Abstract
Objective
Reactive Arthritis (ReA) is an inflammatory spondyloarthritis occurring after infection at a distant site. Chlamydia trachomatis is proposed to be the most common cause of ReA, yet the incidence of sexually-acquired ReA (SARA) has not been well established. We therefore carried out a systematic literature review to collate and critically evaluate the published evidence regarding the incidence of SARA.
Methods
MEDLINE and EMBASE databases were searched using free-text and MeSH terms relating to infection and ReA. The title and abstract of articles returned were screened independently by two reviewers and potentially relevant articles assessed in full. Data was extracted from relevant articles and a risk of bias assessment carried out using a validated tool. Heterogeneity of study methodology and results precluded meta-analysis.
Results
The search yielded a total of 11680 articles, and a further 17 were identified from review articles. After screening, 55 papers were assessed in full, from which 3 met the relevant inclusion criteria for the review. The studies reported an incidence of SARA of 3.0% - 8.1% and were found to be of low to moderate quality.
Conclusions
More studies are required to address the lack of data regarding the incidence of SARA. Specific and sensitive classification criteria must be developed in order for consistent classification and valid conclusions to be drawn. In clinical practice, it is recommended clinicians discuss the possibility of ReA developing at the time of STI diagnosis, and to encourage patients to return if they experience any relevant symptoms.
Introduction
Reactive Arthritis (ReA) is an inflammatory spondyloarthritis occurring after infection at a distant site. It typically occurs in young adults, one to four weeks after infection and can affect axial or peripheral joints and periarticular tissues [1]. While ReA is usually mild and self-limiting, up to 18% of those affected develop a chronic arthritis [2]. In some, but not all studies, the HLA-B27 gene is associated with increased severity and chronicity of ReA [3].
It is reported that ReA can be triggered by infection with bacteria, viruses and parasites [4, 5] with the most frequent microbial triggers infecting the gastrointestinal or genitourinary tracts [1]. Chlamydia trachomatis is proposed to be the most common cause of ReA [4, 6–9], with other sexually transmitted infections (STIs) including Neisseria gonorrhoeae (distinct from its role in septic gonococcal arthritis), and Ureaplasma urealyticum also implicated [10]. ReA triggered by an STI is referred to as sexually acquired reactive arthritis (SARA) [10].
The association of arthritis with STIs has been long recognised. Hippocrates was probably the first to link the presence of arthritis and infection in the genitourinary tract when he observed, at a time when the term “gout” was used to refer to acute arthritis, that “A youth does not suffer from gout until after sexual intercourse” [11, 12]. The first report of joint involvement after venereal disease was in 1715 by Musgrave, and the first clear description given by Swediaur in 1798 and 1809 [13], though the term “reactive arthritis”, referring to nonpurulent arthritis associated with infection, wasn’t introduced until 1969 [14, 15].
Despite this long history and extensive literature, the incidence rate of ReA after STI is not well established. A large proportion of ReA cases are probably unrecognised due to mild symptoms and resolution without treatment [1]. It is also unclear how long after infection ReA can occur, and which infections can cause ReA [16]. This is complicated by conflicting data regarding pathophysiology, where the inciting events could include viable bacteria in the synovium or an immune-mediated mechanism in response to chlamydial antigenic debris at the synovial site [9, 17–19]. As such, there are no validated diagnostic criteria for ReA for clinical use [2] and no universally accepted classification criteria for clinical research which leads to inconsistency in reported incidence rates in the literature [8, 20].
Despite this, many studies have attempted to determine an incidence rate for ReA. The systematic literature reviews to date have focussed on ReA triggered by enteric infection with ReA rates reported as 2.86% - 5.8% [21–23]. Despite chlamydia being widely stated as the most common infection causing ReA, there has not been a systematic review assessing the incidence of SARA. We therefore carried out a systematic literature review to collate and critically evaluate the published evidence regarding the incidence of SARA.
Methods
The methods recommended by the Centre for Reviews and Dissemination (CRD), University of York [24] were used and the data reported following guidelines set out in the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) statement [25]. The protocol for this study is available on request from the authors.
Eligibility criteria
Studies were included that reported an incidence or proportion of ReA cases after any sexually-associated infection, with the exception of Human Immunodeficiency Virus (HIV). HIV was excluded as a trigger for SARA as although ReA is reported in people with HIV infection, it is not clear whether this is due to HIV per se, or as immunocompromised status results in opportunistic infections (such as other STIs) which trigger ReA [26]. The current epidemiological data regarding HIV and ReA are too complex and variable to draw conclusions about the relationship between HIV infection and ReA [26]. As this literature has already been comprehensively reviewed elsewhere, we decided to exclude studies of HIV and ReA from our review.
All prospective studies assessing for ReA after infection, where ReA could be assessed at the time of diagnosis of infection or at follow-up were included. All time periods and populations were included, including any age-group, gender, or country. Only studies with laboratory confirmation of the infection were included to avoid biasing rates due to including subjects with infection based on self-report. Studies that identified ReA and retrospectively tested for STIs were excluded, as were articles not written in English language, as translation facilities were not available.
Information sources
MEDLINE and EMBASE databases (1946-present) were searched to produce a database of abstracts, eliminating any duplicate articles.
Search strategy
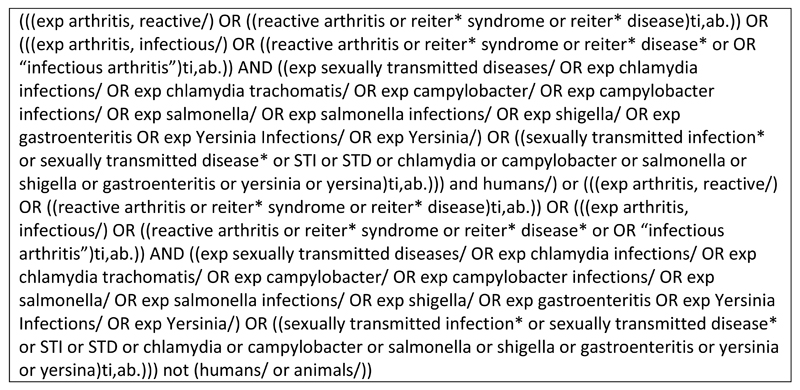
Search terms were generated on the basis of the research objective and included infection terms and ReA terms. Both Medical Subject Headings (MeSH) terms and free-text terms were used. Search terms including enteropathogenic causes of ReA were included to identify papers which may have included STI organisms but not included the title or abstract. Reiter’s syndrome terms were included, as this has been used synonymously with ReA in the past. We sought the input of an information specialist to assist in developing the search strategy to maximise its efficiency at returning relevant articles. The full electronic search strategy is presented in Fig. 1. This search was conducted in June 2014 and saved in the Ovid system for autoalerts to be emailed to the reviewers monthly. These were then screened for additional articles until the end of December 2015. The references of relevant review articles were also checked for studies that may be applicable to the present review.
Fig. 1.
Full electronic search strategy
Study selection
Two reviewers (HD and BC) independently screened the title and abstract of articles returned by the search with a low threshold for inclusion. The final inclusion lists of the two reviewers were then compared and full text of all relevant articles were obtained. The articles were then assessed by one reviewer (HD) to determine if criteria for inclusion in the review were met, and this list was checked by the second reviewer (BC). In case of disagreement, a third reviewer (RG) gave a deciding opinion.
Data collection
Data was extracted from each article by one reviewer (HD) into tables, which were then checked for accuracy by a second reviewer (RG). Information extracted included the following: general details (the reviewer and date); study details (type of study, inclusion/exclusion criteria); study population description; methodology of infection diagnosis; methodology of ReA diagnosis; analysis (statistical techniques used, losses to follow-up); and research results. The principal summary measure recorded to use in this review was incidence rate of ReA, or if no incidence rate was calculated, proportion of infected cases developing ReA.
Risk of bias in individual studies
To assess the methodological quality of the primary research, a risk of bias assessment was conducted independently by two reviewers (HD and BC) using the RTI (Research Triangle Institute) item bank [27]. From the 29 items in the RTI item bank the 14 relevant items were selected, according to the authors’ instructions (Table 3). For each item, criteria relevant to determining the risk of bias were specified to aid the reviewers. An overall judgement of the risk of bias for each study was made based on whether the biases assessed were likely to seriously alter the results. Disagreements were resolved by discussion to reach consensus. The assessment information was used in data synthesis to interpret study results on a background of methodological quality.
Table 3.
Risk of bias assessment using RTI Item Bank tool
| Item | Dimension of bias | Keat et al. [29] | Rich et al. [30] | Carter et al. [31] |
|---|---|---|---|---|
| Is the study design prospective, retrospective, or mixed? | Selection bias; performance bias; detection bias | Prospective | Prospective | Prospective |
| Are critical inclusion/exclusion criteria clearly stated? | Selection bias | Partially | Yes | Yes |
| Are the inclusion/exclusion criteria measured using valid and reliable measures? | Information bias | Partially | Partially | Partially |
| Is the intervention or exposure clearly described in enough detail? | Performance bias | Partially | Partially | Partially |
| Are the important outcomes pre-specified by the researchers? | Reporting bias | Partially | Partially | Yes |
| Are interventions/exposures assessed using valid and reliable measures, implemented consistently across all study participants? | Information bias | Partially | Partially | Partially |
| Are primary outcomes assessed using valid and reliable measures, implemented consistently across all study participants? | Information bias | Cannot determine | Yes | Partially |
| Is the length of time following the intervention/exposure sufficient to support the conclusions of the study regarding primary outcomes? | Attrition bias | Cannot determine | Partially | Partially |
| Did attrition from any group exceed 20 percent? | Attrition bias | Cannot determine | Yes | Yes |
| In cases of high loss to follow-up, is the impact assessed? | Attrition bias | Cannot determine | No | No |
| Are any primary outcomes missing from the results? | Reporting bias | Yes | No | No |
| Are the statistical methods used to assess the primary benefit outcomes appropriate to the data? | Precision | Cannot determine | Yes | Yes |
| Is the source of funding identified? | Reporting bias | Yes | Yes | Yes |
| Are results believable taking study limitations into consideration? | Overall study quality | No | Partially | Partially |
| Overall judgement on risk of bias | - | High risk of bias | Medium risk of bias | Medium risk of bias |
Synthesis of results
Heterogeneity of study methodology and results precluded meta-analysis so data were summarised in tabular and narrative form.
Results
Study selection
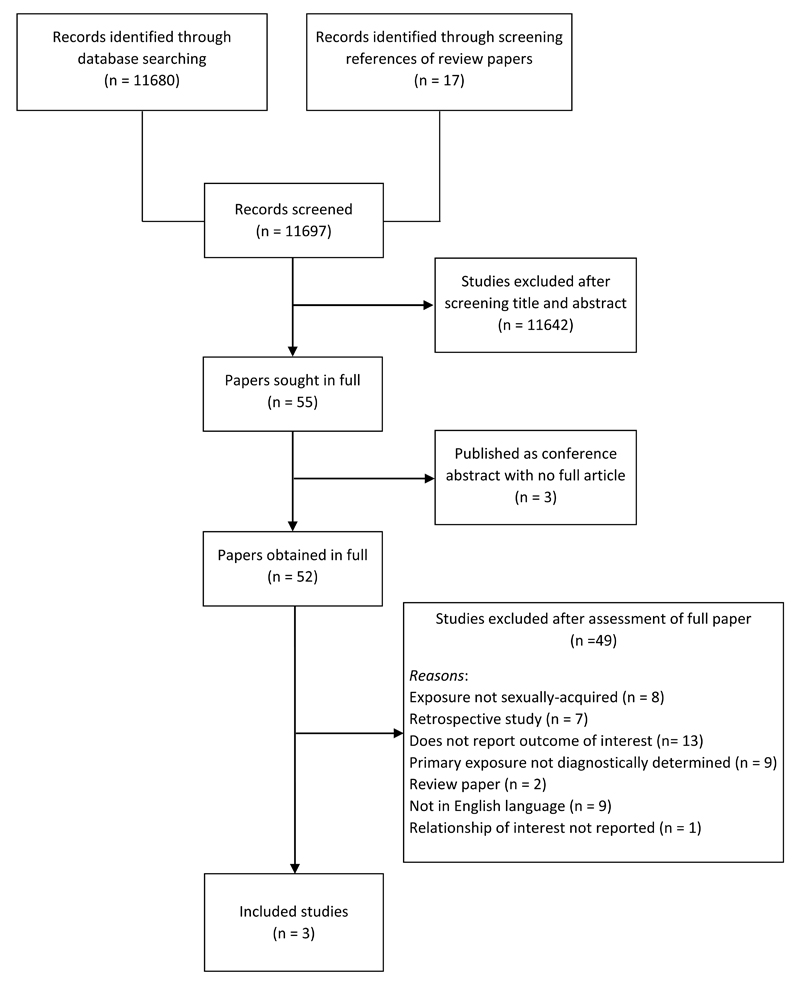
No systematic review addressing the research objective of the present paper was discovered. The database searches yielded a total of 11,680 articles, and a further 17 were identified through screening the references of relevant review articles. After title and abstract screening, 11,642 articles were excluded as they were not relevant. Fifty-five papers were sought in full for further assessment. Three papers were only published as conference abstracts. Forty-nine papers were excluded after assessment of the full text because of the following: they were review papers and did not contain original research (n = 2); they were not available in English language (n = 9); exposure infection was not sexually acquired (n = 8); they had a retrospective design (n = 7); or they did not report ReA incidence (n = 13). Studies where the infection was not laboratory confirmed were also excluded (n = 10). An additional paper [28] was excluded because it reported the number of patients with Reiter’s disease and the number of patients with non-specific genital infection in a group of patients with uveitis, but did not report the relationship of interest (number of patients with genital infection and ReA from the total cohort). Thus, three articles [29–31] were retained for the systematic review (Fig 2).
Fig. 2.
Flow diagram depicting the identification and selection of relevant papers
Study characteristics (table 1)
Table 1.
Characteristics of included studies
| First author | Year of publication | Study location | Type of study | Study size | Study population | Primary exposure(s) (Infection) | Infection diagnosis | Incidence of ReA | Proportion of ReA cases with asymptomatic infection |
|---|---|---|---|---|---|---|---|---|---|
| Carter [31] | 2013 | Florida, USA | Prospective | 149 (from 365 enrolled) | Adults attending communicable disease clinic testing positive for Chlamydia trachomatis | Chlamydia trachomatis | Laboratory diagnosis by gram stain, cell culture or NAAT | 12/149 (8.1%) | 8/12 (66.7%) |
| Rich [30] | 1996 | Alabama, USA | Prospective | 217 | Adults attending a sexually transmitted diseases clinic being treated with doxycycline for a possible or proven Chlamydia trachomatis infection | Genital infection/inflammation | Laboratory diagnosis by cervical cell culture for Neisseria gonorrhoeae and Chlamydia trachomatis. In men, a gram-stained urethral smear and a urethral Neisseria gonorrhoeae culture were obtained. Chlamydia trachomatis genital cultures were obtained for every patient who had objective ReA features. | 9/217 (4.1%) | 7/9 (77.8%) |
| Keat [29] | 1978 | UK | Likely prospective | 531 | Heterosexual men attending sexually transmitted diseases clinic with new episodes of urethritis. | Non-specific urethritis. Cultures for Chlamydia trachomatis taken to investigate association with ReA. | Urethral smear. Non-specific urethritis if over 10 polymorphs found in ≥ three consecutive high-power fields (magnification x 600), if microscopy, culture, and serology excluded gonorrhoea and syphilis. | 16/531 (3.0%) | No details |
The three papers were published over a 35 year period (1978, 1996, 2013), two in the USA [30, 31] and one in the UK [29]. The studies were situated in STI [29, 30] or communicable disease clinics [31]. The mean age of the men and women in the two American studies was 24.4 years [31] and 26 years [30]. Ethnicities varied depending on clinic clientele; participants in the Alabama study were predominantly African American (98.5%) [30] and in the Florida study African American (63%), Hispanic (22%), white (13%) and other (1.3%) [31]. The UK study recruited heterosexual men only and provided no data on age or ethnicity [29].
The primary exposure in the studies varied. The oldest paper, Keat et al, included 531 men with non-specific urethritis [29] while cultures were taken to confirm chlamydia in some participants (n= 384). Rich et al. recruited patients who were being treated with doxycycline for possible or proven chlamydia infection (n=271) and included patients with gram stain or cell culture diagnosis confirmation of genital infection/inflammation (n=217) as the denominator for the ReA incidence analysis [30]. This group included men diagnosed with gonococcal urethritis and nongonococcal urethritis, and women diagnosed with N. gonorrhoeae and C. trachomatis. Carter et al. only recruited patients who tested positive for C. trachomatis (n = 365); 149 were followed up for assessment of ReA symptoms [31].
Two papers identified ReA cases using a screening questionnaire prompting further evaluation [30, 31] (Table 2). One of these used a questionnaire derived from a validated questionnaire [32] and was administered by rheumatology subspecialty residents by phone at six week follow-up only [31]. Rich et al. used a paper questionnaire completed by participants at initial clinic visit and via mail at six weeks [30]. In both studies a positive answer to screening questions prompted invitation to evaluation in person by a rheumatologist. In the study by Carter et al., participants declined to attend a rheumatology evaluation, so the protocol was amended to telephone evaluation only, with all subjects with a positive six-week telephone questionnaire defined as “possible/probable ReA” [31]. The third paper [29] had no detailed data about the process of evaluation.
Table 2.
ReA diagnosis methodology of included studies
| Study | Classification criteria used | Screening tool used | Full evaluation |
|---|---|---|---|
| Carter et al. [31] | The European Spondylarthropathy Study Group (ESSG) criteria [33] (excluding plain radiographs of the sacroiliac joints). | Standardised questionnaire of 13 questions by telephone by rheumatology sub-speciality residents, including new arthritis symptoms or other symptoms of ReA such as conjunctivitis, uveitis and enthesitis. | Details obtained for any positive responses to the questionnaire during the telephone interview. In person review declined by participants. |
| Rich et al. [30] | Features of ReA considered: oligoarthritis, enthesitis in any location, inflammatory axial pain or mucocutaneous inflammation (conjunctivitis, oral ulcers, circinate balanitis, or keratoderma blennorrhagica). | A standardised questionnaire of 8 questions derived from QUEST 2 (Questionnaire Utilizing Epidemic Spondyloarthropathy Traits) [32]. In the last 60 days: joint pain, joint swelling, morning stiffness, heel pain, inflammation of the eyes, oral mucosal lesions, or skin rashes involving the palms or soIes. | Patients with positive answers to any question evaluated by a rheumatologist by history and physical examination with particular attention to features of ReA. |
| Keat et al.[29] | No specific classification criteria given, but state arthritis after a proven or putative infection of the genital tract considered as sexually acquired reactive arthritis (SARA), the arthritis (referred to as “reactive”) being a sterile inflammation of the synovial membrane, tendons, and fascia. | No screening tool described, not clear if used. | Method of full examination not described. Excluded alternative diagnoses, such as gonococcal arthritis, gout, rheumatoid arthritis and septic arthritis, by appropriate investigations. |
Risk of bias assessment
The risk of bias of the articles included in this review was considered medium to high; therefore, the interpretation of the results of these articles is limited (Table 3). Key biases included attrition bias, selection bias, information bias, performance bias and reporting bias. The articles failed to report how many participants were approached to participate in the studies, and how they were selected. Details about the timing of recruitment and assessment were also lacking. Details about participant selection were too vague to interpret to what extent their selection protocol may have affected results.
Infection diagnosis was made using objective and valid diagnostic tools in all studies, either by culture, gram stain or Nucleic Acid Amplification Test (NAAT). However, as the studies were published from 1978 to 2013 the diagnostic techniques vary widely in method and sensitivity, preventing direct comparison.
Only one study completed assessment of ReA by a rheumatologist with physical evaluation [30]. Telephone assessment to define ReA, used in the study by Carter et al. [31], could have introduced bias and limits comparability between the two studies. The final paper [29] did not discuss how ReA was assessed.
In the two studies that reported the number of participants followed-up, there was a high attrition rate which it is likely to have introduced bias [30, 31]. Reliance on return of questionnaires by mail may have resulted in over-reporting of incidence. The article by Keat et al. especially lacked sufficient information about the methods, making it difficult to accurately assess many items on the risk of bias tool [29].
Outcome measure definition
One study used an established criteria for ReA [31], the European Spondyloarthropathy Study Group (ESSG) criteria [33]. Plain radiographs of the sacroiliac joints (one ESSG criterion) was omitted as the evaluation was by telephone. Two patients were classified as ReA cases despite not fully meeting the classification criteria. The other two studies did not use any classification criteria, Rich et al. listed ReA features assessed by rheumatologists [30], and Keat et al. gave a brief definition of SARA in the introduction [29].
ReA incidence rate
A meta-analysis was not possible due to the small number of relevant studies, heterogeneity of methodology between studies, medium to high risk of bias, and the differing ReA diagnostic criteria used. A narrative summary of results is presented.
Rich et al. reported that nine of 217 patients (4.1%) had objective ReA features as assessed by a rheumatologist [30]. Carter et al. reported an incidence rate almost double that of Rich et al., with 12 out of 149 (8.1%) participants having symptoms consistent with reactive arthritis [31]. Ten of these 12 participants met the ESSG diagnostic criteria for spondyloarthritis; the 2 subjects that did not fulfil these criteria were judged to have ReA based on expert opinion. The ratio of female-to-male cases was about 1:1 in both of these mixed sex studies. Keat et al. reported that 16 patients developed ReA from the 531 studied, giving an incidence of 3% [29].
Discussion
Summary of main finding
This systematic review found only three studies, of low to moderate quality, reporting an incidence of SARA of 3.0% - 8.1%. There were insufficient data to perform a meta-analysis.
To our knowledge, this is the first systematic literature review of studies that have assessed the incidence of SARA. It highlights the paucity of data examining the incidence of SARA, which is incongruous since retrospective studies suggest that C. trachomatis is the most common trigger of ReA [4, 7] as well as being a common infection [34, 35].
Although the small number of relevant published studies means the findings of this review need to be interpreted with caution, the incidence of ReA in the included studies is of concern. As chlamydia infection is asymptomatic in approximately 70% of females and 25% of men [36], SARA should be considered in all people presenting with inflammatory arthritis who are at risk for STIs. In two of the included studies 67% to 78% of patients who developed ReA did so after an asymptomatic infection. In addition to the diagnostic consideration for arthritis, an STI screen should be considered for all patients presenting with an inflammatory arthritis that could possibly be ReA, so that any genital infection can be detected and treated. There is some data which suggest this is not common practice [37]. It seems possible that SARA may be overlooked due to the absence of testing, or the lack of diagnostic criteria available to medical practitioners, not the absence of infection.
The included studies also challenge the accepted view that ReA after C. trachomatis is more common in men, as both of the mixed-sex studies [30, 31] found a similar incidence of ReA in men and women. This contrasts population-based and retrospective studies of ReA diagnosed in routine clinical practice, where ReA cases are predominantly males [1, 38]. It is possible that ReA is underdiagnosed in women as genital infections are more likely to be asymptomatic [36].
Strengths and limitations
This review’s strengths include the systematic approach, independent data search and analysis. Limitations include the lack of relevant studies, the medium to high risk of bias assessed to be present within included studies, and the methodological heterogeneity across studies. Additionally, a number of potentially relevant publications were not in English (n=9) and resources were not available to translate these.
Only three studies met the inclusion criteria of assessing the incidence of SARA [29–31]. Furthermore, Keat et al. stated that their study was not specifically designed to provide epidemiological data on the incidence of ReA in the general population or in hospital-based patients with non-specific urethritis [29]. Although the reasons for this statement are unclear, and caution should be applied to the study’s interpretation, the reported incidence of SARA of 3.01% is similar to the incidence reported by Rich et al. (4.1%) [30].
The studies had medium to high risk of bias, meaning results should be interpreted with caution. Reporting of study methods was inconsistent and significantly lacking in the oldest study. Future research should make use of reporting guidelines such as STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) to ensure all the relevant information is available to contextualise the work [39].
The studies used different methods and criteria to identify ReA cases. This is a well-recognised problem in rheumatology where diseases often have heterogeneous presentation and rarely have a single clinical, laboratory, pathological or radiological feature as a “gold standard” for diagnosis and/or classification [40]. Because of this, classification criteria have been developed for use in clinical research. Classification criteria are applicable to groups and are more specific than diagnostic criteria which are applied to individuals. No universal diagnostic or classification criteria have been established for ReA. Classification schemes proposed in aiding the diagnosis of spondyloarthropathies include the Amor criteria [41], the European Spondyloarthropathy Study Group (ESSG) criteria [33] and the Assessment of SpondyloArthritis International Society (ASAS) criteria [42]. Once the diagnosis of spondyloarthropathy is made, further clinical history and symptoms allow identification of the specific spondyloarthropathy, including ReA. There is continued debate in the literature about the performance and suitability of these various classification criteria [43, 44].
In this review, only one study used classification criteria (ESSG) making comparative evaluation of the studies challenging, which is an ongoing problem in ReA [2, 20]. Epidemiological studies of ReA require standardised, validated classification criteria to provide uniformity for inclusion of study subjects. Until these are established, comparability across studies and a full understanding of ReA (including SARA) will not be possible.
Conclusions and recommendations
In summary, there is a lack of data regarding the incidence of SARA. This lack of data feeds into a lack of clinical guidelines and thus physician awareness which perpetuates the problem of underdiagnosis. It is likely that SARA often goes undiagnosed and that only severely affected people are recognised; thus, we cannot determine the true burden of disease. Quantification of the incidence rate of SARA will provide a better evidence-base of the natural history of this condition, as well as allowing improved diagnosis and optimization of treatment. Studies assessing the incidence of ReA after STI are required, using current STI diagnostic tools that are accurate and sensitive. A suggested screening tool, adapted from Townes et al. [45], for use in such future studies is included (Table 4), from which participants with positive answers can be selected for full rheumatological review. In consideration of the attrition problems reported by the included studies in this report, assessment of ReA at the initial sexual health clinic visit is advised, along with follow-up at six weeks and three months to enable capture of ReA that takes longer to develop. Specific and sensitive classification criteria must be developed and universally adopted for use in epidemiological studies in order for consistent classification to take place and valid conclusions to be drawn. In clinical practice, it is recommended that at the time an STI diagnosis is made, clinicians discuss with patients the possibility of ReA developing and to return for assessment if they experience any relevant symptoms.
Table 4.
Screening questionnaire suggested for use in studies of the incidence of SARA, adapted from Townes et al. [45]
| Since developing STI symptoms or since having an STI test, have you experienced pain, swelling or stiffness in any of your joints? This could be in any joints including your fingers, wrists, knees, ankles, toes or the spine. | No |  |
Questionnaire terminates | ||
| Yes |  |
Questionnaire continues | |||
| Since developing STI symptoms or since having an STI test, have you developed any of the following new symptoms? | |||||
| Joint pain or discomfort | No |
|
When did this begin? When did this go away? (provide option for still experiencing symptoms) | ||
| Joint swelling or redness | No | When did this begin? When did this go away? (provide option for still experiencing symptoms) | |||
| Morning joint or back stiffness lasting longer than 1 hour | No | When did this begin? When did this go away? (provide option for still experiencing symptoms) | |||
| Heel pain | No | When did this begin? When did this go away? (provide option for still experiencing symptoms) | |||
| Lower back pain | No | When did this begin? When did this go away? (provide option for still experiencing symptoms) | |||
| Offer rheumatological review if period of symptoms aligns with possible ReA | |||||
Footnotes
Conflict of interest
The authors declare they have no conflicts of interest.
References
- 1.Hamdulay SS, Glynne SJ, Keat A. When is arthritis reactive? Postgrad Med J. 2006;82:446–53. doi: 10.1136/pgmj.2005.044057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hannu T, Inman R, Granfors K, Leirisalo-Repo M. Reactive arthritis or post-infectious arthritis? Best Pract Res Clin Rheumatol. 2006;20:419–33. doi: 10.1016/j.berh.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Pope JE, Krizova A, Garg AX, Thiessen-Philbrook H, Ouimet JM. Campylobacter Reactive Arthritis: A Systematic Review. Semin Arthritis Rheum. 2007;37:48–55. doi: 10.1016/j.semarthrit.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozgul A, Dede I, Taskaynatan MA, Aydogan H, Kalyon TA. Clinical presentations of chlamydial and non-chlamydial reactive arthritis. Rheumatol Int. 2006;26:879–85. doi: 10.1007/s00296-005-0094-z. [DOI] [PubMed] [Google Scholar]
- 5.Espinoza LR, Garcia-Valladares I. Of bugs and joints: the relationship between infection and joints. Reumatol Clin. 2013;9:229–38. doi: 10.1016/j.reuma.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Keat A, Thomas B, Dixey J, Osborn M, Sonnex C, Taylor-Robinson D. Chlamydia trachomatis and reactive arthritis: the missing link. Lancet. 1987;1:72–4. doi: 10.1016/s0140-6736(87)91910-6. [DOI] [PubMed] [Google Scholar]
- 7.Kvien TK, Glennas A, Melby K, Granfors K, Andrup O, Karstensen B, Thoen JE. Reactive arthritis: incidence, triggering agents and clinical presentation. J Rheumatol. 1994;21:115–22. [PubMed] [Google Scholar]
- 8.Singh A, Karrar S. The role of intracellular organisms in the pathogenesis of inflammatory arthritis. Int J Inflam. 2014:158793. doi: 10.1155/2014/158793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris D, Inman RD. Reactive arthritis: developments and challenges in diagnosis and treatment. Curr Rheumatol Rep. 2012;14:390–4. doi: 10.1007/s11926-012-0280-4. [DOI] [PubMed] [Google Scholar]
- 10.Carlin EM, Ziza JM, Keat A, Janier M. 2014 European Guideline on the management of sexually acquired reactive arthritis. Int J STD AIDS. 2014;25:901–12. doi: 10.1177/0956462414540617. [DOI] [PubMed] [Google Scholar]
- 11.Lloyd GE. In: Hippocratic writings. Chadwick J, Mann WN, translators. Pelican Books; New York, N.Y: 1978. p. 229. [Google Scholar]
- 12.Iglesias-Gammara A, Restrepo JF, Valle R, Matteson EL. A Brief History of Stoll-Brodie-Fiessinger-Leroy Syndrome (Reiter's Syndrome) and Reactive Arthritis with a Translation of Reiter's Original 1916 Article into English. Current Rheumatology Reviews. 2005;1:71–9. [Google Scholar]
- 13.Storey GO, Scott DL. Arthritis associated with venereal disease in nineteenth century London. Clin Rheumatol. 1998;17:500–4. doi: 10.1007/BF01451287. [DOI] [PubMed] [Google Scholar]
- 14.Ahvonen P, Sievers K, Aho K. Arthritis Associated with Yersinia Enterocolitica Infection. Acta Rheumatologica Scandinavica. 1969;15(1–4):232–253. doi: 10.3109/rhe1.1969.15.issue-1-4.32. Acta Rheumatologica Scandinavica 15:232-53. [DOI] [PubMed] [Google Scholar]
- 15.Kuipers JG, Kohler L, Zeidler H. Reactive or infectious arthritis. Ann Rheum Dis. 1999;58:661–4. doi: 10.1136/ard.58.11.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Townes JM. Reactive arthritis after enteric infections in the United States: The problem of definition. Clin Infect Dis. 2010;50:247–54. doi: 10.1086/649540. [DOI] [PubMed] [Google Scholar]
- 17.Inman RD, Whittum-Hudson JA, Schumacher HR, Hudson AP. Chlamydia and associated arthritis. Curr Opin Rheumatol. 2000;12:254–62. doi: 10.1097/00002281-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Owlia MB, Eley AR. Is the role of Chlamydia trachomatis underestimated in patients with suspected reactive arthritis? Int J Rheum Dis. 2010;13:27–38. doi: 10.1111/j.1756-185X.2009.01446.x. [DOI] [PubMed] [Google Scholar]
- 19.Selmi C, Gershwin ME. Diagnosis and classification of reactive arthritis. Autoimmun Rev. 2014;13:546–9. doi: 10.1016/j.autrev.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Pacheco-Tena C, Burgos-Vargas R, Vazquez-Mellado J, Cazarin J, Perez-Diaz JA. A proposal for the classification of patients for clinical and experimental studies on reactive arthritis. J Rheumatol. 1999;26:1338–46. [PubMed] [Google Scholar]
- 21.Ajene AN, Fischer Walker CL, Black RE. Enteric pathogens and reactive arthritis: a systematic review of Campylobacter, salmonella and Shigella-associated reactive arthritis. J Health Popul Nutr. 2013;31:299–307. doi: 10.3329/jhpn.v31i3.16515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keithlin J, Sargeant J, Thomas MK, Fazil A. Systematic review and meta-analysis of the proportion of Campylobacter cases that develop chronic sequelae. BMC Public Health. 2014;14:1203. doi: 10.1186/1471-2458-14-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keithlin J, Sargeant JM, Thomas MK, Fazil A. Systematic review and meta-analysis of the proportion of non-typhoidal Salmonella cases that develop chronic sequelae. Epidemiol Infect. 2015;143:1333–51. doi: 10.1017/S0950268814002829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Systematic Reviews: CRD's guidance for undertaking reviews in health care. Centre for Reviews and Dissemination; University of York: 2009. [Accessed 28.03.16]. ISBN 978-1-900640-47-3 https://www.york.ac.uk/crd/guidance/ [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J Clin Epidemiol. 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Lawson E, Walker-Bone K. The changing spectrum of rheumatic disease in HIV infection. Br Med Bull. 2012;103:203–21. doi: 10.1093/bmb/lds022. [DOI] [PubMed] [Google Scholar]
- 27.Viswanathan M, Berkman ND. Development of the RTI item bank on risk of bias and precision of observational studies. J Clin Epidemiol. 2012;65:163–78. doi: 10.1016/j.jclinepi.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Catterall RD. Incidence of chronic genital infection in male patients with uveitis. A preliminary report. Br J Vener Dis. 1958;34:254–5. doi: 10.1136/sti.34.4.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keat AC, Maini RN, Nkwazi GC, Pegrum GD, Ridgway GL, Scott JT. Role of Chlamydia trachomatis and HLA-B27 in sexually acquired reactive arthritis. British Medical Journal. 1978;1:605–7. doi: 10.1136/bmj.1.6113.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rich E, Hook IEW, Alarcon GS, Moreland LW. Reactive arthritis in patients attending an urban sexually transmitted diseases clinic. Arthritis Rheum. 1996;39:1172–7. doi: 10.1002/art.1780390715. [DOI] [PubMed] [Google Scholar]
- 31.Carter JD, Rehman A, Guthrie JP, Gerard HC, Stanich J, Hudson AP. Attack rate of Chlamydia-induced reactive arthritis and effect of the CCR5-Delta-32 mutation: a prospective analysis. J Rheumatol. 2013;40:1578–82. doi: 10.3899/jrheum.130136. [DOI] [PubMed] [Google Scholar]
- 32.Thomson G, Thomson B, Inman R. Validation of a screening questionnaire for epidemic reactive arthritis (QUEST-2) Arthritis Rheum. 1993;36:S. [Google Scholar]
- 33.Dougados M, van der Linden S, Juhlin R, et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991;34:1218–27. doi: 10.1002/art.1780341003. [DOI] [PubMed] [Google Scholar]
- 34.Adams EJ, Charlett A, Edmunds WJ, Hughes G. Chlamydia trachomatis in the United Kingdom: a systematic review and analysis of prevalence studies. Sex Transm Infect. 2004;80:354–62. doi: 10.1136/sti.2003.005454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organisation. Global incidence and prevalence of selected curable sexually transmitted infections 2008. WHO; 2012. [Google Scholar]
- 36.Heymann DL, editor. Control of communicable diseases manual. 19th ed. Washington, DC: American Public Health Association; 2008. [Google Scholar]
- 37.Pease E, Pease B, Pease C. Do rheumatologists think about sex? Reumatol Clin. 2013;9:255. doi: 10.1016/j.reuma.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Collantes E, Zarco P, Munoz E, et al. Disease pattern of spondyloarthropathies in Spain: description of the first national registry (REGISPONSER) extended report. Rheumatology. 2007;46:1309–15. doi: 10.1093/rheumatology/kem084. [DOI] [PubMed] [Google Scholar]
- 39.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–9. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 40.Aggarwal R, Ringold S, Khanna D, et al. Distinctions between diagnostic and classification criteria? Arthritis Care Res (Hoboken) 2015;67:891–7. doi: 10.1002/acr.22583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amor B, Dougados M, Mijiyawa M. Criteria of the classification of spondylarthropathies. Rev Rhum Mal Osteoartic. 1990;57:85–9. [PubMed] [Google Scholar]
- 42.Rudwaleit M, van der Heijde D, Landewe R, et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. 2011;70:25–31. doi: 10.1136/ard.2010.133645. [DOI] [PubMed] [Google Scholar]
- 43.Zeidler H, Amor B. The Assessment in Spondyloarthritis International Society (ASAS) classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general: the spondyloarthritis concept in progress. Ann Rheum Dis. 2011;70:1–3. doi: 10.1136/ard.2010.135889. [DOI] [PubMed] [Google Scholar]
- 44.Zeidler H, Hudson AP. Causality of Chlamydiae in Arthritis and Spondyloarthritis: a Plea for Increased Translational Research. Curr Rheumatol Rep. 2016;18:9. doi: 10.1007/s11926-015-0559-3. [DOI] [PubMed] [Google Scholar]
- 45.Townes JM, Deodhar AA, Laine ES, et al. Reactive arthritis following culture-confirmed infections with bacterial enteric pathogens in Minnesota and Oregon: a population-based study. Ann Rheum Dis. 2008;67:1689–96. doi: 10.1136/ard.2007.083451. [DOI] [PubMed] [Google Scholar]