Abstract
Purpose
Despite advances in the composition of defined embryo culture media, co-culture with somatic cells is still used for bovine in vitro embryo production (IVEP) in many laboratories worldwide. Granulosa cells are most often used for this purpose, although recent work suggests that co-culture with stem cells of adult or embryonic origin or their derived biomaterials may improve mouse, cattle, and pig embryo development.
Materials and methods
In experiment 1, in vitro produced bovine embryos were co-cultured in the presence of two concentrations of bovine adipose tissue-derived mesenchymal cells (b-ATMSCs; 103 and 104 cells/mL), in b-ATMSC preconditioned medium (SOF-Cond), or SOF alone (control). In experiment 2, co-culture with 104 b-ATMSCs/mL was compared to the traditional granulosa cell co-culture system (Gran).
Results
In experiment 1, co-culture with 104 b-ATMSCs/mL improved blastocyst rates in comparison to conditioned and control media (p < 0.05). Despite that it did not show difference with 103 b-ATMSCs/mL (p = 0.051), group 104 b-ATMSCs/mL yielded higher results of blastocyst production. In experiment 2, when compared to group Gran, co-culture with 104 b-ATMSCs/mL improved not only blastocyst rates but also quality as assessed by increased total cell numbers and mRNA expression levels for POU5F1 and G6PDH (p < 0.05).
Conclusions
Co-culture of bovine embryos with b-ATMSCs was more beneficial than the traditional co-culture system with granulosa cells. We speculate that the microenvironmental modulatory potential of MSCs, by means of soluble substances and exosome secretions, could be responsible for the positive effects observed. Further experiments must be done to evaluate if this beneficial effect in vitro also translates to an increase in offspring following embryo transfer. Moreover, this study provides an interesting platform to study the basic requirements during preimplantation embryo development, which, in turn, may aid the improvement of embryo culture protocols in bovine and other species.
Keywords: Bovine, Embryo, IVEP, Co-culture, Mesenchymal stem cells
Introduction
An essential requirement for the success of in vitro production of mammalian embryos (IVEP) is to provide a rich microenvironment for oocyte and embryo culture, mimicking conditions found in the female’s reproductive tract [1–3]. Despite great advances in the design of defined and semidefined embryo culture media [4–6], researchers still seek new media additives and somatic cell-based co-culture systems in an attempt to obtain higher yields of better quality embryos [7–10]. Presumably, somatic cells in a co-culture system may remove toxic components and reactive oxygen species, as well as provide paracrine factors beneficial during early embryonic development [1].
Recently, few reports have presented a new approach based upon the use of stem cells of adult or embryonic origin and their derived biomaterials in a co-culture system with oocytes and/or embryos from mice, pigs, and bovines [11–13]. These studies have been primarily motivated by the fact that stem cells have the ability to secrete a variety of substances such as cytokines, growth factors, and even microRNAs, which are collectively capable of modulating the surrounding microenvironment [14–17]. For instance, in wound repair studies, evidence suggests that most of the therapeutic effects of transferred mesenchymal stem cells may be due to the release of soluble factors that regulate local cellular responses to cutaneous injury; this is further supported by the fact that stem cell-conditioned medium is able to promote tissue repair [18].
With regard to stem cell-enhanced embryo culture systems, some reports have shown a positive effect upon oocyte maturation and/or embryo development. For instance, Ling et al. [19] reported higher mouse oocyte maturation rates when using medium that had been conditioned for 48 h in the presence of bone marrow-derived mesenchymal stem cells. Similarly, supplementation of bovine or porcine embryo culture medium with 10 % of a bioactive material derived from human embryonic [12] or adipose tissue [13] stem cells, respectively, has been shown to be efficient for keeping quantity and quality of in vitro produced embryos in both species. Only one study has addressed the use of stem cells as feeders for embryo culture. In this study, co-culture of in vivo produced two-cell mouse embryos with human umbilical cord-derived stem cells was superior to stem cell-conditioned medium in attenuating the ill effects of light exposure and, consequently, yielded higher blastocyst development rates [11].
Although there is no consensus regarding the use of conditioned media versus co-culture with stem cells, the above studies provide clear evidence of a beneficial effect of stem cell-based culture conditions upon IVEP. Herein, we compared for the first time the use of bovine adipose tissue-derived mesenchymal stem cells (b-ATMSC) either in co-culture or by conditioning culture medium in a bovine IVEP system. This study provides new data that could contribute to the understanding of the basic requirements during preimplantation development of embryos as a means to improve culture media composition.
Materials and methods
All chemicals were purchased from Sigma-Aldrich Chemical Company (Saint Louis, MO, USA), and culture flasks, dishes, and tubes were purchased from TPP-Techno Plastic Products (TPP, Switzerland) unless otherwise stated. Somatic cells and embryos were incubated at 38.5 °C in a 5 % CO2 atmosphere in a humidified air incubator (Thermo Scientific, USA).
Experimental design and embryo evaluations
Experiment 1. Evaluation of conditioned medium and two concentrations of b-ATMSCs in a co-culture system
Initially, we compared two concentrations (103 and 104 cells/mL) of b-ATMSCs (cell passages 3 to 6) and conditioned SOF medium (SOF-Cond). Nonconditioned, without co-culture, SOF medium served as control. In this experiment, the cleavage, blastocyst development (based on the amount of zygotes cultivated), and blastocyst hatching rates (based on the blastocysts obtained) were recorded on the second, seventh, and ninth days of culture, respectively.
Experiment 2. Quantity and quality of bovine embryos produced in vitro in co-culture with b-ATMSCs
Herein, we compared co-culture with b-ATMSCs (cell passages 3 to 6) against the traditional co-culture with granulosa (Gran). Embryo development was recorded as described in the first experiment. Additionally, we analyzed total cell number and gene expression of day 7 blastocysts. Genes analyzed were POU class 5 homeobox 1 (POU5F1 or OCT4), a cell pluripotency marker; glucose-6-phosphate dehydrogenase (G6PDH), a glucose metabolism enzyme; and heat shock protein 70 (HSP70), a gene related to cellular heat stress.
Isolation and culture of b-ATMSCs
To establish the b-ATMSC line, adipose tissue was processed as described previously [20, 21] with minor modifications as follows. Bovine adipose tissue was collected from the thoracic fat tissue of one adult bull at an abattoir. Adipose tissue (∼5 g) was extensively washed, placed in a sterile plastic bottle with PBS, and transported to the laboratory at approximately 4 °C. Once in the laboratory, tissue was minced for 30 min and digested with 1 mg/mL type I collagenase diluted in PBS (Ca++/Mg++ free). Following a 2 h digestion at 38.5 °C, collagenase activity was neutralized by the addition of an equal volume of Iscove’s modified Dulbecco’s medium (IMDM) containing 10 % fetal bovine serum (FBS; Gibco BRL, USA) and 50 μg/mL gentamicin sulfate (IMDM-10 %). The digested tissue was centrifuged at 200×g for 10 min, and the pellet was resuspended in IMDM-10 % and plated on 25-cm2 plastic flasks filled up with 3 mL of IMDM-10 %. Cells were incubated, and once 80–90 % of dish surface was covered by cells, they were dissociated by Tryple Express (Tryple Express, Gibco BRL, USA) and washed in IMDM-10 %. The pellet was resuspended in IMDM-10 %, containing 10 % of DMSO, and cells were frozen (106 cells/mL) in 1.5 mL cryovials (Corning, USA) by plunging in liquid nitrogen. For embryo culture experiments, cells were thawed in a water bath at 37 °C for 5 min and washed in IMDM-10 % by centrifugation at 200×g for 10 min. After determining the percentage of viable cells (trypan blue solution, Gibco, USA), cells were seeded (105 cells/mL) in 25-cm2 plastic flasks filled up with 3 mL of IMDM-10 % (cell passage 1 of b-ATMSCs). b-ATMSCs in between passages 3 and 6 were used for all experiments.
Immunophenotyping and in vitro differentiation assay
The stemness of b-ATMSCs was evaluated at cell passage 4 following the recommendations of the International Society for Cellular Therapy (ISCT) concerning the minimal criteria for defining multipotent mesenchymal stromal cells [22]. For immunophenotyping, b-ATMSCs (cell passage 4) were directly grown in 24-well plates (104 cells/mL) for 24 h and fixed with 4 % paraformaldehyde for 15 min. After washing, fixed cells were co-incubated with a blocking solution (1 % fatty acid free bovine serum albumin plus 0.3 M glycine) and primary antibodies were diluted in PBS overnight at 4 °C. The primary antibodies (Santa Cruz Biotechnology, USA) used were CD90 (goat; sc-6071, 1:100), CD105 (rat; sc-71042, 1:100), CD73 (goat; sc-14682, 1:200), CD34 (goat; sc-7045, 1:200), CD45 (mouse; sc-101839, 1:200), and CD79 (mouse; sc-20064, 1:200). Cells were then washed in PBS and incubated for 45 min at room temperature with Alexa 488- (anti-rat; Thermo Fischer, USA, A-11006), Alexa 594- (anti-goat; Thermo Fischer, USA, A-11080), or FITC-conjugated (anti-mouse; Santa Cruz, sc-2010) secondary antibodies diluted 1:50 in PBS. Cell nuclei were stained with 10 μg/mL Hoechst 33342 in PBS for 15 min at room temperature. Stained cells were examined using an epifluorescence microscope (Nikon Eclipse TE300, Nikon Instruments Inc., Japan).
The differentiation assay was undertaken with cell passage 4 b-ATMSCs, following the instructions from the StemPro Differentiation Kit (Gibco BRL, USA). Briefly, cells were seeded in 24-well dishes in IMDM-10 %. After 3 days, IMDM-10 % was replaced by differentiation medium (chondrogenic, adipogenic, and osteogenic) and the culture continued for 21 days with medium changes every 3 days. Negative control cells were incubated in IMDM-10 % for an equal length of time. To confirm differentiation into the three tissue types, cells were fixed for 20 min at room temperature in 4 % paraformaldehyde and stained for 5 min with 1.25 % Oil Red O to visualize intracellular lipid drops, 2 % (v/v) Alizarin Red S to visualize calcium deposits, and 1 % Alcian blue (v/v) to visualize glycosaminoglycan. Stained cells were examined under bright field microscopy (Nikon Eclipse TE300, Nikon Instruments, Inc., Japan).
Bovine IVEP
Embryo production was undertaken as described by Santana et al. [10]. Briefly, cumulus-oocyte complexes (COCs) were recovered by follicular aspiration from abattoir ovaries. Only compact COCs with good cytoplasm were selected for in vitro maturation for 24 h in tissue culture medium (TCM) 199 (Gibco BRL, USA) supplemented with 2.2 g/L sodium bicarbonate, 11 μg/mL pyruvate, 50 μg/mL gentamicin, 10 % FBS, 0.5 μg/mL follicle-stimulating hormone (Folltropin—Bioniche Animal Health, Canada), and 5.0 μg/mL luteinizing hormone (Lutropin—Bioniche Animal Health, Canada) as described by [10].
Frozen-thawed bull (Bos taurus taurus) semen was prepared for in vitro fertilization (IVF) by density-gradient centrifugation in Percoll. Spermatozoa were diluted to a final concentration of 2 × 106 cells/mL in 80-μL IVF droplets of Tyrode’s albumin-lactate-pyruvate (TALP) medium [10]. Between 15 and 20 expanded COCs were placed into sperm-containing droplets for 24 h. Following IVF, groups of 20 presumptive zygotes were directly transferred to 100-μL drops of synthetic oviductal fluid (SOF) medium supplemented with 5 % FBS, 50 μg/mL gentamicin, and 6 mg/mL bovine serum albumin (BSA) for in vitro culture in an incubator during 7 days without any medium replacement. Embryos were cultured over a feeder cell monolayer according to experimental groups.
Preparation of feeder cells and conditioned medium
Cell passages 3 to 6 of b-ATMSCs and freshly collected cumulus oophorus-derived granulosa cells were used as feeder cells. Twenty-four hours before the beginning of in vitro embryo culture (on the IVF day), cells were seeded at a density of 1 × 103 (103 b-ATMSCs) and 1 × 104 (104 b-ATMSCs) cells/mL in 100-μL drops of SOF culture medium overlaid with paraffin oil. Culture droplets were incubated for 24 h before the addition of zygotes.
Granulosa (Gran) cell co-culture was established from the cumulus oophorus-derived cells that become attached to the bottom of the droplet after in vitro maturation of COCs. For this, droplets with in vitro maturation medium (following the 24-h COC incubation) were washed twice with fresh SOF and replaced with 100 μL of SOF culture medium, and culture dishes were maintained at a CO2 incubator for 24 h awaiting the addition of zygotes.
For the preparation of SOF-conditioned medium (SOF-Cond), 1 × 106 viable b-ATMSCs were seeded into 25-cm2 culture flasks with IMDM-10 %. Previously, we established that when seeded at this concentration, in this cell culture flask, cells reached 80–90 % of confluence in 3 h. After 24 h of incubation, nonadherent cells were removed by washing twice with PBS, and adherent cells were cultured with 6 mL of SOF medium for an additional 24 h. The supernatant was collected, centrifuged at 200×g for 5 min, aliquoted, and frozen at −20 °C. In the experiments, the conditioned medium was thawed and 100-μL culture drops were prepared under paraffin oil, 24 h prior to the beginning of embryo culture.
Blastocyst staining
Following morphological evaluation, blastocysts on day 7 were fixed in 1 % formol-saline solution and stained with the DNA dye Hoechst 33342 (10 μg/mL) in PBS. Total cell number was counted using a fluorescence microscope (Eclipse 50i, Nikon Instruments Inc., Japan).
Gene expression
For relative gene expression quantification, 27 embryos (blastocysts on day 7) were collected per group over the period of the experiment. The embryos were frozen in 5 μL of RNAlater solution (Ambion, Life Technology, USA). Aliquots were kept frozen at −80 °C until RNA extraction, which was performed using 100 μL of TRIzol reagent (Invitrogen, USA), according to the manufacturer’s instructions. Reverse-transcriptase PCR was performed using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, New Zealand), according to the manufacturer’s protocol.
Real-time PCR was performed in a StepOne Real-Time PCR system (Applied Biosystems, New Zealand). Primers were designed (Primer Premier Software 5, Biosoft, USA) covering exon-exon junctions and were validated using complementary DNA (cDNA) samples of bovine blastocysts previously collected. Detailed information is shown in Table 1.
Table 1.
Primers used for real-time PCR quantitative analysis
| Gene | Sequences | Product size (pb) | Accession no. |
|---|---|---|---|
| POU5F1 | F: 5′-GGTGTTCAGCCAAACGACTATC-3′ R: 5′-TCTCTGCCTTGCATATCTCCTG-3′ |
143 | NM174580.1 |
| G6PDH | F: 5′-CGCTGGGACGGGGTGCCCTTCATC-3′ R: 5′-CGCCAGGCCTCCCGCAGTTCATCA-3′ |
347 | XM_0493 |
| HSP70 | F: 5′-GCCAGGAAACCAGAGACAGA-3′ R: 5′-CCTACGCAGGAGTAGGTGGT-3′ |
539 | M98823 |
| SDHA | F: 5′-GCAGAACCTGATGCTTTGTG-3′ R: 5′-CGTAGGAGAGCGTGTGCTT-3′ |
185 | NM_174178 |
| YWHAZ | F: 5′-GCATCCCACAGACTATTTCC-3′ R: 5′-GCAAAGACAATGACAGACCA-3′ |
120 | BM446307 |
POU5F1 POU class 5 homeobox 1; G6PDH glucose-6-phosphate dehydrogenase; HSP70 heat shock protein 70; SDHA succinate dehydrogenase complex, subunit A, flavoprotein (Fp); YWHAZ tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta
Each 20 μL PCR reaction mixture consisted of 10 μL of SYBR Green PCR Master Mix (Applied Biosystems, New Zealand), 0.2 mM of each forward and reverse primer, and 10 μL of a 1:20-diluted cDNA sample. Cycling conditions for initial amplification were as follows: 95 °C for 10 min after 45 cycles of denaturation at 95 °C for 15 s and annealing at 61 °C for 1 min. The PCR specificity was verified by checking the corresponding dissociation curves. The amplification efficiencies of each gene were calculated using the DDCT method, and the efficiency adopted was 2.0 [23].
To ensure equal experimental conditions, the same reagents were used to amplify all genes with assays run simultaneously using the same PCR plate. All the samples were analyzed three times in triplicate, and the average value of the triplicate was used for quantification. Data were normalized to endogenous control genes succinate dehydrogenase complex, subunit A, flavoprotein (Fp: SDHA) and tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta (YWHAZ) [24]. Mean values of relative gene expression were used for statistical analysis and each experiment was performed in triplicate.
Statistical analysis
Statistical analysis was performed using Sigma Plot Version 11.0 software with significance level at p < 0.05. The mean percentage of cleavage and blastocyst among the different treatments was calculated from the number of fertilized oocytes, tested for normal distribution (Shapiro-Wilk test) and compared by ANOVA and Bonferroni’s posttest in experiment 1. t test was used in experiment 2 for comparison of embryo production, total cell number, and gene expression.
Results
Characterization of b-ATMSCs
After enzymatic dissociation and plating, we observed that adherent cells had a very uniform fibroblast-like morphology with a fast proliferating capacity up to the time of freezing (cell passage 1). No effect of the freeze/thaw process was observed on cell morphology and proliferating capacity up to cell passage 7, after which cells were no longer used in the experiments.
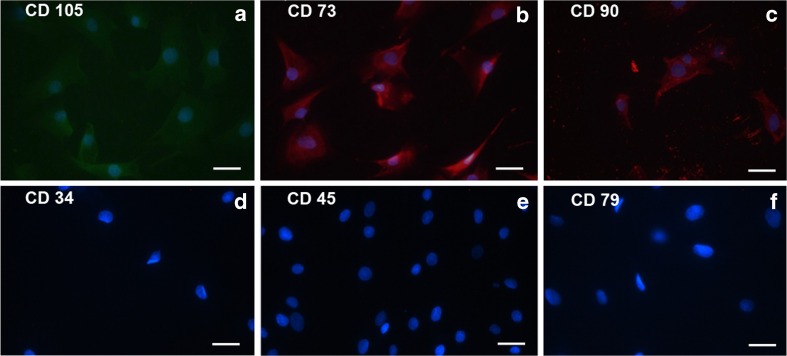
Results of immunophenotyping confirmed the mesenchymal nature of b-ATMSCs as these stained positive for the corresponding markers suggested by ISCT, while they were negative for all hematopoietic markers (Fig. 1). Moreover, following differentiation assay, staining was done for adipose, cartilage, and bone tissues related to multipotency of b-ATMSCs.
Fig. 1.
Immunophenotyping of bovine adipose tissue-derived mesenchymal stem cells (b-ATMSCs). Passage 4 b-ATMSCs were used for these experiments. Mesenchymal stem cell markers were present (a–c) while hematopoietic markers were absent (d–f). Cell nuclei were stained with Hoechst (blue). Scale bar = 15μm
Experiment 1. Evaluation of conditioned medium versus two concentrations of b-ATMSCs in a co-culture system
In this study, the co-culture system improved bovine embryo development. Moreover, co-culture of embryos with 104 b-ATMSCs rendered higher blastocyst rates than the SOF control (p < 0.01) and SOF-conditioned medium (p < 0.01; Table 2). Blastocyst rates were also higher when embryos were co-cultured with 104 than with 103 b-ATMSCs, albeit without reaching significance (p = 0.051).
Table 2.
Development of in vitro produced bovine embryos co-cultured in the presence of two concentrations of adipose tissue-derived mesenchymal stem cells (b-ATMSCs) or cultured with the addition of conditioned medium
| Groups | No. of oocytes | Cleaved (%) | Blastocysts (%) |
|---|---|---|---|
| SOF | 63 | 34 (53.9) a | 12 (19.0) a |
| SOF-Cond | 72 | 58 (80.5) b | 18 (25.0) a |
| 103 b-ATMSCs | 61 | 48 (78.6) b | 20 (32.7) a |
| 104 b-ATMSCs | 61 | 51 (83.6) b | 28 (45.9) b* |
Different lowercase letters within a column denote significant differences (ANOVA; p < 0.05). The mean percentage was calculated from four independent experiments and showed normal distribution (Shapiro-Wilk test)
SOF synthetic oviductal fluid (control), SOF-Cond SOF preconditioned in culture with b-ATMSCs for 48 h, 10 3 or 10 4 b-ATMSCs SOF + co-culture with 103 or 104 cells/mL seeded 24 h earlier
*Comparison with 103 b-ATMSCs showed a p value of 0.051
Experiment 2. Quantity and quality of in vitro produced bovine embryos co-cultured in the presence of b-ATMSCs
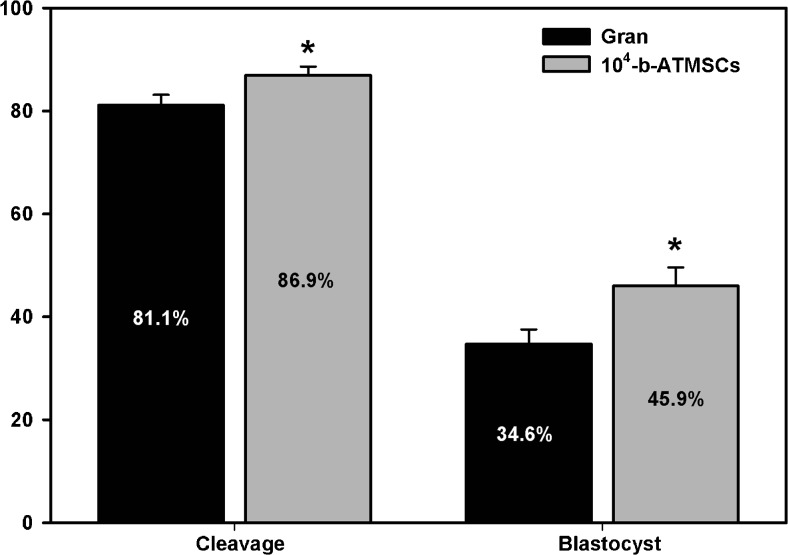
Co-culture with 104 b-ATMSCs increased both cleavage and blastocyst rates (p < 0.05) when compared to the traditional system with co-culture of granulosa cells (Fig. 2).
Fig. 2.
Cleavage and blastocyst rates for in vitro produced bovine embryos co-cultured with either adipose tissue-derived mesenchymal stem cells (b-ATMSCs) or granulosa cells. *p < 0.05 denotes significant differences (t test). The mean percentage was calculated from 12 independent experiments and showed normal distribution (Shapiro-Wilk test)
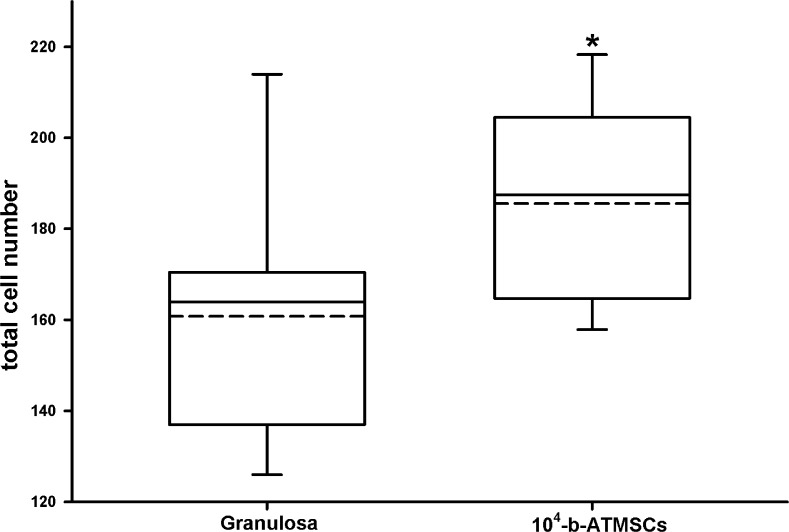
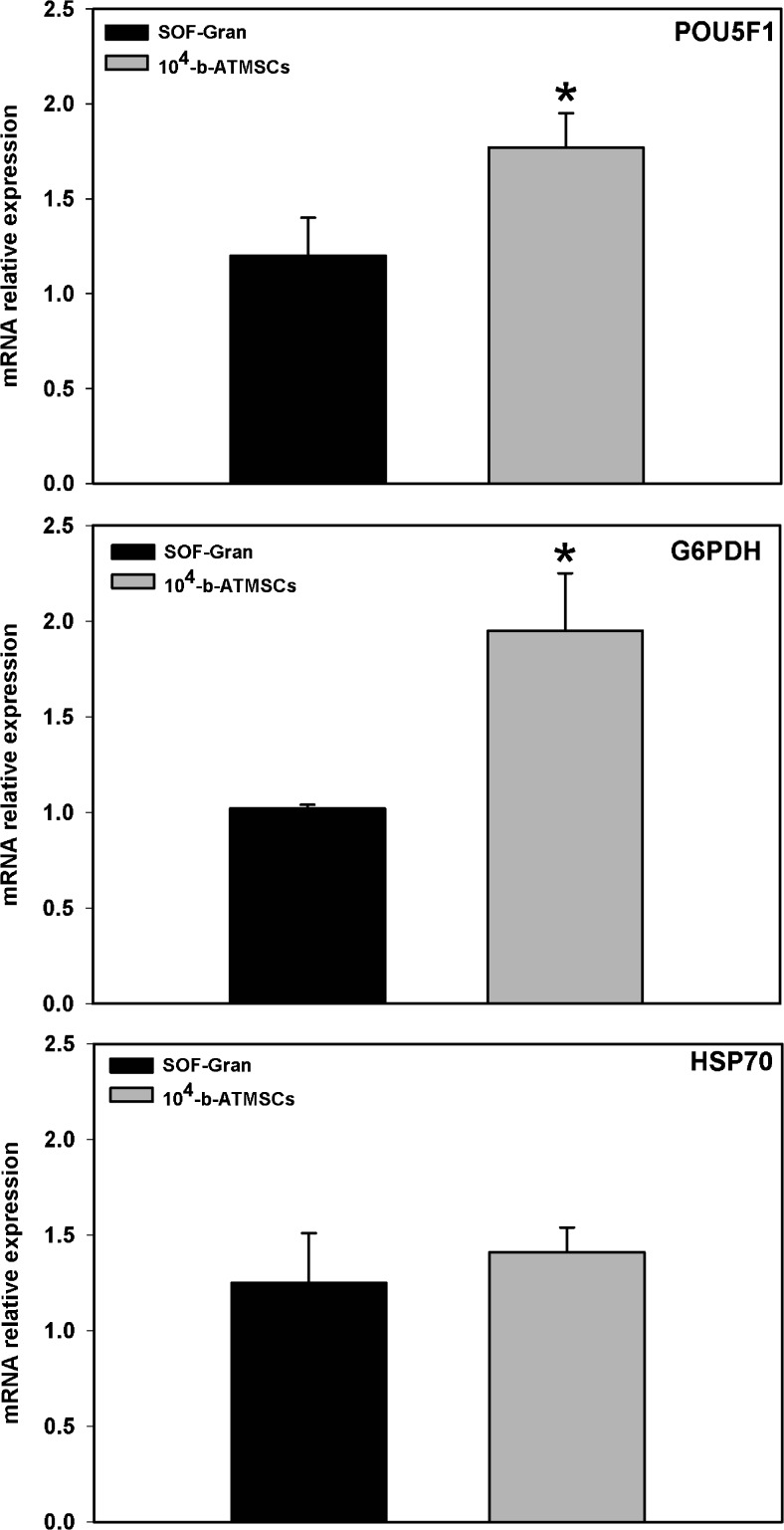
In terms of quality, co-culture with 104 b-ATMSCs rendered blastocysts with an increased total cell number (Fig. 3). In addition, co-culture with 104 b-ATMSCs increased (p < 0.05) the relative expression of the pluripotency (POU5F1) and glucose metabolism (G6PDH) markers but did not affect the expression of the heat stress marker HSP70 (Fig. 4). Altogether, these results support an increase in embryo quality resulting from co-culture with stem cells.
Fig. 3.
Total cell number counts for in vitro produced bovine blastocysts co-cultured with granulosa cells or adipose tissue-derived mesenchymal stem cells (104 b-ATMSCs). *p < 0.05 indicates a significant difference (t test)
Fig. 4.
Gene expression analysis of in vitro produced bovine embryos. Expression of POU5F1 (POU class 5 homeobox 1), G6PDH (glucose-6-phosphate dehydrogenase), and HSP70 (heat shock protein 70) was analyzed in blastocysts derived from a co-culture system with either adipose tissue-derived mesenchymal stem cells (b-ATMSCs; gray bars) or granulosa cells (black bars). *p < 0.05 indicates statistical significance (t test)
Discussion
In this study, we report for the first time the beneficial effect of b-ATMSCs in a co-culture system for in vitro production of bovine embryos. Moreover, we demonstrated that co-culture was more beneficial than the use of media preconditioned with the same stem cells or even than a traditional co-culture system with granulosa cells. Indeed, our findings corroborate previous reports showing a beneficial effect of stem cells in combination with embryo culture [25, 26]. Moshkdanian et al. [11] pioneered this area of study reporting that co-culture of mouse embryos with human umbilical cord mesenchymal cells (h-UCMS) improved the development of embryos previously exposed to light stress. Our study differs in that the stem cells used were from the same species as the embryos cultured.
Moreover, we showed that the stem cell density in co-culture is an important factor with a higher cell concentration further improving embryo development results. Interestingly, Moshkdanian et al. [11] used a stem cell concentration ten times higher (105 h-UCMS) than the highest concentration used in this study. Our choice was based on the fact that bovine embryo culture to the blastocyst stage takes 7 days (versus 4 days in mice), in an attempt to avoid excessive cell growth during embryo culture and hence competition with nutrients in the medium among embryos and feeder cells. Future studies may evaluate whether higher concentrations provide any additional benefits. Alternatively, using inactivated feeder stem cells may avoid the issue of nutrient competition while still providing growth support to embryos in culture.
The effect of ATMSCs on embryo development reported here is likely due to paracrine modulation of the microenvironment, given the reported ability of these cells to exert positive effects in vivo and in vitro on immunomodulation, angiogenesis, and/or tissue homeostasis reported in several studies. Thus, a stem cell-free model may be the desirable approach for cell therapy in the future once authors are showing positive effects of using only stem cell-conditioned medium for therapies which could avoid, for example, the risk of erratic cell divisions (tumors) in patients and also cell membrane-related antigen reactions giving opportunity to interspecies therapy, for example [14, 15, 17].
Growth factors, cytokines, and microRNAs (enclosed in exosomal microvesicles) have been listed as probable effector molecules responsible for the positive results achieved in many cell therapy studies, rather than a direct contribution to tissue regeneration from stem cell differentiation [14, 16, 17]. Exosomes are small vesicles (60 to 200 nm) produced by a variety of cell types, including MSCs, that may fuse with the plasma membrane of target cells to deliver mRNA, microRNAs, and even proteins [27–30]. MSC-derived exosomal microvesicles have been recently documented and may provide a potential explanation for the micromodulation potential observed with stem cells. Moreover, it was recently shown that human and cattle preimplantation embryos also secreted exosomes throughout the in vitro culture period [31, 32]. Therefore, one may also speculate a potential reciprocal effect between b-ATMSCs and embryos in culture that may explain the observed effects. These hypotheses require further investigation.
Notably, co-culture with MSCs not only increased blastocyst rates but also improved embryo quality as assessed by increased total cell numbers and relative gene expression levels. In general terms, considering that heat shock proteins can be stimulated by different stressors (not only overheating) affecting protein folding [33], our results indicated that the use of stem cells in co-culture has not caused any “extra” cellular stress in developing embryos compared to the traditional use of granulose cells. One of the genes positively influenced by co-culture with MSCs was POU5F1 which is considered a transcriptional regulator necessary for the maintenance of pluripotency in the developing embryo and embryonic stem cells of mammals [34, 35]. Similarly, we also observed an increase in G6PDH expression, a gene related to carbohydrate metabolism and NADPH availability for steroid and fatty acid biosynthesis, as well as responsible for increasing the ribose 5-phosphate supply for nucleotide and nucleic acid synthesis [36–38]. Both genes are considered good markers of embryo quality short of the possibility of performing transfer and evaluating postimplantation development [39–44].
Our study also provided a direct comparison of the positive effects of co-culture with stem cells versus the use of preconditioned medium. The use of stem cell-conditioned medium for embryo culture was previously reported [12, 13]. In these studies, supplementation of culture medium with a 10 % addition of human embryonic stem cell- or human adult adipose stem cell-conditioned medium (also termed bioactive material) improved embryo development in bovine or porcine, respectively. Our study differs in that 100 % of the medium used was preconditioned in the corresponding treatments. The time used to precondition the medium in culture with stem cells is not clear in the above referenced studies although, in both, the bioactive material was derived from stem cell cultures that reached ∼90 % confluence [12, 13]. In our study, SOF medium was conditioned for 24 h only. Superior results obtained with SOF-conditioned medium in comparison to SOF control indicate that conditioning was effective, but it may have been suboptimal. Further studies must be done to evaluate if SOF medium conditioned for longer periods than 24 h would further improve embryo development rates to those observed with co-culture with stem cells.
Stem cells derived from adipose tissue were originally isolated in mice and described by Rodbell and Jones in 1966 [45]. Since then, different studies have reported the isolation and characterization of these cells in several species including humans, pigs, dogs, horses, and cats [26, 46–49]. Interestingly, in cattle and buffaloes, ATMSC isolation was not published until recently [21]. We used a similar methodology and adopted the criteria proposed by the International Society for Cellular Therapy for successful isolation and characterization of the multipotent bovine ATMSCs used in this study [22].
Taken together, the results herein showed that co-culture of embryos with b-ATMSCs not only increased the number of embryos produced but appeared to have contributed to improve embryo energy metabolism (G6PDH) not causing cellular stress (HSP70), which could explain the increase in the total number of cells and expression of the pluripotency marker POU5F1. Many groups worldwide have achieved blastocyst rates similar to the ones reported here (∼40 %), indicating that co-culture with stem cells was as efficient as current IVEP protocols in bovines, most of them using supplemented IVC medium[10, 50–54]. Therefore, further identification of molecules produced by stem cells for embryo development could help to improve IVEP not only in bovine but also in other species such as humans. However, the results of transferring these embryos to recipient cows should be evaluated to further confirm any effects of this protocol on postimplantation development.
Conclusion
The use of b-ATMSCs in co-culture with bovine embryos improved both blastocyst rate and quality when compared to traditional co-culture with granulosa cells. Further studies should be conducted to assess whether the positive effects observed translate to in vivo development following embryo transfer and to identify the factors secreted by these stem cells that may be exerting a positive effect on preimplantation embryo development. Also, the results shown here could be tested in the culture of preimplantation embryos of other species.
Acknowledgments
The authors thank the North Paraná State University (UNOPAR—Brazil) for the financial partnership with the authors’ laboratory and the Brazilian Coordination for the Improvement of Higher Education Personnel (CAPES) for the student scholarships.
Footnotes
Capsule
Co-culture of in vitro produced bovine embryos with adult adipose tissue-derived bovine stem cells improved blastocyst development and quality.
References
- 1.Tan X-W, Tan J-H. Co-culture of embryos: influencing factors and mechanisms of action. Sheng Wu Gong Cheng Xue Bao. 2003;19:502–5. [PubMed] [Google Scholar]
- 2.Benkhalifa M, Demirol A, Sari T, Balashova E, Tsouroupaki M, Giakoumakis Y, et al. Autologous embryo-cumulus cells co-culture and blastocyst transfer in repeated implantation failures: a collaborative prospective randomized study. Zygote. 2012;20:173–80. doi: 10.1017/S0967199411000062. [DOI] [PubMed] [Google Scholar]
- 3.Pinyopummintr T, Bavister BD. In vitro-matured/in vitro-fertilized bovine oocytes can develop into morulae/blastocysts in chemically defined, protein-free culture media. Biol Reprod. 1991;45:736–42. doi: 10.1095/biolreprod45.5.736. [DOI] [PubMed] [Google Scholar]
- 4.Sakagami N, Nishino O, Adachi S, Umeki H, Uchiyama H, Ichikawa K, et al. Improvement of preimplantation development of in vitro-fertilized bovine zygotes by glucose supplementation to a chemically defined medium. J Vet Med Sci. 2014;76:1403–5. doi: 10.1292/jvms.13-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L-J, Xiong X-R, Zhang H, Li Y-Y, Li Q, Wang Y-S, et al. Defined media optimization for in vitro culture of bovine somatic cell nuclear transfer (SCNT) embryos. Theriogenology. 2012;78:2110–9. doi: 10.1016/j.theriogenology.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Lim KT, Jang G, Ko KH, Lee WW, Park HJ, Kim JJ, et al. Improved in vitro bovine embryo development and increased efficiency in producing viable calves using defined media. Theriogenology. 2007;67:293–302. doi: 10.1016/j.theriogenology.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Salzano A, Albero G, Zullo G, Neglia G, Abdel-Wahab A, Bifulco G, et al. Effect of resveratrol supplementation during culture on the quality and cryotolerance of bovine in vitro produced embryos. Anim Reprod Sci. 2014;151:91–6. doi: 10.1016/j.anireprosci.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Campelo IS, Pereira AF, Alcântara-Neto AS, Canel NG, Souza-Fabjan JMG, Teixeira DIA, et al. Effect of crotamine, a cell-penetrating peptide, on blastocyst production and gene expression of in vitro fertilized bovine embryos. Zygote. 2014;24:1–10. doi: 10.1017/S0967199414000707. [DOI] [PubMed] [Google Scholar]
- 9.Baldoceda-Baldeon LM, Gagné D, Vigneault C, Blondin P, Robert C. Improvement of bovine in vitro embryo production by vitamin K2 supplementation. Reproduction. 2014;148:489–97. doi: 10.1530/REP-14-0324. [DOI] [PubMed] [Google Scholar]
- 10.Santana PDPB, Silva TVG, da Costa NN, da Silva BB, Carter TF, Cordeiro MDS, et al. Supplementation of bovine embryo culture medium with L-arginine improves embryo quality via nitric oxide production. Mol Reprod Dev. 2014;81:918–27. doi: 10.1002/mrd.22387. [DOI] [PubMed] [Google Scholar]
- 11.Moshkdanian G, Nematollahi-Mahani SN, Pouya F, Nematollahi-Mahani A. Antioxidants rescue stressed embryos at a rate comparable with co-culturing of embryos with human umbilical cord mesenchymal cells. J Assist Reprod Genet. 2011;28:343–9. doi: 10.1007/s10815-010-9529-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim EY, Lee JB, Park HY, Jeong CJ, Riu KZ, Park SP. The use of embryonic stem cell derived bioactive material as a new protein supplement for the in vitro culture of bovine embryos. J Reprod Dev. 2011;57:346–54. doi: 10.1262/jrd.10-113A. [DOI] [PubMed] [Google Scholar]
- 13.Park HY, Kim EY, Lee S-E, Choi H-Y, Moon JJ, Park M-J, et al. Effect of human adipose tissue-derived mesenchymal-stem-cell bioactive materials on porcine embryo development. Mol Reprod Dev. 2013;80:1035–47. doi: 10.1002/mrd.22270. [DOI] [PubMed] [Google Scholar]
- 14.Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 2012;3:359. doi: 10.3389/fphys.2012.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katsuda T, Kosaka N, Takeshita F, Ochiya T. The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics. 2013;13:1637–53. doi: 10.1002/pmic.201200373. [DOI] [PubMed] [Google Scholar]
- 16.Salgado AJ, Reis RL, Sousa NJ, Gimble JM. Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther. 2010;5:103–10. doi: 10.2174/157488810791268564. [DOI] [PubMed] [Google Scholar]
- 17.Kapur SK, Katz AJ. Review of the adipose derived stem cell secretome. Biochimie. 2013;95:2222–8. doi: 10.1016/j.biochi.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Hocking AM, Gibran NS. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res. 2010;316:2213–9. doi: 10.1016/j.yexcr.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ling B, Feng DQ, Zhou Y, Gao T, Wei HM, Tian ZG. Effect of conditioned medium of mesenchymal stem cells on the in vitro maturation and subsequent development of mouse oocyte. Braz J Med Biol Res. 2008;41:978–85. doi: 10.1590/S0100-879X2008005000053. [DOI] [PubMed] [Google Scholar]
- 20.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 21.Sampaio RV, Chiaratti MR, Santos DCN, Bressan FF, Sangalli JR, Sá ALA, et al. Generation of bovine (Bos indicus) and buffalo (Bubalus bubalis) adipose tissue derived stem cells: isolation, characterization, and multipotentiality. Genet Mol Res. 2015;14:53–62. doi: 10.4238/2015.January.15.7. [DOI] [PubMed] [Google Scholar]
- 22.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Goossens K, Van Poucke M, Van Soom A, Vandesompele J, Van Zeveren A, Peelman LJ. Selection of reference genes for quantitative real-time PCR in bovine preimplantation embryos. BMC Dev Biol. 2005;5:27. doi: 10.1186/1471-213X-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linher K, Dyce P, Li J. Primordial germ cell-like cells differentiated in vitro from skin-derived stem cells. PLoS One. 2009;4:e8263. doi: 10.1371/journal.pone.0008263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song S-H, Kumar BM, Kang E-J, Lee Y-M, Kim T-H, Ock S-A, et al. Characterization of porcine multipotent stem/stromal cells derived from skin, adipose, and ovarian tissues and their differentiation in vitro into putative oocyte-like cells. Stem Cells Dev. 2011;20:1359–70. doi: 10.1089/scd.2010.0203. [DOI] [PubMed] [Google Scholar]
- 27.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 28.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 29.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–52. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zomer A, Vendrig T, Hopmans ES, van Eijndhoven M, Middeldorp JM, Pegtel DM. Exosomes. Communicative & Integrative Biology. 2010;3:447–50. doi: 10.4161/cib.3.5.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kropp J, Salih SM, Khatib H. Expression of microRNAs in bovine and human pre-implantation embryo culture media. Front Genet. 2014;5:91. doi: 10.3389/fgene.2014.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenbluth EM, Shelton DN, Wells LM, Sparks AET, Van Voorhis BJ. Human embryos secrete microRNAs into culture media—a potential biomarker for implantation. Fertil Steril. 2014;101:1493–500. doi: 10.1016/j.fertnstert.2014.01.058. [DOI] [PubMed] [Google Scholar]
- 33.Macario AJL, de Macario EC. Sick chaperones, cellular stress, and disease. N Engl J Med. 2005;353:1489–501. doi: 10.1056/NEJMra050111. [DOI] [PubMed] [Google Scholar]
- 34.Sha K, Boyer LA. The chromatin signature of pluripotent cells. In: Stem Book. Cambridge: Harvard Stem Cell Institute; 2009. [PubMed]
- 35.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 36.Lequarre AS, Grisart B, Moreau B, Schuurbiers N, Massip A, Dessy F. Glucose metabolism during bovine preimplantation development: analysis of gene expression in single oocytes and embryos. Mol Reprod Dev. 1997;48:216–26. doi: 10.1002/(SICI)1098-2795(199710)48:2<216::AID-MRD9>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 37.Iwata H, Kimura K, Hashimoto S, Ohta M, Tominaga K, Minami N. Role of G6PD activity on sex ratio and developmental competence of bovine embryos under oxidative stress. J Reprod Dev. 2002;48:447–53. doi: 10.1262/jrd.48.447. [DOI] [Google Scholar]
- 38.Gutiérrez-adan A, Rizos D, Fair T, Moreira PN, Pintado B, de la Fuente J, et al. Effect of speed of development on mRNA expression pattern in early bovine embryos cultured in vivo or in vitro. Mol Reprod Dev. 2004;68:441–8. doi: 10.1002/mrd.20113. [DOI] [PubMed] [Google Scholar]
- 39.Rodríguez-Alvarez L, Manriquez J, Velasquez A, Castro FO. Constitutive expression of the embryonic stem cell marker OCT4 in bovine somatic donor cells influences blastocysts rate and quality after nucleus transfer. In Vitro Cell Dev Biol. 2013;49:657–67. doi: 10.1007/s11626-013-9650-0. [DOI] [PubMed] [Google Scholar]
- 40.Oh HJ, Lee TH, Lee JH, Lee BC. Trichostatin a improves preimplantation development of bovine cloned embryos and alters expression of epigenetic and pluripotency genes in cloned blastocysts. J Vet Med Sci. 2012;74:1409–15. doi: 10.1292/jvms.11-0510. [DOI] [PubMed] [Google Scholar]
- 41.Oliveira CS, de Souza MM, Saraiva NZ, Tetzner TAD, Lima MR, Lopes FL, et al. In vitro culture of bovine embryos in murine ES cell conditioned media negatively affects expression of pluripotency-related markers OCT4, SOX2 and SSEA1. Reprod Domest Anim. 2012;47:428–35. doi: 10.1111/j.1439-0531.2011.01896.x. [DOI] [PubMed] [Google Scholar]
- 42.Khan DR, Dubé D, Gall L, Peynot N, Ruffini S, Laffont L, et al. Expression of pluripotency master regulators during two key developmental transitions: EGA and early lineage specification in the bovine embryo. PLoS One. 2012;7:e34110. doi: 10.1371/journal.pone.0034110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lopes AS, Wrenzycki C, Ramsing NB, Herrmann D, Niemann H, Løvendahl P, et al. Respiration rates correlate with mRNA expression of G6PD and GLUT1 genes in individual bovine in vitro-produced blastocysts. Theriogenology. 2007;68:223–36. doi: 10.1016/j.theriogenology.2007.04.055. [DOI] [PubMed] [Google Scholar]
- 44.Sturmey RG, Bermejo-Alvarez P, Gutierrez-Adan A, Rizos D, Leese HJ, Lonergan P. Amino acid metabolism of bovine blastocysts: a biomarker of sex and viability. Mol Reprod Dev. 2010;77:285–96. doi: 10.1002/mrd.21173. [DOI] [PubMed] [Google Scholar]
- 45.Rodbell M, Jones AB. Metabolism of isolated fat cells. 3. The similar inhibitory action of phospholipase C (Clostridium perfringens alpha toxin) and of insulin on lipolysis stimulated by lipolytic hormones and theophylline. J Biol Chem. 1966;241:140–2. [PubMed] [Google Scholar]
- 46.Aust L, Devlin B, Foster SJ, Halvorsen YDC, Hicok K, du Laney T, et al. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6:7–14. doi: 10.1080/14653240310004539. [DOI] [PubMed] [Google Scholar]
- 47.Pascucci L, Curina G, Mercati F, Marini C, Dall’Aglio C, Paternesi B, et al. Flow cytometric characterization of culture expanded multipotent mesenchymal stromal cells (MSCs) from horse adipose tissue: towards the definition of minimal stemness criteria. Vet Immunol Immunopathol. 2011;144:499–506. doi: 10.1016/j.vetimm.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 48.Webb TL, Quimby JM, Dow SW. In vitro comparison of feline bone marrow-derived and adipose tissue-derived mesenchymal stem cells. J Feline Med Surg. 2012;14:165–8. doi: 10.1177/1098612X11429224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neupane M, Chang C-C, Kiupel M, Yuzbasiyan-Gurkan V. Isolation and characterization of canine adipose-derived mesenchymal stem cells. Tissue Eng Part A. 2008;14:1007–15. doi: 10.1089/ten.tea.2007.0207. [DOI] [PubMed] [Google Scholar]
- 50.Zullo G, Albero G, Neglia G, De Canditiis C, Bifulco G, Campanile G, et al. L-ergothioneine supplementation during culture improves quality of bovine in vitro-produced embryos. Theriogenology. 2016;85:688–97. doi: 10.1016/j.theriogenology.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 51.Remião MH, Lucas CG, Domingues WB, Silveira T, Barther NN, Komninou ER, et al. Melatonin delivery by nanocapsules during in vitro bovine oocyte maturation decreased the reactive oxygen species of oocytes and embryos. Reprod Toxicol. 2016;63:70–81. doi: 10.1016/j.reprotox.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 52.Lucas CG, Remião MH, Komninou ER, Domingues WB, Haas C, de Leon PMM, et al. Tretinoin-loaded lipid-core nanocapsules decrease reactive oxygen species levels and improve bovine embryonic development during in vitro oocyte maturation. Reprod Toxicol. 2015;58:131–9. doi: 10.1016/j.reprotox.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 53.Oseikria M, Elis S, Maillard V, Corbin E, Uzbekova S. N-3 polyunsaturated fatty acid DHA during IVM affected oocyte developmental competence in cattle. Theriogenology. 2016;85:1625–1634.e2. doi: 10.1016/j.theriogenology.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 54.Ortiz-Escribano N, Smits K, Piepers S, Van den Abbeel E, Woelders H, Van Soom A. Role of cumulus cells during vitrification and fertilization of mature bovine oocytes: effects on survival, fertilization, and blastocyst development. Theriogenology. 2016;86:635–41. doi: 10.1016/j.theriogenology.2016.02.015. [DOI] [PubMed] [Google Scholar]