Abstract
In this meeting report, particularly addressing the topic of protection of the cardiovascular system from ischemia/reperfusion injury, highlights are presented that relate to conditioning strategies of the heart with respect to molecular mechanisms and outcome in patients’ cohorts, the influence of co-morbidities and medications, as well as the contribution of innate immune reactions in cardioprotection. Moreover, developmental or systems biology approaches bear great potential in systematically uncovering unexpected components involved in ischemia–reperfusion injury or heart regeneration. Based on the characterization of particular platelet integrins, mitochondrial redox-linked proteins, or lipid-diol compounds in cardiovascular diseases, their targeting by newly developed theranostics and technologies opens new avenues for diagnosis and therapy of myocardial infarction to improve the patients’ outcome.
Keywords: Cardiomyocyte signaling pathways, Cardioprotection, Cardiovascular disease, Co-morbidities, Drug targeting, Endothelial permeability, Extracellular RNA (eRNA), Heart regeneration, Induced pluripotent stem cells, Ischemia–reperfusion injury, Lipid metabolism, MicroRNAs (miRNAs), Mitochondria, Remote ischemic conditioning
Introduction
Despite the progress in our understanding of cellular signaling mechanisms in cardiomyocytes and the cellular communication within the cardiovascular system as well as new treatment options for cardiovascular diseases, ischemic heart disease remains the leading cause of death and disability worldwide [14, 30, 32, 40, 44, 71, 72]. Usually, such events are manifested by acute thrombotic occlusion of a main coronary artery at preselected sites of disturbed blood flow or at ruptured atherosclerotic lesions [18, 56]. Although percutaneous coronary intervention (PCI) is the treatment of choice for reducing the size of a myocardial infarction (MI), the induced reperfusion may not only lead to the recovery of ischemic cardiac tissue but also brings about the paradoxical phenomenon of myocardial “ischemia/reperfusion injury” (IRI).
During the recent 3rd International Symposium on “New Frontiers in Cardiovascular Research” (Singapore), basic researchers and clinicians discussed new biomedical developments as well as new players in cardiovascular diseases, novel targets, and respective interventional strategies, particularly in the area of heart failure. The same holds true for mechanisms of action of the emerging class of atypical chemokines that was focused on at the symposium. Based on novel methods using single-chain antibodies, induced pluripotent stem cells, or miRNAs, novel drugs and technologies, summarized as “Theranostics”, were presented and heavily discussed. In essence, the meeting covered heterogenous and unrelated intra- as well as extracellular molecular targets, which are all linked to the development or prevention of cardiovascular diseases, not only reflecting the complexity of the biological system but also indicating the variety of possible interventional approaches that can be helpful or even lifesaving as cardioprotective strategies.
Ischemia/reperfusion injury and cardiac conditioning: basic mechanisms
While myocardial IRI is associated with an increased death of cardiomyocytes, brief cycles of ischemia and reperfusion (termed “ischemic conditioning”) appear to protect the heart from acute MI and IRI [5, 27, 72]. This phenomenon was discovered 30 years ago and stimulated intense research and clinical trials to understand the mechanisms of myocardial IRI and cardioprotection in patients presenting with ST elevation MI or undergoing cardiac surgery. Yet, experimental animal models as well as clinical studies have presented diverging results of ischemic conditioning in cardiac surgery and PCI or interventions following acute MI [27, 28, 38, 41, 72]. IRI can manifest as reperfusion-induced arrhythmias, myocardial stunning, microvascular obstruction, and cardiomyocyte death, being the main cause of reperfusion-induced myocardial lethality. While the former two situations are more or less self-terminating, the third situation occurs in more than 50 % of patients and is associated with capillary damage, microthrombosis, and cardiomyocyte swelling. Moreover, oxidative stress, calcium overload, and irreversible hypercontracture of cardiomyocytes contribute to lethal myocardial IRI [33, 35]. Myocardial IRI has been investigated in experimental animals, and attenuation of this injury can be obtained by pharmacological and mechanical conditioning strategies, including ischemic pre- and postconditioning and “remote ischemic conditioning” (RIC) [36, 45].
The characterization of the underlying mechanisms and the contributing components in RIC will be a major challenge for future translational work in the cardiovascular field to reduce infarct size and improve clinical outcomes in patients with ischemic heart disease [41, 80]. During tissue injury and upon exposure of tissue towards hypoxia as a source of tissue stress, large amounts of intracellular components as alarming compounds are released that disseminate into the circulation or remain at the site of cell activation/damage and trigger inflammation by activating the innate immune system [109]. In particular, nuclear proteins, such as histones, heat-shock proteins, or amphoterin [56, 79], as well as nuclear DNA [99], mitochondrial DNA [7, 103, 110], ribosomal RNA [86, 87, 111], and miRNAs, become liberated in isolated or complexed form, such as in association with exosomes or microvesicles [14, 15, 17, 25]. The innate immune system senses the signals released by the necrotic cells and activates inflammatory pathways to neutralize such danger signals [75].
In this regard, the endogenous extracellular RNA/RNase system appears to provide new alarmins, primarily present following tissue stress (such as in hypoxia) and cell damage: extracellular RNA (eRNA), together with Tumor Necrosis Factor α (TNF-α), serve as damaging factors in experimental IRI, whereas adminstration of RNase1 had a major therapeutical impact by significantly reducing the infarct area and preventing cardiomyocyte death [16, 17]. Moreover, in a small pilot clinical study, patients undergoing cardiac surgery received initial RIC (4 × 5 min limb ischemia) or a sham procedure. The RIC protocol significantly increased plasma levels of endogenous vascular RNase1 (derived from endothelial cells) with the consequence that circulating eRNA (from arterial and coronary sinus blood) became substantially decreased [15]. Moreover, in rats undergoing the RIC procedure, RNase1 significantly rose in animals receiving buprenorphine or isoflurane, when compared with RIC without these drugs, implying a significant contribution of the RIC-dependent endothelial RNase1 release for improving the outcome of cardiac IRI. Yet, the exact mechanism of RNase1-induced cardioprotection still remains to be uncovered, although split products of RNA, such as nucleotides or nucleosides, may be valuable candidates to confer cardioprotection. It is also possible that medical preconditioning exhausts the conditioning capacity of mammalians, such that RIC may not promote additional preconditioning anymore.
“Macrophage migration inhibition factor” (MIF) family proteins feature overlapping properties with protein-type alarmins, such as high mobility group binding protein-1 (HMGB-1) or RNase1. MIF is known to be a pleiotropic inflammatory cytokine with chemokine-like functions and as such is a prototypical member of the emerging class of atypical or chemokine-like function proteins [95]. MIF is also a major pro-atherogenic factor, but counter-intuitively exhibits protective activities in the heart, damaged by IRI [6, 65]. Interestingly, the cardioprotective effects of MIF are predominant in the early reperfusion phase [74] and are amplified by post-translational modifications, such as S-nitrosylation and N-oxidation. In line with these observations, exogenously administered (“unmodified”) recombinant MIF was unable to convey RIC in a Langendorff heart model [78]. Moreover, MIF produced in the later phase and exacerbated IRI through promoting inflammatory leukocyte infiltration and activation [20, 76]. The role of novel MIF family member MIF-2/D-DT is currently unclear, as its knockout in a mouse model led to an exacerbation of infarct size, while its levels in patients undergoing coronary artery bypass grafting are predictive of ischemia–reperfusion outcome parameters, such as acute kidney injury [92], clearly necessitating the application of additional models on this and to-be-discovered family members, as well as on the MIF/MIF-2 double knockout mouse.
Ischemia/reperfusion injury and cardiac conditioning: clinical applications
The translation of the RIC procedure to patients has been challenging, because improvement of clinical outcome has been observed only in a minority of studies with RIC and findings are not consistent [30, 41, 80]. The explanation for the difficulties in translating the beneficial findings in experimental animals to humans remains unclear and may include confounding factors, such as age, co-morbidity, co-medication and anesthetic regimen in procedures requiring general anesthesia [10, 23, 49]. The intensity of the conditioning procedure also seems important and depends on the number and duration of the conditioning cycles, thereby defining the efficacy of protection by a specific algorithm [36, 45]. The indicated intervention can be initiated already in the ambulance during transportation to the PCI table and increases myocardial salvage leading to improved left ventricular function and outcome when used as an adjunct to primary PCI [90]. In the clinical setting, RIC was found to be effective in patients with diabetes mellitus and other cardiovascular risk factors as well [91, 105].
Yet, in two recent multicentre double-blind randomised controlled clinical trials (RIP-Heart, ERICCA) [64, 105], neutral results were reported upon application of RIC in patients, whereby the type of pre-operative medication appears to have a major impact on the outcome as well [29, 37]. Interestingly enough, both neuronal and vascular factors seem to play an important role in promoting RIC, as demonstrated in experimental animal and human studies [36, 57, 73]. Nevertheless, interventional cardiological procedures in acute angioplasty for ST-segment elevation MI seem to hold the best potential at present.
Despite the successful basic and clinical ischemic-reperfusion research for three decades, no definitive cardioprotective drugs are available yet. The lack of successful translation of experimental results into the clinical setting may be due to (1) the lack of proper co-morbidity experimental models as well as (2) the existence of hypothesis-driven-biased approaches to find molecular targets. Indeed, major cardiovascular co-morbidities, such as hypertension, hyper-lipidemia, diabetes, and their co-medications, interfere with most of the known cardioprotective mechanisms [23]. Moreover, cardiovascular co-morbidities as well as the available conditioning procedures affect the global myocardial gene expression profile at the transcriptional level and the fine-tuning regulators of translation, such as miRNAs [97, 98]. Thus, the comprehensive analysis of the cardioprotective gene expression fingerprint at the transcription and protein level in normal, protected, and in co-morbid probands may lead to the identification of novel molecular targets for cardioprotection by an unbiased non-hypothesis-driven approach.
Like other co-morbidities, chronic kidney disease (CKD) is known as a major risk factor of cardiovascular events and mortality after MI [104]; yet, the contribution of CKD to myocardial IRI remains unclear. In systematic studies, the influence of CKD on cytoprotective signaling was analyzed using a rat model of CKD, particularly addressing the functional role of Akt-phosphorylation. The results indicate that CKD suppresses Akt-activation upon reperfusion with the disruption of protective signaling to mitochondria, associated with infarct size enlargement. Moreover, the impact of CKD on infarct size depends on the severity of CKD that is directly related to insufficient activation of Akt at the time of reperfusion. Chronic treatment with an erythropoietin receptor ligand prevented CKD-induced enlargement of myocardial infarct size by restoration of Akt-mediated signaling possibly via normalized malate-aspartate shuttle flux in cardiomyocytes [69]. These promising data may provide clues for deciphering other co-morbidities and their mechanistic relations to the metabolic status in the context of cardiovascular diseases (Fig. 1).
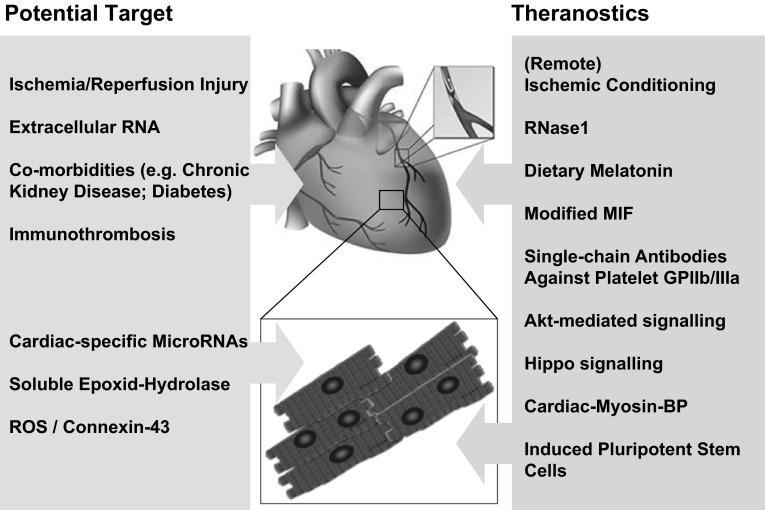
Fig. 1.
Potential new targets and theranostics in cardio-protection. The basic mechanisms, preclinical models and some clinical applications of several cardio-destructive pathologies and cardiovascular diseases (red box) are discussed in the text. Existing and novel antagonistic procedures as well as the related theranostics (green box), both in vitro and in experimental models, were found to promote cardio-protection on different molecular levels, particularly improving the functional status of cardiomyocytes (ROS reactive oxygen species, MIF macrophage migration inhibition factor, GP glycoprotein, BP binding protein)
With regard to the multi-ethnic population in a developing country like Malaysia, a pool of cardiac patients was drawn from both urban and rural areas for the analysis of acute coronary syndrome, being the leading cause of mortality. A striking feature was found in that the majority of patients were presented at a younger age group compared with similar populations in developed countries. While early intervention for ST-elevation MI has more relevance in urban areas with well-staffed care centres [96], substantial data from the voluntary “National Cardiovascular Disease Registry” (launched in 2006 in Malaysia) resulted in the improvement of risk stratification mechanisms for patients from rural areas [3]. Such strategies appear to serve as a role-model and can be relevant to the population at risk in other developing countries as well.
New players in cardiovascular diseases
The integrity and functionality of the monolayer vascular endothelium as a lively and dynamic barrier between the flowing blood and the underlying tissues, including the heart, depend on the natural implementation of distinct, ultralarge protein complexes at cell–cell borders, characteristic for adherens-, tight- und gap-junctions [81]. Newly described, highly dynamic structures, termed “Junction-associated intermittent lamellipodia” (JAIL), which are controlled by the WAVE-WASP/ARP2/3 protein complex, are driven by actin filament rearrangement to provide small plasma membrane protrusions that preferentially appear at junctional sites of endothelial cells with a low level of cadherin-5 (VE-cadherin) [1, 2, 84]. Thrombin as a pro-inflammatory mediator blocks JAIL formation and thus increases endothelial permeability. Such dynamic processes were made visible by stimulated emission depletion (STED) fluorescence microscopy and 3D reconstruction “structured illumination microscopy” (SIM). Since these structures are also disturbed by fluid shear stress and other stimulants, such as hypoxia, it remains to be analyzed in which way JAIL plays a role in, e.g., ischemia-driven disintegration of the endothelium in small and large cardiac vessels or during myocardial IRI.
An emerging new concept relates to innate immunity-related protective mechanism of the heart. In the context of ischemic heart disease, TNF-α, a major player of the immune system, initiates the induction of a cardioprotective signaling pathway [89] that involves the activation of the signal transducer and activator of transcription 3 (STAT-3) [70], designated as the SAFE (“Survivor Activating Factor Enhancement”) pathway [50, 53]. Toll-like receptor 4 (TLR4), sphingosine-1 phosphate, and activation of specific miRNAs are involved in this pathway as well [48]. In particular, TLR4 may trigger the activation of the SAFE pathway to promote cell survival following myocardial IRI [68]. Dietary melatonin, given at a concentration found in red wine, was demonstrated as respective trigger to confer cardioprotection [52] but also to prevent pulmonary hypertension via the activation of the SAFE pathway [59]. Based on the fact that high-density lipoproteins (HDL) can also activate the SAFE pathway, sphingosine-1 phosphate was identified as predominant component of HDL to protect against myocardial IRI [94], ultimately regulating the mitochondrial functions of cardiomyocytes [13]. With the detailed analysis of various lipoprotein subfractions (Lipoprint®), new insights into their composition and functionality in patients suffering from cardiovascular diseases are now available, and this may provide a personal cardiac risk calculator.
So far, microvascular obstruction has been linked to many vascular diseases, including stroke, myocardial infarction, thrombotic microangiopathies, infections, and cancer [39, 85]. Although the pathogenetic relevance of microangiopathies, such as haemolytic-uremic syndrome or disseminated intravascular coagulation, during sepsis has clearly been demonstrated, the pathogenetic mechanism of microvascular thrombosis has remained enigmatic. It has been assumed that the failure to assign a clear pathogenetic role to microvascular thrombosis in many diseases is due to difficulties in its detection and in the inability to assess the efficacy of antithrombotic treatments in the clinical situation. It has recently been shown that during systemic bacterial infections, microvascular thrombosis under certain conditions acts as an instrument of intravascular immunity [22, 62]. In organs, such as the liver and spleen, fibrin-rich microthrombi support the containment and elimination of Escherichia coli inside blood vessels and thereby prevent tissue invasion and dissemination of the pathogens. This mechanism has been termed “Immunothrombosis”. Immunothrombosis is suggested to form a major biological basis of pathological microvascular and macrovascular thrombosis (especially deep vein thrombosis), together with the physiological mechanism arresting bleeding (haemostasis). Immunothrombosis is a transient process as it appears to be normally resolved within 2 days. Pathological forms of microvascular thrombosis during infections, such as disseminated intravascular coagulation, are likely caused by an excessive activation of immunothrombosis and/or by its impaired resolution [82]. Most probably, the formation of microvascular thrombi under non-infectious conditions might equally be able to protect the intravascular compartment from damage as caused by immune complexes, circulating cell fragments, or endothelial damage. This would indicate that the beneficial nature of microvascular thrombosis may also apply to non-infectious conditions. Hence, the failure to document a pathological role for microvascular thrombosis under several pathological conditions could potentially, at least in part, be related to the fact that it is host-protective under those conditions.
Following atherectomy, drug-eluting stents allow a defined local application of anti-proliferative agents and other drugs to reduce neointimal formation and restenosis. Despite the tremendous success of drug-eluting stents using unselective cytostatic substances, their efficacy and safety need further improvement. However, strategies to achieve both of these goals are currently lacking. Likewise, myocardial remodeling and regeneration rely on myocardial capillary density and thus on effective neovascularization after MI. Yet, the mechanisms underlying myocardial angiogenesis and its regulation by miRNAs are not well defined. Comprehensive screens were established to analyze the expression of non-coding RNAs during the development of neointimal hyperplasia in established mouse models of atherogenesis, and particular miRNAs that are regulated in a lesion-, time- and cell-specific manner were identified. As an example, inhibition of miRNA-92a appeared to be safe as an effective treatment to prevent neointima formation as well as to improve re-endothelialization at the same time [19]. In addition, miRNA-146a was upregulated in the ischemic myocardium of mice following ligation of the left anterior descending artery in a time-dependent manner. In vitro, the overexpression of miRNA-146a significantly attenuated endothelial cell proliferation, migration, and abolished endothelial capillary network formation. In contrast, knock-down of miRNA-146a markedly augmented endothelial cell proliferation, migration, network formation, and sprouting (unpublished data). Mechanistically, NOX4, NOTCH1, and nRAS were identified and validated as direct targets of microRNA-146a in endothelial cells (unpublished data). In vivo, antagomirs against miRNA-146a significantly enhanced angiogenesis and re-vascularization in the infarcted myocardium, accompanied by preserved cardiac function and a markedly reduced infarct size (unpublished data). It is expected that additional cardiac-specific miRNAs become characterized that would serve as attractive targets for future therapeutic interventions in the treatment of ischemic heart disease.
Besides the well-known bioactive lipid mediators derived from arachidonic acid by cytochrome-P450-catalyzed reactions, other polyunsaturated fatty acids can be metabolized to epoxides and then diols by the actions of cytochrome P450 enzymes followed by soluble epoxide hydrolase (sEH). These metabolites appear to be ignored in their physiological relevance in the cardiovascular system, although they become generated in high concentrations. Deletion of sEH significantly delayed angiogenesis in the retina, a phenomenon associated with activation of the Notch signaling pathway [43]. sEH-deficient mice are also largely protected against the development of type 2 diabetes and the associated hypertension when fed a high fat diet. This occurs at the expense of the liver, as sEH controls the expression of key enzymes involved in lipid metabolism. Thus, inhibitors of sEH that increase epoxide, but decrease diol levels have potential for the treatment of the metabolic syndrome/type 2 diabetes (influencing cholesterol homeostasis) and its cardiovascular complications [60]. In which way the level of sEH may affect the processes during myocardial IRI as they relate to the disturbance of vascular integrity or the dysfunction of cardiomyocytes remains to be studied (Fig. 1).
Cardiac repair and regeneration
The understanding of developmental and regenerative processes of heart biology and cardiomyocyte proliferation and the underlying mechanisms may lead to their reactivation in diseased hearts postnatally and may have a great potential to protect or improve heart function. It is known that the mammalian heart loses its ability to regenerate, largely due to the fact that after birth cardiomyocytes fail to undergo cytokinesis. Instead, they exit the cell cycle after karyokinesis resulting in bi-nucleated cells, and cardiac tissue further expands by hypertrophy. Moreover, the efficiency in inducing cardiomyocyte proliferation decreases proportionally to cardiomyocyte age [54, 107]. Thus, an understanding how cardiomyocyte cytokinesis is regulated during development might provide new clues towards cardiac regeneration. Interestingly enough, cardiomyocyte centrosome integrity is lost shortly after birth in mammals. This is coupled with the relocalization of various centrosome proteins to the nuclear envelope. Consequently, postnatal cardiomyocytes are unable to undergo ciliogenesis, and the nuclear envelope adopts the function as cellular microtubule organizing center [108]. Loss of centrosome integrity is associated with, and can promote, cardiomyocyte G0/G1 cell cycle arrest, suggesting that centrosome disassembly is developmentally utilized to achieve the post-mitotic state in mammalian cardiomyocytes. In contrast, adult newt and zebrafish maintain the ability to regenerate their hearts through proliferation of cardiomyocytes, which also retain centrosome integrity. Based on these novel results, underlying the post-mitotic state of mammalian cardiomyocytes, potential mechanisms of heart regeneration in zebrafish and newts are testable that may provide clues for the regeneration of mammalian hearts. In addition, using systems biology approaches [24], novel regulators of cardiac development and regulatory networks were identified, integrating large-scale expression datasets. As an example, the joint analysis of a high-resolution temporal expression data set describing heart development and a transcriptomic dataset describing induced cardiomyocyte proliferation were merged and are currently being experimentally validated, eventually leading to novel candidate cytokine genes for cardiac regeneration.
Hippo signaling has been implicated in cardiac development and regeneration after myocardial injury. Genetic deletion of upstream Hippo signaling kinases (Mst1/2, Lats2, or Salvador) leads to an expansion of ventricular myocardium due to increased cardiomyocyte proliferation [31]. Global deletion of Yap results in the early embryonic lethality due to defects in multiple tissues, including yolk sac vasculogenesis, chorioallantoic fusion, and body axis elongation. However, Taz mutant mice are viable but develop glomerulocystic kidney disease and pulmonary disease. Genetic deletion of Yap and Taz both leads to the early embryonic lethality suggesting functional redundancy. Despite the studies described above, a role for Yap and Taz in the epicardium during coronary vasculature development has not been explored. Formation of the coronary vasculature is a complex and precisely coordinated morphogenetic process that begins with the formation of epicardium. The epicardium gives rise to many components of the coronary vasculature, including fibroblasts, smooth muscle cells, and the endothelium. While Hippo signaling mediators Yap and Taz are expressed in proepicardial and epicardial cells, a combination of genetic and pharmacological approaches that inhibited Hippo signaling mediators Yap and Taz also impaired epicardial epithelial-to-mesenchymal transition (EMT) as well as a reduction in epicardial cell proliferation and differentiation into coronary endothelial cells. As a conclusion, Yap and Taz control epicardial cell behavior, in part by regulating Tbx18 and Wt1 expression. These findings show a role for Hippo signaling in epicardial cell proliferation, EMT, and cell fate specification during cardiac organogenesis [88].
Cardiac mitochondria and cardioprotection
Reactive oxygen species (ROS) at high levels do play an adverse role in myocardial IRI, but contribute to endogenous cardioprotection at lower concentrations [35]. Moreover, the aforementioned conditioning protocols appear to recruit complex signaling cascades of activation of cardiomyocyte sarcolemmal receptors, intracellular enzymes, as well as ROS and nitrosative species, to gain mitochondrial stabilisation and finally to protect against cell death. The following questions remain to be addressed: What are the relevant sources of ROS in the cytosol and the mitochondria of cardiomyocytes? Which proteins/enzymes do contribute to ROS formation at different levels and how does the ROS-induced ROS release work in detail? A strong case can be made for the importance of the gap junction protein connexin 43 in the context of myocardial IRI and cardioprotection. Apart from being present at gap-junctions along cardiomyocyte cell borders, connexin 43 is also located at mitochondria and is involved in mitochondrial respiration, ATP generation, and mitochondrial potassium influx [8, 9, 11, 12, 55, 67, 83]. Blockade of connexin 43-formed channels reduces myocardial IRI, but, at the same time, also abolishes cardioprotection induced by ischemic preconditioning. Another redox-linked protein is p66SHC, which translocates into mitochondria where it catalyzes electron transfer from cytochrome c to oxygen resulting in ROS production [34]. Deletion of p66SHC, however, does not reduce infarct size in mice in vivo undergoing 30 min ischemia and 120 min reperfusion (unpublished data), but p66SHC contributes to vascular abnormalities related to diabetes and aging. On the other hand, ROS formation might contribute to self-endogenous defense against mild myocardial IRI [21], whereas p66SHC knockout does not affect endogenous cardioprotection (unpublished data).
It has been shown that the mitochondrial protein NDUFA4L2 plays an essential role in decreasing oxygen consumption and ROS production through inhibition of respiratory chain-complex I [93]. However, even though NDUFA4L2-induced mitochondrial repression has been proven by several research groups [51, 66], its physiological role remains unknown. It appears that the “hypoxia-inducible factor” (HIF)-NDUFA4L2 axis acts as one of the major pathways for cellular adaptation towards hypoxia via mitochondrial activity suppression. Along this line, NDUFA4L2 protein was highly expressed in heart tissue, whereby the cardiac fetal tissues exhibited highest levels when compared with tissues of adult mice. Finally, since the fetal heart is one of the sites that present higher levels of NDUFA4L2, a specific cardiac knockout mouse line was successfully generated by breeding a conditional NDUFA4L2 exon 2-floxed mice line with a with a heart-specific CRE (Nkx 2.5) transgenic line. However, these mice are not embryonically lethal and the role of cardiac NDUFA4L2 in adulthood warrants further investigation.
Diagnosis of cardiac damage and new theranostics
The diagnosis of “Non-ST elevation myocardial infarction” (NSTEMI) is dominated by the need to document an elevation in cardiac troponins I or T above the population-defined 99th centile. Yet, these biomarkers are released only slowly, thereby delaying the rule-in or the rule-out criteria for NSTEMI. The latest ESC guidelines attempt to circumvent this obstacle by adopting a ‘rule-out’ troponin value significantly below the 99th centile and a ‘rule-in’ value well above the 99th centile [77]. However, this leaves the majority of patients in an undefined diagnostic window, requiring repeated testing and further observation. A new cardiac biomarker, the “Cardiac myosin-binding protein C” (cMyC), had been recently introduced, which constitutes an abundant sarcomeric protein with a unique cardiac isoform that is released upon cardiac damage or iatrogenic MI much faster than the troponins [4, 26]. Using newly generated highly specific monoclonal antibodies, an ultrasensitive assay to measure cMyC in biological samples was established with a detection limit of 0.4 ng/l and intra- and inter-series precision around 10 % [61]. In ambulatory patients, cMyC concentration in plasma (median 12.23 ng/l) was linearly correlated with troponin levels, and both parameters showed a similar dependence on age, renal function, or left ventricular activity. In another patient cohort with aortic stenosis, cMyC levels were strongly related to fibrosis (detected by cardiac magnetic resonance imaging) and clinical outcome. It is expected that cMyC will be introduced into the clinics as new diagnostic parameter, e.g., after spontaneous, type 1 MI [46] as well as in other groups of cardiac patients to obtain fast and reliable results on the degree of cardiac damage.
Besides diagnostic markers, there is also need for new prognostic biomarkers. With the rise in obesity and its associated metabolic complications, many more patients will be considered at high risk of cardiovascular disease. Circulating miRNAs have recently evolved as novel players in the field of medicine [102]. Platelets contain and release miRNAs and are a major source of abundant miRNAs in plasma and in serum. There is a striking correlation of miRNAs with platelet activation markers in the general population and platelet-derived miRNAs in plasma correlate with indices of platelet function in patients on dual anti-platelet therapy [47]. Moreover, platelet miRNAs appear to alter their function, most probably by influencing gene expression in megakaryocytes [47]. Since thrombus formation is a key event in triggering the clinical manifestations of atherosclerotic disease, it could be informative to assess platelet activation in the context of cardiovascular risk. Similarly, circulating angiogenic miRNAs have been linked to the onset and progression of retinopathy in patients with type 1 diabetes [106].
To provide an efficient antithrombotic therapy in the context of cardiovascular diseases, the detection and elimination of thrombi and emboli are the major challenge in clinical practice. Here, the site-directed molecular imaging and the associated therapeutic targeting of platelet-specific antigens is a highly promising approach. An established target in the therapy of cardiovascular disease is the integrin αIIb-β3 (GP IIb/IIIa, CD41/CD61), the highly abundant fibrinogen receptor on the activated platelet surface. Since this integrin undergoes a conformational change upon platelet activation, the exposed characteristic epitopes can be used as thrombus-specific targets. Following the screening by phage-display of PCR-cloned human single-chain antibodies, highly specific integrin antagonists were generated. These were coupled by genetic, chemical, or biological approaches to obtain various fusion products, which are available either as therapeutic drugs or in combination with contrast particles. Using these approaches, various anticoagulants, anti-platelet drugs, or fibrinolytics were specifically targeted to the thrombus site to achieve effective thrombolysis and prevention of emboli without any bleeding complications in mice [42]. Moreover, these procedures allow the design of targeted “theranostic compounds”, such as ultrasound micro-bubbles, magnetic resonance nanoparticles, or positron emission tomography tracers, for thrombus detection with high sensitivity and specificity in the respective imaging modality [101]. Compounds are underway that allows the combination of detection and imaging of thrombi with concomitant effective treatment together with the monitoring of success or failure of therapy [100] (Fig. 1).
Human-induced pluripotent stem cell (iPSC)-derived cardiomyocytes present a tremendous opportunity for the study of cardiac arrhythmias in vitro. The characterization of cellular models for major subtypes of inherited channelopathy revealed that these defects were caused by dysfunctional potassium and sodium channels that contribute to the Long QT Syndrome (LQTS) 1, LQTS2, and LQTS3 [63]. In particular, the correction of the trafficking of KCNH2 (LQTS2) potassium channel through intracellular mechanisms restored hERG currents and reduced arrhythmia in LQTS2 patient-derived cardiomyocytes, also documenting the usefulness of iPSC-cardiomyocytes in LQTS2 modeling and drug testing. Moreover, iPSC-cardiomyocytes were used as a tool to evaluate the cardiac toxicity of topical drugs [58]. These applications document the powerful iPSC technology as value creation for understanding the pathomechanisms of cardiac arrhythmias, but also for drug testing and toxicology research.
Acknowledgments
The herein cited work was supported as follows: HACF is funded by a Startup Grant of the “Excellence Cluster Cardio-Pulmonary System” (ECCPS) from the German Research Foundation (DFG, Bonn, Germany) and the “Peter und Traudl Engelhorn-Stiftung” (Weilheim, Germany). Part of the work presented by HACF, WAB, and KTP is supported by the Russian Government Program for competitive growth of Kazan Federal University, Kazan (Russian Federation). JA is supported by Grants from the Ministerio de Economía y Competitividad (SAF2013-46058-R), Comunidad de Madrid/Fondo Social Europeo (S2010/BMD-2542 “Consepoc-CM”) and Red de Cardiovascular (RD12/0042/0065). JB is supported by Deutsche Forschungsgemeinschaft (DFG) Grants BE 1977/9-1, SFB1123 (Project A03), by DFG within the framework of the Munich Cluster for Systems Neurology (EXC 1010 SyNergy), and by Else Kröner-Fresenius Stiftung (EKFS) Grant 2014_A216. HB is supported by the Danish Council for Strategic Research (11-115818), Trygfonden, NovoNordisk Fonden. WAB is funded by NIH Grants HL075677 and HL081863. PF is supported by Grants from the Hungarian Scientific Research Fund (OTKA ANN 107803, OTKA K-105555). This work was supported by the Interdisciplinary Centre for Clinical Research Erlangen (IZKF projects J42 to FF and F3 to FBE), and the Emerging Fields Initiative Cell “Cycle in Disease and Regeneration (CYDER)” (Friedrich-Alexander-University Erlangen-Nürnberg, to FBE). IF is funded by the Deutsche Forschungsgemeinschaft (SFB 1039/A6). SL is funded from Winetech, National Research Foundation. This study was supported by the Interdisciplinary Centre for Clinical Research IZKF Aachen (junior research group to EAL). MMarber is supported by Grants from the Medical Research Council (UK) (G1000737), Guy’s and St Thomas’ Charity (R060701, R100404), British Heart Foundation (TG/15/1/31518), and the UK Department of Health through the National Institute for Health Research Biomedical Research Centre award to Guy’s and St Thomas’ NHS Foundation Trust. SBO is supported by a Khoo Postdoctoral Fellowship Award (Duke-NUS-KPFA/2016/0010) from the Estate of Tan Sri Khoo Teck Puat, Singapore. This work is supported by a German Research Foundation (Cluster of excellence REBIRTH) Grant to DS. MKS is supported by funds from Duke-NUS Medical School Singapore, Goh foundation and Singapore National Research Foundation (NRF) fellowship (NRF-NRFF2016-01). This work is supported by the German Research Council DFG, INST 2105/24-1 and SCHN 430/6-2 to HS. RS is supported by the German Research Foundation (DFG, Bonn, Germany) Schu843/9-1. This work was supported by funds from the National Medical Research Council (NMRC/BNIG/1074/2012), Goh Foundation/Duke-NUS Medical School (GCR/2013/008, GCR/2013/010 and GCR/2013/011), SingHealth Foundation (SHF/FG569P/2014 and SHF/FG630S/2014), Biomedical Research Council Singapore (BMRC13/1/96/19/686A), and Singapore National Research Foundation (NRF) Competitive Research Program (NRF-CRP-2008-02) to WS. MW acknowledges funding support from the Cardiometabolic Board of the Biomedical Research Centre at UCL, funded by the NIHR UK.
Compliance with ethical standards
Conflict of interest
HEB is a shareholder of CellAegis Inc.
References
- 1.Abu Taha A, Schnittler HJ. Dynamics between actin and the VE-cadherin/catenin complex: novel aspects of the ARP2/3 complex in regulation of endothelial junctions. Cell Adh Migr. 2014;8:125–135. doi: 10.4161/cam.28243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu Taha A, Taha M, Seebach J, Schnittler HJ. ARP2/3-mediated junction-associated lamellipodia control VE-cadherin-based cell junction dynamics and maintain monolayer integrity. Mol Biol Cell. 2014;25:245–256. doi: 10.1091/mbc.E13-07-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad WA, Ali RM, Khanom M, Han CK, Bang LH, Yip AF, Ghazi AM, Ismail O, Zambahari R, Hian SK. The journey of Malaysian NCVD-PCI (National Cardiovascular Disease Database-Percutaneous Coronary Intervention) Registry: a summary of three years report. Int J Cardiol. 2013;165:161–164. doi: 10.1016/j.ijcard.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Baker JO, Tyther R, Liebetrau C, Clark J, Howarth R, Patterson T, Mollmann H, Nef H, Sicard P, Kailey B, Devaraj R, Redwood SR, Kunst G, Weber E, Marber MS. Cardiac myosin-binding protein C: a potential early biomarker of myocardial injury. Basic Res Cardiol. 2015;110:23. doi: 10.1007/s00395-015-0478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell RM, Botker HE, Carr RD, Davidson SM, Downey JM, Dutka DP, Heusch G, Ibanez B, Macallister R, Stoppe C, Ovize M, Redington A, Walker JM, Yellon DM. 9th Hatter Biannual Meeting: position document on ischaemia/reperfusion injury, conditioning and the ten commandments of cardioprotection. Basic Res Cardiol. 2016;111:41. doi: 10.1007/s00395-016-0558-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, Dewor M, Georgiev I, Schober A, Leng L, Kooistra T, Fingerle-Rowson G, Ghezzi P, Kleemann R, McColl SR, Bucala R, Hickey MJ, Weber C. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med. 2007;13:587–596. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- 7.Bliksoen M, Mariero LH, Torp MK, Baysa A, Ytrehus K, Haugen F, Seljeflot I, Vaage J, Valen G, Stenslokken KO. Extracellular mtDNA activates NF-kappaB via toll-like receptor 9 and induces cell death in cardiomyocytes. Basic Res Cardiol. 2016;111:42. doi: 10.1007/s00395-016-0553-6. [DOI] [PubMed] [Google Scholar]
- 8.Boengler K, Dodoni G, Rodriguez-Sinovas A, Cabestrero A, Ruiz-Meana M, Gres P, Konietzka I, Lopez-Iglesias C, Garcia-Dorado D, Di Lisa F, Heusch G, Schulz R. Connexin 43 in cardiomyocyte mitochondria and its increase by ischemic preconditioning. Cardiovasc Res. 2005;67:234–244. doi: 10.1016/j.cardiores.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Boengler K, Ruiz-Meana M, Gent S, Ungefug E, Soetkamp D, Miro-Casas E, Cabestrero A, Fernandez-Sanz C, Semenzato M, Di Lisa F, Rohrbach S, Garcia-Dorado D, Heusch G, Schulz R. Mitochondrial connexin 43 impacts on respiratory complex I activity and mitochondrial oxygen consumption. J Cell Mol Med. 2012;16:1649–1655. doi: 10.1111/j.1582-4934.2011.01516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boengler K, Schulz R, Heusch G. Loss of cardioprotection with ageing. Cardiovasc Res. 2009;83:247–261. doi: 10.1093/cvr/cvp033. [DOI] [PubMed] [Google Scholar]
- 11.Boengler K, Stahlhofen S, van de Sand A, Gres P, Ruiz-Meana M, Garcia-Dorado D, Heusch G, Schulz R. Presence of connexin 43 in subsarcolemmal, but not in interfibrillar cardiomyocyte mitochondria. Basic Res Cardiol. 2009;104:141–147. doi: 10.1007/s00395-009-0007-5. [DOI] [PubMed] [Google Scholar]
- 12.Boengler K, Ungefug E, Heusch G, Leybaert L, Schulz R. Connexin 43 impacts on mitochondrial potassium uptake. Front Pharmacol. 2013;4:73. doi: 10.3389/fphar.2013.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brulhart-Meynet MC, Braunersreuther V, Brinck J, Montecucco F, Prost JC, Thomas A, Galan K, Pelli G, Pedretti S, Vuilleumier N, Mach F, Lecour S, James RW, Frias MA. Improving reconstituted HDL composition for efficient post-ischemic reduction of ischemia reperfusion injury. PLoS One. 2015;10:e0119664. doi: 10.1371/journal.pone.0119664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cabrera-Fuentes HA, Alba-Alba C, Aragones J, Bernhagen J, Boisvert WA, Botker HE, Cesarman-Maus G, Fleming I, Garcia-Dorado D, Lecour S, Liehn E, Marber MS, Marina N, Mayr M, Perez-Mendez O, Miura T, Ruiz-Meana M, Salinas-Estefanon EM, Ong SB, Schnittler HJ, Sanchez-Vega JT, Sumoza-Toledo A, Vogel CW, Yarullina D, Yellon DM, Preissner KT, Hausenloy DJ. Meeting report from the 2nd International Symposium on New Frontiers in Cardiovascular Research. Protecting the cardiovascular system from ischemia: between bench and bedside. Basic Res Cardiol. 2016;111:7. doi: 10.1007/s00395-015-0527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cabrera-Fuentes HA, Niemann B, Grieshaber P, Wollbrueck M, Gehron J, Preissner KT, Boning A. RNase1 as a potential mediator of remote ischaemic preconditioning for cardioprotectiondagger. Eur J Cardiothorac Surg. 2015;48(5):732–737. doi: 10.1093/ejcts/ezu519. [DOI] [PubMed] [Google Scholar]
- 16.Cabrera-Fuentes HA, Preissner KT. Abstract 20396: Induction of ischemia–reperfusion injury by extracellular RNA: a case for tumor necrosis factor (TNF-α)—shedding. Circulation. 2014;130:A20396. [Google Scholar]
- 17.Cabrera-Fuentes HA, Ruiz-Meana M, Simsekyilmaz S, Kostin S, Inserte J, Saffarzadeh M, Galuska SP, Vijayan V, Barba I, Barreto G, Fischer S, Lochnit G, Ilinskaya ON, Baumgart-Vogt E, Boning A, Lecour S, Hausenloy DJ, Liehn EA, Garcia-Dorado D, Schluter KD, Preissner KT. RNase1 prevents the damaging interplay between extracellular RNA and tumour necrosis factor-alpha in cardiac ischaemia/reperfusion injury. Thromb Haemost. 2014;112:1110–1119. doi: 10.1160/TH14-08-0703. [DOI] [PubMed] [Google Scholar]
- 18.Cochain C, Zernecke A. Macrophages and immune cells in atherosclerosis: recent advances and novel concepts. Basic Res Cardiol. 2015;110:34. doi: 10.1007/s00395-015-0491-8. [DOI] [PubMed] [Google Scholar]
- 19.Daniel JM, Penzkofer D, Teske R, Dutzmann J, Koch A, Bielenberg W, Bonauer A, Boon RA, Fischer A, Bauersachs J, van Rooij E, Dimmeler S, Sedding DG. Inhibition of miR-92a improves re-endothelialization and prevents neointima formation following vascular injury. Cardiovasc Res. 2014;103:564–572. doi: 10.1093/cvr/cvu162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dayawansa NH, Gao XM, White DA, Dart AM, Du XJ. Role of MIF in myocardial ischaemia and infarction: insight from recent clinical and experimental findings. Clin Sci (Lond) 2014;127:149–161. doi: 10.1042/CS20130828. [DOI] [PubMed] [Google Scholar]
- 21.Di Lisa F, Giorgio M, Ferdinandy P, Schulz R. New aspects of p66Shc in ischemia reperfusion injury and cardiovascular diseases. Br J Pharmacol. 2016 doi: 10.1111/bph.13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 23.Ferdinandy P, Hausenloy DJ, Heusch G, Baxter GF, Schulz R. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev. 2014;66:1142–1174. doi: 10.1124/pr.113.008300. [DOI] [PubMed] [Google Scholar]
- 24.Ferrazzi F, Bellazzi R, Engel FB. Gene network analysis: from heart development to cardiac therapy. Thromb Haemost. 2015;113:522–531. doi: 10.1160/TH14-06-0483. [DOI] [PubMed] [Google Scholar]
- 25.Fischer S, Cabrera-Fuentes HA, Noll T, Preissner KT. Impact of extracellular RNA on endothelial barrier function. Cell Tissue Res. 2014;355:635–645. doi: 10.1007/s00441-014-1850-8. [DOI] [PubMed] [Google Scholar]
- 26.Giannitsis E, Katus HA. A long way to translation: will cMyC survive? Basic Res Cardiol. 2015;110:24. doi: 10.1007/s00395-015-0479-4. [DOI] [PubMed] [Google Scholar]
- 27.Hausenloy DJ, Baxter G, Bell R, Botker HE, Davidson SM, Downey J, Heusch G, Kitakaze M, Lecour S, Mentzer R, Mocanu MM, Ovize M, Schulz R, Shannon R, Walker M, Walkinshaw G, Yellon DM. Translating novel strategies for cardioprotection: the Hatter Workshop Recommendations. Basic Res Cardiol. 2010;105:677–686. doi: 10.1007/s00395-010-0121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hausenloy DJ, Botker HE, Engstrom T, Erlinge D, Heusch G, Ibanez B, Kloner RA, Ovize M, Yellon DM, Garcia-Dorado D. Targeting reperfusion injury in patients with ST-segment elevation myocardial infarction: trials and tribulations. Eur Heart J. 2016 doi: 10.1093/eurheartj/ehw145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, Knight R, Kunst G, Laing C, Nicholas JM, Pepper J, Robertson S, Xenou M, Clayton T, Yellon DM (2016) Effect of remote ischaemic preconditioning on clinical outcomes in patients undergoing coronary artery bypass graft surgery (ERICCA study): a multicentre double-blind randomised controlled clinical trial, Southampton. doi:10.3310/eme03040 [PubMed]
- 30.Hausenloy DJ, Yellon DM. Targeting myocardial reperfusion injury—the search continues. N Engl J Med. 2015;373:1073–1075. doi: 10.1056/NEJMe1509718. [DOI] [PubMed] [Google Scholar]
- 31.Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, Martin JF. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heusch G. Cardioprotection: chances and challenges of its translation to the clinic. Lancet. 2013;381:166–175. doi: 10.1016/S0140-6736(12)60916-7. [DOI] [PubMed] [Google Scholar]
- 33.Heusch G. The coronary circulation as a target of cardioprotection. Circ Res. 2016;118:1643–1658. doi: 10.1161/CIRCRESAHA.116.308640. [DOI] [PubMed] [Google Scholar]
- 34.Heusch G. Mitochondria at the heart of cardiovascular protection: p66shc-friend or foe? Eur Heart J. 2015;36:469–471. doi: 10.1093/eurheartj/ehu409. [DOI] [PubMed] [Google Scholar]
- 35.Heusch G. Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res. 2015;116:674–699. doi: 10.1161/CIRCRESAHA.116.305348. [DOI] [PubMed] [Google Scholar]
- 36.Heusch G, Botker HE, Przyklenk K, Redington A, Yellon D. Remote ischemic conditioning. J Am Coll Cardiol. 2015;65:177–195. doi: 10.1016/j.jacc.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heusch G, Gersh BJ. ERICCA and RIPHeart: two nails in the coffin for cardioprotection by remote ischemic conditioning? Probably not! Eur Heart J. 2016;37:200–202. doi: 10.1093/eurheartj/ehv606. [DOI] [PubMed] [Google Scholar]
- 38.Heusch G, Gersh BJ. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge. Eur Heart J. 2016 doi: 10.1093/eurheartj/ehw224. [DOI] [PubMed] [Google Scholar]
- 39.Heusch G, Kleinbongard P, Skyschally A. Myocardial infarction and coronary microvascular obstruction: an intimate, but complicated relationship. Basic Res Cardiol. 2013;108:380. doi: 10.1007/s00395-013-0380-y. [DOI] [PubMed] [Google Scholar]
- 40.Heusch G, Libby P, Gersh B, Yellon D, Bohm M, Lopaschuk G, Opie L. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet. 2014;383:1933–1943. doi: 10.1016/S0140-6736(14)60107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heusch G, Rassaf T. Time to give up on cardioprotection? A critical appraisal of clinical studies on ischemic pre-, post-, and remote conditioning. Circ Res. 2016;119:676–695. doi: 10.1161/CIRCRESAHA.116.308736. [DOI] [PubMed] [Google Scholar]
- 42.Hohmann JD, Wang X, Krajewski S, Selan C, Haller CA, Straub A, Chaikof EL, Nandurkar HH, Hagemeyer CE, Peter K. Delayed targeting of CD39 to activated platelet GPIIb/IIIa via a single-chain antibody: breaking the link between antithrombotic potency and bleeding? Blood. 2013;121:3067–3075. doi: 10.1182/blood-2012-08-449694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu J, Popp R, Fromel T, Ehling M, Awwad K, Adams RH, Hammes HP, Fleming I. Muller glia cells regulate Notch signaling and retinal angiogenesis via the generation of 19,20-dihydroxydocosapentaenoic acid. J Exp Med. 2014;211:281–295. doi: 10.1084/jem.20131494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ibanez B, Heusch G, Ovize M, Van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol. 2015;65:1454–1471. doi: 10.1016/j.jacc.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 45.Johnsen J, Pryds K, Salman R, Lofgren B, Kristiansen SB, Botker HE. The remote ischemic preconditioning algorithm: effect of number of cycles, cycle duration and effector organ mass on efficacy of protection. Basic Res Cardiol. 2016;111:10. doi: 10.1007/s00395-016-0529-6. [DOI] [PubMed] [Google Scholar]
- 46.Kaier TE, Anand A, Shah AS, Mills NL, Marber M. Temporal relationship between cardiac myosin-binding protein C and cardiac troponin I in type 1 myocardial infarction. Clin Chem. 2016;62:1153–1155. doi: 10.1373/clinchem.2016.257188. [DOI] [PubMed] [Google Scholar]
- 47.Kaudewitz D, Skroblin P, Bender LH, Barwari T, Willeit P, Pechlaner R, Sunderland NP, Willeit K, Morton AC, Armstrong PC, Chan MV, Lu R, Yin X, Gracio F, Dudek K, Langley SR, Zampetaki A, de Rinaldis E, Ye S, Warner TD, Saxena A, Kiechl S, Storey RF, Mayr M. Association of MicroRNAs and YRNAs with platelet function. Circ Res. 2016;118:420–432. doi: 10.1161/CIRCRESAHA.114.305663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kelly-Laubscher RF, King JC, Hacking D, Somers S, Hastie S, Stewart T, Imamdin A, Maarman G, Pedretti S, Lecour S. Cardiac preconditioning with sphingosine-1-phosphate requires activation of signal transducer and activator of transcription-3. Cardiovasc J Afr. 2014;25:118–123. doi: 10.5830/CVJA-2014-016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kleinbongard P, Neuhauser M, Thielmann M, Kottenberg E, Peters J, Jakob H, Heusch G. Confounders of cardioprotection by remote ischemic preconditioning in patients undergoing coronary artery bypass grafting. Cardiology. 2016;133:128–133. doi: 10.1159/000441216. [DOI] [PubMed] [Google Scholar]
- 50.Lacerda L, McCarthy J, Mungly SF, Lynn EG, Sack MN, Opie LH, Lecour S. TNFalpha protects cardiac mitochondria independently of its cell surface receptors. Basic Res Cardiol. 2010;105:751–762. doi: 10.1007/s00395-010-0113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lai RK, Xu IM, Chiu DK, Tse AP, Wei LL, Law CT, Lee D, Wong CM, Wong MP, Ng IO, Wong CC. NDUFA4L2 fine-tunes oxidative stress in hepatocellular carcinoma. Clin Cancer Res. 2016;22:3105–3117. doi: 10.1158/1078-0432.CCR-15-1987. [DOI] [PubMed] [Google Scholar]
- 52.Lamont K, Nduhirabandi F, Adam T, Thomas DP, Opie LH, Lecour S. Role of melatonin, melatonin receptors and STAT3 in the cardioprotective effect of chronic and moderate consumption of red wine. Biochem Biophys Res Commun. 2015;465:719–724. doi: 10.1016/j.bbrc.2015.08.064. [DOI] [PubMed] [Google Scholar]
- 53.Lecour S, James RW. When are pro-inflammatory cytokines SAFE in heart failure? Eur Heart J. 2011;32:680–685. doi: 10.1093/eurheartj/ehq484. [DOI] [PubMed] [Google Scholar]
- 54.Leone M, Magadum A, Engel FB. Cardiomyocyte proliferation in cardiac development and regeneration: a guide to methodologies and interpretations. Am J Physiol Heart Circ Physiol. 2015;309:H1237–H1250. doi: 10.1152/ajpheart.00559.2015. [DOI] [PubMed] [Google Scholar]
- 55.Li X, Heinzel FR, Boengler K, Schulz R, Heusch G. Role of connexin 43 in ischemic preconditioning does not involve intercellular communication through gap junctions. J Mol Cell Cardiol. 2004;36:161–163. doi: 10.1016/j.yjmcc.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 56.Liehn EA, Cabrera-Fuentes HA. Inflammation between defense and disease: impact on tissue repair and chronic sickness. Discoveries. 2015;3(1):e42. doi: 10.15190/d.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lim SY, Yellon DM, Hausenloy DJ. The neural and humoral pathways in remote limb ischemic preconditioning. Basic Res Cardiol. 2010;105:651–655. doi: 10.1007/s00395-010-0099-y. [DOI] [PubMed] [Google Scholar]
- 58.Lu J, Wei H, Wu J, Jamil MF, Tan ML, Adenan MI, Wong P, Shim W. Evaluation of the cardiotoxicity of mitragynine and its analogues using human induced pluripotent stem cell-derived cardiomyocytes. PLoS One. 2014;9:e115648. doi: 10.1371/journal.pone.0115648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maarman G, Blackhurst D, Thienemann F, Blauwet L, Butrous G, Davies N, Sliwa K, Lecour S. Melatonin as a preventive and curative therapy against pulmonary hypertension. J Pineal Res. 2015;59:343–353. doi: 10.1111/jpi.12263. [DOI] [PubMed] [Google Scholar]
- 60.Mangels N, Awwad K, Wettenmann A, Dos Santos LR, Fromel T, Fleming I. The soluble epoxide hydrolase determines cholesterol homeostasis by regulating AMPK and SREBP activity. Prostaglandins Other Lipid Mediat. 2016 doi: 10.1016/j.prostaglandins.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 61.Marjot J, Liebetrau C, Goodson RJ, Kaier T, Weber E, Heseltine P, Marber MS. The development and application of a high-sensitivity immunoassay for cardiac myosin-binding protein C. Transl Res. 2016;170(17–25):e11–e15. doi: 10.1016/j.trsl.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, Brinkmann V, Lorenz M, Bidzhekov K, Khandagale AB, Konrad I, Kennerknecht E, Reges K, Holdenrieder S, Braun S, Reinhardt C, Spannagl M, Preissner KT, Engelmann B. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16:887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 63.Mehta A, Sequiera GL, Ramachandra CJ, Sudibyo Y, Chung Y, Sheng J, Wong KY, Tan TH, Wong P, Liew R, Shim W. Re-trafficking of hERG reverses long QT syndrome 2 phenotype in human iPS-derived cardiomyocytes. Cardiovasc Res. 2014;102:497–506. doi: 10.1093/cvr/cvu060. [DOI] [PubMed] [Google Scholar]
- 64.Meybohm P, Bein B, Brosteanu O, Cremer J, Gruenewald M, Stoppe C, Coburn M, Schaelte G, Boning A, Niemann B, Roesner J, Kletzin F, Strouhal U, Reyher C, Laufenberg-Feldmann R, Ferner M, Brandes IF, Bauer M, Stehr SN, Kortgen A, Wittmann M, Baumgarten G, Meyer-Treschan T, Kienbaum P, Heringlake M, Schon J, Sander M, Treskatsch S, Smul T, Wolwender E, Schilling T, Fuernau G, Hasenclever D, Zacharowski K, Collaborators RIS. A multicenter trial of remote ischemic preconditioning for heart surgery. N Engl J Med. 2015;373:1397–1407. doi: 10.1056/NEJMoa1413579. [DOI] [PubMed] [Google Scholar]
- 65.Miller EJ, Li J, Leng L, McDonald C, Atsumi T, Bucala R, Young LH. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature. 2008;451:578–582. doi: 10.1038/nature06504. [DOI] [PubMed] [Google Scholar]
- 66.Minton DR, Fu L, Mongan NP, Shevchuk MM, Nanus DM, Gudas LJ. Role of NADH dehydrogenase (ubiquinone) 1 alpha subcomplex 4-like 2 in clear cell renal cell carcinoma. Clin Cancer Res. 2016;22:2791–2801. doi: 10.1158/1078-0432.CCR-15-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miro-Casas E, Ruiz-Meana M, Agullo E, Stahlhofen S, Rodriguez-Sinovas A, Cabestrero A, Jorge I, Torre I, Vazquez J, Boengler K, Schulz R, Heusch G, Garcia-Dorado D. Connexin43 in cardiomyocyte mitochondria contributes to mitochondrial potassium uptake. Cardiovasc Res. 2009;83:747–756. doi: 10.1093/cvr/cvp157. [DOI] [PubMed] [Google Scholar]
- 68.Nduhirabandi F, Lamont K, Albertyn Z, Opie LH, Lecour S. Role of toll-like receptor 4 in melatonin-induced cardioprotection. J Pineal Res. 2016;60:39–47. doi: 10.1111/jpi.12286. [DOI] [PubMed] [Google Scholar]
- 69.Nishizawa K, Yano T, Tanno M, Miki T, Kuno A, Tobisawa T, Ogasawara M, Muratsubaki S, Ohno K, Ishikawa S, Miura T. Chronic treatment with an erythropoietin receptor ligand prevents chronic kidney disease-induced enlargement of myocardial infarct size. Hypertension. 2016;68(3):697–706. doi: 10.1161/HYPERTENSIONAHA.116.07480. [DOI] [PubMed] [Google Scholar]
- 70.O’Sullivan KE, Breen EP, Gallagher HC, Buggy DJ, Hurley JP. Understanding STAT3 signaling in cardiac ischemia. Basic Res Cardiol. 2016;111:27. doi: 10.1007/s00395-016-0543-8. [DOI] [PubMed] [Google Scholar]
- 71.Organization WHO (2014) Global status report on noncommunicable diseases (online)
- 72.Pickard JM, Botker HE, Crimi G, Davidson B, Davidson SM, Dutka D, Ferdinandy P, Ganske R, Garcia-Dorado D, Giricz Z, Gourine AV, Heusch G, Kharbanda R, Kleinbongard P, MacAllister R, McIntyre C, Meybohm P, Prunier F, Redington A, Robertson NJ, Suleiman MS, Vanezis A, Walsh S, Yellon DM, Hausenloy DJ. Remote ischemic conditioning: from experimental observation to clinical application: report from the 8th Biennial Hatter Cardiovascular Institute Workshop. Basic Res Cardiol. 2015;110:453. doi: 10.1007/s00395-014-0453-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pickard JM, Davidson SM, Hausenloy DJ, Yellon DM. Co-dependence of the neural and humoral pathways in the mechanism of remote ischemic conditioning. Basic Res Cardiol. 2016;111:50. doi: 10.1007/s00395-016-0568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pohl J, Hendgen-Cotta UB, Rammos C, Luedike P, Mull E, Stoppe C, Julicher K, Lue H, Merx MW, Kelm M, Bernhagen J, Rassaf T. Targeted intracellular accumulation of macrophage migration inhibitory factor in the reperfused heart mediates cardioprotection. Thromb Haemost. 2016;115:200–212. doi: 10.1160/TH15-05-0436. [DOI] [PubMed] [Google Scholar]
- 75.Preissner KT, Boisvert WA, Hausenloy DJ. Surfing on the cardiovascular frontier wave. Thromb Haemost. 2015;113:439–440. doi: 10.1160/TH15-01-0086. [DOI] [PubMed] [Google Scholar]
- 76.Rassaf T, Weber C, Bernhagen J. Macrophage migration inhibitory factor in myocardial ischaemia/reperfusion injury. Cardiovasc Res. 2014;102:321–328. doi: 10.1093/cvr/cvu071. [DOI] [PubMed] [Google Scholar]
- 77.Roffi M, Patrono C, Zamorano JL. CardioPulse: what’s new in the 2015 European Society of Cardiology Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2016;37:206–207. doi: 10.1093/eurheartj/ehv654. [DOI] [PubMed] [Google Scholar]
- 78.Rossello X, Burke N, Stoppe C, Bernhagen J, Davidson SM, Yellon DM. Exogenous administration of recombinant mif at physiological concentrations failed to attenuate infarct size in a Langendorff perfused isolated mouse heart model. Cardiovasc Drugs Ther. 2016 doi: 10.1007/s10557-016-6673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saffarzadeh M, Cabrera-Fuentes HA, Veit F, Jiang D, Scharffetter-Kochanek K, Gille C, Rooijakkers SHM, Hartl D, Preissner KT. Characterization of rapid neutrophil extracellular trap formation and its cooperation with phagocytosis in human neutrophils. Discoveries. 2014;2:e19. doi: 10.15190/d.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sardar P, Chatterjee S, Kundu A, Samady H, Owan T, Giri J, Nairooz R, Selzman CH, Heusch G, Gersh BJ, Abbott JD, Mukherjee D, Fang JC. Remote ischemic preconditioning in patients undergoing cardiovascular surgery: evidence from a meta-analysis of randomized controlled trials. Int J Cardiol. 2016;221:34–41. doi: 10.1016/j.ijcard.2016.06.325. [DOI] [PubMed] [Google Scholar]
- 81.Schnittler H. Between sealing and leakiness: molecular dynamics of the endothelium to maintain and regulate barrier function. Cell Tissue Res. 2014;355:481–483. doi: 10.1007/s00441-014-1838-4. [DOI] [PubMed] [Google Scholar]
- 82.Schulz C, Engelmann B, Massberg S. Crossroads of coagulation and innate immunity: the case of deep vein thrombosis. J Thromb Haemost. 2013;11(Suppl 1):233–241. doi: 10.1111/jth.12261. [DOI] [PubMed] [Google Scholar]
- 83.Schulz R, Gorge PM, Gorbe A, Ferdinandy P, Lampe PD, Leybaert L. Connexin 43 is an emerging therapeutic target in ischemia/reperfusion injury, cardioprotection and neuroprotection. Pharmacol Ther. 2015;153:90–106. doi: 10.1016/j.pharmthera.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Seebach J, Taha AA, Lenk J, Lindemann N, Jiang X, Brinkmann K, Bogdan S, Schnittler HJ. The Cell BorderTracker, a novel tool to quantitatively analyze spatiotemporal endothelial junction dynamics at the subcellular level. Histochem Cell Biol. 2015;144:517–532. doi: 10.1007/s00418-015-1357-8. [DOI] [PubMed] [Google Scholar]
- 85.Serebruany VL, Cherepanov V, Cabrera-Fuentes HA, Kim MH. Solid cancers after antiplatelet therapy: confirmations, controversies, and challenges. Thromb Haemost. 2015;114:1104–1112. doi: 10.1160/TH15-01-0077. [DOI] [PubMed] [Google Scholar]
- 86.Simsekyilmaz S, Cabrera-Fuentes HA, Meiler S, Kostin S, Baumer Y, Liehn EA, Weber C, Boisvert WA, Preissner KT, Zernecke A. Response to letter regarding article “Role of extracellular RNA in atherosclerotic plaque formation in mice”. Circulation. 2014;130:e144–e145. doi: 10.1161/CIRCULATIONAHA.114.012346. [DOI] [PubMed] [Google Scholar]
- 87.Simsekyilmaz S, Cabrera-Fuentes HA, Meiler S, Kostin S, Baumer Y, Liehn EA, Weber C, Boisvert WA, Preissner KT, Zernecke A. Role of extracellular RNA in atherosclerotic plaque formation in mice. Circulation. 2014;129:598–606. doi: 10.1161/CIRCULATIONAHA.113.002562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Singh A, Ramesh S, Cibi DM, Yun LS, Li J, Li L, Manderfield LJ, Olson EN, Epstein JA, Singh MK. Hippo signaling mediators yap and taz are required in the epicardium for coronary vasculature development. Cell Rep. 2016;15:1384–1393. doi: 10.1016/j.celrep.2016.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Skyschally A, Gres P, Hoffmann S, Haude M, Erbel R, Schulz R, Heusch G. Bidirectional role of tumor necrosis factor-alpha in coronary microembolization: progressive contractile dysfunction versus delayed protection against infarction. Circ Res. 2007;100:140–146. doi: 10.1161/01.RES.0000255031.15793.86. [DOI] [PubMed] [Google Scholar]
- 90.Sloth AD, Schmidt MR, Munk K, Kharbanda RK, Redington AN, Schmidt M, Pedersen L, Sorensen HT, Botker HE, Investigators C. Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J. 2014;35:168–175. doi: 10.1093/eurheartj/eht369. [DOI] [PubMed] [Google Scholar]
- 91.Sloth AD, Schmidt MR, Munk K, Schmidt M, Pedersen L, Sorensen HT, Botker HE, Investigators C. Impact of cardiovascular risk factors and medication use on the efficacy of remote ischaemic conditioning: post hoc subgroup analysis of a randomised controlled trial. BMJ Open. 2015;5:e006923. doi: 10.1136/bmjopen-2014-006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stoppe C, Rex S, Goetzenich A, Kraemer S, Emontzpohl C, Soppert J, Averdunk L, Sun Y, Rossaint R, Lue H, Huang C, Song Y, Pantouris G, Lolis E, Leng L, Schulte W, Bucala R, Weber C, Bernhagen J. Interaction of MIF family proteins in myocardial ischemia/reperfusion damage and their influence on clinical outcome of cardiac surgery patients. Antioxid Redox Signal. 2015;23:865–879. doi: 10.1089/ars.2014.6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tello D, Balsa E, Acosta-Iborra B, Fuertes-Yebra E, Elorza A, Ordonez A, Corral-Escariz M, Soro I, Lopez-Bernardo E, Perales-Clemente E, Martinez-Ruiz A, Enriquez JA, Aragones J, Cadenas S, Landazuri MO. Induction of the mitochondrial NDUFA4L2 protein by HIF-1alpha decreases oxygen consumption by inhibiting Complex I activity. Cell Metab. 2011;14:768–779. doi: 10.1016/j.cmet.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 94.Theilmeier G, Schmidt C, Herrmann J, Keul P, Schafers M, Herrgott I, Mersmann J, Larmann J, Hermann S, Stypmann J, Schober O, Hildebrand R, Schulz R, Heusch G, Haude M, von Wnuck Lipinski K, Herzog C, Schmitz M, Erbel R, Chun J, Levkau B. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation. 2006;114:1403–1409. doi: 10.1161/CIRCULATIONAHA.105.607135. [DOI] [PubMed] [Google Scholar]
- 95.Tillmann S, Bernhagen J, Noels H. Arrest functions of the MIF ligand/receptor axes in atherogenesis. Front Immunol. 2013;4:115. doi: 10.3389/fimmu.2013.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tiong WN, Fong AY, Sim EU, Chan HC, Ong TK, Chang BC, Sim KH. Increased serum levels of interleukin-6 and von Willenbrand Factor in early phase of acute coronary syndrome in a young and multiethnic Malaysian population. Heart Asia. 2012;4:146–150. doi: 10.1136/heartasia-2012-010131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Varga ZV, Giricz Z, Bencsik P, Madonna R, Gyongyosi M, Schulz R, Mayr M, Thum T, Puskas LG, Ferdinandy P. Functional genomics of cardioprotection by ischemic conditioning and the influence of comorbid conditions: implications in target identification. Curr Drug Targets. 2015;16:904–911. doi: 10.2174/1389450116666150427154203. [DOI] [PubMed] [Google Scholar]
- 98.Varga ZV, Zvara A, Farago N, Kocsis GF, Pipicz M, Gaspar R, Bencsik P, Gorbe A, Csonka C, Puskas LG, Thum T, Csont T, Ferdinandy P. MicroRNAs associated with ischemia–reperfusion injury and cardioprotection by ischemic pre- and postconditioning: protectomiRs. Am J Physiol Heart Circ Physiol. 2014;307:H216–H227. doi: 10.1152/ajpheart.00812.2013. [DOI] [PubMed] [Google Scholar]
- 99.Vogel B, Shinagawa H, Hofmann U, Ertl G, Frantz S. Acute DNase1 treatment improves left ventricular remodeling after myocardial infarction by disruption of free chromatin. Basic Res Cardiol. 2015;110:15. doi: 10.1007/s00395-015-0472-y. [DOI] [PubMed] [Google Scholar]
- 100.Wang X, Gkanatsas Y, Palasubramaniam J, Hohmann JD, Chen YC, Lim B, Hagemeyer CE, Peter K. Thrombus-targeted theranostic microbubbles: a new technology towards concurrent rapid ultrasound diagnosis and bleeding-free fibrinolytic treatment of thrombosis. Theranostics. 2016;6:726–738. doi: 10.7150/thno.14514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang X, Hagemeyer CE, Hohmann JD, Leitner E, Armstrong PC, Jia F, Olschewski M, Needles A, Peter K, Ahrens I. Novel single-chain antibody-targeted microbubbles for molecular ultrasound imaging of thrombosis: validation of a unique noninvasive method for rapid and sensitive detection of thrombi and monitoring of success or failure of thrombolysis in mice. Circulation. 2012;125:3117–3126. doi: 10.1161/CIRCULATIONAHA.111.030312. [DOI] [PubMed] [Google Scholar]
- 102.Willeit P, Skroblin P, Kiechl S, Fernandez-Hernando C, Mayr M. Liver microRNAs: potential mediators and biomarkers for metabolic and cardiovascular disease? Eur Heart J. 2016 doi: 10.1093/eurheartj/ehw146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang XM, Cui L, White J, Kuck J, Ruchko MV, Wilson GL, Alexeyev M, Gillespie MN, Downey JM, Cohen MV. Mitochondrially targeted Endonuclease III has a powerful anti-infarct effect in an in vivo rat model of myocardial ischemia/reperfusion. Basic Res Cardiol. 2015;110:3. doi: 10.1007/s00395-014-0459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yano T, Miki T, Tanno M, Kuno A, Itoh T, Takada A, Sato T, Kouzu H, Shimamoto K, Miura T. Hypertensive hypertrophied myocardium is vulnerable to infarction and refractory to erythropoietin-induced protection. Hypertension. 2011;57:110–115. doi: 10.1161/HYPERTENSIONAHA.110.158469. [DOI] [PubMed] [Google Scholar]
- 105.Yellon DM, Ackbarkhan AK, Balgobin V, Bulluck H, Deelchand A, Dhuny MR, Domah N, Gaoneadry D, Jagessur RK, Joonas N, Kowlessur S, Lutchoo J, Nicholas JM, Pauvaday K, Shamloll O, Walker JM, Hausenloy DJ. Remote ischemic conditioning reduces myocardial infarct size in STEMI patients treated by thrombolysis. J Am Coll Cardiol. 2015;65:2764–2765. doi: 10.1016/j.jacc.2015.02.082. [DOI] [PubMed] [Google Scholar]
- 106.Zampetaki A, Willeit P, Burr S, Yin X, Langley SR, Kiechl S, Klein R, Rossing P, Chaturvedi N, Mayr M. Angiogenic microRNAs linked to incidence and progression of diabetic retinopathy in type 1 diabetes. Diabetes. 2016;65:216–227. doi: 10.2337/db15-0389. [DOI] [PubMed] [Google Scholar]
- 107.Zebrowski DC, Becker R, Engel FB. Towards regenerating the mammalian heart: challenges in evaluating experimentally induced adult mammalian cardiomyocyte proliferation. Am J Physiol Heart Circ Physiol. 2016;310:H1045–H1054. doi: 10.1152/ajpheart.00697.2015. [DOI] [PubMed] [Google Scholar]
- 108.Zebrowski DC, Vergarajauregui S, Wu CC, Piatkowski T, Becker R, Leone M, Hirth S, Ricciardi F, Falk N, Giessl A, Just S, Braun T, Weidinger G, Engel FB. Developmental alterations in centrosome integrity contribute to the post-mitotic state of mammalian cardiomyocytes. Elife. 2015;4:e05563. doi: 10.7554/eLife.05563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zernecke A, Preissner KT. Extracellular ribonucleic acids (RNA) enter the stage in cardiovascular disease. Circ Res. 2016;118:469–479. doi: 10.1161/CIRCRESAHA.115.307961. [DOI] [PubMed] [Google Scholar]
- 110.Zhang YS, Liu B, Luo XJ, Li TB, Zhang JJ, Peng JJ, Zhang XJ, Ma QL, Hu CP, Li YJ, Peng J, Li Q. Nuclear cardiac myosin light chain 2 modulates NADPH oxidase 2 expression in myocardium: a novel function beyond muscle contraction. Basic Res Cardiol. 2015;110:38. doi: 10.1007/s00395-015-0494-5. [DOI] [PubMed] [Google Scholar]
- 111.Zimmermann-Geller B, Koppert S, Fischer S, Cabrera-Fuentes HA, Lefevre S, Rickert M, Steinmeyer J, Rehart S, Umscheid T, Schonburg M, Muller-Ladner U, Preissner KT, Frommer KW, Neumann E. Influence of extracellular RNAs, released by rheumatoid arthritis synovial fibroblasts, on their adhesive and invasive properties. J Immunol. 2016;197:2589–2597. doi: 10.4049/jimmunol.1501580. [DOI] [PubMed] [Google Scholar]