Abstract
The detection of a brief, sinusoidal probe in a long broadband, simultaneous masker improves as the probe is delayed from the masker's onset. This improvement (“overshoot”) may be mediated by a reduction in cochlear amplifier gain over the timecourse of the masker via the medial olivocochlear (MOC) reflex. Overshoot was measured in younger adults with normal hearing and in older adults with normal and impaired hearing to test the hypothesis that aging and cochlear hearing loss result in abnormal overshoot, consistent with changes in certain structures along the MOC pathway. Overshoot decreased with increasing quiet probe thresholds and was only minimally influenced by increasing age. Marked individual differences in overshoot were observed due to differences in masking thresholds for probes presented near the masker's onset. Model simulations support the interpretation that reduced overshoot in hearing-impaired listeners is due to limited cochlear amplifier gain and therefore less gain to adjust over the timecourse of the masker. Similar overshoot among younger and older adults with normal hearing suggests that age-related changes to mechanisms underlying overshoot do not result in significant differences in overshoot among younger and older adults with normal hearing.
I. INTRODUCTION
Detection of a short sinusoidal probe in a long simultaneous masker improves as the probe's onset is delayed by several tens of milliseconds from the onset of the masker (Zwicker, 1965). This improvement, known as “overshoot,” can be as large as 20 dB, depending on stimulus settings (Bacon and Smith, 1991) and the hearing status of the listener (Bacon and Takahashi, 1992). For example, overshoot is reduced in listeners with cochlear hearing loss (Bacon and Takahashi, 1992; Strickland and Krishnan, 2005) and in normal-hearing (NH) listeners who acquire a temporary cochlear hearing loss from aspirin ingestion (McFadden and Champlin, 1990) or intense noise exposure (Champlin and McFadden, 1989). The reduction in overshoot with permanent or temporary cochlear hearing loss suggests that overshoot is related to the status of the cochlear amplifier (von Klitzing and Kohlrausch, 1994).
For NH listeners, masking thresholds are poorer when the probe is presented near the onset of a long masker rather than tens of milliseconds later. In other words, the signal-to-noise ratio (SNR) at threshold, or critical ratio, is larger for probes near the masker's onset than for probes near the masker's center. von Klitzing and Kohlrausch (1994) demonstrated that poorer thresholds for NH listeners for probes presented at the masker's onset could be explained by the compressive response of the basilar membrane (BM). In their account, the difference in post-cochlear output to the probe+masker and masker alone (i.e., “post-cochlear SNR”) is reduced by compression, resulting in a relatively large critical ratio. They further suggested that the improvement in threshold for NH listeners as the probe is delayed from the onset of the masker could be explained by a reduction in cochlear amplifier gain over the timecourse of the masker. Individuals with cochlear hearing loss have better-than-normal detection thresholds for the probe presented near the masker's onset. These better thresholds, resulting in less overshoot, may be explained by hearing-impaired (HI) listeners having less cochlear amplifier gain than NH listeners and, therefore, less gain to reduce over the timecourse of the masker (Strickland and Krishnan, 2005).
A quantitative framework for explaining overshoot in terms of BM compression and a reduction in cochlear amplifier gain (“gain reduction framework”) was developed by Strickland (2001). In this framework, (1) the probe is detected at a constant post-cochlear SNR through an auditory filter centered on the probe frequency (Fletcher, 1940; Stephens, 1973; Oxenham et al., 1997; Strickland, 2001), (2) the post-cochlear output of the probe+masker and broadband masker alone is determined by the cochlear input/output (IO) function, where growth is linear at low stimulus levels and compressive at mid-to-high stimulus levels (Robles and Ruggero, 2001), and (3) cochlear gain decreases over the timecourse of the masker resulting in reduced compression of part of the mid-level compressive region of the IO function (Cooper and Guinan, 2006), thus improving post-cochlear SNR and critical ratios (for details see Strickland, 2001, 2004, 2008). In addition to providing a quantitative description of overshoot, the gain reduction framework accounts for the dependence of overshoot on several stimulus parameters such as masker level, masker spectrum, and probe frequency (Strickland, 2001, 2004), and the reduction in overshoot with cochlear hearing loss (Strickland and Krishnan, 2005; Jennings et al., 2011). Specifically, the framework predicts overshoot under conditions in which a reduction in gain over the timecourse of the masker results in improved post-cochlear SNRs. Such improvements are not expected at low levels where response growth is linear and gain is applied equally to masker and probe, or at very high levels where gain is minimal.
Several peripheral (Smith and Zwislocki, 1975; Schmidt and Zwicker, 1991; von Klitzing and Kohlrausch, 1994; Strickland, 2001) and central (Carlyon and White, 1992; Fletcher et al., 2015) mechanisms have been proposed to explain overshoot. Of these mechanisms, the medial olivocochlear (MOC) reflex is most consistent with a reduction in cochlear amplifier gain, as suggested by psychophysical studies (Schmidt and Zwicker, 1991; Turner and Doherty, 1997; Bacon and Healy, 2000; Strickland, 2001, 2004, 2008; Jennings et al., 2011). The MOC reflex is known to reduce cochlear amplifier gain in laboratory animals (Cooper and Guinan, 2006) and follows a timecourse similar to the timecourse of overshoot (Bacon and Healy, 2000; Backus and Guinan, 2006; McFadden et al., 2010). Support for the role of the MOC reflex in overshoot in human subjects comes from behavioral studies demonstrating that overshoot is: (1) reduced by contralateral stimulation in some subjects (Turner and Doherty, 1997; Bacon and Liu, 2000), (2) reduced in patients for whom the MOC bundle was cut during surgery (Zeng et al., 2000), and (3) very small (∼2 dB) in adults with cochlear implants (Zeng et al., 2005). The finding that overshoot is reduced in listeners with cochlear hearing loss (Bacon and Takahashi, 1992; Strickland and Krishnan, 2005) is consistent with less available cochlear amplifier gain for the MOC reflex to adjust over the timecourse of the masker. Despite these findings, the association between the MOC reflex and overshoot has yet to be definitively confirmed by otoacoustic emission (OAE)-based studies (Keefe et al., 2009; Walsh et al., 2010), as these have failed to consistently relate overshoot to OAE responses sensitive to MOC activity. Moreover, computational modeling studies suggest that overshoot may be mediated by several mechanisms that influence the dynamic range of the auditory system including the MOC reflex (Jennings et al., 2011).
The pathway of the MOC reflex includes outer hair cells (OHCs), inner hair cells (IHCs), spiral ganglion neurons (SGNs), and brainstem efferent neurons (Guinan, 2006). Among other effects, the aging process results in decreased SGNs (Schuknecht, 1974) and a reduction in the endocochlear potential (EP) (Schmiedt et al., 2002), which may disrupt the normal processing of the MOC reflex pathway and explain reduced contralateral suppression of OAEs in older adults (Kim et al., 2002). Specifically, age-related changes may decrease the input to brainstem MOC neurons and potentially weaken the ability of the MOC reflex to reduce cochlear amplifier gain. Thus, overshoot is expected to be reduced in older adults with NH due to equivalent-to-normal thresholds for the probe near the masker's onset but poorer-than-normal thresholds for the probe near the center of the masker (due to a smaller decrease in cochlear amplifier gain over the timecourse of the masker). Alternatively, age-related loss of SGNs has been shown to diminish the onset response of a population of auditory nerve fibers in laboratory animals (Sergeyenko et al., 2013), which may lead to poorer-than-normal detection of short-duration signals in noise. In this case, overshoot is expected to be (1) unaffected by age if thresholds are elevated equally for probes presented at the onset and temporal center of the masker, or (2) larger than normal in older listeners if thresholds near the onset of the masker are affected more than those at the masker's temporal center. The hypothesized effects of age-related loss of SGNs also apply to older listeners with cochlear hearing loss; however, these effects may be outweighed by the more substantial effects of reduced cochlear amplifier function. Thus, the expectation of poorer-than-normal masking thresholds, which could be attributed to age-related reductions of SGNs, is inconsistent with thresholds for older HI adults being equal to or better than thresholds for NH adults (Bacon and Takahashi, 1992; Strickland and Krishnan, 2005). A primary goal of the current study was to compare overshoot for older adults with normal hearing (ONH) and older adults with impaired hearing (OHI), who are both expected to have age-related changes to SGNs, but who differ in terms of the added effects of age-related hearing loss on hair cell (Gates and Mills, 2005), metabolic (Schmiedt, 2010), and auditory nerve (Lang et al., 2010) function. To provide a comparison group, overshoot was also measured in younger normal-hearing (YNH) listeners.
As noted earlier, it is hypothesized that overshoot in OHI listeners will be reduced, consistent with an auditory system that adapts less to the local sound environment than the normal auditory system. A second goal of the current study was to quantify and compare the gain reduction required to predict overshoot in YNH, ONH, and OHI listeners. Computational model simulations were obtained for the mean data of YNH, ONH, and OHI subjects to estimate gain reduction and test the extent to which overshoot can be explained by a reduction in gain, similar to previous studies (Schmidt and Zwicker, 1991; von Klitzing and Kohlrausch, 1994; Strickland, 2001; Bacon and Savel, 2004). Overshoot is expected to be less than normal for OHI listeners; therefore, it is hypothesized that model-derived estimates of gain reduction will be smaller for OHI listeners than NH listeners.
II. MEASUREMENT OF OVERSHOOT
A. Methods
1. Subjects
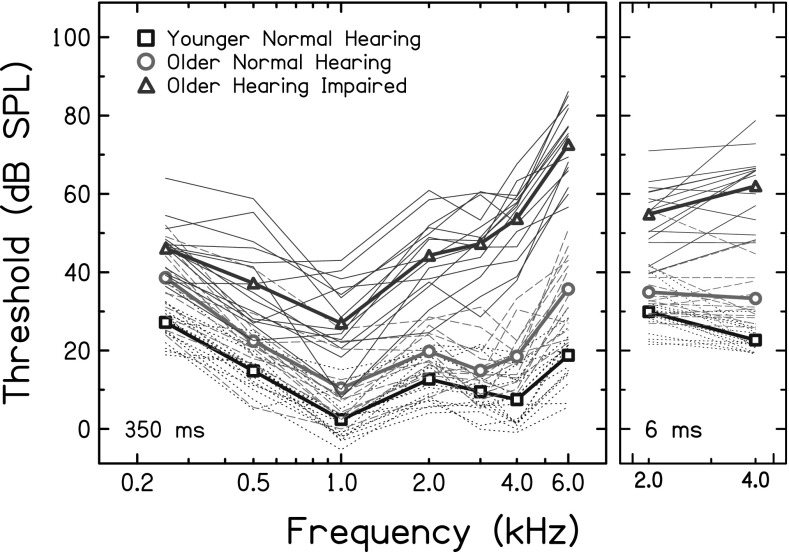
Forty-seven adults, ranging in age from 18 to 81 yrs, participated in this experiment. YNH listeners (n = 17, mean age = 23.3 yrs) were <30 yrs of age, had thresholds ≤ 25 dB hearing level at audiometric frequencies between 0.5 and 6 kHz and were tested at the University of Utah. ONH listeners (n = 15, mean age = 67.6 yrs) were >60 yrs of age with the same audiometric inclusion criteria as YNH listeners. OHI listeners (n = 15, mean age = 73.3 yrs) were >60 yrs of age and had mild-to-moderate sensorineural hearing loss. ONH and OHI listeners were tested at the Medical University of South Carolina (MUSC). The right ear was selected as the test ear if thresholds for both ears were similar; otherwise, the ear that had better average thresholds between 0.25 and 6 kHz was selected as the test ear. Thresholds for 350-ms pure tones at audiometric frequencies for the test ear are shown in Fig. 1 (left panel) for YNH (squares), ONH (circles), and OHI (triangles) groups, where thin and thick lines are individual and mean data, respectively.
FIG. 1.
(Color online) Detection thresholds for 350-ms (left panel) and 6-ms (right panel) pure tone signals. Mean thresholds are shown for the YNH group (squares), ONH group (circles), and OHI group (triangles). Thin lines represent thresholds for individual subjects in these three groups.
2. Overshoot stimuli and apparatus
Probes were 6-ms tone bursts at 2 or 4 kHz and were gated with 3-ms cos2 ramps. The probe's onset was delayed from the onset of a 400-ms broadband (0.1–8 kHz) masker by 2 or 198 ms. The masker was also gated with 3-ms cos2 ramps. Probe level ranged from 50 to 90 dB sound pressure level (SPL) in 10 dB steps for NH listeners (YNH and ONH). For HI listeners, probe levels ranged from 60 to 100 dB SPL, in 10 dB steps, except for conditions in which probe levels were near or below subjects' absolute thresholds. Thresholds for the 6-ms probes are displayed in Fig. 1 (right panel) for the three groups, where thin and thick lines are individual and mean data, respectively.
Stimuli were digitally generated using custom Matlab (The MathWorks, Natick, MA) software (Bidelman et al., 2015) and output through a LynxTWO-B (Lynx Studio Technology, Costa Mesa, CA) sound card (sampling rate, 44.1 kHz; 24-bit resolution) to the right earphone of EARTONE-5 A (3M, Minneapolis, MN) insert phones, which were driven by a headphone buffer (Tucker-Davis-Technologies [TDT] (Tucker-Davis Technologies, Alachua, FL), HB7). Identical equipment was used at both testing sites, with the exception of the model of the headphone buffer (a TDT HB5 was used at MUSC). Pilot testing (not shown) on all overshoot conditions was conducted on three YNH subjects at each data collection site (six listeners total) and confirmed that masking thresholds did not differ significantly between sites [F(1,4) = 0.93, p = 0.39]. Data from this pilot experiment were not included in the final data set.
3. Overshoot procedures
A three-interval, three-alternative forced-choice task, with a 2-up, 1-down adaptive rule, was used to measure the masker level needed to achieve 70.7% correct detection of the fixed-level probe (Levitt, 1971). The step size of the adaptive track was 5 dB for the first two reversals and 2 dB for the remaining ten reversals. Threshold for a given track was defined as the average masker level of the final eight reversals. A familiarization and training period preceded data collection. During familiarization, thresholds were measured for conditions where the probe was delayed by 198 ms from the masker's onset. These thresholds were measured at two probe levels (60 and 90 dB SPL) and for both probe frequencies (2 and 4 kHz). Results from the familiarization task were discarded. During training, three consecutive thresholds were measured on a representative sample of conditions. This sample included both probe frequencies and probe delays and three probe levels. If the standard deviation of the three thresholds obtained during training exceeded 5 dB for a given condition an additional three thresholds were obtained. This occurred a total of seven times in seven different subjects (∼1% of all training measurements). Thresholds measured during training were discarded. During data collection, the first threshold estimate of a given session was treated as a “warm-up” and was discarded. Conditions were blocked by probe frequency and probe delay and randomized by probe level. The order of probe frequency and probe delay blocks were randomly selected for each listener. Three threshold estimates were obtained for each condition and these estimates were averaged to yield the final threshold for the condition. If the standard deviation of the three estimates was greater than 5 dB, a fourth estimate was obtained and the average of all four thresholds estimates was taken as the final threshold. A fourth estimate was measured a total of six times in five different subjects (<1% of measured experimental conditions).
4. DPOAE stimuli, apparatus, and procedures
Distortion product otoacoustic emissions (DPOAEs), with primary frequencies (f1, f2) and levels (L1, L2), were measured for the 2f1–f2 distortion product. f2 ranged from 1.0 to 6.727 kHz (f2/f1 = 1.22), with a resolution of four points per octave, and L1 and L2 were fixed at 65 and 55 dB SPL, respectively. From these values DPOAE “sum” was computed by adding DPOAE levels (exceeding the noise floor by 3 dB) across frequency bands. DPOAEs were collected at the University of Utah using a Biologic Scout® system (Natus Medical Incorporated, Pleasanton, CA). At MUSC, DPOAEs were obtained using an Etymotic ER-10 C® (Etymotic Research Inc., Elk Grove, IL) microphone and custom-designed software [EMAV, Neely and Liu (1994)]. DPOAEs were collected from S.G.J. at both data collection sites revealing a standard error of measurement of 1.7 dB when averaged across distortion product frequencies, which is within the expected test/retest reliability of DPOAEs (Franklin et al., 1992). At both sites, normal middle ear status was confirmed by otoscopic examination and tympanometry (226-Hz probe tone).
B. Results and discussion
1. Detection thresholds for probes in quiet and overall DPOAE response levels
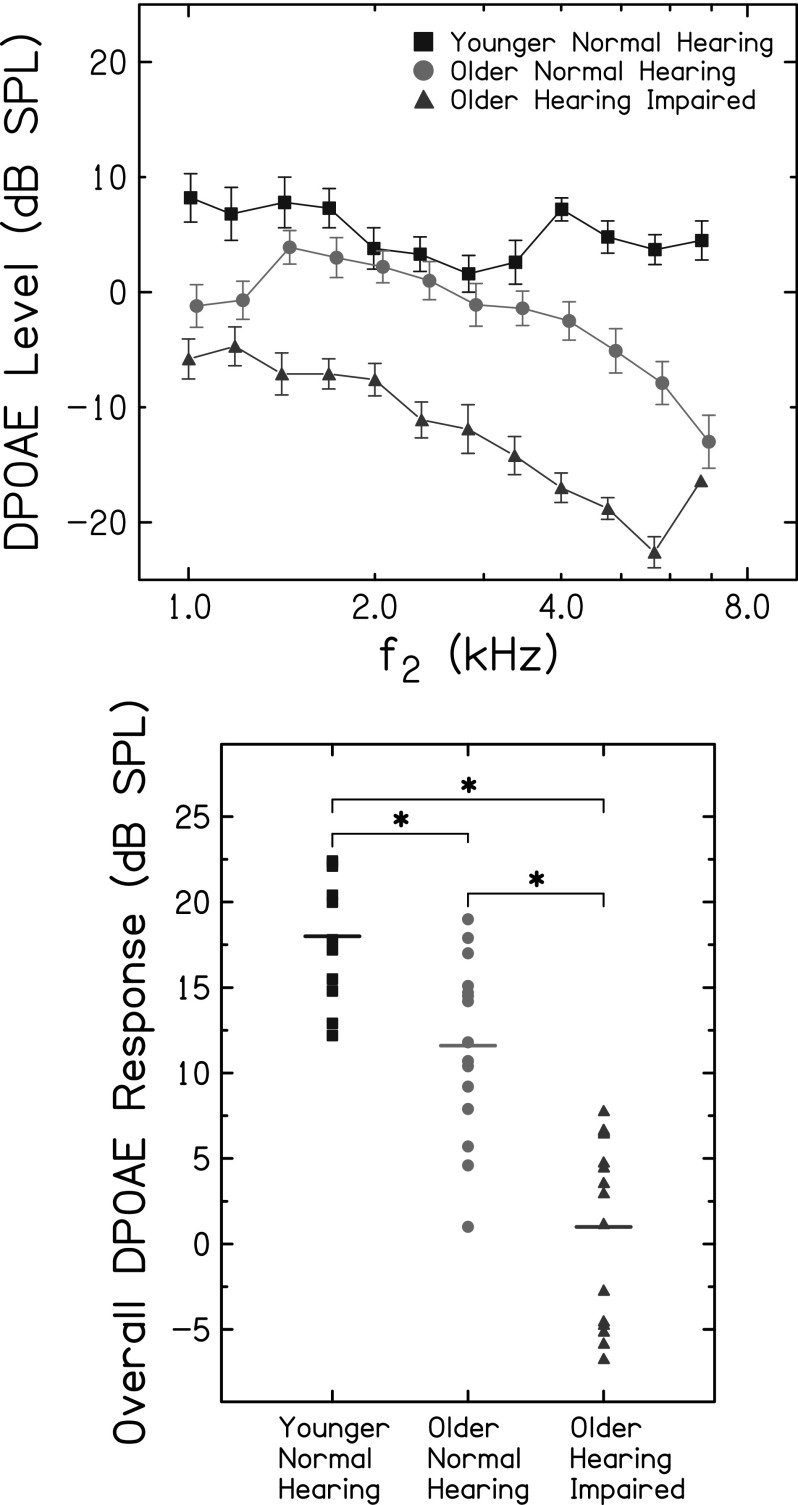
Detection thresholds for the 2- and 4-kHz probes were significantly higher for OHI listeners than for YNH (t = −12.63, p < 0.001) and ONH listeners (t = −8.44, p < 0.001; Bonferroni corrected here and throughout; unless otherwise noted, comparisons not mentioned were not statistically significant). Thresholds for the 4-kHz probe tone were significantly higher for ONH than YNH listeners (t = −5.84, p < 0.001; Fig. 1, right panel). DPOAE levels were greatest for YNH, lowest for OHI, and intermediate for ONH adults across all f2 frequencies (Fig. 2, top panel). Consistent with this finding, YNH listeners had significantly greater overall DPOAE responses than ONH (t = 3.5, p = 0.002) and OHI (t = 9.02, p < 0.001) listeners, and ONH listeners had a significantly greater overall DPOAE response than OHI listeners (t = 5.5, p < 0.001; Fig. 2, bottom panel). When pure tone average (0.5, 1.0, 2.0 kHz) was included as a covariate, overall DPOAE response was not significantly different between groups, for all pairwise comparisons. This suggests that thresholds, rather than age, were the primary factor explaining differences in overall DPOAE response between YNH and ONH groups.
FIG. 2.
(Color online) DPOAE measurements. Top panel: mean DPOAE level as a function of f2, in the YNH group (squares), OHN group (circles), and OHI group (triangles). Error bars are one standard error around the mean. Bottom panel: overall DPOAE response (see text) for each listener is plotted for YNH, ONH, and OHI groups. Asterisks indicate significant differences between groups (p < 0.05).
2. Overshoot: General trends and effects of probe level
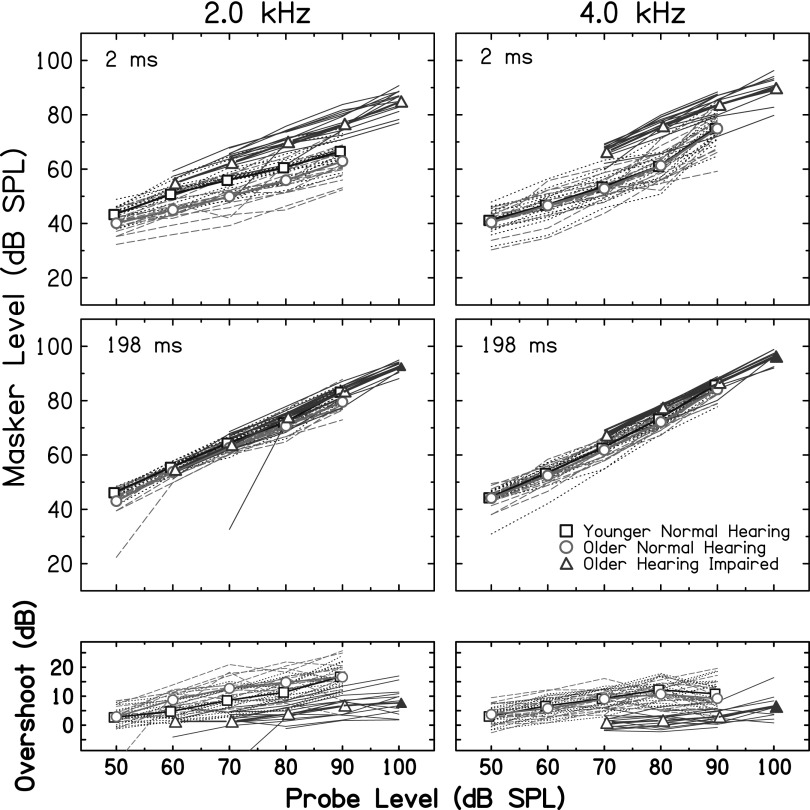
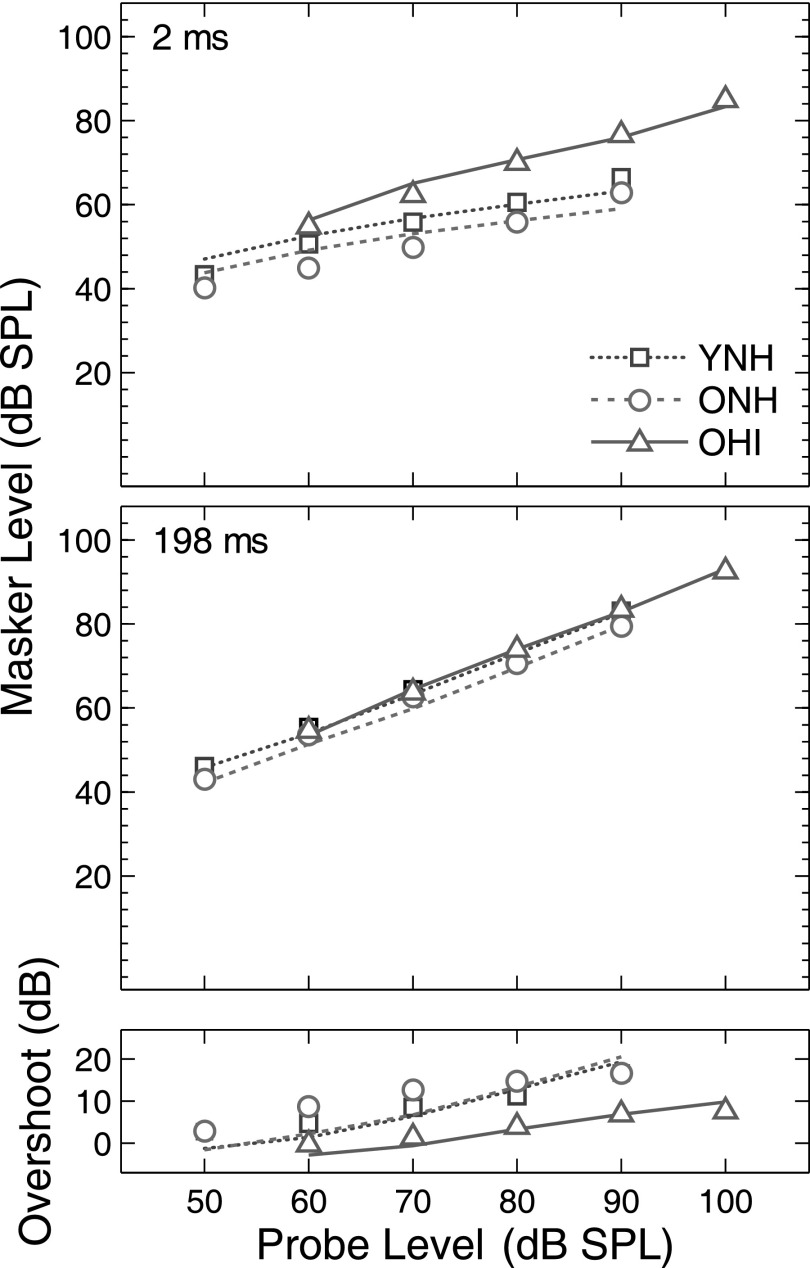
Masking thresholds for 2- and 198-ms delay conditions are plotted in the top and middle panels of Fig. 3, respectively. The bottom panels display overshoot, which was calculated by subtracting thresholds in the 2-ms delay condition from thresholds in the 198-ms delay condition. Masking thresholds in the 198-ms delay condition (Fig. 3, middle panels) are fairly homogeneous among listeners, whereas thresholds are more variable for probes presented near the masker's onset (2-ms delay condition, Fig. 3, top panels). These individual differences are evident regardless of the subject's hearing status. On average, OHI listeners had higher (better) masking thresholds in the 2-ms delay condition than YNH listeners, similar to previous studies (Bacon and Takahashi, 1992; Strickland and Krishnan, 2005).
FIG. 3.
(Color online) Masking thresholds and overshoot. Top and middle panels are masking thresholds for 2− and 198-ms probe delays, respectively. The bottom panels display overshoot, calculated by subtracting the 2-ms thresholds from the 198-ms thresholds. The left and right columns are data for the 2- and 4-kHz probes, respectively. The interpretation of lines is as in Fig. 1. The filled symbols at 100 dB SPL indicate that these thresholds were extrapolated for some OHI subjects due to thresholds being beyond the limits of the equipment.
A repeated-measures analysis of variance (ANOVA) with probe level (50, 60, 70, 80, 90 dB SPL) and probe frequency (2, 4 kHz) as repeated measures was performed on overshoot in YNH listeners. This analysis was restricted to YNH listeners to compare results with those from previous studies in NH listeners where effects of level and probe frequency on overshoot are most fully described. Overshoot varied significantly with probe level [F(1,16) = 90.78, p < 0.001] but not with probe frequency [F(1,16) = 0.37, p = 0.55]; a significant probe level*probe frequency interaction was also observed [F(1,16) = 11.32, p < 0.001].
The increase in overshoot from low to moderate probe levels has been well documented for NH listeners (Bacon, 1990; Strickland, 2001) and is predicted by the gain reduction framework described in Sec. I. A reduction in overshoot was observed at the highest probe level for the 4 kHz probe (Fig. 3; squares, right bottom panel), but not for the 2 kHz probe (Fig. 3; squares, left bottom panel), consistent with the significant probe level*probe frequency interaction. Several studies reported a decrease in overshoot at high probe levels for probe frequencies at and above 4 kHz (Bacon, 1990; Carlyon and White, 1992; Overson et al., 1996). Strickland (2004) modeled this decrease by assuming that the gain of the cochlear amplifier is minimal at high stimulus levels, thus limiting the amount of gain reduction applied to the probe+masker and masker alone. In contrast, overshoot measured with slightly lower probe frequencies (e.g., 2.5 kHz) has been shown to increase and then plateau as a function of probe level (Carlyon and White, 1992), consistent with the current results for the 2-kHz probe (i.e., overshoot did not decrease at high probe levels).
The decrease in overshoot at the highest probe level (90 dB SPL) with a 4-kHz probe is somewhat smaller than previous studies. This may be due to the lower masker levels in this study, or the tracking procedure which adapted on masker level rather than probe level. Indeed, consistent with the current study, Strickland (2004) measured thresholds by adapting on masker level and reported only a modest decrease in overshoot at high probe levels (or no decrease in some subjects) compared to studies that adapted on probe level (Bacon, 1990; Carlyon and White, 1992; Overson et al., 1996). Moreover, the masker level at which maximum overshoot is observed is highly variable among listeners. These individual differences have been reported as a source of discrepancy among studies that did or did not find a decrease in overshoot at high levels (see Overson et al., 1996). The persistence of appreciable overshoot at the highest levels of the 2-kHz probe may appear inconsistent with an MOC-mediated reduction in gain, given that cochlear amplifier gain decreases at mid-to-high levels (e.g., Ruggero et al., 1997). However, for the highest 2-kHz probe level (90 dB SPL), masker levels through an auditory filter were fairly low (∼50–65 dB SPL) for the 2-ms probe delay, suggesting that sufficient cochlear amplifier gain may have been applied to these maskers. If so, the ratio of the response to the probe+masker and the response to masker alone is expected to increase as a result of a reduction in cochlear gain, leading to appreciable overshoot. Alternatively, the large overshoot observed at high levels of the 2-kHz probe may be mediated by non-MOC mechanisms, such as the middle-ear reflex (Feeney and Keefe, 2001).1
3. Overshoot: Effects of hearing loss
To assess the effects of hearing loss, without the confound of age, overshoot for ONH and OHI listeners was submitted to a repeated-measures ANOVA with probe level (80, 90 dB SPL), and probe frequency (2, 4 kHz) as repeated measures, and group (ONH, OHI) as a between subjects factor. Probe levels were limited to 80 and 90 dB SPL, as these were the only levels at which all listeners had a complete data set. The main effects of group [F(1,28) = 78.10, p < 0.001], probe level [F(1,28) = 7.45, p = 0.011], and probe frequency [F(1,28) = 30.53, p < 0.001] were significant, as were the probe level*probe frequency [F(1,28) = 7.27, p = 0.012] and group*probe level [F(1,28) = 5.68, p = 0.024] interactions. The main effect of group stems from reduced or absent overshoot in OHI compared to ONH subjects, which is consistent with previous studies using broadband maskers (Bacon and Takahashi, 1992; Strickland and Krishnan, 2005). The probe level*probe frequency interaction originates from overshoot increasing with probe level (from 80 to 90 dB) for the 2-kHz probe, while remaining constant for the 4-kHz probe. The group*probe level interaction originates from overshoot increasing with probe level (from 80 to 90 dB SPL) in OHI listeners, while remaining relatively constant for ONH listeners, primarily for 4-kHz probes.
Maximum overshoot for a given listener was defined as the largest overshoot across probe level and was used to summarize overshoot at each probe frequency with a single value. Linear regression analyses on data from ONH and OHI listeners revealed that maximum overshoot diminishes as the absolute thresholds of the 2-kHz (r = −0.723, p < 0.001) and 4-kHz (r = −0.673, p < 0.001) probes increase, consistent with Strickland and Krishnan (2005). Slopes of these regression functions were −0.36 and −0.21 dB/dB, that is, maximum overshoot diminishes by 0.36 and 0.21 dB (on average) for each 1 dB increase in probe threshold for 2− and 4-kHz probes, respectively. Less overshoot in listeners with elevated probe thresholds is consistent with reduced cochlear amplifier gain leading to a more favorable post-cochlear SNR and improved thresholds for the probe presented near the masker's onset. If cochlear amplifier gain is already reduced due to cochlear hearing loss, the effect of any additional reduction in gain over the timecourse of the masker may be limited and result in relatively smaller improvements in threshold when the probe is near the temporal center of the masker (i.e., reduced overshoot).
4. Overshoot: Effects of age
To assess the effects of age without the confound of hearing loss, overshoot for ONH and YNH listeners was submitted to a repeated-measures ANOVA with probe level (50, 60, 70, 80, 90 dB SPL) and probe frequency (2, 4 kHz) as repeated measures, and group (YNH, ONH) as a between subjects factor. The effects of probe frequency [F(1,30)= 15.6, p < 0.001], probe level [F(4,27) = 113.31, p < 0.001], and the probe level*probe frequency interaction [F(4,27)= 5.42, p = 0.002] were significant, consistent with the previous analysis between ONH and OHI groups. Additionally, the group*probe frequency interaction was significant [F(1,30)= 9.44, p = 0.004], revealing significantly greater overshoot in ONH listeners than in YNH listeners with the 2-kHz probe, but not with the 4-kHz probe. Overshoot was hypothesized to be smaller in ONH listeners than YNH listeners due to the likelihood of reduced SGNs (Kujawa and Liberman, 2006) and EP (Schmiedt et al., 2002), which may result in less input to MOC neurons and consequently a smaller reduction in cochlear amplifier gain over the timecourse of the masker. Thus, thresholds for ONH subjects were expected to be poorer than normal for the probe near the center of the masker. Alternatively, overshoot was hypothesized to be greater in ONH than YNH listeners due to the likelihood of reduced SGNs, which may diminish the onset response of auditory nerve fibers. If this diminished response has the greatest influence on probes near the onset of the masker, poorer-than-normal thresholds are expected, although auditory nerve responses in aged animals to onsets and increments have not been well studied. Consistent with the latter hypothesis, overshoot in ONH listeners was greater than or equal to YNH listeners, because masking thresholds were poorer than normal when the 2-kHz probe was near the masker's onset.
The significantly greater overshoot in ONH than YNH listeners with the 2-kHz probe but not the 4-kHz probe is difficult to explain. Strickland and Krishnan (2005) found that overshoot was unexpectedly larger in some individuals with a threshold notch in the audiogram; however, there was no evidence of a notch in the audiograms of ONH listeners. Additional analysis revealed that the significant difference in overshoot measured with a 2-kHz probe was attributable to 4 ONH listeners with 3–5 dB greater overshoot than YNH and other ONH listeners. These four ONH listeners did not differ systematically from the rest of the group in any other measure including audiometric thresholds, probe thresholds, temporal integration, growth of masking slopes (discussed in Sec. II B 5), or overall DPOAE responses. Thus, differences in overshoot occurred only with the 2-kHz probe and were driven by results from 4 ONH listeners. It is possible that poorer detection efficiency (perhaps due to reduced SGNs) explains why thresholds were poorer near the onset of the masker than at the temporal center for these listeners compared to other ONH listeners. Skepticism for this interpretation comes from the observation that similar findings (greater overshoot in ONH than YNH) did not occur with the 4-kHz probe. Thus, this potential age-related difference should be explored further in future studies with larger samples of older adults.
5. Overshoot: Growth of masking analysis
Smaller overshoot in OHI than YNH and ONH listeners was due to improved detection of the probe near the masker's onset for OHI listeners, consistent with relatively less compressive BM response growth in OHI than NH listeners (Strickland and Krishnan, 2005). This explanation is supported by growth-of-masking (GOM) slopes, which reveal the relative growth rates of the responses to the probe+masker and masker alone (Nelson and Schroder, 1997). To estimate GOM slopes and intercepts, straight lines were fit to masking thresholds plotted as a function of probe level for each probe delay and probe frequency combination (i.e., 2- and 198-ms probe delays for 2- and 4-kHz probes). For YNH and ONH listeners, masking thresholds for the 4-kHz, 90 dB SPL probe in the 2-ms delay condition were excluded due to the sharp increase in masking thresholds relative to thresholds for the 4-kHz, 80 dB SPL probe (circles and squares in the top right panel of Fig. 3). In general, straight lines provided good fits to data for the 2-ms probe delay [root-mean-square (rms) error: median 1.07 dB; range 0.06–5.10 dB] and the 198-ms probe delay (rms error: median 0.65 dB; range <0.01–8.66 dB).
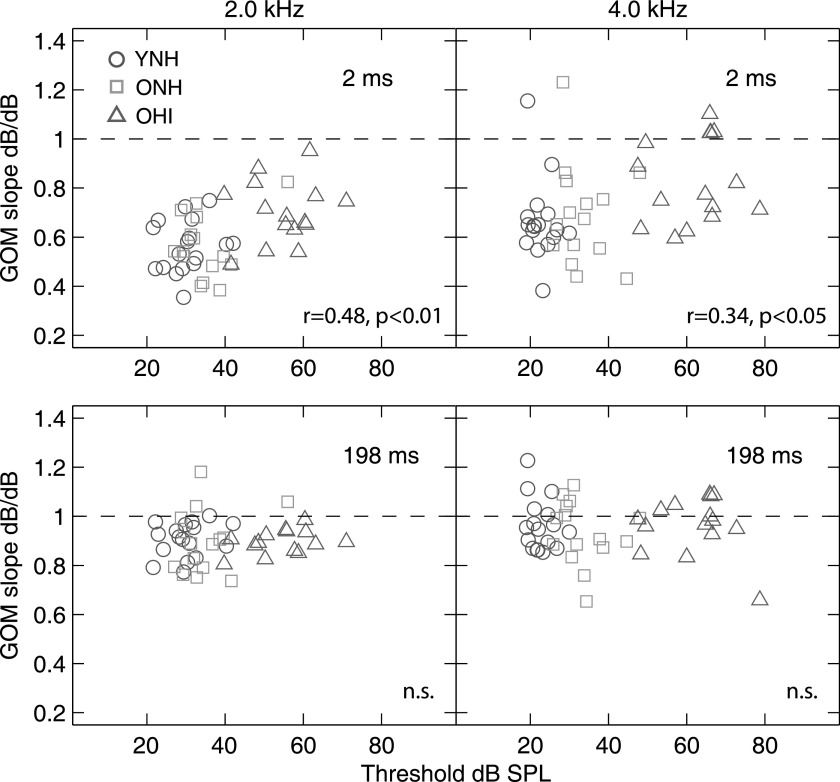
GOM slope as a function of detection thresholds for the 6-ms probe are displayed in Fig. 4. For the 2-ms probe delay, GOM slopes are nearly all <1 dB/dB, with a mean slope of 0.56 dB/dB for NH listeners (YNH, ONH), and 0.70 dB/dB for OHI listeners using a 2-kHz probe (Fig. 4, top left panel). Similarly, for the 4-kHz probe, mean GOM slopes were 0.68 dB/dB for NH listeners and 0.82 dB/dB for OHI listeners (Fig. 4, top right panel). The hypothesis that linear BM response growth led to better post-cochlear SNRs and masking thresholds for OHI listeners in the 2-ms probe delay condition is supported by significant correlations between GOM slopes (2-ms probe delay) and probe thresholds in quiet (2-kHz probe: r = 0.44, p < 0.01; 4-kHz probe: r = 0.33, p < 0.05). That is, GOM slopes for the 2-ms probe delay condition increased toward 1 dB/dB with increasing thresholds at the probe frequency. For the 198-ms delay, mean GOM slopes were ∼1.0 dB/dB for all groups and both probe frequencies (mean slopes were 0.90 and 0.95 dB/dB for NH listeners and 0.95 and 0.96 dB/dB for OHI listeners for 2- and 4-kHz probes, respectively), consistent with a reduction in cochlear gain and a linearization of the cochlear IO function over the timecourse of the masker (Strickland, 2008). Compared to YNH and ONH listeners, GOM slopes for OHI listeners increased by a smaller amount as the probe moved from the onset to the center of the masker, consistent with these listeners having less gain at the masker's onset and therefore less gain to adjust over the timecourse of the masker.
FIG. 4.
(Color online) GOM slopes plotted against quiet threshold (6-ms probes) for the 2-kHz (left panels) and 4-kHz (right panels) probes, where the probe was delayed by 2 ms (top panels) and 198 ms (bottom panels) from the masker's onset. Results from regression analyses (Pearson's correlation coefficient) are shown in panels. Non-significant correlations are denoted by “n.s.”
Differences in the GOM slope between the 2- and 198-ms delay conditions and the finding that overshoot is decreased in listeners with cochlear hearing loss are difficult to explain by theories of overshoot based on confusion effects (Carlyon and White, 1992; Fletcher et al., 2015). These theories posit that overshoot is due to confusion between the onset of the probe and the onset of the masker (i.e., “transient masking”; Bacon and Moore, 1987). The improvement in thresholds for the probe presented near the center of the masker is hypothesized to occur because the probe's onset occurs much later than the masker's onset, thus reducing transient masking. It is unclear how theories based on transient masking account for (1) relatively better masking thresholds in listeners with cochlear hearing loss when the probe is presented at the masker's onset, (2) differences in GOM slopes between the 2- and 198-ms delay conditions, and (3) GOM slopes near 1 dB/dB when the probe is presented near the masker's temporal center. For example, transient masking could predict less overshoot in OHI than NH (YNH, ONH) listeners given that broader auditory filters are expected to produce less ringing, and therefore facilitate better resolution of temporal onsets. However, this reasoning incorrectly predicts that overshoot in NH listeners should be largest at lower probe levels and smallest at higher probe levels, given that auditory filters become progressively broader with increasing stimulus level (e.g., Ruggero et al., 1997). Similarly, transient masking could explain the GOM slope of 1 dB/dB (198-ms condition) if probe+masker and masker alone were equally compressed, as may be the case when probe and masker levels at threshold are very similar (Oxenham and Plack, 1997). Average critical ratios, calculated from the masker level passing through an auditory filter centered on the probe frequency, for NH listeners (YNH, ONH) in the 198-ms condition were 19.28 and 16.35 dB for 2- and 4-kHz probes, respectively. It is unclear whether the response to the probe+masker and the response to the masker alone would be equally compressed despite being processed at different input levels of the cochlear IO function. Alternatively, slopes near 1 dB/dB in the 198-ms condition are consistent with the hypothesis that the improvement in thresholds over the timecourse of the masker is due to linearization of the cochlear IO function.2
6. Overshoot: Individual differences
Overshoot as a function of masker level and probe frequency varied substantially among listeners, even for listeners with similar detection thresholds. Consistent with previous studies, individual differences were large for masking thresholds for the probe near the masker's onset (e.g., Overson et al., 1996; Wright, 1997), but were very small for masking thresholds for the probe near the masker's center. This suggests that mechanisms underlying detection of the probe at the masker's onset play a primary role in individual differences in overshoot. Thus, the contribution of MOC function to individual differences is likely to be minor, as the MOC reflex is assumed to be dormant at the masker's onset. von Klitzing and Kohlrausch (1994) suggested cochlear compression may account for the relatively poorer probe thresholds at the masker's onset than at the masker's temporal center in NH listeners. Consistent with this interpretation, individual differences in overshoot may be partially due to differences in cochlear compression among listeners. In addition, individual differences in detection efficiency (which is assumed here to be constant for a given listener in the 2- and 198-ms conditions) may interact with cochlear compression (Jennings et al., 2014) or contribute independently to account for individual differences in overshoot.
To assess the potential contribution of the cochlear amplifier to individual differences in overshoot a “slope ratio” metric was calculated and regressed on thresholds for the probes in quiet. The slope ratio was defined as the ratio of GOM slopes for the 198- and 2-ms conditions. Slope ratios are larger (from 1.5 to 3.0) for listeners with appreciable overshoot, and approach a value of 1.0 for listeners with little or no overshoot. Slope ratio was significantly positively correlated with probe thresholds in quiet (2-kHz probe: r = 0.29, p = 0.046; 4-kHz probe: r = 0.40, p < 0.01), when all groups (YNH, ONH, OHI) were included in the regression; however, this significant correlation did not survive when regressions were performed separately for YNH, ONH, and OHI groups, despite quiet thresholds spanning ∼20 dB (YNH, ONH), to ∼30 dB (OHI) within groups. These results are consistent with individual differences in overshoot being attributed to differences in cochlear amplifier function across listener groups, but not within groups.
Overshoot may also depend on the status of the cochlear amplifier at cochlear locations slightly above the probe frequency (Bacon et al., 1988; Strickland and Krishnan, 2005). Thus, individual differences may stem from differences in detection thresholds at frequencies remote from the probe frequency. However, slope ratios in the current study were not significantly correlated with audiometric thresholds above or below the two probe frequencies. Thus, individual differences in overshoot measured with a broadband noise in NH and OHI listeners appear to be independent of detection thresholds at frequencies remote from the probe frequency. These results suggest the effect of the cochlear amplifier on individual differences in overshoot is small, or is independent of probe thresholds in quiet, as has been suggested in other studies (Moore et al., 1999; Plack et al., 2004; Lopez-Poveda et al., 2005; Dubno et al., 2007; Jennings et al., 2014). In conclusion, it is unclear the extent to which individual differences in overshoot are related to peripheral processing, central processing, or an interaction between peripheral and central processing.
III. COMPUTATIONAL MODEL SIMULATIONS
As suggested by previous studies (von Klitzing and Kohlrausch, 1994; Strickland, 2001; Jennings et al., 2011) and the earlier analysis of GOM slopes, overshoot may result from a reduction in cochlear gain over the timecourse of the masker. Computational model simulations of average masking thresholds for each subject group (YNH, ONH, OHI) were obtained to quantify gain reduction among subject groups and to determine the extent to which the gain reduction framework is consistent with the current dataset. Reductions in gain were quantified as the decrease in maximum gain (e.g., the gain applied to the masker when presented near absolute threshold), similar to Strickland (2004). Previous gain reduction simulations assumed a constant maximum reduction in gain for all masker levels (Strickland, 2001; Strickland and Krishnan, 2005) or defined the relationship between masker level and maximum gain reduction based on a priori assumptions (Jennings et al., 2011). Similar to Strickland (2008), who measured overshoot in NH listeners, the current simulations estimate the relationship between masker level and maximum gain reduction directly from data from YNH, ONH, and OHI listeners. These estimates are evaluated to determine if maximum gain reduction is weaker and grows more slowly as a function of masker level in OHI listeners than in YNH and ONH listeners. Masking thresholds were not measurable for some OHI subjects at the lowest (60 and 70 dB SPL) and highest (100 dB SPL) probe levels; therefore, the average masking thresholds at these levels were calculated from fewer data points (60 dB SPL: 5/15 points, 70 dB SPL: 14/15 points, 100 dB SPL: 14/15 points) than the averages at other levels. The effects of age were not modeled, as any age-related effects observed appeared to be driven by results from a small number of ONH listeners at one probe frequency.
A. Methods
1. Model structure
Model simulations followed the approach of Jennings and Strickland (2012) and Jennings et al. (2014). Briefly, peripheral processing was simulated using the model of Zilany et al. (2009), based on cat physiology. IHC responses were obtained at the characteristic frequency (CF) centered on the probe frequency (2 kHz) to simulate observation intervals that contained only the masker (“M” interval), or the masker plus the probe (“MP” interval). Detection of the probe via other CFs was not simulated, as pilot experiments revealed that the influence of off-frequency listening is minimal for overshoot measured with a broadband noise masker (Jennings et al., 2011). For central processing it was assumed that the probe was detected at a criterion SNR (Oxenham and Moore, 1994). Before calculating the SNR, IHC responses were squared and then smoothed with a sliding rectangular window, with a width of 5 ms. A similar smoothing process was used in the adaptation model by Oxenham (2001). The SNR was obtained by subtracting the smoothed IHC response to the M interval from that of the MP interval and calculating the maximum difference.
2. Model stimuli
Model simulations were limited to predicting data for the 2-kHz probe.3 Stimuli were similar to those described in the overshoot experiment (Sec. II), except the probe frequency was 5.5 kHz instead of 2 kHz, to account for differences between cat and human cochlear frequency maps (Greenwood, 1990).
3. Model parameters
Three factors were used to predict mean overshoot for YNH, ONH, and OHI listeners: (1) average detection thresholds in quiet (i.e., threshold elevation), (2) detection efficiency, and (3) gain reduction. Threshold elevation was based on the average detection thresholds for a 350-ms, 2-kHz pure tone, while detection efficiency and gain reduction were parameterized to minimize the sum-of-squares error between model predictions and psychophysical data. Threshold elevation (θ) was a constant reduction in OHC gain using the model's “cOHC” setting (see Zilany and Bruce, 2007) and is expressed as the shift in threshold (in dB) relative to normal OHC function (cOHC = 1). IHC dysfunction was always set to cIHC = 1 (normal IHC function), given that OHI listeners generally had only mild/moderate hearing loss, where minimal IHC dysfunction is expected (Lopez-Poveda and Johannesen, 2012). Threshold elevation (θ) was set to 0 dB for NH simulations and 27 dB for OHI simulations, which is the averaged and rounded detection threshold for the 350-ms tone in OHI listeners (43 dB SPL) minus the average and rounded detection thresholds for the 350-ms tone in NH listeners (16 dB SPL; YNH, ONH).
Detection efficiency (k) was modeled as a constant post-cochlear SNR for all predicted thresholds (Oxenham and Moore, 1994; Jennings et al., 2014). Gain reduction was modeled as a decrease in cochlear amplifier gain over the timecourse of the masker. It was assumed that gain reduction peaked by 198-ms, thus only this peak was simulated rather than simulating the timecourse of gain reduction. Although the masker would have elicited a reduction in gain in the 2-ms delay condition, this reduction would have occurred after detection of the probe, based on measurements of the timecourse of the MOC reflex (Backus and Guinan, 2006). Neurophysiological studies of the effects of acoustic stimulation of the MOC reflex show that the magnitude of efferent suppression on auditory nerve responses is proportional to the level of the acoustic elicitor (Liberman, 1989; Warren and Liberman, 1989). For the current simulations, the relationship between masker level and maximum gain reduction was described by a straight line (on dB coordinates) with slope (mGR), and intercept determined by the gain at the lowest probe level tested (bGR). Preliminary simulations revealed a significant correlation between bGR and θ; therefore, bGR was set equal to θ and model predictions were realized through two free parameters: k and mGR.
B. Results and discussion
Lines in Fig. 5 are predicted masking thresholds for the 2-kHz probe presented in the 2-ms delay (top panels) and 198-ms delay (middle panels) conditions; predicted overshoot is plotted in the bottom panel. Mean behavioral data from YNH, ONH, and OHI listeners are replotted from Fig. 4 as squares, circles, and triangles, respectively. Model simulations accurately predicted measured masking thresholds as revealed by the relatively low rms error (1.83, 2.65, and 1.24 dB for YNH, ONH, and OHI groups, respectively). Consistent with other studies (Strickland, 2001; Bacon and Savel, 2004; Strickland, 2004; Jennings et al., 2011), this suggests that overshoot can be effectively modeled as a reduction in cochlear amplifier gain. Moreover, the smaller overshoot in OHI listeners emerges from relatively less compressive IO functions in the 2-ms condition, consistent with the GOM analysis (Sec. II B 5). Model simulations did not predict the larger overshoot at mid-level probes in ONH subjects than YNH subjects. This may be related to the (unknown) factors that resulted in lower (poorer) masking thresholds for the probe near the masker's onset for four ONH listeners, as discussed earlier. Model threshold elevation (θ), average probe thresholds for the 350-ms 2-kHz tone (PT), and the best-fitting model parameters for k and mGR are displayed in Table I. These parameters can be interpreted as follows: k is the constant criterion SNR at threshold in dB and mGR is the rate at which maximum gain is reduced as masker level is increased (dB/dB).
FIG. 5.
(Color online) Predicted masking thresholds (lines) in overshoot conditions for the average behavioral data (symbols) obtained with a 2-kHz probe. Top and middle panels show predictions and measured data in the 2− and 198-ms delay conditions. The bottom panels display overshoot, calculated by subtracting the 2-ms thresholds from the 198-ms thresholds.
TABLE I.
Probe thresholds (PT) and the best-fitting model parameters (θ, k, mGR; see text) for simulations fit to mean data from YNH, ONH, and OHI listeners.
| PT (dB SPL) | θ (dB) | k (dB) | mGR (dB/dB) | |
|---|---|---|---|---|
| YNH | 13.17 | 0.00 | 10.51 | 1.28 |
| ONH | 20.05 | 0.00 | 12.31 | 0.91 |
| OHI | 44.34 | 27.00 | 9.69 | 0.62 |
1. Effects of cochlear hearing loss
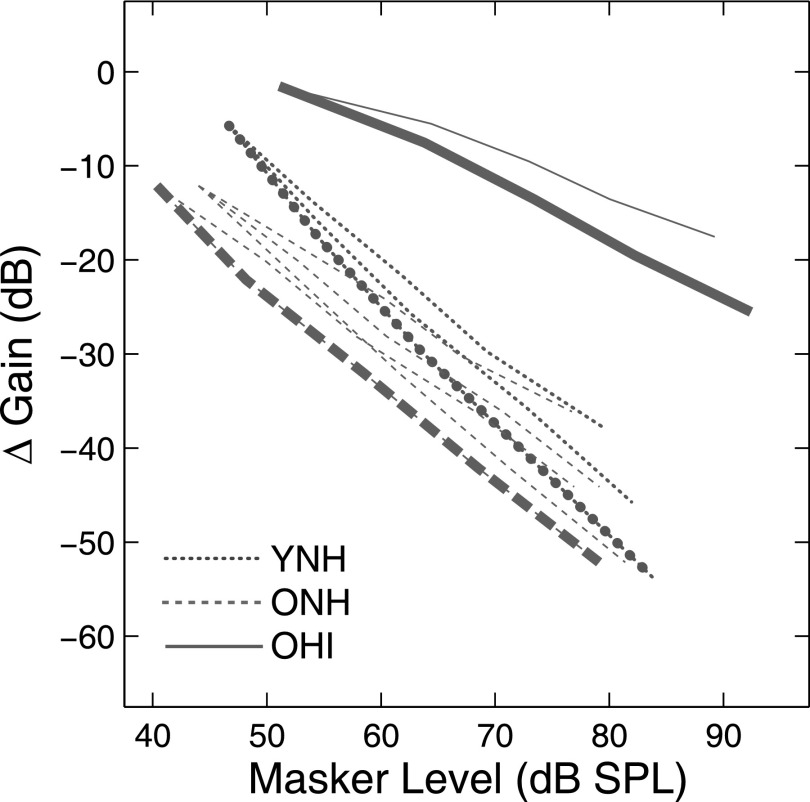
Maximum gain reduction vs masker level functions (calculated from mGR and bGR) are plotted in Fig. 6. Maximum gain was reduced at a rate of −1.28 dB/dB and −0.91 dB/dB with increasing masker level for YNH and ONH listeners; this rate was shallower (−0.62 dB/dB) for OHI listeners (thick dotted, dashed, and solid lines in Fig. 6). The largest reduction in maximum gain was approximately −50 dB for YNH and ONH listeners and −30 dB for OHI listeners for the best-fitting simulations. The gain reduction rate of −1.28 dB/dB observed in YNH listeners is not physiologically realistic as it predicts that cochlear output decreases with increasing probe level. Although the gain reduction rate of −1.28 dB/dB was the best-fitting solution, additional simulations revealed that a range of k and mGR values predicted overshoot within a rms error of 0.5 dB of the best-fitting simulations. These additional simulations involved manually predicting mean overshoot for all combinations of 10 detection efficiency (k) values (4.0, 5.5, 7.0, 8.5, 10.0, 11.5, 13.0, 14.5, 16.0, 17.5 dB) and 10 mGR values (0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4, 1.6, 1.8, 2.0 dB/dB). Additional simulations revealed that rates of gain change from 0.8–1.2 dB/dB, 0.6–1.0 dB/dB, and 0.4–0.6 dB/dB were needed to effectively predict overshoot in YNH, ONH, and OHI subjects, respectively (thin dotted, dashed, and solid lines in Fig. 6). The corresponding largest change in maximum gain for YNH, ONH, and OHI listeners ranged between −37 and −53 dB, −36 and −52 dB, and −17 and −25 dB, suggesting that rate of gain change and the largest gain change are better described by a range of values rather than a single value. Moreover, these ranges for the rate of gain reduction and the largest gain change are more physiologically realistic than the best-fitting simulations. These findings suggest that the ability to adjust cochlear amplifier gain is limited in individuals with cochlear hearing loss.
FIG. 6.
(Color online) The predicted change in maximum OHC gain as a function of masker level based on the model parameter mGR. Thick lines show gain versus level functions for the best-fitting simulations. Thin lines show additional simulations with rms errors within 0.5 dB of the best-fitting simulation. Dotted, dashed, and solid lines represent YNH, ONH, and OHI listeners, respectively.
With current information, physiological bases for reduced ability to adjust cochlear amplifier gain in listeners with cochlear hearing loss remain speculative. Based on the gain reduction framework, the current results could emerge from: (1) reduced cochlear amplifier gain, which diminishes the input to an otherwise healthy MOC reflex, or (2) a dysfunctional MOC reflex that is unable to decrease the residual gain of the cochlear amplifier. If the MOC reflex is otherwise healthy and the cochlear amplifier has some residual gain, overshoot could be measureable as long as the reduction in gain improves the post-cochlear SNR for the probe near the center of the masker. The observation that overshoot was observed in many OHI listeners in the current study is consistent with this possibility. In contrast, overshoot would not be measureable if the MOC reflex is dysfunctional or if cochlear amplifier gain is negligible, consistent with very little or no overshoot reported for some OHI listeners in the current study. Similarly, overshoot is not expected in listeners with a functional MOC reflex and residual cochlear amplifier gain if the reduction in gain over the timecourse of the masker does not improve the post-cochlear SNR.
2. The MOC reflex and other possible mechanisms of overshoot
The hypotheses and the general framework tested in the current study are based on a presumed relationship between the MOC reflex and overshoot, for reasons discussed in Sec. I. However, some OAE studies have failed to confirm this relationship (Keefe et al., 2009). In addition, the current model simulations required −36 to −53 dB change in maximum gain for accurate predictions of overshoot, which is more than the maximum MOC-induced gain reduction (∼30 dB) observed from BM measurements (Murugasu and Russell, 1996; Dolan et al., 1997; Russell and Murugasu, 1997). This suggests that additional mechanisms may contribute to overshoot. For example, previous studies have suggested that neural inhibition plays a role in overshoot (McFadden, 1989; Keefe et al., 2009) and the related effect of masker enhancement (Byrne et al., 2011). Aging and hearing loss have been associated with reduced neural inhibition (Tremblay et al., 2003), which may explain why OHI listeners have better thresholds than YNH listeners in the 2-ms delay condition (because of increased excitation to the probe) and, therefore, less overshoot. Skepticism for this hypothesis comes from the findings that overshoot was not reduced in ONH listeners who are expected to have age-related reduction in neural inhibition (e.g., Caspary et al., 2005). Similarly, firing rate adaptation in auditory nerve fibers has been theorized to be involved in overshoot (Green, 1969; Bacon and Viemeister, 1985b,a); however, adaptation does not account for the magnitude of overshoot observed in psychophysical studies (McFadden, 1989; Bacon and Healy, 2000), nor does adaptation predict overshoot in modeling studies (Jennings et al., 2011).
As discussed by Robinson and McAlpine (2009), many adaptive mechanisms throughout the auditory system work in concert to produce the perceptual dynamic range. In addition to the MOC reflex, these mechanisms may include the stapedial reflex, dynamic range adaptation in the auditory nerve (Wen et al., 2009), inferior colliculus (Dean et al., 2005), and more central efferent pathways (e.g., cortico-collicular efferents). When invoking a specific adaptive mechanism to explain overshoot, the mechanism must account for changes in the GOM slope from nonlinear to linear as the probe is delayed from the onset to the temporal center of the masker (von Klitzing and Kohlrausch, 1994). Currently, it is not clear which adaptive mechanisms (other than the MOC reflex) can account for these changes in GOM slope.
IV. SUMMARY AND CONCLUSIONS
This study measured overshoot in YNH, ONH, and OHI adults. Data analyses were focused on assessing the extent to which dysfunction of certain structures along the MOC reflex pathway could explain age- and hearing-loss-related changes in overshoot. Model simulations were obtained to quantify gain reduction among YNH, ONH, and OHI listeners and determine the extent to which overshoot can be explained by a reduction in cochlear amplifier gain.
Measures of overshoot have the potential to reveal how well the auditory system is able to adjust to ongoing changes in the local soundscape during acoustic stimulation. Moreover, the ability to adjust cochlear amplifier gain in response to environmental sounds may facilitate speech recognition in noisy backgrounds, based on the assumption that MOC reflex activity can enhance the post-cochlear SNR (Kawase et al., 1993). Understanding the mechanisms underlying gain adjustments and how these mechanisms change with aging and cochlear hearing loss may explain reduced speech recognition in noise by older adults with normal and impaired hearing (Summerfield and Assmann, 1989; Bashford et al., 2011).
The results of this study lead to the following conclusions:
-
•
As expected, overshoot was reduced in OHI listeners relative to NH listeners due to better-than-normal thresholds for the probe near the masker's onset. This result is consistent with these listeners having less cochlear amplifier gain than NH listeners and, therefore, less gain to adjust over the timecourse of the masker.
-
•
Individual differences in overshoot were primarily due to individual differences in thresholds for probes presented near the onset of the masker. The extent to which these individual differences are due to peripheral processing, central processing, or an interaction between peripheral and central processing is unknown.
-
•
Overshoot for ONH listeners was similar to that of YNH listeners, contrary to the hypothesis that certain age-related changes in the auditory periphery would lead to increased or reduced overshoot. This suggests that age-related changes to mechanisms presumed to underlie overshoot do not result in significant differences in overshoot among younger and older adults with NH.
-
•
Model simulations of overshoot based on the gain reduction framework suggest that cochlear amplifier gain is reduced less over the timecourse of acoustic stimulation in listeners with cochlear hearing loss than in younger and older listeners with NH.
ACKNOWLEDGMENTS
The authors wish to thank Kelsey Woodard, Nina Pryor, and Jessica Chen (University of Utah), and Tyler Eisenhart and Emily Franko-Tobin (MUSC) for assistance with data collection. Model simulations were conducted using resources from the Center for High Performance Computing at the University of Utah. This work was supported by Grant Nos. R01 DC000184 and P50 DC000422 from NIH/NIDCD; the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the MUSC and NIH/NCRR Grant No. UL1 RR029882. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant No. C06 RR14516 from the National Center for Research Resources, National Institutes of Health. This research was completed while S.G.J. was a mentee in an NIH-sponsored mentoring program for clinician scientists in hearing and balance disorders (U24 DC012079). Comments from Associate Editor Elizabeth Strickland and two anonymous reviewers were helpful in improving the quality of this manuscript.
Portions of this research were presented at the Forty-First Annual Science and Technology Conference of the American Auditory Society in March, 2014 (Scottsdale, AZ) and at the 167th Meeting of the Acoustical Society of America in May, 2014 (Providence, RI).
Footnotes
A wideband noise elicitor results in a change in power level at 2 and 4 kHz, which could influence middle ear transmission of 2- and 4-kHz probes when presented in the temporal center of the masker (Feeney and Keefe, 2001). Reduced transmission of the high-level probe may result in responses to the probe+masker and responses to the masker alone being compressed [assuming that the probe+masker is processed linearly at the highest levels (Johnstone et al., 1986)], which is not expected to improve post-cochlear SNR. Thus, although the middle ear reflex may reduce transmission of the masker and probe, it is not clear how this reduction results in the large overshoot observed.
Previous studies suggest that overshoot involves different mechanisms for on- and off-frequency maskers, where transient masking is most associated with the off-frequency mechanism (e.g., Carlyon and White, 1992). Although the broadband noise included in this study had on- and off-frequency energy, there is little evidence to suggest that transient masking influenced masking thresholds. It is possible that transient masking plays a role in overshoot with off-frequency maskers; however, the current study was not designed to test such a conclusion.
The middle ear filter of the Zilany et al. (2009) model was developed by Bruce et al. (2003) and provides an accurate and stable response out to 10 kHz. The 4-kHz probe used in the current study maps to an 11-kHz characteristic frequency in cat (Greenwood, 1990), which is above the frequency region for which the model's middle ear filter has been validated. Initial simulation using an 11-kHz probe revealed that model thresholds (based on IHC voltage) were roughly 15 dB higher than those for the 5.5 kHz probe. Elevated thresholds at 11 kHz relative to 5.5 kHz are not expected based on cat auditory nerve physiology (Liberman and Kiang, 1978). Thus, elevated thresholds may have been due to the use of a CF that was higher in frequency than the highest frequency for which the model middle ear filter has been validated. These elevated thresholds resulted in an inability to predict overshoot with the 4-kHz probe for YNH and ONH subjects.
References
- 1. Backus, B. C. , and Guinan, J. J. (2006). “ Time-course of the human medial olivocochlear reflex,” J. Acoust. Soc. Am. , 2889–2904. 10.1121/1.2169918 [DOI] [PubMed] [Google Scholar]
- 2. Bacon, S. P. (1990). “ Effect of masker level on overshoot,” J. Acoust. Soc. Am. , 698–702. 10.1121/1.399773 [DOI] [PubMed] [Google Scholar]
- 3. Bacon, S. P. , and Healy, E. W. (2000). “ Effects of ipsilateral and contralateral precursors on the temporal effect in simultaneous masking with pure tones,” J. Acoust. Soc. Am. , 1589–1597. 10.1121/1.428443 [DOI] [PubMed] [Google Scholar]
- 4. Bacon, S. P. , Hedrick, M. S. , and Grantham, D. W. (1988). “ Temporal effects in simultaneous pure-tone masking in subjects with high-frequency sensorineural hearing loss,” Audiology , 313–323. 10.3109/00206098809081602 [DOI] [PubMed] [Google Scholar]
- 5. Bacon, S. P. , and Liu, L. (2000). “ Effects of ipsilateral and contralateral precursors on overshoot,” J. Acoust. Soc. Am. , 1811–1818. 10.1121/1.1290246 [DOI] [PubMed] [Google Scholar]
- 6. Bacon, S. P. , and Moore, B. C. (1987). “ Transient masking and the temporal course of simultaneous tone-on-tone masking,” J. Acoust. Soc. Am. , 1073–1077. 10.1121/1.395125 [DOI] [PubMed] [Google Scholar]
- 7. Bacon, S. P. , and Savel, S. (2004). “ Temporal effects in simultaneous masking with on- and off-frequency noise maskers: Effects of signal frequency and masker level,” J. Acoust. Soc. Am. , 1674–1683. 10.1121/1.1689344 [DOI] [PubMed] [Google Scholar]
- 8. Bacon, S. P. , and Smith, M. A. (1991). “ Spectral, intensive, and temporal factors influencing overshoot,” Q. J. Exp. Psychol. A , 373–399. 10.1080/14640749108400978 [DOI] [PubMed] [Google Scholar]
- 9. Bacon, S. P. , and Takahashi, G. A. (1992). “ Overshoot in normal-hearing and hearing-impaired subjects,” J. Acoust. Soc. Am. , 2865–2871. 10.1121/1.402967 [DOI] [PubMed] [Google Scholar]
- 10. Bacon, S. P. , and Viemeister, N. F. (1985a). “ Simultaneous masking by gated and continuous sinusoidal maskers,” J. Acoust. Soc. Am. , 1220–1230. 10.1121/1.392890 [DOI] [PubMed] [Google Scholar]
- 11. Bacon, S. P. , and Viemeister, N. F. (1985b). “ The temporal course of simultaneous tone-on-tone masking,” J. Acoust. Soc. Am. , 1231–1235. 10.1121/1.392891 [DOI] [PubMed] [Google Scholar]
- 12. Bashford, J. A., Jr. , Warren, R. M. , and Lenz, P. W. (2011). “ Enhancing the intelligibility of high intensity speech: Evidence of inhibition in the lower auditory pathway,” Proc. Meet. Acoust. , 050006. 10.1121/1.3656331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bidelman, G. M. , Jennings, S. G. , and Strickland, E. A. (2015). “ PsyAcoustX: A flexible MATLAB® package for psychoacoustics research,” Front. Psychol. , 1498, 1–11. 10.3389/fpsyg.2015.01498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bruce, I. C. , Sachs, M. B. , and Young, E. D. (2003). “ An auditory-periphery model of the effects of acoustic trauma on auditory nerve responses,” J. Acoust. Soc. Am. , 369–388. 10.1121/1.1519544 [DOI] [PubMed] [Google Scholar]
- 15. Byrne, A. J. , Stellmack, M. A. , and Viemeister, N. F. (2011). “ The enhancement effect: Evidence for adaptation of inhibition using a binaural centering task,” J. Acoust. Soc. Am. , 2088–2094. 10.1121/1.3552880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carlyon, R. P. , and White, L. J. (1992). “ Effect of signal frequency and masker level on the frequency regions responsible for the overshoot effect,” J. Acoust. Soc. Am. , 1034–1041. 10.1121/1.402629 [DOI] [PubMed] [Google Scholar]
- 17. Caspary, D. M. , Schatteman, T. A. , and Hughes, L. F. (2005). “ Age-related changes in the inhibitory response properties of dorsal cochlear nucleus output neurons: Role of inhibitory inputs,” J. Neurosci. , 10952–10959. 10.1523/JNEUROSCI.2451-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Champlin, C. A. , and McFadden, D. (1989). “ Reductions in overshoot following intense sound exposures,” J. Acoust. Soc. Am. , 2005–2011. 10.1121/1.397853 [DOI] [PubMed] [Google Scholar]
- 19. Cooper, N. P. , and Guinan, J. J. (2006). “ Efferent-mediated control of basilar membrane motion,” J. Physiol. , 49–54. 10.1113/jphysiol.2006.114991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dean, I. , Harper, N. S. , and McAlpine, D. (2005). “ Neural population coding of sound level adapts to stimulus statistics,” Nat. Neurosci. , 1684–1689. 10.1038/nn1541 [DOI] [PubMed] [Google Scholar]
- 21. Dolan, D. F. , Guo, M. H. , and Nuttall, A. L. (1997). “ Frequency-dependent enhancement of basilar membrane velocity during olivocochlear bundle stimulation,” J. Acoust. Soc. Am. , 3587–3596. 10.1121/1.421008 [DOI] [PubMed] [Google Scholar]
- 22. Dubno, J. R. , Horwitz, A. R. , and Ahlstrom, J. B. (2007). “ Estimates of basilar-membrane nonlinearity effects on masking of tones and speech,” Ear Hear. , 2–17. 10.1097/AUD.0b013e3180310212 [DOI] [PubMed] [Google Scholar]
- 23. Feeney, M. P. , and Keefe, D. H. (2001). “ Estimating the acoustic reflex threshold from wideband measures of reflectance, admittance, and power,” Ear Hear. , 316–332. 10.1097/00003446-200108000-00006 [DOI] [PubMed] [Google Scholar]
- 24. Fletcher, H. (1940). “ Auditory patterns,” Rev. Mod. Phys. , 47–65. 10.1103/RevModPhys.12.47 [DOI] [Google Scholar]
- 25. Fletcher, M. , de Boer, J. , and Krumbholz, K. (2015). “ Is off-frequency overshoot caused by adaptation of suppression?,” J. Assoc. Res. Otolaryngol. , 241–253. 10.1007/s10162-014-0498-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Franklin, D. J. , McCoy, M. J. , Martin, G. K. , and Lonsbury-Martin, B. L. (1992). “ Test/retest reliability of distortion-product and transiently evoked otoacoustic emissions,” Ear Hear. , 417–429. 10.1097/00003446-199212000-00008 [DOI] [PubMed] [Google Scholar]
- 27. Gates, G. A. , and Mills, J. H. (2005). “ Presbycusis,” Lancet , 1111–1120. 10.1016/S0140-6736(05)67423-5 [DOI] [PubMed] [Google Scholar]
- 28. Green, D. M. (1969). “ Masking with continuous and pulsed sinusoids,” J. Acoust. Soc. Am. , 939–946. 10.1121/1.1911813 [DOI] [PubMed] [Google Scholar]
- 29. Greenwood, D. D. (1990). “ A cochlear frequency-position function for several species—29 years later,” J. Acoust. Soc. Am. , 2592–2605. 10.1121/1.399052 [DOI] [PubMed] [Google Scholar]
- 30. Guinan, J. J. (2006). “ Olivocochlear efferents: Anatomy, physiology, function, and the measurement of efferent effects in humans,” Ear Hear. , 589–607. 10.1097/01.aud.0000240507.83072.e7 [DOI] [PubMed] [Google Scholar]
- 31. Jennings, S. G. , Ahlstrom, J. B. , and Dubno, J. R. (2014). “ Computational modeling of individual differences in behavioral estimates of cochlear nonlinearities,” J. Assoc. Res. Otolaryngol. , 945–960. 10.1007/s10162-014-0486-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jennings, S. G. , Heinz, M. G. , and Strickland, E. A. (2011). “ Evaluating adaptation and olivocochlear efferent feedback as potential explanations of psychophysical overshoot,” J. Assoc. Res. Otolaryngol. , 345–360. 10.1007/s10162-011-0256-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jennings, S. G. , and Strickland, E. A. (2012). “ Evaluating the effects of olivocochlear feedback on psychophysical measures of frequency selectivity,” J. Acoust. Soc. Am. , 2483–2496. 10.1121/1.4742723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johnstone, B. M. , Patuzzi, R. , and Yates, G. K. (1986). “ Basilar membrane measurements and the travelling wave,” Hear. Res. , 147–153. 10.1016/0378-5955(86)90090-0 [DOI] [PubMed] [Google Scholar]
- 35. Kawase, T. , Delgutte, B. , and Liberman, M. C. (1993). “ Antimasking effects of the olivocochlear reflex. II. Enhancement of auditory-nerve response to masked tones,” J. Neurophysiol. , 2533–2549. [DOI] [PubMed] [Google Scholar]
- 36. Keefe, D. H. , Schairer, K. S. , Ellison, J. C. , Fitzpatrick, D. F. , and Jesteadt, W. (2009). “ Use of stimulus-frequency otoacoustic emissions to investigate efferent and cochlear contributions to temporal overshoot,” J. Acoust. Soc. Am. , 1595–1604. 10.1121/1.3068443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim, S. , Frisina, D. R. , and Frisina, R. D. (2002). “ Effects of age on contralateral suppression of distortion product otoacoustic emissions in human listeners with normal hearing,” Audiol. Neurotol. , 348–357. 10.1159/000066159 [DOI] [PubMed] [Google Scholar]
- 38. Kujawa, S. G. , and Liberman, M. C. (2006). “ Acceleration of age-related hearing loss by early noise exposure: Evidence of a misspent youth,” J. Neurosci. , 2115–2123. 10.1523/JNEUROSCI.4985-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lang, H. , Jyothi, V. , Smythe, N. M. , Dubno, J. R. , Schulte, B. A. , and Schmiedt, R. A. (2010). “ Chronic reduction of endocochlear potential reduces auditory nerve activity: Further confirmation of an animal model of metabolic presbyacusis,” J. Assoc. Res. Otolaryngol. , 419–434. 10.1007/s10162-010-0214-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Levitt, H. (1971). “ Transformed up-down methods in psychoacoustics,” J. Acoust. Soc. Am. , 467–477. 10.1121/1.1912375 [DOI] [PubMed] [Google Scholar]
- 41. Liberman, M. C. (1989). “ Rapid assessment of sound-evoked olivocochlear feedback: Suppression of compound action potentials by contralateral sound,” Hear. Res. , 47–56. 10.1016/0378-5955(89)90127-5 [DOI] [PubMed] [Google Scholar]
- 42. Liberman, M. C. , and Kiang, N. Y. (1978). “ Acoustic trauma in cats. Cochlear pathology and auditory-nerve activity,” Acta Otolaryngol. Suppl. , 1–63. [PubMed] [Google Scholar]
- 43. Lopez-Poveda, E. A. , and Johannesen, P. T. (2012). “ Behavioral estimates of the contribution of inner and outer hair cell dysfunction to individualized audiometric loss,” J. Assoc. Res. Otolaryngol. , 485–504. 10.1007/s10162-012-0327-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lopez-Poveda, E. A. , Plack, C. J. , Meddis, R. , and Blanco, J. L. (2005). “ Cochlear compression in listeners with moderate sensorineural hearing loss,” Hear. Res. , 172–183. 10.1016/j.heares.2005.03.015 [DOI] [PubMed] [Google Scholar]
- 45. McFadden, D. (1989). “ Spectral differences in the ability of temporal gaps to reset the mechanisms underlying overshoot,” J. Acoust. Soc. Am. , 254–261. 10.1121/1.397732 [DOI] [PubMed] [Google Scholar]
- 46. McFadden, D. , and Champlin, C. A. (1990). “ Reductions in overshoot during aspirin use,” J. Acoust. Soc. Am. , 2634–2642. 10.1121/1.399056 [DOI] [PubMed] [Google Scholar]
- 47. McFadden, D. , Walsh, K. P. , Pasanen, E. G. , and Grenwelge, E. M. (2010). “ Overshoot using very short signal delays,” J. Acoust. Soc. Am. , 1915–1921. 10.1121/1.3480568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Moore, B. C. , Vickers, D. A. , Plack, C. J. , and Oxenham, A. J. (1999). “ Inter-relationship between different psychoacoustic measures assumed to be related to the cochlear active mechanism,” J. Acoust. Soc. Am. , 2761–2778. 10.1121/1.428133 [DOI] [PubMed] [Google Scholar]
- 49. Murugasu, E. , and Russell, I. J. (1996). “ The effect of efferent stimulation on basilar membrane displacement in the basal turn of the guinea pig cochlea,” J. Neurosci. , 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Neely, S. T. , and Liu, Z. (1994). “ EMAV: Otoacoustic emission averager,” in Technical Memo No. 17 (Boys Town National Research Hospital, Omaha, NE).
- 51. Nelson, D. A. , and Schroder, A. C. (1997). “ Linearized response growth inferred from growth-of-masking slopes in ears with cochlear hearing loss,” J. Acoust. Soc. Am. , 2186–2201. 10.1121/1.418203 [DOI] [PubMed] [Google Scholar]
- 52. Overson, G. J. , Bacon, S. P. , and Webb, T. M. (1996). “ The effect of level and relative frequency region on the recovery of overshoot,” J. Acoust. Soc. Am. , 1059–1065. 10.1121/1.415232 [DOI] [PubMed] [Google Scholar]
- 53. Oxenham, A. J. (2001). “ Forward masking: Adaptation or integration?,” J. Acoust. Soc. Am. , 732–741. 10.1121/1.1336501 [DOI] [PubMed] [Google Scholar]
- 54. Oxenham, A. J. , and Moore, B. C. (1994). “ Modeling the additivity of nonsimultaneous masking,” Hear. Res. , 105–118. 10.1016/0378-5955(94)90014-0 [DOI] [PubMed] [Google Scholar]
- 55. Oxenham, A. J. , Moore, B. C. , and Vickers, D. A. (1997). “ Short-term temporal integration: Evidence for the influence of peripheral compression,” J. Acoust. Soc. Am. , 3676–3687. 10.1121/1.418328 [DOI] [PubMed] [Google Scholar]
- 55. Oxenham, A. J. , and Plack, C. J. (1997). “ A behavioral measure of basilar-membrane nonlinearity in listeners with normal and impaired hearing,” J. Acoust. Soc. Am. , 3666–3675. 10.1121/1.418327 [DOI] [PubMed] [Google Scholar]
- 56. Plack, C. J. , Drga, V. , and Lopez-Poveda, E. A. (2004). “ Inferred basilar-membrane response functions for listeners with mild to moderate sensorineural hearing loss,” J. Acoust. Soc. Am. , 1684–1695. 10.1121/1.1675812 [DOI] [PubMed] [Google Scholar]
- 57. Robinson, B. L. , and McAlpine, D. (2009). “ Gain control mechanisms in the auditory pathway,” Curr. Opin. Neurobiol. , 402–407. 10.1016/j.conb.2009.07.006 [DOI] [PubMed] [Google Scholar]
- 58. Robles, L. , and Ruggero, M. A. (2001). “ Mechanics of the mammalian cochlea,” Physiol. Rev. , 1305–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ruggero, M. A. , Rich, N. C. , Recio, A. , Narayan, S. S. , and Robles, L. (1997). “ Basilar-membrane responses to tones at the base of the chinchilla cochlea,” J. Acoust. Soc. Am. , 2151–2163. 10.1121/1.418265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Russell, I. J. , and Murugasu, E. (1997). “ Medial efferent inhibition suppresses basilar membrane responses to near characteristic frequency tones of moderate to high intensities,” J. Acoust. Soc. Am. , 1734–1738. 10.1121/1.420083 [DOI] [PubMed] [Google Scholar]
- 61. Schmidt, S. , and Zwicker, E. (1991). “ The effect of masker spectral asymmetry on overshoot in simultaneous masking,” J. Acoust. Soc. Am. , 1324–1330. 10.1121/1.400656 [DOI] [PubMed] [Google Scholar]
- 62. Schmiedt, R. A. (2010). “ The physiology of cochlear presbycusis,” in The Aging Auditory System, edited by Gordon-Salant S., Frisina R. D., Popper A. N., and Fay R. R. ( Springer, New York: ), pp. 9–38. [Google Scholar]
- 63. Schmiedt, R. A. , Lang, H. , Okamura, H. O. , and Schulte, B. A. (2002). “ Effects of furosemide applied chronically to the round window: A model of metabolic presbyacusis,” J. Neurosci. , 9643–9650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schuknecht, H. F. (1974). “ Presbyacusis,” in Pathology of the Ear ( Harvard University Press, Cambridge, MA: ), pp. 388–403. [Google Scholar]
- 65. Sergeyenko, Y. , Lall, K. , Liberman, M. C. , and Kujawa, S. G. (2013). “ Age-related cochlear synaptopathy: An early-onset contributor to auditory functional decline,” J. Neurosci. , 13686–13694. 10.1523/JNEUROSCI.1783-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Smith, R. L. , and Zwislocki, J. J. (1975). “ Short-term adaptation and incremental responses of single auditory-nerve fibers,” Biol. Cybern. , 169–182. 10.1007/BF00364166 [DOI] [PubMed] [Google Scholar]
- 67. Stephens, S. D. G. (1973). “ Auditory temporal integration as a function of intensity,” J. Sound Vib. , 109–126. 10.1016/S0022-460X(73)80054-9 [DOI] [Google Scholar]
- 68. Strickland, E. A. (2001). “ The relationship between frequency selectivity and overshoot,” J. Acoust. Soc. Am. , 2062–2073. 10.1121/1.1357811 [DOI] [PubMed] [Google Scholar]
- 69. Strickland, E. A. (2004). “ The temporal effect with notched-noise maskers: Analysis in terms of input-output functions,” J. Acoust. Soc. Am. , 2234–2245. 10.1121/1.1691036 [DOI] [PubMed] [Google Scholar]
- 70. Strickland, E. A. (2008). “ The relationship between precursor level and the temporal effect,” J. Acoust. Soc. Am. , 946–954. 10.1121/1.2821977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Strickland, E. A. , and Krishnan, L. A. (2005). “ The temporal effect in listeners with mild to moderate cochlear hearing impairment,” J. Acoust. Soc. Am. , 3211–3217. 10.1121/1.2074787 [DOI] [PubMed] [Google Scholar]
- 72. Summerfield, Q. , and Assmann, P. F. (1989). “ Auditory enhancement and the perception of concurrent vowels,” Percept. Psychophys. , 529–536. 10.3758/BF03208060 [DOI] [PubMed] [Google Scholar]
- 73. Tremblay, K. L. , Piskosz, M. , and Souza, P. (2003). “ Effects of age and age-related hearing loss on the neural representation of speech cues,” Clin. Neurophysiol. , 1332–1343. 10.1016/S1388-2457(03)00114-7 [DOI] [PubMed] [Google Scholar]
- 74. Turner, C. W. , and Doherty, K. A. (1997). “ Temporal masking and the ‘active process’ in normal and hearing-impaired listeners,” in Modeling Sensorineural Hearing Loss, edited by Jesteadt W. ( Lawrence Erlbaum Associates, Mahwah, NJ: ), pp. 387–396. [Google Scholar]
- 75. von Klitzing, R. , and Kohlrausch, A. (1994). “ Effect of masker level on overshoot in running- and frozen-noise maskers,” J. Acoust. Soc. Am. , 2192–2201. 10.1121/1.408679 [DOI] [PubMed] [Google Scholar]
- 76. Walsh, K. P. , Pasanen, E. G. , and McFadden, D. (2010). “ Overshoot measured physiologically and psychophysically in the same human ears,” Hear. Res. , 22–37. 10.1016/j.heares.2010.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Warren, E. H. III , and Liberman, M. C. (1989). “ Effects of contralateral sound on auditory-nerve responses. II. Dependence on stimulus variables,” Hear. Res. , 105–121. 10.1016/0378-5955(89)90033-6 [DOI] [PubMed] [Google Scholar]
- 78. Wen, B. , Wang, G. I. , Dean, I. , and Delgutte, B. (2009). “ Dynamic range adaptation to sound level statistics in the auditory nerve,” J. Neurosci. , 13797–13808. 10.1523/JNEUROSCI.5610-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wright, B. A. (1997). “ Detectability of simultaneously masked signals as a function of masker bandwidth and configuration for different signal delays,” J. Acoust. Soc. Am. , 420–429. 10.1121/1.417987 [DOI] [PubMed] [Google Scholar]
- 80. Zeng, F. G. , Chen, H. , and Han, S. (2005). “ Temporal masking in electric hearing,” J. Assoc. Res. Otolaryngol. , 390–400. 10.1007/s10162-005-0016-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zeng, F. G. , Martino, K. M. , Linthicum, F. H. , and Soli, S. D. (2000). “ Auditory perception in vestibular neurectomy subjects,” Hear. Res. , 102–112. 10.1016/S0378-5955(00)00011-3 [DOI] [PubMed] [Google Scholar]
- 82. Zilany, M. S. , and Bruce, I. C. (2007). “ Representation of the vowel /ε/ in normal and impaired auditory nerve fibers: Model predictions of responses in cats,” J. Acoust. Soc. Am. , 402–417. 10.1121/1.2735117 [DOI] [PubMed] [Google Scholar]
- 83. Zilany, M. S. , Bruce, I. C. , Nelson, P. C. , and Carney, L. H. (2009). “ A phenomenological model of the synapse between the inner hair cell and auditory nerve: Long-term adaptation with power-law dynamics,” J. Acoust. Soc. Am. , 2390–2412. 10.1121/1.3238250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zwicker, E. (1965). “ Temporal effects in simultaneous masking and loudness,” J. Acoust. Soc. Am. , 132–141. 10.1121/1.1909588 [DOI] [PubMed] [Google Scholar]