Abstract
The decidua has been known as maternal uterine tissue, which plays essential roles in protecting the embryo from being attacked by maternal immune cells and provides nutritional support for the developing embryo prior to placenta formation. However, there are questions that still remain to be answered: (1) How does the decidua supply nutrition and provide a physical scaffold for the growing embryo, before placental vascular connection is established? (2) How is the balance between preventing an anti-embryo immune response and protecting both embryo and mother from infections established? To understand basic personas in decidual tissues, we review the structure of the decidua composed of terminally differentiated uterine stromal cells, blood vessels, and a number of repertoire of uterine local immune cells, including the well-known uterine natural killer (uNK) cells and recently discovered innate lymphoid cells (ILCs). Decidual macrophages and uterine dendritic cells (DCs) are supposed to modulate adaptive immunity via balancing cytokines and promoting generation of regulatory T (Treg) cells. During decidualization, vascular and tissue remodeling in the uterus provide nutritional and physical support for the developing embryo. Secretion of various cytokines and chemokines from both the embryo and the decidual cells activates multiple signaling network between the mother and the embryo upon implantation. Defects in the decidual development during early pregnancy result in loss of pregnancy or complications in later gestational stage.
Keywords: Decidua, Endometrium, Pregnancy, Infertility, Preeclampsia, Vascular remodeling
Introduction
The decidua is a transient but important platform in the uterine tissue, which comprises terminally differentiated endometrial stromal cells, newly generated maternal vascular cells, and maternal blood cells inside and outside the vessels. Development of the decidua after attachment of the blastocyst on uterine wall is a drastic tissue remodeling, involving physical and humoral changes in the residential and recruited immune cells. Indispensability of the decidual tissue for establishing implantation of the embryo and maintaining pregnancy until the stage of placenta formation was first indicated by its physical importance when mice were challenged by blastocyst transfer into the peritoneal cavity and failed [1], and when tubal decidual formation was observed in some cases of human ectopic implantation in the ovary or peritoneal cavity [2], and when embryonic loss was observed by ovariectomy-mediated progesterone withdrawal due to “collapse” of the rat decidua [3]. Thus, decidualization of uterine tissue is essential to establish successful pregnancy, but how is it generated, and how does it affect embryonic growth?
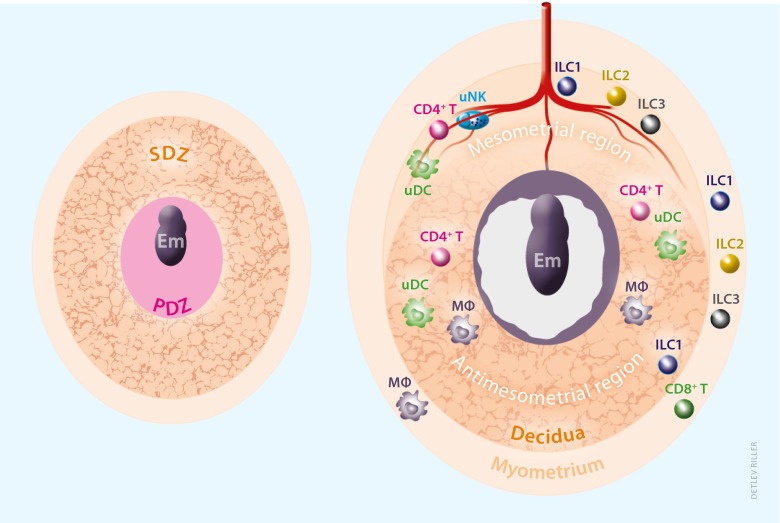
The decidual parenchymal cells, hereinafter called as decidual cells, are derived from uterine stromal fibroblast-like cells in the endometrium. They are large, round, and multi-nuclear polyploid cells, rich in glycogen and lipids, and produce a variety of functional markers such as prolactin and its related family proteins, and insulin-like growth factor binding proteins (IGFBPs). The mouse embryo at blastocyst stage attaches to the uterine lumen on gestational day (gd) 4.0 post-coitum, and the primary decidual zone (PDZ) is immediately established at the endometrial layer closest to the implanted embryo (Fig. 1). This is considered to be the first protective scaffold for the embryonic growth with avascular and tight cellular composition [9]. By gd 5.5, PDZ is completed, while the secondary decidual zone (SDZ), surrounding the embryo and PDZ, is developing into terminal differentiation of the decidual stromal cells, starting from antimesometrial toward mesometrial region. In contrast, mesometrial region seen from gd 6.5 consists of highly dilated large vasculature, and both mesometrial and antimesometrial decidual regions decrease from gd 8.5 along with placenta formation [10]. Concomitant angiogenesis in the decidualizing endometrium and spiral artery remodeling during early pregnancy both in human and rodents strongly suggests that nutrition supplied by maternal blood vessels is essential for early embryonic growth before placental connection.
Fig. 1.
Anatomical localization of maternal immune cells in murine decidua. Due to the difficulty in obtaining specimens from normal pregnant women at the earliest stage of pregnancy, tissue distribution of maternal immune cells in murine uterus at gestational day (gd) 5.5 and 7.5 is shown. The primary decidual zone (PDZ) is avascular and CD45+ cells are scarcely found [4]. Secondary decidual zone (SDZ) at gd 5.5 and antimesometrial decidua at gd 7.5 are rich in small blood vessels, whereas mesometrial region at gd 7.5 is surrounded by lateral dilated large vessels (not shown). In the mesometrial decidua, uNK cells are most abundantly found [5], also see Fig. 2], DCs are confined in the entire decidua [6]. In addition to uILC1 in the decidua, other uILCs are detected in mesometrial region and myometrium [7], but the interaction of uILCs with vasculature or other immune cells have not been reported. Except for the report on suppressed infiltration of cytotoxic effector T cells in the decidua [8], T cell subsets inside the murine decidua have not been well known. Macrophages can be found in the region between trophoblast and uterine stromal cells in association with vascular endothelial cells [4]. Note that the numbers of representative cell types are not consistent with the actual populations
Development of the decidua and its molecular mechanisms
Regulators for decidualization outside and inside uterine stroma
Cyclic fluctuation of endometrial cellular differentiation and subsequent apoptotic death in human uterus are under hormonal control. In the absence of entopic embryo in the uterus, human decidua can be formed routinely and then shed off (like leaves on a deciduous tree, which is the origin of the word “decidua”). In rodents, artificial decidualization can be observed as deciduoma reaction after a mild physical stimulation of pseudo-pregnant uterus without embryos. Compared to the pregnant uterus, various deciduoma models in mice show more expanded sizes of the uterus and slight differences in expression of decidual markers such as alkaline phosphatase 2 and Wnt4 [11]. Signaling between the embryo and uterine luminal epithelia, mediated by adhesion molecules such as integrins and carbohydrate moieties on glycoproteins, leading to luminal epithelial apoptosis and subsequent interaction between the epithelium and the proximal stromal cells, is also considered to be engaged in initial decidual differentiation [12].
By use of artificial deciduoma in rodents stated above, impaired decidualization has been demonstrated in mice deficient of progesterone receptors (PR) or PR-related pathways (Bmp2, Wnt4, Hoxa10, and Hoxa11) [13–16], indicating that the endocrine system plays the major role in decidualization [17]. Other pathways involved in the acceptance of blastocysts by local and systemic regulation in the maternal uterine epithelium and endometrium are reviewed elsewhere [18]. A mouse model deficient in interleukin-11 (IL-11) cytokine signaling (IL-11Rα−/−) [19] shows impaired decidualization, accompanied by reduced uterine stromal cellular proliferation [20]. Once proliferation and differentiation of decidual cells are initiated, they proceed to multi-nuclearization, i.e., DNA replication without cell division (endo-reduplication/polyploidy), which allows expression of multiple genes and secretion of the translated proteins with less energy consumption, and is considered to be an important hallmark of decidual maturation in rodents and humans [21, 22]. In the mouse model deficient in Death effector domain-containing protein (Dedd) [23], bidirectional pathways of Akt signaling and cyclin D3/Cdk4/Cdk6 have been shown to contribute to decidual cellular multi-nuclearization. In the absence of Dedd, protein stability of Akt [23, 24] and Cyclin D3/Cdk4/Cdk6 complexes are reduced, corresponding to lower ratio of multi-nuclearization in decidual cells. As demonstrated in human decidual and endometrial cell cultures [25], Akt signaling is associated with decidual differentiation. Das SK et al. has suggested that cyclin D3, in association with Cdk4, Cdk6, and p21, is an essential cell cycle regulator in the endo-reduplication of multi-nuclearizing murine decidual cells [26]. The expression of cyclin D3 and p21 are also indicated to be downstream of IL-11 signaling [27]. Intriguingly, while Dedd−/− female mice cannot produce any progeny, and while IL-11Rα−/− female mice are severely infertile, any single knockout of either Akt1, Akt2, Akt3, or cyclin D3 does not cause complete infertility, suggesting the importance of Dedd as a master regulator in the upstream of these multiple proteins’ network. The connection between endocrine system and cytokine signaling or Dedd pathway has not been clarified.
Uterine angiogenesis and tissue remodeling
-
Vascular endothelial remodeling modulated via steroid hormones
In menstrual cycles and before implantation occurs, ovarian steroid hormones, 17β-estradiol (E2) and progesterone (P4), modulate the uterine vascular development and functions, resulting in drastic changes of volume, elasticity, and nutrient transportation of the entire uterus. E2 has more effects on vascular permeability via suppression of adhesion molecules such as E-selectin, vascular cell adhesion molecule-1 (VCAM-1), and intercellular adhesion molecule-1 (ICAM-1) in human umbilical vein endothelial cells (HUVEC) [28]. On the other hand, P4 has more angiogenic effects on HUVEC via inducing proliferative factors from endometrial cells, such as vascular endothelial growth factor A (VEGFA), angiopoietin-2 (ANGPT2), and fibroblast growth factor 2 (FGF2) [29]. P4 may also directly suppress ICAM-1 expression in HUVEC [30]; however, this effect seems to be minimal in vivo. VEGF is also expressed in endometrial stroma at the secretory phase and in pregnant decidual cells, both in mice and in humans [31, 32]. Angpt2 and other angiogenic factors are also shown to be regulated under intrauterine E2 [33]. Rapid angiogenesis itself may cause loosened structure of vascular network, leading to local hyper-permeability, e.g., in a tumor lesion [34]. The action of endocrine factors on vascular endothelial and smooth muscle cells may affect vascular stability to modify exchange of substances such as free fatty acids loaded on plasma albumin and blood cells between blood and stromal tissue, and regulate expression of surface molecules to recruit uterine-specific immune cells.
-
Morphological dynamism in the uterine tissue
The decidual cells, not only secrete growth factors but also release tissue inhibitor of matrix metalloproteinases (TIMPs) to suppress trophoblast-derived matrix metalloproteinases (MMPs) [35, 36] and express contact-dependent signaling molecules such as connexin 43 (Cx43) [37]. Although not all of the matrix proteins have been shown to be specifically expressed in either the human or murine decidual cells, α2-macroglobulin, one of potent MMP inhibitors, has been shown as downstream of IL-11-invoked JAK-STAT3 pathway in rodents [38], which is also implicated in human endometrial stromal cells (hESCs) [39]. A murine model using conditional knockout of Cx43 via PR-Cre recombination showed the importance of uterine stromal Cx43 on vascular endothelial proliferation at gd 7.5 [37]. The uterus deficient in Cx43 shows insufficient deciduoma response accompanied by reduced uterine vascular angiogenesis. This effect is possibly related to the gap-junction communication between decidual cells which secretes VEGF and angiopoietins, but the communication between decidua and vascular endothelial cells remains to be investigated.
Classical immunology in the decidua
To challenge the immune privileged feature of the decidua against embryonic graft, the first experimental transplantation of a skin allograft was tested on rodent decidua [40]. The allograft survived longer, however, in the end, the graft was rejected in the pre-immunized pregnant rodent. In contrast to another failure in transplantation of paternal skin allograft tested in the rat choriodecidual junction [41], embryos surrounded by putatively paternal antigen-positive trophoblasts are able to be accepted. Early studies also investigated the existence of immunosuppressive substances from murine decidual culture in vitro [42] and of hormone-dependent suppressor cells regardless of implanted embryos [43]. However, these studies could not identify the cell subset or molecules derived from the decidua.
In the modern era, with the development of technologies in flow cytometry and imaging analysis, a number of studies have described the presence, distribution, and the functions of maternal immune cells in the decidual tissue in the early phase of gestation (Fig. 1, Table 1). The decidua contains a large number of maternal immune cells, which supposedly establish the balance between defense against pathogens and a tolerance of the embryo. The major populations include innate immune cells, i.e., uNK cells and macrophages. However, small populations of ILCs and adaptive immune cells cannot be ignored. Moreover, it is important for the future maternal and fetal health that the decidua holding the embryo can provide protective responses against pathogens, without an excessive inflammation or that would harm the embryo/fetus. Looking at the basic functions and characteristics of these immune cells, tracking the outcomes of deficiency or abnormality in each component of these immune subsets, we may be able to shed light on their roles and importance in normal and pathological conditions.
Table 1.
Composition of uterine immune cells at post-implantation stage in mice
| Virgin | gd 5.5 | gd 6.5 | gd 7.5∼9.5 | |
|---|---|---|---|---|
| uNK | DBA−
10a∼20 %b |
DBA−
<5 %b |
DBA−
8 %a |
DBA−
8 %a |
| DBA+
0 %f |
DBA+
N.D. |
DBA+
<2 %a, f |
DBA+
20 %a |
|
| Mϕ | 8 %b | 20 %b | 30 % f | N.D. |
| DC | 2∼3 %b,d | 5∼6 %d | 15 %f | 15 %e |
| T | <1 % f | N.D. | <1 % f | N.D. |
| uILCs | Very few ILCsc uILC1 is dominant | N.D. | N.D. | Very few ILCsc
uILC3 is dominant |
Uterine cellular composition among CD45+ leukocytes assessed by flow cytometry are shown. During post-implantation stage of murine pregnancy (gestational day (gd) 5.5∼9.5), the ratios of different subsets change in comparison to virgin uterus. uNK: CD3−CD122+ [44] or CD49b+CD11b− [45] cells. Mϕ: CD11b+F4/80+ monocyte-derived cells. DC: CD11c+ cells [45]. T: CD3+CD4+ and CD3+CD8+ T cells. ILCs: defined as in the text [46]. There are no DBA+ cells in non-pregnant uterus and low number of DBA+ cells at gd 6.5 [44], in contrast to increased DBA+ from gd 7.5 [44]
N.D. no data is available
aReference [48]
bReference [55]
cReference [85]
dReference [117]
eReference [47]
fSource: Mori M et al., unpublished data
Uterine NK cells
Natural killer (NK) cells are derived from pluripotent hematopoietic stem cells in the bone marrow and develop as lymphoid but without receptor gene rearrangement like in the case of T cells. NK cells mediate innate cellular immunity against pathogens and cancer cells. Mature NK cells possess both activating and inhibitory receptors for class I MHC such as Ly49 subtypes in mice and killer inhibitory receptors (KIRs) in human [48]. They also have MHC-independent natural cytotoxicity receptors (NCRs). In humans, peripheral and uterine NK cells represent two phenotypically distinct populations (Table 2). The majority of human peripheral NK cells express low density of CD56 (CD56dim) and high levels of the FCγRIII (CD16+) indicating ADCC-mediated cytotoxic functions, while the rest of them express high density of the CD56 (CD56bright) and are CD16-negative corresponding to low cytotoxicity. uNK cells constitute 60 to 70 % of all decidual lymphocytes in the first trimester of human pregnancy [49]. In contrast to peripheral NK subsets, most decidual NK cells are CD16−CD56bright. Further to the phenotypic differences, uterine and peripheral NK cells exert different functions. Peripheral CD16+CD56dim NK cells are granular and cytotoxic, while the minor peripheral NK subset does not contain cytoplasmic granules and is not endowed with a cytotoxic potential but displays an immuno-regulatory role via cytokine production [50]. Decidual NK cells contain cytotoxic granules [51] and selectively overexpress genes of secreted proteins with known immunosuppressive activity [49], as well as perforin and granzymes A and B [52]. In mice, two distinct uNK cell subsets are distinguished by periodic acid-Schiff (PAS) and Dolichos biflorus agglutinin (DBA) reactivity. PAS+DBA− cells produce IFN-γ, which is implicated in maternal spiral arterial remodeling [53], whereas the PAS+DBA+ population produces angiogenic factors [54–56]. Yadi et al. [44] describe two distinct subsets of CD3−CD122+ NK cells in the mid-gestational mouse uterus: a small subset similar to peripheral NK cells, and a larger DBA+ subset that expresses activating receptor NKp46 and inhibitory receptor Ly49s, but not NK1.1 or DX5. It must be mentioned that via maternal blood which perfuses the placenta, both subsets of peripheral blood NK cells could get in contact with fetal tissues. Uterine NK cells have a low spontaneous cytotoxicity, which is in line with the concerted presence of non-classical MHC molecules on the trophoblast and expression of inhibitory receptors on the NK cells, resulting in decreased degranulation [57]. However, in the presence of pathogens, the proportions of activating and inhibitory receptors may shift to promote cytotoxicity [58]. At present, there is no evidence that the cytotoxic potential of NK cells has any direct effect on trophoblast, while maternal MHC but not paternal MHC has been shown to educate uNK cells via matching Ly49 receptors to produce IFN-γ as a vascular-remodeling factor [59]. Murine uNK cell precursors either originate from outside the uterus [60] or differentiate from resident hematopoietic precursors [61] in the presence of other immune cells. In SCID mice, uNK cell differentiation in the decidua basalis was shown to be delayed during the early placentation period due to a lack of functional T and B cells [62]. The number of the resident NK cell population declines between gd 0.5 and 5.5 [45] and is considered to be replaced by recruited extra-uterine precursors which differentiate into uNK cells. DBA+ NK cells are scarce in the decidua at gd 5.5, while from gd 6.5 an increasing number of DBA+ and PAS+DBA− NK cells can be detected ([63], Fig. 2).
Table 2.
The major differences between human peripheral NK cells and uNK cells
| Peripheral NK cells | Uterine NK cells | |
|---|---|---|
| (90 %) | (10 %) | |
| CD56dim | CD56bright | CD56bright |
| CD16+ | CD16− | CD16− |
| Cytotoxic | Non-cytotoxic | Low cytotoxicity |
| Granular | Non-granular, cytokine producing | Granular |
| LFA1+ | CCR7+ | |
| Perforin+ granzyme+ | Perforin+ granzyme+ | |
Fig. 2.
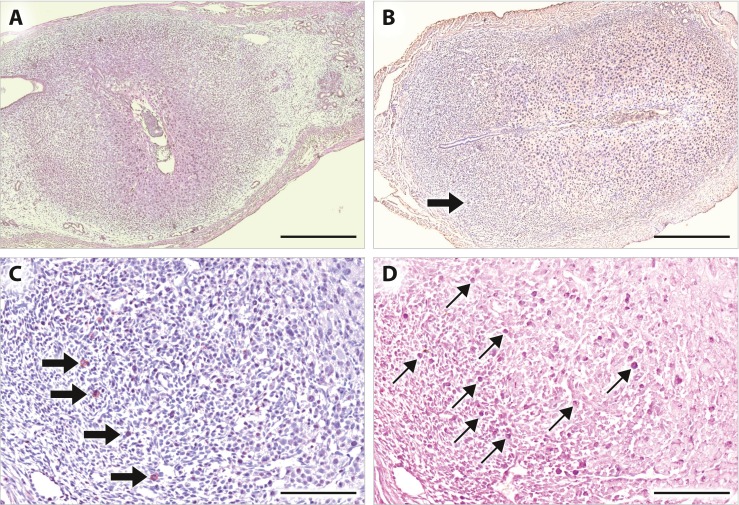
Distribution of uNK cells at early gestational stage in mice. Uterine sections from C57BL/6 female mice at gd 5.5 (a) and gd 6.5 (b−d) were stained with DBA lectin (A−C) or PAS (D). a At gd 5.5, DBA+ NK cells are scarcely detected. b At gd 6.5, DBA+ NK cells are increasingly detected in the mesometrial region. c Higher magnification of b. d Higher number of PAS+ NK cells are detected in the continuous section of c. Thin arrows: PAS+ cells, thick arrows: DBA+ cells. Bars, a, b 500 μm, c, d 100 μm
Interleukin-15 (IL-15) is a critical regulator of NK and uNK cell differentiation [64, 65]. IL-15−/− females lack uNK cells, as well as spiral artery remodeling [66, 67]. A recent study [68], however, compared the gene expression profiles in implantation sites of the uterus during decidualization on gd 7.5 between wild-type and uNK cell-deficient (IL-15−/−) mice, and found no different expression of genes involved in decidualization or angiogenesis, with the exception of Adamts9, which is an anti-angiogenic factor, expressed at a higher rate in IL-15−/− than in wild-type mice. Immune cell-deficient murine models showed the significance of NK cell contribution to implantation and normal pregnancy. A murine alymphoid model, Rag2−/−Δγc, which lack T, B, and NK cells systemically, have deficient early decidual angiogenesis, delayed early embryonic development, and failure of spiral arterial modification at mid-gestation. All these anomalies are corrected by reconstitution with NK+T−B− grafts before mating [69]. Effects of cellular contact of uNK cell with other lymphocytes or other decidual-resident cells are largely unknown, but soluble factors from uNK cells such as IFN-γ has been shown to contribute for spiral artery remodeling [70] in NK-deficient mouse models. In humans, uNK cells also generate an array of angiogenic growth factors, e.g., VEGF, ANGPT-1, 2, transforming growth factor-β (TGF-β) [71], and placental growth factor (PlGF) [72, 73]. As a consequence of inadequate spiral artery remodeling, preeclampsia and intrauterine growth restriction could be expected; however, data from different laboratories are conflicting. This is probably due to differences in the focus on peripheral and decidual NK cells and their subsets, and to diversity of effects derived after preeclampsia and abortion, which may trigger cytotoxic NK activation. A recent report has shown that pregnant women with high Doppler resistance index, indicating impaired spiral artery remodeling and higher risk of preeclampsia, possess non-cytotoxic decidual NK cells as well as normal pregnant women, but with decreased expression of KIR2DL/S1, 3, 5, receptors against canonical class I histocompatibility locus antigen C (HLA-C), and LILRB1 against HLA-G on trophoblast [74]. Controversially, in vivo depletion of NK cells by anti-asialo-ganglio-N-tetraosylceramide (asialo-GM1) antibody reduced abortion rates in the abortion-prone CBA/J female mice mated with DBA/2J male mice [75]. This is considered to be due to the double-face of cytotoxic and vascular-remodeling NK cells, for similar NK depletion of CB17 SCID mice lacking effector T and B cells showed increased abortion rate [76]. This is probably due to lack of innate immunity against indigenous bacteria. Thus, when NK cell are depleted systemically, different cytotoxicity in two subsets in uNK cell should be taken into account.
Herein, cytotoxic NK cells might play a role in pregnancy pathologies. The increased resorption rates of pregnant BALB/c mice induced by anti- progesterone-induced blocking factor (PIBF) antibody were corrected by treating the mice with anti NK-1.1 antibody [77]. In humans, increased number of CD56+ cells were demonstrated in mid-luteal phase endometrial biopsies from patients with idiopathic recurrent miscarriage (RM) [78] but another study (Tuckerman et al., [79]) concluded that numbers of uNK cells in RM do not predict subsequent pregnancy outcome. Among decidual lymphocytes from failed pregnancies, there were less perforin-positive CD56+ cells than in deciduas from normal pregnancies [80], suggesting an increased rate of degranulation taking place in the former cases. In women with RM, a lowered uterine artery resistance to blood flow was demonstrated by Doppler ultrasonography together with an increased percentage of uNK cells during the mid-secretory phase, suggesting a correlation between excessive blood vessel development and the pregnancy failure [81]. Other studies have shown that not the number of endometrial total NK cells but the dominance of CD16−CD56bright uNK cell subset was significantly decreased in favor of CD16+CD56dim uNK cells in recurrent aborters [82]. Patients who miscarried chromosomally normal embryos had decreased percentage of CD16−CD56bright uNK cells compared with those of normal pregnancy [83, 84]. These data suggest that a part of RM with unknown etiology might be explained by deficiency in CD16−CD56bright uNK cells, or alternatively by excess infiltration of CD16+CD56dim NK cells derived from perfusing blood [85]. However, the causal association and precise mechanism have been unknown especially in humans. Taken all these together, further investigation on functions of uNK cells, rather than number or surface markers, is required.
Innate lymphoid cells
Innate lymphoid cells (ILCs) play a role in protection against pathogens, in lymphoid organogenesis, and in tissue remodeling. They are now divided to three subsets on the basis of their phenotypic and functional characteristics [86–88]. Group 1 ILCs can be distinguished from cytotoxic NK cells by lack of the transcription factor Eomes, yet they produce IFN-γ via T-bet transcription factor, rendering ILC1s weakly cytotoxic. Group 2 ILCs express a discriminative IL-33 receptor and also express chemokine receptors CCR4 and CCR5 upon stimulation. In response to IL-25, IL-33, parasites, or tissue injury, they produce Th2 cytokines such as IL-4, IL-5, and IL-13, acting in a regulatory fashion similar to Th2 cells. Group 3 ILCs require the transcription factor retinoic acid-related orphan receptor γt (RORγt) for their generation. They produce IL-22 and also secrete IL-17 in certain circumstances. Though they are non-cytotoxic, a subset of ILC3s expresses an NK activating receptor NCR (NKp46/44). All ILCs share a common precursor expressing the ID2 transcription factor. ILCs play a role in innate defenses against pathogens and in lymphoid tissue organization during fetal life [89], but their most important role is to behave as an intermediary between innate immune responses and T helper functions. Expression of both NK receptors and production of Th1, Th2, Th17, and Th22 cytokines by ILCs suggest that they might play a role in establishing the balance between immunity and tolerance both in innate and adaptive settings (Table 3). During pregnancy, the most important role has been attributed to uNK cells as described in the section above. In addition to this, IL-22-producing non-NK ILCs are also present in the non-pregnant uterine mucosa as well as in the decidua during the second trimester [91]. Recently Doisne et al. have identified uterine ILC subsets (uILCs) in human endometrium in the first trimester and in murine uterus at the beginning of placenta formation [7]. Three subpopulations, uILC2, uILC3, and uterine-specific CD127− uILC1 were found in the murine uterus at gd 9.5, but with different distribution within the implantation site (Fig. 1 and [7]). Both in mouse uterus and in human endometrium, uILC3 seems to be the dominant subset during pregnancy, whereas uILC2 is scarcely detected, and decidual uILC1 consists of CD127− cells similar to intraepithelial lymphocytes (IEL) in the intestine [7] (Table 3).
Table 3.
Comparison of uterine ILC subsets to Th cells and uNK cells
| uILC subsets | Comparable Th subsets | Comparable uNK subsets |
|---|---|---|
| uILC1 (T-bet, (IFN-γ)) | Th1 | IFN-γ (DBA− uNK in mice) |
| uILC2 (IL-33R, IL-7Rα, GATA3, IL-5) | Th2 | |
| uILC3 (RORγt, IL-7Rα, IL-17, 22, partially NCR+CD56+ in human) | Th17, Th22 | Some DBA+IL22+ in mice? NCR+CD16−CD56bright in human? |
To understand the complicated groups of uILCs, subsets are aligned with regard to comparable functions of helper T cells and uNK cell cells. uILC1s produce IFN-γ, but at a lower level compared to that of uNK cells. Unlike ILC2s in other tissues, uILC2s constitutively express IL-5 [89]. uILC3s partially express IL-17 and IL-22 like Th17 or Th22 cells. Interestingly, some DBA+ NK cells are reported to secrete IL-22 [5]. uILC3s in human possess NCRs in addition to CD56 expression, similar to the supported presence of NCR+ NK cells within CD16−CD56bright population [90]
Due to their relatively recent discovery, the role for these cells in reproduction is yet to be established. Mouse models lacking particular subsets of ILCs, without abrogated Th2 cell differentiation seen in ST2- (IL-33R) deficient mice [46], e.g., irradiated wild-type mice of bone marrow chimera with staggerer mutant (RORα sg/sg) mice, are considered as an appropriate model to assess the intrinsic roles of ILC2. RORγt-reporter mice in combination with RAG2−/− genotype are useful to elucidate the roles for ILC3 [92]. Considering the decidua-specific distribution of uILC1s (Fig. 1), PLZF-deficient (lacking all the ILC subsets) mice is also an alternative optional model to investigate the roles of uILCs in the decidua. However, none of these models have been tested for quantitative reproductive capacity, although there has been no report of breeding problems. Further investigation using the ILC-deficient mouse models is demanded to elucidate the specific roles for ILCs in reproduction.
Decidual macrophages
When pregnancy is established, circulating monocytes infiltrate the decidua and develop into macrophages [93], together with uterine resident myeloid cells, constituting 20 to 25 % of human decidual leukocytes [94]. Mice lacking decidual macrophages are not available. Even CSF-I-deficient osteopetronic (op/op) mice show a small number of F4/80-positive cells in the decidua, although seen only at gd 7.5 [95]. However, op/op female mice crossed with op/op males are infertile at implantation stage and any other combination involving op/+ females or op/+ males result in mild subfertility [96], suggesting that a sufficient number of macrophages is necessary to sustain the pregnancy. In op/op females mated with op/+ males, implantation rate was decreased to 60 % with lower number of implantation sites and lower survival rate of implants until term pregnancy, resulting in 39 % fertility in comparison to op/+ females mated with op/+ or op/op males, showing > 92 % implantation rate and > 85 % fertility.
Based on differential expression of the complement receptor CD11c, two distinct subpopulations (CD11chi and CD11clo) have been identified in human decidua [96]. In the first-trimester decidua, the majority of the macrophages are CD11clo. These express genes associated with extracellular matrix formation, muscle regulation, and tissue growth [97]. CD11chi macrophages, constituting approximately one third of the decidual population, express genes associated with antigen-presenting function, e.g., CD1a, CD1c, and CD1d, and process antigens more efficiently than CD11clo macrophages [97].
Decidual macrophages contribute to the “embryo-friendly” immunological environment, which negates surveillance by immunity and permit embryogenesis, in a similar manner to tumor microenvironment that favors neoplastic growth. Based on their cytokine pattern, they are categorized as M1 and M2, respectively [98, 99]. M1 macrophages secrete tumor necrosis factor-α (TNF-α) and IL-12 [100], while M2 macrophages are characterized by a decreased IL-12 production and express IL-1 receptor antagonist [101]. Additionally, M2 macrophages express the macrophage mannose receptor (MMR) that mediates host defense and plays a role in removal of inflammatory by-products [102]. Human decidual macrophages have been shown to inhibit T-cell responses via prostaglandin E2 production [103, 104]. Furthermore, they produce a significant amount of immunosuppressant IL-10 [105, 106], which can reduce the abortion rate in CBA/J x DBA/2J model, and tryptophan metabolites [107–109], which can effectively promote Treg generation. Taken all the above together, it can be speculated that an M2 phenotype is anticipated during normally developing pregnancy. However, there has been no evidence showing that the decidual macrophages have an immunosuppressive phenotype with M2-polarization.
Dendritic cells
Dendritic cells (DCs) constitute an essential player which links innate immunity to adaptive immunity. Following antigen capture at the periphery, they migrate to the regional lymph nodes, where they present peptides to naïve T cells, resulting in antigen-specific immune responses [110–113]. Within adaptive immunity, DCs also control polarization of T helper cell differentiation by cytokine secretion; IL-12 from lymphoid DCs induce development of Th1 cells, whereas no convincing factor has been found to induce Th2 development, and polarization to Th17 or Th22 seems not induced only by DCs [114–116]. DCs as antigen-presenting cells are not only essential for the induction of primary immune response but also play a role in the induction of tolerance. Subtypes of DCs in human decidua are described as immature non-activated (CD209+), immature activated (DEC205+) and mature activated (CD83+) cells [117, 118]. Immature DCs, processing antigens via DEC205 receptor, promote CD8+ T-cell proliferation but suppress cytotoxic IFN-γ production, resulting in tolerance, whereas mature DCs induce T-cell immunity [117].
There are very few CD83+ DCs at the maternal-fetal interface [118], but DCs in pregnant decidua are found CD209+, suggesting that recruited monocyte-derived immature DCs are kept non-activated. Furthermore, DCs present in the mouse decidua are unable to migrate out of this tissue even upon activation [119] (Fig. 1). Secreted factors in the conditioned medium of murine decidual cell culture have been shown to block in vivo antigen presentation by DCs and to inhibit their capacity to induce IFN-γ, but not IL-10 production by primed lymphocytes, suggesting that decidual factors contribute to the development of Th2 dominance, through modulation of DCs function [6]. Myeloid DCs in the decidua produce lower levels of IL-12 than their peripheral blood counterparts do and are somewhat more prone to stimulating Th2 responses in human [120]. These results therefore suggest that decidual DCs might locally present antigen to decidual T cells in ways that minimize Th1 responses.
Recent studies suggest that decidual DCs might play a role in decidual tissue remodeling, e.g., DC-deficient mice showed altered decidual angiogenesis [121, 122]. The data on the role of decidual DCs in human pregnancy pathologies are scarce. Only mild changes in decidual CD83+ DC densities have been described in human pregnancy complications [123, 124]. Askelund et al. reported on significantly higher number of dendritic cells in deciduas from women with RM at 8-week gestation compared to gestational age-matched normal controls [124].
Regulatory T cells
CD4+CD25hiFoxP3+ [125] Treg cells are a component of adaptive immunity, and they function as suppressors of the immune response. By their capacity to downregulate immunological reactions, Treg cells are involved in maintenance of self-tolerance, tumor escape, and transplant tolerance, while during pregnancy, under a certain condition, Treg cells can also contribute to maternal tolerance of the fetus via producing IL-10 [126, 127]. Aluvihare et al. suggested for the first time that Treg cells might mediate maternal tolerance in mice during pregnancy [128]. Indeed, adoptive transfer of Treg cells from BALB/c-mated normal pregnant CBA/J mice prevented fetal loss in the abortion-prone DBA/2J-mated CBA/J female mice, while Treg cells from non-pregnant mice had no effect [129]. Treg cells participate in protection of the fetus by down-regulating inflammatory responses. Treg cells inhibit cytokine production in both CD4+ T cells and CD8+ T cells, cytotoxic activity of NK cells, and dendritic function and maturation, resulting in suppression of local inflammatory activation [127, 130, 131].
The lack of Treg cell-mediated modulation might result in pregnancy failure, or pathologies, but careful distinguish between thymus-derived natural Treg (nTreg) cells and induced Treg (iTreg) cells generated from peripheral CD4+ T cells should be concerned. Reduced frequency of decidual Treg cells was reported in miscarriage with a normal embryo karyotype [132]. In this study, Helios+ Treg cells were assessed within CD4+FoxP3+ population; however, the definition of nTreg cells as Helios+CD4+FoxP3+ is not satisfyingly in consensus. In contrast, Helios− iTreg-deficient murine pregnancies are characterized by an increased resorption rate and defective remodeling of spiral arteries [133]. However, in this mouse model, the total abortion rate was only 10 %; thus, indispensability of iTreg cells is not clear. It should also to be noted that there is no evidence that the lack of Treg cells can cause abortion of embryos expressing paternal alloantigens, the depletion of Treg cells in transgenic mice expressing an artificial MHC II-restricted antigen could invoke fetal resorption at mid-pregnancy.
Non-classical immune communication
Current immunology has revealed intercellular communication not only among lymphoid or myeloid cells but also involving tissue stromal parenchymal or non-parenchymal cells, which can modify the proportion and extracellular paracrine signaling of lymphoid/myeloid cells by means vulnerable to tissue microenvironment. Hereafter, we call this aspect as “non-classical” communication between the embryo and the decidua.
Roles for the uterine stroma and the decidual cells
-
Secreted factors from the decidua
The decidual cells themselves can also play important roles in modulation of immune cells functions. In addition to the diversity of growth factors as decidual markers, cultured endometrial cells secrete cytokines, e.g., IL-6, TNF-α, as well as chemokines, e.g., IL-8, CXCL1, and express the chemokine receptor CXCR4 during normal pregnancy [35, 36]. Miscarriage is characterized by an altered cytokine profile in the human decidua [134]. Uterine stromal fibroblasts produce chemokines, including Cxcl9, Cxcl10, and Ccl5, the production of which is epigenetically downregulated during decidual differentiation upon implantation, by methylation of the promoter regions of the chemokine genes without deactivation of NF-kB or Stat1 signaling, [8]. Therefore, effector cytotoxic CD8+ T cells are not permitted to infiltrate into the decidual region adjacent to the conceptus, even if memory T cells against embryonic antigen were experimentally primed on the day after implantation (Fig. 1). Such an epigenetic suppression of chemokines does not take place in the myometrial fibroblasts and stromal cells in non-implantation sites, which suggests the involvement of blastocyst-derived factors. A mouse model of MHC-restricted rejection of fetus accompanied by T cell infiltration is induced by IDO inhibitor 1-methyltryptophan [135]. However, this compound, working as a competitor with the substrate of IDO, tryptophan, may have a diversity of adverse effects, which still raises questions about alternative activation of immunity overcoming the decidual suppression of chemokines suggested by Erlebacher’s group [8]. Apart from cytokines, the dynamic changes in expression levels and patterns of extracellular matrix proteins during the tissue remodeling in early pregnancy [35, 36] might also induce changes in stromal affinity for uNK cells, as suggested by the tissue distribution of uNK cells (Fig. 1).
-
TLR expression on the decidual cells
There are ten types of human Toll-like receptors (TLRs) with a diversity of specific pattern recognition for pathogens. TLRs are expressed not only in immune cells but also in tissue parenchymal cells such as adipocytes, hepatocytes, and uterine stromal cells. Changes of uterine TLR expression during the menstrual cycle may suggest hormonal regulation [136] (Fig. 3). Although only a few studies have shown the existence of TLR2 and TLR4 in human decidual cells [137], Krikun et al. have reported that LPS stimulation induces secretion of IL-6 and IL-8 in human endometrial cell culture [138]. It cannot be ruled out that if bacterial infection stimulates TLR2/4 on decidual cells during pregnancy and downstream signaling induces cytokine production, endogenous lipid ligands for TLR2/4 present on uterine stromal cells modify the character of these cells during decidualization. The mechanisms for the regulation of cytokine secretion by stromal or decidual cells requires further investigation.
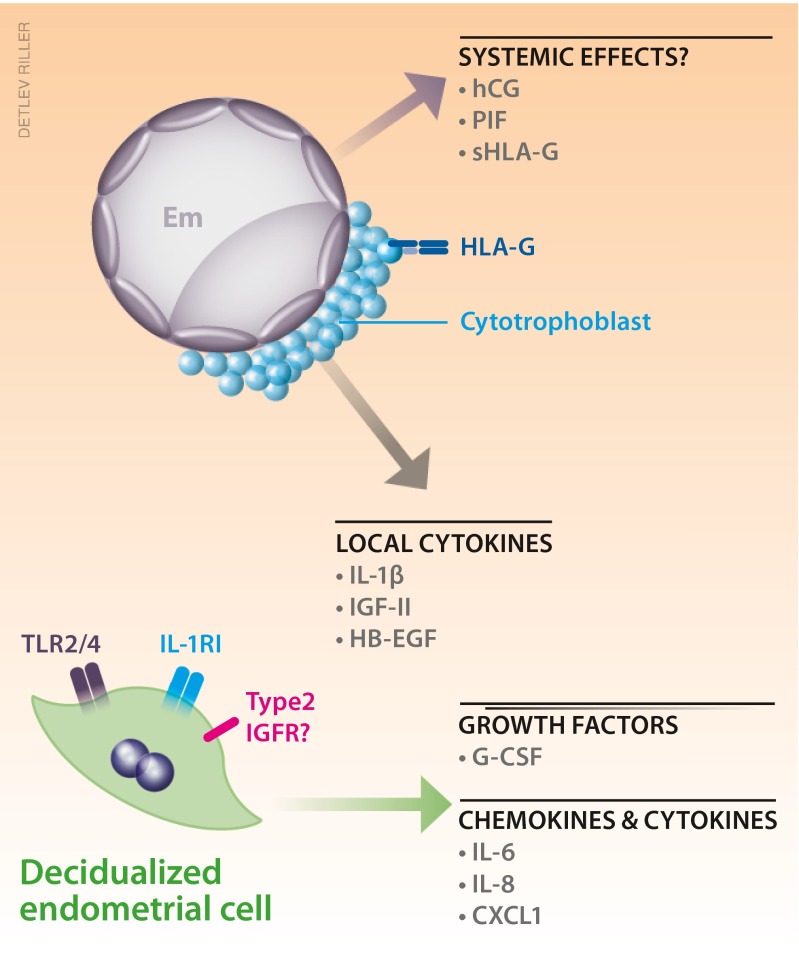
Fig. 3.
Possible human decidua-embryo interaction indicated by in vitro observation. Despite of difficulty in obtaining human specimen during normal peri-implantation stage, a number of studies have utilized in vitro co-culture system to investigate signaling communication between the decidual cells and the embryo. Fertilized eggs secrete interleukin-1β (IL-1β) and growth factors such as IGF-II and HB-EGF, which are indicated to regulate decidual cellular development, in line with expression of IL-1RI and type 2 IGFR in the endometrial cells. Signaling for decidual expression of Toll-like receptors (TLRs) is unknown. Soluble factors from the embryo but detected in maternal peripheral blood, such as human chorionic gonadotropin (hCG), preimplantation factor (PIF), and soluble form of HLA-G (sHLA-G), may have systemic effects such as promoting ovarian progesterone production, balancing cytokines and chemokines secretion in the periphery. Decidual cells also express growth factors such as G-CSF, and cytokines and chemokines such as IL-6, IL-8, and CXCL1. At least some of them are considered to be under the control of signaling from the embryos
Refrained contact from the embryo to maternal immune cells
Importantly, dying or incompetent embryo such as that with abnormal karyotype, seen in humans more frequently than in mice, will be “rejected” by the decidua, the development of which requires calcium signaling via serine proteases secreted from a competent embryo. When endometrial cells are exposed to culture supernatant of incompetent embryos, impaired secretion of prolactin and IGFBP-1 is caused via endoplasmic reticulum (ER) stress and autophagy [139]. The cytotrophoblasts of human embryos at peri-implantation stage also secrete IL-1β (Fig. 3). In baboons, IL-1β has been shown to promote decidual secretion of matrix metalloprotease [140]. Growth factors such as insulin-like growth factor II (IGF-II) and heparin-binding epidermal growth factor (HB-EGF) are indicated to regulate decidual cellular development, in line with expression of IL-1RI and type 2 IGFR in the endometrial cells [140, 141]. Extravillous trophoblast cells, which invade deep into the decidua, express non-canonical class I HLA-E and HLA-G (Fig. 3) in addition to canonical HLAs, which increase Foxp3 expression of Treg cells and generation of CD45RA+ resting Treg cells [142]. However, this does not necessary mean that these HLAs are indispensable to establish uterine receptivity against embryos. Soluble factors from the embryo but detected in maternal peripheral blood, such as human chorionic gonadotropin (hCG), preimplantation factor (PIF), and soluble form of HLA-G (sHLA-G), may have systemic effects such as promoting ovarian progesterone production, balancing cytokine and chemokine secretion in the periphery (Fig. 3) [143–146]. A genomics study revealed that PIF, produced by the embryo post-fertilization, upregulates CX3CL1 expression in the cultured decidual cells from first-trimester pregnant women, and increases interleukin-1 receptor-associated kinase 1-binding protein 1 (IRAK1BP1) expression in both decidual culture and human endometrial stromal cells (hESCs) [147]. However, the gene or enzyme responsible for PIF production is unknown, sustaining studies on PIF KO mice unavailable, which will be necessary to be challenged in order to understand the importance of this peptide.
Conclusions: toward clinical endpoints
Current diagnosis for infertility and failures in treatment
Defective decidua formation in early pregnancy may result in infertility or in a later onset of complications such as preeclampsia, recurrent abortion, and pre-term birth [18, 148, 149]. The definition of infertility by the World Health Organization (WHO) is childlessness within 1 year of active sexual intercourse [150]. Risk factors for female infertility and subfertility including recurrent abortion are uterine disorders such as endometriosis (10 % incidence) [151] and uterine fibroids (70∼80 % women affected, but only a small proportion with huge lesions suffer infertility due to physical unreceptivity) [152], or ovarian problems, e.g., polycystic ovarian syndrome (PCOS) in 4∼8 % women in reproductive age [153] and premature ovarian failure in 0.8∼3.7 % women of various races between 40 and 45 years old [154]. In some cases, infertility is treatable such as surgical resection of fibroids, or metformin administration for PCOS patients with insulin tolerance to reduce androgen’s suppressive effect on luteal development. However, there are still 42 % of RM still unaccounted for (excluding 41 % abnormal embryonic karyotype and recalculated from original literature [155]). According to Japanese governmental reports, the number of successful cases in assisted reproduction technologies (ART) doubled between 2002 and 2012 (15,228 cases in 2002 and 37,953 cases in 2012) [156], while the number of total infertile patients increased four times during the same period (85,664 women in 2002 and 326,426 women in 2012) [156].
Increased number of ART can be attributed to the progress and advances in in vitro fertilization (IVF), intracytoplasmic sperm injection (ICSI), and utilization of frozen eggs. Another effort from immunological view has been made using intravenous immunoglobulin (IVIg) administration to the patients with autoimmune disease represented by anti-phospholipid (aPL) syndrome, systemic lupus erythematosus (SLE), autoimmune thyroid disease (AITD), and type 1 diabetes mellitus [157]. However, this approach has been recognized as the final choice for subgroups of infertile women who were not rescued by other means, because of the high risk of adverse effects and the swelling medical costs [157]. Furthermore, indeed aPL antibodies have affinity to the surface phospholipid exposed on cytotrophoblasts, and indeed IVIg treatment can rescue the RM in AITD patients but in combination with thyroid hormones [157], the mechanisms how autoantibodies affect implantation and how IVIg rescues are unclear. In most cases in autoimmune diseases, ovarian functions and other endocrinal system are also affected. Typical remedy with combination of IVIg and NSAIDs may just suppress cytokine storms, but without understanding the basis it is hard to select appropriate patients.
Better understanding the mechanisms via optimal animal models
A number of molecules, e.g., leukemia inhibitory factor (LIF) and lipid mediator prostaglandins, associated with “uterine receptivity” have been identified in PR and ER pathways and Wnt signaling, via gene-deficient murine models [18]. Immunologists in the field of reproduction have utilized T, B, NK-deficient Rag2−/−Δγc or T, NK-deficient tgε26 to elucidate the role of immune cells in decidual vascular remodeling failure. However, these immunodeficient mice did not show complete pregnancy loss, with only modest changes in uterine vascular remodeling or decidual cellularity, let alone the high resorption rate and placental shrinkage in tgε26 mice [158], probably due to the compensatory functions of other minor immune cellular populations and of uterine stromal cells, or to the adaptively enhanced vascular development, and also to the absence of cytotoxic immune cells in the maternal side. Moreover, only in later placental stage of pregnancy do these mice show increased resorption and abnormal placenta, with an essential caveat of fetal genotype’s effects. Thus, wild-type embryo transfer to these uteri will better clarify the maternal cells roles. In contrast, decidual defects have been reported in several gene-targeted mouse models (Hoxa10, Hoxa11, Bmp2, Wnt4, Dedd, and IL11ra) [13–16, 19, 23]. Several mouse models deficient in cytokine signaling such as Lif−/− or Stat3−/− show infertile phenotype [159, 160], but the defects are already found in implantation rates before decidualization, suggesting more fundamental and pleiotropic roles for the signaling downstream. Intriguingly, uterine conditional Trp53−/− mice present decidual senescence accompanied with excessive terminal differentiation and multi-nuclearization until later gestational period, which results in preterm birth [161]. In order to shed more light on intrinsic and mutual roles for maternal immune cells, uterine vascular and stromal cells, it is necessary to generate new combination of different mouse models. A mouse model with uterine decidual deficiency named as above in combination with NK-deficient models may more clearly explain the functions of uNK cells on decidual development and vice versa, via crossing the two mouse lines and via transferring wild-type NK cells. Likewise, uterine vascular deficiency models might shed light on the role for vascular factors in uNK cell functions. However, embryonic or postnatal lethality of Vegfa−/−, Angpt1−/−, and Angpt2−/− mice demands to assess uterine conditional deletion of these genes, which has not been challenged for female reproductive functions. For instance, PR-Cre x Vegfaflox will delete decidual cell-specific deletion of VEGF-A, and VE-cadherin-Cre-ER x Vegfaflox is possible to delete vascular endothelial VEGF-A production via tamoxifen administration.
Rigorous studies on basic and clinical sides are required to overcome conflicting evidences (which are partially due to the lack of human samples at the peri-implantation stage), in order to obtain a molecular clue for the causes of infertility, and to invent novel diagnostic methods and treatments.
Acknowledgments
This work was supported by the Japan Society for the Promotion of Science (JSPS) to M.M., SROP-4.2.2.A-11/1/KONV-2012-0053 to J.S., SROP-4.2.2.D-15/1/KONV-2015-0004 to J.S., and PTE ÁOK-KA 2015-12 to J.S. We thank Detlev Riller for illustrations.
Footnotes
This article is a contribution to the special issue on Fetomaternal Cross Talk and Its Effect on Pregnancy Maintenance, Maternal and Offspring Health – Guest Editor: Petra Arck
References
- 1.McLaren A, Tarkowski AK. Implantation of mouse eggs in the peritoneal cavity. J Reprod Fertil. 1963;6:385–392. doi: 10.1530/jrf.0.0060385. [DOI] [PubMed] [Google Scholar]
- 2.Spornitz UM. Pseudo-decidualization at the site of implantation in tubal pregnancy. Arch Gynecol Obstet. 1993;253:85–95. doi: 10.1007/BF02768734. [DOI] [PubMed] [Google Scholar]
- 3.Deanesly R. Termination of early pregnancy in rats after ovariectomy is due to immediate collapse of the progesterone-dependent decidua. J Reprod Fertil. 1973;35:183–186. doi: 10.1530/jrf.0.0350183. [DOI] [PubMed] [Google Scholar]
- 4.Douglas NC, Zimmermann RC, Tan QK, Sullivan-Pyke CS, Sauer MV, Kitajewski JK, Shawber CJ. VEGFR-1 blockade disrupts peri-implantation decidual angiogenesis and macrophage recruitment. Vasc Cell. 2014;6:16. doi: 10.1186/2045-824X-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felker AM, Chen Z, Foster WG, Croy BA. Receptors for non-MHC ligands contribute to uterine natural killer cell activation during pregnancy in mice. Placenta. 2013;34:757–764. doi: 10.1016/j.placenta.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zarnani AH, Moazzeni SM, Shokri F, et al. Microenvironment of the feto–maternal interface protects the semiallogenic fetus through its immunomodulatory activity on dendritic cells. Fertil Steril. 2008;90:781–788. doi: 10.1016/j.fertnstert.2007.01.102. [DOI] [PubMed] [Google Scholar]
- 7.Doisne JM, Balmas E, Boulenouar S, Gaynor LM, Kieckbusch J, Gardner L, Hawkes DA, Barbara CF, Sharkey AM, Brady HJ, Brosens JJ, Moffett A, Colucci F. Composition, development, and function of uterine innate lymphoid cells. J Immunol. 2015;195:3937–3945. doi: 10.4049/jimmunol.1500689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nancy P, Tagliani E, Tay CS, Asp P, Levy DE, Erlebacher A. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science. 2012;336:1317–1321. doi: 10.1126/science.1220030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parr MB, Parr EL. Permeability of the primary decidual zone in the rat uterus: studies using fluorescein-labeled proteins and dextrans. Biol Reprod. 1986;34:393–403. doi: 10.1095/biolreprod34.2.393. [DOI] [PubMed] [Google Scholar]
- 10.Croy BA, Yamada AT, De Mayo FJ, Adamson SL. The guide to investigation of mouse pregnancy. London: Academic Press; 2014. [Google Scholar]
- 11.Herington JL, Underwood T, McConaha M, Bany BM. Paracrine signals from the mouse conceptus are not required for the normal progression of decidualization. Endocrinology. 2009;150:4404–4413. doi: 10.1210/en.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimber SJ. Molecular interactions at the maternal-embryonic interface during the early phase of implantation. Semin Reprod Med. 2000;18:237–253. doi: 10.1055/s-2000-12562. [DOI] [PubMed] [Google Scholar]
- 13.Franco HL, Dai D, Lee KY. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J. 2011;25:1176–1187. doi: 10.1096/fj.10-175349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee KY, Jeong JW, Wang J, Ma L, Martin JF, Tsai SY, Lydon JP, DeMayo FJ. Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol. 2007;27:5468–5478. doi: 10.1128/MCB.00342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim H, Ma L, Ma WG, Maas RL, Dey SK. Hoxa-10 regulates uterine stromal cell responsiveness to progesterone during implantation and decidualization in the mouse. Mol Endocrinol. 1999;13:1005–1017. doi: 10.1210/mend.13.6.0284. [DOI] [PubMed] [Google Scholar]
- 16.Gendron RL, Paradis H, Hsieh-Li HM, Lee DW, Potter SS, Markoff E. Abnormal uterine stromal and glandular function associated with maternal reproductive defects in Hoxa-11 null mice. Biol Reprod. 1997;56:1097–1105. doi: 10.1095/biolreprod56.5.1097. [DOI] [PubMed] [Google Scholar]
- 17.Bhurke AS, Bagchi IC, Bagchi MK. Progesterone-regulated endometrial factors controlling implantation. Am J Reprod Immunol. 2016;75:237–245. doi: 10.1111/aji.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18:1754–1767. doi: 10.1038/nm.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robb L, Li R, Hartley L, Nandurkar HH, Koentgen F, Begley CG. Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nat Med. 1998;4:303–308. doi: 10.1038/nm0398-303. [DOI] [PubMed] [Google Scholar]
- 20.Bilinski P, Roopenian D, Gossler A. Maternal IL-11Ralpha function is required for normal decidua and fetoplacental development in mice. Genes Dev. 1998;12:2234–2243. doi: 10.1101/gad.12.14.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das SK. Cell cycle regulatory control for uterine stromal cell decidualization in implantation. Reproduction. 2009;137:889–899. doi: 10.1530/REP-08-0539. [DOI] [PubMed] [Google Scholar]
- 22.Ma X, Gao F, Rusie A, Hemingway J, Ostmann AB, Sroga JM, Jegga AG, Das SK. Decidual cell polyploidization necessitates mitochondrial activity. PLoS One. 2011;6:e26774. doi: 10.1371/journal.pone.0026774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mori M, Kitazume M, Ose R, Kurokawa J, Koga K, Osuga Y, Arai S, Miyazaki T. Death effector domain-containing protein (DEDD) is required for uterine decidualization during early pregnancy in mice. J Clin Invest. 2011;121:318–327. doi: 10.1172/JCI44723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurabe N, Mori M, Kurokawa J, Taniguchi K, Aoyama H, Atsuda K, Nishijima A, Odawara N, Harada S, Nakashima K, Arai S, Miyazaki T. The death effector domain-containing DEDD forms a complex with Akt and Hsp90, and supports their stability. Biochem Biophys Res Commun. 2010;391:1708–1713. doi: 10.1016/j.bbrc.2009.12.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshino O, Osuga Y, Hirota Y, Koga K, Yano T, Tsutsumi O, Taketani Y. Akt as a possible intracellular mediator for decidualization in human endometrial stromal cells. Mol Hum Reprod. 2003;9:265–269. doi: 10.1093/molehr/gag035. [DOI] [PubMed] [Google Scholar]
- 26.Tan J, Raja S, Davis MK, Tawfik O, Dey SK, Das SK. Evidence for coordinated interaction of cyclin D3 with p21 and cdk6 in directing the development of uterine stromal cell decidualization and polyploidy during implantation. Mech Dev. 2002;111:99–113. doi: 10.1016/S0925-4773(01)00614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li F, Devi YS, Bao L, Mao J, Gibori G. Involvement of cyclin D3, CDKN1A (p21), and BIRC5 (Survivin) in interleukin 11 stimulation of decidualization in mice. Biol Reprod. 2008;78:127–133. doi: 10.1095/biolreprod.107.063313. [DOI] [PubMed] [Google Scholar]
- 28.Caulin-Glaser T, Watson CA, Pardi R, Bender JR. Effects of 17beta-estradiol on cytokine-induced endothelial cell adhesion molecule expression. J Clin Invest. 1996;98:36–42. doi: 10.1172/JCI118774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaya HS, Hantak AM, Stubbs LJ, Taylor RN, Bagchi IC, Bagchi MK. Roles of progesterone receptor A and B isoforms during human endometrial decidualization. Mol Endocrinol. 2015;29:882–895. doi: 10.1210/me.2014-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otsuki M, Saito H, Xu X, Sumitani S, Kouhara H, Kishimoto T, Kasayama S. Progesterone, but not medroxyprogesterone, inhibits vascular cell adhesion molecule-1 expression in human vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2001;21:243–248. doi: 10.1161/01.ATV.21.2.243. [DOI] [PubMed] [Google Scholar]
- 31.Shweiki D, Itin A, Neufeld G, Gitay-Goren H, Keshet E. Patterns of expression of vascular endothelial growth factor (VEGF) and VEGF receptors in mice suggest a role in hormonally regulated angiogenesis. J Clin Invest. 1993;91:2235–2243. doi: 10.1172/JCI116450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugino N, Kashida S, Karube-Harada A, Takiguchi S, Kato H. Expression of vascular endothelial growth factor (VEGF) and its receptors in human endometrium throughout the menstrual cycle and in early pregnancy. Reproduction. 2002;123:379–387. doi: 10.1530/rep.0.1230379. [DOI] [PubMed] [Google Scholar]
- 33.Das A, Mantena SR, Kannan A, Evans DB, Bagchi MK, Bagchi IC. De novo synthesis of estrogen in pregnant uterus is critical for stromal decidualization and angiogenesis. Proc Natl Acad Sci USA. 2009;106:12542–12547. doi: 10.1073/pnas.0901647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma S, Godbole G, Modi D. Decidual control of trophoblast invasion. Am J Reprod Immunol. 2016;75:341–350. doi: 10.1111/aji.12466. [DOI] [PubMed] [Google Scholar]
- 36.Hess AP, Hamilton AE, Talbi S, et al. Decidual stromal cell response to paracrine signals from the trophoblast: amplification of immune and angiogenic modulators. Biol Reprod. 2007;76:102–117. doi: 10.1095/biolreprod.106.054791. [DOI] [PubMed] [Google Scholar]
- 37.Laws MJ, Taylor RN, Sidell N, DeMayo FJ, Lydon JP, Gutstein DE, Bagchi MK, Bagchi IC. Gap junction communication between uterine stromal cells plays a critical role in pregnancy-associated neovascularization and embryo survival. Development. 2008;135:2659–2668. doi: 10.1242/dev.019810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bao L, Devi YS, Bowen-Shauver J, Ferguson-Gottschall S, Robb L, Gibori G. The role of interleukin-11 in pregnancy involves up-regulation of alpha2-macroglobulin gene through janus kinase 2-signal transducer and activator of transcription 3 pathway in the decidua. Mol Endocrinol. 2006;20:3240–3250. doi: 10.1210/me.2006-0296. [DOI] [PubMed] [Google Scholar]
- 39.Dimitriadis E, Stoikos C, Tan YL, Salamonsen LA. Interleukin 11 signaling components signal transducer and activator of transcription 3 (STAT3) and suppressor of cytokine signaling 3 (SOCS3) regulate human endometrial stromal cell differentiation. Endocrinology. 2006;147:3809–3817. doi: 10.1210/en.2006-0264. [DOI] [PubMed] [Google Scholar]
- 40.Beer AE, Billingham RE. Host responses to intra–uterine tissue, cellular and fetal allografts. J Reprod Fertil Suppl. 1974;21:59–88. [Google Scholar]
- 41.Clark DA. Controversies in reproductive immunology. Crit Rev Immunol. 1991;11:215–247. [PubMed] [Google Scholar]
- 42.Badet MT, Bell SC, Billington WD. Immunoregulatory activity of supernatants from short-term cultures of mouse decidual tissue. J Reprod Fertil. 1983;68:351–358. doi: 10.1530/jrf.0.0680351. [DOI] [PubMed] [Google Scholar]
- 43.Brierley J, Clark DA. Characterization of hormone-dependent suppressor cells in the uterus of mated and pseudopregnant mice. J Reprod Immunol. 1987;10:201–217. doi: 10.1016/0165-0378(87)90087-8. [DOI] [PubMed] [Google Scholar]
- 44.Yadi H, Burke S, Madeja Z, Hemberger M, Moffett A, Colucci F. Unique receptor repertoire in mouse uterine NK cells. J Immunol. 2008;181:6140–6147. doi: 10.4049/jimmunol.181.9.6140. [DOI] [PubMed] [Google Scholar]
- 45.Takashima A, Ishikawa F, Kuwabara T, Tanaka Y, Kinoshita T, Ito M, Kakiuchi T. Uterine natural killer cells severely decrease in number at gestation day 6 in mice. Biol Reprod. 2013;89:1–8. doi: 10.1095/biolreprod.113.109009. [DOI] [PubMed] [Google Scholar]
- 46.Halim TY, MaClaren A, Romanish MT, Gold MJ, McNagny KM, Takei F. Retinoic-acid-receptor-related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation. Immunity. 2012;37:463–474. doi: 10.1016/j.immuni.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 47.Blois SM, Ilarregui JM, Tometten M, Garcia M, Orsal AS, Cordo-Russo R, Toscano MA, Bianco GA, Kobelt P, Handjiski B, Tirado I, Markert UR, Klapp BF, Poirier F, Szekeres-Bartho J, Rabinovich GA, Arck PC. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med. 2007;13:1450–1457. doi: 10.1038/nm1680. [DOI] [PubMed] [Google Scholar]
- 48.Colucci F, Caligiuri MA, Di Santo JP. What does it take to make a natural killer? Nat Rev Immunol. 2003;3:413–425. doi: 10.1038/nri1088. [DOI] [PubMed] [Google Scholar]
- 49.Koopman LA, Kopcow HD, Rybalov B, et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198:1201–1212. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/S1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 51.King A, Burrows T, Verma S, Hiby S, Loke YW. Human uterine lymphocytes. Hum Reprod Update. 1998;4:480–485. doi: 10.1093/humupd/4.5.480. [DOI] [PubMed] [Google Scholar]
- 52.Bogovic Crncic T, Laskarin G, Juretic K, Strbo N, Dupor J, Srsen S, Randic L, Le Bouteiller P, Tabiasco J, Rukavina D. Perforin and Fas/FasL cytolytic pathways at the maternal-fetal interface. Am J Reprod Immunol. 2005;54:241–248. doi: 10.1111/j.1600-0897.2005.00320.x. [DOI] [PubMed] [Google Scholar]
- 53.Monk JM, Leonard S, McBey BA, Croy BA. Induction of murine spiral artery modification by recombinant human interferon-gamma. Placenta. 2005;26:835–838. doi: 10.1016/j.placenta.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 54.Lima PD, Tu MM, Rahim MM, Peng AR, Croy BA, Makrigiannis AP. Ly49 receptors activate angiogenic mouse DBA+ uterine natural killer cells. Cell Mol Immunol. 2014;11:467–476. doi: 10.1038/cmi.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li XF, Charnock-Jones DS, Zhang E, et al. Angiogenic growth factor messenger ribonucleic acids in uterine natural killer cells. J Clin Endocrinol Metab. 2001;86:1823–343. doi: 10.1210/jcem.86.4.7418. [DOI] [PubMed] [Google Scholar]
- 56.Lash GE, Schiessl B, Kirkley M, Innes BA, Cooper A, Searle RF, Robson SC, Bulmer JN. Expression of angiogenic growth factors by uterine natural killer cells during early pregnancy. J Leukoc Biol. 2006;80:572–580. doi: 10.1189/jlb.0406250. [DOI] [PubMed] [Google Scholar]
- 57.Rukavina D, Rubesa G, Gudelj L, et al. Characteristics of perforin expressing lymphocytes within the first trimester of human pregnancy. Am J Reprod Immunol. 1995;33:394–404. doi: 10.1111/j.1600-0897.1995.tb00908.x. [DOI] [PubMed] [Google Scholar]
- 58.Xu X, Zhao M, Liu X, Jiang Y, Zhang H, Zhai X, Zhang L, Hu X. Toxoplasma gondii infection regulates the balance of activating and inhibitory receptors on decidual natural killer cells. PLoS One. 2013;8:e55432. doi: 10.1371/journal.pone.0055432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kieckbusch J, Gaynor LM, Moffett A, Colucci F. MHC-dependent inhibition of uterine NK cells impedes fetal growth and decidual vascular remodelling. Nat Commun. 2014;5:3359. doi: 10.1038/ncomms4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chantakru S, Miller C, Roach LE, Kuziel WA, Maeda N, Wang WC, Evans SS, Croy BA. Contributions from self-renewal and trafficking to the uterine NK cell population of early pregnancy. J Immunol. 2002;168:22–28. doi: 10.4049/jimmunol.168.1.22. [DOI] [PubMed] [Google Scholar]
- 61.Chiossone L, Vacca P, Orecchia P, Croxatto D, Damonte P, Astigiano S, Barbieri O, Bottino C, Moretta L, Mingari MC. In vivo generation of decidual natural killer cells from resident hematopoietic progenitors. Haematologica. 2014;99:448–457. doi: 10.3324/haematol.2013.091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hiyama M, Kusakabe KT, Kuwahara A, Wakitani S, Khan H, Kiso Y. Differentiation of uterine natural killer cells in pregnant SCID (scid/scid) mice. J Vet Med Sci. 2011;73:1337–1340. doi: 10.1292/jvms.11-0189. [DOI] [PubMed] [Google Scholar]
- 63.Chen Z, Zhang J, Hatta K, Lima PD, Yadi H, Colucci F, Yamada AT, Croy BA. DBA-lectin reactivity defines mouse uterine natural killer cell subsets with biased gene expression. Biol Reprod. 2012;87:81. doi: 10.1095/biolreprod.112.102293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ye W, Zheng LM, Young JD, Liu CC. The involvement of interleukin (IL)-15 in regulating the differentiation of granulated metrial gland cells in mouse pregnant uterus. J Exp Med. 1996;184:2405–2410. doi: 10.1084/jem.184.6.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kennedy MK, Glaccum M, Brown SN, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ashkar AA, Black GP, Wei Q, He H, Liang L, Head JR, Croy BA. Assessment of requirements for IL-15 and IFN regulatory factors in uterine NK cell differentiation and function during pregnancy. J Immunol. 2003;171:2937–2944. doi: 10.4049/jimmunol.171.6.2937. [DOI] [PubMed] [Google Scholar]
- 67.Barber EM, Pollard JW. The uterine NK cell population requires IL-15 but these cells are not required for pregnancy nor the resolution of a Listeria monocytogenes infection. J Immunol. 2003;171:37–46. doi: 10.4049/jimmunol.171.1.37. [DOI] [PubMed] [Google Scholar]
- 68.Bany BM, Scott CA, Eckstrum KS. Analysis of uterine gene expression in interleukin-15 knockout mice reveals uterine natural killer cells do not play a major role in decidualization and associated angiogenesis. Reproduction. 2012;143:359–375. doi: 10.1530/REP-11-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hofmann AP, Gerber SA, Croy BA. Uterine natural killer cells pace early development of mouse decidua basalis. Mol Hum Reprod. 2014;20:66–76. doi: 10.1093/molehr/gat060. [DOI] [PubMed] [Google Scholar]
- 70.Ashkar AA, Di Santo JP, Croy BA. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med. 2000;192:259–270. doi: 10.1084/jem.192.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hanna J, Goldman-Wohl D, Hamani Y, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 72.Chappell LC, Duckworth S, Seed PT, et al. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia: a prospective multicenter study. Circulation. 2013;1238:2121–2131. doi: 10.1161/CIRCULATIONAHA.113.003215. [DOI] [PubMed] [Google Scholar]
- 73.Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 74.Wallace AE, Whitley GS, Thilaganathan B, Cartwright JE. Decidual natural killer cell receptor expression is altered in pregnancies with impaired vascular remodeling and a higher risk of pre-eclampsia. J Leukoc Biol. 2015;97:79–86. doi: 10.1189/jlb.2A0614-282R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clark DA, Chaouat G, Arck PC, Mittruecker HW, Levy GA. Cytokine-dependent abortion in CBA x DBA/2 mice is mediated by the procoagulant fgl2 prothrombinase. J Immunol. 1998;160:545–549. [PubMed] [Google Scholar]
- 76.Clark DA, Quarrington C, Banwatt D, Manuel J, Fulop G. Spontaneous abortion in immunodeficient SCID mice. Am J Reprod Immunol. 1994;32:15–25. doi: 10.1111/j.1600-0897.1994.tb00874.x. [DOI] [PubMed] [Google Scholar]
- 77.Szekeres-Bartho J, Par G, Szereday L, Smart CY, Achatz I. Progesterone and non-specific immunologic mechanisms in pregnancy. Am J Reprod Immunol. 1997;38:176–182. doi: 10.1111/j.1600-0897.1997.tb00295.x. [DOI] [PubMed] [Google Scholar]
- 78.Quenby S, Bates M, Doig T, Brewster J, Lewis-Jones DI, Johnson PM, Vince G. Pre-implantation endometrial leukocytes in women with recurrent miscarriage. Hum Reprod. 1999;14:2386–2391. doi: 10.1093/humrep/14.9.2386. [DOI] [PubMed] [Google Scholar]
- 79.Tuckerman E, Laird SM, Prakash A, Li TC. Prognostic value of the measurement of uterine natural killer cells in the endometrium of women with recurrent miscarriage. Hum Reprod. 2007;22:2208–2213. doi: 10.1093/humrep/dem141. [DOI] [PubMed] [Google Scholar]
- 80.Gulan G, Podack ER, Rukavina D, Gudelj L, Rubesa G, Petrovic O. Perforin-expressing lymphocytes in peripheral blood and decidua of human first-trimester pathological pregnancies. Am J Reprod Immunol. 1997;38:9–18. doi: 10.1111/j.1600-0897.1997.tb00270.x. [DOI] [PubMed] [Google Scholar]
- 81.Quenby S, Nik H, Innes B, Lash G, Turner M, Drury J, Bulmer J. Uterine natural killer cells and angiogenesis in recurrent reproductive failure. Hum Reprod. 2009;24:45–54. doi: 10.1093/humrep/den348. [DOI] [PubMed] [Google Scholar]
- 82.Lachapelle MH, Miron P, Hemmings R, Roy DC. Endometrial T, B, and NK cells in patients with recurrent spontaneous abortion. Altered profile and pregnancy outcome. J Immunol. 1996;156:4027–4034. [PubMed] [Google Scholar]
- 83.Yamada H, Morikawa M, Kato EH, Shimada S, Kobashi G, Minakami H. Pre-conceptional natural killer cell activity and percentage as predictors of biochemical pregnancy and spontaneous abortion with normal chromosome karyotype. Am J Reprod Immunol. 2003;50:351–354. doi: 10.1034/j.1600-0897.2003.00095.x. [DOI] [PubMed] [Google Scholar]
- 84.Aoki K, Kajiura S, Matsumoto Y, Ogasawara M, Okada S, Yagami Y, Gleicher N. Preconceptional natural-killer-cell activity as a predictor of miscarriage. Lancet. 1995;345:1340–1342. doi: 10.1016/S0140-6736(95)92539-2. [DOI] [PubMed] [Google Scholar]
- 85.Karami N, Boroujerdnia MG, Nikbakht R, Khodadadi A. Enhancement of peripheral blood CD56dim cell and NK cell cytotoxicity in women with recurrent spontaneous abortion or in vitro fertilization failure. J Reprod Immunol. 2012;95:87–92. doi: 10.1016/j.jri.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 86.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, Powrie F, Vivier E. Innate lymphoid cells – a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 87.Eberl G, Colonna M, Di Santo JP, McKenzie AN. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science. 2015;348:aaa6566. doi: 10.1126/science.aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moro K, Koyasu S. Innate lymphoid cells, possible interaction with microbiota. Semin Immunopathol. 2015;37:27–37. doi: 10.1007/s00281-014-0470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 90.Siewiera J, Gouilly J, Hocine HR, Cartron G, Levy C, Al-Daccak R, Jabrane-Ferrat N. Natural cytotoxicity receptor splice variants orchestrate the distinct functions of human natural killer cell subtypes. Nat Commun. 2015;6:10183. doi: 10.1038/ncomms10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vacca P, Montaldo E, Croxatto D, et al. Identification of diverse innate lymphoid cells in human decidua. Mucosal Immunol. 2015;8:254–264. doi: 10.1038/mi.2014.63. [DOI] [PubMed] [Google Scholar]
- 92.Sawa S, Lochner M, Satoh-Takayama N, Dulauroy S, Bérard M, Kleinschek M, Cua D, Di Santo JP, Eberl G. RORγt+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat Immunol. 2011;12:320–326. doi: 10.1038/ni.2002. [DOI] [PubMed] [Google Scholar]
- 93.Zhao H, Kalish F, Schulz S, Yang Y, Wong RJ, Stevenson DK. Unique roles of infiltrating myeloid cells in the murine uterus during early to midpregnancy. J Immunol. 2015;194:3713–3722. doi: 10.4049/jimmunol.1401930. [DOI] [PubMed] [Google Scholar]
- 94.Trundley A, Gardner L, Northfield J, Chang C, Moffett A. Methods for isolation of cells from the human fetal-maternal interface. Methods Mol Med. 2006;122:109–122. doi: 10.1385/1-59259-989-3:109. [DOI] [PubMed] [Google Scholar]
- 95.Pollard JW, Hunt JS, Wiktor-Jedrzejczak W, Stanley ER. A pregnancy defect in the osteopetrotic (op/op) mouse demonstrates the requirement for CSF-1 in female fertility. Dev Biol. 1991;148:273–283. doi: 10.1016/0012-1606(91)90336-2. [DOI] [PubMed] [Google Scholar]
- 96.Houser BL, Tilburgs T, Hill J, Nicotra ML, Strominger JL. Two unique human decidual macrophage populations. J Immunol. 2011;186:2633–2642. doi: 10.4049/jimmunol.1003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Houser BL. Decidual macrophages and their roles at the maternal-fetal interface. Yale J Biol Med. 2012;85:105–118. [PMC free article] [PubMed] [Google Scholar]
- 98.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 99.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 100.Devaraj S, Jialal I. C-reactive protein polarizes human macrophages to an M1 phenotype and inhibits transformation to the M2 phenotype. Arterioscler Thromb Vasc Biol. 2011;31:1397–1402. doi: 10.1161/ATVBAHA.111.225508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nagamatsu T, Schust DJ. The contribution of macrophages to normal and pathological pregnancies. Am J Reprod Immunol. 2010;63:460–471. doi: 10.1111/j.1600-0897.2010.00813.x. [DOI] [PubMed] [Google Scholar]
- 102.Chieppa M, Bianchi G, Doni A, et al. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J Immunol. 2003;171:4552–4560. doi: 10.4049/jimmunol.171.9.4552. [DOI] [PubMed] [Google Scholar]
- 103.Mizuno M, Aoki K, Kimbara T. Functions of macrophages in human decidual tissue in early pregnancy. Am J Reprod Immunol. 1994;31:180–188. doi: 10.1111/j.1600-0897.1994.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 104.Parhar RS, Yagel S, Lala PK. PGE2-mediated immunosuppression by first trimester human decidual cells blocks activation of maternal leukocytes in the decidua with potential anti-trophoblast activity. Cell Immunol. 1989;120:61–74. doi: 10.1016/0008-8749(89)90174-3. [DOI] [PubMed] [Google Scholar]
- 105.Heikkinen J, Möttönen M, Komi J, Alanen A, Lassila O. Phenotypic characterization of human decidual macrophages. Clin Exp Immunol. 2003;131:498–505. doi: 10.1046/j.1365-2249.2003.02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thaxton JE, Sharma S. Interleukin-10: a multi-faceted agent of pregnancy. Am J Reprod Immunol. 2010;63:482–491. doi: 10.1111/j.1600-0897.2010.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miwa N, Hayakawa S, Miyazaki S, Myojo S, Sasaki Y, Sakai M, Takikawa O, Saito S. IDO expression on decidual and peripheral blood dendritic cells and monocytes/macrophages after treatment with CTLA-4 or interferon-gamma increase in normal pregnancy but decrease in spontaneous abortion. Mol Hum Reprod. 2005;11:865–870. doi: 10.1093/molehr/gah246. [DOI] [PubMed] [Google Scholar]
- 108.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 109.Renaud SJ, Graham CH. The role of macrophages in utero-placental interactions during normal and pathological pregnancy. Immunol Investig. 2008;37:535–564. doi: 10.1080/08820130802191375. [DOI] [PubMed] [Google Scholar]
- 110.Austyn JM. New insights into the mobilization and phagocytic activity of dendritic cells. J Exp Med. 1996;183:1287–1292. doi: 10.1084/jem.183.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 112.Metlay JP, Witmer-Pack MD, Agger R, Crowley MT, Lawless D, Steinman RM. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J Exp Med. 1990;171:1753–1771. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Witmer MD, Steinman RM. The anatomy of peripheral lymphoid organs with emphasis on accessory cells: light-microscopic immunocytochemical studies of mouse spleen, lymph node, and Peyer’s patch. Am J Anat. 1984;170:465–481. doi: 10.1002/aja.1001700318. [DOI] [PubMed] [Google Scholar]
- 114.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 115.Moser M, Murphy KM. Dendritic cell regulation of TH1–TH2 development. Nat Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 116.Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal MR, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–1186. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 117.Dietl J, Hönig A, Kämmerer U, Rieger L. Natural killer cells and dendritic cells at the human Feto-maternal interface: an effective cooperation? Placenta. 2006;27:341–347. doi: 10.1016/j.placenta.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 118.Kämmerer U, Eggert AO, Kapp M, et al. Unique appearance of proliferating antigen-presenting cells expressing DCSIGN (CD209) in the decidua of early human pregnancy. Am J Pathol. 2003;162:887–896. doi: 10.1016/S0002-9440(10)63884-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Collins MK, Tay CS, Erlebacher A. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J Clin Investig. 2009;119:2062–2073. doi: 10.1172/JCI38714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miyazaki S, Tsuda H, Sakai M, et al. Predominance of Th2-promoting dendritic cells in early human pregnancy decidua. J Leukoc Biol. 2003;74:514–522. doi: 10.1189/jlb.1102566. [DOI] [PubMed] [Google Scholar]
- 121.Plaks V, Birnberg T, Berkutzki T, et al. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J Clin Investig. 2008;118:3954–3965. doi: 10.1172/JCI36682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Krey G, Frank P, Shaikly V, et al. In vivo dendritic cell depletion reduces breeding efficiency, affecting implantation and early placental development inmice. J Mol Med. 2008;86:999–1011. doi: 10.1007/s00109-008-0379-2. [DOI] [PubMed] [Google Scholar]
- 123.Huang SJ, Chen CP, Schatz F, Rahman M, Abrahams VM, Lockwood CJ. Pre-eclampsia is associated with dendritic cell recruitment into the uterine decidua. J Pathol. 2008;214:328–336. doi: 10.1002/path.2257. [DOI] [PubMed] [Google Scholar]
- 124.Askelund K, Liddell HS, Zanderigo AM, et al. CD83+ dendritic cells in the decidua of women with recurrent miscarriage and normal pregnancy. Placenta. 2004;25:140–145. doi: 10.1016/S0143-4004(03)00182-6. [DOI] [PubMed] [Google Scholar]
- 125.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 126.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 128.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 129.Zenclussen AC, Gerlof K, Zenclussen ML, Sollwedel A, Bertoja AZ, Ritter T, Kotsch K, Leber J, Volk HD. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol. 2005;166:811–822. doi: 10.1016/S0002-9440(10)62302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Akbar AN, Vukmanovic-Stejic M, Taams LS, Macallan DC. The dynamic co-evolution of memory and regulatory CD4+ T cells in the periphery. Nat Rev Immunol. 2007;7:231–237. doi: 10.1038/nri2037. [DOI] [PubMed] [Google Scholar]
- 131.Shevach EM. Mechanisms of Foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 132.Inada K, Shima T, Ito M, Ushijima A, Saito S. Helios-positive functional regulatory T cells are decreased in decidua of miscarriage cases with normal fetal chromosomal content. J Reprod Immunol. 2015;107:10–19. doi: 10.1016/j.jri.2014.09.053. [DOI] [PubMed] [Google Scholar]
- 133.Samstein R, Josefowicz S, Arvey A, Treuting P, Rudensky A. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150:29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lash GE, Ernerudh J. Decidual cytokines and pregnancy complications: focus on spontaneous miscarriage. J Reprod Immunol. 2015;108:83–89. doi: 10.1016/j.jri.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 135.Mellor AL, Sivakumar J, Chandler P, Smith K, Molina H, Mao D, Munn DH. Prevention of T cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat Immunol. 2001;2:64–68. doi: 10.1038/83183. [DOI] [PubMed] [Google Scholar]
- 136.Koga K, Izumi G, Mor G, Fujii T, Osuga Y. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy complications. Am J Reprod Immunol. 2014;72:192–205. doi: 10.1111/aji.12258. [DOI] [PubMed] [Google Scholar]
- 137.Krikun G, Lockwood CJ, Abrahams VM, Mor G, Paidas M, Guller S. Expression of Toll-like receptors in the human decidua. Histol Histopathol. 2007;22:847–854. doi: 10.14670/HH-22.847. [DOI] [PubMed] [Google Scholar]
- 138.Krikun G, Trezza J, Shaw J, Rahman M, Guller S, Abrahams VM, Lockwood CJ. Lipopolysaccharide appears to activate human endometrial endothelial cells through TLR-4-dependent and TLR-4-independent mechanisms. Am J Reprod Immunol. 2012;68:233–237. doi: 10.1111/j.1600-0897.2012.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Brosens JJ, Salker MS, Teklenburg G, Nautiyal J, Salter S, Lucas ES, Steel JH, Christian M, Chan YW, Boomsma CM, Moore JD, Hartshorne GM, Sućurović S, Mulac-Jericevic B, Heijnen CJ, Quenby S, Koerkamp MJ, Holstege FC, Shmygol A, Macklon NS. Uterine selection of human embryos at implantation. Sci Rep. 2014;4(3894):1–8. doi: 10.1038/srep03894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fazleabas AT, Kim JJ, Strakova Z (2004) Implantation: embryonic signals and the modulation of the uterine environment--a review. Placenta Suppl A:S26−31 [DOI] [PubMed]
- 141.Hamilton GS, Lysiak JJ, Han VK, Lala PK. Autocrine-paracrine regulation of human trophoblast invasiveness by insulin-like growth factor (IGF)-II and IGF-binding protein (IGFBP)-1. Exp Cell Res. 1998;244:147–156. doi: 10.1006/excr.1998.4195. [DOI] [PubMed] [Google Scholar]
- 142.Tilburgs T, Crespo ÂC, van der Zwan A, Rybalov B, Raj T, Stranger B, Gardner L, Moffett A, Strominger JL. Human HLA-G+ extravillous trophoblasts: Immune-activating cells that interact with decidual leukocytes. Proc Natl Acad Sci U S A. 2015;112:7219–7224. doi: 10.1073/pnas.1507977112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bourdiec A, Shao R, Rao CV, Akoum A. Human chorionic gonadotropin triggers angiogenesis via the modulation of endometrial stromal cell responsiveness to interleukin 1: a new possible mechanism underlying embryo implantation. Biol Reprod. 2012;87:66. doi: 10.1095/biolreprod.112.100370. [DOI] [PubMed] [Google Scholar]
- 144.Barnea ER, Kirk D, Ramu S, Rivnay B, Roussev R, Paidas MJ. PreImplantation Factor (PIF) orchestrates systemic antiinflammatory response by immune cells: effect on peripheral blood mononuclear cells. Am J Obstet Gynecol. 2012;207:313.e1–11. doi: 10.1016/j.ajog.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 145.van der Meer A, Lukassen HG, van Cranenbroek B, Weiss EH, Braat DD, van Lierop MJ, Joosten I. Soluble HLA-G promotes Th1-type cytokine production by cytokine-activated uterine and peripheral natural killer cells. Mol Hum Reprod. 2007;13:123–133. doi: 10.1093/molehr/gal100. [DOI] [PubMed] [Google Scholar]
- 146.Kanai T, Fujii T, Kozuma S, Yamashita T, Miki A, Kikuchi A, Taketani Y. Soluble HLA-G influences the release of cytokines from allogeneic peripheral blood mononuclear cells in culture. Mol Hum Reprod. 2001;7:195–200. doi: 10.1093/molehr/7.2.195. [DOI] [PubMed] [Google Scholar]
- 147.Paidas MJ, Krikun G, Huang SJ, Jones R, Romano M, Annunziato J, Barnea ER. A genomic and proteomic investigation of the impact of preimplantation factor on human decidual cells. Am J Obstet Gynecol. 2010;202:459.e1–8. doi: 10.1016/j.ajog.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.PrabhuDas M, Bonney E, Caron K, Dey S, Erlebacher A, Fazleabas A, Fisher S, Golos T, Matzuk M, McCune JM, Mor G, Schulz L, Soares M, Spencer T, Strominger J, Way SS, Yoshinaga K. Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat Immunol. 2015;16:328–334. doi: 10.1038/ni.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yeh CC, Chao KC, Huang SJ. Innate immunity, decidual cells, and preeclampsia. Reprod Sci. 2013;20:339–353. doi: 10.1177/1933719112450330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.WHO (2012) Preconception care to reduce maternal and childhood mortality and morbidity. http://www.who.int/maternal_child_adolescent/documents/concensus_preconception_care/en/Accessed 16 Feb 2016
- 151.Brown J, Farquhar C. Endometriosis: an overview of cochrane reviews. Cochrane Database Syst Rev. 2014;3:CD009590. doi: 10.1002/14651858.CD009590.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Song H, Lu D, Navaratnam K, Shi G. Aromatase inhibitors for uterine fibroids. Cochrane Database Syst Rev. 2013;10:CD009505. doi: 10.1002/14651858.CD009505.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Franik S, Kremer JAM, Nelen WLDM, Farquhar C. Aromatase inhibitors for subfertile women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2014;2:CD010287. doi: 10.1002/14651858.CD010287.pub2. [DOI] [PubMed] [Google Scholar]
- 154.National Institute for Health and Care Excellence (UK) (2015) Menopause: Full Guideline. Accessed 16 Feb 2016 [PubMed]
- 155.Sugiura-Ogasawara M, Ozaki Y, Katano K, Suzumori N, Kitaori T, Mizutani E. Abnormal embryonic karyotype is the most frequent cause of recurrent miscarriage. Hum Reprod. 2012;27:2297–2303. doi: 10.1093/humrep/des179. [DOI] [PubMed] [Google Scholar]
- 156.Japan Society of Obstetrics & Gynecology (2012) ART Data Book. http://plaza.umin.ac.jp/~jsog-art/2012data.pdf Accessed 24 Feb 2016
- 157.Carp HJ, Selmi C, Shoenfeld Y. The autoimmune bases of infertility and pregnancy loss. J Autoimmun. 2012;38:J266–J274. doi: 10.1016/j.jaut.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 158.Guimond MJ, Luross JA, Wang B, Terhorst C, Danial S, Croy BA. Absence of natural killer cells during murine pregnancy is associated with reproductive compromise in TgE26 mice. Biol Reprod. 1997;56:169–179. doi: 10.1095/biolreprod56.1.169. [DOI] [PubMed] [Google Scholar]
- 159.Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Köntgen F, Abbondanzo SJ. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 160.Nakamura H, Kimura T, Koyama S, Ogita K, Tsutsui T, Shimoya K, Taniguchi T, Koyama M, Kaneda Y, Murata Y. Mouse model of human infertility: transient and local inhibition of endometrial STAT-3 activation results in implantation failure. FEBS Lett. 2006;580:2717–2722. doi: 10.1016/j.febslet.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 161.Hirota Y, Daikoku T, Tranguch S, Xie H, Bradshaw HB, Dey SK. Uterine-specific p53 deficiency confers premature uterine senescence and promotes preterm birth in mice. J Clin Invest. 2010;120:803–815. doi: 10.1172/JCI40051. [DOI] [PMC free article] [PubMed] [Google Scholar]