De Vos A, Janssens R, VandeVelde H, Haentjens P, Bonduelle M, Tournaye H, et al. The type of culture medium and the duration of in vitro culture do not influence birthweight of ART singletons. Hum Reprod. 2015;30:20–27. doi: 10.1093/humrep/deu286. [DOI] [PubMed] [Google Scholar]
- 44.Pelinck MJ, Hadders-Algra M, Haadsma ML, Nijhuis WL, Kiewiet SM, Hoek A, et al. Is the birthweight of singletons born after IVF reduced by ovarian stimulation or by IVF laboratory procedures? Reprod Biomed Online. 2010;21:245–251. doi: 10.1016/j.rbmo.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 45.Morbeck DE, Krisher RL, Herrick JR, Baumann NA, Matern D, Moyer T. Composition of commercial media used for human embryo culture. Fertil Steril. 2014;102:759–766. doi: 10.1016/j.fertnstert.2014.05.043. [DOI] [PubMed] [Google Scholar]
- 46.Bolton VN, Cutting R, Clarke H, Brison DR. ACE consensus meeting report: culture systems. Hum Fertil (Camb) 2014;17:239–251. doi: 10.3109/14647273.2014.944417. [DOI] [PubMed] [Google Scholar]
- 47.Vergouw CG, Kostelijk EH, Doejaaren E, Hompes PG, Lambalk CB, Schats R. The influence of the type of embryo culture medium on neonatal birthweight after single embryo transfer in IVF. Hum Reprod. 2012;27:2619–2626. doi: 10.1093/humrep/des252. [DOI] [PubMed] [Google Scholar]
- 48.Bouillon C, Leandri R, Desch L, Ernst A, Bruno C, Cerf C, et al. Does embryo culture medium influence the health and development of children born after in vitro fertilization? PLoS One. 2016;11:e0150857. doi: 10.1371/journal.pone.0150857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eaton JL, Lieberman ES, Stearns C, Chinchilla M, Racowsky C. Embryo culture media and neonatal birthweight following IVF. Hum Reprod. 2012;27:375–379. doi: 10.1093/humrep/der381. [DOI] [PubMed] [Google Scholar]
- 50.Kleijkers SH, Eijssen LM, Coonen E, Derhaag JG, Mantikou E, Jonker MJ, et al. Differences in gene expression profiles between human preimplantation embryos cultured in two different IVF culture media. Hum Reprod. 2015;30:2303–2311. doi: 10.1093/humrep/dev179. [DOI] [PubMed] [Google Scholar]
- 51.Kleijkers SH, van Montfoort AP, Smits LJ, Viechtbauer W, Roseboom TJ, Nelissen EC, et al. IVF culture medium affects post-natal weight in humans during the first 2 years of life. Hum Reprod. 2014;29:661–669. doi: 10.1093/humrep/deu025. [DOI] [PubMed] [Google Scholar]
- 52.Lemmen JG, Pinborg A, Rasmussen S, Ziebe S. Birthweight distribution in ART singletons resulting from embryo culture in two different culture media compared with the national population. Hum Reprod. 2014;29:2326–2332. doi: 10.1093/humrep/deu188. [DOI] [PubMed] [Google Scholar]
- 53.Nelissen EC, Van Montfoort AP, Coonen E, Derhaag JG, Geraedts JP, Smits LJ, et al. Further evidence that culture media affect perinatal outcome: findings after transfer of fresh and cryopreserved embryos. Hum Reprod. 2012;27:1966–1976. doi: 10.1093/humrep/des145. [DOI] [PubMed] [Google Scholar]
- 54.Nelissen EC, Van Montfoort AP, Smits LJ, Menheere PP, Evers JL, Coonen E, et al. IVF culture medium affects human intrauterine growth as early as the second trimester of pregnancy. Hum Reprod. 2013;28:2067–2074. doi: 10.1093/humrep/det131. [DOI] [PubMed] [Google Scholar]
- 55.Zandstra H, Van Montfoort AP, Dumoulin JC. Does the type of culture medium used influence birthweight of children born after IVF? Hum Reprod. 2015;30:530–542. doi: 10.1093/humrep/deu346. [DOI] [PubMed] [Google Scholar]
- 56.Zhu J, Li M, Chen L, Liu P, Qiao J. The protein source in embryo culture media influences birthweight: a comparative study between G1 v5 and G1-PLUS v5. Hum Reprod. 2014;29:1387–1392. doi: 10.1093/humrep/deu103. [DOI] [PubMed] [Google Scholar]
- 57.Harbin Consensus Conference Workshop Group Improving the reporting of clinical trials of infertility treatments (IMPRINT): modifying the CONSORT statement. Fertil Steril. 2014;102:952–959. doi: 10.1016/j.fertnstert.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 58.Harbin Consensus Conference Workshop Group Improving the reporting of clinical trials of infertility treatments (IMPRINT): modifying the CONSORT statement. Hum Reprod. 2014;29:2075–2082. doi: 10.1093/humrep/deu218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Biggers JD, McGinnis LK, Lawitts JA. One-step versus two-step culture of mouse preimplantation embryos: is there a difference? Hum Reprod. 2005;20:3376–3384. doi: 10.1093/humrep/dei228. [DOI] [PubMed] [Google Scholar]
- 60.Costa-Borges N, Bellés M, Meseguer M, Galliano D, Ballesteros A, Calderón G. Blastocyst development in single medium with or without renewal on day 3: a prospective cohort study on sibling donor oocytes in a time-lapse incubator. Fertil Steril. 2016;105:707–713. doi: 10.1016/j.fertnstert.2015.11.038. [DOI] [PubMed] [Google Scholar]
- 61.Xie Y, Wang F, Puscheck EE, Rappolee DA. Pipetting causes shear stress and elevation of phosphorylated stress-activated protein kinase/jun kinase in preimplantation embryos. Mol Reprod Dev. 2007;74:1287–1294. doi: 10.1002/mrd.20563. [DOI] [PubMed] [Google Scholar]
- 62.Racowsky C, Kovacs P, Martins WP. A critical appraisal of time-lapse imaging for embryo selection: where are we and where do we need to go? J Assist Reprod Genet. 2015;32:1025–1030. doi: 10.1007/s10815-015-0510-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 14.5 kb)
(DOC 14.5 kb)
(DOC 15 kb)
(DOCX 19 kb)