Abstract
The risk of epilepsy among individuals with intellectual disability (ID) is approximately ten times that of the general population. From a cohort of >5,000 families affected by neurodevelopmental disorders, we identified six consanguineous families harboring homozygous inactivating variants in MBOAT7, encoding lysophosphatidylinositol acyltransferase (LPIAT1). Subjects presented with ID frequently accompanied by epilepsy and autistic features. LPIAT1 is a membrane-bound phospholipid-remodeling enzyme that transfers arachidonic acid (AA) to lysophosphatidylinositol to produce AA-containing phosphatidylinositol. This study suggests a role for AA-containing phosphatidylinositols in the development of ID accompanied by epilepsy and autistic features.
Keywords: MBOAT7, LPIAT1, phosphatidylinositol, intellectual disability, epilepsy, autism, lipid, inflammation, arachidonic acid
Main Text
Intellectual disability (ID) is a common neurodevelopmental disorder affecting 1 in 100 children.1, 2 The more severe forms of ID or those with additional signs or symptoms are less common and have a prevalence of roughly 1 in 200.3, 4 Diagnosis of ID is based on the impairment of general mental abilities and activities of daily living.2 In early childhood, the diagnosis of ID is based on global developmental delays affecting speech, motor, and cognitive function in combination with an IQ below 70.2 It has previously been reported that rare de novo or recessive mutations play a major role in severe ID.5, 6 Interestingly, more complex forms of inheritance are thought to be involved in milder cases.2, 7, 8 Although ID has a strong genetic influence, the involvement of non-genetic factors, such as infections, perinatal asphyxia, or environmental exposures, might play a role in the development of other forms.9 To date, over 1,100 genes have been either confirmed or suggested in ID etiology, yet half of ID cases still remain undiagnosed.4, 10, 11, 12
Many individuals with ID also present with other neurological conditions, such as epilepsy13 and autism spectrum disorder (ASD),14 which also have a strong genetic influence. The prevalence of epilepsy is ten times higher in individuals with ID than in the general population.13 As in ID, de novo, recessive, and dominant variants in ASD can contribute to risk; however, a genetic diagnosis can be determined only in a relatively small portion of individuals.15 ASD is characterized by repetitive behavior and varying degrees of impairment of social interaction and communication skills.16 Many ASD-affected individuals show evidence of heritability.17 Genetic evidence suggests the involvement of 200–1,000 genes, including both autosomal-recessive (AR) and de novo variants, in ASD susceptibility.18, 19, 20
In an effort to expand our understanding of the genetic composition of neurodevelopmental disorders with AR inheritance, several centers in the US, Canada, and Germany, in cooperation with colleagues from Egypt, Pakistan, and Jordan, joined efforts to examine and recruit a large number of consanguineous families with affected children. Analyses were performed in accordance with the ethical standards of institutional review boards, and informed consent was obtained for each individual participating in this study. Exome sequencing of our database consisting of >5,000 families with neurodevelopmental disease identified three families affected by biallelic, possibly pathogenic variants in membrane-bound O-acyltransferase family member 7 (MBOAT7 [MIM: 606048]). These three families presented with overlapping clinical signs, including ID frequently acting with epilepsy (7/8 subjects) and autistic features (7/8 subjects). On the basis of exome findings, three additional families were identified from parallel international sequencing efforts through the sharing of gene names among collaborators.
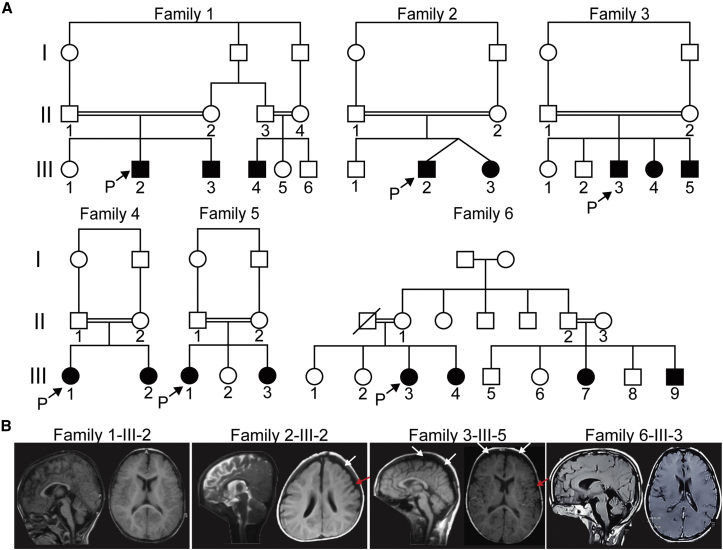
All 16 subjects were born to consanguineous parents (Figure 1A, Table S1, and Supplemental Note). The emerging clinical picture is one of moderate to severe ID given that the majority of subjects are not able to build sentences (14/16) and are non-verbal with delayed motor milestones (9/16). A few of the children (3/16) have never achieved the ability to walk, and the remaining 13 started to walk between the ages of 2 and 7 years. In 6/16 individuals, these clinical signs co-occur with infant-onset epilepsy (mostly focal and multifocal) that has been responsive to antiepileptic drugs. A further two individuals have seizures that began at 1.5 and 2.5 years, whereas another two have febrile seizures. Neurological examination showed that all children have truncal hypotonia and appendicular hypertonia. All subjects have a below-average head size, which is −2 to −3 SDs below the mean in 3/16 affected children, suggesting that microcephaly is not a consistent feature of MBOAT7 variants. ASD was documented in only 7/16 children according to the Childhood Autism Rating Scale, and a further three showed clinical autistic features. Brain imaging was within normal limits, except in two subjects, in whom cortical atrophy was present (Figure 1B). There was some evidence of mild polymicrogyria.
Figure 1.
Consanguineous Families with Variants in MBOAT7, Encoding LPIAT1
(A) Pedigrees of families 1 to 6 show consanguineous marriages (double bars) with a total of 16 affected children. Probands are indicated with “P.”
(B) Brain MRI for one affected individual from each of families 1–3 and 6. White arrows show cortical atrophy, and red arrows show possible polymicrogyria.
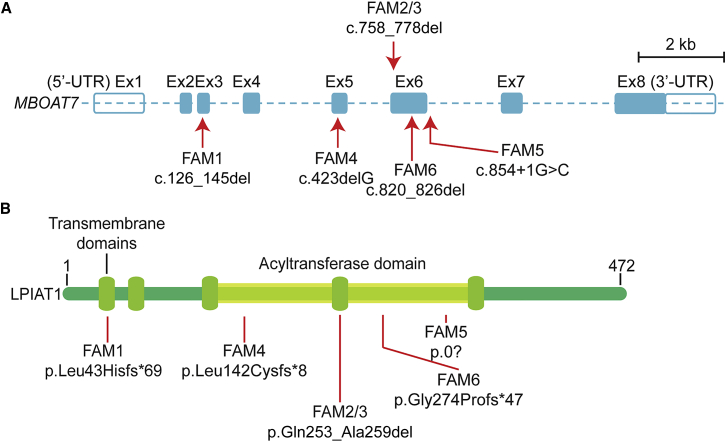
Whole-exome sequencing identified a total of five distinct variants in MBOAT7 (GenBank: NM_024298.3) from six families (Table S2). All variants were prioritized by allele frequency, conservation, blocks of homozygosity, and predicted effect on protein function. All variants were confirmed by Sanger sequencing and segregated with the disease as predicted for a fully penetrant recessive trait within all six families. Family 1, from Egypt, carries a homozygous frameshift deletion (c.126_145del [p.Leu43Hisfs∗69]) in exon 3. Families 2 and 3, from Pakistan, harbor an in-frame deletion in exon 6 (c.758_778del [p.Gln253_Ala259del]). Using actual and inferred sequence data, we estimated the coalescence time of the shared founder mutation for families 2 and 3 to be 9.185 generations (SD ± 4.45 generations), or ∼230 years with a generation time of 25 years. Sequencing data from family 4, from Jordan, revealed a homozygous deletion (c.423delG [p.Leu142Cysfs∗8]) in exon 5. Family 5, from Iraq, carries a biallelic substitution (c.854+1G>C [p.?]) occurring at the canonical splice donor of exon 6. Family 6, from Pakistan, carries a 7 bp frameshift deletion (c.820_826del [p.Gly274Profs∗47]) in exon 6. These variants were not found in dbSNP, the Greater Middle East (GME) Variome, the Exome Aggregation Consortium (ExAC) Browser, or 1000 Genomes and were also not present in our in-house whole-exome database (>5,000 subjects with neurodevelopmental conditions). The ExAC Browser includes over 8,000 South Asian control individuals, almost all Pakistani, from the Pakistan Risk of Myocardial Infarction study. Thus, these disease-related alleles are very rare even in ethnically similar control individuals.
MBOAT7 encodes lysophosphatidylinositol acyltransferase 1 (LPIAT1), which is a member of the MBOAT family of acyltransferases and originates from yeast Ale1p (Figure S1A).21 The human MBOAT family has five members, each of which has a preference toward specific acyl donors and acceptors (Figure S1B).21 LPIAT1 is the only family member that is known to primarily transfer arachidonic acid (AA) from arachidonoyl-CoA to lysophosphatidylinositol (Figure S1C),22 suggesting an essential function. MBOAT7 contains eight exons, resulting in four protein-coding transcripts, and three LPIAT1 isoforms. The five variants described in this study affect all protein-coding transcripts (Figure 2A), interfering with either transmembrane or catalytic domains of the protein (Figure 2B). Balanced translocation in MBOAT1 in one subject has been linked to brachydactyly-syndactyly syndrome.23 None of the other MBOAT genes have had genetic loss-of-function variants linked to human disease.
Figure 2.
Location of Variants and Domains in MBOAT7, Encoding LPIAT1
(A) Genetic structure of MBOAT7. Mutations are indicated by red arrows (exons and numbers as in GenBank: NM_024298.3). In-solution exome capture was performed with the SureSelect Human All Exome 50 Mb Kit (Agilent Technologies) with 125 bp paired-end read sequences generated on a HiSeq2000 or HiSeq2500 (Illumina). Scale bar represents 2 kb.
(B) Structure of LPIAT1, which harbors five transmembrane domains and one catalytic acyltransferase domain. Variants are indicated with red lines. Amino acid numbers are provided above.
LPIAT1 contributes to the regulation of free AA in the cell through the remodeling of phospholipids.24, 25 Free cellular AA is under tight regulation, given that its pro-inflammatory metabolites could be harmful to cellular physiology.26 Enzymes such as lipoxygenase (LOX) and cyclooxygenases (COX) metabolize AA into the pro-inflammatory eicosanoid lipids. The COX enzymes (1 and 2) are known targets of existing non-steroidal anti-inflammatory drugs, such as ibuprofen and aspirin.27 There is compelling evidence linking pro-inflammatory processes to ASD, for instance, the activation of microglia and astrocytes and the overexpression of immune processes in the brains of individuals with ASD.28, 29, 30, 31
A common variant in TMC4 (rs641738), a gene adjacent to MBOAT7, is associated with a 20% increased risk of nonalcoholic fatty-liver disease in individuals of European descent. The variant is predicted to cause a substitution (p.Gly17Glu) early in TMC4.32 Interestingly, this variant is just a few hundred base pairs downstream of the 3′ end of MBOAT7. Carriers of this allele who underwent liver biopsy were found to share reduced MBOAT7 expression and altered phosphatidylinositol levels. None of our affected children or their parents showed evidence of clinically relevant liver disease, but no specific tests were performed. Despite a 49% carrier frequency for the minor allele in the GME Variome, none of our subjects are carriers. Therefore, the connection between this variant and the condition we describe remains uncertain.
In mice, Mboat7 and its encoded protein, LPIAT1, are required for cortical lamination.33 Mboat7 knockout mice are significantly smaller than their littermate controls and show reduced postnatal survival. In a recent study, histological analysis of embryonic day 18.5 Mboat7−/− brains showed a smaller cerebral cortex and hippocampus, abnormal cortical lamination, an increased number of apoptotic cells in the cortex, and dispersed MAP2+ subplate neurons.33 The cerebral cortex showed evidence of gyral structures, whereas normally gyri are absent in the murine cortex, reminiscent of the polymicrogyria we observed in some subjects.
It is recognized that AA-containing phosphatidylinositol is a major lipid in the mammalian brain. It has been shown that Mboat7 is required for cortical lamination is mice.33 In this study, we have linked recessive mutations in MBOAT7 with human neurodevelopmental disease, suggesting a critical role for AA-containing phosphatidylinositol in the developing human brain.
Acknowledgments
We are grateful to the affected individuals and their families for their participation in the study. This study was supported by the Howard Hughes Medical Institute, National Institute of Neurological Disorders and Stroke (R01NS098004 and R01NS048453), Eunice Kennedy Shriver National Institute of Child Health and Human Development (P01HD070494), Qatar National Research Fund (NPRP6-1463), and Simons Foundation Autism Research Initiative (175303 and 275275). We thank the Broad Institute (U54HG003067 to E. Lander and HG00 8900 to D. MacArthur) and the Yale Center for Mendelian Disorders (U54HG006504 to R. Lifton, M. Gunel, M. Gerstein, and S. Mane) for sequencing support and analysis. This study was partially supported by grants from the Deutsche Forschungsgemeinschaft (DFG) to R.A.J. (AB393/2-2 and AB393/4-1), a grant from the Canadian Institutes of Health Research to J.B.V. (MOP-102758), and an award from the Pakistani Higher Education Commission to I.A.
Published: September 8, 2016
Footnotes
Supplemental Data include a Supplemental Note, one figure, and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2016.07.019.
Web Resources
1000 Genomes, http://www.1000genomes.org/
ExAC Browser, http://exac.broadinstitute.org/
GenBank, http://www.ncbi.nlm.nih.gov/genbank/
Genome Analysis Toolkit (GATK, version 2.2), http://www.broadinstitute.org/gatk/
Greater Middle Eastern (GME) Variome Project, http://igm.ucsd.edu/gme/
Mutalyzer, https://mutalyzer.nl/
NHLBI Exome Sequencing Project (ESP) Exome Variant Server, http://evs.gs.washington.edu/EVS/
OMIM, http://www.omim.org
PolyPhen-2, http://genetics.bwh.harvard.edu/pph2/
SeattleSeq (version 134), http://snp.gs.washington.edu/SeattleSeqAnnotation134/
Supplemental Data
References
- 1.Maulik P.K., Mascarenhas M.N., Mathers C.D., Dua T., Saxena S. Prevalence of intellectual disability: a meta-analysis of population-based studies. Res. Dev. Disabil. 2011;32:419–436. doi: 10.1016/j.ridd.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 2.Vissers L.E., Gilissen C., Veltman J.A. Genetic studies in intellectual disability and related disorders. Nat. Rev. Genet. 2016;17:9–18. doi: 10.1038/nrg3999. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association . American Psychiatric Publishing; 2013. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) [Google Scholar]
- 4.Gilissen C., Hehir-Kwa J.Y., Thung D.T., van de Vorst M., van Bon B.W., Willemsen M.H., Kwint M., Janssen I.M., Hoischen A., Schenck A. Genome sequencing identifies major causes of severe intellectual disability. Nature. 2014;511:344–347. doi: 10.1038/nature13394. [DOI] [PubMed] [Google Scholar]
- 5.Hamdan F.F., Srour M., Capo-Chichi J.M., Daoud H., Nassif C., Patry L., Massicotte C., Ambalavanan A., Spiegelman D., Diallo O. De novo mutations in moderate or severe intellectual disability. PLoS Genet. 2014;10:e1004772. doi: 10.1371/journal.pgen.1004772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jansen S., Kleefstra T., Willemsen M.H., de Vries P., Pfundt R., Hehir-Kwa J.Y., Gilissen C., Veltman J.A., de Vries B.B., Vissers L.E. De novo loss-of-function mutations in X-linked SMC1A cause severe ID and therapy resistant epilepsy in females: expanding the phenotypic spectrum. Clin. Genet. 2016 doi: 10.1111/cge.12729. Published online January 11, 2016. [DOI] [PubMed] [Google Scholar]
- 7.Musante L., Ropers H.H. Genetics of recessive cognitive disorders. Trends Genet. 2014;30:32–39. doi: 10.1016/j.tig.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Najmabadi H., Hu H., Garshasbi M., Zemojtel T., Abedini S.S., Chen W., Hosseini M., Behjati F., Haas S., Jamali P. Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature. 2011;478:57–63. doi: 10.1038/nature10423. [DOI] [PubMed] [Google Scholar]
- 9.Kaufman L., Ayub M., Vincent J.B. The genetic basis of non-syndromic intellectual disability: a review. J. Neurodev. Disord. 2010;2:182–209. doi: 10.1007/s11689-010-9055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deciphering Developmental Disorders S., Deciphering Developmental Disorders Study Large-scale discovery of novel genetic causes of developmental disorders. Nature. 2015;519:223–228. doi: 10.1038/nature14135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright C.F., Fitzgerald T.W., Jones W.D., Clayton S., McRae J.F., van Kogelenberg M., King D.A., Ambridge K., Barrett D.M., Bayzetinova T., DDD study Genetic diagnosis of developmental disorders in the DDD study: a scalable analysis of genome-wide research data. Lancet. 2015;385:1305–1314. doi: 10.1016/S0140-6736(14)61705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grozeva D., Carss K., Spasic-Boskovic O., Tejada M.I., Gecz J., Shaw M., Corbett M., Haan E., Thompson E., Friend K., Italian X-linked Mental Retardation Project. UK10K Consortium. GOLD Consortium Targeted Next-Generation Sequencing Analysis of 1,000 Individuals with Intellectual Disability. Hum. Mutat. 2015;36:1197–1204. doi: 10.1002/humu.22901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGrother C.W., Bhaumik S., Thorp C.F., Hauck A., Branford D., Watson J.M. Epilepsy in adults with intellectual disabilities: prevalence, associations and service implications. Seizure. 2006;15:376–386. doi: 10.1016/j.seizure.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Mefford H.C., Batshaw M.L., Hoffman E.P. Genomics, intellectual disability, and autism. N. Engl. J. Med. 2012;366:733–743. doi: 10.1056/NEJMra1114194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen A.S., Berkovic S.F., Cossette P., Delanty N., Dlugos D., Eichler E.E., Epstein M.P., Glauser T., Goldstein D.B., Han Y., Epi4K Consortium. Epilepsy Phenome/Genome Project De novo mutations in epileptic encephalopathies. Nature. 2013;501:217–221. doi: 10.1038/nature12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ronemus M., Iossifov I., Levy D., Wigler M. The role of de novo mutations in the genetics of autism spectrum disorders. Nat. Rev. Genet. 2014;15:133–141. doi: 10.1038/nrg3585. [DOI] [PubMed] [Google Scholar]
- 17.Gaugler T., Klei L., Sanders S.J., Bodea C.A., Goldberg A.P., Lee A.B., Mahajan M., Manaa D., Pawitan Y., Reichert J. Most genetic risk for autism resides with common variation. Nat. Genet. 2014;46:881–885. doi: 10.1038/ng.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J.A., Peñagarikano O., Belgard T.G., Swarup V., Geschwind D.H. The emerging picture of autism spectrum disorder: genetics and pathology. Annu. Rev. Pathol. 2015;10:111–144. doi: 10.1146/annurev-pathol-012414-040405. [DOI] [PubMed] [Google Scholar]
- 19.Berg J.M., Geschwind D.H. Autism genetics: searching for specificity and convergence. Genome Biol. 2012;13:247. doi: 10.1186/gb-2012-13-7-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novarino G., El-Fishawy P., Kayserili H., Meguid N.A., Scott E.M., Schroth J., Silhavy J.L., Kara M., Khalil R.O., Ben-Omran T. Mutations in BCKD-kinase lead to a potentially treatable form of autism with epilepsy. Science. 2012;338:394–397. doi: 10.1126/science.1224631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gijón M.A., Riekhof W.R., Zarini S., Murphy R.C., Voelker D.R. Lysophospholipid acyltransferases and arachidonate recycling in human neutrophils. J. Biol. Chem. 2008;283:30235–30245. doi: 10.1074/jbc.M806194200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee H.C., Inoue T., Imae R., Kono N., Shirae S., Matsuda S., Gengyo-Ando K., Mitani S., Arai H. Caenorhabditis elegans mboa-7, a member of the MBOAT family, is required for selective incorporation of polyunsaturated fatty acids into phosphatidylinositol. Mol. Biol. Cell. 2008;19:1174–1184. doi: 10.1091/mbc.E07-09-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dauwerse J.G., de Vries B.B., Wouters C.H., Bakker E., Rappold G., Mortier G.R., Breuning M.H., Peters D.J. A t(4;6)(q12;p23) translocation disrupts a membrane-associated O-acetyl transferase gene (MBOAT1) in a patient with a novel brachydactyly-syndactyly syndrome. Eur. J. Hum. Genet. 2007;15:743–751. doi: 10.1038/sj.ejhg.5201833. [DOI] [PubMed] [Google Scholar]
- 24.Lands W.E. Metabolism of glycerolipides; a comparison of lecithin and triglyceride synthesis. J. Biol. Chem. 1958;231:883–888. [PubMed] [Google Scholar]
- 25.Zarini S., Hankin J.A., Murphy R.C., Gijón M.A. Lysophospholipid acyltransferases and eicosanoid biosynthesis in zebrafish myeloid cells. Prostaglandins Other Lipid Mediat. 2014;113-115:52–61. doi: 10.1016/j.prostaglandins.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pérez-Chacón G., Astudillo A.M., Balgoma D., Balboa M.A., Balsinde J. Control of free arachidonic acid levels by phospholipases A2 and lysophospholipid acyltransferases. Biochim. Biophys. Acta. 2009;1791:1103–1113. doi: 10.1016/j.bbalip.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Pommery J., Pommery N., Hénichart J.P. Modification of eicosanoid profile in human blood treated by dual COX/LOX inhibitors. Prostaglandins Leukot. Essent. Fatty Acids. 2005;73:411–417. doi: 10.1016/j.plefa.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Anderson A.A., Ushakov D.S., Ferenczi M.A., Mori R., Martin P., Saffell J.L. Morphoregulation by acetylcholinesterase in fibroblasts and astrocytes. J. Cell. Physiol. 2008;215:82–100. doi: 10.1002/jcp.21288. [DOI] [PubMed] [Google Scholar]
- 29.Voineagu I., Wang X., Johnston P., Lowe J.K., Tian Y., Horvath S., Mill J., Cantor R.M., Blencowe B.J., Geschwind D.H. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474:380–384. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young A.M., Campbell E., Lynch S., Suckling J., Powis S.J. Aberrant NF-kappaB expression in autism spectrum condition: a mechanism for neuroinflammation. Front. Psychiatry. 2011;2:27. doi: 10.3389/fpsyt.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young A.M.H., Chakrabarti B., Roberts D., Lai M.-C., Suckling J., Baron-Cohen S. From molecules to neural morphology: understanding neuroinflammation in autism spectrum condition. Mol. Autism. 2016;7:9. doi: 10.1186/s13229-016-0068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mancina R.M., Dongiovanni P., Petta S., Pingitore P., Meroni M., Rametta R., Borén J., Montalcini T., Pujia A., Wiklund O. The MBOAT7-TMC4 Variant rs641738 Increases Risk of Nonalcoholic Fatty Liver Disease in Individuals of European Descent. Gastroenterology. 2016;150:1219–1230.e6. doi: 10.1053/j.gastro.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee H.C., Inoue T., Sasaki J., Kubo T., Matsuda S., Nakasaki Y., Hattori M., Tanaka F., Udagawa O., Kono N. LPIAT1 regulates arachidonic acid content in phosphatidylinositol and is required for cortical lamination in mice. Mol. Biol. Cell. 2012;23:4689–4700. doi: 10.1091/mbc.E12-09-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.