Summary
Memories formed early in life are particularly stable and influential, representing privileged experiences that shape enduring behaviors. Here we show that exposing newly-hatched C. elegans to pathogenic bacteria results in persistent aversion to those bacterial odors, whereas adult exposure generates only transient aversive memory. Long-lasting imprinted aversion has a critical period in the first larval stage, and is specific to the experienced pathogen. Distinct groups of neurons are required during formation (AIB, RIM) and retrieval (AIY, RIA) of the imprinted memory. RIM synthesizes the neuromodulator tyramine, which is required in the L1 stage for learning. AIY memory retrieval neurons sense tyramine via the SER-2 receptor, which is essential for imprinted but not for adult-learned aversion. Odor responses in several neurons, most notably RIA, are altered in imprinted animals. These findings provide insight into neuronal substrates of different forms of memory, and lay a foundation for further understanding of early learning.
Introduction
Although learning can occur at any stage of life, early memories are especially robust and long lasting. An evocative example is imprinting, defined as a process in which a sensory cue presented at a specific time – the critical period or sensitive period – subsequently gains unique access to ecologically-relevant behaviors. Imprinting was first described in newly-hatched birds such as chicks or geese, which form an attachment to the first moving object they see (Lorenz, 1935; Nakamori et al., 2013). It has since been described in a variety of animals, particularly in the olfactory system. For example, young Pacific salmon form an olfactory memory of the natal stream that guides their return years later to spawn (Nevitt et al., 1994), and rodents and other mammals show a strong preference for food odors that they experience perinatally (Wilson and Sullivan, 1994). Each of these imprinted behaviors is critical for an animal’s survival or reproductive success. Among the questions raised by these privileged memories are their sites of formation and retrieval, and the degree to which their mechanisms overlap with those of less specialized memories.
Learning is a universal property of the nervous system. Even the nematode worm Caenorhabditis elegans, whose nervous system consists of 302 neurons, can modify its preferences for sensory cues such as temperature, touch, tastes, and odors based on experience (Ardiel and Rankin, 2010). As in other animals, learning and memory in C. elegans depend on the training regimen. For example, pairing an odor with bacterial food in a single training session results in a short-term preference for that odor, whereas pairing over multiple spaced sessions results in long-term memory that can last for 24 hours (Kauffman et al., 2010; Torayama et al., 2007). Interestingly, pairing odors with food in newly-hatched animals can result in positive imprinting, with increased odor preference in adults and even in the following generation (Remy, 2010; Remy and Hobert, 2005).
C. elegans lives in a complex microbial environment, and its survival depends on its ability to distinguish between nutritious bacteria and pathogenic bacterial species that can infect and kill the animal (Meisel and Kim, 2014). A substantial component of its pathogen defense is behavioral. Within six hours of exposure to a bacterial pathogen, C. elegans learns to avoid that bacterial odor in an associative behavior that resembles conditioned taste aversion, a widespread form of animal learning (Melo and Ruvkun, 2012; Zhang et al., 2005). This associative aversive memory lasts between 12 and 24 hours. Neurons required for naïve bacterial preference as well as learned pathogen aversion have been mapped by laser killing experiments (Figure S1A) (Ha et al., 2010), and molecules required for learning have been identified in genetic studies and mapped back to this circuit (Meisel and Kim, 2014). How these molecules change information processing in the nervous system is unknown. Sensory neuron responses to bacterial odors appear unchanged by aversive learning (Ha et al., 2010), suggesting that the circuit changes occur in integrating neurons, but the neural correlates of memory are obscure.
Neural circuits for learning and memory are studied in many animals, but C. elegans raises unique questions because of its small nervous system. How many forms of learning and memory can be encoded by 302 neurons? How similar are they to each other and to related phenomena in more complex nervous systems? Here we show that pathogen training during the first larval stage results in long-lasting aversive memory that is maintained into adulthood. We characterize distinct groups of neurons required for the formation and retrieval of this privileged aversive memory, define genes required for learning, and identify functional changes in neuronal activity associated with memory. Our results reveal similarities and differences between imprinting and other forms of learning, and suggest a logic for imprinted aversive memory.
Results
Pathogen training early in development gives rise to long-term associative aversion
Learned pathogen aversion is induced by exposing adult C. elegans to pathogenic bacteria for 4–24 hours, or by cultivating animals with both pathogenic and non-pathogenic bacteria (Chen et al., 2013; Zhang et al., 2005). In either case, the aversive memory lasts between 12 and 24 hours. We modified the learning assay by hatching C. elegans eggs on a uniform lawn of the pathogenic bacterium Pseudomonas aeruginosa PA14 (Figure 1A) and forcing exposure to the pathogen for 12 hours during the first larval (L1) stage. This treatment establishes an intestinal infection but does not kill the animals (Tan et al., 1999). Antibiotic washes were then performed to clear the infection (Figure S1B), and animals were grown on non-pathogenic Escherichia coli OP50 until adulthood. When tested in an olfactory choice assay between PA14 and OP50, animals trained as L1 larvae were significantly more likely to migrate away from PA14 than naïve animals (Figure 1B). This shift in preference, measured days after training as a learning index (naïve choice index – trained choice index), resembled the shift in preference of adult animals immediately after training with PA14 (Figure 1A–C). No shift in preference was observed in animals that had been exposed to non-pathogenic P. aeruginosa PA*50E12 (Rahme et al., 1997) or starved for 12 hours as L1 larvae, suggesting that pathogenic infection is required for learning to occur (Figure 1C). The progeny of trained animals returned to the naïve preference (Figure 1C).
Figure 1. Pathogen training at the first larval stage (imprinting) induces long-term aversive memory.
(A) Schematic illustration of bacterial choice assay, imprinting protocol, and adult pathogen training protocol. See also Figure S1.
(B) Olfactory choice preference index of naïve, PA14-imprinted, and adult-trained animals. Each dot represents a single population assay calculated as shown; each line represents the mean value.
(C) Learning index after imprinting on PA14, adult-training, imprinting on non-pathogenic PA*50E12, starving for 12 hours after hatching, and in F1 offspring of PA14-imprinted animals. Boxes represent median and first and third quartiles, and whiskers represent 10th–90th percentiles.
(D) Learning index of mature (two-day old) adults after exposure to PA14 at different developmental stages.
(E) Learning index of animals imprinted either on pathogenic PA14 or on an E. coli BL21 strain expressing the Pseudomonas translational inhibitor ToxA, then tested with choices between PA14/OP50 and ToxA/OP50.
n, number of independent assays, 100–200 animals/assay. P values were generated by ANOVA with the Dunnett correction (B,C,D) or by the nonparametric Mann-Whitney test (E) (*** P <0.001, ** P <0.01, * P <0.05, ns not significant).
To ask whether this long-lasting aversive memory occurs during a critical period, we exposed animals to PA14 at different developmental stages and tested olfactory choice in mature (second-day) adults. Only exposure in the L1 stage for the full 12 hours resulted in stable learned aversion (Figure 1D). In agreement with previous work, animals trained with PA14 as first-day adults did not show learned aversion 24 hours later, nor did animals trained as L2, L3, or L4 larvae. This long-lasting behavioral response will be called “imprinted aversion” to emphasize its early formation, long duration, and the existence of an apparent critical period in the L1 stage.
Imprinted adults appeared healthy as naïve adults, suggesting that the altered behavior is not a consequence of sustained damage from the bacterial infection or early starvation. Their growth, locomotion patterns, and abilities to perform behavioral tasks such as chemotaxis and local search were similar to those of naïve animals, although they had subtle changes in quantitative behavioral assays (Figure S2).
The pathogenicity of PA14 is in part mediated by the toxic translational inhibitor exotoxin A (ToxA) (McEwan et al., 2012). Animals imprinted on an E. coli strain expressing ToxA avoided the ToxA strain as adults in an olfactory choice assay with OP50, showing that imprinted aversion can be induced by a second strain (Figure 1E). ToxA-imprinted animals did not avoid PA14, and conversely, PA14-imprinted animals did not avoid ToxA (Figure 1E). Thus animals selectively avoid the bacterial odors that they experienced during pathogenic infection, a defining property of associative learning.
Formation and retrieval of the imprinted memory require distinct neural circuits
Olfactory chemotaxis in C. elegans is initiated by sensory neurons that converge on common interneurons including AIB and AIY, which synapse with each other and with downstream neurons including RIM and RIA (White et al., 1986) (Figure 2A). Adult learned pathogen aversion requires either AIB or AIY, both RIA and RIM, and several sensory neurons including AWB, AWC, and the serotoninergic neuron ADF (Ha et al., 2010) (Figure S1A). To ask whether the same neurons participate in imprinted aversion, we examined strains expressing the tetanus toxin light chain (TeTx), the gain-of-function potassium channel UNC-103(gf), or a cytotoxic mouse caspase from cell-type selective promoters. These experiments indicated that AIB, AIY, RIA and RIM were all required for imprinted aversion (Figure S3A). Since our behavioral choice assays were different from those in prior circuit work, we confirmed that the AIB::TeTx strain was proficient in adult learning but impaired in imprinted aversion in this assay, whereas RIM::TeTx was impaired in both forms of learning (Figure S3A). Among sensory neurons, AWB and AWC/ASE were required both for imprinted aversion and for adult learned aversion (Ha et al., 2010) (Figure S3A). Thus imprinted aversion and adult learned aversion have similar but not identical neuronal requirements.
Figure 2. Imprinted memory formation and retrieval require distinct circuits.
(A) Weighted wiring diagram of interneurons implicated in imprinted memory formation and retrieval. Synaptic strength is based on the number of chemical synapses from www.wormweb.org. See also Figure S1.
(B) Schematic illustration of neuronal silencing either at the memory formation or memory retrieval stage using cell-specific expression of a histamine-gated chloride channel (HisCl1).
(C–D) Neuronal silencing to identify neurons required either during memory formation (C) or during memory retrieval (D).
n, number of independent assays, 100–200 animals/assay. P values were generated by ANOVA with the Dunnett correction (** P <0.01, * P <0.05, ns not significant). See also Figure S3.
Neurons expressing toxic transgenes or subjected to laser ablations are inactive throughout life. To distinguish the contributions of interneurons in formation and retrieval of the imprinted memory, selected neurons were acutely silenced using the Drosophila histamine-gated chloride channel HisCl1 (Pokala et al., 2014) (Figure 2B). C. elegans does not use histamine as an endogenous transmitter, but absorbs exogenous histamine to rapidly and reversibly silence neurons expressing a HisCl1 transgene. Silencing either AIB or RIM during the L1 learning period abolished imprinted aversion in the adult, suggesting that AIB and RIM are required for formation of the imprinted memory (Figure 2C). Silencing AIB or RIM neurons in adults during the olfactory choice assay spared imprinted aversion, indicating that AIB and RIM are dispensable for memory retrieval (Figure 2C). Conversely, imprinted aversion was robust to silencing AIY or RIA neurons during the L1 learning period, but impaired by silencing AIY or RIA neurons during the olfactory choice assay in adults (Figure 2D). These results identify distinct neurons required during learning and retrieval stages of imprinted aversion.
Imprinting occurs while the nervous system is still developing, but it was not associated with an obvious remodeling of neuronal number, morphology, or fate among AIB, AIY, RIA and RIM neurons (Supplemental Experimental Procedures). The AIY and RIA neurons required for memory retrieval were examined more closely with synaptic markers (Shao et al., 2013). Synaptic regions of AIY and RIA were superficially similar in naïve and imprinted adults, suggesting that imprinting did not lead to major structural changes in these neurons (Figure S4).
Imprinting alters chemotaxis behaviors to the experienced pathogen
The behavioral strategies that give rise to imprinted aversion were examined through a quantitative analysis of chemotaxis parameters. C. elegans approaches attractive chemicals using a biased random walk, in which the frequency of high-angle turns (“pirouettes”) increases when an animal moves down the gradient and decreases when it moves up the gradient (Figure 3A) (Pierce-Shimomura et al., 1999). The turning bias is reversed in a gradient of repellent (Yamazoe-Umemoto et al., 2015). We found that naïve animals turned less when approaching PA14 and more when leaving PA14, as expected for biased random walk attraction, whereas PA14-trained animals expressed the reversed pirouette bias appropriate for aversion (Figure 3B). This effect depended on the learned association, as animals imprinted on PA14 did not change their pirouette bias in response to the non-pathogenic bacteria OP50 (Figure 3B) or the untrained toxic bacterium ToxA (Figure 3C).
Figure 3. Imprinting alters behavioural responses to the experienced pathogen.
(A) A pirouette is a reversal coupled to a high-angle turn. The bearing angle θ is the animal’s direction of movement with respect to the odor source (here, PA14 lawn) before the pirouette. Each choice assay has two bacterial odor sources, which were examined separately (see Supplemental Experimental Procedures).
(B) Normalized pirouette frequency of naïve and imprinted animals at different bearing angles with respect to a PA14 lawn (left) or OP50 lawn (right) in the choice assay. Naïve event frequency was compared to imprinted frequency at each bearing angle; P values were generated by ANOVA with the Sidak correction (* P <0.05).
(C) Normalized pirouette frequency of naïve and PA14-imprinted animals navigating between a novel toxic bacterium, ToxA, and OP50.
(D) Normalized pirouette frequency of naïve and PA14-imprinted animals navigating between PA14 and OP50 with AIY neurons silenced with HisCl1.
Pirouette rates were calculated from 3–5 movies with 40–50 animals each and normalized to average pirouette rates across angles. See also Figure S2.
Silencing the AIY memory retrieval neurons with HisCl1 during the olfactory choice assay eliminated the PA14 pirouette bias in both naïve and imprinted animals (Figure 3D). In addition, imprinting changed the contributions of AIY to basal pirouette regulation, resulting in a stronger AIY effect in chemotaxis assays and a weaker AIY effect during undirected local search (Figure S2A,B). Thus AIY neurons have altered functions after imprinting, which include an acute role in generating the reversed pirouette bias of imprinted aversion.
Imprinted aversion shares genetic requirements with other forms of learning and memory
The overlap between neurons required for adult learned aversion and imprinted aversion suggested that they might share molecular components. Indeed, the serotonin biosynthesis enzyme TPH-1 and serotonin receptor MOD-1 required for adult learned aversion were required for imprinted aversion as well (Zhang et al., 2005) (Figure 4A).
Figure 4. Tyramine in RIM neurons and the tyramine receptor SER-2 in AIY neurons are required for imprinted aversion.
(A) Imprinted aversion and adult learned aversion in mutants for the serotonin biosynthetic enzyme TPH-1, the serotonin receptor MOD-1, the vesicular glutamate transporter EAT-4, the glutamate receptors GLR-1, GLR-3 and NMR-1, the CREB homolog CRH-1 (two alleles), and the orphan G-protein coupled receptor SRA-11. Red bars mark assays with a significant learning deficit.
(B) Biosynthetic pathways for tyramine (produced in RIM and RIC neurons) and octopamine (produced in RIC neurons). Cells of the somatic gonad also make tyramine and octopamine.
(C) Learning index of tyramine/octopamine mutants and rescued strains. See also Figure S3.
(D) Learning index after exogenous tyramine or histamine administration to tdc-1 mutants and RIM::HisCl1 strains.
(E) Learning index of tyramine receptor mutants and rescued strains.
(F) Cell-specific rescue of ser-2 using intersectional promoters. Cre expression and inversion allows ser-2 expression in subsets of ser-2p2-expressing cells.
n, number of independent assays, 100–200 animals/assay. P values were generated by ANOVA with the Dunnett correction. (*** P <0.001, ** P <0.01, * P <0.05, ns not significant).
Glutamate is broadly employed as an excitatory neurotransmitter in vertebrate and invertebrate nervous systems. Glutamatergic signaling and the vesicular glutamate transporter EAT-4 are required for touch habituation in C. elegans, among other behaviors (Lee et al., 1999; Rankin and Wicks, 2000; Rose et al., 2003). We found that adult learned aversion and imprinted aversion both required eat-4, but glutamate receptors distinguished between the two forms of memory. The glutamate receptor GLR-3, which is expressed in RIA, was required for adult learned aversion and for imprinted aversion. However, the AMPA-type glutamate receptor GLR-1 was required for imprinted aversion but not for adult learned aversion, and the NMDA-type glutamate receptor NMR-1 affected adult learned aversion but not imprinted aversion (Figure 4A).
The cAMP response element-binding protein (CREB) is a transcription factor required for long-term memory in Aplysia, C. elegans, Drosophila, and mice (Kauffman et al., 2010; Silva et al., 1998). The C. elegans CREB homolog crh-1 was required for imprinted aversion, but not for adult learned aversion (Figure 4A). Imprinted aversion did not require SRA-11, a G protein-coupled receptor required for positive odor imprinting (Figure 4A) (Remy and Hobert, 2005). Although much remains to be learned about the timing, neuronal site of action, and specificity of these genes, it appears that imprinted aversion has genetic requirements that overlap partly but not entirely with other forms of learning.
Imprinting requires the neuromodulator tyramine in RIM neurons and the tyramine receptor SER-2 in AIY neurons
The RIM neurons release several neurotransmitters, including the monoamine neurotransmitter tyramine (Alkema et al., 2005) (Figure 4B). Invertebrate tyramine and octopamine are analogous to vertebrate epinephrine and norepinephrine, neuromodulators that can act as learning cues (Tully et al., 2007). Synthesis of tyramine and of the related transmitter octopamine requires the tyrosine decarboxylase TDC-1, and we found that tdc-1 mutants were defective both in imprinted aversion and in adult learned aversion (Figure 4C, S3B). tbh-1 mutants, which are deficient in octopamine synthesis, had normal imprinted aversion, suggesting that tyramine is the relevant transmitter (Figure 4C). tdc-1 is expressed in RIM and RIC neurons, and in non-neuronal cells in the gonad (Alkema et al., 2005). Imprinted aversion in tdc-1 mutants was rescued by expressing a tdc-1 cDNA from the RIM-specific gcy-13 promoter, but not from the RIC-specific tbh-1 promoter, indicating that tyramine synthesized by the RIM neurons is sufficient for imprinting (Figure 4C).
Imprinted aversion in tdc-1 mutants was rescued by exogenous tyramine during the L1 stage, when the RIM neurons were required, but not at later times (Figure 4D, left). Direct administration of tyramine during the L1 learning period rescued imprinted aversion when RIM was simultaneously silenced with HisCl1 (Figure 4D, right). The requirement for RIM in imprinted aversion is therefore closely associated with tyramine signaling in the L1 stage. However, L1 supplementation with exogenous tyramine and serotonin was not sufficient to induce imprinted aversion to non-pathogenic bacteria (Figure S3C).
C. elegans senses tyramine through the G-protein coupled receptors TYRA-2, TYRA-3, SER-2, and the tyramine-gated chloride channel LGC-55 (Donnelly et al., 2013; Pirri et al., 2009; Rex et al., 2005; Wragg et al., 2007). tyra-2, ser-2, and lgc-55 were all required for imprinted aversion, but tyra-3 was not (Figure 4E). Among these, ser-2 was required for imprinted aversion but not adult learned aversion (Figure S3B).
The ser-2 gene encodes multiple isoforms from different promoters (Tsalik et al., 2003). A distal promoter fragment (ser-2p2) driving a ser-2e cDNA rescued imprinted aversion in ser-2 mutants, but a proximal promoter fragment (ser-2p1) did not (Figure 4E). Rescuing activity was narrowed down further using an inverted Cre-lox (FLEx) recombination strategy to provide ser-2 to subsets of ser-2p2 neurons (Figure 4F). Expressing the Cre recombinase only in AIY neurons rescued learned aversion almost as well as full ser-2p2 expression, whereas expression in other ser2p2-expressing neurons (RME, SIA, and AIZ) was ineffective (Figure 4F). Thus SER-2 in AIY detects the tyramine produced by RIM, bridging the memory formation and retrieval circuits for imprinted aversion.
Imprinting alters neuronal responses to bacterial chemosensory cues
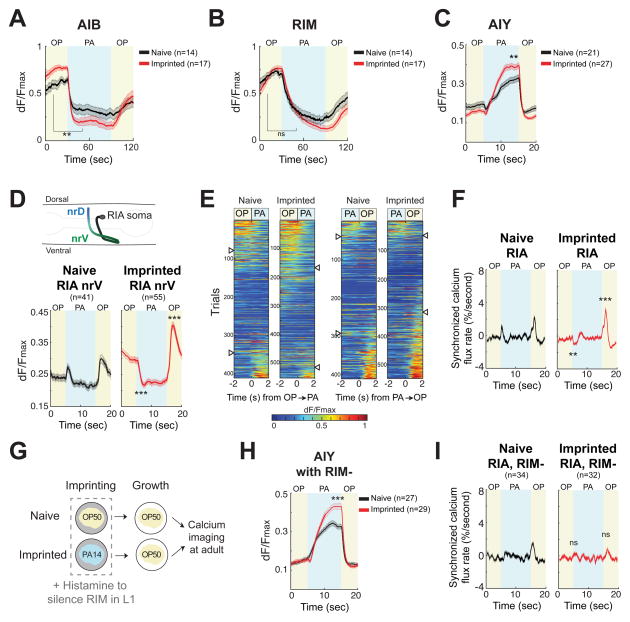
How is imprinted aversion represented in the neural circuit? To assess changes in functional activity, neuronal responses to bacterial odors were examined in animals expressing genetically-encoded calcium indicators in AIB, RIM, AIY, and RIA. All of these neurons respond to odors with calcium transients that are likely correlated with neuronal activity, albeit with relatively low temporal resolution (Chalasani et al., 2007; Gordus et al., 2015; Hendricks et al., 2012). Each neuron was examined in naïve and imprinted animals presented with alternating streams of OP50- and PA14-conditioned medium to imitate the sensory experience associated with a choice between bacterial odors.
In the memory formation neurons AIB and RIM, calcium fell after a transition from OP50- to PA14-conditioned medium and increased after a reciprocal transition from PA14 to OP50 (Figure 5A,B, S5A). Calcium transients were similar in naïve and imprinted adult animals, albeit with a slightly stronger AIB response after imprinting. Therefore, although AIB and RIM are not required for memory retrieval, they are still active and odor-responsive after imprinting (Figure 5, S2C).
Figure 5. Calcium responses of AIB, RIM, AIY and RIA neurons after aversive imprinting.
(A–C) Average (A) AIB (B) RIM and (C) AIY calcium responses to 60 s or 10 s alternations between OP50- and PA14-conditioned medium in naïve (black) and imprinted (red) animals. See also Figure S5.
(D) Altered RIA calcium response after imprinting. Top: illustration of RIA showing nrD and nrV axonal compartments. Calcium dynamics in nrD and nrV are correlated with local input from dorsal and ventral head motor neurons, respectively (Hendricks et al, 2012); odors or bacterial conditioned media acutely synchronize nrD and nrV. Bottom: average calcium responses in RIA nrV compartment of naïve (black) and imprinted (red) animals to alternating 10 s pulses of OP50- and PA14-conditioned medium.
(E) Calcium dynamics of individual RIA nrV responses to odor transitions between PA14- to OP50-conditioned medium. Traces were ordered according to the time derivatives of response at odor transitions (t=0). Arrowhead indicates threshold for calcium activation (dF/dt>0.01 %s−1), suppression (dF/dt <−0.01 %s−1), or no response.
(F) Average synchronous calcium flux rate of nrD and nrV compartments of RIA neurons.
(G–I) AIY and RIA responses in naïve and imprinted animals whose RIM neurons were silenced during the L1 stage. (G) Illustration of the experiment. (H) Average AIY responses. (I) Average synchronous calcium flux rate of RIA.
Blue background: PA14-conditioned medium; yellow: OP50-conditioned medium. Calcium traces were normalized on a 0–1 scale, see Experimental Procedures. Average differences for 10 s (AIB, RIM) or 1 s (AIY, RIA) before and after odor transitions were compared in naïve and imprinted animals. Shaded regions around traces are ±SEM. P values were generated by two-way ANOVA with the Bonferroni correction (*** P <0.001, ** P <0.01, ns not significant). See Figure S6 for further analysis of RIA compartmentalized dynamics and synchrony.
In the AIY memory retrieval neurons, calcium increased after a transition from OP50- to PA14-conditioned medium and fell after the reciprocal transition (Figure 5C). The average response in AIY was significantly stronger in imprinted adults (Figure 5C, S5B), identifying a second functional change associated with memory.
The RIA interneurons have compartmentalized axonal calcium dynamics that integrate sensory input and motor feedback (Hendricks et al., 2012). In naïve animals, average axonal calcium levels in RIA transiently increased each time bacterial odors were exchanged, rising immediately and falling within two seconds (Figure 5D, S6A). In imprinted animals, RIA calcium transients changed in polarity to fall immediately after a switch from OP50- to PA14-conditioned medium, and increased more strongly after a switch from PA14 to OP50 (Figure 5D, S6A). Examination of individual responses demonstrated a systematic shift across the entire distribution of responses: after imprinting, a switch to PA14 elicited more calcium decreases and fewer calcium increases, and a switch to OP50 had the opposite effects (Figure 5E, S6A). Sensory cues such as bacterial medium elicit synchronous calcium responses in both RIA axonal compartments (Hendricks et al., 2012), and these synchronous events also reversed polarity to PA14 presentation after imprinting (Figure 5F). Alterations in RIA activity were restricted to the bacterial choice context (Figure S6C), and to the PA14 bacteria experienced during training (Figure S6D,E).
To ask which changes in AIY or RIA activity correlated with memory, we examined animals whose RIM neurons had been silenced during L1 imprinting, precluding memory formation (Figure 5G). These animals had the same increased AIY responses to bacterial odors as wild-type imprinted animals, but their RIA calcium responses were not remodeled (Figure 5H,I, S6B). Thus imprinted behavioral memory correlates in its circuit requirements with changes in RIA calcium dynamics.
Discussion
C. elegans that are exposed to pathogenic bacteria in the first larval stage form an associative learned aversion to the bacterial odors that is maintained in mature adults. Based on its defined critical period, odor specificity, and potential ecological relevance, this behavior appears to represent a form of olfactory imprinting.
Imprinted aversion differs from classical olfactory imprinting in its valence. Classical olfactory imprinting drives approach behavior – homing to the natal stream for salmon (Nevitt et al., 1994), bonding between mammals and their young (Lorenz, 1935), kin recognition in zebrafish (Gerlach et al., 2008), and acceptance of imprinted foods (Wilson and Sullivan, 1994). Positive imprinting to odors experienced early in life has also been described in C. elegans, although little is known about its mechanisms beyond a requirement for an orphan G protein-coupled receptor sra-11 in AIY neurons (Remy and Hobert, 2005). Unlike positive imprinting, aversive imprinting does not require sra-11 and is not transmitted to the progeny of imprinted animals (Remy, 2010; Remy and Hobert, 2005). The combined value of positive and aversive imprinting, encoded by different mechanisms, may be considerable for an organism like C. elegans whose bacterial environment can be either favourable or life-threatening.
A circuit for imprinted aversion
The formation and retrieval of the imprinted memory depend on distinct groups of interneurons in a sensory processing circuit (Figure 6). These neurons receive input from many sensory neurons that detect pathogenic and benign bacteria. The AWC and AWB neurons, which are required for imprinted aversion and adult learned aversion, detect both E. coli- and PA14-conditioned media (Ha et al., 2010). The ASJ neurons detect PA14 secondary metabolites associated with virulence (Meisel et al., 2014), and also detect E. coli conditioned medium, as do numerous other sensory neurons (Zaslaver et al., 2015). The collective activity of multiple sensory neurons allows discrimination between bacterial odors, providing a substrate for olfactory learning and memory (Harris et al., 2014).
Figure 6. Weighted wiring diagram of imprinting neurons AIB, RIM, AIY, RIA, and their synaptic partners.
Synaptic strengths are based on the number of chemical synapses from www.wormweb.org. The four imprinting interneurons receive input from many sensory neurons (in grey) that represent different sensory modalities, and send output to motorneurons (in brown) to produce behaviors. Many additional neurons are synaptically connected to this network (Figure S1A) (White et al., 1986). Adult learning requires either AIB or AIY neurons, whereas aversive imprinting requires both AIB and AIY. Both adult learning and aversive imprinting appear to require AWC, AWB, ADF, RIM, and RIA neurons. Among the neurons shown, AWC, AIB, RIM, and RIA are glutamatergic; AIY, SMD, and RMD are cholinergic; ADF is serotonergic, RIM is tyraminergic, and all neurons express one or more neuropeptides. The SRA-11 receptor required for positive imprinting is required in AIY.
Tyramine neuromodulation encodes a learning signal
The AIB and RIM neurons are necessary during learning, but dispensable for memory retrieval in the adult. The RIM neurotransmitter tyramine is also necessary for learning during the L1 stage, and tyramine can replace the requirement for RIM activity. These results indicate that tyramine from RIM is an essential learning cue.
AIB and RIM are acutely involved in sensorimotor behaviors, so it was surprising that they were required only during learning. AIB is a synaptic target of many sensory neurons (White et al., 1986) (Figure 6), and AIB and RIM are elements of a coupled network of neurons that is active during most or all reversals (Gordus et al., 2015). After imprinting, AIB and RIM remained responsive to OP50 and PA14 bacterial odors, and AIB could still drive reversals when optogenetically activated. Nonetheless, these sensorimotor functions were dispensable during memory retrieval, whereas AIY and RIA were essential.
Both imprinted aversion and adult learned aversion require tyramine and a second neuromodulator, serotonin (Zhang et al., 2005). These modulators are necessary but not sufficient for imprinted aversion. Although they may relay information about pathogenic infection, they probably do not encode the aversive unconditioned stimulus directly, as certain dopaminergic neurons do in Drosophila olfactory learning (Aso et al., 2010). Instead, we suggest that the role of tyramine from RIM in learning may be related to its role in generating variability in sensory responses. RIM increases the coupling of AIB to the reversal circuit, and decreases its coupling to sensory neurons (Gordus et al., 2015), effectively introducing variability in sensory transmission to motor circuits. Behavioral variability is closely allied with learning, a process that is best understood during auditory-motor learning in songbirds. A young bird learns and practices its tutor song using a specialized brain region called LMAN that is not required for adult song performance (Bottjer et al., 1984). The active generation of variability by LMAN is essential for song learning in juveniles, and can be re-engaged in adult to permit more limited song plasticity (Kao et al., 2005; Olveczky et al., 2005). Like songbird LMAN, C. elegans RIM neurons might generate sensorimotor variability during early learning that gives rise to altered adult circuit function. We speculate that a requirement for variability-generating neurons at the learning stage may be a general feature of sensorimotor pathways for long-term memory.
Retrieval of the imprinted memory
The AIY and RIA neurons are essential for retrieval of the imprinted memory. The G protein-coupled tyramine receptor ser-2 in AIY appears to recognizes the tyramine learning signal from RIM, providing a molecular bridge between the neurons involved in memory formation and memory storage and retrieval.
Of the three tyramine receptors required for aversive learning, SER-2 was required for imprinted aversion but not for adult learned aversion. In motor neurons, SER-2 signals through Gαo to inhibit neurotransmitter release (Donnelly et al., 2013); how it functions in AIY to affect imprinted memory is unknown. Both imprinted aversion and adult learned aversion require two other tyramine receptors, LGC-55 and TYRA-2. LGC-55 is expressed in the forward command neuron AVB and in head motor neurons, and TYRA-2 is expressed in head sensory neurons, suggesting that these neurons could also contribute to aversive memory (Pirri et al., 2009; Rex et al., 2005).
AIY and RIA responses to bacterial odors were altered after imprinting. AIY and RIA have common synaptic inputs, and AIY provides synaptic input to RIA, so these functional changes may be linked (Figure 6). Alterations in AIY were not sufficient for memory, but changes in RIA calcium dynamics correlated with imprinted aversion, were specific to the imprinted bacteria, and depended on RIM learning signals.
RIA is a major integrating interneuron with inputs from multiple neurons required for aversive learning and memory (Figure 6). It is activated by sensory signals and by feedback from motor neurons that guide head movements (Hendricks et al., 2012). One possible model for memory is that RIA receives both excitatory and inhibitory inputs from bacterial odors, and that imprinting changes the relative weights of excitation and inhibition based on odors that are present when RIM is active and tyramine is released. Many neurons required for learning form both direct connections with the RIA neurons (ADF, AWB, AWC, AIB), and indirect connections through AIY and other integrating neurons (Figure 6). The convergence of these signals in RIA is a potential site for the representation of the imprinted memory.
Imprinted aversion and other forms of memory
The existence of this long-lasting C. elegans memory raises a number of questions for future study. One is the nature of the difference between long-lasting imprinted aversion and transient adult learned aversion. These forms of memory have overlapping, but not identical, genetic and circuit requirements. Imprinting has a stronger reliance on the AIB and AIY interneurons, which are individually dispensable for adult learned aversion (Ha et al., 2010). AIB and AIY are immediate targets of sensory neurons, suggesting that imprinting engages early steps of sensory processing. Imprinted aversion also requires three genes that are not required for adult learned aversion, ser-2, glr-1 and crh-1. ser-2 acts in AIY, which is also the site of action of the one gene known to affect positive imprinting to odors, the orphan G protein-coupled receptor sra-11 (Remy and Hobert, 2005). Thus both genetics and circuit analysis point to AIY as an important mediator of imprinted memory. The enhanced AIY response to odor input is not sufficient for imprinted aversion, but the relationship between AIY and pirouette behaviors is altered in several ways in imprinted animals, suggesting that changes in AIY output are an element of the memory.
The requirement for C. elegans CREB (crh-1) in imprinted aversion is consistent with the requirement for this transcription factor in long-term learning in many animals. The site of CRH-1 action and its transcriptional targets may provide further information about imprinting mechanisms. Notably, CREB is required for long-term appetitive olfactory learning and long-term habituation in C. elegans as well as aversive imprinting (Kauffman et al., 2010; Timbers and Rankin, 2011); in each case, CREB is required for memory that lasts at least 24 hours. In long-term appetitive olfactory training, CREB changes the expression of over 1000 genes (Lakhina et al., 2015).
A second question about imprinted aversion concerns the basis of the early critical period. During the L1 stage, the C. elegans nervous system remodels its synaptic connections and neuronal gene expression and incorporates 80 new postembryonic neurons (Hobert, 2010; Kage et al., 2005; Lesch et al., 2009; White et al., 1978). The development of functional connectivity may be modified by L1 experience during imprinting. Silencing RIM led to subtle changes in adult RIA calcium responses in naïve as well as imprinted animals, a hint that RIM activity during early development may affect the maturation or refinement of sensorimotor circuits. A closer examination of structural and functional connections in these circuits, and the relative balance of sensory connections and behaviors driven by different interneurons, might confirm or refute this hypothesis.
A third open question is the site of memory storage in the circuit. Imprinted aversion led to changes in neuronal activity at multiple sites in the circuit, including AIY and RIA memory retrieval neurons, but also including the AIB neurons that are not required for retrieval. This result highlights the fact that a memory can be expressed at many sites in the nervous system that are not active sites of memory storage. At the extreme, if memory were stored in sensory neurons, it could lead to indirect changes in the activity of any neuron downstream of sensory input. The ability to manipulate the activity of individual neurons with precision, while monitoring changes in circuit activity and behavior, should allow a future identification of the essential information in the stored memory.
Experimental Procedures
Standard nematode culture and molecular biology methods were used. A complete strain list and Supplemental Experimental Procedures are provided in Supplemental Information.
Imprinting training
Eggs from young adult hermaphrodites were obtained by bleaching (Stiernagle, 2006), placed on an NGM plate with pathogen or control OP50 bacteria, and incubated at room temperature (22°C). Eggs hatched after ~7 hour, and after 19 hours (12 hours of post-hatching training), both naïve and imprinted L1 larvae were washed off the plate with 200 nM neomycin (Tan et al., 1999) in M9 buffer, rinsed three times, transferred to an OP50-seeded plate, washed again with neomycin solution after 24 hours, and transferred to a second OP50-seeded plate where they were grown at room temperature (22°C).
Food choice assay (modified from (Zhang et al., 2005))
Fresh overnight bacterial cultures were diluted to OD600 = 1, and 20 μL of each bacteria suspension was seeded on a round NGM plate (radius = 5 cm) and incubated at room temperature (22°C) for <2 hours. After this relatively short incubation, chemotaxis is dominated by olfactory cues rather than slowly-diffusing water-soluble cues. To start the assay, young adult hermaphrodites were washed from their growth plate with M9 buffer, rinsed twice, and 100–200 animals were placed in the middle of the assay plate, equidistant from the bacterial lawns. Assays were incubated at room temperature for 60 minutes before being placed in 4°C to end the assay. To test animals bearing transgenic exchromosomal arrays, a COPAS large particle flow cytometry sorter (Union Biometrica) was used to collect the L4 stage animals that expressed the transgenic array one day prior to the food choice assay.
Calcium imaging and data analysis
Bacteria-conditioned medium was prepared on the day of the experiment by filtering a fresh overnight bacterial culture in NGM buffer (with peptone) with a sterile bottle top filter (Nalgene) into amber glass vials (EssVials Inc).
Young transgenic adults expressing GCaMP calcium sensors (Tian et al., 2009) were transferred to a fresh NGM plate, starved for 5 minute, then loaded into a custom PDMS chamber which restrained the animal to allow precise odor stimulation, as previously described (Chalasani et al., 2007). The acetylcholine agonist (−)-tetramisole hydrochloride (Sigma-Aldrich, L9756) at 1 mM was used only during transfer of animals into the chip, but not during imaging, to paralyze body wall muscles and keep animals stationary. Alternating bacteria-conditioned media stimuli were delivered every 60 seconds (AIB, RIM) or 10 seconds (AIY, RIA). 60-s alternation was used in AIB and RIM imaging to capture their slow calcium dynamics (Gordus et al., 2015). Calcium signals were recorded at 10 frame per second using a 40x objective on an upright Axioskop 2 microscope (Zeiss), with Metamorph software (Molecular Devices) and an iXon3 DU-897 EMCCD camera (Andor).
Imaging data were analyzed using custom scripts (ImageJ). MATLAB (MathWorks) was used for subsequent data analysis and display as previously described (Gordus et al., 2015). Bleach-corrected GCaMP fluorescence was divided by the lowest 5% as a baseline value, and then divided by the maximal value in the trace to obtain the normalized calcium response dF/Fmax for each animal. Each animal was normalized only once for data taken throughout a full experiment with many trials. Responses from multiple trials were averaged to obtain a mean population response and SEM. Because of the bimodal responses of AIB and RIM, only the trials in which neurons were in high activity states before PA addition were averaged in Figure 5 (see Supplemental Experimental Procedures). RIA calcium synchrony was calculated as previously described as events in which both axon compartments had time derivatives > 0.005 (% s−1) (influx) or < −0.005 (% s−1) (efflux) (Hendricks et al., 2012).
Supplementary Material
Acknowledgments
We thank Yuichi Iino, Takaaki Hirotsu, Villu Maricq, Daniel Colon-Ramos, Mark Alkema, Fred Ausubel, and the Caenorhabditis Genetics Center (CGC) (NIH P40 OD010440) for strains; Kavita Rangan and Alejandro Lopez-Cruz for experimental advice; the Rockefeller bioimaging facility for technical support; Steve Flavell, Meghan Lockard, Shay Stern, and Richard Axel for comments on the manuscript; and Yun Zhang, Michael Hendricks, Andrew Gordus, Sagi Levy, Steve Serene, and members of our laboratory for advice and discussions. X.J. was supported by an HHMI international predoctoral fellowship. C.I.B. is an Investigator of the Howard Hughes Medical Institute. This work was supported by the Howard Hughes Medical Institute.
Footnotes
Author Contributions
X.J. and C.I.B. designed experiments, X.J. conducted experiments, N.P. developed tracking methods, X.J. and C.I.B. analyzed and interpreted results, and X.J. and C.I.B. wrote the paper.
References
- Alkema MJ, Hunter-Ensor M, Ringstad N, Horvitz HR. Tyramine functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron. 2005;46:247–260. doi: 10.1016/j.neuron.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Ardiel EL, Rankin CH. An elegant mind: learning and memory in Caenorhabditis elegans. Learning & Memory. 2010;17:191–201. doi: 10.1101/lm.960510. [DOI] [PubMed] [Google Scholar]
- Aso Y, Siwanowicz I, Bracker L, Ito K, Kitamoto T, Tanimoto H. Specific dopaminergic neurons for the formation of labile aversive memory. Current Biology. 2010;20:1445–1451. doi: 10.1016/j.cub.2010.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224:901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- Chalasani SH, Chronis N, Tsunozaki M, Gray JM, Ramot D, Goodman MB, Bargmann CI. Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature. 2007;450:63–70. doi: 10.1038/nature06292. [DOI] [PubMed] [Google Scholar]
- Chen Z, Hendricks M, Cornils A, Maier W, Alcedo J, Zhang Y. Two insulin-like peptides antagonistically regulate aversive olfactory learning in C. elegans. Neuron. 2013;77:572–585. doi: 10.1016/j.neuron.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly JL, Clark CM, Leifer AM, Pirri JK, Haburcak M, Francis MM, Samuel AD, Alkema MJ. Monoaminergic orchestration of motor programs in a complex C. elegans behavior. PLoS Biol. 2013;11:e1001529. doi: 10.1371/journal.pbio.1001529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach G, Hodgins-Davis A, Avolio C, Schunter C. Kin recognition in zebrafish: a 24-hour window for olfactory imprinting. Proc Biol Sci. 2008;275:2165–2170. doi: 10.1098/rspb.2008.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordus A, Pokala N, Levy S, Flavell SW, Bargmann CI. Feedback from network states generates variability in a probabilistic olfactory circuit. Cell. 2015;161:215–227. doi: 10.1016/j.cell.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha HI, Hendricks M, Shen Y, Gabel CV, Fang-Yen C, Qin Y, Colon-Ramos D, Shen K, Samuel AD, Zhang Y. Functional organization of a neural network for aversive olfactory learning in Caenorhabditis elegans. Neuron. 2010;68:1173–1186. doi: 10.1016/j.neuron.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G, Shen Y, Ha H, Donato A, Wallis S, Zhang X, Zhang Y. Dissecting the signaling mechanisms underlying recognition and preference of food odors. J Neurosci. 2014;34:9389–9403. doi: 10.1523/JNEUROSCI.0012-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks M, Ha H, Maffey N, Zhang Y. Compartmentalized calcium dynamics in a C. elegans interneuron encode head movement. Nature. 2012;487:99–103. doi: 10.1038/nature11081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O. WormBook, editor. The C. elegans Research Community. WormBook; 2010. Neurogenesis in the nematode Caenorhabditis elegans(October 4 2010) http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kage E, Hayashi Y, Takeuchi H, Hirotsu T, Kunitomo H, Inoue T, Arai H, Iino Y, Kubo T. MBR-1, a novel helix-turn-helix transcription factor, is required for pruning excessive neurites in Caenorhabditis elegans. Curr Biol. 2005;15:1554–1559. doi: 10.1016/j.cub.2005.07.057. [DOI] [PubMed] [Google Scholar]
- Kao MH, Doupe AJ, Brainard MS. Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature. 2005;433:638–643. doi: 10.1038/nature03127. [DOI] [PubMed] [Google Scholar]
- Kauffman AL, Ashraf JM, Corces-Zimmerman MR, Landis JN, Murphy CT. Insulin signaling and dietary restriction differentially influence the decline of learning and memory with age. PLoS Biol. 2010;8:e1000372. doi: 10.1371/journal.pbio.1000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhina V, Arey RN, Kaletsky R, Kauffman A, Stein G, Keyes W, Xu D, Murphy CT. Genome-wide functional analysis of CREB/long-term memory-dependent transcription reveals distinct basal and memory gene expression programs. Neuron. 2015;85:330–345. doi: 10.1016/j.neuron.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RY, Sawin ER, Chalfie M, Horvitz HR, Avery L. EAT-4, a homolog of a mammalian sodium-dependent inorganic phosphate cotransporter, is necessary for glutamatergic neurotransmission in Caenorhabditis elegans. J Neurosci. 1999;19:159–167. doi: 10.1523/JNEUROSCI.19-01-00159.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch BJ, Gehrke AR, Bulyk ML, Bargmann CI. Transcriptional regulation and stabilization of left-right neuronal identity in C. elegans. Genes and Development. 2009;23:345–358. doi: 10.1101/gad.1763509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz K. Der Kumpan in der Umwelt des Vogels. Journal für Ornithologie. 1935;83:137–213. [Google Scholar]
- McEwan DL, Kirienko NV, Ausubel FM. Host translational inhibition by Pseudomonas aeruginosa Exotoxin A triggers an immune response in Caenorhabditis elegans. Cell Host Microbe. 2012;11:364–374. doi: 10.1016/j.chom.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel JD, Kim DH. Behavioral avoidance of pathogenic bacteria by Caenorhabditis elegans. Trends Immunol. 2014;35:465–470. doi: 10.1016/j.it.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Meisel JD, Panda O, Mahanti P, Schroeder FC, Kim DH. Chemosensation of bacterial secondary metabolites modulates neuroendocrine signaling and behavior of C. elegans. Cell. 2014;159:267–280. doi: 10.1016/j.cell.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo JA, Ruvkun G. Inactivation of conserved C. elegans genes engages pathogen- and xenobiotic-associated defenses. Cell. 2012;149:452–466. doi: 10.1016/j.cell.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamori T, Maekawa F, Sato K, Tanaka K, Ohki-Hamazaki H. Neural basis of imprinting behavior in chicks. Dev Growth Differ. 2013;55:198–206. doi: 10.1111/dgd.12028. [DOI] [PubMed] [Google Scholar]
- Nevitt GA, Dittman AH, Quinn TP, Moody WJ., Jr Evidence for a peripheral olfactory memory in imprinted salmon. Proc Natl Acad Sci U S A. 1994;91:4288–4292. doi: 10.1073/pnas.91.10.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olveczky BP, Andalman AS, Fee MS. Vocal experimentation in the juvenile songbird requires a basal ganglia circuit. PLoS Biol. 2005;3:e153. doi: 10.1371/journal.pbio.0030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce-Shimomura JT, Morse TM, Lockery SR. The fundamental role of pirouettes in Caenorhabditis elegans chemotaxis. J Neurosci. 1999;19:9557–9569. doi: 10.1523/JNEUROSCI.19-21-09557.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirri JK, McPherson AD, Donnelly JL, Francis MM, Alkema MJ. A tyramine-gated chloride channel coordinates distinct motor programs of a Caenorhabditis elegans escape response. Neuron. 2009;62:526–538. doi: 10.1016/j.neuron.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokala N, Liu Q, Gordus A, Bargmann CI. Inducible and titratable silencing of Caenorhabditis elegans neurons in vivo with histamine-gated chloride channels. Proc Natl Acad Sci U S A. 2014;111:2770–2775. doi: 10.1073/pnas.1400615111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahme LG, Tan MW, Le L, Wong SM, Tompkins RG, Calderwood SB, Ausubel FM. Use of model plant hosts to identify Pseudomonas aeruginosa virulence factors. Proc Natl Acad Sci U S A. 1997;94:13245–13250. doi: 10.1073/pnas.94.24.13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin CH, Wicks SR. Mutations of the Caenorhabditis elegans brain-specific inorganic phosphate transporter eat-4 affect habituation of the tap-withdrawal response without affecting the response itself. J Neurosci. 2000;20:4337–4344. doi: 10.1523/JNEUROSCI.20-11-04337.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy JJ. Stable inheritance of an acquired behavior in Caenorhabditis elegans. Curr Biol. 2010;20:R877–878. doi: 10.1016/j.cub.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Remy JJ, Hobert O. An interneuronal chemoreceptor required for olfactory imprinting in C. elegans. Science. 2005;309:787–790. doi: 10.1126/science.1114209. [DOI] [PubMed] [Google Scholar]
- Rex E, Hapiak V, Hobson R, Smith K, Xiao H, Komuniecki R. TYRA-2 (F01E11.5): a Caenorhabditis elegans tyramine receptor expressed in the MC and NSM pharyngeal neurons. J Neurochem. 2005;94:181–191. doi: 10.1111/j.1471-4159.2005.03180.x. [DOI] [PubMed] [Google Scholar]
- Rose JK, Kaun KR, Chen SH, Rankin CH. GLR-1, a non-NMDA glutamate receptor homolog, is critical for long-term memory in Caenorhabditis elegans. J Neurosci. 2003;23:9595–9599. doi: 10.1523/JNEUROSCI.23-29-09595.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Watanabe S, Christensen R, Jorgensen EM, Colon-Ramos DA. Synapse location during growth depends on glia location. Cell. 2013;154:337–350. doi: 10.1016/j.cell.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Stiernagle T. WormBook, editor. The C. elegans Research Community. WormBook; 2006. Maintenance of C. elegans February 11 2006. http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan MW, Mahajan-Miklos S, Ausubel FM. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc Natl Acad Sci U S A. 1999;96:715–720. doi: 10.1073/pnas.96.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbers TA, Rankin CH. Tap withdrawal circuit interneurons require CREB for long-term habituation in Caenorhabditis elegans. Behav Neurosci. 2011;125:560–566. doi: 10.1037/a0024370. [DOI] [PubMed] [Google Scholar]
- Torayama I, Ishihara T, Katsura I. Caenorhabditis elegans integrates the signals of butanone and food to enhance chemotaxis to butanone. J Neurosci. 2007;27:741–750. doi: 10.1523/JNEUROSCI.4312-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsalik EL, Niacaris T, Wenick AS, Pau K, Avery L, Hobert O. LIM homeobox gene-dependent expression of biogenic amine receptors in restricted regions of the C. elegans nervous system. Dev Biol. 2003;263:81–102. doi: 10.1016/s0012-1606(03)00447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully K, Li Y, Tsvetkov E, Bolshakov VY. Norepinephrine enables the induction of associative long-term potentiation at thalamo-amygdala synapses. Proc Natl Acad Sci U S A. 2007;104:14146–14150. doi: 10.1073/pnas.0704621104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Albertson DG, Arness MAR. Connectivity changes in a class of motoneurone during the development of a nematode. Nature. 1978;271:764–766. doi: 10.1038/271764a0. [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Neurobiology of associative learning in the neonate: early olfactory learning. Behav Neural Biol. 1994;61:1–18. doi: 10.1016/s0163-1047(05)80039-1. [DOI] [PubMed] [Google Scholar]
- Wragg RT, Hapiak V, Miller SB, Harris GP, Gray J, Komuniecki PR, Komuniecki RW. Tyramine and octopamine independently inhibit serotonin-stimulated aversive behaviors in Caenorhabditis elegans through two novel amine receptors. J Neurosci. 2007;27:13402–13412. doi: 10.1523/JNEUROSCI.3495-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazoe-Umemoto A, Fujita K, Iino Y, Iwasaki Y, Kimura KD. Modulation of different behavioral components by neuropeptide and dopamine signalings in non-associative odor learning of Caenorhabditis elegans. Neurosci Res. 2015 doi: 10.1016/j.neures.2015.05.009. in press. [DOI] [PubMed] [Google Scholar]
- Zaslaver A, Liani I, Shtangel O, Ginzburg S, Yee L, Sternberg PW. Hierarchical sparse coding in the sensory system of Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2015;112:1185–1189. doi: 10.1073/pnas.1423656112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lu H, Bargmann CI. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature. 2005;438:179–184. doi: 10.1038/nature04216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.