Abstract
Background and aims
Physical activity and nutritional supplementation interventions may be used to ameliorate age-related loss of skeletal muscle mass and function. Previous reviews have demonstrated the beneficial effects of resistance exercise training (RET) combined with protein or essential amino acids (EAA) in younger populations. Whether or not older adults also benefit is unclear. The aim of this review was to determine whether regular dietary supplementation with protein/EAA during a RET regimen augments the effects of RET on skeletal muscle in older adults.
Methods
A literature search was conducted in August 2015 using MEDLINE, EMBASE, SPORTDiscus, and CINAHL Plus to identify all controlled trials using a RET regimen with and without protein/EAA supplementation. Outcome variables included muscle strength, muscle size, functional ability, and body composition.
Results
Fifteen studies fulfilled the eligibility criteria, including 917 participants with a mean age of 77.4 years. Studies involving both healthy participants and those described as frail or sarcopenic were included. Overall, results indicated that protein supplementation did not significantly augment the effects of RET on any of the specified outcomes. Exceptions included some measures of muscle strength (3 studies) and body composition (2 studies). Meta-analyses were conducted but were limited because of methodologic differences between studies, and results were inconclusive.
Conclusions
Systematic review and meta-analysis of controlled trials reveal that protein/EAA supplementation does not significantly augment the effects of progressive RET in older adults.
Keywords: Elderly, muscle strength, protein, resistance exercise training, sarcopenia
Sarcopenia is defined as an age-related loss of muscle mass and function, and is associated with frailty and reduced functional ability and independence,1, 2, 3 as well as increased incidence of falls, disability, infection, and mortality.1, 4, 5, 6, 7 Combined with the growing economic impact, demonstrated by US healthcare costs from 2000 which attribute $18.5 billion to sarcopenia and associated health problems,8 sarcopenia is a serious public health issue. Interventions to maintain muscle mass in older adults are a major priority and would be expected to result in both improved quality of life in old age and decreased healthcare costs.
Although the design of effective interventions has been a major goal for 30 years, so far only resistance exercise has shown any real benefit in improving muscle mass in older people. In younger adults, resistance exercise has been shown to acutely sensitize skeletal muscle to the anabolic effects of ingested protein or essential amino acids (EAA).9, 10 Hence, when considered in terms of a chronic response, resistance exercise training (RET) programs and nutritional supplementation (protein, EAA, or leucine) have an additive effect on muscle strength and fat free mass (FFM).11 One might assume, therefore, that an intervention combining RET and supplementation could be an effective strategy against sarcopenia in older adults, however, the presence of an effect in younger adults does not necessarily mean there will be an effect in older adults. The acute anabolic responsiveness to both resistance exercise and protein supplementation is blunted in older adults compared with younger adults,12, 13 and this is thought to translate into a chronic blunting of responsiveness.14 Thus, it is plausible that the chronic response to a combination of these factors may also differ in older adults.
In spite of this anabolic blunting, previous reviews of studies in older adults have found chronic additive effects of RET and protein/EAA supplementation compared with RET only, in terms of FFM11, 15 and muscle strength.11 However, there are issues with the relatively low minimum age limit inclusion criteria for these reviews; one used a cut-off of 50 years of age for the “older” age group, and the oldest participant included was 72 years of age, and in the other the lower age limit was an average of 60 years of age and included studies in which some participants were as young as 50 years of age. These age categories are not necessarily representative of older adults, and a cut-off mean 70 years of age may be more appropriate to define “elderly.” Longitudinal evidence shows that muscle strength and power continue to decline into advanced older age,16 and a dramatic increase in the prevalence of sarcopenia has been observed in the eighth decade of life.5 Hence, it would appear that the muscle of people aged over 70 years is performing differently to that of those aged 50 or 60 years, meaning it is likely that the responsiveness to anabolic factors may also differ between these age ranges. This would mean that the inclusion of studies involving these relatively younger participants may mask any differences in the effects of the combined intervention on the truly older adults. The use of an older cut-off point to define older adults is supported by the studies demonstrating anabolic resistance,12, 13 both of which reported an average age of 70 years, and is likely to give a better representation of the effect of anabolic resistance on chronic responsiveness to RET and protein supplementation. Furthermore, arguably the most important outcomes in terms of a practical impact of an intervention are those related to functional ability. Such outcomes are highly relevant to quality of life and the maintenance of an independent lifestyle, which are key priorities when setting lifestyle recommendations. To date, the combined effects of RET and protein supplementation on functional ability have yet to be addressed within a systematic review.
The aim of this systematic review was to determine whether protein or EAA supplementation can augment the effects of RET in older adults (ie, studies with an average age of 70 years or older). These effects included changes from baseline in muscle strength as the primary outcome, and secondary outcomes of muscle size, body composition, and indicators of functional ability, where functional ability was defined as the ability to perform everyday tasks and activities important for the maintenance of physical independence.
Methods
The systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) report.17 Although this protocol has not been previously published, all procedures were determined in advance.
Selection Criteria
Inclusion criteria were (1) controlled trials in humans implementing a progressive RET regimen in combination with a protein or EAA supplement, and inclusion of a comparison group combining RET with either a placebo/non-protein supplement or no supplement at all [Studies comparing higher versus lower protein diets were accepted providing the low protein diet was equivalent to the US recommended daily allowance (RDA) for protein (0.8 g·kg−1·day−1)]18; (2) studies including participants with a mean age of 70 years or over, both healthy and frail; (3) studies within any publication category and all languages; and (4) outcome measures including muscle strength (primary), muscle size, functional ability (defined as the ability to perform everyday tasks and activities important for the maintenance of physical independence), and body composition (secondary).
Studies were excluded if the intervention was administered with an agent previously shown to result in muscle gains (with the exception of vitamins and minerals), or if it was administered to a specific patient group or with the intention of treating a clinical condition other than frailty or sarcopenia.
Information Sources and Search
An electronic search of online databases was conducted in August 2015, using selected key words, “free text” terms, indexed terms, and Boolean operators (Table 1). Search strategies were constructed using search terms to identify papers on elderly populations with suitable supplementation and training regimens and a search filter to limit retrievals to studies in humans. The search strategies were applied to MEDLINE (1946 to August 2015); EMBASE (1980 to August 2015); CINAHL Plus (1937 to August 2015); SPORTDiscus (1949 to August 2015). Recursive searching of the bibliographies of eligible studies and relevant reviews was performed to identify additional studies.
Table 1.
Example Search Strategy∗
| 1 | Aged/or “aged, 80 and over”/ or frail elderly/ |
| 2 | Aging/ or longevity/ |
| 3 | (old* adj (adult* or age* or people or person* or population*)).tw. |
| 4 | (elder* or old* or ?enarian or aged or ag?ing or senior* or geriatric* or frail).mp. |
| 5 | 1 or 2 or 3 or 4 |
| 6 | Muscles/or muscle, skeletal/ |
| 7 | Exp Muscle Strength/ |
| 8 | Muscle Weakness/ |
| 9 | Muscular atrophy/ or sarcopenia/ |
| 10 | (musc* adj2 (mass or strength or size or cross sectional area or CSA or thick* or power or growth or enlarge* or area or volume or hypertrophy)).tw. |
| 11 | Muscle Development/ |
| 12 | Exercise therapy/ or resistance training/ |
| 13 | (weigh* OR streng* OR resis*) adj2 (train* OR exerc* OR therap*).mp. |
| 14 | Hypertrophy/ |
| 15 | 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 |
| 16 | Exp Dietary Supplements/ |
| 17 | Food, Fortified/ |
| 18 | ((protein* OR amino acid*) adj3 supplement*).tw. |
| 19 | Proteins/ |
| 20 | Exp Amino Acids/ |
| 21 | Exp Dietary Proteins/ |
| 22 | 16 or 17 or 18 or 19 or 20 or 21 |
| 23 | 5 and 15 and 22 |
| 24 | Exp animals/ not humans.sh |
| 25 | 23 not 24 |
CSA, cross-sectional area.
Ovid MEDLINE (R) search, adapted for other databases.
Study Selection
Titles and abstracts were screened for relevance by 1 reviewer (D.T.). Clearly irrelevant titles were removed. Full-text papers were obtained for potentially relevant studies, via a combination of online databases and direct contact with the authors, and these were further evaluated to determine whether they met the inclusion criteria. Two reviewers (D.T. and C.G.) independently assessed full-texts for eligibility, with a third reviewer (D.S.) moderating if necessary. Studies deemed eligible were included in the systematic review.
Data Extraction
Data were extracted from each of the included papers using a standardized data extraction form. Details of interest included various aspects of study design, resistance training regimens, protein/EAA supplements, and the comparison treatment, as well as participant characteristics and baseline protein intake. Data were sought for outcomes of muscle strength, muscle size, functional ability, and body composition. Where necessary, the required data were interpolated from figures or calculated from the reported data. Corresponding authors were contacted if this information could not be obtained from the paper, and if data could not be obtained, the study (or outcome measure) was excluded from meta-analysis.
Quality Assessment
Methodologic quality of included studies was assessed using the Physiotherapy Evidence Database (PEDro) scale.19 A score of 6 or higher indicated moderate to high quality.
Summary Measures and Synthesis of Results
Extracted data were collated and a review was conducted for the primary outcome (ie, muscle strength, as well as the secondary outcomes of muscle size, functional ability and body composition). This included description of studies and tabulation of data presented as mean [standard deviation (SD)] unless stated otherwise.
Meta-analysis was conducted on comparable outcomes reported in a minimum of 2 studies. Similar study protocols were a requisite for comparison using meta-analysis, and, despite all included studies addressing the questioned posed by this review, fundamental differences in their protocols meant that a number of study combinations were unsuitable for meta-analysis. Key criteria for determining study comparability included additional supplementation with vitamin D (which may also influence muscle related outcomes20), the frequency of protein supplementation (ie, daily or only on training days), the timing of supplementation (including number of doses), and the amount of protein supplemented, with consideration also given to the type of protein supplemented and the duration of the study.
Results
Study Selection
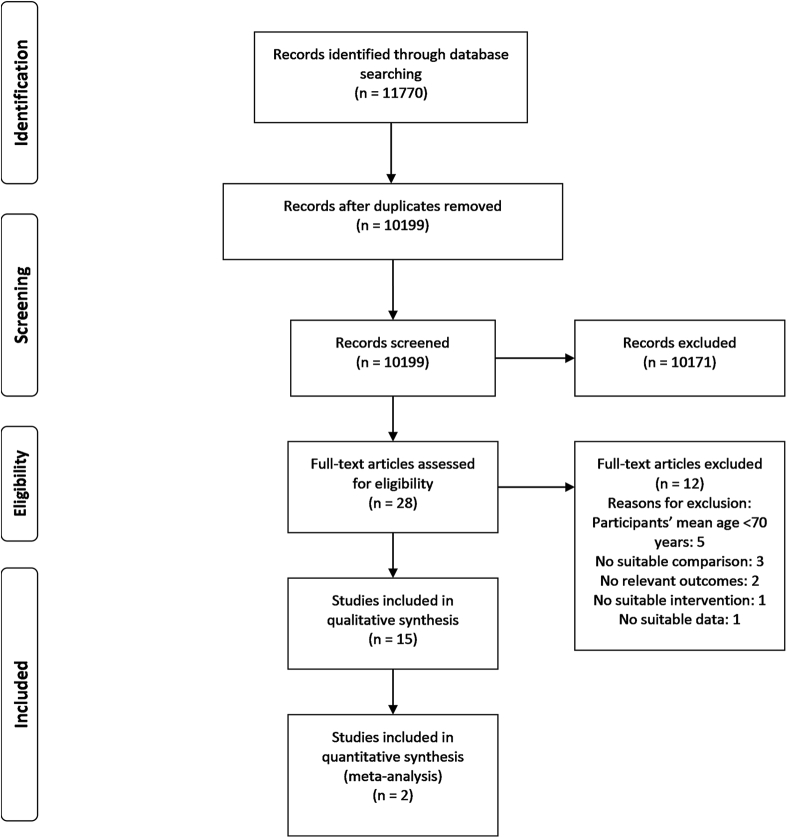
A total of 11,770 publications were identified by the literature search, of which 16 publications including 15 studies met all of the inclusion criteria and were included in the systematic review21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36 (Figure 1). Two publications24, 27 reported results from the Frail Older People-Activity and Nutrition Study in Umeå and, therefore, were considered here as 1 study.24
Fig. 1.
Flow of studies through the review process.
Study Characteristics
The 15 eligible studies included 917 participants with a mean age of 77.4 years (range 60–100 years) (Table 2). Six studies including 400 participants were conducted in older adults described as frail, sarcopenic, or mobility limited.21, 24, 26, 28, 29, 31 Individuals in the remaining 9 studies were categorized as healthy.22, 23, 25, 30, 32, 33, 34, 35, 36 Three studies included only male populations,22, 25, 35 2 included female only populations,28, 33 and the remaining 10 were mixed populations.21, 23, 24, 26, 29, 30, 31, 32, 34, 36 Of the total participants, 32% were male and 68% were female. All studies were published in English.
Table 2.
Study Characteristics
| Author, Year | Participant Details |
Training Details |
Protein/EAA and Placebo Supplement Details |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Participants | Mean Age, Years | Frail/Mobility Limited/Sarcopenic | Baseline Protein Intake (g·kg−1·day−1)∗ | Study Length and RT Frequency | Type of RT | RT Intensity | Functional/Balance Training | Type of Protein | Frequency (Daily/With Training) | Timing of Ingestion | Amount | Control Treatment | |
| Fiatarone et al, 199421 | 50 | 86.7 | Y | NA | 3 d/wk × 10 wk | LL | 80% 1-RM | N | Soy-based | Daily | Evening | 15 g approx. | Water |
| Godard et al, 200222 | 17 | 71.5 | N | 1.14 | 3 d/wk × 12 wk | LL | 80% 1-RM | N | EAA (1.86 g L-lysine, 2.24 g L-leucine, 1.40 g L-valine, 1.86 g L-phenylalanine, 1.76 g L-threonine, 1.30 g L-histidine, 1.2 g L-isoleucine, 0.38 g L-methionine) | Daily | After training | 12 g | Exercise only |
| Bunout et al, 200423 | 47 | 74.1 | N | NA | 2 d/wk × 1 year | LL + UL | Light | N | Undisclosed protein | Daily | Between meals | 15 g | Exercise only |
| Rosendahl et al, 200624† | 91 | 85.2 | Y | NA | 5 d/2 wk × 13 wk | LL | 8–12 RM | Y | Milk-based | With training | After training | 7.4 g | CHO |
| Verdijk et al, 200925 | 28 | 72.0 | N | 1.10 | 3 d/wk × 12 wk | LL | 60%–80% 1-RM | N | Casein | With training | 10 g before, 10 g after training | 20 g | Water |
| Zak et al, 200926 | 40 | 78.7 | Y | NA | 5 d/wk × 7 wk | LL | 80% 1-RM | Y | Undisclosed protein | With training | Before training | 12 g approx. | Water |
| Kim et al, 201228 | 77 | 79.2 | Y | NA | 2 d/wk × 3 months | LL + UL | Moderate | Y | EAA (42.0% leucine, 14.0% lysine, 10.5% valine, 10.5% isoleucine, 10.5% threonine, 7.0% phenylalanine, 5.5% other) | Daily | Twice daily | 6 g | Exercise only |
| Tieland et al, 201229 | 62 | 78.5 | Y | 1.00 | 2 d/wk × 24 wk | LL + UL | 50%–75% 1-RM |
N | Milk-based | Daily | 15 g after breakfast, 15 g after lunch | 30 g | CHO |
| Arnarson et al, 201330 | 161 | 73.9 | N | 1.00 | 3 d/wk × 12 wk | LL + UL | 75%–80% 1-RM | N | Whey | With training | After training | 20 g | CHO |
| Chalé et al, 201331 | 80 | 77.7 | Y | 0.97 | 3 d/wk × 6 months | LL + UL | 80% 1-RM | N | Whey | Daily | 20 g after breakfast, 20 g after dinner | 40 g | CHO |
| Leenders et al, 201332 | 60 | 70.0 | N | 1.15 | 3 d/wk × 24 wk | LL + UL | 60%–80% 1-RM | N | Milk-based (80% Casein, 20% Whey) | Daily | After breakfast | 15 g | CHO |
| Daly et al, 201433 | 100 | 72.8 | N | 1.08 | 2 d/wk × 4 months | LL + UL | Moderate | Y | Red meat | 6 d/wk | Meals, after training | 45 g‡ | CHO |
| Franzke et al, 201534 | 64 | 82.7 | N | NA | 2 d/wk × 6 months | LL + UL | Light to heavy | N | Whey | Daily | Morning and after training | 20.7 g | Exercise only |
| Mitchell et al, 201535 | 16 | 74.4 | N | NA | 3 d/wk × 12 wk | LL + UL | 75%–85% 1-RM | N | Chocolate milk | Daily | After breakfast or after training | 14 g | Placebo drink |
| Trabal et al, 201536 | 24 | 84.5 | N | 1.20 | 3 d/wk × 12 wk | LL | 65% 1-RM | Y | Leucine | Daily | 5 g after lunch, 5 g after dinner | 10 g | CHO |
1-RM, 1-repetition maximum; CHO, carbohydrate; d, days; LL, lower limb; N, no; RT, resistance training; UL, upper limb; wk, weeks; Y, yes.
NA indicates studies did not report baseline protein intake.
Approximately 220 g (raw weight) or 160 g (cooked weight) lean red meat equated to 45 g protein.
Resistance exercise regimens varied in frequency from 2 to 5 occasions per week, with a mean (SD) of 3 (1) per week. Programs lasted between 7 weeks and 1 year, with a mean (SD) of 18 (11) weeks. All studies reported a progressive exercise regimen; 9 comprised both upper and lower limb training,25, 30, 31, 32, 33, 34, 35, 36, 37 and 6 involved training of the lower limbs alone.23, 24, 26, 27, 28, 38 In addition to resistance exercise, participants of 5 studies26, 28, 30, 35, 38 undertook co-interventions including functional and/or balance exercises (Table 2).
A total of 10 studies included daily protein supplementation,21, 22, 23, 28, 29, 31, 32, 34, 35, 36 1 included supplementation on 6 days per week,33 and in the remaining 4, participants received supplements only on the day of training.24, 25, 26, 30 Baseline daily protein intake was reported in 8 studies22, 25, 29, 30, 31, 32, 33, 36 (Table 2), giving a mean (SD) of 1.08 (0.07) g·kg−1·d−1 (range 0.97–1.24 g·kg−1·d−1). The amount of protein supplemented varied from 6 g per day to 45 g per day with a mean (SD) of 19 (11) g (Table 2). Eight supplemented groups24, 25, 29, 30, 31, 32, 34, 35 received protein derived from milk (casein, whey, chocolate milk), 1 group received soy-based protein,21 1 group was supplemented with lean red meat,33 2 studies did not disclose the nature of the protein supplement,23, 26 2 groups received EAA,22, 28 and 1 group received only leucine.36 Timing of ingestion was inconsistent; in 3 studies, the supplement was administered immediately after training22, 24, 30; in 1 study administration was immediately before training26; in 1 study one-half of the supplement was administered before training and half after25; 8 studies administered supplements at a consistent time relative to meals21, 23, 28, 29, 31, 32, 33, 36; the 2 remaining studies used a combination of supplementation after meals and after training.34, 35 In addition to protein, 6 studies also supplemented participants with vitamin D,21, 23, 26, 33, 34, 35 with reported doses ranging from 2 to 25 μg and 2 doses given as approximate proportions of recommendations.
The studies were highly variable in terms of both study characteristics and outcome measures, and as a consequence, only 2 studies were sufficiently similar to be included in a meta-analysis. The results were, thus, inconclusive and are not reported here, although forest plots are available in Supplementary File 1.
Study Quality
The median overall quality score derived using the Physiotherapy Evidence Database scale was 7/10 (range 4–10), and the median score for internal validity was 5/8 (range 2–8) (Table 3). All studies scored 2/2 for statistical reporting.
Table 3.
PEDro Scale for Assessment of Study Quality
| Author, Year | Fiatarone et al, 199421 | Godard et al, 200222 | Bunout et al, 200423 | Rosendahl et al, 200624∗ | Verdijk et al, 200925 | Zak et al, 200926 | Kim et al, 201228 | Tieland et al, 201229 | Arnarson et al, 201330 | Chale et al, 201331 | Leenders et al, 201332 | Daly et al, 201433 | Franzke et al, 201534 | Mitchell et al, 201535 | Trabal et al, 201536 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Eligibility criteria were specified | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 2. Participants were randomly allocated to groups | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 3. Allocation was concealed | N | N | N | Y | N | N | Y | Y | Y | Y | Y | Y | N | N | N |
| 4. The groups were similar at baseline regarding the most important prognostic indicators | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y |
| 5. There was blinding of all participants | N | N | N | N | Y | N | N | Y | Y | Y | Y | N | N | Y | Y |
| 6. There was blinding of all therapists who administered the therapy | N | N | N | N | N | Y | N | Y | Y | Y | Y | N | Y | N | Y |
| 7. There was blinding of all assessors who measured at least 1 key outcome | Y | N | N | Y | Y | Y | Y | N | Y | Y | Y | N | Y | N | N |
| 8. Measures of at least 1 key outcome were obtained from more than 85% of the participants initially allocated to groups | Y | N | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | N |
| 9. All participants for whom outcomes were available received the treatment or control condition as allocated or, where this was not the case, data for at least one key outcome was analyzed by “intention to treat” | Y | N | N | Y | Y | N | N | Y | Y | Y | N | Y | N | N | N |
| Internal Validity | 5 | 2 | 2 | 6 | 6 | 5 | 5 | 7 | 8 | 8 | 6 | 5 | 4 | 4 | 4 |
| 10. The results of between-group statistical comparisons are reported for at least 1 key outcome | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 11. The study provides both point measures of variability for at least 1 key outcome | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Statistical Reporting | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Score/10 (Criterion 1 is not used to calculate PEDro score) | 7 | 4 | 4 | 8 | 8 | 7 | 7 | 9 | 10 | 10 | 8 | 7 | 6 | 6 | 6 |
Effect of Intervention on Muscle Strength
All 15 studies included in the systematic review included a measurement of muscle strength, although a number of different muscle groups were studied (Table 4). Eleven out of 15 studies demonstrated significant improvements from baseline in every measure of muscle strength in all groups undertaking RET with protein/EAA supplementation. Of the remaining studies, 1 demonstrated significant improvements in 6 of the 8 strength measurements included,26 2 reported significant increases in all measurements except handgrip strength,29, 32 and 1 measured only handgrip strength and reported no change.34 Three of the 15 studies reported significant differences between control and supplemented groups, with greater improvements in the supplemented groups in measures of knee extension strength28, 33 and hand grip strength,23 and 1 study reported a trend for greater improvement in leg flexion strength.36
Table 4.
Summary of Outcome Measures and Significant Results
| Author, Year | Outcome Measures | Significant Protein Effect | |
|---|---|---|---|
| Fiatarone et al, 199421 | Muscle strength | 1-RM leg strength (sum of L/R knee and hip extensors) | NS∗ |
| Muscle size | Thigh muscle CSA | ||
| Functional ability | Self-paced gait velocity | ||
| Godard et al 2002,22 | Muscle strength | Knee extensor isometric and isokinetic MVC; 1-RM bilateral knee extension | NS |
| Muscle size | Right thigh whole muscle CSA | ||
| Bunout et al, 200423 | Muscle strength | L/R bicep isometric strength; L/R knee extensor isometric strength; L/R handgrip strength | RH grip strength (P = .031) |
| Muscle size | Midarm, hip, and calf circumference | ||
| Functional ability | 12-minute walk capacity | ||
| Body composition | FFM; FM | ||
| Rosendahl et al, 200624† | Muscle strength | 1-RM leg press | NS |
| Functional ability | Balance test; self-paced and maximum gait velocity; chair-stand test | ||
| Verdijk et al, 200925 | Muscle strength | 1-RM leg press; 1-RM leg extension | NS |
| Muscle size | Quadriceps muscle CSA | ||
| Body composition | LBM; FM; % FM; leg LTM; leg % FM | ||
| Zak et al, 200926 | Muscle strength | L/R 1-RM knee extension; L/R 1-RM knee flexion; L/R 1-RM hip extension; L/R 1-RM hip knee flexion | NS |
| Functional ability | 6-minute walk capacity; POMA | ||
| Carlsson et al, 201127† | Body composition | MM (intra cellular water proxy) | NS |
| Kim et al, 201228 | Muscle strength | Knee extension | Knee extension strength (P = .01) |
| Functional ability | Self-paced and maximum gait velocity | ||
| Body composition | Total MM; appendicular MM; leg MM | ||
| Tieland et al, 201229 | Muscle strength | 1-RM leg press; 1-RM leg extension; handgrip strength | LBM (P = .006); appendicular LTM (P < .001); FM (P = .001) |
| Functional ability | SPPB | ||
| Body composition | LBM; FM; appendicular LTM | ||
| Arnarson et al, 201330 | Muscle strength | Knee extensor isometric MVC | NS |
| Functional ability | Timed up-and-go; 6-minute walk capacity | ||
| Body composition | LBM; appendicular LTM | ||
| Chalé et al, 201331 | Muscle strength | 1-RM leg press; L/R 1-RM knee extension | NS |
| Muscle size | Total muscle CSA of nondominant thigh | ||
| Functional ability | SPPB; stair climb speed; ×10 chair rise time; gait velocity | ||
| Body composition | LBM; FM | ||
| Leenders et al, 201332 | Muscle strength | 1-RM leg press; 1-RM leg extension; handgrip strength | NS |
| Muscle size | Quadriceps muscle CSA | ||
| Functional ability | 5x chair rise time | ||
| Body composition | LBM; FM; % FM; leg LTM; leg FM | ||
| Daly et al, 201433 | Muscle strength | 1-RM leg extension | Leg extension strength (P = .010); LBM (P = .007); leg LTM (P < .05) |
| Muscle size | Femur muscle CSA | ||
| Functional ability | 4-square step test; timed up-and-go; 30-second chair rise test | ||
| Body composition | Total body FM; body fat percentage; LBM; arm LTM, leg LTM | ||
| Franzke et al, 201534 | Muscle strength | Handgrip strength | NS |
| Functional ability | 6-minute walk capacity; 30-second chair rise test | ||
| Mitchell et al, 201535 | Muscle strength | 1-RM leg press; 1-RM leg extension; 1-RM chest press; Knee extension isometric MVC | NS |
| Trabal et al, 201536 | Muscle strength | Leg flexion overcoming isometric strength | Leg flexion (P = .056) |
| Muscle size | Mid upper arm muscle area; triceps skinfold; calf circumference | ||
| Functional ability | Balance test; TUG; ×5 chair rise time; 4m walk time | ||
1-RM, 1-repetition maximum; CSA, cross-sectional area; L/R, right and left; MVC, maximum voluntary contraction; RH, right hand; POMA, Performance Oriented Mobility Assessment; SPPB, Short Physical Performance Battery.
NS indicates no significant differences between the RET and protein supplement and RET only groups. For significant results, the outcome measure and P value, where available, are reported.
Effect of Intervention on Secondary Outcomes
Muscle size
Eight studies investigated the effect of supplementation on muscle size (Table 4). Six studies measured thigh muscle cross-sectional area using computed tomography, another measured midarm, calf, and hip circumference, and the other measured midupper arm muscle area, triceps skinfold, and calf circumference. All but 1 of the studies which used computed tomography to measure cross-sectional area reported significant increases in both supplemented and nonsupplement groups, however, there were no significant differences between the groups. No changes were reported in any other measure of muscle size.
Functional ability
At least 1 functional ability outcome was assessed in 12 out of the 15 included studies, with 27 outcomes assessed in total (Table 4). There was much heterogeneity among outcome measures, which included chair rise ability (6 studies), gait velocity (5 studies), walking capacity (4 studies), the Timed Up and Go (TUG) test (3 studies), balance tests (2 studies), stair climb speed (1 study), and the 4-square step test (1 study). Three studies included a “composite” of some of these physical performance indicators; 2 used the Short Physical Performance Battery (SPPB) which combines balance, gait speed, and chair rise ability,37 and 1 used Tinetti's Performance Oriented Mobility Assessment (POMA) test combining balance and gait.38 Of the 27 functional ability measurements, 21 were significantly improved after the intervention period, although none of these improvements were significantly different with protein/EAA supplementation compared with nonsupplemented controls.
Body composition
Body composition was assessed in 9 studies (Table 4). Body composition was assessed using dual energy X-ray absorptiometry in all studies except 2; one of which used a segmental multifrequency bioelectrical impedance analysis technique, and the other used bioelectrical impedance spectroscopy. Measurements included total lean body mass (LBM) in 6 studies; total body fat mass (FM) in 6 studies; percentage FM in 3 studies; estimated total muscle mass (MM) in 2 studies; and FFM in 1 study. A number of studies also included regional measurements of body composition: leg lean tissue mass (LTM) in 3 studies; appendicular LTM in 2 studies; estimated appendicular MM in 1 study; estimated leg MM in 1 study; leg FM and percentage FM in 1 study; and arm LTM in 1 study. Of the 28 measurements of body composition, 6 demonstrated no significant change during the studies. Improvement in body composition with no difference between treatment groups was shown in 17 measurements, although within-group analysis of 2 of these measurements within 1 study indicated a significant decrease in total FM and body fat percentage in the protein supplemented group but not the control group.33 Five measurements from 2 studies indicated significant differences between groups,29, 33 with greater increases in LBM, leg LTM, appendicular LTM, and FM in the supplemented groups compared with the exercise-only controls.
Discussion
This systematic review presents evidence from 15 studies investigating the additive effects of RET and protein supplementation on skeletal muscle strength and size, body composition, and functional ability in older adults. Studies reported overall improvement from baseline for the majority of outcomes, indicating a positive effect of RET. However, across the 15 studies, these improvements were not significantly different in groups receiving protein/EAA supplements and undergoing RET compared with RET alone.
A previous systematic review has shown that older muscle demonstrates an adaptive response to RET across a range of outcomes,39 hence, RET alone is considered an effective strategy for combatting sarcopenia. Given the anabolic properties of both resistance exercise and protein/EAA ingestion, it is plausible that the combination of these factors in a chronic intervention may have an additive effect and so enhance the responses shown with RET alone. Certainly, this has been shown to be the case in younger adults,11 however, despite individual significant results in strength and body composition outcomes, the overall results of the present review indicate that this does not hold true for older adults.
This overall absence of an additive effect, in contrast to that of younger adults, may be a result of the mechanisms of anabolic resistance in older muscle. For example, the expression and activation of proteins responsible for EAA sensing and signaling are reduced in older people,13 meaning the extent to which the subsequent cascades can be activated is limited, causing a blunted anabolic response compared with younger adults. Thus, if the limit for activation has been reached, any increase in the upstream signal (ie, more amino acids) will not result in any additional response. Where reported, all baseline protein intakes were within the RDA, and with lower sensitivity to higher protein intakes, this may have been sufficient to elicit a maximal protein synthetic response in combination with RET, prior to any supplementation. Certainly, there is evidence to suggest that there is no benefit for older adults in consuming more than the RDA for protein; in a 12-week trial of adults aged 50–80 years, daily protein intake was increased from 0.9 g·kg−1·d−1 to 1.3 g·kg−1·d−1 with no additional response to RET.40 Furthermore, when the effects of consuming the RDA were compared with a higher protein dose in older adults performing RET, the metabolic adaptations to increased protein intake actually reduced the utilization of protein.41 However, data from a recent study in older men do indicate increased phosphorylation of the anabolic signaling molecule p70S6K, indicating a dose-response relationship in an acute setting.42 As this relationship does not appear to translate to a chronic setting, this suggests that the mechanisms of this anabolic effect require further investigation.
An alternative view suggests that older adults actually require more protein than their younger counterparts to protect against sarcopenia. Contrary to the current RDA of 0.8–1.2 g·kg−1·day−1, a recent evidence-based recommendation suggests an intake of 1.0–1.2 g·kg−1·day−1 would be more advisable.43 If the findings of this review are considered from this perspective, most included studies were above the lower limit of this recommendation, and so the idea that baseline intakes were sufficient to maximally stimulate MPS would still apply. However, baseline intakes in 2 studies, while meeting the RDA, were at or below the alternative recommendation,29, 31 and the supplemented groups in these 2 studies saw the greatest increase in protein intake relative to baseline. One of these studies also fell into the small number, which found a significant difference in LBM between supplemented and control groups,29 and the other reported a significant difference in leg press peak power.31 Although not included as an outcome of the review, muscle power is highly relevant in this context, as it is dependent upon muscle mass and also declines with aging, impacting upon functional ability.44, 45 This suggests that, under circumstances of lower baseline protein intake, there may be potential for an additive effect of RET and protein supplementation. This is of particular relevance to older adults who are frail or in institutionalized care, as protein intakes for these groups have been found to be lower than community-dwelling older people, at 1.0 and 0.8 g·kg−1·day−1, respectively.46
In addition to the total daily protein intake, we may also consider the influence of protein supplementation with respect to the size of an individual dose of supplement. Acute studies have demonstrated that older adults require a bolus of at least 20 g of whey protein after resistance exercise to stimulate the MPS level above that of an exercised, unfed state,47 and that an even greater dose of 40 g is required when using a different protein source.48 The studies included in this review used a range of protein doses, some of which were at or above thresholds previously found to be effective in an acute setting to increase MPS. However, there were no consistent differences between the results of these studies compared with those using lower protein doses. Again, acute effects do not appear to translate to a chronic response. This also has implications for total protein intake recommendations for older adults conducting regular exercise, which are partially based on this acute evidence. It is recommended that older adults in this category require more protein than their inactive counterparts, and that they should consume at least 1.2 g·kg−1·day−1 including a 20 g supplement after exercise.43 However, the results from this review indicate that, from the perspective of improvements in muscle mass and function, there may not necessarily be any benefit from the increased protein intake.
The efficacy of protein supplementation in addition to RET has been addressed by previous systematic reviews, with contrasting results. Cermak et al11 found in favor of an additive effect in terms of FFM and muscle strength, concluding that protein/EAA supplementation augmented responsiveness to RET in both older and younger participants, a discrepancy most likely attributable to the different criteria used to define older populations. A mean age of at least 70 years was required for studies to be included in our review, giving an overall mean age of 77.4 years and a range of participant ages between 60 and 100 years, whereas the older cohort included in the previous review was aged between 48 and 72 years. The previous review was also restricted to only healthy participants, whereas we also included participants defined as frail or sarcopenic, and in fact only 1 study was common to both reviews.25 More recently, Finger et al15 considered the effects of RET and protein supplementation in older adults in terms of FFM, and muscle mass and strength. Again, the lower age limit was less than that of the current review at 60 years of age, and included studies with participants as young as 50 years of age. Of the 9 included studies, 5 were also included our review, with the remaining 4 excluded at either the abstract or full text screening stages due to the age criterion. Meta-analysis indicated a significant effect on FFM, which may again be a result of inclusion of younger participants. The meta-analysis is an area in which the methodology differed from the current review; previously, all studies with comparable outcomes were included in meta-analyses, however, in this review, we reviewed the similarity of study protocols based on a number of characteristics prior to meta-analysis, which indicated that very few of the studies were truly comparable. Further methodologic differences also allowed the current review to provide a more comprehensive view of the subject matter; a more extensive list of outcome measures includes measures of functional ability, which are highly relevant when considering the practical effects of an intervention, as well as a greater range of body composition outcomes. Furthermore, the eligibility criteria for the current review were less restrictive, as we did not exclude on the basis of other macronutrients in the supplement, allowing a greater number of studies to be included.
In general, the overall quality of the included studies was moderate to high, although several studies scored poorly for internal validity. In particular, approximately one-half of studies did not report blinding of all participants, and 4 failed to use any placebo in the control groups, meaning these studies may have been susceptible to performance bias.
Limitations
The greatest limitation of this review is the lack of meta-analysis data. Outcome measures showed a high degree of heterogeneity and data were not presented uniformly, and methodologic diversity was high, with variation in protein/EAA supplementation, training protocols, and duration of intervention. As a result, comparable outcome measures were limited, and differences in methodology meant that comparisons between most studies would not have been valid. Ideally, subgroup analysis would have been completed, for example for frail/sarcopenic and healthy groups, and for different distributions and timings of protein intake as the number of doses and proximity to exercise may have affected the response, but methodologic differences did not allow this. However, the vast majority of results indicate no additional effect of protein supplementation, and this did not appear to differ according to population or protocol characteristics, other than that of baseline protein intake discussed above.
The review may also be limited by the sample sizes of the included studies, some of which were relatively low and, therefore, may have lacked sufficient power to identify small differences between groups. Eight of the studies24, 26, 28, 29, 30, 31, 32, 33 reported a power calculation that deemed the sample size to be adequate, and there was no difference in terms of significant results between these and the studies that did not report a power calculation. However, the majority of these were powered for only selected outcome measures, usually body composition, meaning the sample sizes may not have been sufficient for other outcome measures; this is particularly important to consider with respect to more complex outcomes, such as measures of functional ability, which may require larger sample sizes to detect significant differences.
Conclusions
Protein/EAA supplementation does not significantly augment the effects of progressive RET in older adults in terms of muscle strength, muscle size, body composition, or functional ability. The review does, however, support the prescription of RET regimens to maintain and increase muscle mass and strength in older populations, which may help to combat sarcopenia and frailty.
The findings also suggest that there may be an additional benefit of protein supplementation and RET programs in frail older adults who do not regularly consume sufficient protein, particularly those in institutionalized care. Thus, future research may consider exploring this by conducting trials placing greater emphasis on the baseline protein intake of participants. Likewise, little discussion has been given here to the protein supplement characteristics, such as the amount, timing, and distribution of ingestion, and further research may investigate these areas to fully determine whether protein supplementation could be protective against sarcopenia.
Footnotes
Abbreviations used: BIA, bioelectrical impedance analysis; BIS; bioelectrical impedance spectroscopy; CSA, cross-sectional area; CT, computed tomography; DXA, dual-energy X-ray absorptiometry; EAA, essential amino acids; FFM, fat free mass; FM, fat mass; LBM, lean body mass; LTM, lean tissue mass; MM, muscle mass; PEDro, Physiotherapy Evidence Database; POMA, Performance Oriented Mobility Assessment; PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RDA, recommended daily allowance; RET, resistance exercise training; SPPB, Short Physical Performance Battery.
Danielle Thomas is funded by the MRC Arthritis Research UK Center for Musculoskeletal Aging Research.
The authors declare no conflicts of interest.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jamda.2016.07.002.
Supplementary Data
References
- 1.Rosenberg I.H. Sarcopenia: Origins and clinical relevance. J Nutr. 1997;127:990S–991S. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 2.Fielding R.A., Vellas B., Evans W.J. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janssen I., Heymsfield S.B., Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 4.Castillo E.M., Goodman-Gruen D., Kritz-Silverstein D. Sarcopenia in elderly men and women: The Rancho Bernardo study. Am J Prev Med. 2003;25:226–231. doi: 10.1016/s0749-3797(03)00197-1. [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner R.N., Koehler K.M., Gallagher D. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 6.Cosqueric G., Sebag A., Ducolombier C. Sarcopenia is predictive of nosocomial infection in care of the elderly. Br J Nutr. 2006;96:895–901. doi: 10.1017/bjn20061943. [DOI] [PubMed] [Google Scholar]
- 7.Landi F., Cruz-Jentoft A.J., Liperoti R. Sarcopenia and mortality risk in frail older persons aged 80 years and older: Results from ilSIRENTE study. Age Aging. 2013;42:203–209. doi: 10.1093/ageing/afs194. [DOI] [PubMed] [Google Scholar]
- 8.Janssen I., Shepard D.S., Katzmarzyk P.T., Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 9.Biolo G., Tipton K.D., Klein S., Wolfe R.R. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol. 1997;273:E122–E129. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- 10.Moore D.R., Atherton P.J., Rennie M.J. Resistance exercise enhances mTOR and MAPK signalling in human muscle over that seen at rest after bolus protein ingestion. Acta Physiol (Oxf) 2011;201:365–372. doi: 10.1111/j.1748-1716.2010.02187.x. [DOI] [PubMed] [Google Scholar]
- 11.Cermak N.M., Res P.T., de Groot L.C. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: A meta-analysis. Am J Clin Nutr. 2012;96:1454–1464. doi: 10.3945/ajcn.112.037556. [DOI] [PubMed] [Google Scholar]
- 12.Kumar V., Selby A., Rankin D. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 2009;587:211–217. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuthbertson D., Smith K., Babraj J. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 14.Greig C.A., Gray C., Rankin D. Blunting of adaptive responses to resistance exercise training in women over 75 years. Exp Gerontol. 2011;46:884–890. doi: 10.1016/j.exger.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Finger D., Goltz F.R., Umpierre D. Effects of protein supplementation in older adults undergoing resistance training: A systematic review and meta-analysis. Sports Med. 2015;45:245–255. doi: 10.1007/s40279-014-0269-4. [DOI] [PubMed] [Google Scholar]
- 16.Metter E.J., Conwit R., Tobin J., Fozard J.L. Age-associated loss of power and strength in the upper extremities in women and men. J Gerontol A Biol Sci Med Sci. 1997;52:B267–B276. doi: 10.1093/gerona/52a.5.b267. [DOI] [PubMed] [Google Scholar]
- 17.Moher D., Liberati A., Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W64. [DOI] [PubMed] [Google Scholar]
- 18.Food and Nutrition Board of the Institute of Medicine . National Academies Press; Washington DC: 2005. Protein and Amino Acids. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids (Macronutrients) p. 589. [DOI] [PubMed] [Google Scholar]
- 19.The George Institute for Global Health. PEDro Scale. PEDro Physiotherapy Evidence Database [March 2013]. Available at: http://www.pedro.org.au/english/downloads/pedro-scale/. Accessed March 1, 2013.
- 20.Bunout D., Barrera G., Leiva L. Effects of vitamin D supplementation and exercise training on physical performance in Chilean vitamin D deficient elderly subjects. Exp Gerontol. 2006;41:746–752. doi: 10.1016/j.exger.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Fiatarone M.A., O'Neill E.F., Ryan N.D. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 22.Godard M.P., Williamson D.L., Trappe S.W. Oral amino-acid provision does not affect muscle strength or size gains in older men. Med Sci Sports Exerc. 2002;34:1126–1131. doi: 10.1097/00005768-200207000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Bunout B., Barrera G., de la Maza P. Effects of nutritional supplementation and resistance training on muscle strength in free living elders. Results of one year follow. J Nutr Health Aging. 2004;8:68–75. [PubMed] [Google Scholar]
- 24.Rosendahl E., Lindelof N., Littbrand H. High-intensity functional exercise program and protein-enriched energy supplement for older persons dependent in activities of daily living: A randomised controlled trial. Aust J Physiother. 2006;52:105–113. doi: 10.1016/s0004-9514(06)70045-9. [DOI] [PubMed] [Google Scholar]
- 25.Verdijk L.B., Jonkers R.A., Gleeson B.G. Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am J Clin Nutr. 2009;89:608–616. doi: 10.3945/ajcn.2008.26626. [DOI] [PubMed] [Google Scholar]
- 26.Zak M., Swine C., Grodzicki T. Combined effects of functionally-oriented exercise regimens and nutritional supplementation on both the institutionalised and free-living frail elderly (double-blind, randomised clinical trial) BMC Public Health. 2009;9:39. doi: 10.1186/1471-2458-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlsson M., Littbrand H., Gustafson Y. Effects of high-intensity exercise and protein supplement on muscle mass in ADL dependent older people with and without malnutrition: A randomized controlled trial. J Nutr Health Aging. 2011;15:554–560. doi: 10.1007/s12603-011-0017-5. [DOI] [PubMed] [Google Scholar]
- 28.Kim H.K., Suzuki T., Saito K. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: A randomized controlled trial. J Am Geriatr Soc. 2012;60:16–23. doi: 10.1111/j.1532-5415.2011.03776.x. [DOI] [PubMed] [Google Scholar]
- 29.Tieland M., Dirks M.L., van der Zwaluw N. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: A randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2012;13:713–719. doi: 10.1016/j.jamda.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 30.Arnarson A., Gudny Geirsdottir O., Ramel A. Effects of whey proteins and carbohydrates on the efficacy of resistance training in elderly people: Double blind, randomised controlled trial. Eur J Clin Nutr. 2013;67:821–826. doi: 10.1038/ejcn.2013.40. [DOI] [PubMed] [Google Scholar]
- 31.Chalé A., Cloutier G.J., Hau C. Efficacy of whey protein supplementation on resistance exercise-induced changes in lean mass, muscle strength, and physical function in mobility-limited older adults. J Gerontol A Biol Sci Med Sci. 2013;68:682–690. doi: 10.1093/gerona/gls221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leenders M., Verdijk L.B., Van der Hoeven L. Protein supplementation during resistance-type exercise training in the elderly. Med Sci Sports Exerc. 2013;45:542–552. doi: 10.1249/MSS.0b013e318272fcdb. [DOI] [PubMed] [Google Scholar]
- 33.Daly R.M., O'Connell S.L., Mundell N.L. Protein-enriched diet, with the use of lean red meat, combined with progressive resistance training enhances lean tissue mass and muscle strength and reduces circulating IL-6 concentrations in elderly women: A cluster randomized controlled trial. Am J Clin Nutr. 2014;99:899–910. doi: 10.3945/ajcn.113.064154. [DOI] [PubMed] [Google Scholar]
- 34.Franzke B., Halper B., Hofmann M. The effect of six months of elastic band resistance training, nutritional supplementation or cognitive training on chromosomal damage in institutionalized elderly. Exp Gerontol. 2015;65:16–22. doi: 10.1016/j.exger.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell C.J., Oikawa S.Y., Ogborn D.I. Daily chocolate milk consumption does not enhance the effect of resistance training in young and old men: A randomized controlled trial. Appl Physiol Nutr Metab. 2015;40:199–202. doi: 10.1139/apnm-2014-0329. [DOI] [PubMed] [Google Scholar]
- 36.Trabal J., Forga M., Leyes P. Effects of free leucine supplementation and resistance training on muscle strength and functional status in older adults: A randomized controlled trial. Clin Interv Aging. 2015;10:713–723. doi: 10.2147/CIA.S75271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guralnik J.M., Simonsick E.M., Ferrucci L. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 38.Tinetti M.E. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc. 1986;34:119–126. doi: 10.1111/j.1532-5415.1986.tb05480.x. [DOI] [PubMed] [Google Scholar]
- 39.Peterson M.D., Rhea M.R., Sen A., Gordon P.M. Resistance exercise for muscular strength in older adults: A meta-analysis. Ageing Res Rev. 2010;9:226–237. doi: 10.1016/j.arr.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iglay H.B., Apolzan J.W., Gerrard D.E. Moderately increased protein intake predominately from egg sources does not influence whole body, regional, or muscle composition responses to resistance training in older people. J Nutr Health Aging. 2009;13:108–114. doi: 10.1007/s12603-009-0016-y. [DOI] [PubMed] [Google Scholar]
- 41.Campbell W.W., Crim M.C., Young V.R. Effects of resistance training and dietary protein intake on protein metabolism in older adults. Am J Physiol. 1995;268:E1143–E1153. doi: 10.1152/ajpendo.1995.268.6.E1143. [DOI] [PubMed] [Google Scholar]
- 42.D'Souza R.F., Marworth J.F., Figueiredo V.C. Dose-dependent increases in p70S6K phosphorylation and intramuscular branched-chain amino acids in older men following resistance exercise and protein intake. Physiol Rep. 2014;2:1–2. doi: 10.14814/phy2.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bauer J., Biolo G., Cederholm T. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013;14:542–559. doi: 10.1016/j.jamda.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 44.Narici M.V., Maffulli N. Sarcopenia: Characteristics, mechanisms and functional significance. Br Med Bull. 2010;95:139–159. doi: 10.1093/bmb/ldq008. [DOI] [PubMed] [Google Scholar]
- 45.Young A. Ageing and physiological functions. Philos Trans R Soc Lond B Biol Sci. 1997;352:1837–1843. doi: 10.1098/rstb.1997.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tieland M., Borgonjen-Van den Berg K.J., van Loon L.J., de Groot L.C. Dietary protein intake in community-dwelling, frail, and institutionalized elderly people: Scope for improvement. Eur J Nutr. 2012;51:173–179. doi: 10.1007/s00394-011-0203-6. [DOI] [PubMed] [Google Scholar]
- 47.Yang Y., Breen L., Burd N.A. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br J Nutr. 2012;108:1780–1788. doi: 10.1017/S0007114511007422. [DOI] [PubMed] [Google Scholar]
- 48.Yang Y., Churchward-Venne T.A., Burd N.A. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr Metab. 2012;9:57. doi: 10.1186/1743-7075-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.