Abstract
Chromodomain helicase DNA-binding protein 4 (CHD4) is an ATP-dependent chromatin remodeler involved in epigenetic regulation of gene transcription, DNA repair, and cell cycle progression. Also known as Mi2β, CHD4 is an integral subunit of a well-characterized histone deacetylase complex. Here we report five individuals with de novo missense substitutions in CHD4 identified through whole-exome sequencing and web-based gene matching. These individuals have overlapping phenotypes including developmental delay, intellectual disability, hearing loss, macrocephaly, distinct facial dysmorphisms, palatal abnormalities, ventriculomegaly, and hypogonadism as well as additional findings such as bone fusions. The variants, c.3380G>A (p.Arg1127Gln), c.3443G>T (p.Trp1148Leu), c.3518G>T (p.Arg1173Leu), and c.3008G>A, (p.Gly1003Asp) (GenBank: NM_001273.3), affect evolutionarily highly conserved residues and are predicted to be deleterious. Previous studies in yeast showed the equivalent Arg1127 and Trp1148 residues to be crucial for SNF2 function. Furthermore, mutations in the same positions were reported in malignant tumors, and a de novo missense substitution in an equivalent arginine residue in the C-terminal helicase domain of SMARCA4 is associated with Coffin Siris syndrome. Cell-based studies of the p.Arg1127Gln and p.Arg1173Leu mutants demonstrate normal localization to the nucleus and HDAC1 interaction. Based on these findings, the mutations potentially alter the complex activity but not its formation. This report provides evidence for the role of CHD4 in human development and expands an increasingly recognized group of Mendelian disorders involving chromatin remodeling and modification.
Main Text
In the past decade, we have witnessed a dramatic increase in gene discovery of numerous Mendelian disorders associated with intellectual disability. These efforts have shed light on multiple developmental pathways, including the importance of the epigenetic machinery in neuronal development and homeostasis.1, 2, 3 Chromatin remodeling is an epigenetic mechanism that controls DNA accessibility to transcription, replication, and repair machineries. It is driven by nucleosome remodeling complexes that contain ATP-dependent enzymes able to mobilize nucleosomes and modify DNA packaging.4 One of these ATPases is the chromodomain-helicase-DNA-binding protein 4 (CHD4) also known as Mi2-β.5, 6, 7 CHD4 is a core component of the nucleosome remodeling and deacetylase (NuRD) complex, which possesses both chromatin remodeling and histone deacetylation activities.8, 9, 10, 11 Both CHD4 and NuRD have been studied extensively for their role in stem cell differentiation, embryonic development, and oncogenesis.9, 12 For instance, depletion of CHD4 from certain mammalian embryonic tissues resulted in altered development13, 14, 15, 16, 17 and somatic mutations in CHD4 (MIM: 603277) were reported in serous endometrial carcinoma.18, 19 Here we report five individuals with a form of syndromic intellectual disability that carry de novo missense variants in CHD4.
The subjects underwent whole-exome sequencing at four different institutions. They were clinically assessed by experienced clinical geneticists prior to testing and did not have a diagnosis of a known genetic syndrome. Institutional review board-approved consents for whole-exome sequencing were obtained for all subjects. Subject 1 participated in a research project for undiagnosed developmental disorders at the National Human Genome Research Institute (NIH/NHGRI). Subject 2 underwent clinical exome sequencing at the University Medical Center Utrecht, the Netherlands,20 and subject 3 participated in the Deciphering Developmental Disorders (DDD) project in the UK.21 A de novo missense variant in the C-terminal helicase domain of CHD4 was independently selected as the leading candidate variant in these three index subjects based on the gene function, sequence conservation, in silico predictions of deleteriousness, and the absence from the Exome Aggregation Consortium (ExAC) database of 60,700 exomes. The three index subjects were matched using GeneMatcher22 and the Decipher website. We then carefully compared their clinical history and physical exams and verified that all individuals had a similar phenotype. Subsequently, we identified subjects 4 and 5 who previously underwent clinical exome sequencing at the Baylor-Miraca Genetics Laboratories (Baylor College of Medicine [BCM]). For each subject, information on additional candidate variants and previous genetic testing is detailed in the Supplemental Data.
For subject 1, whole-exome sequencing was performed at the NIH Intramural Sequencing Center (NISC) using the SeqCap EZ Exome v.3.0 capture kit (Roche NimbleGen) and the Illumina HiSeq2500 platform. Sequencing data were aligned to the human reference genome using Novoalign (Novocraft Technologies). Variants were called using the in-house MPG genotype caller. Detected variants were annotated and filtered using VarSifter.23 Average coverage attained was 65× with on average 95% of targeted bases covered at 10×. For subject 2, exomes were enriched using the SureSelect XT Human All Exon V5 kit (Agilent Technologies) and sequenced in rapid run mode on the HiSeq2500 sequencing system at a mean target depth of 100× and an average 95% of targeted bases covered at 10×. Reads were aligned to hg19 using BWA (BWA-MEM v.0.7.5a) and variants were called using the GATK haplotype caller (v.2.7-2). Detected variants were annotated, filtered, and prioritized using the Bench lab NGS v.3.1.2 platform (Cartagenia). For subject 3 the methods are described in Firth et al.24 For subjects 4 and 5, whole-exome sequencing and analysis was performed according to the protocol described in Yang et al.25 In summary, exomes were captured with the Roche NimbleGen VCRome reagent and sequenced using Illumina technology. Reads were aligned using the Mercury pipeline and annotated using the Cassandra software. Average coverage attained was 123× for individual 4 and 160× for individual 5, with on average 97.9% and 98.4% of targeted bases covered at 20×, respectively. The variants we detected were c.3380G>A (p.Arg1127Gln) (seen in subjects 1 and 3), c.3443G>T (p.Trp1148Leu), c.3518G>T (p.Arg1173Leu), and c.3008G>A (p.Gly1003Asp) (GenBank: NM_001273.3). All variants were confirmed as de novo by Sanger sequencing using standard methods and are available upon request.
Frequent findings included a history of developmental delay (5/5), hypotonia (4/5), mild to moderate intellectual disability (4/5), and hearing loss (4/5). The brain MRI demonstrated mild to moderate enlargement of the lateral ventricles in all subjects. Physical exam was significant for macrocephaly (4/5), palatal abnormalities (4/5), and similar facial dysmorphisms (5/5) (e.g., wide-spaced eyes, a square-shaped face, and external ear anomalies) (Figure 1). In addition, the three male subjects had hypogonadotrophic hypogonadism. In subjects 3 and 4, there was a history of short stature, and subject 3 was treated for growth hormone deficiency. Additional congenital anomalies that were seen in two subjects include cervical vertebrae fusions, tarsal coalitions, and heart defects. A summary of the clinical findings is shown in Table 1 and detailed case descriptions are in the Supplemental Data. Overall there were similar facial features and clinical histories, but each of the shared clinical finding were relatively non-specific, making it difficult to make a diagnosis without genotypic data. Furthermore, a few subjects had unusual clinical findings not seen in the others, e.g., congenital stroke and moyamoya disease in subject 1, severe developmental and growth delay in subject 4, and eye abnormalities in subject 5. Increased phenotypic heterogeneity has been reported before in Mendelian disorders of the epigenetic machinery and may be the result of genetic variation in downstream targets.1
Figure 1.
Facial Dysmorphism in Subjects Harboring CHD4 Mutations
From left to right: pictures of subjects 1, 2, and 5 at the age of 10 years, 12 months, and 18 years (top) and 1 year (bottom), respectively. There are similar subtle dysmorphic features that include macrocephaly, wide-spaced eyes, fullness of eyelids, a squared face, and low-set, small, or cup-shaped ears.
Table 1.
Clinical Findings in Five Subjects with De Novo Missense Variants in CHD4
| Subject 1 | Subject 2 | Subject 3 | Subject 4 | Subject 5 | |
|---|---|---|---|---|---|
| CHD4 variant | c.3380G>A (p.Arg1127Gln) | c.3518G>T (p.Arg1173Leu) | c.3380G>A (p.Arg1127Gln) | c.3443G>T (p.Trp1148Leu) | c.3008G>A (p.Gly1003Asp) |
| Gender, age at last exam | male, 10 years | female, 16 years | male, 10 years | female, 5 years | male, 18 years |
| Birth weight, OFC | 4 kg, 38 cm | 2.8 kg, ND | 3.7 kg, 37cm | 2.99 kg, 35 cm | 3.06 kg, ND |
| Height, OFC at last exama | 143 cm (75th), 56 cm (>98th) | 161 cm (40th), 62 cm (>98th) | 140 cmb (50th), 56 cm (>98th) | 89.5 cm (<3rd; Z score −5), 49 cm (20th) | 167.5 cm (10th), 52.5 cm at 4 years (90th) |
| Developmental delay | + | + | + | + (severe) | + |
| Intellectual disability | + | + | + (mild) | + | + (mild) |
| Hearing lossc | + | + | + | − | + |
| Undescended testis, micropenis | +, + | NA | +, + | NA | −, + |
| Macrocephalyd | + | + | + | relative to length | +e |
| Widely spaced eyesf | + | + | + | + | + |
| Dysmorphic earsg | + | + | + | + | + |
| Palatal anomalies | +h | − | +i | +i | +i |
| Hypogonadotropic hypogonadism | + | − | + | NT | + |
| Skeletal survey | advanced bone age by 2–3 years | tarsal coalition, cervical vertebrae fusion | falx calcification | scoliosis, platybasia, fusion of C2-C3, bilateral coxa valga, fusion of the cuboid and the 3rd cuneiforms bilaterally, brachymesophalangia | diffusely osteopenic bones |
| Brain MRI | enlarged lateral ventricles, congenital stroke with moyamoya disease | enlarged lateral ventricles, chiari 1 malformation | enlarged lateral ventricles | enlarged ventricles (mild), basilar, invagination and narrow foramen mangum | enlarged lateral and third ventricles |
| Heart | – | – | – | congenital heart defect (PDA s/p ligation, PFO, ASD, and VSD) | ASD, PDA s/p repair, VSD, bicuspid aortic valve, mild dilatation of aortic root |
Abbreviations are as follows: ASD, atrial septal defect; NT, not tested; NA, not applicable; OFC, occipital frontal circumference; PDA, patent ductus arteriosus; VSD, ventricular septal defect.
Data in parentheses are percentiles.
On growth hormone therapy.
Conductive and/or sensorineural hearing loss.
Head circumference >97th percentile for age and sex.
Current OFC unavailable, 90th percentile at the age of 4 years.
Inner canthal distance >97th for age 50.
See a description of ear anomalies in Figure 1.
Bifid uvula.
Hypernasal speech and or velopharyngeal insufficiency/submucosal cleft palate.
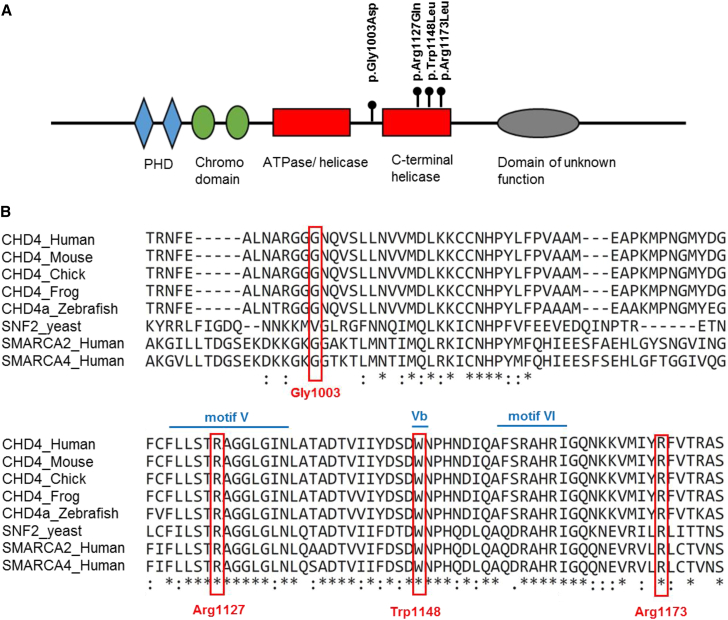
CHD4 belongs to the CHD subfamily II. Similarly to CHD3 and CHD5, CHD4 contains two N-terminal plant homeodomain (PHD) zinc fingers and tandem chromodomains in addition to centrally located ATPase/helicase domains.7 The helicase domains provide the energy necessary for nucleosome remodeling and resemble SNF2, the catalytic subunit of the chromatin remodeling SWI/SNF complex in yeast. The PHD and chromodomains are thought to direct CHD4 to its substrates and regulate the remodeling activity.26, 27 The three variants detected in subjects 1–4 were in the C-terminal helicase domain and subject 5’s variant was between helicase domains (Figure 2A). The involved amino acids are highly conserved across species and other ATP-dependent chromatin remodelers, as shown in Figure 2B. Of note, SNF2 contains conserved motifs that were previously shown to be critical for ATP binding and nucleosome remodeling.28, 29 Specifically, p.Arg1127Gln and p.Trp1148Leu are within motif V and Vb, respectively, and involve residues shown to be crucial for nucleosome remodeling activity in yeast.28 The Combined Annotation Dependent Depletion (CADD) phred score30 was above 26 for all the variants and they were predicted damaging by Provean and SIFT.31, 32 Furthermore, Samocha et al. list CHD4 as one of the top 1,000 genes with excessive constraint to both missense and loss-of-function (LOF) variants.33
Figure 2.
CHD4 Variants and Amino Acid Sequence Conservation
(A) CHD4 protein domains and location of amino acid substitutions. Abbreviation: PHD, plant homeodomain zinc fingers.
(B) Protein alignment of CHD4 orthologs across several vertebrate species and yeast. We also aligned with the ATP-dependent helicase SMARCA2 and SMARCA4. The Arg1127, Trp1148, and Arg1173 positions are conserved down to yeast. The p.Arg1127Gln and p.Trp1148Leu variants are located at the helicase motifs V and Vb, respectively.6, 28
Among the CHD4 paralogs, CHD7 (MIM: 608892), CHD2 (MIM: 615369), and CHD8 (MIM: 610528) have been associated with neurodevelopmental disorders.34, 35, 36 Interestingly, there are several similarities between the CHD4-associated phenotype and CHARGE syndrome (MIM: 214800), which results from haploinsufficiency of CHD7. Those include developmental delay, hearing loss, external ear anomalies, palatal abnormalities, a square-shaped face, and pituitary deficiencies. This correlation may suggest common downstream epigenetic targets, such as TP53, which is downregulated by both CHD7 and CHD4.37, 38 In addition to the CHD protein family, there are other proteins with similar ATP-dependent chromatin remodeling activity, such as the ATP-dependent helicase SMARCA2, SMARCA4, and ATRX, which are associated with neurodevelopmental syndromes.39, 40, 41, 42 The SMARCA4 (MIM: 603254) missense substitution c.3469C>G (p.Arg1157Gln) (GenBank: NM_003072.3) equivalent to p.Arg1127Gln in CHD4 has been previously reported in a person with Coffin-Siris syndrome39 (MIM: 614609), providing additional support for the pathogenicity of substitutions in this amino acid residue.
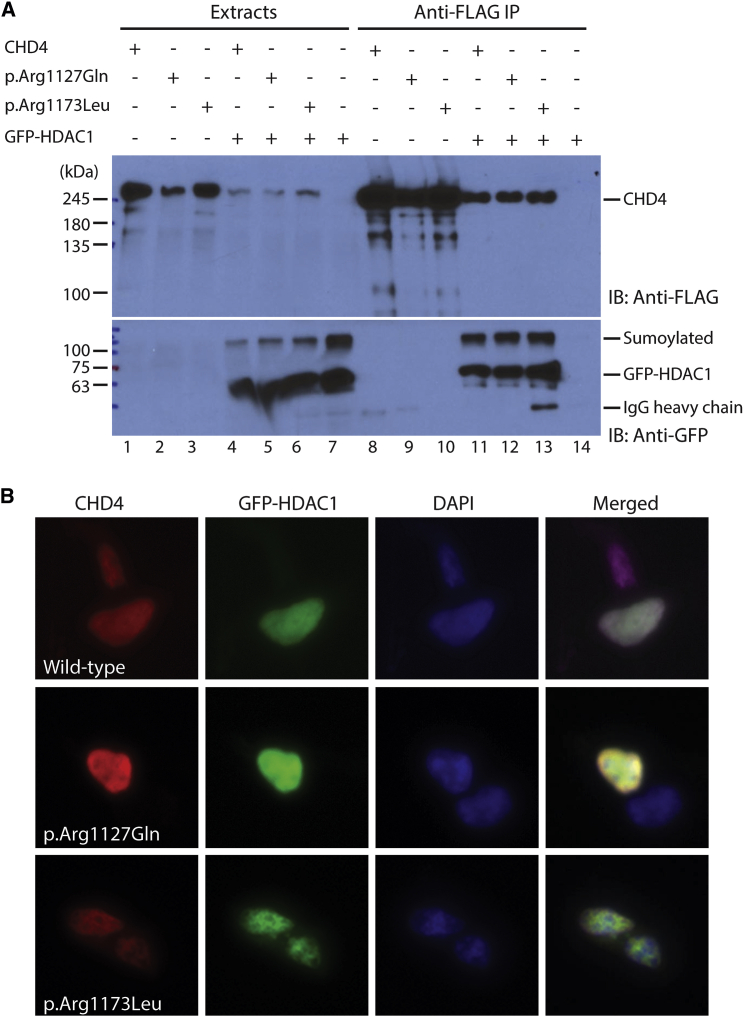
As described above, three of the substitutions are localized to the C-terminal helicase domain of CHD4 (Figure 2A).10 Co-immunoprecipitation and western blot analysis revealed that the c.3380G>A (p.Arg1127Gln) and c.3518G>T (p.Arg1173Leu) substitutions did not affect interaction with HDAC1 (Figure 3A), and immunofluorescence staining showed that similar to the wild-type protein, these mutants localized properly to the nucleus along with HDAC1 (Figure 3B). Based on their results, we do not expect these substitutions to directly affect CHD4 complex formation with HDAC1 and HDAC2. Consistent with this, the substitutions are located within the helicase domain (Figure 2A), away from the PHD fingers that are known to mediate HDAC1/2 binding.10 The substitutions may disrupt the ATPase activity of CHD4, and further experiments will be needed to determine this possibility.
Figure 3.
Comparison of Wild-Type and Mutant CHD4 Proteins by Cell-Based Assays
(A) The two mutations do not change HDAC1 interaction. Wild-type CHD4 and the two mutants were transiently expressed in HEK293 cells as FLAG-tagged proteins with or without GFP-HDAC1. Soluble extracts were prepared ∼36 hr after transfection for immunoprecipitation (IP) on anti-FLAG antibody conjugated to agarose, and bound proteins were eluted with FLAG peptide for immunoblotting with an anti-FLAG monoclonal antibody. After extensive washing, bound proteins were eluted with FLAG peptide for immunoblotting with anti-FLAG and -GFP antibodies as indicated. HDAC1 is known to be efficiently sumoylated.49
(B) Mutations do not affect CHD4 nuclear localization. Wild-type CHD4 and two mutants were expressed in HEK293 cells as FLAG-tagged proteins along with a green fluorescent protein (GFP)-HDAC1 fusion protein. Cells were fixed for indirect immunofluorescence microscopy with the anti-FLAG antibody and a Cy5-conjugated secondary antibody to detect CHD4 and its mutants. Green fluorescence was used an indicator of HDAC1 levels and nuclear DNA was detected with DAPI staining. The merged images are shown at the right column. HEK293 cell transfection, indirect immunofluorescence microscopy, and immunoprecipitation were carried out as described.50
Note: The residual heavy chain on lane 13 is due to some anti-FLAG agarose beads that were incidentally collected when the eluate was transferred out by pipetting.
According to the Mouse Gene Expression Database, Chd4 is broadly expressed in the mouse embryo and highly expressed in the head (brain, ear, and eye), the central nervous system in general, and the genitourinary system. O’Shaughnessy-Kirwan et al. demonstrated that null Chd4 mouse embryos cannot complete the first lineage step at the blastocyst stage.43 In the developing central nervous system of mice, the lack of Chd4 resulted in loss of inhibition of astroglial differentiation and impaired synaptic connectivity.13, 17 Furthermore, the International Mouse Phenotyping Consortium (IMPC) provides phenotypic information on a Chd4 knock-out mouse model resulting from a deletion of the critical exons 11 and 12 in the chromodomains region. Mice homozygous for the targeted deletion are embryonic lethal prior to organogenesis. The heterozygous mice are viable and exhibit several abnormalities that overlap with the phenotype seen in humans. There was decreased hearing with abnormal brainstem auditory evoked potentials at 24 kHz, and abnormal locomotor activation with decreased whole arena average speed that may be secondary to developmental delay. In addition, in some of the mutant mice, there was a significant decrease in the lean body mass and body length, abnormal left ventricle morphology, and abnormal lens morphology. Of note, these results are based on the evaluation of 16 mutants (8 females and 8 males). Although QC was completed and p values were significant, further studies are needed to support these findings.
The phenotype seen in the heterozygous knock-out mice might indicate that the phenotype seen in humans resulted from complete CHD4 loss of function or partial loss of function of the helicase domain. On the other hand, we are not aware of case reports of small microdeletions that include CHD4 or individuals with truncating mutations. Interestingly, mainly nonsynonymous substitutions in the ATP-dependent helicases SMARCA4 and SMARCA2 (MIM: 600014) cause Coffin Siris syndrome and Nicolaides-Baraitser syndrome (MIM: 601358), respectively. The proposed mechanism in those cases is a dominant-negative effect of the abnormal protein on the activity of the SWI/SNF complex.3, 44 If that is the case in the CHD4-related syndrome, we expect to see a different or less severe phenotype in individuals with CHD4 deletions or truncating mutations. Of note, the ExAC database includes six LOF variants in CHD4. These could be explained by sequencing/alignment errors (5/6 are indels) or a mild under-recognized phenotype. As mentioned before, there is significant intolerance to LOF variation relative to the gene’s size, but at this time it is not clear whether carriers of truncating mutations will be similarly affected.
CHD4 and NuRD act mainly but not exclusively through transcriptional repression.45 Several studies have shown that CHD4 has a role in DNA damage response and cell cycle progression either independently or as part of the NuRD complex, and it may also function as an oncogene, a tumor suppressor, or both.37, 46 Le Gallo et al. reported somatic mutations in CHD4 in 17% of endometrial tumors.18 Most of the mutations detected resulted in nonsynonymous substitutions, and roughly half of them clustered in the ATPase/C-terminal helicase domain. Interestingly, when they performed alignments with SMARCAL1 (MIM: 606622), SMARCA4, and SMARCA2, they found that in 2/3 of the cases, the same residues were reported to undergo germline de novo changes causing Schimke immune-osseous dysplasia (MIM: 242900), Coffin-Siris syndrome, or Nicolaides-Baraitser syndrome. This observation led them to speculate that somatic mutations in the C-terminal helicase domain of CHD4 are molecular drivers of endometrial cancer progression. Additionally, Zhao et al.19 reported an increase in the frequency of somatic CHD4 mutations in endometrial tumors. Interestingly, one of the variants (p.Arg1127Gly) affects the same arginine residue seen in two of our subjects. According to the Cosmic database of genetic variations in tumors, the p.Arg1127Gln variant was identified in gastric tumors and an p.Arg1173Trp mutant was reported in hematologic tumors. The subjects in this study do not have a history of cancer, but we cannot discard the possibility that they will develop malignant tumors later in life. Further reports of individuals with germline mutations in CHD4 are required to determine the risk of cancer in these individuals. Of note, somatic mutations in SMARCA2 and SMRACA4 are seen in different types of cancer.47 An increased risk for malignancy in individuals with Coffin-Siris and Nicolaides-Baraitser syndrome has been debated but has not yet been clinically proven.48
In summary, we introduce an intellectual disability syndrome associated with macrocephaly, facial dysmorphisms, hearing loss, ventriculomegaly, hypogonadism, and various congenital anomalies including heart defects and bone fusions. This report provides insight on the role of CHD4 during human development and expands the increasingly recognized group of Mendelian disorders of chromatin remodeling. This is intriguing because CHD4 is not only a chromatin remodeler but also a critical subunit of a multiprotein histone deacetylase complex, suggesting that alteration in chromatin modeling and histone acetylation may be the culprit. Future descriptions of individuals with this condition will be needed to better understand the phenotypic variability and establish genotype-phenotype correlations. In this study we successfully applied the recently available tool of web-based gene matching and the mouse phenotyping consortium. It provides yet another example of the utility of data sharing in facilitating gene discovery in rare syndromes.
Acknowledgments
We are grateful to the patients and their families for consenting to participate in this publication. The DDD study presents independent research commissioned by the Health Innovation Challenge Fund (grant number HICF-1009-003), a parallel funding partnership between the Wellcome Trust and the Department of Health, and the Wellcome Trust Sanger Institute (grant number WT098051). The views expressed in this publication are those of the author(s) and not necessarily those of the Wellcome Trust or the Department of Health. The study has UK Research Ethics Committee approval (10/H0305/83, granted by the Cambridge South REC, and GEN/284/12, granted by the Republic of Ireland REC). The research team acknowledges the support of the National Institute for Health Research, through the Comprehensive Clinical Research Network. P.M.C. is funded by the Canadian Institutes of Health Research Grants RN315908 and RN324373 and the Fonds de Recherche du Québec Santé grant 30647. We are thankful to the NIH Intramural Sequencing Center (NISC) for supporting sequencing in this project. This work was supported by the intramural program of the National Human Genome Research Institute, NIH.
Published: September 8, 2016
Footnotes
Supplemental Data include case reports and one table and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2016.08.001.
Contributor Information
Philippe M. Campeau, Email: p.campeau@umontreal.ca.
Maximilian Muenke, Email: mmuenke@nhgri.nih.gov.
Web Resources
1000 Genomes, http://www.1000genomes.org
COSMIC, http://cancer.sanger.ac.uk/cancergenome/projects/cosmic/
DECIPHER, http://decipher.sanger.ac.uk/
ExAC Browser, http://exac.broadinstitute.org/
GenBank, http://www.ncbi.nlm.nih.gov/genbank/
GeneMatcher, https://genematcher.org/
OMIM, http://www.omim.org/
PROVEAN, http://provean.jcvi.org
UCSC Genome Browser, http://genome.ucsc.edu
UniProt, http://www.uniprot.org/
Supplemental Data
References
- 1.Bjornsson H.T. The Mendelian disorders of the epigenetic machinery. Genome Res. 2015;25:1473–1481. doi: 10.1101/gr.190629.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.López A.J., Wood M.A. Role of nucleosome remodeling in neurodevelopmental and intellectual disability disorders. Front. Behav. Neurosci. 2015;9:100. doi: 10.3389/fnbeh.2015.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santen G.W., Kriek M., van Attikum H. SWI/SNF complex in disorder: SWItching from malignancies to intellectual disability. Epigenetics. 2012;7:1219–1224. doi: 10.4161/epi.22299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hargreaves D.C., Crabtree G.R. ATP-dependent chromatin remodeling: genetics, genomics and mechanisms. Cell Res. 2011;21:396–420. doi: 10.1038/cr.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seelig H.P., Moosbrugger I., Ehrfeld H., Fink T., Renz M., Genth E. The major dermatomyositis-specific Mi-2 autoantigen is a presumed helicase involved in transcriptional activation. Arthritis Rheum. 1995;38:1389–1399. doi: 10.1002/art.1780381006. [DOI] [PubMed] [Google Scholar]
- 6.Woodage T., Basrai M.A., Baxevanis A.D., Hieter P., Collins F.S. Characterization of the CHD family of proteins. Proc. Natl. Acad. Sci. USA. 1997;94:11472–11477. doi: 10.1073/pnas.94.21.11472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall J.A., Georgel P.T. CHD proteins: a diverse family with strong ties. Biochem. Cell Biol. 2007;85:463–476. doi: 10.1139/O07-063. [DOI] [PubMed] [Google Scholar]
- 8.Xue Y., Wong J., Moreno G.T., Young M.K., Côté J., Wang W. NURD, a novel complex with both ATP-dependent chromatin-remodeling and histone deacetylase activities. Mol. Cell. 1998;2:851–861. doi: 10.1016/s1097-2765(00)80299-3. [DOI] [PubMed] [Google Scholar]
- 9.Lai A.Y., Wade P.A. Cancer biology and NuRD: a multifaceted chromatin remodelling complex. Nat. Rev. Cancer. 2011;11:588–596. doi: 10.1038/nrc3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y., LeRoy G., Seelig H.P., Lane W.S., Reinberg D. The dermatomyositis-specific autoantigen Mi2 is a component of a complex containing histone deacetylase and nucleosome remodeling activities. Cell. 1998;95:279–289. doi: 10.1016/s0092-8674(00)81758-4. [DOI] [PubMed] [Google Scholar]
- 11.Wade P.A., Jones P.L., Vermaak D., Wolffe A.P. A multiple subunit Mi-2 histone deacetylase from Xenopus laevis cofractionates with an associated Snf2 superfamily ATPase. Curr. Biol. 1998;8:843–846. doi: 10.1016/s0960-9822(98)70328-8. [DOI] [PubMed] [Google Scholar]
- 12.Basta J., Rauchman M. The nucleosome remodeling and deacetylase complex in development and disease. Transl. Res. 2015;165:36–47. doi: 10.1016/j.trsl.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamada T., Yang Y., Hemberg M., Yoshida T., Cho H.Y., Murphy J.P., Fioravante D., Regehr W.G., Gygi S.P., Georgopoulos K., Bonni A. Promoter decommissioning by the NuRD chromatin remodeling complex triggers synaptic connectivity in the mammalian brain. Neuron. 2014;83:122–134. doi: 10.1016/j.neuron.2014.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denner D.R., Rauchman M. Mi-2/NuRD is required in renal progenitor cells during embryonic kidney development. Dev. Biol. 2013;375:105–116. doi: 10.1016/j.ydbio.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linder B., Mentele E., Mansperger K., Straub T., Kremmer E., Rupp R.A. CHD4/Mi-2beta activity is required for the positioning of the mesoderm/neuroectoderm boundary in Xenopus. Genes Dev. 2007;21:973–983. doi: 10.1101/gad.409507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ingram K.G., Curtis C.D., Silasi-Mansat R., Lupu F., Griffin C.T. The NuRD chromatin-remodeling enzyme CHD4 promotes embryonic vascular integrity by transcriptionally regulating extracellular matrix proteolysis. PLoS Genet. 2013;9:e1004031. doi: 10.1371/journal.pgen.1004031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sparmann A., Xie Y., Verhoeven E., Vermeulen M., Lancini C., Gargiulo G., Hulsman D., Mann M., Knoblich J.A., van Lohuizen M. The chromodomain helicase Chd4 is required for Polycomb-mediated inhibition of astroglial differentiation. EMBO J. 2013;32:1598–1612. doi: 10.1038/emboj.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Gallo M., O’Hara A.J., Rudd M.L., Urick M.E., Hansen N.F., O’Neil N.J., Price J.C., Zhang S., England B.M., Godwin A.K., NIH Intramural Sequencing Center (NISC) Comparative Sequencing Program Exome sequencing of serous endometrial tumors identifies recurrent somatic mutations in chromatin-remodeling and ubiquitin ligase complex genes. Nat. Genet. 2012;44:1310–1315. doi: 10.1038/ng.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao S., Choi M., Overton J.D., Bellone S., Roque D.M., Cocco E., Guzzo F., English D.P., Varughese J., Gasparrini S. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proc. Natl. Acad. Sci. USA. 2013;110:2916–2921. doi: 10.1073/pnas.1222577110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monroe G.R., Frederix G.W., Savelberg S.M., de Vries T.I., Duran K.J., van der Smagt J.J., Terhal P.A., van Hasselt P.M., Kroes H.Y., Verhoeven-Duif N.M. Effectiveness of whole-exome sequencing and costs of the traditional diagnostic trajectory in children with intellectual disability. Genet. Med. 2016;9:999. doi: 10.1038/gim.2015.200. [DOI] [PubMed] [Google Scholar]
- 21.Deciphering Developmental Disorders Study Large-scale discovery of novel genetic causes of developmental disorders. Nature. 2015;519:223–228. doi: 10.1038/nature14135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sobreira N., Schiettecatte F., Valle D., Hamosh A. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum. Mutat. 2015;36:928–930. doi: 10.1002/humu.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teer J.K., Green E.D., Mullikin J.C., Biesecker L.G. VarSifter: visualizing and analyzing exome-scale sequence variation data on a desktop computer. Bioinformatics. 2012;28:599–600. doi: 10.1093/bioinformatics/btr711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Firth H.V., Wright C.F., DDD Study The Deciphering Developmental Disorders (DDD) study. Dev. Med. Child Neurol. 2011;53:702–703. doi: 10.1111/j.1469-8749.2011.04032.x. [DOI] [PubMed] [Google Scholar]
- 25.Yang Y., Muzny D.M., Xia F., Niu Z., Person R., Ding Y., Ward P., Braxton A., Wang M., Buhay C. Molecular findings among patients referred for clinical whole-exome sequencing. JAMA. 2014;312:1870–1879. doi: 10.1001/jama.2014.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morra R., Lee B.M., Shaw H., Tuma R., Mancini E.J. Concerted action of the PHD, chromo and motor domains regulates the human chromatin remodelling ATPase CHD4. FEBS Lett. 2012;586:2513–2521. doi: 10.1016/j.febslet.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watson A.A., Mahajan P., Mertens H.D., Deery M.J., Zhang W., Pham P., Du X., Bartke T., Zhang W., Edlich C. The PHD and chromo domains regulate the ATPase activity of the human chromatin remodeler CHD4. J. Mol. Biol. 2012;422:3–17. doi: 10.1016/j.jmb.2012.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richmond E., Peterson C.L. Functional analysis of the DNA-stimulated ATPase domain of yeast SWI2/SNF2. Nucleic Acids Res. 1996;24:3685–3692. doi: 10.1093/nar/24.19.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouazoune K., Kingston R.E. Chromatin remodeling by the CHD7 protein is impaired by mutations that cause human developmental disorders. Proc. Natl. Acad. Sci. USA. 2012;109:19238–19243. doi: 10.1073/pnas.1213825109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kircher M., Witten D.M., Jain P., O’Roak B.J., Cooper G.M., Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 2014;46:310–315. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi Y., Chan A.P. PROVEAN web server: a tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics. 2015;31:2745–2747. doi: 10.1093/bioinformatics/btv195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar P., Henikoff S., Ng P.C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 2009;4:1073–1081. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 33.Samocha K.E., Robinson E.B., Sanders S.J., Stevens C., Sabo A., McGrath L.M., Kosmicki J.A., Rehnström K., Mallick S., Kirby A. A framework for the interpretation of de novo mutation in human disease. Nat. Genet. 2014;46:944–950. doi: 10.1038/ng.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vissers L.E., van Ravenswaaij C.M., Admiraal R., Hurst J.A., de Vries B.B., Janssen I.M., van der Vliet W.A., Huys E.H., de Jong P.J., Hamel B.C. Mutations in a new member of the chromodomain gene family cause CHARGE syndrome. Nat. Genet. 2004;36:955–957. doi: 10.1038/ng1407. [DOI] [PubMed] [Google Scholar]
- 35.Chénier S., Yoon G., Argiropoulos B., Lauzon J., Laframboise R., Ahn J.W., Ogilvie C.M., Lionel A.C., Marshall C.R., Vaags A.K. CHD2 haploinsufficiency is associated with developmental delay, intellectual disability, epilepsy and neurobehavioural problems. J. Neurodev. Disord. 2014;6:9. doi: 10.1186/1866-1955-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernier R., Golzio C., Xiong B., Stessman H.A., Coe B.P., Penn O., Witherspoon K., Gerdts J., Baker C., Vulto-van Silfhout A.T. Disruptive CHD8 mutations define a subtype of autism early in development. Cell. 2014;158:263–276. doi: 10.1016/j.cell.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polo S.E., Kaidi A., Baskcomb L., Galanty Y., Jackson S.P. Regulation of DNA-damage responses and cell-cycle progression by the chromatin remodelling factor CHD4. EMBO J. 2010;29:3130–3139. doi: 10.1038/emboj.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Nostrand J.L., Brady C.A., Jung H., Fuentes D.R., Kozak M.M., Johnson T.M., Lin C.Y., Lin C.J., Swiderski D.L., Vogel H. Inappropriate p53 activation during development induces features of CHARGE syndrome. Nature. 2014;514:228–232. doi: 10.1038/nature13585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsurusaki Y., Okamoto N., Ohashi H., Kosho T., Imai Y., Hibi-Ko Y., Kaname T., Naritomi K., Kawame H., Wakui K. Mutations affecting components of the SWI/SNF complex cause Coffin-Siris syndrome. Nat. Genet. 2012;44:376–378. doi: 10.1038/ng.2219. [DOI] [PubMed] [Google Scholar]
- 40.Tsurusaki Y., Okamoto N., Ohashi H., Mizuno S., Matsumoto N., Makita Y., Fukuda M., Isidor B., Perrier J., Aggarwal S. Coffin-Siris syndrome is a SWI/SNF complex disorder. Clin. Genet. 2014;85:548–554. doi: 10.1111/cge.12225. [DOI] [PubMed] [Google Scholar]
- 41.Van Houdt J.K., Nowakowska B.A., Sousa S.B., van Schaik B.D., Seuntjens E., Avonce N., Sifrim A., Abdul-Rahman O.A., van den Boogaard M.J., Bottani A. Heterozygous missense mutations in SMARCA2 cause Nicolaides-Baraitser syndrome. Nat. Genet. 2012;44:445–449, S1. doi: 10.1038/ng.1105. [DOI] [PubMed] [Google Scholar]
- 42.Picketts D.J., Higgs D.R., Bachoo S., Blake D.J., Quarrell O.W., Gibbons R.J. ATRX encodes a novel member of the SNF2 family of proteins: mutations point to a common mechanism underlying the ATR-X syndrome. Hum. Mol. Genet. 1996;5:1899–1907. doi: 10.1093/hmg/5.12.1899. [DOI] [PubMed] [Google Scholar]
- 43.O’Shaughnessy-Kirwan A., Signolet J., Costello I., Gharbi S., Hendrich B. Constraint of gene expression by the chromatin remodelling protein CHD4 facilitates lineage specification. Development. 2015;142:2586–2597. doi: 10.1242/dev.125450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sousa S.B., Hennekam R.C., Nicolaides-Baraitser Syndrome International Consortium Phenotype and genotype in Nicolaides-Baraitser syndrome. Am. J. Med. Genet. C. Semin. Med. Genet. 2014;166C:302–314. doi: 10.1002/ajmg.c.31409. [DOI] [PubMed] [Google Scholar]
- 45.Reynolds N., O’Shaughnessy A., Hendrich B. Transcriptional repressors: multifaceted regulators of gene expression. Development. 2013;140:505–512. doi: 10.1242/dev.083105. [DOI] [PubMed] [Google Scholar]
- 46.Smeenk G., Wiegant W.W., Vrolijk H., Solari A.P., Pastink A., van Attikum H. The NuRD chromatin-remodeling complex regulates signaling and repair of DNA damage. J. Cell Biol. 2010;190:741–749. doi: 10.1083/jcb.201001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hodges C., Kirkland J.G., Crabtree G.R. The many roles of BAF (mSWI/SNF) and PBAF complexes in cancer. Cold Spring Harb. Perspect. Med. 2016;6:a026930. doi: 10.1101/cshperspect.a026930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kosho T., Miyake N., Carey J.C. Coffin-Siris syndrome and related disorders involving components of the BAF (mSWI/SNF) complex: historical review and recent advances using next generation sequencing. Am. J. Med. Genet. C. Semin. Med. Genet. 2014;166C:241–251. doi: 10.1002/ajmg.c.31415. [DOI] [PubMed] [Google Scholar]
- 49.David G., Neptune M.A., DePinho R.A. SUMO-1 modification of histone deacetylase 1 (HDAC1) modulates its biological activities. J. Biol. Chem. 2002;277:23658–23663. doi: 10.1074/jbc.M203690200. [DOI] [PubMed] [Google Scholar]
- 50.Walkinshaw D.R., Weist R., Kim G.W., You L., Xiao L., Nie J., Li C.S., Zhao S., Xu M., Yang X.J. The tumor suppressor kinase LKB1 activates the downstream kinases SIK2 and SIK3 to stimulate nuclear export of class IIa histone deacetylases. J. Biol. Chem. 2013;288:9345–9362. doi: 10.1074/jbc.M113.456996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.