Abstract
The design and synthesis of dual aromatase inhibitors/selective estrogen receptor modulators (AI/SERMs) is an attractive strategy for the discovery of new breast cancer therapeutic agents. Previous efforts led to the preparation of norendoxifen (4) derivatives with dual aromatase inhibitory activity and estrogen receptor binding activity. In the present study, some of the structural features of the potent AI letrozole were incorporated into the lead compound (norendoxifen) to afford a series of new dual AI/SERM agents based on a symmetrical diphenylmethylene substructure that eliminates the problem of E,Z isomerization encountered with norendoxifen-based AI/SERMs. Compound 12d had good aromatase inhibitory activity (IC50 = 62.2 nM) while also exhibiting good binding activity to both ER-α (EC50 = 72.1 nM) and ER-β (EC50 = 70.8 nM). In addition, a new synthesis was devised for the preparation of norendoxifen and its analogues through a bis-Suzuki coupling strategy.
Keywords: Aromatase inhibitor; Estrogen receptor; Breast cancer; Antiestrogenic activity ; (E,Z)-Norendoxifen synthesis
Graphical Abstract

2. Introduction
In spite of considerable therapeutic advances, breast cancer remains a significant public health problem.1 Estrogens play a prominent role in stimulating the growth and development of the majority of breast cancers.2, 3 Hence, estrogen receptors (ERs) are important targets for the development of new therapeutic agents for breast cancer treatment. Selective estrogen receptor modulators (SERMs), which were developed in the late 1950’s and early 1960’s, are widely used for the treatment of breast cancer.4 SERMs are able to bind to the estrogen receptors (ER-α and ER-β) in much the same way as estrogens do, and they block ERs in breast cancer cells in breast tissue while stimulating ERs in normal tissues.5 Tamoxifen (1) is a representative antagonist of the ERs in breast tissue and is the most commonly used SERM for the treatment of breast cancer (Figure 1). However, despite the fact that many patients benefit from tamoxifen, resistance to it often emerges, resulting in therapeutic failure.6, 7 Aromatase inhibitors (AIs), which prevent the formation of estradiol, were developed for the treatment of ER-positive breast cancer during the 1980’s.8 They have shown superior clinical efficacy compared to tamoxifen in postmenopausal breast cancer patients,8 and are considered as an alternative strategy for tamoxifen-resistant breast cancer. Unfortunately, the use of AIs is accompanied with significant side effects, including reduction of bone density, severe musculoskeletal pain, and increased frequency of fractures and cardiovascular events.9–12
Figure 1.
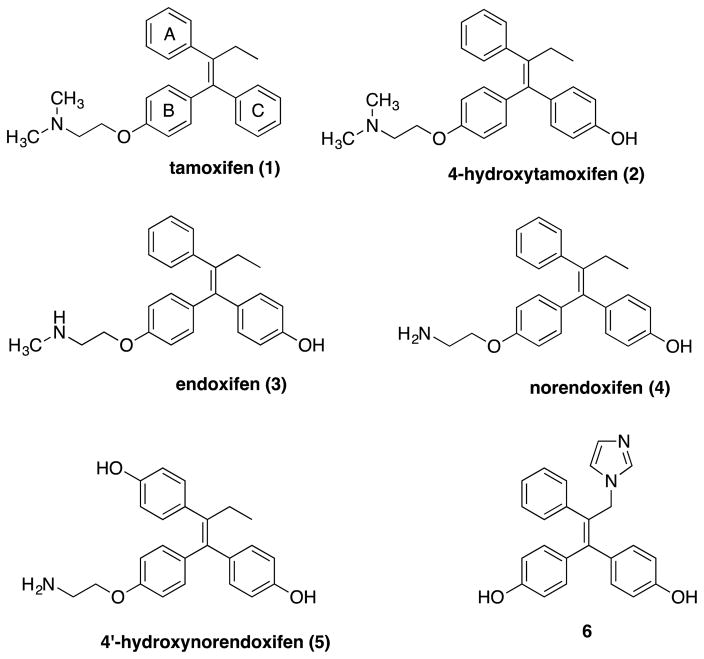
Structures of the SERM tamoxifen (1) and its metabolites 4-hydroxytamoxifen (2), endoxifen (3), norendoxifen (4), and 4′-hydroxynorendoxifen (5), and an active synthetic imidazole analogue 6.
Combination endocrine therapy has emerged as an effective cancer treatment paradigm.13 Several clinical trials have revealed a significant benefit resulting from combination endocrine therapy involving administration of a SERM and an AI.14, 15 However, this approach has some drawbacks. For example, in the ATAC trial, the combination of anastrozole (an AI) and tamoxifen (a SERM) was less effective than anastrozole alone.16 Moreover, a patient who takes a number of different drugs is at greater risk for side effects and drug interactions.
Dual AI/SERMs might be expected to be more effective than the conventional combination of tamoxifen and an AI. The ER blocking activity of a dual AI/SERM in cancer cells might act synergistically with the AI activity to inhibit cancer cell proliferation, while in normal tissues the ER stimulation of a dual AI/SERM would be expected to alleviate the side effects resulting from the global estrogen depletion caused by the AI activity of the dual AI/SERM. This therapeutic hypothesis motivated the search for compounds that inhibit aromatase and bind to estrogen receptors. Norendoxifen (4, Figure 1) was found to be an active tamoxifen metabolite that binds to ERs and is also a potent AI,17, 18 and that discovery has provided a platform for the design and synthesis of dual AI/SERMs based on the structure of norendoxifen.18–20 Subsequent work proved that installation of a 4′-hydroxy group on norendoxifen to make the metabolite 5 increased potency vs. aromatase and the two estrogen receptors.19 More recently, it was determined that the aminoethoxy side chain of norendoxifen can be replaced by a phenolic hydroxyl group and the activity vs. all three receptors (AI, ER-α, and ER-β) maintained as long as the ethyl group is replaced by an imidazolylmethyl moiety (e.g. compound 6) that can coordinate to the iron of aromatases.20 Initial attempts to install a 4′-amino group in norendoxifen derivatives led to mixed results that were generally disappointing with regard to simultaneous binding to all three receptors.20 In spite of that, the present investigation was launched in an attempt to simultaneously optimize activity against aromatase, ER-α, and ER-β by replacement of the hydroxyl groups of 4′-hydroxynorendoxifen (5) derivatives with amino groups or nitro groups and elimination of the 2′-aminoethyl moiety. The hypothesis was that activity against aromatase, ER-α, and ER-β could be maintained in aminated derivatives even in the absence of imidazole and aminoethyl functionality using a structure-based drug design approach that would take advantage of the known structures of the receptors.
Of the third generation AIs, letrozole is 2–5 fold more potent than anastrozole and exemestane in its inhibition of aromatase in noncellular systems and 10–20 fold more potent in cellular systems (Figure 2).21 The structure of letrozole consists of two pharmacophores. One is the triazole ring. The other is the symmetrically substituted diphenylmethane fragment that has two identical substituents incorporated at the 4- and 4′-positions. The incorporation of a basic nitrogen and a symmetrically substituted diphenylmethane fragment into norendoxifen analogues might therefore provide an approach to optimize aromatase inhibition (Figure 3). Therefore, norendoxifen was modified by the removal of the aminoethoxyl side chain and introduction of a nitro or amino group in the para position of the “A” ring (Figure 3). The resulting compounds have no geometrical isomers and, similar to anastrozole and letrozole, they also incorporate hydrogen bond acceptors.
Figure 2.
Structures of the third generation AIs letrozole (7), anastrozole (8) and exemestane (9).
Figure 3.
Design strategy for hybrid AI/SERMs.
3. Results and Discussion
3.1 Synthesis and Evaluation of Triphenylethylenes 12a–d
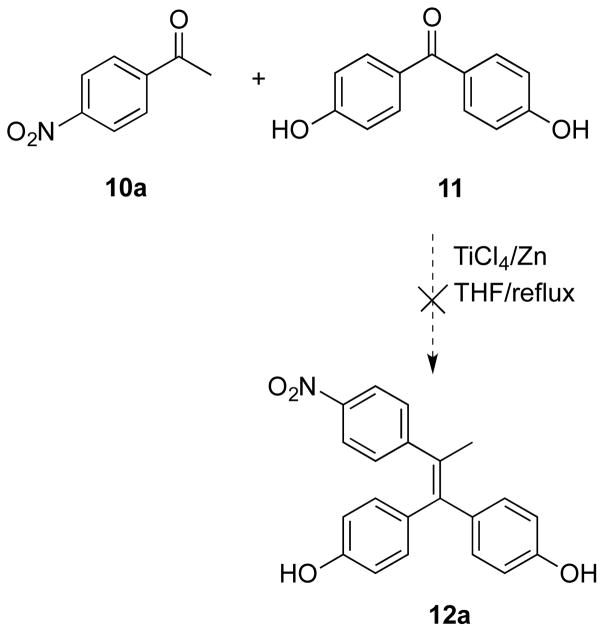
The importance of the nitro group as an H-bond acceptor in potent and selective AIs has been emphasized previously by Gobbi and co-workers.22 Initial attempts were made to synthesize the desired nitrated triphenylethylenes 12 by McMurry coupling of benzophenones with propiophenones using the methodology previously reported by our group and others.23–25 However, when 4-nitroacetophenone 10a was treated with 4,4′-dihydroxybenzophenone 11 in dry THF in the presence of TiCl4 and Zn, none of the desired McMurry product 12a was observed (Scheme 1). Attempts to use BOC-protected 4-aminoacetophenone in place of 4-nitroacetophenone 10a were uniformly unsuccessful.
Scheme 1.
Attempted Preparation of 12a through the McMurry Reaction.
The failure of the McMurry approach led to another strategy to transform 10a into 12a, namely conversion of ketone 10a into a 1,1-dibromo-1-alkene 14a before performing palladium-catalyzed coupling reactions.26, 27 Subsequent coupling of 14a with 4-hydroxyphenylboronic acid under Suzuki conditions proceeded smoothly to provide the desired product 12a in good yield (67%) (Scheme 2).
Scheme 2.
Synthesis of 12a–d. Reagents and conditions: (a) N2H4·H2O, EtOH; (b) CBr4, CuCl, DMSO; (c) 4-(HO)PhB(OH)2 or 4-(H2N)PhB(OH)2, PdCl2(PPh3)2, Na2CO3, THF-H2O.
The analogues 12b–d were prepared by employing the same strategy as that used for the synthesis of compound 12a (Scheme 2). The nitro-substituted ketones 10a and 10b were initially treated with hydrazine hydrate at reflux in EtOH to provide the hydrazones 13a and 13b in 85% and 90% yields, respectively.26 The hydrazones 13a and 13b were reacted with CBr4 in the presence of CuCl to provide the 1,1-dibromo-1-alkenes 14a and 14b in 65% and 50% yields, respectively.27 Finally, the bis-Suzuki arylation of 14a and 14b with 4-hydroxyphenylboronic acid or 4-aminophenylboronic acid in the presence of PdCl2(PPh3)2 at 70 ºC in THF-H2O resulted in the formation of 12a–d in 47–57% yields.
Compounds 12a–d were evaluated for their aromatase inhibitory activities and ER binding affinities (Table 1). The IC50 and EC50 values for the previously reported compounds 13, (E,Z)-norendoxifen, (E)-norendoxifen, and (Z)-norendoxifen are included for comparison.18 The results indicate that nitro-substituted bis-phenol compounds 12a and 12b are very weak AIs with 75% and 78% inhibition at 50 μM, respectively. However, the aniline-type compounds 12c and 12d exhibited remarkably improved inhibitory activity against aromatase and affinity for ER-α and ER-β when compared with the unsubstituted derivative 13 and with the phenols 12a and 12b. They were 113 and 400 times more potent than 13 against aromatase (IC50 220.8 and 62.2 vs 24880 nM), respectively. Compound 12d showed slightly improved aromatase inhibitory activity and slightly decreased binding affinity to both ER-α and ER-β when compared with the lead compound (E,Z-norendoxifen). These results indicate that the replacement of the two phenols of 13 with amino groups and the introduction of a p-nitro group in the A-ring contribute in a positive way to both aromatase inhibition and ER binding affinity. Another conclusion that can be drawn is that the aminoethoxyl side chain is important for the hydroxyl-substituted norendoxifen analogues to retain optimal ER binding affinity and aromatase inhibitory activity, but it is not an essential requirement for amino-substituted analogues.
Table 1.

| |||
|---|---|---|---|
| compd | aromatase (IC50, nM, or percent inhibition) | ER-α (EC50, nM, or percent competition) | ER-β (EC50, nM, or percent competition) |
| 12a | 75% inhibition at 50 μM | 19% competition at 100 μM | 55% competition at 100 μM |
| 12b | 78% inhibition at 50 μM | 40% competition at 100 μM | 66% competition at 100 μM |
| 12c | 220.8 ± 42.2 | 212.9 ± 53.7 | 486.2 ± 239.5 |
| 12d | 62.2 ± 7.8 | 72.1 ± 42.6 | 70.8 ± 5.2 |
| 13 | 24880 ± 7.8 | 80% competition at 100 μM | 306.9 ± 106.4 |
| (E,Z)-norendoxifen | 102.2 ± 32.7 | 26.9 ± 4.8 | 35.2 ± 16.8 |
| (E)-norendoxifen | 76.8 ± 33.3 | 58.7 ± 1.0 | 78.5 ± 57.3 |
| (Z)-norendoxifen | 1029 ± 318 | 17.0 ± 1.9 | 27.5 ± 14.3 |
| Estradiol | 5.7 | 5.6 | |
IC50 values were determined for compounds exhibiting inhibition values higher than 90%.
Percent aromatase inhibition was determined at the concentration of 50000 nM for each compound.
Percent ER competition was determined at the concentration of 100000 nM for each compound.
Molecular modeling was performed in order to investigate the binding mode of 12d in the active sites of aromatase and ER-α. The dianiline 12d was docked in the active sites of aromatase (PDB code 3s7928) and ER-α (PDB code 3ert29) using GOLD 3.0. These studies capitalized on the secure molecular modeling foundation established by previous investigations of the binding of (E)-norendoxifen and several analogues to both aromatase and ER-α, which involved GOLD 3.0 docking, Amber 10 molecular dynamics simulations and Amber parm99 energy minimizations, as well as MM-PBSA binding energy calculations 18–20 The calculated models were consistent with experimentally determined SAR. As shown in Figure 4, the overall pose of the ligand is close to that present in the previously published models of (E)-norendoxifen and its analogues bound to aromatase.18–20 The amino group on the aniline ring that is trans to the nitrophenyl ring hydrogen bonds to the backbone carbonyl of Asp309. In common with the present situation, all of the previously published models involve hydrogen bonding to Asp309. However, the present model is different in that the amino group on the aniline ring that is cis to the nitrophenyl does not hydrogen bond to backbone carbonyl of Leu372. This may be related to the fact that all of the previously modeled compounds were phenols instead of anilines.
Figure 4.
Hypothetical molecular model of 12d bound in the active site of aromatase (PDB code 3s7928).
The molecular model calculated for the binding of 12d to ER-α is displayed in Figure 5. In this case, the amino group on the phenyl ring that is cis to the nitrophenyl ring hydrogen bonds to the hydroxyl of Thr347 while the other amino group hydrogen bonds to the carboxylate of Glu353 and the backbone carbonyl of Phe404. The overall pose and the involvement of Thr347 and Glu353 are similar to that in the previously published model of (Z)-norendoxifen and one of its analogues bound to ER-α.20 On the other hand, the present case differs in the hydrogen bonding to Phe404 instead of Arg394.20
Figure 5.
Hypothetical molecular model of 12d bound in the active site of ER-α (PDB code 3ert29).
3.2 Synthesis and Evaluation of Triphenylethylenes 15a–d
The encouraging findings for 12c and 12d reported in Table 1 led to the preparation of compounds 15a–d to explore the effect of replacing the “A” ring nitro group with an amino group as shown in Scheme 3. Compounds 15a–d were easily obtained in 52–80% yield by reduction of the corresponding nitrosubstituted triphenylalkenes 12a–d with SnCl2 (Scheme 3).
Scheme 3.
Synthesis of 15a–d. Reagents and conditions: (a) SnCl2, EtOH.
The biological results for compounds 15a–d are summarized in Table 2. Replacement of the nitro groups in 12a–d with amino groups produced mixed results on the binding affinity with the ERs while all four amino compounds showed significantly improved inhibitory activity against aromatase. In particular, compound 15b was the most potent of the AIs synthesized in this project with an IC50 value of 8.8 nM, which is close to the widely prescribed AI letrozole (IC50 = 5.3 nM30). These results clearly demonstrate the important role played by the “A” ring amino group in 15b and 15d in increasing the aromatase inhibitory activity compared to norendoxifen, the nitro derivatives 12b and 12d, and unsubstituted compound 13.
Table 2.

| |||
|---|---|---|---|
| compd | aromatase (IC50, nM) | ER-α (EC50, nM, or percent competition) | ER-β (EC50, nM, or percent competition) |
| 15a | 230.0 ± 11.4 | 11036 ± 827 | 857 ± 389 |
| 15b | 8.8 ± 1.6 | 1711 ± 630 | 1263 ± 424 |
| 15c | 177.1 ± 20.2 | 31% competition at 100 μM | 77% competition at 100 μM |
| 15d | 13.4 ± 3.0 | 25% competition at 100 μM | 76% competition at 100 μM |
| Estradiol | 5.7 | 5.6 | |
IC50 values were determined for compounds exhibiting inhibition values higher than 90%.
Percent ER competition was determined at the concentration of 100000 nM for each compound.
Comparing 15b with 15a reveals a significant increase in potency on aromatase from IC50 230 to 8.8 nM and from 11036 to 1711 nM in affinity to ER-α. A similar effect on aromatase was observed for compounds 15c and 15d. Obviously, the ethyl substituent is better than the methyl when tested on aromatase and ER-α, but it is actually slightly worse vs. ER-β when comparing 15a to 15b.
3.3 Synthesis and Evaluation of Triphenylethylenes 16a–d
Compounds 16a–d were prepared in 30–56% yield by treatment of 12a–d with one equivalent of 2-iodoacetamide in the presence of K2CO3 (Scheme 4). This allows comparison of two sets 16a–d and 12a–d with respect to ER binding affinity and aromatase inhibition to determine the effect of the amide side chain on the dual interaction.
Scheme 4.
Synthesis of 16a–d. Reagents and conditions: (a) ICH2CONH2, acetone, K2CO3; (b) LiAlH4, AlCl3, THF.
The biological testing results for compounds 16a–d are summarized in Table 3. IC50 and EC50 values for the previously reported amide 17 are included for comparison.18 Substitution of the amino group in one of the phenyl rings of 12c with the amide side chain in 16c decreased aromatase inhibitory activity (IC50 220.8 vs 645.3 nM). Compound 16d also exhibited decreased aromatase inhibitory activity when compared with 12d (IC50 286.9 vs 62.2 nM). Compounds 16c and 16d exhibited elevated potency against both aromatase and ER when compared with 16a, 16b, and 17. The presence of the side chain produced mixed results on the estrogen receptors. It increased affinity when installed on 12c (compare ER results for 12c and 16c), but it decreased affinity when installed on 12d (compare ER results for 12d and 16d).
Table 3.

| |||
|---|---|---|---|
| compd | aromatase (IC50, nM, or percent inhibition | ER-α (EC50, nM, or percent competition) | ER-β (EC50, nM, or percent competition) |
| 16a | 9724 ± 224 | 0% competition at 100 μM | 0% competition at 100 μM |
| 16b | 76% inhibition at 50 μM | 0% competition at 100 μM | 0% competition at 100 μM |
| 16c | 645.3 ± 246.4 | 164.1 ± 96.9 | 218.4 ± 102.7 |
| 16d | 286.9 ± 31.8 | 451.2 ± 201.2 | 346.4 ± 115.3 |
| 17 | 9257 ± 195 | 57% competition at 100 μM | 71% competition at 100 μM |
| Estradiol | 5.7 | 5.6 | |
IC50 values were determined for compounds exhibiting inhibition values higher than 90%.
Percent aromatase inhibition was determined at the concentration of 50000 nM for each compound.
Percent ER competition was determined at the concentration of 100000 nM for each compound.
3.4 Evaluation of Antiestrogenic Effects in a Functional, Cellular Assay
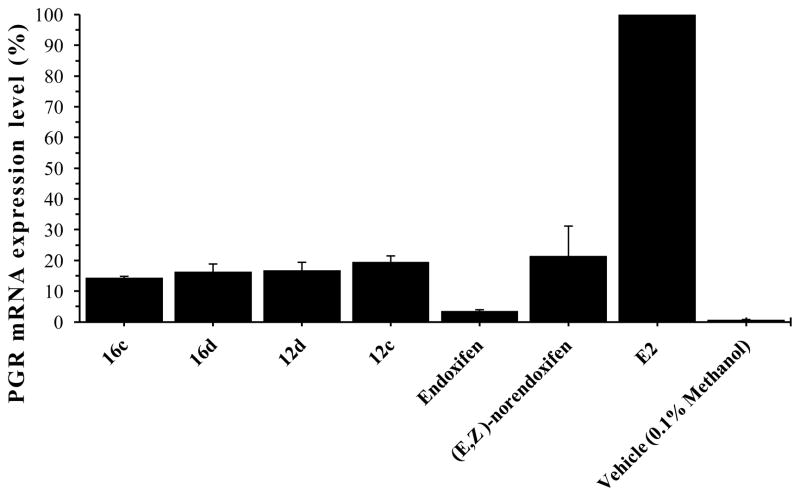
In order to gain information about the behavior of the triphenylethylenes beyond that provided by estrogen receptor affinity studies, compounds 12c, 12d, 16c, and 16d (1 μM) were tested in a functional assay that measured their abilities to block the effects of β-estradiol (10 nM) in MCF-7 human breast cancer cells. These substances were selected for biological testing because of their relatively high affinity for ER-α and ER-β, and their potencies as estradiol antagonists were compared with endoxifen and (E,Z)-norendoxifen. The assay measures progesterone receptor (PGR) mRNA expression level, and the results of the assay are provided in Figure 6. β-Estradiol (10 nM) increased PGR mRNA expression to a level that was assigned the 100% value. Despite the fact that the affinities for ER-α ranged from 451.2 nM (16d) to 72.1 nM (12d) and those for ER-β ranged from 486.2 nM (12c) to 70.8 nM (12d), the % RNA expression levels only ranged from 14% (16c) to 20% (12c) in the functional cellular assay, and the most potent compound in the functional assay (16c) was not one with the highest affinity for ER-α and ER-β (12d was). Endoxifen, the positive control, was able to antagonize PGR mRNA expression in the presence of 10 nM estradiol (E2) to the level of 3.5%, while the level of expression in the presence of (E,Z)-norendoxifen was 22%. These results are generally consistent with those previously reported for structurally related compounds.19, 20, 31, 32
Figure 6.
Abilities of compounds 16c, 16d, 12d, 12c, endoxifen, and (E,Z)-norendoxifen (1 μM) to antagonize β-estradiol (E2, 10 nM)-stimulated progesterone receptor (PGR) mRNA expression in MCF-7 cells. PGR mRNA expression levels relative to estradiol (100%): 16c (14.1%), 16b (16.3%), 16c (16.8%), 16d (19.6%), endoxifen (3.6%), (E,Z)-norendoxifen (21.5%). MCF-7 cells were preconditioned in charcoal-stripped FBS for 72 hours to remove the estrogens. The cells were treated with vehicle (1% methanol), 1 μM endoxifen or 1 μM test compound in the presence of 10 nM β-estradiol (E2) and 10 nM β-estradiol alone for 24 hours. Total RNA was isolated from the cells and cDNA was prepared. The E2-stimulated PGR mRNA expression was quantified using a real-time Taqman® PCR assay.
These data indicate that each of the four compounds effectively inhibited the PGR expression. Future dose response studies will be required to determine if there are differences in potencies toward inhibiting the PGR expression or cell proliferation. Since the affinities for the two estrogen receptors varied quite similarly across the compounds, it will be interesting to determine their effects on cell proliferation in light of the studies showing that ER-α and ER-β have opposing effects on breast cancer cells in vitro.33
3.5 A New Synthesis of (E,Z)-Norendoxifen
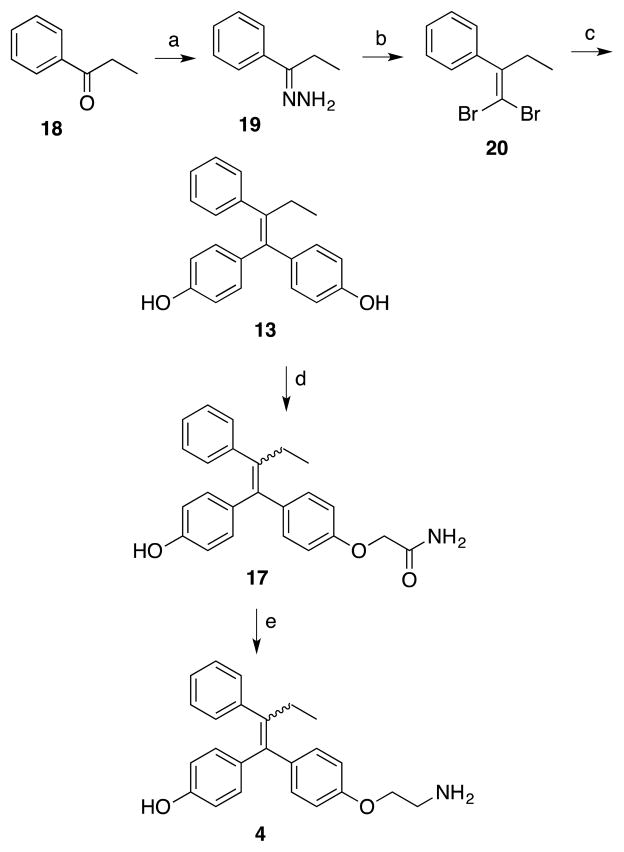
Although the first synthesis of (E,Z)-norendoxifen through the McMurry reaction was recently reported, the method has limited applicability for the direct synthesis of hydroxylated, nitro, and amino derivatives.18 A new synthesis of (E,Z)-norendoxifen was therefore explored as an extension of the present work (Scheme 5). The synthesis of the triphenylalkene framework commenced with the condensation of propiophenone 18 with hydrazine hydrate in EtOH at reflux to provide the hydrazone 19 in excellent yield (88%).34 1-(1,1-Dibromobut-1-en-2-yl)benzene 20 was prepared by reaction of hydrazone 19 with CBr4 (3.0 equiv) in the presence of CuCl (10 mol%) in DMSO in 70% yield.27 The bis-Suzuki arylation of 20 with 4-hydroxyphenylboronic acid in the presence of PdCl2(PPh3)2 (10 mol%) at 70 ºC in THF-H2O resulted in the formation of diphenol 13 in 52% yield. The diphenol 13 was treated with K2CO3 and 2-iodoacetamide (1.1 equiv) to afford the amide 17 as the major product in 32% yield. Finally, the reduction of the amide 17 using LiAlH4 resulted in the generation of (E,Z)-norendoxifen in 64% yield.
Scheme 5.
Synthesis of (E,Z)-Norendoxifen through the Suzuki Coupling Reaction. Reagents and conditions: (a) N2H4·H2O, EtOH; (b) CBr4, CuCl, DMSO; (c) (HO)2BPh-4-OH, PdCl2(PPh3)2, Na2CO3, THF-H2O; (d) ICH2CONH2, acetone, K2CO3; (e) LiAlH4, AlCl3, THF.
4. Conclusion
A series of novel triphenylethylene derivatives based on a symmetrical diphenylmethylene template were designed and synthesized using a new Suzuki bis-arylation approach on an α,α-dibromoalkene. This circumvented problems encountered when the McMurry approach was tried. The para position of the “A” ring of these compounds was substituted with a nitro group or an amino group, while the para position of the “B” and “C” ring was substituted with a hydroxyl group or an amino group. SAR studies demonstrated that the aminoethoxyl side chain is not an essential requirement for tamoxifen (1) analogues to elicit both aromatase inhibitory and ER binding activity despite the fact that many previous reports have argued that it is important for the activity.35–39 The introduction of a para-amino group into the A-ring of the triphenylethylene scaffold led to a remarkable improvement of aromatase inhibitory activity (IC50 24880 nM for 13 vs. 8.8 nM for 15b).
Progress toward the goal to find new classes of AI/SERMs with favorable aromatase inhibitory activity and affinity to ER-α and ER-β was realized with dianiline 12d, which had potent aromatase inhibitory activity (IC50 = 62.2 nM) while also exhibiting high affinity to both ER-α (EC50 = 72.1 nM) and ER-β (EC50 = 70.8 nM). Moreover, compounds 15b (IC50 = 8.8 nM) and 15d (IC50 = 13.4 nM) displayed highly potent aromatase inhibitory activity that was close to that of the marketed drug letrozole (IC50 5.3 nM) while having low affinity for ER-α and ER-β.
The following conclusions about the structure-activity relationships were drawn: (1) The amino groups in the para positions of the “A” ring and “B” ring play key roles in the modulation of the aromatase inhibitory activity. (2) The unsymmetrical diphenylmethylene substructure of norendoxifen can be replaced by a symmetrical diphenylmethylene substructure, thereby eliminating E,Z-isomers of the triphenylethylenes and maintaining activity. (3) The replacement of ethyl side chain with a methyl group produced a negative result, whether for aromatase inhibition or ER binding affinity. (4) The aminoethoxyl side chain in triphenylalkene derivatives is not an essential requirement for optimal interaction with the estrogen receptors and aromatase.
Because of their promising biological activities and no complications arising from the presence of E,Z isomers, the present molecules based on a symmetrical diphenylmethylene template are suitable candidates for further development toward dual AI/SERMs for breast cancer treatment.
5. Experimental Section
5.1 Chemistry
Melting points were determined using capillary tubes with a Mel-Temp apparatus and are uncorrected. Reaction products were obtained in pure form directly from reaction mixtures or after column chromatography and did not require additional recrystallization. 1H NMR and 13C NMR spectra were recorded using a Bruker ARX300 300 MHz spectrometer or a Bruker DPX 400 MHz spectrometer with TMS as internal standard. High-resolution mass spectra (HRMS) were recorded on a double-focusing sector mass spectrometer with magnetic and electrostatic mass analyzers or a Bruker microTOF Q spectrometer. Compound purities were estimated by reversed phase C18 high pressure liquid chromatography (HPLC) with UV detection at 254 nm. The major peak area of each biologically tested compound was ≥95% of the combined total peak area. Cytochrome P450 (CYP) inhibitor screening kits for aromatase (CYP19) inhibition studies were purchased from BD Biosciences (San Jose, CA). ER α and β competitor assay kits were purchased from Invitrogen (Carlsbad, CA).
5.1.1 General Procedure for the Synthesis of Hydrazones (13a and 13b)26
A 98% hydrazine monohydrate solution (1 mL, 20 mmol) was added to a suspension of ketone 10a or 10b (10 mmol) in EtOH (10 mL). The mixture was heated to reflux for 2 h. After cooling to room temperature, the solid was filtered and the crude hydrazone was washed with H2O (20 mL X 2) and dried in vacuo. The product was used in the next step without further purification.
5.1.2 1-[1-(4-Nitrophenyl)ethylidene]hydrazine (13a)
Brick red solid, 85% yield, mp 149–150 °C (lit.26 mp 148–149 °C).
5.1.3 1-(1-(4-Nitrophenyl)propylidene)hydrazine (13b)
Orange crystalline solid, 90% yield, mp 103–104 °C. 1H NMR (300 MHz, CDCl3) δ 8.18 (d, J = 7.1 Hz, 2 H), 7.80 (d, J = 7.1 Hz, 2 H), 5.73 (s, 2 H), 2.64 (q, J = 7.7 Hz, 2 H), 1.18 (t, J = 7.7 Hz, 3 H); 13C NMR (75 MHz, CDCl3) δ 147.9, 146.2, 144.4, 125.8, 123.6, 17.9, 9.44; CIMS m/z 194 (MH+); HRCIMS m/z calcd for C9H12N3O2 (MH+) 194.0924, found 194.0932.
5.1.4 General Procedure for the Synthesis of 1,1-Dibromo-1-alkenes (14a and 14b)27
A 28% aqueous solution of ammonia (1 mL) and CuCl (0.3 mmol) were added to a solution of hydrazones 13a or 13b (3.0 mmol) in DMSO (3 mL). Then CBr4 (9 mmol) in DMSO (5 mL) was added dropwise. The reaction mixture was stirred at room temperature for 16 h and quenched with H2O (30 mL) and extracted with CH2Cl2 (20 mL X 3). After being dried over Na2SO4, the CH2Cl2 was evaporated and the residue was purified by column chromatography (hexane: EtOAc = 2: 1) to afford the product 14a or 14b.
5.1.5 1-(1,1-Dibromoprop-1-en-2-yl)-4-nitrobenzene (14a)
White solid, 65% yield, mp 81–82 °C. 1H NMR (300 MHz, CDCl3) δ 8.23 (d, J = 8.7 Hz, 2 H), 7.41 (d, J = 8.7 Hz, 2 H), 2.23 (s, 1 H); 13C NMR (75 MHz, CDCl3) δ 148.5, 141.1, 128.6, 123.8, 123.4, 89.6, 25.9; CIMS m/z 321 (MH+).
5.1.6 1-(1,1-Dibromobut-1-en-2-yl)-4-nitrobenzene (14b)
White solid, 50% yield: mp 57–58 °C. 1H NMR (300 MHz, CDCl3) δ 8.24 (d, J = 8.7 Hz, 2 H), 7.37 (d, J = 8.7 Hz, 2 H), 2.63 (q, J = 7.5 Hz, 2 H), 0.98 (t, J = 7.4 Hz, 3 H); 13C NMR (75 MHz, CDCl3) δ 147.5, 147.0, 146.9, 129.0, 123.8, 89.3, 32.6, 11.3; CIMS m/z 336 (MH+); HRCIMS m/z calcd for C10H10N1O279Br81Br (MH+) 335.9052, found 335.9048.
5.1.7 General Procedure for the Synthesis of Triphenylalkenes (12a–d)
A solution of 1,1-dibromo-1-alkenes 14a or 14b (1.0 mmol), 4-hydroxyphenylboronic acid or 4-aminophenylboronic acid (4.0 mmol), PdCl2(PPh3)2 (0.1 mmol), and Na2CO3 (3.0 mmol) in THF-H2O (15 mL) was heated to 70 °C under Ar2 for 18 h. After cooling to room temperature, EtOAc (15 mL) and H2O (10 mL) were poured into the reaction mixture. The aqueous layer was extracted with EtOAc (20 mL X 3). The combined organic layers were washed with water and dried, concentrated in vacuo and purified by flash column chromatography (hexane: EtOAc = 2:1) to afford the products 12a–d.
5.1.8 4-(1-(4-Hydroxyphenyl)-2-(4-nitrophenyl)prop-1-enyl)phenol (12a)
Light brown solid, 67% yield: mp 235–236 °C. 1H NMR (300 MHz, CDCl3) δ 8.02 (d, J = 9.0 Hz, 2 H), 7.28 (d, J = 7.8 Hz, 2 H), 7.10 (d, J = 8.1 Hz, 2 H), 6.82 (d, J = 8.7 Hz, 2 H), 6.72 (d, J = 9.0 Hz, 2 H), 6.52 (d, J = 8.4 Hz, 2 H), 4.78 (s, 1 H), 4.62 (s, 1 H), 2.17 (s, 3 H); 13C NMR (75 MHz, CDCl3) δ 154.7, 154.3, 152.1, 145.5, 141.3, 135.2, 134.9, 132.3, 132.1, 131.3, 130.2, 123.2, 115.0, 114.7, 22.9; ESIMS m/z 370 (MNa+); HRESIMS m/z calcd for C21H17NO4Na (MNa+) 370.1055, found 370.1066; HPLC purity, 100% (90% MeOH, 10% H2O).
5.1.9 4-(1-(4-Hydroxyphenyl)-2-(4-nitrophenyl)but-1-enyl)phenol (12b)
Pale yellow solid, 57% yield: mp 111–112 °C. 1H NMR (300 MHz, methanol-d4) δ 7.99 (d, J = 8.7 Hz, 2 H), 7.29 (d, J = 8.7 Hz, 2 H), 7.03 (d, J = 8.7 Hz, 2 H), 6.77 (d, J = 8.1 Hz, 2 H), 6.66 (d, J = 8.4 Hz, 2 H), 6.62 (s, 2 H), 6.44 (d, J = 9.0 Hz, 2 H), 2.54 (q, J = 7.8 Hz, 2 H), 0.90 (t, J = 7.8 Hz, 3 H); 13C NMR (75 MHz, methanol-d4) δ 157.7, 157.1, 152.3, 147.1, 142.8, 139.6, 135.7, 135.3, 133.3, 132.0, 131.6, 128.5, 124.0, 116.8, 116.5, 115.9, 115.5, 29.5, 14.0; negative ion ESIMS m/z 360 (M – H+)−; negative ion HRESIMS m/z calcd for C22H18NO4 (M – H+)− 360.1236, found 360.1237; HPLC purity, 95.18% (90% MeOH, 10% H2O).
5.1.10 4-(1-(4-Aminophenyl)-2-(4-nitrophenyl)prop-1-enyl)benzenamine (12c)
Brick red solid, 55% yield: mp 202–204 °C. 1H NMR (300 MHz, methanol-d4) δ 7.97 (d, J = 6.6 Hz, 2 H), 7.32 (d, J = 7.2 Hz, 2 H), 6.96 (d, J = 6.9 Hz, 2 H), 6.71 (d, J = 6.9 Hz, 2 H), 6.60 (d, J = 6.6 Hz, 2 H), 6.41 (d, J = 6.9 Hz, 2 H), 2.16 (s, 3 H); 13C NMR (75 MHz, methanol-d4) δ 154.3, 147.4, 146.8, 144.1, 134.4, 133.2, 132.0, 131.8, 131.6, 124.0, 116.2, 115.9, 23.0; ESIMS m/z 346 (MH+); HRESIMS m/z calcd for C21H20N3O2 (MH+) 346.1556, found 346.1572; HPLC purity, 100% (90% MeOH, 10% H2O).
5.1.11 4-(1-(4-Aminophenyl)-2-(4-nitrophenyl)but-1-enyl)benzenamine (12d)
Brick red solid, 40% yield: mp 182–183 °C. 1H NMR (300 MHz, methanol-d4) δ 7.99 (d, J = 8.7 Hz, 2 H), 7.32 (d, J = 8.7 Hz, 2 H), 6.95 (d, J = 9.0 Hz, 2 H), 6.70 (d, J = 8.4 Hz, 2 H), 6.57 (d, J = 9.0 Hz, 2 H), 6.38 (d, J = 8.4 Hz, 2 H), 2.58 (q, J = 7.8 Hz, 2 H), 0.92 (t, J = 7.5 Hz, 3 H); 13C NMR (75 MHz, methanol-d4) δ 152.9, 147.9, 143.7, 138.6, 134.1, 133.1, 132.1, 131.4, 124.0, 116.1, 115.6, 29.5, 14.0; ESIMS m/z 360 (MH+); HRESIMS m/z calcd for C22H22N3O2 (MH+) 360.1712, found 360.1723; HPLC purity, 97.74% (90% MeOH, 10% H2O).
5.1.12 General Procedure for the Synthesis of Reduction Products (15a–d)
A solution of nitro-containing compounds 12a or 12b or 12c or 12d (0.3 mmol) and SnCl2 (1.5 mmol) in ethanol (10 mL) was heated at reflux for 5 h. After the reaction mixture was cooled to room temperature, saturated aq K2CO3 solution was slowly added with stirring until the pH was 8–9. Then the mixture was extracted with EtOAc (10 mL X 3), and the combined organic layer was dried. The solvent was evaporated and the residue was purified by flash column chromatography (EtOAc: hexane = 1:1) to afford the products 15a–d.
5.1.13 4-(2-(4-Aminophenyl)-1-(4-hydroxyphenyl)prop-1-enyl)phenol (15a)
White solid, 80% yield: mp 154–156 °C. 1H NMR (300 MHz, DMSO-d6) δ 9.34 (s, 1 H), 9.12 (s, 1 H), 6.91 (d, J = 8.5 Hz, 2 H), 6.75 (d, J = 8.5 Hz, 2 H), 6.70 (d, J = 8.5 Hz, 2 H), 6.60 (d, J = 8.5 Hz, 2 H), 6.42 (d, J = 8.5 Hz, 2 H), 6.32 (d, J = 8.5 Hz, 2 H), 4.97 (s, 2 H), 1.95 (s, 3 H); 13C NMR (75 MHz, DMSO-d6) δ 156.5, 155.8, 147.4, 137.4, 135.7, 133.7, 132.4, 132.2, 131.7, 130.6, 115.7, 115.2, 114.3, 23.9; MALDIMS m/z 317 (M+); HRESIMS m/z calcd for C21H20NO2 (MH+) 318.1494, found 318.1495; HPLC purity, 97.85% (90% MeOH, 10% H2O).
5.1.14 4-(2-(4-Aminophenyl)-1-(4-hydroxyphenyl)but-1-enyl)phenol (15b)
White solid, 71% yield: mp 173–175 °C. 1H NMR (300 MHz, DMSO-d6) δ 9.34 (s, 1 H), 9.10 (s, 1 H), 6.93 (d, J = 8.5 Hz, 2 H), 6.72 (d, J = 8.5 Hz, 2 H), 6.59 (d, J = 8.5 Hz, 2 H), 6.40 (d, J = 8.5 Hz, 2 H), 6.37 (d, J = 8.5 Hz, 2 H), 6.34 (d, J = 8.5 Hz, 2 H), 4.92 (s, 2 H), 2.30 (q, J = 7.4 Hz, 2 H), 0.84 (t, J = 7.4 Hz, 3 H); 13C NMR (75 MHz, DMSO-d6) δ 156.7, 155.7, 147.4, 137.7, 135.8, 135.2, 134.9, 132.3, 131.1, 130.8, 115.8, 115.1, 114.5, 29.3, 14.7; MALDIMS m/z 331 (M+); HRESIMS m/z calcd for C22H22NO2 (MH+) 332.1651, found 332.1651; HPLC purity, 99.59% (90% MeOH, 10% H2O).
5.1.15 4-(1,2-Bis(4-aminophenyl)prop-1-enyl)benzenamine (15c)
White solid, 52% yield: mp 134–135 °C. 1H NMR (300 MHz, DMSO-d6) δ 6.77 (d, J = 8.3 Hz, 2 H), 6.75 (d, J = 8.3 Hz, 2 H), 6.49 (d, J = 8.1 Hz, 2 H), 6.40 (d, J = 8.2 Hz, 2 H), 6.32 (d, J = 8.4 Hz, 2 H), 6.21 (d, J = 8.4 Hz, 2 H), 4.88 (s, 6 H), 1.96 (s, 3 H); 13C NMR (75 MHz, DMSO-d6) δ 147.7, 147.1, 146.9, 138.4, 132.9, 132.2, 131.6, 131.4, 130.6, 114.3, 113.9, 24.1; MALDIMS m/z 315 (M+); HRESIMS m/z calcd for C21H22N3 (MH+) 316.1814, found 316.1814; HPLC purity, 95.50% (90% MeOH, 10% H2O).
5.1.16 4-(1,2-Bis(4-aminophenyl)but-1-enyl)benzenamine (15d)
White solid, 56% yield: mp 145–147 °C. 1H NMR (300 MHz, DMSO-d6) δ 6.77 (d, J = 8.3 Hz, 2 H), 6.73 (d, J = 8.3 Hz, 2 H), 6.49 (d, J = 8.3 Hz, 2 H), 6.45 (d, J = 8.4 Hz, 2 H), 6.34 (d, J = 8.3 Hz, 2 H), 6.20 (d, J = 8.4 Hz, 2 H), 4.89 (s, 6 H), 2.33 (q, J = 7.4 Hz, 2 H), 0.83 (t, J = 7.2 Hz, 3 H); 13C NMR (75 MHz, DMSO-d6) δ 147.7, 147.1, 146.7, 138.6, 133.1, 132.8, 132.1, 131.0, 130.8, 130.7, 129.8, 129.1, 114.5, 114.4, 113.9, 29.2, 14.8; MALDINS m/z 329 (M+); HRESIMS m/z calcd for C22H24N3 (MH+) 330.1970, found 330.1971; HPLC purity, 96.91% (90% MeOH, 10% H2O).
5.1.17 General Procedure for the Synthesis of the Monoalkylated Products (16a–d)
A suspension of the diphenols 12a or 12b or dianilines 12c or 12d (1 mmol) and K2CO3 (3 mmol) in acetone (10 mL) was heated at reflux for 10 min. A solution of 2-iodoacetamide (1.3 mmol) in acetone (6 mL) was added in small portions over 3 h and the reaction mixture was heated at reflux for an additional 1 h. After cooling to room temperature, the solvent was evaporated and the residue was dissolved in saturated NH4Cl solution (30 mL) and extracted with EtOAc (30 mL X 3). The organic layers were combined, dried, concentrated in vacuo and purified by flash column chromatography to provide the products 16a–d.
5.1.18 (E,Z)-2-(4-(1-(4-Hydroxyphenyl)-2-(4-nitrophenyl)prop-1-enyl)phenoxy)-acetamide (16a)
Yellow solid, 41% yield: mp 129–130 °C. 1H NMR (300 MHz, methanol-d4) δ 7.99 (d, J = 9.3 Hz, 2 H, isomer 1), 7.98 (d, J = 8.7 Hz, 2 H, isomer 2), 7.34 (d, J = 9.3 Hz, 2 H, isomer 1), 7.33 (d, J = 8.7 Hz, 2 H, isomer 2), 7.17 (d, J = 8.7 Hz, 2 H, isomer 2), 7.02 (d, J = 8.4 Hz, 2 H, isomer 1), 6.98 (d, J = 8.0 Hz, 2 H, isomer 1), 6.82-6.76 (m, 4 H), 6.66 (d, J = 8.7 Hz, 4 H), 6.46 (d, J = 8.7 Hz, 2 H, isomer 2), 4.51 (s, 2 H, isomer 1), 4.53 (s, 2 H, isomer 2), 2.15 (s, 3 H, isomer 1), 2.13 (s, 3 H, isomer 2); 13C NMR (75 MHz, methanol-d4) δ 174.0, 174.0, 158.2, 157.8, 157.7, 157.4, 153.5, 147.1, 142.8, 137.9, 137.7, 135.2, 135.0, 133.8, 133.6, 133.3, 133.3, 133.1, 132.3, 132.2, 131.6, 124.1, 116.0, 115.6, 115.5, 115.0, 67.9, 67.8, 23.2, 23.0; negative ion ESIMS m/z 403 (M – H+)−; HRESIMS m/z calcd for C23H21N2O5 (MH+) 405.1450, found 405.1460; HPLC purity, 95.55% (90% MeOH, 10% H2O).
5.1.19 (E,Z)-2-(4-(1-(4-Hydroxyphenyl)-2-(4-nitrophenyl)but-1-enyl)phenoxy)-acetamide (16b)
Yellow oil, 56% yield. 1H NMR (300 MHz, methanol-d4) δ 8.01 (d, J = 8.7 Hz, 2 H, isomer 1), 8.00 (d, J = 8.7 Hz, 2 H, isomer 2), 7.32 (d, J = 8.7 Hz, 2 H, isomer 1), 7.31 (d, J = 8.7 Hz, 2 H, isomer 2), 7.15 (d, J = 8.7 Hz, 2 H, isomer 2), 7.00 (d, J = 8.4 Hz, 2 H, isomer 1), 6.97 (d, J = 8.0 Hz, 2 H, isomer 1), 6.79-6.76 (m, 4 H), 6.64 (d, J = 8.7 Hz, 4 H), 6.44 (d, J = 8.7 Hz, 2 H, isomer 2), 4.51 (s, 2 H, isomer 1), 4.34 (s, 2 H, isomer 2), 2.59-2.50 (m, 4 H), 0.94-0.89 (m, 6 H); 13C NMR (75 MHz, methanol-d4) δ 174.1, 158.2, 157.8, 157.2, 152.0, 147.2, 142.2, 140.5, 140.2, 137.9, 137.6, 135.3, 135.0, 133.3, 133.2, 132.1, 131.7, 131.6, 124.1, 116.0, 115.6, 115.0, 68.0 67.8, 29.6, 29.5, 13.9; ESIMS m/z 441 (MNa+); HRESIMS m/z calcd for C24H22N2O5Na (MNa+) 441.1427, found 441.1431; HPLC purity, 97.18% (90% MeOH, 10% H2O).
5.1.20 (E,Z)-2-(4-(1-(4-Aminophenyl)-2-(4-nitrophenyl)prop-1-enyl)phenylamino)acetamide (16c)
Reddish-brown solid, 30% yield: mp 112–114 °C. 1H NMR (300 MHz, methanol-d4) δ 7.98 (d, J = 8.8 Hz, 2 H, isomer 1), 7.97 (d, J = 8.8 Hz, 2 H, isomer 2), 7.33 (d, J = 8.7 Hz, 2 H, isomer 1), 7.32 (d, J = 8.7 Hz, 2 H, isomer 2), 6.97 (d, J = 8.4 Hz, 2 H, isomer 1), 6.95 (d, J = 8.4 Hz, 2 H, isomer 2), 6.70 (d, J = 8.5 Hz, 2 H, isomer 1), 6.65-6.58 (m, 6 H), 6.41 (d, J = 8.5 Hz, 2 H), 6.28 (d, J = 8.5 Hz, 2 H), 3.94 (s, 2 H, isomer 1), 3.75 (s, 2 H, isomer 2), 2.15 (s, 6 H); 13C NMR (75 MHz, methanol-d4) δ 177.1, 154.3, 148.3, 148.0, 146.8, 134.7, 133.9, 133.2, 132.1, 132.0, 131.7, 124.0, 116.1, 115.9, 113.3, 112.9, 23.1; ESIMS m/z 425 (MNa+); HRESIMS m/z calcd for C23H23N4O3 (MH+) 403.1770, found 403.1776; HPLC purity, 97.57% (90% MeOH, 10% H2O).
5.1.21 (E,Z)-2-(4-(1-(4-Aminophenyl)-2-(4-nitrophenyl)but-1-enyl)phenylamino)acetamide (16d)
Reddish-brown solid, 34% yield: mp 132–135 °C. 1H NMR (300 MHz, methanol-d4) δ 7.99 (d, J = 8.8 Hz, 2 H, isomer 1), 7.98 (d, J = 8.8 Hz, 2 H, isomer 2), 7.32 (d, J = 8.7 Hz, 2 H, isomer 1), 7.30 (d, J = 8.7 Hz, 2 H, isomer 2), 7.01 (d, J = 8.4 Hz, 2 H, isomer 1), 7.00 (d, J = 8.4 Hz, 2 H, isomer 2), 6.82 (d, J = 8.5 Hz, 2 H, isomer 1), 6.68-6.58 (m, 6 H), 6.50 (d, J = 8.5 Hz, 2 H), 6.26 (d, J = 8.5 Hz, 2 H), 3.75 (s, 2 H, isomer 1), 3.60 (s, 2 H, isomer 2), 2.61-2.52 (m, 4 H), 0.95-0.88 (m, 6 H); 13C NMR (75 MHz, methanol-d4) δ 177.1, 152.5, 148.6, 148.0, 147.0, 143.1, 139.3, 136.3, 133.7, 133.2, 133.1, 132.1, 131.5, 124.1, 117.4, 117.2, 113.4, 112.9, 29.6, 14.0; ESIMS m/z 417 (MH+); HRESIMS m/z calcd for C24H25N4O3 (MH+) 417.1927, found 417.1935; HPLC purity, 96.48% (90% MeOH, 10% H2O).
5.1.22 1-(1-Phenylpropylidene)hydrazine (19).34
A 98% hydrazine monohydrate solution (2 mL, 40 mmol) was added to propiophenone (18, 2.68 g, 20 mmol) in EtOH (15 mL). The mixture was heated to reflux for 2 h. After cooling to room temperature, the mixture was partitioned between CH2Cl2 and H2O. The aqueous layer was extracted with CH2Cl2 (30 mL X 3). The organic layers were combined, dried, concentrated in vacuo and purified by column chromatography (hexane: EtOAc = 5: 1) to afford the product 19 as yellowish solid (2.60 g, 88% yield): mp 52–53 °C (lit.34 mp 55–57 °C).
5.1.23 1-(1,1-Dibromobut-1-en-2-yl)benzene (20)27
A 28% aqueous solution of ammonia (1 mL) and CuCl (29.7 mg, 0.3 mmol) were added to a solution of 1-(1-phenylpropylidene)hydrazine 19 (444 mg, 3.0 mmol) in DMSO (3 mL). Then CBr4 (2.98 g, 9 mmol) in DMSO (5 mL) was added dropwise. The reaction mixture was stirred at room temperature for 16 h and quenched with H2O (30 mL) and extracted with CH2Cl2 (20 mL X 3). After being dried over Na2SO4, the CH2Cl2 was evaporated and the residue was purified by column chromatography (hexane) to afford the product 12 as a light brown oil (607 mg, 70% yield). 1H NMR (400 MHz, CDCl3) δ 7.39-7.30 (m, 3 H), 7.19-7.16 (m, 2 H), 2.61 (q, J = 7.6 Hz, 2 H), 0.98 (t, J = 7.6 Hz, 3 H); 13C NMR (100 MHz, CDCl3) δ 148.9, 140.9, 128.4, 127.9, 127.7, 87.5, 32.8, 11.4; CIMS m/z 290 (MH+).
5.1.24 4-(1-(4-Hydroxyphenyl)-2-phenylbut-1-enyl)phenol (13)
A solution of 1-(1,1-dibromobut-1-en-2-yl)benzene 20 (288 mg, 1.0 mmol), 4-hydroxyphenylboronic acid (552 mg, 4.0 mmol), PdCl2(PPh3)2 (70 mg, 0.1 mmol), and Na2CO3 (318 mg, 3.0 mmol) in THF-H2O (4:1, 15 mL) was heated to 70 °C under Ar2 for 18 h. After cooling to room temperature, EtOAc (15 mL) and H2O (10 mL) were poured into the reaction mixture. The aqueous layer was extracted with EtOAc (20 mL X 3). The combined organic layers were washed with water and dried, concentrated in vacuo and purified by silica gel flash column chromatography (hexane: EtOAc = 4:1) to afford the product 13 as white solid (164 mg, 52% yield): mp 198–200 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.40 (s, 1 H), 9.15 (s, 1 H), 7.18 (t, J = 7.6 Hz, 2 H), 7.10 (t, J = 7.6 Hz, 3 H), 6.98 (d, J = 8.8 Hz, 2 H), 6.75 (d, J = 8.4 Hz, 2 H), 6.60 (d, J = 8.4 Hz, 2 H), 6.40 (d, J = 8.8 Hz, 2 H), 2.41 (q, J = 7.2 Hz, 2 H), 0.84 (t, J = 7.2 Hz, 3 H); 13C NMR (100 MHz, DMSO-d6) δ 156.8, 155.9, 143.2, 140.1, 139.0, 134.8, 134.6, 132.2, 130.9, 130.2, 128.6, 126.6, 115.7, 115.0, 29.3, 14.2; HRAPCIMS m/z calcd for C22H21O2 (MH+) 317.1542, found 317.1565.
5.1.25 (E,Z)-2-(4-(1-(4-Hydroxyphenyl)-2-phenylbut-1-enyl)phenoxy)-acetamide (17)
The intermediate was prepared as previously described.18
5.1.26 (E,Z)-Norendoxifen
This compound was prepared as previously described.18
5.2 Inhibition of Recombinant Human Aromatase (CYP19) by Microsomal Incubations
These experiments were conducted as previously described.19
5.3 Binding Affinities for Recombinant Human ER-α and ER-β
The binding affinities were determined as previously described.19
5.4 Abilities of Compounds to Antagonize β-Estradiol-stimulated Progesterone Receptor (PGR) mRNA Expression in MCF-7 Cells
The assay was performed as previously described.19
Supplementary Material
Acknowledgments
This research was supported by the Purdue University Center for Cancer Research and the Indiana University Center Joint Funding Award 206330, and by the Purdue Center for Cancer Research grant P30 CA023168.
Abbreviations
- AIs
aromatase inhibitors
- ATAC
arimidex, tamoxifen, alone or in combination
- DMSO
dimethyl sulphoxide
- ER
estrogen receptor
- EtOAc
ethyl acetate
- EtOH
ethanol
- MeOH
methanol
- SAR
structure-activity relationship
- SERM
selective estrogen receptor modulator
- THF
tetrahydrofuran
Footnotes
SMILES molecular strings and PDB files for 12d bound to aromatase and ER-α, as well as 1H and 13C NMR spectra for compounds 12–16 and 20.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amir E, Freedman OC, Seruga B, Evans DG. J Natl Cancer Inst. 2010;102:680. doi: 10.1093/jnci/djq088. [DOI] [PubMed] [Google Scholar]
- 2.Bai Z, Gust R. Arch Pharm. 2009;342:133. doi: 10.1002/ardp.200800174. [DOI] [PubMed] [Google Scholar]
- 3.Ali S, Coombes RC. J Mammary Gland Biol Neoplasia. 2000;5:271. doi: 10.1023/a:1009594727358. [DOI] [PubMed] [Google Scholar]
- 4.Jordan VC, McDaniel R, Agboke F, Maximov PY. Steroids. 2014;90:3. doi: 10.1016/j.steroids.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Komm BS, Mirkin S. J Steroid Biochem Mol Biol. 2014;143:207. doi: 10.1016/j.jsbmb.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Ring A, Dowsett M. Endocr Relat Cancer. 2004;11:643. doi: 10.1677/erc.1.00776. [DOI] [PubMed] [Google Scholar]
- 7.Ali S, Coombes RC. Nat Rev Cancer. 2002;2:101. doi: 10.1038/nrc721. [DOI] [PubMed] [Google Scholar]
- 8.Chumsri S, Howes T, Bao T, Sabnis G, Brodie A. J Steroid Biochem Mol Biol. 2011;125:13. doi: 10.1016/j.jsbmb.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heshmati HM, Khosla S, Robins SP, O’Fallon WM, Melton LJ, Riggs BL. J Bone Miner Res. 2002;17:172. doi: 10.1359/jbmr.2002.17.1.172. [DOI] [PubMed] [Google Scholar]
- 10.Bundred NJ. Br J Cancer. 2005;93:S23. doi: 10.1038/sj.bjc.6602692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bird B, Swain SM. Clin Cancer Res. 2008;14:14. doi: 10.1158/1078-0432.CCR-07-1033. [DOI] [PubMed] [Google Scholar]
- 12.Ewer MS, Gluck S. Cancer. 2009;115:1813. doi: 10.1002/cncr.24219. [DOI] [PubMed] [Google Scholar]
- 13.Borisy AA, Elliott PJ, Hurst NW, Lee MS, Lehar J, Price ER, Serbedzija G, Zimmermann GR, Foley MA, Stockwell BR, Keith CT. Proc Natl Acad Sci USA. 2003;100:7977. doi: 10.1073/pnas.1337088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufmann M, Jonat W, Hilfrich J, Eidtmann H, Gademann G, Zuna I. J Clin Oncol. 2007;25:2664. doi: 10.1200/JCO.2006.08.8054. [DOI] [PubMed] [Google Scholar]
- 15.Boccardo F, Rubagotti A, Guglielmini P, Fini A, Paladini G, Mesiti M, Rinaldini M, Scali S, Porpiglia M, Benedetto C, Restuccia N, Buzzi F, Franchi R, Massidda B, Distante V, Amadori D, Sismondi P trial ITA. Ann Oncol. 2006;17:VII10. doi: 10.1093/annonc/mdl941. [DOI] [PubMed] [Google Scholar]
- 16.Baum M, Buzdar A, Cuzick J, Forbes J, Houghton J, Howell A, Sahmoud T, Group AT. Cancer. 2003;98:1802. doi: 10.1002/cncr.11745. [DOI] [PubMed] [Google Scholar]
- 17.Lu WJ, Xu C, Pei ZF, Mayhoub AS, Cushman M, Flockhart DA. Breast Cancer Res Treat. 2012;133:99. doi: 10.1007/s10549-011-1699-4. [DOI] [PubMed] [Google Scholar]
- 18.Lv W, Liu J, Lu D, Flockhart DA, Cushman M. J Med Chem. 2013;56:4611. doi: 10.1021/jm400364h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lv W, Liu JZ, Skaar TC, Flockhart DA, Cushman M. J Med Chem. 2015;58:2623. doi: 10.1021/jm501218e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lv W, Liu J, Skaar TC, O’Neill E, Yu G, Flockhart DA, Cushman M. J Med Chem. 2016;59:157. doi: 10.1021/acs.jmedchem.5b01677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haynes BP, Dowsett M, Miller WR, Dixon JM, Bhatnagar AS. J Steroid Biochem Mol Biol. 2003;87:35. doi: 10.1016/s0960-0760(03)00384-4. [DOI] [PubMed] [Google Scholar]
- 22.Gobbi S, Zimmer C, Belluti F, Rampa A, Hartmann RW, Recanatini M, Bisi A. J Med Chem. 2010;53:5347. doi: 10.1021/jm100319h. [DOI] [PubMed] [Google Scholar]
- 23.Detsi A, Koufaki M, Calogeropoulou T. J Org Chem. 2002;67:4608. doi: 10.1021/jo0255328. [DOI] [PubMed] [Google Scholar]
- 24.Yu DD, Forman BM. J Org Chem. 2003;68:9489. doi: 10.1021/jo035164n. [DOI] [PubMed] [Google Scholar]
- 25.Uddin MJ, Rao PNP, Knaus EE. Bioorg Med Chem. 2004;12:5929. doi: 10.1016/j.bmc.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 26.Nenajdenko VG, Lenkova ON, Shastin AV, Balenkova ES. Synthesis. 2004:573. [Google Scholar]
- 27.Korotchenko VN, Shastin AV, Nenajdenko VG, Balenkova ES. J Chem Soc Perkin Trans 1. 2002:883. doi: 10.1039/b303221c. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh D, Lo J, Morton D, Valette D, Xi J, Griswold J, Hubbell S, Egbuta C, Jiang W, An J, Davies HML. J Med Chem. 2012;55:8464. doi: 10.1021/jm300930n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, Greene GL. Cell. 1998;95:927. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- 30.Lu WJ, Desta Z, Flockhart DA. Breast Cancer Res Treat. 2012;131:473. doi: 10.1007/s10549-011-1428-z. [DOI] [PubMed] [Google Scholar]
- 31.Lim YC, Desta Z, Flockhart DA, Skaar TC. Cancer Chemother Pharmacol. 2005;55:471. doi: 10.1007/s00280-004-0926-7. [DOI] [PubMed] [Google Scholar]
- 32.Liu JZ, Flockhart PJ, Lu DS, Lv W, Lu WJJ, Han X, Cushman M, Flockhart DA. Drug Metab and Dispos. 2013;41:1715. doi: 10.1124/dmd.113.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paruthiyil S, Parmar H, Kerekatte V, Cunha GR, Firestone GL, Leitman DC. Cancer Res. 2004;64:423. doi: 10.1158/0008-5472.can-03-2446. [DOI] [PubMed] [Google Scholar]
- 34.Lee KJ, Kim JL, Hong MK, Lee JY. J Heterocycl Chem. 1999;36:1235. [Google Scholar]
- 35.Agouridas V, Laios I, Cleeren A, Kizilian E, Magnier E, Blazejewski JC, Leclercq G. Bioorg Med Chem. 2006;14:7531. doi: 10.1016/j.bmc.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Shoda T, Okuhira K, Kato M, Demizu Y, Inoue H, Naito M, Kurihara M. Bioorg Med Chem Lett. 2014;24:87. doi: 10.1016/j.bmcl.2013.11.078. [DOI] [PubMed] [Google Scholar]
- 37.Arsenyan P, Paegle E, Domracheva I, Gulbe A, Kanepe-Lapsa I, Shestakova I. Eur J Med Chem. 2014;87:471. doi: 10.1016/j.ejmech.2014.09.088. [DOI] [PubMed] [Google Scholar]
- 38.Ohta K, Chiba Y, Kaise A, Endo Y. Bioorg Med Chem. 2015;23:861. doi: 10.1016/j.bmc.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 39.Shoda T, Kato M, Harada R, Fujisato T, Okuhira K, Demizu Y, Inoue H, Naito M, Kurihara M. Bioorg Med Chem. 2015;23:3091. doi: 10.1016/j.bmc.2015.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.