Abstract
Purpose
The study aimed to investigate key intrafollicular prognostic factors among various cytokines and angiogenic molecules for prediction of mature oocytes and good-quality embryos in women with endometriosis undergoing in vitro fertilization (IVF).
Methods
Paired follicular fluid and serum samples were collected from 200 women with advanced stage endometriosis and 140 normal ovulating women during oocyte retrieval. The concentrations of cytokines (pro-inflammatory: IL-1β, TNF-α, IL-2, IL-8, IL-12, IFN-γ; anti-inflammatory: IL-4, IL-6, IL-10) and angiogenic molecules (vascular endothelial growth factor (VEGF), adrenomedullin, angiogenin) were determined in follicular fluid and serum using ELISA. Expression of these molecules was subjected to multivariate analysis for the identification of major predictive markers of oocyte and embryo quality. Receiver operating characteristic (ROC) curve was applied to determine the best cutoff point for the discrimination between mature and immature oocytes in these women.
Results
Significant increases in levels of cytokines and angiogenic molecules were observed in women with endometriosis compared to controls (P < 0.001). From the validated partial least squares-discriminant analysis (PLS-DA) model, IL-8, IL-12, and adrenomedullin were identified as the most important factors contributing to endometriosis and were negatively associated with oocyte maturity and embryo quality.
Conclusion
The levels of IL-8, IL-12, and adrenomedullin may be good indicators of embryo and oocyte quality in endometriosis patients undergoing IVF. Further studies are necessary to ascertain the potential of these markers for oocyte and embryo developmental competence which may help improve the chances of a successful IVF in endometriosis patients.
Keywords: Endometriosis, Cytokines, Follicular fluid, Oocytes, Embryos
Introduction
Endometriosis, characterized by benign growth of functional endometrial tissue outside the uterine cavity, affects more than 150 million women worldwide, among which 30–40 % are infertile [1]. An association between endometriosis and infertility has repeatedly been reported in the literature, but an absolute cause–effect relationship is yet to be confirmed. An exhaustive meta-analysis suggests that patients with endometriosis-associated infertility undergoing in vitro fertilization (IVF) have a decreased pregnancy rate as compared with other indications for IVF [2]. The data also suggest that endometriosis affects both the development of the oocyte and embryo and the receptivity of the endometrium [3].
Follicular fluid may be regarded as a biological “window” reflecting metabolic and hormonal processes occurring in the microenvironment of the maturing oocyte before ovulation. It contains several biochemical components including cytokines, chemokines, growth factors, and steroid hormones which are involved in the process of folliculogenesis. It is, therefore, logical to presume that follicular fluid can act as a predictor of IVF outcome parameters, such as oocyte maturity, fertilization rate, embryo quality, and pregnancy rate [4].
It is evidenced that cytokines, as a result of local synthesis in the ovary and from blood plasma, are present in follicular fluid and modulate the processes of follicular growth [5], oocyte maturation [6], ovulation [7], steroidogenesis [8], embryo development, and pregnancy [9]. It is well established that an adequate vascular supply is essential for ovarian follicle development [10]. The angiogenic factors, including adrenomedullin (ADM), vascular endothelial growth factor (VEGF), and angiogenin, present in follicular fluid are reported to play critical roles in the process of folliculogenesis and oocyte maturation due to their vasodilator, vasorelaxant, and angiogenic effects [11, 12].
It is believed that presence of endometriosis may alter the microenvironment of the maturing oocyte, resulting in ovulatory dysfunction, poor oocyte quality, reduced fertilization rate, low-grade embryos, and reduced implantation rates [13, 14]. A recent study also suggests that endometriosis may affect the metabolic and hormonal processes, thereby altering the microenvironment of the follicle and oocyte development [15]. This motivated us to explore intrafollicular cytokines including IL-1β, TNF-α, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, and IFN-γ and angiogenic factors including VEGF, ADM, and angiogenin in serum and follicular fluid of women with endometriosis and relate the findings with oocyte maturation and embryo quality following IVF. Further, three most important prognostic factors for prediction of mature oocytes and good-quality embryos with high accuracy are identified using multivariate analysis.
Material and methods
This prospective case-controlled study was conducted at a tertiary care hospital, the Institute of Reproductive Medicine, Kolkata, India. A part of the study was done at the Reproductive Health Research Unit, School of Medical Science and Technology, Indian Institute of Technology, Kharagpur, India. Approval was obtained from the Institutional Research Ethics Board. Written informed consent was taken from all couples participating in the study.
Subject selection
A total of 340 infertile women (26–40 years) with BMI <25 kg/m2 and duration of infertility >2 years undergoing IVF were included for this study. Out of these, 200 women with endometriosis (stages III and IV) were included as the study group. Endometriosis was confirmed by laparoscopy followed by biopsy and staging done using the revised American Society for Reproductive Medicine guidelines [16]. For controls, 140 normal ovulating women with tubal factor infertility (without endometriosis) were included.
Tubal factor infertility, in this study, refers to women who had fallopian tube(s) removed for tubal pregnancy and proximal tubal obstruction because of low-grade infection or fimbrial occlusion with or without mild peritubal adhesions. Tubal infertility associated with gross hydrosalpingeal changes, dense pelvic adhesions because of endometriosis, polycystic ovary syndrome, uterine anomalies, autoimmune abnormalities, and pelvic inflammatory diseases were excluded. Women with adenomyosis, severe pelvic inflammatory diseases, thyroid disorders, hypoprolactemia, diabetes mellitus, and cardiovascular diseases were also excluded.
Only those women were considered whose partners/donors were normozoospermic with semen parameters normal according to World Health Organization guidelines [17]. It was also ensured that women included had not received any medication other than the standard IVF stimulation protocol since the last 3 months. Baseline follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), and peak E2 levels in serum and various IVF outcome parameters were assessed in both the groups.
Ovarian stimulation protocol
Ovarian stimulation was performed using the downregulation protocol with a gonadotropin-releasing hormone (GnRH) agonist. Briefly, the patients were downregulated with a GnRH agonist (Lupride, Sun Pharmaceuticals, Mumbai, India) mid-luteal phase onwards and, when optimally downregulated, were stimulated with recombinant FSH (Gonal F, Serono, Geneva, Switzerland). Follicular size was monitored regularly by ultrasound and serum estradiol assays. Subcutaneous human chorionic gonadotropin (hCG) (Pregnyl, Organon, the Netherlands) was administered when average diameter of the leading follicles reached at least 18 mm. The oocytes were retrieved under transvaginal ultrasound guidance.
Assessment of oocyte maturity, fertilization, and embryo quality
Oocyte and embryo quality were assessed by the clinical embryologists. Oocyte maturity was evaluated by taking into account cumulus and corona cells, ooplasm granularity, presence or absence of first polar body, and germinal vesicle [18]. After grading, oocyte-cumulus-corona complexes (OCCC) were selected for insemination with freshly prepared swim-up spermatozoa of the male partner. Fertilization was assessed 16–18 h post-insemination. Normal fertilization was considered as when oocytes had two pronuclei and two polar bodies. Embryo morphology was evaluated by taking into account the number, symmetry, and granularity of blastomeres, percentage and type of fragmentation, degree of compaction, and presence of multinucleated blastomeres [18]. For comparison purposes, the retrieved oocytes were divided into two groups: one group consisted of MII oocytes (mature oocytes) and the other group included grade I and II oocytes (immature oocytes). Post-mature or grade IV oocytes were not included in this study. Good (grade I and II) and poor (grade III) quality embryos were classified into two groups in a similar manner. The retrieved oocytes were assessed and corresponding embryos followed and cultured individually. Clinical pregnancy was defined as the presence of a gestational sac with cardiac activity as detected by transvaginal ultrasound at 6–7 weeks of gestation.
Collection and preparation of follicular fluid and serum samples
A total of 1680 follicular fluid samples were carefully aspirated from women with endometriosis (n = 200) and 1400 samples from controls (n = 140) cases during oocyte retrieval and collected in individual sterile containers. Out of these, follicular fluid containing more than one oocyte or no oocytes or contaminated with blood/media was discarded. Follicular fluid containing follicles<17 mm in diameter was also excluded. The remaining 840 samples from endometriosis group and 686 samples from controls containing single oocytes were centrifuged at 800×g for 10 min to remove cellular components; the clear supernatant was divided into aliquots and frozen at −70 °C until further use. Before use, follicular fluid was centrifuged and filtered to remove the precipitate and the protein content was measured.
On the basis of maturity of the retrieved oocytes, 840 follicular fluid samples from endometriosis cases were subdivided into two groups: 607 MII or mature oocytes and 233 immature or grade I and II oocytes. Similarly, 686 follicular fluid samples from the controls were subdivided into 573 MII or mature oocytes and 113 immature or grade I and II oocytes. A total of 306 embryos resulted from 840 oocytes of endometriosis group, and 305 embryos were formed from 686 oocytes of controls. The 306 embryos of endometriosis group were further subdivided into two groups on the basis of the quality of embryos: 209 good-quality embryos (grade I and II) and 97 poor-quality embryos (grade III). Similarly, the 305 embryos of controls were subdivided into 228 good-quality embryos and 77 poor-quality embryos.
Five milliliters of blood was also collected on the day of oocyte retrieval and immediately centrifuged (550×g for 5 min) to separate the serum. Protein content was measured and stored in aliquots at −70 °C until further use.
Enzyme-linked immunosorbent assay (ELISA)
The concentrations of cytokines (pro-inflammatory: IL-1β, TNF-α, IL-2, IL-8, IL-12, IFN-γ; anti-inflammatory: IL-4, IL-6, IL-10) were determined in follicular fluid and serum using commercially available kits (BD Biosciences, USA). Similarly, levels of angiogenic molecules such as VEGF and angiogenin were assessed using quantikine ELISA Kit (R&D Systems Inc., USA). ADM was assessed using DRG ELISA kit (DRG International Inc., USA). All estimations were carried out according to the manufacturer’s instructions. Dilutions were done wherever necessary to obtain readings in the linear range of the assays. All analyses and calibrations were performed in triplicate. The measurements were repeated in cases of very high or low results. The assay sensitivity for IL-1β, TNF-α, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IFN-γ, VEGF, ADM, and angiogenin was 0.8, 2, 1, 2, 2.2, 0.8, 2, 2, 1, 5, 6, and 6 pg/ml, respectively. The intra- and inter-assay variabilities were, respectively, <3.5 and <5 % for IL-1β, <4 and <6 % for TNF-α, <5.5 and <4.5 % for IL-2, <5 and <6 % for IL-4, <5.5 and <7 % for IL-6, <4.5 and <3.5 % for IL-8, <3 and <6 % for IL-10, <5.5 and <7 % for IL-12, <4.5 and <5.8 % for IFN-γ, <6.5 and <7 % for VEGF, <5 and <6 % for ADM, and <4 and <7.5 % for angiogenin.
Statistical analysis
SIMCA 13.0.2 software (Umetrics, Sweden), GraphPad Prism (GraphPad Software, Inc., USA), and GraphPad QuickCalcs were used for statistical analysis. All data were presented as mean ± standard deviation (SD) and median range, wherever appropriate. Data were assessed for normality of distribution using the Shapiro–Wilk test. Comparison between groups was carried out using Student’s t test, Kruskal–Wallis test, and Spearman correlation coefficient, as applicable. Statistical significance was defined as P ≤ 0.05.
Expression of different cytokines and angiogenic factors in endometriosis compared to controls was analyzed using multivariate statistical analysis. Data were preprocessed using normalization and scaling to remove possible bias arising due to sample handling and sample variability. Normalization (by sum) was performed in order to minimize possible differences in concentration between samples. Following normalization, scaling (mean centering and division by the square root of standard deviation of each variable) was done to give all variables equal weight regardless of their absolute value. After data preprocessing, principal component analysis (PCA) and partial least squares-discriminant analysis (PLS-DA) were performed using SIMCA 13.0.2 (Umetrics, Sweden). PCA is an unsupervised method and a data reduction technique that allows the major sources of variation in a multi-dimensional dataset to be analyzed without introducing inherent bias. PLS-DA is a regression extension of the PCA that uses class information to maximize the separation between various groups of observations.
To determine the levels of key contributory molecules in follicular fluid that can discriminate between good-quality and poor-quality oocytes, we applied the receiver operating characteristic (ROC) curve (MedCalc Software, Belgium). To construct the ROC curves, we took into account only two categories: mature (n = 100) and immature (n = 100) oocytes. The ROC curves were constructed by plotting the false-positive rate or (1–specificity) on the x-axis and the true-positive rate or sensitivity on the y-axis. The calculation of the area under the ROC (area under the curve (AUC)–ROC) curve measures the accuracy, i.e., ability of the ROC value to discriminate between mature and immature oocytes.
Results
Demographic values
The main clinical characteristics and IVF outcome parameters of endometriosis and controls are shown in Table 1. Mean age, BMI, duration of infertility, levels of FSH, LH, and E2 were found to be comparable between the groups. Approximately 87 % cases of endometriosis and 18.6 % controls had primary infertility. A maximum of three IVF-ET cycles were attempted in women of both the groups. Total number of follicles aspirated, oocytes retrieved and number of MII oocytes, fertilization rate, and grade I and II embryos formed were significantly less in women with endometriosis as compared with controls (P < 0.01). A total of 68 women from endometriosis group and 57 women from controls became pregnant following IVF. Out of these, 32 and 45.6 % pregnancy occurred after first cycle of IVF-ET in endometriosis and controls, respectively. Although implantation and pregnancy rates were lower in endometriosis than in controls, the differences were not statistically significant.
Table 1.
Clinical characteristics and IVF outcome parameters of women with endometriosis and controls
| Parameters | Endometriosis; n = 200 | Controls; n = 140 |
|---|---|---|
| Age (years) | 32.3 ± 2.98 | 31.7 ± 3.61 |
| BMI (kg/m2) | 22.2 ± 2.67 | 21.6 ± 3.27 |
| Duration of infertility (years) | 7.1 ± 2.28 | 6.7 ± 1.98 |
| Primary infertility (%) | 174 (87) | 26 (18.6)* |
| Secondary infertility (%) | 26 (13) | 114 (81.4)* |
| Basal FSH (mIU/ml) | 6.5 ± 1.65 | 6.3 ± 1.75 |
| Basal LH (mIU/ml) | 5.4 ± 1.32 | 5.2 ± 0.98 |
| Basal E2 (pg/ml) | 48.3 ± 5.54 | 47.6 ± 4.54 |
| Peak E2 (pg/ml) | 2283 ± 945.3 | 2074 ± 1087.7 |
| No. of follicles aspirated | 8.4 ± 2.63 | 10.0 ± 2.81* |
| No. of oocytes retrieved | 7.4 ± 2.67 | 8.3 ± 3.72** |
| Metaphase II (M II) oocyte | 5.2 ± 1.63 | 6.8 ± 1.85* |
| Fertilization rate (%) | 73.2 ± 17.62 | 78.6 ± 18.45** |
| Grade I and II embryos formed | 2.28 ± 1.2 | 3.32 ± 1.3* |
| Number of embryo transferred | 2.2 ± 0.6 | 2.3 ± 0.8 |
| Pregnancy rate (%) | 34 % (68/200) | 40.7 % (57/140) |
| Miscarriage rate (%) | 16 % (32/200) | 12.1 % (17/140) |
Mean ± SD
*P ≤ 0.001; **P ≤ 0.01; others not significant
Levels of cytokines and angiogenic molecules
Intrafollicular and serum levels of cytokines and angiogenic molecules in endometriosis and controls are presented in Table 2. A significant increase in the activities of pro-inflammatory cytokines, anti-inflammatory cytokines, and angiogenic factors in follicular fluid and serum samples was seen in endometriosis as compared with controls. Although levels of these molecules in follicular fluid were significantly lower than in serum, significant correlation was observed between them for individual patients (r = 0.57, P < 0.001 for IL-1β; r = 0.55, P < 0.001 for IL-2; r = 0.68, P < 0.001 for IL-4; r = 0.48, P < 0.001 for IL-6; r = 0.52, P < 0.001 for IL-8; r = 0.63, P < 0.001 for IL-10; r = 0.61, P < 0.001 for IL-12; r = 0.64, P < 0.001 for TNF-α; r = 0.53, P < 0.001 for IFN-γ; r = 0.51, P < 0.001 for VEGF; r = 0.66, P < 0.001 for ADM; r = 0.64, P < 0.001 for angiogenin).
Table 2.
Comparison of various cytokines and angiogenic factors in follicular fluid and serum of women with endometriosis and controls undergoing IVF
| Parameters | Follicular fluid | Serum | ||
|---|---|---|---|---|
| Endometriosis (n = 200)a | Controls (n = 140)b | Endometriosis (n = 200)c | Controls (n = 140)d | |
| IL-β 1 (pg/ml) | 178.67 ± 19.8 | 108.35 ± 14.2 | 247.47 ± 32.6 | 97.43 ± 12.7 |
| TNF-α (pg/ml) | 31.85 ± 3.2 | 17.95 ± 2.5 | 58.24 ± 7.3 | 41.61 ± 3.6 |
| IL-2 (pg/ml) | 144.21 ± 13.4 | 92.36 ± 12.4 | 336.25 ± 28.7 | 248.34 ± 29.6 |
| IL-4 (pg/ml) | 214.24 ± 23.6 | 132.26 ± 17.3 | 467.58 ± 52.6 | 332.44 ± 37.2 |
| IL-6 (pg/ml) | 17.37 ± 2.5 | 11.5 ± 2.4 | 74.56 ± 8.2 | 46.51 ± 5.3 |
| IL-8 (pg/ml) | 13.26 ± 0.9 | 5.61 ± 0.7 | 35.83 ± 4.5 | 21.56 ± 1.3 |
| IL-10 (pg/ml) | 118.65 ± 13.6 | 74.86 ± 10.2 | 374.25 ± 35.2 | 186.54 ± 23.5 |
| IL-12 (pg/ml) | 361.54 ± 27.4 | 168.42 ± 11.5 | 583.28 ± 49.4 | 429.36 ± 45.8 |
| INF-γ (pg/ml) | 78.72 ± 9.6 | 46.28 ± 5.8 | 236.46 ± 25.4 | 121.51 ± 15.5 |
| VEGF (pg/ml) | 571.08 ± 64.08 | 342.82 ± 37.3 | 748.57 ± 65.2 | 584.78 ± 55.3 |
| ADM (pg/ml) | 423.21 ± 35.2 | 216.42 ± 17.2 | 663.53 ± 61.4 | 503.82 ± 53.6 |
| Angiogenin (pg/ml) | 472.39 ± 51.2 | 324.35 ± 32.6 | 732.36 ± 81.5 | 587.35 ± 65.3 |
Mean ± SD
a versus b and c versus d are significant: P < 0.001
Multivariate statistical analyses
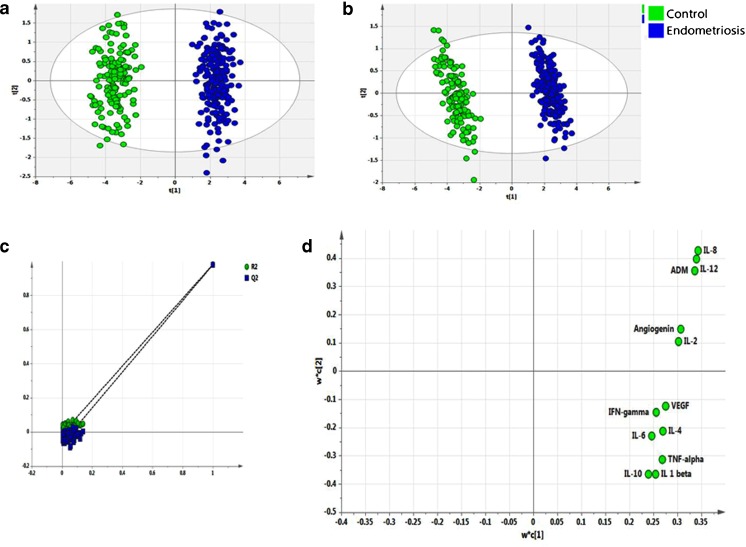
This advanced statistical tool was used to identify the key molecules which significantly affect IVF outcome in women with endometriosis. Exploratory PCA was employed to detect intrinsic clustering and possible outliers. Scatterplot of t1 versus t2 indicates unsupervised (unbiased and having no prior knowledge of sample groups) separation trend between endometriosis and controls (Fig. 1a). The group separation was further maximized by PLS-DA (Fig. 1b). The predictive capability (Q2) and explained variance (R2) were extracted for the PLS-DA model. The model with both R2 and Q2 well above 0.9 indicated a very good predictive ability. Further, validation of the PLS-DA model was performed using permutation test statistics. This validation is performed to compare the R2 (0.981) and Q2 (0.979) of the original model with the R2 and Q2 of several models based on data where the order of the class variable is randomly permuted, while the other data matrix is kept intact. Results of permutation test statistics indicated the original model to be far superior than the entire 100 model generated by random permutation of class variable (Fig. 1c).
Fig. 1.
Multivariate analysis. Score scatterplot of t1 versus t2 after applying a PCA and b PLS-DA to various intrafollicular cytokines and angiogenic growth factors in women with endometriosis (blue) and controls (green). c Results from the permutation test for the endometriosis group suggesting a valid PLS-DA model. The vertical axis comprises of the R2 and Q2 values of each model, and the horizontal axis shows the correlation between the permuted class vectors and the original class vector. The original class has the correlation 1.0 with itself, defining the high point on the horizontal axis. All R2 and Q2 values calculated from the permuted data are lower than the original model in the validation plot. Y-axis intercepts: R2 = (0.0, −0.0114), Q2 = (0.0, −0.0686). The loading scatterplot (d) shows upregulation of all cytokines and angiogenic growth factors in endometriosis
Following validation of the model, PLS-DA loading plot (Fig. 1d) was used to determine the relative contribution of each of the cytokines and angiogenic factors (referred to as the “loading”) for components 1 and 2. Further, a variable lies away from the plot origin, the stronger impact the variable has on the model. Comparing the loading and score plot, all cytokines and angiogenic factors showed positive factor 1 loading, indicating more bias towards endometriosis. Factors with high variable importance in projection (VIP) scores are regarded as significant and, therefore, considered for quantitative analysis of variation. IL-8, IL-12, and ADM in follicular fluid were identified as the prime factors corresponding to three highest VIP scores (Fig. 2). These molecules with higher VIP score denote that these are the three significant factors which can well discriminate between endometriosis and controls.
Fig. 2.
VIP score. Important features identified by PLS-DA and VIP score. IL-8, IL-12, and ADM in follicular fluid show the highest VIP score indicating that these three molecules are the most significant factors in discriminating between endometriosis and controls
ROC curve analysis
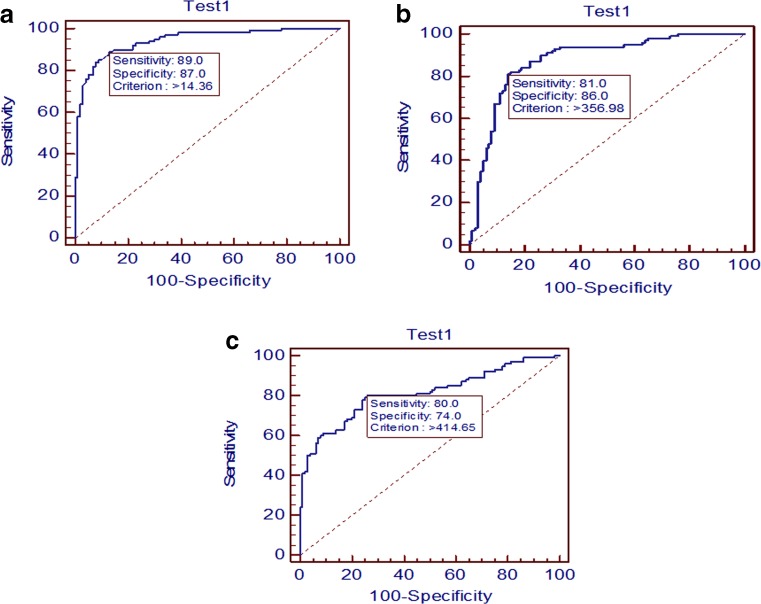
We, therefore, decided to predict the cutoff value for these molecules in follicular fluid beyond which good-quality oocyte formation is not favorable. The accuracy of intrafollicular levels of IL-8, IL-12, and ADM in discriminating between mature and immature oocytes by ROC curve analysis in women with endometriosis is indicated in Fig. 3. IL-8 ≤14.36 pg/ml provided the cutoff value with 89 % sensitivity and 87 % specificity for mature oocytes (Fig. 3a). As a result, it was observed that, above this cutoff value, 178 oocytes out of 200 were of poor quality. IL-12 level ≤356.98 pg/ml provided the cutoff value with 81 % sensitivity and 86 % specificity for mature oocytes (Fig. 3b). In a similar way, above this cutoff value, 162 oocytes out of 200 were of poor quality. Moreover, for ADM, ≤414.65 pg/ml provided the cutoff value with 80 % sensitivity and 74 % specificity for mature oocytes (Fig. 3c), and above this cutoff value, 160 oocytes out of 200 were of poor quality. Interestingly, there were few patients from controls whose cutoff levels for IL-8, IL-12, and ADM were even higher than the endometriosis group for respective cytokines. For example, in case of IL-8, 13 oocytes out of 686 from controls were found to have higher cutoff value than endometriosis group. Similarly, in case of IL-12, 17 oocytes and in case of ADM, 9 oocytes out of 686 from controls were found to have higher cutoff value than endometriosis group. However, these values were very less in number and are non-significant. The AUC for IL-8, IL-12, and ADM was found to be 0.94, 0.87, and 0.81, respectively.
Fig. 3.
Receiver operating characteristic (ROC) curves to predict a IL-8, b IL-12, and c ADM cutoff value in follicular fluid beyond which good-quality oocyte formation is not favorable in endometriosis
Oocyte and embryo quality
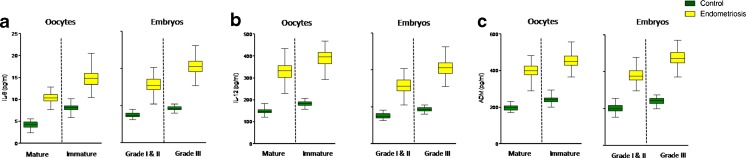
Oocyte maturity and embryo quality were compared with the levels of IL-8, IL-12, and ADM in follicular fluid of women with endometriosis and controls (Fig. 4). These levels were significantly lower (P < 0.001) for MII oocytes as compared with immature oocytes for both the groups. Grade I and II embryo formation was associated with a significant decrease in the levels of these molecules (P < 0.001) as compared with grade III embryos for both the cases. Table 3 shows the IVF outcome in terms of oocyte and embryo quality in endometriosis and controls. Percentage of mature oocyte and good-quality embryos were significantly less in endometriosis as compared with controls. However, IL-8, IL-12, and ADM levels were found to be significantly lower for MII oocytes and good-quality embryos as compared with immature oocytes and poor-quality embryos, respectively, in both the groups (P < 0.001).
Fig. 4.
Box and whisker plots. Follicular fluid concentrations of a IL-8, b IL-12, and c ADM of follicles containing different grades of oocytes (mature and immature) and embryo quality (grades I and II and grade III) in women with controls and endometriosis. The box and whisker plots are constructed from five values: the smallest value, first quartile, median, third quartile, and maximum values (**P < 0.001)
Table 3.
Levels of IL-8, IL-12, and ADM in endometriosis and controls for oocyte and embryo quality
| Parameters | IVF outcome in terms of oocyte and embryo quality | |||||||
|---|---|---|---|---|---|---|---|---|
| Oocyte quality | Embryo quality | |||||||
| Mature oocytes in endometriosis (70.2 %) | Mature oocytes in controls (81.7 %) | Immature oocytes in endometriosis (29.8 %) | Immature oocytes in controls (18.3 %) | Good quality embryos in endometriosis (71.2 %) | Good quality embryos in controls (78.8 %) | Poor quality embryos in endometriosis (28.2 %) | Poor quality embryos in controls (21.2 %) | |
| IL-8 (pg/ml) | 10.42 ± 1.11 | 4.21 ± 0.76 | 14.81 ± 1.62 | 8.13 ± 0.82 | 10.54 ± 1.39 | 4.09 ± 0.60 | 13.77 ± 1.58 | 7.52 ± 0.87 |
| IL-12 (pg/ml) | 330.71 ± 40.60 | 147.97 ± 12.60 | 391.75 ± 35.83 | 182.52 ± 11.78 | 314.86 ± 39.66 | 150.78 ± 13.52 | 410.54 ± 40.32 | 186.06 ± 12.04 |
| ADM (pg/ml) | 397.26 ± 36.75 | 196.51 ± 15.67 | 453.58 ± 35.50 | 240.89 ± 20.56 | 377.38 ± 37.66 | 200.80 ± 22.62 | 473.62 ± 44.10 | 240.84 ± 19.37 |
Discussion
Endometriosis may alter the microenvironment of the follicle, resulting in ovulatory dysfunction, poor oocyte quality, reduced fertilization rate, low-grade embryos, and reduced implantation rates [19]. Several studies demonstrate that intrafollicular levels of cytokines are altered in patients with endometriosis [9, 20, 21]. Elevated levels of cytokines in serum and peritoneal fluid of endometriosis cases are also reported [22, 23]. Increased level of serum-derived cytokines in follicular fluid of women with endometriosis is suggested [24]. IL-8 is suggested to play a role in the pathogenesis of endometriosis by promoting neovascularization, endometrial cell attachment, and cell growth [25]. Patients with severe endometriosis are reported to have higher IL-12 levels in peritoneal fluid [26]. Our findings also indicate a significant increase in the levels of pro-inflammatory (IL-1β, TNF-α, IL-2, IL-8, IL-12, IFN-γ) and anti-inflammatory (IL-4, IL-6, IL-10) cytokines in women with endometriosis undergoing IVF as compared with controls. The expression patterns of all molecules in serum followed a similar trend. The circulating biochemical milieu may be attributed to the fact that follicular fluid is a combined product of those blood plasma constituents that cross the blood follicular barrier and the granulosa and thecal cells’ secretory activity [27].
The levels of cytokines in follicular fluid and their effect on oocyte and embryo formation have been extensively investigated; however, results remain contradictory. Early studies indicate that IL-2 and TNF-α levels are not related to oocyte maturity, fertilization, and likelihood of pregnancy [28]. Another study indicates no correlation between intrafollicular IL-1β level and fertilization and embryo cleavage rates [29]. Presently, there is accumulating evidence suggesting that individual cytokines in human follicular fluid can be used as predictors of IVF outcome [30]. A positive association of IL-6 with oocyte maturation [31] and IL-1β with embryo development and pregnancy [32] is documented. A majority of findings have indicated that IL-12 is negatively associated with folliculogenesis, oocyte quality, and implantation [30, 33, 34]. TNF-α, reported to be significantly higher in follicular fluids corresponding to poor-quality oocytes and embryos, possibly deteriorates the microenvironment in the follicle [35, 36]. While a temporary increase in IFN-γ seems essential for ovulation, levels that exceed normal physiologic concentrations may inhibit ovulation and contribute to early pregnancy loss [37].
Dense vascularization is a characteristic feature of endometriotic lesions and is mediated through the angiogenesis process [38]. Levels of angiogenic molecules in follicular fluid are found to be dysregulated in patients with endometriosis [20, 39]. We found the intrafollicular angiogenic molecules including VEGF, ADM, and angiogenin to be significantly elevated in such women (Table 2).
An adequate blood supply is fundamentally important for the regulation of intrafollicular oxygen levels, nutrients, and determination of oocyte quality [40]. In view of this, various angiogenic factors have been investigated in follicular fluid and their effects on IVF outcome documented. No correlation is reported between VEGF levels and number of oocytes retrieved, embryo development, or pregnancy outcome [41]. Even intrafollicular ADM concentration is suggested to be independent of ovarian function parameters [42]. In contrast, a recent study has established a negative correlation between intrafollicular VEGF and ovarian response and oocyte maturation [43]. Also, increased ADM in follicular fluid is associated with a negative IVF outcome [44]. In another study, increased intrafollicular ADM is suggested to be a marker of decreased ovarian response in IVF [12]. A positive association between elevated follicular fluid angiogenin concentration and oocyte maturity is also established [11].
Next, out of all cytokines and angiogenic molecules, the three main factors having maximum contributory role in endometriosis were identified using multivariate statistical analysis. On plotting t1 × t2, the plots for endometriosis are observed to lie in a principal component plane which is significantly different from the plane where plots for controls are clustered (Fig. 1a). PLS-DA indicates statistically significant separation between endometriosis and controls (Fig. 1b). A quantitative measure of the goodness of fit in the PLS-DA model is given by the parameter R2, which varies between 0 and 1, with 1 representing a perfect fit and 0 no fit at all. However, R2 is inflationary and approaches unity as model complexity increases; model complexity is governed by model parameters and number of components. It is, therefore, not sufficient to have a high R2 value. On the other hand, the goodness of prediction represented by Q2 is less inflationary and does not automatically come close to 1 with increasing model complexity. In short, both R2 and Q2 are important to assess the validity of the model. Our values corresponding to R2 (0.981) and Q2 (0.979) indicate that the model is valid and can predict better than chance. Another validation, showing that the PLS-DA model not only just fits the training set well but also predicts class well for new observations, was performed using permutation test statistics. The permutation test assured the validity of the PLS-DA model with all R2 and Q2 values calculated from the permuted data lower than the original one in the validation plot. Q2 intercepted the y-axis at −0.0686 (Fig. 1c). VIP scores indicate the importance of each variable in the projection used in a PLS-DA model and is often used for variable selection. Variables with top three VIP scores, IL-8, IL-12, and ADM, were identified as the three prime overexpressed factors in women with endometriosis. Further, we observed a negative association between levels of these factors and oocyte maturity and embryo quality.
A receptive endometrium and a good-quality embryo are necessary to establish a successful pregnancy. Impairment in the receptive status of the endometrium in women with endometriosis is well evidenced [45, 46]. It appears that both the endometrium and the oocyte/embryo are affected in endometriosis, and it remains to be unraveled as to which factor is more important in adversely affecting the pregnancy outcome. It was interesting to observe that despite dysregulated levels of cytokines and angiogenic factors and suboptimal quality of embryo/oocytes in patients with endometriosis, pregnancy rates were comparable between the control and experimental group. A possible explanation for this observation is the presence of a healthy embryo in the pool of three embryos transferred for each endometriosis patient. It is, therefore, likely that the overall number of good embryos did not differ significantly in control and endometriosis patients.
It is well recognized that identification of a set of prognostic markers for prediction of IVF success will assist clinicians in patient management. Follicular fluid, being the immediate microenvironment of the developing oocytes, seems to be a good choice for identification of these biomarkers. We found IL-8, IL-12, and ADM levels to be good indicators of embryo and oocyte quality in endometriosis patients undergoing IVF. It is possible that these factors may be prognostic markers for successful IVF outcome, irrespective of the factors contributing to infertility. This may be ascertained by collective evaluation of these factors in future prospective studies in women with various causes of infertility opting for IVF.
The shortcoming of this study is that pregnancy outcome could not be directly correlated with differences in IL-8, IL-12, and ADM levels since two to three embryos are routinely transferred into each patient during IVF; consequently, it was not possible to ascribe whether the fetus in the pregnant woman had developed from the follicle/embryo analyzed. It would have been interesting to include a cohort of early endometriosis women to ascertain whether the intrafollicular factors and the IVF outcome parameters show a similar trend as observed in the advanced stage cases.
Acknowledgments
The first author is grateful to the Government of India, Indian Council of Medical Research for financial support under the ICMR-JRF scheme. The authors are grateful to all the volunteers who participated in this study, as well as to Gunja Bose from IRM, Kolkata for helping in collection of samples.
Footnotes
Capsule
Levels of IL-8, IL-12, and adrenomedullin as good indicators of embryo and oocyte quality in women with endometriosis undergoing IVF
References
- 1.Rizk B, Abdalla H. Epidemiology and pathogenesis. Endometriosis. Oxford: Health Press Limited; 2003. [Google Scholar]
- 2.Barnhart K, Dunsmoor-Su R, Coutifaris C. Effect of endometriosis on in vitro fertilization. Fertil Steril. 2002;77:1148–1155. doi: 10.1016/S0015-0282(02)03112-6. [DOI] [PubMed] [Google Scholar]
- 3.Stilley JA, Birt JA, Sharpe-Timms KL. Cellular and molecular basis for endometriosis-associated infertility. Cell Tissue Res. 2012;349:849–862. doi: 10.1007/s00441-011-1309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das S, Chattopadhyay R, Ghosh S, Goswami SK, Chakravarty BN, et al. Reactive oxygen species level in follicular fluid—embryo quality marker in IVF? Hum Reprod. 2006;21:2403–2407. doi: 10.1093/humrep/del156. [DOI] [PubMed] [Google Scholar]
- 5.Trombly DJ, Woodruff TK, Mayo KE. Roles for transforming growth factor beta superfamily proteins in early folliculogenesis. Semin Reprod Med. 2009;27:14–23. doi: 10.1055/s-0028-1108006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostanin AA, Aizikovich BI, Aizikovich IV, Kozhin AY, Chernykh ER. Role of cytokines in the regulation of reproductive function. Bull Exp Biol Med. 2007;143:75–79. doi: 10.1007/s10517-007-0021-2. [DOI] [PubMed] [Google Scholar]
- 7.Machelon V, Emilie D. Production of ovarian cytokines and their role in ovulation in the mammalian ovary. Eur Cytokine Netw. 1997;8:137–143. [PubMed] [Google Scholar]
- 8.Bornstein SR, Rutkowski H, Vrezas I. Cytokines and steroidogenesis. Mol Cell Endocrinol. 2004;215:135–141. doi: 10.1016/j.mce.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 9.Sarapik A, Velthut A, Haller-Kikkatalo K, Faure GC, Bene MC, et al. Follicular proinflammatory cytokines and chemokines as markers of IVF success. Clin Dev Immunol. 2012;2012:606459. doi: 10.1155/2012/606459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraser HM. Regulation of the ovarian follicular vasculature. Reprod Biol Endocrinol. 2006;4:18. doi: 10.1186/1477-7827-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malamitsi-Puchner A, Sarandakou A, Baka SG, Tziotis J, Rizos D, et al. Concentrations of angiogenic factors in follicular fluid and oocyte-cumulus complex culture medium from women undergoing in vitro fertilization: association with oocyte maturity and fertilization. Fertil Steril. 2001;76:98–101. doi: 10.1016/S0015-0282(01)01854-4. [DOI] [PubMed] [Google Scholar]
- 12.Manau D, Balasch J, Jimenez W, Fabregues F, Civico S, et al. Follicular fluid concentrations of adrenomedullin, vascular endothelial growth factor and nitric oxide in IVF cycles: relationship to ovarian response. Hum Reprod. 2000;15:1295–1299. doi: 10.1093/humrep/15.6.1295. [DOI] [PubMed] [Google Scholar]
- 13.Garrido N, Navarro J, Garcia-Velasco J, Remoh J, Pellice A, et al. The endometrium versus embryonic quality in endometriosis-related infertility. Hum Reprod Update. 2002;8:95–103. doi: 10.1093/humupd/8.1.95. [DOI] [PubMed] [Google Scholar]
- 14.Pellicer A, Albert C, Garrido N, Navarro J, Remohi J, et al. The pathophysiology of endometriosis-associated infertility: follicular environment and embryo quality. J Reprod Fertil Suppl. 2000;55:109–119. [PubMed] [Google Scholar]
- 15.Lo Turco EG, Souza GH, Garcia JS, Ferreira CR, Eberlin MN, et al. Effect of endometriosis on the protein expression pattern of follicular fluid from patients submitted to controlled ovarian hyperstimulation for in vitro fertilization. Hum Reprod. 2010;25:1755–1766. doi: 10.1093/humrep/deq102. [DOI] [PubMed] [Google Scholar]
- 16.ASRM Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817–821. doi: 10.1016/S0015-0282(97)81391-X. [DOI] [PubMed] [Google Scholar]
- 17.WHO . Laboratory manual for the examination of human semen and sperm-cervical mucus interaction. York: Cambridge University Press; 2010. p. 138. [Google Scholar]
- 18.Veeck LL. An atlas of human gametes and conceptuses: an illustrated reference for assisted reproductive technology. New York: Parthenon Publishing; 1999. [Google Scholar]
- 19.Chaudhury K, Chakravarty B. Endometriosis—basic concepts and current research trends. Rijeka: InTech; 2012. [Google Scholar]
- 20.Nouri M, Ghaffari M, Salmasi A, Ghasemzadeh A. Serum and follicular fluid IL-6 and sex steroid hormone levels and their correlation of undergoing IVF-ET with endometriosis and pregnancy rate in women. J Reprod Infertility. 2000;1:55–62. [Google Scholar]
- 21.Pellicer A, Albert C, Mercader A, Bonilla-Musoles F, Remohi J, et al. The follicular and endocrine environment in women with endometriosis: local and systemic cytokine production. Fertil Steril. 1998;70:425–431. doi: 10.1016/S0015-0282(98)00204-0. [DOI] [PubMed] [Google Scholar]
- 22.Podgaec S, Dias Junior JA, Chapron C, Oliveira RM, Baracat EC, et al. Th1 and Th2 immune responses related to pelvic endometriosis. Rev Assoc Med Bras. 2010;56:92–98. doi: 10.1590/S0104-42302010000100022. [DOI] [PubMed] [Google Scholar]
- 23.Yu C, Liu Y, Peng X. IL-8 and IL-10 levels in endometriosis and regulated role of neiyifang on them. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2000;20:603–605. [PubMed] [Google Scholar]
- 24.Galo S, Zubor P, Szunyogh N, Kajo K, Machalekova K, et al. TNF-alpha serum levels in women with endometriosis: prospective clinical study. Ceska Gynekol. 2005;70:286–290. [PubMed] [Google Scholar]
- 25.Arici A. Local cytokines in endometrial tissue: the role of interleukin-8 in the pathogenesis of endometriosis. Ann N Y Acad Sci. 2002;955:101–109. doi: 10.1111/j.1749-6632.2002.tb02770.x. [DOI] [PubMed] [Google Scholar]
- 26.Fairbanks F, Abrao MS, Podgaec S, Dias JA, Jr, de Oliveira RM, et al. Interleukin-12 but not interleukin-18 is associated with severe endometriosis. Fertil Steril. 2009;91:320–324. doi: 10.1016/j.fertnstert.2007.11.060. [DOI] [PubMed] [Google Scholar]
- 27.Gosden RG, Hunter RH, Telfer E, Torrance C, Brown N. Physiological factors underlying the formation of ovarian follicular fluid. J Reprod Fertil. 1988;82:813–825. doi: 10.1530/jrf.0.0820813. [DOI] [PubMed] [Google Scholar]
- 28.Bili H, Tarlatzis BC, Daniilidis M, Fleva A, Bontis J, et al. Cytokines in the human ovary: presence in follicular fluid and correlation with leukotriene B4. J Assist Reprod Genet. 1998;15:93–98. doi: 10.1007/BF02766833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrionuevo MJ, Schwandt RA, Rao PS, Graham LB, Maisel LP, et al. Nitric oxide (NO) and interleukin-1beta (IL-1beta) in follicular fluid and their correlation with fertilization and embryo cleavage. Am J Reprod Immunol. 2000;44:359–364. doi: 10.1111/j.8755-8920.2000.440607.x. [DOI] [PubMed] [Google Scholar]
- 30.Bedaiwy M, Shahin AY, AbulHassan AM, Goldberg JM, Sharma RK, et al. Differential expression of follicular fluid cytokines: relationship to subsequent pregnancy in IVF cycles. Reprod Biomed Online. 2007;15:321–325. doi: 10.1016/S1472-6483(10)60346-X. [DOI] [PubMed] [Google Scholar]
- 31.Kawasaki F, Kawano Y, Kosay Hasan Z, Narahara H, Miyakawa I. The clinical role of interleukin-6 and interleukin-6 soluble receptor in human follicular fluids. Clin Exp Med. 2003;3:27–31. doi: 10.1007/s102380300012. [DOI] [PubMed] [Google Scholar]
- 32.Spandorfer SD, Neuer A, Liu HC, Rosenwaks Z, Witkin SS. Involvement of interleukin-1 and the interleukin-1 receptor antagonist in in vitro embryo development among women undergoing in vitro fertilization-embryo transfer. J Assist Reprod Genet. 2003;20:502–505. doi: 10.1023/B:JARG.0000013650.76052.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ledee N, Lombroso R, Lombardelli L, Selva J, Dubanchet S, et al. Cytokines and chemokines in follicular fluids and potential of the corresponding embryo: the role of granulocyte colony-stimulating factor. Hum Reprod. 2008;23:2001–2009. doi: 10.1093/humrep/den192. [DOI] [PubMed] [Google Scholar]
- 34.Gazvani MR, Bates M, Vince G, Christmas S, Lewis-Jones DI, et al. Follicular fluid concentrations of interleukin-12 and interleukin-8 in IVF cycles. Fertil Steril. 2000;74:953–958. doi: 10.1016/S0015-0282(00)01538-7. [DOI] [PubMed] [Google Scholar]
- 35.Lee KS, Joo BS, Na YJ, Yoon MS, Choi OH, et al. Relationships between concentrations of tumor necrosis factor-alpha and nitric oxide in follicular fluid and oocyte quality. J Assist Reprod Genet. 2000;17:222–228. doi: 10.1023/A:1009495913119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wunder DM, Mueller MD, Birkhauser MH, Bersinger NA. Increased ENA-78 in the follicular fluid of patients with endometriosis. Acta Obstet Gynecol Scand. 2006;85:336–342. doi: 10.1080/00016340500501715. [DOI] [PubMed] [Google Scholar]
- 37.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine Growth Factor Rev. 2001;12:53–72. doi: 10.1016/S1359-6101(00)00015-0. [DOI] [PubMed] [Google Scholar]
- 38.Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389–2398. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hammadeh ME, Fischer-Hammadeh C, Hoffmeister H, Huebner U, Georg T, et al. Fibroblast growth factor (FGF), intracellular adhesion molecule (sICAM-1) level in serum and follicular fluid of infertile women with polycystic ovarian syndrome, endometriosis and tubal damage, and their effect on ICSI outcome. Am J Reprod Immunol. 2003;50:124–130. doi: 10.1034/j.1600-0897.2003.00056.x. [DOI] [PubMed] [Google Scholar]
- 40.Monteleone P, Giovanni Artini P, Simi G, Casarosa E, Cela V, et al. Follicular fluid VEGF levels directly correlate with perifollicular blood flow in normoresponder patients undergoing IVF. J Assist Reprod Genet. 2008;25:183–186. doi: 10.1007/s10815-008-9218-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kung FT, Chang SY, Huang FJ, Lan KC. VEGF, IVF clinical variables and ovarian hyperstimulation syndrome: a prospective study of the preimplantation luteal phase. J Reprod Med. 2007;52:365–374. [PubMed] [Google Scholar]
- 42.Marinoni E, Di Iorio R, Villaccio B, Letizia C, Aragona C, et al. Follicular fluid adrenomedullin concentrations in spontaneous and stimulated cycles: relationship to ovarian function and endothelin-1 and nitric oxide. Regul Pept. 2002;107:125–128. doi: 10.1016/S0167-0115(02)00093-9. [DOI] [PubMed] [Google Scholar]
- 43.Gao MZ, Zhao XM, Lin Y, Sun ZG, Zhang HQ. Effects of EG-VEGF, VEGF and TGF-beta1 on pregnancy outcome in patients undergoing IVF-ET treatment. J Assist Reprod Genet. 2012;29:1091–1096. doi: 10.1007/s10815-012-9833-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marinoni E, Feliciani E, Muzzonigro F, Letizia C, Tranquilli A, et al. Intrafollicular concentration of adrenomedullin is associated with IVF outcome. Gynecol Endocrinol. 2010;26:435–439. doi: 10.3109/09513591003632076. [DOI] [PubMed] [Google Scholar]
- 45.Jana SK, Banerjee P, Mukherjee R, Chakravarty B, Chaudhury K. HOXA-11 mediated dysregulation of matrix remodeling during implantation window in women with endometriosis. J Assist Reprod Genet. 2013;30:1505–1512. doi: 10.1007/s10815-013-0088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei Q, St Clair JB, Fu T, Stratton P, Nieman LK. Reduced expression of biomarkers associated with the implantation window in women with endometriosis. Fertil Steril. 2009;91:1686–1691. doi: 10.1016/j.fertnstert.2008.02.121. [DOI] [PMC free article] [PubMed] [Google Scholar]