Abstract
We describe four families with affected siblings showing unique clinical features: early-onset (before 1 year of age) progressive diffuse brain atrophy with regression, postnatal microcephaly, postnatal growth retardation, muscle weakness/atrophy, and respiratory failure. By whole-exome sequencing, we identified biallelic TBCD mutations in eight affected individuals from the four families. TBCD encodes TBCD (tubulin folding co-factor D), which is one of five tubulin-specific chaperones playing a pivotal role in microtubule assembly in all cells. A total of seven mutations were found: five missense mutations, one nonsense, and one splice site mutation resulting in a frameshift. In vitro cell experiments revealed the impaired binding between most mutant TBCD proteins and ARL2, TBCE, and β-tubulin. The in vivo experiments using olfactory projection neurons in Drosophila melanogaster indicated that the TBCD mutations caused loss of function. The wide range of clinical severity seen in this neurodegenerative encephalopathy may result from the residual function of mutant TBCD proteins. Furthermore, the autopsied brain from one deceased individual showed characteristic neurodegenerative findings: cactus and somatic sprout formations in the residual Purkinje cells in the cerebellum, which are also seen in some diseases associated with mitochondrial impairment. Defects of microtubule formation caused by TBCD mutations may underlie the pathomechanism of this neurodegenerative encephalopathy.
Main Text
Microtubules, cylindrical structures composed of rows of α/β-tubulin heterodimers, are essential components of all eukaryotic cells and are involved in a number of functions such as cell division, morphology, polarization, migration, and intracellular transport.1, 2 Their unique feature of growth (polymerization) and shrinkage (depolymerization) is known as microtubule dynamics.3, 4 α/β-tubulin heterodimers are formed with the aid of tubulin-specific chaperones comprised of five tubulin folding co-factors (TBC): TBCA, TBCB, TBCC, TBCD, and TBCE.5, 6 α- and β-tubulin monomers initially bind with TBCB and TBCA, respectively, and are passed to TBCE and TBCD, respectively, and form complexes together with TBCC and ARL2.5, 7, 8, 9
Of the five TBC genes, only mutations in TBCE (GenBank: NM_003193.4; MIM: 604934) have been found to be associated with an autosomal-recessive hypoparathyroidism-retardation-dysmorphism syndrome (MIM: 241410).10 This is characterized by congenital hypoparathyroidism, microcephaly, hypoplasia of the corpus callosum, intellectual disability, growth failure, and dysmorphic features.11 It is also associated with a similar disease with bone dysplasia, Kenny-Caffey syndrome (MIM: 244460).10 Here, we report biallelic mutations in TBCD (GenBank: NM_005993.4; MIM: 604649) encoding TBCD and the resulting mutant phenotypes in humans.
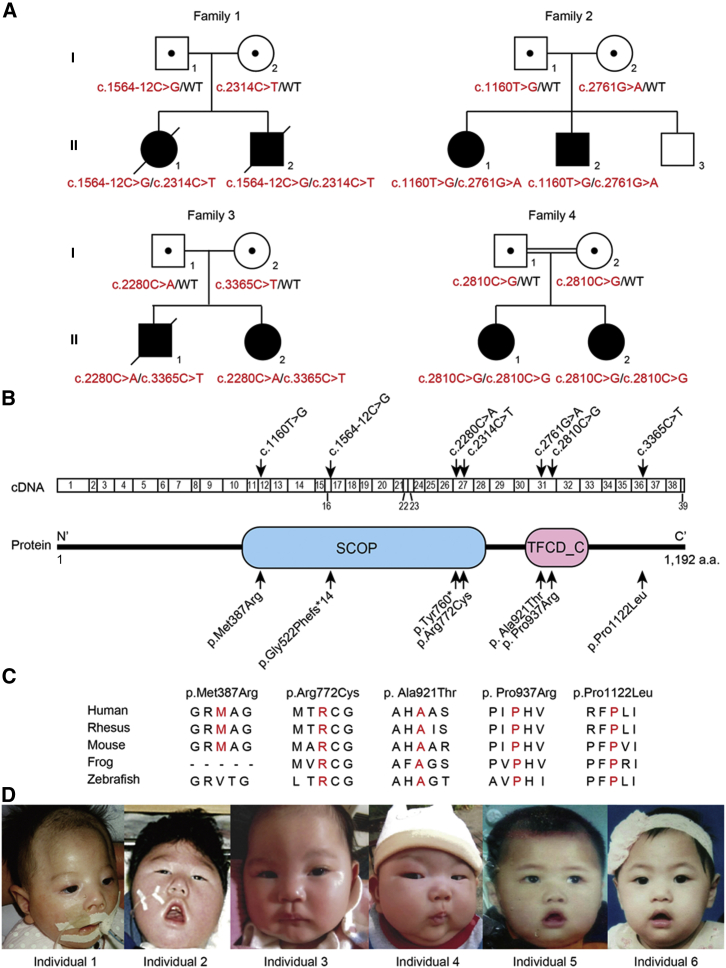
In this study, eight affected individuals from two Japanese families, a Chinese family, and an Israeli family were investigated (Figure 1A). The Israeli family was consanguineous, but the others were not. Genomic DNA was extracted from peripheral leukocytes using QuickGene-610L (Fujifilm) or formalin-fixed paraffin-embedded brain tissue using QIAamp DNA FFPE tissue kit (QIAGEN) (for individual 1: II-1 in family 1 in Figure 1A) according to the manufacturer’s instructions. Blood samples or brain tissue from affected individuals and their families were collected after obtaining written informed consent. The study protocol was approved by the institutional review board of Yokohama City University School of Medicine. To identify the genetic cause of the disease, we performed whole-exome sequencing as described previously.12 The presence of affected siblings in the families implies an autosomal-recessive mode of inheritance (Figure 1A). Therefore, we focused on genes with homozygous or compound heterozygous variants and identified biallelic TBCD mutations in all probands in the four families (Figure 1A and Tables S1–S3). Using Sanger sequencing, we confirmed that the biallelic mutations perfectly co-segregated with the disease status (Figure S1). A total of seven mutations were found in TBCD: five missense, one nonsense, and a splicing mutation (Table 1). These variants were unreported or extremely rare (≤0.0002) in ExAC, EVS, 1000 Genome, HGVD, and our in-house exome database (n = 575). All of the missense variants were predicted to be pathogenic by PolyPhen-2 and MutationTaster. In addition, four altered amino residues due to missense mutations were located within the functional domains (Figure 1B). Five of the amino residues altered by the missense mutations were evolutionally conserved (four from zebrafish to human and one from mouse to human) (Figure 1C). The intronic mutation (c.1564−12C>G) resulted in an 11-bp insertion of intronic sequence between exons 16 and 17, which was confirmed at the cDNA level to cause a frameshift (p.Gly522Phefs∗14) subjected to nonsense-mediated mRNA decay (Figure S2). This genetic evidence strongly indicates that biallelic TBCD mutations may cause this neurodegenerative encephalopathy.
Figure 1.
Familial Pedigrees and TBCD Mutations
(A) The pedigrees of four families with biallelic TBCD mutations. The mutations are shown in red. Affected and unaffected individuals are shown as black and white symbols, respectively. Affected individual identities are as follows: individuals 1 (II-1 in family 1), 2 (II-2 in family 1), 3 (II-1 in family 2), 4 (II-2 in family 2), 5 (II-1 in family 3), 6 (II-2 in family 3), 7 (II-1 in family 4), and 8 (II-2 in family 4).
(B) TBCD cDNA and TBCD protein structures. The p.Met387Arg and p.Arg772Cys variants fall within the SCOP domain, and p.Ala921Thr and p.Pro937Arg within the TFCD_C domain. The seven variants found in the cDNA and protein are indicated by arrows. Functional domains of the TBCD protein were predicted by the SMART program.
(C) The evolutionarily conserved amino acids in the missense variants. The conserved amino residues are marked in red.
(D) The facial features of the affected individuals. From left to right, individual 1 at 3 months, individual 2 at 3 years, individual 3 at 4 months, individual 4 at 3 months, individual 5 at 1 year, and individual 6 at 1 year.
Table 1.
Summary of TBCD Mutations
| Mutation | Amino Acid Change | Position on chr17 (hg19) | dbSNP141 | PolyPhen-2 (Score) | MutationTaster (Score) | ExAC | EVS | 1000 Genomes | HGVD | In-house (n = 575) |
|---|---|---|---|---|---|---|---|---|---|---|
| c.1160T>G | p.Met387Arg | 80,767,595 | no | possibly damaging (0.733) | disease causing (0.996) | no | no | no | no | 0 |
| c.1564−12C>G | p. Gly522Phefs∗14 | 80,851,411 | no | NA | NA | no | no | no | no | 0 |
| c.2280C>A | p.Tyr760∗ | 80,882,834 | no | NA | disease causing (1.000) | no | no | no | no | 0 |
| c.2314C>T | p.Arg772Cys | 80,882,868 | rs181969865 | probably damaging (0.972) | disease causing (1.000) | no | no | 0.0002 | no | 0 |
| c.2761G>A | p. Ala921Thr | 80,887,056 | no | probably damaging (1.000) | disease causing (1.000) | no | no | no | no | 0 |
| c.2810C>G | p.Pro937Arg | 80,887,105 | no | probably damaging (1.000) | disease causing (1.000) | no | no | no | no | 0 |
| c.3365C>T | p.Pro1122Leu | 80,896,008 | no | probably damaging (1.000) | disease causing (1.000) | 0.00003645 | no | no | no | 0 |
The description of the mutation was based on GenBank: NM_005993.4. Abbreviations are as follows: ExAC, Exome Aggregation Consortium; EVS, Exome Variant Server; HGVD, Human Genetic Variation Database; NA, not applicable. Data of minor allele frequencies were examined in May 2016.
The clinical data of all affected individuals are shown in Table 2 and the Supplemental Note. In brief, affected individuals with biallelic TBCD mutations showed an early onset of symptoms (before 1 year of age). The first symptoms varied among the families but were similar in affected siblings from the same family: neonatal asphyxia in family 1, decreased movement and hypotonia at 1 month of age in family 2 and at 5 months in family 3, and seizures at 9–11 months in family 4. The affected individuals commonly had progressive clinical courses with muscle weakness, absent visual tracking, and postnatal microcephaly. More than half of the affected individuals had postnatal growth failure, seizure, respiratory failure, regression, optic nerve atrophy, hypotonia, and muscle atrophy. In addition, two affected individuals had variable blood pressure and two other individuals showed urinary incontinence, one of whom also had fecal incontinence, suggesting an involvement of the autonomic system in some affected individuals. Six affected individuals in three of the families became immobile and five required respiratory management. Three of the eight affected individuals were deceased by 20 years of age. Most affected individuals had muscle weakness and atrophy. A muscle biopsy at 12 days of age (corrected gestational age: 5 days) in individual 2 (family 1, II-2) revealed the following myopathic changes: (1) variation in moderate muscle fiber size, ranging from 5 to 15 μm in diameter (Figure 2A); (2) numerous type 2C fibers; (3) atrophy of type 1 muscle fibers (Figure 2B and 2C); and (4) dot-like staining in the myofiber cytoplasm after the NADH-tetrazolium reductase reaction (Figure 2D). The neuromuscular junction was not included in the section. No evidence of neuropathy was observed. These findings may reflect the immaturity of muscle fibers. However, it was not possible to confirm this because only limited samples were available. Interestingly, four affected individuals had episodes of elevated creatine kinase (CK). The elevation of CK was noticed only at birth (individual 1, 1,338 IU/L; individual 2, 1,345 IU/L) in family 1, and at several time points in individual 3 (max. 285 IU/L at 7 months) and individual 4 (max. 752 IU/L at 11 months) (Table 2, Supplemental Note). We believe that elevated CK was caused by asphyxia and bone fracture in family 1 and by frequent episodes of afebrile seizures in family 2. However, the actual triggers remain unclear. Brain imaging studies from three affected individuals indicated progressive brain atrophy involving the cerebrum, corpus callosum, cerebellum, and brain stem (Figures 3 and S3). As early as 3 months of age, atrophy in the frontal lobe and enlarged ventricles (especially in the frontal horn of the lateral ventricles) were detected. Despite the flat and expressionless faces, downturned corners of the mouth, and open mouths, we did not see any specific dysmorphic features (Figures 1D and S4).
Table 2.
Genetic and Clinical Information of the Affected Individuals with TBCD Mutations
| Family ID | Family 1 | Family 2 | Family 3 | Family 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Ethnicity | Japanese | Japanese | Chinese | Israel | ||||
| Mutations and Variations | c.1564−12C>G (splicing), c.2314C>T (p.Arg772Cys) | c.1160T>G (p.Met387Arg), c.2761G>A (p. Ala921Thr) | c.2280C>A (p.Tyr760∗), c.3365C>T (p.Pro1122Leu) | c.2810C>G (p.Pro937Arg), c.2810C>G (p.Pro937Arg) | ||||
| Sex | female | male | female | male | male | female | female | female |
| Individual ID | II-1 | II-2 | II-1 | II-2 | II-1 | II-2 | II-1 | II-2 |
| Individual number | individual 1 | individual 2 | individual 3 | individual 4 | individual 5 | individual 6 | individual 7 | individual 8 |
| Pregnancy | hydramnion | hydramnion | normal | normal | normal | normal | normal | normal |
| Age of onset | at birth | at birth | 1 month | 1 month | 5 months | 5 months | 11 months | 9 months |
| Initial symptom(s) | asphyxia, bone fracture | asphyxia, bone fracture | paucity of movement | hypotonia | hypotonia, regression | hypotonia, regression | febrile seizure | generalized seizure |
| Age of evaluation | 4 years | 19 years | 9 years | 5 years | 2 years | 3 years | 17 years | 16 years |
| Growth failurea | yes | yes | yes | yes | yes | yes | no | no |
| Seizure | no | no | West syndrome | cataplexy | GTS | GTS | GTS/GTCS | GTS/GTCS |
| Onset age of seizure | ND | ND | 4 months | 6 months | 6 months | 1 years | 11 months | 9 months |
| Respiratory failure | yes | yes | yes | yes | yes | no | no | no |
| Regression | ND | ND | yes | yes | yes | yes | yes | yes |
| Visual tracking | no | no | no | no | no | no | no | no |
| Optic nerve atrophy | no | yes | yes | yes | yes | no | no | no |
| Postnatal microcephaly | yes | yes | yes | yes | yes | yes | yes | yes |
| Ataxia | NDb | NDb | yes | yes | no | no | no | no |
| Hypotonia | yes | yes | yes | yes | yes | yes | no | no |
| Muscle weakness | yes | NDb | yes | yes | yesc | yesc | yes | yes |
| Muscle atrophy | yes | yes | yes | yes | no | no | yes | yes |
| Fasciculationd | no | no | yes | yes | no | no | no | no |
| Urinary incontinence | ND | yese | no | no | no | no | yes | no |
| Fecal incontinence | no | no | no | no | no | no | yes | no |
| Disturbed sweating | no | no | no | no | no | no | no | no |
| Blood-pressure variability | ND | no | moderate | moderate | ND | ND | no | no |
| Elevation of CK | yes | yes | yes | yes | no | no | no | no |
| Prognosis | immobile with ventilation and deceased at 4 years (respiratory failure) | immobile with ventilation and deceased at 19 years (anaphylactic reaction) | immobile with ventilation | immobile with ventilation | immobile with ventilation and deceased at 4 years (pneumonia) | immobile | walking, moderate intellectual disability | walking, moderate intellectual disability |
Abbreviations are as follows: ND, no data; GTS, generalized tonic seizure; GTCS, generalized tonic clonic seizure; CK, creatine kinase.
Judged as height < −2 SD.
Not assessed because individual was immobile.
Floppy and almost immobile.
Fasciculation in tongue.
Neurogenic bladder.
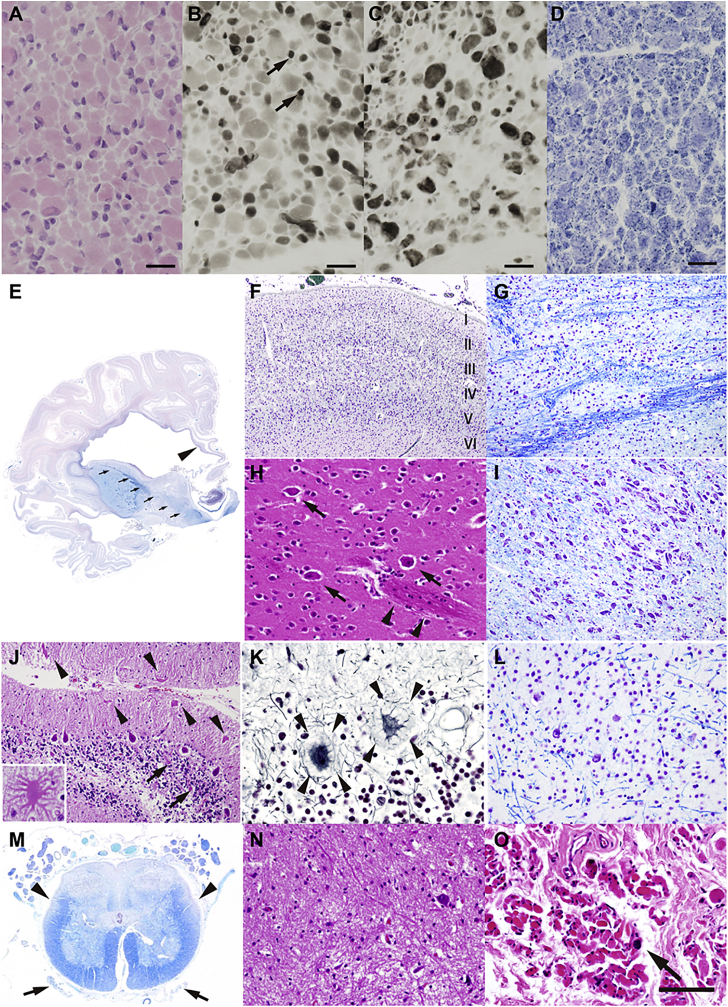
Figure 2.
Histopathological Findings in an Individual with Biallelic TBCD Mutations
(A–D) Histology of a muscle biopsy from individual 2 at the age of 12 days (corrected gestational age: 5 days). Shown are (A) hematoxylin and eosin staining, (B) myosin ATPase (pH 4.2), (C) myosin ATPase (pH 10.5), and (D) NADH-tetrazolium reductase. The arrows indicate type 1 fibers. Scale bars represent 20 μm.
(E) The cerebral cortex of individual 1 showing systemic laminar necrosis, which is more marked in the temporal lobe. The white matter is also atrophic and associated with marked dilatation of the lateral ventricles. The corpus callosum (arrowhead) and posterior limb of the internal capsule (arrows) are thin. The basal ganglia and thalamus are generally well formed.
(F) The frontal cortex has a normal six-layer architecture. Laminar necrosis is evident in the middle cortical layers.
(G) The pontine nucleus with severe neuronal loss and gliosis. Note that both the longitudinal and transverse fibers are degenerative, and longitudinal fibers are almost unrecognizable.
(H) The putamen has a normal composition of medium spiny and large aspiny neurons (arrows). The putamino-pallidal fibers are evident (arrowheads).
(I) Substantia nigra is well preserved in this individual.
(J–L) Loss of Purkinje and granule cells is evident in the cerebellum. The residual Purkinje cells show torpedoes (J, arrows) and asteroid bodies/cactus (J, arrowheads and inset), as well as halo-like amorphous structures (somatic sprouts) (K, arrowheads). Neuron loss is also apparent in the dentate nucleus (L).
(M) Anterior horn volume is reduced in the lumbar cord. The anterior nerve roots are severely atrophic (arrows), whereas the posterior roots are almost preserved. Degeneration of the lateral corticospinal tracts (arrowheads) and posterior column is also evident.
(N) The lumbar anterior horn with severe loss of the lower motor neurons and associated gliosis.
(O) Severe degeneration of the muscle fibers in the diaphragm, with fibrosis and scattered pyknotic nuclear clumps (arrow).
Shown are Klüver-Barrera staining (E, F, G, I, L, M), hematoxylin and eosin staining (H, J, N, O), and Bodian staining (K). Scale bars represent 1 cm (E), 500 μm (F), 200 μm (G, I), 100 μm (H, L, N, O), 160 μm (J), 50 μm (J inset and K), and 1.5 mm (M).
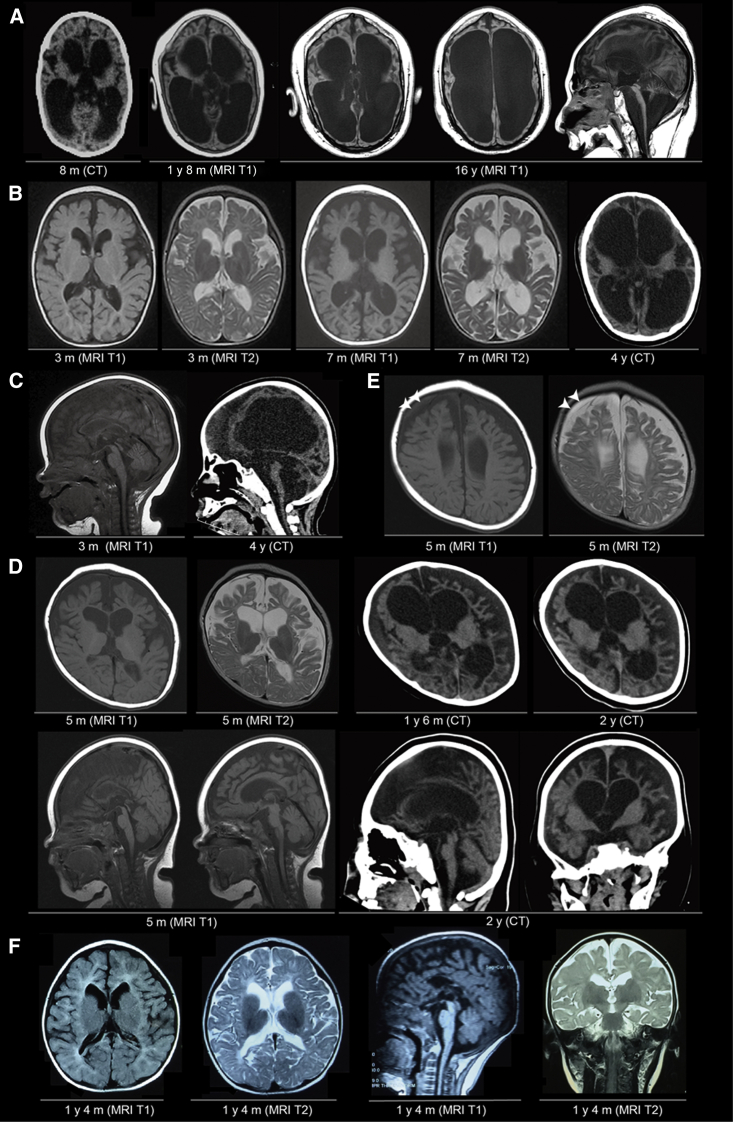
Figure 3.
Neuroimaging of Affected Individuals with Biallelic TBCD Mutations
(A) The neuroimages of individual 2 at 8 months (computed tomography: CT), 1 year 8 months and 16 years (T1-weighted MRI). Severe, progressive enlargement of bilateral ventricles, thin brain stems, and severe cerebellar atrophy are shown.
(B) The axial brain paired T1- and T2-weighted MRI images of individual 3 at 3 months and 7 months and CT at 4 years (right).
(C) The sagittal images of individual 3. T1-weighted MRI at 3 months (left) and CT at 4 years (right). Thinned corpus callosum and enlargement of the great cistern can be seen on the MRI (left).
(D) The serial images from individual 4. From upper left to upper right, axial T1-weighted MRI and T2-weighted MRI at 5 months, CT at 1 year 6 months, and CT at 2 years. Gyral pattern and myelination seemed to be consistent with age, whereas the progressive atrophy of the cerebral cortex (predominantly in the frontal cortex) and enlargement of the ventricles is remarkable. From lower left to lower right, two sections of sagittal T1-weighted MRI at 5 months, sagittal and coronal sections from CT at 2 years.
(E) T1- and T2-weighted MRI images of individual 4 at 5 months. The white arrowheads indicate a chronic subdural hematoma.
(F) T1- and T2-weighted MRI images of individual 6 at 1 year 4 months.
Interestingly, some clinical features overlapped between biallelic TBCD and TBCE mutations: early-onset (<1 year) intellectual disability, postnatal growth failure, hypoplastic corpus callosum, and microcephaly are common in both diseases, while intrauterine growth retardation and hypoparathyroidism were not seen in individuals with TBCD mutations (Table S4).11, 13
TaqMan assays revealed that TBCD was ubiquitously expressed in all tissues, but highly expressed in brain, heart, and skeletal muscle in the fetus and adult (Figure S5). The expression pattern was consistent with the affected organs, including the central nervous system and skeletal muscles, in affected individuals with TBCD mutations.
An autopsy was performed on individual 1 (family 1, II-1). Although no malformation of the internal organs was detected (Table S5), striated muscle volume, including the iliopsoas, was severely decreased and the diaphragm was extremely thin. The brain and spinal cord were generally atrophic. In the cerebrum, there was severe atrophy of the cortex and white matter and the lateral ventricles were markedly dilated (Figure 2E). The corpus callosum was rudimentary, although the basal ganglia and thalamus were generally well formed. Both the anterior and posterior limbs of the internal capsules were severely atrophic.
Histopathological examination revealed systemic laminar necrosis in the cerebral cortex, although a normal six-layer structure was observed with no cytologically abnormal neurons, such as dysmorphic neurons or balloon cells (Figure 2F). Furthermore, the hippocampus, thalamus, pontine nucleus (Figure 2G), globus pallidus, and inferior olivary nucleus had severe neuronal loss and gliosis. Many globular structures of unknown origin were observed in the internal segment of the globus pallidus. Purkinje and granule cells in the cerebellum were severely depleted.
These histological abnormalities might be ascribable, at least in part, to intrapartum asphyxia, in which the pattern of neuronal injury may be almost comparable to that of “full-term” severe asphyxia.14 However, in this individual, other anatomical regions susceptible to asphyxial injury in term infants, including the putamen (Figure 2H), quadrigeminal bodies, substantia nigra (Figure 2I), and dorsal vagal nucleus, had only mild gliosis without apparent neuron loss. Additionally, in the cerebellum, many residual Purkinje cells had focal swellings of the dendrites (cactus/asteroid bodies) (Figure 2J) and some had halo-like amorphous structures around the cell bodies (somatic sprouts) (Figure 2K). Neuronal depletion was severe in the dentate nucleus (Figure 2L). In addition, the brainstem lower motor neuron (LMN) nuclei, including the oculomotor and hypoglossal nuclei, had severe neuronal loss and associated gliosis. In the spinal cord, the lateral corticospinal tracts and dorsal column showed severe degeneration with myelin pallor (Figure 2M). The anterior horns showed severe loss of the LMNs and associated gliosis, which was somewhat more apparent in the lateral nuclear group (Figure 2N). The spinal anterior roots were severely atrophic, whereas the posterior roots were preserved. The diaphragm was severely degenerated with fibrosis and scattered pyknotic nuclear clumps (Figure 2O).
We constructed homology-modeled structures of human TBCD using the Protein Homology/analogY Recognition Engine v.2.0 (Phyre2) server,15 because no experimental structure is available. The resultant models had α-solenoid repeats with pairs of antiparallel alpha helices, which have a deformable nature. We also employed an in silico detector of an α-solenoid repeat based on an amino acid sequence, Alpha-rod Repeat Detector 2 (ARD2)16 to verify the modeled structures. The results by ARD2 were consistent with those by the Phyre2 prediction (data not shown).
In the structures obtained, the side chains of Met387 and Ala921 are buried in the hydrophobic cores of α solenoid repeat (Figure S6A). Therefore, the p.Met387Arg and p.Ala921Thr variants might affect the folding of the α-solenoid repeat. The methylene part of the Arg772 side chain is part of a hydrophobic core and the guanidium group is exposed to the protein surface in the modeled structure (Figure S6A). Thus, the p.Arg772Cys variant might also affect at least the folding structure. Because Pro937 and Pro1122 were predicted to reside within a loop between antiparallel α helices and be exposed to a protein surface (Figure S6A), the p.Pro937Arg and p.Pro1122Leu variants might affect the loop structure and/or interactions with other molecules, although no functional data are available. TBCD functions as a component of the tubulin chaperone multiprotein complex (Figure S6B).17 Therefore, all of the variants described here have the potential to impair complex formation.
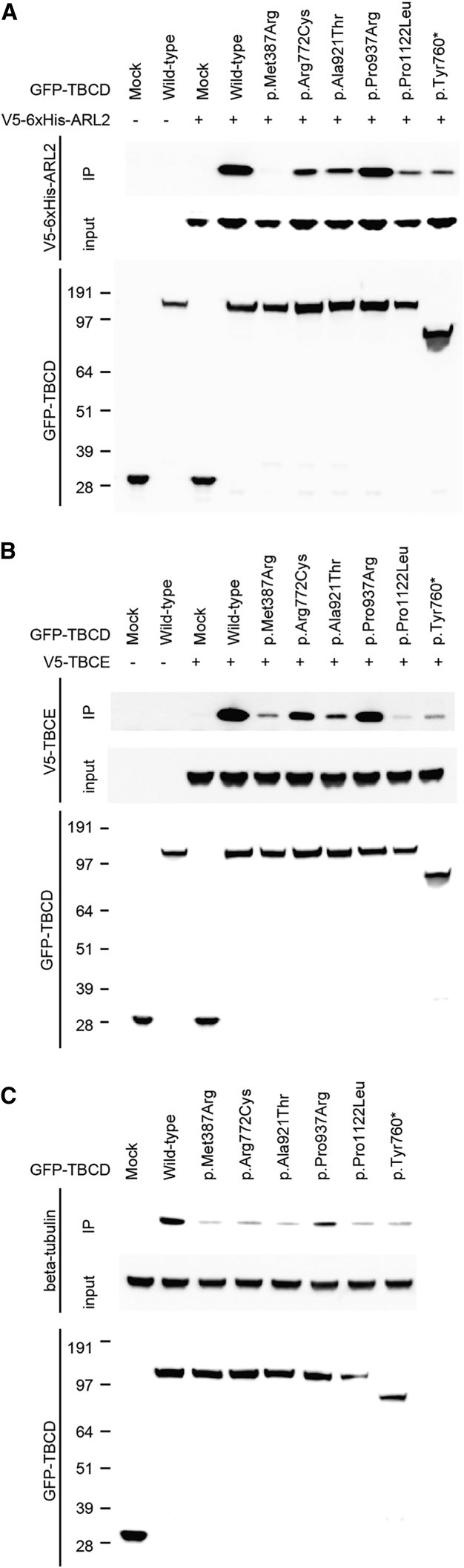
Next, we examined protein-protein interactions between TBCD (wild-type and mutant) and other complex components (α/β-tubulin, TBCE, TBCC, and ARL2) by co-immunoprecipitation. The open reading frames of human TBCD, TBCE, TBCC (GenBank: NM_003192.2), and ARL2 (GenBank: NM_001667.3) were amplified using human cDNA as a template purchased from Clontech. The PCR products were cloned into the Gateway pDONR221 vector (Life Technologies) to create the entry clones, and each sequence was confirmed by Sanger sequencing. The QuickChange II XL Site-Directed Mutagenesis Kit (Agilent Technologies) was used to create mutant constructs. To create the transient mammalian expression vectors, each entry clone was used for LR recombination with pcDNA3.1/nV5-DEST (for N′ V5-tagged TBCC or TBCE), pcDNA-DEST53 (for N′ GFP-fused TBCD), or pcDNA-DEST40 (for C′ V5-6xHis-tagged ARL2). Wild-type TBCD interact with ARL2, TBCE, and β-tubulin (Figure 4). No apparent binding was observed between TBCD and α-tubulin or TBCD and TBCC (data not shown); therefore, we focused on TBCD binding with ARL2, TBCE, and β-tubulin. As for the binding with ARL2, TBCD mutants p.Met387Arg almost completely, and p.Arg772Cys, p.Ala921Thr, p.Pro1122Leu, and p.Tyr760∗ partially, lost binding capacity compared with wild-type TBCD. p.Pro937Arg retained its binding capacity (Figure 4A). Mutant TBCD showed similar impaired binding to TBCE (Figure 4B). All TBCD mutants showed decreased binding to endogenous β-tubulin (Figure 4C). These experiments suggest that all the investigated TBCD mutations may impair the proper formation of microtubule chaperone complexes.
Figure 4.
Intermolecular Associations between TBCD and Its Binding Partners
(A and B) The molecular interaction between human TBCD and ARL2 (A) or TBCE (B). GFP-fused wild-type TBCD (159 kDa) or its mutants were transiently co-overexpressed with V5-6xHis-tagged ARL2 (22 kDa) or V5-tagged TBCE (60 kDa) in HEK293T cells using the X-treme GENE9 DNA transfection kit (Roche). At 48 hr after transfection, the cells were collected and lysed with lysis buffer (25 mM Tris-HCl [pH 7.5], 100 mM NaCl, 2 mM EDTA, 0.5% Triton X-100, and protease inhibitors). GFP-fused TBCD proteins were immunoprecipitated using a rabbit polyclonal anti-GFP antibody (Abcam cat# ab6556, RRID: AB_305564) and Dynabeads Protein-G (Thermo Fisher Scientific). Input and immunoprecipitated samples (IP) were immunoblotted with mouse monoclonal anti-GFP (Roche) and anti-V5 antibodies (Thermo Fisher Scientific cat# R960-25, RRID: AB_2556564).
(C) The binding of human TBCD and endogenous β-tubulin (50 kDa). Wild-type GFP-fused TBCD or its mutants were transiently overexpressed in HEK293T cells. GFP-fused TBCD proteins were immunoprecipitated with a rabbit polyclonal anti-GFP antibody and immunoblotted with mouse monoclonal anti-GFP or anti β-tubulin antibodies (Chemicon International cat# MAB3408). Horseradish peroxidase-conjugated goat anti-mouse IgG antibody (Jackson ImmunoResearch) was used as the secondary antibody. Immunodetection was performed using Supersignal West Dura Extended Duration Substrate (Thermo Fisher Scientific) on a ChemiDoc Touch Imaging system (Bio-Rad Laboratories).
At least three independent experiments were performed to confirm the consistency of results for TBCD binding with ARL2, TBCE, or β-tubulin. Mock indicates only GFP protein (27 kDa). “+” and “−” denote the presence and absence of V5-tagged proteins (or only V5-tag with the size of 1 kDa, which could not be detected due to its small molecular weight), respectively.
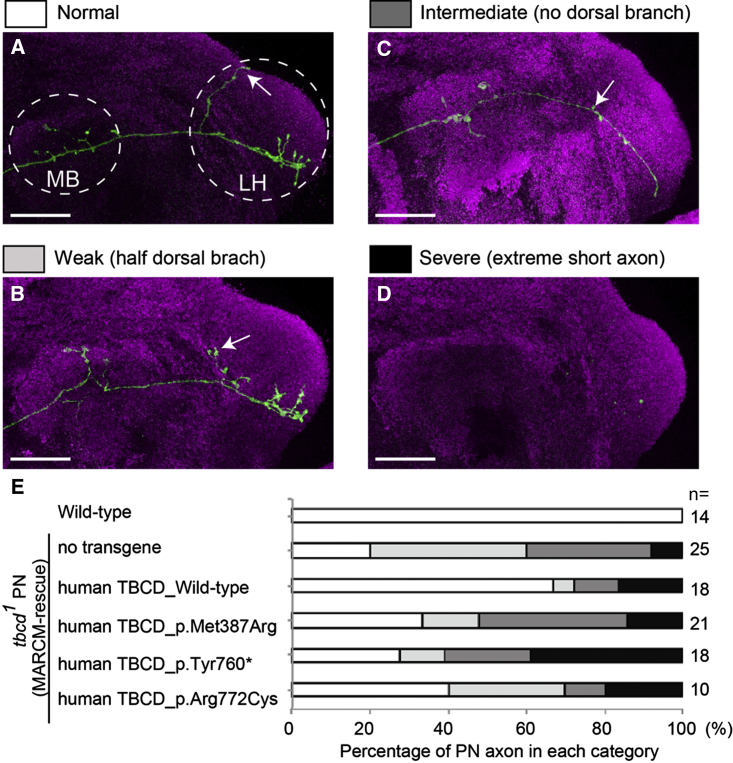
Finally, to investigate the possible effects of disease-associated TBCD mutations in vivo, we took advantage of mosaic analysis with a repressible cell marker (MARCM) in Drosophila, which allowed us to analyze gene function in vivo at single-cell resolution.18 We expressed membrane-tethered GFP (mouse CD8::GFP, mCD8-GFP) to visualize the neuronal morphology of olfactory projection neurons (PNs) in the intact brain (Figure 5).19, 20 The axons of DL1 PNs elongate and form axonal branches at the calyx of the mushroom body and the lateral horn. The dendrites of PNs target a single glomerulus (DL1 glomerulus) in the antennal lobe, the first olfactory center in the Drosophila brain (Figures 5A and S7 for wild-type axon and dendrites, respectively). As we have previously reported,21 PNs homozygous for tbcd1 mutations (tbcd1 PNs), in an otherwise largely heterozygous animal, had shorter axons and/or reduced axonal branches (Figures 5B–5D). Next, we carried out a MARCM-rescue experiment, in which we cell autonomously expressed cDNA of wild-type human TBCD in tbcd1 PNs and found that overexpression of human TBCD significantly suppressed the axonal phenotypes of tbcd1 PNs (Figure 5E). In addition, the dendritic phenotype of tbcd1 PNs also tended to be suppressed by human TBCD overexpression (Figure S7). These results suggest that the function of TBCD in neuronal morphogenesis is partly conserved between Drosophila and humans.
Figure 5.
Human TBCD with Disease-Associated Mutations Impaired Neuronal Morphology
(A–D) The representative axonal morphologies of Drosophila olfactory projection neurons. The axons of DL1 single-cell clones for wild-type Drosophila (A) show the typical branching pattern at the calyx of the mushroom body (MB) and lateral horn (LH). Axons of tbcd1Drosophila PNs show branching and elongation defects (B–D). The dorsal branch at the LH is often affected (arrow in A–C). Axons cannot reach the MB in the severe class (no axon is visible in D). PN morphologies and Bruchpilot (presynaptic marker) are shown in green (anti-CD8 antibody) and magenta (nc82), respectively. Scale bars represent 25 μm.
(E) Quantification of MARCM-rescue experiments. Each gray scale corresponds to the colors in (A)–(D) above. n indicates the number of MARCM clones examined. Because the number of clones we can observe in the Drosophila brain cannot be controlled, we analyzed a variable number of clones (minimum n = 10). Details for the generation of transgenic flies, MARCM clone induction, immunohistochemistry of fly brains, image processing, and quantification methods are available upon request.
To test the effect of disease-associated TBCD mutations, we performed MARCM-rescue experiments with human TBCD carrying disease-associated mutations. To avoid the positional effect that causes unexpected transgene expression differences, we utilized the PhiC31-integrase system to generate UAS transgenic animals and all the UAS transgenes were inserted in an attP2 landing site.22 Human TBCD bearing a disease-associated mutation was less able to suppress the axonal phenotype of tbcd1 PN compared with wild-type human TBCD (Figure 5E). A similar tendency was also observed in PN dendrites (Figure S7). These results indicate that the TBCD disease-associated mutations we tested cause loss of function of the TBCD protein.
In this study, we have reported four families with early-onset neurodegenerative encephalopathy associated with biallelic TBCD mutations. Core clinical features include early-onset progressive diffuse brain atrophy with regression, postnatal microcephaly, postnatal growth delay, muscle weakness/atrophy, and respiratory failure. Our in vitro and in vivo studies showed that seven TBCD mutations are likely to lead to loss of function of the TBCD protein. Interestingly, the clinical course was very similar within individual families, but differed between families. This might be partly explained by the specific mutations in each family. The siblings in family 4 with a homozygous missense mutation (c.2810C>G [p.Pro937Arg]) had a milder phenotype, possibly because binding of the altered TBCD protein with β-tubulin was only mildly affected. In more severe clinical cases (such as the deceased individuals in families 1 and 3), affected individuals carried truncating and missense variants that significantly impaired TBCD binding to ARL2, TBCE, and β-tubulin (Table S6). This indicates that biallelic TBCD mutations may lead to a wide range of clinical severities depending on the level of residual protein function resulting from the specific TBCD mutation.
We neuropathologically examined an autopsied individual (individual 1) in whom the original lesions caused by TBCD mutations could have been masked by asphyxial effects. However, we found the following pathological findings that could not be easily explained by asphyxia: (1) neuronal loss of dentate nuclei of the cerebellum and LMN in the brainstem and anterior horn of the spinal code and (2) somatic sprout and cactus formations in cerebellar Purkinje cells. Neuronal loss in the dentate nuclei and LMN may also be caused by asphyxia.23 However, regions that are known to be susceptible to asphyxia, such as the putamen and quadrigeminal bodies,14 were well preserved in this affected individual. Therefore, the disproportionally severe loss of LMNs in the brainstem, spinal cord, and the cerebellar dentate nucleus may arise from lesions caused by TBCD mutations, rather than asphyxia. In addition, cactus formations are usually observed in Menkes disease and mitochondrial diseases,24, 25, 26 and somatic sprouts have been reported in Menkes disease and spinocerebellar ataxia type 31,27, 28, 29 but not in asphyxia. Notably, mitochondrial dysfunction is also associated with Menkes disease.30
Mitochondria are essential organelles providing energy to all eukaryotic cells. Most mitochondria are newly produced in the soma and are transported to the distal end of axons and dendrites.31, 32, 33 In neurons, mitochondria are distributed unevenly through microtubule trafficking, depending on local energy demand.33, 34, 35, 36 Microtubules are essential for long-distance axonal transport,32, 37 and impaired mitochondrial transport results in neuronal degeneration.33, 38, 39, 40 Reduced levels of TBCD protein affect microtubule network formation and impair microtubule transport in tbcd1 PNs.41 Furthermore, TBCD is also required for axon and dendrite maintenance in PNs.41 Therefore, TBCD depletion may result in an abnormal microtubule network and abnormal microtubule trafficking in the human brain. Consequently, energy supply to neuronal cells is reduced, causing neuronal degradation. The formation of cactus and somatic sprouts might reflect structural and metabolic abnormalities resulting from disrupted mitochondrial transport in neuronal cells caused by TBCD depletion.
In conclusion, we have described an early-onset neurodegenerative encephalopathy caused by biallelic TBCD mutations. We performed in vitro and in vivo experiments which revealed that the described TBCD mutations probably cause loss of function of the TBCD protein. We also investigated a possible genotype-phenotype correlation, particularly with regard to disease severity. Our knowledge of human TBCD mutations is currently limited, and therefore more genetic and clinical studies are required to better understand the clinical consequences of TBCD mutations.
Acknowledgments
The authors appreciate the participation of all the affected individuals and their families in this study. This work was supported by grants for Research on Measures for Intractable Diseases; Comprehensive Research on Disability Health and Welfare, the Strategic Research Program for Brain Science; Initiative on Rare and Undiagnosed Diseases in Pediatrics and Initiative on Rare and Undiagnosed Diseases for Adults from the Japan Agency for Medical Research and Development; Grants-in-Aid for Scientific Research on Innovative Areas (Transcription Cycle and Comprehensive Brain Science Network) from the Ministry of Education, Science, Sports and Culture of Japan; Grants-in-Aid for Scientific Research (B and C) and Challenging Exploratory Research from the Japan Society for the Promotion of Science; Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program in the Project for Developing Innovation Systems from the Japan Science and Technology Agency; the Takeda Science Foundation; the Yokohama Foundation for Advancement of Medical Science; and the Hayashi Memorial Foundation for Female Natural Scientists.
Published: September 22, 2016
Footnotes
Supplemental Data include supplemental note, seven figures, and six tables and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2016.08.005.
Contributor Information
Noriko Miyake, Email: nmiyake@yokohama-cu.ac.jp.
Naomichi Matsumoto, Email: naomat@yokohama-cu.ac.jp.
Web Resources
ExAC Browser (accessed May 2016), http://exac.broadinstitute.org/
GenBank, http://www.ncbi.nlm.nih.gov/genbank/
Human Genetic Variation Database (HGVD), http://www.genome.med.kyoto-u.ac.jp/SnpDB/
NHLBI Exome Sequencing Project (ESP) Exome Variant Server, http://evs.gs.washington.edu/EVS/
Novoalign, http://www.novocraft.com/main/page.php?s=novoalign
OMIM, http://www.omim.org/
Supplemental Data
References
- 1.Nogales E. Structural insight into microtubule function. Annu. Rev. Biophys. Biomol. Struct. 2001;30:397–420. doi: 10.1146/annurev.biophys.30.1.397. [DOI] [PubMed] [Google Scholar]
- 2.Ohi R., Zanic M. Ahead of the curve: new insights into microtubule dynamics. F1000Res. 2016;5 doi: 10.12688/f1000research.7439.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchison T., Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- 4.Desai A., Mitchison T.J. Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 1997;13:83–117. doi: 10.1146/annurev.cellbio.13.1.83. [DOI] [PubMed] [Google Scholar]
- 5.Tian G., Lewis S.A., Feierbach B., Stearns T., Rommelaere H., Ampe C., Cowan N.J. Tubulin subunits exist in an activated conformational state generated and maintained by protein cofactors. J. Cell Biol. 1997;138:821–832. doi: 10.1083/jcb.138.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis S.A., Tian G., Cowan N.J. The alpha- and beta-tubulin folding pathways. Trends Cell Biol. 1997;7:479–484. doi: 10.1016/S0962-8924(97)01168-9. [DOI] [PubMed] [Google Scholar]
- 7.Bhamidipati A., Lewis S.A., Cowan N.J. ADP ribosylation factor-like protein 2 (Arl2) regulates the interaction of tubulin-folding cofactor D with native tubulin. J. Cell Biol. 2000;149:1087–1096. doi: 10.1083/jcb.149.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian G., Bhamidipati A., Cowan N.J., Lewis S.A. Tubulin folding cofactors as GTPase-activating proteins. GTP hydrolysis and the assembly of the alpha/beta-tubulin heterodimer. J. Biol. Chem. 1999;274:24054–24058. doi: 10.1074/jbc.274.34.24054. [DOI] [PubMed] [Google Scholar]
- 9.Nithianantham S., Le S., Seto E., Jia W., Leary J., Corbett K.D., Moore J.K., Al-Bassam J. Tubulin cofactors and Arl2 are cage-like chaperones that regulate the soluble αβ-tubulin pool for microtubule dynamics. eLife. 2015;4 doi: 10.7554/eLife.08811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parvari R., Hershkovitz E., Grossman N., Gorodischer R., Loeys B., Zecic A., Mortier G., Gregory S., Sharony R., Kambouris M., HRD/Autosomal Recessive Kenny-Caffey Syndrome Consortium Mutation of TBCE causes hypoparathyroidism-retardation-dysmorphism and autosomal recessive Kenny-Caffey syndrome. Nat. Genet. 2002;32:448–452. doi: 10.1038/ng1012. [DOI] [PubMed] [Google Scholar]
- 11.Sanjad S.A., Sakati N.A., Abu-Osba Y.K., Kaddoura R., Milner R.D. A new syndrome of congenital hypoparathyroidism, severe growth failure, and dysmorphic features. Arch. Dis. Child. 1991;66:193–196. doi: 10.1136/adc.66.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyake N., Tsukaguchi H., Koshimizu E., Shono A., Matsunaga S., Shiina M., Mimura Y., Imamura S., Hirose T., Okudela K. Biallelic mutations in nuclear pore complex subunit NUP107 cause early-childhood-onset steroid-resistant nephrotic syndrome. Am. J. Hum. Genet. 2015;97:555–566. doi: 10.1016/j.ajhg.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Padidela R., Kelberman D., Press M., Al-Khawari M., Hindmarsh P.C., Dattani M.T. Mutation in the TBCE gene is associated with hypoparathyroidism-retardation-dysmorphism syndrome featuring pituitary hormone deficiencies and hypoplasia of the anterior pituitary and the corpus callosum. J. Clin. Endocrinol. Metab. 2009;94:2686–2691. doi: 10.1210/jc.2008-2788. [DOI] [PubMed] [Google Scholar]
- 14.Volpe J.J. Hypoxic-ischemic encephalopathy. In: Volpe J.J., editor. Neurology of the Newborn. Saunders/Elsevier; Philadelphia: 2008. pp. 347–399. [Google Scholar]
- 15.Kelley L.A., Mezulis S., Yates C.M., Wass M.N., Sternberg M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fournier D., Palidwor G.A., Shcherbinin S., Szengel A., Schaefer M.H., Perez-Iratxeta C., Andrade-Navarro M.A. Functional and genomic analyses of alpha-solenoid proteins. PLoS ONE. 2013;8:e79894. doi: 10.1371/journal.pone.0079894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian G., Cowan N.J. Tubulin-specific chaperones: components of a molecular machine that assembles the α/β heterodimer. Methods Cell Biol. 2013;115:155–171. doi: 10.1016/B978-0-12-407757-7.00011-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee T., Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 19.Jefferis G.S., Hummel T. Wiring specificity in the olfactory system. Semin. Cell Dev. Biol. 2006;17:50–65. doi: 10.1016/j.semcdb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Sakuma C., Anzo M., Miura M., Chihara T. Development of olfactory projection neuron dendrites that contribute to wiring specificity of the Drosophila olfactory circuit. Genes Genet. Syst. 2014;89:17–26. doi: 10.1266/ggs.89.17. [DOI] [PubMed] [Google Scholar]
- 21.Sakuma C., Okumura M., Umehara T., Miura M., Chihara T. A STRIPAK component Strip regulates neuronal morphogenesis by affecting microtubule stability. Sci. Rep. 2015;5:17769. doi: 10.1038/srep17769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groth A.C., Fish M., Nusse R., Calos M.P. Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics. 2004;166:1775–1782. doi: 10.1534/genetics.166.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clancy R.R., Sladky J.T., Rorke L.B. Hypoxic-ischemic spinal cord injury following perinatal asphyxia. Ann. Neurol. 1989;25:185–189. doi: 10.1002/ana.410250213. [DOI] [PubMed] [Google Scholar]
- 24.Okeda R., Gei S., Chen I., Okaniwa M., Shinomiya M., Matsubara O. Menkes’ kinky hair disease: morphological and immunohistochemical comparison of two autopsied patients. Acta Neuropathol. 1991;81:450–457. doi: 10.1007/BF00293467. [DOI] [PubMed] [Google Scholar]
- 25.Tanahashi C., Nakayama A., Yoshida M., Ito M., Mori N., Hashizume Y. MELAS with the mitochondrial DNA 3243 point mutation: a neuropathological study. Acta Neuropathol. 2000;99:31–38. doi: 10.1007/pl00007403. [DOI] [PubMed] [Google Scholar]
- 26.Mori O., Yamazaki M., Ohaki Y., Arai Y., Oguro T., Shimizu H., Asano G. Mitochondrial encephalomyopathy with lactic acidosis and stroke like episodes (MELAS) with prominent degeneration of the intestinal wall and cactus-like cerebellar pathology. Acta Neuropathol. 2000;100:712–717. doi: 10.1007/s004010000209. [DOI] [PubMed] [Google Scholar]
- 27.Hirano A., Llena J.F., French J.H., Ghatak N.R. Fine structure of the cerebellar cortex in Menkes Kinky-hair disease. X-chromosome-linked copper malabsorption. Arch. Neurol. 1977;34:52–56. doi: 10.1001/archneur.1977.00500130072014. [DOI] [PubMed] [Google Scholar]
- 28.Sato N., Amino T., Kobayashi K., Asakawa S., Ishiguro T., Tsunemi T., Takahashi M., Matsuura T., Flanigan K.M., Iwasaki S. Spinocerebellar ataxia type 31 is associated with “inserted” penta-nucleotide repeats containing (TGGAA)n. Am. J. Hum. Genet. 2009;85:544–557. doi: 10.1016/j.ajhg.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Owada K., Ishikawa K., Toru S., Ishida G., Gomyoda M., Tao O., Noguchi Y., Kitamura K., Kondo I., Noguchi E. A clinical, genetic, and neuropathologic study in a family with 16q-linked ADCA type III. Neurology. 2005;65:629–632. doi: 10.1212/01.wnl.0000173065.75680.e2. [DOI] [PubMed] [Google Scholar]
- 30.Rossi L., Lombardo M.F., Ciriolo M.R., Rotilio G. Mitochondrial dysfunction in neurodegenerative diseases associated with copper imbalance. Neurochem. Res. 2004;29:493–504. doi: 10.1023/b:nere.0000014820.99232.8a. [DOI] [PubMed] [Google Scholar]
- 31.Davis A.F., Clayton D.A. In situ localization of mitochondrial DNA replication in intact mammalian cells. J. Cell Biol. 1996;135:883–893. doi: 10.1083/jcb.135.4.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hollenbeck P.J. The pattern and mechanism of mitochondrial transport in axons. Front. Biosci. 1996;1:d91–d102. doi: 10.2741/a118. [DOI] [PubMed] [Google Scholar]
- 33.Sheng Z.H., Cai Q. Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nat. Rev. Neurosci. 2012;13:77–93. doi: 10.1038/nrn3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z., Okamoto K., Hayashi Y., Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Ruthel G., Hollenbeck P.J. Response of mitochondrial traffic to axon determination and differential branch growth. J. Neurosci. 2003;23:8618–8624. doi: 10.1523/JNEUROSCI.23-24-08618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hollenbeck P.J., Saxton W.M. The axonal transport of mitochondria. J. Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grafstein B., Forman D.S. Intracellular transport in neurons. Physiol. Rev. 1980;60:1167–1283. doi: 10.1152/physrev.1980.60.4.1167. [DOI] [PubMed] [Google Scholar]
- 38.Devine M.J., Birsa N., Kittler J.T. Miro sculpts mitochondrial dynamics in neuronal health and disease. Neurobiol. Dis. 2016;90:27–34. doi: 10.1016/j.nbd.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nat. Rev. Neurosci. 2005;6:889–898. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- 40.Lin M.Y., Sheng Z.H. Regulation of mitochondrial transport in neurons. Exp. Cell Res. 2015;334:35–44. doi: 10.1016/j.yexcr.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okumura M., Sakuma C., Miura M., Chihara T. Linking cell surface receptors to microtubules: tubulin folding cofactor D mediates Dscam functions during neuronal morphogenesis. J. Neurosci. 2015;35:1979–1990. doi: 10.1523/JNEUROSCI.0973-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.