Abstract
The apolipoprotein E (apoE) is a classic example of a gene exhibiting pleiotropism. We examine potential pleiotropic associations of the apoE2 allele in three biodemographic cohorts of long-living individuals, offspring, and spouses from the Long Life Family Study, and intermediate mechanisms, which can link this allele with age-related phenotypes. We focused on age-related macular degeneration, bronchitis, asthma, pneumonia, stroke, creatinine, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol, diseases of heart (HD), cancer, and survival. Our analysis detected favorable associations of the ε2 allele with lower LDL-C levels, lower risks of HD, and better survival. The ε2 allele was associated with LDL-C in each gender and biodemographic cohort, including long-living individuals, offspring, and spouses, resulting in highly significant association in the entire sample (beta=-7.1, p=6.6×10-44). This allele was significantly associated with HD in long-living individuals and offspring (relative risk [RR]=0.60, p=3.1×10-6) but this association was not mediated by LDL-C. The protective effect on survival was specific for long-living women but it was not explained by LDL-C and HD in the adjusted model (RR=0.70, p=2.1×10-2). These results show that ε2 allele may favorably influence LDL-C, HD, and survival through three mechanisms. Two of them (HD- and survival-related) are pronounced in the long-living parents and their offspring; the survival-related mechanism is also sensitive to gender. The LDL-C-related mechanism appears to be independent of these factors. Insights into mechanisms linking ε2 allele with age-related phenotypes given biodemographic structure of the population studied may benefit translation of genetic discoveries to health care and personalized medicine.
Keywords: apoE, health span, life span
Introduction
Insights into genetic susceptibility to age-related processes and health traits could be instructive in translational strategies on extending health span and life span (Olshansky et al. 2007; Sierra et al. 2008). Particularly, an attractive idea is to find genes, which could influence not just one health trait, but a major subset of them, and, eventually, life span (Franco et al. 2009; Martin et al. 2007). It is increasingly recognized, however, that genetic influences on complex traits, which are characteristic of post-reproductive age in modern societies, are not straightforward; the complexity is, in large part, due to the elusive role of evolution in these traits (Corella and Ordovas 2014; Kulminski 2013; Vijg and Suh 2005). For example, geriatric diseases are a relatively new phenomenon from the evolutionary viewpoint. This is because of a very modest life span of even our recent predecessors for whom, for example, world record of mean life span in 1840 was about 45 years (Oeppen and Vaupel 2002).
The problem of genetic influences on age-related human traits is complicated by at least two relevant factors. The first is the complexity of gene functions in humans, which has evolved over years to maximize fitness of individuals in different environments. In part, the problem of redundancy can be simplified by focusing on genetically more homogeneous intermediate phenotypes, often called endophenotypes (Matteini et al. 2010; Yashin et al. 2010). The second factor is the elusive role of evolution in these traits. This factor implies that “diseases are not shaped by selection,” (Nesse et al. 2012), i.e., evolution did not fix the molecular basis of age-related disease traits. As a result, genetic influences on these traits can be sensitive to individuals' life course and to compositional changes in populations which often are referred to as biodemographic processes (Vaupel 2010). These two factors may interact because endogenous exposures can influence gene functions related to fitness and diseases. As a result, one should expect complex mechanisms linking genes with age-related traits, which may result in various modes of pleiotropy. In this regard, mechanisms are considered in broad sense encompassing different levels of organization starting from the micro level (e.g., genes) and ending at the macro level (e.g., phenotypic expression in specific biodemographic groups).
The apolipoprotein E (apoE) is a classic example of a pleiotropic gene, which participates in many cellular functions such as plasma lipoprotein metabolism, oxidative processes, inflammation, macrophage, glial cell and neuronal cell homeostasis, adrenal function, central nervous system physiology (Mahley and Rall 2000). While participating in these critical processes, common apoE alleles (ε2, ε3, and ε4) can contribute significantly to the pathobiology of aging and age-related traits but this contribution can be complicated by biodemographic factors discussed above (Kulminski et al. 2014; Kulminski et al. 2013).
Human epidemiological and longitudinal studies suggest that the apoE alleles affect age-related traits (Burt et al. 2008; Mahley et al. 2009; Suri et al. 2013). For example, studies suggest that the apoE2 allele might be protective against cardiovascular diseases (CVD) (Song et al. 2004), Alzheimer disease (AD) (Corder et al. 1994), and mortality (Mahley and Rall 2000). In contrast, the apoE4 allele can confer risk of Alzheimer disease (Corder et al. 1993) and decrease survival chances (Christensen et al. 2006; Kulminski et al. 2014).
The analyses of pleiotropy and mechanisms linking genes with age-related traits is important given the goal of translation of genetic discoveries into health care. Gaining insights into the connections between genes and age-related traits could help in determining population groups who are at risk for these traits. This could help us understand factors and mechanisms mediated by genes and endophenotypes and tailor disease prevention strategies and novel therapeutic approaches.
In this study, we use information on 4,659 genotyped participants of the Long Life Family Study (LLFS), which includes cohorts of long-living parents, their offspring, and spouses of parents and offspring, to address two interlinked questions. First, we investigate whether the apoE2 allele can show pleiotropic effects given a broad array of age-related phenotypes measured in the same study (see Methods). Second, we investigate potential role of endophenotypes in connections between the ε2 allele and downstream phenotypes in different cohorts of the LLFS population.
Methods
Data
The LLFS collected data at four field centers (three in the U.S. and one in Denmark) on families showing exceptional familial longevity. The study eligibility criteria has been described elsewhere (Pedersen et al. 2006; Sebastiani et al. 2009; Yashin et al. 2010). Briefly, in the U.S., the families eligible for the LLFS must have two living siblings aged 80+ years, two living offspring of one or more of the siblings, and a living spouse of one of the offspring. In addition, the family must demonstrate exceptional longevity based on a Family Longevity Selection Score, which is a summary-measure based on the survival experience of the oldest living generation of siblings relative to what would be expected based on birth cohort life tables (Sebastiani et al. 2009).
In Denmark, individuals who would be aged 90+ years during the study recruitment period were first identified in the Danish National Register of Persons (Pedersen et al. 2006). Then, using information on the place of birth and the names, parish registers available in regional archives were searched to locate the parents of the elderly individuals in order to identify sibships. The identified subjects were contacted to further assess the family's eligibility for participation in the LLFS using criteria parallel to that used in the U.S.
Information from the 4954 U.S. and Danish LLFS participants was collected using similar questionnaires and in-home physical examinations at baseline between 2006 and 2009. Information on onsets of diseases was assessed retrospectively at baseline from self-reports and prospectively during ongoing follow up (available currently through 2014). Biospecimens were collected at baseline. Genotyping of the apoE polymorphism was conducted using procedure detailed elsewhere (Schupf et al. 2013). The data include information on the apoE ε2/ε3/ε4 polymorphism for the 4,659 LLFS participants.
Biodemographic structure of the LLFS
LLFS includes long-living (LL) individuals (N=1,384, probands and siblings), their offspring (N=2,321), and 954 spouses of long-living individuals (N=177) and spouses of offspring (N=777). The long-living individuals (mean age and standard deviation are 90.3±6.5 years) were selected based on a history of familial longevity (see the above section). They were also subject to strong survival selection given their old age. Their offsprings were also selected based on a history of familial longevity. Unlike LL individuals and their offspring, however, the spouses were not selected based on a history of familial longevity. Given about the same young-old age of the offspring (60.5±8.2 years) and spouses (65.0±12.1 years), these cohorts were under about the same weak survival selection. Thus, these three groups represent three biodemographic cohorts with potentially different survival chances.
Genotypes
Genotyping of the APOE alleles was conducted at the Biomedical Genomics Center at the University of Minnesota using procedures detailed elsewhere (Schupf et al. 2013). In brief, genotyping of APOE was based on SNPs rs7412 and rs429358 using the Taqman genotyping platform and concordance among genotypes for both SNPs was 100% based on blinded duplicates. We characterize the effects of the apoE2 allele (defined as ε2/ε2, ε2/ε3, or ε2/ε4 genotypes) on a number of traits contrasted by common homozygous genotype ε3/ε3. Distribution of six genotypes of the apoE locus in the entire sample of the LLFS participants as well in subsamples of the LL individuals, offspring, and spouses is given in Supplementary Table 1.
Phenotypes and endophenotypes
The focus of the study was on selected phenotypes (outcomes) and endophenotypes (intermediate phenotypes) including age-related macular degeneration (AMD), bronchitis, asthma, pneumonia, stroke, creatinine, LDL-C, high-density lipoprotein cholesterol (HDL-C), diseases of heart (HD), cancer, and death. HD included coronary heart disease, atrial fibrillation, heart failure, coronary angioplasty, or coronary artery bypass. Cancer included all sites. These traits could be sensitive to the ε2 allele according to prior research (more details are given in the Discussion section). Depending on the analyses used in the study, the same biomarker/disease could be an endophenotype or a phenotype. For example, lipids were outcomes in the analyses of their associations with the ε2 allele but they were endophenotypes in the analyses of the associations of this allele with diseases. Likewise, diseases were endophenotype in the analyses of the associations with risks of death.
HDL-C was measured directly in serum using the Roche HDL-Cholesterol 3rd generation direct method (Roche Diagnostics, Indianapolis, IN 46250) on a Roche Modular P Chemistry Analyzer (Roche Diagnostics Corporation). LDL-cholesterol was calculated by the Friedewald equation Creatinine was measured in serum, EDTA plasma or urine by the Roche enzymatic method (Roche Diagnostics, Indianapolis, IN 46250) on a Roche Modular P Chemistry Analyzer (Roche Diagnostics Corporation). Information on diseases was self-reported.
ApoE-genotype-specific proportions of diseases, deaths, and mean levels of physiological markers are given in Supplementary Table 1.
Analysis
Associations of the ε2 allele with quantitative outcomes were characterized by a mixed effects linear regression model with adjustment for potential familial clustering. Measurements of lipids and creatinine were log transformed to offset potential bias due to skewness of their frequency distributions. We used information on fasting (fasted less than 8 hours or 8+ hours) and lipid-lowering treatment (taken lipid-lowering drugs or not) to investigate whether these conditions modulated the associations. Genotype-specific proportions of these factors are given in Supplementary Table 1.
The risks of the selected diseases and death for carriers of the ε2 allele compared with carriers of the ε3/ε3 common genotype were evaluated using the Cox proportional hazard regression model. Information on both prospective and retrospective onsets of diseases collected in the LLFS was used in these analyses. The use of retrospective onsets in a failure-type model is justified in (Prentice and Breslow 1978). These analyses provide estimates of the effects in a given population. The time variable in the Cox regression analyses was the age at onset of a trait or the age at right censoring. We used robust sandwich estimator of variances in the Cox model to adjust for familial clustering.
The analyses were conducted for men and women separately. Given differences in proportions of carriers of the apoE genotypes across family groups (i.e., LL, offspring, and spouses, see Supplementary Table 1), the analyses were conducted in each family group separately, when applicable. Because of small number of cases of AMD, asthma, bronchitis, and stroke for carriers of the ε2 allele (Supplementary Table 1), the analyses with these diseases were conducted using data for all family groups pooled together.
We evaluated potential mediating role of endophenotypes (e.g., LDL-C) in the associations of the ε2 allele with phenotypes (e.g., diseases of heart) by adjusting the models by the endophenotypes.
All statistical tests were adjusted for field centers and birth cohorts measured by age at baseline. Other adjustments were explicitly stated in the text, when applicable. Statistical analyses were conducted using SAS (release 9.3, Cary, NC, USA).
Results
The following sections provide the associations of the ε2 allele with selected biomarkers as well as with the risks of diseases and mortality highlighting significance of these associations and mediating role of endophenotypes in the downstream phenotypes. We considered: (i) lipids (LDL-C and HDL-C) and lipid-lowering therapy as endophenotypes for diseases of heart and (ii) lipids, lipid-lowering therapy and diseases as endophenotypes for mortality.
Associations of the ε2 allele with diseases of heart
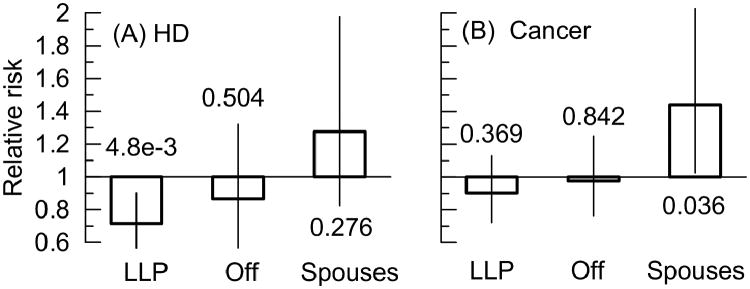
For diseases of heart, we observed protective effects of the ε2 allele in long-living men and women (see Supplementary Table 2). Upon pooling data for men and women, this protective effect in the parents' generation became highly significant (Figure 1A).
Figure 1. Associations of the ε2 allele with the risks of (A) diseases of the heart (HD) and (B) cancer.
Bars show relative risks for men and women combined in each family group. LLP denotes long-living parents. “Off” denotes offspring. Vertical lines show 95% confidence intervals. Numbers show p-values. Other details and numerical estimates are given in Supplementary Table 2.
Associations of the ε2 allele with lipids
In studies of genetic associations with lipids, it was often assumed that fasting and lipid-lowering therapy might modulate genetic associations and, therefore, the analyses should be corrected for these confounding factors a priori. For example, this may be because lipid-lowering therapy may lower total cholesterol and LDL-C levels. However, while these factors might indeed be associated with lipid levels, it was actually unknown whether associations of specific genetic variants with lipids were modulated by fasting and lipid-lowering therapy. Accordingly, we first verified whether fasting and lipid-lowering therapy may modulate associations of the ε2 allele with lipids in the LLFS data.
Our analyses showed that fasting made no difference in the associations of the ε2 allele with LDL-C and high-density lipoprotein cholesterol (HDL-C) levels (Supplementary Table 3). Accordingly, fasting status was disregarded in further analyses. Lipid-lowering therapy, however, did affect the associations of the ε2 allele with LDL-C but not with HDL-C (Supplementary Table 4). For consistency, lipid-lowering therapy was retained in the model.
Table 1 showed highly significant protective associations of the ε2 allele with LDL-C showing the carriers of the ε2 allele having lower LDL-C levels. The effect sizes were remarkably consistent in each family group with somewhat larger absolute sizes in women. Overall, the association of the ε2 allele with LDL-C in the entire LLFS sample was highly significant, beta=-7.09, p=6.6×10-44.
Table 1.
The associations of the ε2 allele with lipids.
| Outcome | Sex | Family group | N | Beta* | SE | p-value |
|---|---|---|---|---|---|---|
| LDL-C | Men | LLP | 557 | -6.89 | 1.28 | 2.4E-07 |
| Offspring | 758 | -6.36 | 1.00 | 4.6E-10 | ||
| Spouses | 333 | -6.49 | 1.56 | 5.9E-05 | ||
| Women | LLP | 622 | -8.14 | 1.38 | 1.2E-08 | |
| Offspring | 1064 | -6.97 | 0.88 | 1.5E-14 | ||
| Spouses | 380 | -7.42 | 1.65 | 1.4E-05 | ||
| M&W | All | 3714 | -7.09 | 0.50 | 6.6E-44 | |
| HDL-C | Men | LLP | 559 | 2.46 | 1.24 | 4.8E-02 |
| Offspring | 772 | 0.89 | 1.05 | 3.9E-01 | ||
| Spouses | 340 | -2.84 | 1.56 | 7.1E-02 | ||
| Women | LLP | 625 | -1.73 | 1.19 | 1.5E-01 | |
| Offspring | 1068 | 0.00 | 0.85 | 1.0E+00 | ||
| Spouses | 381 | 1.73 | 1.54 | 2.6E-01 | ||
| M&W | All | 3745 | 0.07 | 0.47 | 8.8E-01 |
Beta is the estimate of the associations of the ε2 allele with lipids in mixed effects regression model; SE denotes standard error; N denotes sample size. Lipids were represented by low density lipoprotein cholesterol (LDL-C, mg/dL) and high-density lipoprotein cholesterol (HDL-C, mg/dL).
The effect size beta is evaluated for 100×log10(lipids).
The model was adjusted for field centers, birth cohorts, and lipid-lowering therapy, as well as for sex and family groups, when applicable.
M&W is men and women; “All” denotes all family groups combined; LLP denotes long living parents.
Although the associations of the ε2 allele with HDL-C attained conventional and suggestive-effect significance in some family groups, these (and the other non-significant) effects showed opposite trends in few other family groups. Superposition of these antagonistic effects results in negligible effect of the ε2 allele on HDL-C in the entire LLFS population.
The role of lipids in the association of the ε2 allele with diseases of heart
Here we examined whether or not the protective associations of the ε2 allele with LDL-C (Table 1) mediated protective association of this allele with HD (Figure 1A). This analysis helps in clarifying whether or not LDL-C may be in a causal pathway linking the ε2 allele with HD.
Table 2 showed that adjustment of the model for HD by LDL-C amplified protective effects between the ε2 allele and risks of HD in exceptional populations of the long-living parents and their offspring whereas this adjustment weakened the detrimental effect in a population of spouses. These results implied that LDL-C did not mediate the protective effect between the ε2 allele and HD in the exceptional populations whereas in spouses, LDL-C partly mediated (explained) the detrimental effect between the ε2 allele and HD.
Table 2.
Associations of the ε2 allele with the risks of diseases of the heart: the role of LDL-C.
| Sex | Family group | Ntotal | Nevent | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | p-value | RR | 95% CI | p-value | ||||
| Men | LLP | 550 | 267 | 0.75 | 0.55-1.01 | 5.9E-02 | 0.61 | 0.45-0.83 | 1.5E-03 |
| Offspring | 752 | 75 | 0.69 | 0.38-1.25 | 2.2E-01 | 0.54 | 0.31-0.97 | 4.0E-02 | |
| Spouses | 331 | 59 | 1.47 | 0.82-2.62 | 2.0E-01 | 1.32 | 0.73-2.41 | 3.6E-01 | |
| LLP+offspring | 1302 | 342 | 0.72 | 0.55-0.94 | 1.5E-02 | 0.58 | 0.45-0.76 | 7.8E-05 | |
| Women | LLP | 597 | 213 | 0.70 | 0.48-1.03 | 7.2E-02 | 0.59 | 0.40-0.89 | 1.1E-02 |
| Offspring | 1063 | 53 | 0.90 | 0.451.82 | 7.8E-01 | 0.79 | 0.39-1.63 | 5.3E-01 | |
| Spouses | 379 | 45 | 1.23 | 0.60-2.51 | 5.7E-01 | 1.08 | 0.53-2.19 | 8.4E-01 | |
| LLP+offspring | 1660 | 266 | 0.74 | 0.53-1.04 | 7.9E-02 | 0.63 | 0.44-0.89 | 8.7E-03 | |
| M&W | LLP | 1147 | 480 | 0.73 | 0.57-0.92 | 7.5E-03 | 0.60 | 0.47-0.76 | 3.5E-05 |
| M&W | Offspring | 1815 | 128 | 0.78 | 0.50-1.22 | 2.7E-01 | 0.63 | 0.40-1.00 | 4.7E-02 |
| M&W | Spouses | 710 | 104 | 1.31 | 0.84-2.06 | 2.4E-01 | 1.17 | 0.74-1.84 | 5.0E-01 |
| M&W | LLP+offspring | 2962 | 608 | 0.73 | 0.59-0.90 | 2.9E-03 | 0.60 | 0.49-0.74 | 3.1E-06 |
RR is the relative risk evaluated using the Cox proportional hazard regression model.
CI denotes confidence interval.
LLP denotes long living parents.
Model 1: basic adjustment (field center, birth cohorts, as well as sex and family groups, when applicable).
Model 2: basic adjustment + LDL-C.
The results of Model 1 are not the same as in Figure 1A because individuals with missing information on LDL-C (3.5%) were excluded from these analyses.
Ntotal is the sample size; Nevent is the number of events.
Thus, Table 2 demonstrates that: (i) the protective effects of the ε2 allele were more characteristic for the exceptional population of long-living individuals and their offsprings than for spouses and (ii) different mechanisms may link the ε2 allele with HD in general and exceptional populations. Then, pooling data for the long-living individuals and their offspring, we saw an increase in the protective effect of the ε2 allele by about 22% from RR=0.73 (p=2.9×10-3) to RR=0.60 (p=3.1×10-6) in this population (Table 2). Overall, this estimate implied that exceptional survivors carrying the ε2 allele had about 67% lower risk of having HD compared to carriers of a common ε3/ε3 genotype at comparable levels of LDL-C.
The role of lipid-lowering therapy in associations of the ε2 allele with risks of HD
Table 3 showed that lipid-lowering therapy mediates small proportion of the protective association of the ε2 allele with HD in long-living parents (5%) and a larger proportion in their offspring (14%) and in spouses (17%). This therapy explained a larger proportion of the protective effects in men (12%) than in women (3%) from the exceptional populations. Overall, drugs mediated about 8% of the protective association of the ε2 allele with HD in these exceptional populations of men and women combined. The difference in the effects remained, though non-significant as evidenced by overlapping confidence intervals.
Table 3.
Associations of the ε2 allele with the risks of HD: the role of lipid-lowering therapy.
| Sex | Family group | Ntotal | Nevent | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | p-value | RR | 95% CI | p-value | ||||
| M&W | LLP | 1147 | 480 | 0.60 | 0.47-0.76 | 3.5E-05 | 0.63 | 0.49-0.81 | 2.3E-04 |
| M&W | Offspring | 1815 | 128 | 0.63 | 0.40-1.00 | 4.7E-02 | 0.72 | 0.46-1.14 | 1.6E-01 |
| M&W | Spouses | 710 | 104 | 1.17 | 0.74-1.84 | 5.0E-01 | 1.37 | 0.85-2.20 | 1.9E-01 |
| Men | LLP+offspring | 1302 | 342 | 0.58 | 0.45-0.76 | 7.8E-05 | 0.65 | 0.49-0.85 | 1.7E-03 |
| Women | LLP+offspring | 1660 | 266 | 0.63 | 0.44-0.89 | 8.7E-03 | 0.65 | 0.46-0.93 | 1.7E-02 |
| M&W | LLP+offspring | 2962 | 608 | 0.60 | 0.49-0.74 | 3.1E-06 | 0.65 | 0.52-0.80 | 8.6E-05 |
RR is the relative risk evaluated using the Cox proportional hazard regression model.
CI denotes confidence interval.
LLP denotes long living parents; M&W denotes men and women.
Model 1: basic adjustment (field centers, birth cohorts, as well as sex and family groups, when applicable) + LDL-C.
Model 2: basic adjustment + LDL-C + lipid-lowering therapy.
Ntotal is the sample size; Nevent is the number of events.
Associations of the ε2 allele with the risks of death and the role of endophenotypes
Protective effects of the ε2 allele on survival were observed in male offspring, male spouses, long-living women, and female spouses (Supplementary Table 5). However, marginal significance was attained only in a relatively small sample size of male spouses (RR=0.33, p=0.064).
Having observed strong protective effects of the ε2 allele on LDL-C (Table 1) and HD (Table 2), as well as modulating role of lipid-lowering drugs (Table 3), we examined whether or not these factors could modulate the associations of the ε2 allele with risks of deaths. Table 4 showed that LDL-C (Model 2) played an important modulating role improving the protective association of the ε2 allele with risks of death in long-living women. This association attained conventional significance largely due to improving the effect size by about 17% from RR=0.83 (p=0.208) to RR=0.71 (p=0.022). Protective effect was also improved in male spouses (Table 4).
Table 4.
Associations of the ε2 allele with the risks of death: the role of endophenotypes.
| Sex | Family | Ntotal | Nevent | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | RR | 95% CI | p-value | RR | 95% CI | p-value | RR | 95% CI | p-value | |||
| M | LLP | 556 | 301 | 1.21 | 0.93-1.59 | 0.163 | 1.14 | 0.87-1.48 | 0.355 | 1.10 | 0.84-1.44 | 0.492 |
| Off | 758 | 31 | 0.87 | 0.39-1.94 | 0.732 | 0.95 | 0.44-2.05 | 0.895 | 1.06 | 0.48-2.31 | 0.893 | |
| Spouses | 333 | 29 | 0.29 | 0.05-1.58 | 0.153 | 0.22 | 0.04-1.19 | 0.079 | 0.17 | 0.03-1.10 | 0.062 | |
| W | LLP | 622 | 318 | 0.83 | 0.63-1.11 | 0.208 | 0.71 | 0.52-0.95 | 0.022 | 0.70 | 0.52-0.95 | 0.021 |
| Off | 1064 | 29 | 1.15 | 0.46-2.89 | 0.764 | 1.21 | 0.49-3.00 | 0.688 | 1.19 | 0.47-2.96 | 0.716 | |
| Spouses | 380 | 27 | 1.01 | 0.39-2.58 | 0.989 | 1.00 | 0.40-2.48 | 0.994 | 0.83 | 0.33-2.08 | 0.686 | |
RR is the relative risk evaluated using the Cox proportional hazard regression model.
CI denotes confidence interval.
LLP denotes long living parents; “Off” denotes offspring; M denotes men; W denotes women.
Model 1: basic adjustment (field center and birth cohorts);
Model 2: basic adjustment + LDL-C;
Model 3: basic adjustment + LDL-C + diseases of heart + lipid-lowering therapy.
The results of the Model 1 are not the same as in Supplementary Table 5 because individuals with missing information on LDL-C (3.5%) were excluded from these analyses.
Ntotal is the sample size; Nevent is the number of events.
HD and lipid-lowering therapy showed minor effect on the associations of the ε2 allele with risks of death (Table 4, compare Models 2 and 3). Other diseases used in the analyses did not affect the estimates (Supplementary Table 6).
Associations of the ε2 allele with cancer
Interestingly, the ε2 allele was found to be adversely associated with risks of cancer in male and female spouses (Supplementary Table 2). Pooling data for men and women, detrimental effect in spouses attained conventional significance (Figure 1B). Figure 1 shows that protective effects of the ε2 allele (regardless of their significance) against cancer and HD were consistently observed in exceptional population of long living parents and their offspring. Detrimental effects (again, regardless of their significance) were also consistently seen in the spouses.
Moderate associations of the ε2 allele with pneumonia, stroke, and creatinine levels
Marginally significant protective association with risk of stroke was observed in men (RR=0.55, p=0.055) but not in women (RR=1.31, p=0.29) (Supplementary Table 7).
The risks of pneumonia for carriers of the ε2 allele were mixed across sexes and family groups (Supplementary Table 7). Detrimental suggestive-effect association (RR=1.53, p=0.088) was observed in male spouses. Trend on protective effect of the ε2 allele was observed in long living men (RR=0.77, p=0.16).
Similarly, the associations of the ε2 allele with creatinine levels were mixed across sexes and family groups (Supplementary Table 8). The ε2 allele seemed to be associated with decreased creatinine levels in male spouses (beta=-2.09, p=0.031) and, in female offspring (beta=-1.06, p=0.058).
Non-significant associations of the ε2 allele with AMD, asthma, and bronchitis
The analyses did not reveal significant associations of the ε2 allele with the risks of age-related macular degeneration (AMD), asthma, or bronchitis either for males or for females (Supplementary Table 7). The lack of significance could be a result of the sample size due to small number of disease cases.
Discussion
Allelic variations at the apoE locus have been known to influence various age-related traits such as AD, hyperlipidemia, cardiovascular disease, hemorrhagic stroke and survival. Studies in the recent past have indicated that the apoE2 allele might be protective for survival, associated with decreased levels of serum cholesterol, lower risk of cardiovascular diseases, and has neuroprotective effects against AD, infectious diseases, gastric cancer and other age-related disorders. Prior studies have also provided evidence that the apoE2 allele possesses anti-inflammatory and anti-oxidant properties and involved in DNA damage response and repair. Despite the evidence for a strong association of ApoE2 with health span and longevity, fundamental hurdles to the translation of ApoE2 into novel therapeutics include the lack of information on genotype-phenotype relationships and the poor understanding of the functional pathways involved in mediating the protective effects.
In light of these observations, we undertook the current study on the LLFS cohorts to examine two interlinked questions. First, we investigated potential roles of the apoE2 allele in an array of complex age-related traits including AMD, bronchitis, asthma, pneumonia, stroke, creatinine, LDL-C, HDL-C, HD, cancer, and survival. These traits were selected based on published reports on their association with the apoE polymorphism as discussed in sections below. Second, we examined potential mechanisms linking the ε2 allele, endophenotypes, and downstream phenotypes by analyzing modulating role of endophenotypes in the associations of the ε2 allele with phenotypes considering the biodemographic structure of the LLFS population. This structure reflects the study design in the selection of the LLFS participants (see more details in Methods section on “Biodemographic structure of the LLFS”.
No significant effects of the ε2 allele on AMD, asthma, or bronchitis
AMD
Previously, the e4 allele was reported to have a significant association with a decreased risk of AMD and the ε2 allele was reported to be possibly associated with an increased risk of AMD (de Jong 2006; Kulminski et al. 2008; Thakkinstian et al. 2006). Some studies report, however, suggestive-effect protective associations (Klaver et al. 1998) whereas the other show non-significant effect of the ε2 allele (Schultz et al. 2003).
Our analyses show no significant associations of the ε2 allele with AMD in the LLFS. Small number of AMD cases for the ε2 carriers does not allow us to examine whether or not the lack of the detrimental effect of the ε2 allele on AMD could be due to exceptional nature of the LLFS population.
Asthma and bronchitis
The apoE gene has been hypothesized to play a role in asthma and bronchitis based on studies on humanized apoE mice. (Yao et al. 2012). huApoE alleles differentially modified key pathogenic responses to nasal repeated house dust mite challenges, which can be stratified, in rank order of increasing disease severity, ε3<ε4<ε2. Compared with muApoE, the huApoE3 allele confers a protective phenotype with reduced airway hyperreactivity, mucous cell metaplasia, and airway inflammation, whereas the huApoE4 allele has an intermediate asthma phenotype with selectively attenuated AHR and serum IgE production.
However, the results of our analyses do not support connection of the ε2 allele with bronchitis and asthma in the LLFS participants. Again, small number of the disease cases prevents gaining insights on the role of exceptionality of the LLFS population.
The ε2 allele and pneumonia, stroke, and creatinine levels
Pneumonia
There is evidence that apoE could play a role in reducing susceptibility to three categories of infectious agents: viruses, bacteria, and protozoan parasites (Mahley and Rall 2000). Furthermore, the apoE gene may influence both innate and acquired immunity in a mouse model (Roselaar and Daugherty 1998). The susceptibility of apoE knock-out mice to Klebsiella pneumoniae and lowered inhibition of pro-inflammatory cytokine response has also been demonstrated (de Bont et al. 1999).
Our analyses suggest that, potentially, the ε2 allele can influence the risks of pneumonia in the LLFS participants. However, inconsistent effect directions in the LLFS cohorts suggest that these effects can be sensitive to such biodemographic processes as survival selection with stronger sensitivity in men.
Stroke
A meta-analysis of the associations of the apoE genotypes with stroke showed that the ε2/ε3 genotype was associated with reduced risks (Khan et al. 2013). Our analyses support this protective association but only for men carrying the ε2 allele.
Creatinine
ApoE exerts a major role in the pathogenesis and the progression of a variety of renal diseases, as well as in the atherosclerotic complications associated with them. Chronic kidney disease (CKD) as measured by estimated glomerular filtration rate (eGFR) using creatinine and cystatin is known to affect more than 30% of older adults (Coresh et al. 2007). It is believed that development and progression of CKD is multifactorial and contributed by genetic variation, diabetes, hypertension, elevated triglycerides and low HDL-C (Hunsicker et al. 1997). There is still controversy on the effects of apoE alleles in renal diseases. Some studies have provided evidence that apoE2 allele carries a genetic risk for renal diseases whereas others have shown that the apoE4 allele is a risk factor for the progression of renal failure (Araki et al. 2003; Eto et al. 1995; Kimura et al. 1998; Oda et al. 1999). The ε4 allele is found to be associated with a lower risk of CKD in adjusted and unadjusted analyses using creatinine as biomarker (Seshasai et al. 2012). Liberopoulos et al. (Liberopoulos et al. 2004) from their studies on healthy individuals reported that individuals carrying apoE2 allele have increased levels of serum creatinine and reduced GFR compared with both ε3 and ε4 subjects.
Our results provide mixed evidence of the associations of the ε2 allele with creatinine levels across different LLFS cohorts. Significant and marginally significant associations were observed for male spouses and female offspring, respectively, meaning that the ε2 allele is associated with lower creatinine levels. These associations could be interpreted as ε2 being a favorable protective variant for kidney diseases in the LLFS cohort.
Trade-offs in the effects of the ε2 allele on HD and cancer
Diseases of heart
Two major meta-analyses of the associations of apoE with HD show trends towards a protective role of the ε2 allele (Song et al. 2004; Wilson et al. 1996). The results of our analyses, which did not focus on modulating role of endophenotypes, show that the ε2 allele can be protective against HD. A novel important insight was that this protective effect was strongly sensitive to the biodemographic factors. This means that the protective effect was pronounced in the exceptional populations of parents (RR=0.72, p=4.8×10-3 in men and women combined) and male offspring (RR=0.73, p=0.29) whereas in the LLFS spouses (who were not selected for chances of exceptional longevity) the effect of the ε2 allele was detrimental (RR=1.28, p=0.28).
Cancer
Evidence from prior studies on the associations of the apoE gene with cancer are conflicting and sparse with an unclear role of each allele (Anand et al. 2014). The presence of the ε2 allele was hypothesized previously to be a risk factor for cancer (Katan 1986). Some studies supported this hypothesis. Indeed, an increased risk of colon cancer for male carriers of the ε2/ε3 genotype compared to the ε3/ε3 genotype was, reported in (Watson et al. 2003). Other studies did not support that hypothesis. For example, no significant associations of the apoE genotypes with prostate cancer were reported in (Liu et al. 2015). Protective effect of the ε2 allele was reported against gastric cancer (De Feo et al. 2012).
Our results support a detrimental effect of the ε2 allele on cancer in men and women combined. However, this effect was characteristic for a sample of a general population represented by the LLFS spouses (Figure 1B). This finding suggests that the lack of detrimental effect of the ε2 allele on cancer in the exceptional populations can be due to health-protective genetic profiles of individuals in those populations. This protective profile can explain the observed trade-off of the effects of the ε2 allele on HD and cancer seen in a sample of spouses and the exceptional populations.
The e2 allele and lipid levels
The apoE gene is known to influence lipid metabolism (Eichner et al. 2002). The apoE protein is involved in transport of cholesterol and triglycerides in blood as very low density lipoprotein and its conversion to LDL-C (Hauser et al. 2011). The ε2 allele is associated with lower cholesterol levels in most populations (Eichner et al. 2002).
Our results support these findings by showing highly significant associations of the ε2 allele with lower LDL-C levels in different populations regardless of their biodemographic structure (in populations of men and women and in populations characterized by different survival chances, Table 1). However, the ε2 allele shows mostly non-significant population-specific effects on HDL-C.
The ε2 allele, HD, LDL-C, and lipid-lowering therapy
Of the associations with the age related diseases, the ε2 allele shows the most convincing protective associations with HD. This protective effect was, however, limited to the LLFS exceptional populations. In a population of the LLFS spouses, the ε2 allele shows a trend towards adverse association (non-significant) with HD.
The ε2 allele is also found to be strongly associated with lower LDL-C levels suggesting a protection against HD (Eichner et al. 2002). These protective associations of the ε2 allele with LDL-C and HD could imply a causal pathway from ε2 to HD through LDL-C. We show that this is not the case (Table 2). In fact, we see that the protective effect of the ε2 allele on HD strengthened by 22% (RR=0.73 to RR=0.60) and its significance improved by three order of magnitude (p=2.9×10-3 to p=3.1×10-6) in exceptional populations.
Lipid-lowering therapy tends to decrease LDL-C concentrations. Accordingly, it may contribute to causal pathways from ε2 to HD through LDL-C. Our analyses suggest that the effect of this therapy is small explaining about 8% of the protective effect between the ε2 allele and HD in the exceptional populations (Table 3). Accordingly, these results suggest that the lipid-lowering therapy is not a major protective factor against HD in carriers of the ε2 allele in the exceptional populations.
Thus, the ε2 allele is protective against HD in populations of the LLFS parents and their offspring, who were selected according to their chances of exceptional survival, whereas it can be unfavorable for HD in the LLFS spouses, who were not selected for exceptional survival.
Epidemiological studies suggest that long-living individuals can have biological basis to stay in good health and live long lives, although the nature of this basis remains unclear (Govindaraju et al. 2015). In part, good health and long lives can be due to favorable genetic factors (e.g., apoE, apoC3, FOXO3a, CETP, IGF genes). Partly, beneficial biological basis can be due to adaptation to specific environments (e.g., centenarians are found at a greater frequency in specific locations called “blue zones” (Poulain et al. 2013)). The finding of antagonistic effects of the ε2 allele in populations of individuals, who were selected according to different survival chances (i.e., long-living individuals and their offspring vs. spouses) but shared the same environment, suggests that the LLFS long-living individuals and their offspring may have beneficial genetic profile (complementing the apoE2 allele) helping them in enhancing their health span (life span free of HD in the present study). Weak sensitivity of beneficial effects of the ε2 allele to lipid-lowering therapy along with the lack of favorable effect between the ε2 allele and HD in spouses indicates that this beneficial genetic profile is either weakly sensitive to lifestyle and environmental factors or it interacts differently with these exposures (Rajpathak et al. 2011).
The ε2 allele, endophenotypes, lipid-lowering therapy, and life span
Studies show that apoE can be associated with longevity and survival. The ε4 allele is typically associated with higher mortality risks whereas the ε2 allele is considered as protective (Christensen et al. 2006). Survival is a very complex phenotype which summarizes the entire life course of individuals and absorbs a variety of endophenotypes. Accordingly, if the ε2 allele is associated with endophenotypes (HD and LDL-C in this case), then, associations with survival (if any) may or may not be mediated by the associations with these endophenotypes.
We found that the ε2 allele was not significantly associated with survival in any of the LLFS cohorts, although, protective trend was seen in long-living women, male offspring, and spouses. We found, however, that LDL-C strongly moderated the effect of the ε2 allele on survival in long-living women making this protective association to attain nominal significance. This finding suggest that, similarly to the case of HD, this allele acts on LDL-C and on survival through different mechanisms. The lack of a role of other endophenotypes (HD, cancer, stroke, asthma, AMD, bronchitis, and pneumonia) and lipid-lowering therapy in the protective association of the ε2 allele with survival implies that this association is likely mediated by a third mechanism, which is independent of the LDL-C- and HD-related mechanisms.
It should be emphasized that the LLFS is a family-based cohort study examining the genetic and non-genetic factors associated with exceptional familial longevity (see Methods). Long-lived individuals, their siblings and their offspring and spouses were recruited for an examination that characterized key intermediate phenotypes of longevity, including major chronic diseases, risk factors, and physical and cognitive function. The LLFS cohort is, therefore, a highly selected group of exceptional families and not representative of the overall general population. Thus caution is needed when interpreting and extrapolating these results to other populations as they have limited generalizability under the hypothesis of “pure genetic” component in complex traits.
Conclusions
Our analyses of the role of the apoE2 allele in age-related traits showed associations of this allele with many age-related traits revealing both protective and detrimental roles. ApoE2 was found to have no associations with AMD, bronchitis, and asthma in LLFS. It appears that the ε2 allele can influence the risks of pneumonia, but its effects may be sensitive to biodemographic factors. The ε2 allele shows protection against stroke (in men only) and is found to be associated with lower creatinine levels in some biodemographic groups. The ε2 allele shows trade-off effects with protective role against HD and detrimental role in cancer in the LLFS cohorts with different survival chances. The ε2 allele shows favorable associations with LDL-C, HD, and survival through three mechanisms. One mechanism (the LDL-C-related) is independent of biodemographic structure of the LLFS population. The other two (HD- and survival-related) mechanisms are distinctive of the LDL-C-related mechanism because they are sensitive to this structure, i.e., both of them are pronounced in the populations with chances of exceptional survival. The latter two mechanisms are further subdivided with the survival-related mechanism being gender sensitive whereas the HD-related mechanism is not. This implies that, potentially, favorable biomarker profile, longer health span, and longer life can be modulated by the same gene but in an independent manner. The latter implies that the effect of the ε2 allele could be modulated by other factors of genetic and/or non-genetic origin.
Our results indicate an important role of biodemographic factors in the associations of the apoE2 allele with most complex traits considered in this analysis. We believe that by reconciling contradictions on the metabolic effects of ε2 and focusing on the protective functions of apoE may open up new avenues for therapeutic target identification for healthy aging.
Supplementary Material
Supplementary Table 1. Basic characteristics of the 4,659 genotyped LLFS participants.
Supplementary Table 2. Associations of the ε2 allele with the risks of diseases of heart and cancer.
Supplementary Table 3. The role of fasting in the associations of the ε2 allele with lipid levels.
Supplementary Table 4. The role of lipid-lowering therapy in the associations of the ε2 allele with lipids.
Supplementary Table 5. Associations of the ε2 allele with the risks of death.
Supplementary Table 6. Associations of the ε2 allele with the risks of death: the role of diseases used in the analyses.
Supplementary Table 7. Associations of the ε2 allele with the risks of the selected diseases.
Supplementary Table 8. Associations of the ε2 allele with creatinine.
Acknowledgments
The Long Life Family Study is funded by U01AG023749, U01AG023744 and U01AG023712 from the National Institute on Aging. This work was supported by the National Institute on Aging (grant numbers U01 AG023712, P01 AG043352, R01 AG047310).
Footnotes
Author Disclosure Statement. No conflict of interests exist.
References
- Anand R, Prakash SS, Veeramanikandan R, et al. Association between apolipoprotein E genotype and cancer susceptibility: a meta-analysis. J Cancer Res Clin Oncol. 2014;140:1075–1085. doi: 10.1007/s00432-014-1634-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki S, Koya D, Makiishi T, et al. APOE polymorphism and the progression of diabetic nephropathy in Japanese subjects with type 2 diabetes: results of a prospective observational follow-up study. Diabetes Care. 2003;26:2416–2420. doi: 10.2337/diacare.26.8.2416. [DOI] [PubMed] [Google Scholar]
- Burt TD, Agan BK, Marconi VC, et al. Apolipoprotein (apo) E4 enhances HIV-1 cell entry in vitro, and the APOE epsilon4/epsilon4 genotype accelerates HIV disease progression. Proc Natl Acad Sci U S A. 2008;105:8718–8723. doi: 10.1073/pnas.0803526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K, Johnson TE, Vaupel JW. The quest for genetic determinants of human longevity: challenges and insights. Nat Rev Genet. 2006;7:436–448. doi: 10.1038/nrg1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Risch NJ, et al. Protective Effect of Apolipoprotein-E Type-2 Allele for Late-Onset Alzheimer-Disease. Nature Genetics. 1994;7:180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Corella D, Ordovas JM. Aging and cardiovascular diseases: the role of gene-diet interactions. Ageing Res Rev. 2014;18:53–73. doi: 10.1016/j.arr.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- de Bont N, Netea MG, Demacker PN, et al. Apolipoprotein E knock-out mice are highly susceptible to endotoxemia and Klebsiella pneumoniae infection. J Lipid Res. 1999;40:680–685. [PubMed] [Google Scholar]
- De Feo E, Simone B, Persiani R, et al. A case-control study on the effect of Apolipoprotein E genotypes on gastric cancer risk and progression. BMC Cancer. 2012;12:494. doi: 10.1186/1471-2407-12-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong PT. Age-related macular degeneration. N Engl J Med. 2006;355:1474–1485. doi: 10.1056/NEJMra062326. [DOI] [PubMed] [Google Scholar]
- Eichner JE, Dunn ST, Perveen G, et al. Apolipoprotein E polymorphism and cardiovascular disease: a HuGE review. Am J Epidemiol. 2002;155:487–495. doi: 10.1093/aje/155.6.487. [DOI] [PubMed] [Google Scholar]
- Eto M, Horita K, Morikawa A, et al. Increased frequency of apolipoprotein epsilon 2 allele in non-insulin dependent diabetic (NIDDM) patients with nephropathy. Clin Genet. 1995;48:288–292. doi: 10.1111/j.1399-0004.1995.tb04111.x. [DOI] [PubMed] [Google Scholar]
- Franco OH, Karnik K, Osborne G, et al. Changing course in ageing research: The healthy ageing phenotype. Maturitas. 2009;63:13–19. doi: 10.1016/j.maturitas.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Govindaraju D, Atzmon G, Barzilai N. Genetics, lifestyle and longevity: Lessons from centenarians. Appl Transl Genom. 2015;4:23–32. doi: 10.1016/j.atg.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser PS, Narayanaswami V, Ryan RO. Apolipoprotein E: from lipid transport to neurobiology. Prog Lipid Res. 2011;50:62–74. doi: 10.1016/j.plipres.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsicker LG, Adler S, Caggiula A, et al. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int. 1997;51:1908–1919. doi: 10.1038/ki.1997.260. [DOI] [PubMed] [Google Scholar]
- Katan MB. Apolipoprotein E isoforms, serum cholesterol, and cancer. Lancet. 1986;1:507–508. doi: 10.1016/s0140-6736(86)92972-7. [DOI] [PubMed] [Google Scholar]
- Khan TA, Shah T, Prieto D, et al. Apolipoprotein E genotype, cardiovas.cular biomarkers and risk of stroke: systematic review and meta-analysis of 14,015 stroke cases and pooled analysis of primary biomarker data from up to 60,883 individuals. Int J Epidemiol. 2013;42:475–492. doi: 10.1093/ije/dyt034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H, Suzuki Y, Gejyo F, et al. Apolipoprotein E4 reduces risk of diabetic nephropathy in patients with NIDDM. Am J Kidney Dis. 1998;31:666–673. doi: 10.1053/ajkd.1998.v31.pm9531184. [DOI] [PubMed] [Google Scholar]
- Klaver CC, Kliffen M, van Duijn CM, et al. Genetic association of apolipoprotein E with age-related macular degeneration. Am J Hum Genet. 1998;63:200–206. doi: 10.1086/301901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulminski AM. Unraveling genetic origin of aging-related traits: evolving concepts. Rejuvenation Res. 2013;16:304–312. doi: 10.1089/rej.2013.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulminski AM, Arbeev KG, Culminskaya I, et al. Age, gender, and cancer but not neurodegenerative and cardiovascular diseases strongly modulate systemic effect of the apolipoprotein e4 allele on lifespan. PLoS Genet. 2014;10:e1004141. doi: 10.1371/journal.pgen.1004141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulminski AM, Culminskaya I, Arbeev KG, et al. The role of lipid-related genes, aging-related processes, and environment in healthspan. Aging Cell. 2013;12:237–246. doi: 10.1111/acel.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulminski AM, Ukraintseva SV, Arbeev KG, et al. Health-protective and adverse effects of the apolipoprotein E epsilon2 allele in older men. J Am Geriatr Soc. 2008;56:478–483. doi: 10.1111/j.1532-5415.2007.01574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberopoulos E, Siamopoulos K, Elisaf M. Apolipoprotein E and renal disease. Am J Kidney Dis. 2004;43:223–233. doi: 10.1053/j.ajkd.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Liu H, Shui IM, Platz EA, et al. No Association of ApoE Genotype with Risk of Prostate Cancer: A Nested Case-Control Study. Cancer Epidemiol Biomarkers Prev. 2015 doi: 10.1158/1055-9965.EPI-15-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, Rall SC., Jr Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E: structure determines function, from atherosclerosis to Alzheimer's disease to AIDS. J Lipid Res. 2009;50(1):S183–188. doi: 10.1194/jlr.R800069-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GM, Bergman A, Barzilai N. Genetic determinants of human health span and life span: progress and new opportunities. PLoS Genet. 2007;3:e125. doi: 10.1371/journal.pgen.0030125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteini AM, Fallin MD, Kammerer CM, et al. Heritability Estimates of Endophenotypes of Long and Health Life: The Long Life Family Study. J Gerontol A Biol Sci Med Sci. 2010 doi: 10.1093/gerona/glq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesse RM, Ganten D, Gregory TR, et al. Evolutionary molecular medicine. J Mol Med (Berl) 2012;90:509–522. doi: 10.1007/s00109-012-0889-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda H, Yorioka N, Ueda C, et al. Apolipoprotein E polymorphism and renal disease. Kidney Int Suppl. 1999;71:S25–27. doi: 10.1046/j.1523-1755.1999.07107.x. [DOI] [PubMed] [Google Scholar]
- Oeppen J, Vaupel JW. Demography. Broken limits to life expectancy. Science. 2002;296:1029–1031. doi: 10.1126/science.1069675. [DOI] [PubMed] [Google Scholar]
- Olshansky SJ, Perry D, Miller RA, et al. Pursuing the longevity dividend: scientific goals for an aging world. Ann N Y Acad Sci. 2007;1114:11–13. doi: 10.1196/annals.1396.050. [DOI] [PubMed] [Google Scholar]
- Pedersen CB, Gotzsche H, Moller JO, et al. The Danish Civil Registration System. A cohort of eight million persons. Dan Med Bull. 2006;53:441–449. [PubMed] [Google Scholar]
- Poulain M, Herm A, Pes G. The Blue Zones: areas of exceptional longevity around the world. Vienna Yearbook of Population Research. 2013:87–108. [Google Scholar]
- Prentice R, Breslow N. Retrospective studies and failure time models. Biometrika. 1978;65:153–158. [Google Scholar]
- Rajpathak SN, Liu Y, Ben-David O, et al. Lifestyle factors of people with exceptional longevity. J Am Geriatr Soc. 2011;59:1509–1512. doi: 10.1111/j.1532-5415.2011.03498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselaar SE, Daugherty A. Apolipoprotein E-deficient mice have impaired innate immune responses to Listeria monocytogenes in vivo. J Lipid Res. 1998;39:1740–1743. [PubMed] [Google Scholar]
- Schultz DW, Klein ML, Humpert A, et al. Lack of an association of apolipoprotein E gene polymorphisms with familial age-related macular degeneration. Arch Ophthalmol. 2003;121:679–683. doi: 10.1001/archopht.121.5.679. [DOI] [PubMed] [Google Scholar]
- Schupf N, Barral S, Perls T, et al. Apolipoprotein E and familial longevity. Neurobiol Aging. 2013;34:1287–1291. doi: 10.1016/j.neurobiolaging.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastiani P, Hadley EC, Province M, et al. A family longevity selection score: ranking sibships by their longevity, size, and availability for study. Am J Epidemiol. 2009;170:1555–1562. doi: 10.1093/aje/kwp309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshasai RK, Katz R, de Boer IH, et al. Apolipoprotein E and kidney function in older adults. Clin Nephrol. 2012;78:174–180. doi: 10.5414/CN107427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra F, Hadley E, Suzman R, et al. Prospects for Life Span Extension. Annu Rev Med. 2008 doi: 10.1146/annurev.med.60.061607.220533. [DOI] [PubMed] [Google Scholar]
- Song Y, Stampfer MJ, Liu S. Meta-analysis: apolipoprotein E genotypes and risk for coronary heart disease. Ann Intern Med. 2004;141:137–147. doi: 10.7326/0003-4819-141-2-200407200-00013. [DOI] [PubMed] [Google Scholar]
- Suri S, Heise V, Trachtenberg AJ, et al. The forgotten APOE allele: a review of the evidence and suggested mechanisms for the protective effect of APOE varepsilon2. Neurosci Biobehav Rev. 2013;37:2878–2886. doi: 10.1016/j.neubiorev.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Thakkinstian A, Bowe S, McEvoy M, et al. Association between apolipoprotein E polymorphisms and age-related macular degeneration: A HuGE review and meta-analysis. Am J Epidemiol. 2006;164:813–822. doi: 10.1093/aje/kwj279. [DOI] [PubMed] [Google Scholar]
- Vaupel JW. Biodemography of human ageing. Nature. 2010;464:536–542. doi: 10.1038/nature08984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijg J, Suh Y. Genetics of longevity and aging. Annu Rev Med. 2005;56:193–212. doi: 10.1146/annurev.med.56.082103.104617. [DOI] [PubMed] [Google Scholar]
- Watson MA, Gay L, Stebbings WS, et al. Apolipoprotein E gene polymorphism and colorectal cancer: gender-specific modulation of risk and prognosis. Clin Sci (Lond) 2003;104:537–545. doi: 10.1042/CS20020329. [DOI] [PubMed] [Google Scholar]
- Wilson PW, Schaefer EJ, Larson MG, et al. Apolipoprotein E alleles and risk of coronary disease. A meta-analysis. Arterioscler Thromb Vasc Biol. 1996;16:1250–1255. doi: 10.1161/01.atv.16.10.1250. [DOI] [PubMed] [Google Scholar]
- Yao X, Dai C, Fredriksson K, et al. Human apolipoprotein E genotypes differentially modify house dust mite-induced airway disease in mice. Am J Physiol Lung Cell Mol Physiol. 2012;302:L206–215. doi: 10.1152/ajplung.00110.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashin AI, Arbeev KG, Kulminski A, et al. “Predicting” parental longevity from offspring endophenotypes: data from the Long Life Family Study (LLFS) Mech Ageing Dev. 2010;131:215–222. doi: 10.1016/j.mad.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Basic characteristics of the 4,659 genotyped LLFS participants.
Supplementary Table 2. Associations of the ε2 allele with the risks of diseases of heart and cancer.
Supplementary Table 3. The role of fasting in the associations of the ε2 allele with lipid levels.
Supplementary Table 4. The role of lipid-lowering therapy in the associations of the ε2 allele with lipids.
Supplementary Table 5. Associations of the ε2 allele with the risks of death.
Supplementary Table 6. Associations of the ε2 allele with the risks of death: the role of diseases used in the analyses.
Supplementary Table 7. Associations of the ε2 allele with the risks of the selected diseases.
Supplementary Table 8. Associations of the ε2 allele with creatinine.