Abstract
There is growing evidence in support of the involvement of inflammatory response in the pathogenesis of osteoarthritis (OA). Harpagoside, one of the bioactive components of Harpagophytum procumbens (Hp), has been shown to possess anti-inflammatory properties. Here we used an in vitro model of inflammation in OA to investigate the potential of harpagoside to suppress the production of inflammatory cytokines/chemokines such as IL-6 and matrix degrading proteases. We further investigated the likely targets of harpagoside in primary human OA chondrocytes. OA chondrocytes were pre-treated with harpagoside before stimulation with IL-1β. mRNA expression profile of 92 cytokines/chemokines was determined using TaqMan Human Chemokine PCR Array. Expression levels of selected mRNAs were confirmed using TaqMan assays. Protein levels of IL-6 and MMP-13 were assayed by ELISA and immunoblotting. Total protein levels and phosphorylation of signaling proteins were determined by immunoblotting. Cellular localization of IL-6 and c-Fos was performed by immunofluorescence and confocal microscopy. DNA binding activity of c-FOS/AP-1 was determined by ELISA. Harpagoside significantly altered the global chemokine expression profile in IL-1β-stimulated OA chondrocytes. Expression of IL-6 was highly induced by IL-1β, which was significantly inhibited by pre-treatment of OA chondrocytes with harpagoside. Harpagoside did not inhibit the IL-1-induced activation of NF-κB and C/EBPβ transcription factors but suppressed the IL-1β-triggered induction, phosphorylation and DNA binding activity of c-FOS, one of the main components of AP-1 transcription factors. Further, harpagoside significantly inhibited the expression of MMP-13 in OA chondrocytes under pathological conditions. siRNA-mediated knockdown of IL-6 resulted in suppressed expression and secretion of MMP-13 directly linking the role of IL-6 with MMP-13 expression. Taken together, the present study suggests that harpagoside exert a significant anti-inflammatory effect by inhibiting the inflammatory stimuli mediated by suppressing c-FOS/AP-1 activity in OA chondrocytes under pathological conditions.
Keywords: Osteoarthritis, Harpagoside, IL-6, MMP-13, c-FOS/AP-1
Introduction
Osteoarthritis (OA) is a global degenerative disease of the articulating joints and is increasingly recognized to have a significant inflammatory component. Etiology of OA is multifactorial but joint trauma, genetics, age related wear and tear or obesity are believed to be important risk factors. When clinically evident, OA is characterized by joint pain, tenderness, limitation of movement, crepitus, occasional effusion, and variable degrees of inflammation without systemic effects. Innate immune response elicited against the initial damage leads to a state of chronic articular inflammation that further results in propagation and progression of cartilage degradation and develops into clinical OA1–4.
High levels of a number of cellular and soluble factors including inflammatory cytokines, such as IL-1β and chemokines have been shown to be present in the OA joint4. IL-1β plays a pivotal role in OA pathogenesis by triggering a cascade of cartilage damage events such as enhanced production of IL-6, MMPs, COX-2, PGE2 and NO2–6. IL-6 is one of the three major inflammatory cytokine, along with IL-1β and TNF-α, implicated in the cartilage damage due to inflammation7. Significant correlations between levels of IL-6 in the serum as well as in synovial fluid and OA severity have been reported8–10. IL-6 brings about the damage to the cartilage by acting on both anabolic and catabolic mechanisms of cartilage physiology. IL-6 has been found to be a crucial mediator of MMP-13 levels in HIF-2-α induced OA11. In combination with IL-1β and Oncostatin M, IL-6 up-regulates the production of catabolic enzyme MMP-1312. In other studies, IL-6 has been shown to inhibit the expression of type II collagen15.
Extracts of the secondary roots of Harpagophytum procumbens (Devil’s claw) have been used since centuries without notable side effects as a traditional therapy to treat inflammatory diseases16. Recently, in Europe it has been recommended for the management of pain and inflammation in OA17. Several clinical trials have shown good tolerability and anti-inflammatory effects of Hp-containing products18. Harpagoside and harpagide are the two main bioactive components found in the roots of Hp and have been implicated in its ant-inflammatory effects16. Harpagoside has been shown to inhibit indistinctively both COX-1 and COX-2 (37.2 and 29.5%, respectively) activity and greatly inhibited NO production in vitro19. Harpagoside, has also been reported to inhibit the production of IL-1β, IL-6, and TNF-α by RAW 264.7 mouse macrophages20. However, the effect of harpagoside on IL-1β-induced inflammatory response of OA chondrocytes has not been fully elucidated.
In the present study we determined the IL-1β-induced expression profile of chemokines and cytokines in human OA chondrocytes. We found that the expression of IL-6 mRNA and protein was highly increased in IL-1β-stimulated human OA chondrocytes. Next, we studied the effect of harpagoside on IL-1β-induced expression of IL-6 and elucidated the mechanism of IL-6 suppression by harpagoside in human OA chondrocytes.
Materials and Methods
Reagents
Media and other reagents used for cell culture were purchased from HyClone Laboratories (Logan, UT) or from Life Technologies Corp (Carlsbad, CA). Pronase and collagenase A were purchased from Roche Diagnostics (Indianapolis, IN). Recombinant human IL-1β was purchased from R&D Systems (St. Paul, MN, USA). JNK inhibitor SB600125 was from Calbiochem (EMD Millipore, Rockland, MA). c-Fos inhibitor T-5224 was purchased from MedChem Express (Monmouth Junction, NJ). Primary antibodies against IL-6, c-Jun, MMP-13 and β-actin were from Santa Cruz Biotechnologies (Santa Cruz, CA). Primary antibodies against c-FOS, P-c-FOS, P-c-Jun, p65, P-p65, C/EBPβ and P-C/EBPβ were from Cell Signaling Techonology (Beverly, MA). Harpagoside (99.9% pure by HPLC) was purchased from ChromaDex Corp (Irvine, CA) and INDOFINE Chemical Company, Inc., (Hillsborough, NJ). Gene specific TaqMan assays were purchased from Life Technologies Corp.
Human cartilage samples and Preparation of primary human chondrocytes
The study protocol was reviewed and approved by the Institutional Review Board of North East Ohio Medical University, Rootstown, OH as exempt and that no informed consent was needed. Discarded and de-identified knee or hip joint cartilage samples were collected from the donors who underwent total joint replacement surgery at Summa St. Thomas Hospital, Akron, OH. Cartilage was resected from macroscopically unaffected areas and chondrocytes were prepared by sequential digestion with Pronase (1 mg/ml) for 1–2 hr followed by Collagenase A (1 mg/ml) digestion overnight. Chondrocytes (1.5 × 106/60 mm dish) allowed to grow in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% Fetal Calf Serum (FCS), 100 U/ml Pennicillin and 100 mg/ml Streptomycin for 2–3 days after plating and only primary (unpassaged) chondrocytes were used in the experiments. At about 80% confluence, OA chondrocytes were serum starved overnight and were then treated with inhibitors for 2 hrs followed by stimulation with IL-1β.
Cell Viability Assay
To test whether harpagoside shows any adverse effects on cell survival, we performed cell viability assay using CytoTox 96 non-radioactive cytotoxicity assay kit (Promega Corp, Madison, WI) as described by the manufacturer. Briefly, primary human OA chondrocytes were seeded in a 96-well cell culture plate at a density of 18,000 cells/well in 0.2 ml complete culture media. At confluence, cells were serum starved overnight followed by treatment with different concentrations of harpagoside (100, 200, 300 and 400 μg/ml) for 24 h in 0.1 ml of serum free media. Cells treated with DMSO (vehicle) were used as control. Cells were lysed by 1 % Triton X-100. 50 μl of the cell lysate was transferred to a new 96-well assay plate. 50 μl of the supplied substrate mix was added to the cell lysates. The plate was incubated for 30 minutes at room temperature, protected from light. 50 μl of stop solution was added to each well. Absorbance was recorded at 490 nm using Synergy H1 multi-mode plate reader (BioTek Instruments Inc., Winooski, VT).
Global chemokine profiling by TaqMan PCR array
Primary human OA chondrocytes were serum starved overnight and then treated with harpagoside (300 μg/ml) for 2 hrs followed by treatment with IL-1β (10 ng/ml) for 16 hrs. RNA was isolated using RNeasy mini kit (Qiagen, Valencia, CA) and quantitated by spectrophotometry at 260 nm and 500 ng of the RNA was used to synthesize cDNA using High-Capacity cDNA synthesis kit (Life Technologies). Chemokine profiling was performed using TaqMan human chemokine PCR array (Life Technologies) and StepOne Plus real-time PCR system. Data were analyzed using Data Assist software (Life Technologies). We used MyD88 and 18S rRNA for normalization as these genes showed the best stability score calculated using geNorm algorithm.
Western immunoblotting
Western immunoblotting for proteins of interest was performed as previously described21. Briefly, after treatments, OA chondrocytes were washed once with ice-cold PBS and were lysed in RIPA buffer (50 mM Tris-HCl, pH 7.5; 150 mM NaCl; 1% IGEPAL, 4 mM EDTA, 0.1% sodium deoxycholate; 10 mM Na4P2O7, 10 mM NaF, 2 mM Na3VO4, 1 mM PMSF, 1 μg/ml leupeptin, 1 μg/ml aprotinin) in the dishes on ice for 15 minutes. The cell lysates were cleared of cell debris by centrifugation at 13,000 rpm at 4 °C for 5 minutes. Total protein concentration was estimated using DC protein assay kit (BioRad Laboratories, Hercules, CA). Equal amounts of total proteins were resolved by SDS-PAGE and transferred to PVDF membrane. The membranes were blocked using 4% bovine serum albumin (BSA) prepared in 0.1% TBS-T for 1 hr at room temperature. Blocked membranes were incubated overnight at 4 °C with primary antibodies diluted in the blocking buffer. Membranes were washed and then incubated with secondary antibodies for 1 hr at room temperature followed by another wash. Blots were developed using Luminata Western HRP substrate (EMD Millipore). The immunoreactive proteins were visualized by chemiluminescence and imaged using PXi gel imaging system (Syngene, Frederick, MD).
RNA isolation and real time PCR analysis of gene expression
Total RNA was isolated using RNeasy mini kit (Qiagen) on Qiacube automated sample prep platform (Qiagen) according to the manufacturer’s instructions. Total RNA concentration was determined by absorbance at 260 nm using Nanodrop spectrophotometer (Thermo Fisher). cDNA synthesis was performed using the High Capacity cDNA Reverse Transcription kit (Life Technologies Corp) using 250–500 ng of total RNA according to the instructions provided with the kit. One μl from a 20 μl cDNA synthesis reaction mixture was used for TaqMan assays using the StepOne Plus real-time PCR system (Life Technologies Corp). Relative quantification was performed using ΔΔCT method with β-actin as an endogenous control.
Immunofluorescence Staining of OA chondrocytes
Chondrocytes (0.1×106) were seeded in 8-well chamber slide and pre-treated with harpagoside followed by treatment with IL-1β. After completion of treatment cells were fixed with 4% paraformaldehyde and permiabilzed in 0.3% Triton X-100 in phosphate buffered saline. The cells were probed with anti-IL-6 primary antibody (Santa Cruz Biotechnologies) or anti-c-FOS antibodies (Cell Signalling Technologies) followed by anti-mouse or ant-rabbit Alexa Fluor 546 secondary antibodies (Life Technologies). Cells were mounted using antifade mounting medium containing DAPI stain (Vectashield, Burlingame, CA) and visualized by Olympus FV1000 confocal microscope.
IL-6 ELISA
After the treatment of chondrocytes, culture supernatants were collected, cleared by centrifugation (14,000 rpm, 5 min, 4 °C) and frozen at −80°C until assayed. IL-6 concentration was measured in the supernatants by enzyme-linked immunosorbent assay (ELISA; Boster Immunoleader, Pleasanton, CA) according to the manufacturer’s directions.
Measurement of c-FOS/AP-1 DNA binding activity by ELISA
Activation of c-FOS/AP-1 and DNA-binding activity assay was performed using Trans-AM ELISA kit (Active Motif, Carlsbad, CA) according to the manufacturer’s protocol. Briefly, chondrocytes were treated as described above for the indicated times and nuclear extracts were prepared using the nuclear extract kit (Active Motif). After clearing by centrifugation, 20 μg of the nuclear extract was added to the 96-well plates coated with oligonucleotide containing the c-FOS/AP-1 consensus binding site and incubated at room temperature for 1 hr. Wells were washed and primary antibody was added to the wells to bind the target protein in the extract. After incubation for 1 hr, the antibody was removed, and 100 μl of HRP-conjugated secondary antibody was added to the wells and incubated for 1 hr. After thorough washing, 100 μl of developing solution was added, incubated for 2–10 min, and then 100 μl of stop solution was added. The absorbance at 450 nm was determined using the Synergy H1 multi-mode plate reader (BioTek Instruments).
siRNA-mediated depletion of IL-6 expression
Scrambeled as well as anti-IL-6 shRNA clones were purchased from Origene (Rockville, MD). One million primary human articular chondrocytes were transfected with μg the shRNA plasmids using P3 Primary Cell 4D-Nucleofector™ X Kit on 4D-Nucleofector equipment (Lonza, Walkersville, MD) following the instructions provided by the manufacturers. Transfected cells were plated in 6-well plates. Forty-eight hours after the transfections cells were serum starved overnight followed by treatment with IL-1β (0.1 ng/ml) in serum free medium for 16 hrs. Gene expression levels were measured by quantitative PCR using the TaqMan assay system. Protein expression levels of secreted IL-6 and secreted MMP-13 in the culture supernatants were measured by immunoblotting.
Results
Harpagoside had no adverse effects on primary human chondrocytes viability
Treatment of harpagoside at concentrations up to 400 μg/ml did not show any significant cytotoxic effects as compared to vehicle-treated control cells (data not shown).
Harpagoside altered the global chemokine and cytokine expression profile in IL-1β-stimulated OA chondrocytes
Figure 1 shows the profile of 30 most differentially regulated chemokines and related genes in response to IL-1β treatment in the presence and absence of harpagoside. The up-regulation of a number of genes by IL-1β was significantly diminished when cells were pre-treated with harpagoside. Similarly, harpagoside reduced the down-regulation of a number of genes in response to IL-1β. (The detailed list of all the 92 genes and their expression levels is provided as a supplement supplementary data) The PCR array data of a subset of eight genes were validated in primary human OA chondrocytes isolated from four donors and treated with IL-1β and harpagoside as detailed above by TaqMan assay (Table 1). Even though the degree of over-expression of these genes in response to IL-1β varied among the donors, harpagoside showed consistent suppression in the expression of all the genes in all the donors. These results were in agreement with the results obtained by PCR array profiling.
Figure 1. Effect of harpagoside on IL-1β-induced chemokine profile in primary human OA chondrocytes.
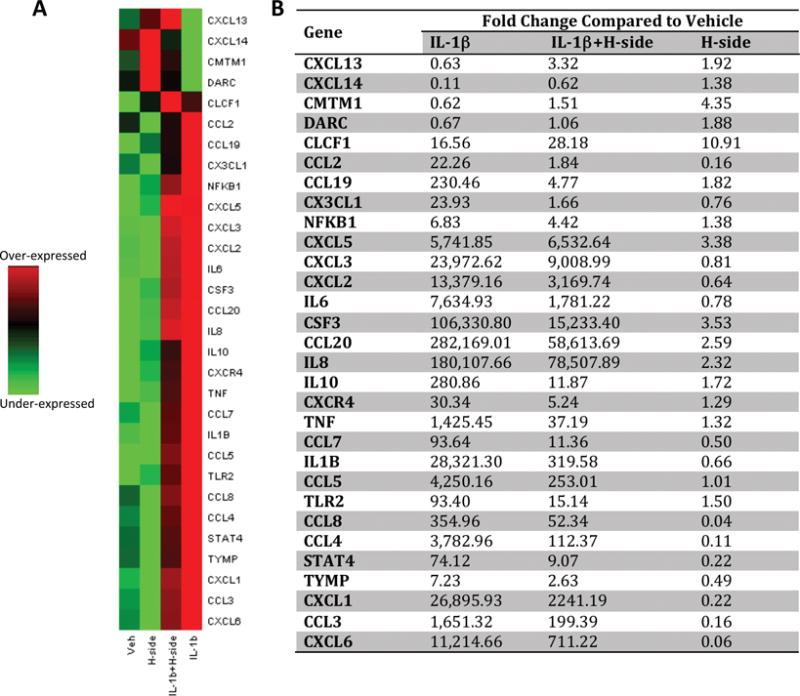
Primary human OA chondrocytes were pre-treated with harpagoside (300 μg/ml) for 2 hrs followed by treatment with IL-1β (10 ng/ml) for 16 hrs. mRNA expression of 92 chemokines and associated genes was profiled by human chemokine PCR array (Life Technologies) using StepOne Plus real-time PCR system. Data were analyzed on DataAssist v3.01 (Life Technologies). MyD88 and 18S rRNA were used for normalization. A. Heat maps of 30 genes that showed high differential expression in response to IL-1β. Assay centric map type is presented here where, for each gene, the middle expression level is set as the mean of all the ΔCT values from all samples for that gene. Green color represents suppressed expression and red color represents increased expression. B. Fold changes calculated by ΔΔCT method in samples treated with IL-1β, IL-1β+harpagside and Harpagoside alone compared to vehicle control are presented. (The detailed list of all the genes and their expression levels is provided as a supplement file).
Table 1.
Relative quantity (RQ) values of eight genes in primary human chondrocytes isolated from four donors in response to IL-1β and harpagoside.
| RQ (Vehicle) | RQ (IL-1β) | RQ (IL-1β+H-side) | RQ (H-side) | |
|---|---|---|---|---|
| CXCL2 | ||||
| Donor 1 | 1.00 | 1607.01 | 384.34 | 0.53 |
| Donor 2 | 1.00 | 5605.98 | 625.12 | 0.44 |
| Donor 3 | 1.00 | 138.16 | 74.18 | 0.58 |
| Donor 4 | 1.00 | 15566.89 | 5525.17 | 1.57 |
| CSF3 | ||||
| Donor 1 | 1.00 | 862.31 | 57.20 | 1.21 |
| Donor 2 | 1.00 | 1773.83 | 123.00 | 2.04 |
| Donor 3 | 1.00 | 3023.85 | 618.84 | 1.21 |
| Donor 4 | 1.00 | 28095.31 | 4923.19 | 4.78 |
| CCL20 | ||||
| Donor 1 | 1.00 | 5010.55 | 1509.45 | 1.25 |
| Donor 2 | 1.00 | 82421.19 | 22632.37 | 1.86 |
| Donor 3 | 1.00 | 7597.00 | 4460.28 | 1.85 |
| Donor 4 | 1.00 | 115619.41 | 24667.53 | 3.19 |
| CCL5 | ||||
| Donor 1 | 1.00 | 136.51 | 21.20 | 0.60 |
| Donor 2 | 1.00 | 2306.85 | 269.56 | 2.16 |
| Donor 3 | 1.00 | 591.73 | 160.91 | 1.39 |
| Donor 4 | 1.00 | 5818.22 | 422.63 | 1.54 |
| CXCL1 | ||||
| Donor 1 | 1.00 | 655.85 | 110.96 | 0.10 |
| Donor 2 | 1.00 | 7169.35 | 392.42 | 0.24 |
| Donor 3 | 1.00 | 2167.72 | 277.70 | 0.20 |
| Donor 4 | 1.00 | 35417.50 | 3368.42 | 0.58 |
| IL-8 | ||||
| Donor 1 | 1.00 | 9315.62 | 4123.86 | 1.27 |
| Donor 2 | 1.00 | 56529.69 | 17000.97 | 3.16 |
| Donor 3 | 1.00 | 9359.40 | 5681.78 | 1.50 |
| Donor 4 | 1.00 | 123804.53 | 65669.66 | 2.84 |
| IL-10 | ||||
| Donor 1 | 1.00 | 58.72 | 5.00 | 0.37 |
| Donor 2 | 1.00 | 295.45 | 20.16 | 0.66 |
| Donor 3 | 1.00 | 67.34 | 16.46 | 0.62 |
| Donor 4 | 1.00 | 449.04 | 26.26 | 2.14 |
| TNF-α | ||||
| Donor 1 | 1.00 | 39.66 | 4.96 | 0.23 |
| Donor 2 | 1.00 | 724.02 | 53.59 | 4.73 |
| Donor 3 | 1.00 | 253.46 | 27.09 | 1.80 |
| Donor 4 | 1.00 | 3188.07 | 98.75 | 3.25 |
Harpagoside inhibited the IL-1β-induced IL-6 expression
As IL-6 has emerged as an important inflammatory factor associated with pathogenesis of OA and was one of the most differentially expressed genes in response to IL-1β that was significantly suppressed by harpagoside (Fig. 1), we focused on the mechanism of suppression of IL-6 expression by harpagoside in human OA chondrocytes. As shown in Figure 2A, pre-treatment of primary human OA chondrocytes with harpagoside significantly blocked the induction of IL-6 mRNA in response to IL-1β, confirming the data obtained using the PCR array experiment. We also found that protein levels of IL-6, both secreted and cytoplasmic, were also significantly suppressed by pre-treatment of human OA chondrocytes with harpagoside (Fig. 2A&2B).
Figure 2. Effect of harpagoside on IL-1β-induced IL-6 expression in primary human chondrocytes.
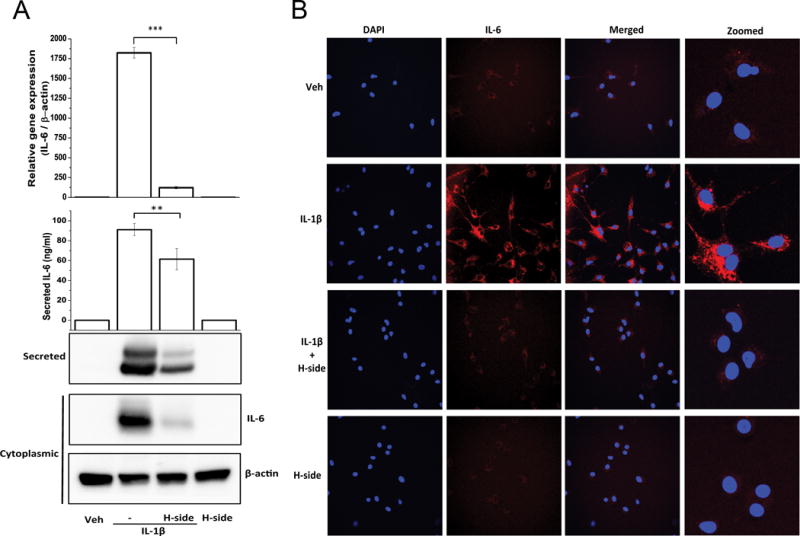
Primary human chondrocytes were pre-treated with harpagoside for 2 hrs followed by treatment with IL-1β (0.1 ng/ml) for 16 hrs. A. Gene expression levels were measured by quantitative PCR using the TaqMan assay system (Life Technologies) (top panel). Secreted levels of IL-6 in the culture supernatant were measured by ELISA and by immunoblotting (middle panel). Cytoplasmic levels of IL-6 were measured by immunoblotting (bottom panel). ELISA and qPCR values are expressed as mean±SD of three different subjects. Immunoblot results are representatives of three blots performed on samples obtained from three individuals. **, p<0.01; ***, p<0.001. B. Cytoplasmic levels of IL-6 were further visualized by immunofluorescence using IL-6-specific antibody on Olympus FV1000 confocal microscope.
Harpagoside blocked the IL-1β-induced activation of c-FOS/AP-1 but not of NF-κB and C/EBPβ
Transcription of IL-6 is regulated by many transcription factors including AP-1, NF-κB, C/EBP and SP-1. Figure 3A shows the binding sites of AP-1, NF-κB and C/EBPβ in the proximal promoter of IL-6 gene. We next checked the effect of harpagoside on the activation of AP-1 in IL-1β-stimulated OA chondrocytes. AP-1 is mainly formed by homo- or hetero-dimerization of c-FOS and c-Jun. There was a significant inhibition in the expression, phosphorylation and binding activity of c-FOS when human chondrocytes were pre-treated with harpagoside followed by IL-1β treatment as compared to IL-1β alone (Fig. 3B & 3C). As shown in figure 3C, c-FOS was mainly localized in the nucleus even without IL-1β treatment. The immunofluorescence analysis also shows the suppression of IL-1β-induced c-FOS expression in the presence of harpagoside. Activation of c-Jun, as shown by its phosphorylation, was not affected by harpagoside treatment. Thus the effect was specific to c-FOS.
Figure 3. Effect of harpagoside on IL-1β-induced activation of AP-1 C/EBPβ and NF-κB.
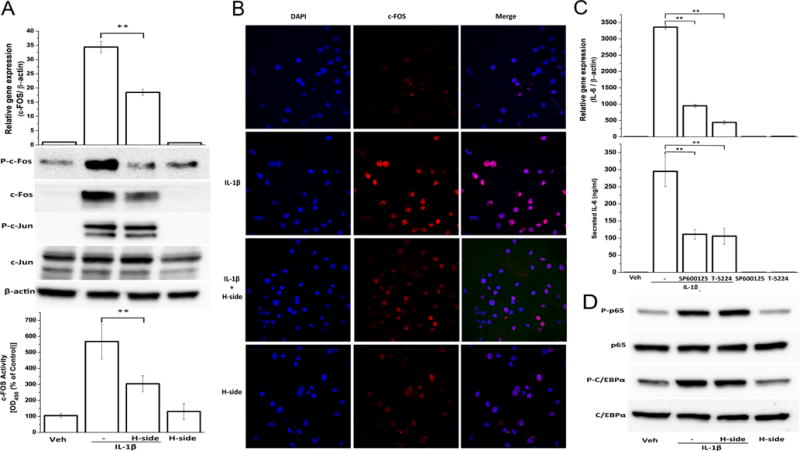
A. Primary human chondrocytes isolated from three individuals were pre-treated with harpagoside for 2 hrs followed by treatment with IL-1β for 45 minutes. RNA levels were quantitated by qPCR. Total protein levels and phosphorylation levels of c-FOS and c-Jun were quantitated by immunoblotting. Binding activity of c-FOS/AP-1 from nuclear extracts of the cells treated as above to its consensus sequence was quantitated by a specific ELISA assay (Active Motif). B. Sub-cellular localization of c-FOS was performed by immunofluorescence using c-FOS-specific antibody on Olympus FV1000 confocal microscope. C. Involvement of AP-1 and, more specifically, c-FOS in the induction of IL-6 by IL-1β was examined by using small molecule inhibitors SP600125, which inhibits AP-1 by inhibiting JNK, and T-5224, a novel selective inhibitor of c-FOS/AP-1. D. Total protein levels and phosphorylated forms of p65/NF-κB and C/EBPβ were quantitated by immunoblotting. **, p<0.01.
In order to confirm the involvement of AP-1 in IL-6 induction in response to IL-1β, we used two small molecule inhibitors of AP-1. SP600125 indirectly inhibits AP-1 activation by inhibiting JNK activity22 and T-5224 is a novel selective inhibitor of AP-1 that specifically binds to c-FOS23. As shown in figure 3D, induction of IL-6 in response to IL-1β was inhibited by both the inhibitors used indicating that AP-1 is essential for IL-6 expression in IL-1β-stimulated OA chondrocytes.
We next checked the effect of harpagoside on the activation of NF-κB and C/EBPβ transcription factors in IL-1β-stimulated OA chondrocytes as these factors have their binding sites in the promoter region of IL-6 gene and are known to regulate its expression in response to IL-1β24. As indicated by phosphorylation, these transcription factors were strongly activated by IL-1β but harpagoside did not inhibit their phosphorylation indicating that activation of NF-κB and C/EBPβ was not impaired in OA chondrocytes pretreated with harpagoside and then stimulated with IL-1β (Fig. 3E).
Involvement of IL-1β-induced ROS generation in the activation of c-FOS and IL-6 expression in primary human OA chondrocytes
As IL-1β is known to stimulate the production of ROS in chondrocytes25 we next studied whether harpagoside exerted its inhibitory effect on the expression of c-FOS and IL-6 via suppression of ROS production. As shown in Fig. 4A, both N-acetyle cysteine (NAC) and diphenyleneiodonium (DPI) had a significant inhibitory effect on IL-1β-induced mRNA expression of IL-6. NAC and DPI also inhibited the expression and phosphorylation level of c-FOS. Figure 4B shows the effect of harpagoside on H2O2-induced IL-6 expression and the expression and activation of c-FOS. Compared to IL-1β, treatment with H2O2 had only a marginal effect on IL-6 expression in human OA chondrocytes and Harpagoside suppressed the induction of both IL-6 and c-FOS in H2O2-stimulated human OA chondrocytes.
Figure 4. Involvement of ROS in IL-6 expression and effect of harpagoside.
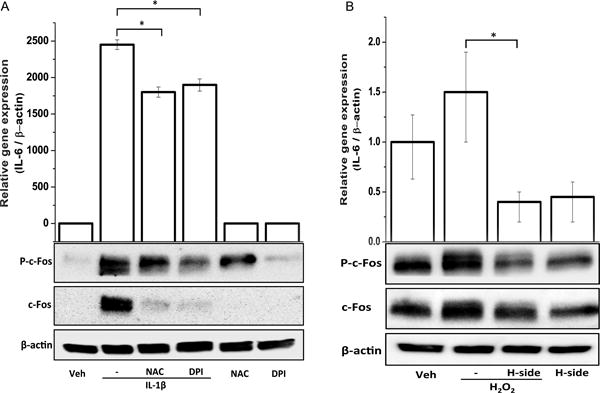
Primary human chondrocytes isolated from three individuals were pre-treated with harpagoside (300 μg/ml), NAC (1 mM) or DPI (10 μM) for 2 hrs followed by treatment with IL-1β (A) or H2O2 (B) overnight. Gene expression levels of IL-6 were measured by quantitative PCR using the TaqMan assay system. Levels of total and phosphorylated forms of c-FOS were determined by Western immunoblotting. *, p<0.05.
Harpagoside inhibited the IL-1β-induced expression of MMP-13
IL-6 is a known inducer of MMP-13 expression in OA chondrocytes14, 26 and MMP-13 has been shown to be one of the most important matrix degradation factors involved in the development of OA27; 28. In this study, we checked whether inhibition of IL-6 expression by harpagoside also results in reduced expression of MMP-13 in human chondrocytes. As shown in Figure 5, OA chondrocytes pre-treated with harpagoside had a dramatic reduction in the expression of MMP-13, both at mRNA and protein levels, in response to IL-1 as compared to control cells. This suggests that harpagoside is also a potent suppressor of MMP-13 in OA chondrocytes under pathological conditions.
Figure 5. Effect of harpagoside on IL-1β-induced expression of MMP-13 in primary human chondrocytes.
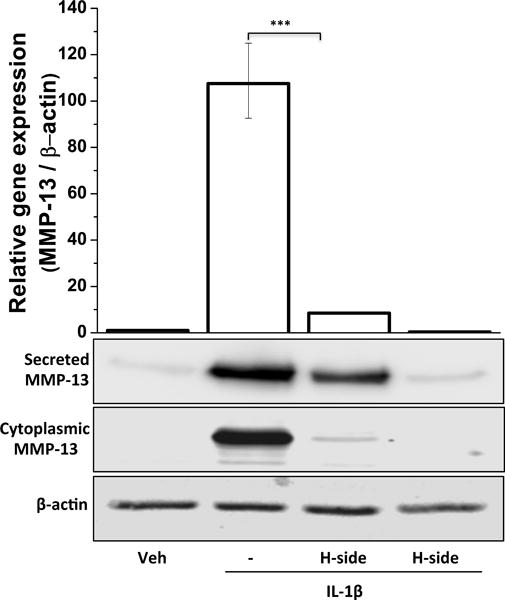
Primary human chondrocytes were pre-treated with harpagoside for 2 hrs followed by treatment with IL-1β (10 ng/ml) for 16 hrs. Gene expression levels were measured by quantitative PCR using the TaqMan assay system (Life Technologies) (top panel). Protein expression levels of MMP-13 were measured by immunoblotting (bottom panel). β-actin was used as a loading control. Immunoblot results are representatives of three blots performed on samples obtained from three individuals. ***, p<0.001.
Expression of IL-6 is necessary for IL-1β-induced expression of MMP-13
As shown in figure 6, all the four shRNAs significantly depleted the IL-1β-induced high level expression of IL-6 mRNA as well as of secreted IL-6 protein in the culture medium. Depletion of IL-6 expression was found to be directly correlated with the suppression of the mRNA and secreted protein levels of MMP-13. These results, along with results reported above, establish a direct role of IL-6 in the IL-1β-induced expression of MMP-13 in OA chondrocytes.
Figure 6. Effect of siRNA-mediated depletion of IL-6 on IL-1β-induced expression of MMP-13 in primary human OA chondrocytes.
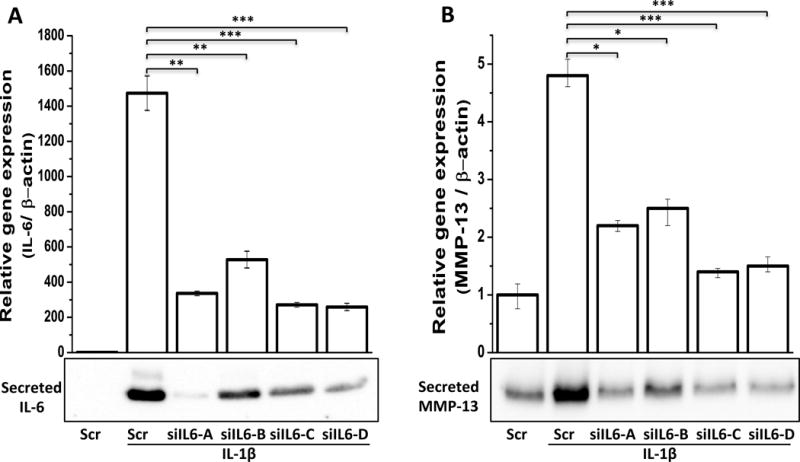
Primary human OA chondrocytes were transfected with plasmids containing scrambeled shRNA or four shRNAs against IL-6 gene (siIL6-A-B). Forty-eight hours after the transfections, OA chondrocytes were serum starved followed by stimulation with IL-1β (0.1 ng/ml) for 16 hrs. Gene expression levels were measured by quantitative PCR using the TaqMan assay (Life Technologies) (top panel). Protein expression levels of secreted IL-6 and secreted MMP-13 were measured by immunoblotting (bottom panel). *, p<0.05; **, p<0.01 ***, p<0.001.
Discussion
The symptoms associated with the degradation of the articular cartilage are pain, stiffness, inflammation of the joint and loss of mobility29. Non-steroidal anti-inflammatory drugs (NSAIDs) currently used in OA to suppress pain and inflammation have deleterious side effects on the gastrointestinal tract, kidney and liver30. Due to the adverse effects of conventional OA drugs such as NSAIDs and corticosteroids, and their inability to suppress the progression of the disease there is immense need for the development of such pharmacological agents that can have less side effects and can suppress the degradation of the joint tissue.
Globally, there is a rise in the use of natural health products to treat OA symptoms31, 32. Use of these products is largely based on anecdotal evidence and their safety, efficacy and mechanism(s) of action are poorly understood. Extracts from the roots of Devil’s Claw (Harpagophytum procumbens) plants, a member of the sesame family found in the Kalahari region in South Africa, is being used to treat degenerative joint disorders in several European and African countries33. This product has been shown to have anti-inflammatory and analgesic effects34 with lesser adverse symptoms compared to NSAIDs making it a potential therapeutic agent in OA35. Harpagoside, a small molecule of iridoid family, is the main bioactive component of the Hp root extracts. In the present study we investigated the anti-inflammatory and chondroprotective effects of harpagoside in IL-1β-induced primary human chondrocytes and elucidated its mechanism of action.
Inflammation has now emerged as a characteristic feature of OA pathophysiology1–4. Inflammatory cytokines such as IL-1β, TNF-α and IL-6 are present at high levels in the OA joint and are the major mediators of disturbed chondrocyte function and cartilage degeneration. IL-1β is considered as the most important cytokine involved in the pathogenesis of OA because of its ability to affect both catabolic and anabolic processes in the joint as well as its ability to stimulate other inflammatory cytokines and chemokines36. In the present study using a PCR array of 92 chemokines we show that IL-1β strongly stimulated the expression of a number of pro-inflammatory cytokines and chemokines and suppressed the expression of anti-inflammatory factors in cultured primary human OA chondrocytes. Harpagoside significantly suppressed the stimulatory or inhibitory effect of IL-1β on the expression of these factors More specifically, when we investigated the role of harpagoside on the expression of IL-6, which was among the highest stimulated factors in response to IL-1β, we noticed a dramatic suppression in the expression of IL-6 in the presence of harpagoside. Since we also discovered that the expression of IL-6 mRNA was suppressed we suspected that harpagoside might be acting through the suppression of its transcription. Earlier studies from other laboratories have established the role of the transcription factors C/EBPβ, NF-κB and AP-1 in the transcriptional regulation of IL-624. Harpagoside had no effect on NF-κB and C/EBPβ activation in IL-1β-stimulated OA chondrocytes. However, a significant suppression in the expression and activation of c-FOS, that is one of the two main components of AP-1 transcription factor, was observed. c-Jun, the other major component of AP-1 was not affected by harpagoside in IL-1β-stimulated OA chondrocytes.
Since earlier studies have shown that ROS stimulates the expression of IL-637 and activates c-FOS38, we checked whether harpagoside suppressed IL-6 expression and c-FOS activation by diminishing cellular ROS levels. We found a very slight increase in the expression of IL-6 in response to hydrogen peroxide, which was significantly suppressed by pre-treatment of the cells with harpagoside. The role of ROS in IL-1β induced IL-6 expression was further confirmed by using N-acetyl cysteine (NAC) and diphenyleneiodonium (DPI), two strong anti-oxidants that inhibit two different ROS producing systems. We found a slight, but significant, reduction in mRNA expression in the presence of NAC and DPI. These data suggest that ROS plays very little role in expression of IL-6 in response to IL-1β and harpagoside’s role in its suppression is not through suppression of cellular ROS. Instead, harpagoside appears to be acting very specifically via suppressing the expression and activation of c-FOS in OA chondrocytes through a different mechanism. This is further supported by the fact that harpagoside, as opposed to total Hp root extracts, shows weak anti-oxidant activity in assays conducted in our lab (data not shown) and reported by others39.
MMPs are a family of matrix degrading enzymes that are produced by chondrocytes and synoviocytes in the articular joints. MMP-13 is one of the most important collagenases involved in the degradation of type II collagen during OA40. High levels of IL-6 have been associated with increased production of MMP-1311–13. Role of AP-1 in the transcriptional regulation of MMP-13 is well-established41. But the interplay between IL-6 and AP-1 in MMP-13 regulation is not clear. Even though earlier studies have shown the ability of IL-6 to induce MMP-13 expression in chondrocytes, we for the first time confirmed the role of IL-6 in IL-1β-induced over-expression by siRNA-mediated knockdown of IL-6 gene. Here we also show a dramatic suppression of MMP-13 expression by harpagoside that may be manifested by the suppression of both AP-1 and IL-6. These in vitro data suggest that harpagoside might protect the cartilage from degradation during OA by acting at multiple targets and may be a potential therapeutic agent to treat OA. But, further in vivo studies will be needed to establish its therapeutic potential.
AP-1 is a major transcription factor that directly controls the expression of several inflammatory cytokines, chemokines and matrix metalloproteinase’s (MMPs) by binding directly to AP-1 motifs in the promoter region of these genes42–48. IL-1β exerts its effects on the expression of a number of genes by mainly activating NF-B and AP-1 transcription factors leading to inflammation and joint destruction. There is extensive cross talk between AP-1 and NF-κB pathways downstream in the IL-1β signaling. These findings make AP-1 an important drug target to treat inflammatory and degenerative diseases like OA. Recently, Aikawa et al reported strong anti-arthritic effects of a specific c-FOS/AP-1 inhibitor, T-5224, by suppressing the production of MMPs and inflammatory cytokines including IL-623. T-5224 showed a high potential of binding to the c-FOS/c-Jun complex, thus inhibiting their DNA binding activity23. In the present study we show the suppression in c-FOS expression by harpagoside in response to IL-1β. Thus harpagoside might be acting via inhibiting the regulatory mechanism of c-FOS transcription and not by binding to c-FOS protein itself. It will be interesting in future studies to find the molecule(s) to which harpagoside might specifically bind and exert its inhibitory effect on c-FOS expression.
In conclusion, we show here that harpagoside exerts anti-inflammatory effect by suppressing the expression of many cytokines/chemokines such as IL-6 and cartilage degrading enzyme MMP-13 by blocking the activation of c-FOS/AP-1 transcription factor in primary human OA chondrocytes (Figure-7). These findings provide further mechanistic insight to support the potential of harpagoside as a therapeutic approach in OA.
Figure 7. Schematic of proposed mechanism of anti-inflammatory effect of harpagoside.
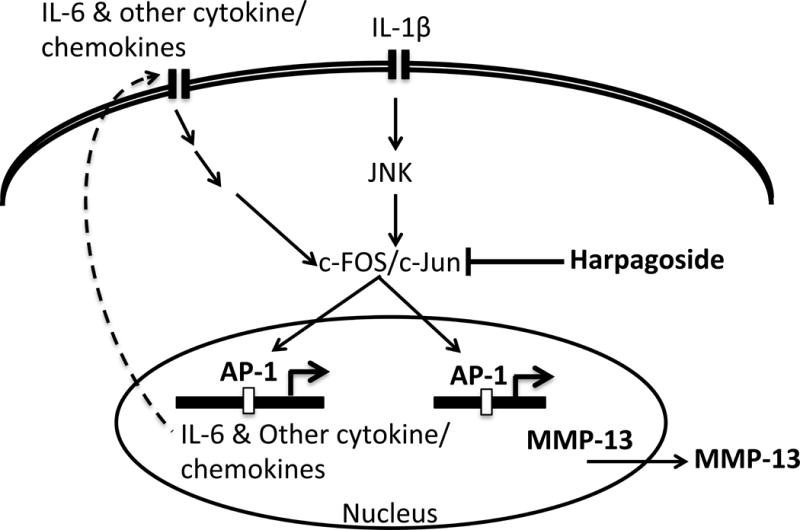
There is an increased production of IL-1β during OA that leads to activation of AP-1 transcription factor that regulates the expression of IL-6 and other cytokines/chemokines as well as MMP-13. IL-1β-induced production of cytokines/chemokine leads to further amplification of inflammatory signal by activating AP-1 and other transcription factors causing the induction of MMP-13 production. In this study we show that harpagoside inhibits the activation of AP-1 in response to IL-1β thus inhibiting the production of IL-6 and MMP-13. Additionally, harpagoside also inhibit the expression of other cytokines/chemokines in IL-1β-stimulated OA chondrocytes.
Supplementary Material
Acknowledgments
This work was supported in part by the National Institute of Health/National Center for Complimentary and Integrative Health grants RO1-AT-007373, RO1-AT-005520, RO1-AR-067056, R21-AR-064890) and funds from the Northeast Ohio Medical University (NEOMED) to TMH.
Footnotes
Author contribution: Abdul Haseeb: Conducted the experiment, interpreted the data and prepared the manuscript.
Mohammad Yunus Ansari: Conducted the experiment and contributed in preparing the manuscript.
Tariq M. Haqqi: Conceived the idea, interpreted the results and revised and edited the manuscript.
All authors have read and approved the final manuscript.
References
- 1.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51:249–257. doi: 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5:77–94. doi: 10.1177/1759720X12467868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felson DT. Clinical practice. Osteoarthritis of the knee. N Engl J Med. 2006;354:841–848. doi: 10.1056/NEJMcp051726. [DOI] [PubMed] [Google Scholar]
- 4.Haseeb A, Haqqi TM. Immunopathogenesis of osteoarthritis. Clin Immunol. 2013;146:185–196. doi: 10.1016/j.clim.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldring MB, Berenbaum F. Emerging targets in osteoarthritis therapy. Curr Opin Pharmacol. 2015;22:51–63. doi: 10.1016/j.coph.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malemud CJ. Biologic basis of osteoarthritis: state of the evidence. Curr Opin Rheumatol. 2015;27:289–294. doi: 10.1097/BOR.0000000000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wojdasiewicz P, Poniatowski LA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators of inflammation. 2014;2014:561459. doi: 10.1155/2014/561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stannus O, Jones G, Cicuttini F, et al. Circulating levels of IL-6 and TNF-alpha are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2010;18:1441–1447. doi: 10.1016/j.joca.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Tsuchida AI, Beekhuizen M, Rutgers M, et al. Interleukin-6 is elevated in synovial fluid of patients with focal cartilage defects and stimulates cartilage matrix production in an in vitro regeneration model. Arthritis research & therapy. 2012;14:R262. doi: 10.1186/ar4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsuda T, Horio F, Osawa T. Cyanidin 3-O-beta-D-glucoside suppresses nitric oxide production during a zymosan treatment in rats. Journal of nutritional science and vitaminology. 2002;48:305–310. doi: 10.3177/jnsv.48.305. [DOI] [PubMed] [Google Scholar]
- 11.Ryu JH, Yang S, Shin Y, et al. Interleukin-6 plays an essential role in hypoxia-inducible factor 2alpha-induced experimental osteoarthritic cartilage destruction in mice. Arthritis and rheumatism. 2011;63:2732–2743. doi: 10.1002/art.30451. [DOI] [PubMed] [Google Scholar]
- 12.Cawston TE, Curry VA, Summers CA, et al. The role of oncostatin M in animal and human connective tissue collagen turnover and its localization within the rheumatoid joint. Arthritis and rheumatism. 1998;41:1760–1771. doi: 10.1002/1529-0131(199810)41:10<1760::AID-ART8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 13.Rowan AD, Koshy PJ, Shingleton WD, et al. Synergistic effects of glycoprotein 130 binding cytokines in combination with interleukin-1 on cartilage collagen breakdown. Arthritis and rheumatism. 2001;44:1620–1632. doi: 10.1002/1529-0131(200107)44:7<1620::AID-ART285>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 14.Hashizume M, Mihara M. Desirable effect of combination therapy with high molecular weight hyaluronate and NSAIDs on MMP production. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2009;17:1513–1518. doi: 10.1016/j.joca.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 15.Poree B, Kypriotou M, Chadjichristos C, et al. Interleukin-6 (IL-6) and/or soluble IL-6 receptor down-regulation of human type II collagen gene expression in articular chondrocytes requires a decrease of Sp1.Sp3 ratio and of the binding activity of both factors to the COL2A1 promoter. The Journal of biological chemistry. 2008;283:4850–4865. doi: 10.1074/jbc.M706387200. [DOI] [PubMed] [Google Scholar]
- 16.Mncwangi N, Chen W, Vermaak I, et al. Devil’s Claw-a review of the ethnobotany, phytochemistry and biological activity of Harpagophytum procumbens. Journal of ethnopharmacology. 2012;143:755–771. doi: 10.1016/j.jep.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Brien S, Lewith GT, McGregor G. Devil’s Claw (Harpagophytum procumbens) as a treatment for osteoarthritis: a review of efficacy and safety. J Altern Complement Med. 2006;12:981–993. doi: 10.1089/acm.2006.12.981. [DOI] [PubMed] [Google Scholar]
- 18.Denner SS. A review of the efficacy and safety of devil’s claw for pain associated with degenerative musculoskeletal diseases, rheumatoid, and osteoarthritis. Holist Nurs Pract. 2007;21:203–207. doi: 10.1097/01.HNP.0000280932.65581.72. [DOI] [PubMed] [Google Scholar]
- 19.Anauate MC, Torres LM, de Mello SB. Effect of isolated fractions of Harpagophytum procumbens D.C. (devil’s claw) on COX-1, COX-2 activity and nitric oxide production on whole-blood assay. Phytother Res. 2010;24:1365–1369. doi: 10.1002/ptr.3124. [DOI] [PubMed] [Google Scholar]
- 20.Inaba K, Murata K, Naruto S, et al. Inhibitory effects of devil’s claw (secondary root of Harpagophytum procumbens) extract and harpagoside on cytokine production in mouse macrophages. J Nat Med. 2010;64:219–222. doi: 10.1007/s11418-010-0395-8. [DOI] [PubMed] [Google Scholar]
- 21.Haseeb A, Chen D, Haqqi TM. Delphinidin inhibits IL-1beta-induced activation of NF-kappaB by modulating the phosphorylation of IRAK-1(Ser376) in human articular chondrocytes. Rheumatology (Oxford) 2013;52:998–1008. doi: 10.1093/rheumatology/kes363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett BL, Sasaki DT, Murray BW, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aikawa Y, Morimoto K, Yamamoto T, et al. Treatment of arthritis with a selective inhibitor of c-Fos/activator protein-1. Nature biotechnology. 2008;26:817–823. doi: 10.1038/nbt1412. [DOI] [PubMed] [Google Scholar]
- 24.Grassl C, Luckow B, Schlondorff D, et al. Transcriptional regulation of the interleukin-6 gene in mesangial cells. J Am Soc Nephrol. 1999;10:1466–1477. doi: 10.1681/ASN.V1071466. [DOI] [PubMed] [Google Scholar]
- 25.Lo YY, Conquer JA, Grinstein S, et al. Interleukin-1 beta induction of c-fos and collagenase expression in articular chondrocytes: involvement of reactive oxygen species. J Cell Biochem. 1998;69:19–29. doi: 10.1002/(sici)1097-4644(19980401)69:1<19::aid-jcb3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki M, Hashizume M, Yoshida H, et al. IL-6 and IL-1 synergistically enhanced the production of MMPs from synovial cells by up-regulating IL-6 production and IL-1 receptor I expression. Cytokine. 2010;51:178–183. doi: 10.1016/j.cyto.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis and rheumatism. 2001;44:585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell PG, Magna HA, Reeves LM, et al. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97:761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377:2115–2126. doi: 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- 30.Berenbaum F. New horizons and perspectives in the treatment of osteoarthritis. Arthritis research & therapy. 2008;10(Suppl 2):S1. doi: 10.1186/ar2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Long L, Soeken K, Ernst E. Herbal medicines for the treatment of osteoarthritis: a systematic review. Rheumatology (Oxford) 2001;40:779–793. doi: 10.1093/rheumatology/40.7.779. [DOI] [PubMed] [Google Scholar]
- 32.Khalife S, Zafarullah M. Molecular targets of natural health products in arthritis. Arthritis research & therapy. 2011;13:102. doi: 10.1186/ar3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ernst E, Chrubasik S. Phyto-anti-inflammatories. A systematic review of randomized, placebo-controlled, double-blind trials. Rheum Dis Clin North Am. 2000;26:13–27, vii. doi: 10.1016/s0889-857x(05)70117-4. [DOI] [PubMed] [Google Scholar]
- 34.Baghdikian B, Lanhers MC, Fleurentin J, et al. An analytical study, anti-inflammatory and analgesic effects of Harpagophytum procumbens and Harpagophytum zeyheri. Planta Med. 1997;63:171–176. doi: 10.1055/s-2006-957638. [DOI] [PubMed] [Google Scholar]
- 35.Chrubasik S, Thanner J, Kunzel O, et al. Comparison of outcome measures during treatment with the proprietary Harpagophytum extract doloteffin in patients with pain in the lower back, knee or hip. Phytomedicine. 2002;9:181–194. doi: 10.1078/0944-7113-00140. [DOI] [PubMed] [Google Scholar]
- 36.Kapoor M, Martel-Pelletier J, Lajeunesse D, et al. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, Johnston G, Stebler B, et al. Hydrogen peroxide activates NFkappaB and the interleukin-6 promoter through NFkappaB-inducing kinase. Antioxid Redox Signal. 2001;3:493–504. doi: 10.1089/15230860152409121. [DOI] [PubMed] [Google Scholar]
- 38.Lo YY, Cruz TF. Involvement of reactive oxygen species in cytokine and growth factor induction of c-fos expression in chondrocytes. The Journal of biological chemistry. 1995;270:11727–11730. doi: 10.1074/jbc.270.20.11727. [DOI] [PubMed] [Google Scholar]
- 39.Betancor-Fernandez A, Perez-Galvez A, Sies H, et al. Screening pharmaceutical preparations containing extracts of turmeric rhizome, artichoke leaf, devil’s claw root and garlic or salmon oil for antioxidant capacity. J Pharm Pharmacol. 2003;55:981–986. doi: 10.1211/0022357021468. [DOI] [PubMed] [Google Scholar]
- 40.Dahlberg L, Billinghurst RC, Manner P, et al. Selective enhancement of collagenase-mediated cleavage of resident type II collagen in cultured osteoarthritic cartilage and arrest with a synthetic inhibitor that spares collagenase 1 (matrix metalloproteinase 1) Arthritis and rheumatism. 2000;43:673–682. doi: 10.1002/1529-0131(200003)43:3<673::AID-ANR25>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 41.Lim H, Kim HP. Matrix metalloproteinase-13 expression in IL-1beta-treated chondrocytes by activation of the p38 MAPK/c-Fos/AP-1 and JAK/STAT pathways. Arch Pharm Res. 2011;34:109–117. doi: 10.1007/s12272-011-0113-4. [DOI] [PubMed] [Google Scholar]
- 42.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 43.Angel P, Imagawa M, Chiu R, et al. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987;49:729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- 44.Schonthal A, Herrlich P, Rahmsdorf HJ, et al. Requirement for fos gene expression in the transcriptional activation of collagenase by other oncogenes and phorbol esters. Cell. 1988;54:325–334. doi: 10.1016/0092-8674(88)90195-x. [DOI] [PubMed] [Google Scholar]
- 45.Gutman A, Wasylyk B. The collagenase gene promoter contains a TPA and oncogene-responsive unit encompassing the PEA3 and AP-1 binding sites. EMBO J. 1990;9:2241–2246. doi: 10.1002/j.1460-2075.1990.tb07394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shiozawa S, Shimizu K, Tanaka K, et al. Studies on the contribution of c-fos/AP-1 to arthritic joint destruction. J Clin Invest. 1997;99:1210–1216. doi: 10.1172/JCI119277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hess J, Porte D, Munz C, et al. AP-1 and Cbfa/runt physically interact and regulate parathyroid hormone-dependent MMP13 expression in osteoblasts through a new osteoblast-specific element 2/AP-1 composite element. The Journal of biological chemistry. 2001;276:20029–20038. doi: 10.1074/jbc.M010601200. [DOI] [PubMed] [Google Scholar]
- 48.Sun Y, Wenger L, Brinckerhoff CE, et al. Basic calcium phosphate crystals induce matrix metalloproteinase-1 through the Ras/mitogen-activated protein kinase/c-Fos/AP-1/metalloproteinase 1 pathway. Involvement of transcription factor binding sites AP-1 and PEA-3. The Journal of biological chemistry. 2002;277:1544–1552. doi: 10.1074/jbc.M100567200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


