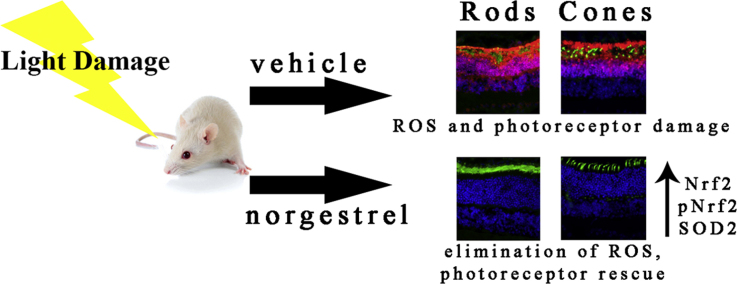
Abstract
Retinitis pigmentosa (RP) is one of the most common retinal degenerative conditions affecting people worldwide, and is currently incurable. It is characterized by the progressive loss of photoreceptors, in which the death of rod cells leads to the secondary death of cone cells; the cause of eventual blindness. As rod cells die, retinal-oxygen metabolism becomes perturbed, leading to increased levels of reactive oxygen species (ROS) and thus oxidative stress; a key factor in the secondary death of cones. In this study, norgestrel, an FDA-approved synthetic analog of progesterone, was found to be a powerful neuroprotective antioxidant, preventing light-induced ROS in photoreceptor cells, and subsequent cell death. Norgestrel also prevented light-induced photoreceptor morphological changes that were associated with ROS production, and that are characteristic of RP. Further investigation showed that norgestrel acts via post-translational modulation of the major antioxidant transcription factor Nrf2; bringing about its phosphorylation, subsequent nuclear translocation, and increased levels of its effector protein superoxide dismutase 2 (SOD2). In summary, these results demonstrate significant protection of photoreceptor cells from oxidative stress, and underscore the potential of norgestrel as a therapeutic option for RP.
Keywords: Retinitis pigmentosa, Photoreceptors, ROS, Nrf2, Norgestrel
Graphical abstract
Highlights
-
•
Norgestrel rescues photoreceptors from structural changes that are characteristic of RP.
-
•
Norgestrel reduces mitochondrial-derived ROS.
-
•
Norgestrel brings about an increase in SOD2 within the photoreceptors.
-
•
Norgestrel brings about increased expression and activation of Nrf2.
1. Introduction
Retinitis pigmentosa (RP) is a group of highly heterogeneous inherited retinal diseases, all of which comprise progressive death of rod and cone photoreceptors via several different signaling cascades, including apoptosis, autophagy and necrosis [1], [2]. Currently, disease causing-mutations have been identified in 60 rod photoreceptor-genes [The Retinal Information Network (RetNet), https://sph.uth.edu/retnet/, July 2016]. Despite the various genes involved, all cases of RP are characterized by death of rod photoreceptors followed by secondary gradual death of cone cells, which eventually leads to total blindness [3], [4]. Oxidative stress has been shown to be a key contributor to the progression of RP and many other retinopathies [5], [6], [7], [8], owing to the fact that photoreceptors are the most metabolically active cells in the body, consuming more oxygen per gram than any other tissue [9]. Many studies both in vivo and in vitro suggest a key reason for secondary-cone cell death in RP is oxidative damage due to the increasing oxygen levels, and thus reactive oxygen species (ROS), as rod cells die [6], [10], [11], [12], [13], [14], [15]. On top of this, it has been demonstrated that ROS produced due to light exposure can potentiate the degeneration of the retina in inherited and age-related retinopathies, including RP [16], [17], [18], [19], [20].
One of the primary cellular responses to oxidative stress is activation of the ubiquitously expressed basic-leucine-zipper transcription factor NF-E2-related factor-2 (Nrf2) [21], [22]. Upon liberation from its repressor, kelch-like ECH-associated protein 1 (KEAP1), Nrf2 translocates into the nucleus where it binds antioxidant response elements (AREs), bringing about the up-regulation of various antioxidant, detoxifying and cytoprotective genes [22], [23], [24], [25], [26]. Studies investigating the anti-oxidant effects of Nrf2 in the retina have demonstrated its importance in protecting retinal ganglion cells following optic nerve crush [27], and in slowing the progression of diabetic retinopathy [28]. Although few studies have focused on RP, a recent investigation by Nakagami and colleagues using a rabbit model demonstrated how activation of Nrf2 and upregulation of its effector proteins delayed the loss of photoreceptor cells [29].
Nrf2 can be regulated by various signaling molecules including progesterone, a pleiotropic neuroprotective hormone. It has also been shown that progestin, as part of hormone replacement therapy (HRT), increases the expression of the Nrf2-regulated superoxide dismutase antioxidant enzymes (SODs) [30], [31], while in the heart, progesterone upregulates the Nrf2 effector NAD(P)H quinone oxidoreductase 1 (NQO1) [32]. Progesterone also exerts antioxidant effects in experimental models of traumatic brain injury, which include increasing SOD activity [33], [34], [35].
We have previously demonstrated the neuroprotective effects of norgestrel, a synthetic analog of progesterone, in both the inherited RP model, and the light-damage model of retinal degeneration. Norgestrel rescues photoreceptors from cell death via a mechanism which includes upregulation of the neurotrophic factors bFGF and LIF [36], [37]. Here, we sought to investigate if the activities of norgestrel in light-induced retinal degeneration include modulation of the redox environment. We provide evidence that norgestrel increases expression and activation of Nrf2 via phosphorylation on serine 40, increases expression of its target antioxidant superoxide dismutase 2 (SOD2), and reduces mitochondrial oxidative stress.
2. Material and methods
2.1. Mice
All animals were maintained and handled in accordance with the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research. Experiments were performed on balb/c mice obtained from Harlan Olac (Bicester, UK). Mice were euthanized by cervical dislocation. All procedures were approved by the Health Products Regulatory Authority (HPRA), Ireland.
2.2. Light damage model
The light-damage model was performed as previously described [37]. Briefly; Balb/c mice were born into and maintained in dim cyclic light less than 10 lx (12 h on/12 h off). At 4–7 weeks of age, mice were dark adapted for 18 h prior to exposure to damaging light. Mice received intraperitoneal injections (i.p.) of 50 μL vehicle (25 μL dimethylsulfoxide/25 μL peanut oil) or 50 μL norgestrel (100 mg/kg), 1 h prior to light-damage. Immediately prior to light-damage, their pupils were dilated with 0.5% cyclopentolate under red light. Retinal light-damage was then induced by exposure to cool white fluorescent light (5000 lx) for 2 h. Following this, the mice were placed in the dark for 6, 24 or 48 h, as indicated, prior to euthanasia.
2.3. Eye sections preparation
Enucleated eyes were fixed in 4% paraformaldehyde (PFA) for 1.5 h followed by cryo-protection in 30% sucrose at 4 °C overnight. Eyes were then frozen in Shandon™ Cyrochrome™ (ThermoFisher Scientific) and 7 µm sections were then cut using a cryostat (Leica CM1950; Leica Co., Meath, Ireland). Sections were stored at −80 °C until use.
2.4. Microscopy
Retinal sections were viewed using a Leica DM LB2 microscope with Nikon Digital Sight DS-U2 camera, using 40× and 100× objectives. Images were taken using the software NIS-Elements version 3.0, Nikon, Japan. Immunofluorescence on retinal sections was performed on at least three mice of each group, at each time-point. Identical microscope settings were used when obtaining images across all timepoints and treatments. Images shown are those taken of the central retina.
2.5. Quantification from immunohistochemically-labeled sections
Quantification of outer nuclear layer (ONL) thickness and fluorescence intensity measurements were carried out using ImageJ software, as previously described [38], [39]. Average ONL thickness was measured by taking measurements from at least 20 sections per mouse. Per section, three distinct measurements were taken and averaged. Nrf2 and SOD2 fluorescence intensity measurements were taken from the same region and in the same orientation, at 40× magnification. These images were used to create plot profiles of the marker of interest and the area under the curve was measured as a readout of fluorescence intensity.
2.6. Apoptosis detection by terminal deoxynucleotidyl transferase-mediated biotinylated dUTP nick end labeling (TUNEL)
DNA strand breaks in photoreceptor nuclei were detected by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL). Frozen sections (7 µm) were incubated with terminal deoxynucleotidyl transferase (Promega, MyBio Ltd., Kilkenny, Republic of Ireland) and fluorescein-12-dUTP (Roche, Lewes, UK) according to manufacturers’ instructions at 37 °C for 1 h. Sections were counter-stained with Hoechst (1 μg/mL) to visualize the nuclei, mounted and viewed under a fluorescence microscope (Leica DM LB2; Leica, Nussloch, Germany).
2.7. Immunofluorescent staining
Frozen sections were brought to room temperature, rehydrated in PBS (137 mM NaCl, 2.7 mM KCL, 10 mM NaPO4, 1.8 mM KH2PO4), permeabilized in PBS with 5% donkey serum and 0.1% Triton X-100 for 30 min at room temperature, followed by blocking in PBS with 5% donkey serum. Subsequently, sections were incubated with primary antibody or lectin solutions overnight at 4 °C (Table 1). Antibody binding was detected by using conjugated secondary antibodies (Alexa Fluor 488; Invitrogen, Carlsbad, CA) at a dilution of 1/500 for 1 h at room temperature. Sections incubated with secondary antibody-only confirmed positive immunolabelling with primary antibodies (data not shown). Hoechst 33,342 (1 μg/mL; Sigma-Aldrich) was used to counterstain the nuclei. Sections were mounted with Mowiol mounting medium (Sigma-Aldrich) and viewed under a fluorescence microscope (Leica DM LB2; Leica).
Table 1.
primary antibodies/lectins used.
| Antibody/Lectin | Supplier | Catalogue # | Dilution/Concentration |
|---|---|---|---|
| PNA-FITC | Vector Laboratories | FL-1071 | 1:500 |
| Rhodopsin | EMDMillipore | AB9279 | 1:200 |
| Nrf2 | Abcam | ab31136 | 1:200 IF |
| 1:1000 western blot | |||
| SOD2 | Abcam | ab13553 | 1:200 |
| SOD1 | Abcam | ab13498 | 1:200 |
| phospho-Nrf2 (S40) | Abcam | Ab76026 | 1:100 IF |
| 1:5000 western blot | |||
| GAPDH | Cell Signaling | 5174 | 1:1000 |
| Histone H3 | Abcam | Ab12079 | 1 µg/mL |
2.8. Assessment of ROS using dihydroethidium (DHE)
Intracellular ROS were evaluated in situ using dihydroethidium (DHE), as previously described [40], [41], [42]. Once in contact with superoxide radicals, DHE is converted to ethidium which intercalates with DNA and emits red fluorescence at approximately 600 nm. Following light-damage, mice received two intraperitoneal injections of 20 mg/kg DHE (Molecular Probes, Life Technologies), 30 min apart. Injections were carried out 3.5/4 h prior to euthanasia, using as little light as possible. Mice were then returned to the dark until euthanized; at 6, 24 or 48 h post-light-damage. Eyes were enucleated, fixed, cryo-sectioned and re-hydrated as described above, followed by counter-staining with primary antibody or TUNEL, and Hoechst; as described in Immunofluorescent Staining above. DHE staining was examined in the red channel; excitation: 543 nm, emission>590 nm.
2.9. Flow cytometry
2.9.1. Single cell suspension preparation and assessment of cell viability
Following euthanasia, the retinas from enucleated eyes were dissected in cold Dulbecco's modified Eagle medium (DMEM) under as little light as possible. Single-cell suspensions (SCSs) were made by digesting each retina in 2.5 mL of Trypsin-EDTA solution (0.25%) containing 50 μL deoxyribonuclease II (DNaseII) (10,000 units/mL) from bovine spleen (both Sigma, Arklow, Ireland) for 15 min at 37 °C. This solution was then decanted, and 2.5 mL DMEM containing 50 μL DNase II was added to retinas. Homogenization was performed by pipetting up and down 10 times using a Pasteur pipette. Large debris was allowed to settle, and 2 mL of single-cell suspension were collected and placed into a FACs tube (BD Biosciences) to be loaded with MitoSox. The cell viability of the SCSs was assessed by tryphan blue-exclusion assay (Sigma, cat.# 15250061) using a haemocytometer.
2.9.2. Assessment of SCS quality
To verify the SCSs comprised intact rather than fragmented cells, cytospin preparations were made using 200 μL of each SCS with a Shandon CytoSpin 11 Cytocentrifuge (Shandon). Cytospins were then incubated with rhodopsin antibody (Table 1) and Hoechst 33,342 (1 μg/mL; Sigma-Aldrich) as described above in Immunofluorescent staining.
2.9.3. MitoSox assessment
Mitochondrial ROS levels in light-damaged retinas were assessed using the intracellular mitochondrial superoxide probe, MitoSox (Molecular probes, Life Technologies). Single cell suspensions were loaded with 5 µM MitoSox for 15 min at 37 °C, and fluorescence was measured using a Becton-Dickinson FACScan flow cytometer. MitoSOX Red was excited by laser at 488 nm, and the data collected at FSC, SSC, 670LP (FL3) channel. 10,000 events were counted in the gated photoreceptor region. Cell debris, as represented by distinct low forward and side scatter, were gated out for analysis. Results were analyzed using FlowJo software (Trustees of Leland Stanford Jr. University).
2.10. RNA isolation, cDNA synthesis, and real-time PCR
Total RNA (tRNA) isolation from retinas was carried out using an RNeasy Midi Kit (Qiagen) according to manufacturer's instruction, including DNase treatment to digest residual genomic DNA. Retinas were homogenized using a pellet pestle cordless motor (Sigma, cat# Z359971). RNA was reverse transcribed using the QuantiTect Reverse Transcription Kit (Qiagen). Real time PCR (rtPCR) was performed using murine QuantiTect Primer Assays (Qiagen) and SYBR Green JumpStart Taq ReadyMix (Sigma-Aldrich) in an ABI Prism 7900HT Sequence Detection System (Applied Biosciences). mRNA values were normalized to the geometric mean of three endogenous reference genes; βactin, gapdh and hprt. Relative changes in gene expression were quantified using the comparative Ct (ΔΔCt) method as described by Livak and Schmittgen [43].
2.11. Western blotting
Subcellular protein fractionation was carried out on snap-frozen retinas using a tissue-specific kit (Thermo Scientific, cat# 87790). 100 mg of retinas (~4 retinas) from each group and each time point were pooled and homogenized using a pellet pestles cordless motor (Sigma, cat# Z359971). Cellular fractions were prepared according to kit instructions, with replacement of the Halt™ Protease Inhibitor Cocktail (included in the kit), with Halt™ Protease and Phosphatase Inhibitor Cocktail (Sigma, cat~78440). Total protein concentration of each fraction was determined by Bradford assay, using BSA as standard. Equivalent amounts of protein were resolved on sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) in 4X Protein Sample Loading Buffer (LI-COR, cat# P/N 928-40004) and then transferred onto nitrocellulose membranes (Schleicher & Schuell, Whatman, Dassel, Germany). Membranes were blocked with Odyssey TBS Blocking Buffer (LI-COR, cat# P/N 927–50000) for 1 h at RT and then incubated at 4 °C overnight with primary antibody (Table 1). Membranes were subsequently washed three times for 5 min in Tris-buffered saline/0.1% Tween-20 (TBST) before adding appropriate Alexa Fluor fluorescent secondary antibodies diluted 1:10,000, in Odyssey TBS Blocking Buffer/TBST solution. Blots were scanned using the Odyssey Infrared Imaging System (LI-COR Biosciences, UK) for fluorescent detection of the secondary antibodies. Fluorescence signal intensity was quantified using Image Studio Lite software (LI-COR Biosciences, UK).
2.12. Statistical analyses
Student's t-test assuming unequal variances was used to determine whether there was a significant difference between the two sample means.
3. Results
3.1. Norgestrel rescues photoreceptor cells from light-induced ROS production and subsequent cell death
We have previously described how norgestrel protects photoreceptor cells from light-induced cell death [37]. To investigate whether norgestrel also reduces light-induced ROS, vehicle or norgestrel treated mice were light-damaged (LD) and subsequently given intraperitoneal injections of dihydroethidium (DHE). DHE is a sensitive indicator of ROS in situ. It is a non-specific superoxide marker, which, once in the presence of ROS, is converted to ethidium bromide and binds DNA, emitting a red fluorescence.
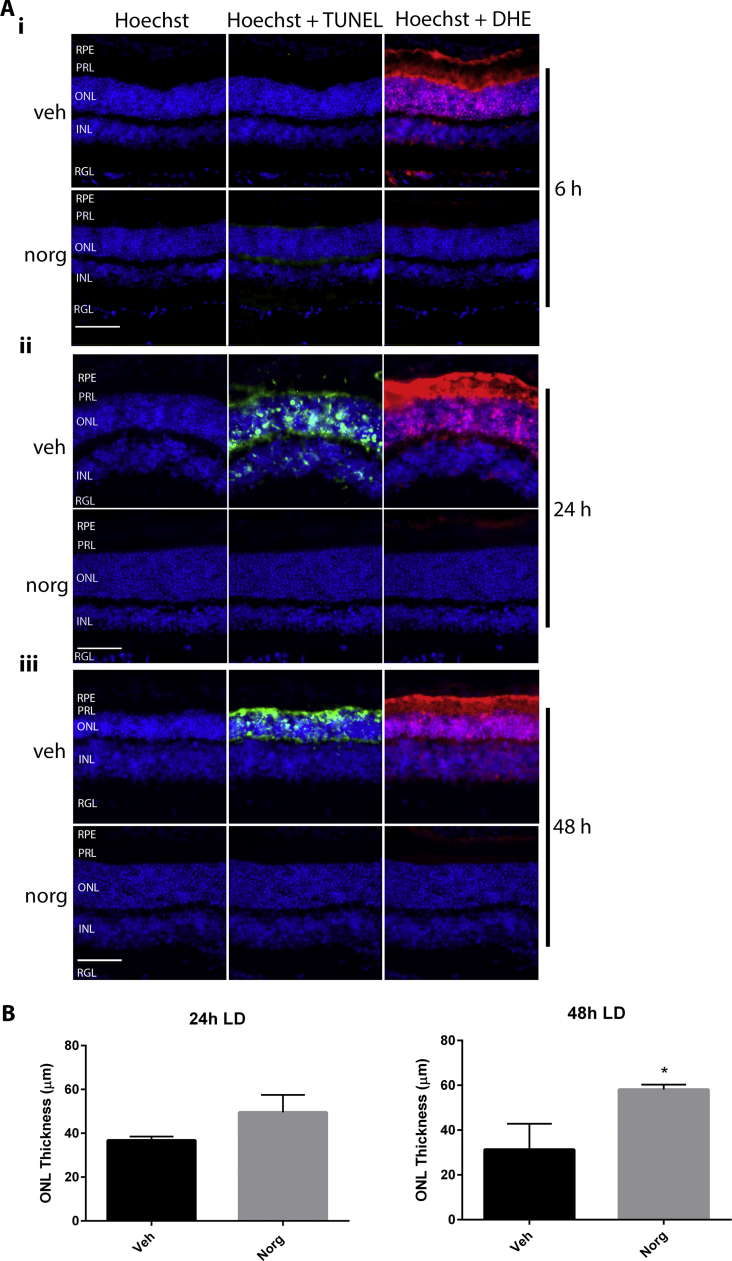
At 6 h (Fig. 1Ai), 24 h (Fig. 1Aii) and 48 h (Fig. 1Aiii) post-LD, vehicle (veh) treated mice are positive for DHE fluorescence, indicative of ROS production. DHE is visible in the photoreceptor layer (PRL), which houses the inner and outer segments of the rods and cones, and the outer nuclear layer (ONL), which comprises the cell bodies of rods and cones. At all time-points, norgestrel (norg) inhibits DHE. Here, and in subsequent figures, DHE fluorescence is also observed within the retinal pigment epithelium (RPE), which is sensitive to light-induced oxidative stress [44]. TUNEL within the ONL shows photoreceptor cell death in vehicle treated mice at 24 h and 48 h post-LD, which is also inhibited by norgestrel, as expected. The ONL is visibly thinner in vehicle treated mice 24 h and 48 h after LD, as assessed by Hoechst staining of cell nuclei. ONL thickness was measured to quantify the reduction in cell death due to norgestrel (Fig. 2B).
Fig. 1.
Norgestrel prevents light-induced ROS production and subsequent cell death. Balb/c mice were given intraperitoneal injections of vehicle (veh) or vehicle containing 100 mg/kg norgestrel (norg) 1 h prior to light damage (LD) and were euthanized at 6 h, 24 h or 48 h post-LD. Approximately 4 h before euthanasia, mice received two intraperitoneal injections of 20 mg/kg dihydroethidine (DHE), 30 min apart. Ocular sections were prepared and assessed by microscopy as described in Methods. A; DHE fluorescence (red), indicative of ROS production, and TUNEL staining (green), indicative of cell death, were assessed in the retinas of mice treated with vehicle (veh) or norgestrel (norg) at 6 h (Ai), 24 h (Aii) and 48 h (Aiii) post-LD. Hoechst staining of retinal nuclei allows orientation of retinal layers, and shows changes in the thickness of the ONL following LD. B; graphical representation of ONL thickness at 24 and 48 h post-LD in vehicle (veh) or norgestrel (norg) treated mice. RPE; retinal pigment epithelium, PRL; Photoreceptor layer, ONL; outer nuclear layer, INL; inner nuclear layer, RGL; retinal ganglion cell layer. Images are representative of at least n=3. Error bars denote SEM from three independent experiments Scale bar=50 µm. *p=<0.05. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
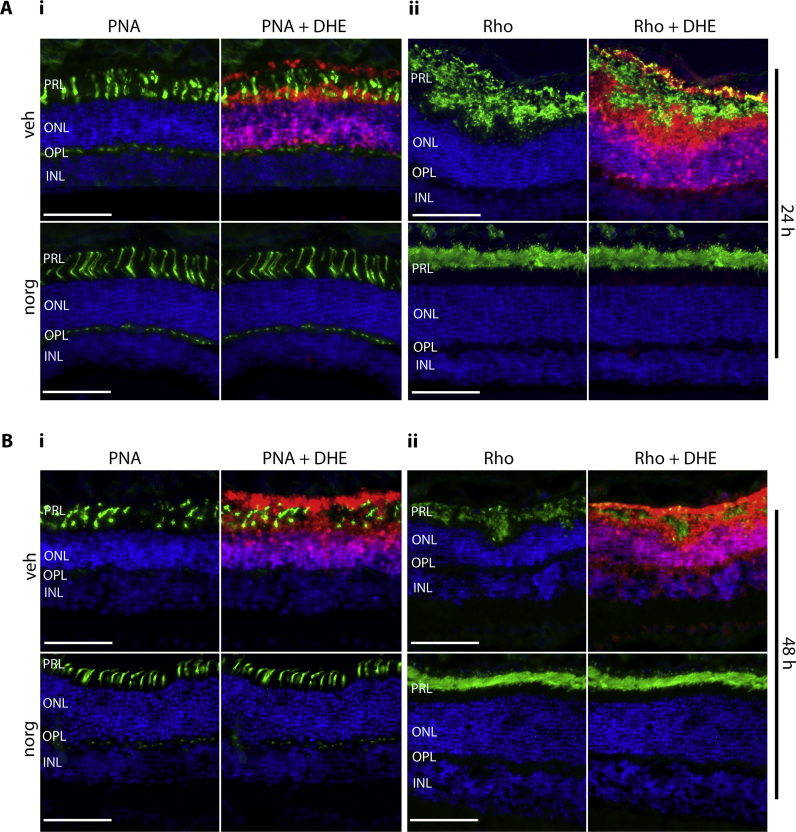
Norgestrel rescues photoreceptors from light-induced structural damage. Balb/c mice were given intraperitoneal injections of vehicle (veh) or vehicle containing 100 mg/kg norgestrel (norg) 1 h prior to light damage (LD) and were euthanized at 24 h or 48 h post-LD. Approximately 4 h before being euthanasia, mice received two intraperitoneal injections of 20 mg/kg dihydroethidine (DHE), 30 min apart. Ocular sections were obtained and assessed by microscopy as described in Methods. A, B; the effect of LD on photoreceptor morphology was assessed in retinas of mice treated with vehicle (veh) or norgestrel (norg) and euthanized 24 (A) or 48 h (B) post-LD. Cone morphology was assessed by peanut agglutinin (PNA) binding (Ai, Bi). Rod morphology was assessed by rhodopsin staining (Aii, Bii). DHE counter-fluorescence (red) is also shown. PRL; Photoreceptor layer, ONL; outer nuclear layer, OPL; outer plexiform layer, INL; inner nuclear layer. Images are representative of at least n=3. Scale bar=50 µm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Norgestrel rescues light-induced photoreceptor morphological damage
We have previously shown that norgestrel preserves photoreceptor morphology in the rd10 model of RP, which translates into preservation of function as measured by electroretinograms [37]. Here, we sought to investigate if light-damage (LD) induces structural damage to the photoreceptors similar to the disease model, whether this correlates with ROS production, and whether norgestrel can prevent such damage. The morphology of the photoreceptors was investigated at 24 and 48 h post-LD (Fig. 2); the times at which cell death is observed (Fig. 1). Rod morphology was assessed by immunofluorescent staining with anti-rhodopsin (Rho), while cone morphology was assessed by staining with the lectin peanut agglutinin (PNA). PNA binds specifically to carbohydrates on the plasma membranes of cone inner and outer segments, as well as their synaptic pedicles that reside within the outer plexiform layer (OPL) [45].
LD markedly reduced photoreceptor cell density in vehicle (veh) treated mice compared to those that received norgestrel (norg), with the remaining photoreceptor layer displaying abnormalities in length and structure (Fig. 2Ai, ii and Bi, ii, upper panels). By 24 h post-LD, cone cells had begun breaking down in vehicle treated mice, becoming fragmented and swollen in appearance (Fig. 2Ai, upper panels), a phenomenon shown to occur in the rd10 model of RP [46]. By 48 h, this aberrant morphology was more advanced, with no synaptic pedicles visible in the OPL (Fig. 2Bi, upper panels). In contrast, cone cell structure was preserved following LD in norgestrel treated mice, with cells retaining their characteristic neuronal morphology (Fig. 2Ai, Bi, lower panels).
At 24 h post-LD rod cell morphology appears more abnormal than that of cones in vehicle treated mice (Fig. 2Aii, upper panels), in accordance with the disease model of RP where cone cell death is secondary to that of rods [3], [4]. Rhodopsin is expressed in rod outer segments, but immunoreactivity appears closer to the ONL in vehicle compared to norgestrel treated mice, (Fig. 2Aii, Bii, top panels). This suggests that rhodopsin has de-localized to the inner segments, a phenomenon also observed during retinal degeneration in inherited RP models [47]. By 48 h, rhodopsin immunoreactivity is reduced in vehicle compared to norgestrel treated mice (Fig. 2Bii), as rod cell death increases. At both time-points, norgestrel restores rod morphology; with uniform rhodopsin staining that is localized further from the ONL (Fig. 2Aii, Bii, lower panels). Counter-staining of DHE with PNA or rhodopsin illustrates that ROS production correlates with morphological changes to the photoreceptors.
3.3. Norgestrel reduces light-induced mitochondrial ROS
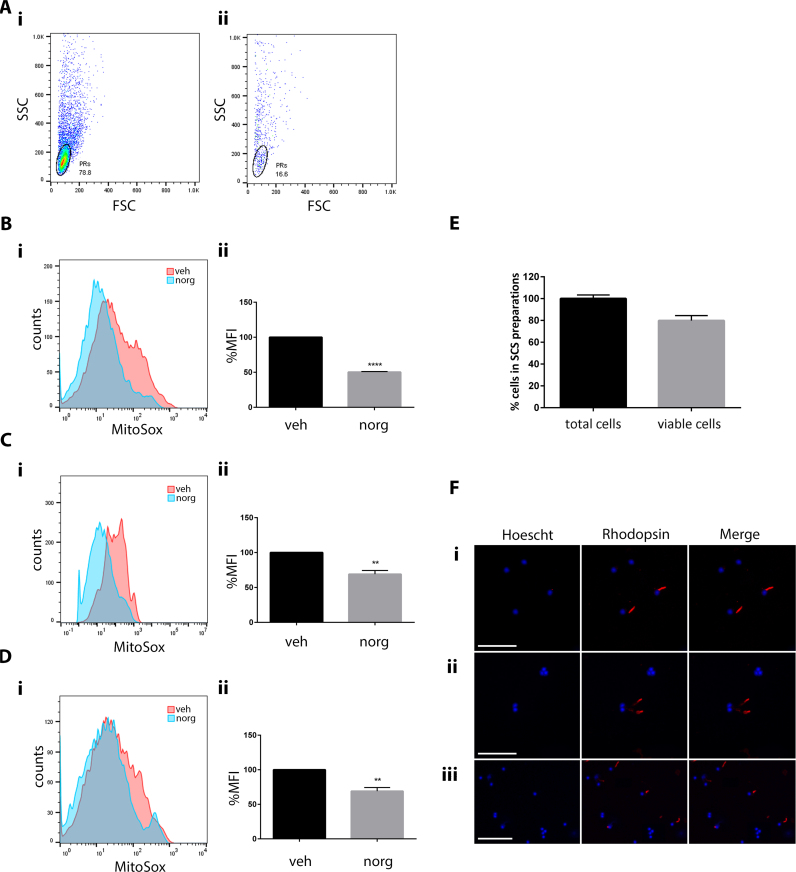
Photoreceptors have a densely packed population of mitochondria within their inner segments, and as such have a high metabolic demand [48]. This demand is met by mitochondrial oxidative phosphorylation (OXPHOS), perturbation of which generates excess levels of ROS and is implicated in various neurodegenerative diseases, including RP [49]. To determine if norgestrel can reduce light-induced mitochondrial-specific ROS, flow cytometry was carried out using single-cell suspensions (SCSs) from retinas of light-damaged (LD) mice loaded with the fluorescent probe MitoSox (Fig. 3). MitoSox is a modified derivative of DHE, which is targeted specifically to the mitochondria [50]. Upon oxidation, MitoSox is converted to mito-2-hydroethidium, which binds mitochondrial DNA and emits a red fluorescence [51]. Unlike DHE, MitoSox cannot be administered to live animals, hence this approach was taken. Initially, the photoreceptor population was identified by carrying out flow cytometry on retinas of untreated LD mice (48 h post-LD) compared with untreated mice which were not subjected to LD (healthy) (Fig. 3A). 10,000 events were counted in both samples. The population present in the healthy cells (78.8%) (figure Ai), that had reduced (16.6%) in the LD mice (figure Aii), was deemed to be photoreceptors based on the results in Fig. 1, in which cell death is exclusively visualized within the ONL by TUNEL assay. This region was gated in all subsequent experiments. Following LD, MitoSox fluorescence is reduced in norgestrel treated mice at 6 h (Fig. 3Bi), 24 h (Fig. 3Ci) and 48 h (Fig. 3Di) as represented by histogram overlays. Taking all experiments, the median fluorescence intensity of MitoSox was significantly different between vehicle and norgestrel treated mice at 6 h; p=<0.001 (Fig. 3Bii), 24 h; p=<0.0042 (Fig. 3Cii) and 48 h p=<0.0044 (Fig. 3Dii).
Fig. 3.
Norgestrel decreases light-induced mitochondrial ROS. Balb/c mice were given intraperitoneal injections of vehicle (veh) or vehicle containing 100 mg/kg norgestrel (norg) 1 h prior to light damage (LD) and were euthanized at 6 h, 24 h or 48 h post-LD. Retinas were removed from the eyes, digested and homogenized into single-cell suspensions (SCSs) and loaded with MitoSox, as described in Methods. MitoSox fluorescence was assessed by flow cytometry. A; initially, the photoreceptor population was identified by counting 10,000 events in SCSs from un-treated healthy mice (i) or un-treated mice 48 h after being subjected to LD (ii). Forward scatter (FSC) plotted against side scatter (SSC) shows the photoreceptor (PR) cell population in healthy mice (78.8%) (Ai) is dramatically reduced (16.6%) in the retinas of LD mice (Aii). B (6 h); C (24 h); D (48 h); representative histogram overlays show MitoSox fluorescence in norgestrel (norg) treated mice compared to vehicle (veh) (i). Graphical representations of the median fluorescence intensity (MFI) of MitoSox in vehicle (veh) or norgestrel (norg) treated mice (ii). E; cell viability of the SCSs was measured by tryphan blue-exclusion assay. F; cytospin preparations of SCSs from healthy mice were assessed for rhodopsin immunoreactivity and visualized at 40× magnification (i, ii) (scale bar=50 µm) or 10× magnification (iii) (scale bar=25 µm). All results are representative of at least n=3. **p=<0.01, ***p=<0.001. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
To ensure the retinal cells remained viable following the SCS preparation tryphan blue-exclusion assays were performed. This test is based on the concept that viable healthy cells do not take up impermeable dyes, whereas dead or compromised cells do. Cell viability was found to be approximately 80% (Fig. 3E).
To further validate the quality of the SCS preparations, and to ensure cells remain intact without becoming fragmented, cytospins were made using SCSs from retinas of healthy mice. Rhodopsin immunoreactivity merged with Hoechst staining verified the photoreceptor cells were indeed intact (Fig. 3Fi–iii).
3.4. Norgestrel increases the expression of Nrf2 and SOD2, but not SOD1
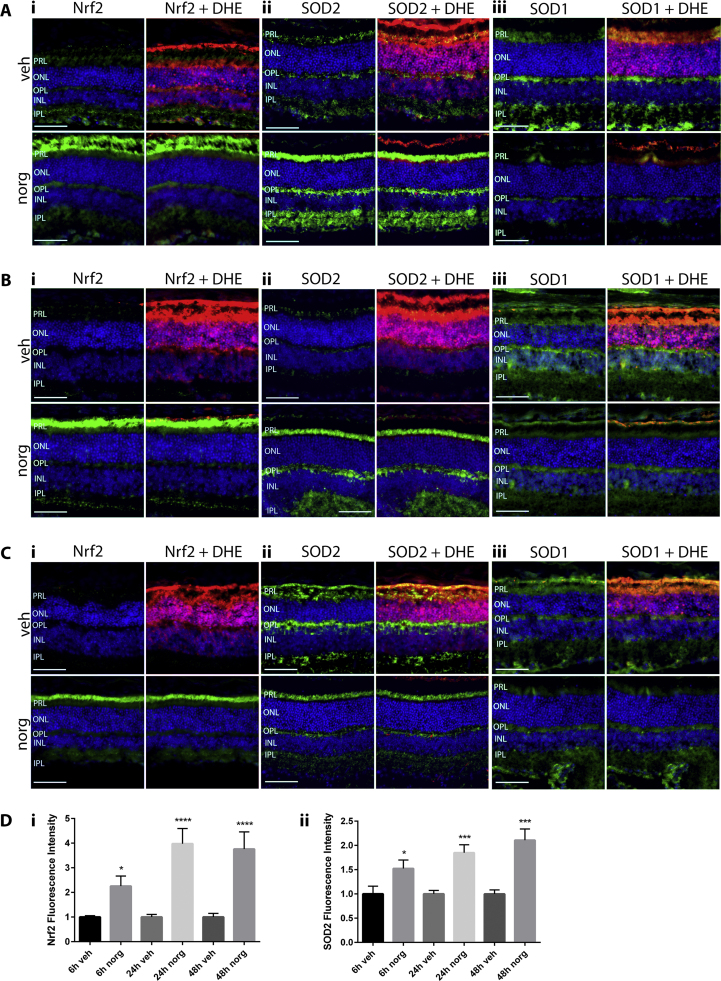
We next sought to investigate if the antioxidant effects of norgestrel following LD include modulation of the expression of Nrf2 and its effector proteins SOD1 and SOD2. Nrf2 was chosen as it is often the first port of call when cells come under oxidative stress, and along with the SODs has been shown to be regulated by progesterone [31], [32], [33], [34], [35]. SOD1 and SOD2 scavenge hydrogen peroxide in the cytosol and mitochondria respectively [52], [53]. Employing immunofluorescent staining of retinal sections, we found that norgestrel markedly increases both Nrf2 and SOD2 expression in photoreceptor cells. Nrf2 Immunoreactivity is increased in the photoreceptor layer (PRL) at 6 h (Fig. 4Ai), 24 h (Fig. 4Bi) and 48 h (Fig. 4Ci) post-LD. SOD2 expression is also increased in the PRL at all time-points studied (Fig. 4A,B,C, ii). Quantification of fluorescence intensity shows that these increases in Nrf2 and SOD2 are significant at all time-points (Fig. 4D). Norgestrel fails to increase the expression of SOD1 any time post-LD (Figs. Aiii, Biii and Ciii). ROS were also visualized by DHE fluorescence.
Fig. 4.
Norgestrel increases the expression of the antioxidants Nrf2 and SOD2, but not SOD1. Balb/c mice were given intraperitoneal injections of vehicle (veh) or vehicle containing 100 mg/kg norgestrel (norg) 1 h prior to light damage (LD) and were euthanized at 6 h, 24 h or 48 h post-LD. Approximately 4 h before being euthanasia, mice received two intraperitoneal injections of 20 mg/kg dihydroethidine (DHE), 30 min apart. Ocular sections were obtained and assessed by microscopy as described in Methods. A; assessment of the expression of Nrf2 (i), SOD2 (ii) and SOD1 (iii) 6 h post-LD in vehicle (veh) or norgestrel (norg) treated mice. B; assessment of the expression of Nrf2 (i), SOD2 (ii) and SOD1 (iii) 24 h post-LD in vehicle (veh) or norgestrel (norg) treated mice. C; assessment of the expression of Nrf2 (i), SOD2 (ii) and SOD1 (iii) 48 h post-LD in vehicle (veh) or norgestrel (norg) treated mice. Counter-fluorescence of DHE (red) is also shown. Hoechst staining (blue) allows orientation of the retinal layers, and shows changes in ONL thickness following LD. PRL; Photoreceptor layer, ONL; outer nuclear layer, OPL; outer plexiform layer, INL; inner nuclear layer, IPL; inner plexiform layer. D; graphical representation of Nrf2 or SOD2 fluorescence intensity in vehicle (veh) or norgestrel (norg) treated mice. Results are representative of at least n=3. Scale bar=50 µm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.5. Norgestrel modulates Nrf2 expression at the post-translational level
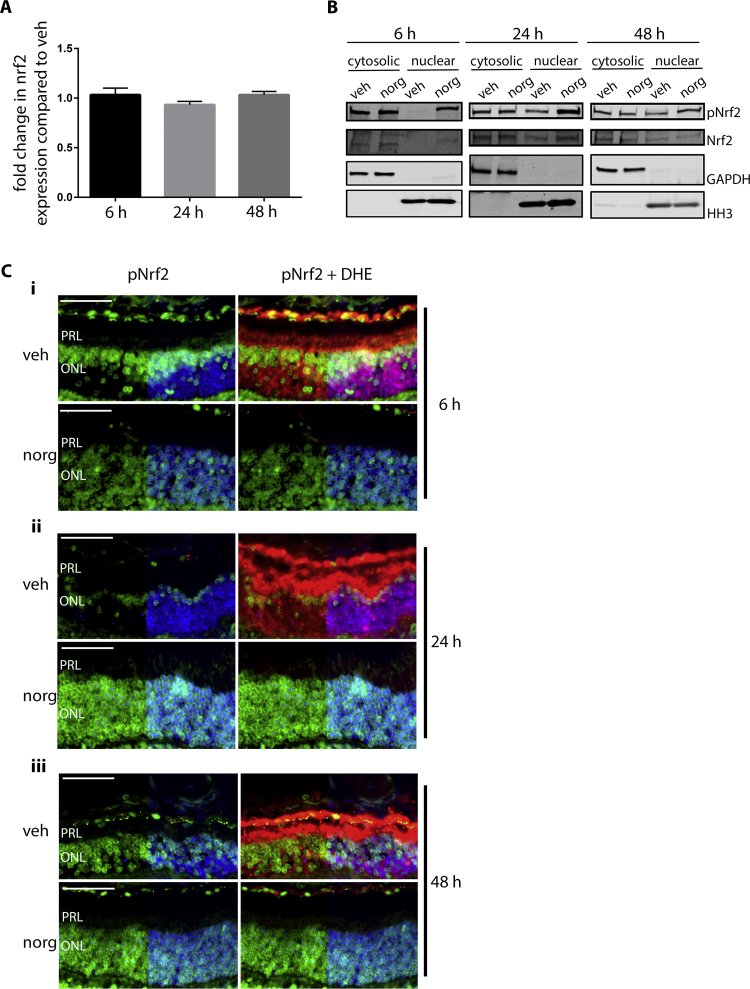
We lastly sought to investigate the mechanism of Nrf2 up-regulation by norgestrel. Firstly, we looked at the effect of norgestrel on Nrf2 transcription by means of quantitative real time PCR (qRT-PCR). At all time-points post-LD, no change was observed in nrf2 expression between vehicle and norgestrel treated mice (Fig. 5A). Next, we investigated the effect of norgestrel on Nrf2 at the post-translational level. Indeed, Nrf2 is regulated mainly at the post-translational level. It is constitutively expressed, and under basal conditions the majority of the population is bound to the inhibitor KEAP1, which sequesters Nrf2 within the cytoplasm and promotes its constant degradation by the ubiquitin protease system (UPS) via the E3 ligase Cul-3 [54]. In response to stimuli Nrf2 can also be activated in various ways, including phosphorylation. Nrf2 contains many potential phospho-sites, which may be targets for different kinases [55]. One verified Nrf2 kinase is protein kinase C (PKC) which phosphorylates Nrf2 on serine 40 (S40), thereby disrupting its interaction with KEAP1, and enabling it to translocate into the nucleus [56], [57]. Firstly, we investigated whether norgestrel has any effect of Nrf2 phosphorylation and thus nuclear translocation. Cytosolic and nuclear fractions from whole retinal lysates of mice treated with vehicle (veh) or norgestrel (norg) prior to LD were probed for pNrf2 (S40) expression by means of western blot analysis (Fig. 5B). pNrf2 is markedly increased in the nuclear fractions at all times post-LD following norgestrel treatment, while total Nrf2 is only marginally increased, or not at all by 48 h. We then employed immunofluorescent staining of retinal sections to look at the expression of pNrf2 in the photoreceptors specifically (Fig. 5C). Compared to vehicle treated mice, norgestrel elevated the levels of pNrf2 within the ONL at 6 h (Fig. 5Ai), 24 h (Fig. 5Aii) and 48 h (Fig. 5Aiii).
Fig. 5.
Norgestrel increases phosphorylation of Nrf2 and its nuclear translocation. Balb/c mice were given intraperitoneal injections of vehicle (veh) or vehicle containing 100 mg/kg norgestrel (norg) 1 h prior to light damage (LD) and were euthanized at 6 h, 24 h or 48 h post-LD. A; LD retinas were assessed for relative changes in nrf2 mRNA expression following vehicle (veh) or norgestrel (norg) treatment. Relative expression was analyzed by means of real time (rt) PCR, with fold change compared to the geometric mean of three endogenous reference genes (gapdh, hprt, β-actin). Results are representatives of n=2. B; sub-cellular fractionation was carried out on retinal lysates from mice treated with vehicle (veh) or norgestrel (norg) and euthanized 6 h, 24 h and 48 h post-LD. Expression of phospho-Nrf2 (pNrf2) or total Nrf2 (Nrf2) in cytosolic and nuclear fractions was assessed by means of western blot analyses. Equal loading of samples and verification of the subcellular fractions were assessed by probing for cytosolic-localized GAPDH and nuclear-localized histone H3 (HH3). Western blot is representative of n=4. C; Approximately 4 h before being euthanized, mice received two intraperitoneal injections of 20 mg/kg dihydroethidine (DHE), 30 min apart. Ocular sections were obtained and assessed by microscopy as described in Methods. pNrf2 expression (green) was assessed in vehicle (veh) or norgestrel (norg) treated mice at 6 h (Ci), 24 h (Cii) and 48 h (Ciii) post-LD. Counter-fluorescence of DHE (red) is also shown. Hoechst staining (blue) allows orientation of the retinal layers, but is removed from half of each image to increase the clarity of pNrf2 staining. PRL; Photoreceptor layer, ONL; outer nuclear layer. Images are representative of at least n=3. Scale bar=50 µm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Photoreceptor cell death is the collective end-point for all cases of RP, irrespective of the underlying genetic cause. As RP progresses, phototransduction is reduced due to the disease-causing mutation and the rod photoreceptors cannot utilize the oxygen available to them. As the photoreceptor layer becomes progressively more disorganized this is potentiated, resulting in hyperoxia within the outer retina and the consequent initiation of cell death pathways [58], [59]. Although the disease-causing mutation resides within rod genes, it is the secondary loss of cones that leads to total blindness. The mechanism behind this is not fully understood, but it is known that oxidative stress is a key factor [6], [7], [8], [41], [60]. Gene therapy is a promising therapeutic approach for RP, however targeting the multitude of RP-related genes is complex and so ROS pathways provide viable indiscriminate therapeutic targets, where the aim is to retard retinal degeneration and preserve vision. If cone cells could be saved, patients could lead a relatively normal life, with good vision in bright conditions.
The steroid hormone progesterone is a potent neuroprotective agent, and has recently been shown to reduce oxidative stress in a model of repeated mild brain injury [35]. We have previously demonstrated how the synthetic progestin norgestrel is neuroprotective in the compromised retina using the rd10 model of RP, and the light-damage model of retinal degeneration [36], [37]. Norgestrel acts via upregulation of the neurotrophic factors bFGF and LIF, the latter being a potent neurotrophic factor which, interestingly, has been shown to have antioxidant effects in the retina [61]. Here, we further expand on the neuroprotective actions of norgestrel in the retina, investigating its effects on oxidative stress using the light-damage model of retinal degeneration, a well-established paradigm [62]. Light-damage caused the production of intracellular ROS in photoreceptor cells, as visualized by DHE fluorescence, which was prevented by pre-treatment with norgestrel (Fig. 1). Due to the non-specificity of DHE [63], we refer here to the detection of ROS, rather than superoxide. In vehicle-only treated mice, total ROS is evident in the PRL and ONL. Within the PRL are the inner segments of the photoreceptor cells which are high in mitochondria [48], thus DHE fluorescence here is suggestive of mitochondrial ROS. As previously reported [37], norgestrel also inhibited light-induced cell death, as visualized by TUNEL assays. Light-induced ROS production preceded cell death, supporting the hypothesis that oxidative stress is a key contributor to photoreceptor loss.
As RP advances and photoreceptors progressively degenerate, the cells undergo various structural changes. Rod cells exhibit de-localization of rhodopsin [47], while cone inner and outer segments become fragmented and swollen [46]. Cone pedicles are also lost, due to perturbed synaptic connectivity within the OPL [64]. These phenomena, characteristic of structural changes during RP, are observed coinciding with ROS production following light-damage (Fig. 2). Moreover, abnormalities in rod cell structure appear more advanced than that of cones at 24 h post light-damage (Fig. 2A), as is observed in RP models, where ROS derived from dying rod cells is a key factor in cone cell death [6], [10], [11]. All structural aberrations were completely prevented by norgestrel; which maintained normal photoreceptor morphology and ONL thickness (Fig. 2).
Having looked at total intracellular ROS using DHE, we sought to investigate whether mitochondrial-derived ROS plays a part in light-induced photoreceptor degeneration (Fig. 3). Indeed, it has been shown to play a major role in the development of other retinopathies, with studies suggesting that mitochondrial-derived superoxide is the primary source of ROS in diabetic tissue, and is responsible for initiating other ROS signaling pathways [65], [66]. We show here that light-damage indeed generates the production of mitochondrial ROS within the photoreceptors which is significantly reduced by norgestrel (Fig. 3B). Unlike the complete inhibition of DHE fluorescence (Fig. 1), norgestrel did not abolish MitoSox fluorescence. The difference here is likely due to that fact that flow cytometry is a far more sensitive application. Also, the preparation of single-cell suspensions may introduce ROS, and the reduction of light-induced mitochondrial-ROS by norgestrel may appear greater if it could be assessed in situ. Again, like DHE, MitoSox is a non-specific superoxide probe [63], and so we refer to the detection of general ROS.
Having established that norgestrel can reduce mitochondrial-ROS within photoreceptors, we sought to investigate the mechanism behind this. Given that the KEAP1-Nrf2 pathway is one of the most important in protecting retinal cells from endogenous- and exogenous-induced oxidative stress [26], and that Nrf2 is paramount to normal mitochondrial function [67], we looked at the expression of Nrf2 in the stressed retina following norgestrel treatment. To date, most studies on Nrf2 in the context of retinopathies have been done using AMD models, where photoreceptor loss is secondary to degeneration of the retinal pigment epithelium (RPE). It has been shown that the aging RPE exhibits dysregulated Nrf2 signaling, leading to impaired induction of antioxidant genes [68], while knockout of nrf2 in otherwise healthy mice increases the susceptibility of the outer retina to age-related degeneration [69]. Similarly, activation of Nrf2 by administration of sulforaphane has been shown to protect RPE cells from photo-oxidative damage [70]. Here, we investigated the expression of Nrf2 in photoreceptor cells following light-induced oxidative stress. While minimal Nrf2 was observed in vehicle treated mice at 6 h post light-damage, with none at 24 h or 48 h, norgestrel induced Nrf2 expression in the photoreceptor layer and sustained it for at least 48 h following the insult (Fig. 4Ai, Bi and Ci).
Following on from this, we examined the expression of two key Nrf2 effectors, SOD1 and SOD2, which catalyze the conversion of superoxide to hydrogen peroxide. SOD1 is cytosolic, whereas SOD2 is localized in the mitochondrial matrix [71]. While SOD1 expression was not increased (Fig. 4 Aiii, Biii and Ciii), SOD2 immunoreactivity was markedly elevated in the photoreceptor inner segments, where the mitochondria reside (Fig. 4 Aii, Bii and Cii). Previous studies have demonstrated the importance of both enzymes in maintaining the retinal redox environment; however SOD2 appears to be more important for photoreceptor homeostasis. In Sod1 knockout mice, Hashizume and colleagues observed delayed changes in the retinal architecture compared to wt mice [72]. Inner nuclear layer (INL) thinning became significant (p<0.01) at 30 weeks of age, while a less significant (p<0.05) decrease in ONL thickness was observed only at 50 weeks of age, suggesting that SOD1 is more important for the health of the INL, rather than the photoreceptors. Indeed, the loss of cells in the outer retina may be a consequence of inner retinal cell death. In comparison to this slow and progressive degeneration which affects mainly the inner layers of the retina, Sod2 knockout mice show photoreceptor degeneration from 9 days old, compared to their wild type counterparts [73]. Further support for this hypothesis comes from a number of studies where SOD1 was found to be an important antioxidant in the retinal ganglion cell (RGC) layer, the innermost layer of the retina. SOD1-deficient mice display increased ROS levels in the RCG layer [74]. In another study, Sod1-null mice in which retinal oxidative stress was induced, displayed TUNEL in the INL and RGCs only [75]. Interestingly, Sod1 overexpressing mice have actually been shown to display increased levels of ROS with accelerated photoreceptor degeneration [5]. The results presented here suggest that mitochondrial-derived ROS is an important consequence of light-induced retinal damage, and that norgestrel can markedly reduce this toxicity via up-regulation of Nrf2 and subsequently SOD2, enabling detoxification within the mitochondria which is necessary for preservation of photoreceptor cell number and structure.
We next sought to investigate the mechanism of norgestrel-induced Nrf2 expression. We first examined nrf2 mRNA expression following norgestrel, but found no change compared to vehicle (Fig. 5A). Although norgestrel is a synthetic steroid hormone, lack of transcriptional activity is not surprising given that it can act through the non-classical receptor; progesterone receptor membrane component 1 (PGRMC1) [76]. Moreover, Nrf2 is known to be regulated almost entirely at the post-translational level, being constitutively expressed and maintained at basal levels by KEAP1-mediated turn-over [54]. Such basal expression enables Nrf2 to elicit a rapid and efficient response to stress stimuli, upon which it escapes KEAP1 and translocates into the nucleus, bringing about transcription of various antioxidant genes. A small population of Nrf2 is also believed to remain in the nucleus, allowing constitutive basal expression of target genes [77], [78]. Cytosolic Nrf2 can be phosphorylated and thus activated by PKC, which disrupts its interaction with KEAP1 [56], [57]. Following light-induced damage, norgestrel treated retinas display an elevated population of phospho-Nrf2 within the nucleus (Fig. 5B). However, the western blot analyses are that of whole retinas. Looking specifically at the photoreceptors, there are marked increases in pNrf2 levels within the ONLs of norgestrel treated mice, which is sustained up to 48 h (Fig. 5C). These data suggest that norgestrel mediates the activation of Nrf2 through PKC-phosphorylation, thereby bringing about an increase in Nrf2 nuclear translocation and consequent antioxidant gene transcription. Further work needs to be carried out to explore the link between norgestrel and PKC, however other studies have shown that progesterone can exert neuroprotective effects via activation of PKC [79], [80].
Nrf2 also contains a phoshodegron, phosphorylation of which promotes nuclear export and a return to basal antioxidant signaling. A central regulator of Nrf2 in this manner is glycogen synthase kinase 3β (GSK3β) [55]. GSK3β phosphorylates and thus activates cytosolic Fyn, allowing it to enter the nucleus where it in turn phosphorylates and brings about the nuclear export of Nrf2 [81], [82], [83], resulting in Nrf2 re-binding KEAP1 and being rapidly degraded. Interestingly, we have previously shown that norgestrel inhibits GSK3β [84], adding weight to the results reported here.
In conclusion, this study supports previous work showing that norgestrel is a potent neuroprotective agent in the compromised retina, and provides strong in vivo evidence that norgestrel is also a powerful antioxidant. We show how norgestrel markedly reduces mitochondrial-derived ROS in the retina via post-translational regulation of the major antioxidant transcription factor Nrf2 and its effector protein SOD2. These results highlight the potential of norgestrel as a therapeutic option for RP and other retinal degenerative conditions, which will not discriminate between genetic etiologies.
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
The authors gratefully acknowledge all members of the Biologic Services Unit (BSU) for technical assistance, and Dr. Francesca Doonan for invaluable advice. This work was supported by Science Foundation Ireland (SFI) (grant no. 13/IA/1783), and Fighting Blindness (registered charity number 20013349).
References
- 1.Lohr H.R. Multiple, parallel cellular suicide mechanisms participate in photoreceptor cell death. Exp. Eye Res. 2006;83(2):380–389. doi: 10.1016/j.exer.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 2.Murakami Y. Receptor interacting protein kinase mediates necrotic cone but not rod cell death in a mouse model of inherited degeneration. Proc. Natl. Acad. Sci. USA. 2012;109(36):14598–14603. doi: 10.1073/pnas.1206937109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartong D.T., Berson E.L., Dryja T.P. Retinitis pigmentosa. Lancet. 2006;368(9549):1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 4.Portera-cailliau C. Apoptotic photoreceptor cell-death in mouse models of retinitis-pigmentosa. Proc. Natl. Acad. Sci. USA. 1994;91(3):974–978. doi: 10.1073/pnas.91.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Usui S. Increased expression of catalase and superoxide dismutase 2 reduces cone cell death in retinitis pigmentosa. Mol. Ther. 2009;17(5):778–786. doi: 10.1038/mt.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen J. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. J. Cell Physiol. 2005;203 doi: 10.1002/jcp.20346. [DOI] [PubMed] [Google Scholar]
- 7.Komeima K. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA. 2006;103(30):11300–11305. doi: 10.1073/pnas.0604056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez-Fernandez de la Camara C. Altered antioxidant-oxidant status in the aqueous humor and peripheral blood of patients with retinitis pigmentosa. PLoS One. 2013;8(9):e74223. doi: 10.1371/journal.pone.0074223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ames A., 3rd Energy requirements of CNS cells as related to their function and to their vulnerability to ischemia: a commentary based on studies on retina. Can. J. Physiol. Pharm. 1992;70(Suppl):S158–S164. doi: 10.1139/y92-257. [DOI] [PubMed] [Google Scholar]
- 10.Yu D.Y. Intraretinal oxygen levels before and after photoreceptor loss in the RCS rat. Invest. Ophthalmol. Vis. Sci. 2000;41(12):3999–4006. [PubMed] [Google Scholar]
- 11.Yu D.Y., Cringle S.J. Retinal degeneration and local oxygen metabolism. Exp. Eye Res. 2005;80(6):745–751. doi: 10.1016/j.exer.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Sharma A.K., Rohrer B. Sustained elevation of intracellular cGMP causes oxidative stress triggering calpain-mediated apoptosis in photoreceptor degeneration. Curr. Eye Res. 2007;32(3):259–269. doi: 10.1080/02713680601161238. [DOI] [PubMed] [Google Scholar]
- 13.Carmody R.J., McGowan A.J., Cotter T.G. Reactive oxygen species as mediators of photoreceptor apoptosis in vitro. Exp. Cell Res. 1999;248(2):520–530. doi: 10.1006/excr.1998.4421. [DOI] [PubMed] [Google Scholar]
- 14.Carmody R.J., Cotter T.G. Oxidative stress induces caspase-independent retinal apoptosis in vitro. Cell Death Differ. 2000;7(3):282–291. doi: 10.1038/sj.cdd.4400646. [DOI] [PubMed] [Google Scholar]
- 15.Sanvicens N. Oxidative stress-induced apoptosis in retinal photoreceptor cells is mediated by calpains and caspases and blocked by the oxygen radical scavenger CR-6. J. Biol. Chem. 2004;279(38):39268–39278. doi: 10.1074/jbc.M402202200. [DOI] [PubMed] [Google Scholar]
- 16.Naash M.L. Light-induced acceleration of photoreceptor degeneration in transgenic mice expressing mutant rhodopsin. Invest Ophthalmol. Vis. Sci. 1996;37(5):775–782. [PubMed] [Google Scholar]
- 17.Chen J. Increased susceptibility to light damage in an arrestin knockout mouse model of Oguchi disease (stationary night blindness) Invest Ophthalmol. Vis. Sci. 1999;40(12):2978–2982. [PubMed] [Google Scholar]
- 18.LaVail M.M. Increased susceptibility to constant light in nr and pcd mice with inherited retinal degenerations. Invest Ophthalmol. Vis. Sci. 1999;40(5):1020–1024. [PubMed] [Google Scholar]
- 19.Paskowitz D.M., LaVail M.M., Duncan J.L. Light and inherited retinal degeneration. Br. J. Ophthalmol. 2006;90(8):1060–1066. doi: 10.1136/bjo.2006.097436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sui G.Y. Is sunlight exposure a risk factor for age-related macular degeneration? A systematic review and meta-analysis. Br. J. Ophthalmol. 2013;97(4):389–394. doi: 10.1136/bjophthalmol-2012-302281. [DOI] [PubMed] [Google Scholar]
- 21.Moi P. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. USA. 1994;91(21):9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itoh K. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13(1):76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Itoh K. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys. Res Commun. 1997;236(2):313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 24.Lee J.M. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J. Biol. Chem. 2003;278(14):12029–12038. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- 25.Xiong W. NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. J. Clin. Invest. 2015;125(4):1433–1445. doi: 10.1172/JCI79735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kensler T.W., Wakabayashi N., Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev. Pharm. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 27.Himori N. Critical role of Nrf2 in oxidative stress-induced retinal ganglion cell death. J. Neurochem. 2013;127(5):669–680. doi: 10.1111/jnc.12325. [DOI] [PubMed] [Google Scholar]
- 28.Xu Z. NRF2 plays a protective role in diabetic retinopathy in mice. Diabetologia. 2014;57(1):204–213. doi: 10.1007/s00125-013-3093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakagami Y. Cytoprotective effects of a novel Nrf2 activator, RS9, in Rhodopsin Pro347Leu Rabbits. Curr. Eye Res. 2015:1–4. doi: 10.3109/02713683.2015.1078362. [DOI] [PubMed] [Google Scholar]
- 30.Unfer T.C. Influence of hormone replacement therapy on blood antioxidant enzymes in menopausal women. Clin. Chim. Acta. 2006;369(1):73–77. doi: 10.1016/j.cca.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Unfer T.C. Estrogen plus progestin increase superoxide dismutase and total antioxidant capacity in postmenopausal women. Climacteric. 2015;18(3):379–388. doi: 10.3109/13697137.2014.964669. [DOI] [PubMed] [Google Scholar]
- 32.Morrissy S. NAD(P)H: quinone oxidoreductase 1 is induced by progesterone in cardiomyocytes. Cardiovasc Toxicol. 2012;12(2):108–114. doi: 10.1007/s12012-011-9144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai J. Progesterone alleviates acute brain injury via reducing apoptosis and oxidative stress in a rat experimental subarachnoid hemorrhage model. Neurosci. Lett. 2015;600:238–243. doi: 10.1016/j.neulet.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 34.N. Lu et al. [Protective effects of progesterone against high intraocular pressure-induced retinal ischemia-reperfusion in rats] Nan Fang. Yi Ke Da Xue Xue Bao, 28(11), 2008, pp. 2026–2029 [PubMed]
- 35.Webster K.M. Progesterone treatment reduces neuroinflammation, oxidative stress and brain damage and improves long-term outcomes in a rat model of repeated mild traumatic brain injury. J. Neuroinflamm. 2015;12:238. doi: 10.1186/s12974-015-0457-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Byrne A.M. The synthetic progestin norgestrel acts to increase LIF levels in the rd10 mouse model of retinitis pigmentosa. Mol. Vis. 2016;22:264–274. [PMC free article] [PubMed] [Google Scholar]
- 37.Doonan F. Enhancing survival of photoreceptor cells in vivo using the synthetic progestin Norgestrel. J. Neurochem. 2011;118(5):915–927. doi: 10.1111/j.1471-4159.2011.07354.x. [DOI] [PubMed] [Google Scholar]
- 38.Roche S.L. Alterations to retinal architecture prior to photoreceptor loss in a mouse model of retinitis pigmentosa. Int J. Dev. Biol. 2016;60(4–6):127–139. doi: 10.1387/ijdb.150400tc. [DOI] [PubMed] [Google Scholar]
- 39.Irannejad R. Conformational biosensors reveal GPCR signalling from endosomes. Nature. 2013;495(7442):534. doi: 10.1038/nature12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oveson B.C. Constituents of bile, bilirubin and TUDCA, protect against oxidative stress-induced retinal degeneration. J. Neurochem. 2011;116(1):144–153. doi: 10.1111/j.1471-4159.2010.07092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Komeima K. Blockade of neuronal nitric oxide synthase reduces cone cell death in a model of retinitis pigmentosa. Free Radic. Biol. Med. 2008;45(6):905–912. doi: 10.1016/j.freeradbiomed.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Usui S. NADPH oxidase plays a central role in cone cell death in retinitis pigmentosa. J. Neurochem. 2009;110(3):1028–1037. doi: 10.1111/j.1471-4159.2009.06195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 44.Zhong Y. X-Box binding protein 1 is essential for the anti-oxidant defense and cell survival in the retinal pigment epithelium. PLoS One. 2012;7(6):e38616. doi: 10.1371/journal.pone.0038616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blanks J.C., Johnson L.V. Specific binding of peanut lectin to a class of retinal photoreceptor cells. A species comparison. Invest Ophthalmol. Vis. Sci. 1984;25(5):546–557. [PubMed] [Google Scholar]
- 46.Murakami Y. Necrotic enlargement of cone photoreceptor cells and the release of high-mobility group box-1 in retinitis pigmentosa. Cell Death Discov. 2015;1:15058. doi: 10.1038/cddiscovery.2015.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao J. Progressive photoreceptor degeneration, outer segment dysplasia, and rhodopsin mislocalization in mice with targeted disruption of the retinitis pigmentosa-1 (Rp1) gene. Proc. Natl. Acad. Sci. USA. 2002;99(8):5698–5703. doi: 10.1073/pnas.042122399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graymore C. Metabolism of the developing retina. III. Respiration in the developing normal rat retina and the effect of an inherited degeneration of the retinal neuroepithelium. Br. J. Ophthalmol. 1960;44:363–369. doi: 10.1136/bjo.44.6.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maleki S. Optical imaging of mitochondrial redox state in rodent model of retinitis pigmentosa. J. Biomed. Opt. 2013;18(1):16004. doi: 10.1117/1.JBO.18.1.016004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robinson K.M. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc. Natl. Acad. Sci. USA. 2006;103(41):15038–15043. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mukhopadhyay P. Simple quantitative detection of mitochondrial superoxide production in live cells. Biochem Biophys. Res Commun. 2007;358(1):203–208. doi: 10.1016/j.bbrc.2007.04.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Son Y.O. Nrf2/p62 signaling in apoptosis resistance and its role in cadmium-induced carcinogenesis. J. Biol. Chem. 2014;289(41):28660–28675. doi: 10.1074/jbc.M114.595496. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Zelko I.N., Mariani T.J., Folz R.J. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002;33(3):337–349. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- 54.Kobayashi A. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell Biol. 2004;24(16):7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rojo A.I. Signaling pathways activated by the phytochemical nordihydroguaiaretic acid contribute to a Keap1-independent regulation of Nrf2 stability: role of glycogen synthase kinase-3. Free Radic. Biol. Med. 2012;52(2):473–487. doi: 10.1016/j.freeradbiomed.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 56.Huang H.C., Nguyen T., Pickett C.B. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF-E2-related factor 2. Proc. Natl. Acad. Sci. USA. 2000;97(23) doi: 10.1073/pnas.220418997. (12475-80) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang H.C., Nguyen T., Pickett C.B. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J. Biol. Chem. 2002;277(45):42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- 58.Bramall A.N. The genomic, biochemical, and cellular responses of the retina in inherited photoreceptor degenerations and prospects for the treatment of these disorders. Annu Rev. Neurosci. 2010;33:441–472. doi: 10.1146/annurev-neuro-060909-153227. [DOI] [PubMed] [Google Scholar]
- 59.Padnick-Silver L. Retinal oxygenation and oxygen metabolism in Abyssinian cats with a hereditary retinal degeneration. Invest Ophthalmol. Vis. Sci. 2006;47(8):3683–3689. doi: 10.1167/iovs.05-1284. [DOI] [PubMed] [Google Scholar]
- 60.Tao Y. Anthocyanin can arrest the cone photoreceptor degeneration and act as a novel treatment for retinitis pigmentosa. Int. J. Ophthalmol. 2016;9(1):153–158. doi: 10.18240/ijo.2016.01.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chollangi S. Preconditioning-induced protection from oxidative injury is mediated by leukemia inhibitory factor receptor (LIFR) and its ligands in the retina. Neurobiol. Dis. 2009;34(3):535–544. doi: 10.1016/j.nbd.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grimm C., Reme C.E. Light damage as a model of retinal degeneration. Method. Mol. Biol. 2013:935. doi: 10.1007/978-1-62703-080-9_6. [DOI] [PubMed] [Google Scholar]
- 63.Kalyanaraman B. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic. Biol. Med. 2012;52(1):1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cuenca N. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog. Retin. Eye Res. 2014;43:17–75. doi: 10.1016/j.preteyeres.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 65.Nishikawa T. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404(6779):787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 66.Giacco F., Brownlee M. Oxidative stress and diabetic complications. Circ. Res. 2010;107(9):1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Holmstrom K.M. Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol. Open. 2013;2(8):761–770. doi: 10.1242/bio.20134853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sachdeva M.M., Cano M., Handa J.T. Nrf2 signaling is impaired in the aging RPE given an oxidative insult. Exp. Eye Res. 2014;119:111–114. doi: 10.1016/j.exer.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao Z. Age-related retinopathy in NRF2-deficient mice. PLoS One. 2011;6(4):e19456. doi: 10.1371/journal.pone.0019456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao X., Talalay P. Induction of phase 2 genes by sulforaphane protects retinal pigment epithelial cells against photooxidative damage. Proc. Natl. Acad. Sci. USA. 2004;101(28):10446–10451. doi: 10.1073/pnas.0403886101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weisiger R.A., Fridovich I. Mitochondrial superoxide dismutase. Site of synthesis and intramitochondrial localization. J. Biol. Chem. 1973;248(13):4793–4796. [PubMed] [Google Scholar]
- 72.Hashizume K. Retinal dysfunction and progressive retinal cell death in SOD1-deficient mice. Am. J. Pathol. 2008;172(5):1325–1331. doi: 10.2353/ajpath.2008.070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sandbach J.M. Ocular pathology in mitochondrial superoxide dismutase (Sod2)-deficient mice. Invest Ophthalmol. Vis. Sci. 2001;42(10):2173–2178. [PubMed] [Google Scholar]
- 74.Yuki K. Retinal ganglion cell loss in superoxide dismutase 1 deficiency. Invest Ophthalmol. Vis. Sci. 2011;52(7):4143–4150. doi: 10.1167/iovs.10-6294. [DOI] [PubMed] [Google Scholar]
- 75.Yuki K. Neuroprotective role of superoxide dismutase 1 in retinal ganglion cells and inner nuclear layer cells against N-methyl-D-aspartate-induced cytotoxicity. Exp. Eye Res. 2013;115:230–238. doi: 10.1016/j.exer.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 76.Jackson A.C. Progesterone receptor signalling in retinal photoreceptor neuroprotection. J. Neurochem. 2016;136(1):63–77. doi: 10.1111/jnc.13388. [DOI] [PubMed] [Google Scholar]
- 77.Nguyen T., Huang H.C., Pickett C.B. Transcriptional regulation of the antioxidant response element. Activation by Nrf2 and repression by MafK. J. Biol. Chem. 2000;275(20):15466–15473. doi: 10.1074/jbc.M000361200. [DOI] [PubMed] [Google Scholar]
- 78.Hayes J.D. The Nrf2 transcription factor contributes both to the basal expression of glutathione S-transferases in mouse liver and to their induction by the chemopreventive synthetic antioxidants, butylated hydroxyanisole and ethoxyquin. Biochem. Soc. Trans. 2000;28(2):33–41. doi: 10.1042/bst0280033. [DOI] [PubMed] [Google Scholar]
- 79.Balasubramanian B. Nonclassical mechanisms of progesterone action in the brain: i. Protein kinase C activation in the hypothalamus of female rats. Endocrinology. 2008;149(11):5509–5517. doi: 10.1210/en.2008-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.He L. Progesterone attenuates Aquaporin-4 expression in an astrocyte model of ischemia/reperfusion. Neurochem. Res. 2014;39(11):2251–2261. doi: 10.1007/s11064-014-1427-7. [DOI] [PubMed] [Google Scholar]
- 81.Jain A.K., Jaiswal A.K. Phosphorylation of tyrosine 568 controls nuclear export of Nrf2. J. Biol. Chem. 2006;281(17):12132–12142. doi: 10.1074/jbc.M511198200. [DOI] [PubMed] [Google Scholar]
- 82.Jain A.K., Jaiswal A.K. GSK-3beta acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF-E2 related factor 2. J. Biol. Chem. 2007;282(22):16502–16510. doi: 10.1074/jbc.M611336200. [DOI] [PubMed] [Google Scholar]
- 83.Rada P. SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol. Cell Biol. 2011;31(6):1121–1133. doi: 10.1128/MCB.01204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wyse Jackson A.C., Cotter T.G. The synthetic progesterone Norgestrel is neuroprotective in stressed photoreceptor-like cells and retinal explants, mediating its effects via basic fibroblast growth factor, protein kinase A and glycogen synthase kinase 3β signalling. Eur. J. Neurosci. 2016;43(7):899–911. doi: 10.1111/ejn.13166. [DOI] [PubMed] [Google Scholar]