Abstract
Objective
The goals of this study were to assess global trends in clinical implementation of Noninvasive Prenatal Testing (NIPT) as commercial tests are marketed increasingly worldwide, and to identify potential challenges for current or future use.
Methods
We surveyed clinicians from 46 countries about the availability of NIPT; their experiences with using NIPT; and their views on clinical, ethical, and legal issues affecting implementation in their countries.
Results
Forty-nine respondents from 28 countries completed the survey. The majority reported that NIPT is available in their country (n=43) and that they offer NIPT in their current practice (n=38). Eighteen respondents from 14 countries reported that there are plans to introduce NIPT into routine antenatal care in their country. Test prices varied widely, ranging from $350–$2900, and several respondents observed that high test prices limited or restricted widespread use of NIPT. Responses varied both across and within countries regarding who is offered NIPT, and what the overall screening protocol should be.
Conclusion
This study provides a snapshot of current use and experiences with NIPT globally. It also highlights differences in service provision that exists both across and within countries, emphasizing the need for developing national and international implementation guidelines for NIPT.
INTRODUCTION
Non-invasive prenatal genetic testing (NIPT) using cell-free fetal DNA (cffDNA) circulating in maternal blood has opened the door to early, accurate, and safe prenatal testing. Traditionally, women identified as high risk following aneuploidy screening require invasive testing for definitive diagnosis, either through chorionic villus sampling (CVS) or amniocentesis conducted around 12–15 weeks gestation. However, these tests carry a small but significant risk of miscarriage.1 The difficulty of decision-making around invasive testing in which a woman has to weigh the desire for a diagnosis against the potential for procedure-related pregnancy loss has been well documented.2–5 NIPT is an attractive option for women because it is procedurally safe for both mother and fetus, it can be conducted as early as ten weeks gestation, and is highly accurate (99% sensitivity and 99.5% specificity for Down syndrome).6 However, the small false positive rate means that NIPT should not be considered diagnostic and invasive testing is recommended to confirm positive NIPT results.7,8 Nevertheless, because of its high sensitivity and positive predictive value relative to serum screening, NIPT has been viewed as a positive advancement in prenatal care by both patients and healthcare professionals,3,4,9–11 as it reduces the overall need for invasive testing.
The widespread availability of NIPT for aneuploidy has been driven by commercialization. NIPT was initially launched through commercial providers in the United States and China/Hong Kong in 2011. It quickly moved into markets in Western Europe, the Middle East, South America, Asia, and Africa, with samples from these countries being sent to the United States or China/Hong Kong for testing.12 Early studies show that uptake in developed countries is high and has significantly reduced the number of invasive tests performed.13–15 Although NIPT is currently available through private providers, it is not yet widely available in publicly funded antenatal services outside of the context of research studies.16–18 Given the rapid global dissemination of NIPT, we conducted a survey to examine the use of NIPT around the world and to get a glimpse of clinical implementation across both public and private sectors in developed and developing countries. This information will be useful to understand global trends in prenatal care, to identify similarities and differences in the use of NIPT, and to reveal current and anticipated challenges for effective clinical implementation of NIPT worldwide.
METHODS
Sample and recruitment
We employed two recruitment strategies for this global survey. Our first approach was to survey colleagues of the authors (CL, SC) or the author’s colleagues who are leaders and experts in the provision of prenatal genetics services in different countries. We believed this “convenience” sampling approach would provide a well-informed overview of NIPT use in various countries by targeting those individuals that we know are actually using NIPT in clinical practice. Moreover, we hoped our survey response rate would be higher by recruiting colleagues who are personal contacts. With this approach we emailed colleagues in 32 different countries (Table 1).
Table 1.
Countries represented in global survey of NIPT use
| Country name | Recruitment method | # invited | # participated |
|---|---|---|---|
| Argentina | Colleague | 1 | 1 |
| Australia | Colleague | 6 | 3 |
| Austria | Colleague | 1 | 0 |
| Belgium | Colleague | 1 | 1 |
| Brazil | Colleague | 1 | 0 |
| Burkina Faso | FIGO society | 1 | 0 |
| Cameroon | FIGO society | 1 | 0 |
| Canada | Colleague | 3 | 2 |
| Chile | Colleague | 2 | 1 |
| Cote d’Ivoire | FIGO society | 1 | 0 |
| Cyprus | Colleague | 1 | 1 |
| Czech Republic | Colleague | 1 | 4 |
| Denmark | Colleague | 2 | 0 |
| Egypt | FIGO society | 1 | 0 |
| Finland | Colleague | 1 | 1 |
| France | Colleague | 1 | 1 |
| Germany | Colleague | 1 | 2 |
| Greece | Colleague | 1 | 1 |
| Hong Kong (China) | Colleague | 1 | 5 |
| Hungary | Colleague | 1 | 1 |
| Iceland | Colleague | 1 | 0 |
| India | Colleague, FIGO society | 6 | 3 |
| Iran | FIGO society | 1 | 0 |
| Ireland | Colleague | 1 | 1 |
| Israel | Colleague | 1 | 2 |
| Italy | Colleague | 3 | 1 |
| Jordan | FIGO society | 1 | 0 |
| Lebanon | Colleague | 1 | 0 |
| Libya | FIGO society | 1 | 0 |
| Malaysia | FIGO society | 1 | 0 |
| Mexico | FIGO society | 1 | 1 |
| Netherlands | Colleague | 2 | 2 |
| Norway | Colleague | 2 | 1 |
| Portugal | Colleague | 3 | 2 |
| Qatar | Colleague | 1 | 1 |
| Saudi Arabia | FIGO society | 1 | 0 |
| Senegal | FIGO society | 1 | 0 |
| Singapore | Colleague | 2 | 1 |
| South Africa | FIGO society | 1 | 0 |
| Spain | Colleague | 1 | 0 |
| Sweden | Colleague | 2 | 1 |
| Switzerland | Colleague | 1 | 1 |
| Tunisia | FIGO society | 1 | 0 |
| Turkey | Colleague | 1 | 1 |
| United Arab Emirates | FIGO society | 1 | 0 |
| United Kingdom | Colleague | 3 | 4 |
| United States | Colleague | 9 | 3 |
|
| |||
| TOTAL | 78 | 49 | |
List of countries included in global survey of NIPT use. Two recruitment methods were used to invite participants to take the survey, direct email invitations to colleagues of the study investigators (LC, SC) (‘colleague’), and email invitations to member societies of the International Federation of Gynecology and Obstetrics (FIGO) (http://www.figo.org/our-members) in which NIPT is marketed12 (‘FIGO society’). Note that there may be more participants in a country than the number who were invited because some participants appear to have forwarded the survey to other colleagues in their country.
In our second approach, designed to expand the reach of our survey, we cross-referenced a list of countries in which NIPT is being marketed12 with the list of member societies of the International Federation of Gynecology and Obstetrics (FIGO) (http://www.figo.org/our-members) that had provided a contact email address, thus identifying 14 additional countries that were eligible for our study. We sent email invitations to these 14 FIGO society members (Table 1). In total, we emailed 78 people from 46 different countries to participate in our study (Table 1).
Survey development and data collection
Our survey was developed to examine three domains of interest: 1) current practice regarding chromosomal aneuploidy screening, 2) current practice regarding invasive diagnostic testing, and 3) current and future practice regarding NIPT. The survey underwent iterative rounds of discussion between all authors until all questions and response options were finalized.
All participants were sent a brief email explaining the study with a link to the survey that was administered with Qualtrics online survey software (Qualtrics, LLC, Provo, UT, USA). The survey was open to participants for a six-week period, from mid-December 2014 to early February 2015, and a reminder email was sent to participants about halfway through the survey period. The 34-question survey (see Supplemental File) consisted of multiple choice and open-ended questions. Respondents were free to skip any questions they did not wish to answer. The survey questions were designed to collect demographic and medical practice information about respondents; information about the availability of prenatal screening and diagnostic tests for chromosomal aneuploidies, pre- and post-test counseling, and termination of pregnancy for chromosomal aneuploidies; and collect details about participants’ clinical experience with NIPT (if applicable). We used skip logic so respondents only answered questions that were applicable to them (e.g., if they did not offer NIPT in their practice, they were not asked follow-up questions about which test(s) they used). Data were analyzed anonymously. The Duke University Institutional Review Board approved this study.
Data analysis
All partially completed surveys (n=1) were excluded from our analysis. For countries where multiple respondents took the survey (n=11 countries), we opted to include all responses obtained from these countries in our analysis. We did so to capture any regional differences in clinical practice or laws/policies that might exist within a country; however, as a result, these countries skew the data more heavily than countries with only one respondent.
Descriptive analysis of the survey data was performed using Qualtrics. Responses to multiple-choice questions are summarized using frequency distributions. Sample sizes vary by question due to the use of skip logic and participants skipping questions. Additionally, several questions gave respondents the ability to select all applicable response options, so frequency percentages for these questions is greater than 100%. Qualitative data collected from open-ended questions about opinions and experiences from respondents and from questions in which participants selected “other” and were given space to provide an answer were analyzed thematically. While we present only illustrative samples or summaries of respondent comments in this paper, we have made sure to present all points of view that were expressed by participants in their responses to open-ended questions.
RESULTS
Survey respondents
Of the 78 individuals who were invited to take our survey, 49 completed it (63% response rate). These 49 individuals came from 28 different countries (Table 1). We had nine respondents from Asia, three from Australia, 23 from Europe, four from the Middle East, six from North America, and four from South America. While the majority of respondents were from high-income countries, we also received responses from four middle-income countries (Argentina, Hungary, India, and Turkey). We noticed that some participants had forwarded the survey to other colleagues in their country because we received more responses per country than originally intended (e.g., Hong Kong, the Czech Republic); we included these extra responses in our analysis.
Table 2 shows the demographic characteristics of respondents. About 70% of respondents were male (n=34) and 30% were female (n=14). On average, respondents had been practicing medicine for 25.7 years (range: 10 – 42 years). The majority of respondents held PhD and/or MD degrees (PhD: n=4, MD: n=12, MD and PhD: n=18). Over half were maternal-fetal medicine specialists (n=27), and almost half practiced medicine in a public hospital (n=24). Respondents reported offering a range of prenatal screening and diagnostic tests in their own medical practices, including NIPT.
Table 2.
Demographic characteristics of survey respondents
| Number | % | |
|---|---|---|
| Sex | ||
| Male | 34 | 69% |
| Female | 15 | 31% |
| Years practicing medicine | ||
| Range | 10–42 | |
| Mean (standard deviation) | 25.7 (9.2) | |
| Highest level of education | ||
| University degree | 8 | 16% |
| Master’s degree | 0 | |
| MD | 12 | 24% |
| PhD | 4 | 8% |
| MD and PhD | 18 | 37% |
| Other | 7 | 14% |
| Specialty area | ||
| General OB/GYN | 10 | 20% |
| Maternal-fetal medicine | 27 | 55% |
| Fetal medicine | 16 | 33% |
| Prenatal genetics | 16 | 33% |
| Obstetric ultrasound | 11 | 22% |
| Other | 7 | 14% |
| Type of medical practice | ||
| Public hospital | 24 | 49% |
| Private hospital | 7 | 14% |
| Private office or practice | 10 | 20% |
| Academic medical center | 24 | 49% |
| Other | 3 | 6% |
| Prenatal tests offered by respondents | ||
| Ultrasound | 43 | 88% |
| RhD testing | 34 | 70% |
| 1st trimester screening (ultrasound) | 28 | 58% |
| 1st trimester screening (ultrasound + biochemistry) | 42 | 86% |
| 2nd semester screening | 28 | 56% |
| Chromosomal microarray | 33 | 68% |
| AFP screening | 24 | 48% |
| CVS | 44 | 90% |
| Amniocentesis | 44 | 90% |
| NIPT | 42 | 86% |
| Other | 8 | 16% |
| None of the above | 1 | 2% |
AFP, alpha-fetoprotein; CVS, chorionic villus sampling; NIPT, non-invasive prenatal testing. The sample size for each question is n=49.
Prenatal Screening
All 49 respondents reported that screening for chromosomal aneuploidies is available in their countries. The majority of respondents reported that it is offered to all women, regardless of age (n=38); followed by women at high risk for fetal chromosomal aneuploidy (n=12); women who request screening tests (n=9); women above a certain age, either 35 years (n=6) or 38 years (n=1); women who can afford screening tests (n=6); and “other” (n=3). The screening risk threshold that was most frequently used to classify a woman as “high risk” for the purposes of offering invasive diagnostic testing is >1:300 (n=12), followed by >1:150 (n=9), >1:250 (n=9), >1:200 (n=4), unsure (n=1), varies by provider (n=3), or “other” (n=11). Responses in the “other” category included 1:100 or 1:380 as additional thresholds used to define “high risk.”
In 35 respondents’ countries, chromosome aneuploidy screening is covered by a public health program; however, it is not universally covered in Argentina, Ireland, India, the Netherlands, Mexico, Qatar, and the United States. Respondents from Australia, the Czech Republic, Greece, and Turkey reported that screening is partly covered and coverage may vary by trimester or be accompanied by additional out-of-pocket expenses for patients. In the majority of respondents’ countries (n=46), counseling from a specialist healthcare provider, like a genetic counselor or midwife, is available for women who screen as high-risk. Respondents from two countries, Chile and Portugal, reported that counseling is not available (although the two respondents from Portugal had conflicting responses), and one respondent was unsure of the availability of counseling. Of the 46 respondents who said counseling is available, 37 said counseling is paid for by a publicly funded health program in their country. Respondents from India, Greece and Qatar reported that counseling was not covered, and there were conflicting responses from Hong Kong and the United States. Respondents from Argentina and Turkey identified “other” forms of payment (limited to certain regions, available through some public hospitals but most patients are managed in private practice).
Prenatal diagnosis
All 49 respondents reported that invasive diagnostic genetic tests, like amniocentesis or CVS, are available in their countries. When asked who is offered such testing, the majority of respondents stated that it is offered to women at high risk for fetal chromosomal aneuploidy (n=44). Other responses to this question included all women who request diagnostic tests (n=15); women of advanced maternal age, above either 35 years (n=10) or 38 years (n=1); women who can afford diagnostic tests (n=6); all women, regardless of age (n=5); or “other” (n=5), including for women with maternal anxiety, ultrasound anomalies, and family history. The majority of respondents reported that invasive diagnostic testing is covered by a publicly funded health program, either for all women (n=15) or for high-risk women (n=25). However, it is not covered in India or Mexico, it is partially covered in Argentina and Greece, and conflicting responses were noted from Hong Kong and the United States.
NIPT implementation
The majority of respondents (n=43) reported that NIPT is available in their country. One respondent was unsure (Finland), one was not familiar with NIPT (Mexico), two reported that NIPT was not available (Cyprus, Norway), and we got conflicting responses about availability of NIPT within the United Kingdom. Thus, a total of 44 respondents were eligible to answer follow-up questions regarding NIPT. When asked where NIPT is available, the majority of respondents (n=41) reported it was available through the private sector. Respondents from Belgium, the Netherlands, and Singapore reported public sector availability, while respondents from Australia, Canada, Israel, and the United States said that the availability is via public and private sector. The respondent from Sweden said that NIPT will be introduced in the public sector in spring 2015, and respondents from France, the Netherlands, and the United Kingdom reported that there are ongoing trials in the public sector. Respondents also provided several comments on implementation plans/approaches for their countries (Table 3).
Table 3.
Selected participant comments on current usage and possible implementation of NIPT for aneuploidy
| Comments on current usage | |
|
| |
| Australia: | “Currently, it is being offered by commercial companies and service providers. Most referrals are to ultrasound practices that will also take the blood after a viability ultrasound. The current charge is $500. They can be referred to such centers if they present to a public hospital, but it is not offered at the public hospital itself.” |
| Canada: | “It is funded in one province for high risk patients, and available in others on a patient-pay basis. Some provinces are trying to get government funding for high risk and others are waiting for the results of the Pegasus study.” |
| Netherlands: | “NIPT is now offered within a study, the Trident study. Currently Dutch laboratories (six) are doing it. It takes long before results are known. There have been some problems (human mistake causing exchange of samples).” |
|
| |
| Comments on implementation | |
|
| |
| Canada: | “The aggressive marketing of NIPT in Canada has led to fragmentation of existing prenatal screening programs, making systematic monitoring impossible. There is an urgent need to address this through either a voluntary or mandatory registry while decisions are being made about implementation models.” |
| India: | “It should be available in view of high detection rate BUT should be backed up by effective pretest and posttest counseling. These tests should not be available as direct to consumer tests. We should try to develop NIPT using other techniques/platforms for which kits can be purchased and the test is run in house.” |
| Israel: | “It is going to be a matter of cost.” |
| Portugal: | “I think people that make decisions are not even thinking or studying this subject.” |
| Qatar: | “It is very early stage and we are not sure what will happen.” |
| Singapore: | “The introduction is being managed in a stepwise manner by academia.” |
| Switzerland: | “MFM Swiss society is currently discussing NIPT issues with the government.” |
When asked about plans to introduce NIPT as part of routine antenatal care, six respondents from Australia, Canada, Greece, Hong Kong, Singapore, and the United States reported that NIPT was already a part of routine prenatal care. Respondents from fourteen countries (n=18) said there were plans to do so in their country; respondents from ten countries (n=11) reported there were no plans to introduce NIPT and from six countries (n=9) were unsure. We observed that additional participants from Australia, Canada, Hong Kong, Singapore and the United States varied in their responses about current availability of NIPT in routine care or future plans to do so. Respondents who were aware of plans to introduce NIPT into routine antenatal care reported that it is anticipated this year (the Czech Republic), in 1–2 years (Belgium and Greece), in 2–3 years (Italy and the United Kingdom), or in 1–5 years (Germany) respectively. Three respondents said they were uncertain about the timeline for introduction of NIPT into routine antenatal care programs (Israel, Sweden, and Switzerland). When asked how their country planned to deliver NIPT (n=33), seven said by ordering tests from existing commercial companies, 10 said through local diagnostic service providers, 13 said this hadn’t been decided yet, 5 didn’t know, and 6 indicated “other” approaches (e.g. combination of “home grown” tests and technology transfer from commercial companies).
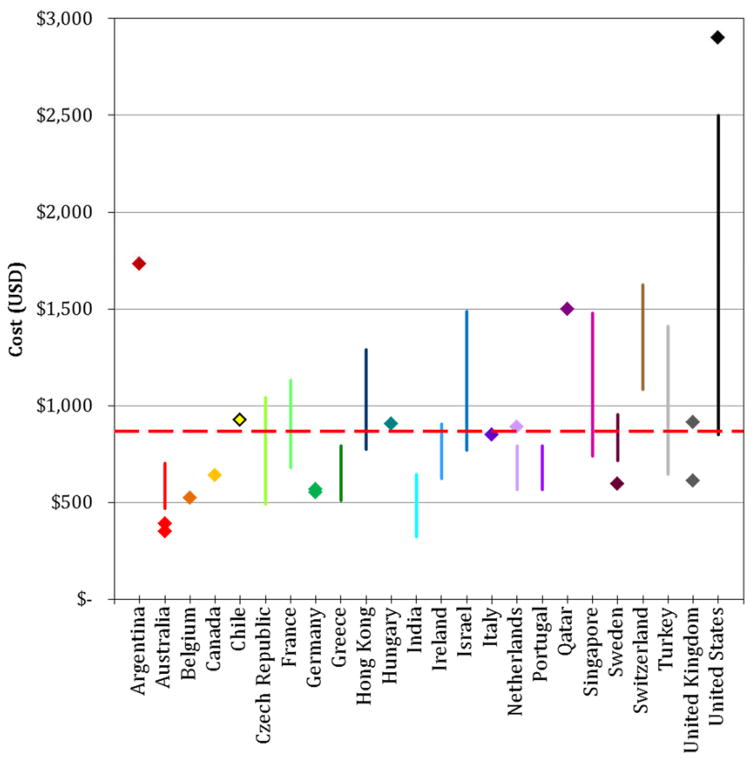
The cost of NIPT reported by respondents varies widely (Figure 1). Test prices provided by 41 respondents ranged from about $350 USD (Australia) to $2900 USD (United States). The average reported price of NIPT was about $874 USD.
Figure 1. Current prices of NIPT around the world.
All costs were provided by respondents in their local currency, and were converted into USD (US dollars) to facilitate comparison. Some respondents provided a range of prices (denoted by a vertical colored line) while others provided a single cost (denoted by a single diamond). For some countries, multiple respondents took the survey; as such, prices and ranges were combined. The average test price reported by respondents, approximately $874 USD, is denoted by a red horizontal dashed line. Countries are graphed from left to right in alphabetical order.
Clinical experience with NIPT
The majority of our respondents (n=38) currently offer NIPT in their practice; five do not, and one was unsure. Thirty nine respondents were therefore asked follow-up questions about their personal experiences with using NIPT in the clinic. When asked which patients they offer NIPT to, 14 said to high-risk women only, eight said to all pregnant women, four said only to women who ask for it, three said only to women who can pay for it, three said only to high-risk women who can pay for it, two said only to high-risk women who ask for it, and seven said “other.” The “other” responses included women who may be “intermediate risk” or are “concerned about invasive testing.” We noted variability in the responses to this question from nearly every country where multiple respondents took the survey: Australia, Canada, the Czech Republic, Hong Kong, India, the Netherlands, the United Kingdom, and the United States. While we did not ask questions about the volume of tests used by respondents in their practices, the respondent from Qatar reported: “I have done so far more than 200 cases in one year on a private basis.”
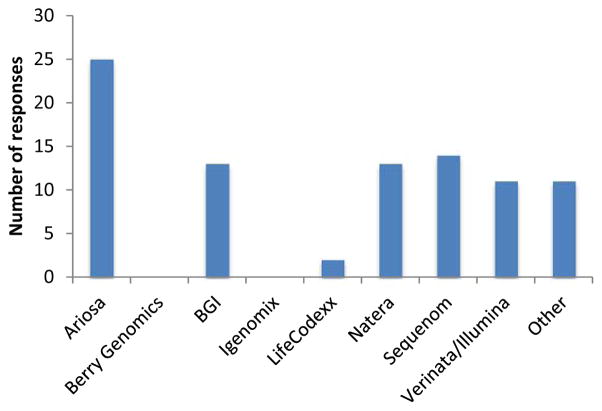
Respondents reported using a variety of test brands, with the most common being the Harmony test from Ariosa (Figure 2). “Other” tests mentioned by respondents included those offered by local laboratories. Several respondents reported that they did not select the particular test used by their patients, but rather that this decision was made either by the patients themselves or by referring to other providers who then make the decision to order a specific test.
Figure 2. Commercial NIPT providers used by respondents in their own medical practices.
Thirty nine respondents answered the question “Which of the following companies’ tests do you offer in your practice?” While respondents were provided with both the company and test name, only the company names are presented here. At the time of the survey, Berry Genomics had ceased all operations in China pending approval from the Chinese Food and Drug Administration.
Twenty-five respondents offer pre-test counseling with a specialist healthcare professional (such as a genetic counselor or midwife), while eight do not and one respondent was unsure. “Other” responses indicated that the patient’s primary care provider is responsible for this counseling or that the respondent is the individual who performs the counseling. In contrast, the vast majority of respondents (n=33) provide post-test counseling to women who receive a positive NIPT result. Only two respondents stated they do not offer post-test counseling and four reported “other” scenarios for post-test counseling that were similar to “other” scenarios described for pre-test counseling.
While respondents generally viewed NIPT positively, they also provided a number of comments when asked to elaborate on their experiences with using NIPT and for any additional thoughts regarding NIPT or prenatal testing/screening in their country. These comments covered a diverse range of topics around clinical implementation such as the cost of NIPT, reimbursement of and access to NIPT, cost effectiveness, policy and practice guidelines, screening protocols for NIPT, performance of tests, counseling, physician education, concerns related to commercialization, and legal and ethical issues (Table 4).
Table 4.
Selected respondent comments on experiences with and views about clinical implementation of NIPT in their countries
| COMMERCIALIZATION | |
|
| |
| Company Behavior | |
|
| |
| Italy: | “Too much business involved. Rewards to doctors prescribing NIPT is not an appropriate model but it is the current one.” |
| India: | “Most obstetricians are still not aware of the test and companies have commercialized the process by offering kickbacks for referring OBs and hence some do the test without understanding the implications or proper counseling.” |
| Hong Kong: | “NIPT can be offered below HKD 2000 and yet the provider (at least one of the Chinese providers) could already make a good profit from it. These companies and their intermediates (such as private doctors and hospitals) are making huge profit by offering the test at HKD 8000, which is not good for the general public.” |
| India: | “Several companies claim their technology is better than others. The best seller wins!!” |
|
| |
| Test Price/Cost | |
|
| |
| Argentina: | “The cost of the test makes it unaffordable for most people, and it is still limited to patients who can afford it.” |
| Israel: | “Still limited use due to high cost.” |
| Australia: | “Cost is the major barrier to uptake.” |
|
| |
| CLINICAL ISSUES | |
|
| |
| Time to return results | |
|
| |
| Australia: | “The current 2 week turn-around offered by most companies is a little concerning (they are generally shipped off-shore for analysis) but I believe in future, the NIPT analysis will be done within Australia.” |
| Greece: | “The shipping to USA is often problematic with delay of results.” |
| Netherlands: | “It takes too long for the result.” |
|
| |
| Test Performance | |
|
| |
| Hong Kong: | “In general, it is good. There is an example that it was wrongly applied to a fetus with thick nuchal >6mm. It turns out to be a false-negative case of Turner's syndrome.” |
| Greece: | “High percentage of false positive results for sex chromosomal abnormalities.” |
| Ireland: | “Low fetal fraction results cause anxiety.” |
|
| |
| Counseling | |
|
| |
| India: | “NIPT is effective after complete understanding of the test by the offering physicians, its advantages and drawbacks and most importantly if it is accompanied with good pretest and posttest counseling.” |
|
| |
| Screening protocol | |
|
| |
| Portugal: | “My opinion is that NIPT should replace screening + invasive testing for aneuploidies in the near future - 2 years?” |
| Canada: | “Ideally will be introduced in a model that preserves beneficial aspects of existing screening programs, especially ultrasound and biochemistry (if further evaluation supports its role in screening for adverse outcome). Will likely be contingent evolving to primary as the cost decreases.” |
| Czech Republic: | “Probably it should be offered for women at the high risk, not for general population.” |
| Netherlands: | “I strongly believe that NIPT should not replace first trimester screening, but run next to it.” |
|
| |
| Physician and Patient Education | |
|
| |
| India: | “At present there is confusion about NIPT availability in India. Awareness about NIPT, its limitations, shortcomings is quite low in medical and general population.” |
| Hong Kong: | “A common algorithm should be introduced to both healthcare providers and pregnant women for better understanding of the role of NIPT and its limitations.” |
| Italy: | “Too much enthusiasm, very little knowledge from professionals. Risk to be patient driven without appropriate counseling and knowledge.” |
|
| |
| Insurance coverage | |
|
| |
| Czech Republic: | “It is still very expensive and it is not covered by health insurance companies.” |
| Chile: | “As a MFM Specialist practicing in a developing country, not all women have Access to NIPT. Chromosomal analysis and ultrasound are available and partially covered by state and private insurance companies. NIPT is not covered at all.” |
| Canada: | “High patient satisfaction but frustrating that only available on a patient- pay basis at present.” |
|
| |
| IMPLEMENTATION | |
|
| |
| Policy/strategy | |
|
| |
| Germany: | “Ongoing discussion whether it will be offered to all or only to high risk women. In case of the latter, it is unclear how the high-risk population will be defined. Maternal age is not state of the art anymore but first trimester screening is only offered on a private basis.” |
| Hong Kong: | “It should be based on women's free choice. A publicly funded coupon will be a much better choice than to be centralized by the government.” |
| Canada: | “I wish we had a national policy. All provinces in Canada practice differently.” |
| Canada: | “Ideally will be introduced in a model that preserves beneficial aspects of existing screening programs especially ultrasound and biochemistry (if further evaluation supports its role in screening for adverse outcome).” |
| India: | “These tests should not be available as direct to consumer tests. We should try to develop NIPT using other techniques/platforms for which kits can be purchased and the test is run in house.” |
|
| |
| Cost effectiveness | |
|
| |
| United Kingdom: | “Unaffordable within NHS budgets at present.” |
| Czech Republic: | “The plan must be financially feasible.” |
| Italy: | “The one with most cost benefit advantages might be in the future recommended for all country from national health service.” |
|
| |
| ETHICAL & LEGAL ISSUES | |
|
| |
| India: | “Due to the serious problem of skewed male female ratio in many parts of India and strict control of PC-PNDT act on the genetic diagnosis and screening programs, there is unwillingness on part of the authorities to give clearance for NIPT. We will require a concerted effort on part of clinicians, counselors, genetic laboratories to explain to the authorities about advantages of NIPT, how it can be safely implemented under the PC-PNDT act guidelines so as to ensure strictly legal and proper use of NIPT. If we can have the NIPT testing in India, it will be easier to convince the authorities that the labs performing NIPT will strictly adhere to PC-PNDT guidelines.” |
| Hong Kong: | “I am a bit worried about "eugenic" in the coming era of next generation sequencing.” |
| Ireland: | “Very exciting and applicable technology; NI specific legislation restricts what can be done about an abnormal result, as we do not have the GB 1967 Abortion Act. NIPT *may* help force a rethink especially in early pregnancy.” |
| Norway: | “Norway was the second country in the world (1986) where the government officially introduced general ultrasound screening at week 18. However, the "Down syndrome debate" has since year 2000 been exceptionally strong. In 2004 a law was introduced making it ILLEGAL to practice a GENERAL OFFER of a full first trimester scan. The first trimester scan is only offered to risk groups and the first trimester scan including NT ++ can only be done at the 5 university hospitals. NIPT is not introduced in the country of Norway for detection of chromosomal abnormalities.” |
DISCUSSION
Our findings provide a glimpse into the experiences of clinicians across the world as they begin to adopt NIPT. Overall, our respondents represented a range of clinical practice experience, areas of specialty, public and private practice, and geography, which we believe enables us to capture a wide range of clinical implementation issues. These data also provide insight on some of the common barriers and challenges providers are facing with NIPT.
One of the most striking findings in our study is the great variability in test prices both between countries and within. We noted that this variability did not necessarily track with the per capita income of the country: Australia had the cheapest tests on average, whereas Argentina had the second highest average price in our dataset. Clearly, large disparities in test price will have a direct impact on global uptake of NIPT. As the respondent from Argentina observed, “[t]he cost of the test makes it unaffordable for most people, and it is still limited to patients who can afford it.” This respondent also estimated that around 0.1% of pregnant women have undergone NIPT since its introduction in Argentina in January 2013 and that “NIPT is not playing a significant role in routine aneuploidy screening.” This statement particularly emphasizes how clinical implementation of NIPT in many countries may be restricted by test prices. Indeed, many respondents commented about high test prices and the unaffordability of NIPT (Table 4).
We observed variation in responses from within the same country; for example, we noted conflicting data from respondents about the availability of NIPT in the UK, with half the respondents reporting that NIPT is not available despite published documentation of availability through the RAPID public sector trial.16 While our limited sample of UK providers cannot reveal nationwide trends, this discrepancy may reflect lack of provider knowledge about NIPT or may reveal uneven availability of NIPT within the UK. We also noted variable responses within countries for questions about whether there are plans to introduce NIPT into routine antenatal care. This suggests that policymakers should hold discussions at a national level in order to integrate the perspectives of different stakeholders, especially healthcare providers who are offering NIPT, and inform all stakeholders about plans for introduction into national programs. Such discussions may also help with the development of clear practice guidelines so that NIPT is used most effectively for each country’s needs. Our preliminary findings also confirm that professional societies, both national and international, can play a valuable role in guiding implementation and appropriate use of this new technology as it enters a growing number of countries. While this study was in progress, the International Society for Prenatal Diagnosis (ISPD) released a position statement providing guidance on the use of NIPT.19 It is not clear whether clinicians, particularly from low- and middle-income countries, are familiar with these and other professional society guidelines and if such guidelines have been adopted in their countries.
Our findings must also be interpreted in the context of limitations to this study. Although reports indicate NIPT may be available in as many as 90 countries,20 we were only able to contact individuals in 46 countries and received responses from 28 countries. The vast majority of these were high-income countries; we have data from three “upper-middle” income countries (Argentina, Hungary, and Turkey) and only one “lower-middle” income country (India).21 Geographically, we have poor coverage of countries in the Middle East and Africa. Our data are hence skewed towards the experiences of practitioners in well-resourced healthcare settings. Our data also should not be considered representative of the current state of NIPT use for an entire country. Our primary goal was to gauge differences and /or similarities in NIPT use across countries, rather than within a particular country. Regional or provincial differences in practice surrounding prenatal care and NIPT use were expected and preliminary findings from analysis of multiple responses from the same country lend support to that. Practices are also expected to be different between private and public sector providers. Moreover, other clinicians from these countries who we did not survey may have different experiences with NIPT use. Finally, some respondents may not have understood that our purpose was to ascertain information about NIPT with respect to fetal chromosomal aneuploidies or microdeletions and there may be confusion regarding the scope of testing specified by the term “NIPT.” For example, one respondent said that NIPT was offered to women with particular Rh blood groups. Currently, none of the commercial providers for NIPT include Rh blood testing on their test panels, although cell-free DNA testing for Rh blood status is available in many countries.22,23
CONCLUSION
This study provides a first glimpse of current uses of and experiences with NIPT globally. It highlights that differences in service provision exist both across and within countries, emphasizing the role for national and international implementation guidelines that can harmonize practice. It is clear that additional empirical research is needed to comprehensively capture the variability of experience with NIPT, challenges encountered, and familiarity with professional society guidelines, especially in low- and middle-income countries. It will also be important to collect data from different countries on additional stakeholders’ experiences, particularly patients, which will be influenced by unique legal and socioeconomic systems that impact the use of and access to prenatal care and genetic services.
What’s already known about this topic?
NIPT is marketed in nearly 90 countries worldwide, but information on clinicians’ experiences emanates largely from the US and Europe
Many countries are considering implementation of NIPT within public health systems
What does this study add?
This study provides insight into world-wide availability of NIPT based on clinicians’ experiences
The price of commercially available NIPT is limiting adoption, creating inequality of access
National and international policy deliberations are required to guide appropriate NIPT implementation worldwide
Acknowledgments
We would like to thank our survey participants, as well as Professor Lyn Chitty for her assistance with survey recruitment and design.
Funding sources: MAM and SC receive salary support from P50HG03391. SP and SC are supported by R01HG007074. CL is funded by the National Institute for Health Research (NIHR) through the Programme Grants for Applied Research (RP-PG-0707-10107). The research funded is independent and the views expressed in the paper are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Footnotes
Conflict of Interest disclosures: MAM, CL, SP, SC: none.
References
- 1.Tabor A, Alfirevic Z. Update on procedure-related risks for prenatal diagnosis techniques. Fetal Diagn Ther. 2010;27(1):1–7. doi: 10.1159/000271995. [DOI] [PubMed] [Google Scholar]
- 2.van Schendel RV, Kleinveld JH, Dondorp WJ, et al. Attitudes of pregnant women and male partners towards non-invasive prenatal testing and widening the scope of prenatal screening. Eur J Hum Genet. 2014;22(12):1345–50. doi: 10.1038/ejhg.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis C, Silcock C, Chitty LS. Non-invasive prenatal testing for Down's syndrome: pregnant women's views and likely uptake. Public Health Genomics. 2013;16(5):223–32. doi: 10.1159/000353523. [DOI] [PubMed] [Google Scholar]
- 4.Yi H, Hallowell N, Griffiths S, et al. Motivations for undertaking DNA sequencing-based non-invasive prenatal testing for fetal aneuploidy: a qualitative study with early adopter patients in Hong Kong. PloS One. 2013;8(11):e81794. doi: 10.1371/journal.pone.0081794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skirton H, Patch C. Factors affecting the clinical use of non-invasive prenatal testing: a mixed methods systematic review. Prenat Diagn. 2013;33(6):532–41. doi: 10.1002/pd.4094. [DOI] [PubMed] [Google Scholar]
- 6.Gil MM, Quezada MS, Revello R, et al. Analysis of cell-free DNA in maternal blood in screening for fetal aneuploidies: updated meta-analysis. Ultrasound Obstet Gynecol. 2015;45(3):249–66. doi: 10.1002/uog.14791. [DOI] [PubMed] [Google Scholar]
- 7.Royal College of Obstetricians & Gynaecologists. RCOG Scientific Impact Paper No. 15. 2014. Non-invasive Prenatal Testing for Chromosomal Abnormality using Maternal Plasma DNA. [Google Scholar]
- 8.Gregg AR, Gross SJ, Best RG, et al. ACMG statement on noninvasive prenatal screening for fetal aneuploidy. Genet Med. 2013;15(5):395–8. doi: 10.1038/gim.2013.29. [DOI] [PubMed] [Google Scholar]
- 9.Alexander E, Kelly S, Kerzin-Storrar L. Non-Invasive Prenatal Testing: UK Genetic Counselors' Experiences and Perspectives. J Genet Counsel. 2014:1–12. doi: 10.1007/s10897-014-9765-9. [DOI] [PubMed] [Google Scholar]
- 10.Tischler R, Hudgins L, Blumenfeld YJ, et al. Noninvasive prenatal diagnosis: pregnant women's interest and expected uptake. Prenat Diagn. 2011;31(13):1292–9. doi: 10.1002/pd.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yotsumoto J, Sekizawa A, Koide K, et al. Attitudes toward non-invasive prenatal diagnosis among pregnant women and health professionals in Japan. Prenat Diagn. 2012;32(7):674–9. doi: 10.1002/pd.3886. [DOI] [PubMed] [Google Scholar]
- 12.Chandrasekharan S, Minear MA, Hung A, et al. Noninvasive prenatal testing goes global. Sci Transl Med. 2014;6(231):231fs15. doi: 10.1126/scitranslmed.3008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larion S, Warsof SL, Romary L, et al. Uptake of noninvasive prenatal testing at a large academic referral center. Am J Obstet Gynecol. 2014;211(6):651, e1–7. doi: 10.1016/j.ajog.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 14.Chan YM, Leung WC, Chan WP, et al. Women's uptake of non-invasive DNA testing following a high-risk screening test for trisomy 21 within a publicly funded healthcare system: findings from a retrospective review. Prenat Diagn. 2014 doi: 10.1002/pd.4544. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Chetty S, Garabedian MJ, Norton ME. Uptake of noninvasive prenatal testing (NIPT) in women following positive aneuploidy screening. Prenat Diagn. 2013;33(6):542–6. doi: 10.1002/pd.4125. [DOI] [PubMed] [Google Scholar]
- 16.Hill M, Wright D, Daley R, et al. Evaluation of non-invasive prenatal testing (NIPT) for aneuploidy in an NHS setting: a reliable accurate prenatal non-invasive diagnosis (RAPID) protocol. BMC Pregnancy Childbirth. 2014;14:229. doi: 10.1186/1471-2393-14-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.EMGO Institute for Health and Care Research. [accessed on 20 March 2015];Non Invasive Prenatal Test (NIPT) now allowed in The Netherlands within TRIDENT study. 2014 [WWW document]. URL http://www.emgo.nl/news-and-events/news/486/non-invasive-prenatal-test-nipt-now-allowed-in-the-netherlands-within-trident-study/
- 18.PEGASUS. [accessed on 20 March 2015];About the PEGASUS project. 2015 [WWW document]. URL http://pegasus-pegase.ca/pegasus/
- 19.Benn P, Borrell A, Chiu R, et al. Position Statement from the Chromosome Abnormality Screening Committee on Behalf of the Board of the International Society for Prenatal Diagnosis [WWW document] [accessed on 11 May 2015]; doi: 10.1002/pd.4608. URL http://www.ispdhome.org/public/news/2015/PositionStatementFinal04082015.pdf. [DOI] [PubMed]
- 20.Ariosa Diagnostics, Inc. [accessed on 20 March 2015];Microarray technology proves superior to sequencing for non-invasive prenatal testing [WWW document] URL http://www.ariosadx.com/news-events/microarray-technology-proves-superior-nipt/
- 21.World Bank. [accessed on 20 March 2015];Country and Lending Groups [WWW document] URL http://data.worldbank.org/about/country-and-lending-groups.
- 22.Clausen FB. Integration of noninvasive prenatal prediction of fetal blood group into clinical prenatal care. Prenat Diagn. 2014;34(5):409–15. doi: 10.1002/pd.4326. [DOI] [PubMed] [Google Scholar]
- 23.Chitty LS, Finning K, Wade A, et al. Diagnostic accuracy of routine antenatal determination of fetal RHD status across gestation: population based cohort study. BMJ. 2014;349:g5243. doi: 10.1136/bmj.g5243. [DOI] [PMC free article] [PubMed] [Google Scholar]