Abstract
Purpose
Not all oncology patients and their family caregivers (FCs) experience the same quality of life (QOL). The purposes of this study were to identify latent classes of oncology patients (n=168) and their FCs (n=85) with distinct physical, psychological, social, and spiritual well-being trajectories from prior to through four months after the completion of radiation therapy and to evaluate for demographic, clinical, and genetic characteristics that distinguished between these latent classes.
Methods
Using growth mixture modelling, two latent classes were found for three (i.e., physical, psychological, and social well-being) of the four QOL domains evaluated.
Results
Across these three domains, the largest percentage of participants reported relatively high well-being scores across the six months of the study. Across these three QOL domains, patients and FCs who were younger, female, belonged to an ethnic minority group, had children at home, had multiple comorbid conditions, or had a lower functional status were more likely to be classified in the lower QOL class. The social well-being domain was the only domain that had a polymorphism in nuclear factor kappa beta 2 (NFKB2) associated with latent class membership. Carrying one or two doses of the rare allele for rs7897947 was associated with a 52% decrease in the odds of belonging to the lower social well-being class (OR (95% CI) = .46 (.21, .99), p=.049).
Conclusions
These findings suggest that a number of phenotypic and molecular characteristics contribute to differences in QOL in oncology patients and their FCs.
Keywords: quality of life, cytokines, genetics, growth mixture modeling, family caregivers, radiation therapy, oncology
Introduction
Unidimensional or multidimensional instruments can be used to evaluate quality of life (QOL). When viewed as a unidimensional concept, QOL is assessed using a single item or reported as a total score from a multi-dimensional instrument. When viewed as a multi-dimensional concept, various domains of QOL are assessed [1]. While the specific QOL domains vary across instruments, the most common domains included are physical, psychological, social, and spiritual well-being [2]. Moreover, while a total score provides an overall assessment of QOL, analysis of each QOL domain can provide valuable information on specific aspects of health and well-being [3].
Studies of the effects of cancer and radiotherapy (RT) on oncology patients’ and their family caregivers’ (FCs) QOL have produced inconsistent findings. In some prospective studies, the physical [4–9] and social [7,9–11] domains of patients’ QOL deteriorated during RT. In four other studies [6,12–14], the psychological domain of QOL improved during RT. In contrast, in five studies [15–19], the physical, psychological, and social domains of QOL did not change during patients’ RT. Only two small prospective studies were found that assessed the spiritual domain of QOL in patients undergoing RT [20,21]. Few changes in spiritual well-being were identified during RT. However, in one study [21], the measurement of spiritual well-being was not reliable. In the second study [20], a conceptual overlap existed between the psychological and spiritual domains. These inconsistent findings suggest that RT may have an impact on various domains of QOL. However, a large amount of inter-individual variability exists in patient’s responses and predictors of these responses warrant investigation.
The QOL of FCs can be affected by the physical, emotional, and financial burden of caring for someone with cancer [22–24]. In addition, many FCs have comorbidities that may affect their QOL [23]. Two reviews of QOL in FCs of oncology patients reported that the majority of studies were cross-sectional and compared patients’ and their FCs’ psychological health [25,26]. Of note, FCs experience similar levels of psychological distress and depressive symptoms as patients [27–34]. In two studies [34,35], the physical and social domains of QOL were negatively impacted because FCs experienced multiple symptoms as well as increased responsibilities and caregiving demands. In terms of the spiritual domain of QOL, two studies reported that lower spiritual well-being was a predictor of FCs’ psychological distress [36,37]. In another study [38], spiritual well-being scores of FCs were higher than the scores on the other domains of QOL.
Most of the studies on the various domains of QOL in oncology patients and FCs have “averaged” the scores on the instruments. This approach does not account for inter-individual variability in the various domains and may contribute to the inconsistent findings reported in the literature. Newer statistical methods for analyzing longitudinal data (e.g., latent class analysis) allow for the identification of subgroups (i.e., classes) of individuals with distinct QOL trajectories [39]. Only three studies were identified that used latent class analysis to identify subgroups of oncology patients with distinct physical, psychological, and social domain trajectories [40–42]. Across these three studies, the majority of patients experienced high and stable trajectories of physical, psychological, and social well-being. However, smaller subgroups of patients were identified who experienced significantly worse trajectories of physical, psychological, and social well-being. No studies were identified that used latent class analysis to evaluate the spiritual domain of QOL or changes in the trajectories of the various domains of QOL in FCs.
Using growth mixture modeling (GMM), we identified two latent classes of oncology patients and their FCs using their total QOL scores [43]. One latent class (61.7%) consisted of individuals with a higher QOL trajectory. The second class consisted of individuals (38.3%) with a lower QOL trajectory. Patients and FCs were more likely to belong to the lower QOL class if they were: younger, identified with an ethnic minority group, had poorer functional status, and had children living at home. In addition, individuals with one or two doses of the rare C allele of interleukin 1 receptor 2 (IL1R2) rs4141134 had a 64% decrease in the odds of belonging to the lower QOL class. In contrast, individuals with two doses of the rare G allele for nuclear factor kappa beta 2 (NFKB2) rs12772374 had a 47.7 fold increase in the odds of belonging to the lower QOL class. These findings add to the growing body of evidence that QOL has a genetic basis [44].
Cytokines are associated with inflammatory responses and sickness behavior [45]. Therefore, one can hypothesize that molecular mechanisms associated with inflammation may influence the various domains of QOL [46]. Only one study of mid- to long-term lung cancer survivors was identified that evaluated the association between cytokine genes and various domains of QOL [47]. In this cross-sectional study, variations in pro- and anti-inflammatory cytokine genes were associated with changes in physical functioning (i.e., IL1B, IL10, IL1 receptor antagonist (IL1RN)), mental health (i.e., IL1RN), emotional role functioning (IL6), and social functioning (i.e., IL6, IL1RN, tumor necrosis factor alpha (TNFA)). While this study provides evidence that variations in cytokine genes are associated with multiple domains of QOL in lung cancer survivors, additional research is warranted to identify these types of associations in patients undergoing active treatment, as well as in their FCs. Therefore, the purposes of this study in a sample of patients who underwent RT and their FCs, were to identify latent classes of individuals with distinct physical, psychological, social, and spiritual QOL trajectories. In addition, this study aimed to evaluate for differences in phenotypic characteristics and genetic variations in pro- and anti-inflammatory cytokine genes between the identified latent classes.
Methods
This descriptive, longitudinal study is part of a larger study that evaluated multiple symptoms in patients who underwent primary or adjuvant RT for breast, prostate, lung, or brain cancer and their FCs [48]. We provide an abbreviated version of the methods below. A more comprehensive description of the methods is described elsewhere [43,48].
Study Procedures
This study was approved by the Committee on Human Research at the University of California, San Francisco and at the second site. Prior to RT, patients and their FCs were recruited from two RT departments. Patients and FCs who met the eligibility criteria and gave written informed consent completed enrollment questionnaires. Participants completed follow-up questionnaires at 4 weeks after the initiation of RT, at the end of RT, and at 4, 8, 12, and 16 weeks after the completion of RT.
Instruments
Participants completed a demographic questionnaire and the Karnofsky Performance Status (KPS) scale [49]. Patients completed the Quality of Life-Scale-Patient Version (QOL-PV) and FCs completed the Quality of Life-Scale-Family Version (QOL-FV) [50,51]. The QOL-PV is a 41-item instrument that measures four domains of QOL in oncology patients as well as a total QOL score. Each item is rated on a 0 to 10 numeric rating scale (NRS) with higher scores indicating better QOL. The QOL-PV has established validity and reliability [50–53]. In the current study, the Cronbach’s alphas for the QOL-PV physical, psychological, social, and spiritual wellbeing subscales were 0.82, 0.94, 0.85, and 0.72, respectively.
The QOL-FV is a 37-item instrument that measures the QOL of a family member who is caring for a patient with cancer on four domains. Each item is rated on a 0 to 10 NRS with higher scores indicating better QOL. The QOL-FV has established validity and reliability [2,53]. In the current study, the Cronbach’s alphas for the physical, psychological, social, and spiritual well-being subscales were 0.72, 0.90, 0.84, and 0.67, respectively. In this study, the QOL scores for each domain, which is the mean score of the items corresponding to each domain, were used in the subsequent analyses.
Methods of analysis for phenotypic data
Data were analyzed using SPSS Version 22 [54] and Mplus Version 6.11 [55]. Descriptive statistics and frequency distributions were generated on the sample characteristics. GMM with robust maximum likelihood estimation was used to identify latent classes (i.e., subgroups of participants) with distinct QOL trajectories (i.e., physical, psychosocial, social, spiritual well-being scores) over the 6 months of the study [39].
The GMM methods are described in detail elsewhere [56]. Briefly, separate GMM analyses were done for each QOL domain. A single growth curve that represented the average change trajectory was estimated for the QOL domain. Then the number of latent classes that best fit the data was identified using published guidelines [57–59]. Model fit was assessed statistically by identifying the model with the lowest Bayesian Information Criterion (BIC), by using the Vuong-Lo-Mendell-Rubin likelihood ratio test (VLMR) [57,58], and by evaluating entropy values, with >.80 being preferred [55,60]. In addition, the best fitting model was visually inspected to determine whether the predicted trajectories “made sense” theoretically and clinically [39].
For each QOL domain, independent sample t-tests and Chi-square analyses were done to evaluate for differences in phenotypic characteristics between the GMM latent classes. Because 65% of the participants were in patient-caregiver dyads, models were estimated with “dyad” as a clustering variable, to ensure that any dependency between the QOL subscale scores for patients and FCs in the same dyad were controlled for in the GMM analyses. Differences in phenotypic characteristics between the latent classes were considered statistically significant at the p <.05 level.
Methods of analysis for genomic data
Blood collection and genotyping
Genomic deoxyribonucleic acid (DNA) was extracted from archived buffy coats using the PUREGene DNA Isolation System (Invitrogen, Carlsbad, CA). Of the 287 participants recruited, DNA was recovered from the archived buffy coats of 253 (i.e., 168 patients and 85 FCs). No differences were found in any demographic and clinical characteristics between participants who did and did not choose to participate in the study or in those participants for whom DNA could not be recovered from archived specimens.
Genotyping was performed blinded to clinical status and positive and negative controls were included. DNA samples were quantitated and normalized to a concentration of 50 nanogram (ng)/microliter (µL). Samples were genotyped using the GoldenGate genotyping platform (Illumina, San Diego, CA) and processed according to the standard protocol using GenomeStudio (Illumina, San Diego, CA).
SNP Selection
A combination of tagging SNPs and literature driven SNPs were selected for analysis. Tagging SNPs were required to be common (defined as having a minor allele frequency (MAF) of ≥0.05) in public databases. In order to ensure robust genetic association analyses, quality control filtering of SNPs was performed. SNPs with call rates of <95% or Hardy-Weinberg p-values of <.001 were excluded.
As shown in Supplementary Table 1, a total of 92 SNPs among the 15 candidate genes (IFNG: 5 SNPs, IFNGR1: 1 SNP; IL1B: 12 SNPs; IL1R1: 5 SNPs; IL1R2: 3 SNPs; IL2: 5 SNPs; IL4: 8 SNPs; IL6: 9 SNPs; IL8: 3 SNPs; IL10: 8 SNPs; IL13: 4 SNPs; IL17A: 5 SNPs; NFKB1: 11 SNPs; NFKB2: 4 SNPs; TNFA: 9 SNPs), that passed all quality control filters, were included in the genetic association analyses.
Statistical Analyses
Allele and genotype frequencies were determined by gene counting. Hardy-Weinberg equilibrium was assessed by the Chi-square. Measures of linkage disequilibrium (i.e., D’ and r2) were computed from the participants’ genotypes with Haploview 4.2. Linkage disequilibrium (LD)-based haplotype block definition was based on D’ confidence interval [61].
For SNPs that were members of the same haplotype, haplotype analyses were conducted in order to localize the association signal within each gene and to determine if haplotypes improved the strength of the association with the phenotype. Haplotypes were constructed using the program PHASE version 2.1 [62]. Only haplotypes that were inferred with probability estimates of >0.85, across five iterations, were retained for downstream analyses. Haplotypes were evaluated assuming a dosage model (i.e., analogous to the additive model).
Ancestry informative markers (AIMS) were used to minimize confounding due to population stratification [63–65]. Homogeneity in ancestry among participants was verified by principal component analysis [66], using Helix Tree (Golden Helix, Bozeman, MT). One hundred and six AIMs were included in the analysis. The first three PCs were selected to adjust for potential confounding due to population substructure (i.e., race/ethnicity) by including the three covariates in all regression models.
For association tests, three genetic models were assessed for each SNP: additive, dominant, and recessive. Barring trivial improvements (i.e., delta <10%), the genetic model that best fit the data, by maximizing the significance of the p-value, was selected for each SNP.
Logistic regression analysis, that controlled for significant covariates, as well as genomic estimates of and self-reported race/ethnicity, was used to evaluate the relationship between genotype and class membership for each domain of QOL. A backwards stepwise approach was used to create a parsimonious model. Except for genomic estimates of and self-reported race/ethnicity, only predictors with a p-value of <.05 were retained in the final model. Genetic model fit and both unadjusted and covariate-adjusted odds ratios were estimated using STATA version 13 [67].
As was done in all of our previous candidate gene studies [48,68–72], based on recommendations in the literature [73,74], the implementation of rigorous quality controls for genomic data, the non-independence of SNPs/haplotypes in LD, and the exploratory nature of the analyses, adjustments were not made for multiple testing. Significant SNPs identified in the bivariate analyses were evaluated using regression analyses that controlled for differences in phenotypic characteristics, potential confounding due to population stratification, and variation in other SNPs/haplotypes within the same gene. Only those SNPs that remained significant were included in the final presentation of the results. Therefore, the significant independent associations reported are unlikely to be due solely to chance. Unadjusted associations are reported for all SNPs passing quality control criteria in Table 1 to allow for subsequent comparisons and meta-analyses.
Table 1.
Fit Indices for the GMM solutions for Physical, Psychological, and Social Well-being Subscales over Seven Assessments, with Dyad as a Clustering Variable
| GMM | LL | AIC | BIC | Entropy | VLMRc |
|---|---|---|---|---|---|
| Physical Well-being | |||||
| 1-Classa | −2268.835 | 4575.669 | 4642.803 | n/a | n/a |
| 2-Classb | −2209.784 | 4457.569 | 4524.703 | 0.675 | 183.719 ** |
| 3-Class | −2191.304 | 4434.607 | 4526.475 | 0.679 | 36.962n.s. |
| Psychological Well-being | |||||
| 1-Classd | −2079.223 | 4196.445 | 4263.580 | n/a | n/a |
| 2-Classe | −2055.050 | 4148.101 | 4215.235 | 0.689 | 105.680 *** |
| 3-Class | −1998.042 | 4048.085 | 4139.746 | 0.723 | 27.755n.s. |
| Social Well-being | |||||
| 1-Classf | −2292.809 | 4617.617 | 4674.151 | n/a | n/a |
| 2-Classg | −2233.520 | 4505.039 | 4572.174 | 0.715 | 150.333 * |
| 3-Class | −2208.160 | 4468.320 | 4560.188 | 0.730 | 50.720n.s. |
p < .05,
p < .01,
p < .001
Random intercepts latent growth curve model with linear and quadratic components; χ2 = 47.222, 23 df, p = .002, CFI = 0.974, RMSEA = 0.065
2-class model was selected, based on its having the smallest BIC and a significant VLMR. Further, the VLMR was not significant for the 3-class model.
This number is the Chi2 statistic for the VLMR. When significant, the VLMR test provides evidence that the K-class model fits the data better than the K-1-class model.
Random intercepts latent growth curve model with linear and quadratic components; χ2 = 22.975, 23 df, p = .460, CFI = 1.000, RMSEA < 0.0005
2-class model was selected, based on a BIC that was smaller than the 1-class model and a significant VLMR. Further, the VLMR was not significant for the 3-class model. When the VLMR is not significant, it indicates that too many classes were extracted. In addition, although the 3-class model has a smaller BIC, the additional class consisted of less than 6% of the sample, indicating that the third class is unlikely to replicate in other samples.
Random intercepts latent growth curve model with linear and quadratic components; χ2 = 67.004, 26 df, p < .0001, CFI = 0.974, RMSEA = 0.079
2-class model was selected, because the BIC was smaller than the 1-class solution and the VLMR was significant. Further, the VLMR was not significant for the 3-class model and no clinically meaningful difference was faced between the two classes that were low on the scale.
Abbreviations: AIC = Akaike information criteria, BIC = Bayesian information criterion, CFI = comparative fit index, df = degrees of freedom, GMM = growth mixture model, LL = log likelihood, n/a = not applicable, n.s. = not significant, RMSEA = root mean square error of approximation, VLMR = Vuong-Lo-Mendell-Rubin likelihood ratio test.
Results
Participant characteristics
The majority of the participants were Caucasian, well educated, and married or partnered. Patients made up 66.4% of the total sample. The mean age of the total sample was 61.4 years. On average, participants had greater than four comorbid conditions and a KPS score of 92. Gender was evenly represented within the total sample with 46.2% male and 53.8% female participants. Approximately 38.1% of the patients had breast cancer, 48.8% had prostate cancer, 7.1% had brain cancer, and 6.0% had lung cancer. The majority of the FCs (92.9%) was the patients’ spouses.
At enrollment, no differences were found between patients’ and FCs’ mean scores for physical well-being (patients: 8.2 (SD=1.8), FCs: 8.1 (SD=1.7), p= .639), psychological well-being (patients: 6.7 (SD=2.0), FCs: 6.6 (SD=1.7), p= .374), or social well-being (patients: 7.2 (SD=2.2), FCs: 7.5 (SD=1.9), p= .280). For spiritual well-being, patients had a mean score at enrollment of 5.4 (SD=2.0) and FCs had a mean score at enrollment of 7.2 (SD=1.5). This between-group difference in spiritual well-being scores was statistically significant (p <.001) and clinically meaningful (effect size, Cohen’s d = 1.01) [75–78].
Physical Well-being
GMM analysis
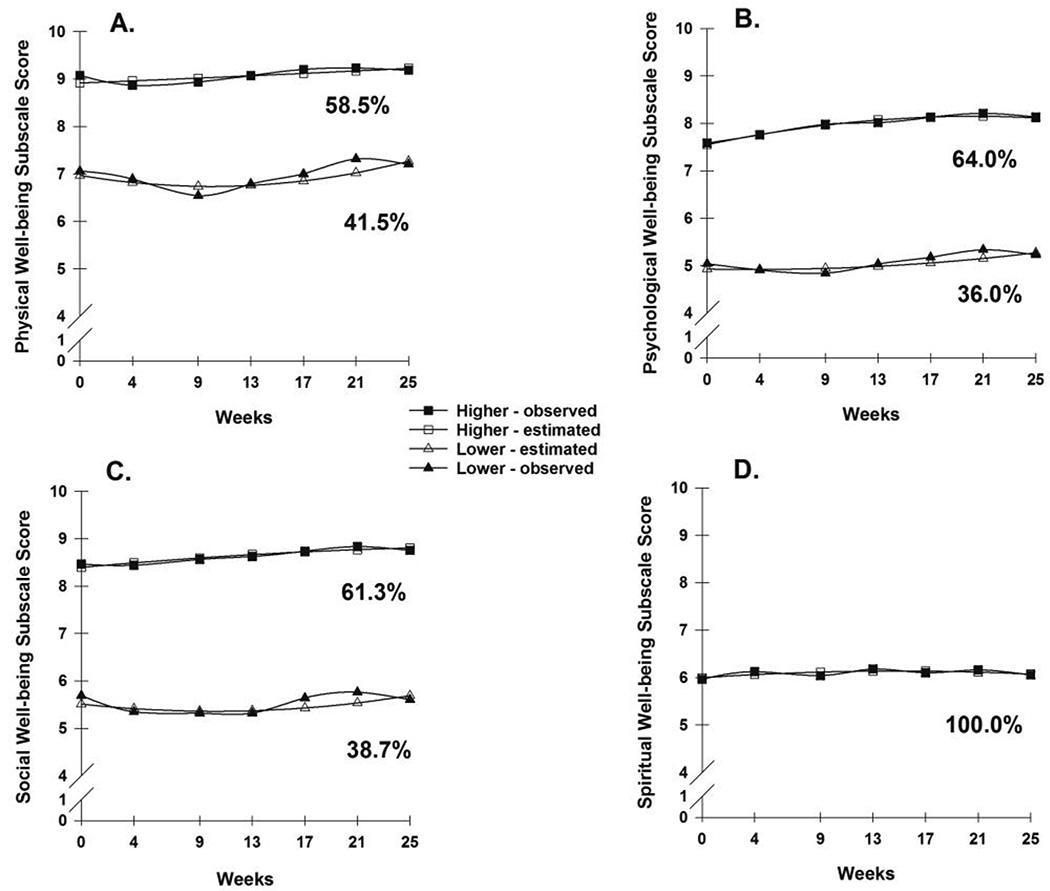
For physical well-being, two distinct latent classes of individuals were identified (Figure 1A). As shown in Table 1, a two-class model was selected because its BIC was smaller than for the one-class and three-class models and by comparisons of the other fit indices. In addition, each class in the two-class model had a reasonable size and interpretability [57]. The parameter estimates for the two latent classes are listed in Table 2. The largest percentage of participants (58.5%) was grouped in the higher physical well-being class. These participants had a mean physical well-being score at enrollment of 9.1 (SD=0.8), that remained relatively stable over time. The lower physical well-being class (41.5%) had a mean physical well-being score at enrollment of 6.9 (SD=1.9) that remained relatively stable over time. The between group difference in physical well-being scores at enrollment was statistically significant (p <.001) and clinically meaningful (effect size, Cohen’s d = 0.81) [75–78].
Figure 1.
A - Observed and estimated physical well-being domain trajectories for participants in each of the latent classes.
B - Observed and estimated psychological well-being domain trajectories for participants in each of the latent classes.
C - Observed and estimated social well-being domain trajectories for participants in each of the latent classes.
D - Observed and estimated spiritual well-being domain trajectory for participants in the latent class.
Table 2.
GMM Parameter Estimates for the Latent Classa Solutions for the Physical, Psychological, and Social Well-being Subscales with 7 Assessments, with Dyad as a Clustering Variable
| Parameter Estimatesb |
Physical Well-being | Psychological Well-being | Social Well-being | |||
|---|---|---|---|---|---|---|
| Higher QOL n = 148 (58.5%) |
Lower QOL n = 105 (41.5%) |
Higher QOL n = 162 (64.0%) |
Lower QOL n = 91 (36.0%) |
Higher QOL n = 155 (61.3%) |
Lower QOL n = 98 (38.7%) |
|
| Mean (SE) | Mean (SE) | Mean (SE) | ||||
| Intercept | 8.919*** (0.142) |
6.968*** (0.228) |
7.552*** (0.220) |
4.930*** (0.221) |
8.396*** (0.361) |
5.511*** (0.317) |
| Linear slope | 0.044n.s. (0.048) |
−0.187* (0.089) |
0.235*** (0.058) |
−0.023n.s. (0.098) |
0.101n.s. (0.088) |
−0.120n.s. (0.224) |
| Characteristic | Physical Well-being | Psychological Well-being | Social Well-being | |||
| Quadratic slope | 0.001n.s. (0.007) |
0.037** (0.013) |
−0.023*** (0.005) |
0.012n.s. (0.014) |
−0.006n.s. (0.012) |
0.024n.s. (0.031) |
| Variances | Variance (SE) | Variance (SE) | Variance (SE) | |||
| Intercept | 0.236* (0.099) |
2.133*** (0.338) |
0.978*** (0.200) |
1.597*** (0.300) |
0.604* (0.289) |
3.058*** (0.477) |
| Linear Slope | 0c | 0.057*** (0.012) |
0c | 0.034*** (0.009) |
0c | 0.046 ** (0.014) |
p < .05,
p < .01,
p < .001
Trajectory group sizes are for classification of individuals based on their most likely latent class probabilities.
Growth mixture model estimates were obtained with robust maximum likelihood, with dyad as a clustering variable to account for dependency between patients and family caregivers within the same dyad. Quadratic slope variances were fixed at zero to improve estimation.
Fixed at zero.
Abbreviations: GMM = Growth mixture model; n.s. = not significant; QOL = quality of life; SE = standard error
Phenotypic analyses
As summarized in Table 3, compared to participants in the higher physical well-being class, participants in the lower class were younger (p <.001), were more likely to be female (p = .003) and members of an ethnic minority group (p = .006), had a higher number of comorbid conditions (p = .002), and had a lower KPS score (p<.001). Post-hoc contrasts found that in comparison to Caucasian participants, participants of Hispanic, mixed ethnic background, or other ethnicity were more likely to be members of the lower physical well-being class (p = .003). Within the higher physical well-being class, no differences in mean physical well-being scores at enrollment were found between patients (9.2 (SD=0.8)) and FCs (9.0 (SD=0.8), p = .159). Within the lower physical well-being class, no differences in mean physical well-being scores at enrollment were found between patients (7.0 (SD=1.9)) and FCs (6.8 (SD=1.8), p = .580).
Table 3.
Differences in Demographic Characteristics between the Latent Classes for the Physical, Psychological, and Social Well-being Subscales
| Higher QOL n =148 |
Lower QOL n =105 |
p-value | Higher QOL n =162 |
Lower QOL n =91 |
p-value | Higher QOL n =155 |
Lower QOL n =98 |
p-value | |
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) |
Mean (SD) |
Mean (SD) |
Mean (SD) |
Mean (SD) |
Mean (SD) |
||||
| Age (years) | 64.5 (9.7) |
57.2 (12.0) |
<.001 | 64.9 (9.2) |
55.3 (12.1) |
<.001 | 64.9 (9.8) |
56.0 (11.4) |
<.001 |
| Education (years) |
16.1 (3.1) |
15.7 (2.9) |
.355 | 16.1 (3.1) |
15.7 (2.9) |
.330 | 15.9 (3.1) |
16.0 (3.0) |
.798 |
| Number of comorbid conditions |
4.2 (2.5) |
5.2 (2.9) |
.002 | 4.5 (2.6) |
4.8 (2.8) |
.420 | 4.3 (2.6) |
5.0 (2.8) |
.033 |
| Weight (pounds) | 175.1 (34.7) |
175.3 (44.2) |
.969 | 179.9 (35.8) |
166.8 (42.8) |
.011 | 176.6 (35.6) |
173.0 (43.5) |
.502 |
| Karnofsky Performance Status score |
96.3 (7.4) |
85.7 (13.4) |
<.001 | 95.5 (7.7) |
85.5 (14.3) |
<.001 | 94.8 (9.1) |
87.4 (13.4) |
<.001 |
| n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
||||
| Gender (% female) |
68 (45.9) |
68 (64.8) |
.003 | 74 (45.7) |
62 (68.1) |
.001 | 78 (50.3) |
58 (59.2) |
.196 |
| Ethnicity | .006; | <.001; | .005; | ||||||
| White (W) | 119 (80.4) |
69 (66.3) |
*HMO> W; | 128 (79.5) |
60 (65.9) |
*AP and , HMO>W and B; | 123 (79.9) |
65 (66.3) |
*HMO>W; p = .003 |
| Asian/Pacific Islander (AP) |
6 (4.1) |
10 (9.6) |
p = .003 | 5 (3.1) |
11 (12.1) |
p <.005 | 7 (4.5) |
9 (9.2) |
|
| Black (B) | 20 (13.5) |
14 (13.5) |
26 (16.1) |
8 (8.8) |
21 (13.6) |
13 (13.3) |
|||
| Hispanic/Mixed background/Other (HMO) |
3 (2.0) |
11 (10.6) |
2 (1.2) |
12 (13.2) |
3 (1.9) |
11 (11.2) |
|||
| Lives alone (% yes) |
29 (30.2) |
25 (34.7) |
.617 | 34 (32.4) |
20 (31.7) |
1.000 | 30 (29.7) |
24 (35.8) |
.500 |
| Married or partnered (% yes) |
108 (73.0) |
66 (64.1) |
.164 | 119 (73.5) |
55 (61.8) |
.063 | 114 (73.5) |
60 (62.5) |
.069 |
| Children at home (% yes) |
18 (14.0) |
18 (21.7) |
.189 | 15 (10.7) |
21 (29.2) |
.002 | 13 (9.7) |
23 (29.5) |
<.001 |
| Older adult at home (% yes) |
2 (1.6) |
5 (5.9) |
.117 | 2 (1.4) |
5 (6.8) |
.050 | 2 (1.5) |
5 (6.3) |
.105 |
| Work for pay (% yes) |
67 (46.2) |
48 (46.6) |
1.000 | 74 (46.5) |
41 (46.1) |
1.000 | 69 (45.1) |
46 (48.4) |
.695 |
| Patient/family caregiver (% Patient) |
96 (64.9) |
72 (68.6) |
.590 | 105 (64.8) |
63 (69.2) |
.492 | 101 (65.2) |
67 (68.4) |
.682 |
Bonferroni post hoc pairwise comparisons; letters refer to ethnic groups. e.g., for Physical Well-Being: HMO>W represents that a higher proportion of participants who self-reported their race/ethnicity as Hispanic, mixed ethnic background, or other ethnicity belonged to the lower QOL class than participants who self-reported their race/ethnicity as White.
Abbreviations: QOL = quality of life, SD = standard deviation
Genotypic analyses
In the bivariate analyses, two SNPs (i.e., IL1R2 rs4141134, IL6 rs4719714), and one haplotype (i.e., IL1R2 HapA1) differed between the two latent classes for physical well-being (Table 4). For IL1R2 rs4141134, a dominant model fit the data best. For IL6 rs4719714, a recessive model fit the data best. However, the association between the physical well-being classes and IL1R2 rs4141134, IL6 rs4719714, and IL1R2 HapA1 did not remain significant in the multivariable logistic regression analyses that included age, gender, number of comorbid conditions, KPS score, and genomic estimates of and self-reported race/ethnicity.
Table 4.
Comparison of Significant Single Nucleotide Polymorphisms in Pro- and Anti-Inflammatory Cytokine Genes and the Growth Mixture Model Analyses for Total Quality of Life Score and Subscale Scores
| Gene | SNP | Alleles | Physical Well-being | Psychological Well-being | Social Well-being | Model | |||
|---|---|---|---|---|---|---|---|---|---|
| Chi2 | p-value | Chi2 | p-value | Chi2 | p-value | ||||
| IFNG | rs2069727 | A>G | FE | 1.000 | FE | .161 | FE | .037 | R |
| IL1R1 | rs2110726 | C>T | 3.986 | .138 | 6.730 | .035 | 0.357 | .836 | A |
| IL1R2 | rs4141134 | T>C | FE | .016 | FE | .053 | FE | .030 | D |
| IL1R2 | HapA1 | 7.141 | .028 | 5.876 | .053 | 2.239 | .326 | ||
| IL1R2 | HapA4 | 5.818 | .055 | 3.846 | .146 | 5.589 | .061 | ||
| IL6 | rs4719714 | A>T | FE | .022 | FE | .704 | FE | .710 | R |
| IL6 | rs2069827 | G>T | FE | .346 | FE | .172 | FE | .034 | D |
| IL6 | rs1800795 | C>G | FE | .116 | FE | .078 | FE | .285 | D |
| IL6 | rs1554606 | G>T | FE | .175 | FE | .026 | FE | .129 | D |
| IL6 | rs2069845 | A>G | FE | .175 | FE | .026 | FE | .129 | D |
| NFKB2 | rs12772374 | A>G | FE | .239 | FE | .670 | FE | .208 | R |
| NFKB2 | rs7897947 | T>G | FE | .082 | FE | .409 | FE | .042 | D |
| TNFA | rs1800610 | C>T | FE | .071 | FE | .029 | FE | .250 | D |
Abbreviations: A = additive model, D = dominant model, FE = Fisher’s exact test, Hap = haplotype, IFNG = interferon gamma, IFNGR = IFNG receptor, IL = interleukin, NFKB = nuclear factor kappa beta, QOL = quality of life, R = recessive model, SNP = single nucleotide polymorphism, TNFA = tumor necrosis factor alpha
Psychological Well-being
GMM Analysis
For psychological well-being, two distinct latent classes of individuals were identified (Figure 1B). As shown in Table 1, a two-class model was selected based on the same criteria used for the physical well-being domain. In addition, each class in the two-class model had a reasonable size and interpretability [57]. The parameter estimates for the two latent classes are listed in Table 2. The largest percentage of participants (64.0%) was in the higher psychological well-being class. These participants had a mean psychological well-being score at enrollment of 7.6 (SD=1.3), which was relatively stable over time. The lower psychological well-being class (36.0%) had a mean psychological well-being score at enrollment of 4.9 (SD=1.5), which was relatively stable over time. The between group difference in psychological well-being scores at enrollment was statistically significant (p <.001) and clinically meaningful (effect size, Cohen’s d = 0.96) [75–78].
Phenotypic Analyses
As summarized in Table 3, compared to participants in the higher psychological well-being class, participants in the lower class were younger (p <.001), were more likely to be female (p = .001), were more likely to be members of an ethnic minority group (p < .001), were more likely to have children at home (p = .002), weighed less (p = .011), and had a lower KPS score (p < .001). Post-hoc contrasts found that in comparison to the Caucasian participants, participants of Asian or Pacific Islander ethnicity or Hispanic, mixed background, or other ethnicity were more likely to be members of the lower psychological well-being class (p = .005 and p <.001, respectively). In addition, compared to African American participants, participants of Asian or Pacific Island ethnicity or Hispanic, mixed background or other ethnicity were more likely to be members of the lower psychological well-being class (p = .004 and p < .001, respectively). Within the higher psychological well-being class, a statistically significant difference in mean psychological scores at enrollment was found between patients (7.7 (SD=1.3)) and FCs (7.3 (SD=1.2), p = .033). While this difference was statistically significant, the effect size was moderate (Cohen’s d = 0.37) [75–78]. Within the lower psychological well-being class, no differences were found in mean psychological well-being scores at enrollment between patients (4.8 (SD=1.6) and FCs (4.9 (SD=1.3), p = .845).
Genotypic analyses
In the bivariate analyses, four SNPs (i.e., IL1R1 rs2110726, IL6 rs1554606, IL6 rs2069845, and TNFA rs1800610) differed significantly between the two psychological well-being latent classes. For IL1R1 rs2110726, an additive model fit the data best. For IL6 rs1554606, IL6 rs2069845, and TNFA rs1800610, a dominant model fit the data best. However, the associations between the psychological well-being class and these four SNPs did not remain significant in the multivariable logistic regression analyses that included age, gender, having children at home, weight, KPS score, and genomic estimates of and self-reported race/ethnicity.
Social Well-being
GMM Analysis
For social well-being, two distinct latent classes of individuals were identified (Figure 1C). As shown in Table 1, a two-class model was selected based on the same criteria used for the physical well-being domain. The parameter estimates for the two latent classes are listed in Table 2. The largest percentage of participants (61.3%) was grouped in the higher social well-being class. These participants had a mean social well-being score at enrollment of 8.5 (SD=1.1), which was relatively stable over time. The lower social well-being class (38.7%) had a mean social well-being score at enrollment of 5.5 (SD=2.0), which was relatively stable over time. The between group difference in social well-being scores at enrollment was statistically significant (p <.001) and clinically meaningful (effect size, Cohen’s d = 0.97) [75–78].
Phenotypic Analyses
As summarized in Table 3, compared to participants in the higher social well-being class, participants in the lower class were younger (p <.001), were more likely to be members of an ethnic minority group (p = .006), were more likely to have children at home (p < .001), had more comorbid conditions (p = .033), and had a lower KPS score (p <.001). Post-hoc contrasts found that in comparison to Caucasian participants, participants of Hispanic, mixed background or other ethnicity were more likely to belong to the lower social well-being class (p = .003). Within the higher social well-being class, no differences in social well-being scores at enrollment were found between patients (8.4, SD 1.2) and FCs (8.5, SD 1.0; p = .482). Within the lower social well-being class, no differences in social well-being scores at enrollment were found between patients (5.4, SD 2.1) and FCs (5.7, SD 1.6; p = .410).
Genotypic analyses
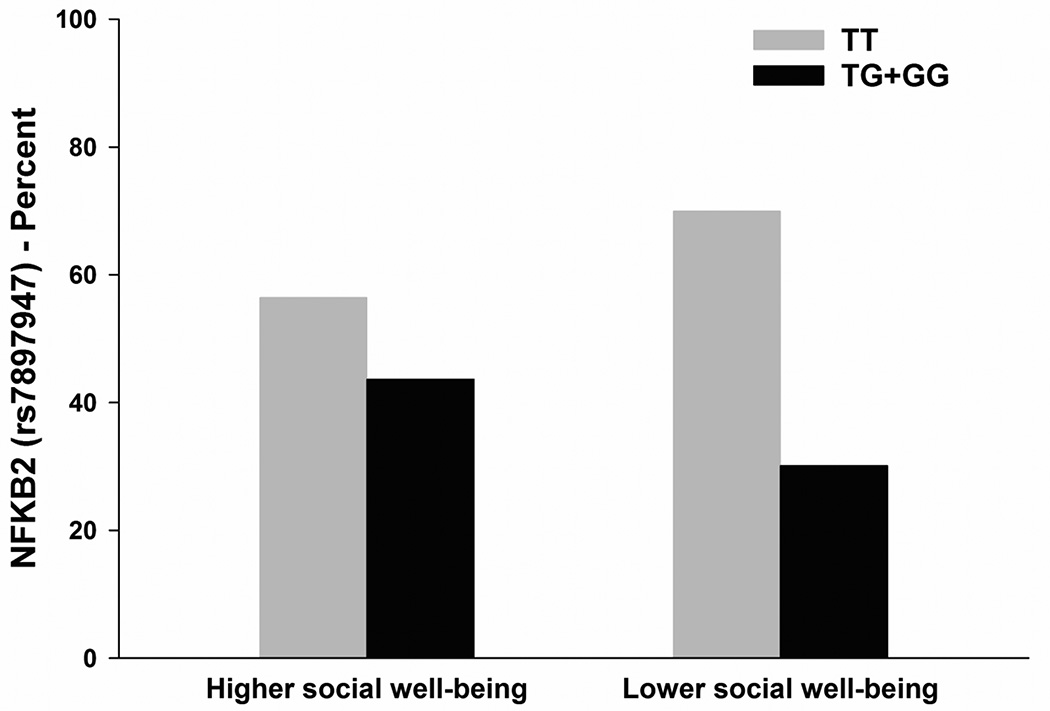
In the bivariate analyses, four SNPs (i.e., IFNG rs2069727, IL1R2 rs4141134, IL6 rs2069827, NFKB2 rs7897947) differed between the two social well-being latent classes (Table 4). For IL1R2 rs4141134, IL6 rs2069827, and NFKB2 rs7897947, a dominant model fit the data best. For IFNG rs2069727, a recessive model fit the data best. Controlling for age, having children at home, number of comorbid conditions, KPS score, and genomic estimates of and self-reported race/ethnicity, the only genetic association that remained significant in the multivariable logistic regression analyses was NFKB2 rs7897947 (Table 5, Figure 2). Carrying one or two doses of the rare G allele (i.e., TT versus TG + GG) was associated with a 54% decrease in the odds of belonging to the lower social well-being class.
Table 5.
Multiple Logistic Regression Analysis for the Association between NFKB2 rs7897947 and Latent Class Membership for the Social Well-being Subscale
| Predictors | Odds Ratio |
Standard Error |
95% CI | Z | p-value |
|---|---|---|---|---|---|
| NFKB2 rs7897947 | 0.46 | 0.181 | 0.214, 0.997 | −1.97 | .049 |
| Age | 0.69 | 0.066 | 0.567, 0.829 | −3.89 | <.001 |
| Children at home | 4.51 | 2.371 | 1.607, 12.638 | 2.86 | .004 |
| Number of comorbid conditions | 1.17 | 0.083 | 1.015, 1.342 | 2.17 | .030 |
| KPS score | 0.57 | 0.097 | 0.412, 0.800 | −3.27 | .001 |
Overall model fit: χ2 = 68.82, p <.0001
Multiple logistic regression analysis of GMM latent classes for social well-being domain QOL scores (0 = higher (n=126), 1 = lower (n=72)). The first three principle components identified from the analysis of ancestry informative markers as well as self-reported race/ethnicity were retained in the model to adjust for potential confounding due to race or ethnicity (data not shown). Predictors evaluated in the model included NFKB2 rs7897947 genotype (TT versus TG + GG)), age (in 5 year increments), children living at home, number of comorbid conditions, and functional status (KPS score in 10 unit increments).
Abbreviations: CI =confidence interval, GMM = growth mixture model, KPS = Karnofsky Performance Status, NFKB2 = nuclear factor kappa beta 2, QOL = quality of life
Figure 2.
Differences between the social well-being latent classes in the percentages of participants who were homozygous for the common T allele (TT), or heterozygous or homozygous for the rare G allele (TG + GG) for rs7897947 in nuclear factor kappa beta 2 (NFKB2).
Spiritual well-being
GMM Analysis
For spiritual well-being, a one-class solution was selected because it had the best model fit and a model with a larger number of classes was not supported (data not shown; Figure 1D).
Discussion
This study is the first to use GMM to identify classes of oncology patients undergoing RT and their FCs who reported distinct trajectories of physical, psychological, and social well-being. For all three QOL domains, a larger class was identified who experienced relatively high and stable trajectories and a smaller class whose trajectories were significantly lower. Of note, only one latent class was identified for the spiritual well-being domain. In terms of the percentages of participants with consistently higher domain scores, our findings are consistent with previous reports [ [40–42]. Specifically, in two of these studies of breast [40] and nasopharyngeal [41] cancer patients, that used the Medical Outcomes Study 36-item short-form health survey (SF-36) and the Functional Assessment of Cancer Therapy–General (FACT-G) respectively, approximately 43% to 85% of the patients were classified as having high stable trajectories across the physical, psychological, and social QOL domains.
In the studies of breast [40] and nasopharyngeal [41] cancer patients, one to three latent classes with lower physical, psychological, and social domain scores were identified. For example, in the study of patients with breast cancer [40], three latent classes with lower Mental Component Summary scores from the SF-36 were found. In contrast, in the study of patients with nasopharyngeal cancer [41], two classes were identified with lower Emotional Subscale scores on the FACT-G. In both studies, the trajectory of one of the lower latent classes improved and another deteriorated over time. In our study, only one class with lower scores on each QOL domain was identified and these scores remained stable overtime. A number of factors may explain these inconsistent findings. First, the length of follow-up varied between six months and fifty-five months. Second, different instruments were used to evaluate the various domains of QOL. Third, in one study, [40] some of the participants had participated in a peer group or educational intervention on adjustment to cancer. Fourth, sample sizes ranged from 253 to 363 which could influence the number of classes identified. Additional research is needed to determine if the number and trajectories of classes identified vary based on participant characteristics or the various phases of the cancer experience (e.g., diagnosis, active treatment, survivorship).
This study is the first to identify a single spiritual well-being class for oncology patients and FCs using GMM. Our finding that compared to patients, FCs reported higher spiritual well-being scores at enrollment contrasts with two previous studies that found spiritual well-being scores of patients and FCs to be comparable [79,80]. However, it is difficult to make direct comparisons across these studies because of differences in sample characteristics, timing of participant recruitment, and measures used to evaluate spiritual well-being. For example, in one study [79] qualitative interviews were done with patients with advanced lung cancer and their FCs to evaluate changes in spiritual well-being over time. In the other study [80], spiritual well-being was evaluated in cancer survivors who were an average of two years post their diagnosis and their FCs. Given the paucity of information on the spiritual well-being of oncology patients and FCs, additional studies are warranted to confirm or refute previous findings.
Common predictors of lower QOL domain class membership
Across the physical, psychological, and social domains, younger age, belonging to an ethnic minority group, and having a lower functional status were associated with membership in the lower QOL classes. In the two studies that used latent class analysis, younger age was associated with lower psychological [42] and social [41,42] well-being trajectories in oncology patients. While research on the predictors of QOL in FCs has focused primarily on psychological well-being, one review of QOL among FCs of oncology patients identified that younger age was a risk factor for poorer psychological outcomes [23]. One potential explanation for the association between age and poorer QOL outcomes is that a diagnosis of cancer and the impact of its treatment are outside of what younger individuals expect to face at this stage in their life. Additionally, younger individuals are more likely to have substantial family and career responsibilities that place an additional strain on their self-care abilities [81].
In this study, participants who self-reported their race/ethnicity as Hispanic/Mixed Background/Other were more likely to be classified in the lower physical and social well-being latent classes. In addition, participants who self-reported their race/ethnicity as Hispanic/Mixed Background/Other or Asian/Pacific Islander were more likely to be classified in the lower psychological well-being class. Previous studies have found a similar relationship between belonging to an ethnic minority group and poorer physical [82], psychological [83], and social well-being [84]. These associations may be related to the negative consequences associated with less education or lower household income [82]. In this study, no associations were found between education or income and latent class memberships. However, our findings need to be interpreted with caution because of the relatively small numbers of individuals in each of the non-white ethnic groups. Alternatively, differences in the type and number of previous cancer treatments or cultural influences (e.g., language barriers, coping styles, lifestyle behaviours, social resources) may contribute to differences in physical, psychological and social well-being latent class memberships [85]. These factors warrant investigation in future studies.
Lower functional status was associated with membership in the lower class for all three QOL domains. Functional status reflects a person’s ability to perform routine tasks (e.g., mobility and self-care) and can affect a person’s QOL [86]. Functional status is not a surrogate for QOL because impaired function is not always associated with poorer QOL [87]. Across all three domains, both latent classes had relatively high KPS scores. However, the differences in KPS scores between the higher and lower classes for the physical, psychological, and social wellbeing classes were clinically meaningful (d = 0.81, d = 0.96, d = 0.97, respectively). These findings suggest that even small differences in functional status are associated with noticeable decrements in physical, psychological, and social well-being.
Unique predictors of lower QOL class membership
In this study, being female was associated with belonging to the lower physical and psychological well-being latent classes. In one [41] of the three previous latent class studies [ [40–42], being female was associated with membership in the lower emotional well-being class. Potential explanations for this finding are that females are more likely to take on too many responsibilities, be more attentive to their own emotions, and use coping strategies associated with negative health outcomes [88].
While the impact of comorbidities on QOL domains was not evaluated in previous latent class analyses [40–42], other studies found that oncology patients and FCs [ [89–92] with a higher number of comorbid conditions reported poorer physical well-being. No studies were identified that found that a higher number of comorbidities had a negative impact on the social well-being of oncology patients and their FCs. However, one review of the association between chronic diseases and QOL suggests that some comorbid conditions are more bothersome in terms of physical well-being (e.g. musculoskeletal disease) while others are more bothersome in terms of social well-being (e.g. cerebrovascular/neurologic disorders) [93]. Future studies need to evaluate the contributions of specific comorbid conditions to each domain of QOL.
While the effect of family responsibilities on QOL was not evaluated in previous latent class analyses [40–42], in this study, having children at home was associated with belonging to the lower psychological and social well-being classes. This finding is consistent with a report on well-being in gynecologic cancer patients who were evaluated pre- and at 12 to 15 months post-treatment [94]. A number of factors may explain these associations. First, having children at home may be a surrogate for other factors like younger age, because younger adults are more likely to have child care responsibilities [95]. Second, having children at home may intensify patients’ and their FCs’ fear of death as they worry about the future prospects for their family [96]. In addition, patients and FCs may neglect their own psychological and social needs and focus on the needs of their family [97]. Lastly, personal needs may be further neglected if the emotional and behavioral needs of a child are increased as a reaction to the changes associated with a parent undergoing cancer treatment [98].
The relationship between lower weight and membership in the lower psychological well-being class requires further exploration. No studies were identified that considered this relationship in oncology patients or their FCs. Lower weight may be a surrogate for other factors associated with psychological well-being. For example, poor nutrition and less enjoyment of eating were associated with poorer psychological well-being in oncology patients [41,99]. However, because nutritional status and enjoyment of eating were not evaluated in the current study, these associations cannot be excluded.
Genomic predictors of lower QOL domain class membership
In this study, while a number of SNPs in cytokine genes were associated with latent class memberships in the bivariate analyses, only one SNP (i.e., NFKB2 rs7897947) remained significant in the multivariable logistic regression analyses for the social well-being domain. Carrying one or two doses of the rare G allele (i.e., TT versus TG + GG) for NFKB2 rs7897947 was associated with a 54% decrease in the odds of belonging to the lower social well-being class. NFKB2 is a gene that belongs to the NFKB family that encodes for transcription factors that contribute to the effective mounting of an immune response as well as to the regulation of cell proliferation, development, and apoptosis [100]. In addition, this gene family appears to be activated in stressful situations and in response to tissue damage, and is linked to inflammatory diseases and cancer [101]. While the function of NFKB2 rs7897947 is not known, it is located in the intron region of NFKB2 and may affect RNA splicing [102]. Alternatively, NFKB2 rs7897947 may be a surrogate for an unmeasured functional SNP that is in linkage disequilibrium with this SNP.
No studies were found that reported on an association between NFKB2 and any QOL domain. In our previous study that evaluated associations between cytokine genes and latent class membership using total QOL scores [43], the relationship between NFKB2 rs7897947 and total QOL class membership was not significant. In contrast, this same SNP was associated with sleep disturbance [48] and trait anxiety [103] in our previous work. In the first study that used GMM to identify latent classes of patients and FCs based on self-reported sleep disturbance prior to, during, and following the completion of RT [48], individuals who carried one or two doses of the rare G allele were more likely to belong to the latent class with lower levels of sleep disturbance. In the second study that investigated anxiety before the initiation of RT [103], individuals with one or two doses of the rare G allele had lower trait anxiety. Taken together, these findings suggest that individuals with the rare allele for NFKB2 rs7897947 may be less susceptible to sleep disturbance, trait anxiety, and poorer social well-being.
These findings support the pathway for genetic influences on social functioning proposed by Ordonana and colleagues [104]. In this proposed pathway, genetic factors may influence health status (e.g., symptom responses) and individual characteristics (e.g., personality) that in turn influence social functioning. Furthermore, given the involvement of NFKB2 in immune responses, our finding is consistent with the propositions inherent in cytokine-induced sickness behavior which suggest that such responses can result in decreased social interactions [45]. Additional research is warranted to confirm the association identified in this study and to explore the potential mechanisms through which NFKB2 rs7897947 may be involved in sleep disturbance, trait anxiety, and social-well-being.
In our previous study that used total QOL scores [43], two SNPs (i.e. IL1R2 rs4141134 and NFKB2 rs12772374) were found to be associated with latent class membership. In the current study, while a significant association was found between the IL1R2 SNP and physical well-being, in the bivariate analyses, it did not persist in the multivariate analyses. The finding that NFKB2 rs12772374 was associated with total QOL but not with any of the QOL domains may reflect that several items across the various subscales that were not concentrated within each subscale of the instrument, contributed to the association found with total QOL scores. In addition, it is possible that the identified associations occurred by chance and replication of these findings is warranted.
Limitations
Findings from this study provide preliminary evidence for two classes of oncology patients and their FCs who report distinctly different physical, psychological and social well-being trajectories. While the sample sizes for these latent class analyses are considered adequate [58,59], analyses with larger samples may identify additional latent classes. Furthermore, additional latent classes may be identified depending on participant characteristics. For example, the major reasons for refusal in this study were being too overwhelmed or too busy, which may have influenced the number of latent classes or range of QOL scores. Additional SNPs in various cytokine genes, as well as serum cytokine levels, warrant evaluation in future studies. The evaluation of circulating levels of cytokines may contribute to a more comprehensive understanding of the functional significance of SNPs identified to be associated with various QOL domains.
Conclusions
Nonetheless, this study adds to the growing body of evidence of genomic involvement in QOL outcomes [44]. The genetic association identified suggests a relationship between a cytokine gene polymorphism and decrements in social well-being. An increased understanding of molecular markers of QOL may lead to the identification of biomarkers that can be used to identify individuals who are at higher risk for poorer outcomes and who warrant supportive care interventions.
Supplementary Material
Footnotes
Declaration of Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Fayers P, Machin D. Quality of Life: The Assessment, Analysis and Interpretation of Patient-reported Outcomes. Hoboken, NJ: Wiley & Sons; 2007. [Google Scholar]
- 2.Ferrell BR, Dow KH, Leigh S, Ly J, Gulasekaram P. Quality of life in long-term cancer survivors. Oncology Nursing Forum. 1995;22(6):915–922. [PubMed] [Google Scholar]
- 3.Sloan JA, Aaronson N, Cappelleri JC, Fairclough DL, Varricchio C. Assessing the clinical significance of single items relative to summated scores. Mayo Clinic Proceedings. 2002;77(5):479–487. [PubMed] [Google Scholar]
- 4.Staff I, Salner A, Bohannon R, Panatieri P, Maljanian R. Disease-specific symptoms and general quality of life of patients with prostate carcinoma before and after primary three-dimensional conformal radiotherapy. Cancer. 2003;98(11):2335–2343. doi: 10.1002/cncr.11805. [DOI] [PubMed] [Google Scholar]
- 5.Monga U, Kerrigan AJ, Thornby J, Monga TN, Zimmermann KP. Longitudinal study of quality of life in patients with localized prostate cancer undergoing radiotherapy. Journal of Rehabilitation Research and Development. 2005;42(3):391–399. doi: 10.1682/jrrd.2004.06.0071. [DOI] [PubMed] [Google Scholar]
- 6.Auchter RM, Scholtens D, Adak S, Wagner H, Cella DF, Mehta MP. Quality of life assessment in advanced non-small-cell lung cancer patients undergoing an accelerated radiotherapy regimen: report of ECOG study 4593. Eastern Cooperative Oncology Group. International Journal of Radiation Oncology, Biology, Physics. 2001;50(5):1199–1206. doi: 10.1016/s0360-3016(01)01604-2. [DOI] [PubMed] [Google Scholar]
- 7.Marchand V, Bourdin S, Charbonnel C, Rio E, Munos C, Campion L, et al. No impairment of quality of life 18 months after high-dose intensity-modulated radiotherapy for localized prostate cancer: a prospective study. International Journal of Radiation Oncology, Biology, Physics. 2010;77(4):1053–1059. doi: 10.1016/j.ijrobp.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Browall M, Ahlberg K, Karlsson P, Danielson E, Persson LO, Gaston-Johansson F. Health-related quality of life during adjuvant treatment for breast cancer among postmenopausal women. European Journal of Oncology Nursing. 2008;12(3):180–189. doi: 10.1016/j.ejon.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Mc Caughan E, Mc Sorley O, Prue G, Parahoo K, Bunting B, Sullivan JO, et al. Quality of life in men receiving radiotherapy and neo-adjuvant androgen deprivation for prostate cancer: results from a prospective longitudinal study. Journal of Advanced Nursing. 2013;69(1):53–65. doi: 10.1111/j.1365-2648.2012.05987.x. [DOI] [PubMed] [Google Scholar]
- 10.Reddy K, Gaspar LE, Kavanagh BD, Waziri A, Damek DM, Ney D, et al. Prospective evaluation of health-related quality of life in patients with glioblastoma multiforme treated on a phase II trial of hypofractionated IMRT with temozolomide. Journal of Neuro-oncology. 2013;114(1):111–116. doi: 10.1007/s11060-013-1159-6. [DOI] [PubMed] [Google Scholar]
- 11.Fransson P, Damber JE, Tomic R, Modig H, Nyberg G, Widmark A. Quality of life and symptoms in a randomized trial of radiotherapy versus deferred treatment of localized prostate carcinoma. Cancer. 2001;92(12):3111–3119. doi: 10.1002/1097-0142(20011215)92:12<3111::aid-cncr10160>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 12.van der Voort van Zyp NC, Prevost JB, van der Holt B, Braat C, van Klaveren RJ, Pattynama PM, et al. Quality of life after stereotactic radiotherapy for stage I non-small-cell lung cancer. International Journal of Radiation Oncology, Biology, Physics. 2010;77(1):31–37. doi: 10.1016/j.ijrobp.2009.04.080. [DOI] [PubMed] [Google Scholar]
- 13.Pijls-Johannesma M, Houben R, Boersma L, Grutters J, Seghers K, Lambin P, et al. High-dose radiotherapy or concurrent chemo-radiation in lung cancer patients only induces a temporary, reversible decline in QoL. Radiotherapy and Oncology. 2009;91(3):443–448. doi: 10.1016/j.radonc.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Lilleby W, Stensvold A, Dahl AA. Intensity-modulated radiotherapy to the pelvis and androgen deprivation in men with locally advanced prostate cancer: A study of adverse effects and their relation to quality of life. The Prostate. 2013;73(10):1038–1047. doi: 10.1002/pros.22651. [DOI] [PubMed] [Google Scholar]
- 15.van Gysen KL, Kneebone AB, Guo L, Vaux KJ, Lazzaro EM, Eade TN. Health-related quality of life using intensity-modulated radiation therapy for post-prostatectomy radiotherapy. Journal of Medical Imaging and Radiation Oncology. 2013;57(1):89–96. doi: 10.1111/j.1754-9485.2012.02464.x. [DOI] [PubMed] [Google Scholar]
- 16.Keilani M, Krall C, Marosi C, Flechl B, Dieckmann K, Widhalm G, et al. Strength of skeletal muscle and self-reported physical performance in Austrian glioblastoma-patients. Wiener klinische Wochenschrift. 2012;124(11–12):377–383. doi: 10.1007/s00508-012-0186-1. [DOI] [PubMed] [Google Scholar]
- 17.Lagerwaard FJ, Aaronson NK, Gundy CM, Haasbeek CJ, Slotman BJ, Senan S. Patient-reported quality of life after stereotactic ablative radiotherapy for early-stage lung cancer. Journal of Thoracic Oncology. 2012;7(7):1148–1154. doi: 10.1097/JTO.0b013e318252cfef. [DOI] [PubMed] [Google Scholar]
- 18.Geinitz H, Thamm R, Scholz C, Heinrich C, Prause N, Kerndl S, et al. Longitudinal analysis of quality of life in patients receiving conformal radiation therapy for prostate cancer. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft. 2010;186(1):46–52. doi: 10.1007/s00066-009-2023-7. [DOI] [PubMed] [Google Scholar]
- 19.Noal S, Levy C, Hardouin A, Rieux C, Heutte N, Segura C, et al. One-year longitudinal study of fatigue, cognitive functions, and quality of life after adjuvant radiotherapy for breast cancer. International Journal of Radiation Oncology, Biology, Physics. 2011;81(3):795–803. doi: 10.1016/j.ijrobp.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 20.Xiaokun L. Quality of life among mastectomy patients receiving radiotherapy. Contemporary Nurse. 2002;13(2–3):198–208. doi: 10.5172/conu.13.2-3.198. [DOI] [PubMed] [Google Scholar]
- 21.Chandra PS, Chaturvedi SK, Channabasavanna SM, Anantha N, Reddy BK, Sharma S, et al. Psychological well-being among cancer patients receiving radiotherapy - A prospective study. Quality of Life Research. 1998;7(6):495–500. doi: 10.1023/a:1008822307420. [DOI] [PubMed] [Google Scholar]
- 22.Sherwood PR, Given BA, Donovan H, Baum A, Given CW, Bender CM, et al. Guiding research in family care: A new approach to oncology caregiving. Psycho-oncology. 2008;17(10):986–996. doi: 10.1002/pon.1314. [DOI] [PubMed] [Google Scholar]
- 23.Girgis A, Lambert S, Johnson C, Waller A, Currow D. Physical, psychosocial, relationship, and economic burden of caring for people with cancer: A review. Journal of Oncology Practice. 2013;9(4):197–202. doi: 10.1200/JOP.2012.000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stenberg U, Ruland CM, Miaskowski C. Review of the literature on the effects of caring for a patient with cancer. Psycho-oncology. 2010;19(10):1013–1025. doi: 10.1002/pon.1670. [DOI] [PubMed] [Google Scholar]
- 25.Kim Y, Given BA. Quality of life of family caregivers of cancer survivors: Across the trajectory of the illness. Cancer. 2008;112(11 Suppl):2556–2568. doi: 10.1002/cncr.23449. [DOI] [PubMed] [Google Scholar]
- 26.Kitrungrote L, Cohen MZ. Quality of life of family caregivers of patients with cancer: A literature review. Oncology Nursing Forum. 2006;33(3):625–632. doi: 10.1188/06.ONF.625-632. [DOI] [PubMed] [Google Scholar]
- 27.Kim Y, Duberstein PR, Sorensen S, Larson MR. Levels of depressive symptoms in spouses of people with lung cancer: Effects of personality, social support, and caregiving burden. Psychosomatics. 2005;46(2):123–130. doi: 10.1176/appi.psy.46.2.123. [DOI] [PubMed] [Google Scholar]
- 28.Manne S, Ostroff J, Winkel G, Goldstein L, Fox K, Grana G. Posttraumatic growth after breast cancer: Patient, partner, and couple perspectives. Psychosomatic Medicine. 2004;66(3):442–454. doi: 10.1097/01.psy.0000127689.38525.7d. [DOI] [PubMed] [Google Scholar]
- 29.Kim Y, Baker F, Spillers RL. Cancer caregivers' quality of life: effects of gender, relationship, and appraisal. Journal of Pain and Symptom Management. 2007;34(3):294–304. doi: 10.1016/j.jpainsymman.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 30.Braun M, Mikulincer M, Rydall A, Walsh A, Rodin G. Hidden morbidity in cancer: Spouse caregivers. Journal of Clinical Oncology. 2007;25(30):4829–4834. doi: 10.1200/JCO.2006.10.0909. [DOI] [PubMed] [Google Scholar]
- 31.Crowe H, Costello AJ. Prostate cancer: perspectives on quality of life and impact of treatment on patients and their partners. Urologic Nursing. 2003;23(4):279–285. [PubMed] [Google Scholar]
- 32.Weitzner MA, McMillan SC, Jacobsen PB. Family caregiver quality of life: Differences between curative and palliative cancer treatment settings. Journal of Pain and Symptom Management. 1999;17(6):418–428. doi: 10.1016/s0885-3924(99)00014-7. [DOI] [PubMed] [Google Scholar]
- 33.Kornblith AB, Herr HW, Ofman US, Scher HI, Holland JC. Quality of life of patients with prostate cancer and their spouses. The value of a data base in clinical care. Cancer. 1994;73(11):2791–2802. doi: 10.1002/1097-0142(19940601)73:11<2791::aid-cncr2820731123>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 34.Kurtz ME, Kurtz JC, Given CW, Given BA. Depression and physical health among family caregivers of geriatric patients with cancer--a longitudinal view. Medical Science Monitor. 2004;10(8):CR447–CR456. [PubMed] [Google Scholar]
- 35.Boyle D, Blodgett L, Gnesdiloff S, White J, Bamford AM, Sheridan M, et al. Caregiver quality of life after autologous bone marrow transplantation. Cancer Nursing. 2000;23(3):193–203. doi: 10.1097/00002820-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Kim Y, Wellisch DK, Spillers RL, Crammer C. Psychological distress of female cancer caregivers: effects of type of cancer and caregivers' spirituality. Supportive Care in Cancer. 2007;15(12):1367–1374. doi: 10.1007/s00520-007-0265-4. [DOI] [PubMed] [Google Scholar]
- 37.Colgrove LA, Kim Y, Thompson N. The effect of spirituality and gender on the quality of life of spousal caregivers of cancer survivors. Annals of Behavioral Medicine. 2007;33(1):90–98. doi: 10.1207/s15324796abm3301_10. [DOI] [PubMed] [Google Scholar]
- 38.Matthews BA, Baker F, Spillers RL. Family caregivers' quality of life: Influence of health protective stance and emotional strain. Psychology and Health. 2004;19(5):625–641. [Google Scholar]
- 39.Muthen BO, Kaplan DW. Latent variable analysis: Growth mixture modeling and related techniques for longitudinal data. In: Kaplan D, editor. The Sage Handbook of Quantitative Methodology for the Social Sciences. Newbury Park, CA: Sage Publications; 2004. pp. 345–368. [Google Scholar]
- 40.Helgeson VS, Snyder P, Seltman H. Psychological and physical adjustment to breast cancer over 4 years: Identifying distinct trajectories of change. Health Psychology. 2004;23(1):3–15. doi: 10.1037/0278-6133.23.1.3. [DOI] [PubMed] [Google Scholar]
- 41.Lam WW, Ye M, Fielding R. Trajectories of quality of life among Chinese patients diagnosed with nasopharynegeal cancer. PLoS One. 2012;7(9):e44022. doi: 10.1371/journal.pone.0044022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunn J, Ng SK, Breitbart W, Aitken J, Youl P, Baade PD, et al. Health-related quality of life and life satisfaction in colorectal cancer survivors: Trajectories of adjustment. Health and Quality of Life Outcomes. 2013;11(1):46. doi: 10.1186/1477-7525-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alexander K, Cooper B, Paul SM, West C, Yates P, Kober KM, et al. Evidence of associations between cytokine gene polymorphisms and quality of life in patients with cancer and their family caregivers. Oncology Nursing Forum. 2014;41(5):E267–E281. doi: 10.1188/14.ONF.E267-E281. [DOI] [PubMed] [Google Scholar]
- 44.Sprangers MA, Thong MS, Bartels M, Barsevick A, Ordonana J, Shi Q, et al. Biological pathways, candidate genes, and molecular markers associated with quality-of-life domains: An update. Quality of Life Research. 2014;23(7):1997–2013. doi: 10.1007/s11136-014-0656-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miaskowski C, Aouizerat BE. Biomarkers: symptoms, survivorship, and quality of life. Seminars in Oncology Nursing. 2012;28(2):129–138. doi: 10.1016/j.soncn.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sprangers MA, Sloan JA, Barsevick A, Chauhan C, Dueck AC, Raat H, et al. Scientific imperatives, clinical implications, and theoretical underpinnings for the investigation of the relationship between genetic variables and patient-reported quality-of-life outcomes. Quality of Life Research. 2010;19(10):1395–1403. doi: 10.1007/s11136-010-9759-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rausch SM, Clark MM, Patten C, Liu H, Felten S, Li Y, et al. Relationship between cytokine gene single nucleotide polymorphisms and symptom burden and quality of life in lung cancer survivors. Cancer. 2010;116(17):4103–4113. doi: 10.1002/cncr.25255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miaskowski C, Cooper BA, Dhruva A, Dunn LB, Langford DJ, Cataldo JK, et al. Evidence of associations between cytokine genes and subjective reports of sleep disturbance in oncology patients and their family caregivers. PLoS One. 2012;7(7):e40560. doi: 10.1371/journal.pone.0040560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karnofsky D, Abelmann WH, Craver LV, Burchenal JH. The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer. 1948;1:634–656. [Google Scholar]
- 50.Padilla GV, Presant C, Grant MM, Metter G, Lipsett J, Heide F. Quality of life index for patients with cancer. Research in Nursing and Health. 1983;6(3):117–126. doi: 10.1002/nur.4770060305. [DOI] [PubMed] [Google Scholar]
- 51.Padilla GV, Ferrell B, Grant MM, Rhiner M. Defining the content domain of quality of life for cancer patients with pain. Cancer Nursing. 1990;13(2):108–115. [PubMed] [Google Scholar]
- 52.Ferrell BR, Dow KH, Grant M. Measurement of the quality of life in cancer survivors. Quality of Life Research. 1995;4(6):523–531. doi: 10.1007/BF00634747. [DOI] [PubMed] [Google Scholar]
- 53.Ferrell BR. The impact of pain on quality of life. A decade of research. Nursing Clinics of North America. 1995;30(4):609–624. [PubMed] [Google Scholar]
- 54.SPSS. IBM SPSS for Windows (Version 22) Chicago, Illinois: SPSS, Inc.; 2010. [Google Scholar]
- 55.Muthen LK, Muthen BO. Mplus User's Guide. 6th. Los Angeles, CA: Muthen & Muthen; 1998–2010. [Google Scholar]
- 56.Dunn LB, Aouizerat BE, Cooper BA, Dodd M, Lee K, West C, et al. Trajectories of anxiety in oncology patients and family caregivers during and after radiation therapy. European Journal of Oncology Nursing. 2012;16:1–9. doi: 10.1016/j.ejon.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jung T, Wickrama KAS. An introduction to latent class growth analysis and growth mixture modeling. Social and Personality Psychology Compass. 2008;2(1):302–317. [Google Scholar]
- 58.Nylund KL, Asparouhov T, Muthen BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling. 2007;14(4):535–569. [Google Scholar]
- 59.Tofighi D, Enders CK. Identifying the correct number of classes in growth mixture models. In: Hancock GR, Samuelsen SM, editors. Advances in Latent Variable Mixture Models. Charlotte, NC: Information Age Publishing; 2008. pp. 317–341. [Google Scholar]
- 60.Celeux G, Soromenho G. An entropy criterion for assessing the number of clusters in a mixture model. Journal of Classification. 1996;13(2):195–212. [Google Scholar]
- 61.Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, et al. The structure of haplotype blocks in the human genome. Science. 2002;296(5576):2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 62.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. American Journal of Human Genetics. 2001;68(4):978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Halder I, Shriver M, Thomas M, Fernandez JR, Frudakis T. A panel of ancestry informative markers for estimating individual biogeographical ancestry and admixture from four continents: utility and applications. Human Mutation. 2008;29(5):648–658. doi: 10.1002/humu.20695. [DOI] [PubMed] [Google Scholar]
- 64.Hoggart CJ, Parra EJ, Shriver MD, Bonilla C, Kittles RA, Clayton DG, et al. Control of confounding of genetic associations in stratified populations. American Journal of Human Genetics. 2003;72(6):1492–1504. doi: 10.1086/375613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tian C, Gregersen PK, Seldin MF. Accounting for ancestry: Population substructure and genome-wide association studies. Human Molecular Genetics. 2008;17(R2):R143–R150. doi: 10.1093/hmg/ddn268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nature Genetics. 2006;38(8):904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 67.StataCorp. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP; 2013. [Google Scholar]
- 68.Illi J, Miaskowski C, Cooper B, Levine JD, Dunn L, West C, et al. Association between pro- and anti-inflammatory cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression. Cytokine. 2012;58(3):437–447. doi: 10.1016/j.cyto.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCann B, Miaskowski C, Koetters T, Baggott C, West C, Levine JD, et al. Associations between pro- and anti-inflammatory cytokine genes and breast pain in women prior to breast cancer surgery. Journal of Pain. 2012;13(5):425–437. doi: 10.1016/j.jpain.2011.02.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Merriman JD, Aouizerat BE, Langford DJ, Cooper BA, Baggott CR, Cataldo JK, et al. Preliminary evidence of an association between an Interleukin 6 promoter polymorphism and self-reported attentional function in oncology patients and their family caregivers. Biological Research for Nursing. 2014;16(2):152–159. doi: 10.1177/1099800413479441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alfaro E, Dhruva A, Langford DJ, Koetters T, Merriman JD, West C, et al. Associations between cytokine gene variations and self-reported sleep disturbance in women following breast cancer surgery. European Journal of Oncology Nursing. 2014;18(1):85–93. doi: 10.1016/j.ejon.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dunn LB, Aouizerat BE, Langford DJ, Cooper BA, Dhruva A, Cataldo JK, et al. Cytokine gene variation is associated with depressive symptom trajectories in oncology patients and family caregivers. European Journal of Oncology Nursing. 2013;17(3):346–353. doi: 10.1016/j.ejon.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. [PubMed] [Google Scholar]
- 74.Hattersley AT, McCarthy MI. What makes a good genetic association study? Lancet. 2005;366(9493):1315–1323. doi: 10.1016/S0140-6736(05)67531-9. [DOI] [PubMed] [Google Scholar]
- 75.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. Journal of Clinical Oncology. 1998;16(1):139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 76.Osoba D. Interpreting the meaningfulness of changes in health-related quality of life scores: lessons from studies in adults. International Journal of Cancer. 1999;(Supplement 12):132–137. doi: 10.1002/(sici)1097-0215(1999)83:12+<132::aid-ijc23>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 77.Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 78.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Medical Care. 2003;41(5):582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 79.Murray SA, Kendall M, Boyd K, Grant L, Highet G, Sheikh A. Archetypal trajectories of social, psychological, and spiritual wellbeing and distress in family care givers of patients with lung cancer: secondary analysis of serial qualitative interviews. British Medical Journal. 2010;340:c2581. doi: 10.1136/bmj.c2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim Y, Carver CS, Spillers RL, Crammer C, Zhou ES. Individual and dyadic relations between spiritual well-being and quality of life among cancer survivors and their spousal caregivers. Psycho-oncology. 2011;20(7):762–770. doi: 10.1002/pon.1778. [DOI] [PubMed] [Google Scholar]
- 81.Dunn J, Steginga SK. Young women's experience of breast cancer: defining young and identifying concerns. Psycho-oncology. 2000;9(2):137–146. doi: 10.1002/(sici)1099-1611(200003/04)9:2<137::aid-pon442>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 82.Krupski TL, Sonn G, Kwan L, Maliski S, Fink A, Litwin MS. Ethnic variation in health-related quality of life among low-income men with prostate cancer. Ethnicity & Disease. 2005;15(3):461–468. [PubMed] [Google Scholar]
- 83.Bowen DJ, Alfano CM, McGregor BA, Kuniyuki A, Bernstein L, Meeske K, et al. Possible socioeconomic and ethnic disparities in quality of life in a cohort of breast cancer survivors. Breast Cancer Research and Treatment. 2007;106(1):85–95. doi: 10.1007/s10549-006-9479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Luckett T, Goldstein D, Butow PN, Gebski V, Aldridge LJ, McGrane J, et al. Psychological morbidity and quality of life of ethnic minority patients with cancer: A systematic review and meta-analysis. Lancet Oncology. 2011;12(13):1240–1248. doi: 10.1016/S1470-2045(11)70212-1. [DOI] [PubMed] [Google Scholar]
- 85.Ashing-Giwa KT, Padilla G, Tejero J, Kraemer J, Wright K, Coscarelli A, et al. Understanding the breast cancer experience of women: A qualitative study of African American, Asian American, Latina and Caucasian cancer survivors. Psychooncology. 2004;13(6):408–428. doi: 10.1002/pon.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. Journal of the American Medical Association. 1995;273(1):59–65. [PubMed] [Google Scholar]
- 87.Sekine R, Ogata M, Uchiyama I, Miyakoshi K, Uruma M, Miyashita M, et al. Changes in and associations among functional status and perceived quality of life of patients with metastatic/locally advanced cancer receiving rehabilitation for general disability. The American Journal of Hospice & Palliative Care. 2015;33(7):695–702. doi: 10.1177/1049909114537871. [DOI] [PubMed] [Google Scholar]
- 88.Li Q, Mak Y, Loke A. Spouses' experience of caregiving for cancer patients: A literature review. International Nursing Review. 2013;60(2):178–187. doi: 10.1111/inr.12000. [DOI] [PubMed] [Google Scholar]
- 89.Sarna L, Brown JK, Cooley ME, Williams RD, Chernecky C, Padilla G, et al. Quality of life and meaning of illness of women with lung cancer. Oncology Nursing Forum. 2005;32(1):E9–E19. doi: 10.1188/05.ONF.E9-E19. [DOI] [PubMed] [Google Scholar]
- 90.Sarna L, Cooley ME, Brown JK, Williams RD, Chernecky C, Padilla G, et al. Quality of life and health status of dyads of women with lung cancer and family members. Oncology Nursing Forum. 2006;33(6):1109–1116. doi: 10.1188/06.ONF.1109-1116. [DOI] [PubMed] [Google Scholar]
- 91.Hofso K, Bjordal K, Diep LM, Rustoen T. The relationships between demographic and clinical characteristics and quality of life during and after radiotherapy in women with breast cancer. Quality of Life Research. 2014;23(10):2769–2777. doi: 10.1007/s11136-014-0736-2. [DOI] [PubMed] [Google Scholar]
- 92.Reeve BB, Chen RC, Moore DT, Deal AM, Usinger DS, Lyons JC, et al. Impact of comorbidity on health-related quality of life after prostate cancer treatment: Combined analysis of two prospective cohort studies. BJU International. 2014;114(6b):E74–E81. doi: 10.1111/bju.12723. [DOI] [PubMed] [Google Scholar]
- 93.Sprangers MA, de Regt EB, Andries F, van Agt HM, Bijl RV, de Boer JB, et al. Which chronic conditions are associated with better or poorer quality of life? Journal of Clinical Epidemiology. 2000;53(9):895–907. doi: 10.1016/s0895-4356(00)00204-3. [DOI] [PubMed] [Google Scholar]
- 94.Eisemann M, Lalos A. Psychosocial determinants of well-being in gynecologic cancer patients. Cancer Nursing. 1999;22(4):303–306. doi: 10.1097/00002820-199908000-00007. [DOI] [PubMed] [Google Scholar]
- 95.Kroenke CH, Rosner B, Chen WY, Kawachi I, Colditz GA, Holmes MD. Functional impact of breast cancer by age at diagnosis. Journal of Clinical Oncology. 2004;22(10):1849–1856. doi: 10.1200/JCO.2004.04.173. [DOI] [PubMed] [Google Scholar]
- 96.Ernst J, Gotze H, Brahler E, Korner A, Hinz A. Quality of life of parents diagnosed with cancer: change over time and influencing factors. European Journal of Cancer Care. 2012;21(4):535–541. doi: 10.1111/j.1365-2354.2012.01330.x. [DOI] [PubMed] [Google Scholar]
- 97.Bloom JR, Stewart SL, Chang S, Banks PJ. Then and now: quality of life of young breast cancer survivors. Psycho-oncology. 2004;13(3):147–160. doi: 10.1002/pon.794. [DOI] [PubMed] [Google Scholar]
- 98.Visser A, Huizinga GA, van der Graaf WT, Hoekstra HJ, Hoekstra-Weebers JE. The impact of parental cancer on children and the family: a review of the literature. Cancer Treatment Reviews. 2004;30(8):683–694. doi: 10.1016/j.ctrv.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 99.Xara S, Amaral T, Parente B. Undernutrition and quality of life in non small cell lung cancer patients. Revista Portuguesa de Pneumologia (English Edition. 2011;17(4):153–158. doi: 10.1016/j.rppneu.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 100.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nature Immunology. 2011;12(8):695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 101.Schmitz ML, Mattioli I, Buss H, Kracht M. NF-kappaB: a multifaceted transcription factor regulated at several levels. Chembiochemistry. 2004;5(10):1348–1358. doi: 10.1002/cbic.200400144. [DOI] [PubMed] [Google Scholar]
- 102.Prasanth KV, Spector DL. Eukaryotic regulatory RNAs: an answer to the 'genome complexity' conundrum. Genes & Development. 2007;21(1):11–42. doi: 10.1101/gad.1484207. [DOI] [PubMed] [Google Scholar]
- 103.Miaskowski C, Cataldo JK, Baggott CR, West C, Dunn LB, Dhruva A, et al. Cytokine gene variations associated with trait and state anxiety in oncology patients and their family caregivers. Supportive Care in Cancer. 2015;23(4):953–965. doi: 10.1007/s00520-014-2443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ordonana JR, Bartels M, Boomsma DI, Cella D, Mosing M, Oliveira JR, et al. Biological pathways and genetic mechanisms involved in social functioning. Quality of Life Research. 2013;22(6):1189–1200. doi: 10.1007/s11136-012-0277-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.