Abstract
The sum of urinary inorganic arsenic (iAs) and methylated arsenic (monomethylarsonate and dimethylarsinate (DMA)) species is the main biomarker of iAs exposure. Assessing iAs exposure, however, is difficult in populations with moderate-to-high seafood intakes. In the present study, we used subsamples from the Multi-Ethnic Study of Atherosclerosis (2000–2002) (n = 310) and the 2003–2006 National Health and Nutrition Examination Survey (n = 1,175). We calibrated urinary concentrations of non–seafood-derived iAs, DMA, and methylarsonate, as well as the sum of inorganic and methylated arsenic species, in the Multi-Ethnic Study of Atherosclerosis and of DMA in the National Health and Nutrition Examination Survey by regressing their original concentrations by arsenobetaine and extracting model residuals. To confirm that calibrated biomarkers reflected iAs exposure but not seafood intake, we compared urinary arsenic concentrations by levels of seafood and rice intakes. Self-reported seafood intakes, estimated n-3 polyunsaturated fatty acid levels, and measured n-3 polyunsaturated fatty acid levels were positively associated with the original urinary arsenic biomarkers. Using the calibrated arsenic biomarkers, we found a marked attenuation of the associations with self-reported seafood intake and estimated or measured n-3 fatty acids, whereas associations with self-reported rice intake remained similar. Our residual-based method provides estimates of iAs exposure and metabolism for each participant that no longer reflect seafood intake and can facilitate research about low-to-moderate levels of iAs exposure in populations with high seafood intakes.
Keywords: arsenic, arsenobetaine, dimethylarsinate, food frequency questionnaire, methylarsonate, omega-3 fatty acids, rice, seafood
Chronic exposure to inorganic arsenic (iAs) levels above 10 µg/L (the standard in many countries, including the United States) in drinking water has been related to higher risks of cardiovascular disease, diabetes, respiratory disease, adverse neurodevelopmental outcomes, and some cancers (1–11). Most populations around the world are exposed to iAs levels in water that are lower than 10 µg/L (12, 13). In those populations, foods such as rice and grains are the major source of low chronic exposure to iAs (14–18); air pollution can also contribute (19–21). Although investigation of the health implications of low chronic arsenic exposure is needed, epidemiologic research of these low levels is limited by challenges in assessment of arsenic exposure.
After a person is exposed to iAs (arsenite, arsenate), it is methylated to mono and dimethylated arsenic compounds (monomethylarsonate (MMA) and dimethylarsinate (DMA)), which are excreted in the urine together with unchanged iAs (Figure 1) (22). In populations with low seafood intakes, the sum of inorganic and methylated arsenic species levels in urine correlates well with arsenic intake from drinking water and dietary sources and is an accepted biomarker of iAs exposure (23–25). In addition to iAs, rice may also contain DMA, which is also excreted through the urine. Seafood, including fish, shellfish, and seaweed, are important sources of organic arsenicals (arsenobetaine, arsenosugars, and arsenolipids), which are believed to have low toxicity (26–29). Arsenobetaine is rapidly cleared from the blood stream and excreted unchanged via the kidneys, thereby contributing to total arsenic levels in urine (30–32). Seaweed, mollusks (e.g., scallops, mussels), and fatty fishes are rich in arsenosugars and/or arsenolipids that are metabolized into several arsenic species, including DMA, dimethylated thio arsenic species, and possibly MMA (30–33). Therefore, in populations with moderate-to-high fish intakes, the sum of inorganic and methylated arsenic species levels in urine cannot be used as a biomarker of iAs intake.
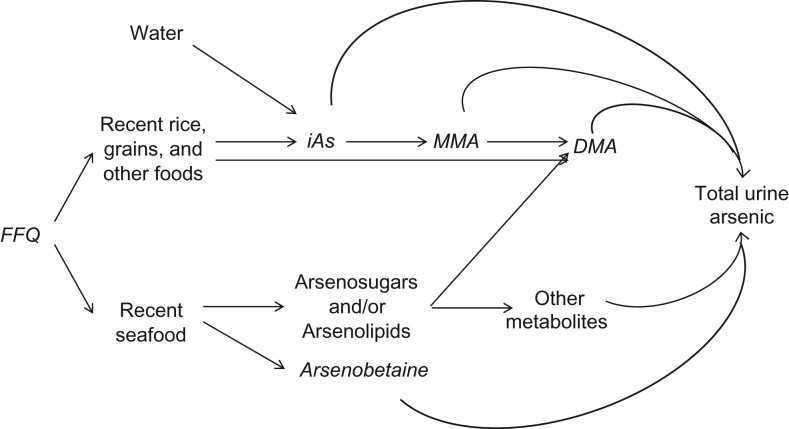
Figure 1.
Contribution of major forms of arsenic in foods to total urine arsenic after food intake. In the Multi-Ethnic Study of Atherosclerosis (MESA), data were available for inorganic arsenic (iAs), monomethylarsonate (MMA), dimethylarsinate (DMA), and arsenobetaine measured in urine (shown in italics). In the National Health and Nutrition Examination Survey (NHANES), only urine DMA and total arsenic measures were available. The food frequency questionnaire (FFQ), administered in both in MESA and NHANES, provided information on dietary patterns in the past year. NHANES also has information on dietary intake in the past 24 hours. Other sources of inorganic arsenic, for example, inhalation in occupational settings and ambient air pollution, are not represented here. To block the contribution of seafood arsenicals (arsenosugars and arsenolipids) to DMA and total urine arsenic, we used arsenobetaine, a specific biomarker of seafood intake that is directly excreted through the urine.
In the present study, our objective was to estimate iAs exposure in populations in which seafood consumption is common. In our proposed approach, we calibrated urinary concentrations of iAs, MMA, and DMA by regressing their original concentrations by arsenobetaine concentrations and extracting the arsenobetaine-independent model residuals. We hypothesized that calibrated biomarkers would remain associated with intake of rice, a source of iAs and DMA, but would no longer be associated with seafood intake because arsenobetaine, which is not metabolized, can remove at least part of the contribution of seafood arsenicals to total arsenic estimates in urine.
METHODS
Study populations
To develop an arsenic biomarker that is minimally affected by seafood intake, we used data from the Multi-Ethnic Study of Atherosclerosis (MESA), a population-based study that enrolled 6,814 white, black, Hispanic, and Chinese participants who were 45–84 years of age and free of cardiovascular disease from 6 communities in the United States (Winston-Salem, North Carolina; New York, New York; Baltimore, Maryland; St. Paul, Minnesota; Chicago, Illinois; and Los Angeles, California) between 2000 and 2002 (33). White participants were recruited from all 6 study communities; black participants were recruited from all communities except St. Paul; Hispanic participants were only recruited in Los Angeles, New York, and St. Paul; and Chinese participants were only recruited in Chicago and Los Angeles. After excluding 999 participants who were not eligible because of limited biospecimen sample availability and 1,588 participants with missing information on food frequency questionnaire (FFQ) responses, smoking status, alcohol intake or urine creatinine, we used a random-stratified strategy to select 310 participants for urine arsenic analysis. The goal was to obtain a study population with a predefined number of participants of each race/ethnicity and from each study community: 90 white participants (15 in each community), 75 black participants (15 in each community), 75 Hispanic participants (25 in each community), and 70 Chinese participants (35 in each community).
In addition, we used the 2003–2006 National Health and Nutrition Examination Survey (NHANES), a study conducted by the US National Center for Health Statistics in which investigators used a multistage sampling design to obtain a representative sample of the US population. We restricted NHANES data to those available from 2003–2006 because although urinary arsenic concentrations were available in NHANES data from 2003–2012, the FFQ information was only available for NHANES data from 2003–2006. To match the age distribution of MESA participants, we restricted our analysis to adults 45–84 years of age in NHANES. Of the 5,108 adults who were 45–84 years of age in NHANES, urine arsenic measures were available for 1,602. We excluded 375 participants with missing FFQ information on seafood intake, 36 with missing information on rice intake, and 16 with missing information on other covariates of interest, leaving 1,175 participants for this study.
Urine arsenic
MESA participants provided spot urine samples as part of the baseline examination in 2000–2002. Urine samples were stored at −70°C or colder. In 2013, 1 mL of urine per participant was shipped on dry ice to the Trace Element Laboratory at the University of Graz (Graz, Austria). Urine arsenic species (iAs, MMA, DMA, and arsenobetaine plus other cations (hereafter referred to as arsenobetaine because arsenobetaine is the most common cation)) were measured using anion-exchange high-performance liquid chromatography (Agilent 1100, Agilent Technologies, Waldbronn, Germany) coupled with inductively coupled plasma mass spectrometry (Agilent 7700x ICPMS, Agilent Technologies) following an established protocol (34). Arsenite was oxidized to arsenate with hydrogen peroxide to assess overall iAs. The limits of detection were 0.1 µg/L for arsenobetaine, DMA, MMA, and arsenate (Web Table 1, available athttp://aje.oxfordjournals.org/). The percentages of participants with urinary concentrations below the limit of detection was 3.9%, 0%, 14.2%, and 45.8% for arsenobetaine, DMA, MMA, and arsenate, respectively. The interassay coefficients of variation for iAs, MMA, DMA, and arsenobetaine were 6.0%, 6.5%, 5.9%, and 6.5%, respectively (34).
In NHANES, spot urine samples for arsenic analysis were collected, shipped on dry ice, and stored at −70°C or colder (35). Arsenic species were measured at the Environmental Health Sciences Laboratory of the National Center for Environmental Health following a standardized protocol (36). Arsenite, arsenate, DMA, MMA, and arsenobetaine were measured using high-performance liquid chromatography coupled to inductively coupled plasma dynamic reaction cell-mass spectrometry on a Perkin-Elmer ELAN 6100 DRCPLUS or ELAN DRC II ICPMS (Perkin Elmer SCIEX, Concord, Ontario, Canada). In NHANES, the limits of detection were 0.4 µg/L for arsenobetaine and 1.7 µg/L for DMA, with 27.1% of participants having urinary arsenobetaine levels below the limit of detection and 15.4% having urinary DMA levels below the limit of detection (Web Table 1). The limits of detection for arsenate and MMA were too high for use in the NHANES population, and most participants were below the limit of detection for these species (94.3% for arsenate and 68.1% for MMA); therefore, these species could not be considered (Web Table 1). NHANES investigators also measured arsenocholine (an arsenic cation that is commonly measured but often undetectable), which was undetectable in 97.1% of the study participants. For MESA and NHANES participants with urine arsenic species below the limit of detection, we assigned a level equal to the limit of detection divided by the square root of 2.
Other variables
Demographic characteristics (age, sex, and race/ethnicity) and dietary intake were assessed using questionnaires at the MESA and NHANES examinations. Information on intakes of rice and seafood were assessed using FFQ in both studies. In MESA, information on usual food intakes, including intakes of seafood and rice during the past year, was assessed using a 120-item FFQ (37–39) that included Chinese and Hispanic foods to accommodate the MESA study population. Frequency of intake for MESA participants was classified in 9 categories ranging from “rare or never” to “≥2 times/day.” To ascertain information on frequency of food intake during the past year in NHANES, a 124-item FFQ was mailed to participants’ homes (40, 41). In NHANES, frequency of intake was classified in 11 categories ranging from “never” to “≥2 times/day.” For the present study, we categorized rice and seafood consumption as never/rare (≤1 time per month), 2–4 times per month, or ≥2 times per week. In NHANES, seafood intake in the 24 hours preceding the interview was obtained from a dietary recall interview and categorized based on US Department of Agriculture food codes.
In addition to self-reported seafood intake, we also used the level of n-3 polyunsaturated fatty acids (PUFAs; levels were determined as the sum of eicosapentaenoic acid and docosahexaenoic acid). In MESA, intake of nutrients including n-3 PUFAs was estimated for each FFQ item using the Minnesota Nutrition Data System NDS software (version 4.02/30) (42). A subset of MESA participants (n = 120) also had n-3 PUFAs measured in fasting plasma samples. Phospholipid fatty acids were extracted from plasma using a chloroform/methanol extraction method and then subsequently separated from cholesterol esters, triglycerides, and free fatty acids using thin layer chromatography (43). Fatty acids from the phospholipids were converted to methyl esters and detected by gas chromatography with flame ionization detection. The detected fatty acids were expressed as a percentage of total fatty acids. In NHANES, n-3 PUFAs were estimated for each food listed in the 24-hour dietary recall conducted during the day of the examination using the US Department of Agriculture Food and Nutrient Database for Dietary Studies, 5.0 (44). Measured or estimated n-3 PUFAs were categorized in tertiles based on the distributions in the study populations. In NHANES, tertile cutoffs were based on the weighted distributions.
In both studies, body mass index was calculated by dividing measured weight in kilograms by measured height in meters squared. Urine creatinine, used to adjust for urine dilution in spot urine samples in statistical models, was determined using a Jaffe rate reaction measured with the Vitros 950IRC instrument (Johnson & Johnson Clinical Diagnostics, Inc., Rochester, New York) in MESA and a CX3 analyzer (Beckman Instruments, Brea, California) in NHANES.
Statistical analysis
Urine arsenic species were right skewed and log-transformed for the analyses. Urinary arsenobetaine concentrations were used as a marker of exposure to seafood arsenicals. Urinary concentrations of iAs, MMA and DMA from exposure to iAs were obtained by regressing the measured concentrations of iAs, MMA, and DMA on arsenobetaine concentrations using a log-log regression model as follows:
for i in 1,…,N, where arsenical corresponds to iAs, MMA, or DMA, β0 and β1 correspond to the intercept and slope coefficients, and εi represents the residuals in the ith individual of N total individuals. The residuals reflect inorganic and methylated arsenic species from exposure to iAs not explained by recent seafood intake. The method of utilizing residuals to obtain adjusted estimates has been extensively used in the literature (45–51). To have levels of exposure that are meaningful for the population, are easy to communicate, and represent arsenic concentrations after removing the influence of seafood, we added the mean levels of the corresponding arsenic species (iAs, MMA, or DMA estimated from participants with low arsenobetaine (<1 µg/L)) (52) to the residuals, assuming that iAs exposure levels not derived from seafood are similar in participants with low and high arsenobetaine concentrations.
To evaluate whether the calibrated biomarkers no longer reflected organic arsenicals from seafood, we estimated the ratios of the geometric means (GMs) of the calibrated urinary concentrations of iAs, MMA, and DMA individually and added them together to obtain the sum of the calibrated biomarkers across categories of self-reported seafood intake and tertiles of n-3 PUFAs. We then compared them to the corresponding ratios obtained using the measured urinary arsenic concentrations. In NHANES, we only conducted models for DMA because iAs and MMA were undetectable in most participants. We hypothesized that self-reported seafood intake and n-3 PUFA concentrations would no longer be associated with the calibrated urinary concentrations of iAs, MMA, and DMA or the sum of inorganic and methylated arsenic species. We also compared the ratios of the geometric means using the measured and calibrated urinary arsenic concentrations across categories of intake of rice, which is a source of iAs and DMA (Figure 1). We hypothesized that self-reported rice intake would remain associated with the calibrated urinary biomarkers. All models were adjusted for sex, age (continuous), body mass index (continuous), and urine creatinine (log-transformed). Analyses were stratified by study (MESA and NHANES). We conducted a sensitivity analysis in which we excluded participants with estimated n-3 PUFA concentrations in the 95th percentile or higher in order to compare the performance of the model when we excluded participants with very high seafood intakes. We also repeated the analyses with further adjustment for smoking status, as well as stratification by the median arsenobetaine levels (above and below), with similar results (data not shown). Statistical analyses were performed using R statistical software, version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria). In NHANES, we used the survey package (version 3.29.5) in R to account for the complex sampling design.
RESULTS
Participant characteristics and urine arsenic concentrations
Compared with participants in NHANES, MESA participants were slightly older and more likely to be men (Table 1). The median concentration of urinary arsenobetaine was 5.2 μg/L (interquartile range (IQR), 1.1–18.7) in MESA versus 1.6 μg/L (IQR, 0.3–6.9) in NHANES, which was consistent with the higher seafood intake in the MESA study population (Table 2), although the maximum arsenobetaine concentrations were higher in NHANES participants (755 µg/L) than in MESA participants (402 µg/L). Arsenobetaine concentrations were strongly and positively associated with seafood intake in both MESA and NHANES (Web Table 2). Before calibration, the median urine concentrations in MESA were 0.2 μg/L (IQR, 0.1–0.5) for iAs, 0.7 μg/L (IQR, 0.3–1.3) for MMA, 6.7 μg/L (IQR, 3.4–13.6) for DMA, and 8.1 μg/L (IQR, 3.8–15.5) for the sum of inorganic and methylated arsenic species (Table 2). After calibration, the median urinary arsenic concentrations corrected for arsenobetaine in MESA were 0.1 μg/L (IQR, 0.05–0.3) for iAs, 0.3 μg/L (IQR, 0.2–0.6) for MMA, 2.5 μg/L (IQR, 1.4–4.3) for DMA, and 3.1 μg/L (IQR, 1.7–5.5) for the sum of inorganic and methylated arsenic species. In NHANES, the median for DMA was 3.5 μg/L (IQR, 2.0–5.9) before calibration and 2.5 μg/L (IQR, 1.6–4.1) after calibration (Table 2). Scatterplots and log-log regression models showing the relationship of urinary arsenobetaine concentrations with the original and calibrated urinary arsenic biomarkers are shown in Web Figure 1.
Table 1.
Characteristics of Participants Stratified by Study, Multi-Ethnic Study of Atherosclerosis (2000–2002) and National Health and Nutrition Examination Survey (2003–2006)
| Characteristic | MESA (n = 310) | NHANESa (n = 1,175) | ||||
|---|---|---|---|---|---|---|
| No. | % | Mean (SE) | No. | % | Mean (SE) | |
| Sex | ||||||
| Male | 176 | 56.8 | 592 | 45.7 | ||
| Female | 134 | 43.2 | 583 | 54.3 | ||
| Age, years | 61.4 (0.5) | 59.4 (0.4) | ||||
| Race/ethnicity | ||||||
| White | 90 | 29.0 | 718 | 81.9 | ||
| Black | 75 | 24.2 | 213 | 8.5 | ||
| Hispanic | 75 | 24.2 | 186 | 3.7 | ||
| Chinese | 70 | 22.6 | N/A | N/A | ||
| Other | N/A | N/A | 58 | 6.0 | ||
| Body mass indexb | 27.4 (0.3) | 29.1 (0.3) | ||||
| Smoking status | ||||||
| Never | 140 | 45.2 | 540 | 46.4 | ||
| Former | 128 | 41.3 | 427 | 35.1 | ||
| Current | 42 | 13.5 | 208 | 18.5 | ||
| Self-reported seafood intake | ||||||
| Rare/never | 60 | 19.4 | 719 | 61.1 | ||
| 2–4 times per month | 159 | 51.3 | 364 | 31.2 | ||
| ≥2 times per week | 91 | 29.4 | 92 | 7.6 | ||
| Seafood consumed in the past 24 hours | ||||||
| No | N/A | N/A | 978 | 82.9 | ||
| Yes | N/A | N/A | 197 | 17.1 | ||
| Estimated n-3 PUFAs, g | 0.14 (0.01) | 0.07 (0.01) | ||||
| Measured n-3 PUFAs, %c | 5.5 (0.2) | N/A | ||||
| Rice intake | ||||||
| Rare/never | 43 | 13.9 | 500 | 42.0 | ||
| 1–4 times/month | 105 | 33.9 | 394 | 36.2 | ||
| ≥2 times/week | 162 | 52.3 | 281 | 21.8 | ||
| Urine creatinine, mg/dL | 122 (4.1) | 107 (2.6) | ||||
Abbreviations: MESA, Multi-Ethnic Study of Atherosclerosis; N/A, not applicable; NHANES, National Health and Nutrition Examination Survey; PUFA, polyunsaturated fatty acid; SE, standard error.
a Percentages and standard errors for NHANES represent weighted values.
b Weight (kg)/height (m)2.
c Measured in plasma in 120 MESA participants and expressed as a percent of total fatty acids.
Table 2.
Median Urine Arsenic Measures Before and After Calibration, Stratified by Study, Multi-Ethnic Study of Atherosclerosis (2000–2002) and National Health and Nutrition Examination Survey (2003–2006)
| Study and Arsenic Measure | Measured Concentrationa (µg/L), Median (IQR) | Calibrated Concentrationb (µg/L), Median (IQR) |
|---|---|---|
| MESA | ||
| Arsenobetaine | 5.2 (1.1–18.7) | |
| iAs | 0.2 (0.1–0.5) | 0.1 (0.05–0.3) |
| MMA | 0.7 (0.3–1.3) | 0.3 (0.2–0.6) |
| DMA | 6.7 (3.4–13.6) | 2.5 (1.4–4.3) |
| ∑As | 8.1 (3.8–15.5) | 3.1 (1.7–5.5) |
| Arsenic species %c | ||
| iAs % | 3.1 (1.6–5.0) | 4.2 (2.2–6.7) |
| MMA % | 8.4 (5.2–12.4) | 10.3 (6.5–14.8) |
| DMA % | 87.5 (83.2–91.9) | 85.4 (79.6–89.6) |
| NHANES | ||
| Arsenobetaine | 1.6 (0.3–6.9) | |
| DMA | 3.5 (2.0–5.9) | 2.5 (1.6–4.1) |
Abbreviations: DMA, dimethylarsinate; iAs, inorganic arsenic; IQR, interquartile range; MESA, Multi-Ethnic Study of Atherosclerosis; MMA, methylarsonate; NHANES, National Health and Nutrition Examination Survey; ∑As, sum of inorganic and methylated arsenic species.
a Measured concentrations represent originally measured urine arsenic concentrations.
b Calibrated concentrations represent urine arsenic concentrations corrected for arsenobetaine concentrations.
c iAs %, MMA %, and DMA % are estimated as their concentrations divided by the sum of the inorganic and methylated arsenic species.
Comparison of measured and calibrated biomarkers by self-reported seafood intake
In MESA, 19.4% of participants reported rarely or never eating seafood compared with 51.3% who ate seafood 2–4 times per month and 29.4% who ate seafood 2 or more times per week (Table 1). Participants who frequently consumed seafood had higher concentrations of all urine arsenic measures using the measured biomarker (Table 3). The GM ratios for eating seafood 2 times or more per week compared with rarely or never eating it were 1.48 (95% confidence interval (CI): 1.10, 1.98) for iAs, 1.83 (95% CI: 1.39, 2.41) for MMA, 1.93 (95% CI: 1.50, 2.48) for DMA, and 1.91 (95% CI: 1.50, 2.43) for the sum of inorganic and methylated arsenic species. Using the calibrated estimates, the corresponding GM ratios were 1.06 (95% CI: 0.79, 1.43) for iAs, 1.27 (95% CI: 0.96, 1.69) for MMA, 1.04 (95% CI: 0.83, 1.30) for DMA, and 1.08 (95% CI: 0.87, 1.33) for the sum of inorganic and methylated arsenic species. The results with the calibrated biomarker were similar after excluding participants with n-3 PUFA concentrations in the 95th percentile or higher (data not shown).
Table 3.
Ratio of Geometric Mean Urine Arsenic Concentrations by Seafood Intake Before and After Calibration, Stratified by Studya, Multi-Ethnic Study of Atherosclerosis (2000–2002) and National Health and Nutrition Examination Survey (2003–2006)
| Arsenic Measure and Frequency of Seafood Consumption | Measured Biomarker | Calibrated Biomarker | ||
|---|---|---|---|---|
| GM Ratio | 95% CI | GM Ratio | 95% CI | |
| MESA (n = 310)b | ||||
| iAs | ||||
| Rare/never | 1.00 | Referent | 1.00 | Referent |
| 2–4 times per month | 1.11 | 0.85, 1.45 | 0.95 | 0.73, 1.25 |
| ≥2 times per week | 1.48 | 1.10, 1.98 | 1.06 | 0.79, 1.43 |
| MMA | ||||
| Rare/never | 1.00 | Referent | 1.00 | Referent |
| 2–4 times per month | 1.32 | 1.03, 1.70 | 1.11 | 0.86, 1.44 |
| ≥2 times per week | 1.83 | 1.39, 2.41 | 1.27 | 0.96, 1.69 |
| DMA | ||||
| Rare/never | 1.00 | Referent | 1.00 | Referent |
| 2–4 times per month | 1.27 | 1.01, 1.59 | 0.95 | 0.78, 1.16 |
| ≥2 times per week | 1.93 | 1.50, 2.48 | 1.04 | 0.83, 1.30 |
| ∑As | ||||
| Rare/never | 1.00 | Referent | 1.00 | Referent |
| 2–4 times per month | 1.26 | 1.02, 1.57 | 0.97 | 0.80, 1.18 |
| ≥2 times per week | 1.91 | 1.50, 2.43 | 1.08 | 0.87, 1.33 |
| NHANES (n = 1,175)c | ||||
| DMA | ||||
| Rare/never | 1.00 | Referent | 1.00 | Referent |
| 2–4 times per month | 1.20 | 1.07, 1.34 | 1.00 | 0.90, 1.12 |
| ≥2 times per week | 1.68 | 1.40, 2.01 | 1.23 | 1.05, 1.43 |
| Seafood in the past 24 hours | ||||
| No | 1.00 | Referent | 1.00 | Referent |
| Yes | 1.99 | 1.77, 2.25 | 1.25 | 1.12, 1.39 |
| NHANES With Exclusions (n = 1,119)d, e | ||||
| DMA | ||||
| Rare/never | 1.00 | Referent | 1.00 | Referent |
| 2–4 times per month | 1.15 | 1.02, 1.29 | 0.98 | 0.88, 1.09 |
| ≥2 times per week | 1.44 | 1.17, 1.78 | 1.10 | 0.94, 1.29 |
| Seafood in the past 24 hours | ||||
| No | 1.00 | Referent | 1.00 | Referent |
| Yes | 1.74 | 1.54, 1.97 | 1.10 | 0.98, 1.24 |
Abbreviations: CI, confidence interval; DMA, dimethylarsinate; GM, geometric mean; iAs, inorganic arsenic; MESA, Multi-Ethnic Study of Atherosclerosis; MMA, methylarsonate; NHANES, National Health and Nutrition Examination Survey; ∑As, sum of iAs and methylated species.
a All models were adjusted for age, sex, body mass index, and urine creatinine.
b Numbers of participants in each category are as follows: for rare/never, n = 60; for 2–4 times per month, n = 159; and for ≥2 times per week, n = 91.
c Numbers of participants in each category are as follows: for rare/never, n = 719; for 2–4 times per month, n = 364; for ≥2 times per week, n = 92; for no seafood in the past 24 hours, n = 978; and for seafood in the past 24 hours, n = 197.
d Numbers of participants in each category are as follows: for rare/never, n = 695; for 2–4 times per month, n = 343; for ≥2 times per week, n = 81; for no seafood in the past 24 hours, n = 978; and for seafood in the past 24 hours, n = 141.
e Participants with estimated n-3 polyunsaturated fatty acid levels in the 95th percentile or were excluded. The 95th percentile in NHANES was 0.37 g.
In NHANES, 61.1% of participants reported rarely or never eating seafood compared with 31.2% who ate seafood 2–4 times per month and 7.6% who ate seafood 2 or more times per week (Table 1). Seventeen percent of participants reported eating seafood in the 24 hours preceding the NHANES interview (Table 1). The GM ratios for eating seafood 2 times or more per week compared with rarely or never eating it were 1.68 (95% CI: 1.40, 2.01) for the traditional DMA biomarker, 1.23 (95% CI: 1.05, 1.43) for calibrated DMA, and 1.10 (95% CI: 0.94, 1.29) for calibrated DMA after excluding participants with n-3 PUFA concentrations in the 95th percentile or higher (Table 3). Associations of DMA with seafood intake in the past 24 hours were also markedly attenuated when using the calibrated levels compared with the measured levels (using the measured biomarker, GM ratio: 1.99, 95% CI: 1.77, 2.25; using the calibrated biomarker, GM ratio: 1.25, 95% CI: 1.12, 1.39; and using the calibrated biomarker excluding participants with n-3 PUFA concentrations in the 95th percentile or higher, GM ratio: 1.10, 95% CI: 0.98, 1.24) (Table 3).
Comparison of measured and calibrated biomarkers by concentrations of n-3 PUFAs
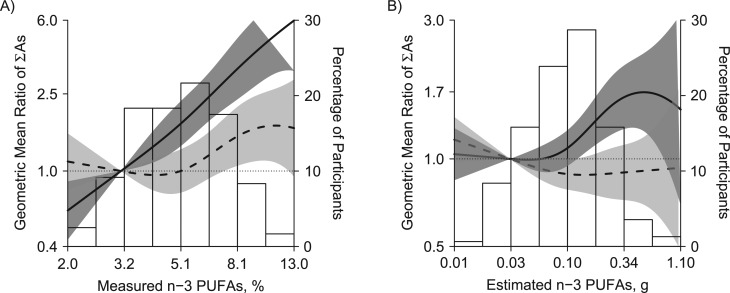
The mean estimated n-3 PUFA concentration per day in MESA was 0.14 g compared with 0.07 g in NHANES (Table 1), although the maximum concentration of estimated n-3 PUFAs was higher in NHANES (2.69 g) than in MESA (1.23 g). In MESA, urinary concentrations of DMA and the sum of inorganic and methylated arsenic species were significantly higher in participants in the third tertile of estimated n-3 PUFAs compared with participants in the first tertile (for DMA, GM ratio: 1.57, 95% CI: 1.26, 1.94; and for the sum of inorganic and methylated arsenic species, GM ratio: 1.54, 95% CI: 1.25, 1.89) (Table 4). Among the 120 MESA participants with measured n-3 PUFA concentrations, urinary arsenic concentrations were also significantly higher in participants with higher levels of n-3 PUFAs using the measured biomarker, and the association was stronger than with estimated n-3 PUFAs (Table 4 and Figure 2). Using the calibrated biomarker, urine arsenic measures were no longer associated with estimated or measured n-3 PUFA concentration (Table 4). In flexible dose-response models (Figure 2), the association between n-3 PUFAs and the sum of inorganic and methylated arsenic species was also markedly attenuated when comparing the calibrated biomarker to the measured biomarker, although for measured n-3 PUFAs, the association increased again and became statistically significant with n-3 PUFAs levels above 8%.
Table 4.
Ratio of Geometric Mean Urine Arsenic Concentrations by Tertile of n-3 Polyunsaturated Fatty Acids Before and After Calibration, Stratified by Studya, Multi-Ethnic Study of Atherosclerosis (2000–2002) and National Health and Nutrition Examination Survey (2003–2006)
| Arsenic Measure and n-3 PUFA concentration | Measured Biomarker | Calibrated Biomarker | ||
|---|---|---|---|---|
| GM Ratio | 95% CI | GM Ratio | 95% CI | |
| MESA: Estimated n-3 PUFAs, g (n = 310)b | ||||
| iAs | ||||
| ≤0.0670 | 1.00 | Referent | 1.00 | Referent |
| 0.0671–0.1440 | 1.01 | 0.79, 1.30 | 0.86 | 0.67, 1.10 |
| >0.144 | 1.20 | 0.94, 1.54 | 0.90 | 0.70, 1.15 |
| MMA | ||||
| ≤0.0670 | 1.00 | Referent | 1.00 | Referent |
| 0.0671–0.1440 | 1.14 | 0.90, 1.44 | 0.95 | 0.74, 1.21 |
| >0.144 | 1.23 | 0.97, 1.56 | 0.89 | 0.70, 1.13 |
| DMA | ||||
| ≤0.0670 | 1.00 | Referent | 1.00 | Referent |
| 0.0671–0.1440 | 1.17 | 0.94, 1.44 | 0.86 | 0.71, 1.03 |
| >0.144 | 1.57 | 1.26, 1.94 | 0.91 | 0.76, 1.10 |
| ∑As | ||||
| ≤0.0670 | 1.00 | Referent | 1.00 | Referent |
| 0.0671–0.1440 | 1.17 | 0.95, 1.43 | 0.87 | 0.73, 1.04 |
| >0.144 | 1.54 | 1.25, 1.89 | 0.92 | 0.77, 1.11 |
| MESA: Measured n-3 PUFAs, % (n = 120)c | ||||
| iAs | ||||
| ≤4.12 | 1.00 | Referent | 1.00 | Referent |
| >4.12–6.08 | 1.43 | 0.92, 2.21 | 1.03 | 0.67, 1.57 |
| >6.08 | 1.69 | 1.08, 2.63 | 1.10 | 0.71, 1.69 |
| MMA | ||||
| ≤4.12 | 1.00 | Referent | 1.00 | Referent |
| >4.12–6.08 | 1.79 | 1.22, 2.63 | 1.25 | 0.86, 1.82 |
| >6.08 | 1.66 | 1.12, 2.45 | 1.03 | 0.71, 1.51 |
| DMA | ||||
| ≤4.12 | 1.00 | Referent | 1.00 | Referent |
| 4.13–6.08 | 2.17 | 1.55, 3.06 | 1.18 | 0.85, 1.63 |
| >6.08 | 3.07 | 2.17, 4.34 | 1.38 | 0.99, 1.91 |
| ∑As | ||||
| ≤4.12 | 1.00 | Referent | 1.00 | Referent |
| 4.13–6.08 | 2.09 | 1.50, 2.92 | 1.19 | 0.87, 1.62 |
| >6.08 | 2.86 | 2.04, 4.01 | 1.34 | 0.97, 1.83 |
| NHANES: Estimated n-3 PUFAs, g (n = 1,175)d | ||||
| DMA | ||||
| ≤0.002 | 1.00 | Referent | 1.00 | Referent |
| 0.003–0.023 | 1.07 | 0.96, 1.20 | 1.05 | 0.96, 1.15 |
| >0.023 | 1.53 | 1.33, 1.74 | 1.16 | 1.03, 1.30 |
| NHANES: Estimated n-3 PUFAs With Exclusions (n = 1,119)e, f | ||||
| DMA | ||||
| ≤0.002 | 1.00 | Referent | 1.00 | Referent |
| 0.003–0.023 | 1.06 | 0.95, 1.18 | 1.06 | 0.97, 1.15 |
| >0.023 | 1.35 | 1.19, 1.52 | 1.07 | 0.97, 1.19 |
Abbreviations: CI, confidence interval; DMA, dimethylarsinate; GM, geometric mean; iAs, inorganic arsenic; MESA, Multi-Ethnic Study of Atherosclerosis; MMA, methylarsonate; NHANES, National Health and Nutrition Examination Survey; PUFA, polyunsaturated fatty acid; ∑As, sum of iAs and methylated species.
a All models were adjusted for age, sex, body mass index, and urine creatinine. n-3 PUFA concentrations are the sum of eicosapentaenoic acid and docosahexaenoic acid.
b Numbers of participants in each category are as follows: for ≤0.0670 g, n = 104; for 0.0671–0.1440 g, n = 102; and for >0.1440 g, n = 104.
c Numbers of participants in each category are as follows: for ≤4.12%, n = 41; for 4.13%–6.08%, n = 39; and for >6.08%, n = 40.
d Numbers of participants in each category are as follows: for ≤0.002 g, n = 389; for 0.003–0.023 g, n = 404; and for >0.023 g, n = 382.
e Numbers of participants in each category are as follows: for ≤0.002 g, n = 389; for >0.002–0.023 g, n = 404; and for >0.023 g, n = 326.
f Participants with estimated n-3 PUFA concentrations in the 95th percentile or were excluded. The 95th percentile in NHANES was 0.37 g.
Figure 2.
Sum of inorganic and methylated arsenic species (∑As) by measured (A) and estimated (B) n-3 polyunsaturated fatty acids (n-3 PUFA; sum of eicosapentaenoic acid a docosahexaenoic acid), Multi-Ethnic Study of Atherosclerosis, 2000–2002. Lines represent geometric mean ratios using measured (solid line) and calibrated (dotted line) urinary arsenic biomarkers based on restricted quadratic spline models for log-transformed ∑As with 3 knots. Dark gray and light gray shaded areas represent 95% confidence intervals of geometric mean ratios for measured and calibrated biomarkers, respectively. Geometric mean ratios were adjusted for age (continuous), sex, body mass index (continuous), and urine creatinine concentration (log-transformed).
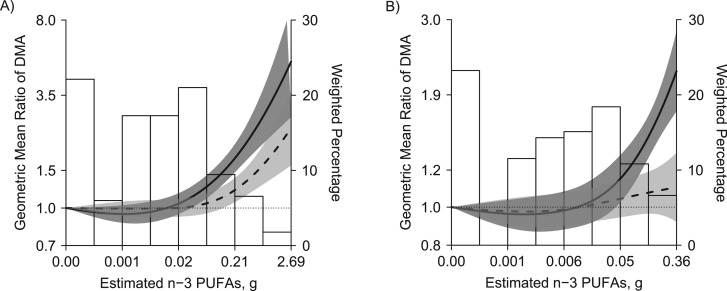
In NHANES, the GM ratios for DMA when comparing the highest estimated n-3 PUFA concentration tertiles with the lowest were 1.53 (95% CI: 1.33, 1.74) for the measured biomarker, 1.16 (95% CI: 1.03, 1.30) for the calibrated biomarker, and 1.07 (95% CI: 0.97, 1.19) for the calibrated biomarker excluding participants with n-3 PUFA concentrations in the 95th percentile or higher (Table 4). In flexible dose-response models (Figure 3), the association between n-3 PUFAs and DMA was attenuated when comparing the calibrated biomarker to the measured biomarker, especially when excluding participants with n-3 PUFA concentrations in the 95th percentile or higher.
Figure 3.
Urinary dimethylarsinate (DMA) by estimated omega-3 polyunsaturated fatty acids (n-3 PUFA; sum of eicosapentaenoic acid and docosahexaenoic acid) in National Health and Nutrition Examination Survey (NHANES) participants (A; n = 1,175) and excluding NHANES participants with n-3 PUFA concentrations in the 95th percentile or higher (B; n = 1,119), NHANES, 2003–2006. Lines represent geometric mean ratios of measured (solid line) and calibrated (dotted line) DMA based on restricted quadratic spline models for log-transformed DMA with 3 knots. Dark gray and light gray shaded areas represent 95% confidence intervals of geometric mean ratios for measured and calibrated DMA, respectively. Geometric mean ratios were adjusted for age (continuous), sex, body mass index (continuous), and urine creatinine (log-transformed).
Comparison of measured and calibrated biomarkers by self-reported rice intake
The frequency of rice consumption was greater among MESA participants than among NHANES participants. In MESA, 13.9% of participants reported eating rice rarely or never, compared with 33.9% who ate rice 2–4 times per month and 52.3% who ate rice 2 or more times per week (Table 1). In NHANES, 42.0% of participants reported eating rice rarely or never, compared with 36.2% who ate rice 2–4 times per month and 21.8% who ate rice 2 or more times per week (Table 1). Rice intake was associated with arsenic concentrations in urine, and the association was similar between the measured and calibrated biomarkers in both MESA and NHANES (Table 5).
Table 5.
Ratio of Geometric Mean Urine Arsenic Concentrations by Rice Intake Before and After Calibration, Stratified by Studya, Multi-Ethnic Study of Atherosclerosis (2000–2002) and National Health and Nutrition Examination Survey (2003–2006)
| Arsenic Measure and Rice Intake | Measured Biomarker | Calibrated Biomarker | ||
|---|---|---|---|---|
| GM Ratio | 95% CI | GM Ratio | 95% CI | |
| MESA (n = 310)b | ||||
| iAs | ||||
| Rare/never | 1.00 | Referent | 1.00 | Referent |
| 2–4 times per month | 1.04 | 0.76, 1.41 | 1.06 | 0.77, 1.46 |
| ≥2 times per week | 1.59 | 1.18, 2.14 | 1.43 | 1.06, 1.93 |
| MMA | ||||
| Rare/never | 1.00 | Referent | 1.00 | Referent |
| 2–4 times per month | 1.21 | 0.90, 1.62 | 1.24 | 0.91, 1.68 |
| ≥2 times per week | 1.86 | 1.40, 2.46 | 1.66 | 1.24, 2.21 |
| DMA | ||||
| Rare/never | 1.00 | Referent | 1.00 | Referent |
| 2–4 times per month | 1.25 | 0.96, 1.62 | 1.31 | 1.04, 1.65 |
| ≥2 times per week | 2.15 | 1.67, 2.76 | 1.77 | 1.42, 2.20 |
| ∑As | ||||
| Rare/never | 1.00 | Referent | 1.00 | Referent |
| 2–4 times per month | 1.23 | 0.96, 1.58 | 1.29 | 1.03, 1.61 |
| ≥2 times per week | 2.10 | 1.65, 2.67 | 1.75 | 1.41, 2.16 |
| NHANES (n = 1,175)c | ||||
| DMA | ||||
| Rare/never | 1.00 | Referent | 1.00 | Referent |
| 2–4 times per month | 1.09 | 0.99, 1.19 | 1.08 | 1.00, 1.18 |
| ≥2 times per week | 1.41 | 1.25, 1.60 | 1.33 | 1.21, 1.47 |
| NHANES With Exclusions (n = 1,119)d, e | ||||
| DMA | ||||
| Rare/never | 1.00 | Referent | 1.00 | Referent |
| 2–4 times per month | 1.08 | 0.99, 1.18 | 1.08 | 1.00, 1.18 |
| ≥2 times per week | 1.34 | 1.22, 1.48 | 1.29 | 1.19, 1.41 |
Abbreviations: CI, confidence interval; DMA, dimethylarsinate; GM, geometric mean; iAs, inorganic arsenic; MESA, Multi-Ethnic Study of Atherosclerosis; MMA, methylarsonate; NHANES, National Health and Nutrition Examination Survey; ∑As, sum of iAs and methylated arsenic species.
a All models were adjusted for age, sex, body mass index and urine creatinine.
b Numbers of participants in each category are as follows: for rare/never, n = 43; for 2–4 times per month, n = 105; and for ≥2 times per week, n = 162.
c Numbers of participants in each category are as follows: for rare/never, n = 500; for 2–4 times per month, n = 394; and for ≥2 times per week, n = 281.
d Numbers of participants in each category are as follows: for rare/never, n = 480; for 2–4 times per month, n = 376; and for ≥2 times per week, n = 263.
e Participants with estimated n-3 polyunsaturated fatty acid levels in the 95th percentile or were excluded. The 95th percentile in NHANES was 0.37 g.
DISCUSSION
Using data from MESA and NHANES, we obtained an estimation of urinary of iAs exposure that greatly removed the influence of arsenicals from low-toxicity seafood compared with the originally measured arsenic concentrations. Our method takes advantage of the fact that urinary arsenobetaine, a specific biomarker of seafood intake, is excreted unchanged in urine (29, 53, 54). In MESA, although the measured urinary concentrations of iAs, MMA, and DMA were associated with seafood consumption measured by self-report or by estimated or measured n-3 PUFA concentrations, the calibrated biomarkers were not associated. In NHANES, the association between DMA and seafood consumption was also markedly attenuated after calibration, although the association was only completely removed after excluding some outliers with estimated n-3 PUFA concentrations in the 95th percentile or higher. The associations of urinary concentrations of iAs, MMA, and DMA with intake of rice, a source of iAs and DMA, remained when using the calibrated biomarkers, which supports the hypothesis that the calibrated biomarker reflects exposure to iAs and DMA from sources other than seafood.
iAs is a widespread toxicant and carcinogen that may play a role in cardiovascular disease and diabetes development. Appropriate assessment of exposure to iAs in epidemiologic studies is critical to understanding the health consequences of arsenic, especially in general populations exposed to low chronic arsenic levels through diet, water, and air. In populations with high seafood intakes, assessment of iAs using biomarkers is difficult because some nontoxic seafood arsenicals contribute to DMA and total arsenic concentrations in urine. Previous epidemiologic studies have relied on methods such as excluding participants who reported recent seafood intake or adjusting for seafood intake (using self-reported seafood intake or urine arsenobetaine) in regression models (54, 55). In populations with high seafood intakes, excluding participants who report recent seafood consumption is problematic. First, it reduces the available sample size. Second, this exclusion would be nonrandom and may be differential with respect to participant characteristics, such as race/ethnicity, geographic location, and disease risk. Also, the exclusion of participants using this method depends on self-reported seafood intake, which has potential for incomplete recall from participants, incomplete seafood assessment in FFQs, and the presence of seafood arsenicals in unknown or difficult-to-collect sources for which investigators would not account (54).
An alternative method to restricting participants to those who do not eat seafood is to adjust for urinary arsenobetaine concentrations in regression models. Adjustment for arsenobetaine in the regression model is very similar to our proposed residual-based approach; it allows for control for seafood arsenicals and informs on the health consequences of arsenic not derived from seafood (52, 54, 56). The residual-based method, however, has the advantage of providing estimations of the biomarker concentrations for each participant that no longer reflect seafood intake and of facilitating the evaluation of exposure and metabolism of iAs in the population. This residual-based method additionally allows for the estimation of the percentages of each arsenic species relative to their sum (iAs %, MMA %, and DMA %), which are measures of arsenic metabolism. Having an estimation of metabolism for inorganic arsenic is important because it allows for the investigation of genetic and nongenetic determinants of arsenic metabolism (57, 58) and of the role of arsenic metabolism in disease development.
The differences in the behaviors of the calibrated biomarkers in MESA versus NHANES could be related to the different distributions of seafood intake in both populations. Average seafood intake was higher in MESA than in NHANES; however, variability was higher for NHANES than for MESA. For instance, the 95th percentile for estimated n-3 PUFAs was slightly higher in NHANES than in MESA (0.37 g vs. 0.34 g), whereas the maximum was markedly higher (2.7 g vs. 1.2 g). This is consistent with a more diverse population in NHANES than in MESA. Outlier participants with markedly higher n-3 PUFA concentrations compared with the rest of the population could have acted as influential points and levels of seafood intake that might be difficult to correct even with the proposed method. After excluding participants with estimated n-3 PUFA concentrations in the 95th percentile or higher in NHANES, both long-term and recent seafood intake were no longer associated with urine DMA, results that were more similar to those found in MESA. In MESA, excluding participants with estimated n-3 PUFA concentrations in the 95th percentile or higher resulted in similar findings, possibly because those values were not as extreme compared with values in the rest of the population. Also, the higher limits of detection for arsenobetaine and DMA in NHANES compared with MESA and the higher proportion of participants with levels below the limits of detection may affect the estimation of the calibrated biomarker and its ability to fully account for seafood intake in NHANES. In NHANES, 15.4% of participants had concentrations of urine DMA below the limit of detection (vs. 0% in MESA), and 27.1% had concentrations of urine arsenobetaine below the limit of detection (vs. 3.9% in MESA).
Strengths and limitations
The strengths of the present study include the analytical methods used for measuring urine arsenic in NHANES and MESA, as well as the large sample size and the fact that the NHANES sample was representative of the US population. We assessed consumption of seafood using both self-reported intake in the past year and estimated concentrations of n-3 PUFAs (measured n-3 PUFAs in a subset in MESA), which may reflect recent intake of seafood (59). Additionally, the n-3 PUFAs examined were limited to docosahexaenoic acid and eicosapentaenoic acid, which are concentrated in seafood, and did not include other n-3 fatty acids that are found primarily in foods such as grains and nuts/seeds. Sensitivity analyses excluding MESA participants who reported using dietary supplements containing n-3 fatty acids at least once per month (n = 11) resulted in similar findings.
There were some limitations to our study. First, we were unable to evaluate urinary concentrations of iAs and MMA in NHANES participants because of the large proportion of participants with values below the limit of detection. The likely reason for a much higher percentage of samples with undetectable iAs and MMA species concentrations in NHANES is the higher limits of detection in NHANES compared with MESA, although for iAs the percentage of samples with undetectable levels in MESA was still substantial. Second, information on seafood and rice intakes was self-reported; however, we were able to additionally evaluate seafood intake using measured concentrations of n-3 PUFAs in a MESA subsample. Urinary arsenic biomarkers reflect exposure to arsenic from multiple sources, including water, rice, seafood, other foods, and air; we did not have measured concentrations of arsenic in those sources. Fourth, spot urine samples were used for assessment of urine arsenic and urine creatinine. Although our method informs on exposure to iAs not derived from seafood, the calibrated biomarkers do not allow investigation of the health consequences of iAs, MMA, and DMA, as well as other arsenic species such as thioarsenicals, arsenosugars, and arsenolipids that are also contained in or derived from seafood. More sophisticated arsenic speciation would be needed to assess the health consequences of seafood arsenicals. Although this is an unintended consequence of our proposed strategy, the advantages are still large given the need to investigate the health consequences of exposure to iAs from drinking water, rice, and other grains in general populations and the smaller contribution of seafood intake to iAs and MMA. Finally, because toenail arsenic levels mostly reflect iAs exposure, a possible validation method would be to evaluate the relationship of our calibrated biomarker with toenail arsenic. Data on levels of arsenic in toenails, however, are not available in MESA, and toenail arsenic does not allow for the evaluation of arsenic metabolism.
Conclusion
Using data from MESA, a study with a population with 4 different racial/ethnic groups from 6 different geographical areas of the United States and a range of seafood intakes, and NHANES, a study with a population representative of the US population, we were able to develop urine arsenic biomarkers that do not reflect seafood intake. Our proposed residual-based method provides estimates of iAs exposure and metabolism for each participant with minimized influence from seafood intake and can thus facilitate arsenic research in populations characterized by frequent seafood intake and exposure to low-to-moderate levels of iAs through water, food, and air.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Miranda R. Jones, Eliseo Guallar, Wendy S. Post, Ana Navas-Acien); Department of Environmental Health Sciences, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Maria Tellez-Plaza, Maria Grau, Ana Navas-Acien); INCLIVA Biomedical Research Institute, Hospital Clinico Universitario de Valencia, University of Valencia, Valencia, Spain (Maria Tellez-Plaza); Department of Medicine, School of Medicine, Johns Hopkins University, Baltimore, Maryland (Dhananjay Vaidya, Eliseo Guallar, Wendy S. Post); Institute of Chemistry-Analytical Chemistry, University of Graz, Graz, Austria (Kevin A. Francesconi, Walter Goessler); Welch Center for Prevention, Epidemiology and Clinical Research, Johns Hopkins Medical Institutions, Baltimore, Maryland (Eliseo Guallar, Wendy S. Post, Ana Navas-Acien); Department of Environmental and Occupational Health Sciences, School of Public Health, University of Washington, Seattle, Washington (Joel D. Kaufman); and Department of Oncology, School of Medicine, Johns Hopkins University, Baltimore, Maryland (Ana Navas-Acien).
The Multi-Ethnic Study of Atherosclerosis (MESA) was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute (NHLBI) and by grants UL1-TR-000040 and UL1-TR-001079 from the National Center for Research Resources. Arsenic analyses were supported by grant R01HL090863 from NHLBI and by grant R01ES021367 from the National Institute of Environmental Health Sciences. M.R.J. was supported by a National Cancer Institute National Research Service Award (T32CA009314). M.T.-P. was supported by the Strategic Action for Research in Health Sciences (grant CP12/03080) from the Spanish Ministry of Economy and Competitiveness and the European Funds for Regional Development.
We thank the other investigators and the staff of MESA for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Conflict of interest: none declared.
REFERENCES
- 1.Chen Y, Wu F, Liu M, et al. A prospective study of arsenic exposure, arsenic methylation capacity, and risk of cardiovascular disease in Bangladesh. Environ Health Perspect. 2013;121(7):832–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Research Council Critical Aspects of EPA's IRIS Assessment of Inorganic Arsenic: Interim Report. Washington, DC: National Academy Press; 2014. [Google Scholar]
- 3.Gilbert-Diamond D, Li Z, Perry AE, et al. A population-based case-control study of urinary arsenic species and squamous cell carcinoma in New Hampshire, USA. Environ Health Perspect. 2013;121(10):1154–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.James KA, Byers T, Hokanson JE, et al. Association between lifetime exposure to inorganic arsenic in drinking water and coronary heart disease in Colorado residents. Environ Health Perspect. 2015;123(2):128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.James KA, Marshall JA, Hokanson JE, et al. A case-cohort study examining lifetime exposure to inorganic arsenic in drinking water and diabetes mellitus. Environ Res. 2013;123:33–38. [DOI] [PubMed] [Google Scholar]
- 6.Karagas MR, Tosteson TD, Morris JS, et al. Incidence of transitional cell carcinoma of the bladder and arsenic exposure in New Hampshire. Cancer Causes Control. 2004;15(5):465–472. [DOI] [PubMed] [Google Scholar]
- 7.Moon KA, Guallar E, Umans JG, et al. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease. A prospective cohort study. Ann Intern Med. 2013;159(10):649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parvez F, Chen Y, Brandt-Rauf PW, et al. A prospective study of respiratory symptoms associated with chronic arsenic exposure in Bangladesh: findings from the Health Effects of Arsenic Longitudinal Study (HEALS). Thorax. 2010;65(6):528–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parvez F, Chen Y, Yunus M, et al. Arsenic exposure and impaired lung function. Findings from a large population-based prospective cohort study. Am J Respir Crit Care Med. 2013;188(7):813–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinmaus C, Ferreccio C, Yuan Y, et al. Elevated lung cancer in younger adults and low concentrations of arsenic in water. Am J Epidemiol. 2014;180(11):1082–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinmaus C, Yuan Y, Bates MN, et al. Case-control study of bladder cancer and drinking water arsenic in the western United States. Am J Epidemiol. 2003;158(12):1193–1201. [DOI] [PubMed] [Google Scholar]
- 12.Chappells H, Parker L, Fernandez CV, et al. Arsenic in private drinking water wells: an assessment of jurisdictional regulations and guidelines for risk remediation in North America. J Water Health. 2014;12(3):372–392. [DOI] [PubMed] [Google Scholar]
- 13.Tsuji JS, Perez V, Garry MR, et al. Association of low-level arsenic exposure in drinking water with cardiovascular disease: a systematic review and risk assessment. Toxicology. 2014;323:78–94. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert-Diamond D, Cottingham KL, Gruber JF, et al. Rice consumption contributes to arsenic exposure in US women. Proc Natl Acad Sci USA. 2011;108(51):20656–20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes MF, Beck BD, Chen Y, et al. Arsenic exposure and toxicology: a historical perspective. Toxicol Sci. 2011;123(2):305–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson BP, Taylor VF, Karagas MR, et al. Arsenic, organic foods, and brown rice syrup. Environ Health Perspect. 2012;120(5):623–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nordstrom DK. Public health. Worldwide occurrences of arsenic in ground water. Science. 2002;296(5576):2143–2145. [DOI] [PubMed] [Google Scholar]
- 18.Smith AH, Steinmaus CM. Arsenic in drinking water. BMJ. 2011;342:d2248. [DOI] [PubMed] [Google Scholar]
- 19.Chung JY, Yu SD, Hong YS. Environmental source of arsenic exposure. J Prev Med Public Health. 2014;47(5):253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.García-Aleix JR, Delgado-Saborit JM, Verdú-Martín G, et al. Trends in arsenic levels in PM10 and PM 2.5 aerosol fractions in an industrialized area. Environ Sci Pollut Res Int. 2014;21(1):695–703. [DOI] [PubMed] [Google Scholar]
- 21.Lewis AS, Reid KR, Pollock MC, et al. Speciated arsenic in air: measurement methodology and risk assessment considerations. J Air Waste Manag Assoc. 2012;62(1):2–17. [DOI] [PubMed] [Google Scholar]
- 22.National Research Council Arsenic in Drinking Water. Washington, DC: National Academy Press; 1999. [Google Scholar]
- 23.Ahsan H, Perrin M, Rahman A, et al. Associations between drinking water and urinary arsenic levels and skin lesions in Bangladesh. J Occup Environ Med. 2000;42(12):1195–1201. [DOI] [PubMed] [Google Scholar]
- 24.Calderon RL, Hudgens E, Le XC, et al. Excretion of arsenic in urine as a function of exposure to arsenic in drinking water. Environ Health Perspect. 1999;107(8):663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pellizzari ED, Clayton CA. Assessing the measurement precision of various arsenic forms and arsenic exposure in the National Human Exposure Assessment Survey (NHEXAS). Environ Health Perspect. 2006;114(2):220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cannon JR, Edmonds JS, Francesconi KA, et al. Isolation, crystal structure and synthesis of arsenobetaine, a constituent of the western rock lobster, the dusky shark, and some samples of human urine. Aust J Chem. 1981;34(4):787–789. [Google Scholar]
- 27.Cullen WR, Reimer KJ. Arsenic speciation in the environment. Chem Rev. 1989;89(4):713–764. [Google Scholar]
- 28.Edmonds JS, Francesconi KA, Cannon JR, et al. Isolation, crystal-structure and synthesis of arsenobetaine, arsenical constituent of western rock lobster panulirus-longipes-cygnus george. Tetrahedron Lett. 1977(18):1543–1546. [Google Scholar]
- 29.Francesconi KA, Edmonds JS. Arsenic and marine organisms. Adv Inorg Chem. 1996;44:147–189. [Google Scholar]
- 30.Brown RM, Newton D, Pickford CJ, et al. Human metabolism of arsenobetaine ingested with fish. Hum Exp Toxicol. 1990;9(1):41–46. [DOI] [PubMed] [Google Scholar]
- 31.Francesconi KA, Tanggaar R, McKenzie CJ, et al. Arsenic metabolites in human urine after ingestion of an arsenosugar. Clin Chem. 2002;48(1):92–101. [PubMed] [Google Scholar]
- 32.Le XC, Cullen WR, Reimer KJ. Human urinary arsenic excretion after one-time ingestion of seaweed, crab, and shrimp. Clin Chem. 1994;40(4):617–624. [PubMed] [Google Scholar]
- 33.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. [DOI] [PubMed] [Google Scholar]
- 34.Scheer J, Findenig S, Goessler W, et al. Arsenic species and selected metals in human urine: validation of HPLC/ICPMS and ICPMS procedures for a long-term population-based epidemiological study. Anal Methods. 2012;4(2):406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caldwell KL, Jones RL, Verdon CP, et al. Levels of urinary total and speciated arsenic in the US population: National Health and Nutrition Examination Survey 2003-2004. J Expo Sci Environ Epidemiol. 2009;19(1):59–68. [DOI] [PubMed] [Google Scholar]
- 36.National Center for Environmental Health NHANES 2003–2006: Laboratory Component: Total Arsenic and Speciated Arsenics. Atlanta, GA: CDC/National Center for Environmental Health; 2004. [Google Scholar]
- 37.Block G, Woods M, Potosky A, et al. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43(12):1327–1335. [DOI] [PubMed] [Google Scholar]
- 38.Mayer-Davis EJ, Vitolins MZ, Carmichael SL, et al. Validity and reproducibility of a food frequency interview in a Multi-Cultural Epidemiology Study. Ann Epidemiol. 1999;9(5):314–324. [DOI] [PubMed] [Google Scholar]
- 39.Nettleton JA, Rock CL, Wang Y, et al. Associations between dietary macronutrient intake and plasma lipids demonstrate criterion performance of the Multi-Ethnic Study of Atherosclerosis (MESA) food-frequency questionnaire. Br J Nutr. 2009;102(8):1220–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Subar AF, Thompson FE, Kipnis V, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: The Eating at America's Table Study. Am J Epidemiol. 2001;154(12):1089–1099. [DOI] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention, National Center for Health Statistics NHANES 2003–2004. Food Frequency Questionnaire—Output from Dietcalc Software. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 42.Minnesota Nutritional Coordinating Center Minnesota Nutrition Data System (NDS). Version 4.02; nutrient database version 30. Minneapolis, MN: Nutrition Coordinating Center, University of Minnesota; 1999. [Google Scholar]
- 43.Cao J, Schwichtenberg KA, Hanson NQ, et al. Incorporation and clearance of omega-3 fatty acids in erythrocyte membranes and plasma phospholipids. Clin Chem. 2006;52(12):2265–2272. [DOI] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention, National Center for Health Statistics National Health and Nutrition Examination Survey Questionnaire (or Examination Protocol, or Laboratory Protocol). Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2013. [Google Scholar]
- 45.Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149(6):531–540. [DOI] [PubMed] [Google Scholar]
- 46.Michels KB, Bingham SA, Luben R, et al. The effect of correlated measurement error in multivariate models of diet. Am J Epidemiol. 2004;160(1):59–67. [DOI] [PubMed] [Google Scholar]
- 47.Rhee JJ, Cho E, Willett WC. Energy adjustment of nutrient intakes is preferable to adjustment using body weight and physical activity in epidemiological analyses. Public Health Nutr. 2014;17(5):1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slager SL, Iturria SJ. Genome-wide linkage analysis of systolic blood pressure: a comparison of two approaches to phenotype definition. BMC Genet. 2003;4(suppl 1):S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124(1):17–27. [DOI] [PubMed] [Google Scholar]
- 50.Willett WC. Nutritional Epidemiology. 2nd ed New York, NY: Oxford University Press; 1998. [Google Scholar]
- 51.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 suppl):1220S–1228S. [DOI] [PubMed] [Google Scholar]
- 52.Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, et al. Rejoinder: arsenic exposure and prevalence of type 2 diabetes: updated findings from the National Health Nutrition and Examination Survey, 2003-2006. Epidemiology. 2009;20(6):816–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Francesconi KA. Arsenic species in seafood: origin and human health implications. Pure Appl Chem. 2010;82(2):373–381. [Google Scholar]
- 54.Navas-Acien A, Francesconi KA, Silbergeld EK, et al. Seafood intake and urine concentrations of total arsenic, dimethylarsinate and arsenobetaine in the US population. Environ Res. 2011;111(1):110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wei Y, Zhu J, Nguyen A. Rice consumption and urinary concentrations of arsenic in US adults. Int J Environ Health Res. 2014;24(5):459–470. [DOI] [PubMed] [Google Scholar]
- 56.Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, et al. Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA. 2008;300(7):814–822. [DOI] [PubMed] [Google Scholar]
- 57.Pierce BL, Kibriya MG, Tong L, et al. Genome-wide association study identifies chromosome 10q24.32 variants associated with arsenic metabolism and toxicity phenotypes in Bangladesh. PLoS Genet. 2012;8(2):e1002522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tellez-Plaza M, Gribble MO, Voruganti VS, et al. Heritability and preliminary genome-wide linkage analysis of arsenic metabolites in urine. Environ Health Perspect 2013;121(3):345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chung H, Nettleton JA, Lemaitre RN, et al. Frequency and type of seafood consumed influence plasma (n-3) fatty acid concentrations. J Nutr. 2008;138(12):2422–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.