Abstract
To safeguard the cell from the accumulation of potentially harmful metabolic intermediates, specific repair mechanisms have evolved. APOA1BP, now renamed NAXE, encodes an epimerase essential in the cellular metabolite repair for NADHX and NADPHX. The enzyme catalyzes the epimerization of NAD(P)HX, thereby avoiding the accumulation of toxic metabolites. The clinical importance of the NAD(P)HX repair system has been unknown. Exome sequencing revealed pathogenic biallelic mutations in NAXE in children from four families with (sub-) acute-onset ataxia, cerebellar edema, spinal myelopathy, and skin lesions. Lactate was elevated in cerebrospinal fluid of all affected individuals. Disease onset was during the second year of life and clinical signs as well as episodes of deterioration were triggered by febrile infections. Disease course was rapidly progressive, leading to coma, global brain atrophy, and finally to death in all affected individuals. NAXE levels were undetectable in fibroblasts from affected individuals of two families. In these fibroblasts we measured highly elevated concentrations of the toxic metabolite cyclic-NADHX, confirming a deficiency of the mitochondrial NAD(P)HX repair system. Finally, NAD or nicotinic acid (vitamin B3) supplementation might have therapeutic implications for this fatal disorder.
Keywords: metabolite repair, energy metabolism, NAD(P)HX, mitochondrial
Main Text
Whereas chemical modification or degradation and repair of macromolecules such as DNA and proteins are well-recognized processes, damage and repair of metabolites only recently has become an emerging field of research. Aberrant metabolites can arise either spontaneously or by enzymatic side reactions and can result in neutral or harmful species. To prevent toxic effects on the cell, damage-control systems have developed that either remove or recycle the aberrant metabolite.1 Nicotinamide adenine dinucleotide (reduced form, NADH; oxidized form, NAD+) and nicotinamide adenine dinucleotide phosphate (reduced form, NADPH; oxidized form, NADP+) are major redox equivalents in the cell involved in either catabolic or anabolic reactions as well as reactions involved in protection against reactive oxygen species (ROS). One of the double bonds of the nicotinamide ring present in NADH and NADPH is prone to hydration. This hydration can occur either spontaneously at mildly acidic pH or at elevated temperature (already relevant at 37°C) or enzymatically, e.g., as a side reaction of the glyceraldehyde 3-phosphate dehydrogenase (GAPDH). NAD(P)HX is formed as R- and S-epimers.2, 3 Neither NADHX nor NADPHX can act as electron donors or acceptors and have been shown to inhibit several dehydrogenases, making the aberrant metabolites not only dispensable but toxic. NAD(P)HX can further spontaneously react to cyclic-NAD(P)HX in an irreversible way (for schematic overview see Figure 1). Whereas no repair system is known for the cyclic form of NAD(P)HX, reconversion of NAD(P)HX to NAD(P)H is catalyzed by an ATP-dependent dehydratase.4 This dehydratase, now renamed NAXD (previously CARKD [MIM: 615910]), reacts only with the S-epimer of NAD(P)HX. In order to eliminate the R-epimer, a specific epimerase, which is encoded by NAXE in humans (MIM: 608862), catalyzes the conversion to S-NADHX. Deficiency of the epimerase in S. cerevisiae strains did not affect viability and growth.4, 5 Even in the multicellular organism A. thaliana, knockdown of the dehydratase resulted in no observable phenotype.6 Therefore, the physiological importance of this repair system remains unclear in these species. Effects of an enzyme deficiency in humans have not been analyzed so far.
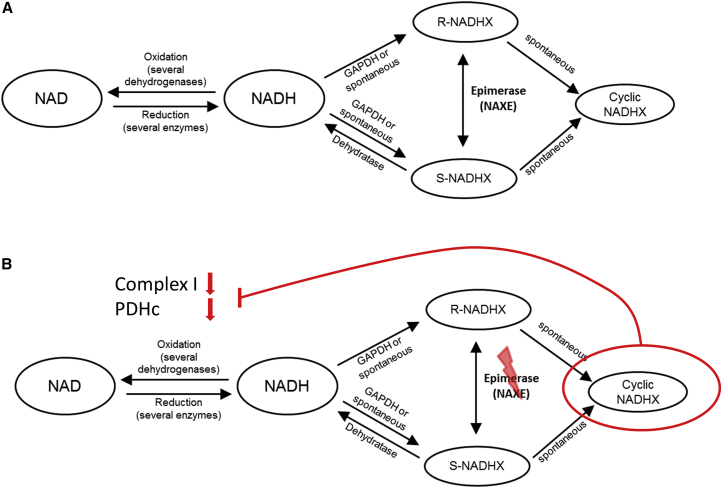
Figure 1.
Schematic Overview of NAD/NADH Metabolism and the NAD(P)HX Repair System
(A) NAD(P)HX repair system at metabolic equilibrium under normal/healthy conditions.
(B) Putative consequences of NAXE deficiency, leading to increased formation of cyclic NADHX, a toxic inhibitor of cellular NADH dehydrogenases.
In this study, we report on a joint investigation of whole-exome sequencing (WES) datasets of a cohort of 600 individuals recruited within the German and European network for mitochondrial disorders (mitoNET and GENOMIT) to elucidate the molecular basis of the disease using a previously described bioinformatic filtering pipeline7, 8 that identified three affected individuals carrying rare biallelic variants in NAXE. A fourth family with NAXE mutations was discovered by WES independently. The predominant clinical features found in these subjects include (sub-) acute onset of ataxia, cerebellar edema, spinal myelopathy, and skin lesions (summarized in Table 1). Neuroimaging findings and skin manifestations are depicted in Figure 2 and detailed case reports as well as additional neuroimaging findings are provided in the Supplemental Data. Written informed consent was obtained from the guardians of all individuals investigated. The study was approved by the local ethics committees.
Table 1.
Genetic and Clinical Findings in Individuals with Mutations in NAXE
| ID | Sex |
NAXE Nutations |
Clinical Features and Neuroimaging Findings |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| cDNA (NM_144772.2); Protein (NP_658985.2) | Age at Onset | Age at Death | Trigger at Initial Presentation | First Clinical Symptoms | Clinical Course | Cerebellar Edema/Brain Herniation | Myelopathy on MRI | Skin Manifestations | ||
| #1-1 | M | c.[177C>A];[177C>A]; p.[Tyr59∗];[Tyr59∗] | 20 months | 21 months | fever/infection | ataxia, torticollis, subacute tetraparesis, respiratory insufficiency | undulating disease course with episodes of stabilization, finally rapidly progressive with fatal outcome | yes | yes | yes |
| #1-2 | F | ND | 19 months | 24 months | fever/infection | acute onset ataxia, subacute tetraparesis, respiratory insufficiency | undulating disease course with episodes of stabilization, finally rapidly progressive with fatal outcome | unclear | yes | yes |
| #2 | F | c.[196C>T];[516+1G>A]; p.[Gln66∗];[?] | 15 months | 24 months | fever/infection | delayed development, muscular hypotonia and nystagmus, sudden deterioration with respiratory failure | undulating disease course with episodes of stabilization, finally rapidly progressive with fatal outcome | yes | yes | yes |
| #3 | M | c.[804_807delinsA];[804_807delinsA]; p.[Lys270del];[Lys270del] | 16 months | 18 months | fever/infection | delayed development, muscular hypotonia, subacute-onset ataxia and nystagmus | rapidly progressive with fatal outcome | yes | ND | no |
| #4-1 | M | c.[653A>T];[743delC]; p.[Asp218Val];[Ala248Glufs∗26] | 16 months | 16 months | unclear | acute-onset ataxia, nystagmus, muscle hypotonia, dysarthria | undulating disease course with episodes of stabilization, finally rapidly progressive with fatal outcome | yes | ND | yes |
| #4-2 | M | c.[653A>T];[743delC]; p.[Asp218Val];[Ala248Glufs∗26] | 8 months | 28 months | unclear | delayed development, seizures, muscular hypotonia | undulating disease course with episodes of stabilization, finally rapidly progressive with fatal outcome | unclear | ND | no |
Abbreviation is as follows: ND, not determined.
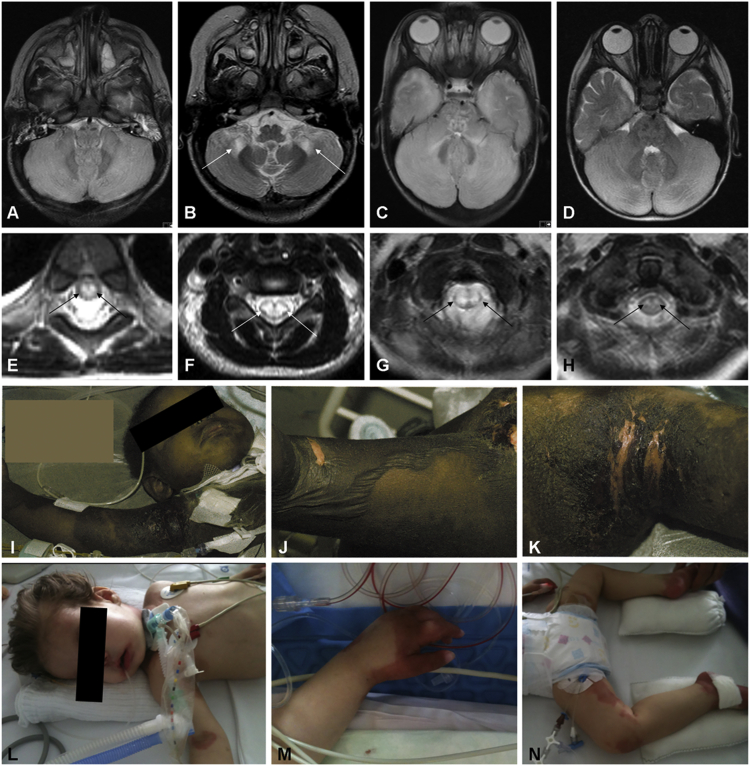
Figure 2.
Unifying Neuroimaging Findings and Skin Manifestations in Children with NAXE Mutations
(A) Brain MRI (axial view, T2-weighted) of individual #1-1, showing diffuse cerebellar edema.
(B) Brain MRI of individual #1-2 (axial view, T2-weighted), demonstrating symmetrical signal alterations in cerebellar white matter and cerebellar peduncles.
(C) Brain MRI (axial view, T2-weighted) in individual #2 showing diffuse cerebellar edema comparable to the findings in (A), leading to nearly fatal brain herniation.
(D) Brain MRI (axial view, T2-weighted) of individual #4-1, also demonstrating diffuse cerebellar edema.
(E–H) Spinal MRI (axial view, T2-weighted) in individuals #1-1 (cervical spine) (E), #1-2 (thoracic spine) (F), #2 (cervical spine) (G), and #4-1 (cervical spine) (H), showing symmetrical signal abnormalities of the spinal cord, indicating various degrees of myelopathy. Please note that additional imaging findings can be found in Figure S1.
(I–K) Extensive skin lesions in individual #1-2. Onset of skin manifestation was sub-acute within 2–3 weeks after the start of neurological symptoms. Large bullous skin lesions developed (J shows popliteal fossa), partially leading to erosion and eruption of the skin (K shows axilla).
(L–N) Skin lesions in individual #2.
Please also note the critical clinical condition of both affected individuals as indicated in (I) and (L).
Exome sequencing of affected individuals from families F1, F2, and F3 was performed in Germany using the SureSelect Human All Exon 50 Mb kit (Agilent) for in-solution enrichment of exonic regions followed by sequencing as 100 base pair (bp) paired-end runs on a HiSeq2500 (Illumina).7, 8 Read alignment to the human genome (UCSC Genome Browser build hg19) was done with Burrows-Wheeler Aligner (BWA, v.0.7.5a)9 and single-nucleotide variants and small insertions and deletions were detected with BWA (v.0.7.5a) and SAMtools (version 0.1.19). We achieved an average fold coverage between 134 and 148 with 97.5% of the exome covered at least 20-fold. Sequencing analysis of individuals #1-1, #2, and #3 did not identify mutations in disease-associated genes that would account for the phenotype. Per individual, ∼11,000 non-synonymous single-nucleotide variants (SNVs) were detected and subsequently filtered for a minor allele frequency < 0.1% in in-house database comprising 7,000 control exomes and for biallelic variants assuming a recessive mode of inheritance. We identified 567, 14, and 13 genes in individuals #1-1, #2, and #3, respectively, carrying potentially biallelic variants with NAXE variants being identified in all three (Figure 3 and Table 1). The high number of variants in #1-1 reflects the African ancestry of this individual and limited efficacy of the frequency filter based on mainly Central European control subjects. In individual #1-1 we detected a homozygous c.177C>A (p.Tyr59∗) variant, in individual #2 a compound heterozygous c.196C>T (p.Gln66∗) and a 516+1G>A splice variant, and in individual #3 a homozygous c.804_807delinsA (p.Lys270del) variant. In individuals #2 and #3, NAXE was the only gene encoding a protein with a reported mitochondrial localization.10 In the 7,000 in-house control exomes, no other individual was found with predicted pathogenic biallelic variants in NAXE. For all three index case subjects, fibroblast cell lines were available for functional studies. Very recently, a fourth subject with a suspected mitochondrial disorder was exome sequenced in Poland. The library was prepared using the Nextera Rapid Capture Exome kit (Illumina) followed by sequencing as 2× 75 bp paired-end reads on the HiSeq1500 achieving on average 49-fold coverage, with 92.7% of the exome covered more than 10-fold. Data were processed as described.11 In individual #4-1 a compound heterozygous frameshift (c.743delC) and missense mutation (c.653A>T) in NAXE was discovered (Figure 3). In this case, ∼13,000 non-synonymous single-nucleotide variants (SNVs) were detected and subsequently filtered for variants with a minor allele frequency < 0.1% in both in-house and public databases, identifying 147 variants. Filtering for predicted biallelic variants led to no identification of genes harboring variants in a homozygous state and only one gene harboring predicted pathogenic variants in a compound heterozygous state, NAXE.
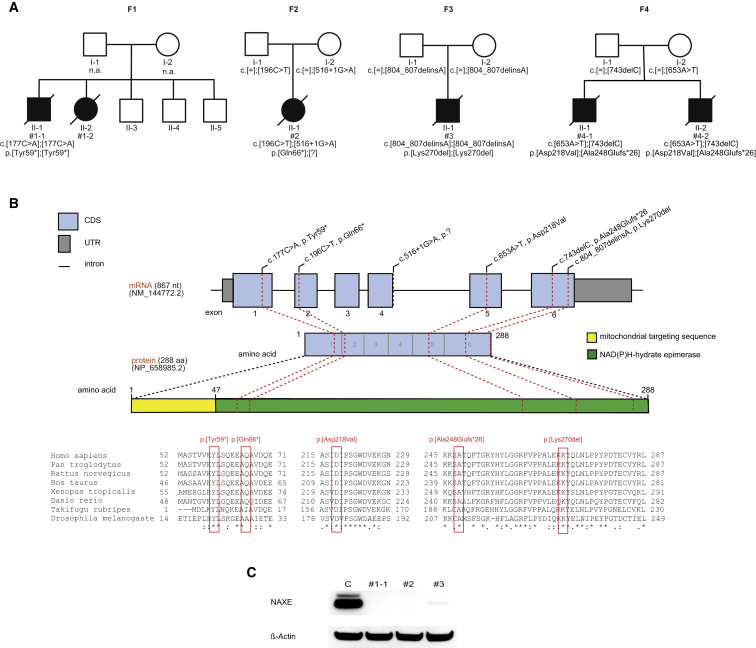
Figure 3.
Genetic Findings in Four NAXE-Deficient Families with Consequences on the Protein Level
(A) Pedigrees of four families identified with recessive inherited mutations in NAXE.
(B) Genomic organization of NAXE with known conserved protein domains in the gene product, location of mutations within NAXE, and phylogenetic conservation of amino acid residues affected by mutations; positions of mutations are highlighted in red.
(C) Western blot analysis of fibroblast from index case subjects with NAXE mutations and a control cell line. Fibroblasts were cultured in Dulbecco’s Modified Eagle’s medium (Life Technologies) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (both: Life Technologies) at 37°C in an atmosphere of 5% CO2 in air. Whole cell lysates were prepared with cell lysis reagent CelLytic M (Sigma Aldrich). A polyclonal antibody against NAXE detecting amino acid sequence 218 to 286 (HPA048164, Sigma Aldrich) was used. A monoclonal antibody against beta-actin (Sigma Aldrich) served as loading control. Secondary antibodies were anti-mouse and anti-rabbit (GE Healthcare). Signal was detected using BM chemiluminescence blotting substrate (Roche).
The homozygous loss-of-function variant in individual #1-1, the homozygous deletion of Lys270 in individual #3, and the two variants found in individual #4-1 were absent from public databases. The c.196C>T (p.Gln66∗) nonsense and c.516+1G>A splice variant detected in individual #2 were found four and one times only, respectively, in a heterozygous state in ∼120,000 alleles of the Exome Aggregation Consortium (ExAC) Server (Cambridge, MA [09/2015]). All variants were confirmed by Sanger sequencing with parents being heterozygous carriers shown for families #2, #3, and #4.
Taken together, the identification of biallelic NAXE mutations in five individuals from four families with a strikingly similar phenotype establishes NAXE variants to be confidently implicated in this infancy-onset disease characterized by (sub-) acute-onset ataxia, cerebellar edema, spinal myelopathy, and skin lesions, induced by febrile infections, leading to coma and finally to death within the first 3 years of life.
Whereas the NAXE mutations found in individuals #1-1 and #2 are expected to result in a truncated protein being non-functional, the consequence of the Lys70 deletion on protein stability was unclear. Western blot analysis on fibroblasts derived from individuals #1, #2, and #3 revealed loss of NAXE in #1 and #2 and considerably reduced levels of NAXE in #3 (Figure 3). We therefore proceeded to use the fibroblast cell lines to study the consequences caused by the NAXE deficiency. Mutations in NAXE resulting in disrupted NAXE function are expected to impair the NAD(P)HX repair, which in turn should result in accumulation of the abnormal metabolites. We therefore measured levels of R-NADHX, S-NADHX, cyclic-NADHX, NAD, and NADH in fibroblast cell lines of two affected individuals and in two independent control cell lines. For optimization of the LC-MS/MS method, the compounds S-NADHX, R-NADHX, and cyclic-NADHX were synthesized from NADH under acidic pH conditions as published.6 Finally, for each individual sample, two T-75 cm2 flasks containing fibroblasts (∼90% confluency) were dissociated using TrypLE (Invitrogen) and cells were washed three times with ice-cold PBS. A portion of each sample was used for protein determination (BCA Protein Assay, Pierce). Next, cells were centrifuged at 300 × g and 4°C for 5 min. Stable isotope d4-nicotinamide was added as an internal standard prior to the ethanolic extraction procedure. Cell pellets were resuspended in 1 mL hot (80°C) 80% ethanol containing 20 mM HEPES (pH 7) and incubated at 80°C for 2 min followed by cooling on ice for 3 min. Next, cells were homogenized for 30 s using a Minilys homogenizer (Bertin). Samples were centrifuged at 15,000 × g and 4°C for 3 min. Supernatants were collected. For further use, a volume of 500 μL of the ethanolic extracts was gently dried under nitrogen stream. The dried sample was dissolved in 50 μL of 50 mM ammonium acetate (pH 7). After centrifugation, a volume of 30 μL was analyzed by HPLC-MS/MS system. The system consisted of an Acquity UPLC- I Class (Waters, UK) coupled to a Xevo TQ-S tandem mass spectrometer (Waters, UK) which is equipped with an ESI source operating in the negative ion mode for the both epimers S- and R-NADHX, NADH, and cyclic-NADHX and in positive ion mode for NAD and d4-nicotinamide (internal standard). Quantitative data were conducted in the multiple reaction monitoring (MRM) mode. The chromatographic separation was performed on a Polaris 3 C18-A 150 × 2.0 mm HPLC column (Agilent) using acetonitrile and 50 mM ammonium acetate (pH 7) as mobile phases. A gradient elution mode was used for rapid separation of the analytes.
Target compounds were identified and quantified based on their retention times (4.47 min S-NADHX, 4.94 min R-NADHX, 5.34 min NADH, 5.79 and 6.07 min cyclic-NADHX, 4.78 min d4-nicotinamide, and 4.73 min NAD) and MS/MS fragments (664.1 > 158.8 m/z, 664.1 > 79 m/z, and 664.1 > 664.2 m/z for both NADH and cyclic-NADHX; 682.2 > 158.8 m/z, 682.2 > 79 m/z, and 682.2 > 682.1 m/z for both epimers S-NADHX and R-NADHX; 127.1 > 84.1 m/z for d4-nicotinamide; 664.1 > 136.1 m/z and 664.1 > 427.8 m/z for NAD).
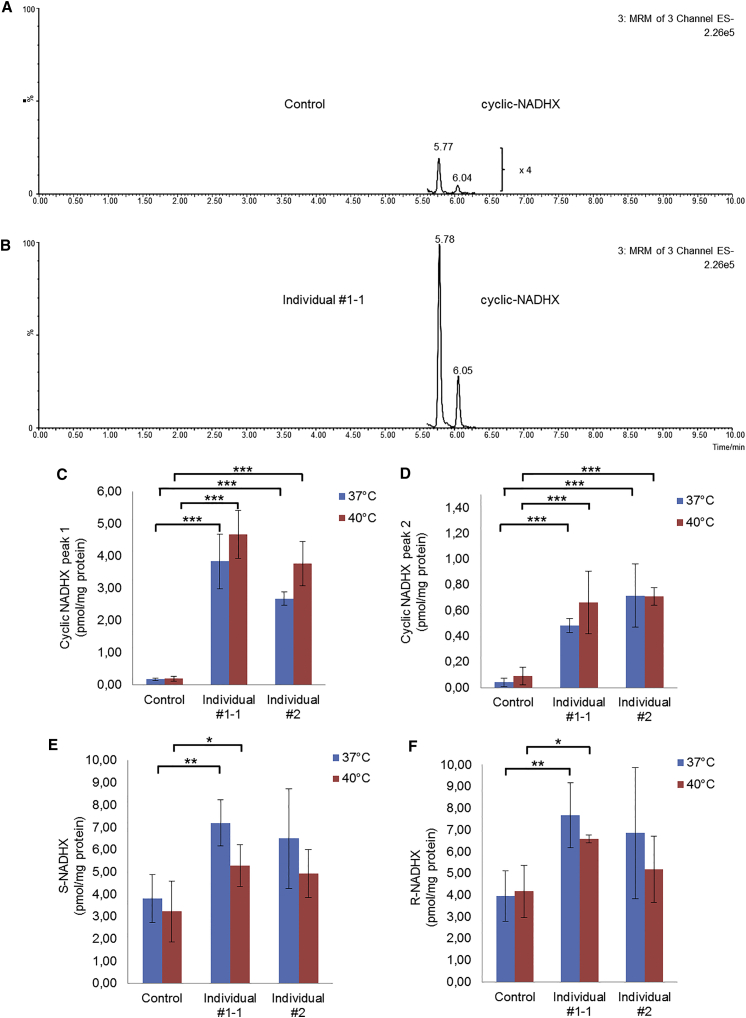
Fibroblasts with loss-of-function mutations in NAXE showed a significant increase in cyclic-NADHX levels compared to controls (Figure 4). Upon heat stress, we observed a further gradual increase in NAXE-deficient cells. The levels of R-NADHX and S-NADHX were also increased, but to a lesser extent than observed for cyclic-NADHX. The levels of NAD and NADH were not significantly altered (data not shown).
Figure 4.
Cellular Consequences of NAXE Deficiency
(A and B) Typical examples of chromatograms of cyclic-NADHX analyzed by HPLC-ESI-MS/MS in a control cell line (A) and in the affected individual #1-1 (B). Chromatograms clearly show that the levels of cyclic-NADHX are increased in NAXE-deficient cells compared to controls. The two peaks that we measured for cyclic-NADHX most likely depict the R- and the S-form of the metabolite. In the control cell line (B), the peaks were additionally 4-fold magnified for better visualization.
(C and D) Quantitative results of the cyclic-NADHX measurements under normal (37°C) and heat-stressed (40°C) conditions for 24 hr.
(E and F) Quantitative results of S- and R-NADHX measurements. Control bar graphs depict pooled data obtained from two independent healthy cell lines.
All experimental data were obtained in at least three independent experiments. Student’s t test was used to determine the statistical significance. Statistics: ∗∗∗p < 0.001, ∗∗p < 0.01, and ∗p < 0.05 relative to controls. Error bars indicate standard deviation.
Toxic metabolites or damaged cofactors can occur due to instability of cellular substances or (undesirable) moonlighting reactions of enzymes. To avoid the accumulation of such metabolites and to prevent cellular damage, “support systems” dedicated to metabolite repair have developed during evolution.1 The clinical importance of such systems was mostly unnoticed until the first inherited metabolic diseases related to deficient repair enzymes were described (e.g., D-2-hydroxyglutaric aciduria type II12 [MIM: 613657]). These studies highlighted the importance of metabolite repair systems for mammalian development and viability. Here, the identification of six individuals with a strikingly uniform clinical phenotype with biallelic NAXE variants in four families, including two homozygous loss-of-function variants, establishes NAXE variants as cause of a human disease of metabolite repair. NAXE is an essential component of the NAD(P)HX repair system. Its deficiency causes a detectable accumulation of the toxic metabolite cyclic-NADHX, which has been shown to inhibit various cellular NADH dehydrogenases.3, 13 The inhibition of NADH dehydrogenases is therefore a likely pathomechanism of NAXE deficiency. The decreased activity of complex I (MIM: 252010) and pyruvate oxidation in the investigation of intact mitochondria from fresh muscle biopsies of individuals #1-1 and #3 (Table S1) support this hypothesis.
NADH and NADPH are major cofactors of biochemical reactions with more than 250 entries in the Uniprot database for enzymes in human metabolism that rely on one of these two cofactors. These reactions are localized in all parts of the cell, many of them are found in mitochondria, especially those with high turnover rates. Elevated lactate levels in cerebrospinal fluid, as found in all NAXE-deficient individuals, are suggesting a deficiency/disturbance in mitochondrial energy metabolism. Reduced activities in intact mitochondria found in muscle biopsies of individuals #1-1 and #3 also indicate the pathogenic effects of NAXE mutations on the mitochondrial oxidative phosphorylation.
Of note, disturbed NAD metabolism as a cause of human disease is not entirely novel. The best-known condition is certainly pellagra,14 an acquired condition caused by a chronic lack of nicotinic acid (vitamin B3). In view of the skin manifestations in our affected individuals, it is tempting to speculate about similarities in the underlying pathomechanisms. However, skin lesions in our individuals were not typically located (e.g., not primarily affecting sun-exposed areas), appeared bullous/Lyell-like (e.g., not scaly or desquamating), and neurological (especially cerebellar) signs were dominating the clinical picture.
An inherited disorder indirectly affecting NAD metabolism is the so-called Hartnup disease (MIM: 234500).15 The disorder is caused by mutations in SLC6A19 (MIM: 608893), which encodes the sodium-dependent neutral amino acid transporter B(0)AT1.16 The defect impairs the absorption of neutral amino acids like tryptophan, which further serves as an important precursor in the biosynthesis of nicotinic acid in humans. Strikingly, Hartnup disease is characterized by a pellagra-like light-sensitive rash in combination with cerebellar ataxia. In addition to classical Hartnup disease, other “pellagra-like” conditions (MIM: 260650) have also been described. Freundlich et al.17 and Salih et al.18 both reported about familial disorders with pellagra-like skin lesions associated with neurologic abnormalities and cerebellar symptoms (MIM: 260650). Also in these disorders, a disturbance of tryptophan metabolism was suggested. In contrast to Hartnup disease, these disorders had an onset in early childhood and were lethal in most of the cases (one child described by Freundlich et al. died at the age of 15 months; none of the ten affected children described by Salih et al. survived beyond 2 years of age).17, 18 Interestingly, one of the three affected individuals reported by Freundlich et al. had a milder phenotype and symptoms of pellagra first at the age of 4 years, which could be successfully treated by nicotinamide. He experienced several crises with pellagra but also combined with ataxia and even with coma at the age of 14 years, but he recovered with nicotinamide treatment (100 mg/day).17 These findings suggest similarities to the disorder reported here. The reason for the cerebellar/neurological symptoms in Hartnup disease and associated conditions is still enigmatic. It was speculated whether a reduced synthesis of 5-hydroxytryptamine in the nervous system or the production of an abnormal by-product in tryptophan degradation pathway might be responsible for the neurological problems.17, 18
Importantly, in classical pellagra as well as in the inherited pellagra-like conditions, administration of nicotinic acid has a therapeutic effect.14, 17, 18 Therefore, it is tempting to speculate whether nicotinic acid supplementation might be beneficial in affected individuals with NAXE mutations. Further in vitro studies will be required to investigate this potential treatment strategy.
In general, early fever control might be beneficial in affected individuals. Intriguingly, in all children reported here, onset of symptoms and/or clinical deterioration was triggered by infections with high fever. This observation gains importance in view of evidence from basic research, indicating that NADPHX especially arises at high temperatures or acidic pH.2 Accordingly, in our cell studies, we also observed a gradual increase of cyclic-NADHX levels after NAXE-deficient cells were exposed to heat stress. In that respect, early and sustained antipyretic therapy might help to mitigate metabolic decompensation in NAXE-deficient individuals and should be accompanied by general supportive measures (e.g., adequate caloric supply, infusion therapy, etc.). Importantly, because of the combination of high fever and acute neurological deterioration, encephalitis, meningitis, or autoimmune-mediated inflammation was initially suspected in the children. Our findings suggest that NAXE deficiency should be considered as an important differential diagnosis in such cases. Additional evidence is provided by a report that was published during the review process of this article. The authors describe a family with five affected siblings with a similar clinical presentation as described here and biallelic variants in NAXE.19
In summary, we describe a disorder of metabolite repair, placing great importance to safeguarding the cell from the accumulation of potentially harmful metabolic intermediates. A defective NAD(P)HX repair system resulted in elevated concentrations of toxic cyclic-NADHX along with rapidly progressive ataxia, spinal myelopathy, skin lesions, and cerebellar edema leading to coma and finally to death within the first 3 years of life.
Acknowledgments
We thank all families for their participation and for providing important samples for the present research study. For their work on individual #3, we thank Mrs. Ira Brandstetter (mitochondrial respiratory chain biochemistry), Mrs. Julia Emmerich (fibroblast isolation from muscle biopsy), and Dr. Benedikt Schoser (assessment of muscle morphology and ultrastructure). We thank Prof. Marjo van der Knaap for the assessment of the MRI from individual #2. The study was supported by the German Bundesministerium für Bildung und Forschung (BMBF) through the German Network for mitochondrial disorders (mitoNET, 01GM1113A-E to L.S.K., K.D., W.M.-F., T.M., T.K., J.A.M., F.D., and H.P.), the E-Rare project GENOMIT (01GM1603 to J.A.M. and H.P.), the Juniorverbund in der Systemmedizin “mitOmics” (FKZ 01ZX1405C to T.B.H.), and the Forschungskommission of the Medical Faculty of the Heinrich-Heine-University Düsseldorf (F.D. and K.D.). This study was further supported by the CMHI project S136/13.
Published: September 8, 2016
Footnotes
Supplemental Data include detailed case reports, one figure and one table and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2016.07.018.
Contributor Information
Felix Distelmaier, Email: felix.distelmaier@med.uni-duesseldorf.de.
Holger Prokisch, Email: prokisch@helmholtz-muenchen.de.
Web Resources
ExAC Browser, http://exac.broadinstitute.org/
GenBank, http://www.ncbi.nlm.nih.gov/genbank/
OMIM, http://www.omim.org/
UniProt, http://www.uniprot.org/
Supplemental Data
References
- 1.Linster C.L., Van Schaftingen E., Hanson A.D. Metabolite damage and its repair or pre-emption. Nat. Chem. Biol. 2013;9:72–80. doi: 10.1038/nchembio.1141. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida A., Dave V. Inhibition of NADP-dependent dehydrogenases by modified products of NADPH. Arch. Biochem. Biophys. 1975;169:298–303. doi: 10.1016/0003-9861(75)90344-6. [DOI] [PubMed] [Google Scholar]
- 3.Marbaix A.Y., Tyteca D., Niehaus T.D., Hanson A.D., Linster C.L., Van Schaftingen E. Occurrence and subcellular distribution of the NADPHX repair system in mammals. Biochem. J. 2014;460:49–58. doi: 10.1042/BJ20131482. [DOI] [PubMed] [Google Scholar]
- 4.Marbaix A.Y., Noël G., Detroux A.M., Vertommen D., Van Schaftingen E., Linster C.L. Extremely conserved ATP- or ADP-dependent enzymatic system for nicotinamide nucleotide repair. J. Biol. Chem. 2011;286:41246–41252. doi: 10.1074/jbc.C111.310847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giaever G., Chu A.M., Ni L., Connelly C., Riles L., Véronneau S., Dow S., Lucau-Danila A., Anderson K., André B. Functional profiling of the Saccharomyces cerevisiae genome. Nature. 2002;418:387–391. doi: 10.1038/nature00935. [DOI] [PubMed] [Google Scholar]
- 6.Niehaus T.D., Richardson L.G., Gidda S.K., ElBadawi-Sidhu M., Meissen J.K., Mullen R.T., Fiehn O., Hanson A.D. Plants utilize a highly conserved system for repair of NADH and NADPH hydrates. Plant Physiol. 2014;165:52–61. doi: 10.1104/pp.114.236539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haack T.B., Haberberger B., Frisch E.M., Wieland T., Iuso A., Gorza M., Strecker V., Graf E., Mayr J.A., Herberg U. Molecular diagnosis in mitochondrial complex I deficiency using exome sequencing. J. Med. Genet. 2012;49:277–283. doi: 10.1136/jmedgenet-2012-100846. [DOI] [PubMed] [Google Scholar]
- 8.Haack T.B., Kopajtich R., Freisinger P., Wieland T., Rorbach J., Nicholls T.J., Baruffini E., Walther A., Danhauser K., Zimmermann F.A. ELAC2 mutations cause a mitochondrial RNA processing defect associated with hypertrophic cardiomyopathy. Am. J. Hum. Genet. 2013;93:211–223. doi: 10.1016/j.ajhg.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elstner M., Andreoli C., Ahting U., Tetko I., Klopstock T., Meitinger T., Prokisch H. MitoP2: an integrative tool for the analysis of the mitochondrial proteome. Mol. Biotechnol. 2008;40:306–315. doi: 10.1007/s12033-008-9100-5. [DOI] [PubMed] [Google Scholar]
- 11.Ploski R., Pollak A., Müller S., Franaszczyk M., Michalak E., Kosinska J., Stawinski P., Spiewak M., Seggewiss H., Bilinska Z.T. Does p.Q247X in TRIM63 cause human hypertrophic cardiomyopathy? Circ. Res. 2014;114:e2–e5. doi: 10.1161/CIRCRESAHA.114.302662. [DOI] [PubMed] [Google Scholar]
- 12.Kranendijk M., Struys E.A., van Schaftingen E., Gibson K.M., Kanhai W.A., van der Knaap M.S., Amiel J., Buist N.R., Das A.M., de Klerk J.B. IDH2 mutations in patients with D-2-hydroxyglutaric aciduria. Science. 2010;330:336. doi: 10.1126/science.1192632. [DOI] [PubMed] [Google Scholar]
- 13.Prabhakar P., Laboy J.I., Wang J., Budker T., Din Z.Z., Chobanian M., Fahien L.A. Effect of NADH-X on cytosolic glycerol-3-phosphate dehydrogenase. Arch. Biochem. Biophys. 1998;360:195–205. doi: 10.1006/abbi.1998.0939. [DOI] [PubMed] [Google Scholar]
- 14.Hegyi J., Schwartz R.A., Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. Int. J. Dermatol. 2004;43:1–5. doi: 10.1111/j.1365-4632.2004.01959.x. [DOI] [PubMed] [Google Scholar]
- 15.Baron D.N., Dent C.E., Harris H., Hart E.W., Jepson J.B. Hereditary pellagra-like skin rash with temporary cerebellar ataxia, constant renal amino-aciduria, and other bizarre biochemical features. Lancet. 1956;271:421–428. doi: 10.1016/s0140-6736(56)91914-6. [DOI] [PubMed] [Google Scholar]
- 16.Kleta R., Romeo E., Ristic Z., Ohura T., Stuart C., Arcos-Burgos M., Dave M.H., Wagner C.A., Camargo S.R., Inoue S. Mutations in SLC6A19, encoding B0AT1, cause Hartnup disorder. Nat. Genet. 2004;36:999–1002. doi: 10.1038/ng1405. [DOI] [PubMed] [Google Scholar]
- 17.Freundlich E., Statter M., Yatziv S. Familial pellagra-like skin rash with neurological manifestations. Arch. Dis. Child. 1981;56:146–148. doi: 10.1136/adc.56.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salih M.A., Bender D.A., McCreanor G.M. Lethal familial pellagra-like skin lesion associated with neurologic and developmental impairment and the development of cataracts. Pediatrics. 1985;76:787–793. [PubMed] [Google Scholar]
- 19.Spiegel R., Shaag A., Shalev S., Elpeleg O. Homozygous mutation in the APOA1BP is associated with a lethal infantile leukoencephalopathy. Neurogenetics. 2016;17:187–190. doi: 10.1007/s10048-016-0483-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.