Abstract
Limbal stem cell deficiency (LSCD) is a pathologic condition caused by the dysfunction and/or destruction of stem cell precursors of the corneal epithelium, typified clinically by corneal conjunctivalization. The purpose of this review is to critically discuss a less well-known cause of limbal stem cell disease: contact lens (CL) wear. A literature search was conducted to include original articles containing patients with CL-induced LSCD. This review describes epidemiology, diagnostic strategies, pathogenesis, differential diagnosis, and treatment modalities for this condition.
Keywords: corneal conjunctivalization, contact lens, limbal stem cell deficiency, limbal stem cells
I. Introduction
Research over the last few decades has significantly advanced our knowledge of limbal stem cells and their function in maintaining and regenerating the corneal epithelium during normal and disease states. Limbal stem cells (LSCs) provide a regenerative source of transient amplifying cells (progenitor cells) that terminally differentiate and replace corneal epithelium.1–4 LSCs reside in the basal limbal region, which provides a nourishing niche necessary for their function.2 Although many describe the true niche of corneal epithelial stem cells at the limbus,1–4 some recent publications suggest that corneal epithelial stem cells are also found elsewhere on the ocular surface.2,5,6
Limbal stem cell deficiency (LSCD) is a pathologic condition caused by the dysfunction and/or destruction of stem cell precursors of the corneal epithelium.2,7–12 There are numerous congenital and acquired etiologies of LSCD, including but not limited to aniridia, ectodermal dysplasia, chemical or thermal injuries, Stevens-Johnson syndrome, iatrogenic disease (secondary to ocular medications or surgery), and contact lens (CL) wear.2 Of these, CL wear is the less well-known, and often asymptomatic, cause of LSCD.11,13–15
While the manifestations of CL-induced LSCD are often not as severe as in LSCD from some of the other causes, the basic pathologic process is similar.7,11,14 Historically, CL-induced LSCD has been described by other names in the literature, including CL-induced keratopathy, chronic CL-associated epitheliopathy, advancing wave-like epitheliopathy, hurricane keratopathy, and CL-induced superior limbic keratoconjunctivitis (CL-SLK).10,16–20 Given our improved understanding of its pathophysiology, CL-induced LSCD is now the most appropriate and widely accepted term to describe the aforementioned conditions.
II. Method of Literature Search
The literature search for this review was conducted on PubMed with the search phrase “limbal stem cell deficiency” and the reference lists from included papers were also evaluated for inclusion in the literature review. Original studies and case reports were included if they included at least 1 case of CL-induced LSCD or were landmark papers describing the first descriptions of conditions and/or treatments associated with LSCD. A recent book chapter on the basics of LSCD was also included to provide a foundation of background information on the condition.
III. Epidemiology
There are an estimated 125 million CL lens wearers worldwide, with 37 million in the United States.21 An estimated 2.4–5% of CL wearers develop signs of LSCD,13,22 and 15% of LSCD cases are attributed to CL use.23 This may be an underestimation, as mild cases are often asymptomatic.13,14 A positive correlation exists between LSCD and duration of CL use.11,13–15,22 Although the mean duration of CL use associated with LSCD is 14.2–17.6 years and 12.5–16.25 hours per day, patients have been reported to develop LSCD after as little as 6–12 months of CL use.11,13,14,16,24 One retrospective review on CL-induced LSCD identified a mean age at diagnosis of 42 years, with 50% of cases presenting with bilateral findings and 58% with previously diagnosed ocular disorders.11 Disease in CL wearers is frequently bilateral yet asymmetric, commonly because of subclinical disease in the fellow eye.10,11,14–16
In the reports that identify the type of CL used at LSCD diagnosis, nearly all cases involved exclusive soft CL use,7,8,11–13,15–19,23–26 although one case series reported on patients with rigid gas permeable lens wear.20 The risk with hybrid lenses or rigid mini-scleral/scleral lenses is not known at this time. Although not all reports indicate lens wearing schedules (daily versus extended wear), those that provide this information show that CL-induced LSCD increases with both daily-wear12,13,18,20,25 and extended-wear25 use.
Approximately 65% of CL wearers in the United States are female, which is consistent with the worldwide proportion, ranging between 59–73%.27 Thus, as expected, women represent the majority (67–93%) of patients with CL-induced LSCD.11,13,14,24 The explanation for this slight female overrepresentation beyond their proportionate representation of CL users is unclear but may be related to the increased prevalence of dry eyes in females secondary to hormonal regulatory factors.11,14 Other underlying discrepancies in gender susceptibility are in need of further elucidation.
IV. Diagnostic Strategies
A. History
Patients with CL-induced LSCD often have a history of many years of daily soft CL use worn for many hours per day.11,13,14,16,24 Patients are usually asymptomatic initially, as were 71.4% of patients in a recent chart review of CL-induced LSCD with corneal conjunctivalization.13 When symptomatic, patients often describe nonspecific symptoms such as pain, photophobia, decreased vision, tearing, dryness, blepharospasm, redness and irritation.2,7,8,10,11,13–15,20,26
B. Physical Examination
Combined with history, clinical examination is often sufficient for diagnosis.2 Since many patients are initially symptom-free, and because early diagnosis and treatment can improve outcomes, regular screening by slit lamp examination should be performed in all CL wearers.11,13,14 Patients wearing CLs, particularly soft CLs, should be annually examined with a high degree of suspicion,14 with particular attention to the superior cornea and its staining pattern.7–9,13,14,16–20,22,24,26,28 CL-induced LSCD can be reversible by medical treatment if recognized early.7,14,19,24
Specific attention should be given to the underside of the upper lid, as the most common location for CL-induced LSCD is the superior limbus, followed by the inferior limbus (Table 1).7–9,13,14,16–20,22,24,26,28 LSCD can be either partial or total.7,11,13–15 In a group of patients with more advanced disease, Chan and Holland found an average 10-clock hours of limbal involvement (range of 6–12 clock hours), with entire limbal involvement in 61% of eyes (11 of 18) and in 50% with bilateral disease.11 The presentation is usually asymmetric, with one eye more affected than the other, but usually the disease is bilateral in patients wearing lenses in both eyes.10,11,14–16 The absence of palisades of Vogt on slit lamp examination may be an indicator of LSCD.2,13 However, palisades of Vogt are not reliably visible in all patients or in all quadrants29; thus, their absence cannot confirm LSCD, but their presence can help rule out LSCD.
Table 1.
Location of Disease Involvement in CL-induced LSCD
| Reference | Superior Involvement | Inferior Involvement | Superior & Inferior Involvement | Other |
|---|---|---|---|---|
| Kim et al7 | 44% | 6% | 19% | - 6% superior/temporal/nasal - 6% superior/temporal - 19% subtotal |
| Martin et al13 | 79% | 12% | — | — |
| Jeng et al14 | 72.2% | — | 27.8% | — |
This table provides the breakdown of the reported localization of disease in CL-induced LSCD from three papers. The values are percentages of eyes that presented with the associated location of disease presentation.
When the LSC barrier is absent, the conjunctiva invades the cornea,2 making conjunctivalization the key slit lamp finding of LSCD.2,11,14,15,30 Conjunctival epithelium is more opaque than corneal epithelium, which is most noticeable when both types of epithelium are present on the cornea.7,14,30 Since epithelium grows in a spiral pattern from the limbus onto the cornea, whorls of opaque epithelium are seen on examination (Figures 1 and 2).11,14 The diagnosis of LSCD is significantly aided by a characteristic late-staining fluorescein pattern of conjunctivalization (Figures 1 and 2).2,7,9,11,13,14,31,32 The late-staining pattern in LSCD primarily occurs because conjunctival epithelium is more permeable to fluorescein than corneal epithelium.15,31 More specifically, the tight junctions in conjunctival epithelium are “less tight” than in normal corneal epithelium, so fluorescein dye can penetrate through the intercellular junctions more easily and cause the epithelium to show greater uptake.30
Figure 1. Contact lens-induced limbal stem cell deficiency.
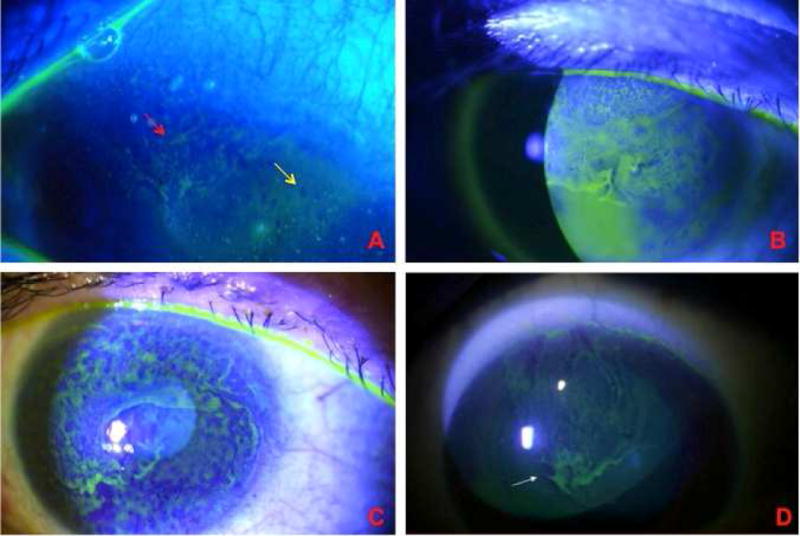
A. Early involvement of the superior cornea. There is whorl-like epithelium (red arrow) adjacent to an area of punctate staining (yellow area), which is the earliest sign of LSCD. B Superior involvement of the cornea, characteristic of moderate CL-induced LSCD. C. Late-staining fluorescein diffusely in a whorled pattern as a confluent sheet across the cornea in late-stage CL-induced LSCD. D. Characteristic superior involvement of corneal epithelium.
Figure 2. Contact lens-induced limbal stem Cell deficiency - 360° disease.
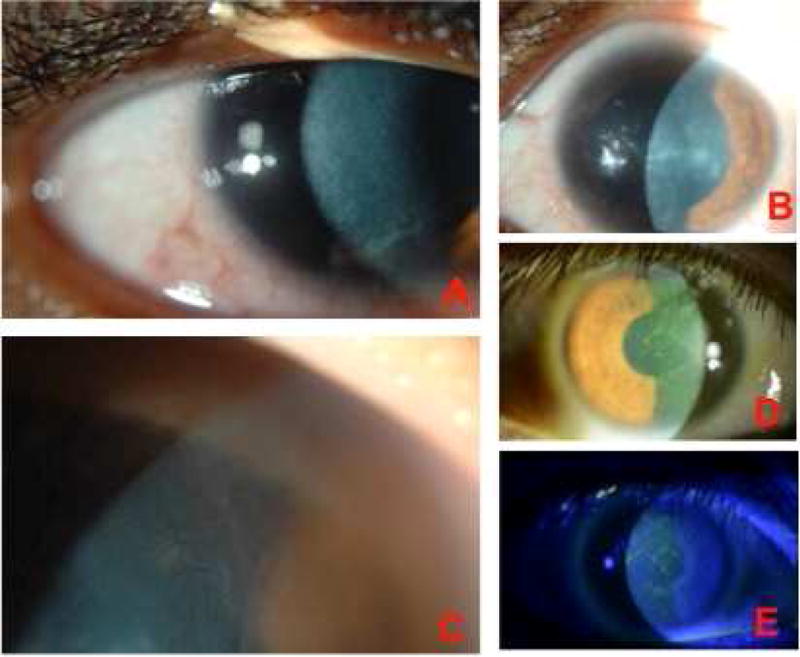
A. CL-induced LSCD with whorl-like epithelium throughout the cornea. B. Another example of diffuse (360 degrees) disease along with stromal changes. C. 360° disease with superficial neovascularization. D, E. Fluorescein staining in a patient with diffuse CL-induced LSC disease (two weeks after discontinuing CL wear).
The clinical findings of various stages of disease progression have been described.11,17,32 In early CL-induced LSCD, only punctate fluorescein staining (following a curve-like path) may be seen in the superior cornea.7,8,17,18,20,32 As the disease progresses, punctate changes coalesce in a more linear pattern, and later a confluent sheet spreads centrally.7,17,24,33 With chronic disease and further loss of the limbal barrier, a sheet of conjunctival-type epithelium spreads across the cornea, which, along with neovascularization, may be loosely referred to as corneal pannus (Figure 2).7,11,12,17,32 Superficial neovascularization is a later finding in CL-induced LSCD and often encroaches peripherally.30,34 Corneal neovascularization likely develops, as conjunctival epithelium does not have the same anti-angiogenic properties as the corneal epithelium and is promoted by inflammation.35 At end-stages of disease, there can be recurrent/persistent epithelial defects, scarring, and profound loss of vision.17,18,32
C. Laboratory and Imaging Findings
1. Impression Cytology
Since conjunctivalization is the most reliable sign of LSCD, impression cytology is useful in cases not clinically diagnosable but with high suspicion for disease.9,22 In CL-induced LSCD, impression cytology demonstrates conjunctival goblet cells on the cornea (through detection of mucin), not normally present in patients with functioning limbal stem cells.2,9,13,31 Because first-test sensitivity in detecting goblet cells is low,9 a negative cytologic analysis does not rule out LSCD, and cytology may need to be repeated.2 Impression cytology utilizes in vivo (clinical) markers; for example, the cytokeratin in conjunctival epithelial cells (K19) differs from that in corneal epithelial cells (K3), making cytokeratin markers useful for diagnosis.13,23 One potential use for this technique in CL-induced LSCD is to evaluate for subclinical disease in a fellow eye.
2. Imaging
In vivo confocal microscopy can be used to identify LSCD by amplifying specific microstructural changes not visible on slit lamp examination, particularly goblet cells associated with conjunctivalization.2,32,36 This imaging modality provides information on disease severity and can be used in both diagnosis and monitoring of LSCD (Figure 3).32,36 However, it has several limitations, including expense, difficulty of use, and small field of view, and its use currently is limited to academic centers.2,36 Optical coherence tomography has also shown promise, as it allows a noninvasive, wide view of the eye’s surface.2,37
Figure 3. Confocal microscopy images of limbal stem cell deficiency.
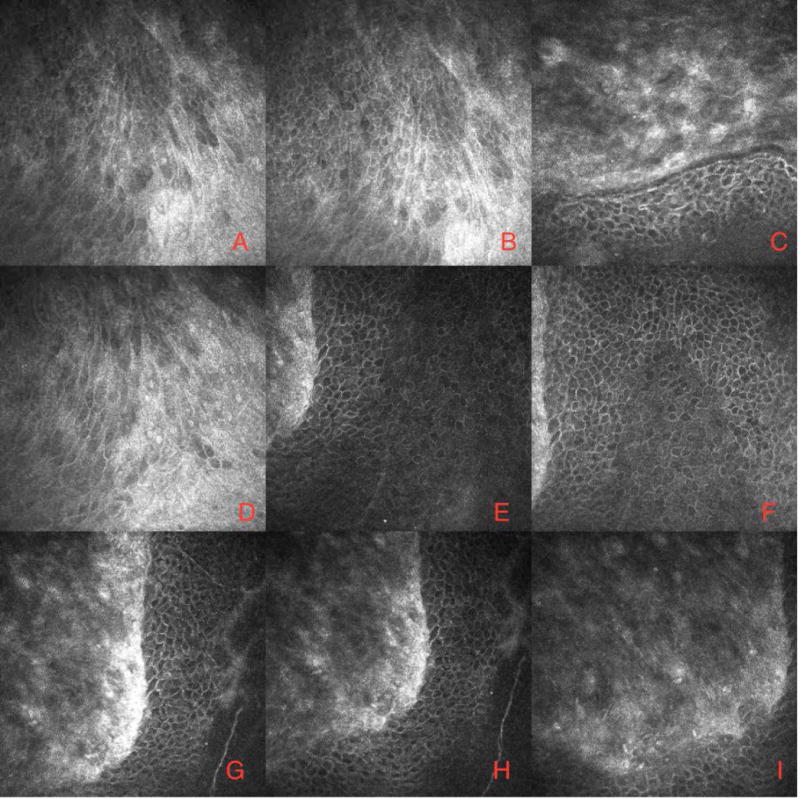
In vivo confocal microscopic appearance of the cornea in CL-induced LS CD. Confocal images demonstrating the presence of conjunctival type epithelium adjacent to the more organized basal corneal epithelial cells. (Courtesy of Drs. Alessandro Abbouda and Pedram Hamrah.)
3. Histologic Markers
While many histologic, or in vitro, markers, for LSCs have been proposed, identifying a definitive, specific marker for LSCs is an area of ongoing research.2 LSCs can be distinguished from corneal epithelium by staining characteristics. They stain positively for markers such as ABCG2 ABCB5, and DeltaNp63, and stain negatively for K3/K12 and Cx43.38 These markers, however, are not specific for stem cells in the limbus, and they are also seen in other epithelial cells (such as transit amplifying cells).38 Therefore, there is no reliable and specific marker that can be used clinically to assess the health of LSCs. Future research should lead to the identification of such a marker to allow determination of the status of LSCs more quantitatively and specifically.
V. Pathogenesis
The presence of conjunctival goblet cells on the cornea, as determined by impression cytology, is considered a diagnostic sign of LSCD.13,23 In severe LSCD, there is a significant loss of the LSCs, but milder cases result from persistent but dysfunctional LSCs.7,8,17 Typically, such partial LSCD has a mixed epithelial phenotype consisting of both corneal- and conjunctival-type epithelium on the cornea (Figure 1).7 In more advanced cases, a superficial fibrovascular pannus develops, inducing destruction of the basement membrane, neo-collagen deposition, and, ultimately, scarring of the corneal stroma (Figure 2).8,17
Recent experimental studies have shown that damage to the corneal epithelium near the limbus alters the niche, causing LSCs to inappropriately differentiate into “corneal goblet cells.”39 Therefore, it is possible that the goblet cells characteristic of “conjunctivalization” in LSCD are not entirely from invading conjunctiva but are also from abnormal differentiation of LSCs due to the altered limbal niche.7,39
The etiopathogenesis of CL-induced LSCD is likely multifactorial (Figure 4).7,11,13–15 Factors underlying and contributing to LSCD development may include mechanical trauma, dry eye, lens disinfecting solutions and/or their preservatives, and hypoxia. Inflammation plays a central role in LSCD, serving pathophysiologically as both a cause and a consequence of LSCD.7,11,35,40 In the case of CL-induced LSCD, inflammation, which is often subclinical, likely contributes to the disruption and loss of the limbal niche.7
Figure 4. Etio-pathogenesis of contact lens-induced limbal stem cell deficiency.
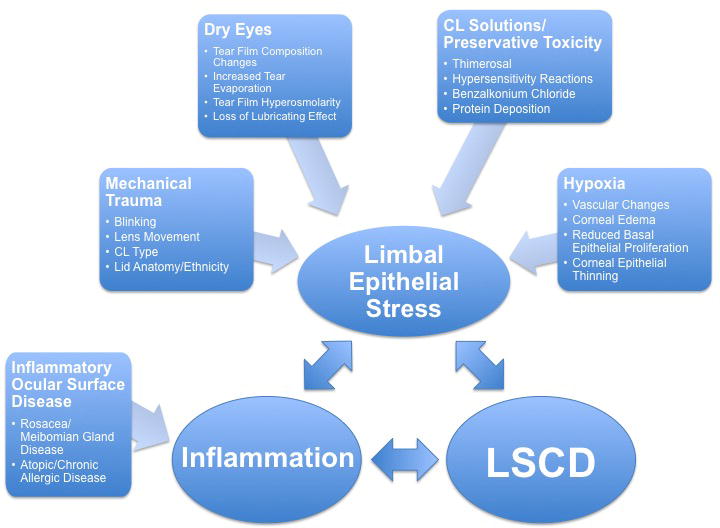
Multifactorial etiology of CL-induced LSCD. A key component is limbal epithelial stress, which may stem from a combination of dry eye, CL solutions/preservative toxicity, mechanical trauma and hypoxia. Inflammation can develop secondarily or can be due to other conditions such as rosacea/MGD and atopy.*LSCD = Limbal Stem Cell Deficiency.*CL = Contact Lens.*MGD = meibomian gland dysfunction.
A. Mechanical Trauma
Mechanical trauma due to CLs plays a central role in many cases of CL-induced LSCD. A CL moves against the ocular surface, even when properly fitted, with a movement of 0.1 mm–0.4 mm, a rate reportedly comfortable for wearers.41 Inadequate lens movement can lead to increased dryness due to decreased tear flow behind the lens causing more friction.41 When the lids move against the CL, they push the lens against the ocular surface, moving tears away, drying the surface, and creating friction.42 Blinking causes lens movement across high-pressure areas of the cornea – most notably at the superior limbus – which can induce chronic trauma.7,8,13,14,17,18,22,40 CLs less often induce epithelial trauma at the inferior limbus due to reduced eyelid pressure in this region, which may explain why LSCD is less commonly inferiorly located.13
Potentially, anatomic differences in eye and eyelid anatomy, as is often associated with ethnicity,43 could affect the extent of mechanical trauma associated with CL use. However, reports describing an ethnic disparity in CL-induced LSCD has not been reported to the best of the authors’ knowledge. It has been reported that Asian CL users tend to have higher rates of discomfort, dryness, and corneal staining than do non-Asian CL users, due to differences in anatomy and physiology.41,44,45 Tighter lids with more narrow palpebral apertures in Asian populations may increase the force of CL movement against a less steep cornea.41,44–46 Asian patients may also have physiologically dryer eyes with decreased tear film with CL use.41,45 Other patient characteristics may also contribute to the mechanical forces on the ocular surface; for example, patients with steeper corneas tend to have less CL movement.42
Certain CL characteristics can increase mechanical trauma at the ocular surface, e.g., lenses made from less flexible material (such as silicon hydrogel lenses), bulky lenses (such as those with increased oxygen permeability, knife-edge lenses (which are more closely opposed to the ocular surface than are rounded edge lenses), lenses with a steepened base curve (whose pressure is focused on the region surrounding the cornea), and poorly fitted lenses.13,42,44 Further research is needed to determine if specific types of lenses (single vision, toric, multifocal lenses) or the power of lenses are associated with increased mechanical trauma leading to LSCD. Larger diameter scleral CLs have a role in the treatment of LSCD and epithelial diseases.7,12
B. Dry Eye
Tears preserve the proper functioning of corneal epithelial cells by lubricating the eye, producing mucin and antimicrobial factors, and providing oxygen and nutrients important for corneal health.2,47 A “co-dependence” exists between tear film stability and CL interaction with the corneal surface such that a problem with either one will cause problems with the other. The decreased tear film in dry eye potentiates the traumatic effect of the CL on the ocular surface; however, the CL itself can cause disruption of the tear film as it rests on the ocular surface.47 CLs can disrupt the tear film by the following mechanisms: creating a thinner, less stable lipid layer with delayed spread, changing the tear film osmolarity (initially hypo-osmolar, then hyper-osmolar), and altering the composition of the tear film (including amounts of certain lipids, proteins [i.e., inflammatory mediators-MMP-9s], mucin, glycocalyx, antioxidants, and neutrophils).47 The amount of tear exchange around the lens is variable and based on lens and patient characteristics, including lens diameter, lens movement, blink, and the rate of tear production.47 As numerous lens characteristics (e.g., material, solutions, lens design, lens-to-cornea fitting relationship) can influence tear film, and the matrix of available combinations is nearly endless, it is difficult to fully appreciate the exact nature of how each CL affects tear film.
C. Lens Disinfecting Solutions and Preservatives
The lens cleaning solution preservative thimerosal has been strongly suspected to contribute to LSCD development in CL wearers.8,11,16,17,20,48 Thimerosal has been known to cause a number of corneal epitheliopathies, including superior limbic/diffuse keratoconjunctivitis, keratitis, and pseudodendritic lesions. Because of these adverse side effects, thimerosal use in CL disinfecting solutions was phased out in the 1980s.48 Thimerosal toxicity may, in fact, be a more severe entity distinguishable from the more typical CL-induced LSCD by cytologic analysis; squamous metaplasia is more likely to be associated with thimerosal exposure, whereas conjunctival goblet cells on the cornea are typically seen in patients with classic CL-induced LSCD.8,9
Preservatives other than thimerosal can produce a hypersensitivity reaction or epithelial disturbance with prolonged use of lens disinfecting solutions.8,17–20,24,33,49 Cleaning solutions and preservatives can adversely affect the corneal epithelium both from direct toxic effects and by inducing secondary inflammation.17,34
A few studies have directly compared lens cleaning solutions with PHMB preservatives to hydrogen peroxide- based solutions and found significantly more corneal staining in the preservative-containing lens solutions.33 It is interesting to note that patients using the same lens disinfecting solution for bilateral CLs often present with asymmetric LSCD, suggesting that other factors related to the ocular surface and/or the CL contribute to disease development.16,48 Furthermore, solutions with the same percentage of these preservatives, such as polyhexamthylene biguanide (PHMB) and its equivalents, can produce different staining patterns in patients due to other ingredients in the solutions and their interactions with various lens properties.33,49 Other ocular solutions containing preservatives, such as benzalkonium chloride (BAK), which is used in many antiglaucoma medications and ophthalmic solutions, are well known to have toxic effects on the epithelium and limbal niche, potentially leading to the development of LSCD.7,50
D. Hypoxia
Oxygen is needed to maintain and regenerate healthy corneal epithelium.51 CLs create physical barriers that prevent oxygen from reaching the cornea, leading to hypoxia,34,52 which is suggested by an elevated lactate dehydrogenase level in the tear film of CL wearers.53 Hypoxia due to CL use has been proposed as an underlying cause of LSCD.8,13,14,40,51 Hypoxia with CL use is most significant underneath the upper lid, explaining localization of disease to the superior limbus.8,14,22,40,52 We propose that this finding may be more significant with higher minus-powered lenses, which are thicker peripherally.54 The pathogenesis of hypoxia’s contribution to CL-induced LSCD is likely secondary to the limbal stress and inflammation caused by hypoxia, which are detrimental to the limbal niche.7,11,13–15 However, as discussed earlier, hypoxia by itself may not be enough to induce LSCD, and it likely becomes more significant in the setting of mechanical trauma and/or toxicity from solutions.13,14,44
E. Multifactorial Pathogenesis
Based on our understanding of how CLs cause mechanical trauma,13,41,42,44 disrupt the tear film,47 create hypoxia,34,52,53 promote inflammation,34 and are associated with irritating effects of preservatives,33,49 we propose the following hypothesis on the multifactorial pathogenesis of CL-induced LSCD.
We believe that an inadequate tear film in conjunction with CL wear contributes to LSCD development by the following mechanisms: 1) The degree of limbal trauma is increased in part due to the loss of the lubricating effect of the tears, which increases friction between the CL and the ocular surface, 2) producing more prolonged exposure to the CL solutions/preservatives due to the loss of the irrigating effect of the tears, and 3) increasing inflammation due to hypoxia, dessication, and hyperosmolarity, further contributing to the loss of a normal limbal niche. Further research is warranted to test this hypothesis.
It is unclear why some CL users develop LSCD and some do not. Chan and Holland described a “second hit” hypothesis, in which predisposing factors, such as an underlying disease process, contribute to the development of LSCD in CL wearers.11 Table 2 lists factors that may be associated with the development of CL-induced LSCD.
Table 2.
Factors that may be associated with CL-induced LSCD*
| Diseases that lead to chronic inflammation at the ocular surface (i.e. meibomian gland disease/rosacea or chronic allergic disease)7,11 |
| Duration of CL use and hours of daily wear11,13–15,22 |
| Female gender11,13–15,24 |
| Corneal surface differences (i.e. less steep cornea)42 |
| Eyelid anatomy differences (i.e tighter lids with more narrow palpebral apertures in Asian populations)41,44–46 |
In an attempt to understand why some CL wearers develop CL-induced LSCD, and others do not, we have developed a list of patient factors that may predispose to disease development. Some of these factors have been correlated with CL-induced LSCD (underlying diseases,7,11 duration of CL use and hours of daily wear,11,13–15,22 female gender,11,13–15,24) while other factors (corneal surface42 and eyelid anatomy differences41,44–46) have shown to increase some of the believed pathophysiologic causes of CL-induced LSCD (such as mechanical trauma). Further research is needed to explore these potential factors to determine if they do predispose to CL-induced LSCD.
We propose that with multiple processes contributing to the development of CL-induced LSCD, the exact pathogenesis of disease among patients is not identical. We believe that the specific constellation of risk factors and inciting causes vary from patient to patient, and the degree and localization of CL-induced LSCD differs based on each patient’s unique situation. For example, we hypothesize that patients with more diffuse 360-degree disease may be more likely to have solution toxicity, whereas patients whose disease is related to mechanical trauma from the CL may have more focal involvement of the superior cornea. It is nonetheless possible that solution toxicity would only manifest in the superior limbus, given the added stress of hypoxia in that area. Likewise, it is theoretically possible that certain lens designs/edges can contribute to mechanical trauma of the entire limbus and, hence, the development of 360-degree disease. These theories are yet to be evaluated by clinical research.
VI. Differential Diagnosis
The differential diagnosis of CL-induced LSCD includes chronic corneal epitheliopathy, superior limbic keratoconjunctivitis, and conjunctival/corneal intraepithelial neoplasia.8,13,17,18,55 Superior limbic keratoconjunctivitis (SLK), collectively described by Theodore in the 1960s (before the widespread use of CLs), has many similarities to LSCD.56 SLK and CL-induced LSCD are both characterized by limbal inflammation and superficial punctate keratitis in the superior limbus. Likewise, while the pathogenesis of both conditions involves trauma and dry eye,8,17,56 SLK differs from CL-induced LSCD in that SLK is more consistently bilateral, often associated with thyroid dysfunction (not CL use), and resolves with silver nitrate/cauterization of the conjunctiva, large diameter bandage CLs, or superior perilimbal conjunctival resection.8 Conjunctival/corneal intraepithelial neoplasia presents often as a raised, circumscribed vascularized growth, which can be diagnosed by exfoliative cytology or impression cytology with findings of hyperplastic, dysplastic, or anaplastic cells.57
It is important to distinguish corneal neovascularization that takes place in LSCD from CL-induced peripheral neovascularization, in which the corneal epithelium is typically normal; furthermore, up to 1–2 mm of peripheral neovascularization is not considered abnormal in CL wearers.34,58,59 Peripheral neovascularization, which is primarily due to hypoxia, inflammation, and edema, does not directly induce changes in the epithelium34,59 unless there is co-existing LSCD. Corneal neovascularization can also occur secondary to other, non-CL-related, causes, such as congenital disease (i.e., aniridia), inflammatory conditions (i.e., Stevens-Johnson syndrome), infections, degenerative diseases, and trauma.58
LSCD is only one disease process that stains positively with fluorescein, as this staining technique is often used to identify corneal defects. However, as opposed to delineated defects visualized with staining corneal defects, such as ulcers, LSCD has a characteristic late-staining, often whorl-like, fluorescein pattern of conjunctivalization (Figures 1 and 2).2,7,9,11,13,14,31,32 The clinician should be aware that CL users might have abnormal fluorescein staining in a punctate pattern up to 4–6 hours after inserting a CL because of the preservatives in lens cleaning solutions.60,61
VII. Conservative/Medical Management
Randomized controlled trials designed to establish guidelines for CL-induced LSCD management are lacking. Reported outcomes following conservative/medical management in several studies and case reports on CL-induced LSCD vary widely, possibly because of the wide range of disease severity.
A. Discontinuance of Contact Lens Wear
Conservative treatment includes first and foremost complete discontinuation of CL use.7,11,13,14 Jeng et al found that 11 of 18 eyes stabilized or improved following cessation of CL wear.14 Stenson reported 4 cases of CL-induced LSCD (some with thimerosal or other preservative exposure) that responded to discontinuation of CL use.19 Fuerst et al reported on 13 patients with CL-induced LSCD who had slow symptomatic improvement with discontinuation of CL use; 54% of these patients also received silver nitrate treatment.24
Conservative management alone is not always sufficient to reverse LSCD. In one series, only 1 of 6 patients with CL-induced LSCD associated with thimerosal use improved following cessation of CL use,16 and in another 12 of 12 patients with severe CL-induced LSCD failed conservative treatment.11
B. Treatment of Dry Eye
Dry eye is a significant risk factor for CL-induced LSCD, and tear film dysfunction is often seen in patients with LSCD.2,47 Dry eye should be treated aggressively in any patient with CL-induced LSCD with preservative-free lubrication to promote epithelial repair and improve the ocular surface milieu.7,8,11,14,20 Achong and Caroline reported one case in which cessation of CL use and artificial tear application led to full symptom resolution.8 Sendele et al reported 40 patients with CL-induced LSCD exposed to thimerosal who showed resolution within weeks to months with conservative treatment including CL discontinuation and artificial tear use.20 In those with blepharitis and meibomian gland dysfunction, aggressive treatment with lid hygiene/warm compresses as well as medications, such as doxycycline, are useful.7 Recent evidence has also shown improvement in dry eye symptoms and tear film breakup time in CLs wearers given oral omega-3 fatty acid supplementation.62
In patients who have failed these first line therapies for dry eye, more advanced treatments that promote epithelial health may be considered. These include topical vitamin A (0.01%) and autologous serum tears.7,11,14 The mechanism of action of these treatments provides insight into their efficacy in treating LSCD: vitamin A (retinoic acid) induces epithelial differentiation,63 while serum tears contain growth factors and “nutrients” that enhance epithelial function.64 In a series by Kim et al, almost all the patients had tear film deficiencies requiring treatment with preservative-free tears and lid hygiene, sometimes also with vitamin A ointment (2 eyes with CL-induced LSCD) or punctal/cautery occlusion (4 patients with CL-induced LSCD).7 While generally efficacious in the treatment of dry eye, retinoic acid has not demonstrated efficacy in all cases of CL-induced LSCD,10,16 suggesting that additional therapies may be necessary.
C. Anti-inflammatory Treatment
Since inflammation contributes to CL-induced LSCD, anti-inflammatory medications, including topical corticosteroids and topical cyclosporine, can be utilized.7,11,14,26 Kim et al reported success with use of corticosteroids and cyclosporine in 13 eyes with CL-induced LSCD.7 The authors also described effective treatment of 2 patients with CL-induced LSCD and rosacea with lid hygiene and oral doxycycline, both of which can improve the tear film and counteract inflammation. Lim and Wei reported a case in which discontinuation of CL use and 5 months of topical steroids led to full bilateral symptom resolution.26 Jeng et al reported 5 eyes stabilizing or improving with cessation of CL wear and topical corticosteroids.14 Bloomfield et al reported a patient who initially responded to discontinuation of CL use and administration of fluorometholone, but resumed CL use and was lost to follow-up for one year.17 She returned with an inflammatory pannus covering nearly the entire cornea. Her irritation and photophobia improved with patching and topical cortisone, but she ultimately required penetrating keratoplasty to correct the permanent structural changes and improve acuity. Of note, the diagnosis of LSCD was unconfirmed in this case,17 and, as discussed in section VIII.G, penetrating keratoplasty is not an effective monotherapy for LSCD. Sendele et al discontinued use of antibiotics and corticosteroids when they found that they did not affect outcomes in patients with CL-SLK.20
D. Changing Contact Lens Type and/or Care
Initially, patients should wear glasses for refractive error. Some patients who have complete resolution of their LSCD may be able to return to soft CL use, particularly if the offending agent can be eliminated.19,20 For instance, patients who developed the disease while using thimerosal-containing cleaning solutions may be able to return to soft CL use after switching to preservative-free cleaning solutions or those that are heat-based.20 Changing the type of lens, ensuring proper fit of CLs, and/or decreasing usage time may also allow resumption of CL use.19 Disposable silicone hydrogel13,52 and rigid gas permeable13 lenses have been used for refractive error management after CL-induced LSCD. CL use should be promptly discontinued at any sign of disease recurrence.19,24 Refractive surgery, including laser in situ keratomileusis (LASIK), has been undertaken safely in a few reported patients who had previously undergone treatment for CL-induced LSCD.13,14,26
In our opinion, the main type of CL that may have a therapeutic role in CL-induced LSCD are scleral lenses, particularly those large enough to vault the limbus and avoid limbal trauma. In the series by Kim et al, 1 of 16 eyes with medically reversible LSCD in soft CL users was treated with a PROSE lens.7 Schornack reported successful treatment of CL-induced LSCD with a scleral lens in one patient.12 We have collectively treated at least 10 patients with CL-induced LSCD using scleral/PROSE lenses with good results, substantiating their therapeutic utility (unpublished data).
E. Summary
Conservative management is a reasonable first step in treating patients with CL-induced LSCD, as many cases are medically reversible.7,8,14,19,20,24,26 In addition to discontinuing CL wear bilaterally, maintaining an adequate tear film is essential for treatment.7,8,11,14,20 For mild cases, aggressive treatment of the tear film with preservative-free artificial tears should be initiated, along with treatment of lid disease, followed next by topical corticosteroids, then by cyclosporine (during steroid wean), punctal plugs, vitamin A, and autologous serum tears.7 For more severe disease, simultaneous use of multiple treatments can be considered, with a regimen including preservative-free artificial tears, vitamin A, topical steroids, oral doxycycline, and topical cyclosporine.15 Conservative and medical treatment can be continued for months to years, as long as the patient exhibits continued evidence of clinical improvement.7 In most cases that respond to therapy, there is progressive regression of the opaque, late-staining epithelium (Figure 5). When patients fail to respond to therapy, more invasive treatments are warranted. Supplemental medical treatments, especially those targeted at improving the tear film and inflammation, should be continued, as patients with dry eye and inflammation have poorer surgical outcomes.2,7,15
Figure 5. Response to treatment in CL-induced LSCD.
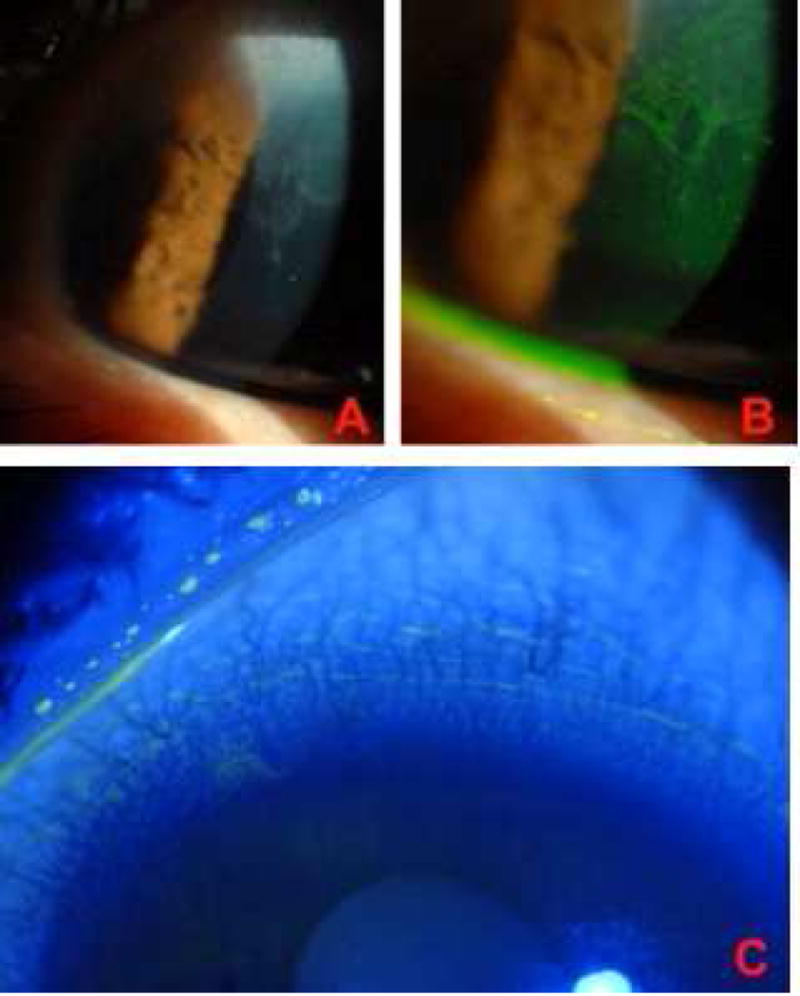
A, B. Patient with CL-induced LSCD presenting with opaque epithelial sheet from superior limbus extending into visual axis with visual acuity reduced to 20/100. C. The same patient after 6 months of treatment which included completely stopping CL wear, topical steroids, cyclosporine and punctal occlusion. Final visual acuity was 20/25.
VIII. Surgical Management
When conservative management alone fails, surgical management may be considered. Surgical management options for LSCD include mechanical debridement, amniotic membrane transplant, autologous LSC transplant, allograft LSC transplant, ex vivo cultivated LSC, phototherapeutic keratectomy, and penetrating keratoplasty (Table 3).
Table 3.
Surgical Treatment Options for CL-induced LSCD
| Surgical Treatment | Use for CL-induced LSCD |
|---|---|
| Mechanical Debridement | Adjuvant treatment to remove corneal conjunctivalization10,14,20,28,66 |
| Amniotic Membrane Transplant (AMT) | Primary surgical treatment for partial LSCD or adjuvant treatment to limbal transplant for total LSCD14,28,31,48,66 |
| Autologous Limbal Stem Cell Transplant | - Contralateral Eye Donor: Use with caution and proper patient counseling; in clinically unilateral disease, the fellow eye often has subclinical disease. Consider simple limbal epithelial transplant (SLET) technique, which harvests less donor tissue.10,14,16,25,65,68,69 - Ipsilateral Eye Donor: Use in patients with partial deficiency localized to one area (oftentimes the disease is superior and the donor site is inferior)70 |
| Allograft Limbal Stem Cell Transplant | Donor tissue from cadaver or living relative. Monotherapy or in conjunction with AMT or PK for severe disease10,11,16,48,66 |
| Cultivated Limbal Epithelial Stem Cells | Considered at facilities that have the resources to expand in vitro donor epithelial cells in the same patient population of other limbal stem cell transplant patients.75–77 |
| Phototherapeutic keratectomy (PTK) | Adjuvant laser therapy to improve superficial stromal scarring78,79 |
| Penetrating Keratoplasty (PK) | Adjuvant corneal transplant completed only after limbal stem cell disease has resolved or has been treated with limbal transplantation8,9,11,17,31,48,66,69 |
A. Mechanical Debridement
Mechanical debridement, also known as superficial keratectomy, is a procedure performed in an office setting under topical anesthesia. It has not been shown to be consistently effective as monotherapy for CL-induced LSCD; although patients may have immediate symptomatic improvement, recurrence is frequent.14,18 Mechanical debridement may be useful in conjunction with other treatment modalities, such as lubrication and topical steroids20 or other surgical treatments10,14,28,65,66 to help restore a more normal ocular surface environment.
B. Amniotic Membrane Transplantation
Amniotic membrane transplantation (AMT) for LSCD was first described by Kim and Tseng in 1995.67 Human amniotic membranes provide a nourishing environment for the growth of LSCs and beneficial anti-inflammatory properties.28,31 Although the exact mechanism is unclear, amniotic membranes appear to help repair the limbal niche, returning proper functioning to LSCs. AMT may be utilized alone for partial LSCD or in conjunction with limbal transplantation for severe cases of complete LSCD.2,31
The AMT procedure for CL-induced LSCD involves first making a conjunctival peritomy at the affected limbus, followed by scraping of the abnormal epithelium over the involved cornea.28 The amniotic membrane is then draped over the conjunctiva and denuded cornea and either sutured in place28 or, preferably, attached with fibrin glue.14
Multiple reports have documented success in treating CL-induced LSCD with AMT.14,28,31,48 AMT without limbal graft was shown to improve CL-induced LSCD in one casereport (also with debridements and subconjunctival bevacizumab)14 and in two mildly affected eyes in a second study.31 Anderson et al noted long-term symptomatic relief and improved visual acuity in one patient with bilateral CL-induced LSCD who received superficial keratectomy of the conjunctivalization followed by AMT.28 AMT was used successfully in conjunction with a keratolimbal allograft followed by penetrating keratoplasty in one case of CL-induced LSCD associated with thimerosal use.48 Tseng et al reported a patient with severe CL-induced LSCD who received AMT, limbal allograft, and penetrating keratoplasty and ultimately developed irreversible PK rejection.31 Solomon et al also reported using AMT in addition to limbal allograft for their LSCD patients, including two patients with CL-induced LSCD.66
The main advantages of AMT are reduced risk of rejection and avoidance of donor eye complications and systemic immunosuppression.8,28,31 AMT requires at least partial preservation of LSCs and thus should not be utilized as the sole treatment for complete LSCD.8,28,31
C. Autologous Limbal Stem Cell Transplantation
The first successful reports of autologous limbal transplantation were by Kenyon and Tseng in 1989. The procedure is used for unilateral LSCD, with donor limbus being harvested from the patient’s healthy contralateral eye and transplanted onto the diseased eye.68 The initial report included 26 cases of LSCD. Of these, 3 were secondary to CL wear, and all 3 showed improvement in visual acuity following transplantation.68 There have been some reported successes with autologous conjunctival transplantation as well, given that the transplanted conjunctiva may contain some LSCs.14,25 Clinch and colleagues reported two cases of CL-induced corneal epithelial abnormalities that improved following autologous limbal conjunctival transplantation.25 Jeng and colleagues reported a good visual outcome in a patient who underwent superficial keratectomy combined with conjunctival autograft.14
Other studies have shown high complication rates with autologous limbal transplantation for CL-induced LSCD. Tan et al reported 18 cases, 6 of which were CL-induced. Four were treated with autologous limbal transplantation with initial improvement. At follow-up, 2 of 4 had developed complications in the recipient eye and 1 of 4 had developed superior epithelial abnormalities in the donor eye.10 Similarly, Jenkins reported initial visual improvement in 4 of 4 patients treated for thimerosal-associated CL-induced LSCD with limbal autograft, but, at follow-up, 3 of 4 patients demonstrated conjunctivalization in the recipient eye and 1 of 4 had complications in the donor eye.16 The only patient in that study who had a favorable response to autologous transplant wore a CL unilaterally in the affected eye only. Dua and Azuara-Blanco reported 6 cases of autograft for LSCD, with 1 case associated with CL wear and multiple ocular surgeries.69 This patient also underwent penetrating keratoplast6y with simultaneous LSC transplantation and had multiple complications from the initial surgery, including glaucoma and corneal graft failure in the recipient eye and filamentary keratitis in the donor eye.
A major benefit of autologous LSC transplantation is that it does not require systemic immunosuppression. However, this method does present the risk of damage to the patient’s donor eye, including possible conjunctivalization and LSCD.10,16,69 Moreover, the patient’s donor eye may have subclinical LSCD, which is quite likely in patients who have worn CLs bilaterally.10,11,16 Subclinical LSCD worsened by surgery may account for the reported complications seen in the donor eyes of autologous limbal transplantation for patients with CL-induced LSCD.10,11,16,69 As a result, autologous transplantation is generally not recommended for patients with LSCD who have worn CLs bilaterally.11,15
Other autologous transplantation options include ipsilateral limbal grafting from the inferior (presumably less affected area) to the superior limbus. This may be accomplished with a smaller graft that does not significantly compromise the health of the remaining limbus. Ipsilateral limbus grafting for LSCD was described by Nishiwaki-Dantas et al, who effectively treated five patients with LSCD secondary to chemical burns.70 The newly described technique of simple limbal epithelial transplantation (SLET) may be considered, as it minimizes the amount of donor tissue that needs to be harvested. Sangwan et al described the SLET technique used for 6 patients with LSCD secondary to burns, in which less donor tissue from the contralateral eye is taken, divided, and placed on amniotic membrane prior to transplantation to the affected eye.65
D. Allograft Limbal Stem Cell Transplantation
Keratoepithelioplasty, a precursor to allograft limbal transplant, was described by Thoft and colleagues in 1984 and 1990 for the treatment of persistent epithelial defects.71,72 Cadaver donor tissue from the peripheral corneal and limbal epithelium was transplanted at the limbus on the recipient eye following superficial keratectomy.
Allograft limbal transplantation has also been reported for the treatment of CL-induced LSCD.10,11,15,16,48,66 In this method of limbal transplantation, donor tissue is taken from either a cadaver (keratolimbal allograft, KLAL) or a living relative (HLA-matched living-related conjunctival limbal allograft, lr-CLAL), and thus may be used in patients with bilateral disease.2,10,16 In a 1993 study by Jenkins et al, 1 patient with CL-induced LSCD underwent living related allograft transplantation for 1 eye.16 There was concern that the epithelium was failing at 1-month follow-up, but with intensive topical steroid therapy the epithelium improved and the patient had improved visual acuity at 3-month follow-up. In a 1996 study by Tan et al, 2 of 6 patients with CL-induced LSCD had improved visual acuities following allograft LSC transplantation: 1 with a living related donor and 1 with a cadaver donor.10 Chan and Holland reported 12 of 14 patients with CL-induced LSCD who received limbal allograft (4 with living-related donor, 10 with cadaver donor), resulting in alleviation of symptoms and improved visual acuities.11 Nguyen reported one patient with CL-induced LSCD who had successful treatment with KLAL and AMT followed by PK 17 months later, resulting in visual acuity of 20/30.48 Shen et al described 9 patients (14 eyes), with living-related (29%) donor or cadaver donor (71%) keratolimbal allograft surgery for CL-induced LSCD, with 86% of eyes having significant clinical improvement and best-corrected visual acuity of at least 20/30.15
Cadaver allograft limbal transplantation eliminates the risk of inducing LSCD in a relative’s donor eye.16 Complications following allograft transplantation include acute or chronic rejection, ocular hypertension, and complications from prolonged immunosuppression.10,16,73 The most important factor in the success of allograft transplantation is proper use of systemic immunosuppressive therapy, which has an acceptably low risk of adverse effects and toxicity when managed in close collaboration with an organ transplantation specialist.2,10,11,15,16,73,74 One study reported an average of 42.1 months of immunosuppressive therapy in LSCD patients treated with allograft limbal transplantation; the most common agents used were tacrolimus, mycophenolate mofetil, and a short course of prednisone.73
E. Cultivated Limbal Epithelial Cells
Lindberg and Pellegrini first described in vitro expanded limbal epithelial cell transplantation for LSCD.75,76 In this technique, donor limbal epithelial cells (from the patient, living relative, or cadaver) are expanded as an epithelial sheet ex vivo to be transplanted.2,77 In other studies, limbal epithelial cells have been cultivated from other anatomic stem cell sources, including oral mucosal epithelium and conjunctival epithelium (among others).2 Basu et al conducted a retrospective review on allogeneic cultivated limbal epithelial transplantation for bilateral and total LSCD (2 of 21 due to CL-induced LSCD) with an overall success rate of 71.4%.77
Advantages of cultivated limbal epithelial cells include decreased requirement for donor tissue, shortened epithelialization time following transplantation, and a paucity of immune factors in the transplanted cells leading to a decreased risk of autoimmune reaction.2,77 The primary disadvantage of this technique is the time and cost required to cultivate limbal epithelial cells in a specialized facility.2
F. Phototherapeutic Keratectomy
Superficial stromal scarring that can develop with LSCD can be treated with laser therapy in an attempt to avoid keratoplasty.78,79 Phototherapeutic keratectomy (PTK) using an excimer laser has been shown to remove anterior corneal scars, including those due to LSCD.78,79 In our opinion, it is best to use PTK after LSCD has been treated, in part because stromal scarring may largely regress after a stable corneal epithelium has been restored.
G. Penetrating Keratoplasty
Corneal transplantation monotherapy for LSCD provides only temporary improvement due to the absence of LSCs in the graft and hence an increased risk of failure due to recurrent corneal conjunctivalization.8,9,11 However, penetrating keratoplasty (PK) can be used as adjuvant therapy following other treatments that improve the limbal niche.17,31,48,66,69 Bloomfield et al reported the successful treatment of a patient with possible CL-induced LSCD treated with corticosteroids and PK (the limitations of this study were discussed in Section VII.C).17 Nguyen at al reported a patient with CL-induced LSCD, who improved with limbal transplantation and AMT followed by PK.48 A study by Tseng et al included a patient with severe limbal deficiency secondary to CL use, who received AMT followed by allograft limbal transplantation and PK with eventual PK rejection.31 Dua and Azuara-Blanco’s study included one patient with CL-induced LSCD who had PK at the time of autologous limbal transplantation and then required a second PK. Although outcome was initially favorable, the patient later developed retinal detachment with significant visual loss.69 In general, because patients with CL-induced LSCD have normal endothelium, if keratoplasty is necessary for the scarring, a deep anterior lamellar keratoplasty would be preferred, but only after the LSC disease has resolved or has been treated with limbal transplantation.
H. Summary
If no improvement is seen or the disease continues to progress with medical management, surgical treatments should be considered while conservative measures aimed at nourishing the limbal niche are continued.7 If a patient has partial LSCD, AMT may be considered to improve the limbal niche,14,28,31 or AMT can be used as adjuvant therapy to limbal transplantation in more severe disease.31,47,66 Autologous limbal transplantation from the contralateral eye is generally not recommended, even in seemingly unilateral cases, due to the high risk of subclinical LSCD in the donor eye with a history of bilateral CL wear.10,11,14–16 When LSCD is total and bilateral, allogeneic limbal transplantation (from cadaver or living-related donor) is often the treatment of choice.10,11,15,16,48,66 If corneal clarity does not return after treatment of LSCD, supplementary procedures include mechanical debridement,10,14,20,28,66 phototherapeutic keratectomy,77,78 and deep anterior lamellar keratoplasty.17,31,48,66,69 Our proposed treatment algorithm for CL-induced LSCD is depicted in Figure 6. Further research is needed to test the efficacy of our treatment model.
Figure 6. Proposed treatment algorithm for CL-induced LSCD.
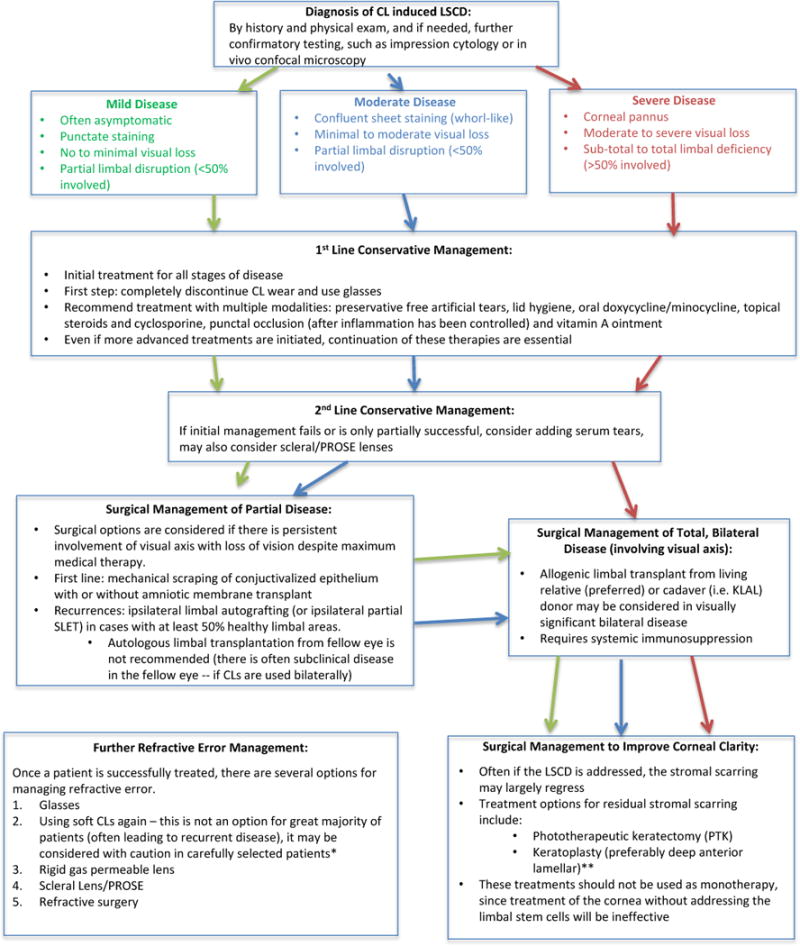
*Return to soft CL use can be considered in patients who will comply with good follow-up and most often in patients where disease is suspected to develop secondary to preservative-containing lens cleaning solutions. These patients should use preservative-free cleaning methods (such as hydrogen peroxide solution), switch to disposable daily lenses, ensure proper fit, and decrease amount of daily wear time. Any recurrence of disease should lead to prompt discontinuation of wear.
**Keratoplasty will only be successful long-term in patients with a healthy limbal niche; it cannot be utilized until LSCD has resolved or been fully treated with limbal transplant.
Note: Further research is needed to test the efficacy of our treatment model.
IX. Limitations of This Review
The major limitation of this review paper is the paucity of bench and clinical research on CL-induced LSCD. The exact pathogenesis of the disease is yet to be proven. We have provided relevant research on the effects of CL wear on the ocular surface and have hypothesized in regard to the multifactorial pathogenesis of CL-induced LSCD. Further research is warranted to evaluate the validity of our hypothesis. We have presented the research on the efficacy of conservative and surgical treatments of CL-induced LSCD and have provided a suggested treatment approach. More research is needed to test various treatment modalities and proposed algorithms. Avenues for further research on CL-induced LSCD include evaluation of underlying pathogenesis, creation of a standardized severity grading, and development of treatment guidelines to better understand, identify, and treat this disease.
X. Conclusion
CL-induced LSCD is a lesser-known condition due to its often asymmetric, asymptomatic, and subclinical presentation. Thus, it is essential that patients wearing CLs, particularly soft CLs, receive annual examinations with a high degree of suspicion for the condition. The true pathogenesis is unknown, but it is likely multifactorial and leads to a wide range of disease severity with varying response to treatment. Medical treatment consists of discontinuing CL use, restoring tear film, and decreasing inflammation. More advanced treatment methods include AMT or LSC transplantation, in addition to adjuvant therapy to improve corneal clarity. More research is needed to further understand the true prevalence and pathogenesis of the disease and to develop a standardized treatment regimen.
Acknowledgments
We would like to acknowledge Margaret Chervinko, MFA, MLIS, for her help in locating and retrieving publications for the literature review.
This work was supported by R01 EY024349-01A1 to ARD, a core grant EY01792 from National Institute of Health and an unrestricted grant from Research to Prevent Blindness. ARD is the recipient of a Career Development Award from Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no commercial or proprietary interest in any concept or product discussed in this article.
References
- 1.Schermer A, Galvin S, Sun T-T. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Utheim TP. Limbal epithelial cell therapy: past, present, and future. In: Wright B, Connon CJ, editors. Methods In Molecular Biology: Cornea Regenerative Medicine: Methods and Protocols. New York, NY: Humana Press; 2013. pp. 3–43. [DOI] [PubMed] [Google Scholar]
- 3.Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983;24:1442–3. [PubMed] [Google Scholar]
- 4.Kruse FE, Chen JJY, Tsai RJF, Tseng SCG. Conjunctival transdifferentiation is due to the incomplete removal of limbal basal epithelium. Invest Ophthalmol Vis Sci. 1990;31:1903–13. [PubMed] [Google Scholar]
- 5.Majo F, Rochat A, Nicolas M, et al. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature. 2008;13(7219):456. 250–4. doi: 10.1038/nature07406. [DOI] [PubMed] [Google Scholar]
- 6.Dua HS, Miri A, Alomar T, et al. The role of limbal stem cells in corneal epithelial maintenance: testing the dogma. Ophthalmology. 2009;116:856–63. doi: 10.1016/j.ophtha.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 7.Kim BY, Riaz KM, Bakhtiari P, et al. Medically reversible limbal stem cell disease: clinical features and management strategies. Ophthalmology. 2014;121:2053–8. doi: 10.1016/j.ophtha.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Achong RA, Caroline P. Limbal stem cell deficiency in a contact lens-related case. Clin Eye Vis Care. 1999;11:191–7. [Google Scholar]
- 9.Puangsricharern V, Tseng SC. Cytologic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology. 1995;102:1476–85. doi: 10.1016/s0161-6420(95)30842-1. [DOI] [PubMed] [Google Scholar]
- 10.Tan DT, Ficker LA, Buckley RJ. Limbal transplantation. Ophthalmology. 1996;103:29–36. doi: 10.1016/s0161-6420(96)30737-9. [DOI] [PubMed] [Google Scholar]
- 11.Chan CC, Holland EJ. Severe limbal stem cell deficiency from contact lens wear: patient clinical features. Am J Ophthalmol. 2013;155:544–9. doi: 10.1016/j.ajo.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Schornack MM. Limbal stem cell disease: management with scleral lenses. Clin Exp Optom. 2011;94:592–4. doi: 10.1111/j.1444-0938.2011.00618.x. [DOI] [PubMed] [Google Scholar]
- 13.Martin R. Corneal conjunctivalisation in long-standing contact lens wearers. Clin Exp Optom. 2007;90:26–30. doi: 10.1111/j.1444-0938.2006.00083.x. [DOI] [PubMed] [Google Scholar]
- 14.Jeng BH, Halfpenny CP, Meisler DM, Stock EL. Management of focal limbal stem cell deficiency associated with soft contact lens wear. Cornea. 2011;30:18–23. doi: 10.1097/ICO.0b013e3181e2d0f5. [DOI] [PubMed] [Google Scholar]
- 15.Shen C, Chan CC, Holland EJ. Limbal stem cell transplantation for soft contact lens wear-related limbal stem cell deficiency. Am J Ophthalmol. 2015;160:1142–9. doi: 10.1016/j.ajo.2015.07.038. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins C, Tuft S, Liu C, Buckley R. Limbal transplantation in the management of chronic contact-lens-associated epitheliopathy. Eye (Lond) 1993;7(Pt 5):629–33. doi: 10.1038/eye.1993.145. [DOI] [PubMed] [Google Scholar]
- 17.Bloomfield SE, Jakobiec FA, Theodore FH. Contact lens-induced keratopathy: a severe complication extending the spectrum of keratoconjunctivitis in contact lens wearers. Ophthalmology. 1984;91:290–4. doi: 10.1016/s0161-6420(84)34308-1. [DOI] [PubMed] [Google Scholar]
- 18.D’Aversa G, Luchs JL, Fox MJ, et al. Advancing wave-like epitheliopathy: Clinical features and treatment. Ophthalmology. 1997;104:962–9. doi: 10.1016/s0161-6420(97)30199-7. [DOI] [PubMed] [Google Scholar]
- 19.Stenson S. Superior limbic keratoconjunctivitis associated with soft contact lens wear. Arch Ophthalmol. 1983;101:402–4. doi: 10.1001/archopht.1983.01040010402010. [DOI] [PubMed] [Google Scholar]
- 20.Sendele DD, Kenyon KR, Mobilia EF, et al. Superior limbic keratoconjunctivitis in contact lens wearers. Ophthalmology. 1983;90:616–22. doi: 10.1016/s0161-6420(83)34507-3. [DOI] [PubMed] [Google Scholar]
- 21.Nichols JJ. Contact lenses 2013. Contact Lens Spectr. 2013;29:22–8. [Google Scholar]
- 22.Bhatia RP, Srivastava R, Ghosh A. Limbal stem cell study in contact lens wearers. Ann Ophthalmol (Skokie) 2009 Summer;41(2):87–92. [PubMed] [Google Scholar]
- 23.Donisi PM, Rama P, Fasolo A, Ponzin D. Analysis of limbal stem cell deficiency by corneal impression cytology. Cornea. 2003;22:533–8. doi: 10.1097/00003226-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Fuerst DJ, Sugar J, Worobec S. Superior limbic keratoconjunctivitis associated with cosmetic soft contact lens wear. Arch Ophthalmol. 1983;101:1214–6. doi: 10.1001/archopht.1983.01040020216010. [DOI] [PubMed] [Google Scholar]
- 25.Clinch TE, Goins KM, Cobo LM. Treatment of contact lens-related ocular surface disorders with autologous conjunctival transplantation. Ophthalmology. 1992;99:634–8. doi: 10.1016/s0161-6420(92)31925-6. [DOI] [PubMed] [Google Scholar]
- 26.Lim L, Wei RH. Laser in situ keratomileusis treatment for myopia in a patient with partial limbal stem cell deficiency. Eye Contact Lens. 2005;31:67–9. doi: 10.1097/01.icl.0000146302.00152.47. [DOI] [PubMed] [Google Scholar]
- 27.Morgan PB, Efron N, Woods CA, et al. International contact lens prescribing in 2005. Contact Lens Spectr. 2006;21:35–9. [Google Scholar]
- 28.Anderson DF, Ellies P, Pires RT, Tseng SC. Amniotic membrane transplantation for partial limbal stem cell deficiency. Br J Ophthalmol. 2001;85:567–75. doi: 10.1136/bjo.85.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldberg MF, Bron AJ. Limbal palisades of Vogt. Trans Am Ophthalmol Soc. 1982;80:155–71. [PMC free article] [PubMed] [Google Scholar]
- 30.Dua HS, Gomes JA, Singh A. Corneal epithelial wound healing. Br J Ophthalmol. 1994;78:401–8. doi: 10.1136/bjo.78.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tseng SCG, Prabhawat P, Barton K, et al. Aminiotic membrane transplantaton with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998;116:431–41. doi: 10.1001/archopht.116.4.431. [DOI] [PubMed] [Google Scholar]
- 32.Deng SX, Sejpal KD, Tang Q, et al. Characterization of limbal stem cell deficiency by in vivo laser scanning confocal microscopy: a microstructural approach. Arch Ophthalmol. 2012;130:440–5. doi: 10.1001/archophthalmol.2011.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garofalo RJ, Dassanayake N, Carey C, et al. Corneal staining and subjective symptoms with multipurpose solutions as a function of time. Eye Contact Lens. 2005;31:166–74. doi: 10.1097/01.icl.0000152489.99455.db. [DOI] [PubMed] [Google Scholar]
- 34.Arentsen JJ. Corneal neovascularization in contact lens wearers. Int Ophthalmol Clin. 1986 Spring;26(1):15–23. doi: 10.1097/00004397-198602610-00005. [DOI] [PubMed] [Google Scholar]
- 35.Lim P, Fuchsluger TA, Jurkunas UV. Limbal stem cell deficiency and corneal neovascularization. Semin Ophthalmol. 2009;24:139–48. doi: 10.1080/08820530902801478. [DOI] [PubMed] [Google Scholar]
- 36.Miri A, Alomar T, Nubile M, et al. In vivo confocal microscopic findings in patients with limbal stem cell deficiency. Br J Ophthalmol. 2012;96:523–9. doi: 10.1136/bjophthalmol-2011-300551. [DOI] [PubMed] [Google Scholar]
- 37.Espana EM, Djalilian AR, Yoo SH, Romano A. En face optical coherence tomography imaging of corneal limbal stem cell niche. In: Lumbroso B, Huang D, Romano A, et al., editors. Clinical En Face OCT Atlas. New Delhi, India: Jaypee Brothers Medical Publishers (P) Ltd; pp. 77–9. [Google Scholar]
- 38.Schlötzer-Schrehardt U, Kruse FE. Identification and characterization of limbal stem cells. Exp Eye Res. 2005;81:247–64. doi: 10.1016/j.exer.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 39.Pajoohesh-Ganji A, Pal-Ghosh S, Tadvalkar G, Stepp MA. Corneal goblet cells and their niche: implications for corneal stem cell deficiency. Stem Cells. 2012;30:2032–43. doi: 10.1002/stem.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holland EJ, Schwartz GS. Iatrogenic limbal stem cell deficiency. Trans Am Ophthalmol Soc. 1997;95:95–110. doi: 10.1007/0-387-21570-0_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Truong TN, Graham AD, Lin MC. Factors in contact lens symptoms: evidence from a multistudy database. Optom Vis Sci. 2014;91:133–41. doi: 10.1097/OPX.0000000000000138. [DOI] [PubMed] [Google Scholar]
- 42.Holden BA, Stephenson A, Stretton S, et al. Superior epithelial arcuate lesions with soft contact lens wear. Optom Vis Sci. 2001;78:9–12. doi: 10.1097/00006324-200101010-00008. [DOI] [PubMed] [Google Scholar]
- 43.Craig JP, Wang MTM, Dabin K, et al. Exploring the predisposition of the Asian eye to development of dry eye. Ocul Surf. 2016;14:385–92. doi: 10.1016/j.jtos.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Lin MC, Soliman GN, Song MJ, et al. Soft contact lens extended wear affects corneal epithelial permeability: hypoxic or mechanical etiology? Cont Lens Anterior Eye. 2003;26:11–6. doi: 10.1016/S1367-0484(02)00088-7. [DOI] [PubMed] [Google Scholar]
- 45.Tran N, Graham AD, Lin MC. Ethnic differences in dry eye symptoms: Effects of corneal staining and length of contact lens wear. Cont Lens Anterior Eye. 2013;36:281–8. doi: 10.1016/j.clae.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 46.Joslin CE, Wu CS, Torres M, Varma R. Racial/ethnic differences in corneal power. Optom Vis Sci. 2013;90 E-abstract 130265. [Google Scholar]
- 47.Craig JP, Willcox MD, Argüeso P, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the contact lens interactions with the tear film subcommittee. Invest Ophthalmol Vis Sci. 2013;54:TFOS123–56. doi: 10.1167/iovs.13-13235. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen DQ, Srinivasan S, Hiscott P, Kaye SB. Thimerosal-induced limbal stem cell failure: report of a case and review of the literature. Eye Contact Lens. 2007;33:196–8. doi: 10.1097/01.icl.0000247636.10720.19. [DOI] [PubMed] [Google Scholar]
- 49.Lebow KA, Schachet JL. Evaluation of corneal staining and patient preference with use of three multi-purpose solutions and two brands of soft contact lenses. Eye Contact Lens. 2003;29:213–20. doi: 10.1097/01.icl.0000081601.75812.03. [DOI] [PubMed] [Google Scholar]
- 50.Lin Z, He H, Zhou T, et al. A mouse model of limbal stem cell deficiency induced by topical medication with the preservative benzalkonium chloride. Invest Ophthalmol Vis Sci. 2013;54:6314–25. doi: 10.1167/iovs.12-10725. [DOI] [PubMed] [Google Scholar]
- 51.Rosenthal P, Cotter JM, Baum J. Treatment of persistent corneal epithelial defect with extended wear of a fluid-ventilated gas-permeable scleral contact lens. Am J Ophthalmol. 2000;130:33–41. doi: 10.1016/s0002-9394(00)00379-2. [DOI] [PubMed] [Google Scholar]
- 52.Chun M, Kageyama J. Corneal pannus resolved with silicone hydrogel contact lenses: a case series. Int Contact Lens Clin. 2000;27:170–4. [Google Scholar]
- 53.Papas EB. The significance of oxygen during contact lens wear. Cont Lens Anterior Eye. 2014;37:394–404. doi: 10.1016/j.clae.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 54.Edrington TB, Schornack . Chapter 8 Initial Evaluation. In: Bennett ES, Weissman BA, editors. Clinical Contact Lens Practice. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. p. 211. [Google Scholar]
- 55.Di Pascuale M, Espana E, Tseng SCG. A case of conjunctiva-cornea intraepithelial neoplasia successfully treated with topical Mitomycin C and Interferon Alfa-2b in cycles. Cornea. 2004;23:89–92. doi: 10.1097/00003226-200401000-00016. [DOI] [PubMed] [Google Scholar]
- 56.Theodore FH. Superior limbic keratoconjunctivitis. Eye Ear Nose Throat Mon. 1963;42:25–8. [PubMed] [Google Scholar]
- 57.Hamam R, Bhat P, Foster CS. Conjunctival/corneal intraepithelial neoplasia. Int Ophthalmol Clin. 2009 Winter;49(1):63–70. doi: 10.1097/IIO.0b013e3181924ec3. [DOI] [PubMed] [Google Scholar]
- 58.Abdelfattah NS, Amgad M, Zayed AA, et al. Clinical correlates of common corneal neovascular diseases: a literature review. Int J Ophthalmol. 2015;8(1):182–93. doi: 10.3980/j.issn.2222-3959.2015.01.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chun WK, Weissman BA. Corneal pannus associated with contact lens wear. Am J Ophthalmol. 1996;121:540–6. doi: 10.1016/s0002-9394(14)75428-5. [DOI] [PubMed] [Google Scholar]
- 60.Bron AJ, Argüeso P, Irkec M, Bright FV. Clinical staining of the ocular surface: mechanisms and interpretations. Prog Retin Eye Res. 2015;44:36–61. doi: 10.1016/j.preteyeres.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 61.Fonn D, Peterson R, Woods C. Corneal staining as a response to contact lens wear. Eye Contact Lens. 2010;36:318–21. doi: 10.1097/ICL.0b013e3181f35d54. [DOI] [PubMed] [Google Scholar]
- 62.Bhargava R, Kumar P. Oral omega-3 fatty acid treatment for dry eye in contact lens wearers. Cornea. 2015;34:413–20. doi: 10.1097/ICO.0000000000000386. [DOI] [PubMed] [Google Scholar]
- 63.Kim SW, Seo KY, Rhim T, Kim EK. Effect of retinoic acid on epithelial differentiation and mucin expression in primary human corneal limbal epithelial cells. Curr Eye Res. 2012;37:33–42. doi: 10.3109/02713683.2011.620728. [DOI] [PubMed] [Google Scholar]
- 64.Geerling G, Maclennan S, Hartwig D. Autologous serum eye drops for ocular surface disorders. Br J Ophthalmol. 2004;88:1467–74. doi: 10.1136/bjo.2004.044347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sangwan VS, Basu S, MacNeil S, Balasubramanian D. Simple limbal epithelial transplantation (SLET): a novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br J Ophthalmol. 2012;96:931–4. doi: 10.1136/bjophthalmol-2011-301164. [DOI] [PubMed] [Google Scholar]
- 66.Solomon A, Ellies P, Anderson DF, et al. Long-term outcome of keratolimbal allograft with or without penetrating keratoplasty for total limbal stem cell deficiency. Ophthalmology. 2002;109:1159–66. doi: 10.1016/s0161-6420(02)00960-0. [DOI] [PubMed] [Google Scholar]
- 67.Kim JC, Tseng SC. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995;14:473–84. [PubMed] [Google Scholar]
- 68.Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96:709–22. doi: 10.1016/s0161-6420(89)32833-8. discussion 722-3. [DOI] [PubMed] [Google Scholar]
- 69.Dua HS, Azuara-Blanco A. Autologous limbal transplantation in patients with unilateral corneal stem cell deficiency. Br J Ophthalmol. 2000;84:273–8. doi: 10.1136/bjo.84.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nishiwaki-Dantas MC, Dantas PE, Reggi JR. Ipsilateral limbal translocation for treatment of partial limbal deficiency secondary to ocular alkali burn. Br J Ophthalmol. 2001;85:1031–3. doi: 10.1136/bjo.85.9.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thoft RA. Keratoepithelioplasty. Am J Ophthalmol. 1984;97:l–6. doi: 10.1016/0002-9394(84)90438-0. [DOI] [PubMed] [Google Scholar]
- 72.Turgeon PW, Nauhein RC, Roat MI, et al. Indications of keratoepithelioplasty. Arch Ophthalmol. 1990;108:233–36. doi: 10.1001/archopht.1990.01070040085036. [DOI] [PubMed] [Google Scholar]
- 73.Holland EJ, Mogilishetty G, Skeens HM, et al. Systemic immunosuppression in ocular surface stem cell transplantation: results of a 10-year experience. Cornea. 2012;31:655–61. doi: 10.1097/ICO.0b013e31823f8b0c. [DOI] [PubMed] [Google Scholar]
- 74.Krakauer M, Welder JD, Pandya HK, et al. Adverse effects of systemic immunosuppression in keratolimbal allograft. J Ophthalmol. 2012;2012:576712. doi: 10.1155/2012/576712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lindberg K, Brown ME, Chaves HV, et al. In vitro propagation of human ocular surface epithelial cells for transplantation. Invest Ophthalmol Vis Sci. 1993;34:2672–9. [PubMed] [Google Scholar]
- 76.Pellegrini G, Traverso CE, Franzi AT, et al. Longterm restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997 Apr 5;349(9057):990–3. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 77.Basu S, Fernandez MM, Das S, et al. Clinical outcomes of xeno-free allogeneic cultivated limbal epithelial transplantation for bilateral limbal stem cell deficiency. Br J Ophthalmol. 2012;96:1504–9. doi: 10.1136/bjophthalmol-2012-301869. [DOI] [PubMed] [Google Scholar]
- 78.Stasi K, Chuck RS. Update on phototherapeutic keratectomy. Curr Opin Ophthalmol. 2009;20:272–5. doi: 10.1097/ICU.0b013e32832b4f44. [DOI] [PubMed] [Google Scholar]
- 79.Vinciguerra P, Albè E, Rosetta P, et al. Custom phototherapeutic keratectomy and autologous fibrin-cultured limbal stem cell autografting: a combined approach. J Refract Surg. 2008;24:323–4. doi: 10.3928/1081597X-20080401-02. [DOI] [PubMed] [Google Scholar]


