Abstract
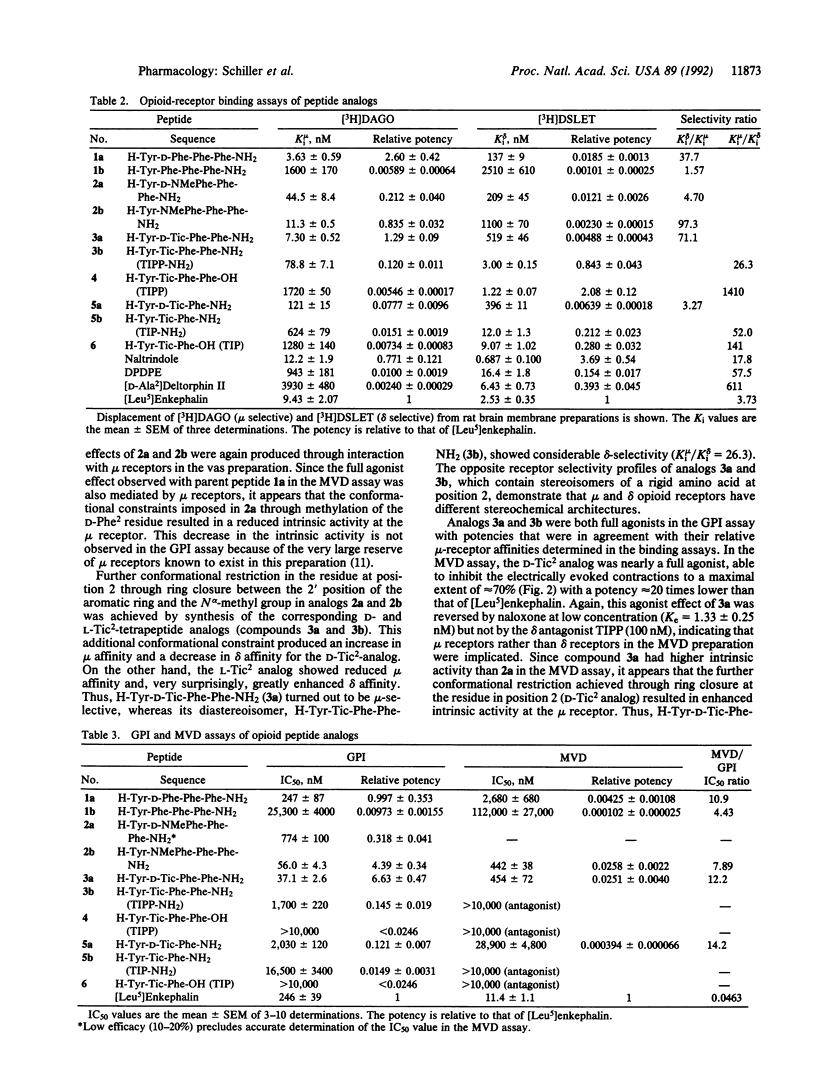
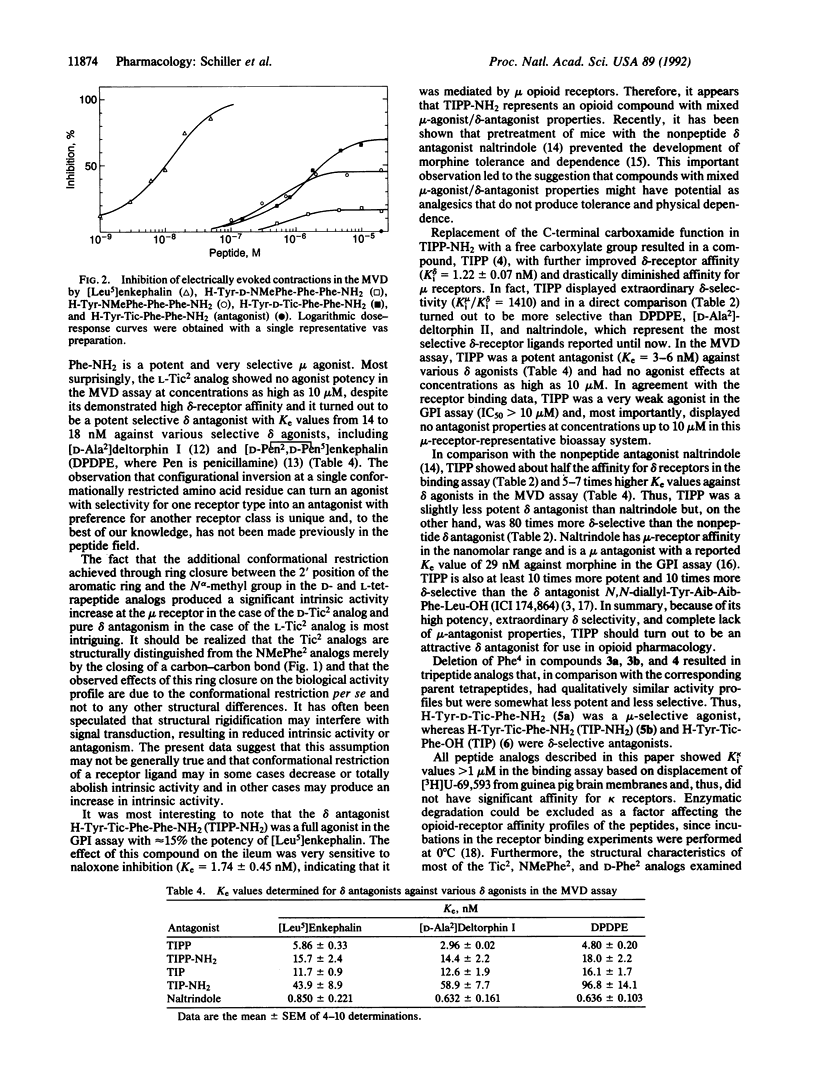
Opioid peptide analogs consisting entirely of aromatic amino acid residues and containing conformationally restricted phenylalanine derivatives in position 2 of the peptide sequence were synthesized and pharmacologically characterized in vitro. Both diastereoisomers of H-Tyr-(D or L)-NMePhe-Phe-Phe-NH2 (NMePhe is N alpha-methylphenylalanine) were mu-receptor-selective, were full agonists in the mu-receptor-representative guinea pig ileum assay, and were partial agonists in the mouse vas deferens assay, with the L-NMePhe2 analog displaying somewhat higher intrinsic activity than the D-NMePhe2 analog. Further conformational restriction at position 2 in the sequence, as achieved through substitution of D- or L-tetrahydro-3-isoquinoline carboxylic acid (Tic), produced a configuration-dependent differential effect on receptor selectivity and intrinsic activity, leading to a potent mu-selective mu agonist (the D-Tic2 analog) with increased intrinsic activity in the mouse vas deferens assay and to a potent delta-selective delta antagonist (the L-Tic2 analog). These results demonstrate that imposition of conformational constraints in a peptide not only may alter receptor selectivity but also may decrease, totally abolish, or even enhance intrinsic activity. The tetrapeptide H-Tyr-Tic-Phe-Phe-NH2 was a moderately potent full agonist in the guinea pig ileum assay and, thus, represents a compound with mixed mu-agonist/delta-antagonist properties. The corresponding peptide with a free C-terminal carboxyl group H-Tyr-Tic-Phe-Phe-OH showed high delta-receptor affinity (Ki delta = 1.2 nM), unprecedented delta selectivity (Ki mu/Ki delta = 1410), high potency as delta antagonist (Ke = 3-8 nM against various delta agonists in the mouse vas deferens assay) and, unlike other delta antagonists, had no mu-antagonist properties. The tripeptides H-Tyr-Tic-Phe-OH and H-Tyr-Tic-Phe-NH2 were also delta antagonists.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdelhamid E. E., Sultana M., Portoghese P. S., Takemori A. E. Selective blockage of delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J Pharmacol Exp Ther. 1991 Jul 1;258(1):299–303. [PubMed] [Google Scholar]
- Chavkin C., Goldstein A. Reduction in opiate receptor reserve in morphine tolerant guinea pig ilea. Life Sci. 1982 Oct 18;31(16-17):1687–1690. doi: 10.1016/0024-3205(82)90186-2. [DOI] [PubMed] [Google Scholar]
- Chavkin C., James I. F., Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982 Jan 22;215(4531):413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Corbett A. D., Gillan M. G., Kosterlitz H. W., McKnight A. T., Paterson S. J., Robson L. E. Selectivities of opioid peptide analogues as agonists and antagonists at the delta-receptor. Br J Pharmacol. 1984 Sep;83(1):271–279. doi: 10.1111/j.1476-5381.1984.tb10143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton R., Giles M. G., Miller L., Shaw J. S., Timms D. ICI 174864: a highly selective antagonist for the opioid delta-receptor. Eur J Pharmacol. 1984 Jan 27;97(3-4):331–332. doi: 10.1016/0014-2999(84)90470-9. [DOI] [PubMed] [Google Scholar]
- DiMaio J., Schiller P. W. A cyclic enkephalin analog with high in vitro opiate activity. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7162–7166. doi: 10.1073/pnas.77.12.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erspamer V., Melchiorri P., Falconieri-Erspamer G., Negri L., Corsi R., Severini C., Barra D., Simmaco M., Kreil G. Deltorphins: a family of naturally occurring peptides with high affinity and selectivity for delta opioid binding sites. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5188–5192. doi: 10.1073/pnas.86.13.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. A., Waterfield A. A., Hughes J., Kosterlitz H. W. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977 Jun 9;267(5611):495–499. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- Mosberg H. I., Hurst R., Hruby V. J., Gee K., Yamamura H. I., Galligan J. J., Burks T. F. Bis-penicillamine enkephalins possess highly improved specificity toward delta opioid receptors. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5871–5874. doi: 10.1073/pnas.80.19.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portoghese P. S. An approach to the design of receptor-type-selective non-peptide antagonists of peptidergic receptors: delta opioid antagonists. J Med Chem. 1991 Jun;34(6):1757–1762. doi: 10.1021/jm00110a001. [DOI] [PubMed] [Google Scholar]
- Portoghese P. S., Sultana M., Takemori A. E. Naltrindole, a highly selective and potent non-peptide delta opioid receptor antagonist. Eur J Pharmacol. 1988 Jan 27;146(1):185–186. doi: 10.1016/0014-2999(88)90502-x. [DOI] [PubMed] [Google Scholar]
- Schiller P. W., DiMaio J. Opiate receptor subclasses differ in their conformational requirements. Nature. 1982 May 6;297(5861):74–76. doi: 10.1038/297074a0. [DOI] [PubMed] [Google Scholar]
- Schiller P. W., Nguyen T. M., Chung N. N., Lemieux C. Dermorphin analogues carrying an increased positive net charge in their "message" domain display extremely high mu opioid receptor selectivity. J Med Chem. 1989 Mar;32(3):698–703. doi: 10.1021/jm00123a035. [DOI] [PubMed] [Google Scholar]
- Schiller P. W., Weltrowska G., Nguyen T. M., Lemieux C., Chung N. N., Marsden B. J., Wilkes B. C. Conformational restriction of the phenylalanine residue in a cyclic opioid peptide analogue: effects on receptor selectivity and stereospecificity. J Med Chem. 1991 Oct;34(10):3125–3132. doi: 10.1021/jm00114a023. [DOI] [PubMed] [Google Scholar]
- Wilkes B. C., Schiller P. W. Theoretical conformational analysis of a mu-selective cyclic opioid peptide analog. Biopolymers. 1987 Aug;26(8):1431–1444. doi: 10.1002/bip.360260817. [DOI] [PubMed] [Google Scholar]


