Abstract
We investigated long-lasting changes in endothelial and vascular function in adult rat survivors of severe sepsis induced by cecal ligation and puncture (CLP) model. For this, male Wistar rats (200–350 g) had their cecum punctured once (non-transfixing hole) with a 14-gauge needle. Performed in this way, a mortality rate around 30% was achieved in the first 72 h. The survivors, together with age-matched control rats (not subjected to CLP), were maintained in our holding room for 60 days (S60 group) and had the descending thoracic aorta processed for functional, histological, biochemical or molecular analyses. Endothelium-intact aortic rings obtained from sepsis-surviving S60 group displayed increased angiotensin II-induced contraction, accompanied by decreased activity of the endogenous superoxide dismutase, augmented reactive oxygen species generation, and increased levels of tyrosine nitration compared with vessels from control group. The superoxide scavengers superoxide dismutase and tempol, and the antioxidant apocynin, were able to avoid this enhanced contractility to angiotensin II in aortic rings from the S60 group. In addition, aortic rings from the S60 group presented reduced sensitivity to Y-27632, a Rho-kinase (ROCK) inhibitor. Immunoblot analyses revealed augmented RhoA and ROCK II, and high levels of phosphorylation of myosin phosphatase target subunit 1 in vessels from S60 rats. In conclusion, aortic rings from sepsis-surviving rats display endothelial dysfunction mediated by the increased production of reactive oxygen species, which in turn reduces the bioavailability of nitric oxide and increases the formation of peroxynitrite, and enhances RhoA-ROCK-mediated calcium sensitization, leading to augmented contractile responses to angiotensin II. Notably, this is the first study demonstrating long-term dysfunction in the vasculature of sepsis-surviving rats, which take place or remain beyond the acute septic insult.
Abbreviations: AII, angiotensin II; AT1R, angiotensin II type 1 receptor; CAT, catalase; CLP, cecal ligation and puncture; CT, control; DHE, dihydroethidium; GSH, reduced glutathione; GST, glutathione S-transferase; LOOH, lipid hydroperoxide; MYPT-1, subunit 1 of the myosin light chain phosphatase; Nox, NAD(P)H oxidase; RNS, reactive nitrogen species; ROCK, Rho-kinase; ROS, reactive oxygen species; S60, surviving 60 days; SOD, superoxide dismutase
Keywords: Vascular tone, Calcium sensitization, NAD(P)H oxidase, Septic shock
Graphical abstract
Highlights
-
•
Vessels from sepsis-surviving rats develop enhanced contractile responses to angiotensin II.
-
•
This hyperreactivity is avoided by the superoxide scavengers superoxide dismutase and tempol.
-
•
Generation of reactive oxygen species by angiotensin II is enhanced in these vessels.
-
•
Vessels from sepsis-surviving rats present an augmented degree of tyrosine nitration.
-
•
The activity of the RhoA-Rho-kinase pathway is enhanced in the aorta from sepsis-surviving rats.
1. Introduction
In spite of the acute nature of sepsis, several epidemiological investigations have revealed that the mortality rate among those patients who survive the episode of severe sepsis or septic shock is strikingly higher when compared with age-matched people who never had sepsis [1], [2]. For instance, a pioneering study published by Quartin et al. (1997), disclosed that 80% of the 1505 survivors of the septic episode evaluated in their study died in less than 8 years [3]. Although these findings clearly indicate that a single episode of sepsis is predictive of an increased risk of death in a few years, the physiological and/or pathological events that occur beyond the period of sepsis remain poorly understood. It is important to note that despite a number of studies having confirmed the decreased life expectancy among those who survived sepsis [4], [5], [6], [7], [8], most of the animal studies in this field were conducted focusing only in the first hours or days after the induction of sepsis, with few exception regarding the investigation of long-term cognitive deficits, mainly involving learning and memory impairments [9], [10], [11].
It is well known that the functionality of the cardiovascular system is depressed by sepsis. This includes the inability of vessels to keep their tone and development of systemic hypotension, which results in inadequate blood perfusion and oxygenation of several tissues, and has a major role in the dysfunction of multiple organs [12]. Interestingly, if on the one hand pre-existing cardiovascular diseases have been shown to contribute to the progression to heart failure and higher mortality rates among individuals diagnosed with septic shock [13], on the other hand, recent clinical data revealed an increased risk of cardiovascular diseases, including myocardial infarction and stroke, among survivors of severe sepsis [14]. In this regard, we hypothesized that in spite of the reestablishment of normal blood pressure, the vascular biology of survivors from severe sepsis remains impaired for prolonged periods.
According to our knowledge, our study is one of the few evaluating the late consequences of sepsis in a period exceeding 30 days, and the first to explore long-term dysfunctions in the vascular system. Here we present an animal model of long-term endothelial and vascular dysfunction that can be described as sequelae of the septic insult. The results presented also reveal that NAD(P)H oxidase-derived reactive oxygen species (ROS) and reactive nitrogen species (RNS), as well as the RhoA/Rho-associated protein kinase (ROCK) signaling pathway, contribute to the hyper-contractility evoked by angiotensin II (AII) in the vasculature of sepsis-surviving rats.
2. Materials and methods
2.1. Animals and experimental model of sepsis
Two hundred male Wistar rats (3 months) supplied by Universidade Federal do Paraná (UFPR, Curitiba, PR, Brazil) were used in the experiments. All procedures described in this study have been carried out in accordance with Guidelines for the Care and Use of Laboratory Animals from U.S. National Institutes of Health [15], and were approved by the Institutional Animal Care and Use Committee of UFPR (authorization number 527). The protocol used in the cecal ligation and puncture (CLP) model was followed according to Rittirsch and coworkers [16], with minor modifications. The rats were anesthetized with ketamine/xylazine (100/20 mg/kg, ip). A skin midline incision was performed in the abdomen, the cecum was exposed and ligated (comprising 75% of the cecum). Since the mortality rate obtained in this model is fully dependent on the size of the needle used and number of punctures performed in the cecum (Supplementary Results – Fig. S1), the animals used in this study had their cecum punctured once (non-transfixing hole) with a 14-gauge needle. A small amount of stool was expelled from the puncture to ensure leakage of the intestinal content, and the incision was closed in layers with sutures applied in the abdominal muscle and skin. Finally, all animals received a postoperative fluid resuscitation by injecting sterile saline solution (3 mL/100 g, s.c.) to prevent dehydration. Performed in this way, a mortality rate around 30% was achieved in the first 72 h (Supplementary Results – Fig. S1, 1 hole 14 -gaugegroup). The survivors (n=88), together with age-matched control (CT) rats (not subjected to CLP; n=90), were maintained in our holding room under controlled conditions (12/12 h light-dark cycle, and temperature of 22±2 °C), with free access to chow and water for 30 days (S30 group; n=12), or 60 days (S60 group; n=76). At the selected time-point, the animals were subjected to general anesthesia induced by ketamine/xylazine (100/20 mg/kg, ip), and had the descending thoracic aorta carefully removed, cleaned of connective tissue and processed for functional, histological, biochemical or molecular analyses.
2.2. Functional study in organ baths
In these experiments the aortas were mounted in classical organ baths for measurement of contractile force generation via isometric transducers. The details regarding the isolation of tissue and experimental design, including the addition of pharmacological tools are detailed in the Supplementary Methods.
2.3. Immunoblot analyses
The protein levels of AT1 receptors, Rho-A, ROCK-I, ROCK-II, total and phosphorylated MYPT-1 in the aorta of CT and S60 groups were assessed by Western blot analysis, as previously detailed [17] and presented in the Supplementary Methods.
2.4. Biochemical analyses of oxidative stress
Samples of aortas from CT and S60 groups were processed for determination of lipid hydroperoxide (LOOH) levels [18], reduced glutathione (GSH) contents [19], as well as for measurement of the activity of superoxide dismutase (SOD) [20], [21], catalase (CAT) [22], and glutathione S-transferase (GST) [23]. In addition, sections of aortas were allocated in microscope slides and loaded with dihydroethidium (DHE), with or without exposure to angiotensin II (40 nM) for assessment of reactive oxygen species generation. Different slides were incubated with primary antibody against nitrotyrosine followed by secondary antibody conjugated with phycoerythrin for detection of tyrosine nitration. The steps followed for each set of experiments are presented in the Supplementary Methods.
2.5. Statistical analysis
The results are expressed as mean±standard error of mean (S.E.M.). Statistical significance was determined using two-way analysis of variance (ANOVA) followed by Bonferroni'spost-test, or Student's t-test, when applicable. A p value less than 0.05 was considered statistically significant. The graphs were drawn and the statistical analyses were performed using GraphPad Prism version 6 for Mac (GraphPad Software, La Jolla, CA, USA).
3. Results
3.1. Standard control and experimental groups
Preliminary data revealed that at 6 h after the cecal ligation and puncture (CLP) surgery to induce sepsis the animals (n=12) presented piloerection, porphyrin secretion (eyes and nose) and lethargy, as well as leukocytopenia, thrombocytopenia and granulocytosis, high levels of blood nitrate and nitrite (an indicative of exacerbated production of nitric oxide), hypotension, and both in vivo and in vitro refractoriness to vasoconstrictors, confirming the ability of the CLP model to induce several aspects commonly found in severe sepsis. None of these signs were recorded in sham-operated or in age-matched control animals (CT groups; data not shown). Importantly, all animals that survived to CLP were fully recovered after 5 days and remained undistinguished from CT animals during the 60 days of follow-up, including parameters such as body weight, water and chow consumption (Supplementary Results – Table S1).
3.2. Increased AII-induced contraction in vessels from sepsis-surviving rats
To explore our hypothesis that sepsis-surviving rats present long-term changes in vascular reactivity, thoracic aortas were tested in organ baths for development of contractile force induced by vasoactive agents commonly used in studies of vascular biology. The aortic rings obtained from the S60 group did not present any difference in the reactivity to acetylcholine, sodium nitroprusside, KCl, phenylephrine and angiotensin I, compared with vessels from CT rats (Supplementary Results – Fig. S2). However, the contractile effect induced by cumulative concentrations of AII was significantly increased in endothelium-intact aortic rings from the S60 group (Fig. 1A). For instance, the maximal increase in vascular tone induced by AII, reached when the final the concentration of 3 μM was added, varied from 0.65±0.08 to 1.26±0.16 g in CT and S60 groups, respectively. Similarly, the non-cumulative addition of 3 μM AII increased the contractile force of aortic rings from CT and S60 groups by 0.58±0.14 and 1.22±0.09 g, indicating that the maximal effects induced by cumulative concentrations of AII was not being reduced by tachyphylaxys.
Fig. 1.
Enhanced AII-induced vasoconstriction in the aorta of sepsis-surviving rats and involvement of AT1 receptors. The vessels were obtained from control animals (CT; age-matched naïve rats) or sepsis-surviving rats at 60 days after the CLP (S60), as indicated in each panel. AII-induced contraction in A) endothelium-intact rat aortic rings, B) endothelium-denuded rat aortic rings, C) endothelium-intact rat aortic rings previously incubated with l-NAME (100 μM), D) and E) endothelium-intact rat aortic rings previously incubated with vehicle or the AT1R antagonist losartan. F) Representative images and densitometric analysis (in arbitrary units, A.U.) of levels of AT1R protein in aortas from CT and S60 groups. The results show the mean±SEM of preparations obtained from 6 to 8 animals per group (panels A–E), or samples from 4 different animals per group (panel F). Statistical analyses were performed by means of two-way analysis of variance (ANOVA) followed by Bonferroni's post-test (panels A–E) or unpaired Student t-test (panel F). * Indicates p<0.05 compared with the respective CT group.
Our experiments did not reveal any difference in the vascular reactivity between aortic rings obtained from the S30 group and its respective CT group (Supplementary Results – Fig. S3). For this, the next steps of this study were conducted only with animals from the S60 group.
3.3. Lack of influence of endothelium removal, and effect of nitric oxide synthase (NOS) inhibition on AII-induced contraction in aortic rings from sepsis-surviving rats
The increased responsiveness to AII was found only in endothelium-intact aortic rings (Fig. 1A), as the mechanical damage of the endothelial layer was completely without effect on AII-induced contraction in vessels from the S60 group (Fig. 1B). On the other hand, incubation with the NOS inhibitor l-NAME raised the contractile responses to AII in aortic rings from both CT and S60 groups (Fig. 1C).
3.4. Participation of angiotensin II type 1 receptor (AT1R) in the enhanced effects of AII in aortas from sepsis-surviving rats
AII-induced contraction was reduced to similar levels by losartan in both the CT and S60 groups (Fig. 1D and E). However, the Western blot analysis in the aortas from CT and S60 groups did not reveal any difference in the protein levels of the AT1R (Fig. 1F).
3.5. Absence of histological changes in aortas from sepsis-surviving rats
As shown in the Supplementary Fig. S5, there were no signals of morphological changes, inflammatory cell migration, edema, hypertrophy, or collagen deposition in histological sections from either the CT or S60 groups.
3.6. Impairment of endogenous antioxidant machinery in the vasculature of sepsis-surviving rats
In spite of the lack of differences in the activity of catalase (CAT) and glutathione S-transferase (GST), the activity of superoxide dismutase (SOD) in aortic homogenates of the S60 group was decreased by 45% in the S60 group. Furthermore, the levels of glutathione (GSH) and lipid hydroperoxide (LOOH) were increased by 98% and 24%, respectively, compared with the CT group (Table 1), indicating that the endogenous antioxidant machinery was impaired in vessels from S60 group.
Table 1.
Impaired antioxidant system in the aorta of sepsis-surviving rats.
| Control group | S60 group | |
|---|---|---|
| Lipid hydroperoxide (µmol/mg protein) | 11.2±0.5 | 13.9±1* |
| Reduced glutathione (µg/g of tissue) | 277.2±54.6 | 548.9±107.5* |
| Superoxide dismutase (U/mg protein) | 28.8±3 | 15.8±0.8* |
| Catalase (µmol/min/mg protein) | 5.6±0.4 | 4.7±0.6 |
| Glutathione S-transferase (nmol/min/mg protein) | 429.3±63.6 | 432.6±79.8 |
The values show the mean±S.E.M. of 6–8 samples per group, obtained from different animals. Statistical analyses were performed using Student's t-test. S60 group, sepsis-surviving rats at 60 days after the CLP.
Indicates p<0.05 when compared with the respective control group (age-matched naïve animals).
3.7. Superoxide scavengers avoided AII-induced augmented contraction in aortic rings from sepsis-surviving rats
To determine whether the imbalance between endogenous antioxidant and ROS production would contribute to increase the contractile effects of AII, we performed functional experiments in the presence of exogenous superoxide scavengers and antioxidants. The incubation of aortic rings with SOD, or tempol (a SOD mimetic agent), did not change the contractile effects of AII in vessels from CT animals, but abolished the hyper-reactivity to AII in preparations obtained from S60 group (Fig. 2A–D). Similarly, the commonly used inhibitor of NAD(P)H oxidases, apocynin, prevented the exacerbated responses to AII in aortas from the S60 group, without modify AII-induced contraction in CT vessels (Fig. 2E and F).
Fig. 2.
Inhibition of the enhanced contractile effects of AII in aortic rings from sepsis-surviving rats by apocynin, SOD and tempol. Aortic rings obtained from control (CT group; left panels) or sepsis-surviving rats (S60 group; right panels) had their reactivity to AII evaluated after incubation with superoxide dismutase (SOD; A and B), tempol (C and D), or apocynin (E and F). For comparative purposes, the contractile effects of AII were measured in aortic rings from both CT and S60 groups previously incubated with vehicle (closed circles). The results show the mean±SEM of preparations obtained from 4 to 6 animals per group. Statistical analyses were performed by means of two-way analysis of variance (ANOVA) followed by Bonferroni's post-test. * Indicates p<0.05 compared with the respective vehicle-treated group.
3.8. Augmented oxidative and nitrosative stress in aortas from sepsis-surviving rats
Experiments using the fluorescent probe dyhydroethidium (DHE) revealed that in spite of unchanged basal levels, the generation of ROS under stimulation by AII was augmented (up to 30%) in vessels from the S60 group, compared with the CT group (Fig. 3A and B), reinforcing the hypothesis of an increased oxidant status in vessels from sepsis-surviving rats. As the superoxide anion may readily interact with NO to form the strong RNS peroxynitrite, we evaluated the amounts of nitrotyrosine present in aortas from the S60 group as an indicator of exposure to peroxynitrite. Notably, aortas from the S60 group presented more than a 3-fold increase in the degree of tyrosine nitration, compared with samples from CT animals, indicating exacerbated production of peroxynitrite in vessels from sepsis-surviving rats (Fig. 3C and D).
Fig. 3.
Increased ROS and RNS levels in the aorta of sepsis-surviving rats. A) Representative images and B) analysis of the relative fluorescence intensity (in arbitrary units, A.U.) obtained with the fluorescent dye dyhydroethidium (DHE) in aortic sections from control (CT) and sepsis-surviving rats at 60 days after the CLP (S60) under stimulation with vehicle or angiotensin II, as indicate inside the panels. C) Representative images and D) analysis of the relative fluorescence intensity (in arbitrary units, A.U.) of tyrosine nitration detected by fluorescent antibody against nitrotyrosine in aortic sections from control (CT) and sepsis-surviving rats at 60 days after the CLP (S60). The blue color in the right images represents the nuclei stained with Hoeschst blue. The images were acquired using magnification of x40. The results show the mean±SEM of samples obtained from 4 different animals per group. Statistical analysis was performed by unpaired Student's t-test. ns indicates not significant; # Indicates p<0.05 compared with the S60 group exposed to vehicle only; * Indicates p<0.05 compared with the respective CT group. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
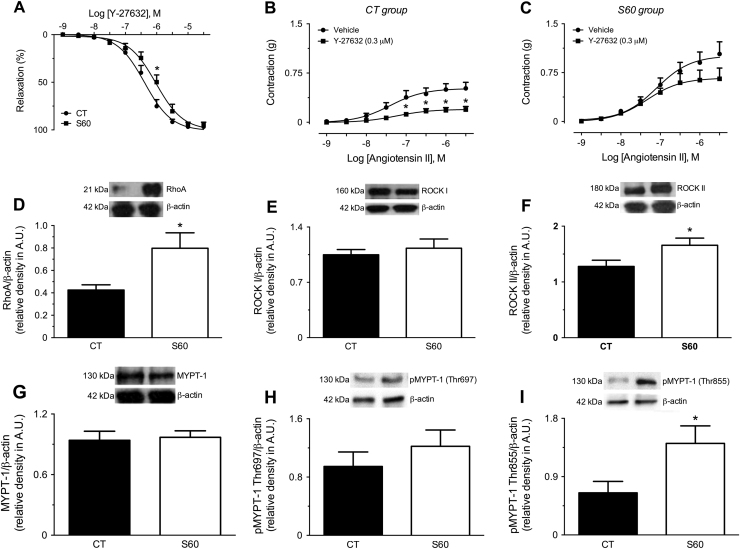
3.9. Reduced effects of the ROCK inhibitor Y-27632 on AII-induced contraction in aortas from sepsis surviving rats
To explore a possible mechanism contributing to the AII-induced hyper-contractility in vessels from sepsis-surviving rats, we used the selective ROCK inhibitor Y-27632 in functional experiments. The analyses of the relaxation induced by Y-27632 in phenylephrine-contracted aortic rings from S60 animals revealed a parallel rightward shift in the cumulative concentration-response curve for this agent, with a corresponding increase in the half maximal effective concentration (EC50) for relaxation from 0.43 (0.33–0.55) to 1.03 (0.76–1.40) µM, in CT and S60 groups, respectively (Fig. 4A). Moreover, aortic rings from CT animals had their reactivity to AII reduced by 60% after incubation with 0.3 µM Y-27632 (Fig. 4B), but this same concentration of ROCK inhibitor failed to do the same in vessels from the S60 group (Fig. 4C). Importantly, 1 µM Y-27632 prevented AII-induced contraction in both the CT and S60 groups (data not shown).
Fig. 4.
Reduced sensitivity of aortic rings from sepsis-surviving rats to the ROCK inhibitor Y-27632 and augmented levels of components of the RhoA/Rho-kinase pathway. The vessels were obtained from control animals (CT; age-matched naïve rats) or sepsis-surviving rats at 60 days after the CLP (S60), as indicated in each panel. A) Relaxation induced by Y-27632 in endothelium-intact aortic rings. B) and C) Angiotensin II-induced contraction in endothelium-intact aortic rings from CT and S60 groups, respectively, previously incubated with vehicle or the ROCK inhibitor Y-27631. The results show the mean±SEM of preparations obtained from 4 to 6 animals per group. Statistical analyses were performed by means of two-way analysis of variance (ANOVA) followed by Bonferroni's post-test. * Indicates p<0.05 compared with the respective vehicle-treated group; # Indicates p<0.05 compared with CT. Representative images and densitometric analyses of D) RhoA, E) ROCK I, F) ROCKMethods of enzymatic analysis II, G) MYPT-1, H) pMYPT-1 (at Thr697) and I) pMYPT-1 (at Thr855) in aortas from CT and S60 groups. The results show the mean±SEM of 4–6 samples or preparations per group, obtained from different animals. Statistical analyses were performed by means of two-way analysis of variance (ANOVA) followed by Bonferroni's post-test (panels A-C), or unpaired Student's t-test (panels D–I). * Indicates p<0.05 when compared with the respective control group.
3.10. Expression of Rho-A, ROCK II and phosphorylated MYPT-1 are increased in the aorta from sepsis-surviving rats
The immunoblot data revealed a remarkable increase in the expression of RhoA in aortas from the S60 group (Fig. 4D). Moreover, compared with the CT group, aortas from the S60 group presented augmented levels of ROCK II (Fig. 4F), without any change in ROCK I (Fig. 4E), or in total MYPT-1 (Fig. 4G). The RhoA/ROCK system is well known as a calcium-sensitization pathway due its ability to inhibit the activity of myosin phosphatase through phosphorylation of the myosin phosphatase target subunit 1 (MYPT-1) [24]. In particular, our results revealed hyperphosphorylation of MYPT-1 at Thr-855 (Fig. 4I), but not at Thr-697 (Fig. 4H), in homogenates of aortas obtained from the S60 group.
4. Discussion
The first important finding in our study was that despite the recovery from the vascular dysfunction provoked by sepsis, and the lack of detectable abnormalities at 30 days after the CLP surgery, aortic rings from sepsis-surviving rats presented enhanced contractile responses to AII at 60 days after the septic insult. Although this data cannot be used to state that the entire vascular system from sepsis-surviving rats is more sensitive to AII-induced contraction, and additional studies mainly with resistance vessels remain to be carried out, the contractile responses evoked by AII were also augmented in endothelium-intact carotid rings obtained from the S60 group (Supplementary Results – Fig. S4), indicating that this hyperresponsiveness to AII occurs not only in aorta but also in other conductance vessels.
The lack of changes in the effects of phenylephrine, a selective agonist of α1A-adrenoceptors, suggested that the impairment of vascular reactivity to AII could be associated with regulatory changes in AT1 or AT2 receptors in the vasculature. Indeed, increased expression of AT1R has been described in several cardiovascular diseases, in both animals and humans [25], and AT2 receptors are well known for their counter-regulatory actions against activation of the AT1 receptors [26]. The inhibitory effect of the selective antagonist losartan found in our experiments clearly indicates that the enhanced reactivity to AII found in aortas from the S60 group was fully dependent on the activation of AT1R. Notably, we have not found differences in the level of AT1R in aortas from the S60 group, compared with samples from the CT group, a data suggesting that the enhanced AII-induced vasoconstriction in vessels from the S60 group was not associated with an augmented expression of these receptors, but instead it was fully dependent on AII-evoked intracellular signaling.
Physiologically, the tone of vessels is maintained by a rigorous balance between hormonal and paracrine factors that mediate either vascular dilation or constriction. Among these factors, NO produced mainly by endothelial cells through the endothelial NOS (eNOS) has been accepted as the major regulator of vascular tone [27]. Our data showing that endothelium removal was completely unable to change the vascular reactivity to AII in aortic rings from the S60 group, in spite of the well described ability of this approach to increase the vascular reactivity to contractile agents in control preparations (as found in our CT group), was a suggestion of impairment of the endothelial function in the aorta from the S60 group. Interestingly, inhibition of NOS by l-NAME raised the vascular reactivity of aortic rings from both CT and S60 groups, indicating that the production and the role of NO in the control of vascular tone was, at least in part, maintained in vessels from the S60 group, a finding that may explain why acetylcholine-induced relaxation was not impaired in these vessels (Supplementary Results – Fig. S2A). Importantly, a number of clinical investigations have described endothelial dysfunction and the reduction in the bioavailability of NO as an important predictor of cardiovascular risk [28], [29].
Several mechanisms may contribute to the reduced bioavailability of NO in the vascular system, including reduced expression, dimerization, or activation of the eNOS [30], and its enhanced breakdown by increased oxidative stress [31]. However, it is very important to note that the disrupted endothelial function found in vessels from the S60 group was fully dependent on stimulation by angiotensin II. It is now well established that AT1 receptors, mainly in endothelial cells, may be coupled to the membrane-associated NAD(P)H oxidase (Nox) enzymes, stimulating the production of superoxide in the vessels. It is interesting to mention that several previous studies associated endothelial dysfunction and hypertension induced by AII with superoxide generation in the vascular system [32]. Taking into account that superoxide interacts with and inactivates NO, mainly in endothelial cells, the normalization of AII-induced vasoconstriction in the aortic rings from the S60 group after incubation with SOD, or the SOD mimetic tempol, or apocynin, and the evidence of increased oxidative stress obtained with the fluorescent dye DHE in aortas stimulated by AII, the second most important finding of our study was that aortic rings from sepsis-surviving rats present endothelial dysfunction, characterized by the augmented production of superoxide anion, and putative reduction in the bioavailability of NO when stimulated by AII. Although our results do not allow us to clearly state the molecular mechanisms involved in this process, the reduction in the activity (or availability) of SOD found in aorta homogenates from the S60 group may account, at least in part, for the accumulation of superoxide in the aorta.
SOD is a major player in the endogenous antioxidant system. This enzyme catalyzes the dismutation of the superoxide anion to hydrogen peroxide, which in turn reacts with a number of enzymes, including catalases, peroxiredoxins, glutathione and peroxidases, as well as cellular thiol-containing compounds, which play a major role in the redox control of cell function [33]. Impaired production of any ROS and/or disruption of redox homeostasis can lead to oxidative stress, promoting or amplifying inflammatory processes [34], platelet aggregation [35], activity of matrix metalloproteinases [36], and the proliferation of vascular smooth muscle [37], among others. Although we did not explore any of these possibilities, one consequence of oxidative stress found in the aortas of sepsis-surviving rats was the augmented lipid peroxidation, which can induce the oxidative degradation of membrane lipids [38], as evidenced by increased levels of LOOH. We have not explored the activity of enzymes such as glutathione peroxidase and glutathione reductase, nor the levels of oxidized GSH (GSSH) in our experiments, but the higher amounts of reduced glutathione (GSH) measured in aortas of the S60 group, which were not correlated with changes in the activity of GST, confirmed the impaired redox status and may be associated with a compensatory mechanism to confer protection against oxidative damage in these vessels. For instance, a number of studies have indicated that the expression of glutamylcysteine synthetase, responsible for glutathione synthesis, is sensitive to oxidative stress [39], [40], [41]. In spite of the lack of changes in the vascular reactivity to angiotensin II at earlier stages after the septic insult (i.e. 30 days after sepsis; Supplementary Results – Fig. S3F), the onset of the changes in the markers of oxidative stress in vessels from survivors of sepsis deserves to be investigated, since early changes in the redox state may have a crucial role in the development of the vascular dysfunction found at 60 days after sepsis.
The accumulation of superoxide deregulates its diffusion-controlled reaction with NO, reducing the bioavailability of NO and augmenting the generation of peroxynitrite, a highly reactive oxidizing and nitrating agent. Increased levels of peroxynitrite in vessels over long periods have been described as an important mechanism involved in the development of various forms of cardiovascular diseases, including stroke, myocardial infarction, chronic heart failure and circulatory shock [42]. Accordingly with the increased nitration of tyrosine found in our experiments, it is reasonable to conclude that the aortas from the S60 group present an increased production of superoxide, which results in the excessive suppression of endothelium-derived NO and the accumulation of peroxynitrite. Peroxynitrite was previously associated with a significant increase in RhoA activity in the coronary arteries of diabetic rats [43]. Further, peroxynitrite-mediated nitration at Tyr-34 in RhoA after LPS challenge resulted in the 2-fold increased activity of RhoA in human lung microvascular endothelial cells [44]. RhoA is a small G protein expressed in a wide range of cells. Importantly, RhoA and its downstream effector ROCK mediate several effects of AII [45]. After stimulation by RhoA, ROCK phosphorylates the regulatory subunit 1 of the myosin light chain phosphatase (MYPT-1), inhibiting this enzyme [46]. For this, the RhoA/ROCK pathway is often described as a calcium sensitization system, and is an important regulator of vascular tone in both health and disease [24]. The third important point disclosed in our study was that the RhoA/ROCK pathway was more active in vessels from the S60 group, as suggested by the reduced sensitivity to the ROCK inhibitor Y-27632 found in our functional experiments in organ baths, the increased protein levels of both RhoA and ROCK II, and the augmented phosphorylated MYPT-1, as detected by Western-blot assays. Interestingly, phosphorylation of MYPT-1 at Thr-855 in rats, as found in the S60 group, has been previously associated with myogenic responses mediated by RhoA/ROCK pathway after agonist stimulation, independently of phosphorylation at Thr-697 [47]. Abnormal activation of the RhoA/ROCK pathway was previously implicated in cytoskeleton reorganization, focal adhesion and migration of vascular smooth muscle cells during the development of several cardiovascular diseases. We did not disclose any evidence of histological, morphological, or inflammatory-associated impairment in the aortic rings of sepsis-surviving rats from the S60 group. However, taking into account the augmented activity of the RhoA/ROCK pathway, the enhanced responsiveness to AII, the ROS- and RNS-associated endothelial dysfunction described in this study, and the putative relationship between these factors and several cardiovascular diseases, the development of histological changes including but not restrict to vascular remodeling in later periods after sepsis, or in specific vascular beds, deserves further consideration.
In summary, this is the first study describing that sepsis-surviving rats present long-term impairment in the vascular system, including endothelial dysfunction, which was characterized by the increased production of superoxide and increased generation of peroxynitrite. This oxidative and nitrosative environment may contribute to the augmented activity of the RhoA/ROCK pathway, which in turn plays a major role in the enhanced responsiveness to AII, among other potentially deleterious effects that remain to be investigated. Importantly, the acute events that occur during the septic insult and contribute for such late changes in the vascular function remain to be characterized. Moreover, additional studies using both in vitro and in vivo approaches, and including more prolonged periods of time, also must be carried out to investigate the behavior of the cardiovascular system after a septic insult.
Funding
This work received grants from Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC, SC, Brazil; TR2012000367 and TR201200078). Priscila de Souza received a Ph.D. fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil).
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2016.09.016.
Appendix A. Supplementary material
Supplementary material
References
- 1.Laupland K.B., Zygun D.A., Doig C.J., Bagshaw S.M., Svenson L.W., Fick G.H. One-year mortality of bloodstream infection-associated sepsis and septic shock among patients presenting to a regional critical care system. Intensive Care Med. 2005;31:213–219. doi: 10.1007/s00134-004-2544-6. [DOI] [PubMed] [Google Scholar]
- 2.Sands K.E., Bates D.W., Lanken P.N., Graman P.S., Hibberd P.L., Kahn K.L., Parsonnet J., Panzer R., Orav E.J., Snydman D.R., Black E., Schwartz J.S., Moore R., Johnson B.L., Jr., Platt R., Academic Medical Center Consortium Sepsis Project Working G. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA. 1997;278:234–240. [PubMed] [Google Scholar]
- 3.Quartin A.A., Schein R.M., Kett D.H., Peduzzi P.N. Magnitude and duration of the effect of sepsis on survival. Department of veterans affairs systemic sepsis cooperative studies group. JAMA. 1997;277:1058–1063. [PubMed] [Google Scholar]
- 4.Westphal G.A., Vieira K.D., Orzechowski R., Kaefer K.M., Zaclikevis V.R., Mastroeni M.F. Analysis of quality of life following hospital discharge among survivors of severe sepsis and septic shock. Rev. Panam. Salud Publica. 2012;31:499–505. doi: 10.1590/s1020-49892012000600008. [DOI] [PubMed] [Google Scholar]
- 5.Contrin L.M., Paschoal V.D., Beccaria L.M., Cesarino C.B., Lobo S.M. Quality of life of severe sepsis survivors after hospital discharge. Rev. Lat. Am. Enferm. 2013;21:795–802. doi: 10.1590/S0104-11692013000300020. [DOI] [PubMed] [Google Scholar]
- 6.Cuthbertson B.H., Elders A., Hall S., Taylor J., MacLennan G., Mackirdy F., Mackenzie S.J., Scottish Critical Care Trials G., Scottish Intensive Care Society Audit G. Mortality and quality of life in the five years after severe sepsis. Crit. Care. 2013;17:R70. doi: 10.1186/cc12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavon A., Binquet C., Kara F., Martinet O., Ganster F., Navellou J.C., Castelain V., Barraud D., Cousson J., Louis G., Perez P., Kuteifan K., Noirot A., Badie J., Mezher C., Lessire H., Quantin C., Abrahamowicz M., Quenot J.P., Group E.Po.S.S.S. Profile of the risk of death after septic shock in the present era: an epidemiologic study. Crit. Care Med. 2013;41:2600–2609. doi: 10.1097/CCM.0b013e31829a6e89. [DOI] [PubMed] [Google Scholar]
- 8.Zhang K., Mao X., Fang Q., Jin Y., Cheng B., Xie G., Li H., Yu L., Zhu T., Wang H., Liu X., Zhang Y., Jin Y., Zhang N., Lou T., Fang X.M. Impaired long-term quality of life in survivors of severe sepsis: Chinese multicenter study over 6 years. Anaesthesist. 2013;62:995–1002. doi: 10.1007/s00101-013-2257-8. [DOI] [PubMed] [Google Scholar]
- 9.Barichello T., Martins M.R., Reinke A., Feier G., Ritter C., Quevedo J., Dal-Pizzol F. Long-term cognitive impairment in sepsis survivors. Crit. Care Med. 2005;33:1671. doi: 10.1097/01.ccm.0000170192.54682.c1. [DOI] [PubMed] [Google Scholar]
- 10.Hernandes M.S., D’Avila J.C., Trevelin S.C., Reis P.A., Kinjo E.R., Lopes L.R., Castro-Faria-Neto H.C., Cunha F.Q., Britto L.R., Bozza F.A. The role of Nox2-derived ROS in the development of cognitive impairment after sepsis. J. Neuroinflamm. 2014;11:36. doi: 10.1186/1742-2094-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mina F., Comim C.M., Dominguini D., Cassol-Jr O.J., Dall Igna D.M., Ferreira G.K., Silva M.C., Galant L.S., Streck E.L., Quevedo J., Dal-Pizzol F. Il1-beta involvement in cognitive impairment after sepsis. Mol. Neurobiol. 2014;49:1069–1076. doi: 10.1007/s12035-013-8581-9. [DOI] [PubMed] [Google Scholar]
- 12.Angus D.C., van der Poll T. Severe sepsis and septic shock. N. Engl. J. Med. 2013;369:2063. doi: 10.1056/NEJMc1312359. [DOI] [PubMed] [Google Scholar]
- 13.Scott E.C., Ho H.C., Yu M., Chapital A.D., Koss W., Takanishi D.M., Jr. Pre-existing cardiac disease, troponin I elevation and mortality in patients with severe sepsis and septic shock. Anaesth. Intensive Care. 2008;36:51–59. doi: 10.1177/0310057X0803600109. [DOI] [PubMed] [Google Scholar]
- 14.Yende S., Linde-Zwirble W., Mayr F., Weissfeld L.A., Reis S., Angus D.C. Risk of cardiovascular events in survivors of severe sepsis. Am. J. Respir. Crit. Care Med. 2014;189:1065–1074. doi: 10.1164/rccm.201307-1321OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NRC, I.f.L.A.R, Guide for the Care and Use of Laboratory Animals, 8, 2010, ed2011.
- 16.Rittirsch D., Huber-Lang M.S., Flierl M.A., Ward P.A. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guarido K.L., Goncalves R.P., Junior A.G., da Silva-Santos J.E. Increased activation of the Rho-A/Rho-kinase pathway in the renal vascular system is responsible for the enhanced reactivity to exogenous vasopressin in endotoxemic rats. Crit. Care Med. 2014;42:e461–e471. doi: 10.1097/CCM.0000000000000313. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Z.Y., Woollard A.C., Wolff S.P. Lipid hydroperoxide measurement by oxidation of Fe2+ in the presence of xylenol orange. Comparison with the TBA assay and an iodometric method. Lipids. 1991;26:853–856. doi: 10.1007/BF02536169. [DOI] [PubMed] [Google Scholar]
- 19.Sedlak J., Lindsay R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 20.Gao R.M., Yuan Z.B., Zhao Z.Q., Gao X.R. Mechanism of pyrogallol autoxidation and determination of superoxide dismutase enzyme activity. Bioelectrochem. Bioenerg. 1998;45:41–45. [Google Scholar]
- 21.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 22.Aebi H. Catalase. In: Bergmeyer H.U., editor. Methods of Enzymatic Analysis. Academic Press; New York and London: 1974. pp. 673–677. [Google Scholar]
- 23.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 24.Uehata M., Ishizaki T., Satoh H., Ono T., Kawahara T., Morishita T., Tamakawa H., Yamagami K., Inui J., Maekawa M., Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 25.Mehta P.K., Griendling K.K. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 26.Higuchi S., Ohtsu H., Suzuki H., Shirai H., Frank G.D., Eguchi S. Angiotensin II signal transduction through the AT1 receptor: novel insights into mechanisms and pathophysiology. Clin. Sci. 2007;112:417–428. doi: 10.1042/CS20060342. [DOI] [PubMed] [Google Scholar]
- 27.Moncada S. Nitric oxide: discovery and impact on clinical medicine. J. R. Soc. Med. 1999;92:164–169. doi: 10.1177/014107689909200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suwaidi J.A., Higano H.S., Nishimura S.T., Holmes R.A., DR Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:7. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 29.Halcox J.P.J., Schenke W.H., Zalos G., Mincemoyer R., Prasad A., Waclawiw M.A., Nour K.R.A., Quyyumi A.A. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–658. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 30.Tejero J., Stuehr D. Tetrahydrobiopterin in nitric oxide synthase. IUBMB Life. 2013;65:358–365. doi: 10.1002/iub.1136. [DOI] [PubMed] [Google Scholar]
- 31.Kurowska E.M. Nitric oxide therapies in vascular diseases. Curr. Pharm. Des. 2002;8:155–166. doi: 10.2174/1381612023396429. [DOI] [PubMed] [Google Scholar]
- 32.Lassegue B., Clempus R.E. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 33.Manea A. NADPH oxidase-derived reactive oxygen species: involvement in vascular physiology and pathology. Cell Tissue Res. 2010;342:325–339. doi: 10.1007/s00441-010-1060-y. [DOI] [PubMed] [Google Scholar]
- 34.Lavrovsky Y., Chatterjee B., Clark R.A., Roy A.K. Role of redox-regulated transcription factors in inflammation, aging and age-related diseases. Exp. Gerontol. 2000;35:521–532. doi: 10.1016/s0531-5565(00)00118-2. [DOI] [PubMed] [Google Scholar]
- 35.Yao S.K., Ober J.C., Gonenne A., Clubb F.J., Jr., Krishnaswami A., Ferguson J.J., Anderson H.V., Gorecki M., Buja L.M., Willerson J.T. Active oxygen species play a role in mediating platelet aggregation and cyclic flow variations in severely stenosed and endothelium-injured coronary arteries. Circ. Res. 1993;73:952–967. doi: 10.1161/01.res.73.5.952. [DOI] [PubMed] [Google Scholar]
- 36.Rajagopalan S., Meng X.P., Ramasamy S., Harrison D.G., Galis Z.S. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J. Clin. Invest. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griendling K.K., Ushio-Fukai M. Redox control of vascular smooth muscle proliferation. J. Lab. Clin. Med. 1998;132:9–15. doi: 10.1016/s0022-2143(98)90019-1. [DOI] [PubMed] [Google Scholar]
- 38.Coskun S., Peker E.G.G., Balabanli B., Ahiska S., Acarturk F. Effect of transforming growth factor beta 1 (TGF-beta 1) on nitric oxide production and lipid peroxidation in oral mucosal wound healing. Med. Chem. Res. 2011;20:23–28. [Google Scholar]
- 39.Moellering D., McAndrew J., Patel R.P., Cornwell T., Lincoln T., Cao X., Messina J.L., Forman H.J., Jo H., Darley-Usmar V.M. Nitric oxide-dependent induction of glutathione synthesis through increased expression of gamma-glutamylcysteine synthetase. Arch. Biochem. Biophys. 1998;358:74–82. doi: 10.1006/abbi.1998.0854. [DOI] [PubMed] [Google Scholar]
- 40.Ray S., Watkins D.N., Misso N.L., Thompson P.J. Oxidant stress induces gamma-glutamylcysteine synthetase and glutathione synthesis in human bronchial epithelial NCI-H292 cells. Clin. Exp. Allergy. 2002;32:571–577. doi: 10.1046/j.0954-7894.2002.01294.x. [DOI] [PubMed] [Google Scholar]
- 41.Sekhar K.R., Spitz D.R., Harris S., Nguyen T.T., Meredith M.J., Holt J.T., Gius D., Marnett L.J., Summar M.L., Freeman M.L. Redox-sensitive interaction between KIAA0132 and Nrf2 mediates indomethacin-induced expression of gamma-glutamylcysteine synthetase. Free Radic. Biol. Med. 2002;32:650–662. doi: 10.1016/s0891-5849(02)00755-4. [DOI] [PubMed] [Google Scholar]
- 42.Szabo C., Ischiropoulos H., Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Disco. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 43.El-Remessy A.B., Tawfik H.E., Matragoon S., Pillai B., Caldwell R.B., Caldwell R.W. Peroxynitrite mediates diabetes-induced endothelial dysfunction: possible role of Rho kinase activation. Exp. Diabetes Res. 2010;2010:247861. doi: 10.1155/2010/247861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rafikov R., Dimitropoulou C., Aggarwal S., Kangath A., Gross C., Pardo D., Sharma S., Jezierska-Drutel A., Patel V., Snead C., Lucas R., Verin A., Fulton D., Catravas J.D., Black S.M. Lipopolysaccharide-induced lung injury involves the nitration-mediated activation of RhoA. J. Biol. Chem. 2014;289:4710–4722. doi: 10.1074/jbc.M114.547596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamakawa T., Tanaka S., Numaguchi K., Yamakawa Y., Motley E.D., Ichihara S., Inagami T. Involvement of Rho-kinase in angiotensin II-induced hypertrophy of rat vascular smooth muscle cells. Hypertension. 2000;35:313–318. doi: 10.1161/01.hyp.35.1.313. [DOI] [PubMed] [Google Scholar]
- 46.Kimura K., Ito M., Amano M., Chihara K., Fukata Y., Nakafuku M., Yamamori B., Feng J., Nakano T., Okawa K., Iwamatsu A., Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho- kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 47.Johnson R.P., El-Yazbi A.F., Takeya K., Walsh E.J., Walsh M.P., Cole W.C. Ca2+ sensitization via phosphorylation of myosin phosphatase targeting subunit at threonine-855 by Rho kinase contributes to the arterial myogenic response. J. Physiol. 2009;587:2537–2553. doi: 10.1113/jphysiol.2008.168252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material