Abstract
Characterized by pain, cartilage degradation, and inflammation, osteoarthritis is often treated with anti-inflammatory therapies that provide short-term relief but can have adverse side effects; intra-articular drug delivery systems with controlled release of anti-inflammatory peptides using degradable poly(N-isopropylacrylamide) (pNIPAM) nanoparticles could prolong relief and minimize these side effects. Nanoparticles provide a biocompatible drug carrier that can protect encapsulated therapeutics from enzymatic degradation and increase payload delivery upon encountering a degradation stimulus. Here we demonstrate passive targeting of inflamed cartilage ex vivo by uptake of PEGylated pNIPAM nanoparticles with degradable disulfide crosslinks (abbreviated as NGPEGSS) into chondrocytes and subsequent intracellular release of an anti-inflammatory peptide KAFAKLAARLYRKALARQLGVAA (KAFAK). The KAFAK-loaded NGPEGSS treatment reduced ex vivo inflammation to a greater extent compared to its non-degradable counterparts. This study highlights a nanoparticle system that delivers therapeutics intracellularly with improved efficacy by triggered degradation and suppresses inflammation in multiple cell types within an inflamed joint.
Keywords: N-isopropylacrylamide, Thermosensitive polymer, Anti-inflammatory peptides, Inflammation, Osteoarthritis
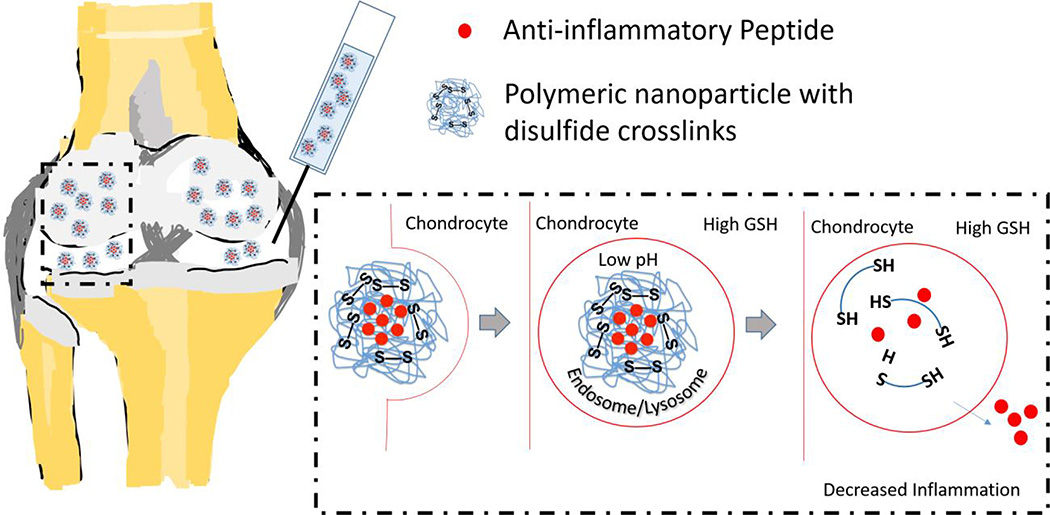
Graphical Abstract
The anti-inflammatory MK2 inhibiting peptide, KAFAKLAARLYRKALARQLGVAA (KAFAK), has shown promise as a therapy for osteoarthritis; however its susceptibility to proteases in the blood and synovial fluid limit its therapeutic potential. Here we investigate a thermosensitive nanoparticle system to protect KAFAK from premature extracellular degradation. The nanoparticles are stable in the extracellular environment, but degrade upon uptake into cells through reduction of disulfide crosslinks. This study shows that these nanoparticles are taken up into chondrocytes, delivering KAFAK and suppressing inflammation to a greater extent than non-degradable nanoparticle counterparts.
INTRODUCTION
Osteoarthritis, affecting ~27 million Americans (1, 2), is the leading cause of chronic disability in the United States (3). It is primarily characterized by a progressive cycle of chronic inflammation (4) and cartilage degradation, causing joint pain and stiffness. In response to sustained levels of pro-inflammatory cytokines, chondrocytes synthesize proteolytic enzymes that degrade extracellular matrix components (5, 6), including aggrecan (7), stimulating additional inflammation and facilitating penetration of inflammation and degradation factors into the cartilage. Osteoarthritis is treated with anti-inflammatory therapies that provide short-term relief and can have adverse side effects. Locally-delivered, degradable, controlled release, anti-inflammatory drug delivery systems could prolong relief and minimize side effects.
Brugnano et al. (8) investigated an anti-inflammatory cell-penetrating peptide KAFAKLAARLYRKALARQLGVAA, (KAFAK), which inhibits MAPK-activated protein kinase 2 (MK2). MK2 controls the synthesis of many pro-inflammatory cytokines, including IL-1, TNF-α, and IL-6 (6, 9–11). The MK2 pathway is triggered in osteoarthritis and promotes release of enzymes that degrade cartilage (6). KAFAK treatments are susceptible to peptidases in the blood and synovial fluid. To limit systemic exposure and premature degradation of KAFAK, our laboratory has designed polyN-isopropylacrylamide (pNIPAM) nanoparticle delivery systems to increase the peptide’s therapeutic lifetime (12–16).
pNIPAM has numerous applications in drug delivery due to its lower critical solution temperature (LCST) between 31°C and 33°C (17, 18). pNIPAM nanoparticles swell in aqueous solutions at temperatures below the LCST where they can be rapidly loaded with agents by diffusion, and collapse at temperatures above the LCST thus entrapping diffusible agents (13, 14) followed by slow release. To tailor pNIPAM nanoparticles to load and release cationic peptides, co-polymerization of 2-acrylamido-2-methyl-1-propanesulfonic acid (AMPS), an anionic sulfated monomer has been used (13, 14). Further, co-polymerization with polyethyleneglycol (PEG) can decrease non-specific protein binding (19), and endolytic degradation can be instilled using the degradable crosslinker bis(acryloyl)cystamine (BAC).
We previously reported a one-pot synthesis incorporating NIPAM, AMPS, BAC, and PEG monomers, with fluorescein o-acrylate for visualization, to develop a system (NGPEGSS) that degraded in the low-pH and reducing environment of endolysosomes. In RAW 264.7 macrophages, an approximately 6-fold greater KAFAK release and improved anti-inflammatory response was seen with NGPEGSS compared to non-degradable NGPEGMBA counterparts (16). While Bartlett et al. reported delivery of KAFAK to damaged cartilage in pH sensitive pNIPAm nanoparticles, the nanoparticles continuously degraded before reaching the target cell, thus limiting the therapeutic potential of the system (15).
As a step toward developing an improved KAFAK delivery system for osteoarthritis, we investigated the ability of the NGPEGSS nanoparticles to selectively penetrate damaged cartilage. We also investigated the ability of NGPEGSS, which show enhanced extracellular stability and improved KAFAK retention and release, to effectively suppress inflammation via chondrocyte uptake in an ex vivo model of osteoarthritis.
METHODS
Nanoparticle and peptide synthesis were performed and characterized as previously reported with no modifications (16). Details of experimental methods are provided in supplementary materials.
RESULTS
Chondrocyte Nanoparticle Uptake and Cytotoxicity
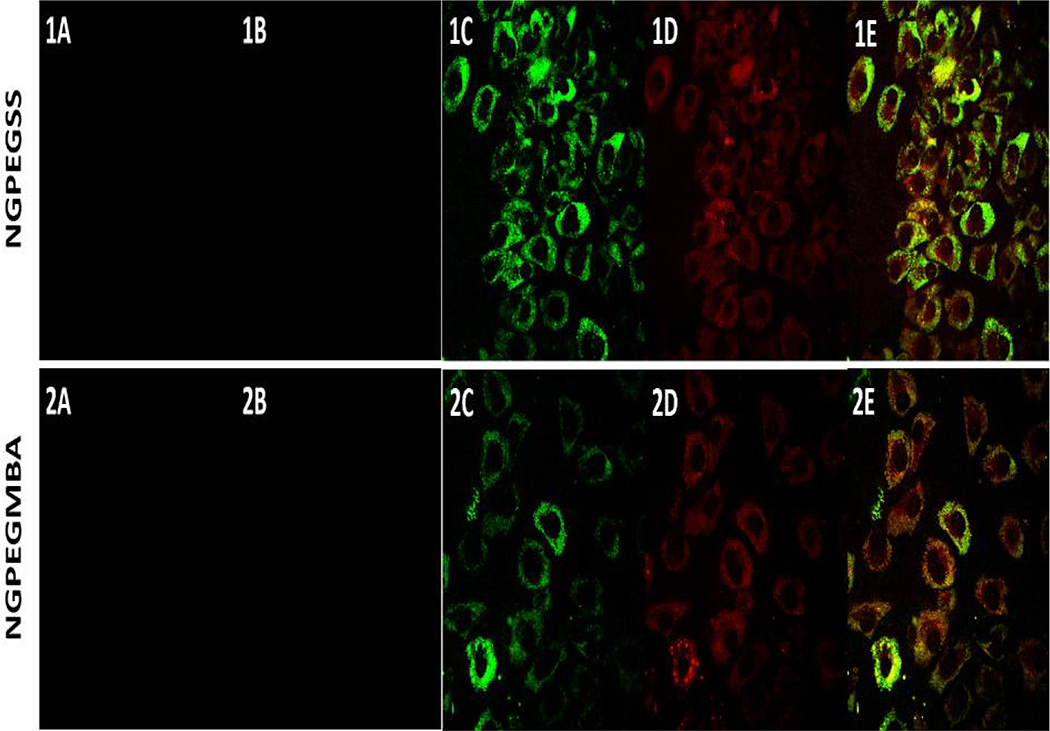
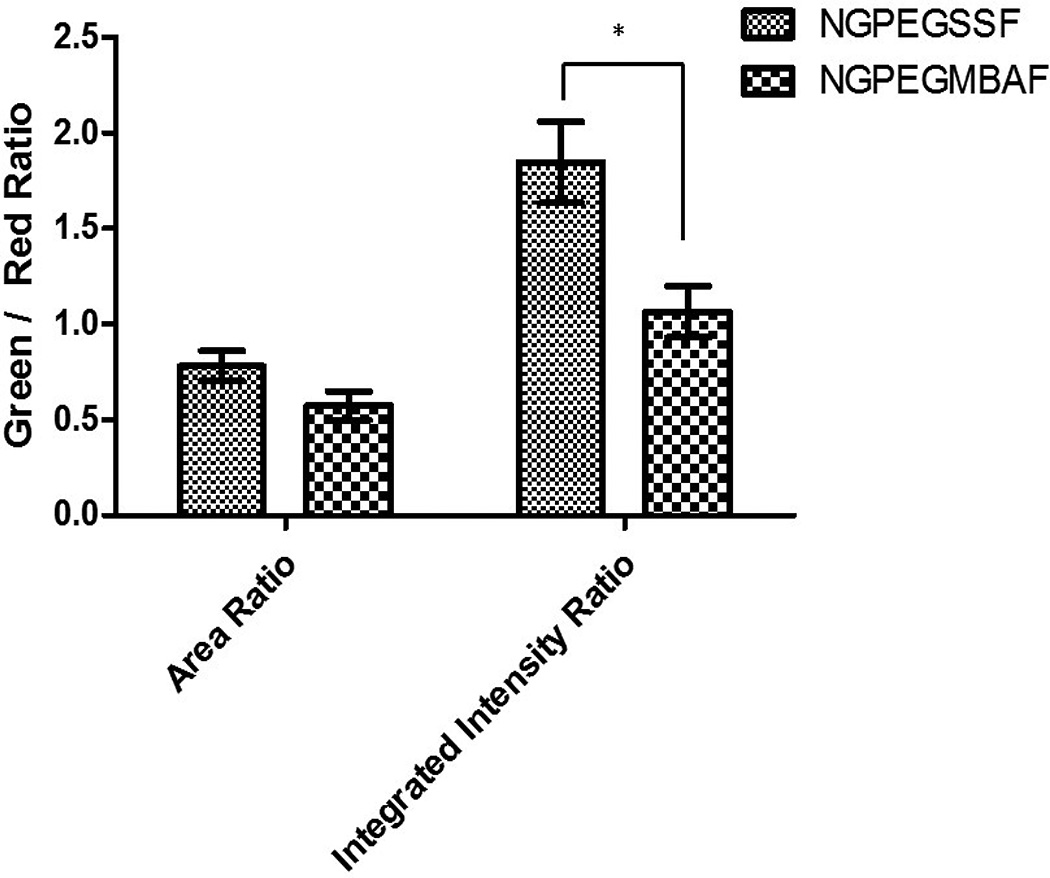
Confocal microscopy confirmed the uptake of both fluorescent degradable NGPEGSSF and non-degradable NGPEGMBAF nanoparticles into primary chondrocytes after 24 h incubation but not after 4 h incubation (Figure 1). Figure 1 also demonstrates co-localization of green fluorescent nanoparticles with red endolysosomal compartments for both MBA and BAC crosslinked nanoparticles. Semi-quantitative green/red fluorescence ratio analysis in Figure 2 suggests that the co-localization of nanoparticles in endolysosomal compartments is similar, as determined by the area ratio, but the total number of nanoparticles taken up into these intracellular compartments is significantly greater for the NGPEGSS as compared to the NGPEGMBA nanoparticles as evaluated by the integrated intensity ratio.
Figure 1. Confocal microscopy of primary bovine chondrocytes incubated at 37°C with (1) NGPEGMBA and (2) NGPEGSS.
(A) non-fluorescent nanoparticles as negative controls; (B&C) fluorescent nanoparticles after (B) 4 h and (C) 24 h incubation; (D) Lysotracker DND-99; (E) green nanoparticle overlay on red endolysosomes.
Figure 2. Semi-quantitative fluorescence analysis of green nanoparticles and red lysotracker-labeled endolysosomes co-localization ratios by pixel area (left) and total integrated intensities (right).
Bars represent averages ± sample standard deviations for 3 images for each condition. The area is the number of pixels above a threshold background value in the green and red fluorescent channels. There is no statistical significance in the area ratio differences. * denotes statistical significance (p < 0.05) between NGPEGSSF and NGPEGMBAF in integrated intensity ratio.
The CellTiter assay showed no significant cytotoxicity after incubation for 48 h with NGPEGSS, NGPEGMA, or free KAFAK peptide (Supplementary Figure 1).
Cartilage Penetration by Nanoparticles
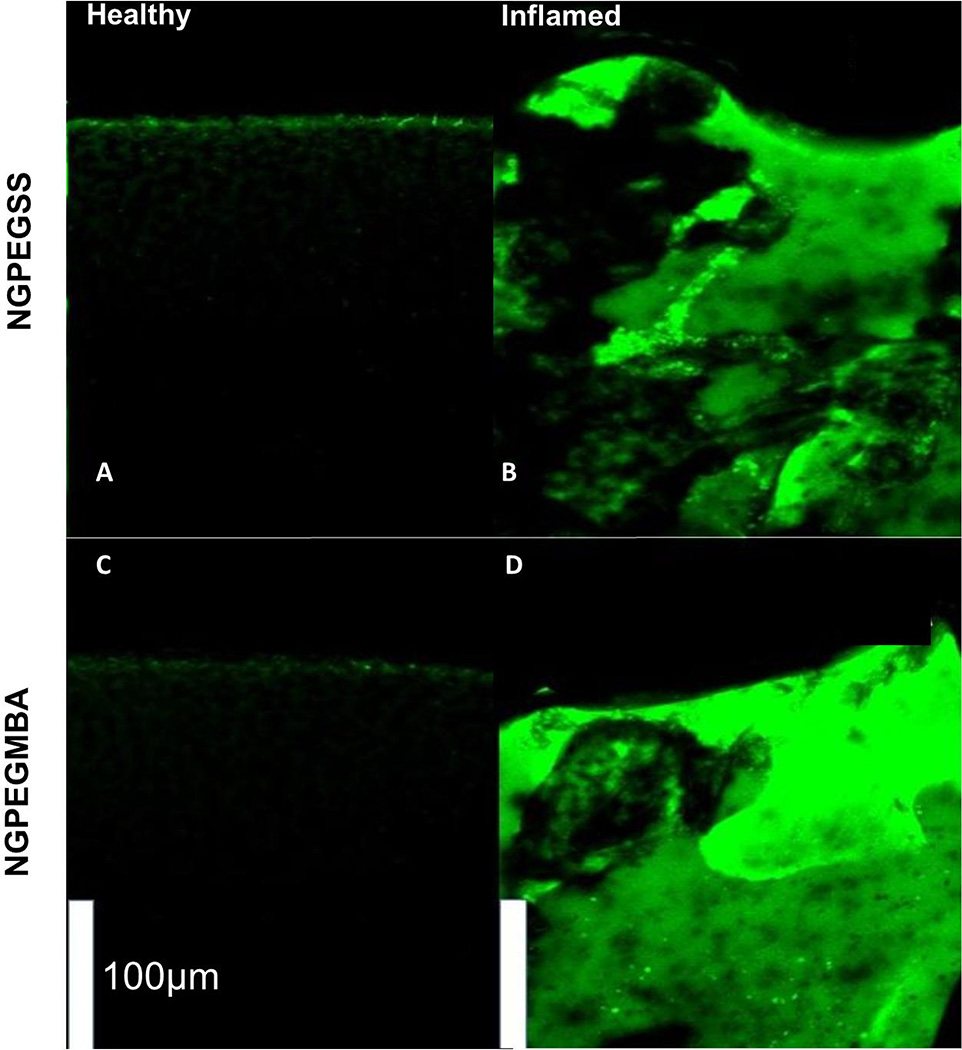
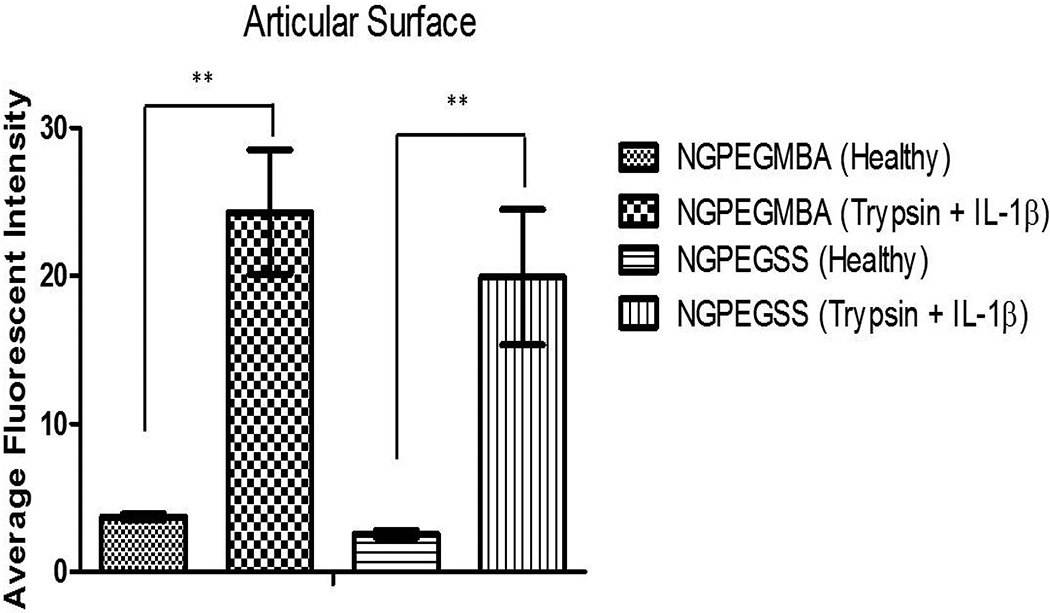
Incubation of cartilage explants for 24 h with FITC-labeled nanoparticles revealed greater penetration of nanoparticles throughout inflamed, aggrecan-depleted cartilage plugs compared to their healthy counterparts (Figures 3 & 4).
Figure 3. Mid-sagittal cross-sections of bovine knee explants from the load-bearing region of the femoral condyles with the top representing the articular surface after 24 h incubation with FITC-labeled nanoparticles.
(Top) NGPEGSSF and (Bottom) NGPEGMBAF. Left panel represents normal healthy cartilage incubated with fluorescent (A) NGPEGSSF or (C) NGPEGMBAF showing minimal diffusion of fluorescent particles. Right panel represents (B) NGPEGSSF and (D) NGPEGMBAF diffusing through the inflamed, aggrecan-depleted ex vivo cartilage explants. Scale bar represents 100 µm.
Figure 4. Average intensity analysis of the articular surface incubated with fluorescent nanoparticles indicates significantly higher fluorescence observed in inflamed, aggrecan-depleted cartilage (Trypsin + IL-1β) demonstrating greater penetration of nanoparticles.
Significance denoted as ** with p < 0.01. Bars represent intensity averages ± SEM of 3 independent areas at the articular surface.
IL-6 Expression in Cartilage Explants Treated with KAFAK-loaded Nanoparticles
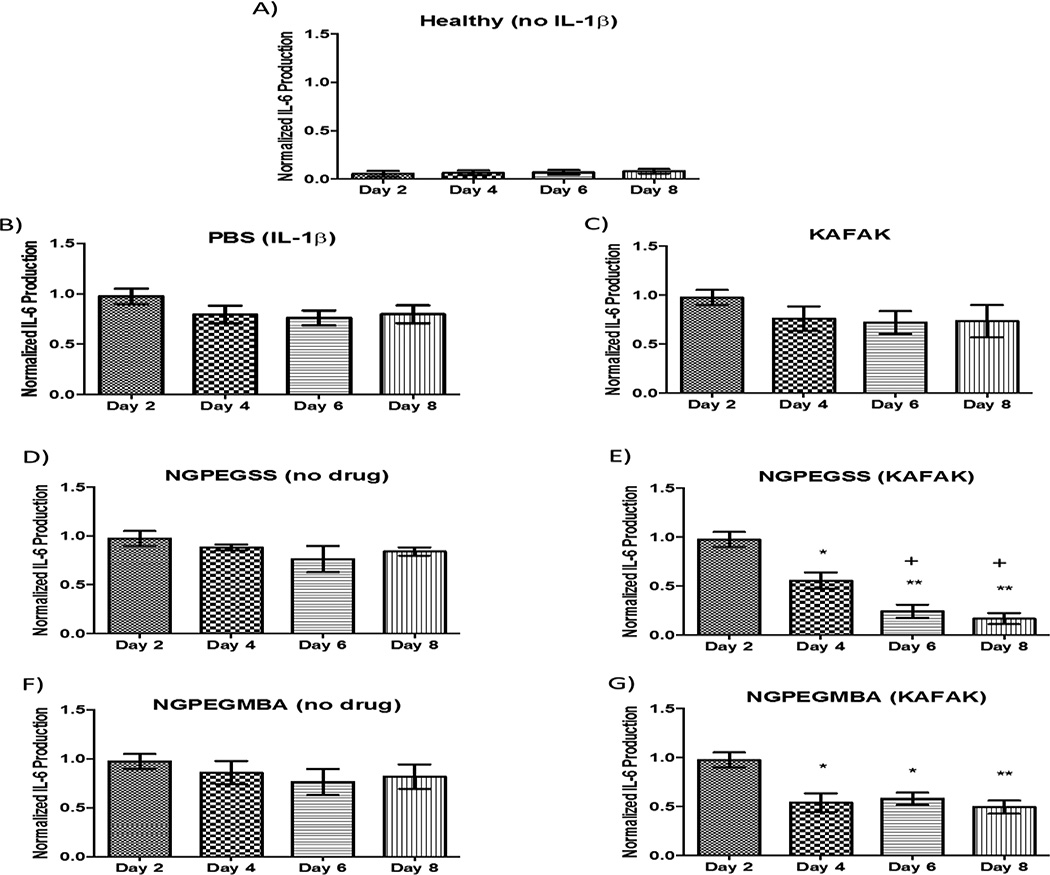
Inflamed, aggrecan-depleted cartilage explants treated with KAFAK-loaded NGPEGSS or NGPEGMBA exhibited significant suppression of IL-6 secretion on days 4, 6, and 8 (plots E & G). Furthermore, NGPEGSS decreased IL-6 production by a greater degree compared to NGPEGMBA on days 6 and 8. These anti-inflammatory effects can be ascribed to KAFAK released from the nanoparticles, as the empty nanoparticles do not suppress IL-6 secretion (plots D & F). As demonstrated by plot C, free KAFAK treatment was not effective in inflammatory suppression presumably due to rapid degradation by peptidases present in serum-supplemented media.
DISCUSSION
To develop a clinically relevant nanoparticle therapy for intra-articular injection to treat osteoarthritis, it is imperative that the nanoparticles do not aggregate in aqueous solutions and effectively deliver their payload to the target cells. As described previously, in aqueous solutions pNIPAM nanoparticles with 5% AMPS co-monomer remain spherical, well dispersed, and capable of delivering therapeutic peptides (14, 16). KAFAK-loaded pNIPAM nanoparticles with N,O-dimethacryloyl hydroxylamine (DMHA) crosslinks were reported to impart anti-inflammatory activity in cartilage explants and to penetrate deeply into aggrecan-depleted cartilage (15). However, the extracellular instability of the DMHA-crosslinked nanoparticles resulted in rapid release of KAFAK and may not have efficiently protected the KAFAK from extracellular proteases. In contrast NGPEGSS nanoparticles were stable and released less than 10% of the loaded KAFAK over 96 h at pH 7.4; however up to ~30% of loaded KAFAK was released within the same 96 h period in a reducing environment where the particles degraded (16). Limiting the extracellular KAFAK release may further improve payload delivery by protecting KAFAK from proteases within synovial fluid.
In exploring efficacy of the NGPEGSS nanoparticle system for treatment of osteoarthritis, uptake in macrophages was confirmed (16), but no studies confirming cartilage penetration, nor uptake in chondrocytes were reported. In studies presented here, as expected based on chondrocyte uptake of other nanoparticle carriers (chitosan (20), iron oxide (21, 22), and PLGA (23)) ranging from 50 nm to 300 nm, we observed nanoparticle uptake into endolysosomal compartments within chondrocytes (Figures 1 & 2). Further we observed reduced chondrocyte expression of inflammatory cytokines upon uptake of KAFAK-loaded nanoparticles. Importantly for treatment of osteoarthritis, these nanoparticles reduce cytokines secreted by macrophages (16) as well as chondrocytes. This suggests that our sulfated PNIPAM nanoparticle system penetrates inflamed cartilage and then gets taken up not only by macrophages that have evolved to endocytose foreign objects, but also by chondrocytes; therefore this delivery system shows significant promise for treatment of articular cartilage via intraarticular injection.
While the disulfide crosslinked nanoparticles exhibit the majority of their KAFAK release within 24 h of encountering a degradation stimulus, they remain stable and release less than 10% of the loaded peptide prior to entering endolysosome-like environments (16). Further, they significantly prolonged suppression of the secretion of IL-6 by inflamed, aggrecan-depleted cartilage. Their ability to diffuse into this damaged cartilage, get taken up by chondrocytes and macrophages, and maintain the majority of their payload prior to cell uptake may make these systems ideal for treatment of osteoarthritis.
Supplementary Material
Figure 5. Normalized IL-6 production by IL-1β inflamed, aggrecan-depleted cartilage plugs dosed with nanoparticles loaded with and without KAFAK.
Treatments were added on day 2 after IL-1β stimulation. (A) Negative control, healthy cartilage without IL-1β stimulation. (B-E) Aggrecan-depleted plugs dosed with IL-1β and treated with (B) PBS, (C) free KAFAK, (D) empty NGPEGSS, (E) KAFAK-loaded NGPEGSS, (F) empty NGPEGMBA, and (G) KAFAK-loaded NGPEGMBA nanoparticles. Plots are normalized to individual plug weight and IL-6 production from the control and represent average ± SEM (n = 3). * denotes p < 0.05 and ** p < 0.01 with respect to the IL-1β positive control (plot B). + denotes a significant difference (p < 0.05) in IL-6 production between KAFAK-loaded NGPEGSS and NGPEGMBA nanoparticles on days 6 and 8.
Acknowledgments
We wish to thank Dr. Rush Bartlett II, Dr. Shaili Sharma, Nelda Vazquez Portalatin, and James McMasters for their valuable assistance.
Funding Sources: This work was supported by NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases grant R01AR065398 and support for JBL by DK101001.
ABBREVIATIONS
- NG
pNIPAM-AMPS
- BAC
N,N’-bis(acryloyl)cystamine
- SS
disulfide crosslinker
- NGSS
pNIPAM-AMPS-disulfide
- NGPEGSS
PEGylated-pNIPAM-AMPS-disulfide
- NGPEGSS
PEGylated-pNIPAM-AMPS-disulfide
- NGSSF
pNIPAM-AMPS-disulfide-fluorescein
- NGPEGSSF
PEGylated-pNIPAM-AMPS-disulfide-fluorescein
- MBA
N,N’-methylenebisacrylamide
- NGMBA
non-degradable pNIPAM-AMPS-MBA
- NGPEGMBA
nondegradable PEGylated-pNIPAM-AMPS-MBA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Moerae Matrix, Inc. has a worldwide exclusive license to the MK2 inhibitor peptide. A. Panitch owns greater than 5% of Moerae Matrix, Inc.
REFERENCES
- 1.Van Manen MD, Nace J, Mont MA. Management of primary knee osteoarthritis and indications for total knee arthroplasty for general practitioners. J Am Osteopath Assoc. 2012;112(11):709–715. Epub 2012/11/10. PubMed PMID: 23139341. [PubMed] [Google Scholar]
- 2.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!) Osteoarthritis Cartilage. 2013;21(1):16–21. doi: 10.1016/j.joca.2012.11.012. Epub 2012/12/01. PubMed PMID: 23194896. [DOI] [PubMed] [Google Scholar]
- 3.Prevalence of disabilities and associated health conditions among adults--United States, 1999. MMWR Morb Mortal Wkly Rep. 2001;50(7):120–125. Epub 2001/06/08. PubMed PMID: 11393491. [PubMed] [Google Scholar]
- 4.Daghestani HN, Pieper CF, Kraus VB. Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis Rheumatol. 2015;67(4):956–965. doi: 10.1002/art.39006. Epub 2014/12/30. PubMed PMID: 25544994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44(6):1237–1247. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. Epub 2001/06/16. PubMed PMID: 11407681. [DOI] [PubMed] [Google Scholar]
- 6.Arner EC, Hughes CE, Decicco CP, Caterson B, Tortorella MD. Cytokine-induced cartilage proteoglycan degradation is mediated by aggrecanase. Osteoarthritis and cartilage. 1998;6(3):214–228. doi: 10.1053/joca.1998.0114. [DOI] [PubMed] [Google Scholar]
- 7.Pratta MA, Yao W, Decicco C, Tortorella MD, Liu RQ, Copeland RA, Magolda R, Newton RC, Trzaskos JM, Arner EC. Aggrecan protects cartilage collagen from proteolytic cleavage. J Biol Chem. 2003;278(46):45539–45545. doi: 10.1074/jbc.M303737200. Epub 2003/08/02. PubMed PMID: 12890681. [DOI] [PubMed] [Google Scholar]
- 8.Brugnano JL, Chan BK, Seal BL, Panitch A. Cell-penetrating peptides can confer biological function: regulation of inflammatory cytokines in human monocytes by MK2 inhibitor peptides. Journal of Controlled Release. 2011;155(2):128–133. doi: 10.1016/j.jconrel.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiology and molecular biology reviews. 2004;68(2):320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engel K, Ahlers A, Brach MA, Herrmann F, Gaestel M. MAPKAP kinase 2 is activated by heat shock and TNF-α: In vivo phosphorylation of small heat shock protein results from stimulation of the MAP kinase cascade. Journal of cellular biochemistry. 1995;57(2):321–330. doi: 10.1002/jcb.240570216. [DOI] [PubMed] [Google Scholar]
- 11.Belka C, Ahlers A, Sott C, Gaestel M, Herrmann F, Brach M. Interleukin (IL)-6 signaling leads to phosphorylation of the small heat shock protein (Hsp) 27 through activation of the MAP kinase and MAPKAP kinase 2 pathway in monocytes and monocytic leukemia cells. Leukemia. 1995;9(2):288–294. [PubMed] [Google Scholar]
- 12.Craik DJ, Fairlie DP, Liras S, Price D. The future of peptide-based drugs. Chemical biology & drug design. 2013;81(1):136–147. doi: 10.1111/cbdd.12055. [DOI] [PubMed] [Google Scholar]
- 13.Bartlett RL, 2nd, Medow MR, Panitch A, Seal B. Hemocompatible poly(NIPAm-MBAAMPS) colloidal nanoparticles as carriers of anti-inflammatory cell penetrating peptides. Biomacromolecules. 2012;13(4):1204–1211. doi: 10.1021/bm300173x. PubMed PMID: 22452800. [DOI] [PubMed] [Google Scholar]
- 14.Bartlett RL, 2nd, Panitch A. Thermosensitive nanoparticles with pH-triggered degradation and release of anti-inflammatory cell-penetrating peptides. Biomacromolecules. 2012;13(8):2578–2584. doi: 10.1021/bm300826v. PubMed PMID: 22852804. [DOI] [PubMed] [Google Scholar]
- 15.Bartlett RL, 2nd, Sharma S, Panitch A. Cell-penetrating peptides released from thermosensitive nanoparticles suppress pro-inflammatory cytokine response by specifically targeting inflamed cartilage explants. Nanomedicine : nanotechnology, biology, and medicine. 2013;9(3):419–427. doi: 10.1016/j.nano.2012.09.003. PubMed PMID: 23041412; PMCID: 4006693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poh S, Lin JB, Panitch A. Release of Anti-inflammatory Peptides from Thermosensitive Nanoparticles with Degradable Cross-Links Suppresses Pro-inflammatory Cytokine Production. Biomacromolecules. 2015;16(4):1191–1200. doi: 10.1021/bm501849p. PubMed PMID: 25728363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyon LA, Meng Z, Singh N, Sorrell CD, John AS. Thermoresponsive microgel-based materials. Chemical Society Reviews. 2009;38(4):865–874. doi: 10.1039/b715522k. [DOI] [PubMed] [Google Scholar]
- 18.Karg M, Hellweg T. New “smart” poly (NIPAM) microgels and nanoparticle microgel hybrids: properties and advances in characterisation. Current Opinion in Colloid & Interface Science. 2009;14(6):438–450. [Google Scholar]
- 19.Gulati N, Rastogi R, Dinda AK, Saxena R, Koul V. Characterization and cell material interactions of PEGylated PNIPAAM nanoparticles. Colloids and surfaces B, Biointerfaces. 2010;79(1):164–173. doi: 10.1016/j.colsurfb.2010.03.049. Epub 2010/05/08. PubMed PMID: 20447809. [DOI] [PubMed] [Google Scholar]
- 20.Zhao X, Yu SB, Wu FL, Mao ZB, Yu CL. Transfection of primary chondrocytes using chitosan-pEGFP nanoparticles. J Control Release. 2006;112(2):223–228. doi: 10.1016/j.jconrel.2006.01.016. PubMed PMID: 16556468. [DOI] [PubMed] [Google Scholar]
- 21.Ramaswamy S, Greco JB, Uluer MC, Zhang Z, Fishbein KW, Spencer RG. Magnetic resonance imaging of chondrocytes labeled with superparamagnetic iron oxide nanoparticles in tissue-engineered cartilage. Tissue Eng Part A. 2009;15(12):3899–3910. doi: 10.1089/ten.tea.2008.0677. PubMed PMID: 19788362; PMCID: PMC2792067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J, Wang F, Zhang Y, Jin X, Zhang L, Feng Y, Lin X, Yang L. In vivo tracking of superparamagnetic iron oxide nanoparticle labeled chondrocytes in large animal model. Ann Biomed Eng. 2012;40(12):2568–2578. doi: 10.1007/s10439-012-0621-5. PubMed PMID: 22810839. [DOI] [PubMed] [Google Scholar]
- 23.Park JS, Yang HN, Jeon SY, Woo DG, Kim MS, Park KH. The use of anti-COX2 siRNA coated onto PLGA nanoparticles loading dexamethasone in the treatment of rheumatoid arthritis. Biomaterials. 2012;33(33):8600–8612. doi: 10.1016/j.biomaterials.2012.08.008. PubMed PMID: 22910222. [DOI] [PubMed] [Google Scholar]
- 24.Niikura T, Reddi AH. Differential regulation of lubricin/superficial zone protein by transforming growth factor beta/bone morphogenetic protein superfamily members in articular chondrocytes and synoviocytes. Arthritis Rheum. 2007;56(7):2312–2321. doi: 10.1002/art.22659. PubMed PMID: 17599751. [DOI] [PubMed] [Google Scholar]
- 25.Poole AR, Pidoux I, Reiner A, Tang LH, Choi H, Rosenberg L. Localization of proteoglycan monomer and link protein in the matrix of bovine articular cartilage: An immunohistochemical study. J Histochem Cytochem. 1980;28(7):621–635. doi: 10.1177/28.7.6156200. Epub 1980/07/01 PubMed PMID: 6156200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.