Abstract
The bottom-up synthesis of ligand-stabilized functional nanoparticles from molecular precursors is widely applied but is difficult to study mechanistically. Here we use 31P NMR spectroscopy to follow the trajectory of phosphinate ligands during the synthesis of a range of ligated zinc oxo clusters, containing 4, 6 and 11 zinc atoms. Using an organometallic route, the clusters interconvert rapidly and self-assemble in solution based on thermodynamic equilibria rather than nucleation kinetics. These clusters are also identified in situ during the synthesis of phosphinate-capped zinc oxide nanoparticles. Unexpectedly, the ligand is sequestered to a stable Zn11 cluster during the majority of the synthesis and only becomes coordinated to the nanoparticle surface, in the final step. In addition to a versatile and accessible route to (optionally doped) zinc clusters, the findings provide an understanding of the role of well-defined molecular precursors during the synthesis of small (2–4 nm) nanoparticles.
 Ligands and surfactants play an important part in the synthesis of
nanoparticles from molecular precursors although their exact roles are poorly
understood. Here, the authors isolate a range of intermediate sized zinc clusters and
are able to spectroscopically probe the self-assembly and ligand effects.
Ligands and surfactants play an important part in the synthesis of
nanoparticles from molecular precursors although their exact roles are poorly
understood. Here, the authors isolate a range of intermediate sized zinc clusters and
are able to spectroscopically probe the self-assembly and ligand effects.
Zinc oxide nanoparticles are used in a huge range of contexts, ranging from wide band gap semiconductors and photo-electronic materials1,2,3, to catalysts and photoactive antimicrobial surfaces4,5,6,7,8. Individualized nanoparticles are synthesized in solution via a similar broad range of techniques including sol-gel chemistry and hydrolysis/thermolysis routes9,10,11,12,13,14,15. Regardless of the synthesis method, ZnO and indeed other nanoparticles are frequently capped by surfactants or organic ligands, commonly alkyl amines or carboxylic acids, to maximize solubility during growth and use. In these cases, as with many nanoparticle systems, a ligand is usually implicitly considered to control the synthesis, even if exchanged later16,17. Despite the importance of such systems, the detailed mechanism by which discrete, often monometallic, molecular precursors and ligands are combined to form functionalized nanoparticles is not well understood18,19. One very attractive approach to ZnO nanoparticle synthesis, pioneered by Chaudret and colleagues10, hydrolyses organo-zinc reagents, at room temperature in organic solvents, to form crystalline Wurztite products12,20. This route provides small particles and is compatible with thermally sensitive organic/polymer chemistry21; it has enabled the preparation of high-performance composite photovoltaic (PV) cells22,23, colloidal catalysts20,24 and antimicrobial plastics4. In the context of the mechanistic studies reported below, the route provides the opportunity for detailed control of precursor/ligand stoichiometry, as excess ligand can be avoided12, and the extent of reaction is limited by water supply.
Metallic cluster complexes can grow to more than 1 nm in size and bridge between the domains of molecules and nanoparticulates25; they frequently show unusual and impressive optical, electronic and magnetic properties26,27. In contrast, the field of zinc-oxo cluster chemistry is relatively less developed, although it is known that alkyl zinc alkoxide/carboxylate clusters are useful precursors to form ZnO nanoparticles and thin films9,11,12,13,15,18,28. Doped materials may also be prepared from heterobimetallic clusters, with improved properties attributed to intimate mixing of the two metals13,28. Furthermore, tetrahedral zinc carboxylate clusters, [Zn4O(CO2R)6], are ubiquitous vertices in metal organic frameworks (MOFs), showing outstanding gas sorption and separation characteristics, although they can be sensitive to attack from water or donor solvents29,30,31,32,33. The metal-oxygen framework structures of reported precursors do not generally map directly onto the Wurzite–ZnO structure10,12; therefore, their transformations into nanoparticulate ZnO probably involves significant molecular rearrangement. In the case of [RZnOR]4 complexes, alkoxide is lost before ZnO nucleation (to a Wurzite structure) and the relationship of the ligands to the growing nanoparticle is not clear. Here we show that ligands may coordinate to cluster species, which act as spectators, while ZnO nucleation occurs and act as a ‘ligand reservoir' that is only consumed at the end of the synthesis procedure.
Although the alkyl zinc cluster chemistry using ligands such as alkoxide or carboxylate shows significant promise13,34,35,36,37,38,39, utilization of alternative ligands is less well explored. Furthermore, attempts to analyse the speciation during carboxylate/alkoxide precursor transformations result in product mixtures and broadened, complex nuclear magnetic resonance (NMR) spectra. In contrast, ligands coordinated with a P-containing group provide a 31P NMR spectroscopic handle that allows for simple identification of individual cluster geometries even when a complicated mixture is present. Various discrete complexes of zinc phosphonate [RPO3]2– or dialkylphosphate [(RO)2PO2]– are known as viable precursors to nanomaterials40,41,42. One stand-out example is a well-defined Zn12 cluster, which contains a Zn4O core surrounded by Zn−Et fragments supported by eight phosphonate ligands41. This cluster is proposed to be a viable precursor to porous zincophosphonate materials. Phosphinate ([R2PO2]–) ligands are iso-electronic with carboxylates and could be attractive alternatives. The parent phosphinic acids are more acidic than carboxylic acids (for example, pKa: diphenylphosphinic acid (DPPA-H), 2.3; benzoic acid, 4.2) and thus, although the bonding to zinc is slightly weaker, they should be less susceptible to hydrolysis. Furthermore, having two R groups increases their steric protection and enhances hydrophobicity. Stability to hydrogenation and good solubilizing properties make dioctylphosphinate an interesting ligand for supporting nanoparticles used for quasi-homogeneous hydrogenation catalysis (for example, the hydrogenation of CO2 to MeOH)20. Despite their promise as ligands, well-defined zinc complexes and clusters coordinated by monoanionc phosphinate ligands are hardly studied43.
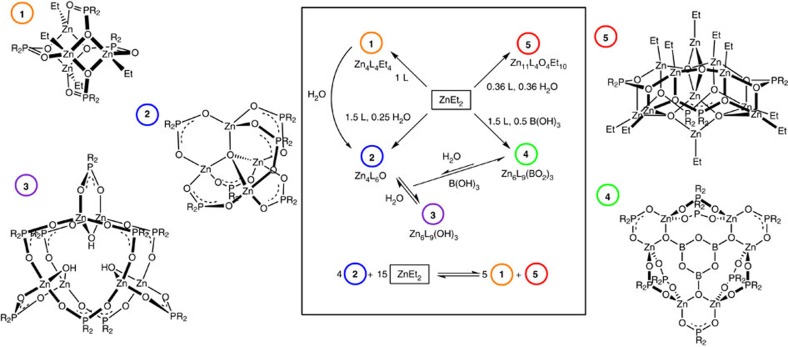
Here, the reactions of simple, commercially-available diethyl zinc with various equivalents of phosphinic acids and water are used to reproducibly prepare a series of new clusters, which are all fully characterized, including by X-ray diffraction (XRD; Fig. 1). Alongside more typical alkyl zinc ligand and tetrahedral Zn4O(ligand)6 clusters, some very unusual larger structures are identified, including partially hydrolysed zinc clusters and those containing hydroxy or boroxine cores. Interestingly, these species all show equilibrium relationships with each other and other small molecules, suggesting the clusters may readily interconvert in solution. With the detailed understanding of these species, it is possible to directly identify them in situ during the synthesis of ZnO nanoparticles.
Figure 1. Zinc cluster complexes.
The zinc cluster structures and their interconversions, where L=R2PO2H, R=Ph, C6H4OMe or C8H17 (4 only formed with R=Ph).
Results
Synthesis of zinc phosphinate cluster complexes
The first part of the study focused on understanding and characterizing the species present during simple reactions between diethyl zinc and DPPA-H (as a model ligand). Thus, the reaction between equimolar quantities of ZnEt2 and DPPA-H forms a new tetra-zinc cluster, 1A. Its 31P{1H} NMR spectrum shows a sharp singlet (23.2 p.p.m.) and the 1H NMR spectrum shows a 1:1 ratio of ethyl:DPPA resonances (Supplementary Figs 1 and 2). Although the structures of alkyl zinc phosphinate complexes are not yet reported, alkyl zinc carboxylates adopt a range of chemical structures44, including hexa-35 or pentanuclear complexes37,38,39. Crystals of 1A, analysed by XRD, show a distorted cubic structure [Zn4Et4(DPPA)4] with a tetrahedral arrangement of zinc atoms (Fig. 2 and Supplementary Figs 3,4). Each zinc is singly coordinated to a P=O oxygen (P=O range, 1.492(2)–1.497(2) Å) and each P−O– oxygen atom (P–O range, 1.531(2)–1.534(2) Å) bridges between two zinc centres. The shape of 1A is most closely related to the ‘cubane' structures of alkyl zinc alkoxides but with the phosphinate ligand adopting bidentate chelation13.
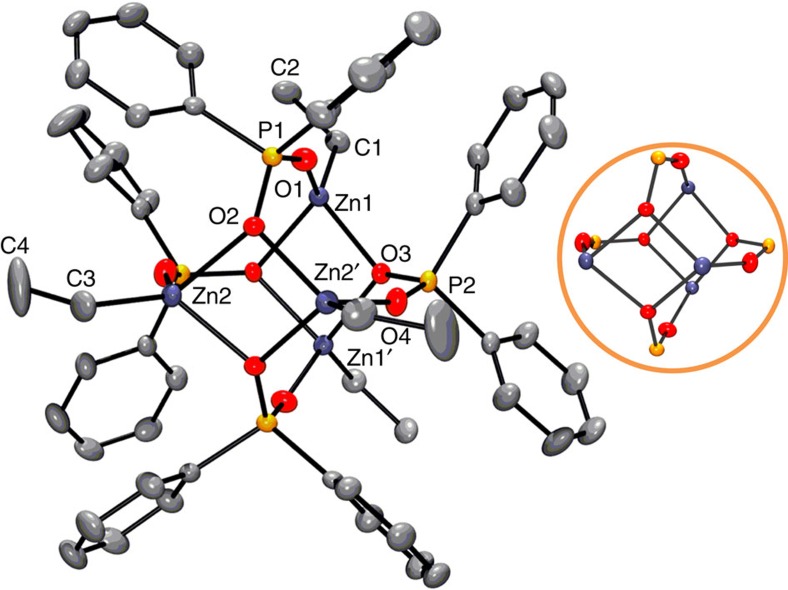
Figure 2. Solid-state structure of 1A.
H-atoms omitted for clarity; a view of the Zn cluster core structure, with the phenyl/ethyl groups omitted, is provided within the coloured circle.
Compound 1A is highly moisture sensitive and the addition of ∼2 eq. of water (allowing full hydrolysis of all Zn−Et bonds) forms a new species, 2A, which also exhibits a single peak in the 31P{1H} NMR spectrum (32.9 p.p.m.; Supplementary Figs 5–8). Complex 2A is also a tetrazinc cluster of the form [Zn4(μ4-O)(DPPA)6], featuring a central μ4-oxo atom and six phosphinate ligands (Supplementary Fig. 9); the excess zinc/ethyl is likely to be hydrolysed to form NMR-silent insoluble ZnO particles. Compound 2A can also be directly prepared, in quantitative yield (NMR spectroscopy), by reaction of a 4:6:1 ratio of ZnEt2:DPPA-H:water, in toluene or CH2Cl2 (Fig. 3). The matrix-assisted laser desorption/ionization–time of flight (MALDI–ToF) mass spectrum shows a peak for [Zn4O(DPPA)5]+ in keeping with the expected cluster formula (Supplementary Fig. 7). The 1H NMR spectrum shows a single environment for the phenyl substituents in 2A (Supplementary Fig. 6). The crystal structures of 2A, grown either from toluene or CH2Cl2/hexane, show four independent molecules in the asymmetric unit. The phenyl groups could not be located to acceptable degrees of accuracy; however, [Zn4(μ4-O)(O2P)6] units were observed (Supplementary Figs 9, 10). By using bis(4-methoxyphenyl)phosphinic acid, a fully resolvable crystal structure of 2B was obtained, a complex with an analogous structure to 2A (Fig. 3a). The structure of 2B shows a tetrahedral core of Zn4O capped by six bidentate ligands, which show equal P−O bond lengths (within error), indicating a delocalized coordination mode (Supplementary Figs 11–15). Although such structures are not yet known for phosphinate ligands, they are commonly observed for other anionic ligands and the benzene dicarboxylate Zn4O cluster is a common construct in MOFs29,30,45.
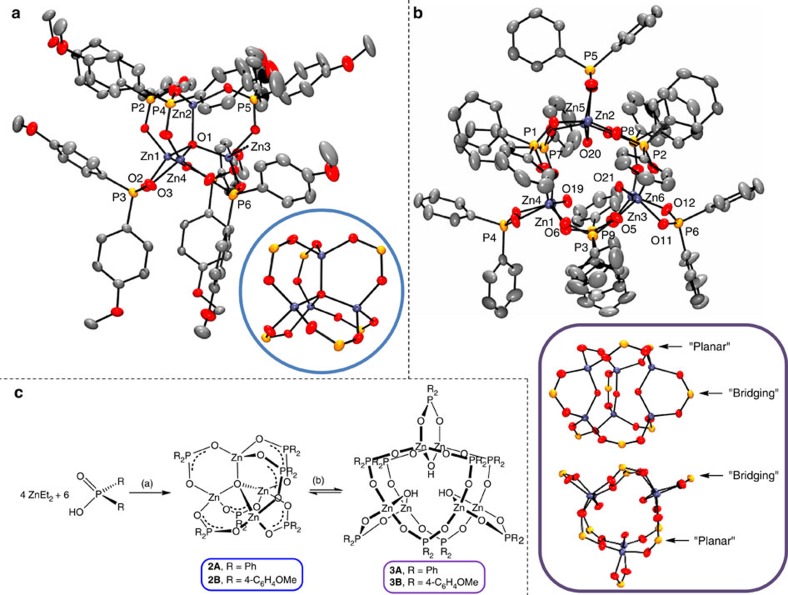
Figure 3. Synthetic path and solid-state structures of 2B and 3A.
Structures: (a) 2B and (b) 3A (H-atoms omitted for clarity). Views of the Zn cluster core structures, with aromatic groups omitted, are provided inside the coloured circle/box c) Synthesis and equilibrium of 2A/B and 3A/B. Reagents:(a) 1 eq. H2O, toluene or CH2Cl2. (b) 5 eq. H2O.
Samples of 2A exposed to moisture led to the formation of a new complex, 3A, which displays two 31P NMR signals in a 2:1 ratio (30.1 (1P), 24.2 (2P) p.p.m.; Supplementary Figs 16–20). The addition of 5 eq. (versus 2A) of water to the solution results in a mixture comprising a relative molar ratio 2A:3A=2:3. Solvated water is also observed in the NMR spectrum, suggesting equilibration between 2A, water and 3A (Fig. 3). It is important to emphasize that 3A reproducibly forms on addition of water to chloroform, toluene or tetrahydrofuran (THF) solutions of 2A.
The formula of 3A is [Zn6(μ2-OH)3(DPPA)9], established by XRD analysis of single crystals (Fig. 3b and Supplementary Fig. 18). Solid 3A can also be isolated in quantitative yield by direct reaction, in this case of a 1:1.5:0.75 ratio of ZnEt2:DPPA-H:water. The product has a pseudo trigonal prismatic shape, previously unknown for such zinc clusters. It also features three bridging zinc hydroxide ligands. The trigonal prismatic shape results from two planar triangular units of Zn3(DPPA)3, which are bridged by three further DPPA units and three hydroxides. In all cases, P–O bond lengths are similar, suggesting delocalized bonding.
The stability of the cluster may stem from the hydroxide groups being positioned just far enough from each other to hinder any further condensation reactions (Supplementary Fig. 21). Attenuated total reflection–infrared spectroscopy of a crystalline sample of 3A (dried under vacuum) shows a weak signal at 3,644 cm−1 attributed to O–H stretches (not present for 2A), consistent with values reported for other bridging Zn2(μ2-OH) units; a second broad signal at 3,410 cm−1 could be a different OH stretching mode or traces of adsorbed moisture (Supplementary Fig. 18)46,47,48,49. The 1H NMR spectrum of 3A shows three sets of phenyl environments in a 1:1:1 ratio, which are assigned using correlation spectroscopy NMR (Supplementary Fig. 17). The solution structure is consistent with the solid-state structure, assuming that there is some flexibility enabling a D3H symmetry in solution. The six ‘planar' DPPA units are assigned to two phenyl environments: one pointing approximately in the plane of the triangle and the other perpendicular. The third phenyl environment is attributed to the three ‘bridging' DPPA units in which both phenyl groups occupy identical environments. The hydroxide protons are also observable as a sharp signal at 3.67 p.p.m., with a relative integral of 3H.
The formation of 3A is unexpected; the isolation and characterization of well-defined Zn-hydroxide complexes is usually challenging, often requiring the use of bulky, multi-dentate ligands for stabilization46,49. Zinc-hydroxy species are of interest in a range of contexts, including as putative intermediates during ZnO nanoparticle synthesis and as models for a range of zinc-dependent metalloenzymes11,45,46,47,50,51,52,53,54.
Given interest in similar carboxylate-ligated Zn clusters, the different structures and reactivity observed here with phosphinate ligands is notable. The solid-state structure of 2B shows Zn−phosphinate bond lengths ranging from 1.917(2) to 1.960(2) Å, with an average (1.936(2) Å) slightly greater than that of the analogous Zn-benzoate structure Zn4O(O2CPh)6 (average=1.926 Å)35, in line with slightly weaker bonding from the phosphinate. The Zn−(μ4-O) bonds in 2B are also lengthened (average 2B, 1.989(2) Å; Zn4O(O2CPh)6 1.946 Å), presumably as a result of the larger size of the phosphinate chelate compared with a carboxylate (average P−O (2B), 1.512(2) Å; average C−O (Zn4O(O2CPh)6), 1.258 Å)35, which allows for an expansion of the Zn4O cluster. Compounds 2A/B react with water to form well-defined zinc hydroxide complexes, whereas the carboxyate analogue [Zn4O(CO2Ph)6] reacts as a Lewis acid towards water to form an aqua complex [Zn4(μ4-O)(OOCPh)6(H2O)(THF)]31. Thus, complexes with phosphinate ligands undergo disruption of the Zn4O core. The Zn−OH bonds in 3A are shorter on average (1.935(2) Å) than the Zn−(μ4-O) bonds in 2B (1.989(2) Å); it may be that owing to the larger size of the phosphinate ligand, effective bonding to the oxo/hydroxo ligand is favoured in the expanded Zn6 structure.
Equilibrium studies
To explore the factors controlling the equilibration of zinc-oxo and zinc-hydroxy clusters, variable-temperature NMR spectroscopy was applied, using a solution containing a starting 2:3 ratio of 2A:3A, over the temperature range 288–328 K (Supplementary Fig. 24). At each temperature, the equilibrium was rapidly established, as confirmed by an identical second spectrum, obtained after ∼15 min. The ratio of 2A:3A is easily determined from the 31P{1H} NMR spectra (see Supplementary Methods), with 2A being the major species at temperatures above 318 K. Under the experimental conditions, the concentration of water is low (0.059 M) but is all fully dissolved with no downfield signal, which would be expected from separated water droplets. Van't Hoff analysis showed that ΔHr=−108±3 kJ mol−1 and ΔSr=−238±9 J K−1 mol−1 (Supplementary Fig. 25 and Supplementary Methods). Clearly, the hydroxo structure 3A is enthalpically favoured, but the entropic advantage results in 2A becoming dominant at higher temperatures. A similar equilibrium exists between zinc-oxo cluster 2B, water and 3B (Supplementary Figs 22 and 23). The equilibrium lies more towards the zinc oxo species, 2B, than in the 2A/3A system (2B/3B=1:0.23 cf. 2A:2B=1:0.9, 2.3 eq. water added). Van't Hoff analysis revealed that ΔHr=−97±3 kJ mol−1 and ΔSr=−234±9 J K−1 mol−1 (Supplementary Fig. 26 and Supplementary Table 1). Compared with 2A/3A, the entropy of reaction is unchanged (within error), but the zinc hydroxyl cluster, 3B, is slightly less enthalpically favoured (Supplementary Table 1). These results provide a thermodynamic rationale for the equilibration between the clusters and demonstrate the importance of the phosphinate ligand in controlling the relative stabilities of the clusters.
Synthesis of a zinc-boroxine cluster
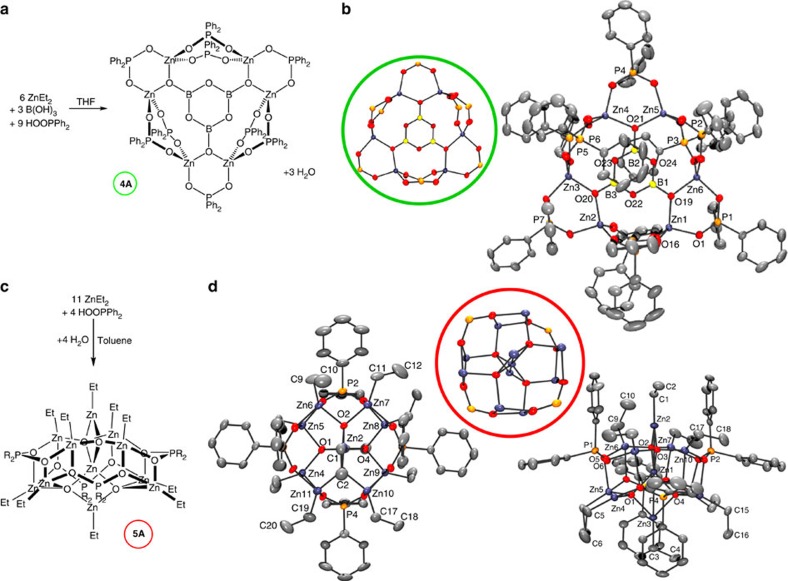
The proximity of the three hydroxyl groups in 3A suggests the intriguing possibility of coordination of further atoms/molecules in the centre of the cluster (O−centroid distances 1.5–1.8 Å, Supplementary Fig. 21). In a different system and geometry, partially condensed trisilanol silsequioxanes have been widely used to bind heteroatoms for catalytic and other studies55. The reactivity of 3A with organometallic reagents (such as AlEt3) is challenging, especially given the presence of water in the solution equilibrium, which results in preferential hydrolysis of the organometallic species, driving the equilibrium back towards 2A. An alternative approach is to use a different oxygen source to form the Zn–O–X moieties. In this regard, boric acid (B(OH)3) is attractive for its aqueous stability and trigonal planar shape. Boric acid clearly reacts with a THF solution of 2A/3A, leading to the formation of a product 4A (Supplementary Figs 27–29). Compound 4A can also be prepared in quantitative yield (31P NMR) by the direct reaction of a 2:3:1 ratio of ZnEt2, DPPA-H and boric acid, in THF (Fig. 4). Again, an equilibrium exists between 4A, 2A and 3A (Supplementary Fig. 33); when 17 eq. of water was added to a solution of pure 4A, a molar ratio of 89:7:4 for 4A:2A:3A formed, showing that 4A is favoured even under wet conditions. Crystals of 4A, grown from THF/hexane, showed the structure as [Zn6B3O3(DPPA)9] (Fig. 4b and Supplementary Figs 30 and 32). The planar cluster contains six zinc atoms surrounding a B3O3 core. Each zinc atom is tetrahedrally coordinated to three bridging phosphinate ligands and a μ3-oxo ligand. The oxo ligands are each also coordinated to the boroxine core. Two phenyl substituents align above and below this boroxine core, suggesting some π–π stacking exists in the solid state, it is well known that boroxines exhibit partial aromaticity56. The structure of 4A is quite different to that of 2A or 3A and it is proposed that the spontaneous self-assembly is driven by the planar boroxine core. The Zn6B3O3 cluster planarity may also be relevant for the construction of more complex two-dimensional materials, including MOFs. The structure of 4A is maintained in solution; two singlet signals in the 31P NMR spectrum are observed in a 2:1 ratio (22.7, 29.3 p.p.m.) as expected from the two environments (in and out of the plane) in the solid-state structure (Supplementary Fig. 27). The 1H NMR spectrum shows three sets of phenyl resonances in a 1:1:1 ratio (Supplementary Fig. 28).
Figure 4. Synthetic path and solid-state structures of 4A and 5A.
Schemes showing synthesis of (a) 4A and (c) 5A. Solid-state structures of (b) 4A and (d) 5A (2 views shown) (views of the Zn cluster core structures, with the phenyl/ethyl groups omitted, are provided inside the coloured circles).
Synthesis of a partially hydrolysed Zn11 cluster
It is of interest to consider what role clusters such as 1–3 might take during the formation of phosphinate-coordinated zinc oxide nanoparticles by hydrolysis routes. We have previously reported the potential to introduce sub-stoichiometric quantities of carboxylic acid/phosphinic acid during ZnEt2 hydrolysis, to deliver surface-ligated crystalline ZnO nanoparticles with well-defined sizes (2–4 nm). The capped nanoparticles show good solubility in organic solvents and have been used as quasi-homogeneous catalysts as well as in the preparation of high-loading fraction ZnO-polymer composites19,20. In general, there is significant interest in the preparation of ZnO nanoparticles by the controlled hydrolysis of organozinc reagents, including ZnEt2, as it provides a room-temperature method to crystalline nanoparticles and a route to useful inorganic hybrid materials10,12,16,57. So far, however, the mechanism and intermediates implicated in the hydrolysis of well-defined organometallic reagents, with or without capping ligands, to nanoparticles is not at all well understood19. As a starting point to understanding how the particles form, we proposed that there may be some partly hydrolysed clusters present. The hydrolysis reaction occurs in solutions, often of inert organic solvents; thus, it is beneficial to apply solution-based spectroscopic techniques. A particular benefit of phosphinate ligands, as noted above, is the facility to apply 31P{1H} NMR spectroscopy. Previous studies of ZnO nanoparticles have shown they approach surface saturation with ligand, when a mixture of 5 eq. of ZnEt2 with one equivalent of ligand (typically dioctylphosphinic acid) is hydrolysed20. Introducing the water gradually allows the speciation during this process to be probed. Using DPPA as a model ligand and adding only one equivalent of water to this 5:1 mixture, a new phosphorus-containing cluster compound was identified by NMR spectroscopy (Supplementary Figs 34–36). By adjusting the ratios to favour this new species, we were able to form crystals from an 11:4:4 mixture of ZnEt2, H2O and DPPA-H. The isolated crystals revealed a cluster containing 11 zinc atoms, [Zn11Et10O4(DPPA)4]; elemental analysis was also in good agreement (Fig. 4d and Supplementary Fig. 37). Compound 5A, [Zn11Et10O4(DPPA)4], can be thought of as an extension of 1A in which 6 extra Zn−Et groups are added along with a central ZnO4 tetrahedron. Unlike 1A, the bonding within the phosphinate ligand is now delocalized with equivalent P−O bonds throughout. Compound 5A has approximate D2d point symmetry, with eight Zn−Et groups coordinated by bridging phosphinate ligands surrounding a central ZnO4 tetrahedron. A further two Zn−Et groups are located above and below the central ZnO4 core, without any bonds to phosphinate ligands; these two zinc atoms are three coordinate (trigonal planar). The phosphinate–Zn bonds are somewhat variable (1.870(2)–2.094(2) Å; cf. 2B, 1.917(2)–1.960(2) Å), suggesting the central core dictates the geometry. In solution, the 1H NMR spectrum indicates a similar structure, with two different zinc-coordinated ethyl environments in a 4:1 ratio (Supplementary Fig. 35). The two ethyl ligands at the three coordinate zinc centres are significantly shifted (–1.48, 0.23 p.p.m.) presumably due to proximity to electron-deficient zinc centres (Supplementary Fig. 36). The other zinc ethyl ligands show diastereotopic methylene proton signals, due to chirality at those zinc centres.
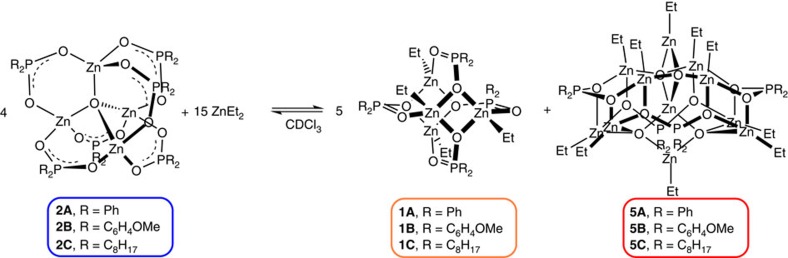
To understand the cluster interconversions, 15 eq. of ZnEt2 were added to 4 eq. of 2A, leading to a 5:1 ratio of 1A:5A, together with residual 2A and ZnEt2. Increasing the temperature drives the backward reaction and increases the relative proportions of 2A and ZnEt2, indicating that an equilibrium exists between the four species (Fig. 5 and Supplementary Figs 38–43). Establishing the same equilibrium from 2B also revealed a 5:1 ratio of new clusters (that is, the analogues 1B and 5B; Supplementary Figs 40 and 41, and Supplementary Table 2), highlighting the generality of these reactions with different phosphinate ligands. Furthermore, all the room-temperature 1H NMR spectra of 3A, 3B and 4A show broadening of the phenyl environments for the phosphinates in asymmetrical environments, indicating that ligand rotation allows exchange between phenyl environments. This rotation may suggest that the flexible ligand coordination of phosphinate ligands enable rearrangements to the thermodynamic products.
Figure 5. Cluster equilibrium.
Equilibrium between 2, 1 and 5. Complexes 1B, 5B, 1C and 5C identified by NMR spectroscopy only.
In situ identification of clusters in nanoparticle synthesis
Having established the structures of clusters 1–5A and the generality to other related model ligands, their roles in ZnO nanoparticle synthesis was explored. In particular, the hydrolysis of ZnEt2 was performed in the presence of the di-octylphosphinate (DOPA) ligands, which are representative of systems used to sterically stabilize nanoparticles. The long alkyl chains hinder crystallization, but the utility of the 31P NMR handle allows characterization. Using analogous procedures to the preparation of 1A/B and 2A/B, the clusters 1C (Zn4Et4(DOPA)4) and 2C (Zn4O(DOPA)6) were easily identified following the reactions of appropriate ratios of ZnEt2 with DOPA-H (and water for 2C) (Supplementary Figs 44–48). Addition of an excess of water (∼6 eq.) to 2C leads to two 31P signals (which sum to 1% of the total integral considering residual 2C) indicative of the formation of 3C (Zn6(OH)3(DOPA)9) (Supplementary Fig. 49). However, in this case, the equilibrium strongly favours 2C, likely to be due to the greater steric hindrance from the bulky ligand. Nevertheless, the speciation using the long-chain phosphinate maps onto the model clusters already structurally characterized.
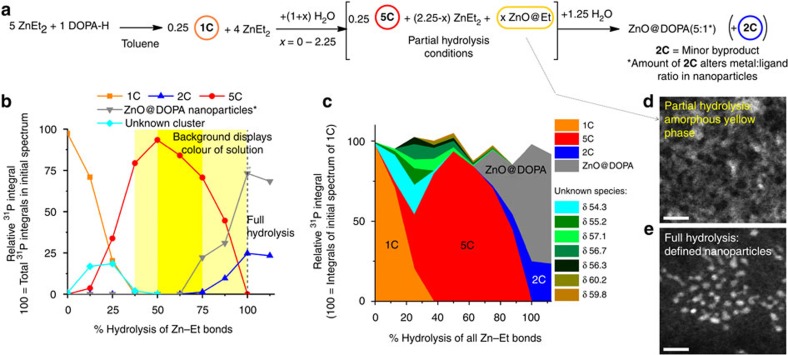
For the synthesis of functionalized nanoparticles, a Young's tap NMR tube was loaded with a 5:1 ratio of ZnEt2 and DOPA-H solvated in d8-toluene, similar to conditions established previously20. Water was added in sequential 0.5 μl aliquots to the NMR tube (each aliquot making up 12.5% of the 4 μl required for total hydrolysis) under a flow of N2. NMR spectra were recorded at each point along the hydrolysis pathway (after ∼30 min reaction time at each point, Supplementary Figs 50–54). An internal standard (PPh3 in a capillary tube) was used to allow calculation of relative integrals from the 31P{1H} NMR spectra and to monitor the evolution of the cluster species during the reaction (Fig. 6b,c). It is worth noting that the reactions described above were performed at stoichiometries targeted to particular clusters, whereas the reactions on the path to nanoparticles occur in the presence of excess diethyl zinc. Initially, in the absence of water, 1C was observed alongside the excess ZnEt2, as expected. Initial hydrolysis of the remaining Zn−Et bonds results in the loss of signal for 1C and the formation of a signal at 61.0 p.p.m., assigned to complex 5C [Zn11Et10O4(DOPA)4] (see Supplementary Note 1), accompanied by various low-intensity new signals. Compound 5C can be independently synthesized and also forms an equilibrium with 1C, 2C and ZnEt2 as shown in Fig. 5 (Supplementary Figs 55–58). The other minor species (shown in Fig. 6c) are currently not assigned; the strongest signal is tracked in Fig. 6b and disappears after 38% total hydrolysis, leaving 5C as the dominant ligated species until hydrolysis nears completion (Fig. 6). In the accompanying series of 1H NMR spectra (Supplementary Fig. 51), the sharp ethyl signals for 5C also grow in and persist almost until hydrolysis is completed. The initially exchange-broadened signals for free ZnEt2 sharpen as 1C and other clusters are consumed and then disappear as hydrolysis proceeds. Although the NMR spectra change little between 38 and 63% hydrolysis, a distinctive yellow colour emerges. It appears that the extra water reacts with ZnEt2 to produce species that are silent in the 1H and 31P NMR spectra. This same yellow colour is observed during hydrolysis of ZnEt2 in the absence of ligands and has been noted by other researchers preparing ZnO nanoparticles, although the speciation remains unexplored57. Transmission electron microscopy (TEM) and powder XRD of this ‘yellow ZnO' derived from a reaction of ZnEt2 with 0.75 eq. of H2O in toluene revealed a mainly amorphous polydispersed and agglomerated nanoparticulate material displaying a very broad, weak ZnO diffraction pattern (Fig. 6d and Supplementary Figs 59–61). Elemental analysis confirmed that some ethyl groups were also maintained, although no sharp signals attributable to ethyl moieties were observed in the 1H NMR spectra. To monitor the formation of crystalline ZnO, the hydrolysis reaction, in the presence of DOPA ligands, was also followed by ultraviolet spectroscopy (290–400 nm; Supplementary Fig. 62). No optical absorption was observed in either the starting molecular precursors or 25% hydrolysed mixtures, confirming that the yellow colour is not associated with ligated species, 1C or 5C, but rather the product of partial ZnEt2 hydrolysis, as noted above57. At 50% hydrolysis, a strong absorption is observed, which reduces smoothly, from 290–350 nm, but with no visible band edge typically associated with a crystalline ZnO species; this absorption is similar but stronger at 75% hydrolysis, as consumption of ZnEt2 continues, and is attributed to highly defective/disordered amorphous ZnO nanoparticles, as discussed above58. However, at 100% hydrolysis, the ultraviolet absorption drops significantly (consistent with the observed loss of yellow colour), giving a typical ZnO band edge signal, corresponding to nanoparticles with a diameter of ∼3 nm (Supplementary Fig 63)59.
Figure 6. In situ study of ZnO nanoparticle synthesis.
(a) Scheme showing the synthesis of ZnO nanoparticles. (b) Relative integrals and (c) area graph showing sum of integrals from 31P{1H} NMR spectra of the P-containing species, formed on increasing amounts of added water to an original 5:1 ZnEt2: DOPA-H mixture (*the signal for the ZnO@DOPA nanoparticles is broad, leading to less accurate integration). Alongside the unknown cluster displayed in b, small quantities of other unknown species (always <10% total integral) were also observed between 0–82% hydrolysis (shown in c and labelled by their 31P NMR signal (Supplementary Figs 50–53 for spectra and Supplementary Fig. 54 for %Zn speciation plots)). Area graph (c) shows that the sum of relative integrals remains close to 100% of the initial spectrum (1C only species) throughout the reaction. (d,e) Representative scanning TEM (STEM) images (in annular dark field mode) of the partially hydrolysed ZnEt2 (ZnO@Et yellow phase) and fully hydrolysed ZnO@DOPA nanoparticles at the same level of magnification (scale bar, 10 nm; note, Zn-containing phase appears pale on a dark background).
The final product of hydrolysis is colourless ZnO nanoparticles (2–3 nm) capped by di(octylphosphinate) ligands (ZnO@DOPA), extensively characterized previously (and here shown in Fig. 6e and Supplementary Figs 64–72), showing well-isolated crystalline ZnO cores in TEM (Supplementary Fig. 64), clear XRD features of Wurtzite ZnO (Supplementary Fig. 69), an identifiable organic content in elemental analysis, and maintaining a high solubility in toluene (unlike the hydrolysis product in the absence of phosphinate)20. Both NMR and ultraviolet spectroscopy studies indicate that the ligand-capped ZnO@DOPA nanoparticles only form towards the end of the hydrolysis reaction (Fig. 6 and Supplementary Fig. 62). The nanoparticles are observed by 31P NMR spectroscopy as a broad signal after 75% hydrolysis (52 p.p.m. (full width at half maximum ∼1,800 Hz)); the signal breadth is typical for ligands coordinated to nanoparticle surfaces (Supplementary Figs 66 and 67)17. After all the Zn−Et bonds are hydrolysed, a second new species (2C) is observed along with the nanoparticles in the 31P NMR spectra (Fig. 6b,c). Although the majority of phosphinate is bound to nanoparticles, the zinc oxo cluster 2C forms as a significant byproduct (25% of total ligand). The presence of 2C, which has a high DOPA:Zn ratio, is consistent with the liberation of DOPA on the final hydrolysis of 5C and agglomeration of ZnO units (note: 3C was not observed here as it only forms as a very minor equilibrium partner with 2C in the presence of excess moisture). Compound 2C is soluble in acetone, unlike ZnO@DOPA nanoparticles, and thus can be easily removed, allowing the isolation of pure nanoparticles. It is notable also that Chaudret and colleagues16, and Mayer and colleagues7 have recently both reported that amine-ligated zinc oxide surfaces also exhibit exchange between coordinated and ‘free' ligands; it is interesting to consider whether well-defined molecular zinc cluster complexes may also be present in these cases. However, low temperature and diffusion-ordered NMR spectroscopy studies (Supplementary Figs 66–68) showed no evidence of ligand exchange between the nanoparticles and 2C.
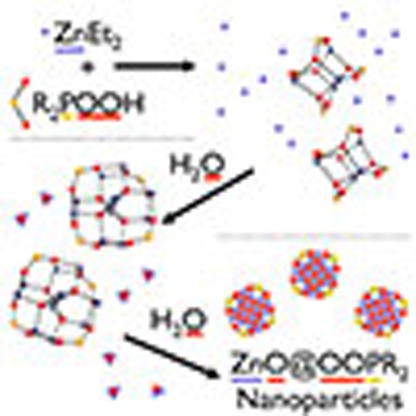
This reaction trajectory is quite unexpected. The highly moisture sensitive alkyl–zinc complex 5C forms rapidly and is maintained throughout the majority of the hydrolysis reaction, sequestering essentially all the available ligand, whereas residual ZnEt2 is consumed to form unligated ZnO nanoparticle precursors. A 50% hydrolysed mixture was monitored and found to be unchanged after 15 h, indicating that the system is in thermodynamic equilibrium, with 93% of the total phosphinate supply incorporated in the form of a stable cluster (minor unidentified species make up the balance to ∼100% relative to the internal standard; Fig. 6c and Supplementary Figs 52 and 53). It is only near-full hydrolysis of all other Zn−Et species that 5C reacts with the ‘yellow' ZnO precursors to form the phosphinate capped ZnO nanoparticles. Unlike previous reports in the literature, proposing cluster compounds as molecular building blocks that directly map onto the final nanoparticle (NP) crystal structure60, here the cluster compounds do not obviously relate to Wurtzite and instead appear to act only as a reservoir of ligand. This fresh insight into ligand behaviour during nanoparticle synthesis has implications for the concepts of nanoparticle growth and stabilization.
The formation of ZnO nanoparticles by hydrolysis, under the same conditions, but in the absence of any ligand produces insoluble nanoparticles, with average particle sizes of ∼3.5 nm (by XRD; Supplementary Fig. 73). The similarity in size range to the particles prepared using the DOPA ligands (2–3 nm by XRD) indicates that the ligand is not critical for particle size control, in keeping with the distribution of ligands only at the end of the reaction. Previous studies using carboxylate ligands and a similar synthetic protocol also found that ZnO particles were consistently formed within the 3–5 nm size range, regardless of the nature or loading of the ligand applied12. Nonetheless, the ligands are important to produce well-dispersed and soluble nanoparticles, as they prevent aggregation observed in their absence. Furthermore, ligands are likely to have a significant impact on subsequent ripening and ageing of the nanoparticles12,19.
Discussion
This study exploits an organometallic route to nanoparticles that delivers only the stoichiometric quantity of ligand. By avoiding the excess uncoordinated ligand used in many liquid-phase nanoparticle syntheses, the fate of the ligand at various stages of the reaction can be directly determined. Nanoparticle nucleation is very often considered to be a non-equilibrium process, requiring high degrees of super-saturation and high concentrations of active surfactants to minimize the size of the critical nuclei, with particle size often controlled by kinetics, requiring hot injection, fast mixing and the like61. Alternatively, sol-gel approaches often involve irreversible condensation reactions20. The presence of ligands during nanoparticle synthesis is usually assumed to reduce the nucleation barrier and critical nucleus size, by reducing surface energy of the nascent nanoparticle. Smaller particles can therefore form, which are sterically stabilized against coalescence by the coordinated ligand62,63,64. Here, this model is completely subverted as the ligands are observed to only interact at the end of the reaction. The behaviour found in this ZnO system may well be observed in other cases, where ligand supply is limited65,66, or in systems with analogous structures, such as (Zn, Cd) (S, Se, Te) capped by coordinating ligands (carboxylates, phosphinates or phosphonates).
This study shows that equilibrium cluster interconversions, including of oxo-bridged species, can play a key role in the distribution of ligand on growing nanoparticles. Understanding the mechanisms by which the nanoparticle core is formed and then decorated by ligands is likely to help in the formation of (surface) doped nanoparticles crucial for many applications1,67,68, especially in (opto)electronics, and in forming mixed ligand layers, which may allow unusual wettability or adaptive behaviour69,70.
In conclusion, the reactions between diethyl zinc, phosphinic acids and water lead to a rich variety of new clusters. Using equimolar diethyl zinc and phosphinic acid yields an organometallic cubic structure, 1; the species is hydrolysed, by water to a Zn4O cluster, 2. The Zn4O cluster equilibrates with excess water to produce hexa-zinc tris(hydroxide) trigonal prismatic complexes, 3. The equilibrium is very unusual, yet provides a simple means to prepare zinc hydroxide clusters; such species are usually significantly more challenging to prepare, yet are important structures in bio-inorganic and other processes. A new planar Zn6B3 cluster, 4A, is formed with B(OH)3 taking the role of the water in the ‘hydrolysis' of the Zn–Et bonds. Finally, 5A, a cluster containing 11 zinc atoms, was prepared by partial hydrolysis of Zn–Et bonds. Its analogue, coordinated by a long-chain di(octyl phosphinate) ligand, 5C plays a crucial role in the synthesis of ZnO@DOPA nanoparticles. Interestingly the reactive cluster is retained as a spectator after its formation during the initial hydrolysis, sequestering all the available DOPA ligand in a stable form, leaving water to react directly with residual unligated ethyl-zinc species. Only on approaching total hydrolysis of all zinc alkyl functionalities is the ligand delivered to the growing nanoparticle surface. The final re-equilibration step converts disordered polydispersed nanoparticle precursors into the well-defined ligand-capped ZnO product.
In addition to the rich cluster chemistry identified, using simple, commercial reagents, relevant as single-source precursors to prepare (optionally doped) nanomaterials or as nodes in new families of metal-organic frameworks, this study highlights a number of useful principles. First, phosphinates are under-utilized ligands, which provide a diagnostic 31P NMR handle; a strong and distinct NMR signal from the binding group is extremely helpful for navigating complex mixtures to identify the correct stoichiometries for pure products. Second, the cluster species form through a series of reversible equilibria as hydrolysis proceeds, but specific products can be isolated directly once their composition is identified. These equilibrium processes should allow further investigation and interpretation of the nucleation and growth of nanoparticles.
Methods
Experimental details
All manipulations were undertaken using a nitrogen filled glovebox or using a Schlenk line, unless otherwise stated. DPPA and bis(4-methoxyphenyl) phosphinic acid were used directly from suppliers and di-octylphosphinic acid was prepared using an established literature route71. ZnO@DOPA nanoparticles were prepared using a literature route20, and synthetic details and characterization of ZnO@DOPA, ZnO and ‘yellow ZnO' are included in the Supplementary Methods. ZnEt2 is pyrophoric (caution!) and was added to samples in a nitrogen-filled glovebox. As a liquid, all additions of ZnEt2 were transferred by syringe (measured by negative weight of donor flask). THF was dried by refluxing over sodium and benzophenone, and stored under nitrogen. Hexane and toluene were pre-dried over potassium hydroxide and then further dried by refluxing over sodium (benzophenone, for hexane) and stored under nitrogen. ‘Extra-dry' acetone was purchased from Acros Organics. All dry solvents and reagents were degassed by three freeze–pump–thaw cycles and stored under nitrogen. Solvents were tested for moisture content by Karl Fischer Titration (Mettler Toledo): toluene, 3.8 p.p.m.; THF, 4.1 p.p.m.; dichloromethane, 1.7 p.p.m.
NMR spectra were recorded on Bruker AV-400 or AV-500 instruments and all chemical shifts reported in p.p.m. Solid-state Fourier transform infrared spectra were recorded using a Perkin-Elmer Spectrum 100 FT-IR spectrometer with a Universal ATR Sampling Accessory. Ultraviolet spectroscopy was recorded using a PerkinElmer Lambda 950 spectrophotometer, from toluene solutions. All mass spectrometry measurements were performed using a MALDI micro MX micromass instrument. Isotope patterns were compared with predicted patterns using mMass. Elemental Analysis was determined by Stephen Boyer at London Metropolitan University. Thermo-gravimetric analysis was undertaken under an air atmosphere, using a Mettler/Toledo TGA/DSC 1LF/UMX instrument at a heating rate of 10 K min−1. Powder XRD was performed using an X'Pert Pro diffractometer (PANalytical B. V., The Netherlands) and X'Pert Data Collector software, version 2.2b. The instrument was used in the theta/theta reflection mode, fitted with a nickel filter, 0.04 rad Soller slit, 10 mm mask, 1/4° fixed divergence slit and 1/2° fixed antiscatter slit. The diffraction patterns were analysed using Fityk (version 0.9.0; Marcin Wojdyr, 2010), the peaks were fitted to a SplitPearson7 function and the particle size was calculated using the fitted full-width half-maximum using the Scherrer equation. Scanning TEM images, conventional TEM images and electron diffraction patterns were acquired on an FEI Titan 80–300 microscope operated at 300 kV. For air-sensitive samples, the sample solution was deposited on a 400-mesh copper holey carbon grid with an ultra-thin 3 nm-thick carbon support (Agar Scientific AGS187-4), while in a glove box. The grid was then loaded into a Gatan environmental holder to prevent any exposure to air prior to TEM imaging. Further details of Van't Hoff analysis, NMR equilibrium studies, in situ experiments and single crystal XRD are included in the Supplementary Methods and supplementary Table 3.
Syntheses and characterization of 1A
Zn4Et4(DPPA)4: DPPA-H (88.3 mg, 0.405 mmol) was placed in a Young's tap flask and dissolved in CH2Cl2 (∼3 ml). To this, ZnEt2 (50 mg, 0.405 mmol) was added and the evolution of ethane gas was observed. Hexane was layered onto the solution, allowing the growth of white crystalline 1A over several days (Isolated yield: 35 mg, 23%). Alternatively, toluene can be used as the reaction solvent and 1A precipitates out directly as a powder in this case (42% yield). Compound 1A is highly moisture sensitive and traces of 2A can be observed in its NMR spectra if there is any contamination by trace moisture. 31P{1H} NMR (162 MHz, CDCl3): δ 23.2 (s, 4P) p.p.m.; 1H NMR (400 MHz, CDCl3): δ 0.41 (q, CH2, JHH=8 Hz, 8H), 1.33 (t, CH3, JHH=8 Hz, 12H), 7.08 (td, DPPA, JHH=8 Hz, 3 Hz, 16H), 7.26 (m, DPPA, 8H), 7.34 (m, DPPA, 16H); anal. calcd for: Zn4P4O8C56H60=C, 53.96; H, 4.85%; found C, 53.82; H, 4.76%.
Syntheses and characterization of 1C
Zn4Et4(DOPA)4: 47 mg (0.162 mmol) dioctylphosphinic acid was placed in a Young's cap NMR tube and dissolved in CDCl3 (0.5 ml). To this, 20 mg (0.162 mmol) of ZnEt2 was added and the evolution of ethane gas was observed. The product was analysed by NMR spectroscopy directly and 1C was identified as ∼68% of 31P NMR signal with the remainder as broad unidentified products. If an excess of ZnEt2 is present, 1C forms as the sole 31P-containing species. 31P{1H} NMR (162 MHz, CDCl3): δ 50.9 (s, 4P); 1H NMR (400 MHz, CDCl3): δ 0.03 (q, Et CH2, JHH=8 Hz, 8H), 0.91 (t, DOPA CH3, JHH=7 Hz, 24H), 1.14 (t, Et CH3, JHH=8 Hz, 12H), 1.30 (br, DOPA CH2, 80H), 1.53 (br, DOPA β-CH2, 16H), 1.68 (m, DOPA α-CH2, 16H) (400 MHz, d8-toluene): δ 0.62 (q, Et CH2, JHH=8 Hz, 8H), 0.91 (t, DOPA CH3, JHH=7 Hz, 24H), 1.31 (br, DOPA CH2, 80H), 1.66 (t, Et CH3, JHH=8 Hz, 12H), 1.8 (br, DOPA β-CH2, 16H), 1.9 (m, DOPA α-CH2, 16H)
Syntheses and characterization of 2A
Zn4(μ4-O)(DPPA)6: DPPA-H (265 mg, 1.21 mmol) was placed in a Schlenk flask with a stirrer bar and suspended in toluene (∼15 ml). To this, ZnEt2 (100 mg, 0.81 mmol) was added and the evolution of ethane gas was observed. The solution was stirred for 30 min, before addition of a 3.6 μl (0.2 mmol) of water by Eppendorf pipette, under a flow of nitrogen, and the mixture stirred overnight. A precipitate formed from the reaction solution; gentle heating allowed re-solvation of this material and then the flask was allowed to stand at room temperature, to allow the formation of a colourless crystalline material (isolated yield=264 mg, 83%). Alternatively, 2A can be synthesized using CH2Cl2 as the reaction solvent and crystals can then be formed by layering the solution with hexane. It is notable that if there is any excess of water then traces of 3A are observable. 31P{1H} NMR (162 MHz, CDCl3): δ 32.9 (s, 6P) p.p.m.; 1H NMR (400 MHz, CDCl3): δ 7.08 (td, DPPA, JHH=8 Hz and 3 Hz, 24H), 7.31 (m, DPPA, 12H), 7.51 (m, DPPA, 24H) p.p.m.; m/z (MALDI–ToF, matrix=9-nitroanthracene, solvent=CHCl3): 1363.2 {[Zn4O(DPPA)5]+ calcd 1362.9}, 1619.3 {[Zn4O(DPPA)6.K]+ calcd 1618.9} amu; anal calcd for Zn4P6O13C72H60=C, 54.71; H, 3.83%; found C, 54.33; H, 4.23% (note: traces of CH2Cl2 and hexane were observed by NMR spectroscopy when these crystals were solvated even after thorough drying under vacuum).
Syntheses and characterization of 2B
Zn4(μ4-O)(DMeOPPA)6: Bis(4-methoxyphenyl)-phosphinic acid (337.9 mg, 1.21 mmol) was placed in a Schlenk flask with a stirrer bar and dissolved in CH2Cl2 (20 ml). To this, ZnEt2 (100 mg, 0.81 mmol) was added and the evolution of ethane gas was observed. The solution was stirred for 30 min, before addition of a solution of water (4 μl, 0.22 mmol) in dry acetone (0.1 ml). The volume of solvent was reduced to ∼7 ml, by vacuum, before layering the solution with hexane (∼40 ml), to allow the formation of crystals (isolated yield=210 mg, 80%). Alternatively, 2B was synthesized in toluene solvent and crystals formed directly from the reaction medium. This product, which crystallizes with two molecules of toluene, was rather insoluble once formed. 31P{1H} NMR (162 MHz, CDCl3): δ 32.8 (s, 6P) p.p.m.; 1H NMR (400 MHz, CDCl3): δ 3.75 (s, OMe, 36H), 6.58 (m, C6H4OMe, 24H), 7.47 (m, C6H4OMe, 24H) p.p.m.; m/z (MALDI–ToF, matrix=9-nitroanthracene, solvent=CHCl3): 1663.4, {[Zn4O(DMeOPPA)5]+ calcd 1663.0} amu.; anal. calcd for Zn4P6O25C84H84=C, 51.98; H, 4.36%; found C, 51.84; H, 4.43%; anal. calcd for (product isolated from toluene) Zn4P6O25C84H84.2(C7H8)=C, 55.39; H, 4.74%; found C, 55.24; H, 4.98%.
Syntheses and characterization of 2C
Zn4(μ4-O)(DOPA)6: (529 mg, 1.82 mmol) dioctylphosphinic acid was placed in a Schlenk flask with a stirrer bar and dissolved in toluene (∼12 ml). To this, 150 mg (1.21 mmol) of ZnEt2 was added and the evolution of ethane gas was observed. The solution was stirred for 30 min before addition of a solution of water in acetone (6 μl (0.33 mmol) water in 0.2 ml acetone). The solvent may be removed to leave an oily colourless product. 31P{1H} NMR (162 MHz, CDCl3): δ 56.5 (s, 6P);1H NMR (400 MHz, CDCl3): δ 0.87 (t, DOPA CH3, JHH=7 Hz, 18H), 1.25 (br, DOPA CH2, 60H), 1.57 (br, DOPA α and β CH2, 24H); m/z (MALDI–ToF, matrix=trans-2-[3-(4-tert-butylphenyl)-2-methyl-2-properylidene] malononitrile, solvent=toluene): 1724.2 {[Zn4O(DPPA)5]+ calcd 1723.9} amu;
Syntheses and characterization of 3A
Zn6(μ2-OH)3(DPPA)9: ZnEt2 (100 mg, 0.809 mmol) was added to a toluene (8 ml) suspension of DPPA-H (265 mg, 1.21 mmol). After stirring for 30 min, a slight excess of water (10 μl, 0.56 mmol) in acetone (0.1 ml) was added. The resulting solution yielded a white precipitate, which could be isolated by removal of the solvent by evacuation (311 mg, 96% yield), which was deemed pure by powder XRD. Alternatively, single crystals of 3A could be grown by gently warming the original toluene solution/suspension and then allowing it to cool to room temperature. 31P{1H} NMR (162 MHz, CDCl3): δ 24.2 (s, 6P), 30.1 (s, 3P) p.p.m.; (162 MHz, d8-THF): δ 23.7 (s, 6P), 29.2 (s, 3P) p.p.m.; 1H NMR (400 MHz, 273 K, CDCl3): δ 3.68 (s, OH, 3H), 6.57 (m, C6H5 ‘planar (1)', 12H), 6.89 (t, C6H5 ‘planar (1)', JHH=7 Hz, 6H), 6.94 (m, C6H5 ‘planar (2)', 12H), 7.20 (t, C6H5 ‘planar (1)', JHH=7 Hz, 6H), 7.3–7.52 (m, C6H5 ‘bridging, planar (1) and (2)', 42H), 7.81 (dd, C6H5 ‘bridging', JHH=7 and 12 Hz, 12H) p.p.m.; anal. calcd for (from toluene) Zn6P9O21C122H109.2(C7H8)=C, 56.75; H, 4.25%; found C, 56.71; H, 4.36%.
Syntheses and characterization of 3B
Zn6(μ2-OH)3(DMeOPPA)9: 3B formed as the minor product of an equilibrium with 2B, when excess moisture is present in a hydrophobic solvent such as CDCl3. For example, water (0.5 μl) was added to 2B (9 mg, 0.0046, mmol) in CDCl3 (0.5 ml). 31P{1H} NMR (162 MHz, CDCl3): δ 24.8 (s, 6P), 30.6 (s, 3P) p.p.m.; 1H NMR (400 MHz, CDCl3): δ 3.61 (br, OMe, 18H), 3.70 (br, OMe, 18H), 3.77 (s, OMe, 18H), 3.81 (s, OH, 3H), 6.22 (br C6H4OMe, 12H), 6.46 (br, C6H4OMe, 12H), 6.71 (m, C6H4OMe, 12H), 7.33 (m, C6H4OMe, 2 × 12H), 7.64 (m, C6H4OMe, 12H).
Syntheses and characterization of 3C
Zn6(μ2-OH)3(DOPA)9: 3C forms as the minor product of an equilibrium with 2C when excess moisture is present. 31P{1H} NMR (162 MHz, CDCl3): δ 49.9 (s, 6P), 53.9 (s, 3P); 1H NMR (400 MHz, CDCl3): δ 3.48 (s (OH), 3H), DOPA peaks overlap with 2C.
Syntheses and characterization of 4A
Zn6B3O6(DPPA)9: DPPA-H (265 mg, 1.21 mmol) and B(OH)3 (25 mg, 0.40 mmol) were placed in a Young's flask with a stirrer bar. To this, THF (10 ml) was added and ZnEt2 (100 mg, 0.81 mmol) was then added dropwise, while stirring. The solution was stirred overnight. A small amount of white precipitate may form, which is expected to be 3A from reaction with liberated water, the solution was thus filtered and precipitated using hexane to yield a white powder, which was dried under vacuum (200 mg, 60% yield). The bulk powder from rapid precipitation appears amorphous by powder XRD but single crystals could be grown by slow diffusion of hexane into a THF solution of 4a. 31P{1H} NMR (162 MHz, CDCl3): δ 22.7 (s, 6P), 29.3 (s, 3P) p.p.m.; (162 MHz, d8-THF): δ 22.5 (s, 6P), 29.0 (s, 3P) p.p.m.;1H NMR (400 MHz, CDCl3): δ 6.79 (br, ‘Asym' DPPA, 12H), 6.86 (br, ‘Asym' DPPA, 6H), 7.21 (br, ‘Asym' DPPA, 12H), 7.38 (m, ‘Asym and Sym' DPPA, 36H), 7.55 (m, ‘Asym' DPPA, 12H), 8.13 (m, ‘Sym' DPPA, 12H); m/z (MALDI–ToF, matrix=trans-2-[3-(4-tert-butylphenyl)-2-methyl-2-properylidene] malononitrile, solvent=toluene): 2257.2, {[Zn6O6B3(DPPA)8]+ calcd 2256.9} amu; anal. calcd for: Zn6P9B3O18C114H102=C, 55.58; H, 4.17%; found C, 55.32; H, 3.94%.
Syntheses and characterization of 5A
Zn11Et10O4(DPPA)4: DPPA-H (96.4 mg, 0.442 mmol) was placed in a Schlenk flask, with a stirrer bar. To this, toluene (10 ml) was added and ZnEt2 (150 mg, 1.21 mmol) was then added dropwise, while stirring. To this solution, water (7.95 μl, 0.442 mmol) was added, by Eppendorf syringe, under a stream of N2. The solution was stirred overnight, to ensure all water had been dissolved. Hexane was added and the product isolated as crystals (isolated yield=77 mg, 36%). 31P{1H} NMR (162 MHz, CDCl3): δ 33.1 (s, 4P) p.p.m.; (162 MHz, C6D6): δ 33.7 (s, 4P) p.p.m.; 1H NMR (400 MHz, CDCl3): δ –1.48 (q, CH2, JHH=8 Hz, 4H), −0.07 (dm, CH2*, 16H), 0.23 (q, CH3, JHH=8 Hz, 6H), 1.24 (t, CH3, JHH=8 Hz, 24H), 7.41 (m, DPPA, 8H), 7.50 (m, DPPA, 16H), 7.98 (m, DPPA, 16H) p.p.m.; anal. calcd for: Zn11P4O12C68H90=C, 42.05; H, 4.67%; found C, 42.11; H, 4.52%. *Symmetrical multiplet, suggesting diasterotopic Et CH2 protons. Both halves of multiplet couple to the CH3 signal (δ 1.24) in the 1H-1H correlation spectroscopy spectrum.
Syntheses and characterization of 5C
Zn11Et10O4(DOPA)4: (64.1 mg, 0.221 mmol) of dioctylphosphinic acid was placed in a Young's tap flask with a stirrer bar. To this, 10 ml of toluene was added and 75 mg (0.607 mmol) of ZnEt2 was then added dropwise, while stirring. To this solution, 3.9 μl (0.217 mmol) of water was added by Eppendorf syringe under a stream of N2. The solution was stirred for 1 h followed by brief sonication to ensure all water had been incorporated. The product was isolated by evacuation of the solvent to give an air-sensitive colourless oil. 31P{1H} NMR (162 MHz, CDCl3): δ 60.8 (s, 4P); (162 MHz, d8-toluene): δ 61.1 (s, 4P) (162 MHz, h8-toluene): δ 61.1 (s, 4P); 1H NMR (400 MHz, CDCl3): δ 0.14 (q, Et CH2, JHH=8 Hz, 16H), 0.45 (q, Et CH2, JHH=8 Hz, 4H), 0.89 (br, DOPA CH3, 24H), 1.14 (t, Et CH3, JHH=8 Hz, 24H), 1.2–1.9 (m, DOPA CH2, 112H); (400 MHz, h8-toluene): δ 0.72 (q, Et CH2, JHH=8 Hz, 16H), 0.91 (t, DOPA CH3, 24H), 0.95 (q, Et CH2, JHH=8 Hz, 4H), 1.29–1.52 (br, DOPA CH2, 112H), 1.67 (t, Et CH3, JHH=8 Hz, 24H), *CH3 signal for the minor ethyl group is not located, probably obscured.
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information or are available from the authors. The Crystallographic data have been deposited with the Cambridge Crystallographic Data Centre under CCDC 1432882–1432886. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Full bond length and bond angle data may be found in the CIFs, which are available as Supplementary Data 1–5.
Additional information
How to cite this article: Pike, S. D. et al. Simple phosphinate ligands access zinc clusters identified in the synthesis of zinc oxide nanoparticles. Nat. Commun. 7, 13008 doi: 10.1038/ncomms13008 (2016).
Supplementary Material
Supplementary Figures 1-73, Supplementary Tables 1-3, Supplementary Note 1, Supplementary Methods and Supplementary References.
crystallographic information files for structure 1A
crystallographic information files for structure 2B
crystallographic information files for structure 3A
crystallographic information files for structure 4A
crystallographic information files for structure 5A
Acknowledgments
The EPSRC are acknowledged for funding (EP/K035274/1, EP/M013839/1 and EP/H046380/1).
Footnotes
Author contributions S.D.P. conducted all experimental work. E.R.W. conducted all TEM characterization and associated analysis. S.D.P., M.S.P.S and C.K.W. conceived the experiments. All authors were involved in writing the manuscript.
References
- Cohn A. W., Kittilstved K. R. & Gamelin D. R. Tuning the potentials of “extra” electrons in colloidal n-type ZnO nanocrystals via Mg2+ substitution. J. Am. Chem. Soc. 134, 7937–7943 (2012). [DOI] [PubMed] [Google Scholar]
- Schrauben J. N. et al. Titanium and zinc oxide nanoparticles are proton-coupled electron transfer agents. Science 336, 1298–1301 (2012). [DOI] [PubMed] [Google Scholar]
- Valdez C. N., Braten M., Soria A., Gamelin D. R. & Mayer J. M. Effect of protons on the redox chemistry of colloidal zinc oxide nanocrystals. J. Am. Chem. Soc. 135, 8492–8495 (2013). [DOI] [PubMed] [Google Scholar]
- Noimark S. et al. Dual-mechanism antimicrobial polymer–ZnO nanoparticle and crystal violet-encapsulated silicone. Adv. Funct. Mater. 25, 1367–1373 (2015). [Google Scholar]
- Fortunato E., Barquinha P. & Martins R. Oxide semiconductor thin-film transistors: a review of recent advances. Adv. Mater. 24, 2945–2986 (2012). [DOI] [PubMed] [Google Scholar]
- Zhou Y., Eck M. & Krueger M. Bulk-heterojunction hybrid solar cells based on colloidal nanocrystals and conjugated polymers. Energy Environ. Sci. 3, 1851–1864 (2010). [Google Scholar]
- Schimpf A. M., Gunthardt C. E., Rinehart J. D., Mayer J. M. & Gamelin D. R. Controlling carrier densities in photochemically reduced colloidal ZnO nanocrystals: size dependence and role of the hole quencher. J. Am. Chem. Soc. 135, 16569–16577 (2013). [DOI] [PubMed] [Google Scholar]
- Behrens M. et al. The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts. Science 336, 893–897 (2012). [DOI] [PubMed] [Google Scholar]
- Boyle T. J. et al. Precursor structural influences on the final ZnO nanoparticle morphology from a novel family of structurally characterized zinc alkoxy alkyl precursors. Chem. Mater. 16, 3279–3288 (2004). [Google Scholar]
- Kahn M. L. et al. Size- and shape-control of crystalline zinc oxide nanoparticles: a new organometallic synthetic method. Adv. Funct. Mater. 15, 458–468 (2005). [Google Scholar]
- Lizandara-Pueyo C., van den Berg M. W. E., De Toni A., Goes T. & Polarz S. Nucleation and growth of ZnO in organic solvents - an in situ study. J. Am. Chem. Soc. 130, 16601–16610 (2008). [DOI] [PubMed] [Google Scholar]
- Orchard K. L., Shaffer M. S. P. & Williams C. K. Organometallic route to surface-modified ZnO nanoparticles suitable for in situ nanocomposite synthesis: bound carboxylate stoichiometry controls particle size or surface coverage. Chem. Mater. 24, 2443–2448 (2012). [Google Scholar]
- Heitz S., Aksu Y., Merschjann C. & Driess M. Unprecedented alkylzinc–magnesium alkoxide clusters as suitable organometallic precursors for magnesium-containing ZnO nanoparticles. Chem. Eur. J. 17, 3904–3910 (2011). [DOI] [PubMed] [Google Scholar]
- Cozzoli P. D., Kornowski A. & Weller H. Colloidal synthesis of organic-capped ZnO nanocrystals via a sequential reduction−oxidation reaction. J. Phys. Chem. B 109, 2638–2644 (2005). [DOI] [PubMed] [Google Scholar]
- Kim C. G., Sung K., Chung T. -M., Jung D. Y. & Kim Y. Monodispersed ZnO nanoparticles from a single molecular precursor. Chem. Commun. 2068–2069 (2003). [DOI] [PubMed] [Google Scholar]
- Coppel Y. et al. Full characterization of colloidal solutions of long-alkyl-chain-amine-stabilized ZnO nanoparticles by NMR spectroscopy: surface state, equilibria, and affinity. Chem. Eur. J. 18, 5384–5393 (2012). [DOI] [PubMed] [Google Scholar]
- Valdez C. N., Schimpf A. M., Gamelin D. R. & Mayer J. M. Low capping group surface density on zinc oxide nanocrystals. ACS Nano 8, 9463–9470 (2014). [DOI] [PubMed] [Google Scholar]
- Briois V. et al. Dynamical study of ZnO nanocrystal and Zn-HDS layered basic zinc acetate formation from sol−gel route. J. Phys. Chem. C 111, 3253–3258 (2007). [Google Scholar]
- Ludi B. & Niederberger M. Zinc oxide nanoparticles: chemical mechanisms and classical and non-classical crystallization. Dalton Trans. 42, 12554–12568 (2013). [DOI] [PubMed] [Google Scholar]
- Brown N. J., Weiner J., Hellgardt K., Shaffer M. S. P. & Williams C. K. Phosphinate stabilised ZnO and Cu colloidal nanocatalysts for CO2 hydrogenation to methanol. Chem. Commun. 49, 11074–11076 (2013). [DOI] [PubMed] [Google Scholar]
- Kango S. et al. Surface modification of inorganic nanoparticles for development of organic-inorganic nanocomposites-a review. Prog. Polym. Sci. 38, 1232–1261 (2013). [Google Scholar]
- Beek W. J. E., Slooff L. H., Wienk M. M., Kroon J. M. & Janssen R. A. J. Hybrid solar cells using a zinc oxide precursor and a conjugated polymer. Adv. Funct. Mater. 15, 1703–1707 (2005). [Google Scholar]
- Koster L. J. A. et al. Morphology and efficiency: the case of polymer/ZnO solar cells. Adv. Energy Mater. 3, 615–621 (2013). [Google Scholar]
- Sliem M. A. et al. Preparation, microstructure characterization and catalytic performance of Cu/ZnO and ZnO/Cu composite nanoparticles for liquid phase methanol synthesis. Phys. Chem. Chem. Phys. 14, 8170–8178 (2012). [DOI] [PubMed] [Google Scholar]
- Jadzinsky P. D., Calero G., Ackerson C. J., Bushnell D. A. & Kornberg R. D. Structure of a thiol monolayer-protected gold nanoparticle at 1.1Å resolution. Science 318, 430–433 (2007). [DOI] [PubMed] [Google Scholar]
- Freitag K. et al. The sigma-aromatic clusters Zn-3 (+) and Zn2Cu: embryonic brass. Angew. Chem. Int. Ed. 54, 4370–4374 (2015). [DOI] [PubMed] [Google Scholar]
- Cui P. et al. A multicentre-bonded Zn-I (8) cluster with cubic aromaticity. Nat. Commun. 6, 6331–6336 (2015). [DOI] [PubMed] [Google Scholar]
- Hill M. R., Russell J. J. & Lamb R. N. High-quality ZnxMg1−xO thin films deposited from a single molecular source. intimate mixing as a means to improved film properties. Chem. Mater. 20, 2461–2467 (2008). [Google Scholar]
- Deng H. X. et al. Multiple functional groups of varying ratios in metal-organic frameworks. Science 327, 846–850 (2010). [DOI] [PubMed] [Google Scholar]
- Mason J. A., Sumida K., Herm Z. R., Krishna R. & Long J. R. Evaluating metal-organic frameworks for post-combustion carbon dioxide capture via temperature swing adsorption. Energy Environ. Sci. 4, 3030–3040 (2011). [Google Scholar]
- Bury W., Justyniak I., Prochowicz D., Wrobel Z. & Lewinski J. Oxozinc carboxylates: a predesigned platform for modelling prototypical Zn-MOFs' reactivity toward water and donor solvents. Chem. Commun. 48, 7362–7364 (2012). [DOI] [PubMed] [Google Scholar]
- Yang J., Grzech A., Mulder F. M. & Dingemans T. J. Methyl modified MOF-5: a water stable hydrogen storage material. Chem. Commun. 47, 5244–5246 (2011). [DOI] [PubMed] [Google Scholar]
- Greathouse J. A. & Allendorf M. D. The interaction of water with MOF-5 simulated by molecular dynamics. J. Am. Chem. Soc. 128, 10678–10679 (2006). [DOI] [PubMed] [Google Scholar]
- Olmstead M. M., Power P. P. & Shoner S. C. Synthesis and characterization of novel quasiaromatic zinc sulfur aggregates and related zinc oxygen complexes. J. Am. Chem. Soc. 113, 3379–3385 (1991). [Google Scholar]
- Lewinski J. et al. Alkylzinc carboxylates as efficient precursors for zinc oxocarboxylates and sulfidocarboxylates. Angew. Chem. Int. Ed. 47, 573–576 (2008). [DOI] [PubMed] [Google Scholar]
- Polarz S. et al. A systematic study on zinc oxide materials containing group I metals (Li, Na, K)-synthesis from organometallic precursors, characterization, and properties. Chem. Mater. 21, 3889–3897 (2009). [Google Scholar]
- Orchard K. L., White A. J. P., Shaffer M. S. P. & Williams C. K. Pentanuclear complexes for a series of alkylzinc carboxylates. Organometallics 28, 5828–5832 (2009). [Google Scholar]
- Redshaw C. et al. Enhancement of H-2 uptake via fluorination but not lithiation for Zn4N8 and Zn4N6O type clusters. Chem. Commun. 46, 9055–9057 (2010). [DOI] [PubMed] [Google Scholar]
- Orchard K. L., Harris J. E., White A. J. P., Shaffer M. S. P. & Williams C. K. Solvent dependence of the structure of ethylzinc acetate and its application in CO(2)/epoxide copolymerization. Organometallics 30, 2223–2229 (2011). [Google Scholar]
- Lugmair C. G., Tilley T. D. & Rheingold A. L. Zinc di(tert-butyl)phosphate complexes as precursors to zinc phosphates. manipulation of zincophosphate structures. Chem. Mater. 9, 339–348 (1997). [Google Scholar]
- Yang Y., Pinkas J., Noltemeyer M., Schmidt H.-G. & Roesky H. W. [Zn2(thf)2(EtZn)6Zn4(μ4-O)(tBuPO3)8]: a dodecanuclear zincophosphonate aggregate with a Zn4(μ4-O) Core. Angew. Chem. Int. Ed. 38, 664–666 (1999). [DOI] [PubMed] [Google Scholar]
- Murugavel R., Walawalkar M. G., Dan M., Roesky H. W. & Rao C. N. R. Transformations of molecules and secondary building units to materials: a bottom-up approach. Acc. Chem. Res. 37, 763–774 (2004). [DOI] [PubMed] [Google Scholar]
- Coates G. E. & Ridley D. 341. Alkoxy-, thio-, and amino-derivatives of methylzinc. J. Chem. Soc. (Resumed) 1870–1877 (1965). [Google Scholar]
- Grala A. et al. Structural diversity of ethylzinc carboxylates. Organometallics 34, 4959–4964 (2015). [Google Scholar]
- Prochowicz D., Sokolowski K. & Lewinski J. Zinc hydroxides and oxides supported by organic ligands: synthesis and structural diversity. Coord. Chem. Rev. 270, 112–126 (2014). [Google Scholar]
- Looney A. et al. Monomeric alkyl and hydride derivatives of zinc supported by poly(pyrazoly)hydroborato ligation: synthetic, structural, and reactivity studies. Organometallics 14, 274–288 (1995). [Google Scholar]
- He C. & Lippard S. J. modeling carboxylate-bridged dinuclear active sites in metalloenzymes using a novel naphthyridine-based dinucleating ligand. J. Am. Chem. Soc. 122, 184–185 (2000). [Google Scholar]
- Kaminskaia N. V., He C. & Lippard S. J. Reactivity of μ-hydroxodizinc(II) centers in enzymatic catalysis through model studies. Inorg. Chem. 39, 3365–3373 (2000). [DOI] [PubMed] [Google Scholar]
- Schulz S., Spielmann J., Blaser D. & Wolper C. X-ray crystal structure of a heterobimetallic Al-Zn-oxide complex. Chem. Commun. 47, 2676–2678 (2011). [DOI] [PubMed] [Google Scholar]
- Bergquist C. et al. Factors influencing the thermodynamics of zinc alkoxide formation by alcoholysis of the terminal hydroxide complex, Tp(But,Me) ZnOH: an experimental and theoretical study relevant to the mechanism of action of liver alcohol dehydrogenase. J. Am. Chem. Soc. 122, 12651–12658 (2000). [Google Scholar]
- Bury W. et al. tert-Butylzinc hydroxide as an efficient predesigned precursor of ZnO nanoparticles. Chem. Commun. 47, 5467–5469 (2011). [DOI] [PubMed] [Google Scholar]
- Cadenbach T. et al. Macrocyclic platforms for the construction of tetranuclear oxo and hydroxo zinc clusters. Organometallics 34, 2608–2613 (2015). [Google Scholar]
- Reger D. L., Pascui A. E., Pellechia P. J. & Smith M. D. Zinc(II) and cadmium(II) monohydroxide bridged, dinuclear metallacycles: a unique case of concerted double berry pseudorotation. Inorg. Chem. 52, 11638–11649 (2013). [DOI] [PubMed] [Google Scholar]
- Sokołowski K. et al. tert-Butyl(tert-butoxy)zinc hydroxides: hybrid models for single-source precursors of ZnO nanocrystals. Chem. Eur. J. 21, 5488–5495 (2015). [DOI] [PubMed] [Google Scholar]
- Duchateau R. Incompletely condensed silsesquioxanes: versatile tools in developing silica-supported olefin polymerization catalysts. Chem. Rev. 102, 3525–3542 (2002). [DOI] [PubMed] [Google Scholar]
- Beckett M. A. et al. π-Bonding in B–O ring species: Lewis acidity of Me3B3O3, synthesis of amine Me3B3O3 adducts, and the crystal and molecular structure of Me3B3O3.NH2iBu·MeB(OH)2. J. Organomet. Chem. 585, 7–11 (1999). [Google Scholar]
- Pagès C., Coppel Y., Kahn M. L., Maisonnat A. & Chaudret B. Self-assembly of ZnO nanocrystals in colloidal solutions. ChemPhysChem 10, 2334–2344 (2009). [DOI] [PubMed] [Google Scholar]
- van Craeynest F., Maenhout-Van Der Vorst W. & Dekeyser W. Interpretation of the yellow colour of heat treated ZnO powder. Phys. Stat. Solid. B 8, 841–846 (1965). [Google Scholar]
- Meulenkamp E. A. Synthesis and growth of ZnO nanoparticles. J. Phys. Chem. B 102, 5566–5572 (1998). [Google Scholar]
- Spanhel L. Colloidal ZnO nanostructures and functional coatings: a survey. J. Sol-Gel Sci. Technol. 39, 7–24 (2006). [Google Scholar]
- Na K., Zhang Q. & Somorjai G. Colloidal metal nanocatalysts: synthesis, characterization, and catalytic applications. J. Clust. Sci. 25, 83–114 (2014). [Google Scholar]
- Viswanatha R. & Sarma D. D. in Nanomaterials Chemistry 139–170Wiley-VCH Verlag GmbH & Co. KGaA (2007). [Google Scholar]
- Bullen C. R. & Mulvaney P. Nucleation and growth kinetics of CdSe nanocrystals in octadecene. Nano Lett. 4, 2303–2307 (2004). [Google Scholar]
- Kim B. H., Hackett M. J., Park J. & Hyeon T. Synthesis, characterization, and application of ultrasmall nanoparticles. Chem. Mater. 26, 59–71 (2014). [Google Scholar]
- Park J. et al. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 3, 891–895 (2004). [DOI] [PubMed] [Google Scholar]
- Barriere C. et al. Ligand effects on the air stability of copper nanoparticles obtained from organometallic synthesis. J. Mater. Chem. 22, 2279–2285 (2012). [Google Scholar]
- Mocatta D. et al. Heavily doped semiconductor nanocrystal quantum dots. Science 332, 77–81 (2011). [DOI] [PubMed] [Google Scholar]
- Schwartz D. A., Norberg N. S., Nguyen Q. P., Parker J. M. & Gamelin D. R. Magnetic quantum dots: synthesis, spectroscopy, and magnetism of Co2+- and Ni2+-doped ZnO nanocrystals. J. Am. Chem. Soc. 125, 13205–13218 (2003). [DOI] [PubMed] [Google Scholar]
- Centrone A. et al. The role of nanostructure in the wetting behavior of mixed-monolayer-protected metal nanoparticles. Proc. Natl Acad. Sci. USA 105, 9886–9891 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Hu Y. & Stellacci F. Mixed-ligand nanoparticles as supramolecular receptors. Small 7, 1961–1966 (2011). [DOI] [PubMed] [Google Scholar]
- Wang F., Tang R. & Buhro W. E. The trouble with TOPO; identification of adventitious impurities beneficial to the growth of cadmium selenide quantum dots, rods, and wires. Nano Lett. 8, 3521–3524 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-73, Supplementary Tables 1-3, Supplementary Note 1, Supplementary Methods and Supplementary References.
crystallographic information files for structure 1A
crystallographic information files for structure 2B
crystallographic information files for structure 3A
crystallographic information files for structure 4A
crystallographic information files for structure 5A
Data Availability Statement
The data supporting the findings of this study are available within the article and its Supplementary Information or are available from the authors. The Crystallographic data have been deposited with the Cambridge Crystallographic Data Centre under CCDC 1432882–1432886. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Full bond length and bond angle data may be found in the CIFs, which are available as Supplementary Data 1–5.