Abstract
Purpose
The purpose of this study was to evaluate the best protocol to prepare endometrium for frozen embryo replacement (FER) cycles.
Methods
This study is a systematic review and meta-analysis. Following PubMed and OvidSP search, a total of 1166 studies published after 1990 were identified following removal of duplicates. Following exclusion of studies not matching our inclusion criteria, a total of 33 studies were analyzed. Primary outcome measure was live birth. The following protocols, including true natural cycle (tNC), modified natural cycle (mNC), artificial cycle (AC) with or without suppression, and mild ovarian stimulation (OS) with gonadotropin (Gn) or aromatase inhibitor (AI), were compared.
Results
No statistically significant difference for both clinical pregnancy and live birth was noted between tNC and mNC groups. When tNC and AC without suppression groups are compared, there was a statistically significant difference in clinical pregnancy rate in favor of tNC, whereas it failed to reach statistical significance for live birth. When tNC and AC with suppression groups are compared, there was a statistically significant difference in live birth rate favoring the latter. Similar pregnancy outcome was noted among mNC versus AC with or without suppression groups. Similarly, no difference in clinical pregnancy and live birth was noted when ACs with or without suppression groups are compared.
Conclusions
There is no consistent superiority of any endometrial preparation for FER. However, mNC has several advantages (being patient-friendly; yielding at least equivalent or better pregnancy rates when compared with tNC and AC with or without suppression; may not require LPS). Mild OS with Gn or AI may be promising.
Keywords: Frozen embryo replacement, Endometrial preparation, Thawed embryo transfer, Assisted reproduction, Meta-analysis
Introduction
There has been a recent significant increase in the frozen embryo replacement (FER) cycles not only due to the availability of surplus embryos but also due to cryo-all cycles to avoid the risk of ovarian hyperstimulation syndrome [1], pre-implantation genetic screening [2], and concerns for detrimental effect of controlled ovarian stimulation on endometrial receptivity in a fresh cycle [3]. The 2011 results, published in 2016, generated from European registers by ESHRE reported that the proportion of FER cycles was 32.3 % [4] compared to 28 % in 2010 [5]. As concordant with the European data, the proportion of FER cycles was 24.5 % as reported by the most recent US nationwide database [6]. Improved laboratory techniques and especially enhanced survival and implantation rates achieved by vitrification have also contributed to the increasing trend for performing FER cycles.
Despite the increased interest in FER and personalized approaches in reproductive medicine, the best-individualized approach to prepare endometrium for FER is still a matter of debate [7, 8]. Furthermore, although the data are lacking, one may need to take the etiology of infertility (e.g., endometriosis) into account while assigning the FER protocol. The available protocols to be used for FER include (i) true natural cycle (tNC), (ii) modified natural cycle (mNC), (iii) artificial cycle (AC) without suppression, (iv) AC with suppression, and (v) mild ovarian stimulation (OS) with gonadotropins (Gns) or aromatase inhibitor (AI).
True natural cycle can only be employed in regularly cycling women. In a tNC, ultrasonographic and endocrine monitoring is performed to delineate the timing of spontaneous ovulation. Endocrine monitoring should be performed in such cycles to verify the timing of luteinizing hormone (LH) surge. When a rise in serum LH levels is noted, it is assumed that ovulation will occur in 36–40 h later [9]. Urinary LH kits might be patient-friendly but might be misleading [10]. Therefore, it is better to monitor circulating LH levels daily during the time of ovulation. There is paucity of data on the need for luteal phase support (LPS) in tNC [11].
Frequent endocrine and ultrasonographic monitoring may be cumbersome in tNC. Hence, mNC may be more patient-friendly. In mNC, following a baseline scan on day 2/3 of menses, ultrasonographic monitoring is generally started on day 8–10 and continued on alternate days or daily until the dominant follicle reached 16–20 mm in diameter during which human chorionic gonadotropin (hCG) triggering is employed. Since hCG has a long half-life and has a sustained luteotropic effect in the early luteal phase up to 7 days following administration [12], LPS might not be needed in mNC [13, 14].
Artificial cycles may be performed in all women regardless of the menstrual regularity and offer the greatest flexibility for timing of FER. Although AC with suppression is robust to avoid ovulation, AC without suppression is more patient-friendly. However, premature ovulation leading to cycle cancellation, encountered in 1.9 to 7.4 % of the cycles, is the main drawback of AC without suppression [15, 16]. Luteal phase should be supported in AC with or without suppression.
Another option to prepare endometrium in FER is to employ mild stimulation with either exogenous gonadotropins or oral agents. In regularly cycling women, it has been hypothesized that ovarian stimulation with either oral agents or exogenous gonadotropins (Gns) may improve certain defects in the follicular and subsequent luteal phase, resulting in a better endometrial preparation for embryo implantation [17, 18]. However, it has also been claimed that ovarian stimulation may lead to decreased endometrial receptivity [3, 19, 20].
There is a paucity of well-designed randomized controlled trials (RCTs) and systematic reviews [8, 21] to evaluate the best protocol(s) to prepare endometrium for FER. In this systematic review and meta-analysis, we overviewed the available evidence in this context.
Material and methods
In this systematic review, we strictly incorporated the guidelines by the PRISMA statement.
Definition of protocols
True natural cycle was defined when frozen embryo transfer had been performed after documentation of spontaneous ovulation with ultrasonographic and/or endocrine monitoring. Studies employing endocrine monitoring with either serum or urinary LH levels were included. Modified natural cycle was defined when the leading follicle (16–20 mm) was triggered with hCG. Regarding tNC and mNC, studies that did or did not employ LPS were included.
In AC cycles, oral estrogen was commenced on the first, second, or third day of the cycle with the aim of supporting endometrial proliferation and suppressing follicle growth. Estrogen was used either at a fixed dose (6 mg daily) or in an incremental fashion (2 to 6 mg daily). After 12–14 days of estrogen use, vaginal ultrasound examination was performed to confirm that no dominant follicle had emerged and to measure endometrial thickness. When the endometrial thickness exceeded 7 mm, progesterone supplementation was commenced and FER was scheduled accordingly. This group was further divided according to addition of a gonadotropin-releasing hormone (GnRH) analog as AC with suppression or not as AC without suppression. Mild ovarian stimulation was defined when the follicle cohort was induced either with <150 IU Gn daily or with an AI.
Search strategy and study selection
Criteria for inclusion in the study were established before literature search. Inclusion was limited to studies that were published of RCTs or prospective/retrospective cohort studies, comparing different FER endometrial preparation protocols. A thorough search of PubMed and OvidSP databases was performed using the keywords (endometrial preparation, frozen embryo transfer, cryo-thawed, natural cycle frozen embryo transfer, modified natural cycle embryo transfer, artificial frozen cycle, artificial frozen cycle with gonadotropin suppression) and MeSH terms (cryopreservation and pregnancy).
After screening from the title and abstract, we excluded the data published as abstract, meeting proceeding, book chapter, or review articles and irrelevant studies that did not give any information for the preparation of the endometrium in FER cycles. Case-series, case-control studies, abstracts, and articles published in languages other than English were excluded. Studies before January 1990 and donor-recipient cycles were excluded. The primary end-point was taken as live birth rate, and the secondary outcome measure was taken as clinical pregnancy rate.
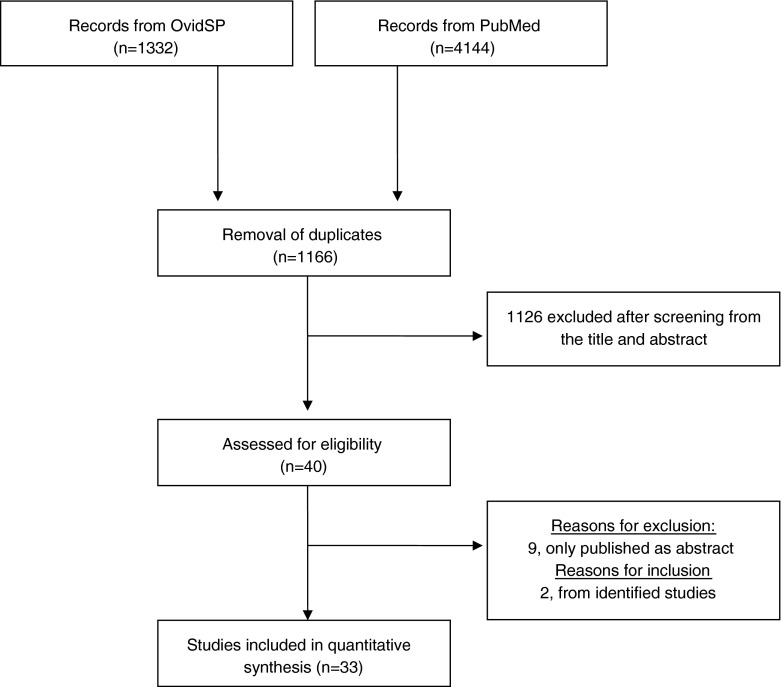
An extensive literature search was performed up to April 2016 in PubMed and OvidSP by two blind investigators (S.M. and M.P.) to generate the meta-analyses. The search strategy yielded a total of 4144 and 1332 references, from PubMed and OvidSP, respectively (Fig. 1). After removal of duplicates (n = 1166), all remaining studies were examined in detail from the title and abstract. We excluded the data published as abstract, meeting proceeding, book chapter, or review articles and irrelevant full-article studies that did not give any information on FER cycles (n = 1126). Of the remaining 40 studies, 9 were excluded due to being abstract only [22–30]. Literature search was also performed on references from identified studies, and two new studies [31, 32] were retrieved, making a total of 33 studies (Fig. 1).
Fig. 1.
PRISMA flow diagram depicting the study selection
The meta-analysis was performed employing RevMan 5.3 software (The Nordic Cochrane Centre, The Cochrane Collaboration). The I 2 statistics was used to assess the statistical heterogeneity, and >50 % was considered to assign heterogeneity. A random-effect model was used in case of statistical heterogeneity whereas a fixed-effect model was used in the absence of heterogeneity. To weigh the scores of individual studies, the inverse-variance method was employed. Odds ratios (ORs) were used to pool available data regardless of the design of the included studies. The Mantel-Haenszel method was applied to estimate the pooled effect size. A priori specified subgroup analyses were performed. A two-sided significance level of p < 0.05 was used.
Results
Studies included for meta-analyses
A total of 40 studies were left after screening from title, abstract, or manuscript, and 33 were finally included for quantitative final analysis (Fig. 1). Of the 33 studies included in this meta-analysis, only 11 were RCTs. The remaining 22 studies were retrospective cohort studies with their inherent selection bias. The detailed description of the included studies is given in Table 1.
Table 1.
Overview of included studies in the current meta-analysis
| Study, year | Design | Study population; cycle | Allocation | Outcomes | Luteal phase support | Results |
|---|---|---|---|---|---|---|
| tNC vs. mNC | ||||||
| Weissman A, 2009 | Retrospective cohort | Ovulatory patients; tNC = 71/mNC = 61 |
Preference | CP/LB | No LPS | No difference |
| Fatemi HM, 2010 | RCT | Ovulatory patients; tNC = 61/mNC = 63 |
Computer generated randomization | CP | No LPS | CP: tNC > mNC, p = 0.025 |
| Weissman A, 2011 | RCT | Ovulatory patients; tNC = 30/mNC = 25 |
Computer generated randomization | CP/LB | tNC: vaginal progesterone gel 1 × 90 mg or vaginal micronized progesterone 2 × 100 mg/day mNC: vaginal progesterone gel 1 × 90 mg or vaginal micronized progesterone 2 × 100 mg/day |
No difference |
| Chang EM, 2011 | Retrospective cohort | Ovulatory patients; tNC = 310/mNC = 134 |
Convenience, cost | CP | tNC: vaginal micronized progesterone 600 mg/day mNC: vaginal micronized progesterone 600 mg/day |
No difference |
| Tomas C, 2012 | Retrospective cohort | Ovulatory patients; tNC = 1168/mNC = 444 |
Preference, cycle characteristics | CP/LB | tNC: vaginal natural progesterone 3 × 200 mg/day or 2 × 400 mg/day or vaginal progesterone gel 2 × 90 mg/day mNC: No LPS |
No difference |
| tNC vs. AC without suppression | ||||||
| Loh SK, 1999 | Retrospective cohort | Ovulatory patients; tNC = 51/AC wo-s = 161 |
Preference | CP/LB | NA | LB: tNC > AC wo-s, p = 0.073 |
| Morozov V, 2007 | Retrospective cohort | Ovulatory patients; tNC = 68/AC wo-s = 174 |
Not stated | CP | tNC: vaginal micronized progesterone 4 × 200 mg/day AC wo-s: progesterone in oil 1 × 50mg/day i.m. |
CP: tNC > AC wo-s, p = 0.0298 |
| Chang EM, 2011 | Retrospective cohort | Ovulatory patients; tNC = 310/AC wo-s = 204 |
Convenience, cost | CP | tNC: vaginal micronized progesterone 600 mg/day AC wo-s: vaginal micronized progesterone 600 mg/day |
CP: tNC > AC wo-s, p = 0.008 |
| Xiao Z, 2011 | Retrospective cohort | Ovulatory and anovulatory patients; tNC = 380/AC wo-s = 646 |
Preference, cycle characteristics | CP | tNC: progesterone in oil 1 × 40 mg/day i.m. AC wo-s: progesterone in oil 1 × 40–60 mg/day i.m. |
No difference |
| Tomas C, 2012 | Retrospective cohort | Ovulatory and anovulatory patients; tNC = 1168/AC wo-s = 2858 |
Cycle characteristics | CP/LB | tNC: vaginal natural progesterone 3 × 200 mg/day or 2 × 400 mg/day or vaginal progesterone gel 2 × 90 mg/day AC wo-s: vaginal natural progesterone 2 × 400 mg/day or vaginal progesterone gel 2 × 90 mg/day |
No difference |
| Veleva Z, 2013 | Retrospective cohort | Ovulatory and anovulatory patients; tNC = 1276/AC wo-s = 312 |
Cycle characteristics | LB | tNC: No LPS: 302, with LPS: 974; vaginal micronized progesterone 200 mg/day AC wo-s: vaginal micronized progesterone 800 mg/day |
LB: tNC > AC wo-s, p < 0.0001 |
| Levron J, 2014 | Retrospective cohort | Not stated; tNC = 798/AC wo-s = 437 |
Not Stated | CP | tNC: No LPS AC wo-s: vaginal micronized progesterone 3 × 300 mg/day |
CP: tNC > AC wo-s, p < 0.02 |
| Orvieto R, 2016 | Retrospective cohort | Ovulatory and anovulatory patients; 2012–2014 tNC = 74/ 2014–2015 tNC = 59 2012–2014 AC wo-s = 113/ 2014–2015 AC wo-s = 54 |
Preference | CP | 2012–2014 tNC: vaginal micronized progesterone 600 mg/day or vaginal progesterone gel 1 × 90 mg/day 2014–2015 tNC: rhCG 250 μg the day of ET and triptorelin 0.1 mg 4 days after from ET 2012–2014 and 2014–2015 AC wo-s: vaginal micronized progesterone 3 × 300 mg/day or vaginal progesterone gel 2 × 90 mg/day |
2014–2015 tNC > 2014–2015 AC wo-s, p < 0.001 |
| tNC vs. AC-with suppression | ||||||
| Al Shawaf T, 1993 | RCT | Ovulatory and anovulatory patients; tNC = 77/AC w-s = 72 |
Age, cycle characteristics | CP/LB | tNC: No LPS AC w-s: NA |
No difference |
| Queenan JT, 1994 | Retrospective cohort | Ovulatory and anovulatory patients; tNC = 398/AC w-s = 230 |
Cycle characteristics | CP | tNC: NA AC w-s: NA |
No difference |
| Tanos V, 1996 | Quasi-randomized | Ovulatory and anovulatory patients; tNC = 219/AC w-s = 85 |
Preference, cycle characteristics | CP | tNC: NA AC w-s: NA |
No difference |
| Gelbaya TA, 2006 | Retrospective cohort | Ovulatory patients; tNC = 212/AC w-s = 205 |
Preference | CP/LB | tNC: No LPS AC w-s: micronized progesterone pessaries 200–800 mg/day |
No difference |
| Hill MJ, 2010 | Retrospective cohort | Ovulatory and anovulatory patients; NC = 240/AC w-s = 1151 |
Preference | CP/LB | tNC: progesterone in oil 1 × 50 mg/day i.m. AC w-s: progesterone in oil 1 × 50 mg/day i.m. |
CP/LB: AC w-s > tNC, p < 0.01/p < 0.01 |
| Mounce G, 2015 | RCT | Ovulatory and anovulatory patients; NC = 80/AC w-s = 79 |
Randomized using minimization algorithm | CP/LB | tNC: No LPS AC w-s: vaginal progesterone pessaries 2 × 400 mg/day |
No difference |
| mNC vs. AC-without suppression | ||||||
| Kawamura T, 2007 | Retrospective cohort | Ovulatory patients; NC = 720/AC wo-s = 136 |
CP | mNC: 2 × 0.5 mg/day chlormadinone acetate, AC wo-s: 2 × 222 mg/day vaginal progesterone and hydroxyl-progesterone caproate 1 × 125 mg/week i.m. |
No difference | |
| Givens CR, 2009 | Retrospective cohort | Ovulatory and anovulatory patients; NC = 862/AC wo-s = 205 |
Preference, cycle characteristics | CP/LB | mNC: vaginal micronized progesterone 2 × 200 mg/day AC wo-s: progesterone in oil 50 mg/day i.m. |
CP: AC wo-s > mNC, p = 0.011 |
| Chang EM, 2011 | Retrospective cohort | Ovulatory patients; mNC = 134/AC wo-s = 204 |
Convenience, cost | CP | mNC: vaginal micronized progesterone 600 mg/day AC wo-s: vaginal micronized progesterone 600 mg/day |
CP: mNC > AC wo-s, p = 0.032 |
| Tomas C, 2012 | Retrospective cohort | Ovulatory and anovulatory patients; mNC = 444/AC wo-s = 2858 |
Cycle characteristics | CP/LB | mNC: No LPS, AC wo-s: vaginal natural progesterone 2 × 400 mg/day or vaginal progesterone gel 2 × 90 mg/day |
No difference |
| Hancke K, 2012 | Retrospective cohort | Ovulatory and anovulatory patients; mNC = 148/AC wo-s = 55 |
Preference | CP/LB | mNC: vaginal micronized progesterone 3 × 200 mg/day AC wo-s: vaginal micronized progesterone 3 × 200 mg/day |
No difference |
| Groenewoud ER, 2016 | RCT | Ovulatory patients; mNC = 495/AC wo-s = 464 |
Web based randomization module | CP/LB | mNC: No LPS AC wo-s: vaginal micronized progesterone 3 × 200 mg/day |
No difference |
| mNC vs. AC-with suppression | ||||||
| Lathi RB, 2015 | Retrospective cohort | Ovulatory and anovulatory patients; mNC = 519/AC w-s = 106 |
Cycle characteristics | CP/LB | mNC: vaginal micronized progesterone 2 × 100 mg/day AC w-s: progesterone in oil 50 mg/day i.m. and vaginal micronized progesterone 3 × 200 mg/day |
No difference |
| Konc J, 2010 | Retrospective cohort | Ovulatory and anovulatory patients; mNC = 315/AC w-s = 234 |
Not stated | CP | mNC: vaginal micronized progesterone 600 mg/day AC w-s: vaginal micronized progesterone 600 mg/day |
No difference |
| AC-with suppression vs. AC-without suppression | ||||||
| Simon A, 1998 | RCT | Ovulatory and anovulatory patients; AC w-s = 53/AC wo-s = 53 |
Cycle characteristics | CP | AC w-s: vaginal micronized progesterone 3 × 300 mg/day AC wo-s: vaginal micronized progesterone 3 × 300 mg/day |
No difference |
| Dal Prato L, 2002 | Prospective randomized | Ovulatory patients; AC w-s = 146/AC wo-s = 150 |
Randomization | CP | AC w-s: progesterone in oil 100 mg/day i.m. AC wo-s: progesterone in oil 100 mg/day i.m. |
No difference |
| El Toukhy T, 2004 | RCT | Ovulatory patients; AC w-s = 117/AC wo-s = 117 |
Computer generated randomization | CP/LB | AC w-s: vaginal progesterone pessaries 2 × 400 mg/day AC wo-s: vaginal progesterone pessaries 2 × 400 mg/day |
CP/LB: AC w-s > AC wo-s, p = 0.01/p = 0.01 |
| van de Vijver A, 2014 | Retrospective cohort | Not stated; AC w-s = 280/AC wo-s = 849 |
Preference | CP/LB | AC w-s: vaginal micronized progesterone 3 × 200 mg/day AC wo-s: vaginal micronized progesterone 3 × 200 mg/day |
No difference |
| Nekoo EA, 2014 | RCT | Ovulatory patients; AC w-s = 93/AC wo-s = 83 |
Computer generated randomization | CP | AC w-s: vaginal micronized progesterone 2 × 400 mg/day AC wo-s: vaginal micronized progesterone 2 × 400 mg/day |
No difference |
| NC vs. OS with Gn | ||||||
| Dor J, 1991 | Retrospective cohort | NA; NC = 56/OS = 44 |
NA | CP/LB | NC: NA OS: NA |
No difference |
| Imthum B, 1996 | Retrospective cohort | NA; NC = 16/OS = 8 |
NA | CP | NC: NA OS: NA |
No difference |
| Tanos V, 1996 | Retrospective cohort | NA; NC = 219/OS = 77 |
NA | CP | NC: NA OS: NA |
No difference |
| Konc J, 2010 | Retrospective cohort | Ovulatory and anovulatory patients; NC = 315/OS = 282 |
Preference | CP | NC: vaginal micronized progesterone 600 mg/day OS: vaginal micronized progesterone 600 mg/day |
No difference |
| Peeraer K, 2015 | RCT | Ovulatory patients; NC = 291/OS = 288 |
Randomization | CP/LB | NC: vaginal micronized progesterone 3 × 200 mg/day OS: vaginal micronized 3 × 200 mg/day |
No difference |
| AC vs. OS with Gn or AI | ||||||
| Dor J, 1991 | Retrospective cohort | NA; AC = 42/OS = 44 |
NA | CP/LB | NA | No difference |
| Konc J, 2010 | Retrospective cohort | Ovulatory and anovulatory patients; AC = 234/OS = 282 |
Preference | CP | AC: vaginal micronized progesterone 600 mg/day OS: vaginal micronized progesterone 600 mg/day |
No difference |
| Li SJ, 2014 | RCT | Ovulatory and anovulatory patients; AC = 354/AI = 359 |
Randomization | CP/LB | AC: progesterone in oil 60 mg/day i.m. AI: dydrogesterone 2 × 10 mg/day oral plus 20–40 mg/day progestin i.m. |
CP/LB: AI > AC, p = 0.043/p = 0.002 |
| Yu J, 2015 | RCT | Ovulatory and anovulatory patients; AC = 291/OS = 285 |
Randomization | CP/LB | AC: vaginal micronized progesterone 2 × 200 mg/day OS: vaginal micronized progesterone 2 × 200 mg/day |
No difference |
RCT randomized controlled trial, NA not available, CP clinical pregnancy, LB live birth, LPS luteal phase support, tNC true natural cycle, mNC modified natural cycle, AC artificial cycle, w-s with suppression, wo-s without suppression, OS ovarian stimulation, Gn gonadotropin, AI aromatase inhibitor
tNC versus mNC
A total of five studies, two RCTs [33, 34] and three retrospective cohort studies [35–37], making a total of 2081 embryo transfer (ET) cycles, were included.
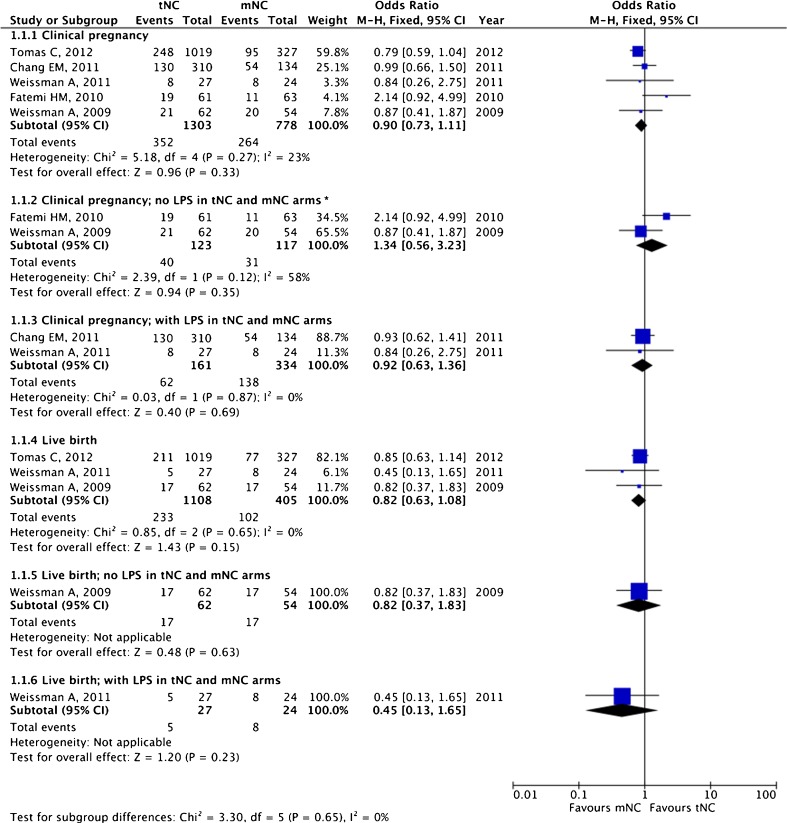
The pooled estimates for clinical pregnancy (OR 0.90, 95 % CI 0.73–1.12; five studies) and live birth (OR 0.82, 95 % CI 0.63–1.08; three studies) are given in Fig. 2. No statistically significant difference for both clinical pregnancy and live birth was noted between tNC and mNC groups.
Fig. 2.
True natural cycle (tNC) versus modified natural cycle (mNC): pooled results of all studies and subgroup analysis based on luteal phase support (LPS). Asterisk donates random effect
In two studies, no LPS was employed in both tNC and mNC arms [33, 37]. However, LPS was administered to both tNC and mNC arms in another two studies [34, 35]. In the study by Tomas et al. [36], no LPS was given to the mNC group whereas LPS in the form of vaginal progesterone gel or micronized progesterone tablet was administered to the tNC group. Among available studies that employed or did not employ LPS, comparable clinical pregnancy rates were noted between the tNC and mNC groups (Fig. 2). With LPS, the OR for clinical pregnancy was 0.92 (95 % CI 0.63–1.36; two studies); the OR for the only available study reporting live birth was 0.45 (95 % CI 0.13–1.65; one study). Without LPS, the OR for clinical pregnancy was 1.34 (95 % CI 0.56–3.23; two studies); with the only available study reporting live birth, the OR was 0.82 (95 % CI 0.37–1.83; one study).
tNC versus AC without suppression
A total of eight studies, all being retrospective cohort studies [35, 36, 38–43], making a total of 8762 ET cycles, were included.
The pooled estimates for clinical pregnancy and live birth are given in Fig. 3. There was a statistically significant difference in clinical pregnancy rate in favor of tNC (OR 1.46, 95 % CI 1.07–1.99; seven studies), whereas it failed to reach statistical significance for live birth (OR 1.80, 95 % CI 0.92–3.49; three studies).
Fig. 3.
True natural cycle (tNC) versus artificial cycle (AC) without suppression: pooled results of all studies and subgroup analysis based on luteal phase support (LPS). Asterisk denotes fixed effect
In the most recent study by Orvieto et al. [41], different LPS strategies have been employed during two time periods; in the tNC arm, during 2012–2014, micronized progesterone soft gel vaginal capsule at a dose of 3 × 200 mg or vaginal bioadhesive gel at 90 mg (8 %) was employed whereas during 2014–2015, in addition to vaginal progesterone, luteal rhCG 250 mcg and 0.1 mg triptorelin were administered. Hence, the time periods employing different LPS strategies have been enrolled as two separate data in the forest plot analysis (Fig. 3).
In two studies, no LPS was administered in the tNC arm [35, 38]; there was a statistically significant difference in clinical pregnancy rate favoring tNC against AC without suppression (OR 1.63, 95 % CI 1.24–2.14; two studies). Luteal phase support was given in five studies [36, 40–43]; the ORs for clinical pregnancy and live birth rates were not significantly different (OR 1.32 (95 % CI 0.90–1.93; four studies) and 1.47 (95 % CI 0.72–3.03; two studies), respectively).
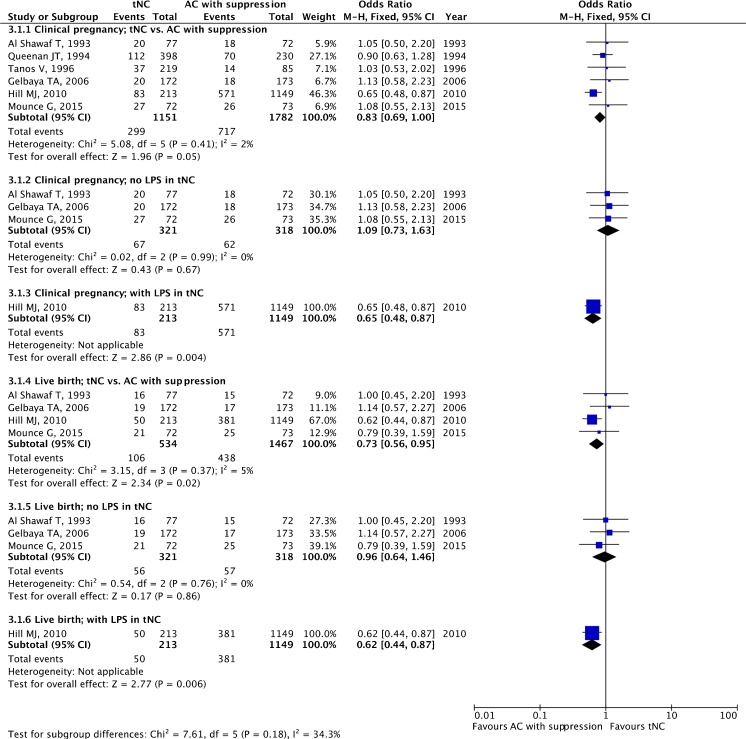
tNC versus AC with suppression
A total of six studies, one RCT [44] and one quasi-randomized [45] and four retrospective cohort studies [46–49], making a total of 2933 ET cycles, were included.
The pooled estimates for clinical pregnancy and live birth are given in Fig. 4. There was a significant difference favoring AC with suppression for live birth (OR 0.73, 95 % CI 0.56–0.95; four studies), whereas it failed to reach statistical significance for clinical pregnancy (OR 0.83, 95 % CI 0.69–1.00; six studies). The study by Hill et al. [48], being a retrospective cohort study, with a total of 1391 ET cycles, dominated the analyses for both the clinical pregnancy and live birth rates.
Fig. 4.
True natural cycle (tNC) versus artificial cycle (AC) with suppression: pooled results of all studies and subgroup analysis based on luteal phase support (LPS)
In three studies, LPS was not employed in the tNC arm [44, 46, 47]. In only one study, LPS was administered in the tNC arm [48]. Without LPS, there was no significant difference for clinical pregnancy (OR 1.09, 95 % CI 0.73–1.63; three studies) or live birth rates (OR 0.96 95 % CI 0.64–1.46; three studies). With LPS, based on a single study, the ORs for clinical pregnancy and live birth were 0.65 (95 % CI 0.48–0.87; one study) and 0.62 (95 % CI 0.44–0.87; one study), respectively, favoring AC with suppression (Fig. 4).
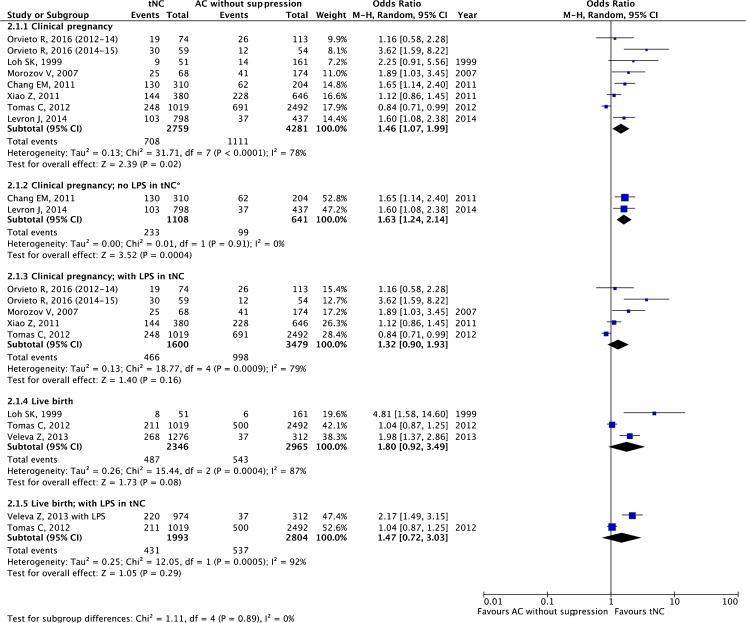
mNC versus AC without suppression
A total of six studies, one RCT [50] and five retrospective cohort studies [32, 35, 36, 51, 52] making a total of 6074 ET cycles, were included.
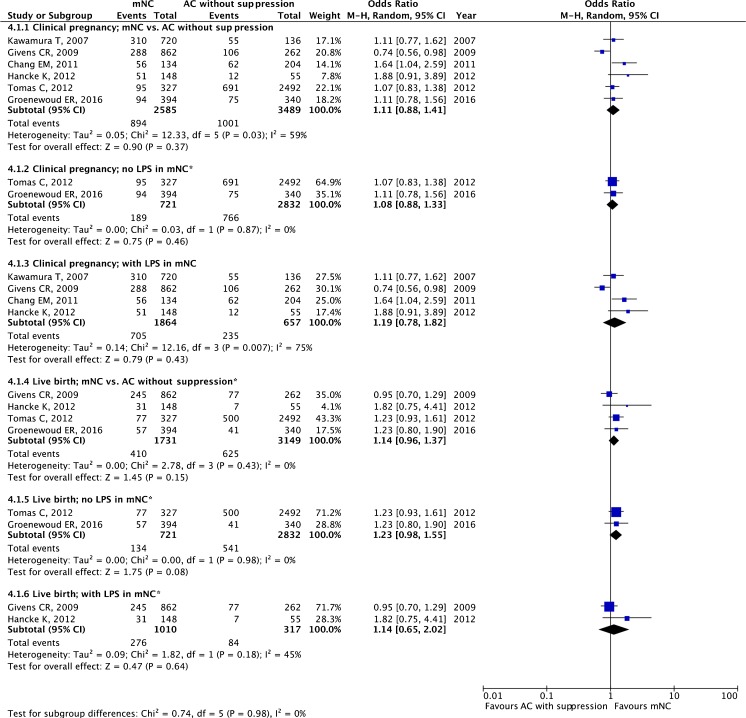
The pooled estimates for clinical pregnancy and live birth are given in Fig. 5. There was no statistically significant difference between these two protocols regarding clinical pregnancy (OR 1.11, 95 % CI 0.88–1.41; six studies) and live birth rates (OR 1.14, 95 % CI 0.96–1.37; four studies).
Fig. 5.
Modified natural cycle (mNC) versus artificial cycle (AC) without suppression: pooled results of all studies and subgroup analysis based on luteal phase support (LPS). Asterisk denotes fixed effect
In two studies, no LPS was employed in the mNC arm [36, 50]. Without LPS, there was no significant difference for clinical pregnancy (OR 1.08, 95 % CI 0.88–1.33; two studies) or live birth rates (OR 1.23 95 % CI 0.98–1.55; two studies). In four studies, LPS was administered in the mNC arm [32, 35, 51, 52] With LPS, the ORs for clinical pregnancy and live birth were 1.19 (95 % CI 0.78–1.82; four studies) and 1.14 (95 % CI 0.65–2.02; two studies), respectively.
mNC versus AC with suppression
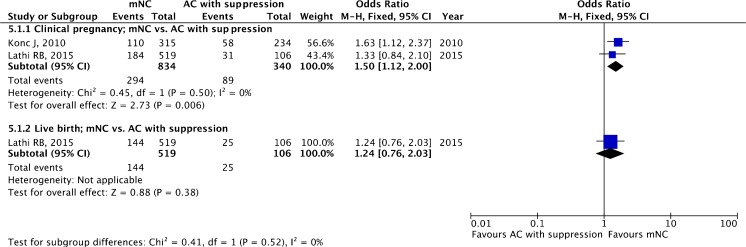
A total of two retrospective cohort studies [53, 54] with a total of 1174 ET cycles were included. LPS has been administered in the mNC arm in both studies.
The pooled estimates for clinical pregnancy (OR 1.50, 95 % CI 1.12–2.00; two studies) and live birth (OR 1.24, 95 % CI 0.76–2.03; one study) are given in Fig. 6.
Fig. 6.
Modified natural cycle (mNC) versus artificial cycle (AC) with suppression: pooled results of all studies
AC with suppression versus AC without suppression
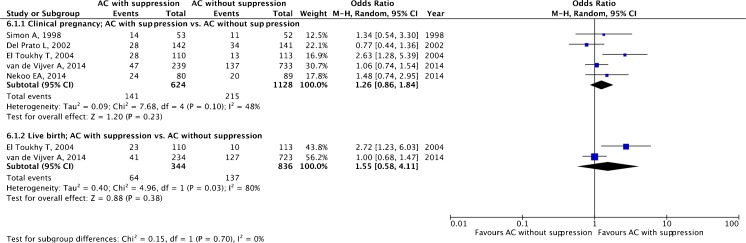
A total of five studies, four RCTs [15, 55–57] and one retrospective cohort study [16], making a total of 1752 ET cycles, were included.
The pooled estimates for clinical pregnancy (OR 1.26, 95 % CI 0.86–1.84; five studies) and live birth (OR 1.55, 95 % CI 0.58–4.11; two studies) are given in Fig. 7 with no statistically significant difference for both outcomes.
Fig. 7.
Artificial cycle (AC) with versus without suppression: pooled results of all studies
Natural cycle (either tNC or mNC) versus mild OS with Gn
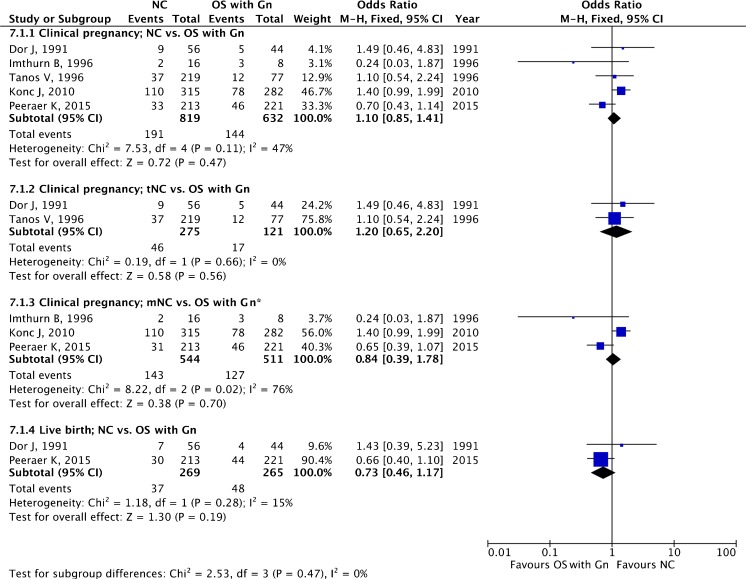
A total of five studies, one RCT [18] and four retrospective cohort studies [45, 53, 58, 59], making a total of 1451 ET cycles, were included. In two studies tNC [45, 58] was employed, and in three studies mNC [18, 53, 59] was employed.
The pooled estimates for clinical pregnancy (OR 1.10, 95 % CI 0.85–1.41; five studies) and live birth (OR 0.73, 95 % CI 0.46–1.17; two studies) are given in Fig. 8. When subgroup analysis was performed comparing tNC versus OS with Gn, and mNC versus OS with Gn, the pooled estimates for clinical pregnancy were OR 1.20, 95 % CI 0.65–2.20 (two studies), and OR 0.84, 95 % CI 0.39–1.78 (three studies), respectively.
Fig. 8.
Natural cycle versus mild ovarian stimulation with gonadotropin (OS with Gn): pooled results of all studies and subgroup analysis based on type of NC. Asterisk donates random effect
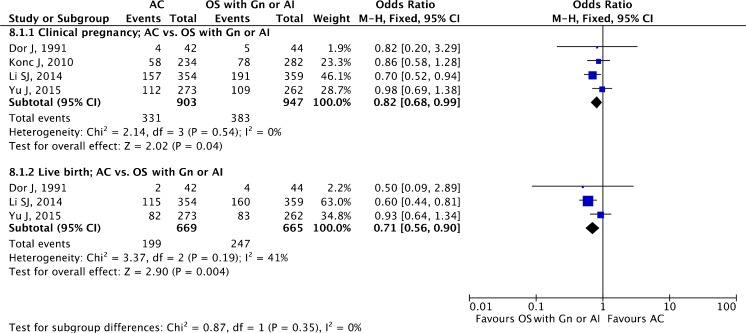
AC (with or without suppression) versus mild OS with Gn or AI (letrozole)
A total of four studies, two RCTs [60, 61] and two retrospective cohort studies [53, 58], making a total of 1850 ET cycles, were included.
The pooled estimates for clinical pregnancy (OR 0.82 95 % CI 0.68–0.99; four studies) and live birth (OR 0.71, 95 % CI 0.56–0.90; three studies) are given in Fig. 9. There appears to be increased clinical pregnancy and live birth rates employing OS with Gn or AI (letrozole).
Fig. 9.
Artificial cycle versus mild ovarian stimulation with gonadotropin (OS with Gn or AI): pooled results of all studies
Discussion
In the current meta-analysis, we failed to observe consistent superiority of a particular protocol to prepare endometrium for FER cycles when different available protocols were compared, in concordant with previous two meta-analyses [8, 21]. The best evidence was available for the comparison of AC with or without suppression (four RCTs), and no difference in clinical pregnancy and live birth rates were noted. However, although statistical significance was noted at several two-sided comparisons of the available protocols, none of them was noted to be consistently superior. Of note, mNC appeared to yield at least equivalent or better pregnancy rates when compared with tNC and AC with or without suppression. Of the available RCTs, the ANTARCTICA trial had the largest sample size and concluded that mNC and AC without suppression had similar live birth rates. Endometrial preparation with ovarian stimulation with either Gn or AI (letrozole) may be promising and warrants further powerful RCTs.
In the current meta-analysis, vast majority of the data is achieved from retrospective studies. Obviously, combining retrospective cohort studies with RCTs in the same meta-analysis may introduce selection bias. On the other hand, it is of interest that despite an increasing trend in the performance of FER cycles in the last decade, there is still a paucity of well-designed, powerful RCTs to delineate the best protocol to prepare endometrium.
Although the lack of use of any medication is an advantage of tNC, the need of frequent endocrine and ultrasonographic monitoring is a drawback. Obviously, mNC is performed with less such monitoring. In this meta-analysis, tNC and mNC appear to be associated with similar clinical pregnancy and live birth rates. In this comparison, there are two RCTs [33, 34], with opposing results. Fatemi et al. [33] concluded that tNC is superior to mNC whereas Weissman et al. [34] reported a similar pregnancy outcome. The differences in study protocols might have contributed to contradictory results. In the study by Weisman et al. [34], if an LH surge was noted, hCG was not administered and the cycle was cancelled. An LH surge at the time of hCG administration was not a cancellation criterion in the study by Fatemi et al. [33]; it is of note that, in this study, among 23 patients with impending spontaneous LH surge and hCG administration, only 1 (4.3 %) conceived [33]. However, the detrimental effect of spontaneous LH surge at the time of hCG administration is controversial [62, 63]. Another contributory factor for discordant results might be the administration of LPS or not; LPS had been administered in the study by Weismann et al. [34], whereas no LPS was given in the study by Fatemi et al. [33].
The need for LPS in tNC is controversial; there is only one RCT evaluating the impact of LPS with vaginal progesterone administration on live birth rates in tNC cycles [11]. In this study by Bjuresten et al., 435 women undergoing tNC were randomized to either vaginal progesterone (400 mg vaginal micronized progesterone bid) or no LPS [11]. The primary outcome measure was live birth rate. Administration of LPS was associated with statistically significant increase in live birth rate (30 versus 20 %; p = 0.027). Luteal phase support may not be required in mNC cycles due to sustained luteotropic effect of hCG used [12]. In the current study, when subgroup analysis was performed with administration of LPS or not, no significant difference with regard to clinical pregnancy and live birth was noted among the tNC and mNC arms.
When tNC and AC without suppression are compared, there appears to be a statistical significance for clinical pregnancy favoring tNC. Of the included seven studies, there was no RCT. Inferior pregnancy outcome in AC cycles without suppression might be due to escape from pituitary suppression, which is encountered in 1.9 to 7.4 % of such cycles [15, 16] Of the seven included studies, both endocrine and ultrasonographic monitoring was performed in only two studies in the AC without suppression arm, which might have contributed to inferior pregnancy outcome in our meta-analysis [38, 41]. Finally, lack of homogenous assignment of either anovulatory or ovulatory patients to both arms, with their different propensity for escape from ovulation, may introduce allocation bias. In two out of seven studies included in this analysis, only anovulatory patients had been enrolled in the AC without suppression arm, whereas ovulatory women had been enrolled in the tNC arm [38, 43].
When tNC and AC with suppression are compared, the latter is associated with significantly higher live birth rate and marginally significant difference regarding clinical pregnancy rate. Of the six studies included, there is one RCT [44] and one quasi-randomized trial [45] with both studies reporting no difference.
There appears to be no significant difference in clinical pregnancy and live birth rates among the mNC and AC without suppression groups. Of the six studies included, there is one powerful, multi-center RCT (ANTARCTICA trial) reporting comparable live birth rates [50]. However, significantly more cycles had been cancelled in the AC without suppression group (124/464 versus 101/495, OR 1.4, 95 % CI 1.1–1.9, p = 0.02). Apart from inadequate embryo survival, the main reasons for cycle cancellation in the mNC and AC without suppression groups were ovulation prior to hCG injection (21/101, 20.8 %) and insufficient endometrial thickness (37/124, 29.8 %), respectively. The costs of two endometrial preparation methods were comparable.
There are only two retrospective studies comparing mNC and AC with suppression [53, 54]. There appears to be a significant difference favoring mNC regarding clinical pregnancy, which was unexpected. However, since the number of available studies is limited, no definitive conclusion can be made. There appears to be no difference in clinical pregnancy and live birth rates when the AC with and without suppression groups are compared. Of the five studies included, four are RCTs [15, 31, 55, 57].
If natural cycle, either tNC or mNC, is planned, female age might also be taken into consideration, since unexpected aberrant ovulatory patterns, from anovulation to premature ovulation from 14–16–mm-sized follicles, may be observed in women with advanced female age. In such women, AC with or without suppression might be preferred to avoid such difficulties in timing of FER.
To prepare endometrium for FER, one may consider mild OS with either Gn or AI (letrozole) to overcome subtle defects in folliculogenesis and hence luteal phase in a spontaneous cycle. When NC and OS with Gn groups are compared, there appears to be no difference regarding clinical pregnancy and live birth rates. Of the five studies included, there is one RCT and four retrospective studies. When subgroup analysis is performed splitting NC as tNC and mNC, there is still no difference in clinical pregnancy.
When AC (with or without suppression) is compared with OS with Gn or AI, there appears to be a significant difference in clinical pregnancy and live birth favoring the latter group. Of the four studies included, two are RCTs [60, 61] and the remaining two are retrospective cohort studies [53, 58]. These data suggest that mild stimulation with either Gn or AI may overcome subtle defects in folliculogenesis, potentially correct luteal phase, and hence improve endometrial receptivity. Since high endometrial aromatase P450 mRNA expression is associated with poor IVF outcome [64], correction of such subtle defects with AI might contribute to better pregnancy rates with such treatment. However, further powerful RCTs are warranted to make definitive conclusions.
To add more clinical heterogeneity to the available studies to prepare endometrium for FER in AC (with or without suppression), different approaches may be employed to prime endometrium with estrogen and to support the luteal phase. Estrogen priming may be done with different routes (oral or transdermal) and dose schemes (incremental or fixed dosing). Most studies have used oral micronized estradiol (E2) 4–6 mg per day. To our knowledge, there is no study comparing oral and transdermal estrogen in non-donor FER cycles. However, in a recent large-scale retrospective case-control study encompassing 8362 fresh ET in donor cycles, no significant difference in live birth rate was found between oral (32.9 %) and transdermal (33.2 %) routes [65].
The need for endocrine and ultrasonographic monitoring in AC without suppression is controversial. In such cycles, the risk of cycle cancellation due to escape from suppression and hence premature ovulation has been reported to vary from 1.9 to 7.4 % [15, 16]. A delay in estrogen initiation [66] or an insufficient estrogen dose [57] might be associated with a higher risk of such premature ovulation. An additional preventive measure to further reduce the incidence of premature progesterone rise might be the use of higher estrogen starting doses (e.g., 6 mg daily from day 1 to day 3 of the cycle onwards), to further suppress gonadotropin release and prevent the occurrence of follicular dominance and excessive LH secretion [57]. However, in the above-mentioned large-scale retrospective case-control study encompassing 8362 fresh ET in donor cycles, employing AC with suppression, no significant difference in live birth rate was found between incremental or constant estrogen dosing [65].
With regard to LPS, different approaches might be employed. Although progesterone is the main hormone used for LPS, other approaches such as hCG and GnRH agonist might be employed in the luteal phase [41]. Furthermore, the use of hCG in mNC not only triggers final oocyte maturation but also has luteotrophic effect in the early luteal phase. Although there is substantial evidence that intramuscular and vaginal routes have comparable ongoing pregnancy/live birth rates [67] in fresh autologous cycles, there is controversy in FER cycles. In FER cycles, some studies reported better pregnancy outcome with intramuscular route [68, 69], whereas the majority of the studies reported similar pregnancy outcome [70–72].
The endometrial thickness and pattern were not taken as outcome measures in our meta-analysis. The impact of endometrial thickness and pattern on pregnancy rates in FER cycles is controversial. In a retrospective observational study, an endometrial thickness of 9–14 mm on the day of progesterone supplementation was associated with significantly higher implantation and pregnancy rates compared with an endometrial thickness of 7–8 mm [73]. However, neither endometrial thickness nor endometrial pattern had any impact on implantation and pregnancy rates following euploid blastocyst transfer [74].
The type of freezing method, slow freezing versus vitrification, might have an impact on post-thaw embryo development and metabolism [75, 76]. A higher post-thaw developmental rate of vitrified embryos might entail the need to change the endometrial preparation protocol, in particular the timing of progesterone commencement in artificial cycles, in order to obtain a better synchrony between embryos and endometrium. However, to our knowledge, there is no available data that compares different progesterone commencement timings and pregnancy outcome based on the type of freezing method employed.
In conclusion, with the best available evidence, there is no consistent superiority of any endometrial preparation for FER. However, mNC has several advantages including the following: (i) being patient-friendly; (ii) yields at least equivalent or better pregnancy rates when compared with tNC and AC with or without suppression; (iii) may not require LPS. Mild OS with Gn or AI may be promising. Further, powerful RCTs are warranted not only to delineate the best protocol to prepare endometrium for FER in selected circumstances but also to evaluate cost-effectiveness and patient convenience.
Footnotes
Capsule
There is no consistent superiority of any endometrial preparation for FER.
References
- 1.D’Angelo A. Ovarian hyperstimulation syndrome prevention strategies: cryopreservation of all embryos. Semin Reprod Med. 2010;28(6):513–8. doi: 10.1055/s-0030-1265679. [DOI] [PubMed] [Google Scholar]
- 2.Taylor TH, Patrick JL, Gitlin SA, Michael Wilson J, Crain JL, Griffin DK. Outcomes of blastocysts biopsied and vitrified once versus those cryopreserved twice for euploid blastocyst transfer. Reprod Biomed Online. 2014;29(1):59–64. doi: 10.1016/j.rbmo.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Bourgain C, Devroey P. The endometrium in stimulated cycles for IVF. Hum Reprod Update. 2003;9(6):515–22. doi: 10.1093/humupd/dmg045. [DOI] [PubMed] [Google Scholar]
- 4.European IVFMC, European Society of Human R. European IVFMC, European Society of Human R, Embryology. Kupka MS, D’Hooghe T, Ferraretti AP, et al. Assisted reproductive technology in Europe, 2011: results generated from European registers by ESHRE. Hum Reprod. 2016;31(2):233–48. doi: 10.1093/humrep/dev319. [DOI] [PubMed] [Google Scholar]
- 5.Kupka MS, Ferraretti AP, de Mouzon J, Erb K, D’Hooghe T, Castilla JA, et al. Assisted reproductive technology in Europe, 2010: results generated from European registers by ESHREdagger. Hum Reprod. 2014;29(10):2099–113. doi: 10.1093/humrep/deu175. [DOI] [PubMed] [Google Scholar]
- 6.CDC. Assisted reproductive technology, National summary report. 2013.
- 7.Glujovsky D, Dominguez M, Fiszbajn G, Papier S, Lavolpe M, Sueldo C. A shared egg donor program: which is the minimum number of oocytes to be allocated? J Assist Reprod Genet. 2011;28(3):263–7. doi: 10.1007/s10815-010-9511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groenewoud ER, Cantineau AE, Kollen BJ, Macklon NS, Cohlen BJ. What is the optimal means of preparing the endometrium in frozen-thawed embryo transfer cycles? A systematic review and meta-analysis. Hum Reprod Update. 2013;19(5):458–70. doi: 10.1093/humupd/dmt030. [DOI] [PubMed] [Google Scholar]
- 9.Andersen AG, Als-Nielsen B, Hornnes PJ, Franch AL. Time interval from human chorionic gonadotrophin (HCG) injection to follicular rupture. Hum Reprod. 1995;10(12):3202–5. doi: 10.1093/oxfordjournals.humrep.a135888. [DOI] [PubMed] [Google Scholar]
- 10.Park SJ, Goldsmith LT, Skurnick JH, Wojtczuk A, Weiss G. Characteristics of the urinary luteinizing hormone surge in young ovulatory women. Fertil Steril. 2007;88(3):684–90. doi: 10.1016/j.fertnstert.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 11.Bjuresten K, Landgren BM, Hovatta O, Stavreus-Evers A. Luteal phase progesterone increases live birth rate after frozen embryo transfer. Fertil Steril. 2011;95(2):534–7. doi: 10.1016/j.fertnstert.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 12.Fauser BC, de Jong D, Olivennes F, Wramsby H, Tay C, Itskovitz-Eldor J, et al. Endocrine profiles after triggering of final oocyte maturation with GnRH agonist after cotreatment with the GnRH antagonist ganirelix during ovarian hyperstimulation for in vitro fertilization. J Clin Endocrinol Metab. 2002;87(2):709–15. doi: 10.1210/jcem.87.2.8197. [DOI] [PubMed] [Google Scholar]
- 13.Casper RF, Yanushpolsky EH. Optimal endometrial preparation for frozen embryo transfer cycles: window of implantation and progesterone support. Fertil Steril. 2016;105(4):867–72. doi: 10.1016/j.fertnstert.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Eftekhar M, Rahsepar M, Rahmani E. Effect of progesterone supplementation on natural frozen-thawed embryo transfer cycles: a randomized controlled trial. Int J Fertil Steril. 2013;7(1):13–20. [PMC free article] [PubMed] [Google Scholar]
- 15.Dal Prato L, Borini A, Cattoli M, Bonu MA, Sciajno R, Flamigni C. Endometrial preparation for frozen-thawed embryo transfer with or without pretreatment with gonadotropin-releasing hormone agonist. Fertil Steril. 2002;77(5):956–60. doi: 10.1016/s0015-0282(02)02960-6. [DOI] [PubMed] [Google Scholar]
- 16.van de Vijver A, Polyzos NP, Van Landuyt L, De Vos M, Camus M, Stoop D, et al. Cryopreserved embryo transfer in an artificial cycle: is GnRH agonist down-regulation necessary? Reprod Biomed Online. 2014;29(5):588–94. doi: 10.1016/j.rbmo.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Van der Auwera I, Meuleman C, Koninckx PR. Human menopausal gonadotrophin increases pregnancy rate in comparison with clomiphene citrate during replacement cycles of frozen/thawed pronucleate ova. Hum Reprod. 1994;9(8):1556–60. doi: 10.1093/oxfordjournals.humrep.a138748. [DOI] [PubMed] [Google Scholar]
- 18.Peeraer K, Couck I, Debrock S, De Neubourg D, De Loecker P, Tomassetti C, et al. Frozen-thawed embryo transfer in a natural or mildly hormonally stimulated cycle in women with regular ovulatory cycles: a RCT. Hum Reprod. 2015;30(11):2552–62. doi: 10.1093/humrep/dev224. [DOI] [PubMed] [Google Scholar]
- 19.Ezoe K, Daikoku T, Yabuuchi A, Murata N, Kawano H, Abe T, et al. Ovarian stimulation using human chorionic gonadotrophin impairs blastocyst implantation and decidualization by altering ovarian hormone levels and downstream signaling in mice. Mol Hum Reprod. 2014;20(11):1101–16. doi: 10.1093/molehr/gau065. [DOI] [PubMed] [Google Scholar]
- 20.Horcajadas JA, Minguez P, Dopazo J, Esteban FJ, Dominguez F, Giudice LC, et al. Controlled ovarian stimulation induces a functional genomic delay of the endometrium with potential clinical implications. J Clin Endocrinol Metab. 2008;93(11):4500–10. doi: 10.1210/jc.2008-0588. [DOI] [PubMed] [Google Scholar]
- 21.Glujovsky D, Pesce R, Fiszbajn G, Sueldo C, Hart RJ, Ciapponi A. Endometrial preparation for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes. Cochrane Database Syst Rev. 2010;1:CD006359. doi: 10.1002/14651858.CD006359.pub2. [DOI] [PubMed] [Google Scholar]
- 22.El Bahja D. Frozen embryo transfer protocol: does spontaneous cycle give good results? Gynecol Obstet Fertil. 2012. [DOI] [PubMed]
- 23.Yishia D. Do we need to artificially prepare the endometrium for frozen embryo transfer in normal cycling women? A controlled study. Fertil Steril. 2001;76:112. [Google Scholar]
- 24.Belaisch-Allart J. Clinical management of a frozen–thawed embryo transfer cycle. Abstracts of the 10th Annual Meeting of the ESHRE Brussels. 1994:138-9.
- 25.Gonzales J. Natural cycle and hormonal replacement in FET: implantation and pregnancy rates. Abstract Book of the 48th Meeting of the American Fertility Society. 1992:42.
- 26.Cattoli M. Arandomized prospective studyon cryopreserved-thawedembryo transfer: natural versus homrone replacement cycles. Abstracts of the 10th Annual Meeting of the ESHRE Brussels. 1994;356:139.
- 27.Alama P. Higher ongoing pregnancy rates in blastocyst transfer of frozen-thawed embryos in natural cycles than in hormone replacement therapy cycles. Fertil Steril. 2007;88:161. [Google Scholar]
- 28.Dolan P. Natural cycles and estrogen/progestone induced cycles produce an equallly receptive endometrium for implantation of cryopreserved embryos. Hum Reprod (Oxford, England) 1991:16.
- 29.Lee S. Comparison of clinical outcome of frozen–thawed embryo transfer cycles between natural and artificial (hormone-treated) cycles. Hum Reprod. 2008;23:217. [Google Scholar]
- 30.Spandorfer S. Blastocyst frozen embryo transfer (FET): comparison of outcome with replacement in natural or programmed/medicated cycle. Fertil Steril. 2004;82:154. [Google Scholar]
- 31.Azimi Nekoo E, Chamani M, Shahrokh Tehrani E, Hossein Rashidi B, Davari Tanha F, Kalantari V. Artificial endometrial preparation for frozen-thawed embryo transfer with or without pretreatment with depot gonadotropin releasing hormone agonist in women with regular menses. J Fam Reprod Health. 2015;9(1):1–4. [PMC free article] [PubMed] [Google Scholar]
- 32.Kawamura T, Motoyama H, Yanaihara A, Yoramitsu TAA, Karasawa K, et al. Clinical outcomes of two different endometrial preparation methods for cryopreserved-thawed embryo transfer in patients with a normal menstrual cycle. Reprod Med Biol. 2007;6(1):53–7. doi: 10.1111/j.1447-0578.2007.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fatemi HM, Kyrou D, Bourgain C, Van den Abbeel E, Griesinger G, Devroey P. Cryopreserved-thawed human embryo transfer: spontaneous natural cycle is superior to human chorionic gonadotropin-induced natural cycle. Fertil Steril. 2010;94(6):2054–8. doi: 10.1016/j.fertnstert.2009.11.036. [DOI] [PubMed] [Google Scholar]
- 34.Weissman A, Horowitz E, Ravhon A, Steinfeld Z, Mutzafi R, Golan A, et al. Spontaneous ovulation versus HCG triggering for timing natural-cycle frozen-thawed embryo transfer: a randomized study. Reprod Biomed Online. 2011;23(4):484–9. doi: 10.1016/j.rbmo.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Chang EM, Han JE, Kim YS, Lyu SW, Lee WS, Yoon TK. Use of the natural cycle and vitrification thawed blastocyst transfer results in better in-vitro fertilization outcomes : cycle regimens of vitrification thawed blastocyst transfer. J Assist Reprod Genet. 2011;28(4):369–74. doi: 10.1007/s10815-010-9530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomas C, Alsbjerg B, Martikainen H, Humaidan P. Pregnancy loss after frozen-embryo transfer—a comparison of three protocols. Fertil Steril. 2012;98(5):1165–9. doi: 10.1016/j.fertnstert.2012.07.1058. [DOI] [PubMed] [Google Scholar]
- 37.Weissman A, Levin D, Ravhon A, Eran H, Golan A, Levran D. What is the preferred method for timing natural cycle frozen-thawed embryo transfer? Reprod Biomed Online. 2009;19(1):66–71. doi: 10.1016/s1472-6483(10)60048-x. [DOI] [PubMed] [Google Scholar]
- 38.Levron J, Yerushalmi GM, Brengauz M, Gat I, Katorza E. Comparison between two protocols for thawed embryo transfer: natural cycle versus exogenous hormone replacement. Gynecol Endocrinol : Off J Int Soc Gynecol Endocrinol. 2014;30(7):494–7. doi: 10.3109/09513590.2014.900032. [DOI] [PubMed] [Google Scholar]
- 39.Loh SK, Leong NK. Factors affecting success in an embryo cryopreservation programme. Ann Acad Med Singap. 1999;28(2):260–5. [PubMed] [Google Scholar]
- 40.Morozov V, Ruman J, Kenigsberg D, Moodie G, Brenner S. Natural cycle cryo-thaw transfer may improve pregnancy outcome. J Assist Reprod Genet. 2007;24(4):119–23. doi: 10.1007/s10815-006-9100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orvieto R, Feldman N, Lantsberg D, Manela D, Zilberberg E, Haas J. Natural cycle frozen-thawed embryo transfer—can we improve cycle outcome? J Assist Reprod Genet. 2016;33(5):611–5. doi: 10.1007/s10815-016-0685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veleva Z, Orava M, Nuojua-Huttunen S, Tapanainen JS, Martikainen H. Factors affecting the outcome of frozen-thawed embryo transfer. Hum Reprod. 2013;28(9):2425–31. doi: 10.1093/humrep/det251. [DOI] [PubMed] [Google Scholar]
- 43.Xiao Z, Zhou X, Xu W, Yang J, Xie Q. Natural cycle is superior to hormone replacement therapy cycle for vitrificated-preserved frozen-thawed embryo transfer. Syst Biol Reprod Med. 2012;58(2):107–12. doi: 10.3109/19396368.2011.646047. [DOI] [PubMed] [Google Scholar]
- 44.Mounce G, McVeigh E, Turner K, Child TJ. Randomized, controlled pilot trial of natural versus hormone replacement therapy cycles in frozen embryo replacement in vitro fertilization. Fertil Steril. 2015;104(4):915–20. doi: 10.1016/j.fertnstert.2015.07.1131. [DOI] [PubMed] [Google Scholar]
- 45.Tanos V, Friedler S, Zajicek G, Neiger M, Lewin A, Schenker JG. The impact of endometrial preparation on implantation following cryopreserved-thawed-embryo transfer. Gynecol Obstet Investig. 1996;41(4):227–31. doi: 10.1159/000292274. [DOI] [PubMed] [Google Scholar]
- 46.al-Shawaf T, Dave R, Harper J, Linehan D, Riley P, Craft I. Transfer of embryos into the uterus: how much do technical factors affect pregnancy rates? J Assist Reprod Genet. 1993;10(1):31–6. doi: 10.1007/BF01204437. [DOI] [PubMed] [Google Scholar]
- 47.Gelbaya TA, Nardo LG, Hunter HR, Fitzgerald CT, Horne G, Pease EE, et al. Cryopreserved-thawed embryo transfer in natural or down-regulated hormonally controlled cycles: a retrospective study. Fertil Steril. 2006;85(3):603–9. doi: 10.1016/j.fertnstert.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 48.Hill MJ, Miller KA, Frattarelli JL. A GnRH agonist and exogenous hormone stimulation protocol has a higher live-birth rate than a natural endogenous hormone protocol for frozen-thawed blastocyst-stage embryo transfer cycles: an analysis of 1391 cycles. Fertil Steril. 2010;93(2):416–22. doi: 10.1016/j.fertnstert.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 49.Queenan JT, Jr, Veeck LL, Seltman HJ, Muasher SJ. Transfer of cryopreserved-thawed pre-embryos in a natural cycle or a programmed cycle with exogenous hormonal replacement yields similar pregnancy results. Fertil Steril. 1994;62(3):545–50. doi: 10.1016/s0015-0282(16)56943-x. [DOI] [PubMed] [Google Scholar]
- 50.Groenewoud ER, Cohlen BJ, Al-Oraiby A, Brinkhuis EA, Broekmans FJ, de Bruin JP, et al. A randomized controlled, non-inferiority trial of modified natural versus artificial cycle for cryo-thawed embryo transfer. Hum Reprod. 2016 doi: 10.1093/humrep/dew120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Givens CR, Markun LC, Ryan IP, Chenette PE, Herbert CM, Schriock ED. Outcomes of natural cycles versus programmed cycles for 1677 frozen-thawed embryo transfers. Reprod Biomed Online. 2009;19(3):380–4. doi: 10.1016/s1472-6483(10)60172-1. [DOI] [PubMed] [Google Scholar]
- 52.Hancke K, More S, Kreienberg R, Weiss JM. Patients undergoing frozen-thawed embryo transfer have similar live birth rates in spontaneous and artificial cycles. J Assist Reprod Genet. 2012;29(5):403–7. doi: 10.1007/s10815-012-9724-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Konc J, Kanyo K, Varga E, Kriston R, Cseh S. The effect of cycle regimen used for endometrium preparation on the outcome of day 3 frozen embryo transfer cycle. Fertil Steril. 2010;94(2):767–8. doi: 10.1016/j.fertnstert.2009.09.053. [DOI] [PubMed] [Google Scholar]
- 54.Lathi RB, Chi YY, Liu J, Saravanabavanandhan B, Hegde A, Baker VL. Frozen blastocyst embryo transfer using a supplemented natural cycle protocol has a similar live birth rate compared to a programmed cycle protocol. J Assist Reprod Genet. 2015;32(7):1057–62. doi: 10.1007/s10815-015-0499-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.El-Toukhy T, Taylor A, Khalaf Y, Al-Darazi K, Rowell P, Seed P, et al. Pituitary suppression in ultrasound-monitored frozen embryo replacement cycles. A randomised study. Hum Reprod. 2004;19(4):874–9. doi: 10.1093/humrep/deh183. [DOI] [PubMed] [Google Scholar]
- 56.Nekoo EA, Chamani M, Tehrani ES, Rashidi BH, Tanha FD, Kalantari V. Artificial endometrial preparation for frozen-thawed embryo transfer with or without pretreatment with depot gonadotropin releasing hormone agonist in women with regular menses. J Fam Reprod Health. 2014;9(1):1–4. [PMC free article] [PubMed] [Google Scholar]
- 57.Simon A, Hurwitz A, Zentner BS, Bdolah Y, Laufer N. Transfer of frozen-thawed embryos in artificially prepared cycles with and without prior gonadotrophin-releasing hormone agonist suppression: a prospective randomized study. Hum Reprod. 1998;13(1O):2712–7. doi: 10.1093/humrep/13.10.2712. [DOI] [PubMed] [Google Scholar]
- 58.Dor J, Rudak E, Davidson A, Levran D, Ben-Rafael Z, Mashiach S. Endocrine and biological factors influencing implantation of human embryos following cryopreservation. Gynecol Endocrinol : Off J Int Soc Gynecol Endocrinol. 1991;5(3):203–11. doi: 10.3109/09513599109028442. [DOI] [PubMed] [Google Scholar]
- 59.Imthurn B, Macas E, Rosselli M, Keller PJ. Effect of a programmed short-term stimulation protocol on the replacement of cryopreserved embryos. J Assist Reprod Genet. 1996;13(9):709–12. doi: 10.1007/BF02066423. [DOI] [PubMed] [Google Scholar]
- 60.Li SJ, Zhang YJ, Chai XS, Nie MF, Zhou YY, Chen JL, et al. Letrozole ovulation induction: an effective option in endometrial preparation for frozen-thawed embryo transfer. Arch Gynecol Obstet. 2014;289(3):687–93. doi: 10.1007/s00404-013-3044-0. [DOI] [PubMed] [Google Scholar]
- 61.Yu J, Ma Y, Wu Z, Li Y, Tang L, Li Y, et al. Endometrial preparation protocol of the frozen-thawed embryo transfer in patients with polycystic ovary syndrome. Arch Gynecol Obstet. 2015;291(1):201–11. doi: 10.1007/s00404-014-3396-0. [DOI] [PubMed] [Google Scholar]
- 62.Griesinger G, Weig M, Schroer A, Diedrich K, Kolibianakis EM. Mid-cycle serum levels of endogenous LH are not associated with the likelihood of pregnancy in artificial frozen-thawed embryo transfer cycles without pituitary suppression. Hum Reprod. 2007;22(10):2589–93. doi: 10.1093/humrep/dem207. [DOI] [PubMed] [Google Scholar]
- 63.Groenewoud ER, Kollen BJ, Macklon NS, Cohlen BJ. Spontaneous LH surges prior to HCG administration in unstimulated-cycle frozen-thawed embryo transfer do not influence pregnancy rates. Reprod Biomed Online. 2012;24(2):191–6. doi: 10.1016/j.rbmo.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 64.Brosens J, Verhoeven H, Campo R, Gianaroli L, Gordts S, Hazekamp J, et al. High endometrial aromatase P450 mRNA expression is associated with poor IVF outcome. Hum Reprod. 2004;19(2):352–6. doi: 10.1093/humrep/deh075. [DOI] [PubMed] [Google Scholar]
- 65.Madero S, Rodriguez A, Vassena R, Vernaeve V. Endometrial preparation: effect of estrogen dose and administration route on reproductive outcomes in oocyte donation cycles with fresh embryo transfer. Hum Reprod. 2016 doi: 10.1093/humrep/dew099. [DOI] [PubMed] [Google Scholar]
- 66.Remohi J, Vidal A, Pellicer A. Oocyte donation in low responders to conventional ovarian stimulation for in vitro fertilization. Fertil Steril. 1993;59(6):1208–15. doi: 10.1016/s0015-0282(16)55978-0. [DOI] [PubMed] [Google Scholar]
- 67.van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev. 2015;7:CD009154. doi: 10.1002/14651858.CD009154.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haddad G, Saguan DA, Maxwell R, Thomas MA. Intramuscular route of progesterone administration increases pregnancy rates during non-downregulated frozen embryo transfer cycles. J Assist Reprod Genet. 2007;24(10):467–70. doi: 10.1007/s10815-007-9168-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaser DJ, Ginsburg ES, Missmer SA, Correia KF, Racowsky C. Intramuscular progesterone versus 8% Crinone vaginal gel for luteal phase support for day 3 cryopreserved embryo transfer. Fertil Steril. 2012;98(6):1464–9. doi: 10.1016/j.fertnstert.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, He Y, Zhao X, Ji X, Hong Y, Wang Y, et al. Crinone gel for luteal phase support in frozen-thawed embryo transfer cycles: a prospective randomized clinical trial in the Chinese population. PLoS One. 2015;10(7):e0133027. doi: 10.1371/journal.pone.0133027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shapiro DB, Pappadakis JA, Ellsworth NM, Hait HI, Nagy ZP. Progesterone replacement with vaginal gel versus i.m. injection: cycle and pregnancy outcomes in IVF patients receiving vitrified blastocysts. Hum Reprod. 2014;29(8):1706–11. doi: 10.1093/humrep/deu121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leonard PH, Hokenstad AN, Khan Z, Jensen JR, Stewart EA, Coddington CC. Progesterone support for frozen embryo transfer: intramuscular versus vaginal suppository demonstrates no difference in a cohort. J Reprod Med. 2015;60(3-4):103–8. [PubMed] [Google Scholar]
- 73.El-Toukhy T, Coomarasamy A, Khairy M, Sunkara K, Seed P, Khalaf Y, et al. The relationship between endometrial thickness and outcome of medicated frozen embryo replacement cycles. Fertil Steril. 2008;89(4):832–9. doi: 10.1016/j.fertnstert.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 74.Gingold JA, Lee JA, Rodriguez-Purata J, Whitehouse MC, Sandler B, Grunfeld L, et al. Endometrial pattern, but not endometrial thickness, affects implantation rates in euploid embryo transfers. Fertil Steril. 2015;104(3):620–8. doi: 10.1016/j.fertnstert.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Balaban B, Urman B, Ata B, Isiklar A, Larman MG, Hamilton R, et al. A randomized controlled study of human Day 3 embryo cryopreservation by slow freezing or vitrification: vitrification is associated with higher survival, metabolism and blastocyst formation. Hum Reprod. 2008;23(9):1976–82. doi: 10.1093/humrep/den222. [DOI] [PubMed] [Google Scholar]
- 76.Cercas R, Villas C, Pons I, Brana C, Fernandez-Shaw S. Vitrification can modify embryo cleavage stage after warming. Should we change endometrial preparation? J Assist Reprod Genet. 2012;29(12):1363–8. doi: 10.1007/s10815-012-9881-0. [DOI] [PMC free article] [PubMed] [Google Scholar]