Abstract
Dorsal root ganglia (DRG) are critical anatomical structures involved in nociception. Intraganglionic (IG) drug delivery is therefore an important route of administration for novel analgesic therapies. Although IG injection in large animal models is highly desirable for pre-clinical biodistribution and toxicology studies of new drugs, no method to deliver pharmaceutics into the DRG has been reported in any large species. The present study describes a minimally invasive technique of IG agent delivery in domestic swine, one of the most common large animal models. The technique utilizes computed tomography (CT) guidance for DRG targeting, and a custom-made injection assembly for convection-enhanced delivery (CED) of therapeutic agents directly into DRG parenchyma. The DRG were initially visualized by CT-myelogram to determine the optimal access route to the DRG. The subsequent IG injection consisted of three steps. First, a commercially available guide needle was advanced to a position dorso-lateral to the DRG, and the dural root sleeve was punctured, leaving the guide needle contiguous with, but not penetrating, the DRG. Second, the custom-made stepped stylet was inserted through the guide needle into the DRG parenchyma. Third, the stepped stylet was replaced by the custom-made stepped needle, which was used for the IG CED. Initial dye injections performed in pig cadavers confirmed the accuracy of DRG targeting under CT guidance. IG administration of adeno-associated virus in vivo resulted in a unilateral transduction of the injected DRG, with 33.5% DRG neurons transduced. Transgene expression was also found in the dorsal root entry zones at the corresponding spinal levels. The results thereby confirm the efficacy of CED by the stepped needle and a selectivity of DRG targeting. Imaging based modeling of the procedure in humans suggests that IG CED may be translatable to the clinical setting.
Keywords: Dorsal root ganglia, computed tomography, intraganglionic injection, convection enhanced delivery, gene therapy, pain
Introduction
Dorsal root ganglia (DRG) contain the first order neurons of all sensory pathways and are therefore essential structures in pain signaling. Targeted delivery of therapeutic agents into the DRG in animal models is consequently a critical technique to investigate novel analgesic treatments in preclinical pharmacology. In rodents, DRG exposure by open surgical procedure and intra-DRG administration of agents has been demonstrated15,6,23. While rodent species are used for initial drug discovery and proof-of-concept studies, large animals models are often needed for preclinical toxicity and biodistribution testing of novel drugs. However, no method describing DRG targeting and intra-DRG drug delivery in large animals has been reported.
In the clinical setting, the epidural space adjacent to the DRG is frequently accessed under C-arm fluoroscopy or computed tomography (CT) guidance in the performance of transforaminal epidural steroid injections, which are effective in treatment of radicular pain4,13,21 . This minimally invasive approach may also be occasionally used for radio-frequency modulation of the dorsal, sensory portion of the DRG to treat select pain syndromes7. The interventional pain management techniques, however, have not been used to insert the needle tip or to deliver therapeutic agents into the DRG parenchyma.
In order to optimize the mechanics of intraparenchymal drug administration, convection-enhanced delivery (CED) has been successfully used in the central nervous system (CNS), and in the peripheral nerve distal to the DRG3,16. CED uses bulk flow, as opposed to simple diffusion, to enhance distribution of drugs in solid tissues3. Studies investigating the needle design needed for efficient CED in the CNS have demonstrated that the stepped needle, consisting of a sharp transition from the wider needle shaft to a narrow tip, improves the volume of distribution of the injectate, allows higher flow rates, and prevents reflux of the injectate along the needle path18,22,11.
We have developed a CT-guided technique to advance a needle percutaneously into the lumbar DRG in the pig. Successful intraganglionic (IG) drug administration is evidenced by robust transduction of the DRG neurons achieved by convection-enhanced delivery (CED) of adeno-associated virus (AAV). Preliminary clinical translatability of the IG injection is demonstrated by imaging based modeling of the procedure in humans.
Materials and methods
Animals
Farm pigs of mixed Landrace background (Manthei Hog Farm, Elk River, MN, USA) weighing between 20 and 30 kg were used. All procedures were performed in accordance to the Guide for the Care and Use of Laboratory Animals17 and approved by the Institutional Animal Care and Use Committee of the Mayo Clinic (protocol number A18810). The animals were sedated by intramuscular injection of Telazol (tiletamine and zolazepam, 5 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA, USA), Xylazine (2 mg/kg; AnaSed, Akorn, Decatur, IL, USA), and Glycopyrrolate (0.01 mg/kg; Baxter Healthcare, Deerfield, IL, USA). The pigs were then intubated and general anesthesia maintained by 1.5–2% isoflurane (Terrel; Piramal Healthcare, Bethlehem, PA, USA).
Imaging
A clinical CT scanner (Definition DS, Siemens Healthcare, Forchheim, Germany) with interventional CT / fluoroscopy (CTF) hardware and software packages were used. Topograms, pre-procedural spiral CT scans, and intra-procedural CT fluoroscopy (CTF) images were captured using identical acquisition settings as reported previously14.
Intraganglionic (IG) injection assembly
Overview of the assembly components
The IG injection was performed using an outer guide needle (22 G, 152.7 mm, Quincke tip) and three insets (Fig. 1). The insets were passed consecutively through the guide needle in the following order: (1) the proprietary stylet of the guide needle, used to access the intrathecal (IT) space adjacent to the DRG under CT guidance; (2) the stepped stylet, used to penetrate the DRG; and (3) the stepped needle, used to for IG CED. The guide needle with its first inset (proprietary stylet) was commercially available (Kimberley-Clark, Roswell, GA, USA). The other two insets (the stepped stylet and needle) were custom made, with design adopted from Krauze et al.12 and modified for use in the IG paradigm.
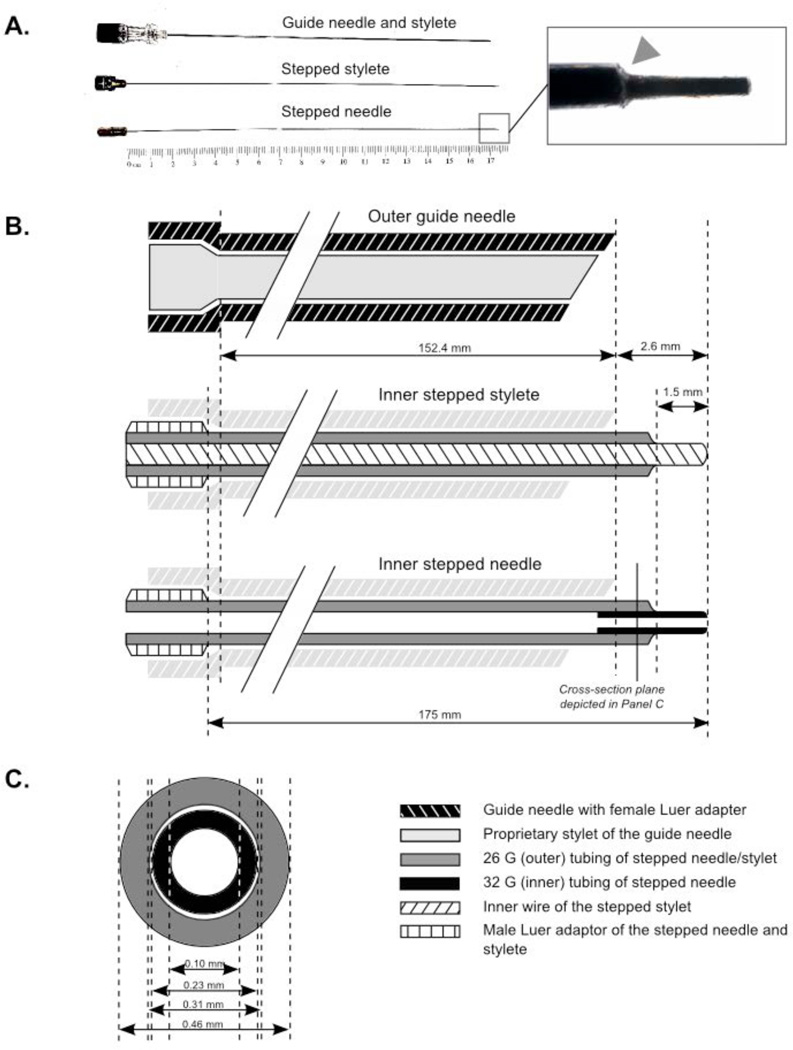
Figure 1. Injection assembly used for DRG targeting and intraganglionic (IG) convection enhanced delivery (CED).
The 22 G 6’’ guide needle and three insets (proprietary stylet of the guide needle, custom made stepped stylet, and custom made stepped needle) were used throughout the 3-step procedure detailed in the main text.
A. Overview of the guide needle (with its proprietary stylet in place), the stepped stylet, and the stepped needle. The term “step” (arrowhead) refers to the sharp transition between the wide needle/stylet shaft and their narrow tips.
B. Longitudinal section of the guide needle with its three insets in place: proprietary stylet (top), custom made stepped stylet (middle); or custom made stepped needle (bottom).
C. Cross-section of the stepped needle.
Parameters of the custom stepped stylet and needle
The 175 mm shafts of both the stepped stylet and needle were made of 26 G stainless steel tubing (outer diameter, OD: 0.01825”; inner diameter, ID: 0.01224”; wall thickness: 0.003”; Small Parts, Logansport, IN, USA) and their proximal ends were welded to Luer style male hubs. For the stylet, solid stainless steel wire (OD: 0.009’’; Small Parts, Logansport, IN, USA) was welded inside the 26 G tubing to form a 1.5 mm stepped tip at the distal end of the stylet. For the stepped needle, 5 mm of 32 G stainless steel tubing (OD: 0.009”; ID: 0.0041”; wall thickness: 0.0025”; Small Parts, Logansport, IN, USA) was welded inside the 26 G tubing to also form a 1.5 mm stepped tip at its distal end. The diameter and length of both the stepped stylet and needle were selected to fit inside the guide needle while exceeding its length by 2.6 mm at its distal end.
Infusion apparatus
The male Luer hub of the stepped needle was linked to a male Luer hub of a 100 μl glass injection syringe (Hamilton Company, Reno, NV, USA) by a compression-fitted coupler. The coupler was made of 100 cm of polyethylene tubing (PE/5; Scientific Commodities, Lake Havasu City, AZ, USA) and flanked on both sides by female Luer to 1/16’’ hose barb, nylon adaptors (Cole-Parmer, Vernon Hill, IL, USA). The infusate was delivered at a controlled flow rate by a syringe pump (Chemyx, Stafford, TX, USA).
In vitro testing of the stepped needle
Efficacy of CED by the stepped needle was compared to that by the guide needle alone in agarose gel. The needle was attached to a stereotactic frame and inserted 5’’ (127 mm) into 0.5% w/v agarose gel (Life Technologies, Carlsbad, CA, USA). Next, 50 μl of 0.4% Evans Blue dye (Sigma-Aldrich, St. Louis, MO, USA) was injected at flow rates between of 2 and 20 μl/min. The needle was left in place for an additional 3 min after the injection had been completed.
DRG targeting under CT guidance
Planning of the IG injection trajectory by CT / myelogram
The myelogram was obtained by administration of 0.5 mL diluted contrast media (300 mgI/ml; Omnipaque; Novation, Chicago, IL) into the dorsal subarachnoid space14. The contrast opacified the lumbar thecal sac, including its lateral root sleeves that extend into the intervertebral foramina, and visualized the DRG residing in the lateral IT sleeves. The myelogram therefore allowed determination of the optimal skin entry point, length, and direction of the IG injection path before the IG procedure was initiated (Fig. 2A). Generally, an angle of 60 – 70 degrees relative to the sagittal plane was found to facilitate access to the DRG.
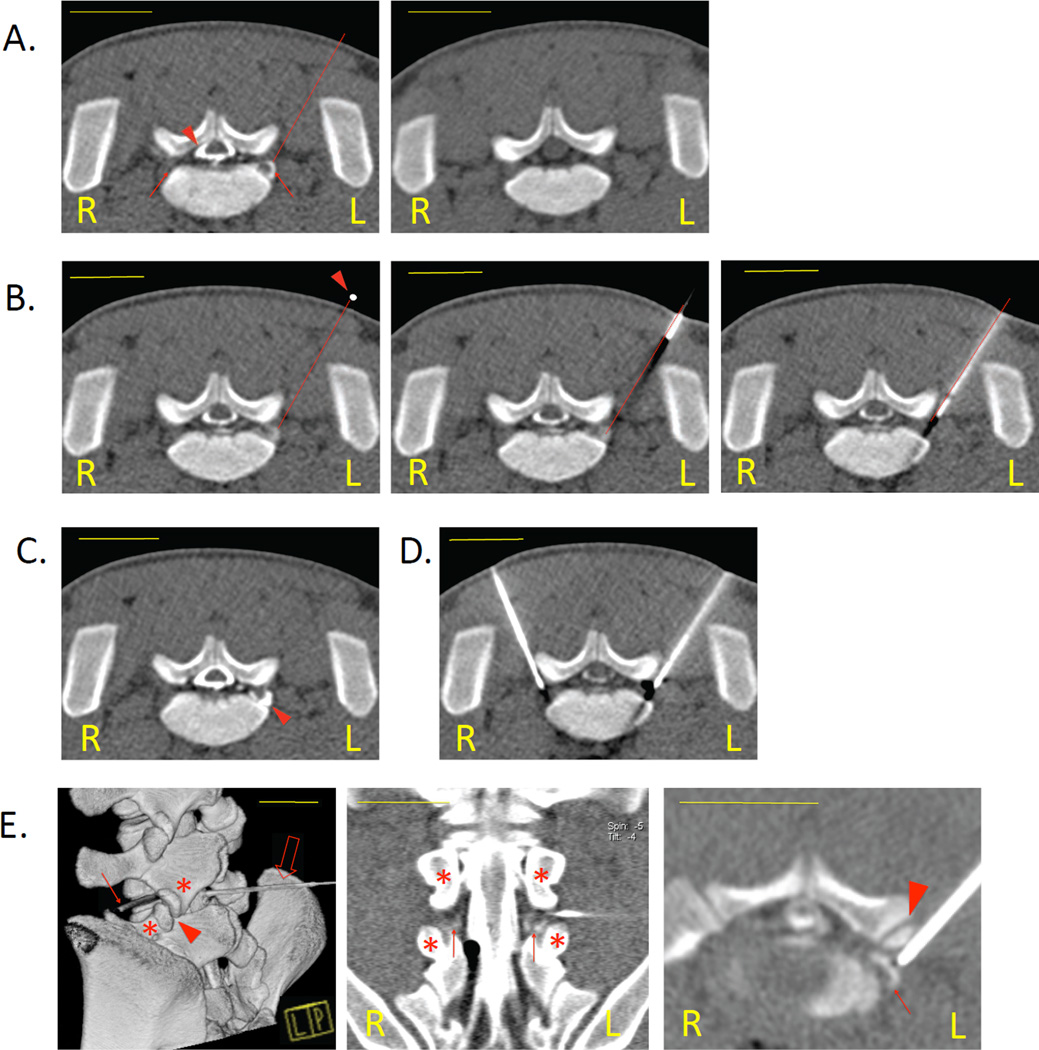
Figure 2. Targeting the DRG under computed tomography (CT) guidance in the pig model.
CT imaging was used to visualize the pertinent spinal anatomy, determine the optimal needle path, and monitor the advancement of the guide needle to the DRG.
A. DRG visualization and planning of the injection route. A CT myelogram (left) opacified the thecal sac (arrowhead) and visualized the DRG (arrows). The myelogram was used to determine the optimal trajectory of the needle (dotted line), here shown for the left L6 DRG. CT image of the same spinal level without IT contrast is shown for comparison (right).
B. Placement of the guide needle under CTF guidance. The skin entry point determined by the myelogram was first matched with the corresponding point on the body surface of the animal by placing a radiopaque lead marker (arrowhead) on the skin. The needle was then advanced in increments along its predetermined path (middle) until its tip was located directly adjacent to the dorsal surface of the DRG (right).
C. Confirmation of the guide needle placement. The additional contrast delivered to the left lateral IT sleeve was visualized by CTF as a crescent-shaped hyperdense area (arrowhead), further outlining the targeted DRG. The guide needle on an adjacent slice is not shown to allow better comparison with the myelogram alone presented in Panel A.
D. Bilateral DRG targeting. Once the first needle reached the lateral sleeve of the IT space, a second needle could be advanced to the contralateral DRG using the same technique. The DRG could be safely targeted bilaterally at up to 3 spinal levels during one session with no adverse effects.
E. Needle path and neighboring skeletal structures. Volume-rendered reconstruction (left) provides an overview of the trajectory of the guide needle (solid arrow). The lumbar puncture needle, used for obtaining the myelogram, is also shown (empty arrow). Coronal view (center) shows the cauda equina and L6 DRG (arrows) bilaterally. The tip of the guide needle was passed between the articular processes (asterisks) and into the L5-L6 intervertebral foramen. Oblique axial view (right), parallel with the long axis of intervertebral foramen, details the position of the tip of the guide needle immediately dorsal to the DRG (arrow) and ventral to the facet joint (arrowhead).
Scale bars: 2.5 cm.
Advancement of the guide needle to the lateral recess of the IT space under CTF guidance
The guide needle, with its proprietary stylet (first inset) in place, was passed through the skin lateral to the midline and incrementally advanced ventro-medially towards the DRG until its tip reached the lateral sleeve of the IT space at a site directly dorsal to the DRG. Intra-procedural CTF imaging monitored advancement of the needle (Fig. 2B) and any deviations from the optimal trajectory were corrected.
Verification of the guide needle placement by contrast injection
When the needle tip was visualized directly adjacent to the dorsal aspect of the DRG, the stylet of the guide needle was withdrawn and a small volume (less than 0.1 mL) of the contrast media was injected. CTF showed the spread of the contrast media within the cerebrospinal fluid surrounding the DRG (Fig. 2C) and verified that the needle tip had reached the lateral recess of the IT space while not penetrating the DRG itself.
IG placement of the stepped needle
Once the correct position of the guide needle was verified, the custom-made stepped stylet (second inset) was inserted through the guide needle. The length of the stepped stylet exceeded the length of the guide needle and therefore only the stepped tip of the stylet but not the Quincke tip of the guide needle penetrated the DRG parenchyma. The stepped stylet was then withdrawn and replaced by the stepped needle (third inset). The prior insertion of the stepped stylet prevented clogging of the narrow needle tip.
Adeno-associated virus (AAV) preparation
Self-complementary AAV serotype 1 (AAV1) expressing enhanced green fluorescent protein (EGFP) reporter gene under control of CMV promoter/enhancer and rBG polyA sequence was used 19. The vector was produced at the Penn Vector Core (University of Pennsylvania, Philadelphia, PA, USA).
Detection of AAV transduction
The animals were euthanized by intravenous injection of pentobarbital. The thoracic aorta and the common iliac arteries were clamped and the isolated segment perfused under pressure with 2L of PBS followed by 2L of 4% paraformaldehyde in phosphate-buffered saline (PBS). The harvested tissue samples were viewed for direct EGFP fluorescence by laser scanning microscopy as reported previously20. The proportion of the DRG neurons transduced was determined as described by Jacques et al9.
Imaging in humans
To assess possible application to the human DRG, random clinical cases of CT / myelography were chosen from the daily imaging schedule for evaluation. Specifically, cases were sought which had both fat-saturated, gadolinium enhanced MRI images and a high quality CT / myelogram.
Results
The stepped needle showed effective CED in agarose gel
Comparison of the stepped needle with a regular, non-stepped needle in agarose gel showed superior performance of the stepped needle. Use of the stepped needle resulted in homogenous distribution of the injectate around the needle tip for flow rates up to 20 μL/min and volumes up to 100 μL (Fig. 3A). In contrast, the non-stepped spinal needle led to tracking of the dye along the needle path.
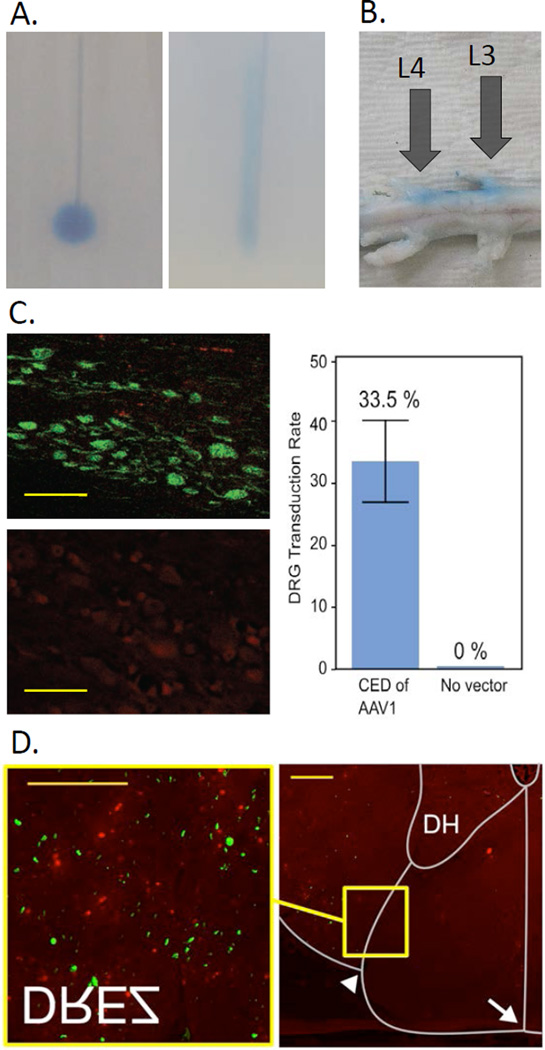
Figure 3. Validation of the IG injectate delivery.
Efficacy of CED and accuracy of DRG targeting was first verified by dye injection both in vitro and post mortem, and then confirmed in vivo by IG administration of adeno-associated virus (AAV).
A. Performance of the stepped needle used for CED (left) was compared to a conventional needle (22G spinal needle with Quincke tip; right) in vitro by administration of Evans blue dye into agarose gel, here shown for the flow rate of 10 μL/min.
B. Accurate radiographic visualization of the DRG and the needle was verified in a pig cadaver. Unilateral administration of Chicago Blue dye at two spinal levels confirmed correct targeting of the DRG and the adjacent spinal root.
C. CED of AAV1 via the stepped needle transduced 33.5% of DRG sensory neurons (right), as evidenced by EGFP expression (top left). The transduced cells demonstrated morphology characteristic of primary sensory neurons. 7.5×1010 genome copies of the vector suspended in 50 μl PBS were delivered into the left L6 DRG at the flow rate of 10 μL/min. Transduction was detected 4 weeks later by laser scanning microscopy. No transduction was found in the DRG that were not injected (bottom left), suggesting that no spillage of the vector to the cerebrospinal fluid had occurred. Scale bars: 100 μm.
D. Transduction of the spinal cord was found in the dorsal root entry zones (DREZ), corresponding to the centripetal axons of the DRG sensory neurons. The transduction was thereby restricted to the cells whose cell bodies or axons came into a direct contact with the transducing agent within the DRG, indicating that no trans-synaptic spread of the vector had occurred. DH, dorsal horn; arrow, posterior median sulcus; arrowhead; postero-lateral sulcus. Scale bars: 200 μm.
All microscopic images were acquired in the lambda stack mode, and linear unmixing was used to distinguish the specific EGFP signal (green) from non-specific autofluorescence (red). Original magnification:×200 for the DRG and the spinal cord inset;×50 for the spinal cord overview.
CT-guided injection accurately targeted the DRG in pig cadaveric studies
In addition to imaging, correct IG placement of the needle tip was verified by administration of the Chicago Blue dye in pig cadavers. Fig. 3B shows the dye observed in the DRG and spinal roots of the injected spinal levels.
CED led to a widespread distribution of the injectate in the DRG of live pigs
When administered into the DRG in vivo, CED of AAV1 resulted in a robust transduction of the sensory neurons of the injected DRG, with a mean transduction rate of 33.5% (Fig. 3C). Transgene expression was also found in the posterior nerve root and the dorsal root entry zone in the posterior horn of the spinal cord (Fig. 3D), reflecting the anatomical pattern of primary sensory neuron transduction previously observed in rodents. Transduction of axons of the anterior nerve root was present and may have been related to the close proximity of the anterior nerve root to the DRG and the known ability of AAV1 to transduce both neuronal bodies and axons. An absolute degree of anatomical specificity was confirmed in terms of the level and laterality of the targeted DRG; there was no transduction of neighboring spinal levels or of DRG on the contralateral side.
Imaging based modeling in humans supported clinical translatability of the IG technique
Magnetic resonance imaging (MRI) of the human lumbar spine identified the DRG by the presence of gadolinium enhancement (Fig. 4A). The DRG enhances because it lacks a blood-nerve barrier. The other contents of the neural foramen (the nerve roots found proximally and the spinal nerve and its rami found distally) have an intact barrier and therefore show no signs of gadolinium enhancement.
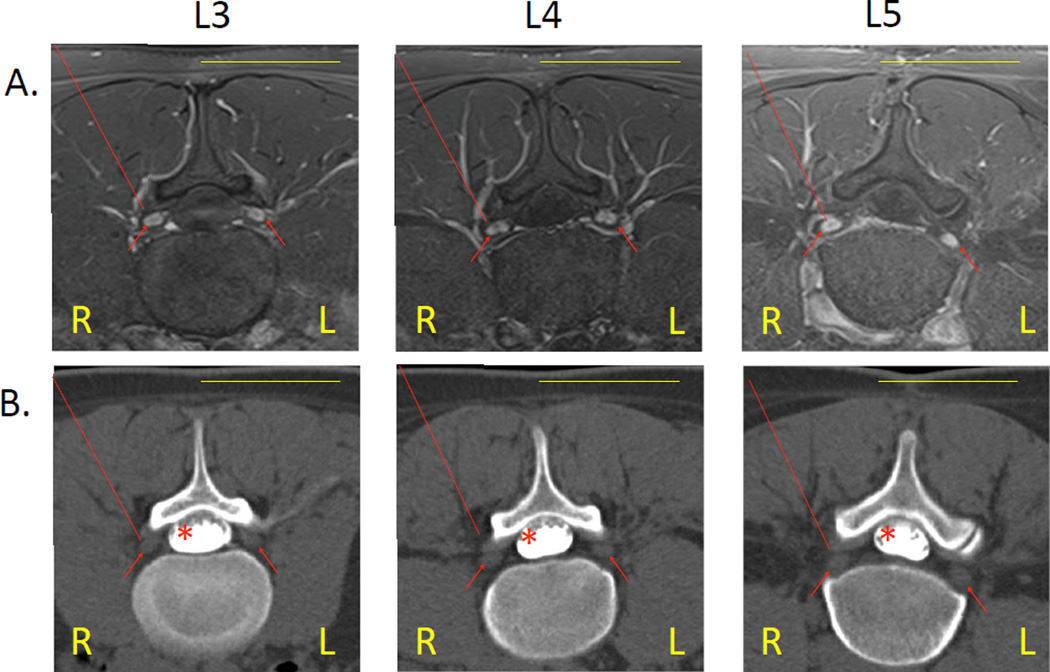
Figure 4. Modeling the IG injection in humans.
Analysis of human spinal anatomy by MRI and CT-myelography sustains the feasibility of DRG targeting from a postero-lateral approach under CTF guidance in human patients.
A. MRI images (T1 weighted, fat saturated) of the human lumbar spine, here shown for L3, L4, and L5 levels, identified the DRG by gadolinium enhancement (arrows).
B. CT-myelography of the corresponding segments showed unobstructed access to the DRG. The normodense contours of the DRG (arrows) stood out from the hypodense background of the epidural fat; the IT contrast did not spread into the root sleeves and therefore did not further outline the DRG. The optimal trajectory for accessing the DRG is indicated by a dotted line.
Scale bars: 5 cm.
CT imaging at the corresponding planes showed that there was no skeletal barrier to access the DRG by the postero-lateral vector established in the pig model (Fig. 4B). Compared to the pigs, human posterior elements are less bulky, with the lamina and facet joints terminating more medially, facilitating the access to the DRG. Although CT / myelography opacified the lumbar thecal sac, the contrast media did not spread to encompass the DRG, presumably due to the meninges sealing about the nerve roots more proximally in humans than in the pigs. MRI was therefore used to provide a physiologic cross-reference for the DRG position, which allowed certain identification of the DRG tissue on the CT / myelogram.
Discussion
Recent development of analgesic therapies directly targeting the primary sensory neurons creates a need for selective drug delivery into the DRG. An important example explored in the present study is AAV based gene therapy for pain, which has been found to be efficacious in rodents when the vector was delivered IT20. However, subsequent large animal studies have shown that at least some commonly used AAV subtypes can lead to promiscuous transduction of distant structures, such as spinal cord or brain, which was not observed in rodents8,5. The IG administration markedly reduces this risk associated with IT delivery by minimizing transduction outside the injected DRG. It also allows for a lower therapeutic dose of the virus. The present work demonstrates the efficacy of IG delivery of AAV by CED, while achieving the desired anatomical specificity.
In addition to gene therapy, IG injection might provide an important alternative route of delivery for several other novel pharmacological agents that have so far been investigated only in the IT paradigm. Examples include resiniferatoxin and P-saporin, analgesic neurotoxins exerting their therapeutic effect by selective deletions of specific cell populations critical in pain signaling1,10. Delivery of either drug by the IG route might be of future interest and could be tested in the described pig model.
In humans, the IG injection is expected to be more straightforward than in the pig model because the human posterior elements tend to be more compact and the intervertebral foramina more readily accessible. Therefore, the IG injection of novel drugs tested in the pig model should be translatable to the clinical setting.
Acknowledgments
The study was supported by funds from the Schulze Family Foundation (to A.S.B) and by NIH construction grant, C06 RR018898 (CT imaging equipment).
References
- 1.Allen JW, Mantyh PW, Horais K, Tozier N, Rogers SD, Ghilardi JR, et al. Safety evaluation of intrathecal substance P-saporin, a targeted neurotoxin, in dogs. Toxicol Sci. 2006;91:286–98. doi: 10.1093/toxsci/kfj143. [DOI] [PubMed] [Google Scholar]
- 2.Allen JW, Mantyh PW, Horais K, Tozier N, Rogers SD, Ghilardi JR, et al. Safety evaluation of intrathecal substance P-saporin, a targeted neurotoxin, in dogs. Toxicol Sci. 2006;91:286–298. doi: 10.1093/toxsci/kfj143. [DOI] [PubMed] [Google Scholar]
- 3.Bobo RH, Laske DW, Akbasak a, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogduk N, editor. Practice Guidelines for Spinal Diagnostic and Treatment Procedures. San Francisco, Calif: International Spine Intervention Society; 2004. [Google Scholar]
- 5.Federici T, Taub JS, Baum GR, Gray SJ, Grieger JC, Matthews Ka, et al. Robust spinal motor neuron transduction following intrathecal delivery of AAV9 in pigs. Gene Ther. 2011:1–8. doi: 10.1038/gt.2011.130. [DOI] [PubMed] [Google Scholar]
- 6.Fischer G, Kostic S, Nakai H, Park F, Sapunar D, Yu H, et al. Direct injection into the dorsal root ganglion: technical, behavioral, and histological observations. J Neurosci Methods. 2011;199:43–55. doi: 10.1016/j.jneumeth.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geurts JW, van Wijk RM, Stolker RJ, Groen GJ. Efficacy of radiofrequency procedures for the treatment of spinal pain: a systematic review of randomized clinical trials. Reg Anesth Pain Med. 2001;26:394–400. doi: 10.1053/rapm.2001.23673. [DOI] [PubMed] [Google Scholar]
- 8.Gray SJ, Nagabhushan Kalburgi S, McCown TJ, Jude Samulski R. Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates. Gene Ther. 2013:1–10. doi: 10.1038/gt.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacques SJ, Ahmed Z, Forbes A, Douglas MR, Vigenswara V, Berry M, et al. AAV8(gfp) preferentially targets large diameter dorsal root ganglion neurones after both intra-dorsal root ganglion and intrathecal injection. Mol Cell Neurosci. 2012;49:464–474. doi: 10.1016/j.mcn.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Karai L, Brown DC, Mannes AJ, Connelly ST, Brown J, Gandal M, et al. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest. 2004;113:1344–52. doi: 10.1172/JCI20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krauze MT, Saito R, Noble C, Tamas M, Bringas J, Park JW, et al. Reflux-free cannula for convection-enhanced high-speed delivery of therapeutic agents. J Neurosurg. 2005;103:923–929. doi: 10.3171/jns.2005.103.5.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krauze MT, Saito R, Noble C, Tamas M, Bringas J, Park JW, et al. Reflux-free cannula for convection-enhanced high-speed delivery of therapeutic agents. J Neurosurg. 2005;103:923–929. doi: 10.3171/jns.2005.103.5.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacVicar J, King W, Landers MH, Bogduk N. The effectiveness of lumbar transforaminal injection of steroids: a comprehensive review with systematic analysis of the published data. Pain Med. 2013;14:14–28. doi: 10.1111/j.1526-4637.2012.01508.x. [DOI] [PubMed] [Google Scholar]
- 14.Pleticha J, Maus TP, Jeng-Singh C, Marsh MP, Al-Saiegh F, Christner JA, et al. Pig lumbar spine anatomy and imaging-guided lateral lumbar puncture: A new large animal model for intrathecal drug delivery. J Neurosci Methods. 2013;216:10–15. doi: 10.1016/j.jneumeth.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puljak L, Kojundzic SL, Hogan QH, Sapunar D. Targeted delivery of pharmacological agents into rat dorsal root ganglion. J Neurosci Methods. 2009;177:397–402. doi: 10.1016/j.jneumeth.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ratliff JK, Oldfield EH. Convection-enhanced delivery in intact and lesioned peripheral nerve. J Neurosurg. 2001;95:1001–1011. doi: 10.3171/jns.2001.95.6.1001. [DOI] [PubMed] [Google Scholar]
- 17.Research I for AL, Council NR. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- 18.Rosenbluth KH, Luz M, Mohr E, Mittermeyer S, Bringas J, Bankiewicz KS. Design of an in-dwelling cannula for convection-enhanced delivery. J Neurosci Methods. 2011;196:118–123. doi: 10.1016/j.jneumeth.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 19.Storek B, Harder NM, Banck MS, Wang C, McCarty DM, Janssen WG, et al. Intrathecal long-term gene expression by self-complementary adeno-associated virus type 1 suitable for chronic pain studies in rats. Mol Pain. 2006;2:4. doi: 10.1186/1744-8069-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Storek B, Reinhardt M, Wang C, Janssen WGM, Harder NM, Banck MS, et al. Sensory neuron targeting by self-complementary AAV8 via lumbar puncture for chronic pain. Proc Natl Acad Sci U S A. 2008;105:1055–1060. doi: 10.1073/pnas.0708003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wald JT, Maus TP, Geske JR, Carter RE, Diehn FE, Kaufmann TJ, et al. Safety and efficacy of CT-guided transforaminal cervical epidural steroid injections using a posterior approach. AJNR Am J Neuroradiol. 2012;33:415–419. doi: 10.3174/ajnr.A2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin D, Forsayeth J, Bankiewicz KS. Optimized cannula design and placement for convection-enhanced delivery in rat striatum. J Neurosci Methods. 2010;187:46–51. doi: 10.1016/j.jneumeth.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu H, Fischer G, Ferhatovic L, Fan F, Light AR, Weihrauch D, et al. Intraganglionic AAV6 Results in Efficient and Long-Term Gene Transfer to Peripheral Sensory Nervous System in Adult Rats. PLoS One. 2013;8:e61266. doi: 10.1371/journal.pone.0061266. [DOI] [PMC free article] [PubMed] [Google Scholar]