Abstract
ATPase family AAA-domain containing protein 3A (ATAD3A) is a nuclear-encoded mitochondrial membrane protein implicated in mitochondrial dynamics, nucleoid organization, protein translation, cell growth, and cholesterol metabolism. We identified a recurrent de novo ATAD3A c.1582C>T (p.Arg528Trp) variant by whole-exome sequencing (WES) in five unrelated individuals with a core phenotype of global developmental delay, hypotonia, optic atrophy, axonal neuropathy, and hypertrophic cardiomyopathy. We also describe two families with biallelic variants in ATAD3A, including a homozygous variant in two siblings, and biallelic ATAD3A deletions mediated by nonallelic homologous recombination (NAHR) between ATAD3A and gene family members ATAD3B and ATAD3C. Tissue-specific overexpression of borR534W, the Drosophila mutation homologous to the human c.1582C>T (p.Arg528Trp) variant, resulted in a dramatic decrease in mitochondrial content, aberrant mitochondrial morphology, and increased autophagy. Homozygous null bor larvae showed a significant decrease of mitochondria, while overexpression of borWT resulted in larger, elongated mitochondria. Finally, fibroblasts of an affected individual exhibited increased mitophagy. We conclude that the p.Arg528Trp variant functions through a dominant-negative mechanism that results in small mitochondria that trigger mitophagy, resulting in a reduction in mitochondrial content. ATAD3A variation represents an additional link between mitochondrial dynamics and recognizable neurological syndromes, as seen with MFN2, OPA1, DNM1L, and STAT2 mutations.
Keywords: whole-exome sequencing, mitochondrial dynamics, ATAD3A, de novo variant, neuropathy, optic atrophy, cardiomyopathy, CNV, dominant negative
Introduction
Remodeling of the mitochondrial membrane through continuous cycles of fusion and fission promotes mixing of mitochondrial proteins and DNA, ensuring proper function.1, 2, 3 Pathogenic variation in genes encoding fusion and fission factors, including MFN2 (MIM: 608507),4, 5, 6, 7 OPA1 (MIM: 605290),8, 9 DNM1L (MIM: 603850),10, 11 STAT2 (MIM: 600556),12 SLC25A46 (MIM: 610826),13 and GDAP1 (MIM: 606598)14 have been associated with recognizable human syndromes including optic atrophy, peripheral neuropathy, cardiomyopathy, brain malformations, and developmental delay as recurrent features.
ATAD3A (MIM: 612316) is ubiquitously expressed and encodes an ATPase family AAA-domain containing protein 3A (ATAD3A) that simultaneously interacts with the outer and inner mitochondrial membranes. In vitro studies show that ATAD3A is involved in diverse cellular processes, including mitochondrial dynamics,15 mitochondrial DNA (mtDNA) maintenance and replication,16 channeling of cholesterol for steroidogenesis,17, 18 and resistance of cancer cells to therapy.19, 20 Co-immunoprecipitation in lung adenocarcinoma cell lines revealed evidence for interaction of ATAD3A with MFN2, OPA1, and DNM1L,21 suggesting that ATAD3A may affect mitochondrial dynamics via interaction with these proteins.
The ATAD3 gene family in humans includes three paralogs (ATAD3A, ATAD3B [MIM: 612317], and ATAD3C) positioned in tandem on chromosome 1p36.33 and appears to have recently evolved by duplication of a single ancestral gene.22, 23 Disruption of the Drosophila ortholog (bor), which has ∼70% similarity to human ATAD3A, results in growth arrest during larval development.15 The ortholog in C. elegans is essential for mitochondrial activity and development.24 Atad3 homozygous null mice are embryonic lethal at day E7.5 due to growth retardation and defective development of the trophoblast lineage, whereas heterozygotes for a loss-of-function allele seem unaffected.22 RNAi studies of ATAD3A in HeLa and lung cancer cells have documented increased mitochondrial fragmentation and a decreased co-localization of mitochondria and endoplasmic reticulum (ER).21 Despite multiple studies in vitro and in animal models, the precise function of ATAD3A has not been elucidated to date.
We identified an identical de novo heterozygous variant in ATAD3A in five individuals. In addition, we identified two families with biallelic single-nucleotide variants (SNVs) or copy-number variants (CNVs) involving ATAD3A. Our data indicate that both monoallelic and biallelic pathogenic variation in ATAD3A led to the observed neurological phenotypes in these seven families. Functional studies in fibroblasts from an affected individual and in D. melanogaster revealed significant mitochondrial abnormalities and indicate that ATAD3A is required to maintain the proper size and number of mitochondria in neurons and muscles.
Material and Methods
Subjects
Families 1, 2, 3, 5, and 7 provided consent according to the Baylor-Hopkins Center for Mendelian Genomics (BHCMG) research protocol H-29697, approved by the Institutional Review Board (IRB) at Baylor College of Medicine (BCM). Families 4 and 6 provided consent according to protocol M0010, approved by the IRB at Meyer Children Hospital, Florence, and protocol 13084, approved by the IRB at AUSL, Bologna, respectively. Subjects are described further in Tables 1 and S1.
Table 1.
Comparison of the Phenotypic Features of Seven Individuals with ATAD3A Variants
|
Monoallelic |
Biallelic |
|||||||
|---|---|---|---|---|---|---|---|---|
| Family 1, II-2 | Family 2, II-4 | Family 3, II-1 | Family 4, II-1 | Family 5, II-1 | Family 6, II-1 | Family 6, II-2 | Family 7, II-1 | |
| Age at last exam | 9 years | 5 years | 3 years | 5 years | 23 months | 26 years | 24 years | 5 days |
| Gender | F | F | M | F | M | F | M | F |
| DD | + | + | + | + | + | + | + | NR |
| ID | + (mild) | + | + | + | + | + (mild) | − | NR |
| Seizures | − | − | − | − | − | + | + | + |
| Optic atrophy | + | − | + | + | − | − | − | − |
| Congenital cataract | − | − | − | − | − | + | + | − |
| Corneal clouding | − | − | − | − | − | − | − | + |
| Hypotonia | + | + | + | + | + | + | + | + |
| Spasticity | + | + | + | + | − | − | − | + |
| Pectus carinatum | − | − | + | − | − | − | + | − |
| Peripheral neuropathy | + | + | + | + | borderline | − | − | NR |
| Hypertrophic cardiomyopathy | − | − | + | + | − | − | − | mild RVH with septal hypertrophy (newborn) |
| Elevated plasma lactate | − | + (mild) | + (intermittent) | + | NR | NR | + | NR |
| Cerebellar atrophy/ hypoplasia | − | − | − | − | − | + (moderate) | + (mild) | + (severe) |
| MR spectroscopy | normal | NR | lactate peak | NR | NR | reduction of left cerebellar hemisphere NAA | normal | reduction of NAA, increased choline |
| Other | ADHD | deficiency complex I+III | methyl-glutaconic aciduria; growth hormone deficiency; dystonia | methyl-glutaconic aciduria; deficiency complex II+III, II+CS | 47,XXY | delayed puberty, hyposomia | delayed puberty, delayed bone age, hyposomia, cryptorchidism | smooth sulcal/gyral pattern, hypoplasia of posterior fossa structures, intubated at birth; deceased at 13 days |
Abbreviations are as follows: ADHD, attention deficit hyperactivity disorder; CS, citrate synthase; DD, developmental delay; F, female; ID, intellectual disability; IQ, intelligence quotient; M, male; MR, magnetic resonance; NAA, N-acetyl aspartate; NR, not reported; RVH, right ventricular hypertrophy.
Whole-Exome Sequencing
Family 1, II-2 (proband), family 5 (parents and proband), and family 7 (proband) had whole-exome sequencing (WES) at Baylor Genetics (BG, BCM) according to the protocol described in Yang et al.25 After clinical WES results were unrevealing for a potential etiologic molecular diagnosis in known disease genes, parental exome sequencing for family 1 was obtained through the BHCMG research initiative. In brief, DNA samples were prepared into Illumina paired-end libraries and underwent whole-exome capture with the BCM-HGSC core design (52 Mb, Roche NimbleGen), followed by sequencing on the Illumina HiSeq 2000 platform (Illumina). Data produced were aligned and mapped to the human genome reference sequence (hg19) by the Mercury pipeline.26 Single-nucleotide variants (SNVs) were called with the ATLAS (an integrative variant analysis pipeline optimized for variant discovery) variant calling method and annotated by the in-house-developed “Cassandra” annotation pipeline that uses ANNOVAR (Annotation of Genetic Variants) and additional tools and databases.27, 28, 29 De novo variants were calculated by an in-house developed pipeline (DNM-Finder) for in silico subtraction of parental variants from the proband’s variants in vcf files while accounting for the read number information extracted from BAM files. Bioinformatic tools predicted conservation and pathogenicity of candidate variants, and variants were compared against both an internal database (∼5,000 exomes) and publicly available databases such as the 1000 Genomes Project, the Exome variant server, NHLBI GO Exome Sequencing Project (ESP), the Atherosclerosis Risk in Communities Study (ARIC) Database, and the Exome Aggregation Consortium (ExAC) database. Families 5 and 7 were identified through an internal database. The GeneMatcher tool facilitated identification of four additional families with variants of interest in ATAD3A.30 Detailed sequencing methods for these families can be found in the Supplemental Data.
PCR Amplification and Sanger Sequencing
An amplicon containing the ATAD3A variant was amplified from genomic DNA by conventional PCR, with a primer design that ensured specific amplification of ATAD3A and not its homolog ATAD3B. Representative primers for family 1, II-2 were ATAD3A-F1 (5′-CCC CTC CAA AGA GGA TGT TT-3′) and ATAD3A-R1 (5′-CAA CTG AGC ATC TCC ACA GC-3′). Additionally, the homologous exon of ATAD3B was sequenced with a specific intronic primer: ATAD3B-F1 (5′-TGC CTC ACT TGG GAA CTC C-3′) and ATAD3B-R1 (5′-ACA GAA GCT CCC ACA TGA CA-3′). PCR products were purified by ExoSAP-IT (Affymetrix) and analyzed by standard Sanger di-deoxy nucleotide sequencing (DNA Sequencing Core Facility, Baylor College of Medicine). Details for Sanger validation and segregation of variants in other individuals can be found in the Supplemental Data.
3D Modeling of Protein Structure
The 3D protein structure model of ATAD3A (GenBank: NM_001170535.1) was predicted by I-TASSER.31, 32 The side chain orientation of the mutant residue was obtained by the PyMol Molecular Graphics System, v.1.5 Schrodinger, LLC. Amino acid conservation was obtained from the Consurf server based on sequence analysis.33
Biochemistry
The activities of mitochondrial respiratory chain complexes (I-IV) and Citrate Synthase (CS) were measured in skeletal muscle homogenates and digitonin-treated cultured skin fibroblasts as described previously.34 Activities are expressed relative to CS for the muscle or relative to mitochondrial protein for fibroblasts.
Detection of CNV from WES Read Depth Analysis
Internal databases were screened for homozygous CNVs using two in-house-developed algorithms. The first method utilized a dual normalization approach consisting of principal component analysis (PCA)-XHMM35 intermediates and read per kilobase per million mapped reads (RPKM). The former normalized raw read depth values with PCA, which were then centered by exon target on a Z-score scale, whereas the latter was normalized at the sample level (RPKM). The intersection of two orthogonal normalization approaches (target based and sample based) allowed for an enhancement in signal and reduction in noise. In each method, we removed exons with extreme GC content and low complexity biases from analysis. Regions with either very high or very low GC content are more likely to be captured poorly by WES. Complete homozygous and hemizygous exon deletions were called by strict filtering criteria, including exon target Z-scores less than −2 and RPKM equal or near 0. The second algorithm, in the R programming language, was also based on the total number of reads (TR) in each exon and normalized read depth values (RPKM).
High-Density Array Comparative Genomic Hybridization
To further characterize the CNVs involving the ATAD3A genomic region, we designed an 8× 60K oligonucleotide array with ∼200 bp per probe spacing from Agilent Technologies (AMADID 082882). DNA from HapMap individual NA15510 was used as hybridization control. Scanned array images were processed using Agilent Feature Extraction software (v.10) and extracted files were analyzed using Agilent Genomic Workbench (v.7.0.4.0). Array designs and sequence alignment for breakpoint analysis were based on the February 2009 genome build (GRCh37/hg19 assembly).
Evaluation of CNV Boundaries and Breakpoint Junction Analysis
Droplet digital PCR (ddPCR) was performed using the QX200 AutoDG Droplet Digital PCR System from Bio-Rad according to manufacturer’s protocols. In brief, a 20 μL mixture was set up for each PCR reaction, containing 10 μL of 2× Q200 ddPCR EvaGreen Supermix, 0.25 μL of each primer (10 μM) and 20 ng of genomic DNA. Reaction mixture was subjected to automatic droplet generation, followed by PCR reaction and droplet reading. Cycling conditions for PCR were as follows: 5 min at 95°C, 40 cycles of 30 s at 95°C/1 min at 67.1°C/1 min at 72°C, 5 min at 4°C, 5 min at 90°C and final hold at 4°C. Ramp rate was set for 2°C per s for all steps. Data were analyzed with QuantaSoft Software (Bio-Rad), and concentrations of positive droplets (number of positive droplets per μL of reaction) were obtained for each PCR reaction. Raw data of ddPCR and primer sequences are shown as Table S2.
The breakpoint junctions were amplified with either TaKaRa LA Taq (Clontech) or QIAGEN HotStarTaq DNA Polymerase according to the manufacturer’s protocol. The breakpoint junction of BAB8733 (family 7, II-2) was amplified with the following primers: BAB8733_del_F1 (5′-TTG GAG TTC TGT GGT CCT GG-3′) and BAB8733_del_R1 (5′-CAG GCC CAC ACT GCT GAC-3′), while the wild-type allele was amplified with BAB8733_wt_F2 (5′-ATG GGC ACA GTC ACA GGT TT-3′) and BAB8733_del_R1. The breakpoint junction of BAB8734 (family 7, II-1) was amplified with the primers BAB8734_del_F3 (5′-GAG AGC GGA GTC CAC ACC-3′) and BAB8734_del_R3 (5′-GGA AAC CAA CCA CAC ACG G-3′), while the wild-type allele was amplified with BAB8734_del_F3 and BAB8734_wt_R4 (5′-CTG ATC CAC CGA CAG AAG CAG-3′).
Miropeats
The Miropeats program descriptively illustrates the genomic architecture by plotting the intra-species alignments of the reference genome. ICAass (v.2.5) algorithm performed DNA sequence comparisons, and Miropeats (v.2.01) was then applied for converting the comparisons into graphical display based on the position and matching quality.36 A threshold of 200 was set for comparison of human paralogs.
Fly Strains and Maintenance
The following stocks were obtained from the Bloomington Stock Center at Indiana University (BDSC):
w1118; PBac{PB}borc05496/TM6B, Tb1
w1118; Df(3R)Exel7329/TM6B, Tb137
y1 w∗; tubulin-Gal4/TM3, Sb1 Ser1
w∗; Ubi-Gal4 /CyO
yw;+; n-syb-Gal4
w; D42-Gal4 UAS-mito-HA-GFP e/TM6B, Tb (from Bill Saxton).
w; C57-Gal4
All flies were maintained at room temperature (21°C). All crosses were kept at 21°C except those for C57-Gal4 (25°C).
Cloning and Transgenesis
LD30988, an EST of bor (Drosophila Genomics Resource Center), contains a mutation that causes a substitution of an amino acid (D40 to Y) and an insertion of 72 additional nucleotides near the 3′ end. These mutations were corrected by site-directed PCR using primers bor Y40→D-F (5′-acg gcc ggc gag aag tcc ggg Gat tcg caa ctg agc cgg gcg-3′) and bor Y40→D-R (5′-cgc ccg gct cag ttg cga atC ccc gga ctt ctc gcc ggc cgt-3′); LD30988-F (5′-CAG CCT GCC GCT GCG GGT GCC AAG cgg ttc aaa ctg gac acc ttt gat-3′) and LD30988-R (5′-atc aaa ggt gtc cag ttt gaa ccg CTT GGC ACC CGC AGC GGC AGG CTG-3′). All final clones were confirmed by sequencing.
To generate bor p.Arg534Trp (R534W) (equivalent to human p.Arg528Trp), we performed site-directed mutagenesis using primers 5′-ctg tgc gag ggt atg tcg ggt TgG gaa atc tcc aag ctg ggc-3′ and 5′-gcc cag ctt gga gat ttc CcA acc cga cat acc ctc gca cag-3′. The corrected bor EST clone and bor (R534W) clone were amplified by PCR using primers bor-F BamHI (5′-cat ttt GGA TCC aaa ATG TCG TGG CTT TTG GGC AGG-3′) and bor-R NotI (5′-tgt aaG CGG CCG CTT ACA GTT TCT TTG CAG TTA G-3′). The PCR products were subcloned into BglII/NotI sites in a pUASTattB38 vector to generate pUASTattB-borWT and pUASTattB-borR534W. The pUASTattB constructs were injected into y,w,ΦC31; VK37 embryos39 and transgenic flies were selected.
Larvae Dissection and Immunostaining
For muscle and axon staining, third instar larvae were dissected as described in Bellen and Budnik.40 In brief, third instar larvae were fixed in 4% formaldehyde for 30 min at room temperature, rinsed in PBS twice, and washed in PBS containing 0.3% Triton X-100 three times. The primary antibodies were used at the following dilutions: mouse anti-ATP5A 1:500 (abcam), chick anti-GFP 1:1,000 (abcam), mouse anti-Dlg 1:250 (mAb 4F3, DSHB; developed by C.S. Goodman, Renovis), and rabbit anti-HRP 1:1,000 (Jackson ImmunoResearch). Alexa 488-conjugated (Invitrogen) and Cy3- or Cy5-conjugated secondary antibodies (Jackson ImmunoResearch) were used at 1:120. Samples were mounted in Vectashield (Vector Labs). Alexa Fluor 568 phalloidin (ThermoFisher) was used at 1:1,000.
Bright-Field Imaging and Transmission Electron Microscopy of Flies
Drosophila larval muscles and neuro-muscular junctions (NMJs) were imaged according to standard electron microscopy procedures using a Ted Pella Bio Wave processing microwave with vacuum attachments. In brief, whole first instar larvae were dissected at room temperature under modified Karnovski’s fixative consisting of 2% paraformaldehyde, 2.5% glutaraldehyde, in 0.1 M sodium cacodylate buffer at pH 7.2. The NMJ muscle samples were filleted under modified Karnovski’s fixative and allowed to sit pinned under fixative for 40 min. After dissection the first instar larvae and NMJs were fixed for 2 days in fresh modified Karnovski’s fixative rotating in a 4°C cold room. After 2 days in fixative, the samples were irradiated using the Ted Pella Biowave Microwave, rinsed three times with Millipore water, post-fixed with 1% aqueous Osmium Tetroxide, and rinsed again three times with Millipore water.
Concentrations from 30%–100% of ethanol were used for the initial dehydration series, followed with propylene oxide as the final dehydrant. The samples were gradually infiltrated with 3 ratios of propylene oxide and Embed 812, finally going into three changes of pure resin all under vacuum. Samples were allowed to infiltrate in pure resin overnight on a rotator. The samples were embedded into flat silicone molds and cured in the oven at 62°C for 3 days. The polymerized samples were thin-sectioned at 50 nm and stained with 1% uranyl acetate for 12 min followed by lead citrate for 2 min before TEM examination. Grids were viewed in a JEOL 1400 Plus transmission electron microscope at 80 kV. Micrographs were captured by an AMT XR-16 mid-mount 16 mega-pixel digital camera.
Fibroblast Culture and TEM
Human fibroblasts were grown in DMEM (10569-010, Life Technologies) supplemented with 10% fetal bovine serum (FBS) and penicillin-streptomycin (Sigma) at 37°C. For fixation, cells were placed in a 50:50 media/fix solution with Karnovski’s fixative (2% paraformaldehyde and 2.5% glutaraldehyde in 1% sodium cacodylate buffer) for 10 min, followed by 100% Karnovski’s fixative for 45 min. Cells were scraped and placed into micro-centrifuge tubes, spun down at 1,500 × g for 3 min, and left in fresh fixative for 15 min after an additional centrifugation. The cell pellets were then subjected to a normal processing procedure as above.
Immunoblotting Analysis
Protein concentration was quantitated using the Bio-Rad DC protein assay. 10 μg of total lysate or DDM-treated crude mitochondria protein were analyzed by electrophoresis in NuPage Novex 4%–12% bis-tris gel (Invitrogen). After electrophoresis, proteins were transferred electrophoretically to a PVDF membrane and probed with the following antibodies: rabbit polyclonal anti-ATAD3A (1:1,000, Novus Biological), anti-GAPDH (1:10,000, Abcam), or anti-VDAC1 (1:10,000, Abcam). HRP-conjugated anti-rabbit or anti-mouse IgG secondary antibodies (Promega) were used at a dilution of 1:3,000. The protein bands were visualized by chemiluminescence, using reagents purchased from GE Healthcare.
Results
De Novo ATAD3A Variant Is Associated with Optic Atrophy and Peripheral Neuropathy
The clinical findings of five affected individuals from five families with an identical, recurrent de novo variant in ATAD3A (g.chr1:1464679C>T [hg19]; GenBank: NM_001170535.1; c.1582C>T [p.Arg528Trp], Figures 1 and S1–S4) are summarized in Tables 1 and S1; detailed case reports can be found in the Supplemental Data and photographs are available in Figure 2. The phenotypic spectrum included developmental delay (5/5), hypotonia (5/5), optic atrophy (3/5), peripheral neuropathy (4/5), and hypertrophic cardiomyopathy (2/5). Mitochondrial dysfunction was suggested in different cases by intermittently or mildly elevated plasma lactate, a lactate peak on MR spectroscopy, slightly increased excretion of 3-methylglutaconic acid, and/or deficiency in respiratory chain activities on muscle homogenate (Supplemental Data). These phenotypic features bear striking resemblance to those encountered with pathogenic variation in other mitochondrial fission and fusion proteins, including DNM1L,10, 11 MFN2,5, 7 OPA1,8, 9 and SLC25A4613 (Figure S5).
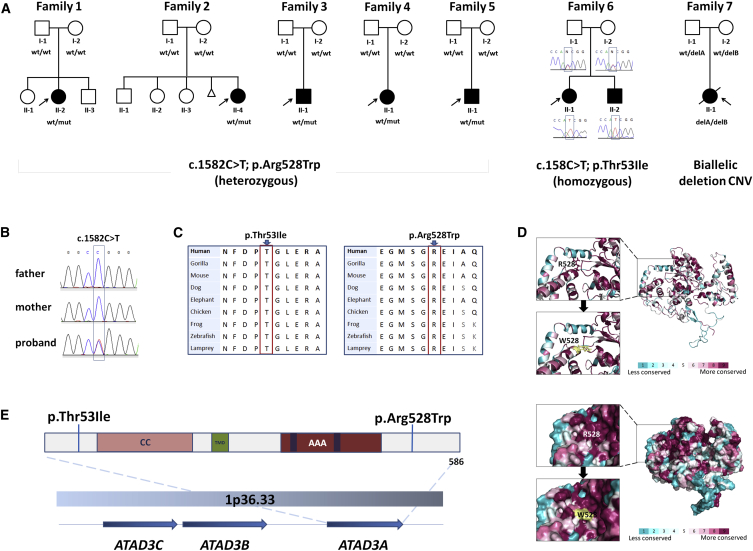
Figure 1.
Pedigrees and ATAD3A Variant Details
(A) Pedigrees of studied families, indicating the recurrent de novo single-nucleotide variant (SNV) in ATAD3A in families 1–5, a homozygous SNV in family 6, and compound heterozygous deletion copy-number variants (CNVs) in family 7.
(B) Sanger validation of the ATAD3A variant in family 1.
(C) The p.Thr53Ile and p.Arg528Trp substitutions alter conserved residues.
(D) Protein structure prediction shows replacement of the arginine long side chain by a flat aromatic ring of tryptophan. Color spectrum indicates high conservation of the Arg528 residue. Surface structure modeling indicates that the arginine at position 528 resides in a conserved pocket, possibly a functional site. The mutation alters the configuration of the pocket.
(E) Schematic representation of ATAD3A (isoform 2) with indication of the coiled-coil domain (CC), transmembrane domain (TMD), and AAA domain including Walker A and Walker B ATP-binding and ATPase domains, respectively. Localization of the altered residues in families 1–6 is indicated. The three human ATAD3 genes are located in tandem on chromosome 1p36.33.
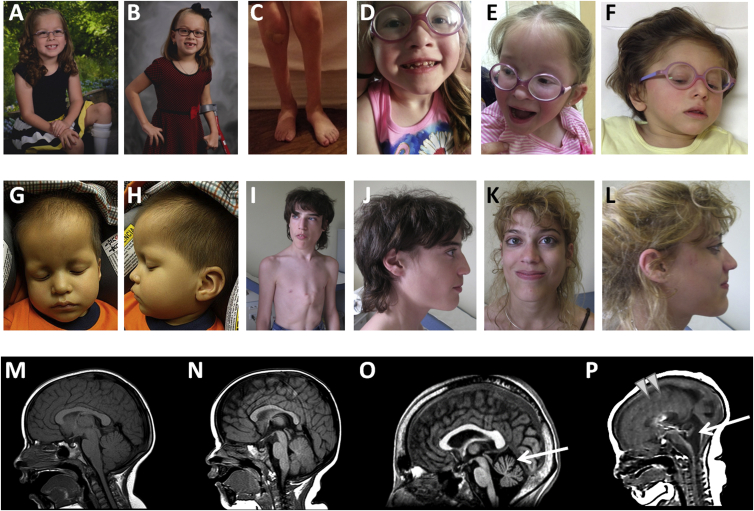
Figure 2.
Clinical Findings of Affected Individuals
(A–C) Family 1, II-2 at 6 years (A) and 8 years (B), showing high forehead (B) and marked atrophy of the lower legs (C).
(D and E) Family 2, II-4 at 5 years with high forehead, small nose, and thin hair.
(F) Family 4, II-1 at 5 years with triangular facies, micrognathia, and low-set ears.
(G and H) Family 5, II-1 at 23 months, with frontal bossing, deep set eyes, and micrognathia.
(I–L) Individuals II-2 (I, J) and II-1 (K, L) from family 6, demonstrating elongated face, triangular nose and prognathism, and pectus carinatum (I).
(M–P) Mid-sagittal T1-weighted brain magnetic resonance imaging (MRI) images from affected individuals.
(M and N) Individuals with heterozygous recurrent de novo variant show either no significant abnormalities (M, family 1, II-2 at 6 years) or prominent extra-axial spaces and incomplete myelination (N, family 5, II-1 at 8 months).
(O) Family 6, II-1, harboring a homozygous ATAD3A missense variant, had moderate atrophy of the cerebellum (arrow) at age 26 years.
(P) MRI of family 7, II-1, with biallelic deletion of ATAD3A, showed very poor gyration and sulcation (arrowheads) and hypoplastic cerebellum, vermis, and brainstem (arrow).
The c.1582C>T variant has robust bioinformatic damaging predictions (Table S3), arose at a CpG dinucleotide (Figure 1B),41, 42, 43 and was absent from internal and publicly available databases, including the ExAC database of ∼60,000 unrelated individuals. 3D structural modeling further supported pathogenicity, as the long side chain of arginine at position 528 is replaced by the flat aromatic ring of tryptophan and alters the configuration of a conserved pocket, possibly a functional site (Figure 1D). To ensure that the variant was specific to ATAD3A and to investigate the possibility of gene conversion from the highly homologous ATAD3B, primer design was targeted to specific intronic sequences flanking the exon. No variation was seen in ATAD3B, suggesting that the c.1582C>T variant is specific to ATAD3A. This argues against gene conversion as a mutational mechanism (Figure S1).
Sanger sequencing of the maternal sample in family 4 suggested low-level mosaicism in genomic DNA derived from blood (Figure S3). Nerve conduction velocities of the mother (family 4, I-2) were within normal range; however, she had cardiac arrhythmia diagnosed in childhood and required a pacemaker, which might be a clinical phenotype relevant to the ATAD3A variation given the cardiac phenotype in two individuals with the recurrent de novo variant and our inability to assess the degree of mosaicism in the mother’s heart tissue. The proband in family 5 had a dual molecular diagnosis, including Klinefelter syndrome and the ATAD3A variant. Dual molecular diagnoses have been documented in ∼4.6%–7.2% of molecularly diagnosed individuals.25, 44, 45, 46 Both of these clinical situations, parental mosaicism and dual molecular diagnoses, require specific consideration with respect to prognosis and recurrence risk for the families.47, 48
Homozygous ATAD3A Single-Nucleotide Variant Identified in Siblings with Congenital Cataract, Ataxia, and Seizures
In a sixth family (Figures 1A and 2I–2L), two siblings born to distantly related parents presented with motor developmental delay, speech delay, congenital cataracts, seizures, ataxia, hypotonia, and reduced muscle strength in the extremities (Tables 1, S1, Supplemental Data). Magnetic resonance imaging (MRI) revealed hypoplasia of the optic nerves and cerebellar atrophy (Figures 2O and S6), and magnetic resonance spectroscopy (MRS) revealed a moderate reduction of N-acetyl aspartate to creatine (NAA/Cr) ratio in the cerebellum, indicating neuronal-axonal degeneration. No CSF lactate was detected. Muscle biopsy of the male sibling showed slight increase of Oil Red O (ORO) and sub-sarcolemmal COX activities. Mitochondrial respiratory chain enzyme analysis in muscle homogenate and in skin fibroblasts revealed normal activities (Table S4). The variant with the strongest bioinformatic predictions shared by the siblings was a homozygous ATAD3A variant (g.chr1:1447806C>T [hg19]; GenBank: NM_001170535.1; c.158C>T [p.Thr53Ile]), found in an ∼700 kb run of homozygosity (ROH). Bioinformatic predictions and in silico modeling supported pathogenicity (Table S3, Figure S7). The genetic data suggest that biallelic variation in ATAD3A might lead to a distinct neurological phenotype as a recessive disease trait.
Biallelic Deletion of ATAD3A Is Associated with Infantile Lethality
The high homology between the three ATAD3 paralogs, as depicted by Miropeats alignment of this region to itself (Figure 3A), provides an endogenous genome architecture as substrate for nonallelic homologous recombination (NAHR) in this region and is predicted to make this locus susceptible to genomic instability.49, 50 Indeed, multiple heterozygous CNVs at the ATAD3 locus have been reported in the Database of Genomic Variants (DGV, Figure S8A), yet no biallelic CNVs are reported.
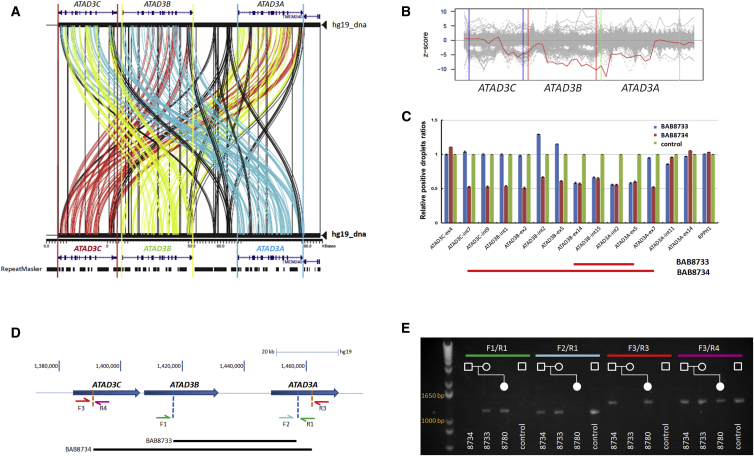
Figure 3.
Compound Heterozygous Copy-Number Variation in Family 7
(A) Miropeats alignment of the ATAD3 region indicates homology of the three ATAD3 paralogs. ATAD3C reference sequence is indicated in red, ATAD3B reference sequence is indicated in green, and ATAD3A reference sequence is indicated in blue.
(B) Target Z score of PCA-normalized read depths for 44 exon targets of the three ATAD3 genes. Comparative read depth data obtained from 2,634 WES samples are shown in gray (clinical collection). Family 7, II-1 is shown in red. Target Z score of PCA-normalized read depths for 16 exons (x axis) spanning portions of ATAD3B and ATAD3A have large negative Z scores (y axis), indicating a copy loss of both alleles.
(C) Droplet Digital PCR (ddPCR) detected a larger heterozygous deletion in BAB8734 (father) than the heterozygous deletion in BAB8733 (mother). Primer pairs targeting various exonic or intronic regions of ATAD3C, ATAD3B, and ATAD3A and a control primer pair targeting RPPH1 were used to perform ddPCR in BAB8733 (mother), BAB8734 (father), and a control individual N/A10851. RPPH1 is a gene known to have exactly two copies in the human genome. Ratios of concentrations of positive droplets were plotted for each amplicon tested against the control individual. For a region without deletion, the expected ratio is ∼1, whereas for heterozygous deletion, the expected ratio is ∼0.5 (one deleted copy-number versus two copies). Corresponding raw data of ddPCR and primer sequences are shown in Table S1.
(D) Schematic of primer design targeting maternal and paternal deletions and wild-type alleles. PCR amplicons indicating segregation of maternal deletion allele (F1/R1) and paternal deletion allele (F3/R3). Note that proband (BAB8780) lacks wild-type allele amplified by F2/R1 but does have wild-type allele amplified by F3/R4 since the latter is positioned outside the maternal deletion and can be amplified from this allele.
(E) Results of PCR breakpoint analysis. Proband inherited both the maternal and paternal deletions.
Through analysis of WES data read depth, we identified a case subject with a biallelic deletion involving exons 1–5 of ATAD3A (Figure 3B). The proband (BAB8780 or family 7, II-1 in Figure 1A) had reduced fetal movement, respiratory failure requiring intubation at birth, cloudy corneas, seizures, and cerebellar and brainstem hypoplasia (Figure 2P). He died at 13 days. Targeted array comparative genomic hybridization (aCGH) (Figures S9A and S9B) and droplet digital PCR on parental samples (Figure 3C), followed by amplification of the breakpoint junctions (Figures 3D and 3E), revealed two distinct deletions. In the maternal sample (BAB8733), deletion of a ∼38 kb segment was mediated by NAHR between the highly homologous fifth introns of ATAD3B and ATAD3A (Figure 4A). The approximate breakpoint junctions are located within chr1: 1,416,438–1,417,192 and 1,454,542–1,455,296 (hg19). Sanger sequencing showed a stretch of 754 bp with 100% identity at the breakpoint junctions, including an AluSx1 element (Figures S8B and S10A). In the paternal sample (BAB8734), deletion of a ∼68 kb segment was mediated by NAHR between the highly homologous region spanning exons 7 and 11 in ATAD3C and ATAD3A, respectively (Figure 4). The approximate breakpoint junctions are located within chr1: 1,391,287–1,392,247 and chr1: 1,459,379–1,460,339 (hg19). Sanger sequencing revealed a stretch of 960 bp with 99.7% homology at the breakpoint junction and 273 bp with 100% identity (Figure S10B). The paternal deletion was not found in 100 population-matched white controls, as evaluated by breakpoint junction PCR (data not shown). The proband inherited both deletions (Figure 3E), presumably leading to loss of both functional copies of ATAD3A. A heterozygous deletion similar to that observed in the mother was identified previously in 1/15,767 individuals (dbVar: nsv545010),51 and a heterozygous deletion similar to that of the father—spanning from ATAD3C to ATAD3A—was present in 13/873 (∼1.5%) individuals (dbVAR: dgv2e212).52 The latter frequency suggests that homozygous or compound heterozygous deletions of ATAD3A may be a source of morbidity in humans. This outcome may be under-recognized, perhaps due to genomic complexity at the locus, issues with the ability to uniquely map sequencing reads to the reference sequencing reads because of the presence of the gene family at this locus, poor coverage on some clinically available aCGH platforms, and small size of these intergenic deletion CNVs.
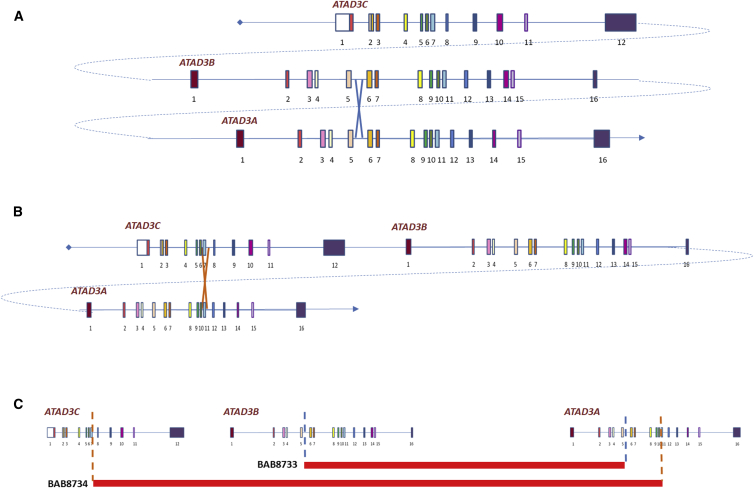
Figure 4.
CNVs in Family 7 Are Mediated by NAHR
(A) Homologous exons of the three ATAD3 genes are represented by similar colors. The deletion in BAB8733 (mother) is mediated by nonallelic homologous recombination (NAHR) between the fifth introns of ATAD3B and ATAD3A.
(B) The deletion in BAB8734 (father) is mediated by NAHR between the region of exon 7 of ATAD3C and exon 11 of ATAD3A.
(C) The boundaries of both deletions are represented by dotted lines.
We also identified a subject (BAB761) with an apparent homozygous deletion of ATAD3C and ATAD3B, not involving ATAD3A and confirmed by aCGH and ddPCR (Figures S9C, S9D, and S11). BAB761 had previously been assigned a clinical and molecular diagnosis of Smith-Magenis syndrome (SMS [MIM: 182290]), associated with a second, heterozygous deletion on chromosome 17p11.2 encompassing RAI1 (MIM: 607642).53 Hence, we concluded that homozygous deletion of ATAD3C and ATAD3B does not have a substantial impact on phenotype and narrowed the critical region for the ATAD3 locus to ATAD3A.
The Fly p.Arg534Trp Variant Causes a Dramatic Loss of Mitochondria in Flies
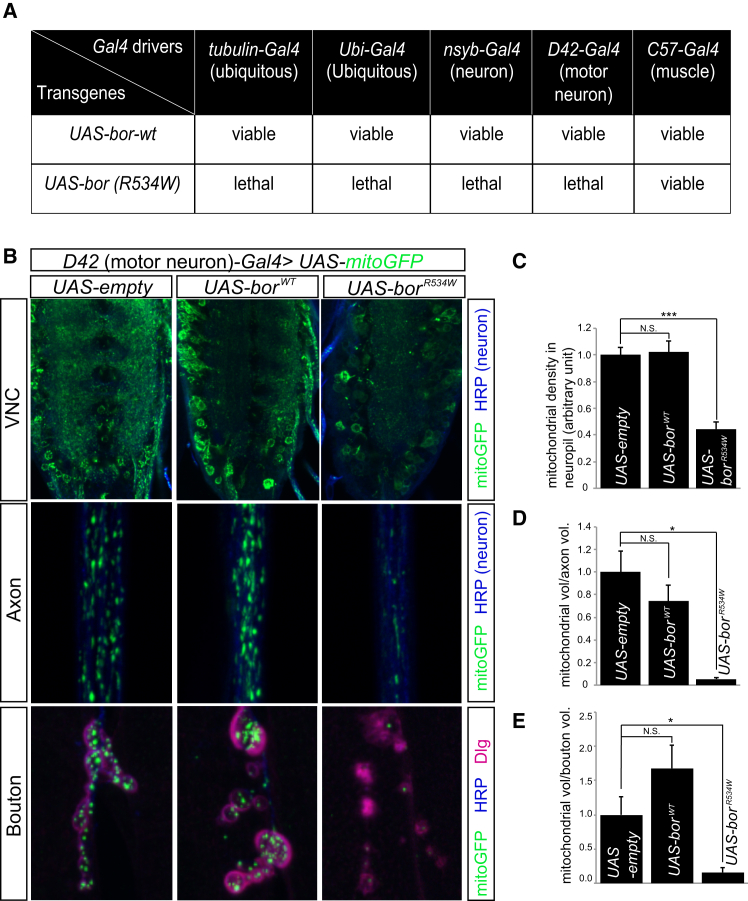
To functionally evaluate the potential pathogenicity of the recurrent de novo p.Arg528Trp variant, we generated transgenic flies harboring a wild-type or variant UAS-cDNA copy (p.Arg534Trp, equivalent to human p.Arg528Trp) of bor, the fly homolog of ATAD3A (UAS-borWT and UAS-borR534W). This allowed us to drive these cDNA with tissue-specific Gal4 drivers. Ubiquitous expression of borR534W (tub- or Ubi-Gal4 > UAS-borR534W), as well as pan-neuronal (n-syb-panneuronal-Gal4 > UAS-borR534W) and motor neuron-specific (D42-motorneurons-Gal4 > UAS-borR534W) expression, all caused lethality, whereas borWT expression (tub-, Ubi-Gal4, n-syb-panneuronal-Gal4, or D42-motorneurons-Gal4 > UAS-borWT) did not affect viability or cause any other obvious phenotype (Figure 5A). Muscle-specific expression of borR534W (C57-muscles-Gal4 > UAS- borR534W) led to ∼90% lethality (Figure 5A). Hence, borR534W expression causes dramatic reductions in survival when expressed in all neurons, motor neurons, or muscles, suggesting that the protein is highly toxic. It may therefore act as either a dominant-negative or a gain-of-function mutation.
Figure 5.
Expression of Drosophila ATAD3A Ortholog bor with p.Arg534Trp Leads to a Decrease in Mitochondria in Motor Neurons
(A) Effects of expression of wild-type bor and borR534W, driven by different Gal4 drivers, on viability.
(B) Confocal micrographs of ventral nerve cord (VNC), axons, and boutons from third instar larvae carrying D42-Gal4 and UAS-mito-GFP (green) together with UAS-empty (control), UAS-borWT, or UAS-borR534W. Neurons are labeled by HRP (blue) and boutons by Dlg (red).
(C) Quantification of mitochondrial density in VNC neuropil in control, UAS-borWT, or UAS-borR534W larvae carrying D42-Gal4, UAS-mito-GFP together with the respective vectors.
(D and E) Quantification of mitochondrial volume in axons (D) and in boutons (E) of larvae carrying D42-Gal4, UAS-mito-GFP together with UAS-empty, UAS-borWT, or UAS-borR534W.
(C–E) Error bars indicate standard error of the mean (SEM). p values were calculated by Student’s t test. ∗p < 0.05, ∗∗∗p < 0.001. N.S. indicates not statistically significant.
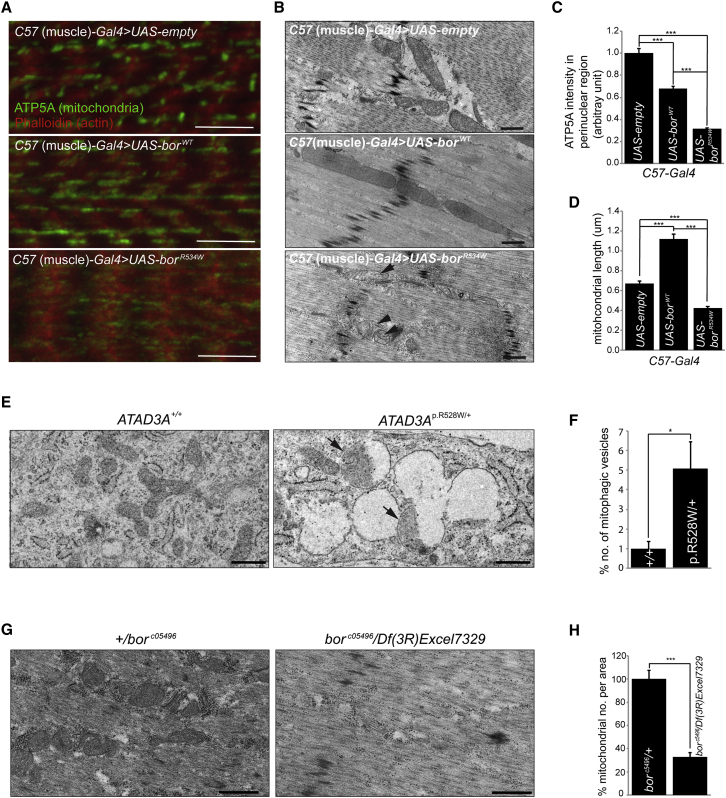
At the cellular level, we noted that motor neurons expressing borR534W exhibited a dramatic reduction in mitochondria in cell bodies in the ventral nerve cord (VNC), axons, and synaptic boutons (Figures 5B–5E), suggesting that expression of the variant severely affects mitochondrial biogenesis54 or mitochondrial dynamics, leading to mitophagy. When expressed specifically in larvae body wall muscles, the signal intensity of the mitochondrial marker ATP5A in borR534W-expressing muscles was significantly reduced when compared to borWT-expressing muscles or wild-type controls, consistent with our observation in motor neurons (Figures 6A and 6C). Transmission electron microscopy (TEM) revealed that muscles expressing borR534W contain very few small mitochondria with highly aberrant cristae (Figure 6B, arrowheads) and display a substantive increase in autophagic intermediates (Figure S12). In contrast, overexpression of borWT resulted in an increase in the number of large elongated mitochondria (Figures 6B and 6D). These data indicate that BorWT promotes fusion or inhibits fission, or affects both, whereas BorR534W inhibits fusion or promotes fission.
Figure 6.
Expression of Drosophila ATAD3 Ortholog bor with p.Arg534Trp Variant in Muscle Causes a Decrease of Mitochondria
(A) Confocal micrographs of muscle from third instar larvae carrying C57-Gal4 together with UAS-empty (control), UAS-borWT, or UAS-borR534W. ATP5A (green) labels mitochondria. Phalloidin (red) labels actin. Scale bars represent 10 μm.
(B) TEM of muscle from third instar larvae carrying C57-Gal4 together with UAS-empty (control), UAS-borWT, or UAS-borR534W. Scale bars represent 500 nm.
(C) Quantification of ATP5A signal intensity in muscle of larvae carrying C57-Gal4, together with UAS-empty, UAS-borWT, or UAS-borR534W from confocal images (A).
(D) Quantification of mitochondrial length in muscle of larvae carrying C57-Gal4, together with UAS-empty, UAS-borWT, or UAS-borR534W from TEM images (B).
(E) TEM of fibroblasts from an affected individual (BAB8644, ATAD3Ap.R528W/+) and control (ATAD3A+/+) fibroblasts. Scale bars represent 500 nm.
(F) Quantification of mitophagic vesicles (E, arrows) among all vesicles in affected (BAB8644, ATAD3AR528W/+) and control (ATAD3A+/+) fibroblasts.
(G) TEM of muscle from first instar larvae for bor mutants (borc05496/Df (3R)Excel7329) and control (borc05496/+). Scale bars represent 500 nm.
(H) Quantification of mitochondria per area in bor mutants (borc05496/Df (3R)Excel7329) and control (borc05496/+).
(C, D, F, H) Error bars indicate SEM. p values were calculated by Student’s t test. ∗p < 0.05. ∗∗∗p < 0.001. N.S. indicates not statistically significant.
Fibroblasts from individual II-2 in family 1 at age 9 years showed a significant increase in mitophagy (Figures 6E, 6F, and S13) and a reduction, though not significant, in mitochondrial content (Figure S14). In conjunction with the fly data, this indicates that the p.Arg528Trp variant interferes with mitochondrial dynamics by either promoting fission or inhibiting fusion, which in turn may trigger mitophagy.
To assess the loss-of-function phenotypes associated with bor, we performed TEM on first instar larvae that lack bor just prior to their death and identified a dramatic decrease in mitochondrial content, similar to overexpression of BorR534W (Figures 6G and 6H). Given the similarities between the phenotypes associated with the loss of function of bor and the overexpression of borR534W, we propose that BorR534W acts as a strong dominant-negative mutation. This is supported by our finding that steady-state levels of ATAD3A protein in fibroblasts of the proband in family 4 are comparable to controls, when assayed both in total lysate and in crude mitochondria (Figure S15).
Discussion
We report eight individuals from seven families with monoallelic or biallelic variation involving both SNVs and CNVs at the ATAD3A locus, all presenting with a primarily neurologic disease. The clinical syndrome associated with the recurrent de novo c.1582C>G variant, observed in five families, manifests within a phenotypic and metabolic spectrum including global developmental delay, hypotonia, optic atrophy, axonal neuropathy, hypertrophic cardiomyopathy, lactic acidosis, and/or increased excretion of Krebs cycle intermediates. Several of these features overlap with phenotypes associated with other mitochondrial fission and fusion proteins (Figure S5), as exemplified by DNM1L (severe developmental delay, optic atrophy, peripheral neuropathy, lactic acidemia [MIM: 614388]),10, 11 MFN2 (axonal peripheral neuropathy and variable optic atrophy [MIM: 609260]),5, 7 OPA1 (optic atrophy, variable peripheral neuropathy,8 and recently described hypertrophic cardiomyopathy [MIM: 165500 and 125250]),9 and SLC25A46 (axonal peripheral neuropathy and optic atrophy [MIM: 616505]).13
The recurrence of the de novo c.1582C>T variant in five distinct probands is likely attributable to a mutational hotspot at a CpG dinucleotide.41, 42, 43 However, it is unclear from this initial study whether this is the only variant that can lead to a dominant phenotype, or if the bias is due to the CpG mutational hotspot. Three of the most highly mutable nucleotides known in the human genome are at CpG sites (FGFR3 [MIM: 100800], DMD [MIM: 310200], and FGFR2 [MIM: 101200]),55 with two more recent examples (KCNC1 [MIM: 616187]56 and PACS1 [MIM: 607492]).57
The features associated with the biallelic SNV identified in two siblings include ataxia, epilepsy, and congenital cataracts, while the compound heterozygous CNVs conferred a more severe phenotype of respiratory insufficiency at birth, cloudy corneas, hypoplastic cerebellum and brainstem, and seizures. Monoallelic and biallelic pathogenic variation at a single locus has been observed for numerous genes, including mitochondrial genes (e.g., OPA1 [MIM: 605290], SLC25A4 [MIM: 103220], AARS [MIM: 601065], EMC1 [MIM: 616846], MAB21L2 [MIM: 604357], and NALCN [MIM: 611549]).9, 58, 59, 60, 61, 62, 63 There is often a difference in severity of phenotype between the monoallelic and biallelic forms of disease, although in many cases, the same organ systems are involved. In the case of ATAD3A, there is primarily involvement of the nervous system and the eye in both monoallelic and biallelic forms.
The ExAC constraint metric for loss of function is 0.04, indicating a high tolerance for heterozygous loss-of-function alleles. Indeed, individuals harboring a heterozygous deletion involving the ATAD3 genes (i.e., parents in family 7) are unaffected. These data suggest a dominant-negative pathogenic mechanism or a gain-of-function mechanism for the recurrent de novo variant rather than haploinsufficiency. The data from Drosophila show that overexpression of a wild-type cDNA, one form of a gain-of-function experiment, does not affect viability and leads to an expansion of mitochondrial size. However, expression of the de novo variant in many tissues in flies is toxic and leads to death. The observed phenotype in these tissues phenocopies the null mutant and yields effects opposite to those seen with overexpression of the wild-type cDNA. Hence, experimental evidence indicates that ATAD3A p.Arg528Trp behaves as a dominant-negative, or antimorphic, allele. This conclusion is supported further by the proposed oligomeric formation of ATAD3 proteins.15
Although most species have only one ATAD3 gene locus, primates have three ATAD3 paralogs (ATAD3A, ATAD3B, ATAD3C), which appear to have evolved by duplication of a single precursor gene. In humans, the three genes are located in tandem on chromosome 1p36.33 (Figure 1E). Multiple isoforms of the three ATAD3 genes are generated by transcriptional and post-transcriptional events. The major ATAD3A isoform, p66, is ubiquitously expressed, whereas the major ATAD3B isoform, p67, was specifically detected in embryo muscle, placenta, and adult brain and heart. ATAD3C is missing four exons as compared to ATAD3A, suggesting that it may be a pseudogene. In the mouse, a single Atad3 gene is located on murine chromosome 4 and encodes a major ubiquitous isoform and several smaller tissue- and/or temporal-specific isoforms.23 ATAD3 function is essential in the mouse for early post-implantation development. ATAD3-deficient embryos die around E7.5 due to growth retardation and a defective development of the trophoblast lineage; heterozygotes seem unaffected.22 Given the severe phenotype and respiratory insufficiency of family 7, II-1, it may be surmised that biallelic deletions involving ATAD3A will lead to fetal demise. The potential salvage of lethality in this individual during development by a theoretical fusion protein extending from ATAD3B to ATAD3A and under control of the ATAD3B promoter, active during embryonic development,23, 64 warrants further investigation. Moreover, the potential embryonic lethality of the homozygous ∼68 kb ATAD3C-ATAD3A allele could distort Mendelian expectations despite the observed relatively high (∼1.5%) carrier frequency in the white population.
ATAD3A has two coiled-coil domains with high oligomerization probability within the N terminus, followed by a predicted transmembrane segment in the central part of the molecule and a conserved ATPase domain in the C terminus (Figure 1E).15, 23 Oligomerization of ATAD3A monomers is further supported by findings showing that other AAA proteins assemble as hexameric rings.65 Studies in human U373 cells demonstrated that defective ATP-binding ATAD3A mutants interfere with normal oligomer functions and lead to fragmentation of mitochondria.15 In the current study, we observed a dramatic loss of mitochondria in motor neurons of transgenic mutant flies (Figures 5B–5E), highly aberrant mitochondrial morphology, and an increase in autophagic intermediates in the fly muscle (Figure S12). Moreover, overexpression of wild-type bor resulted in an increased number of large elongated mitochondria (Figures 6B and 6D) whereas TEM of homozygous null flies showed a dramatic decrease in size and number of mitochondria (Figures 6G and 6H). Finally, fibroblasts from an affected individual harboring the p.Arg528Trp variant showed a significant increase in mitophagy (Figures 6E, 6F, and S13) and a reduction, though not statistically significant, in mitochondrial content (Figure S14). The aggregate data suggest that the production of small aberrant mitochondria probably results in increased mitophagy and a significant reduction of mitochondrial content. The molecular mechanisms remain to be elucidated.
In summary, we have identified monoallelic and biallelic variation involving both SNVs and CNVs at the ATAD3A locus. We propose that the dominant p.Arg528Trp variant disturbs ATAD3A function by a dominant-negative mechanism, while the recessive CNVs lead to infantile lethality through loss of ATAD3A function. These data suggest an allelic series with decreasing severity: c.1582C>T (p.Arg528Trp) (antimorph) > ATAD3A deletion CNV (null) > c.158C>T (p.Thr53Ile) (hypomorph). The previously described function of ATAD3A in mitochondrial dynamics and its co-immunoprecipitation with MFN2 and OPA1, in conjunction with the phenotypic and functional data presented herein, indicates that ATAD3A is essential in humans.
Conflicts of Interest
J.R.L. has stock ownership in 23andMe, is a paid consultant for Regeneron Pharmaceuticals, has stock options in Lasergen, Inc., is a member of the Scientific Advisory Board of Baylor Genetics, and is a co-inventor on multiple United States and European patents related to molecular diagnostics for inherited neuropathies, eye diseases, and bacterial genomic fingerprinting. The Department of Molecular and Human Genetics at Baylor College of Medicine derives revenue from the chromosomal microarray analysis (CMA) and clinical exome sequencing offered at Baylor Genetics.
Acknowledgments
We thank the families for their collaboration and participation in this study. This work was supported in part by the US National Human Genome Research Institute/National Heart Lung and Blood Institute Baylor Hopkins Center for Mendelian Genomics (NHGRI/NHLBI, U54HG006542), the Intellectual Developmental Disabilities Research Center, IDDRC (NICHD HD083092, Clinical Translational Core), and the NHGRI/NHLBI University of Washington Center for Mendelian Genomics (2UM1HG006493). This work was also supported in part by the National Institute of Neurological Disease and Stroke (NINDS, RO1NS058529) to J.R.L. T.H. and J.E.P. are supported by the NIH/NIGMS T32 GM07526 Medical Genetics Research Fellowship Program. C. Garone is supported by European Commission Project 705560, Marie Curie Individual Fellowship. H.J.B. is supported by the Undiagnosed Diseases Network (UDN, 1U54NS093793-01). R.A.L. is supported in part by the Genetic Resource Association of Texas (GReAT), Houston, Texas. D.M. Martin is supported by the NIH (R01 DC009410) and the Dointa B. Sullivan, MD Research Professorship.
Published: September 15, 2016
Footnotes
Supplemental Data include detailed clinical case reports, 15 figures, and 4 tables and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2016.08.007.
Contributor Information
Tamar Harel, Email: harel.tamar@gmail.com.
James R. Lupski, Email: jlupski@bcm.edu.
Accession Numbers
The ClinVar accession numbers for the DNA variant data reported in this paper are SCV000267601, SCV000267602, SCV000267603, and SCV000267604.
Web Resources
1000 Genomes, http://www.1000genomes.org
Atherosclerosis Risk in Communities Study (ARIC), http://drupal.cscc.unc.edu/aric/
Baylor Genetics, http://www.bmgl.com
ConSurf, consurf.tau.ac.il
Database of Genomic Variants (DGV), http://dgv.tcag.ca/dgv/app/home
Ensembl Genome Browser, http://www.ensembl.org/index.html
ExAC Browser, http://exac.broadinstitute.org/
GeneMatcher, https://genematcher.org/
HMZDelFinder, https://github.com/BCM-Lupskilab/HMZDelFinder
NHLBI Exome Sequencing Project (ESP) Exome Variant Server, http://evs.gs.washington.edu/EVS/
OMIM, http://www.omim.org/
PyMOL, http://www.pymol.org
R statistical software, http://www.r-project.org/
Supplemental Data
References
- 1.Mishra P., Chan D.C. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat. Rev. Mol. Cell Biol. 2014;15:634–646. doi: 10.1038/nrm3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niemann A., Ruegg M., La Padula V., Schenone A., Suter U. Ganglioside-induced differentiation associated protein 1 is a regulator of the mitochondrial network: new implications for Charcot-Marie-Tooth disease. J. Cell Biol. 2005;170:1067–1078. doi: 10.1083/jcb.200507087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pareyson D., Saveri P., Sagnelli A., Piscosquito G. Mitochondrial dynamics and inherited peripheral nerve diseases. Neurosci. Lett. 2015;596:66–77. doi: 10.1016/j.neulet.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Züchner S., Mersiyanova I.V., Muglia M., Bissar-Tadmouri N., Rochelle J., Dadali E.L., Zappia M., Nelis E., Patitucci A., Senderek J. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat. Genet. 2004;36:449–451. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]
- 5.Züchner S., De Jonghe P., Jordanova A., Claeys K.G., Guergueltcheva V., Cherninkova S., Hamilton S.R., Van Stavern G., Krajewski K.M., Stajich J. Axonal neuropathy with optic atrophy is caused by mutations in mitofusin 2. Ann. Neurol. 2006;59:276–281. doi: 10.1002/ana.20797. [DOI] [PubMed] [Google Scholar]
- 6.Verhoeven K., Claeys K.G., Züchner S., Schröder J.M., Weis J., Ceuterick C., Jordanova A., Nelis E., De Vriendt E., Van Hul M. MFN2 mutation distribution and genotype/phenotype correlation in Charcot-Marie-Tooth type 2. Brain. 2006;129:2093–2102. doi: 10.1093/brain/awl126. [DOI] [PubMed] [Google Scholar]
- 7.Rouzier C., Bannwarth S., Chaussenot A., Chevrollier A., Verschueren A., Bonello-Palot N., Fragaki K., Cano A., Pouget J., Pellissier J.F. The MFN2 gene is responsible for mitochondrial DNA instability and optic atrophy ‘plus’ phenotype. Brain. 2012;135:23–34. doi: 10.1093/brain/awr323. [DOI] [PubMed] [Google Scholar]
- 8.Yu-Wai-Man P., Griffiths P.G., Gorman G.S., Lourenco C.M., Wright A.F., Auer-Grumbach M., Toscano A., Musumeci O., Valentino M.L., Caporali L. Multi-system neurological disease is common in patients with OPA1 mutations. Brain. 2010;133:771–786. doi: 10.1093/brain/awq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spiegel R., Saada A., Flannery P.J., Burté F., Soiferman D., Khayat M., Eisner V., Vladovski E., Taylor R.W., Bindoff L.A. Fatal infantile mitochondrial encephalomyopathy, hypertrophic cardiomyopathy and optic atrophy associated with a homozygous OPA1 mutation. J. Med. Genet. 2016;53:127–131. doi: 10.1136/jmedgenet-2015-103361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chao Y.H., Robak L.A., Xia F., Koenig M.K., Adesina A., Bacino C.A., Scaglia F., Bellen H.J., Wangler M.F. Missense variants in the middle domain of DNM1L in cases of infantile encephalopathy alter peroxisomes and mitochondria when assayed in Drosophila. Hum. Mol. Genet. 2016;25:1846–1856. doi: 10.1093/hmg/ddw059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waterham H.R., Koster J., van Roermund C.W., Mooyer P.A., Wanders R.J., Leonard J.V. A lethal defect of mitochondrial and peroxisomal fission. N. Engl. J. Med. 2007;356:1736–1741. doi: 10.1056/NEJMoa064436. [DOI] [PubMed] [Google Scholar]
- 12.Shahni R., Cale C.M., Anderson G., Osellame L.D., Hambleton S., Jacques T.S., Wedatilake Y., Taanman J.W., Chan E., Qasim W. Signal transducer and activator of transcription 2 deficiency is a novel disorder of mitochondrial fission. Brain. 2015;138:2834–2846. doi: 10.1093/brain/awv182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abrams A.J., Hufnagel R.B., Rebelo A., Zanna C., Patel N., Gonzalez M.A., Campeanu I.J., Griffin L.B., Groenewald S., Strickland A.V. Mutations in SLC25A46, encoding a UGO1-like protein, cause an optic atrophy spectrum disorder. Nat. Genet. 2015;47:926–932. doi: 10.1038/ng.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niemann A., Wagner K.M., Ruegg M., Suter U. GDAP1 mutations differ in their effects on mitochondrial dynamics and apoptosis depending on the mode of inheritance. Neurobiol. Dis. 2009;36:509–520. doi: 10.1016/j.nbd.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Gilquin B., Taillebourg E., Cherradi N., Hubstenberger A., Gay O., Merle N., Assard N., Fauvarque M.O., Tomohiro S., Kuge O., Baudier J. The AAA+ ATPase ATAD3A controls mitochondrial dynamics at the interface of the inner and outer membranes. Mol. Cell. Biol. 2010;30:1984–1996. doi: 10.1128/MCB.00007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He J., Cooper H.M., Reyes A., Di Re M., Sembongi H., Litwin T.R., Gao J., Neuman K.C., Fearnley I.M., Spinazzola A. Mitochondrial nucleoid interacting proteins support mitochondrial protein synthesis. Nucleic Acids Res. 2012;40:6109–6121. doi: 10.1093/nar/gks266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Issop L., Fan J., Lee S., Rone M.B., Basu K., Mui J., Papadopoulos V. Mitochondria-associated membrane formation in hormone-stimulated Leydig cell steroidogenesis: role of ATAD3. Endocrinology. 2015;156:334–345. doi: 10.1210/en.2014-1503. [DOI] [PubMed] [Google Scholar]
- 18.Rone M.B., Midzak A.S., Issop L., Rammouz G., Jagannathan S., Fan J., Ye X., Blonder J., Veenstra T., Papadopoulos V. Identification of a dynamic mitochondrial protein complex driving cholesterol import, trafficking, and metabolism to steroid hormones. Mol. Endocrinol. 2012;26:1868–1882. doi: 10.1210/me.2012-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teng Y., Ren X., Li H., Shull A., Kim J., Cowell J.K. Mitochondrial ATAD3A combines with GRP78 to regulate the WASF3 metastasis-promoting protein. Oncogene. 2016;35:333–343. doi: 10.1038/onc.2015.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.You W.C., Chiou S.H., Huang C.Y., Chiang S.F., Yang C.L., Sudhakar J.N., Lin T.Y., Chiang I.P., Shen C.C., Cheng W.Y. Mitochondrial protein ATPase family, AAA domain containing 3A correlates with radioresistance in glioblastoma. Neuro-oncol. 2013;15:1342–1352. doi: 10.1093/neuonc/not077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang H.Y., Chang C.L., Hsu S.H., Huang C.Y., Chiang S.F., Chiou S.H., Huang C.H., Hsiao Y.T., Lin T.Y., Chiang I.P. ATPase family AAA domain-containing 3A is a novel anti-apoptotic factor in lung adenocarcinoma cells. J. Cell Sci. 2010;123:1171–1180. doi: 10.1242/jcs.062034. [DOI] [PubMed] [Google Scholar]
- 22.Goller T., Seibold U.K., Kremmer E., Voos W., Kolanus W. Atad3 function is essential for early post-implantation development in the mouse. PLoS ONE. 2013;8:e54799. doi: 10.1371/journal.pone.0054799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S., Lamarche F., Charton R., Delphin C., Gires O., Hubstenberger A., Schlattner U., Rousseau D. Expression analysis of ATAD3 isoforms in rodent and human cell lines and tissues. Gene. 2014;535:60–69. doi: 10.1016/j.gene.2013.10.062. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann M., Bellance N., Rossignol R., Koopman W.J., Willems P.H., Mayatepek E., Bossinger O., Distelmaier F. C. elegans ATAD-3 is essential for mitochondrial activity and development. PLoS ONE. 2009;4:e7644. doi: 10.1371/journal.pone.0007644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Y., Muzny D.M., Xia F., Niu Z., Person R., Ding Y., Ward P., Braxton A., Wang M., Buhay C. Molecular findings among patients referred for clinical whole-exome sequencing. JAMA. 2014;312:1870–1879. doi: 10.1001/jama.2014.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reid J.G., Carroll A., Veeraraghavan N., Dahdouli M., Sundquist A., English A., Bainbridge M., White S., Salerno W., Buhay C. Launching genomics into the cloud: deployment of Mercury, a next generation sequence analysis pipeline. BMC Bioinformatics. 2014;15:30. doi: 10.1186/1471-2105-15-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Challis D., Yu J., Evani U.S., Jackson A.R., Paithankar S., Coarfa C., Milosavljevic A., Gibbs R.A., Yu F. An integrative variant analysis suite for whole exome next-generation sequencing data. BMC Bioinformatics. 2012;13:8. doi: 10.1186/1471-2105-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R., 1000 Genome Project Data Processing Subgroup The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang K., Li M., Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sobreira N., Schiettecatte F., Valle D., Hamosh A. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum. Mutat. 2015;36:928–930. doi: 10.1002/humu.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang J., Yan R., Roy A., Xu D., Poisson J., Zhang Y. The I-TASSER Suite: protein structure and function prediction. Nat. Methods. 2015;12:7–8. doi: 10.1038/nmeth.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ashkenazy H., Erez E., Martz E., Pupko T., Ben-Tal N. ConSurf 2010: calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res. 2010;38:W529-33. doi: 10.1093/nar/gkq399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bugiani M., Invernizzi F., Alberio S., Briem E., Lamantea E., Carrara F., Moroni I., Farina L., Spada M., Donati M.A. Clinical and molecular findings in children with complex I deficiency. Biochim. Biophys. Acta. 2004;1659:136–147. doi: 10.1016/j.bbabio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Fromer M., Moran J.L., Chambert K., Banks E., Bergen S.E., Ruderfer D.M., Handsaker R.E., McCarroll S.A., O’Donovan M.C., Owen M.J. Discovery and statistical genotyping of copy-number variation from whole-exome sequencing depth. Am. J. Hum. Genet. 2012;91:597–607. doi: 10.1016/j.ajhg.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parsons J.D. Miropeats: graphical DNA sequence comparisons. Comput. Appl. Biosci. 1995;11:615–619. doi: 10.1093/bioinformatics/11.6.615. [DOI] [PubMed] [Google Scholar]
- 37.Parks A.L., Cook K.R., Belvin M., Dompe N.A., Fawcett R., Huppert K., Tan L.R., Winter C.G., Bogart K.P., Deal J.E. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat. Genet. 2004;36:288–292. doi: 10.1038/ng1312. [DOI] [PubMed] [Google Scholar]
- 38.Bischof J., Maeda R.K., Hediger M., Karch F., Basler K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl. Acad. Sci. USA. 2007;104:3312–3317. doi: 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Venken K.J., He Y., Hoskins R.A., Bellen H.J. P[acman]: a BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science. 2006;314:1747–1751. doi: 10.1126/science.1134426. [DOI] [PubMed] [Google Scholar]
- 40.Bellen H., Budnik V. Cold Spring Harbor Laboratory Press; New York: 2000. The Neuromuscular Junction. [Google Scholar]
- 41.Cooper D.N., Mort M., Stenson P.D., Ball E.V., Chuzhanova N.A. Methylation-mediated deamination of 5-methylcytosine appears to give rise to mutations causing human inherited disease in CpNpG trinucleotides, as well as in CpG dinucleotides. Hum. Genomics. 2010;4:406–410. doi: 10.1186/1479-7364-4-6-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cooper D.N., Krawczak M. Cytosine methylation and the fate of CpG dinucleotides in vertebrate genomes. Hum. Genet. 1989;83:181–188. doi: 10.1007/BF00286715. [DOI] [PubMed] [Google Scholar]
- 43.Coulondre C., Miller J.H., Farabaugh P.J., Gilbert W. Molecular basis of base substitution hotspots in Escherichia coli. Nature. 1978;274:775–780. doi: 10.1038/274775a0. [DOI] [PubMed] [Google Scholar]
- 44.Posey J.E., Rosenfeld J.A., James R.A., Bainbridge M., Niu Z., Wang X., Dhar S., Wiszniewski W., Akdemir Z.H., Gambin T. Molecular diagnostic experience of whole-exome sequencing in adult patients. Genet. Med. 2016;18:678–685. doi: 10.1038/gim.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farwell K.D., Shahmirzadi L., El-Khechen D., Powis Z., Chao E.C., Tippin Davis B., Baxter R.M., Zeng W., Mroske C., Parra M.C. Enhanced utility of family-centered diagnostic exome sequencing with inheritance model-based analysis: results from 500 unselected families with undiagnosed genetic conditions. Genet. Med. 2015;17:578–586. doi: 10.1038/gim.2014.154. [DOI] [PubMed] [Google Scholar]
- 46.Yang Y., Muzny D.M., Reid J.G., Bainbridge M.N., Willis A., Ward P.A., Braxton A., Beuten J., Xia F., Niu Z. Clinical whole-exome sequencing for the diagnosis of mendelian disorders. N. Engl. J. Med. 2013;369:1502–1511. doi: 10.1056/NEJMoa1306555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campbell I.M., Shaw C.A., Stankiewicz P., Lupski J.R. Somatic mosaicism: implications for disease and transmission genetics. Trends Genet. 2015;31:382–392. doi: 10.1016/j.tig.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Campbell I.M., Stewart J.R., James R.A., Lupski J.R., Stankiewicz P., Olofsson P., Shaw C.A. Parent of origin, mosaicism, and recurrence risk: probabilistic modeling explains the broken symmetry of transmission genetics. Am. J. Hum. Genet. 2014;95:345–359. doi: 10.1016/j.ajhg.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carvalho C.M., Lupski J.R. Mechanisms underlying structural variant formation in genomic disorders. Nat. Rev. Genet. 2016;17:224–238. doi: 10.1038/nrg.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dittwald P., Gambin T., Szafranski P., Li J., Amato S., Divon M.Y., Rodríguez Rojas L.X., Elton L.E., Scott D.A., Schaaf C.P. NAHR-mediated copy-number variants in a clinical population: mechanistic insights into both genomic disorders and Mendelizing traits. Genome Res. 2013;23:1395–1409. doi: 10.1101/gr.152454.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cooper G.M., Coe B.P., Girirajan S., Rosenfeld J.A., Vu T.H., Baker C., Williams C., Stalker H., Hamid R., Hannig V. A copy number variation morbidity map of developmental delay. Nat. Genet. 2011;43:838–846. doi: 10.1038/ng.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uddin M., Thiruvahindrapuram B., Walker S., Wang Z., Hu P., Lamoureux S., Wei J., MacDonald J.R., Pellecchia G., Lu C. A high-resolution copy-number variation resource for clinical and population genetics. Genet. Med. 2015;17:747–752. doi: 10.1038/gim.2014.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greenberg F., Lewis R.A., Potocki L., Glaze D., Parke J., Killian J., Murphy M.A., Williamson D., Brown F., Dutton R. Multi-disciplinary clinical study of Smith-Magenis syndrome (deletion 17p11.2) Am. J. Med. Genet. 1996;62:247–254. doi: 10.1002/(SICI)1096-8628(19960329)62:3<247::AID-AJMG9>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 54.Li S., Yao Y., Xu R., Pesenti S., Cottet-Rousselle C., Rieusset J., Tokarska-Schlattner M., Liao K., Schlattner U., Rousseau D. ATAD3 is a limiting factor in mitochondrial biogenesis and adipogenesis of white adipocyte-like 3T3-L1 cells. Mol. Cell. Biol. 2014 doi: 10.1128/MCB.01170-13. Published online April 14, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buzin C.H., Feng J., Yan J., Scaringe W., Liu Q., den Dunnen J., Mendell J.R., Sommer S.S. Mutation rates in the dystrophin gene: a hotspot of mutation at a CpG dinucleotide. Hum. Mutat. 2005;25:177–188. doi: 10.1002/humu.20132. [DOI] [PubMed] [Google Scholar]
- 56.Muona M., Berkovic S.F., Dibbens L.M., Oliver K.L., Maljevic S., Bayly M.A., Joensuu T., Canafoglia L., Franceschetti S., Michelucci R. A recurrent de novo mutation in KCNC1 causes progressive myoclonus epilepsy. Nat. Genet. 2015;47:39–46. doi: 10.1038/ng.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schuurs-Hoeijmakers J.H., Oh E.C., Vissers L.E., Swinkels M.E., Gilissen C., Willemsen M.A., Holvoet M., Steehouwer M., Veltman J.A., de Vries B.B. Recurrent de novo mutations in PACS1 cause defective cranial-neural-crest migration and define a recognizable intellectual-disability syndrome. Am. J. Hum. Genet. 2012;91:1122–1127. doi: 10.1016/j.ajhg.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaukonen J.A., Amati P., Suomalainen A., Rötig A., Piscaglia M.-G., Salvi F., Weissenbach J., Fratta G., Comi G., Peltonen L., Zeviani M. An autosomal locus predisposing to multiple deletions of mtDNA on chromosome 3p. Am. J. Hum. Genet. 1996;58:763–769. [PMC free article] [PubMed] [Google Scholar]
- 59.Palmieri L., Alberio S., Pisano I., Lodi T., Meznaric-Petrusa M., Zidar J., Santoro A., Scarcia P., Fontanesi F., Lamantea E. Complete loss-of-function of the heart/muscle-specific adenine nucleotide translocator is associated with mitochondrial myopathy and cardiomyopathy. Hum. Mol. Genet. 2005;14:3079–3088. doi: 10.1093/hmg/ddi341. [DOI] [PubMed] [Google Scholar]
- 60.Simons C., Griffin L.B., Helman G., Golas G., Pizzino A., Bloom M., Murphy J.L., Crawford J., Evans S.H., Topper S. Loss-of-function alanyl-tRNA synthetase mutations cause an autosomal-recessive early-onset epileptic encephalopathy with persistent myelination defect. Am. J. Hum. Genet. 2015;96:675–681. doi: 10.1016/j.ajhg.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harel T., Yesil G., Bayram Y., Coban-Akdemir Z., Charng W.L., Karaca E., Al Asmari A., Eldomery M.K., Hunter J.V., Jhangiani S.N., Baylor-Hopkins Center for Mendelian Genomics Monoallelic and biallelic variants in EMC1 identified in individuals with global developmental delay, hypotonia, scoliosis and cerebellar atrophy. Am. J. Hum. Genet. 2016;98:562–570. doi: 10.1016/j.ajhg.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rainger J., Pehlivan D., Johansson S., Bengani H., Sanchez-Pulido L., Williamson K.A., Ture M., Barker H., Rosendahl K., Spranger J., UK10K. Baylor-Hopkins Center for Mendelian Genomics Monoallelic and biallelic mutations in MAB21L2 cause a spectrum of major eye malformations. Am. J. Hum. Genet. 2014;94:915–923. doi: 10.1016/j.ajhg.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chong J.X., McMillin M.J., Shively K.M., Beck A.E., Marvin C.T., Armenteros J.R., Buckingham K.J., Nkinsi N.T., Boyle E.A., Berry M.N., University of Washington Center for Mendelian Genomics De novo mutations in NALCN cause a syndrome characterized by congenital contractures of the limbs and face, hypotonia, and developmental delay. Am. J. Hum. Genet. 2015;96:462–473. doi: 10.1016/j.ajhg.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Merle N., Féraud O., Gilquin B., Hubstenberger A., Kieffer-Jacquinot S., Assard N., Bennaceur-Griscelli A., Honnorat J., Baudier J. ATAD3B is a human embryonic stem cell specific mitochondrial protein, re-expressed in cancer cells, that functions as dominant negative for the ubiquitous ATAD3A. Mitochondrion. 2012;12:441–448. doi: 10.1016/j.mito.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 65.Langer T., Käser M., Klanner C., Leonhard K. AAA proteases of mitochondria: quality control of membrane proteins and regulatory functions during mitochondrial biogenesis. Biochem. Soc. Trans. 2001;29:431–436. doi: 10.1042/bst0290431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.