Abstract
Objective
To estimate the probability of complete clinical response and toxicity of paclitaxel as second-line chemotherapy in measurable disease patients with malignant tumors of the ovarian stroma, and to evaluate the value of inhibin for predicting response.
Methods
Thirty-one patients with histologically confirmed ovarian stromal tumor were enrolled from 2000 to 2013. Patients were required to have measurable recurrent disease, and to have received only one prior chemotherapy regimen. Paclitaxel 175 mg/m2 was administered over a 3 hour infusion, cycling every 21 days. Inhibin levels were drawn within two weeks of initiation of treatment.
Results
Of 31 women enrolled, there was only one complete response (3.2%), and partial response in eight of 31 cases (25.8%). The pretreatment inhibin level for the single patient who had complete response was 88 pg/mL. Median progression-free survival was 10.0 months and overall survival was 73.6 months. Myelosuppression was common with 12 of 31 patients (38.7%) suffering grade 3 or 4 neutropenia, leukopenia, or anemia.
Conclusion
There were too few complete responses to warrant continued evaluation of paclitaxel as a single agent treatment for women with recurrent malignant ovarian stromal tumors with measurable disease according to the primary objective of the study. Toxicity of the regimen was acceptable. Pretreatment inhibin is not a reliable tumor marker as it was not elevated in the majority of patients.
Keywords: Stromal tumor of ovary, Paclitaxel
1. Introduction
Malignant sex cord stromal cell tumors comprise 2–8% of all ovarian cancers [1]. Stromal tumors can be benign or malignant and express a variety of markers and hormones. Granulosa cell tumors are the most common malignant stromal tumor. The less common ovarian sex cord stromal tumors include Sertoli–Leydig types which may produce androgenic hormones and span benign to malignant phenotypes. Rare stromal tumors include granulosa cell–theca cell tumor, steroid cell tumor, gynandroblastoma, unclassified sex cord stromal tumors, and sex cord tumor with annular tubules.
Patients with stage I malignant stromal tumors have a favorable prognosis with surgical treatment alone, however, women with advanced and recurrent disease have a poor prognosis and are commonly treated with chemotherapy. Most studies have focused on the behavior of these tumors which present in early stage when adjuvant therapy is not needed. However, these tumors are on occasion indolent and late recurrences can occur.
Due to the rarity of these tumors, defining adjuvant and therapeutic regimens has been difficult. Studies have evaluated agents such as 5-fluorouracil, vincristine, dactinomycin, and cyclophosphamide. None of these agents attained significant clinical response [2, 3]. Case reports and series have described varying degrees of success with combinations of cisplatin, doxorubicin, cyclophosphamide, and altretamide [4–7]. In 1996, Gershenson et al. proposed treatment with a combination of bleomycin, cisplatin, and etoposide (BEP) [8]. In that study of nine patients, of the six with measurable disease there was a 33% complete response and 50% partial response observed. In 1999, a Gynecologic Oncology Group (GOG) study by Homesley et al. evaluated this similar regimen with 16 women with primary disease and 41 with recurrent disease, and confirmed activity of this combination. The end point used for response was second-look laparotomy. Of patients undergoing second-look laparotomy, 37% (14/38) had negative findings [9]. As a result of this study, BEP became the standard of care for treatment of stromal cell tumors. A few clinical trials have studied the more rare androgen-based sex cord stromal tumors and none resulted in clear clinical recommendation for treatment. Consequently, androgenbased sex cord stromal tumors have been combined with granulosa cell tumors for the purpose of study and treatment.
Paclitaxel, originally derived from the Pacific Yew tree, belongs to a class of drugs whose prime mechanism of action is the disruption of microtubule activity and thus is considered a mitotic inhibitor. In the pivotal study by McGuire et al., a taxane/platin regimen emerged as the standard of care for treatment of epithelial ovarian cancer [10]. GOG-187 was designed to evaluate the clinical response and toxicity of single agent paclitaxel as second-line therapy in patients with measurable recurrent malignant stromal tumor and to evaluate the value of inhibin A and inhibin B as predictors of tumor response.
2. Materials and methods
Women diagnosed with histologically confirmed ovarian stromal tumor were eligible for enrollment. Initially, patients with any measurable disease were enrolled including those receiving first-line therapy. The protocol was later amended, requiring patients to have recurrent disease and to have received only one previous chemotherapy regimen. Measurable disease was required as defined by GOG Response Evaluation Criteria in Solid Tumors (RECIST) criteria [11]. Stromal histology included: granulosa cell tumor, granulosa cell-theca cell tumor, Sertoli–Leydig cell tumor, steroid cell tumor, gynandroblastoma, unclassified sex cord stromal tumors, and sex cord tumor with annular tubules. Patients were required to have GOG performance status of 0, 1, or 2. Histologic confirmation was performed by central review by the GOG Pathology Committee. Adequate bone marrow function was required as defined by: absolute neutrophil counts (ANC) greater than or equal to 1500/l, platelet count greater than 100,000/l, hepatic function (bilirubin ≤ 1.5 times institutional upper limit of normal), and SGOT and alkaline phosphatase ≤ 2.5 times institutional upper limit of normal.
The GOG protocol and consent were approved by each participating institution. The protocol was reviewed annually by the Institutional Review Board. Patients were enrolled after obtaining informed consent. Each patient had been treated with first-line therapy as chosen by her physician. Patients with persistent/stable disease or progression were eligible for trial entry. Serum inhibin A and inhibin B were drawn to monitor response to therapy. Paclitaxel at 175 mg/m2 was administered over a 3 hour infusion, cycling every 21 days until progression or adverse events prohibited additional treatment. When a patient had a complete response, two additional courses of paclitaxel were administered. Maximum body surface area calculated for dosing was 2.0 m2 as per GOG Chemotherapy procedures manual. CT of the chest, abdomen and pelvis was performed within 28 days of initiation of treatment and repeated every other cycle. If disease was followed by physical exam, measurements were repeated every cycle while on therapy. Inhibin levels were drawn within two weeks of initiation of treatment.
Patients received paclitaxel administered at 175 mg/m2 IV over 3 h on day one of a 21-day cycle. Pre-medication for paclitaxel infusion was at the discretion of the investigator, however, oral/IV dexamethasone, IV diphenhydramine, and IV H2 blockers were recommended.
Patients continued receiving treatment if white blood cell count was N 3000/mcl and platelet counts were ≥ 100,000/mcl. Modification was made for patients with febrile neutropenia or sepsis. For these patients, a dose reduction of one level, to a dose of 135 mg/m2, was initiated. Use of G-CSF was allowed at the discretion of the investigator. A delay of greater than two weeks resulted in a dose reduction to 135 mg/m2. Patients who were delayed more than three weeks were removed from study. Re-escalation was allowed if a dose reduction was initiated due to low white count or platelet count in a previous cycle or for patients who experienced Grade 0/1 hematologic toxicity without other organ toxicity. In these cases, dosing was escalated by one level for each subsequent course until maximum dosing was reached. Grade 3 or grade 4 GI toxicity required a two dose level reduction, to a dose of 110 mg/m2, if the toxicities were due to mucositis or diarrhea. Any other grade 4 GI toxicity was accompanied by a dose reduction to 135 mg/m2. In regard to hepatic toxicity, grade 3 or grade 4 hepatic toxicity required one dose level reduction in subsequent courses. For renal toxicity of grade 2 or greater, not clearly due to obstructive uropathy, a dose reduction to 135 mg/m2 was performed.
The primary measure of treatment efficacy in this study is complete clinical response. This study implemented an optimal and flexible two-stage accrual design [12]. The number of individuals enrolled during each stage of the study was allowed to vary in order to account for the complexities of managing a multi-institutional study or the potential for enrolling patients who could eventually be deemed ineligible following centralized review. During the first stage of accrual between 15 and 22 patients were to be enrolled, and at least one complete response out of the 15–16 enrolled or two complete responses out of the 17–22 enrolled was required in order to advance to the second stage of accrual. If the second stage of accrual was warranted, then enrollment would continue until a total of 30–37 eligible patients were enrolled onto the study. If there were more than two complete responses out of 30–31 enrolled or three complete responses out of 32–37, then the regimen would be deemed sufficiently active to warrant further investigation. This design provides an average of 90% power for detecting a treatment that has a true 20% complete response probability, when the type I error is set to 10% for a one-sided test of the null hypothesis that the true complete response rate is no more than 5%. These type I and II errors are averaged over all allowable enrollment configurations [12].
3. Results
3.1. Patient characteristics
During the first stage of accrual 15 patients were enrolled from 2000 to 2004 and one complete responder was reported in this cohort. Therefore, a second stage of accrual was initiated during which 16 additional patients were enrolled from 2005 to 2012. In total, 31 patients, all with recurrent disease, were enrolled in this study. Patient characteristics are summarized in Table 1. The majority of patients were non-Hispanic white (18/31). The majority had a performance status of 0 (25/31). Three patients had been treated with prior radiation, and one patient had had prior immunotherapy. Twenty-four patients had received prior chemotherapy, and three patients had had previous hormone therapy. Pretreatment inhibin levels were available for 30 of 31 patients, and they ranged from 1.8 to 1062 pg/mL with the majority (12/30, 40%) having initial levels of ≤ 10 pg/mL. Twelve of 30 (40.0%) patients with measured inhibin levels had pretreatment levels of ≤ 10 pg/mL. Ten of 23 (33.3%) had a value in the range of 11–100 pg/mL. Eight patients (26.7%) had a pretreatment inhibin value of N 100 pg/mL. The pretreatment inhibin level for the single patient who experienced a complete response was 88 pg/mL. Over half of the patients enrolled received greater than five cycles of treatment. The range of treatment cycles was 1–46.
Table 1.
Patient characteristics.
| Characteristics | No. of cases | % of cases |
|---|---|---|
| Age | ||
| <40 | 7 | 22.6 |
| 40–49 | 8 | 25.8 |
| 50–59 | 9 | 29.0 |
| ≥60 | 7 | 22.6 |
| Race | ||
| Non-Hispanic Black | 7 | 22.6 |
| Non-Hispanic White | 18 | 58.1 |
| Hispanic | 2 | 6.4 |
| Asian/Pacific Islander | 4 | 12.9 |
| Disease status | ||
| Previously untreated | 0 | 0.0 |
| Recurrent disease | 31 | 100.0 |
| Performance status | ||
| 0 | 25 | 80.6 |
| 1 | 5 | 16.1 |
| 2 | 1 | 3.2 |
| Prior radiotherapy | ||
| Yes | 3 | 9.7 |
| No | 28 | 90.3 |
| Prior immunotherapy | ||
| Yes | 1 | 3.2 |
| No | 30 | 96.8 |
| Prior chemotherapy | ||
| Yes | 24 | 77.4 |
| No | 7 | 22.6 |
| Prior hormone therapy | ||
| Yes | 3 | 9.7 |
| No | 28 | 90.3 |
| Pretreatment Inhibin (pg/mL) | ||
| ≤10 | 12 | 38.7 |
| 11–100 | 10 | 32.2 |
| >100 | 1 | 3.2 |
3.2. Patient response
There was one complete response (3.2%, 80% CI: [0.74%–15.3%]) and 8 (25.8%) partial responses. Fifteen (48.4%) patients had stable disease while six (19.3%) patients progressed on treatment. One patient was indeterminate. At the time of analysis, 27 of 31 (87.1%) had progressed. Sixteen (51.6%) were dead of disease and 11 (35.5%) were alive with progression. Only four (12.9%) were alive without progression of disease. The median duration of follow-up among those who were alive at last contact was 5.6 years. Median progression-free survival (PFS) was 10.0 months and overall survival was 73.6 months. Treatment response is shown in Table 2, and survival curves are shown in Fig. 1.
Table 2.
Clinical response.
| Characteristics | No. of cases | % of cases |
|---|---|---|
| Response | ||
| Complete response | 1 | 3.2 |
| Partial response | 8 | 25.8 |
| Stable disease | 15 | 48.4 |
| Increasing disease | 6 | 19.3 |
| Indeterminate | 1 | 3.2 |
Fig. 1.
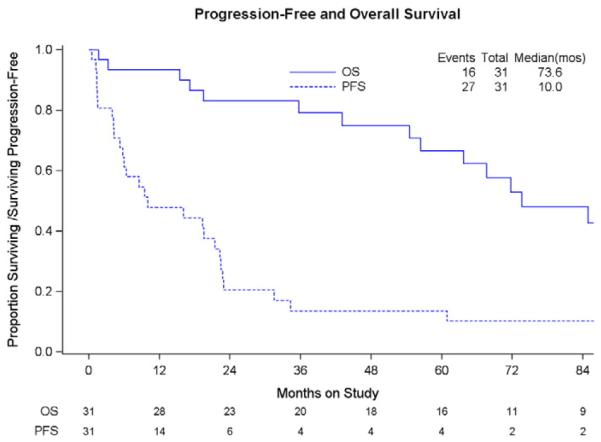
Median duration of follow-up for patients who were alive at last contact is 5.6 years.
3.3. Toxicity
Adverse events were classified and graded based on Common Toxicity Criteria version 2.0. Myelosuppression was common, with 12 of the 31 patients (38.7%) experiencing at least one instance of grade 3 or grade 4 neutropenia (11 individuals), leukopenia (2 individuals), or anemia (1 individual). Grade 2 dermatologic adverse events were reported in 19/31 (61.3%). There were no deaths attributed to the study treatment. Overall, the treatment was well-tolerated.
4. Discussion
In our study of 31 patients only one had a complete response. According to the primary objective of the study, our data do not justify continuing to evaluate single agent paclitaxel administered every three weeks for women with recurrent measurable malignant stromal tumor of the ovary. Alternatively, paclitaxel may be evaluated with a different administration schedule. We delivered paclitaxel on day one every three weeks at 175 mg/m2. In epithelial ovarian cancer, the Japanese GOG demonstrated that weekly (dose-dense) paclitaxel improved median PFS and 3 year survival, with a continuation of this trend at a median follow-up of 6.4 years [13,14]. Weekly paclitaxel may have an anti-angiogenic effect in epithelial ovarian cancer. A similar anti-angiogenic effect due to dose-dense paclitaxel may also occur in stromal cell tumors of the ovary. A recent GOG phase 2 trial demonstrated the efficacy and safety of bevacizumab in recurrent sex cord stromal ovarian tumors with a 16.7% partial response rate and 77.8% patients with stable disease [15]. In this GOG phase 2 trial, patients were allowed unlimited prior therapy with the exception of bevacizumab. Thirty-three of 36 patients (91.7%) had received prior chemotherapy with a median of two prior regimens. 6 patients had had prior radiation therapy. This trial concluded that bevacizumab had activity in recurrent sex cord stromal tumors. It may be reasonable for future trials to include a dose-dense approach to paclitaxel administration in an effort to mimic the anti-tumorigenic effects of biologics such as bevacizumab, but with a different toxicity profile.
Brown et al. compared BEP to taxane-based therapy. In this retrospective study the authors found that while patients treated with BEP had a higher response rate than those treated with taxanes, the difference was not statistically significant. However, there was a better toxicity profile for the group who received taxane as a single agent. Additionally, they suggested that the taxane group may have had a more durable response. However, when the taxane arm excluded those patients that received platinum in combination, the response rate was considerably lower (18% versus 60%) [16]. This retrospective study evaluated patients who received platinum combination therapy with those who received single agent taxane therapy. At the same time, our prospective trial was underway to evaluate single agent paclitaxel without the addition of a platinum agent.
The prognostic factors associated with survival in stromal tumors of the ovary range widely, from tumor size to stage. Prior studies generally included only small numbers of patients, and therefore there is much variation when drawing conclusions about prognostic factors.
Inhibin A and inhibin B as well as anti-Mullerian hormone may be effective tumor markers for recurrence or progression of disease in sex cord stromal tumors of the ovary [17,18]. Pretreatment inhibin levels were available in 30 patients. Twelve of 30 patients had pretreatment inhibin levels at ≤ 10 pg/mL. Thus, inhibin may not always be an effective tumor marker particularly if it not elevated at the time of recurrence. As there is no prospective trial of stromal tumors that report inhibin levels, future trials should investigate inhibin A and inhibin B levels prior to treatment and the correlation of levels with response to treatment.
Suri et al. found that diabetes had an associated increased risk of recurrent ovarian granulosa cell tumor with a hazard ratio of 3.19 [19]. In their study, 20% of the population had diabetes, so the data may not have been applicable to a larger, more diverse population. Metformin, an anti-diabetic medication from the biguanide class, is at the center of exciting and relatively new investigation. Recent epidemiologic evidence suggests that metformin use lowers cancer risk and reduces cancer deaths among diabetic patients. [20–22]. Metformin has an anti-tumorigenic which effect may be due to the reduction of circulating insulin levels, or a direct action in the inhibition of the mTOR pathway. The GOG is currently studying patients with advanced or recurrent endometrial cancer. Many stromal tumors of the ovary are similarly hormone-driven, therefore, it is possible that metformin has a role in the adjuvant treatment of this rare tumor.
A variety of novel biologic agents are currently being evaluated for activity in stromal cell tumors. Enzatulamide is a new generation of anti-androgenic agent approved for prostate cancer [23]. Enzatulamide's target is steroidogenesis by way of CYP17 inhibition. Total ablation of androgen production by potent CYP17 inhibition is the goal of enzatulamide and many other agents [24]. Androgen receptors are abundant in granulosa cell tumors. When the FOXL2 gene is mutated, it causes increased CYP17 expression and subsequent increase in androgen synthesis. As the mutated FOXL2 gene results in an increased CYP17 expression, it follows that inhibition of this enzyme may reverse the effect of the FOXL2 gene mutation. Additional inhibition of CYP17 may be achieved by other novel agents such as abiraterone, also used in prostate cancer, as well as ketoconazole, which has been used in granulosa cell ovarian tumors [24,25]. Bevacizumab and other antiangiogenesis agents such as cediranib are also being studied in recurrent stromal tumors of the ovary [26].
As the armamentarium of active agents against advanced or recurrent stromal ovary tumors increases, cytotoxic agents remain among the most active. While single agent paclitaxel did not produce a robust response in the patients enrolled in this trial, there may be a role for dose-dense paclitaxel or the combination of paclitaxel and novel biologic agents in the treatment of advanced or recurrent stromal tumor of the ovary. We reaffirm that the toxicity of paclitaxel at the dose and regimen described is acceptable. In the case of malignant stromal ovarian tumors, rarity coupled with the indolent nature and overall good prognosis in early stage, makes prospective studies difficult. It took 13 years to accrue 31 patients with measurable recurrent disease. We must continue to work within the constraints that are inherent in studying the rare and indolent tumor that is stromal tumor of the ovary. Current clinical trials are decreasing the length of time for accrual by fostering international relationships and scientific collaboration [27]. Among other methods, international collaboration will allow more rapid study of rare tumors which are inherently difficult to study.
HIGHLIGHTS.
Paclitaxel has activity in recurrent, malignant stromal tumor of the ovary.
There was a complete response in 3.2% and partial response in 25.8% of women.
• Inhibin is not a reliable tumor marker.
Footnotes
Conflicts of interest
Dr. Mark Brady reports that he receives funding from the NCI (CA 27469, CA 37517, 1-U10 CA180822, 1-U10 CA180868). All co-authors have no conflicts of interest to declare.
This study was supported by the National Cancer Institute grants to Gynecologic Oncology Group Administrative Office (CA 27469), Gynecologic Oncology Group Statistical Office (CA 37517), NRG Oncology (1U10 CA180822) and NRG Operations (1U10 CA180868). The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: University of Alabama, Abington Memorial Hospital, University of Mississippi Medical Center, University of California at Los Angeles, University of North Carolina, Wake Forest University, Rush University, University of Kentucky, Cleveland Clinic Foundation, University of Oklahoma, University of Virginia, Moffitt Cancer Center, and Saitama Medical University.
References
- [1].Ayhan A, Tuncer ZS, Tuncer R, Mercan R, Yuce K, Ayhan A. Granulosa cell tumor of the ovary. A clinicopathological evaluation of 60 cases. Eur. J. Gynaecol. Oncol. 1994;15:320–324. [PubMed] [Google Scholar]
- [2].Schwartz PE, Smith JP. Treatment of ovarian stromal tumors. Obstet. Gynecol. 1976;125:402–411. doi: 10.1016/0002-9378(76)90577-9. [DOI] [PubMed] [Google Scholar]
- [3].Tavassoli FA, Norris HJ. Sertoli tumors of the ovary: a clinicopathologic study of 28 cases with ultrastructural observations. Cancer. 1980;46:2281–2297. doi: 10.1002/1097-0142(19801115)46:10<2281::aid-cncr2820461028>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- [4].Jacobs AJ, Deppe G, Cohen CJ. Combination chemotherapy of ovarian granulosa cell tumor with cisplatin and doxorubicin. Gynecol. Oncol. 1982;14:294–297. doi: 10.1016/0090-8258(82)90102-0. [DOI] [PubMed] [Google Scholar]
- [5].Camlibel FT, Caputo TA. Chemotherapy of granulosa cell tumors. Am. J. Obstet. Gynecol. 1983;145:763–765. doi: 10.1016/0002-9378(83)90589-6. [DOI] [PubMed] [Google Scholar]
- [6].Neville AJ, Gilchrist KW, Davis TE. The chemotherapy of granulosa cell tumors of the ovary: experience of the Wisconsin clinical cancer center. Med. Pediatr. Oncol. 1984;12:397–400. doi: 10.1002/mpo.2950120608. [DOI] [PubMed] [Google Scholar]
- [7].Kaye ST, Davies E. Cyclophosphamide, Adriamycin, and cis-platinum for the treatment of advanced granulosa cell tumor, using serum estradiol as a tumor marker. Gynecol. Oncol. 1986;24:261–264. doi: 10.1016/0090-8258(86)90035-1. [DOI] [PubMed] [Google Scholar]
- [8].Gershenson DM, Morris M, Burke TW, Levenback C, Matthew CM, Wharton JT. Treatment of poor-prognosis sex cord-stromal tumors of the ovary with the combination of bleomycin, etoposide, and cisplatin. Obstet. Gynecol. 1996;87:527–531. doi: 10.1016/0029-7844(95)00491-2. [DOI] [PubMed] [Google Scholar]
- [9].Homesley HD, Bundy BN, Hurteau JA, Roth LM. Bleomycin, etoposide, and cisplatin combination therapy of ovarian granulosa cell tumors and other stromal malignancies: a Gynecologic Oncology Group Study. Gynecol. Oncol. 1999;72:131–137. doi: 10.1006/gyno.1998.5304. [DOI] [PubMed] [Google Scholar]
- [10].McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N. Engl. J. Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- [11].Rustin GJS, Vergote I, Eisenhauer E, Pujade-Lauraine E, Quinn M, Thigpen T, et al. Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA125 agreed by the Gynecological Cancer Intergroup (GCIG) Int. J. Gynecol. Cancer. 2011;21:419–423. doi: 10.1097/IGC.0b013e3182070f17. [DOI] [PubMed] [Google Scholar]
- [12].Chen TT, Ng T. Optimal flexible designs in phase II clinical trials. Stat. Med. 1998;17:2301–2312. doi: 10.1002/(sici)1097-0258(19981030)17:20<2301::aid-sim927>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- [13].Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomized controlled trial. Lancet. 2009;374:1331–1338. doi: 10.1016/S0140-6736(09)61157-0. [DOI] [PubMed] [Google Scholar]
- [14].Katsumata N, Yasuda M, Isonishi S, Takahashi F, Michimae H, Kimura E, et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomized, controlled, openlabel trial. Lancet Oncol. 2013 Sep;14(10):1020–1026. doi: 10.1016/S1470-2045(13)70363-2. [DOI] [PubMed] [Google Scholar]
- [15].Brown J, Brady WE, Schink J, Van Le L, Leitao M, Yamada SD, et al. Efficacy and safety of bevacizumab in recurrent sex cord-stromal ovarian tumors: results of a phase 2 trial of the Gynecologic Oncology Group. Cancer. 2014;120:344–351. doi: 10.1002/cncr.28421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Brown J, Shvartsman HS, Deavers MT, Ramondetta LM, Burke TW, Munsell MF, et al. The activity of taxanes compared with bleomycin, etoposide, and cisplatin in the treatment of sex cord-stromal ovarian tumors. Gynecol. Oncol. 2005;97:489–496. doi: 10.1016/j.ygyno.2005.01.011. [DOI] [PubMed] [Google Scholar]
- [17].Lyubimova NV, Beyshembaev AM, Kushlinskiy DN, et al. Granulosa cell tumors of the ovary and inhibin B. Bull. Exp. Biol. Med. 2011;150:635–638. doi: 10.1007/s10517-011-1209-z. [DOI] [PubMed] [Google Scholar]
- [18].Geerts I, Vergote I, Neven P, et al. The role of inhibins B and antimullerian hormone for diagnosis and follow-up of granulosa cell tumors. Int. J. Gynecol. Cancer. 2009;19:847–855. doi: 10.1111/IGC.0b013e3181a702d1. [DOI] [PubMed] [Google Scholar]
- [19].Suri A, Carter EB, Horowitz N, Denslow S, Gehrig PA. Factors associated with an increased risk of recurrence in women with ovarian granulosa cell tumors. Gynecol. Oncol. 2013;131:321–324. doi: 10.1016/j.ygyno.2013.08.013. [DOI] [PubMed] [Google Scholar]
- [20].Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–258. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- [22].Libby G, Donnelly LA, Donnan PT, Alessi DR, Morri AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620–1625. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, et al. For the PREVAIL Investigators. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 2014;371:424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vasaitis TS, Bruno RB, Njar VCO. CYP17 inhibitors for prostate cancer therapy. J. Steroid Biochem. Mol. Biol. 2011;125:23–31. doi: 10.1016/j.jsbmb.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Garcia-Donas J, Hurtado A, Garcia-Casado Z, Albareda J, Lopex-Guerrero JA, Alemany I, et al. Cytochrome P17 inhibition with ketoconazole as treatment for advanced granulosa cell ovarian tumor. J. Clin. Oncol. 2013;31:e165–e166. doi: 10.1200/JCO.2012.45.0346. [DOI] [PubMed] [Google Scholar]
- [26].Sahebjam S, Bedard PL, Castonguay V, Chen Z, Reedijk M, Liu G, et al. A phase I study of the combination of ro4929097 and cediranib in patients with advanced solid tumours (PJC-004/NCI 8503) Br. J. Cancer. 2013;109:943–949. doi: 10.1038/bjc.2013.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ray-Coquard I, Brown J, Harter P, Provencher D, Fong P, Maenpaa J, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for ovarian sex cord stromal tumors. Int. J. Gynecol. Cancer. 2014;24(9):S42–S47. doi: 10.1097/IGC.0000000000000249. [DOI] [PubMed] [Google Scholar]


