Abstract
In pre-clinical safety studies, drug-induced vascular injury (DIVI) is defined as an adverse response to a drug characterized by degenerative and hyperplastic changes of endothelial cells and vascular smooth muscle cells. Inflammation may also be seen, along with extravasation of red blood cells into the smooth muscle layer (i.e., hemorrhage). Drugs that cause DIVI are often discontinued from development after considerable cost has occurred. An in vitro vascular model has been developed using endothelial and smooth muscle cells in co-culture across a porous membrane mimicking the internal elastic lamina. Arterial flow rates of perfusion media within the endothelial chamber of the model induce physiologic endothelial cell alignment. Pilot testing with a drug known to cause DIVI induced extravasation of red blood cells into the smooth muscle layer in all devices with no extravasation seen in control devices. This engineered vascular model offers the potential to evaluate candidate drugs for DIVI early in the discovery process. The physiologic flow within the co-culture model also makes it candidate for a wide variety of vascular biology investigations.
I. INTRODUCTION
An in vitro model of small diameter blood vessels would be a useful tool for the study of a variety of vascular diseases, physiological mechanisms, and toxicological responses. Drug-induced vascular injury (DIVI) in pre-clinical toxicology studies of pharmaceutical agents in particular, involves both the endothelium and tunica media of small vessels to medium sized vessels, but little is known about its mechanism or inciting factors agent.1,2 In animals, DIVI is diagnosed by postmortem histopathologic examination of vessels from animals exposed to new chemical entities,3 usually as part of pre-clinical in vivo safety studies. DIVI is characterized by degenerative and hyperplastic changes of endothelial cells (ECs) and vascular smooth muscle cells (SMCs), sometimes accompanied by inflammation.3 More specifically, there can be degeneration, apoptosis, necrosis, hyperplasia, and/or hypertrophy in ECs. Changes in vascular SMCs can include hyalinization, apoptosis, necrosis, hypertrophy, and/or hyperplasia. Inflammation associated with DIVI is characterized by the severity and the specific leukocytes present in the inflammatory infiltrate. The types of inflammation in DIVI are granulocytic (neutrophils and/or eosinophils), mixed, and mononuclear (histiocytic and/or lymphocytic). Occasionally, edema or hemorrhage including extravasation of red blood cells (RBCs) into the tunica media layer of blood vessels4 is seen; analysis of these vessels reveals a relatively undisturbed endothelial layer but a widespread deposition of RBCs and few white blood cells into the vessel wall. With some drugs, particular vascular beds in certain species, such as mesenteric vessels in rats (diameter ≈ 200 μm) and coronary arteries in dogs (diameter ≈ 0.5–1 mm), appear to be more predisposed to these lesions5–7 although DIVI can occur in many different vascular beds in these species. Injury to both ECs and SMCs leading to subsequent inflammatory response has been implicated in DIVI pathogenesis, but the individual contributions from each cell type is unclear.8 SMC apoptosis or necrosis is a classic finding in DIVI caused by compounds that cause vasodilation or vasoconstriction.3
DIVI occurs in approximately 10% of candidate drugs that reach the in vivo testing stage of development. Although DIVI is a relatively common finding in pre-clinical toxicology studies in dogs and rats, similar findings have not been documented in human clinical trials with drugs that cause DIVI in dogs and rats, making relevance to human risk unclear. Nonetheless, compounds that cause DIVI in pre-clinical settings are often stopped from further development, or the drug development is delayed. By the time in vivo studies have begun and DIVI is identified, large sums of money have already been spent on a candidate drug. DIVI remains an issue of additional concern for the pharmaceutical industry because no diagnostic biomarkers exist to manage the finding clinically and no in vitro models are currently available to screen candidate compounds or chemical series for DIVI risk in drug development. Therefore, opportunities exist for the development of in vitro screens to allow identification of compounds that have the potential to cause DIVI early in the drug discovery process.
Because DIVI affects both the endothelial and tunica medial layers of vascular beds in vivo, an in vitro vascular model is required that can not only mimic the architecture of the vessel, but also demonstrate an appropriate mechanical response with extravasation of blood in response to treatment with a DIVI inducing compound known to induce microscopic hemorrhage in the pathogenesis of injury. Using a microfluidics-based platform, a bilayer small diameter in vitro vascular model has been developed. The model was designed to mimic geometry and flow dynamics of a mesenteric vessel of a rat, a typical animal model used in toxicology studies on candidate compounds and a common vascular bed that is pre-disposed to DIVI. The model was then evaluated for its ability to detect one feature of DIVI seen with some drugs: extravasation of RBCs into the smooth muscle layer of the vessel.
II. MATERIALS AND METHODS
A. Microfluidic vascular device development
The mechanical phenomenon of extravasation of RBCs into the vascular tunica media without significant disruption of the endothelial layer posed a challenge to creation of a model that could recapitulate this process. A key element in the DIVI phenomenon may be the internal elastic lamina (IEL). Rather than functioning as an impermeable sheath, the internal elastic lamina is porous.9,10 In analysis of IEL images,9 the pores of the IEL range from 2.3 to 13.1 μm, with a mean pore size of 6.4 μm and a total porosity of 11%. The diameter of holes in the IEL is large enough to permit extravasation of RBCs, and we hypothesize that this is their route of passage into the tunica media in DIVI. The microfluidic vascular model was designed with a porous membrane separating the endothelial layer and the tunica media layer, mimicking the naturally porous IEL. The porous membrane in the model is a track-etched polycarbonate membrane with 10 μm pore size and 7.9% total porosity (Isopore™ Membrane Filters, Millipore, Billerica, MA).
Other significant design criteria for the model included an endothelial chamber on the order of 200 μm in diameter, designed to replicate the size of rat mesentery vessels affected by DIVI.1,4 To reproduce the confluent, flow aligned EC layer present in normal blood vessels, it was necessary for the ECs to form an adherent monolayer on the porous membrane and to sustain perfusion media flow rates that achieved physiologic levels of shear stress in the model. The range of physiologic arterial shear stress11 is 10 to 70 dynes/cm2, so a shear stress target of 15 dynes/cm2 was chosen for the model. The tunica media layer of the model was required to be positioned opposite the ECs, and to grow into a multi-layer tissue in a chamber with no flow. The model needed to be translucent to allow monitoring of the cells growth (particularly during model development) and for testing for RBC extravasation.
Using these design criteria, the model was developed as a microfluidic device consisting of three principal components: a 200 × 200 μm (height and width) vascular channel, a porous membrane mimicking the IEL, and a smooth muscle chamber (Fig. 1). The mold for the vascular channel was created by patterning a silicon wafer using SU-8 photolithographic techniques. The vascular chamber of the device was made using poly(dimethylsiloxane) (PDMS) (Sylgard 184, Dow Corning, Midland, MI) and soft lithographic techniques. The smooth muscle chamber was made with PDMS using a stamp method over a machined mold with the central channel feature to create a thin layer. The chamber portion of the smooth muscle chamber was formed by casting PDMS in a machined mold. A thin glass cover slip formed the lid of the device. Microbore silicone tubing (Dow Corning) was connected to the device to allow perfusion media to flow through the endothelial chamber and was also used for cell seeding. Tubing connections to the smooth muscle chamber allowed perfusion media changes and extraction of perfusion media for analysis. Devices were assembled using PDMS silicone glue and stamp gluing techniques. Both sides of the polycarbonate membrane were coated with type I collagen (Trevigen Inc., Gaithersburg, MD) prior to ethylene oxide sterilization.
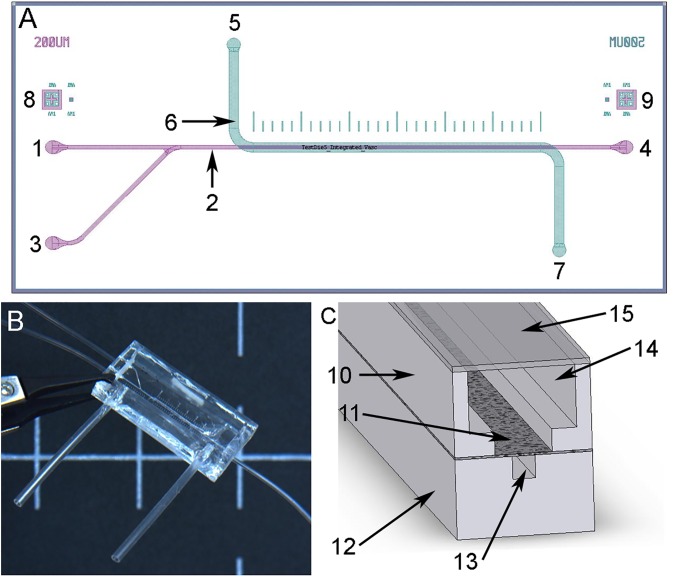
FIG. 1.
The vascular model consists of a vascular channel, porous membrane, and smooth muscle cell (SMC) chamber. (a) Device design with vascular channel layer (purple) and smooth muscle layer (green) overlaid. The endothelial layer inlet flow channel (1) is continuous with the vascular channel (2). The endothelial cells (EC) were seeded through the seeding inlet channel (3), flown through the vascular channel (2) and exited the device at the outlet (4). The smooth muscle layer of the device has an inlet channel (5) into the SMC chamber (6) with an outlet (7). Markings above the channel (500 μm between marks) were used for measurement during microscopic imaging. Alignment features (8) and (9) on opposite sides of the device were used for precision alignment of the EC vascular channel and SMC layers during manufacturing. (b) Photo of the assembled device with attached tubing. Scale bar, 1.0 cm. (c) Cross-section of device showing the lower EC layer (12) with EC channel (13) (200 μm × 200 μm), the upper SMC layer (10) with SMC chamber (14) with lid (15), and the porous layer between that mimics the internal elastic lamina (11).
The complete system of the model included a programmable syringe pump (PHD series, Harvard Apparatus, Holliston, MA) to flow perfusion media through the endothelial channel of the device and a collection chamber to collect the perfusion media. A particular timeline for the device seeding sequence and endothelial flow ramp up was developed to achieve cell growth and appropriate endothelial cell (EC) and smooth muscle cell (SMC) phenotype within the model (Fig. 2(a)). An extensive iterative design process was required to develop the device features and microfluidic cell culture methodology to achieve stable and phenotypically appropriate cell growth in the device. Device design was modeled in 3D using SolidWorks (SolidWorks Corp., Concord, MA) and analyzed with computational fluid dynamics (CFD) in FloWorks (SolidWorks Corp). CFD analysis was used to determine the appropriate flow rates to achieve physiologic shear stress.
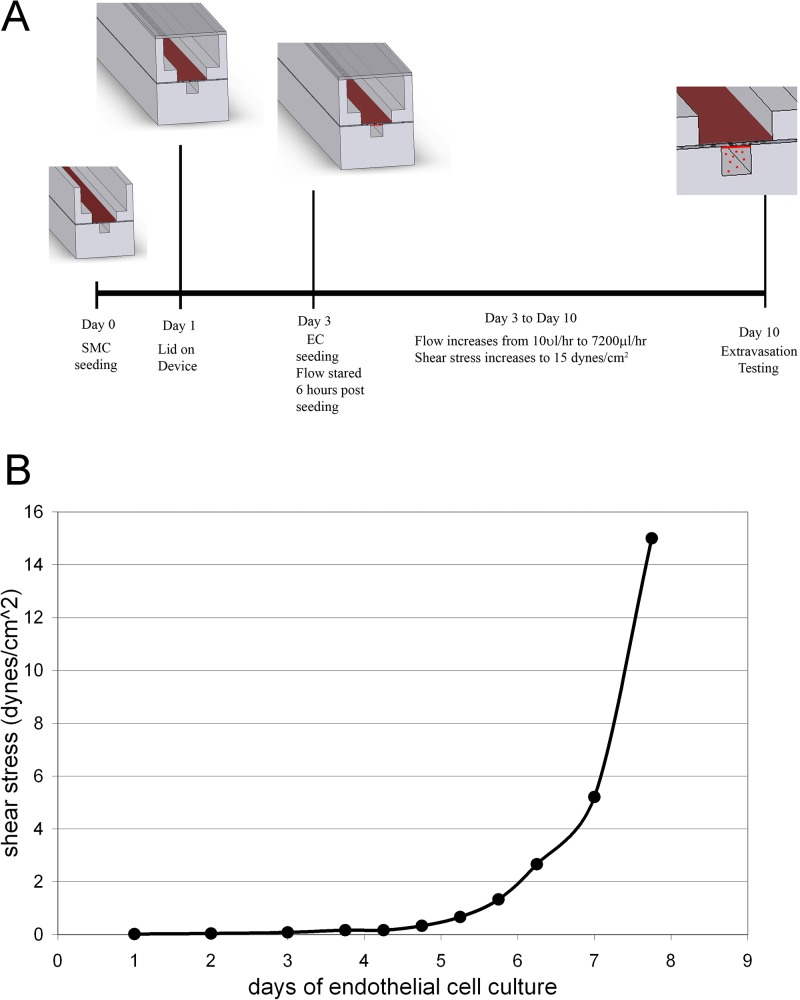
FIG. 2.
Sequence of cell seeding and flow ramp up was developed to achieve cell attachment and growth and appropriate EC and SMC phenotypes within the model. (a) Cell seeding sequence and timeline for device maturation. (b) Flow rate ramp scheme to achieve a confluent layer of flow-aligned ECs with flow increase from 10 μl/h to 7200 μl/h (15 dynes/cm2) over eight days.
B. Device cell culture
Primary rat aortic endothelial cells (VEC Technologies, Rensselaer, NY) passage 4–12 and primary rat aortic smooth muscle cells (VEC Technologies) passage 4–12 were used in the model. ECs and SMCs were expanded, sequentially seeded and cultured within the device in Endothelial Cell Growth Medium-2 (EGM-2) (Lonza, Walkersville, MD) and DMEM (Dulbecco's Modified Eagle Medium) with 10% fetal bovine serum and 1% pen-strep, respectively. After priming the endothelial channel with EGM-2, SMCs were seeded (35 μl at 3 × 106 cells/ml) within the open channel of the SMC chamber and attached to the exposed membrane within the channel. Unattached SMCs were removed after 4 h and the perfusion media changed. After one day of SMC culture, the cover glass lid was glued to the device using silicone glue (Sil-Poxy, Smooth On Inc, Easton, PA). After three days of SMC culture, ECs were then seeded in the 200 μm EC channel via the primed EC seeding port (10 μl at 3 × 106 cells/ml). The devices were flipped over for 2 to 3 h to facilitate EC attachment to the membrane. 6 h after EC seeding, flow within the 200 × 200 μm channel was initiated at 10 μl/h using the syringe pump. Flow was gradually increased to a flow rate that corresponded to normal arterial shear stress (15 dynes/cm2, 7200 μl/h) over 7 days (Fig. 2(b)).
C. Immunohistochemical evaluation of cell confluence and phenotype
After devices had achieved full endothelial flow, SMC phenotype and qualitative cell density was evaluated by staining SMCs on the membrane with LIVE/DEAD stain (calcein AM, ethidium homodimer-1, Invitrogen, Carlsbad, CA). In other devices, cells were fixed with 4% paraformaldehyde (PFA) and stained for F-actin (Alexa Fluor 568 phalloidin, Invitrogen), alpha smooth muscle actin (αSMA) (M0851, Dako, Carpinteria, CA) with secondary antibody of Alexa Fluor 594 (Invitrogen) and calponin (M3556, Dako) with secondary antibody of Alexa Fluor 488 (Invitrogen) and DAPI (4′,6-diamidino-2-phenylindole) counterstain. EC coverage and alignment was evaluated by fixing cells on membrane with 4% paraformaldehyde (PFA) and staining for CD31 (clone TLD-3A12, Millipore) with Alexa Fluor 488 secondary antibody or von Willebrand Factor (vWF) (M0616, Dako) with Alexa Fluor 594 secondary antibody with DAPI counterstain.
D. Scanning electron microscopy (SEM) evaluation of cell co-culture
Cellular phenotype, confluency, and EC alignment in devices were further evaluated by scanning electron microscopy (SEM) analysis. Cells in devices were fixed with 4% PFA and dehydrated in a series of ethanol washes. The devices were carefully disassembled and the membranes with ECs and SMCs still attached were gold sputter coated and imaged.
E. Microsphere extravasation analysis and pilot DIVI compound testing
Once arterial shear stress was achieved, confluency of the cell layers was demonstrated by flowing 6 μm diameter fluorescent microspheres (Peak Flow Flow Cytometry Reference Beads, Invitrogen) through the device for two minutes. Bead extravasation into or through the SMC layer was visually determined with fluorescent microscopy and with Fluorescence-activated cell sorting (FACS) analysis of the fluid extracted from the SMC chamber. Devices without EC or SMC (n = 6) were compared to devices with visually intact EC and SMC (n = 8).
Pilot testing was performed to evaluate the devices with a drug known to cause DIVI (CI-1044, Pfizer Inc., Groton, CT). The objective was to reproduce in the model, the extravasation through the endothelium and into the SMC layer that occurs in DIVI. To prepare for device testing with CI-1044, the EC and SMC co-cultures were evaluated using serum free medium (Opti-MEM, Invitrogen) for 24 h and evaluated with CD31 staining of EC. Complete devices were matured with increasing flow rates until arterial flow rate was achieved. DIVI pilot test devices (n = 3) were perfused with Opti-MEM containing CI-1044 (50 μM) for 8 h at arterial flow rates prior to extravasation testing. Control devices (n = 2) were perfused for 8 h with Opti-MEM at arterial flow rates prior to extravasation testing. During extravasation testing, whole porcine blood (Lampire Biologics, Pipersville, PA) at 37 °C was made to flow through the device at levels approximating an arterial shear rate (15 dynes/cm2) for 30 min. Extravasation of porcine RBC (4–8 μM in diameter) from the endothelial channel into the smooth muscle chamber was evaluated in real time using light microscopy imaging. Statistical significance for microsphere extravasation was determined using Student's t-test. was considered statistically significant.
III. RESULTS AND DISCUSSION
A. Microfluidic vascular device development
A small diameter in vitro blood vessel model with flow was created recapitulating the normal spatial arrangement of ECs adjacent to SMCs. In this model, a porous polycarbonate membrane mimics the naturally porous internal elastic lamina and permits extravasation of RBCs after exposure to pharmaceutical compounds. The multilayer microfluidic device design achieved the design criteria and established a model of a 200 μm diameter blood vessel with 2 cm length of an endothelial luminal surface with a porous membrane opposite a smooth muscle chamber. Optical alignment features integrated into the molds facilitated layer alignment, which was performed under direct microscopy.
B. EC and SMC growth and evaluation
An iterative approach was required to optimize the culture environments for effective seeding of both ECs and SMCs. In a first iteration, the SMC chamber and EC channel were similar dimensions. However, the SMCs were unable to achieve confluence or an appropriate phenotype. When the volume of the SMC chamber was increased significantly to (>1 ml compared to 1.6 μl), SMCs thrived in the SMC chamber with normal growth patterns and a normal phenotype compared to standard in vitro culture conditions. Presumably, the small volume of perfusion media above the SMC cells was not well circulated and hampered the cell growth.
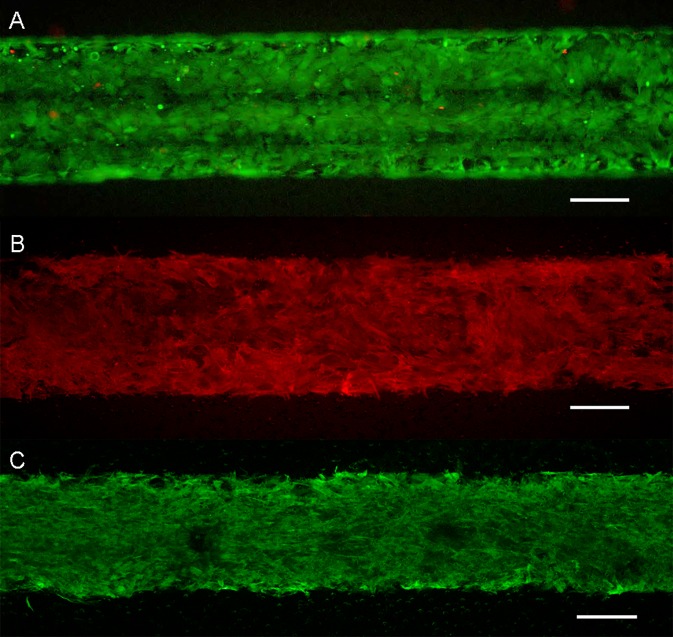
SMCs were reliably seeded and grown to confluence for three days prior to EC seeding. After the device was matured with arterial shear stress achieved in the endothelial channel, SMCs demonstrated viability with the LIVE/DEAD stain (Fig. 3(a)) and appropriate phenotype and confluency in the SMC chamber with F-actin (not shown), αSMA, and calponin (Fig. 3).
FIG. 3.
Evaluation of SMC phenotype in the device after full device maturation. (a) LIVE/DEAD (green/red). (b) αSMA expression (c) Calponin expression. Scale is 200 μm for the entire figure.
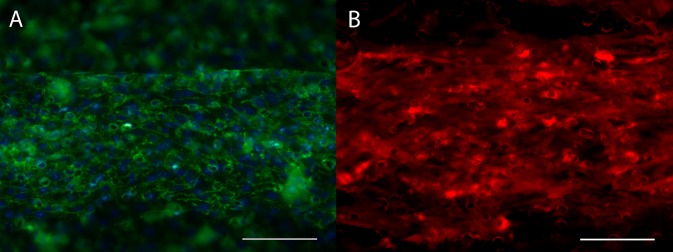
ECs sufficiently attached to the collagen coated polycarbonate membrane and tolerated low flow within 6 h of seeding. ECs grew to a confluent monolayer after approximately three days in culture with a modest increase in flow during that period. Over the remaining four days of device maturation, flow was increased to achieve low arterial shear stress rates of 15 dynes/cm2 within the endothelial channel (Fig. 2). During device validation testing, arterial shear stress of over 70 dynes/cm2 was tolerated without identifiable EC loss (data not shown). The ECs formed a monolayer that was evaluated after seven days of flow when arterial shear stress was achieved. There was considerable alignment of the ECs in the direction of flow as evidenced by CD31 staining, as shown in Fig. 4(a). The ECs also appropriately stained for vWF, Fig. 4(b).
FIG. 4.
ECs formed a monolayer on the membrane in the EC channel of the device. (a) CD31 staining (green) with DAPI counterstain (cyan) demonstrates a confluent coverage and evidence of cell alignment in the direction of flow (left to right). (b) von Willebrand expression in ECs. Scale is 200 μm for the entire figure.
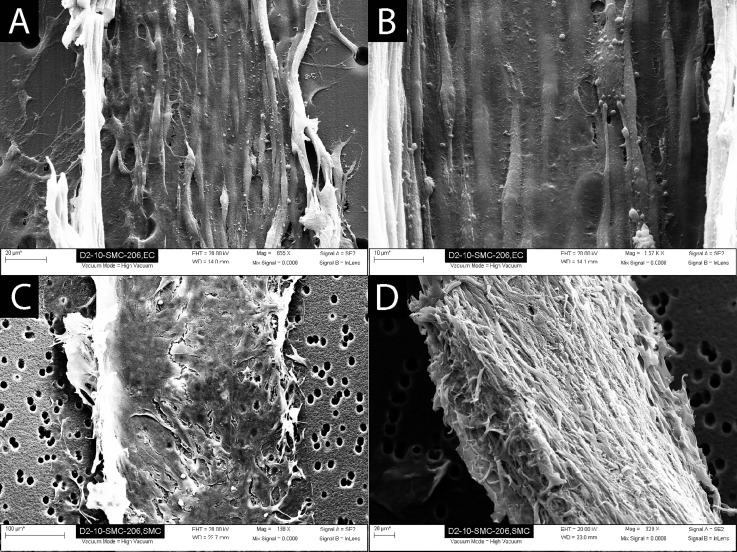
SEM analysis of the devices demonstrated confluent coverage of the membrane with ECs (Figs. 5(a) and 5(b)). ECs demonstrated a normal phenotype and a uniform cell alignment in the direction of flow. SMCs formed a multi-layer cell covering over the membrane in the SMC chamber side (Figs. 5(c) and 5(d)).
FIG. 5.
Scanning electron microscopy analysis of the devices demonstrated a confluent coverage of the endothelial channel side of the membrane with ECs, and confluent coverage of the SMC channel side of the membrane with SMCs. (a) EC layer (edges of cell layer peeled up during SEM sample preparation). (b) Higher magnification of EC layer demonstrating uniform EC coverage and alignment. (c) SMCs attached to the porous membrane. (d) SMCs removed from the membrane to demonstrate thickness of the cell layer (∼30 μm) and cell morphology.
C. Microsphere extravasation analysis and pilot DIVI compound testing
Microsphere flow analysis demonstrated a minor extravasation of microspheres in devices with visually confluent cellular layers without mechanical defects. Cellularized devices had an average extravasation of 6.6 beads over 2 min (range 0 to 20, stdev 8.3) which was significantly less (p < 0.001) than devices without cells that had an average extravasation of 84.7 beads (range 54 to 133, stdev 26.5). Microspheres in this diameter range may be easily procured and deployed from off the shelf sources. They are a useful pre-qualification of the device to test for cell confluency prior to further testing for DIVI or other studies which may involve expensive drugs or cellular preparations.
Although microspheres were used to evaluate devices for confluency, it was expected that whole blood would be required for the testing of potential DIVI compounds. One of the poorly understood aspects of DIVI, with some compounds, is the extravasation of RBCs through the endothelium during exposure of an animal to a DIVI compound but with no apparent damage to the endothelium during later histological evaluation of the vessels.3 We hypothesized that there is either a temporary separation of the ECs or an increase in permeability of the ECs such that the pores in the IEL are exposed, allowing extravasation of RBCs into the tunica media that has been demonstrated.4 Accordingly, it is expected that the deformability of RBCs is important in their extravasation, and thus it is critical to use whole blood for the in vitro evaluation of DIVI compounds using this model.
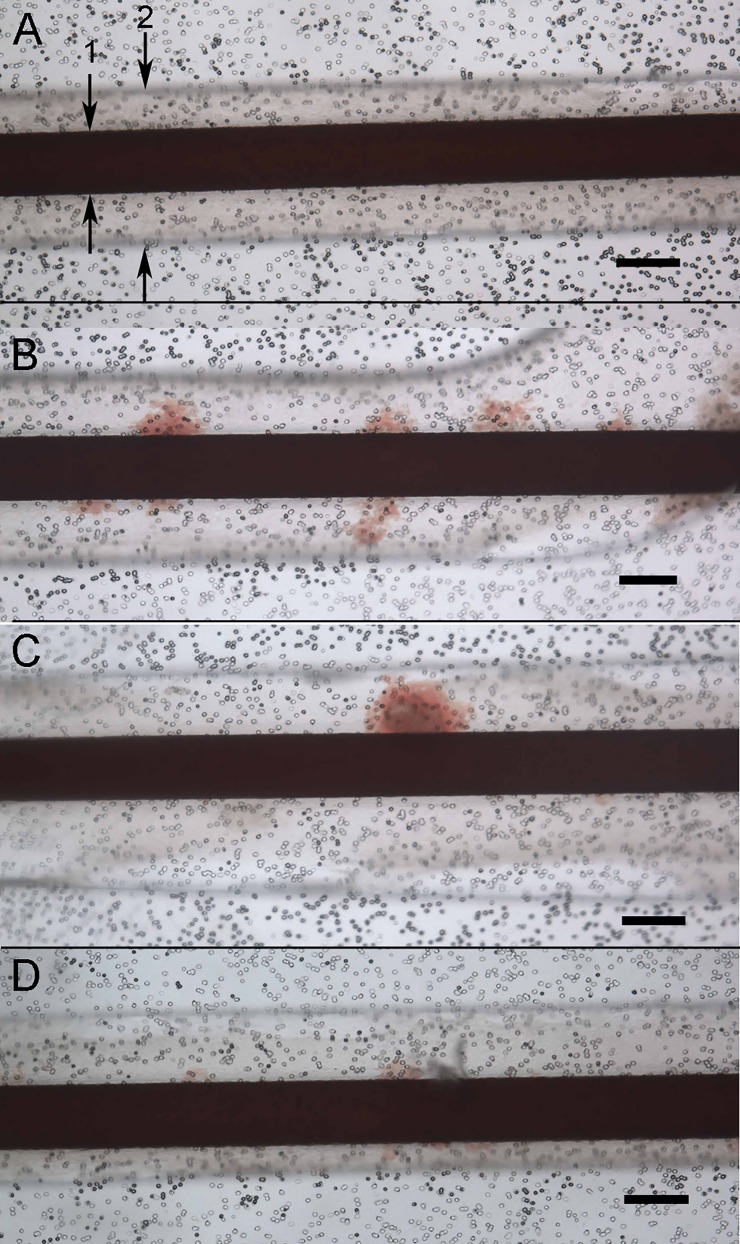
A pilot test of a candidate drug (CI-1044) known to cause DIVI12–15 was performed. In the two control devices, whole blood flowed through the devices at arterial shear without any visual extravasation of RBCs, as shown in Fig. 6(a). In all (n = 3) devices treated with 50 μM CI-1044 for 8 h (known toxic window of treatment 2–24 h), subsequent perfusion of whole blood at arterial flow rates caused extravasation of RBCs. RBC extravasation after CI-1044 treatment may have been due to deleterious effects of the compound on the endothelial junction, or other EC stress response, such as vascular oxidant stress and inflammation as reported in the previous in vivo studies of CI-1044.13 Future studies will include immunohistochemical analysis and permeability assays to further evaluate mechanisms underlying RBC extravasation in DIVI.
FIG. 6.
Whole blood flowed through the devices at arterial shear stress (15 dynes/cm2) in control device and pilot testing with known DIVI compound CI-1044. (a) Control device with blood flow in endothelial channel (1 noted with arrows) with overlying SMC chamber visible (2) demonstrating no extravasation of RBCs. (b) CI-1044 test device 1 demonstrating areas of extravasation of RBC into SMC chamber. (c) CI-1044 test device 2 demonstrating areas of extravasation of RBC into SMC chamber. (d) CI-1044 test device 3 demonstrating areas of extravasation of RBCs into SMC chamber. Scale bars for the entire figure are 200 μm. Black dots are the membrane pores.
The extravasation of the RBCs was only evaluated qualitatively as present or absent in the devices. Patchy occurrence of the extravasation (not uniform along the length of the model) is consistent with DIVI lesions seen during in vivo testing.4 Importantly, no free extravasation of RBCs was visually observed into the perfusion media of the smooth muscle chamber above the cells in either the control or DIVI devices. In the DIVI devices, the RBCs appeared to be present within the SMC layer.
Future testing of RBC extravasation will include RBC count analysis of the cell culture medium above the SMCs and SEM evaluation of the SMC cells to demonstrate the presence of the RBCs lodged within the SMCs as demonstrated in animal models.4 Another potential quantitative method could include flushing the model with isotonic saline to allow quantification of the percent area of extravasation into the SMC cell layer.
IV. CONCLUSIONS AND FUTURE WORK
Drug-induced vascular injury has been elusive in that although inflammatory biomarkers have some correlation with DIVI, no vascular-specific biomarkers have been identified that reproducibly correlate with the formation of DIVI despite an exhaustive investigation of molecular3,7,8,13,16 and imaging markers.15 This model is the first to incorporate the co-culture of ECs and SMCs with flow and a porous internal elastic lamina to evaluate drugs for DIVI. Further validation of this model to predict DIVI will be pursued with an array of known DIVI compounds and appropriate controls. In addition to RBC extravasation, the model can be investigated for the occurrence of biomarkers associated with extravasation. This may support some candidate biomarkers that have been identified or identify new potential biomarkers.
Rat aortic ECs were used in the model despite the occurrence of DIVI in specific vasculatures including rat mesentery and dog coronary arteries. Despite our experience and expertise with primary cell isolation and culture, we were unsuccessful in our attempt to reliably isolate rat mesentery ECs. While our preliminary results suggest that extravasation is observed with aortic ECs, the sensitivity and specificity of these cells to DIVI causing agents remain to be determined.
This vascular model does have some limitations in its current form. Flow through the device is generated by a syringe pump and consequently is laminar and non-pulsatile. Although this is ideal for many applications, it does not fully reproduce the vascular pulsatile flow that may contribute to DIVI. Hemodynamic changes have been described with DIVI in the rat15 and the dog model. Tachycardia and systemic hypotension have occurred after doses with DIVI compounds6,7,17 in beagle dogs. These hemodynamic changes may play a role in the development of DIVI lesions. Future iterations of the model will include both pulsatile flow as well as precise hemodynamic control to be able to separate the hemodynamic changes from other variables as DIVI is further investigated. Additionally, the SMCs in the model do not undergo mechanical stresses as they do in resistance vessels and subsequently it is expected that they have a primarily synthetic rather than contractile phenotype. Design changes that would incorporate a distensible membrane into the model as a means to transmit mechanical forces to the SMCs to retain a contractile phenotype are another future aim.
Several other in vitro models of EC and SMC co-culture have been described including static direct culture of ECs on SMCs18 and ECs and SMCs grown on opposite sides of a microporous membrane in a transwell model.19,20 In vitro models have also studied ECs in a shear model using a parallel plate design.21,22 Other recent publications have described the use of a porous membrane with different cell types grown on each side to reproduce some of the features of human lung and gut tissue.23,24 An innovative co-culture model utilizing a combination of parallel plates with flow and a transwell with EC and SMC co-culture has been developed.25 Rather than a vascular channel, this model has a wide flow field that is well suited for molecular biological analysis given the larger cell numbers but less ideal for investigating hemodynamic changes within the model and invoking a possible extravasation of RBCs.
Although it was designed to study DIVI, the model described in this paper has wide potential applications for studying vascular pathophysiology. In particular, the complex relationship and signaling between ECs and SMCs could be investigated. The ability to control shear stress in a channel design and dose additional cells and compounds in the vascular channel may serve as an excellent test bed for cancer metastasis cell adhesion and migration through a vessel wall. Fibroblasts could be added on top of the SMC cells to create a three-layer vascular model with new options for studying cell-cell interactions. Finally, the model could be used as an endothelial blood-brain barrier model without the SMCs. The ability to sample the upper chamber for compounds could be valuable in determining the selective compound permeability of this endothelium.
Utilizing a microfluidic platform, a co-culture model of a 200 μm blood vessel was developed with a biomimetic internal elastic lamina that had confluent alignment of ECs at arterial flow rates. Testing with a known DIVI compound demonstrated extravasation of RBCs into the SMC layer, a finding associated in vivo with some compounds that cause DIVI. Additional endpoints that focus on EC and SMC health and function will need to be evaluated to determine if the model can detect other features of DIVI, including degenerative and hyperplastic changes of ECs and SMCs. With further development, this model may have utility as an in vitro screening test of candidate drug compounds for DIVI. The physiologic nature of the model also gives it great flexibility for a variety of vascular related research applications.
ACKNOWLEDGMENTS
This study was supported by a generous research gift from Pfizer, Inc. B.F. is an employee of Pfizer. The authors gratefully acknowledge the critical review provided by Mike Lawton and Bradley Enerson from Pfizer, Inc. The authors also acknowledge the support from the NIH (F32 DK076349-01, DMH).
References
- 1. Kerns W. D., Arena E., and Morgan D. G., Am. J. Pathol. , 339 (1989). [PMC free article] [PubMed] [Google Scholar]
- 2. Louden C. and Morgan D. G., Pharmacol. Toxicol. , 158 (2001). 10.1111/j.0901-9928.2001.890404.x [DOI] [PubMed] [Google Scholar]
- 3. Mikaelian I., Cameron M., Dalmas D. A., Enerson B. E., Gonzalez R. J., Guionaud S., Hoffmann P. K., King N. M., Lawton M. P., Scicchitano M. S., Smith H. W., Thomas R. A., Weaver J. L., and Zabka T. S., Toxicol. Pathol. , 635 (2014). 10.1177/0192623314525686 [DOI] [PubMed] [Google Scholar]
- 4. Joseph E. C., Toxicol. Lett. , 537 (2000). 10.1016/S0378-4274(99)00221-0 [DOI] [PubMed] [Google Scholar]
- 5. Mecklenburg L., Heuser A., Juengling T., Kohler M., Foell R., Ockert D., Tuch K., and Bode G., Toxicol. Lett. , 54 (2006). 10.1016/j.toxlet.2005.09.037 [DOI] [PubMed] [Google Scholar]
- 6. Clemo F. A., Evering W. E., Snyder P. W., and Albassam M. A., Toxicol. Pathol. , 25 (2003). 10.1080/01926230390174904 [DOI] [PubMed] [Google Scholar]
- 7. Louden C., Brott D., Katein A., Kelly T., Gould S., Jones H., Betton G., Valetin J. P., and Richardson R. J., Toxicol. Pathol. , 19 (2006). 10.1080/01926230500512076 [DOI] [PubMed] [Google Scholar]
- 8. Kerns W., Schwartz L., Blanchard K., Burchiel S., Essayan D., Fung E., Johnson R., Lawton M., Louden C., MacGregor J., Miller F., Nagarkatti P., Robertson D., Snyder P., Thomas H., Wagner B., Ward A., and Zhang J., Toxicol. Appl. Pharmacol. , 62 (2005). 10.1016/j.taap.2004.08.001 [DOI] [PubMed] [Google Scholar]
- 9. Gonzalez J. M., Briones A. M., Somoza B., Daly C. J., Vila E., Starcher B., McGrath J. C., Gonzalez M. C., and Arribas S. M., Am. J. Physiol. Heart Circ. Physiol. , H804 (2006). 10.1152/ajpheart.01262.2005 [DOI] [PubMed] [Google Scholar]
- 10. Megens R. T., Reitsma S., Schiffers P. H., Hilgers R. H., De Mey J. G., Slaaf D. W., oude Egbrink M. G., and van Zandvoort M. A., J. Vasc. Res. , 87 (2007). 10.1159/000098259 [DOI] [PubMed] [Google Scholar]
- 11. Malek A. M., Alper S. L., and Izumo S., JAMA , 2035 (1999). 10.1001/jama.282.21.2035 [DOI] [PubMed] [Google Scholar]
- 12. Dagues N., Pawlowski V., Guigon G., Ledieu D., Sobry C., Hanton G., Freslon J. L., and Chevalier S., Toxicol. Appl. Pharmacol. , 52 (2007). 10.1016/j.taap.2006.10.018 [DOI] [PubMed] [Google Scholar]
- 13. Dagues N., Pawlowski V., Sobry C., Hanton G., Borde F., Soler S., Freslon J. L., and Chevalier S., Toxicol. Sci. , 238 (2007). 10.1093/toxsci/kfm161 [DOI] [PubMed] [Google Scholar]
- 14. Hanton G., Sobry C., Dagues N., Provost J. P., Le Net J. L., Comby P., and Chevalier S., Toxicol. Lett. , 15 (2008). 10.1016/j.toxlet.2008.03.009 [DOI] [PubMed] [Google Scholar]
- 15. Swanson T. A., Conte T., Deeley B., Portugal S., Kreeger J. M., Obert L. A., Joseph E. C., Wisialowski T. A., Sokolowski S. A., Rief C., Nugent P., Lawton M. P., and Enerson B. E., Toxicol. Pathol. , 784–791 (2014). 10.1177/0192623314525687 [DOI] [PubMed] [Google Scholar]
- 16. Brott D., Gould S., Jones H., Schofield J., Prior H., Valentin J. P., Bjurstrom S., Kenne K., Schuppe-Koistinen I., Katein A., Foster-Brown L., Betton G., Richardson R., Evans G., and Louden C., Toxicol. Appl. Pharmacol. , 441 (2005). 10.1016/j.taap.2005.04.028 [DOI] [PubMed] [Google Scholar]
- 17. Enerson B. E., Lin A., Lu B., Zhao H., Lawton M. P., and Floyd E., Toxicol. Pathol. , 27 (2006). 10.1080/01926230500512068 [DOI] [PubMed] [Google Scholar]
- 18. Wallace C. S. and Truskey G. A., Am. J. Physiol. Heart Circ. Physiol. , H338 (2010). 10.1152/ajpheart.01029.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang H. Q., Bai L., Shen B. R., Yan Z. Q., and Jiang Z. L., Eur. J. Cell Biol. , 51 (2007). 10.1016/j.ejcb.2006.09.001 [DOI] [PubMed] [Google Scholar]
- 20. Rose S. L. and Babensee J. E., Ann. Biomed. Eng. , 1382 (2007). 10.1007/s10439-007-9311-0 [DOI] [PubMed] [Google Scholar]
- 21. Daculsi R., Grellier M., Remy M., Bareille R., Pierron D., Fernandez P., and Bordenave L., J. Biomech. , 2781 (2008). 10.1016/j.jbiomech.2008.06.003 [DOI] [PubMed] [Google Scholar]
- 22. Brown M. A., Wallace C. S., Angelos M., and Truskey G. A., Tissue Eng. Part A , 3575 (2009). 10.1089/ten.tea.2008.0444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huh D., Matthews B. D., Mammoto A., Montoya-Zavala M., Hsin H. Y., and Ingber D. E., Science , 1662 (2010). 10.1126/science.1188302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim H. J., Huh D., Hamilton G., and Ingber D. E., Lab Chip , 2165 (2012). 10.1039/c2lc40074j [DOI] [PubMed] [Google Scholar]
- 25. Wang Y. H., Yan Z. Q., Qi Y. X., Cheng B. B., Wang X. D., Zhao D., Shen B. R., and Jiang Z. L., Ann. Biomed. Eng. , 729 (2010). 10.1007/s10439-009-9896-6 [DOI] [PubMed] [Google Scholar]