Abstract
Introduction
List learning tasks are powerful clinical tools for studying memory, yet have been relatively underutilized within the functional imaging literature. This limits understanding of regions such as the Papez circuit which support memory performance in healthy, non-demented adults.
Method
The current study characterized list learning performance in 40 adults who completed a Semantic List Learning Task (SLLT) with a Brown-Peterson manipulation during functional MRI (fMRI). Cued recall with semantic cues, and recognition memory were assessed after imaging. Internal reliability and convergent and discriminant validity were evaluated.
Results
Subjects averaged 38% accuracy in recall (62% for recognition), with primacy but no recency effects observed. Validity and reliability were demonstrated by showing that the SLLT was correlated with the California Verbal Learning test (CVLT), but not with executive functioning tests, and high intraclass correlation coefficient across lists for recall (.91). fMRI measurements during Encoding (vs. Silent Rehearsal) revealed significant activation in bilateral hippocampus, parahippocampus, and bilateral anterior and posterior cingulate cortex. Post-hoc analyses showed increased activation in anterior and middle hippocampus, subgenual cingulate, and mammillary bodies specific to Encoding. In addition, increasing age was positively associated with increased activation in a diffuse network, particularly frontal cortex and specific Papez regions for correctly recalled words. Gender differences were specific to left inferior and superior frontal cortex.
Conclusions
This is a clinically relevant list learning task that can be used in studies of groups for which the Papez circuit is damaged or disrupted, in mixed or crossover studies at imaging and clinical sites.
Keywords: Papez circuit, list learning, fMRI, imaging, memory, hippocampus
1. Introduction
Memory functions are thought to depend upon the integrity of the Papez circuit (Papez, 1937), which includes the hippocampus, parahippocampal gyrus, medial temporal lobe (MTL), mammillary bodies, insula, and cingulate gyrus. Damage in portions of this circuit from lesions and disease can produce dysphoria and memory difficulties. In particular, widespread damage to these circuits, such as in Alzheimer’s disease, can result in amnesia or memory loss so severe that it interferes with an individual’s ability to engage in many independent activities of daily life (Scoville & Milner, 1957; Zola-Morgan, Squire, & Amaral, 1986). Given the important role of clinical memory evaluations, it is essential to design tasks that are sensitive and specific to functional aberrations within the Papez circuit. Unfortunately, traditional clinical measures of learning and memory have been underutilized in functional neuroimaging research, making it difficult to infer the relationship between brain activity and memory functions. A deeper understanding of the specific anatomical pathways that are engaged when performing clinically relevant memory tasks is important for improving diagnoses of specific types of memory difficulties, as well as for localizing affected regions for future treatments.
1.1. Long-Term and Short-Term Memory
Researchers have drawn distinctions between different forms of memory, such as short-term memory (STM) versus long-term memory (LTM; Glanzer & Cunitz, 1966; Atkinson & Shiffrin, 1968). This separation of STM and LTM is often referred to as the dual store model of memory (Baddeley, 1996; Clark & Wagner, 2003; Moscovitch, 1992; Talmi, Grady, Goshen-Gottstein, & Moscovitch, 2005). STM involves the temporary, short-term encoding and storage of a few items of information lasting seconds to minutes. STM plays a role in the process of rehearsing items held in memory, and is incorporated within the broader construct of executive functions such as organizing information to be encoded. In contrast, LTM is characterized as the encoding of information in long-term storage, lasting hours to a lifetime.
1.2. Neural Bases of Long-Term Memory
Functional imaging studies of list learning and LTM have implicated Papez circuit activity (e.g. MTL) during encoding (Axmacher, Elger, & Fell, 2009; Fernandez et al., 1998; Kim & Cabeza, 2007, Starkman, Giordani, Gebarski, & Schteingart, 2003; Strange, Otten, Josephs, Rugg, & Dolan, 2002; Wagner et al., 1998), consistent with lesion work in animals and humans (Baddeley et al., 2000; Broadbent, Clark, Zola, & Squire, 2002). Experiments using STM tasks often report frontal and parietal activation during encoding (Baker, Sanders, Maccotta, & Buckner, 2001; Dupont, Samson, Le Bihan, & Baulac, 2002; Fletcher, Stephenson, Carpenter, Donovan, & Bullmore, 2003; Guerin & Miller, 2009; Heinze, Sartory, Müller, de Greiff, Forsting, & Juptner, 2006; Marvel & Desmond, 2010), but not within MTL/hippocampus. A recent meta-analysis found that most subsequent memory studies have reported activation only in STM circuits, or those thought to be involved in both LTM and STM (Kim, 2011). Thus, there is a lack of clarity on how neuroimaging probes, that could be ecologically translated to and valid in clinical settings, relate to activation of the Papez circuit and performance in STM and LTM.
1.3. Considering Impact of Encoding Strategies on Memory Performance Variability
LTM is impacted negatively by many diseases; in order to serve more specific clinical purposes, tasks that help to focus on LTM processes are highly desirable. As STM and LTM operate together in healthy individuals, designing experiments to specifically target Papez circuit activity supporting LTM (e.g., Strange, Otten, Josephs, Rugg, & Dolan, 2002) is an important step. This can lead to validated behavioral paradigms for use in clinical populations, either with or without functional imaging. Several strategies have previously been used to focus on LTM functions and diminish the influence of STM functions on performance, including minimizing the variance in task performance that could be attributed to executive functioning or short term memory. To this end, the current study provided semantic cues during list learning to help organize encoding and retrieval. This promotes more consistent memory performance by minimizing individual variability in the contributions of executive functioning (STM) to subsequent recall (Bermingham, Hill, Woltz, & Gardner, 2013). Semantic organization and processing strategies are also consistent with deeper encoding and more robust hippocampal activation (Craik and Lockhart, 1972; Flegal, Marín-Gutiérrez, Ragland, & Ranganath, 2014; Fliessbach, Buerger, Trautner, Elger, & Weber, 2010; Otten, Henson, & Rugg, 2001; Park, Uncapher, & Rugg, 2008; Woodard et al., 2005). We sought to further enhance the clinical utility of the current paradigm by including a behavioral measure of subsequent memory in which a wide range of performance is possible, thereby ensuring the sensitivity of our task to memory impairments.
1.4. Task Development Strategies to Focus on Individual Differences in LTM
The current study also employed a Brown-Peterson (BP) paradigm, in which subjects performed a distractor task following each initial list learning phase, in order to prevent immediate rehearsal and reduce STM processes that might facilitate consolidation of later items in the list (Berman, Jonides, & Lewis, 2009; Jarrold, Tam, Baddeley, & Harvey, 2010; Lewandowsky, Geiger, & Oberauer, 2008; Luria, 1971). This has been shown to reduce memory performance advantages for later list items (known as the recency effect), while leaving the performance advantage for early list items (primacy effect) intact (Brown, 1958; Peterson & Peterson, 1959). The recency effect is believed to depend strongly on STM, while the primacy effect is believed to depend more significantly on LTM (Glanzer & Cunitz, 1966; Atkinson & Shiffrin, 1968). Reducing the recency effect allows for greater a contribution from LTM during behavioral evaluations of subsequent memory. Moreover, the BP manipulation allowed us to more deeply explore the relationship between behavioral performance measures (e.g. subsequent recall, primacy effect) and functional activation related to encoding.
We hypothesized that the Semantic List Learning Task (SLLT) would reveal robust activation of the Papez circuit (especially in MTL regions) during encoding of words. By comparing patterns of brain activation during encoding with those observed during silent rehearsal, we sought to identify brain regions specific to LTM encoding relative to STM processes during rehearsal and retrieval. We also expected that greater MTL activation during encoding would predict words that would later be recalled. Additionally, when comparing behavioral data from our task and other list learning/cognitive measures used in clinical settings, we expected to show convergent validity with memory tasks and discriminant validity with executive functioning tasks. We also evaluated the hypothesis that increasing age, even in a healthy and relatively younger sample, would be associated with greater frontal activation, including more diffuse patterns of activation.
2. Materials and Methods
2.1. Participants
Forty healthy adult subjects (21 males, 19 females) between the ages of 18 and 65 completed a semantic list-learning task during fMRI. Participants were a mean age of 38.4 years (SD = 17.3), with a mean level of education of 15.7 years (SD = 1.7). For the majority of individuals, intellectual functioning (mean estimated IQ = 114, SD = 10) was assessed using the Shipley Institute of Living Scale-Vocabulary Test (Shipley, 1940). Demographic information is provided separately for males and females in Supplemental Table 1. Participants were recruited through a university website designed to recruit local subjects, and through print advertisements in the community. Participants gave their written informed consent prior to participation in the study. A battery of neuropsychological tests was completed before scanning, and subjects were compensated $30–60, depending on the combined duration of both sessions. Subjects were screened for neurological (e.g., cognitive decline, dementia), psychiatric (DSM-IV), and medical disorders consistent with our prior work (Langenecker et al., 2007a; Langenecker & Nielson, 2003). Additionally, subjects were screened for low/no nicotine use (less than 10 cigarettes per week) and minimal alcohol use (fewer than 14 alcoholic drinks per week).
2.2. The Semantic List Learning Task
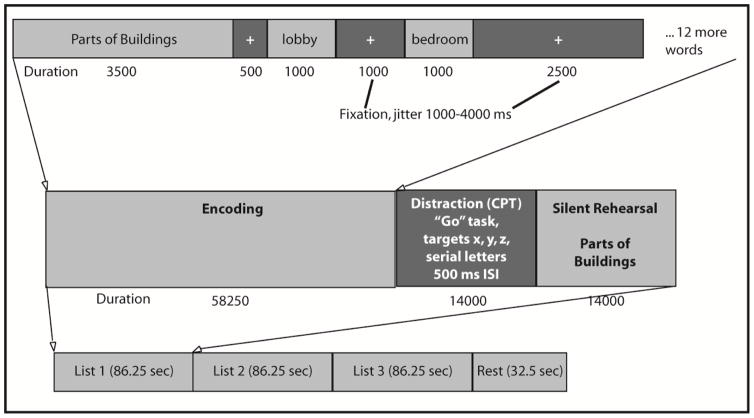
The Semantic List Learning Task (SLLT) has three block types: Encoding, Distraction, and Silent Rehearsal (see Figure 1). During each of 15 Encoding blocks, subjects were presented with 14 words from one of 15 different semantic categories. Lists were derived from work by Winograd (1968), and included five each of low, medium, and high frequency word categories. Lists were then respectively matched for average number of syllables (Table 1), and we ensured that each semantic category had sufficient items for both within list targets and same list distractors (for the recognition portion of the task). Lists were presented in a random order, as were words within each list, to avoid confounding effects of having more common/obscure, shorter/longer words appear for all subjects in a particular order within and between lists.
Figure 1.
Semantic List Learning Task (SLLT) design.
Table 1.
Frequency and Syllable Characteristics for the Semantic List Learning Task
| List | Frequency | Syllables | Recall | Recall | |
|---|---|---|---|---|---|
| Mean | Median | Mean | Mean (SD) | Correlation | |
| Parts of Buildings | 65 | 22 | 2.3 | 4.7 (2.4) | .46* |
| Countries | 72 | 33 | 2.3 | 6.1 (2.8) | .73* |
| Types of Music | 51 | 19 | 2.3 | 5.6 (2.3) | .61* |
| Weapons | 51 | 24 | 2.3 | 4.5 (2.3) | .66* |
| Kinds of Cloth | 69 | 18 | 2.9 | 3.8 (1.6) | .60* |
| Type of Vehicles | 74 | 25 | 1.9 | 4.3 (2.8) | .48* |
| Four-Footed Animals | 89 | 46 | 2.5 | 3.6 (1.8) | .77* |
| Types of Footgear | 80 | 37 | 2.4 | 5.7 (2.6) | .79* |
| Types of Disease | 60 | 39 | 1.8 | 4.9 (2.4) | .64* |
| Human Dwellings | 67 | 30 | 2.2 | 6.1 (2.4) | .74* |
| Sports | 83 | 30 | 2.4 | 5.3 (2.2) | .64* |
| Alcoholic Beverages | 100 | 35 | 2.1 | 6.6 (2.3) | .67* |
| Vegetables | 110 | 71 | 2.1 | 6.1 (2.5) | .75* |
| Snakes | 90 | 50 | 2.2 | 7.0 (2.5) | .66* |
| Precious Stones | 121 | 61 | 2.1 | 6.9 (2.5) | .61* |
Correlation of the individual lists with total list recall.
p < .05
In this task, a prompt with the name of the semantic category being studied was displayed for 3.5 seconds. Words from that category were then presented for one second each with a 1750–4250 ms jittered inter-stimulus interval during which a fixation cross was presented. The total time for each encoding block was 58.25 seconds. Subjects then completed the “Go” distractor task in which they had to make a button-press response each time they saw the letters “x,” “y,” or “z” presented in a visual stream (500 msec per letter, 14 seconds total per block). This BP distraction block was intended to reduce the effect of recency (fewer recalled items) during recall/recognition by preventing rehearsal of list items held in STM (Brown, 1958; Peterson & Peterson, 1959). The final portion of each list sequence consisted of a silent rehearsal period that lasted for 14 seconds. Here, participants saw the category prompt and were asked to silently recall and rehearse words that were just presented during the previous encoding phase. Runs (functional scans) included 3 word lists in separate Encoding blocks, each followed by Distraction and Silent Rehearsal blocks. At the end of each run there was a 32.5 second rest period. Five runs were completed in each scanning session. Figure 1 displays the SLLT as described.
Measures of convergent and discriminant validity were also included to evaluate and contextualize this task within the broader memory literature. To investigate convergent validity, we chose the California Verbal Learning Test-2nd edition (CVLT-II; Delis, Kramer, Kaplan, & Ober, 2000), which is a widely used list-learning task in clinical settings (Rabin, Barr, & Burton, 2005). We focused on the performance in trial 1 of the CVLT-II to compare the shape of the serial position curve (SPC), as words from the SLLT are presented only once. We also used the long delay free recall performance as the standard measure of memory for convergent validity, given the similar delay between learning and recall in the CVLT-II and SLLT and the fact that this is the most commonly reported metric for delayed recall in the task. To investigate discriminant validity, we included executive functioning indices from the Trail Making Test (Reitan, 1958, 1992), Wisconsin Card Sorting Test (Grant & Berg, 1948; Heaton, 1981), and the Parametric Go/No-Go Test (Votruba and Langenecker, 2013). Subjects completed these tasks on a separate day, prior to scanning.
2.3. Experimental Procedure
Participants were first verbally introduced to the task by the experimenter prior to entering the scanner. They were told they would observe lists of categorically related words presented one at a time, and that they should silently read and remember the words to the best of their ability, utilizing the list category as a semantic encoding strategy. They were informed that a different (distractor) task would then appear. This was the “Go” portion of the “Parametric Go/No-Go” paradigm previously reported (Langenecker et al., 2005; Langenecker et al., 2007b), on which participants were trained prior to scanning. Lastly, they were told that a silent rehearsal phase would occur, during which they would be asked to rehearse the words from the list that appeared just prior to the distraction phase without vocalization or movement of the lips. Total task duration was approximately 25 minutes.
After scanning, subjects were asked to first complete a cued recall task outside the scanner in which they wrote down all of the words they could remember for each of the semantic categories presented. Category name prompts were provided as recall cues (in alphabetical order). Subjects then completed a cued recognition task in which they had to discern words seen inside the scanner from a list of correct words amongst semantically-related and unrelated distractors. The recognition task included 210 targets and 228 distractors, with the latter divided evenly into within category and out of category (also presented in alphabetical order). Correctly recalled words were used as regressors in an event-related analysis of the fMRI data.
2.3.1. fMRI Procedures
fMRI procedures were similar in detail and followed the method used in our previous work (Langenecker et al., 2007a; Langenecker et al., 2012; Weisenbach et al., 2012; Weisenbach et al., 2014). Briefly, a GE Signa 3T scanner (release VH3) was used to conduct whole brain fMRI. Thirty contiguous oblique-axial slices, each four millimeters thick, composed the fMRI series. Data were acquired using a forward-reverse spiral sequence. The image matrix was 64 × 64 over a 24 cm field of view for a 3.75 × 3.75 × 4 mm voxel. Slices were acquired serially at 1750 msec temporal resolution for a total of 770 time points across the five runs. The task was presented inside the scanner using E-Prime (Psychology Software Tools, Pittsburgh, PA) backward projection and prism glasses attached to the head coil, and visual acuity correction was used when necessary. Subjects lay supine, and responses for the distractor task were recorded via index finger using a five button key-press apparatus attached to the right hand. Participants wore earplugs inside the scanner in order to reduce the noise experienced from 95 dB to well below 75 dB. We attempted to minimize head motion inside the scanner using foam padding and a Velcro fixation strap, and motion was specifically evaluated to be certain that movement in x, y, and z planes was well below 1/2 voxel width. High resolution T1 spoiled gradient recalled (SPGR) anatomical images were obtained after SLLT administration. One individual contributed data for behavioral analyses only, as there was loss of image signal in the dorsal cortex resulting in a warping/normalization failure, which led us to exclude this subject from fMRI data analyses.
2.4. Statistical Analyses
Total hits, false positives, and percent accuracy for Primacy, Middle, and Recency portions of both recall and recognition tasks comprised the behavioral data entered into SPSS for statistical analysis, with which a repeated measures analysis of variance (rmANOVA) was performed. Functional imaging data were processed and analyzed using MATLAB (The MathWorks, Natick, MA) and SPM 8 and FSL software (Friston, Ashburner, Frith, Poline, Heather, & Frackowiak, 1995). The Encoding, Silent Rehearsal, Distraction, and Rest blocks were entered into first level models to determine global activation patterns for list learning, and contrast images were used in second level analyses (i.e., Encoding-Silent Rehearsal). A second set of contrast images was also derived from behavioral data for memory hits for both the recall and recognition models in an event-related analysis. One sample t-tests were performed for all contrasts. Functional images were normalized to fit a Montreal Neurological Institute (MNI) canonical template using DARTEL and were smoothed with a 5 mm FWHM kernel. AlphaSim correction (1000 iterations) was used for all whole brain analyses, balancing height (p < 0.005) and extent (440 mm3) thresholds to achieve a whole brain correction of p < 0.05. The MarsBaR toolbox (Brett, Anton, Valabregue, & Poline, 2002) was used to extract mean signal change during all blocks in regions of interest (ROIs) to evaluate Papez circuit specific ROIs in relationship to condition. Mean activation in each region (10), by side (2), and by condition (4), were analyzed in an rmANOVA in SPSS (alpha set at p < .05). Of note, age was significantly positively correlated with false positives during both recall, r(40) = 0.44, p < 0.01, and recognition, r(40) = 0.35, p < 0.05 and was used as a covariate in these analyses. Age was also specifically evaluated as a supplementary analysis given the well-known age effects on memory in the literature (hyperactivation, more diffuse activation, decreased memory performance).
3. Results
3.1. Hits and False Positives for Recall and Recognition
After the scanning session, list learning behavioral performance was assessed outside the scanner using semantically cued recall and recognition paradigms (see Methods). Participants had a mean of 38% accuracy for recall with 10.6 false positives on average (SD = 14.4), and 62% accuracy for recognition with 22.0 false positives on average (SD = 27.2). A 2 (recollection mode: recall vs. recognition) × 2 (recollection variable: hits vs. false positives) rmANOVA was conducted, with age entered as a covariate. There was a significant effect of recollection mode, such that more words were recognized than recalled, F (1,38) = 12.20, p = 0.001 (Figure 2A). A significant effect of recollection variable was also observed, with more correct hits than false positives overall, F (1,38) = 67.06, p < 0.001. Age was not a significant covariate in these analyses, F (1,38) = 0.54, n.s. and there was no significant difference in recall or recognition accuracy between males and females (Supplemental Table 1). Intraclass correlation coefficient was calculated for recall of each of fifteen lists (ICC = .91, p < .01). In addition, correlation of each lists recall with total recall was significant (rs > .46, ps < .01, Table 1)
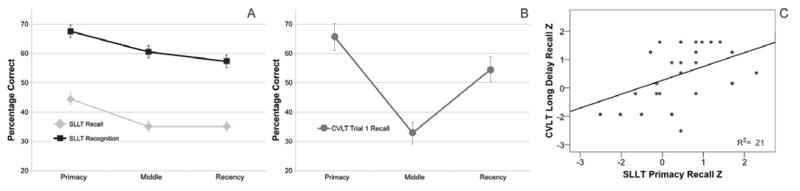
Figure 2.
Phases of Memory Recall for the SLLT and CVLT-II. Panel A includes the serial position curve modified by the SLLT with Brown-Peterson adjustment to the curve, removing the recency effect. Panel B includes the immediate recall for the CVLT-II. Panel C shows the correlation between SLLT Primacy Recall and CVLT-II Long Delay Free Recall Z scores.
3.2. Serial Position Curve for Recall and Recognition
During cued recall, participants averaged 44% accuracy for words in the Primacy position (first four words, SD = 13%) and 35% accuracy for words in both the Middle (six words, SD = 12%) and Recency positions (last four words, SD = 11%) of the SPC. Recognition accuracy averaged 68%, 61%, and 57% in Primacy, Middle, and Recency positions (SD = 14, 13, and 14%), respectively. The serial position effect was assessed with a 2 (recollection mode: recall vs. recognition) × 3 (Primacy, Middle, Recency positions) rmANOVA with false positives and age entered as covariates. A significant overall effect of serial position was observed, F (2,72) = 21.39, p < 0.001. Post-hoc paired samples t-tests showed that Recall was significantly higher for words presented during the Primacy phase of each category list (see Figure 2A), greater than for the Middle, t (39) = 6.51, p < 0.001, and Recency periods, t (39) = 5.80, p < 0.001. Recall performance during Middle and Recency periods was not significantly different, t (39) = −0.03, n.s. Post-hoc paired samples t-tests also confirmed the serial position effect for recognition. Recognition performance for the Primacy period was greater than that during both the Middle, t (39) = 5.09, p < 0.001, and Recency, t (39) = 7.22, p < 0.001, periods. Recognition performance during the Middle period was significantly greater than that observed during the Recency period, t (39) = 2.48, p = 0.02. The same significant effect of recollection mode was again observed when examining SLLT performance in each serial position separately (i.e., as above, more words were recognized than recalled, F (1, 36= 38.61, p < .001). However, no significant interaction between serial position and recollection mode was observed, F (2, 72) = 0.67, n.s. We present the SPC for Trial 1 of the CVLT-II recall (Figure 2B) for comparison to SLLT recall (Figure 2A), to demonstrate how the SPC differs when a BP distraction paradigm is used. As the SLLT recall is substantially later (about 40 minutes, compared to immediate recall for CVLT-II Trial1), one cannot compare the actual percent recalled by phase, merely the presence of a primacy effect in both tasks and the presence of a recency effect on the CVLT-II only.
3.3. Convergent and Discriminant Validity of the SLLT
We explored convergent validity between the SLLT and the CVLT-II. In both tasks there was a significant correlation in performance for Primacy words (Table 2). There was also a significant correlation between SLLT Primacy performance and CVLT-II Long Delay Free Recall (see Figure 2C), as well as CVLT-II Long Delay Cued Recall. Discriminant validity was examined between the SLLT and executive functioning indices from the Trail Making Test (Reitan, 1958, 1992), Wisconsin Card Sorting Test (Heaton, 1981; Grant & Berg, 1948), and the Parametric Go/No-Go Test (Langenecker et al., 2007b). None of the accuracy measures of executive functioning correlated with SLLT performance; however, correlations were observed between these executive functioning measures and CVLT-II Trial 1 performance. We did observe an inverse correlation between mean reaction time (processing speed) on the Parametric Go/No-Go (PGNG) test and performance in each serial position phase of the SLLT. Age was positively correlated with Trail Making A and response time for Go trials on the PGNG, and negatively correlated with Primacy words recalled in the SLLT and Go accuracy for the PGNG. Recall accuracy was higher among females versus males for Middle words in the SLLT (Supplemental Table 1). Accuracy for SLLT Primacy words and SLLT recognition were both positively correlated with estimated IQ scores (Supplemental Table 2).
Table 2.
Correlations of SLLT Performance with Other Memory, Processing Speed, and Executive Functioning Measures
| SLLT % Accuracy |
CVLT-II Trial 1 % Recall |
Age | ||||||
|---|---|---|---|---|---|---|---|---|
| Primacy | Middle | Recency | Primacy | Middle | Recency | |||
|
SLLT % Recall |
Primacy | 1 | 0.75** | 0.67** | 0.42* | 0.12 | 0.01 | −0.34* |
| Middle | -- | 1 | 0.78** | 0.35 | 0.21 | 0.08 | −0.17 | |
| Recency | -- | -- | 1 | 0.28 | 0.08 | −0.03 | −0.08 | |
|
CVLT-II Trial 1 % Recall |
Primacy | -- | -- | -- | 1 | 0.11 | −0.22 | 0.02 |
| Middle | -- | -- | -- | -- | 1 | −0.01 | −0.20 | |
| Recency | -- | -- | -- | -- | -- | 1 | −0.01 | |
|
| ||||||||
| CVLT-II | Long Delay Free Recall- % Recall | 0.46* | 0.37 | 0.28 | 0.45* | 0.26 | −0.04 | −0.12 |
| Long Delay Cued Recall- % Recall | 0.42* | 0.32 | 0.17 | 0.34 | 0.12 | 0.11 | −0.19 | |
| Recognition Discrimination | −0.11 | 0.04 | 0.15 | 0.23 | 0.15 | 0.39* | 0.27 | |
| Semantic Clustering | 0.22 | 0.15 | −0.02 | 0.29 | 0.53** | −0.09 | 0.05 | |
|
| ||||||||
| Attention | PGNG % Correct | 0.31 | 0.17 | 0.07 | −0.02 | 0.29 | 0.29 | −0.48** |
|
| ||||||||
| Inhibition | PGNG % Correct Inhibitions | 0.04 | 0.13 | −0.01 | 0.14 | 0.56** | 0.49* | −0.12 |
|
| ||||||||
| Set-Shifting | WCST % Correct | 0.24 | 0.08 | 0.02 | 0.35 | −0.08 | 0.24 | −0.03 |
| WCST Perseverative Errors |
−0.06 | 0.05 | −0.01 | 0.32 | −0.17 | 0.05 | 0.15 | |
| Trails B time | 0.29 | 0.01 | 0.26 | 0.14 | −0.39* | −0.15 | 0.29 | |
|
| ||||||||
| Processing Speed | PGNG mean RT | −0.60** | −0.53** | −0.46* | −0.48* | −0.16 | 0.16 | 0.54** |
| Trails A Time | 0.02 | −0.02 | 0.09 | −0.21 | −0.41* | 0.05 | 0.38 | |
p < 0.05.
p < 0.01.
3.4. Imaging Results
3.4.1. Block Design, Encoding – Silent Rehearsal
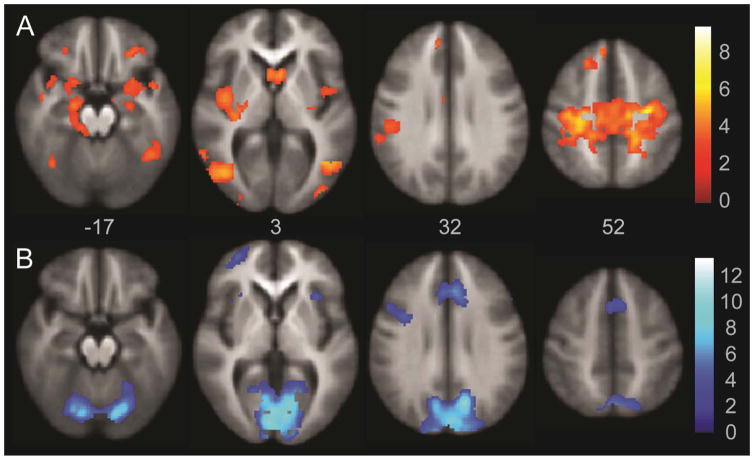
In order to assess the role of Papez circuit regions during long term memory encoding, we compared fMRI activation during encoding to silent rehearsal in our SLLT paradigm. There were significant activation differences during Encoding relative to the Silent Rehearsal blocks in several areas, including the Papez circuit, as illustrated in Figure 3 (top, in orange scale) and listed in Table 3. These included bilateral amygdala, hippocampus and parahippocampal gyrus, orbital frontal cortex, ventral and posterior insula, and uncus. In addition, bilateral superior frontal gyrus and superior dorsal motor cortex were more active during Encoding relative to Silent Rehearsal. Bilateral posterior inferior temporal gyri, and para- and postcentral gyri were also significantly more active during Encoding relative to Silent Rehearsal.
Figure 3.
Encoding-Silent Rehearsal Whole Brain Activation. Activation in orange indicates significant foci of activation in the Encoding-Silent Rehearsal contrast. The areas of greater activation in the Silent Rehearsal block relative to the Encoding block are illustrated in blue. Images are whole brain, AlphaSim corrected at p < 0.05.
Table 3.
Activation for Encoding versus Silent Rehearsal Conditions
| Lobe | Foci | BA | x | y | z | mm3 | Z |
|---|---|---|---|---|---|---|---|
| Encoding Minus Silent Rehearsal | |||||||
| Occipital | Inferior Occipital, Fusiform | 18/19/37 | −38 | −69 | −6 | 11848 | 6.49 |
| 17/19 | 27 | −93 | −9 | 7688 | 5.88 | ||
| Temporal | Superior Temporal, Middle Temporal, Uncus, Hippocampal Formation, Amygdala | 21/38 | 36 | 14 | −31 | 11752 | 4.46 |
| Middle Temporal | 39 | −43 | −67 | 14 | 560 | 3.1 | |
| Frontal | Precentral, Cingulate | 4/31 | 26 | −19 | 45 | 90840 | 5.5 |
| Superior Frontal, Middle Frontal | 8/9 | −13 | 30 | 49 | 3536 | 3.7 | |
| Inferior Frontal | 9 | 43 | −1 | 21 | 440 | 3.73 | |
| 47 | −25 | 34 | −7 | 624 | 3.29 | ||
| Inferior Frontal, Middle Frontal | 11/47 | 22 | 30 | −13 | 1440 | 3.71 | |
| Subcortical | Thalamus (Pulvinar) | N/A | 10 | −32 | 12 | 616 | 3.42 |
| Silent Rehearsal Minus Encoding | |||||||
| Frontal | Cingulate | 32 | 6 | 16 | 36 | 11928 | 5.42 |
| Middle Frontal | 9/46 | 43 | 24 | 24 | 608 | 4.16 | |
| Middle Frontal, Inferior Frontal | 10 | −31 | 49 | 4 | 1672 | 3.87 | |
| Precentral, Inferior Frontal | 9 | −38 | 4 | 32 | 2648 | 3.93 | |
| Limbic | Posterior Cingulate | 23 | −1 | −30 | 18 | 1440 | 4.38 |
| Subcortical | Claustrum, Insula | N/A | 29 | 18 | 1 | 1000 | 3.93 |
| N/A | −40 | 12 | −3 | 736 | 3.11 | ||
| Cerebellum | Declive, Culmen | N/A | 3 | −81 | −10 | 103240 | 7.67 |
BA = Brodmann’s area; mm3 = volume of activation in cubic millimeters; Z = cluster Z score; N/A = Not Applicable. Voxels showing differential activation between blocks were assessed at a whole brain significance threshold of AlphaSim corrected p value < 0.05.
3.4.2. Block Design Encoding – Distraction
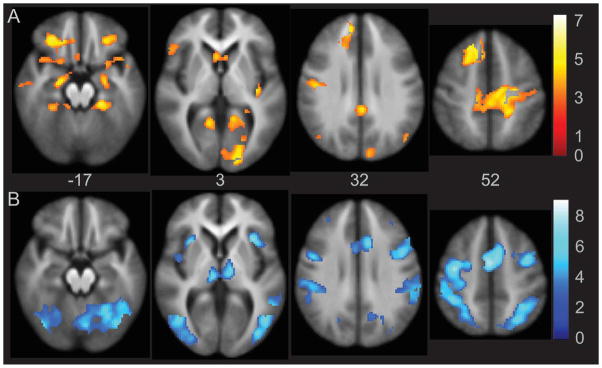
The Encoding-Distraction contrast was used as a confirmatory analysis to verify that increased activation observed in the Encoding-Silent Rehearsal contrast was associated with learning and encoding processes specific to LTM, as brain areas related to STM processing should be similarly negated when subtracting either Silent Rehearsal or Distraction conditions. To this extent, the activation foci for Encoding-Distraction overlapped significantly with Encoding-Silent Rehearsal. Activation foci are listed in Supplemental Table 3 and shown in Figure 4 (top, in orange scale), as they are highly redundant with those for Encoding versus Silent Rehearsal in Figure 3 and Table 3.
Figure 4.
Activation analysis of Encoding-Distraction. Activation in orange indicates significant foci of activation in the Encoding-Distraction contrast. The areas of greater activation in the Distraction block relative to the Encoding block are illustrated in blue. Images are whole brain, AlphaSim corrected at p < 0.05.
3.4.3. Block Design Silent Rehearsal – Encoding
Significant activation was observed within the Silent Rehearsal-Encoding contrast in executive control and early visual areas, specifically in an extensive dorsal and middle cingulate cluster, and across early visual cortex, extending to the precuneus and cerebellum. This information is presented in Table 3 and Figure 3 (bottom, in blue scale).
3.4.4. Block Design Distraction – Encoding
Activation that was significantly greater during Distraction blocks than during Encoding encompassed well-known executive control and motor circuits (see Supplemental Table 3 and Figure 4, bottom, in blue scale), including bilateral middle frontal and cingulate gyrus. There was also significant activation in the Distraction condition relative to the Encoding condition in bilateral fusiform gyrus and anterior insula.
3.4.5. Planned Analysis of Activation in Papez Circuit Subcomponents for Encoding, Distraction, and Silent Rehearsal Relative to Rest
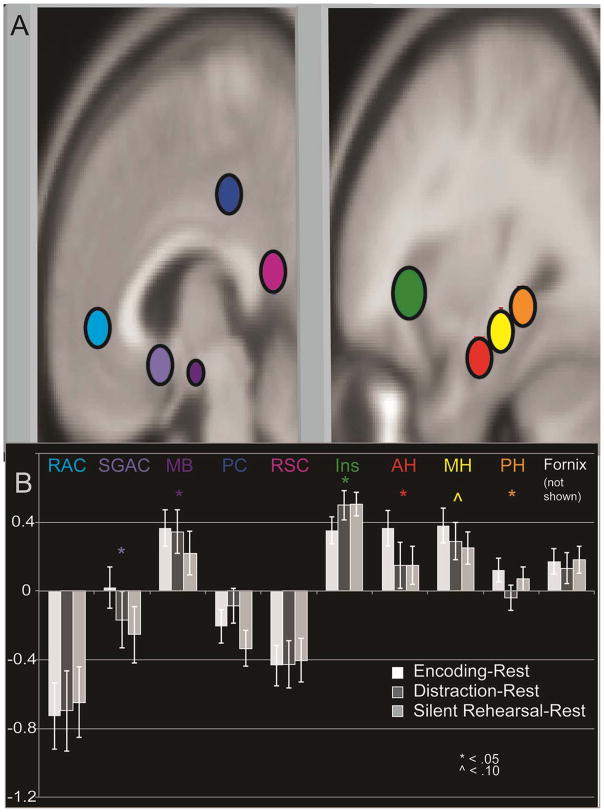
Planned analyses were conducted to evaluate the relative contributions of different Papez circuit regions to the Encoding process and in relation to Silent Rehearsal and Distraction. A total of ten spherical ROIs were created bilaterally in MarsBaR (Brett et al., 2002), and mean signal was extracted for each of four conditions (Encoding, Distraction, Silent Rehearsal, Rest). Three spherical ROIs were created on the long axis of the hippocampus in twelve millimeter intervals, using MNI coordinates (30, −12,−18; 32, −24, −11; and 31, −36, −4). These coordinates were confirmed on the MNI brain visually, as well as upon an average T1 brain of all subjects. The anterior hippocampal ROI radius was visually confirmed to have minimal overlap with the amygdala on the average brain. In addition, activation was extracted for a number of other regions that are believed to be part of the Papez circuit. To determine ROI positions, we used activation foci from a recent meta-analysis (Kim, 2011), and examined similar findings in other published memory imaging work in at least two independent studies (Beason-Held, Spinnler, Vallar, & Zanobia, 2005; Braskie, Small, & Bookheimer, 2009). In some cases, we could also confirm the coordinates using ROIs from the Wake Forest PickAtlas (Maldjian, Laurienti, Burdette, & Kraft, 2003; Maldjian, Laurienti, & Burdette, 2004). A 5 mm radius spherical ROI was used for most regions except two that were smaller (mammillary bodies and fornix = 3 mm radius), and one that was larger (anterior insula = 7 mm radius). These ROIs are displayed in Figure 5, except for the fornix.
Figure 5.
Activation in Papez Circuit Sub-regions by SLLT Condition. Regions used to extract activation, overlaid onto the MNI template. Signal change to illustrate differences between Encoding and Silent Rehearsal in the Papez circuit regions. Abbreviations: rostral anterior cingulate (RAC), subgenual anterior cingulate (SGAC), mammillary bodies (MB), posterior cingulate (PC), retrosplenial cingulate (RSC), insula (Ins), anterior hippocampus (AH), middle hippocampus (MH), posterior hippocampus (PH).
Mean activation in each region (10), by side (2), and by condition (4), were analyzed in a rmANOVA in SPSS with Greenhouse-Geisser correction for sphericity. There was not a significant main effect of condition F (1.99,75.58) = 0.38, p = 0.69. The primary interaction of interest was ROI by condition, which was significant F (6.50,246.89) = 10.48, p < 6.11 × 10−11, and these results are displayed in Figure 5, Panel C. Anterior and middle hippocampus, as well as mammillary bodies and subgenual anterior cingulate ROIs showed significantly higher activation in Encoding than in Distraction or Silent Rehearsal blocks, while less activity was observed during Encoding in the insula. The interaction between condition, side, and region was significant, F (8.09,307.55) = 2.48, p = 0.01, driven by higher activation in hippocampus and rostral anterior cingulate on the left for all conditions, and higher activation in the right mammillary bodies for all conditions. Anterior insula activation was higher on the left for Encoding and Silent Rehearsal, and higher on the right for Distraction.
3.4.6. Post-hoc Analysis of Distinctiveness of Primacy-Related Activation Compared to Middle- and Recency-Related Activation
One benefit of the present task design was the ability to investigate the specificity of activation within the Primacy portion of the serial position curve, in order to identify foci of activation that might help explain why subsequent memory is better for information presented early in a list. To achieve this aim, a post-hoc model was created that subdivided activation by the phase of encoding, within the Papez circuit ROIs depicted in Figure 5. A 2 side × 10 ROI × 3 phase rmANOVA was computed with Greenhouse-Geisser correction. There was a significant interaction between phase and Papez region, F (6.99,265.53) = 2.68, p = 0.01. Posterior cingulate cortex, retrosplenial cingulate cortex, anterior insula, and mammillary bodies all had greater activation later in the SPC, typically with Recency greater than Primacy. No areas within the Papez circuit had more activation in Primacy relative to the other 2 phases of the SPC. Activation was present for all 3 conditions in all parts of the hippocampus, mammillary bodies, posterior cingulate cortex, and subgenual anterior cingulate.
3.4.7. Encoding during Successfully Recalled (or Recognized) Words
The SLLT design includes jittered presentation of words to allow for event-related analyses based on subsequent memory performance. During encoding of successfully recalled words, significant activation was observed in many of the same areas identified in the Encoding minus Silent Rehearsal analysis. Activation for later recalled words included bilateral amygdala and hippocampus, parahippocampal gyrus, uncus, lateral orbital frontal cortex, left inferior and superior frontal gyrus, medial and right pre- and paracentral gyrus (see Supplemental Table 4 and Supplemental Figure 1). The areas missing in the recalled contrast (but present in Encoding block analyses) were insula and posterior middle temporal gyrus. Encoding of successfully recognized words yielded significant activations in slightly more extensive, but highly overlapping regions relative to the recalled words (see Supplemental Table 5).
3.4.8. Effects of Age on Encoding and Successfully Recalled Words
There are well-known age effects of decline in memory, even in healthy individuals. There are also well published findings of increased activation during performance of a number of tasks in healthy older adults, sometimes referred to as compensation, but also as dedifferentiation, reserve, etc. (Bartres-Faz & Arenaza-Urquijo, 2011; Han, Bangen, & Bondi, 2009; Langenecker & Nielson, 2003). We tested positive and negative associations of activation with age for the Encoding-Silent rehearsal and Recall contrasts. Increasing activation with increasing age was observed for Recalled items in the event-related analysis in primarily right frontal areas (Table 4, middle, inferior frontal gyris, anterior cingulate) as well as left superior frontal gyrus, and a few parietal (precuneus, postcentral), occipital (middle temporal, inferior occipital), temporal (superior temporal) and subcortical (left lateral globus pallidus) areas. There were no negatively correlated activations with age for recalled items. No positive or negative age associations were observed with activation for the Encoding-Silent Rehearsal contrast.
Table 4.
Increasing Activation for the Recalled Words with Increasing Age and Education, and Differences between Men and Women
| Lobe | Foci | BA | x | y | z | mm3 | Z |
|---|---|---|---|---|---|---|---|
| Age Positive Correlation | |||||||
|
| |||||||
| Frontal | Middle Frontal Gyrus | 6 | 34 | 10 | 54 | 2624 | 3.44 |
| Anterior Cingulate | 24 | 10 | 34 | 8 | 2544 | 3.82 | |
| Inferior Frontal | 47 | 26 | 18 | −16 | 648 | 3.79 | |
| 6 | 62 | 6 | 18 | 456 | 3.13 | ||
| Superior Frontal | 6 | −26 | 16 | 54 | 472 | 3.29 | |
| Parietal | Precuneus | 31 | −4 | −64 | 32 | 792 | 3.45 |
| Postcentral | 43 | 66 | −8 | 18 | 664 | 3.59 | |
| Temporal | Superior Temporal | 22 | −48 | −18 | −4 | 656 | 3.4 |
| Occipital | Middle Temporal | 19 | 56 | −58 | 20 | 3216 | 3.6 |
| Inferior Occipital | 19 | −44 | −80 | 2 | 2224 | 3.78 | |
| Subcortical | Lateral Globus Pallidus | -- | −22 | −2 | −2 | 1648 | 3.58 |
|
| |||||||
| Education _ Negative Correlation | |||||||
|
| |||||||
| No foci | |||||||
|
| |||||||
| Education – Positive Correlation | |||||||
|
| |||||||
| No foci | |||||||
|
| |||||||
| Men greater than Women | |||||||
|
| |||||||
| Frontal | Inferior Frontal | 44 | −48 | −3 | 22 | 472 | 3.22 |
|
| |||||||
| Women greater than Men | |||||||
|
| |||||||
| Frontal | Inferior Frontal/Insula | 45/13 | −36 | 14 | 15 | 1552 | 3.58 |
| Superior Frontal | 6 | −11 | 30 | 35 | 1184 | 3.65 | |
BA = Brodmann’s area; mm3 = volume of activation in cubic millimeters; Z = cluster Z score. Voxels showing differential activation between blocks were assessed at a whole brain significance threshold of AlphaSim corrected p value < 0.05.
We further evaluated relationships between activation for recalled items in the Papez circuit and age. We used activation for Encoding-Silent Rehearsal to predict age in a backward multiple regression. This analysis first examined whether age significantly influenced activation across all 10 Papez circuit ROIs, and then iteratively and automatically removed the ROI for which the influence of age was the weakest. The model was not significant with all 10 Papez ROIs entered (F(10,28)=0.81, p = .62), nor was it significant after 7 ROIs were removed (F(3, 35)=2.40, p = .08). We then used activation for the event-related analysis of recalled items to predict age using the same backward multiple regression procedure. The full model was not significant (F(10,28)=1.88, p = .09), whereas the model significantly predicted age at steps 3 through 7, with the optimal model at step 6 (F(5,33)=3.36, p = .01, R2 = .34), including the following 5 ROIs: subgenual anterior cingulate (t = −1.45, B = −.25, p = .16), anterior insula (t = 2.08, B = .31, p = .04), retrosplenial posterior cingulate cortex (t = 2.29, B = .36, p = .03), posterior hippocampus (t = 3.43, B = .86, p = .002), and fornix (t = −3.10, B = −.74, p = .004).
4. Discussion
The present study successfully provided an initial characterization of behavioral and fMRI responses during the Semantic List Learning Task. (SLLT). The SLLT demonstrates convergent validity with the California Verbal Learning Test-2nd edition (Delis et al., 2000), with better discriminant validity than the CVLT-II when compared with executive functioning and problem solving measures. Use of semantic cues for organization of learning and retrieval resulted in satisfactory later recall (44% accuracy), and engagement of a distracting BP paradigm resulted in a diminished behavioral recency effect (only 35%). fMRI activation during encoding was observed across a broad neural network, the Papez circuit, which supports the encoding process. This circuit was also highlighted in the event-related analysis that examined activation during encoding of correctly recalled and recognized items only. We also observed more nuanced effects, including decreased activation for Primacy words in Papez circuit regions that are part of the default mode network (rostral and posterior cingulate; Greicius, Krasnow, Reiss, & Menon, 2003).
4.1. Serial Position Effects within the SLLT
The significant behavioral primacy effect that was observed in the present study is in line with a wealth of research on serial position effects (Atkinson & Shiffrin, 1968; Glanzer & Cunitz, 1966; Rundus, 1971; Talmi et al., 2005). The facilitation of memory for early list items is caused by superior encoding of early items into LTM, possibly as the result of less competition for rehearsal early in the task (Atkinson & Shiffrin, 1968). The absence of a significant recency effect was expected as a result of the BP manipulation (Brown, 1958; Peterson & Peterson, 1959). Taken together, these results indicate that we were successful in minimizing facilitation of recent memory items by STM. Recency may have been reduced by memory decay via prevention of immediate rehearsal (Brown, 1958; Jarrold, et al., 2010; Peterson & Peterson, 1959) and/or by interference from items in the distractor task (Berman et al., 2009; Lewandowsky et al., 2008; Luria, 1971). As a result of the BP manipulation, a greater portion of the behavioral performance in the SLLT might be attributed to LTM processes.
4.2. Encoding and Papez Circuit Activation
Significant activation of the Papez circuit, including MTL structures, was observed during the Encoding block relative to both Silent Rehearsal and Distraction. This included bilateral anterior and middle hippocampus, similar to previous studies (Fernandez et al., 1998; Flegal, et al., 2014; Strange et al., 2002; Talmi et al., 2005). Activation observed in MTL during semantic list learning was predominantly driven by encoding of verbal material into LTM, as it was significantly greater compared with Silent Rehearsal or Distraction blocks. Significant activation was also observed in bilateral middle/posterior cingulate cortex during Encoding relative to Silent Rehearsal, and these areas have also been previously implicated in the encoding process (Burianova, McIntosh, & Grady, 2010; McDermott et al., 1999). These results suggest that select Papez circuit structures were important for LTM encoding during word list learning, and thus play a vital role in a clinically-relevant test of human memory.
4.3. Specificity of Activation to LTM versus STM in Papez Regions of Interest
While we cannot rule out the involvement of regions subserving LTM in Silent Rehearsal based on our block design analysis, our planned ROI analyses revealed that hippocampal and cingulate ROIs were not significantly activated during Silent Rehearsal when compared with Rest. Thus, some Papez circuit regions typically associated with LTM do not seem strongly engaged during the Silent Rehearsal block. Retrieval and consolidation (both correct and incorrect) were likely taking place during Silent Rehearsal, and recall and rehearsal behavior are believed to strongly engage STM (Atkinson & Shiffrin, 1968; Jarrold et al., 2010). By subtracting this activation from that seen during Encoding, we believe that the resulting activation pattern highlights regions more heavily involved in LTM encoding. This result was corroborated when studying the effect of correctly recalled and recognized items only.
Many fMRI experiments that have focused on list learning or related memory paradigms observed activation bilaterally in ventrolateral frontal cortex during encoding, but failed to find encoding activation in hippocampus or parahippocampus (Dupont et al., 2002; Fletcher et al., 2003). Engagement and performance variance related to STM processes may have contributed to the frontal activation observed in these studies (see Axmacher, et al., 2009), and the focus on shorter delays may have minimized influence of LTM regions. In this experiment, a left superior frontal cluster was the only prominent lateral frontal activation observed in the Encoding minus Silent Rehearsal contrast. Inferior frontal activation was not present in the Encoding minus Silent Rehearsal contrast but was observed during Encoding minus Distraction, suggesting that this activation could be related to sub-vocal rehearsal and/or semantic processing (which would have been minimal during Distraction), and not activation related to unelaborated encoding process per se (Wagner et al., 1998; Thompson-Schill, D’Esposito, Aguirre, & Farah, 1997).
Planned ROI analyses for the block design explored the relationship between Papez circuit activation and different blocks (i.e., Encoding, Distraction, and Silent Rehearsal) within the SLLT. To assure clinical utility for future studies, it would be important for encoding to result in significant activation of Papez circuit subcomponents, such as the hippocampus, during this task. We confirmed this specificity within the anterior and middle hippocampal ROIs, as well as in subgenual anterior cingulate and mammillary bodies. Post-hoc analyses also revealed significantly higher activation in Papez circuit regions such as posterior cingulate, retrosplenial cingulate, and anterior insula for the Middle and Recency portions of the SPC. Suppressed activity in these regions during Primacy may facilitate better encoding, and later activation might be related to default mode network re-engagement (Greicius et al., 2003). This may reflect greater ability to filter out distracting information during the Primacy phase, or a higher level of alertness and/or task engagement during primacy.
4.4. Other Potential Causes for Medial Temporal Activation During Encoding
Temporal lobe structures in the immediate vicinity of the hippocampi such as parahippocampal, fusiform, and perirhinal cortices are known to play important roles in visual perception, in addition to the roles of these regions in memory (Buckley, 2005; Epstein, Harris, Stanley, & Kanwisher, 1999; Kanwisher & Yovel, 2006; Libby, Hannual, & Ranganath, 2014). Therefore, one might posit that the MTL activity observed in the Encoding minus Silent Rehearsal contrast was related to perception of the large number of visual stimuli presented in the former but not the latter block, rather than being driven by LTM encoding. However, very similar MTL activation was observed in the Encoding minus Distraction contrast, with dynamic visual stimuli presented during both blocks. Furthermore, we found a large cluster of activation within visual cortex for the Silent Rehearsal minus Encoding contrast, contrary to what would be predicted if the large number of dynamic visual stimuli were exclusively responsible for driving activity during Encoding.
4.5. Limitations
There are some limitations of the current study. First, as is well-established, the block design effects were slightly more powerful than those seen in the event-related analysis, which led us to focus primarily on those activation foci that were present in both the block and event-related analyses. Future studies could lengthen the jitter between individual words to facilitate analysis of activation specific for words that are later recalled relative to non-recalled words. However, it is not clear whether variability in the length of the inter-stimulus interval may affect subsequent memory performance in this paradigm. Second, we provided cues for use of a specific encoding and retrieval strategy, namely semantic organization cues and semantically related word lists. Although less likely, subjects could have used other encoding and retrieval strategies, as we did not query subjects after the scan about whether they used the provided strategies or not. Thus, variability in encoding and retrieval strategies could have weakened our analyses – but this would result in greater type II error. Future experiments might more thoroughly explore the influence of different encoding and retrieval strategies on SLLT performance by comparing behavioral and functional data between runs during which different strategies were encouraged or through the use of post-scanning questionnaires. Also, our knowledge of how consolidation occurs in relation to the BOLD response remains quite naïve. Thus, silent rehearsal could reflect consolidation, rote rehearsal, or deep encoding (with the semantic cue, Bontempi et al., 1999). Third, we did not observe activation in the posterior hippocampus, contrary to Fernandez and colleagues (1998). Our observations of activation at the individual level has been that there are greater age, sex, and other individual variation in the medial-lateral-inferior/superior location of the posterior hippocampus. While location was verified based upon the mean anatomy for all participants in the sample, it is possible that greater anatomical variability in this area contributed to an absence of observed effects.
Fourth, sex differences have been found on performance for word-list learning tasks (Herlitz, Nilsson, & Backman, 1997), particularly among young adults (Kramer, Yaffe, Lengenfelder, & Delis, 2003). The greater use of a clustering strategy during encoding is thought to underlie sex differences (Andreano & Cahill, 2009), especially for longer word lists (Sunderaraman, Blumen, DeMatteo, Apa, & Cosentino, 2013). Because semantic encoding is implicit in the design of the SLLT, we did not include the study of sex differences as a primary variable of interest, though this does not negate the presence of a sex effect. In post hoc analyses, women did have greater recall than men for items in the middle of the word lists, and there were three regions demonstrating sex differences between females and males during Encoding relative to Recall. These sex effects are minor, and do not detract from the overall findings, but should be considered in any future work with the task. We also did not control for phase of menstrual cycle, exclude for use of hormonal contraceptives, or measure levels of estrogen/progesterone in our sample of women, who are likely to range from pre- to peri- and post-menopausal. Studies have demonstrated that estrogen facilitates verbal memory among women (Phillips & Sherwin, 1992; Sherwin, 1988; Sherwin & Tulandi, 1996). At the same time, verbal explicit memory may be less prone to changes across the menstrual cycle, relative to other forms of memory (Maki, Rich, & Rosenbaum, 2002; O’Reilly, Cunningham, Lawlor, Walsh, & Rowan, 2004; Phillips and Sherwin, 1992), and oral contraceptives, during the active phase, may facilitate explicit verbal memory performance (Mordecai, Rubin, & Maki, 2008). Future studies might assess sex effects on SLLT performance and activation, in addition to explicitly examining or controlling for levels of estrogen and progesterone.
In addition, the large age range used may also have weakened the task specific effects that could be observed. We observed similar results when more stringent age cut-offs were used (below age 55), and thus opted to include all subjects within the 18–65 age range. Further, we also demonstrated robust behavioral and imaging aging effects, above and beyond task effects The current study aimed to validate the SLLT for use across a wide age range, and we include specific age-related findings in this healthy sample as an additional source of convergent validity. Our subject pool was also relatively well educated, which may have limited the range of performance and the corresponding range of activation. A final limitation is of order effects. The CVLT-II was always administered prior to the SLLT, as our neuropsychological battery was routinely performed prior to fMRI. A lack of counterbalancing of the CVLT-II and SLLT is a limitation, as order effects may have confounded relationships between these measures, as well as with other executive functioning measures.
5. Conclusion
In order to better understand conditions in which memory is compromised (e.g. dementia, mental illness), it is essential to study the neural circuits that support LTM in healthy participants during list learning tasks. In addition, it is important to develop tests with robust and specific behavioral performance metrics, so that analogues of the imaging paradigms can be translated into clinical applications and studies. Such translational tasks will improve the ability of clinicians to assess the functional integrity of the neural circuits engaged during clinical and diagnostic memory tests. The Papez circuit is a neuroanatomical pathway that plays an important role in human emotion and memory, and is sensitive to disruption by trauma and disease (Papez, 1937). Tasks that assess its function through behavioral and neuroimaging methods will be of great value in both clinical and research contexts. To this end, the current study has defined the relationship between Papez circuit activity, list learning, and subsequent memory performance during the SLLT using fMRI.
Supplementary Material
Acknowledgments
This work was supported by a K23 Award (MH074459, SAL), a GCRC grant (SAL and MSN, # MO1 RR00042), a NARSAD Young Investigator award (SAL), Rachel Upjohn Clinical Scholars Awards (SAL and SLW), a Psychiatry Research Committee grant (SAL), and fMRI pilot scans (SAL and SLW). A subset of these individuals was used in the senior honors thesis of the lead author (MPS), which is available online through http://deepblue.lib.umich.edu.
References
- Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learning and Memory. 2009;16:248–266. doi: 10.1101/lm.918309. [DOI] [PubMed] [Google Scholar]
- Atkinson RC, Shiffrin RM. Human memory: A proposed system and its control processes. In: Spence KW, Spence JT, editors. The psychology of learning and motivation. New York: Academic Press; 1968. pp. 89–195. [Google Scholar]
- Axmacher N, Elger CE, Fell J. Working memory-related hippocampal deactivation interferes with long-term memory formation. Journal of Neuroscience. 2009;29:1052–1060. doi: 10.1523/JNEUROSCI.5277-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley AD. The fractionation of working memory. Proceedings of the National Academy of Science. 1996;93:13468–13472. doi: 10.1073/pnas.93.24.13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A, Bueno O, Cahill L, Fuster JM, Izquierdo I, McGaugh JL, et al. The brain decade in debate: I. Neurobiology of learning and memory. Brazilian Journal of Medical and Biological Research. 2000;33:993–1002. doi: 10.1590/s0100-879x2000000900002. [DOI] [PubMed] [Google Scholar]
- Baker JT, Sanders AL, Maccotta L, Buckner RL. Neural correlates of verbal memory encoding during semantic and structural processing tasks. NeuroReport. 2001;12:1251–1257. doi: 10.1097/00001756-200105080-00039. [DOI] [PubMed] [Google Scholar]
- Bartres-Faz D, Arenaza-Urquijo E. Structural and Functional Imaging Correlates of Cognitive and Brain Reserve Hypotheses in Healthy and Pathological Aging. Brain Topography. 2011;24:340–357. doi: 10.1007/s10548-011-0195-9. [DOI] [PubMed] [Google Scholar]
- Beason-Held LL, Golski S, Kraut MA, Esposito G, Resnick SM. Brain activation during encoding and recognition of verbal and figural information in older adults. Neurobiology of Aging. 2005;26(2):237–250. doi: 10.1016/j.neurobiolaging.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Berman MG, Jonides J, Lewis RL. In search of decay in verbal short-term memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2009;35:317–333. doi: 10.1037/a0014873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham D, Hill RD, Woltz D, Gardner MK. Cognitive strategy use and measured numeric ability in immediate- and long-term recall of everyday numeric information. PLOS ONE. 2013;8:1–10. doi: 10.1371/journal.pone.0057999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi B, Laurent-Demir C, Destrade C, Jaffard R. Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nature. 1999;400:671–675. doi: 10.1038/23270. [DOI] [PubMed] [Google Scholar]
- Braskie MN, Small GW, Bookheimer SY. Entorhinal cortex structure and functional MRI response during an associative verbal memory task. Human Brain Mapping. 2009;30:3981–3992. doi: 10.1002/hbm.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-P. Region of interest analysis using an SPM toolbox. Poster presented at the 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. Jun 2–6, 2002. [Google Scholar]
- Broadbent NJ, Clark RE, Zola S, Squire LR. The medial temporal lobe and memory. In: Squire LR, Schacter DL, editors. Neuropsychology of memory. New York: The Guilford Press; 2002. pp. 3–23. [Google Scholar]
- Brown JA. Some tests of the decay theory of immediate memory. Quarterly Journal of Experimental Psychology. 1958;10:12–21. [Google Scholar]
- Buckley MJ. The role of the perirhinal cortex and hippocampus in learning, memory, and perception. The Quarterly Journal of Experimental Psychology. 2005;35B:246–268. doi: 10.1080/02724990444000186. [DOI] [PubMed] [Google Scholar]
- Burianova H, McIntosh AR, Grady CL. A common functional brain network for autobiographical, episodic, and semantic memory retrieval. Neuroimage. 2010;49:865–874. doi: 10.1016/j.neuroimage.2009.08.066. [DOI] [PubMed] [Google Scholar]
- Clark D, Wagner AD. Assembling and encoding word representations: fMRI subsequent memory effects implicate a role for phonological control. Neuropsychologia. 2003;41:304–317. doi: 10.1016/s0028-3932(02)00163-x. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Lockhart RS. Level of processing: A framework for memory research. Journal of Verbal Learning and Verbal Behavior. 1972;11:671–684. [Google Scholar]
- Delis D, Kramer J, Kaplan E, Ober B. California Verbal Learning Test-II. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- Dupont S, Samson Y, Le Bihan D, Baulac M. Anatomy of verbal memory: A functional MRI study. Surgical and Radiologic Anatomy. 2002;24:57–63. doi: 10.1007/s00276-002-0005-x. [DOI] [PubMed] [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N. The parahippocampal place area: Recognition, navigation, or encoding? Neuron. 1999;23:115–125. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- Fernandez G, Weyerts H, Schrader-Bolsche M, Tendolkar I, Smid H, Tempelmann C, et al. Successful verbal encoding into episodic memory engages the posterior hippocampus: A parametrically analyzed functional magnetic resonance imaging study. Journal of Neuroscience. 1998;18:1841–1847. doi: 10.1523/JNEUROSCI.18-05-01841.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KE, Marín-Gutiérrez A, Ragland JD, Ranganath C. Brain mechanisms of successful recognition through retrieval of semantic context. Journal of Cognitive Neuroscience. 2014;26:1694–1704. doi: 10.1162/jocn_a_00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliessbach K, Buerger C, Trautner P, Elger CE, Weber B. Differential effects of semantic processing on memory encoding, Human Brain Mapping. 2010;31:1653–1664. doi: 10.1002/hbm.20969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Stephenson CME, Carpenter A, Donovan T, Bullmore ET. Regional brain activations predicting subsequent memory success: An event-related fMRI study of the influence of encoding tasks. Cortex. 2003;39:1009–1026. doi: 10.1016/s0010-9452(08)70875-x. [DOI] [PubMed] [Google Scholar]
- Friston K, Ashburner J, Frith C, Poline JB, Heather J, Frackowiak R. Spatial registration and normalization of images. Human Brain Mapping. 1995;2:165–189. [Google Scholar]
- Glanzer M, Cunitz AR. Two storage mechanisms in free recall. Journal of Verbal Learning and Verbal Behavior. 1966;5:351–360. [Google Scholar]
- Grant DA, Berg E. A behavioral analysis of degree of reinforcement and ease of shifting to new responses in a Weigl-type card-sorting problem. Journal of Experimental Psychology. 1948;38:404–411. doi: 10.1037/h0059831. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin SA, Miller MB. Lateralization of the parietal old/new effect: An event-related fMRI study comparing recognition memory for words and faces. Neuroimage. 2009;44:232–242. doi: 10.1016/j.neuroimage.2008.08.035. [DOI] [PubMed] [Google Scholar]
- Han SD, Bangen KJ, Bondi MW. Functional Magnetic Resonance Imaging of Compensatory Neural Recruitment in Aging and Risk for Alzheimer’s Disease: Review and Recommendations. Dementia, Geriatrics, and Cognitive Disorders. 2009;27:1–10. doi: 10.1159/000182420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK. A manual for the Wisconsin card sorting test. Western Psychological Services; 1981. [Google Scholar]
- Heinze S, Sartory G, Müller BW, de Greiff A, Forsting M, Jüptner M. Neural encoding correlates of high and low verbal memory performance. Journal of Psychophysiology. 2006;20:68–78. [Google Scholar]
- Herlitz A, Nilsson LG, Bäckman L. Gender differences in episodic memory. Memory & Cognition. 1997;25:801–811. doi: 10.3758/bf03211324. [DOI] [PubMed] [Google Scholar]
- Jarrold C, Tam H, Baddeley AD, Harvey CE. The nature and position of processing determines why forgetting occurs in working memory tasks. Psychonomic Bulletin & Review. 2010;17:772–777. doi: 10.3758/PBR.17.6.772. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Yovel G. The fusiform face area: a cortical region specialized for the perception of faces. Philosophical Transactions of the Royal Society. 2006;361:2109–2128. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. Neural activity that predicts subsequent memory and forgetting: A meta-analysis of 74 fMRI studies. Neuroimage. 2011;54:2446–2461. doi: 10.1016/j.neuroimage.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Kim H, Cabeza R. Differential contributions of prefrontal, medial temporal, and sensory-perceptual regions to true and false memory formation. Cerebral Cortex. 2007;17:2143–2150. doi: 10.1093/cercor/bhl122. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Yaffe K, Lengenfelder J, Delis DC. Age and gender interactions on verbal memory performance. Journal of the International Neuropsychological Society. 2003;9:97–102. doi: 10.1017/s1355617703910113. [DOI] [PubMed] [Google Scholar]
- Langenecker S, Bieliauskas L, Rapport L, Zubieta J, Wilde E, Berent S. Face emotion perception and executive functioning deficits in depression. Journal of Clinical and Experimental Neuropsychology. 2005;27:320–333. doi: 10.1080/13803390490490515720. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Kennedy SE, Guidotti LM, Briceno EM, Own LS, Hooven T, et al. Frontal and limbic activation during inhibitory control predicts treatment response in major depressive disorder. Biological Psychiatry. 2007a;62:1272–1280. doi: 10.1016/j.biopsych.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenecker SA, Nielson KA. Frontal recruitment during response inhibition in older adults replicated with fMRI. NeuroImage. 2003;20:1384–1392. doi: 10.1016/S1053-8119(03)00372-0. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Weisenbach SL, Giordani B, Briceno EM, Guidotti LM, Schallmo MP, Leon HM, Noll DC, Zubieta J-K, Schteingart DE, Starkman MN. Impact of chronic hypercortisolism on affective processing. Neuropharmacology. 2012;62:217–225. doi: 10.1016/j.neuropharm.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenecker SA, Zubieta JK, Young EA, Akil H, Nielson KA. A task to manipulate attentional load, set-shifting, and inhibitory control: Convergent validity and test-retest reliability of the Parametric Go/No-Go Test. Journal of Clinical and Experimental Neuropsychology. 2007b;29:842–853. doi: 10.1080/13803390601147611. [DOI] [PubMed] [Google Scholar]
- Lewandowsky S, Geiger SM, Oberauer K. Interference-based forgetting in verbal short-term memory. Journal of Memory and Language. 2008;59:200–222. [Google Scholar]
- Libby LA, Hannula DE, Ranganath C. Medial temporal lobe coding of item and spatial information during relational binding in working memory. Journal of Neuroscience. 2014;34:14233–14242. doi: 10.1523/JNEUROSCI.0655-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria AR. Memory disturbances in local brain lesions. Neuropsychologia. 1971;9:367–375. doi: 10.1016/0028-3932(71)90001-7. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH. Precentral Gyrus Discrepancy in Electronic Versions of the Talairach Atlas. Neuroimage. 2004;21(1):450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA. An Automated Method for Neuroanatomic and Cytoarchitectonic Atlas-based Interrogation of fMRI Data Sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Marvel CL, Desmond JE. The contributions of cerebro-cerebellar circuitry to executive verbal working memory. Cortex. 2010;46:880–895. doi: 10.1016/j.cortex.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott KB, Ojemann JG, Petersen SE, Ollinger JM, Snyder AZ, Akbudak E, Conturo TE, Raichle ME. Direct comparison of episodic encoding and retrieval of words: An event-related fMRI study. Memory. 1999;7:661–678. doi: 10.1080/096582199387797. [DOI] [PubMed] [Google Scholar]
- Maki PM, Rich JB, Rosenbaum RS. Implicit memory varies across the menstrual cycle: estrogen effects in young women. Neuropsychologia. 2002;40:518–529. doi: 10.1016/s0028-3932(01)00126-9. [DOI] [PubMed] [Google Scholar]
- Marvel CL, Desmond JE. The contributions of cerebro-cerebellar circuitry to executive verbal working memory. Cortex. 2010;46:880–895. doi: 10.1016/j.cortex.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordecai KL, Rubin LH, Maki PM. Effects of menstrual cycle phase and oral contraceptive use on verbal memory. Hormones and Behavior. 2008;54:286–293. doi: 10.1016/j.yhbeh.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Memory and working-with-memory: A component process model based on modules and central systems. Journal of Cognitive Neuroscience. 1992;4:257–267. doi: 10.1162/jocn.1992.4.3.257. [DOI] [PubMed] [Google Scholar]
- O’Reilly MA, Cunningham CJ, Lawlor BA, Walsh CD, Rowan MJ. The effect of the menstrual cycle on electrophysiological and behavioral measures of memory and mood. Psychophysiology. 2004;41:592–603. doi: 10.1111/j.1469-8986.2004.00194.x. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Henson RN, Rugg MD. Depth of processing effects on neural correlates of memory encoding: relationship between findings from across- and within-task comparisons. Brain. 2001;124:399–412. doi: 10.1093/brain/124.2.399. [DOI] [PubMed] [Google Scholar]
- Papez JW. A proposed mechanism of emotion. Archives of Neurology and Psychiatry. 1937;7:103–112. doi: 10.1176/jnp.7.1.103. [DOI] [PubMed] [Google Scholar]
- Park H, Uncapher MR, Rugg MD. Effects of study task on the neural correlates of source encoding. Learning & Memory. 2008;15:417–425. doi: 10.1101/lm.878908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson LR, Peterson MJ. Short-term retention of individual verbal items. Journal of Experimental Psychology. 1959;58:193–198. doi: 10.1037/h0049234. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB. Variations in memory function and sex steroid hormones across the menstrual cycle. Psychoneuroendocrinology. 1992;17:497–506. doi: 10.1016/0306-4530(92)90008-u. [DOI] [PubMed] [Google Scholar]
- Rabin LA, Barr WB, Burton LA. Assessment practices of clinical neuropsychologists in the United States and Canada: A survey of INS, NAN, and APA division 40 members. Archives of Clinical Neuropsychology. 2005;20:33–65. doi: 10.1016/j.acn.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- Reitain RM. Trail making test. Reitan Neuropsychology Laboratory; 1992. [Google Scholar]
- Rundus D. Analysis of rehearsal process in free recall. Journal of Experimental Psychology. 1971;89:63–77. [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery & Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–357. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Tulandi T. “Add-back” estrogen reverses cognitive deficits induced by gonadotropin-releasing hormone agonist in women with leiomyomata uteri. Journal of Clinical Endocrinology and Metabolism. 1996;81:2545–2549. doi: 10.1210/jcem.81.7.8675575. [DOI] [PubMed] [Google Scholar]
- Shipley WC. A self-administering scale for measuring intellectual impairment and deterioration. The Journal of Psychology: Interdisciplinary and Applied. 1940;9:371–377. [Google Scholar]
- Starkman MN, Giordani B, Gebarski SS, Schteingart DE. Improvement in learning associated with increase in hippocampal formation volume. Biological Psychiatry. 2003;53:233–238. doi: 10.1016/s0006-3223(02)01750-x. [DOI] [PubMed] [Google Scholar]
- Strange BA, Otten LJ, Josephs O, Rugg MD, Dolan RJ. Dissociable human perirhinal, hippocampal, and parahippocampal roles during verbal encoding. Journal of Neuroscience. 2002;22:523–528. doi: 10.1523/JNEUROSCI.22-02-00523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderaraman P, Blumen HM, DeMatteo D, Apa Z, Cosentino S. Task demand influences relationships among sex, clustering strategy, and recall: 16-word versus 9-word list learning tests. Cognitive & Behavioral Neurology. 2013;26:78–84. doi: 10.1097/WNN.0b013e31829de450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmi D, Grady CL, Goshen-Gottstein Y, Moscovitch M. Neuroimaging the serial position curve: a test of single-store versus dual-store models. Psychological Science. 2005;16:716–723. doi: 10.1111/j.1467-9280.2005.01601.x. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proceedings of the National Academy of Sciences. 1997;94(26):14792–14794. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votruba KL, Langenecker SA. Age- and Education-based Normative Data for the Parametric Go/No-go Task. Journal of Clinical and Experimental Neuropsychology. 2013;35(2):132–46. doi: 10.1080/13803395.2012.758239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votruba KL, Langenecker SA. Factor structure, construct validity, and age- and education-based normative data for the Parametric Go/No-Go Test. Journal of Clinical and Experimental Neuropsychology. 2013;35:132–146. doi: 10.1080/13803395.2012.758239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Weisenbach SL, Kassel MT, Rao J, Weldon AL, Avery ET, Briceno EM, et al. Differential prefrontal and subcortical circuitry engagement during encoding of semantically related words in patients with late-life depression. International Journal of Geriatric Psychiatry. 2014;29:1104–1115. doi: 10.1002/gps.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenbach SL, Rapport LJ, Briceno EM, Haase BD, Vederman AC, Bieliauskas LA, Welsh RC, Starkman MN, McInnis MG, Zubieta JK, Langenecker SA. Reduced emotion processing efficiency in healthy males relative to females. Social Cognitive and Affective Neuroscience. 2012 doi: 10.1093/scan/nss137. Epub November 29, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winograd E. List differentiation as a function of frequency and retention interval. Journal of Experimental Psychology. 1968;76:1–18. doi: 10.1037/h0025380. [DOI] [PubMed] [Google Scholar]
- Woodard JL, Seidenberg M, Miller SK, Leveroni CL, Nielson KA, Franczak M, Antuono P, Rao SM. Medial temporal lobe activity for recognition of recent and remote famous names: An event-related fMRI study. Neuropsychologia. 2005;43:693–703. doi: 10.1016/j.neuropsychologia.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: Enduring memory impairment following bilateral lesion limited to field CA1 of the hippocampus. Journal of Neuroscience. 1986;6:2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.