Abstract
Brain arteriovenous malformation (bAVM), characterized by tangled dysplastic vessels, is an important cause of intracranial hemorrhage in young adults, and its pathogenesis and progression are not fully understood. Patients with haploinsufficiency of transforming growth factor-β (TGF-β) receptors, activin receptor-like kinase 1 (ALK1) or endoglin (ENG) have a higher incidence of bAVM than the general population. However, bAVM does not develop effectively in mice with the same haploinsufficiency. The expression of integrin β8 subunit (ITGB8), another member in the TGF-β superfamily, is reduced in sporadic human bAVM. Brain angiogenic stimulation results at the capillary level of vascular malformation in adult Alk1 haploinsufficient (Alk1+/−) mice. We hypothesized that deletion of Itgb8 enhances bAVM development in adult Alk1+/− mice. An adenoviral vector expressing Cre recombinase (Ad-Cre) was co-injected with an adeno-associated viral vector expressing vascular endothelial growth factor (AAV-VEGF) into the brain of Alk1+/−;Itgb8-floxed mice to induce focal itgb8 gene deletion and angiogenesis. We showed that compared with Alk+/− mice (4.75±1.38/mm2), the Alk1+/−;Itgb8-deficient mice had more dysplastic vessels in the angiogenic foci (7.14±0.68/mm2, P=0.003). More severe hemorrhage was associated with dysplastic vessels in the brain of Itgb8-deleted Alk1+/−, as evidenced by larger Prussian blue-positive areas (1278±373 pixels/mm2 vs. Alk1+/−: 320±104/mm2; P=0.028). These data indicate that both Itgb8 and Alk1 are important in maintaining normal cerebral angiogenesis in response to VEGF. Itgb8 deficiency enhances the formation of dysplastic vessels and hemorrhage in Alk1+/− mice.
Keywords: Activin receptor-like kinase 1, Brain arteriovenous malformations, Brain hemorrhage, Integrin β8, Mouse model
Introduction
Brain arteriovenous malformation (bAVM) is a vascular disease with tangles of dysplastic, dilated vessels that shunt blood directly from arteries to veins [1, 2]. These abnormal vessels are prone to rupture, causing life-threatening intracranial hemorrhage. The annual rupture rate is 2–4% [3]. While most bAVMs occur sporadically, a few are associated with genetic disorders, such as Osler-Weber-Rendu syndrome, also called hereditary hemorrhagic telangiectasia (HHT). HHT is the most common familial disorder associated with bAVMs [4]. It is an autosomal dominant vascular disease mainly caused by haploinsufficiency of the genes in the transforming growth factor-β (TGF-β)/bone morphogenic protein (BMP) superfamily: endoglin (ENG, type 1 HHT), activin receptor-like kinase 1 (ALK1, type 2 HHT) [5, 6], and SMAD4 (JPHT, HHT and juvenile polyposis). Common polymorphisms in ALK1 are associated with sporadic bAVM [7–9]; however, few spontaneous bAVMs develop in Alk1+/− (similar to HHT2 genotype) mice [10]. Dysmorphic vessels at the capillary level developed in the brain only after focal angiogenic stimulation [11, 12], suggesting that other factors are required for bAVM development.
We showed previously that compared with normal brain tissue, there is a reduced level of integrin β8 (ITGB8) in human bAVM tissue [13]. ITGB8 is expressed primarily in neuroepithelial cells, serving as a major pathway for transforming growth factor β (TGF-β) activation and signaling in vivo [14, 15]. ITGB8 in perivascular astrocytes plays a central role in regulating brain vessel homeostasis through modulation of TGF-β activation and expression of TGF-β-responsive genes that promote vessel differentiation and stabilization [16]. Focal deletion of Itgb8 in the mouse brain reduces the activation of TGF-β and induces microscopic vascular dysplasia in the angiogenic foci of the VEGF-stimulated adult mouse brain [13]. Similar to Alk1+/− brain, the dysplasia is found primarily in capillaries.
In this study, we tested the hypothesis that deletion of Itgb8 in the brain of Alk1+/− mice enhances bAVM development. We found that focal deletion of Itgb8 in the adult Alk1+/− brain enhanced the formation of dysplastic vessels and hemorrhage.
Methods
Animals and Experimental Groups
The experimental protocols involving animal usage were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of California, San Francisco (UCSF) and conformed to National Institutes of Health (NIH) guidelines. The staff of the Animal Core Facility and the IACUC of UCSF provided animal husbandry under the guidance of supervisors who are certified Animal Technologists, and IACUC faculty members and veterinary residents located on the San Francisco General Hospital campus provided veterinary care.
Adult male and female mice (8 to10 weeks old) that have one allele of the Alk1 gene deleted (Alk1+/−) and have the exon 4 of Itgb8 gene flanked by loxP sides (Itgb8-floxed) [13] were used (Table 1). Mouse genotypes were determined through PCR genomic DNA isolated from tails using the following primer sets. For Alk1+/−: 5′-CCACTCATTCCTCCTGGGTA-3′ (wildtype allele), 5′-ACTTCCTGACTAGGGGAGGAGTAG-3′ (common), and 5′-GTGTTCATGGTAGTGGGTGAT-3′ (knockout allele). For Itgb8-floxed locus: 5′-GTGGTTAAGAGCACCGATTG-3′ and 5′-CACTTTAGTATGCTAATGATGG-3′.
Table 1.
Sample size and mice gender*
| Viral vector-injected
|
Male (n=6) | Female (n=12) | Total (n=17) | ||
|---|---|---|---|---|---|
| Ad-Cre | AAV-VEGF | ||||
| Group A | + | + | 1(20.0) | 4(80.0) | 5(100) |
| Group B | − | + | 1(25.0) | 3(75.0) | 4(100) |
| Group C | + | − | 3(50.0) | 3(50.0) | 6(100) |
| Group D | − | − | 1(33.3) | 2(66.7) | 3(100) |
Table entries are No. (%)
The following vectors were injected into the brain to induce vascular dysplasia: (1) Ad-Cre, adenoviral vector with CMV promoter driving Cre recombinase expression, to mediate deletion of floxed sequence in Itgb8; (2) Ad-GFP, adenoviral vector with green fluorescent protein (GFP) gene, as vector control for Ad-Cre; (3) AAV-VEGF, adeno-associated viral vector expressing vascular endothelial growth factor (VEGF), to induce brain angiogenesis; and (4) AAV-LacZ, adeno-associated viral vector expressing β-galactosidase (LacZ), as vector control for AAV-VEGF. Brain focal angiogenesis was induced because our previous study showed that cerebral vascular dysplasia occurs more robustly in the Alk1+/− brain after angiogenic stimulation [12]. We chose an adenoviral vector to deliver the Cre gene since protein production following transduction peaks earlier than with AAV [17]. Therefore, we co-injected Ad-Cre with AAV-VEGF to favor Cre-mediated Itgb8 deletion before the peak of VEGF stimulation [18]. AAV-VEGF and AAV-LacZ were prepared as described previously [18, 19]. Ad-Cre and Ad-GFP were purchased from Vector Biolabs (Philadelphia, PA).
Mice were randomly assigned to the following groups (Table 2) and were injected with: (A) Ad-Cre+AAV-VEGF; (B) Ad-GFP+AAV-VEGF; (C) Ad-Cre+AAV-LacZ; and (D) Ad-GFP+AAV-LacZ.
Table 2.
Experimental groups*
| Viral vector-injected
|
Itgb8 gene deletion | Angiogenesis | ||
|---|---|---|---|---|
| Ad-Cre | AAV-VEGF | |||
| Group A | + | + | Yes | Yes |
| Group B | − | + | No | Yes |
| Group C | + | − | Yes | No |
| Group D | − | − | No | No |
Mice in all groups are Alk1 +/− (loss of a single allele of Alk1).
Ad-Cre: adenoviral vector expressing Cre recombinase gene; AAV-VEGF: adeno-associated viral vectors expressing vascular endothelial growth factor (VEGF).
Stereotactic Injection of Viral Vectors
After being anesthetized with 2% isoflurane inhalation, the mice were placed in a stereotactic frame with a holder (David Kopf Instruments, Tujunga, CA), and a burr hole was drilled in the pericranium 2 mm lateral to the sagittal suture and 1 mm posterior to the coronal suture. Three μl viral suspension containing 2×107 plaque forming unit (PFU) of adenoviral vectors and 2×109 genome copies (gcs) of AAV viral vectors were stereotactically injected into the right basal ganglia at a rate of 0.2 μl/minute; the needle was withdrawn after 10 minutes and the wound was closed with a suture [20].
Cerebrovascular Casting
Using casting technique, we assessed whether dysplastic vessels in mice resemble the AVM nidus in humans. We explored the cerebrovasculature with Microfil cast (Flow Tech, Inc. Carver, MA) 8 weeks after vector injection, as previously described [21]. Briefly, mice were anesthetized using isoflurane inhalation, perfused with 37°C PBS plus heparin (5 unit/ml) through the left ventricle of the heart to wash out blood from the circulation using a Masterflex Pump Controller (Cole Parmer Instrument, Chicago, IL) at 4 ml/min, followed by perfusion with Microfil. This flow rate generated a pressure approximate to murine physiological blood pressure [22]. Brain samples were collected and fixed overnight in 4% paraformaldehyde (PFA) at 4°C, then clarified using an alcohol-methyl salicylate clearing sequence according to the manufacturer’s instructions. Vasculature was imaged under a dissecting microscope (Leica MZFL III microscope, Leica Microsystems, Bannockburn, IL).
Quantitative Assessment of Vessel Morphology
The Microfil-cast brain samples were embedded in paraffin. Coronal sections (7 μm thick) were cut using a microtome (Leica, RM2155, Germany). Two brain coronal sections from each mouse were chosen for vessel quantification, 0.5 mm anterior and 0.5 mm posterior to the needle track. Sections were incubated overnight with fluorescein lycopersicon esculentum lectin (Vector Laboratories Burlingame, CA), 2 g/ml at 4 °C, and then coverslipped with Vectashield mounting medium with 4′-6-diamidino-2-phenylinidole (Vector Laboratories, Burlingame, CA) to label cell nuclei. Three areas (to the right and left of and below the injection site) within the angiogenic region of each section were imaged under the 20× microscopic objective lens for quantification. Vessel density was quantified using NIH Image 1.63 software and reported as mean vessels counts/mm2. The dysplasia index (number of vessels larger than 15 μm in diameter per mm2) was calculated as previously described [12, 23–25]. All quantification was performed by three blinded investigators.
Prussian Blue Staining
Two sections per brain, selected as described above, were used for detecting iron deposition with an Iron Stain Kit (Sigma-Aldrich, St. Louis, MO). Slides were incubated in a freshly prepared working iron stain solution for 15 minutes, washed in distilled water, and then counterstained with pararosaniline solution for 3 minutes. The positive stain area was quantified using NIH Image 1.63 software. Data are presented as total Prussian blue positive pixel numbers (pixels) in the entire area of the hemisphere (mm2).
Statistical Analyses
Data are presented as mean ± standard deviation (SD). One-way analysis of variance was used to determine statistical significance among multiple groups, followed by pairwise multiple comparisons using the post-hoc Tukey test. We measured the linear relationship among vascular density, vascular dysplasia and Prussian blue-positive area using Pearson correlation coefficient. The linear relationship was determined using a simple linear regression analysis, which generates the corresponding R2 estimate. A P value of < 0.05 was considered statistically significant. Sample sizes are shown in Table 1.
Results
Itgb8 Deletion Enhances Vascular Dysplasia in the Angiogenic Foci of Adult Alk1+/− Brain
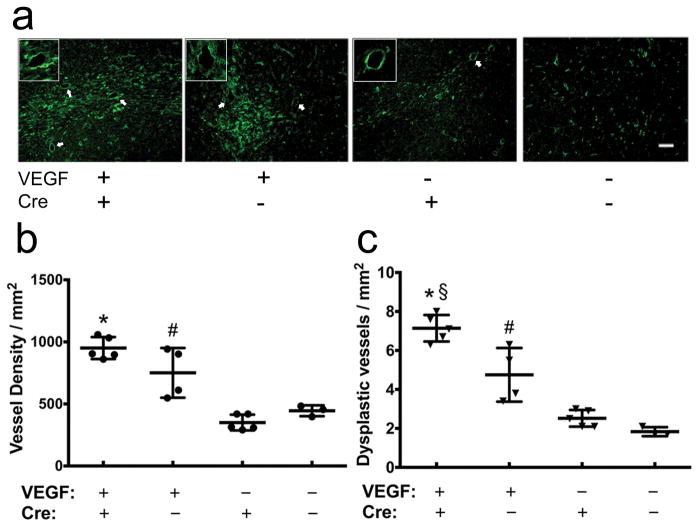
Deletion of Itgb8 plus angiogenic stimulation in the Alk1+/− brain did not induce a similar level of cerebrovascular dysplasia as we have observed in the angiogenic-stimulated Alk1 homozygous deleted brain [23] (Fig. 1). Injection of AAV-VEGF induced a similar level of angiogenesis in the Alk1+/− brain with or without Itgb8 deletion (Fig. 2a and b). However, deletion of Itgb8 increased the number of dysplastic capillaries in the brain angiogenic foci (7.14±0.68/mm2), compared with Alk1+/− mice (4.75±1.38/mm2, P=0.003) (Fig. 2a and b). Compared with AAV-VEGF-injected-Alk1+/− brain with Itgb8 deletion, there were few dysplasia vessels in the AAV-LacZ-injected Alk1+/− brain (1.83 ±0.23/mm2) and Itgb8-deleted Alk1+/− brain (2.52 ±0.43/mm2, P<0.0001) (Fig. 2a and b).
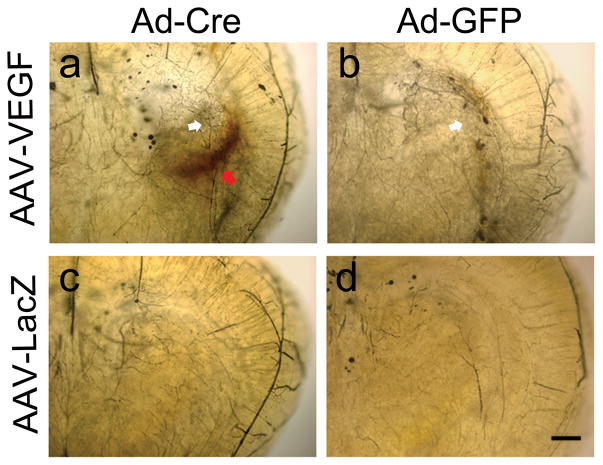
Fig. 1. Representative images of Microfil-cast brain.
a. Itgb8-deleted Alk1+/− brain with AAV-VEGF injection. b. Alk1+/− brain with AAV-VEGF injection. c. Itgb8-deleted Alk1+/− brain with AAV-LacZ injection. d. Alk1+/− brain with AAV-LacZ injection. No tangled abnormal vessel was detected. Increased vascular density (white arrow) was observed around the AAV-VEGF injected sites (a & b) Itgb8-deleted Alk1+/− brain showed significant hemorrhage (red arrow) around the AAV-VEGF injection site. Scale bar=500μm.
Fig. 2. Deletion of Itgb8 increases vascular dysplasia in the angiogenic foci of Alk1+/− brain.
a. Representative lectin-stained sections. Vessels are labeled by green fluorescence. White arrows indicate dysplastic vessels. The insets show enlarged images of dysplastic vessels. Scale bar= 50μm. b. Quantification of vessel density. c. Quantification of dysplasia index. Error bars indicate SD. VEGF: AAV-VEGF treatment. Cre: Ad-Cre treatment. *P<0.0001 versus brain injected with AAV-LacZ and Ad-Cre; §P=0.003 versus brain injected with AAV-VEGF and Ad-GFP; # P<0.05 versus brain injected with AAV-LacZ and Ad-GFP.
Itgβ8 Deletion Increases Hemorrhage in the Brain Angiogenic Foci of Alk1+/− Mice
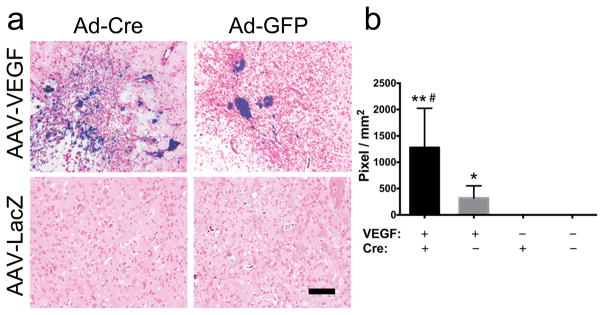
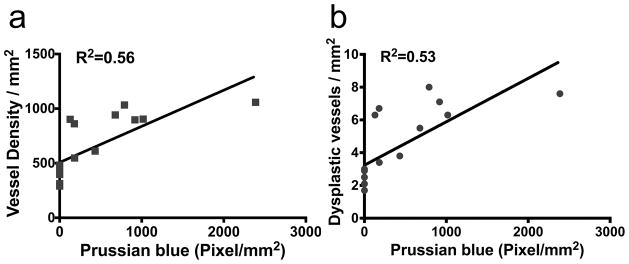
Alk1+/− mice had microhemorrhage in the brain angiogenic foci. Deletion of itgb8 enhanced the hemorrhage (Fig. 1). No hemorrhage was observed in AAV-LacZ-injected brain (Fig. 1). Prussian blue-positive areas were ≈4-fold larger in the angiogenic region of Itgb8-deleted Alk1+/− brain (1278±373 pixels/mm2) than in the Alk1+/− brain (320±104 pixels/mm2; P=0.028) (Fig. 3a and b). The Prussian blue-positive area positively correlated with vascular density (R2=0.56, P=0.0006, Fig. 4a) and vascular dysplasia (R2=0.53, P=0.001, Fig. 4b). No Prussian blue-positive area was detected in the brain injected with AAV-LacZ (Fig. 3a and b).
Fig. 3. Deletion of Itgb8 increases hemorrhage in the angiogenic foci in Alk1+/− brain.
a. Representative images of Prussian blue stained sections. The iron depositions are stained blue. Scale bar=100μm. b. Quantification of Prussian blue-positive area. VEGF: AAV-VEGF treatment. Cre: Ad-Cre treatment. * P=0.008 and ** P=0.003 versus brain injected with AAV-LacZ and Ad-Cre or Ad-GFP; # P =0.028 versus brain injected with AAV-VEGF and Ad-GFP.
Fig. 4. Correlation between Prussian blue-positive area and vascular features.
a. Positive correlation of Prussian blue-positive area with vessel density (R2=0.56, P<0.001). b. Positive correlation of Prussian blue-positive area with vascular dysplasia (R2=0.53, P=0.001).
Discussion
Using mice with focal Itgb8 deletion combined with haploinsufficiency of Alk1, we demonstrated a role for Itgb8 in vascular dysplasia in the angiogenic foci. The vascular abnormalities were in the capillaries, as we have observed in the Alk1+/− brain [12]. However, deletion of Itgb8 in the Alk1+/− brain caused a significant increase of microhemorrhage in the angiogenic foci (Fig. 5a). The degree of microhemorrhage positively correlated with vessel density and the number of abnormal vessels.
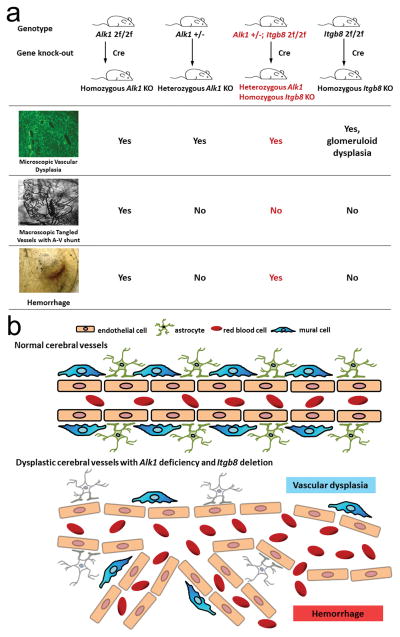
Fig. 5. Summary of vascular phenotypes in the Alk1-deficient brain and depiction of underlying mechanisms.
a. Cerebrovascular phenotype of Alk1 and/or Itgb8 knock-out (KO) mice in the brain angiogenic foci. Homozygous Alk1 KO in the brain causes microscopic vascular dysplasia and macroscopic tangled vessels with arteriovenous (A-V) shunt [23]; heterozygous Alk1 KO or homozygous Itgb8 KO results in microscopic level of vascular dysplasia only. Heterozygous Alk1 KO plus Itgb8 homozygous deletion cause microscopic vascular dysplasia and hemorrhage. Alk12f/2f: exons 4–6 of both alleles of Alk1 gene are flanked by loxP sides and are deleted when Cre is expressed [23, 47]; Alk1 +/−: one allele of Alk1 is deleted [12]; Itgb8 2f/2f: exon 4 of both alleles of Itgb8 gene is flanked by loxP sides and is deleted when Cre is expressed [13]. b. Depiction of the mechanisms of Alk1 and Itgb8 deletion in the vascular malformation and hemorrhage. Normal cerebrovascularture is covered with mural cells (including pericytes and smooth muscle cells, top panel). Alk1 deletion in endothelial cells (reduced color intensity, bottom panel) reduces vascular mural cell coverage [46], and Itgb8 deletion in astrocytes (white color) results in abnormal sprouting [42]. Therefore, deletion of Itgb8 in the Alk1+/− brain enhances vascular dysplasia and hemorrhage.
It has been observed that HHT patients with the same mutated gene have different phenotypes. For example, brain [26, 27] and pulmonary [26–29] AVMs are more common among patients with ENG mutation, while AVM prevalence and hemorrhage risk in the same organ vary among these patients [30]. The variability of phenotypes cannot be explained by global heterozygous gene deletion. Haploinsufficiency of the HHT causative gene is not sufficient to cause spontaneous lesion development in mice [31]. Inactivation of the remaining WT allele appears to have a powerful effect, irrespective of the mechanism by which it is inactivated, e.g., loss of heterozygosity or loss of protein during inflammation [32]. For example, the loss of a single allele of one of the HHT causative genes, such as Eng or Alk1, reproduces certain aspects of the human disease in animal models, primarily in older animals [10, 33]. In contrast, the loss of both alleles of any HHT causative gene is embryonically lethal in mice [34, 35], and conditional (tissue/time-specific) homozygous deletion of Eng [32] or Alk1 [36, 37] results in striking vascular malformations resembling the AVMs found in HHT. This has also been demonstrated in our previous studies in modeling bAVM in mice [23, 38–40]. AVM development in an HHT gene-deficient mouse model is influenced by mouse strains, which have various levels of plasma TGF-β1 [41]. Therefore, modifier genes involved in the regulation of TGF-β1 expression may act in combination with a single functional copy of Eng or Alk1. Our data suggest that Itgb8 could be one of the modifiers that influence the AVM progression.
ITGB8 is expressed in the perivascular cell process surrounding cerebral blood vessels, serving as a major pathway for TGFβ activation and signaling in vivo [16, 42, 43]. Perivascular astrocytes interact with vascular endothelial cells to regulate brain vessel differentiation and stabilization through integrin β8-mediated activation of TGF-β1 [16]. Itgb8 deletion reduces activated TGF-β1 in both the developmental and adult brain [13, 42]. Excessive vascular sprouting and vascular dysplasia are obvious in the embryonic brain with Itgb8 deletion [42]. Increased cerebral blood vessel densities that appear to be the remnants of abnormal vessels occurring during development are also observed in Itgb8 homozygous knockout mice that survive to adulthood [43]. In the adult mouse brain, conditional deletion of Itgb8 does not increase vascular density or cause vascular dysplasia [44]; however, triggering angiogenesis in the Itgb8-deleted adult brain induces vascular dysplasia [13]. Therefore, integrins containing the β8 subunit appear to be important in regulating vascular development.
We showed previously that VEGF stimulation induced vascular dysplasia in the capillaries in the brain with focal Itgb8 deletion and in the brain of Alk1+/− mice (Fig. 5a) [12, 13]. To test if mutation of both Itgb8 and Alk1 could enhance bAVM formation, we crossbred Itgb8-floxed mice with Alk1+/− mice. Although deletion of Itgb8 increased the number of abnormal vessels in the angiogenic foci of the Alk1+/− brain, the abnormality was still at capillary level, and no macroscopic level of dysplasia was formed (Fig. 5a). These data suggest that Itgb8 is not a key component for bAVM development.
Brain vessels in the Itgb8-deficient embryo show defective anastomotic connections and increased endothelial cell proliferation resulting in “glomeruloid” vascular malformations, which are associated with extensive cerebral hemorrhage [45]. The primary ultrastructural defect in the Itgb8-null developmental mouse brain appears to be abnormal astrocyte end-feet; the perivascular cells fail to associate with endothelial cells [45]. However, selective ablation of Itgb8 in endothelial cells or neurons does not cause cerebral hemorrhage [44]. Deletion of Itgb8 in astrocytes causes cerebral hemorrhage in the embryonic brain, although the phenotype is less severe than in mice with Itgb8-deleted ubiquitously. Cerebral hemorrhage in the embryonic brain with targeted deletion of Itgb8 in neuroepithelium correlates with decreased active TGF-β [42]. However, deletion of Itgb8 in the adult brain does not cause significant vascular dysplasia and cerebral hemorrhage [44]. In homozygous Itgb8-null mice with outbred background that survive to adulthood, cerebral hemorrhage is obvious in newborn mice but surprisingly absent in the adults [43]. These data indicate that the expression of Itgb8 in neuroepithelial cells and correlated TGF-β activation are essential for maintaining normal cerebrovascular structure in the setting of active angiogenesis, thereby preventing cerebral hemorrhage.
We have shown in this study that Itgb8 deletion induces severe hemorrhage in the angiogenic foci of Alk1+/− adult mouse brain. The degree of hemorrhage as quantified by Prussian blue staining positively correlates with both vascular density and vascular dysplasia. Therefore, integrins containing the β8 subunit appear to be important for maintaining cerebrovascular stability and preventing hemorrhage in the angiogenic foci of Alk1+/− mice.
Our previous study indicated that ALK1 could play a role in maintaining the integrity of vessels in the adult brain during angiogenesis [46]. Cerebral hemorrhage in Alk1 homozygous mutant mice has been found to be associated with impaired vascular integrity and blood-brain barrier (BBB) dysfunction. In the angiogenic foci of Alk1-null brain, the dysplastic vessels have fewer pericytes and smooth muscle cells than normal cerebrovasculature (Fig. 5b), positively correlating with the degree of BBB leakage [46]. However, there is no evidence of BBB leakage in the Itgb8-deficient embryo before cerebral hemorrhage [42]. The numbers of perivascular smooth muscle cells and pericytes are normal in the brain of Itgb8-null embryo [44]. A strong spatial-temporal association of sprouting abnormal angiogenesis and cerebral hemorrhage in Itgb8-deficient embryo indicates that abnormal angiogenesis, not BBB leakage, causes hemorrhage in the Itgb8-deficient brain [42]. Therefore, the brain hemorrhage we have observed in the angiogenic foci of Itgβ8-deleted and Alk1+/− brain might be due to both abnormal angiogenic sprouting and loss of vascular mural cells (Fig. 5b). Future studies should investigate the interaction of ITGB8 and ALK1 in maintaining vascular integrity.
In summary, our data suggest that both Itgb8 and Alk1 play important roles in maintaining normal vascular structure during brain angiogenesis in adult mice, and that Itgb8 deficiency could enhance vascular dysplasia and hemorrhage in brain vascular malformations in Alk1+/− mice, which, in turn, might be associated with bAVM hemorrhage.
Acknowledgments
This study was supported by grants to HS from the National Institutes of Health (R01 NS027713, R01 HL122774 and R21 NS083788), and from the Michael Ryan Zodda Foundation and the UCSF Research Evaluation and Allocation Committee (REAC). We thank members of the UCSF BAVM Study Project (http://avm.ucsf.edu) for their support, and Voltaire Gungab for his assistance with manuscript preparation.
Funding: This study was supported by grants to Hua Su from the National Institutes of Health (R01 NS027713, R01 HL122774 and R21 NS083788), and from the Michael Ryan Zodda Foundation and the UCSF Research Evaluation and Allocation Committee (REAC).
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Compliance with Ethical Standards
Ethical Approval: All procedures performed in studies involving animals were approved by and were in accordance with the ethical standards of the Institutional Animal Care and Use Committee of the University of California, San Francisco.
References
- 1.Arteriovenous malformations of the brain in adults. N Engl J Med. 1999;340(23):1812–8. doi: 10.1056/NEJM199906103402307. [DOI] [PubMed] [Google Scholar]
- 2.Rangel-Castilla L, Russin JJ, Martinez-Del-Campo E, Soriano-Baron H, Spetzler RF, Nakaji P. Molecular and cellular biology of cerebral arteriovenous malformations: a review of current concepts and future trends in treatment. Neurosurg Focus. 2014;37(3):E1. doi: 10.3171/2014.7.FOCUS14214. [DOI] [PubMed] [Google Scholar]
- 3.Rutledge WC, Ko NU, Lawton MT, Kim H. Hemorrhage rates and risk factors in the natural history course of brain arteriovenous malformations. Transl Stroke Res. 2014;5(5):538–42. doi: 10.1007/s12975-014-0351-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedlander RM. Clinical practice. Arteriovenous malformations of the brain. N Engl J Med. 2007;356(26):2704–12. doi: 10.1056/NEJMcp067192. [DOI] [PubMed] [Google Scholar]
- 5.McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet. 1994;8(4):345–51. doi: 10.1038/ng1294-345. [DOI] [PubMed] [Google Scholar]
- 6.Johnson DW, Berg JN, Baldwin MA, Gallione CJ, Marondel I, Yoon SJ, et al. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet. 1996;13(2):189–95. doi: 10.1038/ng0696-189. [DOI] [PubMed] [Google Scholar]
- 7.Pawlikowska L, Tran MN, Achrol AS, Ha C, Burchard EG, Choudhry S, et al. Polymorphisms in transforming growth factor-beta-related genes ALK1 and ENG are associated with sporadic brain arteriovenous malformations. Stroke. 2005;36(10):2278–80. doi: 10.1161/01.STR.0000182253.91167.fa. [DOI] [PubMed] [Google Scholar]
- 8.Simon M, Franke D, Ludwig M, Aliashkevich AF, Koster G, Oldenburg J, et al. Association of a polymorphism of the ACVRL1 gene with sporadic arteriovenous malformations of the central nervous system. J Neurosurg. 2006;104(6):945–9. doi: 10.3171/jns.2006.104.6.945. [DOI] [PubMed] [Google Scholar]
- 9.Boshuisen K, Brundel M, de Kovel CG, Letteboer TG, Rinkel GJ, Westermann CJ, et al. Polymorphisms in ACVRL1 and endoglin genes are not associated with sporadic and HHT-related brain AVMs in Dutch patients. Transl Stroke Res. 2013;4(3):375–8. doi: 10.1007/s12975-012-0231-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srinivasan S, Hanes MA, Dickens T, Porteous ME, Oh SP, Hale LP, et al. A mouse model for hereditary hemorrhagic telangiectasia (HHT) type 2. Hum Mol Genet. 2003;12(5):473–82. doi: 10.1093/hmg/ddg050. [DOI] [PubMed] [Google Scholar]
- 11.Hao Q, Su H, Marchuk DA, Rola R, Wang Y, Liu W, et al. Increased tissue perfusion promotes capillary dysplasia in the ALK1-deficient mouse brain following VEGF stimulation. Am J Physiol Heart Circ Physiol. 2008;295(6):H2250–6. doi: 10.1152/ajpheart.00083.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hao Q, Zhu Y, Su H, Shen F, Yang GY, Kim H, et al. VEGF induces more severe cerebrovascular dysplasia in Endoglin+/− than in Alk1+/− mice. Transl Stroke Res. 2010;1(3):197–201. doi: 10.1007/s12975-010-0020-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su H, Kim H, Pawlikowska L, Kitamura H, Shen F, Cambier S, et al. Reduced expression of integrin alphavbeta8 is associated with brain arteriovenous malformation pathogenesis. Am J Pathol. 2010;176(2):1018–27. doi: 10.2353/ajpath.2010.090453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, et al. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002;157(3):493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishimura SL, Boylen KP, Einheber S, Milner TA, Ramos DM, Pytela R. Synaptic and glial localization of the integrin alphavbeta8 in mouse and rat brain. Brain Res. 1998;791(1–2):271–82. doi: 10.1016/s0006-8993(98)00118-8. [DOI] [PubMed] [Google Scholar]
- 16.Cambier S, Gline S, Mu D, Collins R, Araya J, Dolganov G, et al. Integrin alpha(v)beta8-mediated activation of transforming growth factor-beta by perivascular astrocytes: an angiogenic control switch. Am J Pathol. 2005;166(6):1883–94. doi: 10.1016/s0002-9440(10)62497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu D, Sullivan CC, Weitzman MD, Du L, Wolf PL, Jamieson SW, et al. Direct comparison of efficiency and stability of gene transfer into the mammalian heart using adeno-associated virus versus adenovirus vectors. J Thorac Cardiovasc Surg. 2003;126(3):671–9. doi: 10.1016/s0022-5223(03)00082-5. [DOI] [PubMed] [Google Scholar]
- 18.Shen F, Su H, Liu W, Kan YW, Young WL, Yang GY. Recombinant adeno-associated viral vector encoding human VEGF165 induces neomicrovessel formation in the adult mouse brain. Front Biosci. 2006;11:3190–8. doi: 10.2741/2042. [DOI] [PubMed] [Google Scholar]
- 19.Su H, Lu R, Kan YW. Adeno-associated viral vector-mediated vascular endothelial growth factor gene transfer induces neovascular formation in ischemic heart. Proc Natl Acad Sci U S A. 2000;97(25):13801–6. doi: 10.1073/pnas.250488097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang GY, Zhao Y, Davidson BL, Betz AL. Overexpression of interleukin-1 receptor antagonist in the mouse brain reduces ischemic brain injury. Brain Res. 1997;751:181–8. doi: 10.1016/s0006-8993(96)01277-2. [DOI] [PubMed] [Google Scholar]
- 21.Walker EJ, Shen F, Young WL, Su H. Cerebrovascular casting of the adult mouse for 3D imaging and morphological analysis. J Vis Exp. 2011;57(v):e2958. doi: 10.3791/2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janssen BJ, De Celle T, Debets JJ, Brouns AE, Callahan MF, Smith TL. Effects of anesthetics on systemic hemodynamics in mice. Am J Physiol Heart Circ Physiol. 2004;287(4):H1618–24. doi: 10.1152/ajpheart.01192.2003. [DOI] [PubMed] [Google Scholar]
- 23.Walker EJ, Su H, Shen F, Choi EJ, Oh SP, Chen G, et al. Arteriovenous malformation in the adult mouse brain resembling the human disease. Ann Neurol. 2011;69(6):954–62. doi: 10.1002/ana.22348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi EJ, Walker EJ, Shen F, Oh SP, Arthur HM, Young WL, et al. Minimal homozygous endothelial deletion of Eng with VEGF stimulation is sufficient to cause cerebrovascular dysplasia in the adult mouse. Cerebrovasc Dis. 2012;33(6):540–7. doi: 10.1159/000337762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi EJ, Walker EJ, Degos V, Jun K, Kuo R, Su H, et al. Endoglin deficiency in bone marrow is sufficient to cause cerebrovascular dysplasia in the adult mouse after vascular endothelial growth factor stimulation. Stroke. 2013;44(3):795–8. doi: 10.1161/STROKEAHA.112.671974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabba C, Pasculli G, Lenato GM, Suppressa P, Lastella P, Memeo M, et al. Hereditary hemorragic telangiectasia: clinical features in ENG and ALK1 mutation carriers. J Thromb Haemost. 2007;5(6):1149–57. doi: 10.1111/j.1538-7836.2007.02531.x. [DOI] [PubMed] [Google Scholar]
- 27.Bayrak-Toydemir P, McDonald J, Markewitz B, Lewin S, Miller F, Chou LS, et al. Genotype-phenotype correlation in hereditary hemorrhagic telangiectasia: mutations and manifestations. Am J Med Genet A. 2006;140(5):463–70. doi: 10.1002/ajmg.a.31101. [DOI] [PubMed] [Google Scholar]
- 28.Letteboer TG, Mager JJ, Snijder RJ, Koeleman BP, Lindhout D, Ploos van Amstel JK, et al. Genotype-phenotype relationship in hereditary haemorrhagic telangiectasia. J Med Genet. 2006;43(4):371–7. doi: 10.1136/jmg.2005.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lesca G, Olivieri C, Burnichon N, Pagella F, Carette MF, Gilbert-Dussardier B, et al. Genotype-phenotype correlations in hereditary hemorrhagic telangiectasia: Data from the French-Italian HHT network. Genet Med. 2007;9(1):14–22. doi: 10.1097/gim.0b013e31802d8373. [DOI] [PubMed] [Google Scholar]
- 30.Kim H, Nelson J, Krings T, terBrugge K, McCulloch CE, Lawton MT, et al. Hemorrhage rates from brain arteriovenous malformation in patients with hereditary hemorrhagic telangiectasia. Stroke. 2015;46(5):1362–4. doi: 10.1161/STROKEAHA.114.007367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bourdeau A, Cymerman U, Paquet ME, Meschino W, McKinnon WC, Guttmacher AE, et al. Endoglin expression is reduced in normal vessels but still detectable in arteriovenous malformations of patients with hereditary hemorrhagic telangiectasia type 1. Am J Pathol. 2000;156(3):911–23. doi: 10.1016/S0002-9440(10)64960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahmoud M, Allinson KR, Zhai Z, Oakenfull R, Ghandi P, Adams RH, et al. Pathogenesis of arteriovenous malformations in the absence of endoglin. Circ Res. 2010;106(8):1425–33. doi: 10.1161/CIRCRESAHA.109.211037. [DOI] [PubMed] [Google Scholar]
- 33.Bourdeau A, Faughnan ME, Letarte M. Endoglin-deficient mice, a unique model to study hereditary hemorrhagic telangiectasia. Trends Cardiovasc Med. 2000;10(7):279–85. doi: 10.1016/s1050-1738(01)00062-7. [DOI] [PubMed] [Google Scholar]
- 34.Urness LD, Sorensen LK, Li DY. Arteriovenous malformations in mice lacking activin receptor-like kinase-1. Nat Genet. 2000;26(3):328–31. doi: 10.1038/81634. [DOI] [PubMed] [Google Scholar]
- 35.Sorensen LK, Brooke BS, Li DY, Urness LD. Loss of distinct arterial and venous boundaries in mice lacking endoglin, a vascular-specific TGFbeta coreceptor. Dev Biol. 2003;261(1):235–50. doi: 10.1016/s0012-1606(03)00158-1. [DOI] [PubMed] [Google Scholar]
- 36.Milton I, Ouyang D, Allen CJ, Yanasak NE, Gossage JR, Alleyne CH, Jr, et al. Age-dependent lethality in novel transgenic mouse models of central nervous system arteriovenous malformations. Stroke. 2012;43(5):1432–5. doi: 10.1161/STROKEAHA.111.647024. [DOI] [PubMed] [Google Scholar]
- 37.Park SO, Wankhede M, Lee YJ, Choi EJ, Fliess N, Choe SW, et al. Real-time imaging of de novo arteriovenous malformation in a mouse model of hereditary hemorrhagic telangiectasia. J Clin Invest. 2009;119(11):3487–96. doi: 10.1172/JCI39482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi EJ, Chen W, Jun K, Arthur HM, Young WL, Su H. Novel brain arteriovenous malformation mouse models for type 1 hereditary hemorrhagic telangiectasia. PLoS One. 2014;9(2):e88511. doi: 10.1371/journal.pone.0088511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen W, Sun Z, Han Z, Jun K, Camus M, Wankhede M, et al. De novo cerebrovascular malformation in the adult mouse after endothelial Alk1 deletion and angiogenic stimulation. Stroke. 2014;45(3):900–2. doi: 10.1161/STROKEAHA.113.003655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen W, Choi EJ, McDougall CM, Su H. Brain arteriovenous malformation modeling, pathogenesis and novel therapeutic targets. Transl Stroke Res. 2014;5(3):316–29. doi: 10.1007/s12975-014-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bourdeau A, Faughnan ME, McDonald ML, Paterson AD, Wanless IR, Letarte M. Potential role of modifier genes influencing transforming growth factor- beta1 levels in the development of vascular defects in endoglin heterozygous mice with hereditary hemorrhagic telangiectasia. Am J Pathol. 2001;158(6):2011–20. doi: 10.1016/s0002-9440(10)64673-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arnold TD, Niaudet C, Pang MF, Siegenthaler J, Gaengel K, Jung B, et al. Excessive vascular sprouting underlies cerebral hemorrhage in mice lacking alphaVbeta8-TGFbeta signaling in the brain. Development. 2014;141(23):4489–99. doi: 10.1242/dev.107193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mobley AK, Tchaicha JH, Shin J, Hossain MG, McCarty JH. Beta8 integrin regulates neurogenesis and neurovascular homeostasis in the adult brain. J Cell Sci. 2009;122(Pt 11):1842–51. doi: 10.1242/jcs.043257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Proctor JM, Zang K, Wang D, Wang R, Reichardt LF. Vascular development of the brain requires beta8 integrin expression in the neuroepithelium. J Neurosci. 2005;25(43):9940–8. doi: 10.1523/JNEUROSCI.3467-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu J, Motejlek K, Wang D, Zang K, Schmidt A, Reichardt LF. beta8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002;129(12):2891–903. doi: 10.1242/dev.129.12.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen W, Guo Y, Walker EJ, Shen F, Jun K, Oh SP, et al. Reduced mural cell coverage and impaired vessel integrity after angiogenic stimulation in the Alk1-deficient brain. Arterioscler Thromb Vasc Biol. 2013;33(2):305–10. doi: 10.1161/ATVBAHA.112.300485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park SO, Lee YJ, Seki T, Hong KH, Fliess N, Jiang Z, et al. ALK5- and TGFBR2-independent role of ALK1 in the pathogenesis of hereditary hemorrhagic telangiectasia type 2 (HHT2) Blood. 2008;111(2):633–42. doi: 10.1182/blood-2007-08-107359. [DOI] [PMC free article] [PubMed] [Google Scholar]