Abstract
It is widely held that in response to high salt diets, normal individuals are acutely and chronically resistant to salt-induced hypertension because they rapidly excrete salt and retain little of it so that their blood volume, and therefore blood pressure, does not increase. Conversely, it is also widely held that salt-sensitive individuals develop salt-induced hypertension because of an impaired renal capacity to excrete salt that causes greater salt retention and blood volume expansion than that which occurs in normal salt-resistant individuals. Here we review results of both acute and chronic salt-loading studies that have compared salt-induced changes in sodium retention and blood volume between normal subjects (salt-resistant normotensive controls) and salt-sensitive subjects. The results of properly controlled studies strongly support an alternative view: during acute or chronic increases in salt intake, normal salt-resistant subjects undergo substantial salt retention and do not excrete salt more rapidly, retain less sodium, or undergo lesser blood volume expansion than do salt-sensitive subjects. These observations: 1) directly conflict with the widely held view that renal excretion of sodium accounts for resistance to salt-induced hypertension, and 2) have implications for contemporary understanding of how various genetic, immunologic, and other factors determine acute and chronic blood pressure responses to high salt diets.
Keywords: Hypertension, blood pressure, kidney, salt, sodium, sodium chloride, salt-resistance, salt-sensitivity
According to Hall, raising salt intake in individuals with normal kidney function "usually does not increase arterial pressure much because the kidneys rapidly eliminate the excess salt and blood volume is hardly altered."1 This view holds that normal subjects are usually salt-resistant, i.e., resistant to the pressor effects of both acute and chronic increases in salt intake, because they are able to rapidly eliminate excess salt and avoid substantial sodium retention and volume expansion. Further, as recently stated by Crowley and Coffman, "classic Guytonian models suggest that a defect in sodium excretion by the kidney is the basis for salt sensitivity, with impaired elimination of sodium during high-salt feeding leading directly to expanded extracellular fluid volume, which promotes increased blood pressure."2
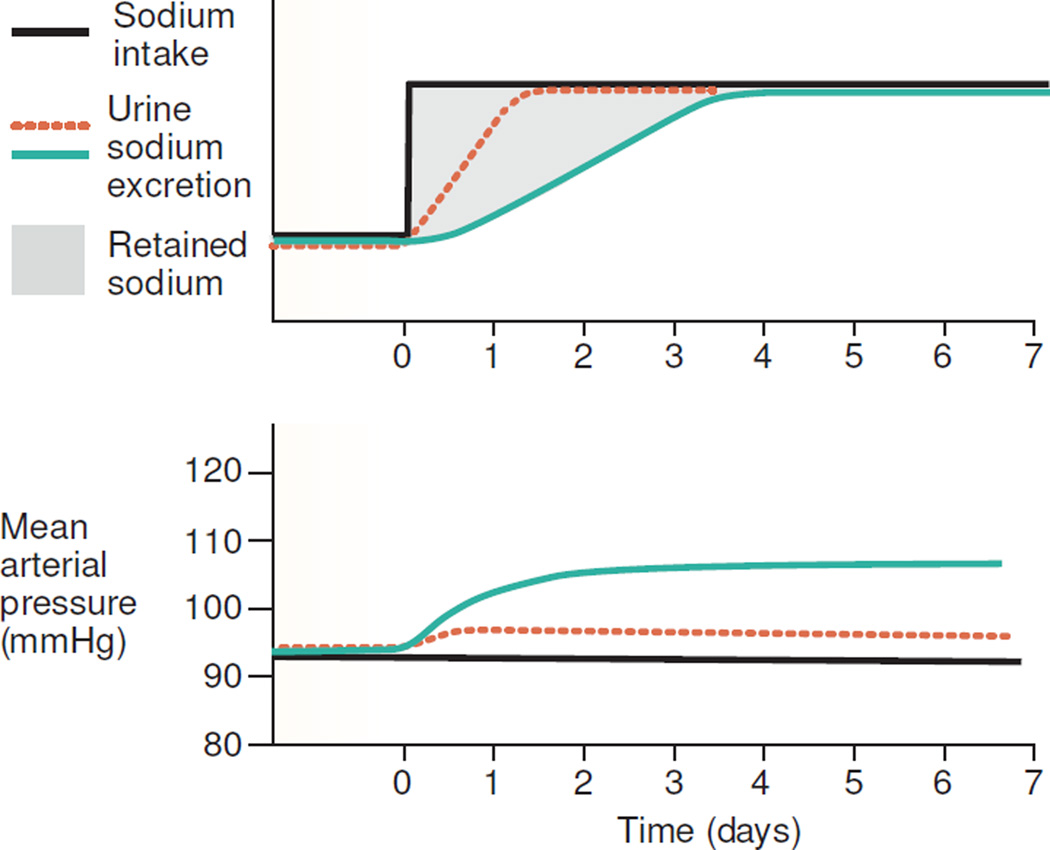
The widely held view,1–8 that normal salt-resistant subjects are protected from salt-induced increases in blood pressure because they rapidly excrete a salt load9 and retain little sodium, is illustrated by the hypothetical study results presented in a recent review by Brands (Figure 1).8 This view is incorporated, in full or in part, into many contemporary accounts of how various genetic, immunologic, and other factors determine acute or chronic blood pressure responses to a high salt intake.1, 2, 6, 8, 10–15 In addition, Lifton and others have proposed that in the general population, genetic variants associated with decreased activity of sodium transporters in the renal tubule, contribute to resistance from hypertension by promoting increased salt excretion and decreased external salt balance.16–18
Figure 1.
Hypothetical experimental results reflecting the widely held view1–4, 6, 8 that normal subjects excrete a salt load more rapidly and retain less sodium than salt-sensitive subjects (adapted from Brands8). The figure shows presumed changes in sodium balance, i.e., changes in sodium balance envisioned to occur during salt loading in normal subjects and salt-sensitive subjects.8 Note that these hypothetical sodium balance results envisioned by Brands8 differ from the actual sodium balance results generated in published salt-loading studies of normal subjects and salt-sensitive subjects (shown in Figures 2–6). In referring to the hypothetical results in the figure for salt-resistant normal subjects (depicted by the dotted, orange line), Brands states that the "line shows that sodium excretion normally rises rapidly to match an increase in sodium intake, such that blood pressure changes only minimally."8 In referring to the hypothetical results in the figure for salt-sensitive subjects (depicted by the solid, light green line), Brands notes that the line "shows that when renal sodium excretory capability is impaired, sodium balance takes longer to achieve. The result is an increase in extracellular fluid volume and blood pressure."8
Recently, we noted19 that the results of acute salt-loading studies in normotensive salt-resistant subjects seem to be at odds with the widely-held view that normal subjects are usually salt-resistant because they excrete a salt load rapidly9 and undergo very little blood volume expansion.1 Here we systematically review the results of studies that investigate whether normal subjects are usually resistant to the pressor effects of either acute or chronic salt loading because they rapidly excrete the excess salt and their blood volume is hardly altered.1 While many investigators have discussed salt-loading studies in salt-resistant hypertensive subjects, few if any investigators have systematically considered the results of both acute and chronic salt-loading studies in normal individuals (salt-resistant normotensive controls).
For purposes of discussion, we refer to "acute" (short-term) salt-loading studies as those in which the salt-loading is carried out for a matter of days to no more than a week or two. We define "chronic" (long-term) salt-loading studies as those in which the salt-loading is carried out for at least three weeks, and in some cases up to a year. Because we are focused here on mechanisms that mediate resistance to salt-induced hypertension, we review studies of the effects of increasing salt intake rather than studies of dietary salt restriction or diuretic-induced salt depletion. We emphasize the results of clinical studies in which the levels of salt intake tested are within ranges observed to be consumed by humans in non-research environments. We begin by discussing the effects of acute and chronic salt-loading on external sodium balance, and then discuss the effects of acute and chronic salt-loading on blood volume.
DURING ACUTE SALT LOADING, NORMAL SALT-RESISTANT SUBJECTS USUALLY RETAIN LARGE AMOUNTS OF SODIUM AND DO NOT RAPIDLY EXCRETE IT
In humans and in animals, the results of sodium balance studies consistently demonstrate that in response to short-term salt-loading over approximately one week, normal salt-resistant subjects retain substantial amounts of sodium,20–34 just as much and sometimes more than the amounts of sodium retained by either normotensive or hypertensive salt-sensitive subjects (Figures 2, 3, 4, 5).21, 23–25, 29–32 Yet, blood pressure increases little if at all in normal salt-resistant subjects (Figures 2, 3, 4, 5). This observation, that normal salt-resistant subjects acutely retain large amounts of sodium like salt-sensitive subjects, has been made not only in salt-loading studies in which normal salt-resistant controls have been compared to humans or animals with spontaneous forms of salt-sensitivity (Figures 2, 3, 4),21, 23–25, 29–31 but also in salt-loading studies in which normal controls have been compared to animals rendered salt-sensitive by surgical reduction of renal mass (Figure 5).32 During acute salt-loading sufficient to raise blood pressure in salt-sensitive subjects, normal salt-resistant individuals undergo large increases in external salt balance and continue to retain the excess sodium throughout the period of salt-loading.21, 23–25, 29–32 These observations in acute salt loading studies directly contradict the view that raising salt intake in normal individuals usually has little effect on blood pressure because their kidneys rapidly excrete the excess salt.1
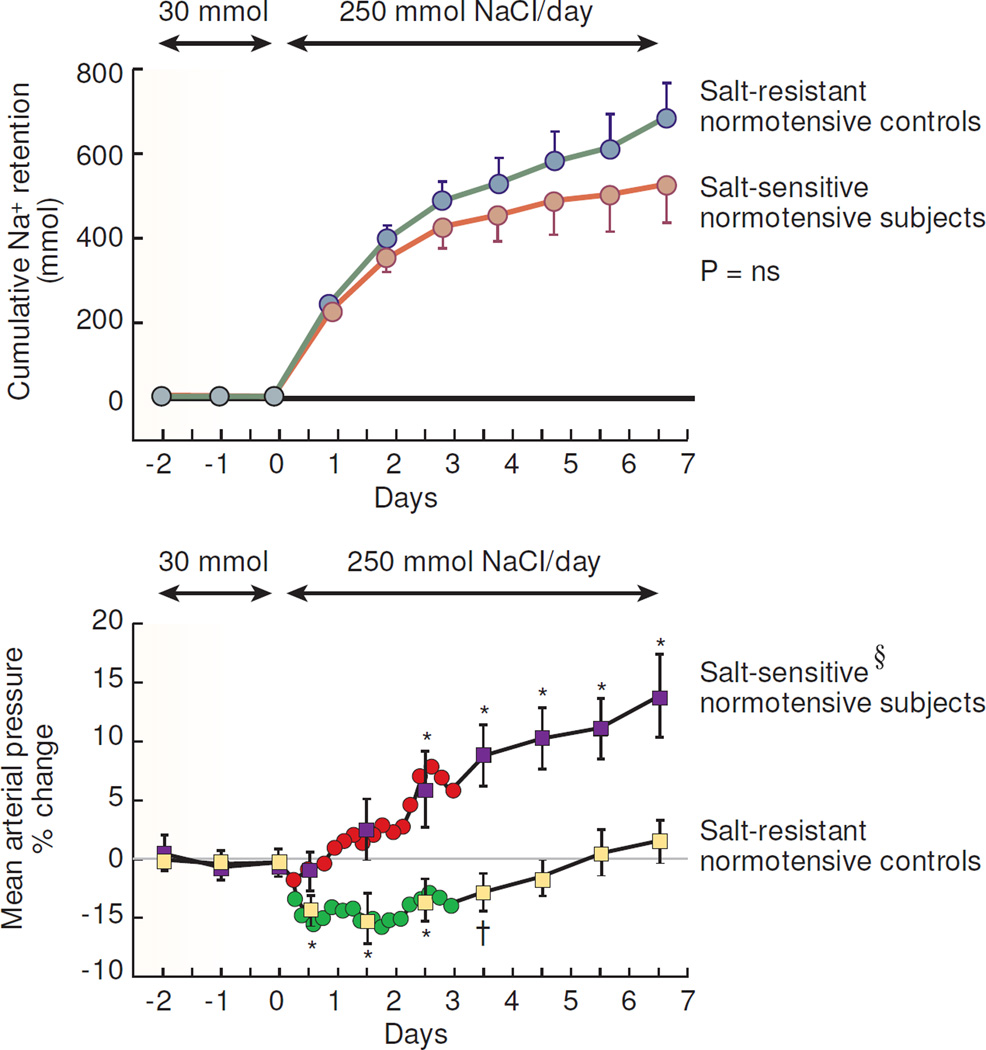
Figure 2.
Results of studies in normotensive African Americans in which Schmidlin et al measured cumulative sodium retention and mean arterial pressure before and after increasing dietary intake of NaCl from 30 mmol/day to 250 mmol/day (adapted from Schmidlin et al).25 During the last 3 days of the low NaCl diet, the salt-resistant normotensive control subjects (n=18) did not differ from the salt-sensitive normotensive subjects (n=19) with respect to mean arterial pressure or cumulative sodium balance. § denotes significant difference between the salt-induced change in arterial pressure in salt-sensitive subjects versus salt-resistant normal controls on day 1 of high NaCl intake (P < .05) and on day 2 to 7 of high NaCl intake (P < .001). ns, no significant difference in cumulative sodium balance between salt-sensitive subjects and salt-resistant normal controls throughout the 7 day period of salt loading. Significant difference compared to period of low NaCl intake denoted by †, P < .05; *, P < .01. Changes in blood pressure are shown at 4 hour intervals for the first 72 hours and then at 24 hour intervals thereafter. Results are displayed as means and 95% confidence intervals.
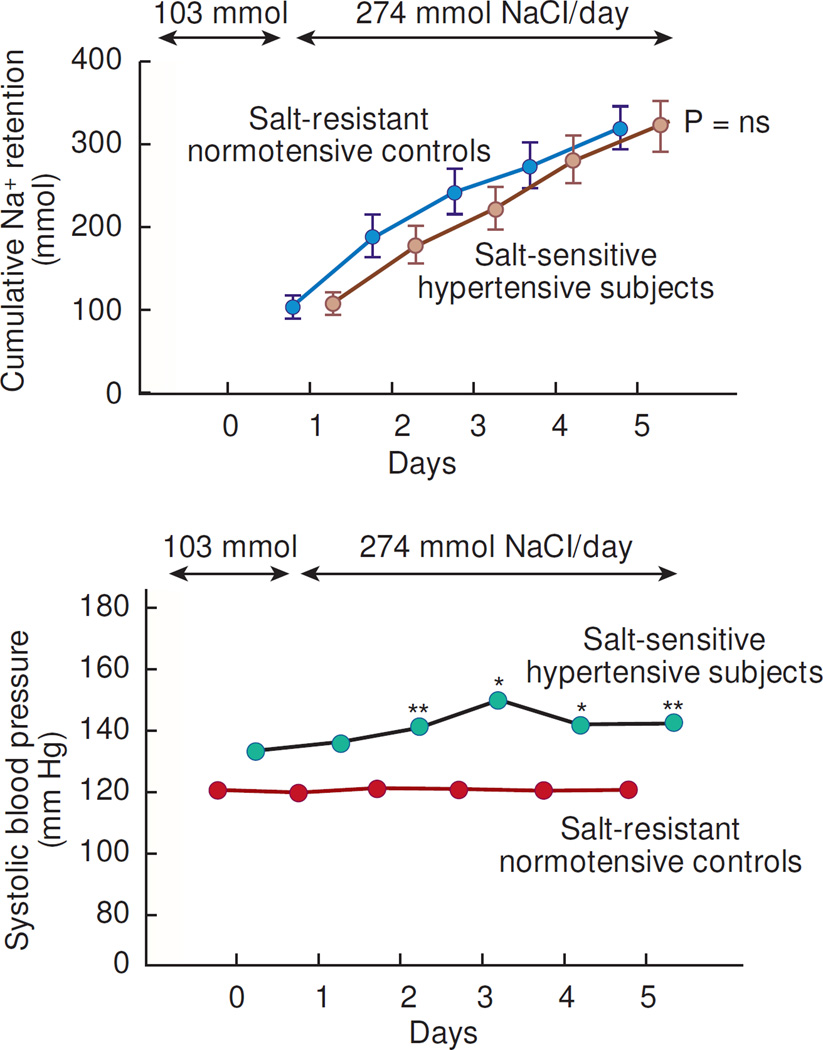
Figure 3.
Results of studies in Japanese in which Ishii et al measured cumulative sodium retention and systolic blood pressure in normal salt-resistant subjects and salt-sensitive subjects when dietary intake of NaCl was increased from 6 grams/day (approximately 103 mmol/day) to 15 grams/day (approximately 274 mmol/day) (adapted from Ishii et al.).21 ns, no significant difference in cumulative sodium balance between salt-resistant normal controls, 315 ± 23 mmol, and salt-sensitive hypertensive subjects, 319 ± 32 mmol, (means ± SEM). In each group, sample size is n = 11. ** p < .05 and * p < .01 denote significant differences in blood pressure versus day 0 before salt loading.
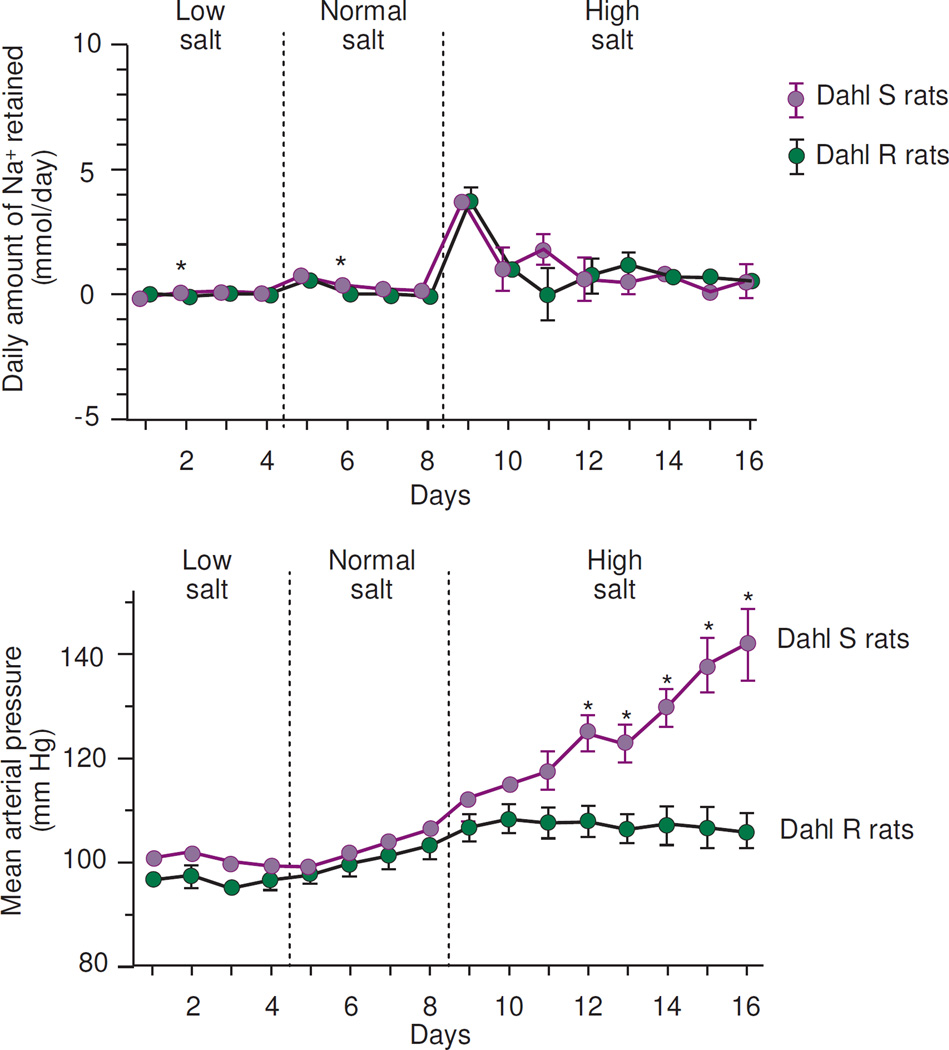
Figure 4.
Results of studies in which Hu and Manning measured daily sodium balance and mean arterial pressure before and after increasing salt intake in Dahl salt-resistant (R) rats (n=12) and Dahl salt-sensitive (S) rats (n=8) (adapted from Hu and Manning).31 Low salt intake was provided by a "low-sodium" diet plus intravenous (IV) administration of 0.64 mmol Na+/day as 0.3% NaCl, the normal salt intake was provided by a "low-sodium" diet plus IV administration of 1.57 mmol Na+/day as 0.9% NaCl, and the high salt intake was provided by the "low-sodium" diet plus IV administration of 12.6 mmol Na+/day as 8% NaCl. * P < .01 when S rats are compared with R rats in the same salt intake period.
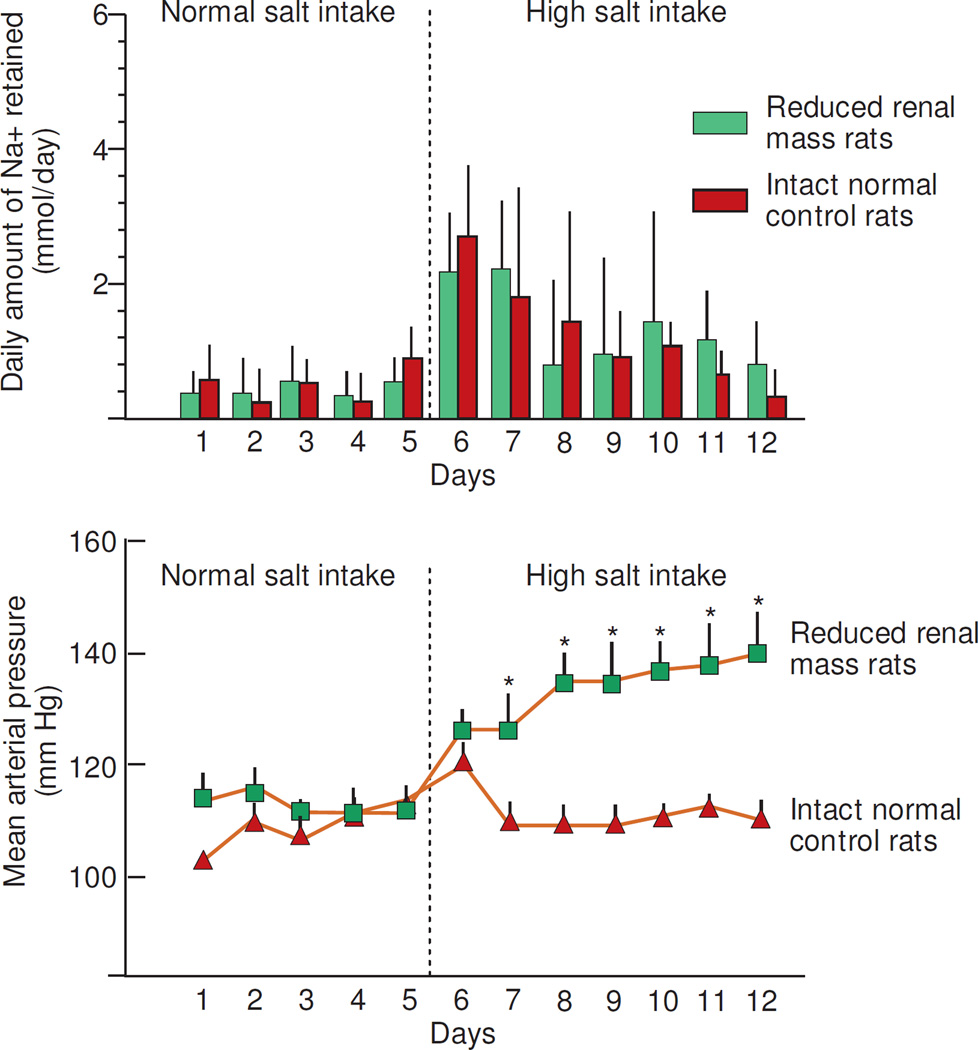
Figure 5.
Results of studies in which Kanagy and Fink measured daily sodium balance and mean arterial pressure before and after increasing salt intake in Sprague Dawley normal control rats with intact kidneys (n=8) and in Sprague Dawley rats with surgically reduced renal mass (reduced by 80%) (n=7) (adapted from Kanagy and Fink).32 Normal salt intake was provided by a "sodium-deficient" chow plus IV administration of 2 mmol NaCl per day in 5 mL of water and the high salt intake was provided by the "sodium-deficient" chow plus IV administration of 6 mmol NaCl per day in 5 mL of water. * denotes P <.05 group differences in blood pressure.
According to Hall, during the onset of salt-induced increases in blood pressure (BP), impaired salt excretion in salt-sensitive subjects compared to normal salt-resistant subjects may be subtle and difficult to detect because "in the presence of high BP, the natriuretic handicap disappears, and renal excretory function appears to be normal."35 This view implies that before high BP is present in salt-sensitive subjects, the natriuretic handicap should be apparent. However, in normotensive salt-sensitive subjects, at no time during salt-loading, either before or after the onset of high BP, are the levels of salt excretion and sodium retention different from those in salt-loaded normal salt-resistant controls.21, 23–25, 29–32 Furthermore, the view expressed by Hall does not explain why blood pressure increases minimally in normal salt-resistant subjects compared to salt-sensitive subjects even when they are given more salt and retain more sodium than salt-sensitive subjects.36
THE VIEW THAT INCREASES IN BLOOD PRESSURE ARE "REQUIRED" FOR SALT-SENSITIVE SUBJECTS TO ACHIEVE AND MAINTAIN SODIUM BALANCE
Some investigators contend that in response to increases in salt intake, increases in blood pressure are required for salt-sensitive subjects, but not normal salt-resistant subjects, to achieve and maintain sodium balance (sodium output = sodium intake).37, 38 It is held that in salt-sensitive subjects ingesting high salt diets, an underlying impairment in renal sodium excretion (abnormal pressure natriuresis) necessitates39 a compensatory increase in blood pressure to overcome the altered sodium excretion in order to maintain net sodium balance37 and prevent volume expansion.38 However, such a teleological view does not address how a high salt intake increases blood pressure. Nor does it explain why, in normotensive salt-resistant subjects, retention of very large amounts of salt is usually associated with little or no increase in blood pressure.20–34
IN NORMAL SALT-RESISTANT SUBJECTS, SUBSTANTIAL INCREASES IN TOTAL BODY SODIUM OCCUR DURING CHRONIC SALT LOADING
In chronic metabolic studies, Titze et al performed measurements of total body sodium and blood pressure in normal salt-resistant Dahl rats, normal salt-resistant Sprague Dawley rats, and salt-sensitive Dahl rats that were fed measured amounts of either a low salt diet or a high salt diet for 4 weeks.36 In response to the same given increment in total body sodium, blood pressure increased by a factor of 1.5 to 1.8 more in salt-sensitive rats than in normal control rats.36 Furthermore, Titze et al found that normal salt-resistant rats fed a high salt diet had greater levels of total body sodium and lower levels of blood pressure than salt-sensitive rats fed a low salt diet (Figure 6).36 Thus, it appears that blood pressure can remain chronically lower in normal salt-resistant rats than in Dahl salt-sensitive rats even when they chronically retain greater amounts of sodium than salt-sensitive rats.36
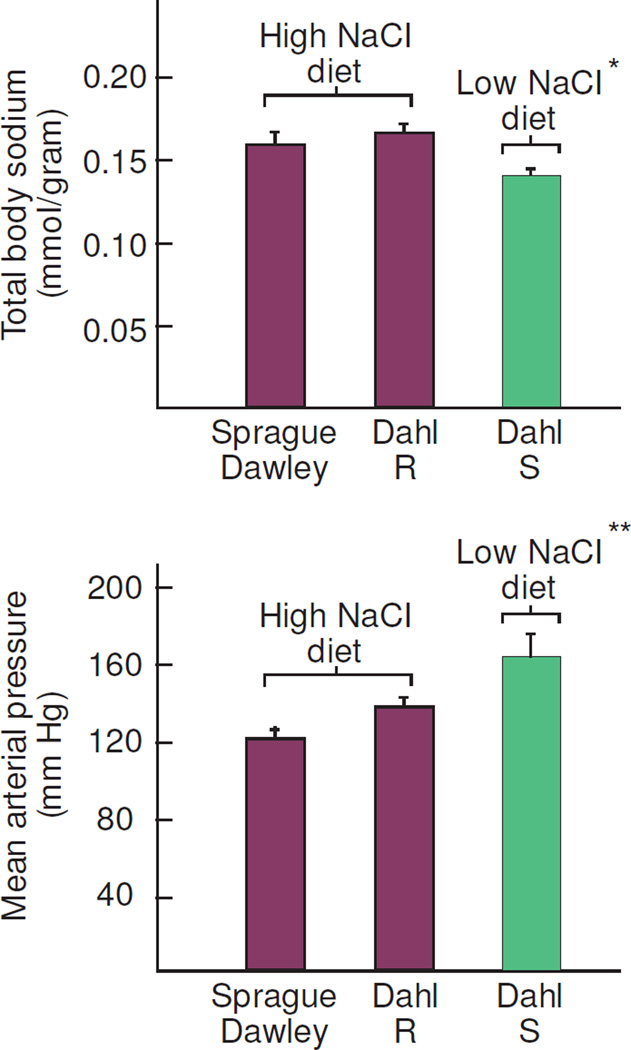
Figure 6.
Studies by Titze et al demonstrating that lower blood pressure in normal Sprague Dawley rats (n=10) or normal Dahl salt-resistant (R) rats (n=10) than in Dahl salt-sensitive (S) rats (n=10) does not depend on chronic retention of lower amounts of sodium in the normal rats (adapted from Titze et al).36 Blood pressure was lower in salt-resistant normal rats (Sprague Dawley rats or Dahl R rats) fed a high salt diet than in Dahl S rats fed a low salt diet, even though the salt-resistant rats had significantly greater levels of total body sodium. * denotes P < .005 compared to Sprague Dawley rats or Dahl R rats and ** denotes P < .005 compared to Sprague Dawley rats and P <.05 compared to Dahl R rats by Holm Sidak analysis.
These observations in normotensive salt-resistant rats are supported by the results of detailed metabolic balance studies of humans in which high salt diets were administered for 24 days. Specifically, Heer et al reported that normal subjects chronically retained very large amounts of sodium (1,704 ± 309.8 meq, mean ± SEM) with little or no increase in blood pressure.27 Furthermore, in long-term salt-loading studies in normal humans conducted over weeks to months, Titze's group found that variations in total body sodium were dissociated from variations in blood pressure.40
IN NORMAL SALT-RESISTANT SUBJECTS, SUBSTANTIAL INCREASES IN BLOOD VOLUME OCCUR DURING ACUTE SALT-LOADING
In normal salt-resistant animals and humans, short-term salt-loading for a few days to a few weeks, has often been shown to induce substantial increases (~10%) in blood volume27, 41, 42 and plasma volume.26, 33, 34, 43–45 One exception was reported in a short-term salt-loading study in normal dogs (n=6) in which blood volume did not appear to be increased based on only one measurement time point on a single day.22 However, the meaning of the one-time measurement of blood volume is questionable because in the same dogs, measurements of total body water, stroke volume, and cardiac output were found to be increased on multiple days of the study, including on the first day of salt loading when blood volume was not measured.22 Furthermore, in normal subjects, acute salt-induced increases in blood volume,41 stroke volume,24, 25, 41, 46 and cardiac output,24, 25, 41, 45, 46 are usually just as great as those in salt-sensitive subjects. Thus, with short-term salt-loading, blood volume, stroke volume, and cardiac output increase substantially in normal salt-resistant subjects, just as in salt-sensitive subjects, but blood pressure increases little in the normal subjects. These observations in acute salt-loading studies are directly at odds with the contention that raising salt intake in normal individuals usually has little effect on blood pressure because they rapidly eliminate the excess salt and undergo little expansion of blood volume.1
IN NORMAL SALT-RESISTANT SUBJECTS, SUBSTANTIAL INCREASES IN BLOOD VOLUME OCCUR DURING CHRONIC SALT-LOADING
In normal, salt-resistant dogs, Gupta et al47 have reported that chronic administration of a high salt diet (delivering 12.4 mmol Na+/kg body weight/day) for six weeks causes very large increases in blood volume (compared to the level of blood volume in normal dogs fed a diet that contains the recommended dietary allowance for sodium in dogs of 0.55 mmol Na+/kg body weight/day).48 In the normal dogs studied by Gupta, chronic salt-loading was observed to increase blood volume by > 50% without inducing much if any increase in blood pressure.47
Chronic salt-loading studies also indicate that in normal salt-resistant humans, salt-induced increases in blood volume may be chronically sustained during prolonged intake of high salt diets. In normal salt-resistant humans, Heer et al found that after salt intake had been increased from 220 mmol/day up to 660 mmol/day over a 24 day period, blood volume had increased by more than 10% (724 ± 180 mL, P < .05).27 Yet, during this chronic salt-loading, the increased blood volume was not associated with any chronic effects on systolic or diastolic blood pressure.27 Thus, the results of both acute and chronic salt-loading studies appear to be at odds with the view that in normal subjects, increasing salt intake induces little increase in blood pressure because their kidneys rapidly excrete the excess salt and their blood volume is hardly altered.1
It should also be noted that in normal salt-resistant humans studied for up to 1 year after switching from a low salt intake to a high salt intake, Sullivan et al found substantial salt-induced increases (~20 – 30%) in cardiac index, stroke volume, and end diastolic volume that were sustained for 12 months without increases in mean arterial pressure.49, 50 In salt-sensitive subjects, salt-induced increases in cardiac index similar in magnitude to those in normal salt-resistant subjects did not appear to be sustained for 12 months.50 Although Sullivan did not report measurements of blood volume, the cardiac function measurements in the normal salt-resistant subjects suggest that chronic salt loading caused either a sustained or cyclical increase in total blood volume, or redistribution of blood into the central vascular compartment. In acute salt-loading studies in normotensive humans conducted over 7 days, Schmidlin et al observed substantial increases in cardiac output in both salt-resistant and salt-sensitive subjects that were not sustained beyond 3 to 4 days.25 However, the effects of chronic salt-loading on cardiac output were not reported. In light of the chronic salt-loading studies of Sullivan et al in normal salt-resistant subjects,49, 50 it is possible that in the normal salt-resistant subjects studied by Schmidlin et al.,25 additional increases in cardiac output might have occurred with continued ingestion of a high salt diet beyond 7 days.
IN SALT-SENSITIVE SUBJECTS, SUBSTANTIAL INCREASES IN BLOOD VOLUME DO NOT APPEAR TO OCCUR DURING CHRONIC SALT-LOADING
In dogs rendered salt-sensitive by surgical reduction of renal mass, Manning, Guyton, and colleagues reported that massive intravenous salt loading (29 mmol Na+/kg body weight/day) induced large increases in blood volume over 3 days that were not sustained and could no longer be detected after 7 days or 13 days of chronic salt loading.51 In other studies carried out in reduced renal mass dogs given 1.2% salt (NaCl) water to drink, Guyton and colleagues were able to detect large increases in blood volume during the first 14 days of oral salt loading that were not sustained and could no longer be detected after 3 to 5 weeks of chronic salt loading.52 Despite having failed to show significantly greater fluid volume than normal, Guyton and colleagues concluded that in the chronic state in salt-loaded dogs with reduced renal mass, "almost un-measurable increases in extracellular fluid volume, blood volume, and cardiac output seem to be sufficient to cause marked elevation of the arterial pressure."5 As previously noted, in the salt-loading studies of Sullivan et al in salt-sensitive humans, salt-induced increases in cardiac index also did not appear to be chronically sustained.50
It should be noted that Hall and colleagues have indicated that in cases of hypertension induced by administration of a vasoconstrictor, cumulative sodium retention and blood volume may not be greater in hypertensive subjects than in normal controls.53–55 However, these investigators1, 3–5 and others1, 2, 6, 8, 10–15 contend that in cases in which the hypertension is induced by increased salt intake, (e.g., reduced renal mass models of salt-sensitivity or spontaneous forms of salt-sensitivity), salt-sensitive subjects do undergo greater salt-induced increases in sodium balance and blood volume than normal. Yet, the results of acute and chronic studies in humans and animals consistently demonstrate that in normal salt-resistant subjects, the salt-induced increases in sodium retention, blood volume, and cardiac output are similar to, or greater than, those in salt-sensitive subjects.21, 23–25, 29–32, 41, 45, 46, 49, 50
MODELING THE EFFECTS OF ACUTE AND CHRONIC SALT LOADING ON SODIUM BALANCE AND BLOOD VOLUME IN NORMAL SALT-RESISTANT SUBJECTS
"HumMod" is a Windows-based model of integrative human physiology that was developed by investigators in the physiology department established by Dr. Arthur Guyton.56 We tested whether HumMod (downloaded April 4, 2015 from www.hummod.org) provides results in accordance with the view that raising salt intake in individuals with normal kidneys usually induces little increase in blood pressure because the kidneys rapidly excrete the excess salt and blood volume is little altered.1 The HumMod simulation of salt-loading in a normal subject shows that a substantial increase in salt intake (from 30 mmol day to 500 mmol/day for approximately 1 week) induces substantial increases in sodium balance, blood volume, and cardiac output without inducing much of an effect on blood pressure (Figure 7). When the simulation is extended, the increased levels of sodium balance, blood volume, and cardiac output are largely sustained for at least 60 days (data not shown). Thus, in accordance with the results of short-term and long-term salt-loading studies, the HumMod simulation indicates that the failure of salt-loading to increase blood pressure much in normal subjects, either acutely or chronically, is not due to lack of either substantial sodium retention or volume expansion in normal subjects. Further, HumMod simulations indicate that salt-loading increases blood pressure less in normal salt-resistant subjects than in salt-sensitive subjects even when salt-resistant subjects are given ~ three-times more salt than salt-sensitive subjects and undergo greater increases in sodium balance, blood volume, and cardiac output than salt-sensitive subjects (Figure 7). Thus, the results of this computer simulation, like the results of the experimental studies in humans and animals,21, 23–25, 29–32, 36, 40, 41, 47, 52 indicate that the extent to which acute or chronic salt-loading induces increases in sodium retention or blood volume can be dissociated from the extent to which it induces increases in blood pressure.
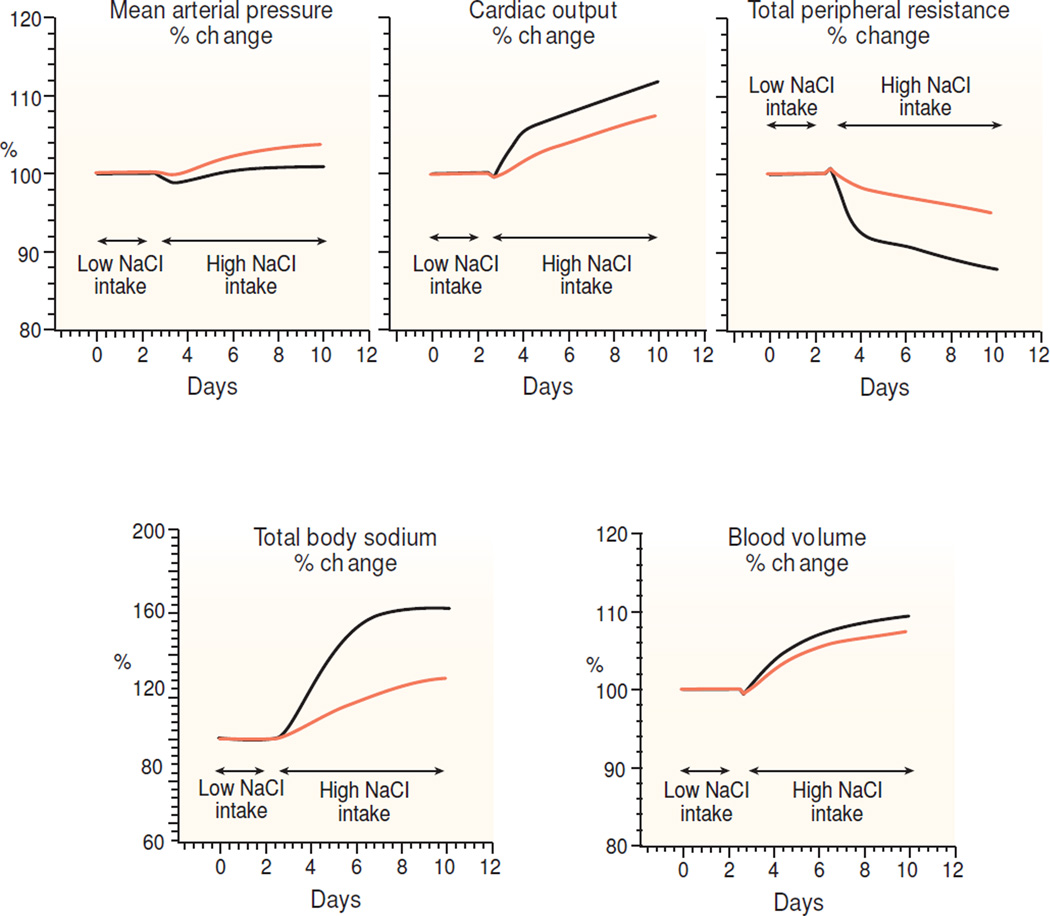
Figure 7.
Results of a computer model simulation with the "HumMod" program56 (downloaded April 4, 2015 from www.hummod.org) of a salt-loading experiment in a normal subject (denoted by solid black lines) and in a subject in which nephron number was reduced to 30% of normal (denoted by light orange lines). Before initiating the salt-loading, both subjects were stabilized on a 30 mmol NaCl diet for 90 days. Measurements of mean arterial pressure, cardiac output, total peripheral resistance, blood volume, and total body sodium were recorded during the baseline period on the low salt diet (30 mmol NaCl/day). In the simulated normal subject with intact kidneys, dietary salt intake was increased to 500 mmol/day and the high salt intake continued as shown in the Figure. In the simulated normal subject, blood pressure increased relatively little despite very large increases in total body sodium, blood volume, and cardiac output. In the simulated subject with reduced renal mass, dietary salt intake was increased to 150 mmol/day and the high salt intake continued as shown in the Figure. Note that blood pressure increased less in the normal subject (solid black line) than in the subject with reduced nephron mass (light orange line), even though the normal subject retained more sodium than did the subject with reduced nephron mass.
IN NORMAL INDIVIDUALS, HOW CAN ACUTE AND CHRONIC INCREASES IN SODIUM BALANCE AND BLOOD VOLUME BE DISSOCIATED FROM EFFECTS ON BLOOD PRESSURE?
According to Crowley and Coffman, "dissociation of blood pressure from fluid volumes likely reflects complexities of sodium handling not accounted for by Guyton’s hypothesis."2 However, the dissociation of salt-induced changes in blood pressure from salt-induced changes in blood volume could be explained by alterations in vascular resistance that may not necessarily depend on alterations in renal or extra-renal sodium handling.19, 57 Normal salt-resistant subjects, acutely20–34, 41, 42, 45, 46 and chronically,27, 40, 47, 49, 50 can sustain large salt-induced increases in sodium balance, blood volume, and cardiac output without increases in blood pressure because they vasodilate and substantially reduce vascular resistance in response to high salt diets.19, 24, 25, 41, 45, 46, 49, 50 Thus, in normal individuals, resistance to the pressor effects of acute or chronic increases in salt intake is not usually due to an ability to rapidly excrete a salt load and prevent substantial increases in sodium balance, blood volume, and cardiac output.19 Rather, it may be due to reductions in systemic vascular resistance in response to acute and chronic salt-loading19, 24, 25, 41, 45, 46, 49, 50 mediated largely, or only by, reductions in renal vascular resistance.58, 59
It is well recognized that in certain pathophysiologic conditions such as heart failure, increases in sodium balance and fluid volumes do not cause increases in blood pressure.1, 4 However, it is not widely appreciated that in most normal individuals, substantial increases in sodium balance and blood volume also do not cause increases in blood pressure. In normal individuals, it seems to be the ability to vasodilate and reduce vascular resistance in response to a high salt diet that usually protects them from either acute or chronic salt-induced increases in blood pressure.19, 24, 25, 41, 45, 46, 49, 50
Remarkably, in response to increases in salt intake, normal salt-resistant humans can retain substantial amounts of sodium and undergo substantial increases in blood volume, without retaining commensurate amounts of water and without undergoing increases in extracellular fluid volume.27 Heer et al have suggested that normal subjects might be able to retain substantial amounts of sodium without retaining substantial amounts of water because of a capacity to store sodium in interstitial spaces where it may be osmotically inactive.27 Further, Kusche-Vihrog and others have proposed that non-osmotic storage of sodium in the glycocalyx lining of endothelial surfaces may protect against salt-induced increases in blood pressure by impeding sodium ions in plasma from entering endothelial cells.60, 61 Such impedance of sodium movement may prevent salt-induced increases in endothelial cell sodium concentration that would otherwise cause increases in cell stiffness that interfere with flow-mediated increases in nitric oxide activity and endothelial dependent vasodilation.60, 61 In addition, Titze and others have indicated that alterations in the storage of non-osmotically active sodium in skin may affect cutaneous vasoreactivity and that this could impact total peripheral resistance and thereby contribute to salt-sensitive hypertension.62, 63
HOW DID THE MISTAKEN VIEW ARISE THAT NORMAL SALT-RESISTANT SUBJECTS RETAIN LESS SODIUM THAN SALT-SENSITIVE SUBJECTS?
Several factors have contributed to the widely held view that subjects with normal kidneys usually excrete a salt load more rapidly and retain less sodium than salt-sensitive subjects. For example, this view has been, and continues to be prominently supported by influential publications of Lifton, Guyton, and others.1, 3, 6, 8, 9 However, the studies of these investigators do not compare measurements of cumulative sodium balance between salt-sensitive subjects and normal salt-resistant controls studied during salt-loading.6, 8, 51, 52, 64, 65 Studies of salt-sensitivity by other prominent investigators also do not include measurements of cumulative sodium balance in normal controls but instead compare salt-sensitive subjects to salt-resistant control subjects with hypertension,66, 67 or to a collection of salt-resistant hypertensive subjects and salt-resistant normal subjects.68 Studies by Kawasaki et al66, 67 and Fujita et al66, 67 demonstrated that salt-sensitive hypertensive subjects retain more of a salt load than salt-resistant hypertensive subjects, but those studies did not include normal controls, i.e., salt-resistant subjects with normal blood pressure. However, as originally pointed out by Campese et al.,69 and as we have noted elsewhere,19 comparisons of salt-sensitive subjects to salt-resistant hypertensive subjects cannot be used to judge whether the amount of a sodium load retained by salt-sensitive subjects is normal or abnormal. To make this judgment, it is necessary to compare the results of salt-loading studies in salt-sensitive subjects with those in normal controls (salt-resistant subjects with normal blood pressure).
IMPLICATIONS FOR THE PATHOGENESIS OF SALT-INDUCED HYPERTENSION
The results of both acute20–34, 41, 42, 45, 46 and chronic salt-loading studies27, 40, 47, 49, 50 are at odds with the traditional view that normal subjects are salt-resistant because they rapidly excrete the excess salt and undergo very little expansion of blood volume.1 Further, the results of appropriately controlled studies indicate that salt-induced hypertension is not usually initiated and maintained by the occurrence of greater sodium retention, volume expansion, and increases in cardiac output in salt-sensitive subjects than in salt-loaded normal subjects.21, 23–25, 29–32, 41, 45, 46, 49, 50 Nevertheless, many investigators1, 2, 6, 10–15 seek to align the results of contemporary genetic, immunologic, and other studies of salt-induced hypertension with traditional views on the mechanisms of salt-resistance and salt-sensitivity. The current observations suggest that it might be more useful to align such contemporary research findings with hypotheses19, 70–74 which do not hold that normal subjects are resistant to salt-induced hypertension because they undergo little or no increase in sodium balance or blood volume in response to a high salt diet.
Acknowledgments
None
SOURCES OF FUNDING
This work was supported by the General Clinical Research Center, Moffitt-Long Hospital, University of California, San Francisco, with funds provided by the National Center for Research Resources, M0 RR-00079, US Public Health Service. Additional support was provided by a Praemium Academiae award of the Czech Academy of Sciences to MP, National Institutes of Health/National Heart, Lung and Blood Institute grant RO1-HL64230, and gifts from the Emil Mosbacher, Jr Foundation, the Antel Foundation, and the Maier Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES – The authors have no financial interests to disclose.
REFERENCES
- 1.Hall JE. Guyton and Hall Textbook of Medical Physiology. 13th edition. Philadelphia: Elsevier; 2015. [Google Scholar]
- 2.Crowley SD, Coffman TM. The inextricable role of the kidney in hypertension. J Clin Invest. 2014;124:2341–2347. doi: 10.1172/JCI72274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guyton AC. Circulatory Physiology III: Arterial Pressure and Hypertension. Philadelphia: W.B. Saunders; 1980. [Google Scholar]
- 4.Guyton AC, Manning RD, Jr, Hall JE, Norman RA, Jr, et al. The pathogenic role of the kidney. J Cardiovasc Pharmacol. 1984;6(Suppl 1):S151–S161. doi: 10.1097/00005344-198400061-00025. [DOI] [PubMed] [Google Scholar]
- 5.Guyton AC, Hall JE, Coleman TG, Manning RD, et al. The Dominant Role of the Kidneys in Long-Term Arterial Pressure Regulation in Normal and Hypertensive States. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, Diagnosis, and Management. 2nd edition. Vol. 1. New York: Raven Press, Ltd.; 1995. pp. 1311–1326. [Google Scholar]
- 6.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 7.Cruz DN, Simon DB, Nelson-Williams C, Farhi A, et al. Mutations in the Na-Cl cotransporter reduce blood pressure in humans. Hypertension. 2001;37:1458–1464. doi: 10.1161/01.hyp.37.6.1458. [DOI] [PubMed] [Google Scholar]
- 8.Brands MW. Chronic blood pressure control. Compr Physiol. 2012;2:2481–2494. doi: 10.1002/cphy.c100056. [DOI] [PubMed] [Google Scholar]
- 9.Guyton AC. Long-term arterial pressure control: an analysis from animal experiments and computer and graphic models. Am J Physiol. 1990;259:R865–R877. doi: 10.1152/ajpregu.1990.259.5.R865. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Iturbe B, Romero F, Johnson RJ. Pathophysiological mechanisms of salt-dependent hypertension. Am J Kidney Dis. 2007;50:655–672. doi: 10.1053/j.ajkd.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 11.Carey RM. Pathophysiology of Primary Hypertension. In: Tuma RF, Duran WN, Ley K, editors. Handbook of Physiology, Microcirculation. San Diego: Academic Press; 2008. pp. 794–895. [Google Scholar]
- 12.Scholl UI, Lifton RP. Inherited disorders of renal salt homeostasis: Insights from molecular genetics studies. In: Alpern RJ, Moe OW, Caplan M, editors. Seldin and Giebisch's The Kidney. 5th edition. III. Vol. 1. London: Elsevier; 2013. pp. 1213–1240. [Google Scholar]
- 13.Ando K, Fujita T. Pathophysiology of salt sensitivity hypertension. Ann Med. 2012;44(Suppl 1):S119–S126. doi: 10.3109/07853890.2012.671538. [DOI] [PubMed] [Google Scholar]
- 14.Rossier BC, Staub O, Hummler E. Genetic dissection of sodium and potassium transport along the aldosterone-sensitive distal nephron: importance in the control of blood pressure and hypertension. FEBS Lett. 2013;587:1929–1941. doi: 10.1016/j.febslet.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 15.McDonough AA, Nguyen MT. Maintaining Balance Under Pressure: Integrated Regulation of Renal Transporters During Hypertension. Hypertension. 2015;66:450–455. doi: 10.1161/HYPERTENSIONAHA.115.04593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji W, Foo JN, O'Roak BJ, Zhao H, et al. Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet. 2008;40:592–599. doi: 10.1038/ng.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Acuna R, Martinez-de-la-Maza L, Ponce-Coria J, Vazquez N, et al. Rare mutations in SLC12A1 and SLC12A3 protect against hypertension by reducing the activity of renal salt cotransporters. J Hypertens. 2011;29:475–483. doi: 10.1097/HJH.0b013e328341d0fd. [DOI] [PubMed] [Google Scholar]
- 18.Subramanya AR, Welling PA. Toward an understanding of hypertension resistance. Am J Physiol Renal Physiol. 2011;300:F838–F839. doi: 10.1152/ajprenal.00078.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris RC, Schmidlin O, Sebastian A, Tanaka M, et al. Vasodysfunction that involves renal vasodysfunction, not abnormally increased renal retention of sodium, accounts for the initiation of salt-induced hypertension. Circulation. 2016;137:881–893. doi: 10.1161/CIRCULATIONAHA.115.017923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinberger MH, Luft FC, Bloch R, Henry DP, et al. The blood pressure-raising effects of high dietary sodium intake: racial differences and the role of potassium. J Am Coll Cardiol. 1982;1:139–148. doi: 10.1080/07315724.1982.10718981. [DOI] [PubMed] [Google Scholar]
- 21.Ishii M, Atarashi K, Ikeda T, Hirata Y, et al. Role of the aldosterone system in the salt-sensitivity of patients with benign essential hypertension. Jpn Heart J. 1983;24:79–89. doi: 10.1536/ihj.24.79. [DOI] [PubMed] [Google Scholar]
- 22.Krieger JE, Liard JF, Cowley AW., Jr Hemodynamics, fluid volume, and hormonal responses to chronic high-salt intake in dogs. Am J Physiol. 1990;259:H1629–H1636. doi: 10.1152/ajpheart.1990.259.6.H1629. [DOI] [PubMed] [Google Scholar]
- 23.Wedler B, Brier ME, Wiersbitzky M, Gruska S, et al. Sodium kinetics in salt-sensitive and salt-resistant normotensive and hypertensive subjects. J Hypertens. 1992;10:663–669. [PubMed] [Google Scholar]
- 24.Schmidlin O, Sebastian AF, Morris RC., Jr What initiates the pressor effect of salt in salt-sensitive humans? Observations in normotensive blacks. Hypertension. 2007;49:1032–1039. doi: 10.1161/HYPERTENSIONAHA.106.084640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidlin O, Forman A, Leone A, Sebastian A, et al. Salt sensitivity in blacks: evidence that the initial pressor effect of NaCl involves inhibition of vasodilatation by asymmetrical dimethylarginine. Hypertension. 2011;58:380–385. doi: 10.1161/HYPERTENSIONAHA.111.170175. [DOI] [PubMed] [Google Scholar]
- 26.Heer M, Frings-Meuthen P, Titze J, Boschmann M, et al. Increasing sodium intake from a previous low or high intake affects water, electrolyte and acid-base balance differently. Br J Nutr. 2009;101:1286–1294. doi: 10.1017/S0007114508088041. [DOI] [PubMed] [Google Scholar]
- 27.Heer M, Baisch F, Kropp J, Gerzer R, et al. High dietary sodium chloride consumption may not induce body fluid retention in humans. Am J Physiol Renal Physiol. 2000;278:F585–F595. doi: 10.1152/ajprenal.2000.278.4.F585. [DOI] [PubMed] [Google Scholar]
- 28.Sagnella GA, Markandu ND, Buckley MG, Miller MA, et al. Hormonal responses to gradual changes in dietary sodium intake in humans. Am J Physiol. 1989;256:R1171–R1175. doi: 10.1152/ajpregu.1989.256.6.R1171. [DOI] [PubMed] [Google Scholar]
- 29.Roman RJ, Osborn JL. Renal function and sodium balance in conscious Dahl S and R rats. Am J Physiol. 1987;252:R833–R841. doi: 10.1152/ajpregu.1987.252.5.R833. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura K, Cowley AW., Jr Sequential changes of cerebrospinal fluid sodium during the development of hypertension in Dahl rats. Hypertension. 1989;13:243–249. doi: 10.1161/01.hyp.13.3.243. [DOI] [PubMed] [Google Scholar]
- 31.Hu L, Manning RD., Jr Role of nitric oxide in regulation of long-term pressure-natriuresis relationship in Dahl rats. Am J Physiol. 1995;268:H2375–H2383. doi: 10.1152/ajpheart.1995.268.6.H2375. [DOI] [PubMed] [Google Scholar]
- 32.Kanagy NL, Fink GD. Losartan prevents salt-induced hypertension in reduced renal mass rats. JPharmacolExpTher. 1993;265:1131–1136. [PubMed] [Google Scholar]
- 33.Damgaard M, Norsk P, Gustafsson F, Kanters JK, et al. Hemodynamic and neuroendocrine responses to changes in sodium intake in compensated heart failure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1294–R1301. doi: 10.1152/ajpregu.00738.2005. [DOI] [PubMed] [Google Scholar]
- 34.Damgaard M, Gabrielsen A, Heer M, Warberg J, et al. Effects of sodium intake on cardiovascular variables in humans during posture changes and ambulatory conditions. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1404–R1411. doi: 10.1152/ajpregu.00198.2002. [DOI] [PubMed] [Google Scholar]
- 35.Hall JE. Renal dysfunction, rather than non-renal vascular dysfunction, mediates salt-induced hypertension. Circulation. 2016;137:894–907. doi: 10.1161/CIRCULATIONAHA.115.018526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Titze J, Krause H, Hecht H, Dietsch P, et al. Reduced osmotically inactive Na storage capacity and hypertension in the Dahl model. Am J Physiol Renal Physiol. 2002;283:F134–F141. doi: 10.1152/ajprenal.00323.2001. [DOI] [PubMed] [Google Scholar]
- 37.Kotchen TA, Cowley AW, Jr, Frohlich ED. Salt in health and disease--a delicate balance. N Engl J Med. 2013;368:1229–1237. doi: 10.1056/NEJMra1212606. [DOI] [PubMed] [Google Scholar]
- 38.Landsberg L. On Rounds: 1000 Internal Medicine Pearls. Lippincott Williams & Wilkins; 2015. [Google Scholar]
- 39.Hall JE, Mizelle HL, Hildebrandt DA, Brands MW. Abnormal pressure natriuresis: A cause or a consequence of hypertension? Hypertension. 1990;15:547–559. doi: 10.1161/01.hyp.15.6.547. [DOI] [PubMed] [Google Scholar]
- 40.Rakova N, Juttner K, Dahlmann A, Schroder A, et al. Long-term space flight simulation reveals infradian rhythmicity in human Na(+) balance. Cell Metab. 2013;17:125–131. doi: 10.1016/j.cmet.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 41.Greene AS, Yu ZY, Roman RJ, Cowley AW., Jr Role of blood volume expansion in Dahl rat model of hypertension. Am J Physiol. 1990;258:H508–H514. doi: 10.1152/ajpheart.1990.258.2.H508. [DOI] [PubMed] [Google Scholar]
- 42.Brown WJJ, Brown FK, Krishan E. Exchangeable sodium and blood volume in normotensive and hypertensive humans on high and low sodium intake. Circulation. 1971;43:508–519. doi: 10.1161/01.cir.43.4.508. [DOI] [PubMed] [Google Scholar]
- 43.Rocchini AP, Cant JR, Barger AC. Carotid sinus reflex in dogs with low- to high-sodium intake. Am J Physiol. 1977;233:H196–H202. doi: 10.1152/ajpheart.1977.233.2.H196. [DOI] [PubMed] [Google Scholar]
- 44.Lyons RH, Jacobson SD, Avery NL. Increases in the plasma volume following the administration of sodium salts. Am J Med Sci. 1944;208:148–154. [Google Scholar]
- 45.West SG, Light KC, Hinderliter AL, Stanwyck CL, et al. Potassium supplementation induces beneficial cardiovascular changes during rest and stress in salt sensitive individuals. Health Psychol. 1999;18:229–240. doi: 10.1037//0278-6133.18.3.229. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan JM, Prewitt RL, Ratts TE, Josephs JA, et al. Hemodynamic characteristics of sodium-sensitive human subjects. Hypertension. 1987;9:398–406. doi: 10.1161/01.hyp.9.4.398. [DOI] [PubMed] [Google Scholar]
- 47.Gupta BN, Linden RJ, Mary DA, Weatherill D. The influence of high and low sodium intake on blood volume in the dog. Q J Exp Physiol. 1981;66:117–128. doi: 10.1113/expphysiol.1981.sp002539. [DOI] [PubMed] [Google Scholar]
- 48.National Research Council (U.S.). Ad Hoc Committee on Dog and Cat Nutrition. Nutrient requirements of dogs and cats. Washington, D.C.: National Academies Press; 2006. [Google Scholar]
- 49.Sullivan JM, Ratts TE. Hemodynamic mechanisms of adaptation to chronic high sodium intake in normal humans. Hypertension. 1983;5:814–820. doi: 10.1161/01.hyp.5.6.814. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan JM. Salt sensitivity. Definition, conception, methodology, and long-term issues. Hypertension. 1991;17:I61–I68. doi: 10.1161/01.hyp.17.1_suppl.i61. [DOI] [PubMed] [Google Scholar]
- 51.Manning RD, Jr, Coleman TG, Guyton AC, Norman RA, Jr, et al. Essential role of mean circulatory filling pressure in salt-induced hypertension. Am J Physiol. 1979;236:R40–R47. doi: 10.1152/ajpregu.1979.236.1.R40. [DOI] [PubMed] [Google Scholar]
- 52.Douglas BH, Guyton AC, Langston JB, Bishop VS. Hypertension Caused by Salt Loading. Ii. Fluid Volume and Tissue Pressure Changes. Am J Physiol. 1964;207:669–671. doi: 10.1152/ajplegacy.1964.207.3.669. [DOI] [PubMed] [Google Scholar]
- 53.Hall JE, Guyton AC, Brands MW. Pressure-volume regulation in hypertension. Kidney Int Suppl. 1996;55:S35–S41. [PubMed] [Google Scholar]
- 54.Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41:625–633. doi: 10.1161/01.HYP.0000052314.95497.78. [DOI] [PubMed] [Google Scholar]
- 55.Hall JE, Granger JP, do Carmo JM, da Silva AA, et al. Hypertension: physiology and pathophysiology. Compr Physiol. 2012;2:2393–2442. doi: 10.1002/cphy.c110058. [DOI] [PubMed] [Google Scholar]
- 56.Hester RL, Brown AJ, Husband L, Iliescu R, et al. HumMod: A Modeling Environment for the Simulation of Integrative Human Physiology. Front Physiol. 2011;2:12. doi: 10.3389/fphys.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kurtz TW, Dominiczak AF, DiCarlo SE, Pravenec M, et al. Molecular based mechanisms of Mendelian forms of salt-dependent hypertension: Questioning the prevailing theory. Hypertension. 2015;65:932–941. doi: 10.1161/HYPERTENSIONAHA.114.05092. [DOI] [PubMed] [Google Scholar]
- 58.Hall JE, Guyton AC, Smith MJ, Jr, Coleman TG. Blood pressure and renal function during chronic changes in sodium intake: role of angiotensin. Am J Physiol. 1980;239:F271–F280. doi: 10.1152/ajprenal.1980.239.3.F271. [DOI] [PubMed] [Google Scholar]
- 59.Bech JN, Nielsen CB, Ivarsen P, Jensen KT, et al. Dietary sodium affects systemic and renal hemodynamic response to NO inhibition in healthy humans. Am J Physiol. 1998;274:F914–F923. doi: 10.1152/ajprenal.1998.274.5.F914. [DOI] [PubMed] [Google Scholar]
- 60.Kusche-Vihrog K, Jeggle P, Oberleithner H. The role of ENaC in vascular endothelium. Pflugers Arch. 2014;466:851–859. doi: 10.1007/s00424-013-1356-3. [DOI] [PubMed] [Google Scholar]
- 61.Olde Engberink RH, Rorije NM, Homan van der Heide JJ, van den Born BJ, et al. Role of the vascular wall in sodium homeostasis and salt sensitivity. J Am Soc Nephrol. 2015;26:777–783. doi: 10.1681/ASN.2014050430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Helle F, Karlsen TV, Tenstad O, Titze J, et al. High-salt diet increases hormonal sensitivity in skin pre-capillary resistance vessels. Acta Physiol (Oxf) 2013;207:577–581. doi: 10.1111/apha.12049. [DOI] [PubMed] [Google Scholar]
- 63.Johnson RS, Titze J, Weller R. Cutaneous control of blood pressure. Curr Opin Nephrol Hypertens. 2016;25:11–15. doi: 10.1097/MNH.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Langston JB, Guyton AC, Douglas BH, Dorsett PE. Effect of changes in salt intake on arterial pressure and renal function in partially nephrectomized dogs. Circ Res. 1963;12:508–513. [Google Scholar]
- 65.Coleman TG, Guyton AC. Hypertension caused by salt loading in the dog. 3. Onset transients of cardiac output and other circulatory variables. Circ Res. 1969;25:153–160. doi: 10.1161/01.res.25.2.153. [DOI] [PubMed] [Google Scholar]
- 66.Kawasaki T, Delea CS, Bartter FC, Smith H. The effect of high-sodium and low-sodium intakes on blood pressure and other related variables in human subjects with idiopathic hypertension. Am J Med. 1978;64:193–198. doi: 10.1016/0002-9343(78)90045-1. [DOI] [PubMed] [Google Scholar]
- 67.Fujita T, Henry WL, Bartter FC, Lake CR, et al. Factors influencing blood pressure in salt-sensitive patients with hypertension. Am J Med. 1980;80:234. doi: 10.1016/0002-9343(80)90002-9. [DOI] [PubMed] [Google Scholar]
- 68.Weinberger MH, Stegner JE, Fineberg NS. A comparison of two tests for the assessment of blood pressure responses to sodium. Am J Hypertens. 1993;6:179–184. [PubMed] [Google Scholar]
- 69.Campese VM, Romoff MS, Levitan D, Saglikes Y, et al. Abnormal relationship between sodium intake and sympathetic nervous system activity in salt-sensitive patients with essential hypertension. Kidney Int. 1982;21:371–378. doi: 10.1038/ki.1982.32. [DOI] [PubMed] [Google Scholar]
- 70.Leenen FH. The central role of the brain aldosterone-"ouabain" pathway in salt-sensitive hypertension. Biochim Biophys Acta. 2010;1802:1132–1139. doi: 10.1016/j.bbadis.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 71.Beard DA, Pettersen KH, Carlson BE, Omholt SW, et al. A computational analysis of the long-term regulation of arterial pressure. F1000Res. 2013;2:208. doi: 10.12688/f1000research.2-208.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Averina VA, Othmer HG, Fink GD, Osborn JW. A new conceptual paradigm for the haemodynamics of salt-sensitive hypertension: a mathematical modelling approach. J Physiol. 2012;590:5975–5992. doi: 10.1113/jphysiol.2012.228619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Averina VA, Othmer HG, Fink GD, Osborn JW. A mathematical model of salt-sensitive hypertension: the neurogenic hypothesis. J Physiol. 2015;593:3065–3075. doi: 10.1113/jphysiol.2014.278317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blaustein MP, Leenen FH, Chen L, Golovina VA, et al. How NaCl raises blood pressure: a new paradigm for the pathogenesis of salt-dependent hypertension. Am J Physiol Heart Circ Physiol. 2012;302:H1031–H1049. doi: 10.1152/ajpheart.00899.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]