Abstract
The objective of the study was to evaluate differences in gait mechanics 5 years after unilateral anterior cruciate ligament reconstruction surgery, for non-osteoarthritic (n = 24) versus osteoarthritic (n = 9) subjects. For the involved knee, the osteoarthritic group demonstrated significantly lower peak knee flexion angles (non-osteoarthritic = 24.3 ± 4.6°, osteoarthritic = 19.1 ± 2.9°, p = 0.01) and peak knee flexion moments (non-osteoarthritic = 5.3 ± 1.2% Body Weight × Height, osteoarthritic = 4.4 ± 1.2% Body Weight × Height, p = 0.05). Differences in peak knee adduction moment approached significance, with a higher magnitude for the osteoarthritic group (non-osteoarthritic = 2.4 ±0.8% Body Weight × Height, osteoarthritic = 2.9 ± 0.5% Body Weight × Height, p = 0.09). Peak medial compartment joint load was evaluated using electromyography-informed neuromusculoskeletal modeling. Peak medial compartment joint load in the involved knee for the two groups was not different (non-osteoarthritic = 2.4 ± 0.4 Body Weight, osteoarthritic = 2.3 ± 0.6 Body Weight). The results suggest that subjects with dissimilar peak knee moments can have similar peak medial compartment joint load magnitudes. There was no evidence of inter-limb asymmetry for either group. Given the presence of inter-group differences (non-osteoarthritic vs. osteoarthritic) for the involved knee, but an absence of inter-limb asymmetry in either group, it may be necessary to evaluate how symmetry is achieved, over time, and to differentiate between good versus bad inter-limb symmetry, when evaluating knee gait parameters.
Keywords: knee, osteoarthritis, ACL, ACLR, musculoskeletal modeling
400,000 estimated individuals sustain an ACL injury annually in the United States, and at least 100,000 individuals undergo ACL reconstruction (ACLR) each year.1,2 Premature development of knee osteoarthritis (OA) despite ACLR is a growing concern.3 Estimates of radiographic knee OA 5–7 years after ACLR range from 12% to over 60%,4–6 and as high as 74% at 10–15 years after ACLR.3 While the mechanism leading to knee OA remains yet to be identified, excessive joint loading is believed to be one of reasons that causes degeneration of cartilage.7 Multiple factors, including initial insult due to injury,8 have been correlated to onset and development of premature knee OA after ACL injury and surgery (ACLR).9 One factor associated with medial compartment knee OA in subjects after ACLR is altered knee gait mechanics after surgery.10–13 The prevalence of medial compartment knee OA, compared to lateral compartment knee OA, has been explained, at least in part, by the greater load in the medial versus lateral knee compartment during gait.14,15
In the sagittal plane, subjects with unilateral ACLR demonstrate a smaller peak knee flexion angle in the ACLR versus contralateral knee during the weight acceptance phase of gait (i.e., the weight bearing period during the first 25% of stance phase).16 Also in the sagittal plane, a lower peak knee flexion moment after ACLR has been correlated to unfavorable morphological changes in the medial tibial cartilage.17 In the frontal plane, a higher peak knee adduction moment during gait has been suggested as a potential mechanism for higher medial compartment loading and OA development after ACLR.13
In the sagittal plane, external knee flexion moment during gait is balanced by muscle co-contraction, which induces a compressive knee joint load.18 While knee flexion moment influences the magnitude of the total knee joint load, knee adduction moment modulates the distribution of the total knee joint load between medial and lateral knee compartments. However, it is not clear whether flexion and adduction moments are correlated in ACLR subjects, or whether a correlation exists for both non-OA as well as OA groups.
Neuromusculoskeletal (NMS) models that utilize information derived from electromyography (EMG) signals during gait have enhanced the capability of traditional gait analysis, by allowing an estimation of loading in the medial compartment of the knee.19,20 Utilizing these methods, studies have shown that 6 months after ACLR, subjects who go on to eventually develop knee OA demonstrate a lower medial compartment load in the ACLR knee, compared to those who do not develop knee OA.6 Inter-limb differences as well as inter-group (non-OA vs. OA) differences in gait mechanics are also present 6 months after ACLR.6 However, these inter-limb and group differences diminish in magnitude 1–2 years after ACLR.6 Given that not all subjects develop knee OA after ACLR, comparing the nature and progression of joint biomechanics in non-OA versus OA ACLR groups may be the key to develop rehabilitation strategies, to delay the progression of the disease.
With that background, the objective of the study was to evaluate whether inter-group (non-OA vs. OA) and inter-limb differences in knee gait mechanics exist 5 years after ACLR. The following hypotheses were evaluated 5 years after ACLR surgery, for the ACLR knee during the weight acceptance phase of gait: Compared to the non-OA group, the OA group will demonstrate lower peak knee flexion angle and moment. For both groups, the peak knee flexion angle and moment will be positively correlated. Compared to the non-OA group, the OA group will demonstrate greater peak knee adduction moment. For both groups, the peak knee flexion and adduction moments will be negatively correlated. For both groups, the magnitude of peak medial compartment load will be similar. Finally, we also evaluated inter-limb asymmetry for each parameter mentioned above, for both groups.
METHODS
The current study design provides level III evidence. The study population included 40 subjects from part of a larger trial of individuals after ACLR who had undergone progressive, pre-operative rehabilitation training.21 The 40 subjects were included as they had completed radiographs 5 years after ACLR, which were required to determine the presence/absence of radiographic knee OA. The study was approved by the Institutional Review Board at the University of Delaware. All subjects were provided with written consent forms for the study. Each subject was a regular participant in level I–II cutting and pivoting activities prior to ACL injury. Minimal knee joint effusion, full knee range of motion and quadriceps strength criteria of at least 70% of the contralateral limb were used for inclusion.22 Exclusion criteria encompassed concomitant repairable meniscus injuries, grade III injury to other knee ligaments, and full-thickness articular cartilage lesions greater than 1cm2 diagnosed before ACLR or contralateral ACL injury after ACLR. Either a four-bundle semitendinosus-gracilis autograft or soft tissue allograft was used for surgery. Signs of knee OA were evaluated from posterior-to-anterior bent knee (30°) radiographs 5 years after ACLR. SigmaView software (Agfa HealthCare Corporation, Greenville, SC) and the Kellgren-Lawrence (KL) system were used to grade levels of OA in each tibiofemoral compartment. The presence of OA in each compartment was operationally defined as a KL grade ≥2. Twenty-four subjects did not have knee OA in the ACLR knee, and were included in the non-OA group (Fig. 1). Nine subjects had medial compartment knee OA in the ACLR knee, and were included in the OA group (Fig. 1). Seven subjects were excluded for this analysis due to presence of OA only in the contralateral knee (n = 3) and only in the lateral knee compartment (n = 4). For all subjects with medial compartment knee OA, the osteophyte was located near the medial joint margin (Fig. 2).
Figure 1.
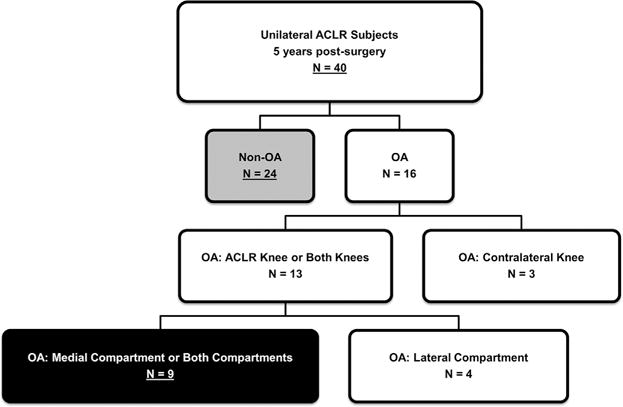
Study population of unilateral ACLR subjects 5 years after surgery (ACLR, anterior cruciate ligament reconstruction; OA, osteoarthritis).
Figure 2.
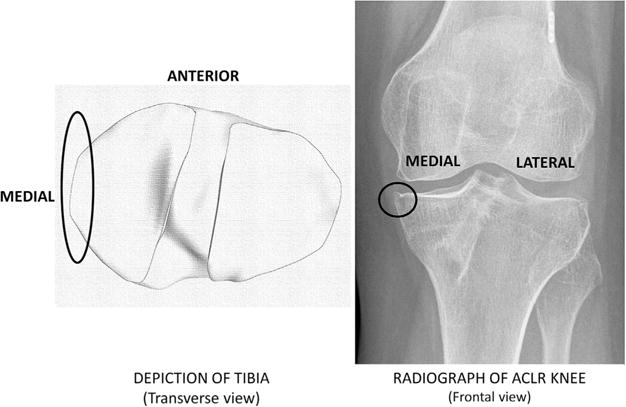
Osteophyte location near medial knee joint margin 5 years after ACLR (ACLR, anterior cruciate ligament reconstruction).
Five years after ACLR, each subject performed multiple gait trials wearing shoes. Kinematic parameters were recorded using an eight infrared camera setup (Vicon, Oxford Metrics Limited, London, UK) and retroreflective markers at a sampling rate of 120 Hz. Retroreflective markers were placed on bony landmarks at each lower extremity, with rigid marker shells placed at the pelvis, thighs, and shanks. Subjects walked at a self-selected speed along a 6 m walkway. Walking speed was maintained within ± 5% during the testing session. Kinetic parameters during gait were recorded using a force platform (Bertec Corporation, Worthington, OH) at a sampling rate of 1080 Hz. Stance phase knee kinematics/kinetics were processed using inverse dynamics in Visual3D (C-Motion, Germantown, MD). Knee joint angles and moments were time-normalized to 100% of stance phase and knee moments, expressed in the tibial coordinate system, were normalized to % Body Weight × Height (% BW × HT).
The testing protocol also included surface EMG data collection during gait.25 EMG was band-pass filtered (20–500 Hz) prior to sampling, and then sampled at 1080 Hz using a MA-300 EMG system (Motion Lab Systems, Baton Rouge, LA). EMG electrodes were placed over muscle bellies of seven muscles crossing the knee joint, for each limb. The flexor muscles included semimembranosus, long head of biceps femoris and medial/lateral gastrocnemii, while extensor muscles included rectus femoris and medial/lateral vasti. For each muscle, EMG data was high-pass filtered (2nd order Butterworth, cutoff = 30Hz), rectified, low-pass filtered (cutoff = 6Hz) to create linear envelopes, and normalized to maximum EMG found during maximum voluntary isometric contractions or gait trails. EMG for semitendinosus and short head of biceps femoris were set to be equal to linear envelopes of semimembranosus and long head of biceps femoris, respectively. EMG for vastus intermedius was calculated as the average of medial and lateral vasti linear envelopes. Next, linear envelopes from the muscles were used as input in a previously validated EMG-informed NMS model, described in detail elsewhere.19,26 EMG-informed NMS modeling involves subject-specific anatomical scaling (for muscle moment arm estimation) and calibration (for muscle force estimation) to minimize the squared difference between net internal and external sagittal plane knee moments. Subject-specific anatomical scaling is based on marker data from a standing trial, and it enables muscle-tendon length and moment arm estimations using stance phase kinematics (SIMM 6.0, Musculographics Inc., Chicago, IL). Subject-specific calibration involves adjustment of two global strength coefficients, as well as adjustment of parameters in the muscle activation and muscle contraction subcomponents. The muscle activation sub-component transforms EMG signal to a muscle activation measure, and is characterized by four adjustable parameters. The muscle contraction sub-component transforms muscle activation to muscle force. It is a modified Hill-type representation of a muscle fiber in series with a tendon and is characterized by two adjustable parameters (optimal fiber length and tendon slack length). Optimized muscle forces and frontal plane moment arms are then used to balance the external frontal plane knee moment.27 This allows for subject-specific prediction of medial compartment joint loads, averaged for three predicted walking trials. The medial compartment joint loads thus obtained were normalized to body weight (BW). Normalization of joint load and joint moment aids in reducing effects of body weight, height, and gender, when comparing differences between (non-OA vs. OA) groups.28
Chi-square tests were used to test non-OA versus OA group differences for sex and graft type, while independent t-tests were used to test group differences for age, mass, height, and walking speed during gait trials. The key parameters of interest were peak knee flexion angle, peak knee flexion moment, peak knee adduction moment, and peak medial compartment force, during weight acceptance. Independent t-tests were performed to test for differences between non-OA versus OA groups. Mean ± standard deviations (SD), 95% confidence intervals (CI) and effect size (Cohen’s d) were computed. For both groups, the following linear correlations and regressions were also evaluated: Peak knee flexion angle versus peak knee flexion moment, and peak knee flexion moment versus peak knee adduction moment. Statistical analysis was conducted using JMP (Cary, NC). Statistical significance for all tests was set at α ≤ 0.05. Gait data from 12 control subjects was used to determine meaningful inter-limb difference (MILD) thresholds for the key parameters of interest, using the methodology for estimating minimum detectable change. The control group consisted of five women and seven men, with the following subject characteristics (Mean ± standard deviation): age = 21 ± 3 years, mass = 75 ± 18kg, height = 1.73 ± 0.1m, walking speed = 1.6 ± 0.2 m/s. An inter-limb difference that was greater than the MILD threshold was interpreted as a reliable indication of inter-limb asymmetry.
RESULTS
The non-OA versus OA group demonstrated no differences for sex, graft type, age, mass, height, and walking speed 5 years after unilateral ACLR (Table 1).
Table 1.
Non-OA Versus OA Subject Population Characteristics
| Parameter | Non-OA (n = 24) (Mean ± SD Where Applicable) |
OA (n = 9) (Mean ± SD Where Applicable) |
p-Value |
|---|---|---|---|
| Sex | 16 men, 8 women | 4 men, 5 women | 0.25 |
| Graft type | 16 allograft, 8 autograft | 4 allograft, 5 autograft | 0.25 |
| Age | 32 ± 11 years | 32 ± 13 years | 0.90 |
| Mass | 88 ± 16 kg | 86 ± 21 kg | 0.79 |
| Height | 1.7 ± 0.1m | 1.7 ± 0.1m | 0.65 |
| Walking speed | 1.6 ± 0.1 m/s | 1.5 ± 0.1 m/s | 0.19 |
OA, osteoarthritis; SD, standard deviation; kg, kilogram; m, meter; s, second.
Sagittal Plane Kinematics and Kinetics
For the ACLR knee, the OA group demonstrated a significantly lower peak knee flexion angle (effect size = 1.23, Fig. 3). 95%CI: Non-OA = 22.5–26.1°, OA = 17.3–21.0°.
Figure 3.
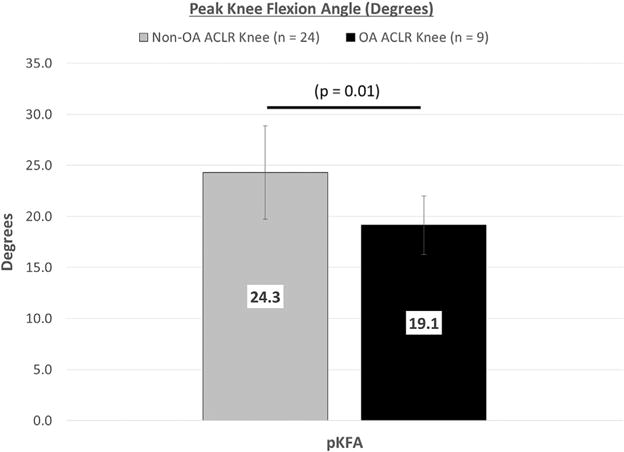
Peak knee flexion angle during gait for non-OA versus OA subjects, Mean ± SD (pKFA, peak knee flexion angle; OA, osteoarthritis; ACLR, anterior cruciate ligament reconstruction; SD, standard deviation).
The OA group also demonstrated a significantly lower external peak knee flexion moment (effect size = 0.78, Fig. 4). 95%CI: Non-OA = 4.8–5.8 % BW × HT, OA = 3.5–5.2 % BW × HT.
Figure 4.

Peak knee joint moment during gait for non-OA versus OA subjects, Mean ± SD (BW, body weight; HT, height; OA, osteoarthritis; ACLR, anterior cruciate ligament reconstruction; pKFM, peak knee flexion moment; pKAM, peak knee adduction moment; SD, standard deviation).
For each group, the peak knee flexion angle and peak knee flexion moment demonstrated a significant positive correlation (Non-OA: R2 = 0.48, p = 0.01, OA: R2 = 0.64, p = 0.01).
Frontal Plane Kinetics
For the ACLR knee, the difference in external peak knee adduction moment between groups approached significance, with the OA group demonstrating a greater peak knee adduction moment, compared to the non-OA group (effect size =0.69, Fig. 4). 95%CI: Non-OA = 2.1–2.8 % BW × HT, OA = 2.6–3.3% BW × HT.
Correlation Between Sagittal and Frontal Plane Kinetics
For the ACLR knee, the OA group demonstrated a significant negative correlation between external peak knee flexion and adduction moments (R2 = 0.59, p = 0.02). For the non-OA group, there was no correlation (R = 0.05, p = 0.28). The correlations are shown in Figure 5.
Figure 5.
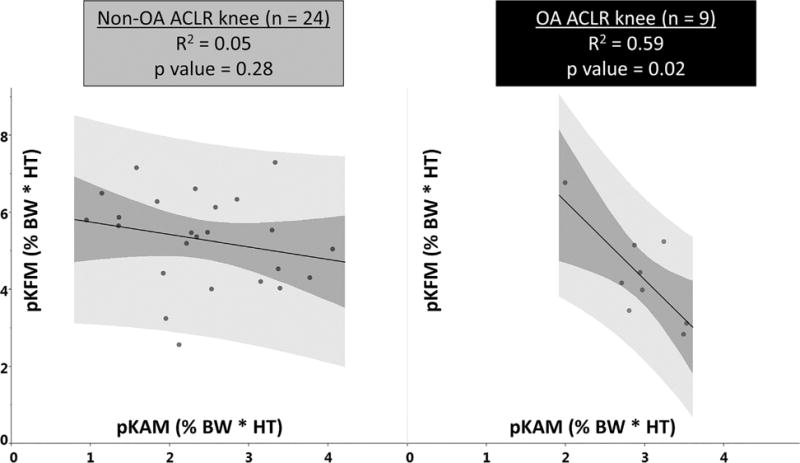
Peak knee flexion (y-axis) versus adduction (x-axis) moments during gait for non-OA versus OA subjects (OA, osteoarthritis; ACLR, anterior cruciate ligament reconstruction; BW, body weight; HT, height; pKFM, peak knee flexion moment; pKAM, peak knee adduction moment; dark color band, 95% confidence interval for fitted line; light color band, confidence region for individual predicted values).
Peak Medial Compartment Knee Joint Force
For the ACLR knee, the difference in medial compartment knee joint force was not significant between groups (effect size = 0.07, Fig. 6). 95%CI: Non-OA = 2.2–2.5 BW, OA= 1.9–2.7 BW.
Figure 6.
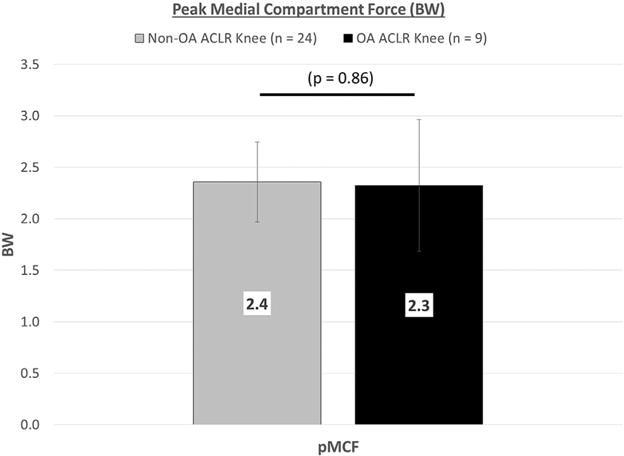
Peak medial compartment force during gait for non-OA versus OA subjects, Mean ± SD (BW, body weight; pMCF, peak medial compartment force; OA, osteoarthritis; ACLR, anterior cruciate ligament reconstruction; SD, standard deviation).
Inter-Limb Difference
Three out of nine subjects in the OA group had knee OA in the contralateral knee, in addition to the ACLR knee. These three subjects were not included in the evaluation of inter-limb differences. For both non-OA and OA groups, the mean inter-limb difference was less than the meaningful inter-limb difference (MILD) threshold for each parameter, that is, both groups demonstrated symmetry between limbs, for each parameter (Table 2).
Table 2.
Absolute Inter-Limb Difference (|ACLR—contralateral|) in Biomechanical Parameters, Compared to MILD Thresholds Calculated From 12 Healthy Subjects
| |ACLR—Contralateral| | Non-OA (n = 24) Mean ± SD | OA (n = 6) Mean ± SD | MILD Threshold (n = 12) |
|---|---|---|---|
| pKFA (degrees) | 0.2 ± 2.9 | 0.2 ± 1.7 | 4.0 |
| pKFM (% BW × HT) | 0.1 ± 0.8 | 0.1 ± 0.3 | 0.9 |
| pKAM (% BW × HT) | 0.3 ± 0.7 | 0.2 ± 1.0 | 0.6 |
| pMCF (BW) | 0.1 ± 0.4 | 0.3 ± 0.7 | 0.4 |
An inter-limb difference greater than the MILD threshold is considered asymmetrical (ACLR, anterior cruciate ligament reconstruction; MILD, meaningful inter-limb difference; OA, osteoarthritis; SD, standard deviation; BW, body weight; HT, height; pKFA, peak knee flexion angle; pKFM, peak knee flexion moment; pKAM, peak knee adduction moment; pMCF, peak medial compartment force).
To summarize, the hypotheses pertaining to smaller peak values for sagittal plane kinematics and kinetics in the OA (vs. non-OA) group, was supported. Both groups also demonstrated a positive correlation between peak values for sagittal plane kinematics and kinetics. For frontal plane kinetics, the OA group did not demonstrate significantly higher peak knee adduction moment (although it approached significance), and the hypothesis was not supported. The hypothesis pertaining to a negative correlation between peak knee flexion and adduction moment was supported for the OA group, but not for the non-OA group. Both groups did demonstrate similar peak values of medial compartment load. Finally, both groups demonstrated symmetry between limbs for each parameter.
DISCUSSION
In the current study, for the ACLR knee, the OA group demonstrated a significantly lower peak knee flexion angle during the weight acceptance phase of gait. This observation is in line with previous reports that investigated the changes in sagittal plane kinematics in relation to OA.29 A lower peak knee flexion angle has also been observed for subjects who undergo ACLR and demonstrate quadriceps weakness, when compared to uninjured controls.30 The rationale as to why a smaller peak knee flexion angle during weight acceptance would be detrimental for the cartilage is the potential shift in contact location. Between 0° and 30° of knee flexion, joint contact in the medial compartment can shift posteriorly with an increasing knee flexion angle.31,32 Hence, the smaller the peak knee flexion angle, the more anterior the contact location in the medial compartment of the knee. Knowing that the medial tibial cartilage in the anterior region is thinner compared to the weight bearing region, even a normal medial compartment load magnitude can induce high stresses in the thinner anterior region of the cartilage.33
For peak external flexion knee moment during gait, studies have shown smaller values during weight acceptance in relation to OA progression, based on inter-group (non-OA vs. OA) and inter-limb differences, up to 1 year after surgery.30,34–36 These differences diminish in magnitude and are not found 3 years after surgery.35 In the current study, the OA group demonstrated a smaller external peak knee flexion moment in the ACLR knee during weight acceptance, compared to the non-OA group. Both non-OA and OA groups demonstrated a positive correlation between peak knee flexion angle and moment, similar to other findings.14 Also, a high peak knee flexion moment at baseline has been correlated to a reduction in medial tibial cartilage thickness over 5 years.37 Considering these findings, the lower magnitude of the peak knee flexion moment in ACLR knee of subjects with OA, by itself, may not be linked to a detrimental effect on cartilage thickness. It is also difficult to estimate whether a smaller peak knee external flexion moment corresponds to an increase or decrease in medial compartment load, as net external flexion moment does not provide any insight about the level of muscle co-contraction.
In the frontal plane, a larger knee adduction moment is linked to an increase in the medial compartment load. Compared to healthy controls, individuals who develop knee OA demonstrate greater knee adduction moments.14,38 However, up to 3 years after surgery, there is limited and conflicting evidence about the knee adduction moment being higher in the ACLR knee, compared to the contralateral knee and healthy controls.11,13,35 In the current study, the peak knee adduction moment during weight acceptance was not different for the non-OA versus OA group. The knee adduction angle during gait can also be linked to the contact location as well as the medial versus lateral compartment load distribution.39 While an accurate in vivo measurement of the knee adduction angle is difficult in a standard gait experiment, there is some evidence in literature that peak knee adduction moment is positively correlated to the peak knee adduction angle.40 Although not significant, the OA group did demonstrate a greater peak knee adduction moment compared to the non-OA group in the current study. Hence, if the peak knee adduction angle in these OA subjects were also greater, the increased varus angle during gait would shift contact closer to medial margin of the medial compartment. It is in this region where radiographic osteophytes were observed in subjects with OA (Fig. 2).
Subjects with large magnitudes for both peak knee flexion and adduction moments tend to have a large medial compartment load, while subjects with small magnitudes for both parameters have a small medial compartment load. The latter condition is more likely to be true when pain reduction may be the goal. However, it is not clear whether peak knee flexion and adduction moments are correlated in subjects that demonstrate evidence of radiographic knee OA without pain. In the current study, the non-OA group did not demonstrate a significant correlation. Interestingly, however, the OA group demonstrated a significant negative correlation between external peak knee flexion and adduction moments in the ACLR knee 5 years after surgery. Evaluating the correlation in a larger number of subjects with knee OA will help verify or reject this possibility. It is not clear as to why such a relationship may exist, but since some level of inter-limb symmetry is desirable for smooth gait, the relationship between peak knee flexion and adduction moment could be a consequence of neuromuscular control aimed at maintaining symmetry. The OA group may be maintaining the magnitude of the medial compartment load either through a combination of high peak flexion moment and low peak knee adduction moment, or, a combination of low peak knee flexion moment and high peak knee adduction moment. This implies that a high peak knee adduction moment may not always result in a high peak medial compartment load, at least in the OA group. In the current study, the peak medial compartment load magnitudes were similar for both non-OA and OA groups. For the two groups, peak extensor muscle force (sum of extensor muscle forces at peak knee flexion moment24) was not significantly different. 95% CI: non-OA = 2.6–3.2 BW, OA = 2.0–2.8 BW, p = 0.12, effect size = 0.64. Peak flexor muscle force (sum of flexor muscle forces at peak knee flexion moment24) was also not significantly different between groups. 95%CI: non-OA = 0.9–1.2 BW, OA = 0.8–1.7 BW, p = 0.21, effect size = 0.52. Although not significant, the OA (vs. non-OA) group demonstrated lower peak extensor muscle forces and higher peak flexor muscle forces. Thus, despite differences in net external peak knee moment between groups, the peak medial compartment load magnitude was not different, based on muscle activation, muscle co-contraction and muscle forces during gait.
Pertaining to inter-limb symmetry, smaller peak knee flexion angles during the weight acceptance phase have been reported in the ACLR versus contralateral knee 6–12 months after ACLR.16 At 1–3 years after ACLR, there is evidence of no difference in peak knee flexion angle between the ACLR and contralateral knees.35 Our study also did not show any inter-limb differences for either group, at 5 years after ACLR. These data suggest that inter-limb differences in peak knee flexion angle during weight acceptance are resolved over time. However, for the ACLR knee, as noted above, the OA (vs. non-OA) group demonstrated a smaller peak knee flexion angle during weight acceptance at 5 years after surgery. It is plausible that inter-limb symmetry in non-OA versus OA groups is achieved in different ways after ACLR, over time. This is in line with the notion that maintenance of some level of symmetry between two legs is desirable for smooth gait, and to maintain the same, changes may be seen in the uninjured contralateral knee, in addition to changes in the ACLR knee.41 A similar argument could apply to inter-limb symmetry observed for all other gait parameters. For the OA group, this could mean an increased risk of knee OA in the uninjured contralateral knee over time, in addition to the ACLR knee. Hence, rather than evaluating just the inter-limb symmetry, it may be necessary to differentiate between good versus bad inter-limb symmetry, when evaluating knee gait parameters. In this context, good symmetry would mean that the contralateral knee gait parameter magnitude (at an early time point after ACLR) is the goal, and over time, the ACLR knee tries to match the contralateral knee. On the other hand, bad symmetry would mean that the ACLR knee gait parameter magnitude is the goal, and over time, the contralateral knee tries to match the ACLR knee. Long term follow-up evidence over multiple time points after ACLR can help verify this possibility,6,15 and MILD thresholds can be used to evaluate symmetry. Further work needs to be done, possibly using a combination of modalities used for studying the knee, including magnetic resonance imaging (MRI),42,43 finite element (FE) modeling,44 and biplanar fluoroscopy studies.45 This would help verify whether the kinematic, kinetic, and joint load magnitudes observed after ACLR in subjects with medial compartment OA, are, in fact, associated with a detrimentally high cartilage stress close to the region where osteophytes are observed.
Our study findings should be interpreted with limitations in mind. In the current study, we used radiographs, and not MRI, to detect the presence of OA. Compared to radiographs, OA related changes in knee cartilage can be located and detected sooner using MRI.46 Based on a priori power analysis with peak medial compartment load magnitude as the key variable of interest (α = 0.05 and effect size based on minimum detectable change25), the sample size of the current study was sufficient to yield a power of 0.8. Further work with a larger OA cohort and greater power may better evaluate differences in all knee gait parameters between non-OA versus OA groups. Due to the sample size, differences based on sex were not considered. In the current study, 5 out of 13 women (38%) versus 4 out of 20 men (20%) showed signs of medial compartment knee OA. While pre-clinical animal studies have shown that sex can effect biomechanical outcomes of ACLR,47 clinical follow-up using radiographs in human studies indicate that sex may not be a determining factor in the development of knee OA after ACLR.15 The effect of sex on incidence of knee OA after ACLR is an on-going debate and more evidence is required to verify this claim. Differences in the transverse plane48 between groups were not included in the current study. A recent systematic analysis and meta-review has failed to draw any conclusions about changes in transverse plane kinematics and kinetics after ACLR.35 Transverse plane knee parameters are typically smaller in magnitude (compared to the sagittal plane), and future studies that include biplanar fluoroscopy may be better suited to identify group differences, if present. While medial compartment knee OA is more common than lateral compartment knee OA (which was also true for the current study), cases of lateral compartment knee OA after ACLR have been reported.49 Changes in gait mechanics related to medial versus lateral compartment knee OA are likely different. Due to the smaller number of subjects with lateral compartment knee OA, those subjects were excluded from the analysis. Further studies with a larger sample size that include subjects with lateral compartment as well as medial compartment knee OA may provide insight related to differences between the two (medial vs. lateral compartment) OA groups, in comparison to the non-OA group.
It should be noted that the results of the current study demonstrate differences in (non-OA vs. OA) groups and correlations in gait parameters, but not causality. The observed differences may be due to underlying biological changes during onset and progression of knee OA. In conclusion, 5 years after unilateral ACLR, the OA group demonstrated significantly lower peak knee flexion angle and moment, compared to the non-OA group. The peak knee flexion and adduction moments were negatively correlated for the OA group, while peak medial compartment joint load for the two groups was similar. Given the presence of inter-group differences (non-OA vs. OA) for the ACLR knee, but an absence of inter-limb asymmetry in either group, it may be necessary to differentiate between good versus bad inter-limb symmetry, when evaluating knee gait parameters.
Acknowledgments
Grant sponsor: National Institutes of Health; Grant sponsor: National Institute of General Medical Sciences; Grant number: P30 GM103333; Grant sponsor: National Institute of Arthritis and Musculoskeletal and Skin Diseases; Grant numbers: R01 AR046386, R01 AR048212; Grant sponsor: Eunice Kennedy Shriver National Institute of Child Health and Human Development; Grant number: R01 HD087459-01.
Footnotes
Conflict of interest: None.
AUTHORS’ CONTRIBUTIONS
All authors hereby certify that they have read and approved the final submitted manuscript. AK contributed to research design, data analysis and interpretation, drafting the paper and incorporating revisions. KM contributed to data analysis, interpretation and critical review. EW and JC contributed to data acquisition, analysis and critical review. LS-M and TSB contributed to research design, data interpretation, and critical review.
References
- 1.Gardinier ES, Manal K, Buchanan TS, et al. Altered loading in the injured knee after ACL rupture. J Orthop Res. 2013;31:458–464. doi: 10.1002/jor.22249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray M. The ACL handbook, chapter 2: history of ACL treatment and current gold standard of care. New York, NY: Springer New York; 2013. pp. 19–28. [Google Scholar]
- 3.Oiestad BE, Holm I, Aune AK, et al. Knee function and prevalence of knee osteoarthritis after anterior cruciate ligament reconstruction: a prospective study with 10 to 15 years of follow-up. Am J Sports Med. 2010;38:2201–2210. doi: 10.1177/0363546510373876. [DOI] [PubMed] [Google Scholar]
- 4.Frobell RB, Roos HP, Roos EM, et al. Treatment for acute anterior cruciate ligament tear: five year outcome of randomised trial. BMJ. 2013;346:f232. doi: 10.1136/bmj.f232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li RT, Lorenz S, Xu Y, et al. Predictors of radiographic knee osteoarthritis after anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39:2595–2603. doi: 10.1177/0363546511424720. [DOI] [PubMed] [Google Scholar]
- 6.Wellsandt E, Gardinier ES, Manal K, et al. Decreased knee joint loading associated with early knee osteoarthritis after anterior cruciate ligament injury. Am J Sports Med. 2016;44:143–151. doi: 10.1177/0363546515608475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frost HM. Perspectives: a biomechanical model of the pathogenesis of arthroses. Anat Rec. 1994;240:19–31. doi: 10.1002/ar.1092400103. [DOI] [PubMed] [Google Scholar]
- 8.Simon D, Mascarenhas R, Saltzman BM, et al. The relationship between anterior cruciate ligament injury and osteoarthritis of the knee. Adv Orthop. 2015;2015:928301. doi: 10.1155/2015/928301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andriacchi TP, Favre J, Erhart-Hledik JC, et al. A systems view of risk factors for knee osteoarthritis reveals insights into the pathogenesis of the disease. Ann Biomed Eng. 2014;43:376–387. doi: 10.1007/s10439-014-1117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleming BC, Hulstyn MJ, Oksendahl HL, et al. Ligament injury, reconstruction and osteoarthritis. Curr Opin Orthop. 2005;16:354–362. doi: 10.1097/01.bco.0000176423.07865.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zabala ME, Favre J, Scanlan SF, et al. Three-dimensional knee moments of ACL reconstructed and control subjects during gait, stair ascent, and stair descent. J Biomech. 2013;46:515–520. doi: 10.1016/j.jbiomech.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao B, Zheng N. Alterations in three-dimensional joint kinematics of anterior cruciate ligament-deficient and -reconstructed knees during walking. Clin Biomech. 2010;25:222–229. doi: 10.1016/j.clinbiomech.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Butler RJ, Minick KI, Ferber R, et al. Gait mechanics after ACL reconstruction: implications for the early onset of knee osteoarthritis. Br J Sports Med. 2009;43:366–370. doi: 10.1136/bjsm.2008.052522. [DOI] [PubMed] [Google Scholar]
- 14.Baliunas A, Hurwitz D, Ryals A, et al. Increased knee joint loads during walking are present in subjects with knee osteoarthritis. Osteoarthr Cartil. 2002;10:573–579. doi: 10.1053/joca.2002.0797. [DOI] [PubMed] [Google Scholar]
- 15.Barenius B, Ponzer S, Shalabi A, et al. Increased risk of osteoarthritis after anterior cruciate ligament reconstruction: a 14-year follow-up study of a randomized controlled trial. Am J Sports Med. 2014;42:1049–1057. doi: 10.1177/0363546514526139. [DOI] [PubMed] [Google Scholar]
- 16.Di Stasi SL, Logerstedt D, Gardinier ES, et al. Gait patterns differ between ACL-reconstructed athletes who pass return-to-sport criteria and those who fail. Am J Sports Med. 2013;41:1310–1318. doi: 10.1177/0363546513482718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scanlan S, Blazek K, Schmidt Joshua, et al. Relationship between knee flexion moment and early cartilage changes in the ACL reconstructed knee. Proc Am Soc Biomech. 2007;252:3–4. [Google Scholar]
- 18.Creaby MW. It’s not all about the knee adduction moment: the role of the knee flexion moment in medial knee joint loading. Osteoarthritis Cartilage. 2015;23:1038–1040. doi: 10.1016/j.joca.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 19.Manal K, Buchanan TS. An electromyogram-driven musculoskeletal model of the knee to predict in vivo joint contact forces during normal and novel gait patterns. J Biomech Eng. 2013;135:021014. doi: 10.1115/1.4023457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lloyd DG, Besier TF. An EMG-driven musculoskeletal model to estimate muscle forces and knee joint moments in vivo. J Biomech. 2003;36:765–776. doi: 10.1016/s0021-9290(03)00010-1. [DOI] [PubMed] [Google Scholar]
- 21.Hartigan EH, Axe MJ, Snyder-Mackler L. Time line for noncopers to pass return-to-sports criteria after anterior cruciate ligament reconstruction. J Orthop Sport Phys Ther. 2010;40:141–154. doi: 10.2519/jospt.2010.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurd WJ, Axe MJ, Snyder-Mackler L. A 10-year prospective trial of a patient management algorithm and screening examination for highly active individuals with anterior cruciate ligament injury: part 1, outcomes. Am J Sports Med. 2008;36:40–47. doi: 10.1177/0363546507308190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gardinier ES, Manal K, Buchanan TS, et al. Gait and neuromuscular asymmetries after acute anterior cruciate ligament rupture. Med Sci Sports Exerc. 2012;44:1490–1496. doi: 10.1249/MSS.0b013e31824d2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardinier ES, Manal K, Buchanan TS, et al. Minimum detectable change for knee joint contact force estimates using an EMG-driven model. Gait Posture. 2013;38:1051–1053. doi: 10.1016/j.gaitpost.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buchanan TS, Lloyd DG, Manal K, et al. Neuromusculoskeletal modeling: estimation of muscle forces and joint moments and movements from measurements of neural command. J Appl Biomech. 2004;20:367–395. doi: 10.1123/jab.20.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winby CR, Lloyd DG, Besier TF, et al. Muscle and external load contribution to knee joint contact loads during normal gait. J Biomech. 2009;42:2294–2300. doi: 10.1016/j.jbiomech.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 28.Moisio KC, Sumner DR, Shott S, et al. Normalization of joint moments during gait: a comparison of two techniques. J Biomech. 2003;36:599–603. doi: 10.1016/s0021-9290(02)00433-5. [DOI] [PubMed] [Google Scholar]
- 29.Astephen JL, Deluzio KJ, Caldwell GE, et al. Biomechanical changes at the hip, knee, and ankle joints during gait are associated with knee osteoarthritis severity. J Orthop Res. 2008;26:332–341. doi: 10.1002/jor.20496. [DOI] [PubMed] [Google Scholar]
- 30.Lewek M, Rudolph K, Axe M, et al. The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin Biomech. 2002;17:56–63. doi: 10.1016/s0268-0033(01)00097-3. [DOI] [PubMed] [Google Scholar]
- 31.Masouros SD, Bull AMJ, Amis AA. (i) Biomechanics of the knee joint. Orthop Trauma. 2010;24:84–91. [Google Scholar]
- 32.Wretenberg P, Ramsey DK, Nemeth G. Tibiofemoral contact points relative to flexion angle measured with MRI. Clin Biomech. 2002;17:477–485. doi: 10.1016/s0268-0033(02)00036-0. [DOI] [PubMed] [Google Scholar]
- 33.Koo S, Gold GE, Andriacchi TP. Considerations in measuring cartilage thickness using MRI: factors influencing reproducibility and accuracy. Osteoarthritis Cartilage. 2005;13:782–789. doi: 10.1016/j.joca.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Webster KE. Gait patterns after anterior cruciate ligament reconstruction are related to graft type. Am J Sports Med. 2005;33:247–254. doi: 10.1177/0363546504266483. [DOI] [PubMed] [Google Scholar]
- 35.Hart HF, Culvenor AG, Collins NJ, et al. Knee kinematics and joint moments during gait following anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Br J Sports Med. 2015:1–17. doi: 10.1136/bjsports-2015-094797. [DOI] [PubMed] [Google Scholar]
- 36.Astephen JL, Deluzio KJ, Caldwell GE, et al. Biomechanical changes at the hip, knee, and ankle joints during gait are associated with knee osteoarthritis severity. J Orthop Res. 2008;26:332–341. doi: 10.1002/jor.20496. [DOI] [PubMed] [Google Scholar]
- 37.Chehab EF, Favre J, Erhart-Hledik JC, et al. Baseline knee adduction and flexion moments during walking are both associated with 5 year cartilage changes in patients with medial knee osteoarthritis. Osteoarthr Cartil. 2014;22:1833–1839. doi: 10.1016/j.joca.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reeves ND, Bowling FL. Conservative biomechanical strategies for knee osteoarthritis. Nat Rev Rheumatol. 2011;7:113–122. doi: 10.1038/nrrheum.2010.212. [DOI] [PubMed] [Google Scholar]
- 39.Adouni M, Shirazi-Adl A. Partitioning of knee joint internal forces in gait is dictated by the knee adduction angle and not by the knee adduction moment. J Biomech. 2014;47:1696–1703. doi: 10.1016/j.jbiomech.2014.02.028. [DOI] [PubMed] [Google Scholar]
- 40.Barrios JA, Royer TD, Davis IS. Dynamic versus radiographic alignment in relation to medial knee loading in symptomatic osteoarthritis. J Appl Biomech. 2012;28:551–559. doi: 10.1123/jab.28.5.551. [DOI] [PubMed] [Google Scholar]
- 41.Decker LM, Moraiti C, Stergiou N, et al. New insights into anterior cruciate ligament deficiency and reconstruction through the assessment of knee kinematic variability in terms of nonlinear dynamics. Knee Surgery Sport Traumatol Arthrosc. 2011;19:1620–1633. doi: 10.1007/s00167-011-1484-2. [DOI] [PubMed] [Google Scholar]
- 42.Culvenor AG, Collins NJ, Guermazi A, et al. Early knee osteoarthritis is evident one year following anterior cruciate ligament reconstruction: a magnetic resonance imaging evaluation. Arthritis Rheumatol. 2015;67:946–955. doi: 10.1002/art.39005. [DOI] [PubMed] [Google Scholar]
- 43.DeFrate LE, Sun H, Gill TJ, et al. In vivo tibiofemoral contact analysis using 3D MRI-based knee models. J Biomech. 2004;37:1499–1504. doi: 10.1016/j.jbiomech.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 44.Kiapour A, Kiapour AM, Kaul V, et al. Finite element model of the knee for investigation of injury mechanisms: development and validation. J Biomech Eng. 2014;136:011002. doi: 10.1115/1.4025692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van de Velde SK, Bingham JT, Hosseini A, et al. Increased tibiofemoral cartilage contact deformation in patients with anterior cruciate ligament deficiency. Arthritis Rheum. 2009;60:3693–3702. doi: 10.1002/art.24965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Ginckel A, Verdonk P, Witvrouw E. Cartilage adaptation after anterior cruciate ligament injury and reconstruction: implications for clinical management and research? A systematic review of longitudinal MRI studies. Osteoarthritis Cartilage. 2013;21:1009–1024. doi: 10.1016/j.joca.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 47.Kiapour AM, Fleming BC, Proffen BL, et al. Sex influences the biomechanical outcomes of anterior cruciate ligament reconstruction in a preclinical large animal model. Am J Sports Med. 2015;43:1623–1631. doi: 10.1177/0363546515582024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scanlan SF, Chaudhari AMW, Dyrby CO, et al. Differences in tibial rotation during walking in ACL reconstructed and healthy contralateral knees. J Biomech. 2010;43:1817–1822. doi: 10.1016/j.jbiomech.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hart HF, Collins NJ, Ackland DC, et al. Gait characteristics of people with lateral knee osteoarthritis after ACL reconstruction. Med Sci Sports Exerc. 2015;47:2406–2415. doi: 10.1249/MSS.0000000000000671. [DOI] [PubMed] [Google Scholar]


