Abstract
Patients with chronic kidney disease (CKD) develop increased levels of the phosphate-regulating hormone, fibroblast growth factor (FGF) 23, that are associated with a higher risk of mortality. Increases in inflammatory markers are another common feature of CKD that predict poor clinical outcomes. Elevated FGF23 is associated with higher circulating levels of inflammatory cytokines in CKD, which can stimulate osteocyte production of FGF23. Here, we studied whether FGF23 can directly stimulate hepatic production of inflammatory cytokines in the absence of α-klotho, an FGF23 co-receptor in the kidney that is not expressed by hepatocytes. By activating FGF receptor isoform 4 (FGFR4), FGF23 stimulated calcineurin signaling in cultured hepatocytes, which increased the expression and secretion of inflammatory cytokines, including C-reactive protein. Elevating serum FGF23 levels increased hepatic and circulating levels of C-reactive protein in wild-type mice, but not in FGFR4 knockout mice. Administration of an isoform-specific FGFR4 blocking antibody reduced hepatic and circulating levels of C-reactive protein in the 5/6 nephrectomy rat model of CKD. Thus, FGF23 can directly stimulate hepatic secretion of inflammatory cytokines. Our findings indicate a novel mechanism of chronic inflammation in patients with CKD and suggest that FGFR4 blockade might have therapeutic anti-inflammatory effects in CKD.
Keywords: chronic kidney disease, hepatocytes, inflammation, FGF23, calcineurin
Introduction
Fibroblast growth factor (FGF) 23 is a hormone produced by osteocytes that regulates phosphate homeostasis (1). In its classic target organs such as kidney and parathyroid glands, FGF23 binds FGF receptor (FGFR)/α-klotho co-receptor complexes to enhance phosphate excretion (2, 3), inhibit parathyroid hormone (PTH) secretion, and decrease levels of active vitamin D (4, 5). In patients with chronic kidney disease (CKD), serum levels of FGF23 rise progressively (6–8). While compensatory increases in FGF23 help to maintain normal serum phosphate levels despite severely reduced renal function (7), several clinical studies have confirmed a concentration-dependent association between elevated FGF23 levels and higher risks of major cardiovascular events and mortality (9–12). These findings establish FGF23 as a potent risk factor of death in CKD.
FGFRs are receptor tyrosine kinases (13). Following ligand-induced auto-phosphorylation of the receptor, FGF receptor substrate (FRS) 2α undergoes tyrosine phosphorylation and stimulates Ras/mitogen-activated protein kinase (MAPK) signaling (14). In contrast to FRS2α, which is constitutively bound to FGFR independent of the receptor’s activation state (15), phosphoslipase Cγ (PLCγ) can be recruited to bind directly to the FGFR cytoplasmic tail (16, 17). Activated PLCγ increases cytoplasmic Ca2+ levels leading to an activation of the protein phosphatase calcineurin and its substrate, nuclear factor of activated T cells (NFAT) (14). The mammalian genome encodes for four different receptor isoforms, FGFR1-4 (13). We previously reported that in the absence of α-klotho, FGF23 can directly target cardiac myocytes in an FGFR4-dependent manner and thereby contribute to cardiac hypertrophy and injury in CKD (18, 19). Whereas α-klotho-expressing cells respond to FGF23 by activating the Ras/MAPK cascade (2), FGF23’s effects on cultured cardiac myocytes are mediated by PLCγ and calcineurin, indicating that FGF23 activates the pro-hypertrophic PLCγ/calcineurin/NFAT signaling pathway in the heart (18, 19). This suggested the concept of α-klotho independent, direct end-organ effects of FGF23 in non-classic target tissues, and a potential causative role of FGFR4 in complications of CKD.
In patients with CKD, systemic inflammation contributes to morbidity and mortality (20, 21). Serum levels of inflammatory cytokines such as C-reactive protein (CRP), interleukin 6 (IL-6), and tumor necrosis factor α (TNFα) are significantly elevated and are strong predictors of poor clinical outcome in CKD (22–25). In a previous clinical study, we reported that elevated FGF23 is independently associated with higher serum levels of CRP, IL-6 and TNFα, and greater odds of severe inflammation (26). Furthermore, we recently showed that inflammation increases FGF23 transcription in osteocytes (27), indicating a causative interconnection between inflammation and FGF23 production. Since hepatocytes express high levels of FGFR4, but lack α-klotho (28–30), we tested the hypothesis that FGF23 stimulates hepatic production of inflammatory cytokines through an α-klotho-independent signaling pathway, similar to the recently described mechanism of FGF23-induced signaling in cardiac myocytes (18, 19). We postulate that FGF23’s direct actions on hepatocytes might contribute to systemic inflammation in CKD and further elevations of FGF23 production.
Results
Hepatocytes express high levels of FGFR4 and β-klotho but lack α-klotho
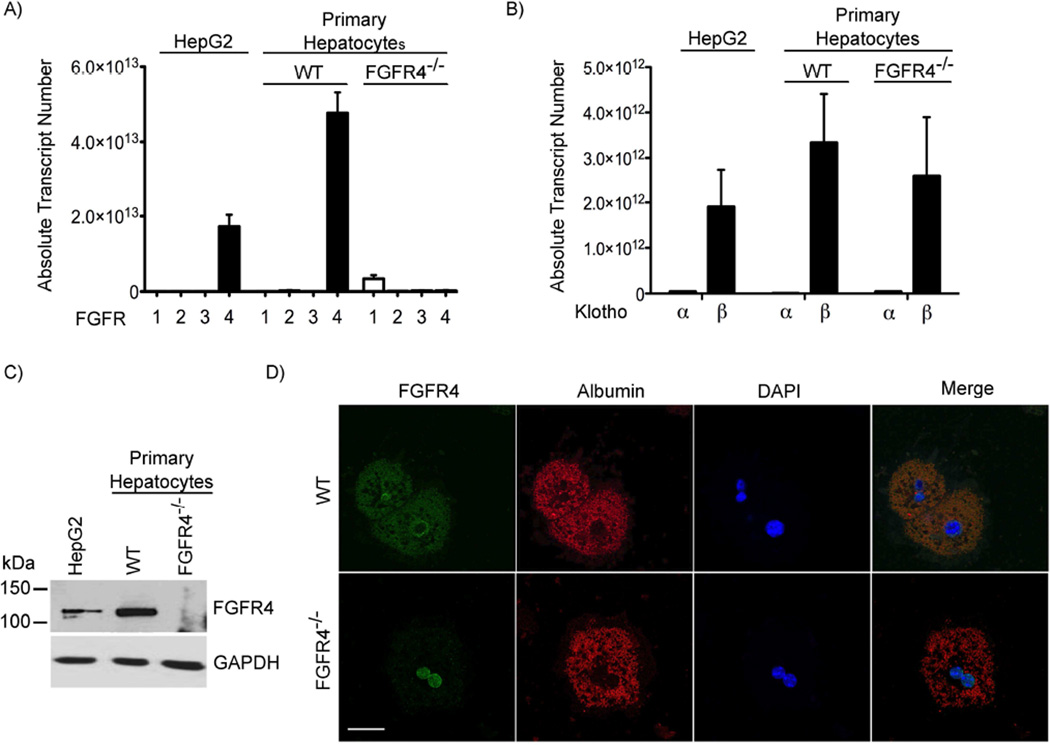
We investigated the expression profile of the four FGFR isoforms and the two klotho isoforms in HepG2 cells, a human hepatocellular carcinoma cell line, and in isolated primary mouse hepatocytes by real-time PCR. Both HepG2 cells and primary hepatocytes expressed high levels of FGFR4 and β-klotho but lacked FGFR1, FGFR2, FGFR3 and α-klotho (Figure 1A, B). In contrast, we could not detect FGFR4 in hepatocytes that were isolated from constitutive FGFR4−/− mice (31) (Figure 1A). FGFR4 deletion did not induce the expression of α-klotho or alter β-klotho levels in mouse hepatocytes (Figure 1B).
Figure 1. Hepatocytes express FGFR4 but lack α-klotho.
(A-B) Quantitative RT-PCR expression analysis of FGFR isoforms FGFR1-4 and klotho isoforms α and β in HepG2 cells, and primary hepatocytes isolated from wild-type (WT) and FGFR4 knockout (FGFR4−/−) mice (n = 3). (C) Immunoblot analysis of protein lysates from HepG2 cells, and primary WT and FGFR4−/− mouse hepatocytes with anti-FGFR4 reveals the expected 110-kDa signal for FGFR4 that is absent in extracts from FGFR4−/− hepatocytes. GAPDH shows equal protein loading. (D) Immunofluorescence microscopy analysis shows that FGFR4 (green) is expressed in WT mouse hepatocytes but absent in FGFR4−/− hepatocytes. Co-immunolabeling for albumin (red) identifies hepatocytes, and DAPI (blue) shows nuclei (original magnification, 100×; scale bar, 25 µm).
To confirm FGFR4 protein expression, we performed Western blot analyses. HepG2 cells and primary hepatocytes from wild-type mice showed a signal with the expected size for FGFR4 of approximately 110 kDa (32), which was absent in FGFR4−/− hepatocytes (Figure 1C). Immunocytochemical analyses revealed a membrane-associated and cytoplasmic localization pattern of FGFR4 in wild-type hepatocytes, which was not detected in FGFR4−/− hepatocytes (Figure 1D).
Overall, our findings confirm that hepatocytes express β-klotho but lack α-klotho, and that FGFR4 appears to be the only FGFR isoform present in hepatocytes. This result is consistent with previous reports that hepatic expression of FGFR1, FGFR2 and FGFR3 is limited to non-parenchymal cells and hepatocytes progenitors, whereas only FGFR4 is present in mature hepatocytes (28, 29).
FGF23 activates FGFR4 and induces PLCγ/calcineurin/NFAT signaling in cultured hepatocytes
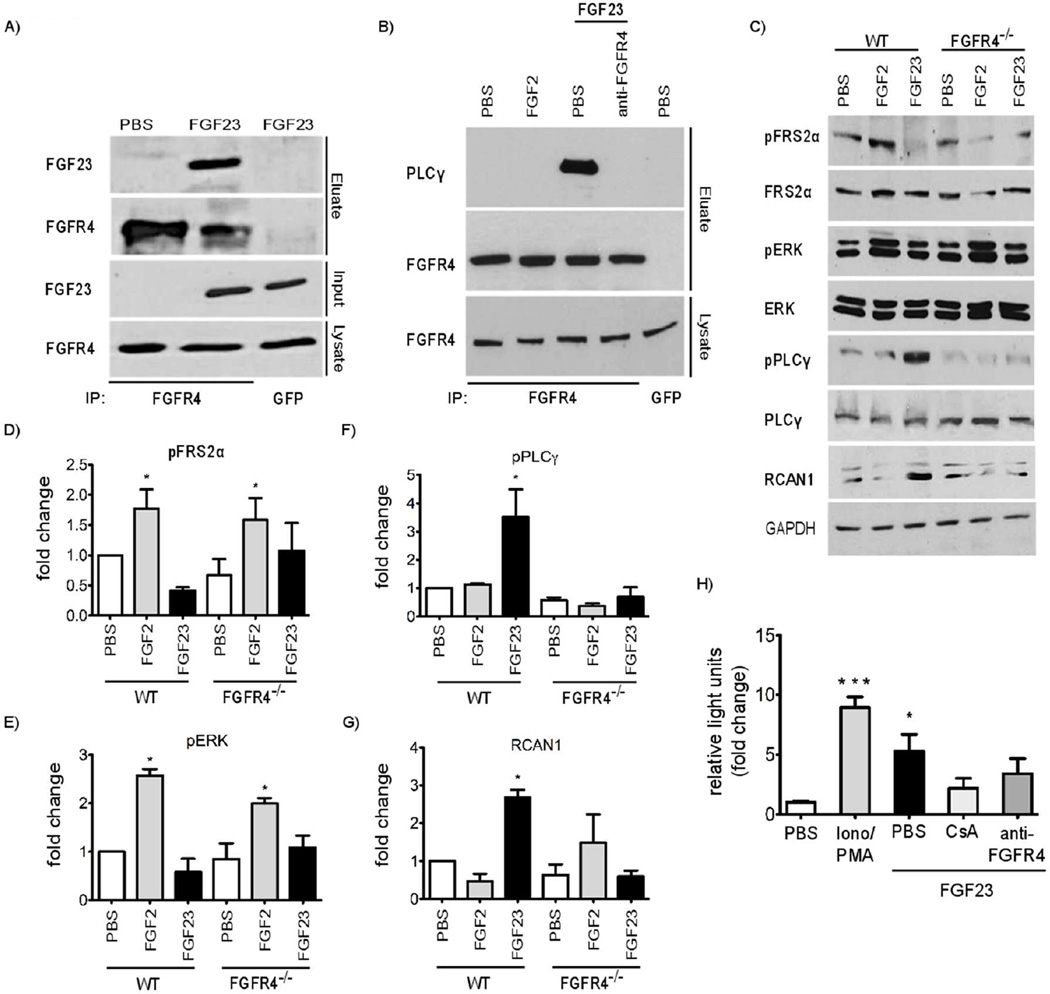
To determine if FGF23 can bind FGFR4 on hepatocytes, we incubated primary mouse hepatocytes in culture medium containing FGF23 for 20 minutes. We immunoprecipitated endogenous FGFR4 from total protein extracts, and analyzed eluates by immunoblotting with an anti-FGF23 antibody. FGF23 co-precipitated with FGFR4, but this effect was not detected in eluates derived from control hepatocytes that were cultured in regular medium (Figure 2A).
Figure 2. FGF23 activates FGFR4 and downstream PLCγ/calcineurin/NFAT signaling in primary mouse hepatocytes.
(A) FGF23 was overexpressed in HEK293 cells for 48 hours. Cell supernatants containing FGF23 were transferred onto primary mouse hepatocyte cultures for 30 minutes followed by immunoprecipitation of FGFR4 from total protein extracts. FGF23 only co-precipitates with FGFR4 in hepatocytes that were cultured in FGF23-containing medium. Immunoprecipitation with control antibody (anti-GFP) does not result in isolation of FGFR4 or FGF23. (B) Wild-type mouse hepatocytes were co-treated with PBS, FGF2 or FGF23 and an FGFR4-specific blocking antibody (anti-FGFR4) for 30 min followed by immunoprecipitation of FGFR4. PLCγ only co-precipitates with FGFR4 in FGF23-treated cells, and anti-FGFR4 treatment blocks this interaction. Immunoprecipitation with control antibody (anti-GFP) does not result in isolation of FGFR4 or PLCγ. (C) Primary hepatocytes from wild-type (WT) and FGFR4 knockout (FGFR4−/−) mice were treated with PBS, FGF2 or FGF23 for 30 min and total protein extracts were analyzed by immunoblotting. (D-G) Quantification of immunoblot signals including representative blot in (C) by densitometry. In WT hepatocytes, FGF2 increases phosphorylation of FRS2α and ERK, whereas FGF23 increases phosphorylation levels of PLCγ and expression levels of RCAN1. FGF23 effects are not observed in FGFR4−/−. (H) Hepatocytes were isolated from transgenic NFAT-luciferase reporter mice and stimulated with FGF23 for 2 h. Treatment with ionomycin and phorbol 12-myristate 13-acetate (Iono/PMA) for 24 h was used as positive control for NFAT activation. FGF23 treatment significantly increases luciferase activity compared to PBS-treated hepatocytes. The effect is blocked in cells that were pre-treated with cyclosporine A (CsA) or anti-FGFR4 for 1 h. All values represent fold change ± SEM compared to PBS-treated control cells; n = 3–4; *p < 0.05, ***p < 0.001 (1-way ANOVA with Bonferroni's Multiple Comparison Test).
To test whether FGF23 can activate FGFR4 in hepatocytes, we analyzed binding of PLCγ to FGFR4 in primary mouse hepatocytes after treatment with recombinant FGF23 or solvent for 15 minutes. We immunoprecipitated endogenous FGFR4 and analyzed eluates by immunoblotting with an anti-PLCγ antibody. Compared to eluates derived from control cells that were treated with vehicle solution, levels of co-purified PLCγ were elevated in FGF23-treated hepatocytes while total PLCγ and FGFR4 expression levels were unchanged (Figure 2B). When cells were co-treated with FGF23 and an isoform-specific FGFR4 blocking antibody (anti-FGFR4), we could not detect an interaction between FGFR4 and PLCγ. FGF2, a prototypical paracrine member of the FGF family, did not induce binding of PLCγ to FGFR4 (Figure 2B). In HepG2 cells, we found that FGF23 increases tyrosine phosphorylation and thereby activation of FGFR4 when compared to PBS-treated control cells (Supplementary Figure 1A).
Next, we analyzed the Ras/MAPK and calcineurin/NFAT signaling pathways in FGF23-treated primary mouse hepatocytes, as they are two major signaling branches activated by FGF23 in cells expressing or lacking α-klotho, respectively (2, 18, 19). FGF2, a known activator of Ras/MAPK signaling (14), was used as control. Hepatocytes were treated with FGF23 or FGF2 for 30 minutes, followed by Western blot analyses of total protein extracts for phosphorylated FRS2α and ERK. FGF2, but not FGF23, activated Ras/MAPK signaling (Figure 2C-E). In contrast, FGF23 increased levels of phosphorylated PLCγ (Figure 2C, F), indicating that FGF23 activates PLCγ signaling, consistent with our immunoprecipitation results (Figure 2B). Furthermore, FGF23 elevated expression of regulator of calcineurin (RCAN) 1–4 (Figure 2C, G), the promoter of which is exquisitely responsive to transcription factors of the NFAT family (33). These effects were not observed in FGFR4−/− hepatocytes (Figure 2C-G). Consistent with our results in primary mouse hepatocytes, FGF23 increased the phosphorylation of PLCγ and elevated RCAN1-4 expression levels in HepG2 cells, whereas FGF2 activated FRS2α and ERK (Supplementary Figure 1).
Finally, we conducted chemiluminescence reporter assays in primary hepatocytes that were isolated from a transgenic mouse line with a NFAT binding site-dependent luciferase reporter (34). After 2 hours of treatment, FGF23 caused a significant elevation in luciferase activity that was blocked by cyclosporine A and anti-FGFR4 (Figure 2H). Combined, our findings indicate that in cultured hepatocytes, FGF23 binds and activates FGFR4 resulting in the recruitment of PLCγ to the receptor, phosphorylation of PLCγ and subsequent activation of calcineurin/NFAT signaling. In contrast, FGF2 activates Ras/MAPK signaling, which also requires FGFR4.
FGF23 induces expression of inflammatory cytokines in cultured hepatocytes
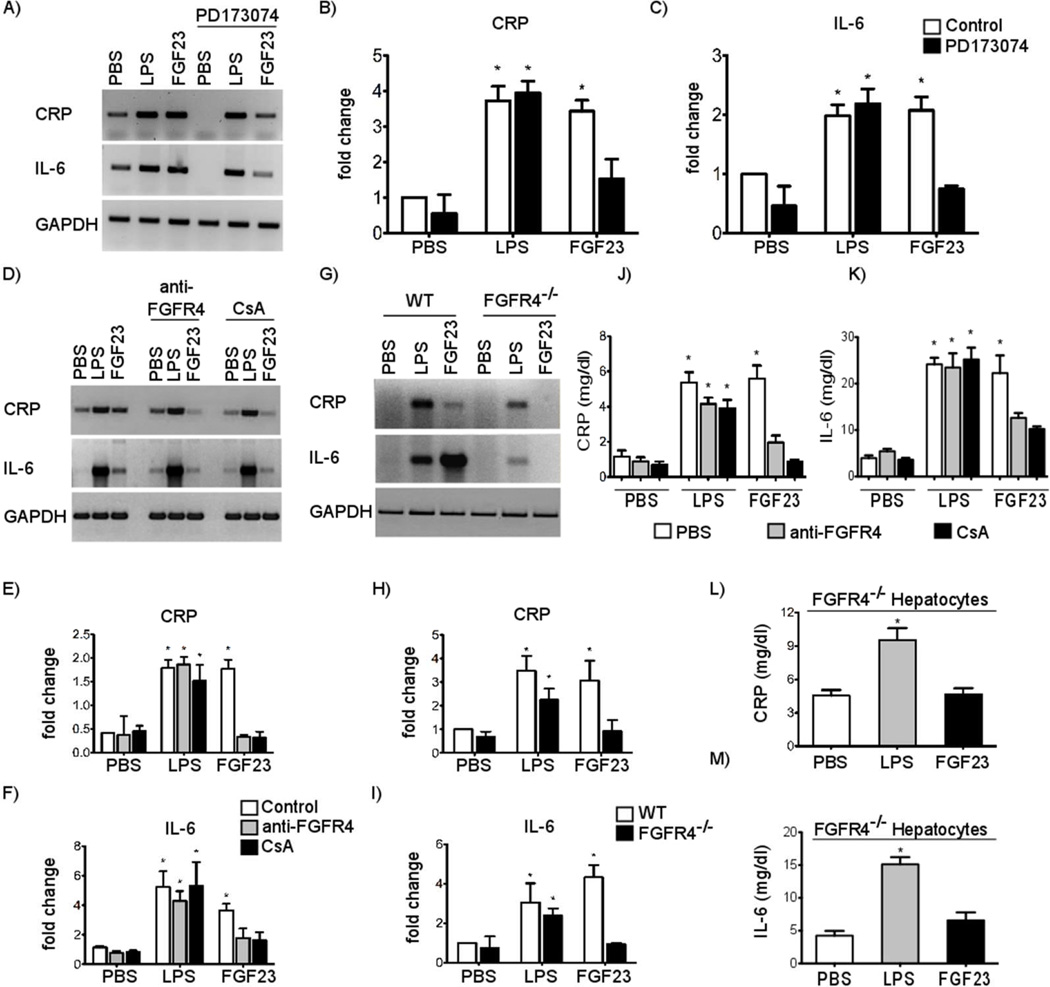
To investigate functional consequences of FGF23-induced FGFR4 signaling in hepatocytes, we analyzed expression of NFAT-regulated inflammatory cytokines (35). Serum-starved primary mouse hepatocytes were treated with FGF23 for 24 hours followed by RT-PCR analysis. As a positive control for the induction of the inflammatory response, we also treated cells with lipopolysaccharide (LPS), which signals via Toll-like receptor 4 and NFκB (36). FGF23 and LPS treatments significantly increased expression levels of CRP and IL-6 (Figure 3A-C). This effect of FGF23 on CRP and IL-6 expression was dose-dependent, as revealed by real-time PCR (Supplementary Figure 2). Co-treatment with the pan-FGFR inhibitor PD173074 only blocked the FGF23-induced effects (Figure 3A-C), indicating that the FGF23-mediated increase in cytokine expression requires FGFR activation. Since our previous findings suggest that FGF23 specifically activates FGFR4 and induces PLCγ/calcineurin/NFAT signaling, we co-treated hepatocytes with the anti-FGFR4 blocking antibody or cyclosporine A. FGF23-induced expression of both cytokines was inhibited in the presence of both inhibitors, but neither inhibitor altered the effects of LPS (Figure 3D-F). Furthermore, when we treated FGFR4−/− hepatocytes, only LPS but not FGF23 elevated mRNA levels of CRP and IL-6 (Figure 3G-I). FGF23 also increased mRNA levels of CRP and IL-6 in HepG2 cells which could be significantly reduced by inhibition of FGFRs or calcineurin but not by ERK blockade (Supplementary Figure 3).
Figure 3. FGF23 increases expression and secretion of inflammatory cytokines in primary mouse hepatocytes in an FGFR4- and calcineurin-dependent manner.
(A-C) RT-PCR expression analysis and quantification of DNA gel electrophoresis signals for CRP and IL-6 in primary mouse hepatocytes that were stimulated with LPS at 50 µg/ml or FGF23 at 25 ng/ml for 24 h. Pre-treatment with the pan-FGFR inhibitor PD173074 at 10 nM for 1 h significantly reduces FGF23-, but not LPS-induced elevations in CRP and IL-6 expression levels (values are expressed as fold change ± SEM; n = 3; *p < 0.05 compared with PBS control; #p < 0.01 compared to FGF23 without PD173074). (D-F) RT-PCR analysis of CRP and IL-6 expression in primary mouse hepatocytes pretreated with an FGFR4-specific blocking antibody (anti-FGFR4) at 10 µg/m or cyclosporine A (CsA) at 0.83 µM for 1 h followed by the treatment with LPS or FGF23. Anti-FGFR4 and CsA inhibit effects of FGF23, but not of LPS. (G-I) RT-PCR analysis of CRP and IL-6 expression in primary hepatocytes isolated from wild-type (WT) or FGFR4 knockout (FGFR4−/−) mice and treated with LPS or FGF23 for 24 h. FGFR4−/− hepatocytes are protected from FGF23- but not LPS-induced effects. (J-K) Quantification of CRP and IL-6 protein levels in supernatants from primary mouse hepatocytes pretreated with anti-FGFR4 or CsA for 1 h followed by the treatment with LPS or FGF23 by ELISA. (L-M) Quantification of CRP and IL-6 protein levels in supernatants of primary FGFR4−/− hepatocytes. Cells are protected from FGF23-, but not LPS-induced increases in CRP and IL-6 protein levels. Values represent CRP and IL-6 concentrations in mg/dl per 500,000 cells. All values are expressed as fold change ± SEM; n = 3 independent isolations of primary hepatocytes; *p < 0.05 compared with PBS-treated control cells (1-way ANOVA with Bonferroni's Multiple Comparison Test).
Consistent with our RT-PCR analysis, supernatants from primary mouse hepatocytes showed significantly higher protein levels of CRP and IL-6 upon FGF23 or LPS treatment when compared to PBS-treated control cells (Figure 3J-K). When hepatocytes were treated with anti-FGFR4 or cyclosporine A, only LPS but not FGF23 increased levels of secreted CRP and IL-6 in cell supernatants. Similarly, in FGFR4−/− mouse hepatocytes only LPS but not FGF23 elevated protein levels of CRP and IL-6 in supernatants (Figure 3L-M). Taken together, our findings indicate that FGF23 induces expression and release of inflammatory cytokines in cultured hepatocytes via activation of FGFR4 and calcineurin.
FGF23 increases hepatic and serum levels of inflammatory cytokines in an FGFR4-dependent manner in mice
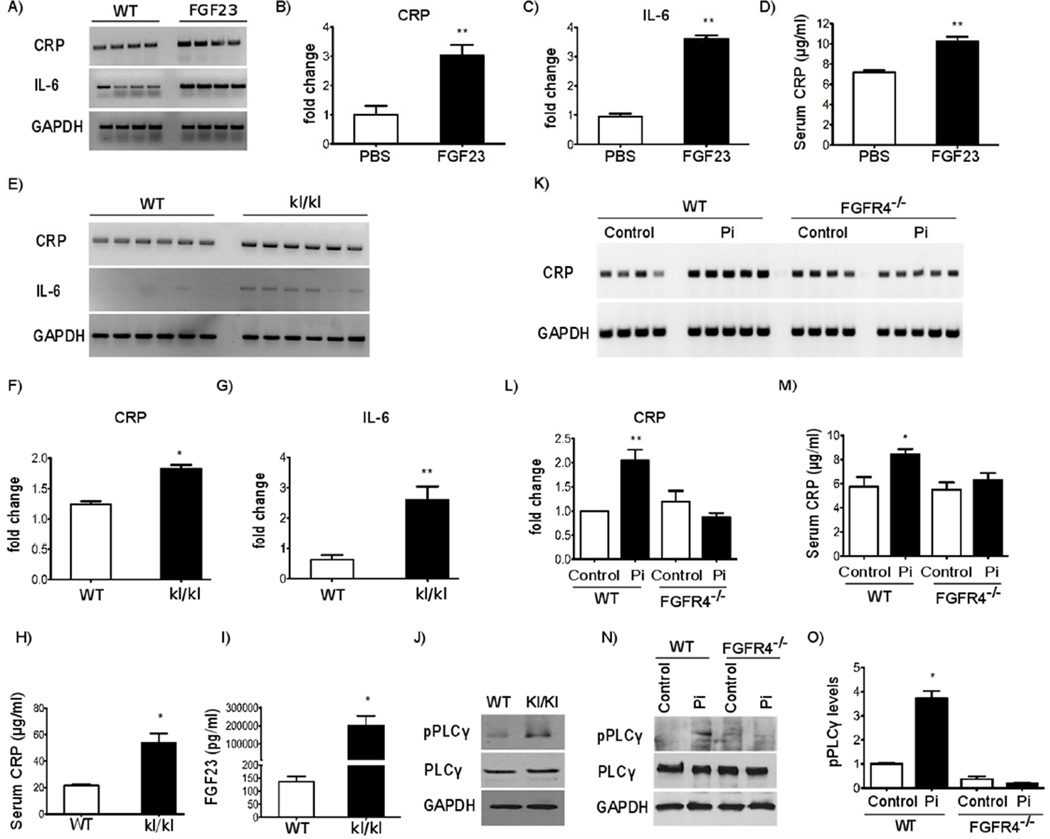
To test if FGF23 can increase CRP and IL-6 levels in vivo, we administered recombinant FGF23 protein intravenously in adult wild-type mice twice daily for 5 days, with 8 hours between daily doses. Control animals underwent the same injection schedule using solvent alone. On the morning of day 6, mice were sacrificed and serum and liver tissue were collected. In this model, serum FGF23 levels were significantly elevated by about 4-fold and mice developed cardiac hypertrophy (18). Compared to PBS injected mice, hepatic CRP and IL-6 expression, and serum CRP levels increased significantly in mice that were administered FGF23 (Figure 4A-D).
Figure 4. Mice with elevated FGF23 levels have increased hepatic and serum levels of CRP and IL-6 that is not detectable in mice lacking FGFR4.
(A-C) RT-PCR expression analysis and quantification of DNA gel electrophoresis signals for CRP and IL-6 in the liver of wild-type mice with intravenous injections of FGF23 at 40 µg/kg twice daily for 5 days. CRP and IL-6 mRNA levels are significantly elevated in FGF23-injected mice (n = 4–6; *p < 0.01; compared with PBS-injected mice). (D) Serum CRP levels, as determined by ELISA, are significantly elevated in wild-type mice that were injected with FGF23 (n = 4; *p < 0.05 compared with PBS-injected mice). (E-G) Analysis of CRP and IL-6 mRNA levels in livers of wild-type (WT) and hypomorphic klotho (kl/kl) mice by RT-PCR. CRP and IL-6 expression is elevated in kl/kl mice (n = 6; *p < 0.05 **p < 0.001 compared with WT mice). (H, I) Serum levels of CRP and FGF23 are increased in kl/kl mice (n = 6; *p < 0.05 compared with WT mice). (J) Representative Western blot analysis of PLCγ expression and phosphorylation in liver extracts. Compared to WT littermates, kl/kl mice show increased levels of phosphorylated PLCγ (pPLCγ) while total PLCγ levels are not altered. GAPDH shows equal protein loading. (K-L) CRP expression in the liver of WT and FGFR4 knockout (FGFR4−/−) mice on normal chow (Control) or on high phosphate diet (Pi) were analyzed by RT-PCR. High Pi elevates hepatic CRP expression in WT, but not in FGFR4−/− mice (n=4–5; **p < 0.001; compared with WT mice on normal diet). (M) Serum CRP levels in WT and FGFR4−/− mice on control or high Pi diet were analyzed by ELISA. High Pi elevates serum CRP levels in WT, but not in FGFR4−/− mice (n = 4–6; *p < 0.05 compared with WT mice on normal chow). (N) Representative Western blot analysis of PLCγ expression and phosphorylation in liver extracts from WT and FGFR4−/− mice. Compared to control chow, high Pi diet increases hepatic pPLCγ levels in WT but not in FGFR4−/− mice. Total PLCγ levels are not altered among groups. GAPDH shows equal protein loading. (O) Quantification of immunoblot signals including representative blot in (M) by densitometry. Levels of pPLCγ were determined in relation to total PLCγ levels that were normalized to GAPDH (n = 4–6; *p < 0.05 compared with WT mice in normal diet). All values are expressed as fold change ± SEM (1-way ANOVA with Bonferroni's Multiple Comparison Test).
To confirm this finding, we analyzed hepatic expression levels of inflammatory cytokines in hypomorphic α-klotho mice. Homozygous mice (kl/kl) that lack functional α-klotho develop hyperphosphatemia, and high FGF23 levels and die prematurely (37). Livers from 8-week old kl/kl mice showed significantly increased mRNA levels of IL-6 (Figure 4E, G), which is consistent with the reported elevation of serum IL-6 in this mouse model (38, 39). Compared to wild-type littermates, kl/kl mice also developed elevated CRP expression levels in the liver (Figure 4E, F) and in the circulation (Figure 4H). As expected, serum FGF23 levels were significantly elevated in kl/kl mice (Figure 4I). Finally, we analyzed PLCγ activity in liver extracts by immunoblot analysis. Compared to wild-type littermates, kl/kl mice showed increased levels of phosphorylated PLCγ while overall PLCγ expression was not altered (Figure 4J).
To determine if these effects were FGFR4-dependent, we elevated serum FGF23 levels by chronic dietary phosphate loading (40) of wild-type and constitutive FGFR4−/− mice (31). Six-month old wild-type and FGFR4−/− mice were administered a high phosphate (2%) diet for 12 weeks. Wild-type and FGFR4−/− mice on normal chow were used as negative controls. We have recently shown that compared to control mice, serum phosphate and FGF23 levels increased significantly in both wild-type and FGFR4−/− mice on high phosphate diet in the absence of changes in kidney function (19). In response to high phosphate diet, hepatic CRP expression and serum CRP levels increased significantly in wild-type mice, but not in FGFR4−/− mice (Figure 4K-M), despite their similar degree of serum FGF23 elevation (19). FGFR4 deletion by itself did not affect CRP expression, since we could not detect significant differences between wild-type and FGFR4−/− mice on normal diet (Figure 4K-M). Furthermore, high phosphate diet increased hepatic levels of phosphorylated PLCγ in wild-type mice, when compared to mice on normal chow, without affecting overall PLCγ expression levels (Figure 4N, O). In contrast, FGFR4−/− mice did not show an elevation of phospho-PLCγ levels in the liver in response to high phosphate diet. We conclude that elevated FGF23 can increase expression of CRP and IL-6 in the liver. This process requires FGFR4, involves the activation of hepatic PLCγ and occurs in the presence of intact kidney function.
Pharmacologic FGFR4 blockade reduces hepatic and serum CRP levels in a rat model of CKD
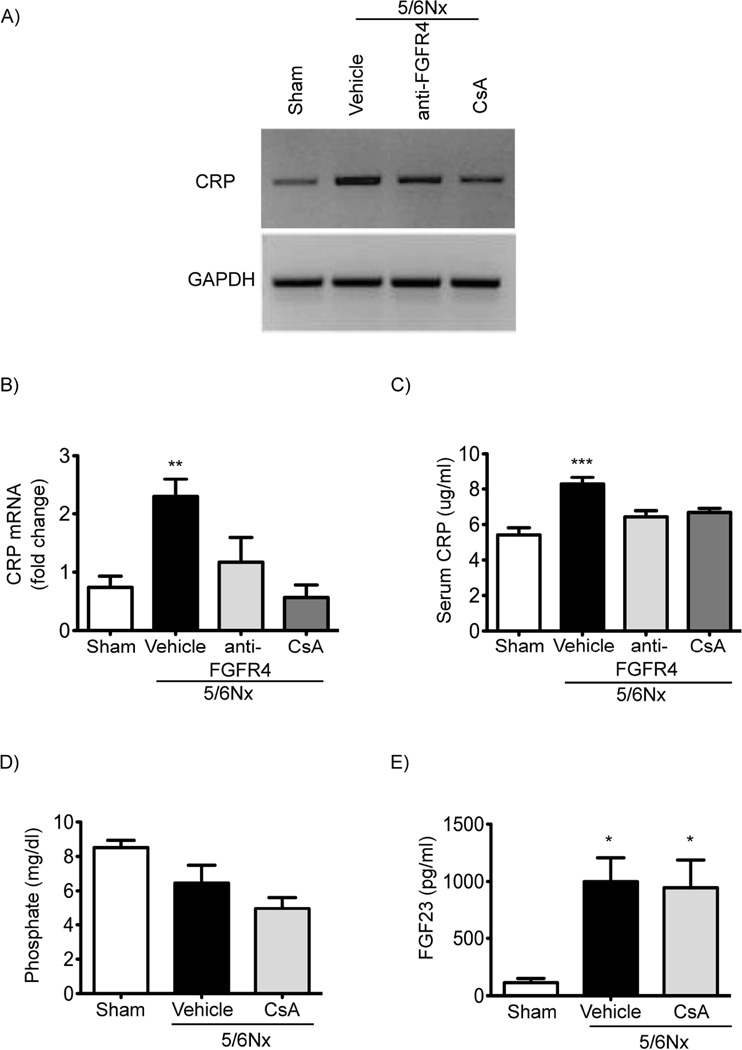
Since FGF23 elevations in mice increase CRP levels in an FGFR4-dependent manner, we tested if FGFR4 blockade could reduce CRP expression in the 5/6 nephrectomy rat model of CKD, which is known to manifest high serum levels of FGF23 (18) and inflammatory cytokines (41). We administered a specific anti-FGFR4 blocking antibody (25 mg/kg body weight via intraperitoneal injection) beginning one hour after nephrectomy and then every 3 days through day 12. At day 14 after nephrectomy, we collected blood for serological analyses and liver tissue to examine CRP expression by RT-PCR. We previously reported that anti-FGFR4 treatment inhibited development of LVH in 5/6 nephrectomized rats (19). Renal function was significantly impaired and blood pressure and serum FGF23 levels significantly elevated in the animals that underwent 5/6 versus sham nephrectomy, but there were no significant differences between 5/6 nephrectomized animals that received anti-FGFR4 antibodies or PBS (19). Of note, pharmacologic FGFR4 inhibition did not cause liver injury, as indicated by unchanged serum levels of alanine aminotransferase, aspartate aminotransferase and alkaline phosphatase (18). Compared with sham-operated rats, 5/6 nephrectomized animals that received vehicle showed increased CRP expression in the liver (Figure 5A, B) and elevated CRP levels in the circulation (Figure 5C). In contrast, hepatic and serum CRP levels did not significantly increase in 5/6 nephrectomized rats that were injected with anti-FGFR4 when compared to sham-operated control animals (Figure 5A-C).
Figure 5. FGFR4 and calcineurin blockade reduce hepatic and serum CRP levels in CKD rats.
(A-B) RT-PCR analysis of CRP expression in the liver of 5/6 nephrectomy rats (5/6Nx) two weeks post-surgery. A sub-group of 5/6Nx rats received an FGFR4-specific blocking antibody (anti-FGFR4) at 25 mg/kg/day or cyclosporine A (CsA) at 5 mg/kg/day, beginning 1 hour after surgery and continued every 3 days for a total of 14 days. Anti-FGFR4 and CsA inhibit elevations in hepatic CRP expression. (C) ELISA-based quantification of CRP levels in the serum of 5/6Nx rats that were injected with anti-FGFR4 or CsA. Anti-FGFR4 and CsA inhibit elevations of serum CRP. (D, E) Quantitative analysis of serum phosphate and FGF23 levels in 5/6Nx rats injected with vehicle or CsA. Elevated serum FGF23 levels of 5/6Nx rats are not reduced by CsA injections. All values are expressed as fold change ± SEM in comparison with Sham-operated rats; n = 5–25; *p < 0.05 **p < 0.001 ***p < 0.0001 (1-way ANOVA with Bonferroni's Multiple Comparison Test).
Finally, since we observed that FGF23 activates calcineurin/NFAT signaling in cultured hepatocytes and that cyclosporine A blocks FGF23-induced cytokine expression and secretion in vitro, we tested if calcineurin inhibition affects CRP levels in 5/6 nephrectomized rats. We administered cyclosporine A (5 mg/kg body weight via intraperitoneal injection) daily beginning one hour after nephrectomy. At day 14 after surgery, we collected blood and liver tissue. We previously reported that cyclosporine A did not improve renal function or reduce blood pressure, but ameliorated cardiac hypertrophy and fibrosis (42). Here we find that the increase in hepatic and serum CRP levels did not occur in 5/6 nephrectomized rats that were treated with cyclosporine A (Figure 5A-C). Calcineurin inhibition did not affect serum phosphate or FGF23 levels (Figure 5D, E) in 5/6 nephrectomized rats. These data indicate that pharmacologic blockade of FGFR4 or calcineurin reduces CRP levels in a well-established animal model of CKD without lowering blood pressure or FGF23 levels or improving kidney function.
Discussion
In the present study, we show that FGF23 can directly activate FGFR4 and calcineurin/NFAT signaling in hepatocytes in the absence of its classic co-receptor α-klotho leading to increased expression and secretion of inflammatory cytokines. Our in vitro observations in an established tumor cell line and in primary mouse hepatocytes are supported by complementary in vivo analyses of four animal models with elevated FGF23. First, we demonstrate that injections of recombinant FGF23 increase CRP expression and serum levels in wild-type mice. Second, hypomorphic kl/kl mice, an established animal model for senescence with high serum levels of phosphate and FGF23, have elevated hepatic and circulating levels of CRP and IL-6. Third, we show that the administration of a high phosphate diet for three months causes a significant increase in serum FGF23 levels and elevates hepatic expression and serum levels of CRP in wild-type but not in FGFR4−/− mice. Fourth, as a therapeutic proof-of-concept experiment, we injected 5/6 nephrectomized rats with an isoform-specific FGFR4 blocking antibody for two weeks. While this treatment did not lower serum FGF23 levels or blood pressure or improve kidney function, it reduced hepatic CRP production and circulating CRP levels.
CKD is a state of chronic inflammation, and in patients with CKD, elevated FGF23 is independently associated with higher serum levels of inflammatory cytokines and with substantially greater odds of manifesting severe inflammation (26, 43, 44). Our experimental data provide novel mechanistic insights into a potentially causative association between increased serum levels of FGF23 and increased inflammatory cytokine expression in the liver. Our results suggest that inflammation might be one underlying mechanism of the higher FGF23 levels observed in glomerular diseases (45, 46), and that future studies should examine the relationship between FGF23 and inflammation in different CKD models and different etiologies of human CKD. Our results are also broadly consistent with a previous genome-wide analysis of FGF23-regulated genes in a mouse model of CKD that suggested inflammatory cytokine genes as FGF23 targets (47), and with studies in which FGF23 stimulated TNFα expression in macrophages (48, 49) and in the spleen (50). Importantly, FGF23 excess induced hepatic production of inflammatory mediators in the absence of increased liver enzymes, which is expected based on the clinical observation that presence of severe CKD per se does not promote liver injury despite marked increases in circulating FGF23 levels in the vast majority of CKD patients.
Associations between serum levels of FGF23 and inflammatory cytokines have been also reported in adults without CKD and in the elderly, despite their significantly lower FGF23 levels relative to patients with CKD (51–53). This suggests a general pro-inflammatory role of FGF23 that is independent of reduced kidney function, but further research is needed to determine whether physiologic concentrations of FGF23 can stimulate hepatocyte cytokine expression, or if only high FGF23 levels observed in advanced CKD are capable of directly inducing the pro-inflammatory effects. Additional studies are also needed to determine whether FGF23 can stimulate inflammatory cytokine expression from other known reservoirs such as adipocytes.
Inflammatory cytokines can directly increase production of FGF23 in bone (27, 54, 55). When considered together with our current findings, it is possible that FGF23 and inflammatory cytokines are part of a positive feedback loop in which FGF23 promotes expression of inflammatory cytokines, which in turn increases bone expression of FGF23. Such a vicious cycle could help explain the 100- to 1000-fold FGF23 elevations often observed in advanced CKD. Our finding that treatment of 5/6 nephrectomized rats with cyclosporine A or anti-FGFR4 does not reduce serum FGF23 levels significantly while preventing a significant elevation of hepatic and circulating levels of CRP indicates that in this rat model of CKD this particular cytokine and/or this particular degree and duration of cytokine elevation is not sufficient to increase FGF23 production in bone. Therefore, the postulated liver-bone axis of CRP/IL-6/FGF23 might be only one of many mechanisms that links inflammation to FGF23 elevations. Furthermore, mechanisms promoting FGF23 production in CKD are multifactorial, including hyperphosphatemia, anemia and hypoxia, and are not understood in detail.
Similar to cardiac myocytes, our molecular analysis revealed that FGF23 stimulates the PLCγ/calcineurin/NFAT signaling cascade in the absence of α-klotho in hepatocytes. In contrast to the heart, where calcineurin and NFAT are components of an established pathway that regulates cardiac remodeling (56), little is known about the function of calcineurin/NFAT signaling in the liver (57, 58). Our study suggests that calcineurin/NFAT activation in hepatocytes induces an inflammatory response. Since NFAT induces expression of many cytokines in different cell types, including IL-2, IL-4 and TNF-α in T cells and IL-6 in mast cells (35), our finding that NFAT activation in hepatocytes causes an elevation in the production of inflammatory cytokines is not surprising. Calcineurin inhibitors, like cyclosporine A, are established drugs in immunosuppressive therapies, but potential anti-inflammatory actions in patients with CKD have not been described.
Our study also suggests the induction of expression and secretion of inflammatory cytokines as a novel effect of klotho-independent FGFR4 activation in the liver. Previous studies in constitutive FGFR4−/− mice and in mice overexpressing constitutively active FGFR4 in hepatocytes revealed the role of FGFR4 in regulating cholesterol and lipid metabolism and bile acid synthesis (59–61). These FGFR4-dependent effects are induced by FGF19 and FGF21, two members of the family of endocrine FGFs that require β-klotho as co-receptor (62). Furthermore, it has been reported that FGFR4−/− mice are more inclined to carbon tetrachloride (CCl4)-induced liver injury and fibrosis (63), and that an inducible FGFR4 knockdown in mice impairs hepatocyte proliferation after hepatectomy (64), suggesting an important role of FGFR4 in liver regeneration. FGFR4 has also been proposed to play a role in the induction of hepatocyte proliferation and carcinogenesis, and overexpression of FGFR4 has been reported in hepatic tumors (65, 66). Interestingly, patients with CKD have higher rates of mortality due to liver cancer (67), but whether FGF23-FGFR4 signaling has mitogenic effects on hepatocytes and could contribute to carcinogenesis is unknown.
While our in vitro experiments show direct effects of FGF23 on hepatocytes, our animal models with systemic FGF23 elevations and global FGFR4 blockade or deletion cannot definitively establish a direct causal effect of FGF23 to induce hepatic expression of inflammatory cytokines. Clearly, elevating FGF23 levels in a mouse model with a hepatocyte-specific FGFR4 deletion is required to determine a direct involvement of hepatic FGFR4 activity in FGF23-mediated inflammation. However, floxed FGFR4 mice that are required to generate a tissue-specific gene knockout are currently not available. Nevertheless, since systemic FGF23 elevations correlate with significant increases in liver and serum CRP levels in wild-type but not in FGFR4−/− mice, and plasma CRP is predominantly produced and secreted by hepatocytes (68), we conclude that FGFR4 in the liver is required for FGF23’s stimulating effects on CRP expression.
Furthermore, we cannot exclude that in our animal models other paracrine or endocrine factors than FGF23 cause an increase in expression levels of inflammatory proteins in the liver. Since FGF23−/− mice develop an aging phenotype and die within 12 weeks after birth (69), and prolonged FGF23 blocked in rats induces cardiovascular injury (70), in vivo studies in an FGF23 null state are not feasible. However, since a high-phosphate diet does not reduce kidney function, we postulate that other pro-inflammatory factors that could be induced by kidney injury and failure are not altered in our mouse model. Since hepatic and systemic CRP levels are decreased by FGFR4 depletion, it is likely that elevated serum FGF23 is causatively involved in the inflammatory response.
In conclusion, our study suggests that elevations of serum FGF23 levels, as found in patients with CKD, can activate FGFR4 in the liver in the absence of α-klotho and trigger PLCγ/calcineurin/NFAT signaling to stimulate an inflammatory response. By demonstrating direct pro-inflammatory effects of FGF23 on hepatocytes, our findings uncover a novel mechanism of inflammation in CKD and a potential new target for clinical intervention. We postulate that FGFR4 blockade could be effective in reducing chronic inflammation and improving overall survival of CKD patients. Whether FGF23-mediated activation of FGFR4 in hepatocytes also regulates other aspects of liver function needs further investigation. Combined with our previous analyses in cardiac myocytes, our data highlight the importance of FGFR4 function in facilitating α-klotho independent effects of FGF23. Since FGFR4 is expressed in several tissues (19, 71), widespread actions of FGF23 that may contribute to a variety of pathologies associated with CKD seem possible.
Methods
Fibroblast growth factors and lipopolysaccharide
Recombinant mouse FGF23 (6His-tagged Tyr25-Val251 [Arg179Gln]; 26.1 kDa), and FGF2 (Ala11-Ser154; 16.2 kDa) (R&D Systems) were used as described before (18, 19). In cell culture studies, FGF23 and FGF2 were used at 25 ng/ml with varying incubation times, i.e. 30 minutes to determine activity of FGFR4 and downstream signal mediators, and 24 hours to study the expression and secretion of cytokines. As positive control for cytokine expression analyses, lipopolysaccharide from E.coli (O111:B4; Invivo Gen) was used at 100 µg/ml for 24 hours.
Primary mouse hepatocyte cultures
Primary hepatocytes were isolated from 12–16 week old mice by retrograde perfusion and consecutive digestion of the liver. In brief, a catheter needle (BD Angiocath Autogaurd 24G and 19 mm) was used to cannulate the inferior vena cava (IVC). To ensure complete perfusion, the IVC was ligated between the liver and the heart using surgical silk (4–0), and the portal vein was perforated allowing complete drainage of perfusion media. The liver was perfused using 10 ml pre-heated Hepatocyte Liver Perfusion Medium (Gibco) followed by 30 ml Hepatocyte Liver Digest Medium (Gibco) at a constant flow of 1 ml/min. Digested liver lobes were transferred to a petri-dish containing 10 ml of Hepatocyte Liver Digestion Medium. The central region of the liver was gently exposed working through the lobes using fine-tipped forceps. Once torn apart, the remaining section of the liver was shook gently to free residual cells. After gentle trituration, cells were filtered through a 70-micron cell strainer, pelleted by centrifugation at 50 g for 2 minutes, and resuspended in 25 ml ice-cold isolation medium (Williams’ Media E + plating and thawing supplement CM3000 Gibco) for a total of 3 washes. Cells were tested for viability by counting on a hemocytometer using trypan blue staining.
Intravenous injections of FGF23 protein in mice
Twelve-week-old C57BL/6 mice underwent tail vein injections using standard protocols as previously described for the systemic delivery recombinant FGF23 (18). Briefly, mice were placed in a restrainer and 40 µg/kg FGF23 dissolved in 200 µl PBS were injected into the tail vein of 6 mice twice daily with 8 hours between injections for 5 consecutive days. Six mice underwent the same injection schedule using 200 µl PBS alone as negative control. On the morning of the sixth day, 16 hours after the final tail vein injections, animals were sacrificed, liver tissue was isolated and serum was collected.
Klotho hypomorphic mice
We analyzed six homozygous klotho-deficient (kl/kl) mice in SV129 background (37) and wild-type littermates. At 6–7 weeks of age, animals were sacrificed for RNA isolation and for serological analysis as described before.
High phosphate diet in FGFR4−/− mice
Six-month old wild-type and FGFR4−/− mice in the C57Bl/6 background were randomly divided into four experimental groups of five mice each and fed normal chow or a 2.0% phosphate diet (Teklad 08020, Harlan) for 12 weeks, as done before (19). Mice were given free access to chow and distilled water. At the end of the experimental period, all mice were euthanized under anesthesia and blood and liver were collected for analyses.
5/6 nephrectomy rat model of CKD
Kidney injury was induced in Sprague Dawley rats using the 5/6 nephrectomy method as described previously (18, 19). Rats were randomized into three groups, with six animals per group: sham nephrectomy or 5/6 nephrectomy plus 14 daily intraperitoneal injections of vehicle (PBS), anti-FGFR4 blocking antibody (human monoclonal, U3Pharma) at 25 mg/kg/day or cyclosporine A at 5 mg/kg every 3 days beginning 1 hour after surgery. On day 14, animals were sacrificed, and the livers were isolated and prepared for molecular and serological analyses.
Supplementary Material
Acknowledgments
Source of support
This study was supported by the Stifterverband für die Deutsche Wissenschaft and Simon-Claussen-Stiftung (H 1405409999915626 to M.B.), the Deutsche Forschungs Gemeinschaft (GR 4228/1-1 to A.G.), Roche (A.G.), the American Heart Association (A.G., C.F.), the American Diabetes Association (C.F.), U3 Pharma GmbH, Germany (C.F.), and grants F31DK10236101 (K.S.), R01DK044234 (M.J.C.), R01DK061498 (M.J.C.), R01AA022601 (M.J.C.) R01DK076116 (M.W.), K24DK093723 (M.W.) and R01HL128714 (C.F.) from the National Institutes of Health.
Footnotes
Disclosure
Dr. Faul has served as a consultant or received honoraria from Ultragenyx and has received research support from U3 Pharma GmbH. Dr. Czaja has served as a consultant or received honoraria from Genzyme/Sanofi and Verlyx Pharma and has received research support from Verlyx Pharma. Dr. Wolf has served as a consultant or received honoraria from Ultragenyx, Amgen, Keryx, Lutipold, Opko, Pfizer and Sanofi. All other authors have declared that no conflict of interest exists. Drs. Bartz and Abraham are employees of U3Pharma GmbH.
References
- 1.Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J Clin Invest. 2008;118:3820–3828. doi: 10.1172/JCI36479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 3.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf M. Forging forward with 10 burning questions on FGF23 in kidney disease. J Am Soc Nephrol. 2010;21:1427–1435. doi: 10.1681/ASN.2009121293. [DOI] [PubMed] [Google Scholar]
- 6.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Juppner H, Wolf M. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 7.Isakova T, Wahl P, Vargas GS, Gutierrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370–1378. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimada T, Urakawa I, Isakova T, Yamazaki Y, Epstein M, Wesseling-Perry K, Wolf M, Salusky IB, Juppner H. Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. J Clin Endocrinol Metab. 2010;95:578–585. doi: 10.1210/jc.2009-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Juppner H, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jean G, Terrat JC, Vanel T, Hurot JM, Lorriaux C, Mayor B, Chazot C. High levels of serum fibroblast growth factor (FGF)-23 are associated with increased mortality in long haemodialysis patients. Nephrol Dial Transplant. 2009;24:2792–2796. doi: 10.1093/ndt/gfp191. [DOI] [PubMed] [Google Scholar]
- 11.Parker BD, Schurgers LJ, Brandenburg VM, Christenson RH, Vermeer C, Ketteler M, Shlipak MG, Whooley MA, Ix JH. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Ann Intern Med. 2010;152:640–648. doi: 10.1059/0003-4819-152-10-201005180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutierrez OM, Steigerwalt S, He J, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2:REVIEWS3005. doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Kouhara H, Hadari YR, Spivak-Kroizman T, Schilling J, Bar-Sagi D, Lax I, Schlessinger J. A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell. 1997;89:693–702. doi: 10.1016/s0092-8674(00)80252-4. [DOI] [PubMed] [Google Scholar]
- 16.Mohammadi M, Honegger AM, Rotin D, Fischer R, Bellot F, Li W, Dionne CA, Jaye M, Rubinstein M, Schlessinger J. A tyrosine-phosphorylated carboxy-terminal peptide of the fibroblast growth factor receptor (Flg) is a binding site for the SH2 domain of phospholipase C-gamma 1. Mol Cell Biol. 1991;11:5068–5078. doi: 10.1128/mcb.11.10.5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vainikka S, Joukov V, Wennstrom S, Bergman M, Pelicci PG, Alitalo K. Signal transduction by fibroblast growth factor receptor-4 (FGFR-4). Comparison with FGFR-1. J Biol Chem. 1994;269:18320–18326. [PubMed] [Google Scholar]
- 18.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grabner A, Amaral AP, Schramm K, Singh S, Sloan A, Yanucil C, Li J, Shehadeh LA, Hare JM, David V, et al. Activation of Cardiac Fibroblast Growth Factor Receptor 4 Causes Left Ventricular Hypertrophy. Cell Metab. 2015 doi: 10.1016/j.cmet.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimmel PL, Phillips TM, Simmens SJ, Peterson RA, Weihs KL, Alleyne S, Cruz I, Yanovski JA, Veis JH. Immunologic function and survival in hemodialysis patients. Kidney Int. 1998;54:236–244. doi: 10.1046/j.1523-1755.1998.00981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stenvinkel P. Inflammation in end-stage renal disease--a fire that burns within. Contrib Nephrol. 2005;149:185–199. doi: 10.1159/000085525. [DOI] [PubMed] [Google Scholar]
- 22.Barreto DV, Barreto FC, Liabeuf S, Temmar M, Lemke HD, Tribouilloy C, Choukroun G, Vanholder R, Massy ZA European Uremic Toxin Work, G. Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int. 2010;77:550–556. doi: 10.1038/ki.2009.503. [DOI] [PubMed] [Google Scholar]
- 23.Pereira BJ, Shapiro L, King AJ, Falagas ME, Strom JA, Dinarello CA. Plasma levels of IL-1 beta, TNF alpha and their specific inhibitors in undialyzed chronic renal failure, CAPD and hemodialysis patients. Kidney Int. 1994;45:890–896. doi: 10.1038/ki.1994.117. [DOI] [PubMed] [Google Scholar]
- 24.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E Pravastatin or Atorvastatin, E., and Infection Therapy-Thrombolysis in Myocardial Infarction, I. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 25.Shlipak MG, Fried LF, Crump C, Bleyer AJ, Manolio TA, Tracy RP, Furberg CD, Psaty BM. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003;107:87–92. doi: 10.1161/01.cir.0000042700.48769.59. [DOI] [PubMed] [Google Scholar]
- 26.Munoz Mendoza J, Isakova T, Ricardo AC, Xie H, Navaneethan SD, Anderson AH, Bazzano LA, Xie D, Kretzler M, Nessel L, et al. Fibroblast growth factor 23 and Inflammation in CKD. Clin J Am Soc Nephrol. 2012;7:1155–1162. doi: 10.2215/CJN.13281211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.David V, Martin A, Isakova T, Spaulding C, Qi L, Ramirez V, Zumbrennen-Bullough KB, Sun CC, Lin HY, Babitt JL, et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 2015 doi: 10.1038/ki.2015.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kan M, Wu X, Wang F, McKeehan WL. Specificity for fibroblast growth factors determined by heparan sulfate in a binary complex with the receptor kinase. J Biol Chem. 1999;274:15947–15952. doi: 10.1074/jbc.274.22.15947. [DOI] [PubMed] [Google Scholar]
- 29.Lin BC, Wang M, Blackmore C, Desnoyers LR. Liver-specific activities of FGF19 require Klotho beta. J Biol Chem. 2007;282:27277–27284. doi: 10.1074/jbc.M704244200. [DOI] [PubMed] [Google Scholar]
- 30.Fon Tacer K, Bookout AL, Ding X, Kurosu H, John GB, Wang L, Goetz R, Mohammadi M, Kuro-o M, Mangelsdorf DJ, et al. Research resource: Comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol. 2010;24:2050–2064. doi: 10.1210/me.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinstein M, Xu X, Ohyama K, Deng CX. FGFR-3 and FGFR-4 function cooperatively to direct alveogenesis in the murine lung. Development. 1998;125:3615–3623. doi: 10.1242/dev.125.18.3615. [DOI] [PubMed] [Google Scholar]
- 32.Partanen J, Makela TP, Eerola E, Korhonen J, Hirvonen H, Claesson-Welsh L, Alitalo K. FGFR-4, a novel acidic fibroblast growth factor receptor with a distinct expression pattern. EMBO J. 1991;10:1347–1354. doi: 10.1002/j.1460-2075.1991.tb07654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang J, Rothermel B, Vega RB, Frey N, McKinsey TA, Olson EN, Bassel-Duby R, Williams RS. Independent signals control expression of the calcineurin inhibitory proteins MCIP1 and MCIP2 in striated muscles. Circ Res. 2000;87:E61–E68. doi: 10.1161/01.res.87.12.e61. [DOI] [PubMed] [Google Scholar]
- 34.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 35.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 36.Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 38.Liu F, Wu S, Ren H, Gu J. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat Cell Biol. 2011;13:254–262. doi: 10.1038/ncb2167. [DOI] [PubMed] [Google Scholar]
- 39.Eren M, Boe AE, Murphy SB, Place AT, Nagpal V, Morales-Nebreda L, Urich D, Quaggin SE, Budinger GR, Mutlu GM, et al. PAI-1-regulated extracellular proteolysis governs senescence and survival in Klotho mice. Proc Natl Acad Sci U S A. 2014;111:7090–7095. doi: 10.1073/pnas.1321942111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu MC, Shi M, Cho HJ, Adams-Huet B, Paek J, Hill K, Shelton J, Amaral AP, Faul C, Taniguchi M, et al. Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2014050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korish AA, Arafah MM. The potential anti-inflammatory effect of tetrahydrobiopterin administration in renal mass reduction-induced chronic renal failure in rats. Saudi Med J. 2007;28:1803–1809. [PubMed] [Google Scholar]
- 42.Di Marco GS, Reuter S, Kentrup D, Ting L, Grabner A, Jacobi AM, Pavenstadt H, Baba HA, Tiemann K, Brand M. Cardioprotective effect of calcineurin inhibition in an animal model of renal disease. Eur Heart J. 2011;32:1935–1945. doi: 10.1093/eurheartj/ehq436. [DOI] [PubMed] [Google Scholar]
- 43.Nasrallah MM, El-Shehaby AR, Osman NA, Fayad T, Nassef A, Salem MM, Sharaf El Din UA. The Association between Fibroblast Growth Factor-23 and Vascular Calcification Is Mitigated by Inflammation Markers. Nephron Extra. 2013;3:106–112. doi: 10.1159/000356118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manghat P, Fraser WD, Wierzbicki AS, Fogelman I, Goldsmith DJ, Hampson G. Fibroblast growth factor-23 is associated with C-reactive protein, serum phosphate and bone mineral density in chronic kidney disease. Osteoporos Int. 2010;21:1853–1861. doi: 10.1007/s00198-009-1142-4. [DOI] [PubMed] [Google Scholar]
- 45.Bacchetta J, Dubourg L, Harambat J, Ranchin B, Abou-Jaoude P, Arnaud S, Carlier MC, Richard M, Cochat P. The influence of glomerular filtration rate and age on fibroblast growth factor 23 serum levels in pediatric chronic kidney disease. J Clin Endocrinol Metab. 2010;95:1741–1748. doi: 10.1210/jc.2009-1576. [DOI] [PubMed] [Google Scholar]
- 46.Portale AA, Wolf M, Juppner H, Messinger S, Kumar J, Wesseling-Perry K, Schwartz GJ, Furth SL, Warady BA, Salusky IB. Disordered FGF23 and mineral metabolism in children with CKD. Clin J Am Soc Nephrol. 2014;9:344–353. doi: 10.2215/CJN.05840513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dai B, David V, Martin A, Huang J, Li H, Jiao Y, Gu W, Quarles LD. A comparative transcriptome analysis identifying FGF23 regulated genes in the kidney of a mouse CKD model. PLoS One. 2012;7:e44161. doi: 10.1371/journal.pone.0044161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Masuda Y, Ohta H, Morita Y, Nakayama Y, Miyake A, Itoh N, Konishi M. Expression of Fgf23 in activated dendritic cells and macrophages in response to immunological stimuli in mice. Biol Pharm Bull. 2015;38:687–693. doi: 10.1248/bpb.b14-00276. [DOI] [PubMed] [Google Scholar]
- 49.Han X, Li L, Yang J, King G, Xiao Z, Quarles LD. Counter-regulatory paracrine actions of FGF-23 and 1,25(OH)2 D in macrophages. FEBS Lett. 2016;590:53–67. doi: 10.1002/1873-3468.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamauchi M, Hirohashi Y, Torigoe T, Matsumoto Y, Yamashita K, Kayama M, Sato N, Yotsuyanagi T. Wound healing delays in alpha-Klotho-deficient mice that have skin appearance similar to that in aged humans - Study of delayed wound healing mechanism. Biochem Biophys Res Commun. 2016 doi: 10.1016/j.bbrc.2016.03.138. [DOI] [PubMed] [Google Scholar]
- 51.Holecki M, Chudek J, Owczarek A, Olszanecka-Glinianowicz M, Bozentowicz-Wikarek M, Dulawa J, Mossakowska M, Zdrojewski T, Skalska A, Wiecek A. Inflammation but not obesity or insulin resistance is associated with increased plasma fibroblast growth factor 23 concentration in the elderly. Clin Endocrinol (Oxf) 2015;82:900–909. doi: 10.1111/cen.12759. [DOI] [PubMed] [Google Scholar]
- 52.Hanks LJ, Casazza K, Judd SE, Jenny NS, Gutierrez OM. Associations of fibroblast growth factor-23 with markers of inflammation, insulin resistance and obesity in adults. PLoS One. 2015;10:e0122885. doi: 10.1371/journal.pone.0122885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.di Giuseppe R, Kuhn T, Hirche F, Buijsse B, Dierkes J, Fritsche A, Kaaks R, Boeing H, Stangl GI, Weikert C. Potential Predictors of Plasma Fibroblast Growth Factor 23 Concentrations: Cross-Sectional Analysis in the EPIC-Germany Study. PLoS One. 2015;10:e0133580. doi: 10.1371/journal.pone.0133580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ito N, Wijenayaka AR, Prideaux M, Kogawa M, Ormsby RT, Evdokiou A, Bonewald LF, Findlay DM, Atkins GJ. Regulation of FGF23 expression in IDG-SW3 osteocytes and human bone by pro-inflammatory stimuli. Mol Cell Endocrinol. 2015;399:208–218. doi: 10.1016/j.mce.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 55.Pathak JL, Bakker AD, Luyten FP, Verschueren P, Lems WF, Klein-Nulend J, Bravenboer N. Systemic Inflammation Affects Human Osteocyte-Specific Protein and Cytokine Expression. Calcif Tissue Int. 2016 doi: 10.1007/s00223-016-0116-8. [DOI] [PubMed] [Google Scholar]
- 56.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 57.Kalkan Y, Tomak Y, Altuner D, Tumkaya L, Bostan H, Yilmaz A, Unal D, Kara A, Turan A. Hepatic effects of ketamine administration for 2 weeks in rats. Hum Exp Toxicol. 2014;33:32–40. doi: 10.1177/0960327112472990. [DOI] [PubMed] [Google Scholar]
- 58.Wang S, Kang X, Cao S, Cheng H, Wang D, Geng J. Calcineurin/NFATc1 pathway contributes to cell proliferation in hepatocellular carcinoma. Dig Dis Sci. 2012;57:3184–3188. doi: 10.1007/s10620-012-2255-8. [DOI] [PubMed] [Google Scholar]
- 59.Yu C, Wang F, Kan M, Jin C, Jones RB, Weinstein M, Deng CX, McKeehan WL. Elevated cholesterol metabolism and bile acid synthesis in mice lacking membrane tyrosine kinase receptor FGFR4. J Biol Chem. 2000;275:15482–15489. doi: 10.1074/jbc.275.20.15482. [DOI] [PubMed] [Google Scholar]
- 60.Yu C, Wang F, Jin C, Huang X, McKeehan WL. Independent repression of bile acid synthesis and activation of c-Jun N-terminal kinase (JNK) by activated hepatocyte fibroblast growth factor receptor 4 (FGFR4) and bile acids. J Biol Chem. 2005;280:17707–17714. doi: 10.1074/jbc.M411771200. [DOI] [PubMed] [Google Scholar]
- 61.Huang X, Yang C, Luo Y, Jin C, Wang F, McKeehan WL. FGFR4 prevents hyperlipidemia and insulin resistance but underlies high-fat diet induced fatty liver. Diabetes. 2007;56:2501–2510. doi: 10.2337/db07-0648. [DOI] [PubMed] [Google Scholar]
- 62.Owen BM, Mangelsdorf DJ, Kliewer SA. Tissue-specific actions of the metabolic hormones FGF15/19 and FGF21. Trends Endocrinol Metab. 2015;26:22–29. doi: 10.1016/j.tem.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu C, Wang F, Jin C, Wu X, Chan WK, McKeehan WL. Increased carbon tetrachloride-induced liver injury and fibrosis in FGFR4-deficient mice. Am J Pathol. 2002;161:2003–2010. doi: 10.1016/S0002-9440(10)64478-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Padrissa-Altes S, Bachofner M, Bogorad RL, Pohlmeier L, Rossolini T, Bohm F, Liebisch G, Hellerbrand C, Koteliansky V, Speicher T, et al. Control of hepatocyte proliferation and survival by Fgf receptors is essential for liver regeneration in mice. Gut. 2014 doi: 10.1136/gutjnl-2014-307874. [DOI] [PubMed] [Google Scholar]
- 65.Ho HK, Pok S, Streit S, Ruhe JE, Hart S, Lim KS, Loo HL, Aung MO, Lim SG, Ullrich A. Fibroblast growth factor receptor 4 regulates proliferation, anti-apoptosis and alpha-fetoprotein secretion during hepatocellular carcinoma progression and represents a potential target for therapeutic intervention. J Hepatol. 2009;50:118–127. doi: 10.1016/j.jhep.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 66.Mellor HR. Targeted inhibition of the FGF19-FGFR4 pathway in hepatocellular carcinoma; translational safety considerations. Liver Int. 2014;34:e1–e9. doi: 10.1111/liv.12462. [DOI] [PubMed] [Google Scholar]
- 67.Weng PH, Hung KY, Huang HL, Chen JH, Sung PK, Huang KC. Cancer-specific mortality in chronic kidney disease: longitudinal follow-up of a large cohort. Clin J Am Soc Nephrol. 2011;6:1121–1128. doi: 10.2215/CJN.09011010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shalhoub V, Shatzen EM, Ward SC, Davis J, Stevens J, Bi V, Renshaw L, Hawkins N, Wang W, Chen C, et al. FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J Clin Invest. 2012;122:2543–2553. doi: 10.1172/JCI61405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seitzer N, Mayr T, Streit S, Ullrich A. A single nucleotide change in the mouse genome accelerates breast cancer progression. Cancer Res. 2010;70:802–812. doi: 10.1158/0008-5472.CAN-09-3239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.