Abstract
Background
Coenzyme Q10 has antioxidative and free radical scavenging effects. CoQ10 supplementation is known to have neuroprotective effects in some neurodegenerative diseases, such as Parkinson’s disease and Huntington’s disease.
Objectives
The aim of this study was to evaluate both histopathologic and behavioral whether Coenzyme Q10 is protective against trimethyltin chloride (TMT) induced hippocampal damage.
Materials and Methods
This was an experimental study. Thirty-six Balb/c mice were divided into four groups, as follows: 1) control group; 2) sham group of mice that received a 100 µL intraperitoneal injection (IP) of sesame oil; 3) TMT group of mice that received a single 2.5 mg/kg/day IP injection of TMT; and 4) TMT + CoQ10 group of mice that received a 10 mg/kg IP injection of CoQ10. Body weight and Morris water maze (MWM) responses were investigated. In addition, the dentate gyrus neurons of the hippocampus were evaluated histopathologically by light and electron microscopes.
Results
This study revealed that the body weight scale was found to be significantly higher in the CoQ10 group (21.39 ± 2.70), compared to the TMT group (19.39 ± 2.74) (P < 0.05). In the TMT group, the animals showed body a weight loss that was significantly lower than that of the control group (22.33 ± 3.06) (P < 0.05). Our results showed that CoQ10 provided protection against MWM deficits. Furthermore, TMT impaired the ability of mice to locate the hidden platform, compared to the control group (P < 0.05). Microscopic studies showed that TMT caused histopathological changes in the dentate gyrus and increased the number of necrotic neurons (476 ± 78.51), compared to the control group (208 ± 40.84) (P < 0.001). But, CoQ10 significantly attenuated (31 9 ± 60.08) the density of necrotic neurons compared to TMT (P < 0.05).
Conclusions
The results of the present study indicate that Coenzyme Q10 diminished neuronal necrosis and improved learning memory. Part of its beneficial effect is due to its potential to discount oxidative stress.
Keywords: Trimethyltin Chloride, CoQ10, Dentate Gyrus, Learning and Memory
1. Background
In the central nervous system, hippocampal neurons are highly vulnerable to damage. In rodent models of trimethyltin chloride (TMT) toxicity, both necrotic and apoptotic neurons in the dentate gyrus, CA1, CA3 and hilus of the hippocampus have been observed (1). Thus, trimethyltin-induced hippocampal degeneration is a tool that can be used to investigate neurodegenerative processes (2). TMT is an organotin compound that is used in agricultural and industrial settings. Acute exposure of mice to TMT results in extensive damage to dentate granular cells (3).
TMT toxicity has been proposed to inhibit mitochondrial ATP synthesis, raise Ca2+, and decrease neurotransmitter uptake (4). The mechanisms by which TMT produces selective neuronal degeneration are not understood. However, the action of TMT is affected by a series of molecular events and cellular pathways, such as activation of various kinases (e.g., JNK and PKC) and stress proteins that lead to a cytotoxic response (5). The other mechanisms that can be explained by the neurotoxic actions of TMT include glutamate excitotoxicity, intracellular calcium overload, and enhancement of ROS formation (6). TMT damage of hippocampal neurons increases the rate of corticosterone and learning impairment in rats (7). Kim and Diamond have shown that corticosterone and stress alter hippocampal dendritic morphology and inhibit neurogenesis in the adult brain, which could also have an impact on memory-related functions (8). However, cell death and many neurological deficits can be reduced by antioxidants. Coenzyme Q10 is considered as a neuroprotective agent that prevents the cascade of cell death events in order to maintain cellular integration and restore neuronal function. Recently, Li et al. reported that CoQ10 plays a critical role as an intrinsic free radical-scavenging and antioxidant enzyme that acts against the H2O2-induced oxidative damage in PC12 cells (9). Furthermore, CoQ10 is a key component of the mitochondrial respiratory chain, and it has a fundamental role in oxidative phosphorylation (10). Against the toxicant agent cisplatin, CoQ10 significantly compensated deficits in the antioxidant defense mechanisms (reduced glutathione level and superoxide dismutase activity), decreased the elevations of TNF-α, and suppressed lipid peroxidation (11). CoQ10 treatment also reduced the activity of P53 and caspase-3 which may be due to its free radical-scavenging activity, anti-inflammatory performance, and attenuation of expression of NF-kappa B (11). Ishrat et al. demonstrated that supplementation with CoQ10 (10 mg/kg) may have an important therapeutic effect in the treatment of Alzheimer’s type dementia (12). Another research study found that there are neuroprotective effects of CoQ10 against the loss of dopamine and tyrosine hydroxylase in the dopaminergic neurons of the substantia nigra (13). In experimental models of Alzehimer’s disease and Parkinson’s disease, CoQ10 has been reported to improve cognitive deficits by improving electron transport from complex-I to III in the mitochondrial membrane (14). Furthermore, CoQ10 has been shown to pay a key role in the mitochondrial respiratory chain that enhances the production of ATP (15).
2. Objectives
The present investigation aimed to evaluate the antioxidant and protective efficacies of CoQ10 against TMT induced neurotoxicity by evaluation of spatial memory and histological values in the dentate gyrus of the hippocampus.
3. Materials and Methods
Thirty-six, 8-week-old adult male Balb/c mice (18 - 21 grams) purchased from the Pharmacology Department of Tehran University of Medical Science were used for the present experiment. The animals were housed under controlled conditions (12 hours light/dark cycle at 21 - 23°C) with access to food and water ad libitum. All the procedures used in this study were approved by the committee of ethics in animal research (Code: 90-4-11-12500, date: 17/9/1391) of the Iran University of Medical Sciences (Tehran, Iran). Sample size determination was conducted in accordance with the results of the study of Ishrat et al. (12), by considering the mean ± SD of the two groups as 58.26 ± 6.5 and 48.42 ± 3.62, respectively, and, according to the power of study equal to 80% (1-β = 0.80) and α = 0.05, the minimum sample size of each group was calculated as 5. The used formula is presented below (Equation 1):
| Equation 1. |
The animals were randomly divided into the following four groups: 1) control group (n = 9) in which the mice were given an IP injection of normal saline (vehicle of TMT); 2) sesame oil group (n = 9) in which the mice were given an IP injection of TMT and sesame oil (Sigma, La Jolla, CA, USA ) for two weeks (vehicle of CoQ10); 3) TMT group (n = 9) in which the mice received a single IP injection of TMT (Catalogue number 808729; Merck, Darmstadt, Germany); and 4) TMT + CoQ10 group (n = 9) in which the mice were given a single IP dose (10 mg/kg body weight) of TMT and CoQ10 (Sigma-Aldrich, St. Louis, MO, USA) dissolved in sesame oil for two weeks.
3.1. Body Weight Assessment
In toxicological studies, the evaluation of body weight and organs is one of the most important procedures (16). In the present study, after TMT injection, the body weight of each mouse was recorded daily with a precision digital scale.
3.2. Morris Water Maze Performance
At the end of the treatment with CoQ10, the animals were examined using the Morris water maze task for assessment of learning and spatial memory. The apparatus consisted of a water tank 110 cm in diameter filled with water to within 35 cm of the top. A platform with an 11 cm top surface was placed in one of the quadrants, and the top surface of the platform was submerged about 1.5 cm below the surface of the water. The animals were trained to escape from water by swimming to the hidden platform. An animal could find the platform, which was under the water and served as a “rescue” from the stress situation, by using visual extra-maze cues. The animals were subjected to 4 days of hidden platform trials (four trials of 60 seconds each).
Each training trial was started randomly in one of four selected locations corresponding to each quadrant of the tank. On 5th day, the time spent and latency to reach the platform were compared among all groups across the trial days. For visual platform trials, a video camera (Nikon, Melville, NY, USA) linked to a computer was used to monitor the water maze tank and record both the time taken to reach the hidden platform (escape latency) and the length of the swim path (traveled distance) for each mouse. All trials were analyzed with EthoVision (Noldus Information Technology, Leesbury, Virginia, USA) software.
3.3. Tissue Preparation and Histological Study
At the end of the experiment, for histological assessment of the dentate gyrus, all animals were anesthetized under IP injection of ketamine/xylazine (60 mg/kg and 6 mg/kg, respectively). Next, the animals were perfused transcardially with saline solution, followed by 110 mL of phosphate-buffered saline (0.1 M), pH 7.4, and 4% paraformaldehyde. Then, the brains were removed from the skulls and post-fixed in 10% formalin and embedded in paraffin. Coronal brain sections (5-μm-thick) were cut on a microtome (Leica Microsystems, Wetzlar, Germany) from -2.2, -3.4 mm posterior to the bregma (17). Subsequently, sections were deparaffinized, rehydrated in graded alcohols, and stained by 0.1 % cresyl violet solution (Sigma-Aldrich, St. Louis, MO, USA). The sections were examined under a light microscope (Olympus Ax70, Tokyo, Japan) using at magnification of 400x. The average number of necrotic neurons was determined by stereological methods from sections taken from the dentate gyrus of the hippocampus. A1000 μm2 counting frame was used. The mean number of necrotic neurons per unit area (NA) in the dentate gyrus was calculated using the following formula (Equation 2):
| Equation 2. |
∑p = the sum of counted particle in sections.
a/f = the area associated with each frame.
∑Q = the sum of frame associated points hitting space.
3.4. Electron Microscopy
After removal of the hippocampal tissues samples, they were immersed in 2.5% glutaraldehyde fixative and then transferred to a post-fixative agent for 24 hours. The sections were trimmed to produce a dentate gyrus (DG) block of tissue. After the specimens were washed in PBS, they were post-fixed in 1% osmium tetroxide. Next, they were dehydrated in ascending graded ethanol, embedded in Epon 812 resin (TAAB, Berkshire, UK), and polymerized at 55°C. After that, semi-thin sections (0.3 μm) were stained with toluidine blue to accurately identify the DG region. Subsequently, ultra-thin sections (70 nm) were cut on an ultra-microtome (Leica Ultracut; Leica, Bensheim, Germany), placed on copper grids, and then stained with uranyl acetate and 1% lead citrate. The sections were studied with a transmission electron microscope (LEO 906; LEO, Oberkochen, Germany) for characteristics of apoptosis that included chromatin condensation, decrease of the crista of the mitochondria, and cytoplasm vacuolization.
3.5. Statistical Analysis
The body weight measurement data analyses between groups were performed by using the one-way analysis of variance (ANOVA) bootstrap method, followed by Tukey’s post hoc test. Data obtained over the training days from the MWM test were analyzed by the ANOVA bootstrap method and followed by Tukey’s test for multiple comparison. The differences between the mean numbers of necrotic neurons in the DG were also determined by the ANOVA bootstrap method. In all calculations, the significance level was set at P < 0.05.
4. Results
4.1. Results of Body Weight Measurement
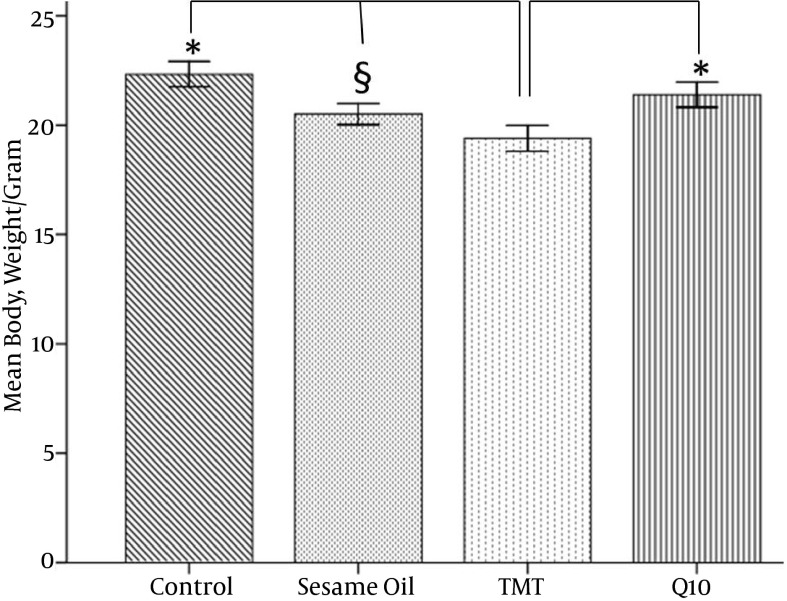
Effects of CoQ10 administration on body weight were evaluated and summarized in Figure 1. CoQCoQ10 treatment was effective in ameliorating weight loss as compared to the TMT group (P < 0.05). In the TMT group, the body weight by 5 days after the injection had significantly decreased. In addition, there were no significant differences between the body weight of the CoQ10 and sesame oil groups.
Figure 1. Mean Body Weight of Animals After TMT Toxicity.
In the CoQ10 treated group, retention of weight significantly improved compared to the TMT group *(P < 0.05).The mean body weight of the TMT group significantly decreased compared to the control §(P < 0.01).
4.2. Results of Morris Water Maze Performance
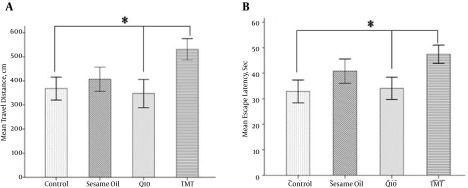
The changes in learning and memory were assessed by using the MWM test onto a hidden platform over 4 days of training. In the TMT group, mean travel distance (Figure 2A, Table 1) was significantly prolonged as compared to the CoQ10 and control groups (P < 0.05), showing a failure in learning performance due to TMT toxicity. This poorer function of the TMT group was significantly improved with CoQ10 treatment in the CoQ10 group (P < 0.05). The results of the MWM test concerning escape latency are indicated in Figure 2B and Table 1. CoQ10 administration caused a significant decrease in escape latency compared to the TMT group (P < 0.05). The TMT treated group showed a significant increase in the escape latency compared to the control (P < 0.05). The group treated with sesame oil alone showed an insignificant decrease in the escape latency compared to the CoQ10 group.
Figure 2. Morris Water Maze Learning in Adult Animals.
A, mean travel distance (cm) to reach the platform during 4 days of training; B, mean escape latency (sec) to reach the hidden platform. *P < 0.05 and n = 7 for each group.
Table 1. Effect of CoQ10 on the Weight, Spatial Memory, and Number of Necrotic Neurons in a Model of Hippocampal Injurya.
| Variablesb | Control (CI 95%) | Sesame Oil (CI 95%) | TMT (CI 95%) | CoQ10 (CI 95%) | Bootstrap Method | P Value (Tukey’s Post Hoc Test) |
|---|---|---|---|---|---|---|
| Weight, g | ||||||
| 22.34 ± 3.08 | 20.50 ± 2.56 | 19.37 ± 2.74 | 21.56 ± 2.37 | Control vs Sesame oil | 0.000 | |
| 21.77 - 22.86 | 20.04 - 20.98 | 18.82 - 19.95 | 21.4 - 22.09 | Control vs TMT | 0.000 | |
| Control vs CoQ10 | 0.205 | |||||
| Sesame oil vs TMT | 0.020 | |||||
| Sesame oil vs CoQ10 | 0.037 | |||||
| TMT vs CoQ10 | 0.000 | |||||
| Escape latency, s | ||||||
| 32.84 ± 9.56 | 40.79 ± 10.23 | 47.43 ± 9.26 | 34.06 ± 10.36 | Control vs Sesame oil | 0.003 | |
| 29.53 - 36.25 | 37.55 - 43.57 | 44.45 - 50.27 | 30.81 - 37.64 | Control vs TMT | 0.000 | |
| Control vs CoQ10 | 0.928 | |||||
| Sesame oil vs. TMT | 0.018 | |||||
| Sesame oil vs CoQ10 | 0.020 | |||||
| TMT vs. Q10 | 0.001 | |||||
| Travel distance, cm | ||||||
| 368.07 ± 95.68 | 406.92 ± 107.1 | 530.86 ± 103.7 | 347.37 ± 121.1 | Control vs. Sesame oil | 0.359 | |
| 337.58 - 398.25 | 388.81 - 436.41 | 494.09 - 565.14 | 310.01 - 389.24 | Control vs TMT | 0.000 | |
| Control vs CoQ10 | 0.835 | |||||
| Sesame oil vs TMT | 0.000 | |||||
| Sesame oil vs CoQ10 | 0.070 | |||||
| TMT vs CoQ10 | 0.000 | |||||
| Number of necrotic neurons | ||||||
| 208 ± 40.84 | 417 ± 81.7 | 476 ± 78.51 | 319 ± 60.08 | Control vs Sesame oil | 0.000 | |
| 183.33 - 231.24 | 372.05 - 461.74 | 425.19 - 528.25 | 283.62 - 353.79 | Control vs TMT | 0.001 | |
| Control vs CoQ10 | 0.044 | |||||
| Sesame oil vs TMT | 0.448 | |||||
| Sesame oil vs Co Q10 | 0.085 | |||||
| TMT vs CoQ10 | 0.003 |
aValues are expressed as mean ± SD.
bCI denotes upper and lower boundary of the confidence interval.
4.3. Results of Microscopic Studies
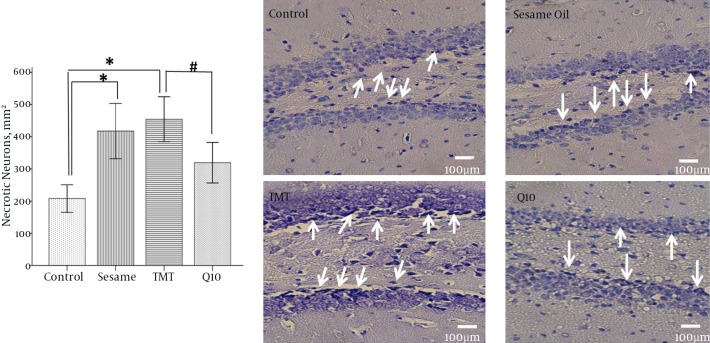
In this study, the number of necrotic neurons in the granular layer of the DG of the hippocampus were counted and compared among groups (Figure 3, Table 1). T results showed that TMT induced a dramatic and significant degeneration of neurons in the DG of the TMT group versus the control group (P < 0.001). In this regard, the neurodegeneration in the hippocampus was typified by an apparent dark nucleus in the DG of the TMT injured model. CoQ10 treatment showed a significant decrease in the number of necrotic neurons as compared to the TMT group (P < 0.05).
Figure 3. Photomicrograph of Coronal Sections Through the Upper and Lower Blade of Dentate Gyrus by Nissl Staining (Right Panel).
Number of Nissl stained necrotic neurons in dentate gyrus of the hippocampus (left panel). *P < 0.001, P < 0.05.
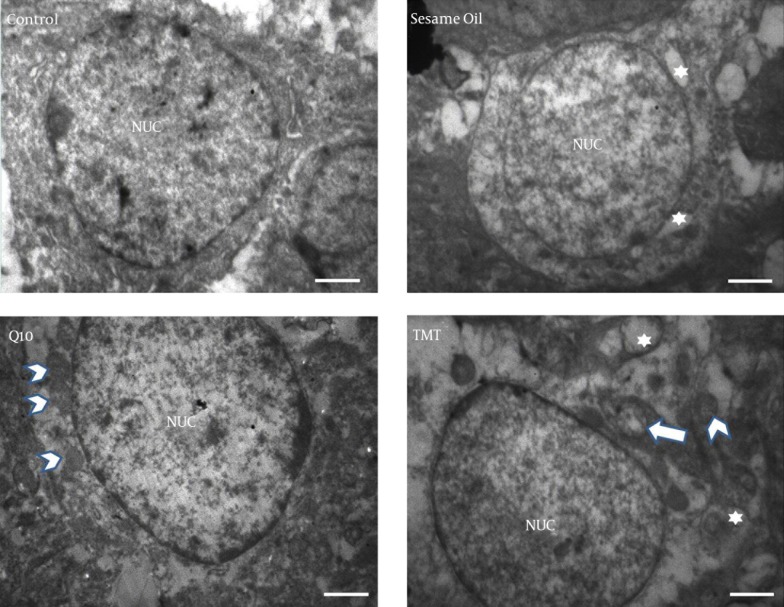
Electron microscopy findings showed that, in the control group, the granular cells of the DG had oval nuclei with homogenate dispersed chromatin and that the nuclear membrane was intact (Figure 4). In the injured neurons of the TMT group, it was observed that several vacuoles in the cytoplasm and crista of the mitochondria were reduced. In the sesame oil group, there were some vacuoles in the cytoplasm but they were decreased in comparison to those in the TMT group. In the CoQ10 group, these changes were fewer, and more intact neurons in comparison with the TMT group were observed.
Figure 4. TEM Electron Microscopy of a Typical Granular Neuron of Dentate Gyrus.
In the TMT group there are some vacuoles in the cytoplasm (white star), and the crista of the mitochondria are reduced (arrow). The arrow shows intact mitochondria. In the CoQ10 group, there were no vacuoles. Scale bar equals 600 nm.
5. Discussion
This study showed that the TMT group exhibited significantly less weight loss compared to the control and CoQ10 groups. TMT induced transient decreases in water and food intake with a concomitant decrease in body weight (18). In a recent study, it was reported that treatment with a dimethyltin (DMT) organotin compound in high doses, in both male and female rats, caused damage to the digestive tract and liver and caused stomach distention (19). Following an oral organolead dose, dilation of the gastric mucosal microcirculation and desquamation of surface mucous cells were observed, which resulted in erosion of the gastric glands. Starvation and dehydration may be important factors related to body weight loss and death as effects of organotin and organolead compounds (20).
We also examined whether CoQ10 treatment was effective in ameliorating weight loss with the TMT group (In In the study of Schilling et al., their findings showed increases of body weight in Huntington’s transgenic mice that received CoQ10 + remacemide (21). CoQ10 treatment increased the body weight of diabetic mice toward that of the normal control group (22). CoQ10 has been reported to improve mitochondrial function and facilitate the synthesis of ATP (23). Therefore, CoQ10 may improve energy supplementation and prevent body weight loss. We also assessed the efficiency of CoQ10 in aiding mice in the location of the hidden platform by measuring the average length of swimming time and the distance needed to arrive at the platform over the four trials of each session. The CoQ10 group had a shorter path length as a function of the sessions during the learning phase, and their performance improved during this phase. Also, it has been shown that treatment with CoQ10 for three weeks in streptozotocin infused rats significantly improved the learning and memory deficits (12). This may be attributed to the ability of CoQ10 to up-regulate the mitochondrial functions for ATP synthesis. Another study suggested that CoQ10 improved spatial learning and attenuated oxidative damage when administered in relatively high doses and also delayed early senescence (24). Therefore, CoQ10, through its antioxidant effects, could improve the cognitive deficit that occurs with TMT intoxication.
The results of our study showed that TMT increased the distance the animals needed to reach hidden platform. In agreement with our result, the study of Earley et al. revealed a significant impairment of escape latency in TMT treated rats (25). The study of Mignini on TMT induced neurodegeneration highlights the relationship between hippocampal dopamine receptors and transporters. He suggested that TMT neurotoxicity reduced the expression of dopaminergic markers in the hippocampus and impaired performance of rats in spatial memory (26). The study of Ishikawa presented the idea that TMT induced histological changes with reduction of intrahippocampal concentrations of acetylcholine and glutamate (27). TMT has also been shown to induce glutamate excitotoxicity, intracellular calcium overload, and impairment of neurotransmission (28). Calcium overload generates oxidative stress and causes neurodegeneration associated with necrosis (29). In a study on streptozotocine-induced diabetic mice, it was shown that disturbed Ca2+ homeostasis adversely affected behavioral performance of animals in learning and memory (30).
The results of this study also revealed the significant increase of the number of necrotic neurons in the TMT and sesame oil groups. TMT induced chromatin condensation in hippocampal granular neurons is one of the most characteristic features of apoptosis (31). Geloso has reported that TMT increases the necrotic neurons in the brain of experimental animals (29). The mechanism of neuronal death induced by TMT has been linked to inflammation, necrosis, and apoptosis (31). The TMT produced neuronal loss in the hippocampus of rodents is accompanied by behavioral alterations. In addition, administration of TMT, in the granular cell layer of the DG with the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining procedure detects DNA fragmentation and apoptotic bodies (29). In this study, we also observed that treatment with CoQ10 decreased DG necrotic neuronal cells. It is now established that CoQ10 has significant neuroprotection properties and that it may protect neurons against neuronal damage in neurodegenerative disorders (32). The results of the study of Li et al. showed that water-soluble CoQ10 prevents the accumulation reactive oxygen species, improves the mitochondrial membrane potential, inhibits apoptosis induced factors, and, finally, reduces cell death (33). Pretreatment with CoQ10 (300 mg/kg for 5 days) in a model of spinal cord contusion improved neurological functions and retention of normal motor neurons (34). CoQ10 also dose-dependently preserved the hippocampal catalase, glutathione peroxidase, and hippocampal malondialdehyde, which indicated the potential of the protective effects of CoQ10 against hippocampal lipid peroxidation and DNA damage (35). There is also evidence that CoQ10 protected neural stem cells against hypoxia-reperfusion by inhibiting free radical formation. Treatment with CoQ10 increased the viability of primary cultured cortical neurons exposed to amyloid beta by inhibition of a specific inhibitor of the PI3K pathway (36).
In conclusion, our study demonstrated that treatment with CoQ10 reduces hippocampal injuries and improves cognitive deficits. We suggest that CoQ10 might be a supplementary therapeutic strategy for neurotoxicity agents, although more research is necessary to take into account all the potential therapeutic effects of CoQ10.
Acknowledgments
We wish to thank the vice-chancellor of research of Iran University of Medical Science, M.C., for financial support.
References
- 1.Harry GJ, Lefebvre d'Hellencourt C. Dentate gyrus: alterations that occur with hippocampal injury. Neurotoxicology. 2003;24(3):343–56. doi: 10.1016/S0161-813X(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 2.Geloso MC, Corvino V, Michetti F. Trimethyltin-induced hippocampal degeneration as a tool to investigate neurodegenerative processes. Neurochem Int. 2011;58(7):729–38. doi: 10.1016/j.neuint.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Reuhl KR, Smallridge EA, Chang LW, Mackenzie BA. Developmental effects of trimethyltin intoxication in the neonatal mouse. I. Light microscopic studies. Neurotoxicology. 1983;4(1):19–28. [PubMed] [Google Scholar]
- 4.Mailman RB, Krigman MR, Frye GD, Hanin I. Effects of postnatal trimethyltin or triethyltin treatment on CNS catecholamine, GABA, and acetylcholine systems in the rat. J Neurochem. 1983;40(5):1423–9. doi: 10.1111/j.1471-4159.1983.tb13585.x. [DOI] [PubMed] [Google Scholar]
- 5.Kassed CA, Butler TL, Patton GW, Demesquita DD, Navidomskis MT, Memet S, et al. Injury-induced NF-kappaB activation in the hippocampus: implications for neuronal survival. FASEB J. 2004;18(6):723–4. doi: 10.1096/fj.03-0773fje. [DOI] [PubMed] [Google Scholar]
- 6.Florea AM, Splettstoesser F, Dopp E, Rettenmeier AW, Busselberg D. Modulation of intracellular calcium homeostasis by trimethyltin chloride in human tumour cells: neuroblastoma SY5Y and cervix adenocarcinoma HeLa S3. Toxicology. 2005;216(1):1–8. doi: 10.1016/j.tox.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 7.Tsutsumi S, Akaike M, Arimitsu H, Imai H, Kato N. Circulating corticosterone alters the rate of neuropathological and behavioral changes induced by trimethyltin in rats. Exp Neurol. 2002;173(1):86–94. doi: 10.1006/exnr.2001.7824. [DOI] [PubMed] [Google Scholar]
- 8.Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3(6):453–62. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Du J, Lian Y, Zhang Y, Li X, Liu Y, et al. Protective Effects of Coenzyme Q10 Against Hydrogen Peroxide-Induced Oxidative Stress in PC12 Cell: The Role of Nrf2 and Antioxidant Enzymes. Cell Mol Neurobiol. 2016;36(1):103–11. doi: 10.1007/s10571-015-0224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sattarinezhad E, Shafaroodi H, Sheikhnouri K, Mousavi Z, Moezi L. The effects of coenzyme Q10 on seizures in mice: the involvement of nitric oxide. Epilepsy Behav. 2014;37:36–42. doi: 10.1016/j.yebeh.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 11.Fouad AA, Al-Sultan AI, Refaie SM, Yacoubi MT. Coenzyme Q10 treatment ameliorates acute cisplatin nephrotoxicity in mice. Toxicology. 2010;274(1-3):49–56. doi: 10.1016/j.tox.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Ishrat T, Khan MB, Hoda MN, Yousuf S, Ahmad M, Ansari MA, et al. Coenzyme Q10 modulates cognitive impairment against intracerebroventricular injection of streptozotocin in rats. Behav Brain Res. 2006;171(1):9–16. doi: 10.1016/j.bbr.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Cleren C, Yang L, Lorenzo B, Calingasan NY, Schomer A, Sireci A, et al. Therapeutic effects of coenzyme Q10 (CoQ10) and reduced CoQ10 in the MPTP model of Parkinsonism. J Neurochem. 2008;104(6):1613–21. doi: 10.1111/j.1471-4159.2007.05097.x. [DOI] [PubMed] [Google Scholar]
- 14.Sandhir R, Sethi N, Aggarwal A, Khera A. Coenzyme Q10 treatment ameliorates cognitive deficits by modulating mitochondrial functions in surgically induced menopause. Neurochem Int. 2014;74:16–23. doi: 10.1016/j.neuint.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Littarru GP, Tiano L. Bioenergetic and antioxidant properties of coenzyme Q10: recent developments. Mol Biotechnol. 2007;37(1):31–7. doi: 10.1007/s12033-007-0052-y. [DOI] [PubMed] [Google Scholar]
- 16.Mastropaolo JP, Dacanay RJ, Luna BH, Tuck DL, Riley AL. Effects of trimethyltin chloride on differential-reinforcement-of-low-rate responding. Neurobehav Toxicol Teratol. 1984;6(3):193–9. [PubMed] [Google Scholar]
- 17.Franklin KBJ, Paxinos G. Mouse brain in stereotaxic coordinates. Academic press; 1997. [Google Scholar]
- 18.Morita Y, Yanagida D, Shintani N, Ogita K, Nishiyama N, Tsuchida R, et al. Lack of trimethyltin (TMT)-induced elevation of plasma corticosterone in PACAP-deficient mice. Ann N Y Acad Sci. 2006;1070:450–6. doi: 10.1196/annals.1317.060. [DOI] [PubMed] [Google Scholar]
- 19.Tang X, Wu X, Dubois AM, Sui G, Wu B, Lai G, et al. Toxicity of trimethyltin and dimethyltin in rats and mice. Bull Environ Contam Toxicol. 2013;90(5):626–33. doi: 10.1007/s00128-013-0975-x. [DOI] [PubMed] [Google Scholar]
- 20.Verschoyle RD, Little RA. The acute toxicity of some organolead and organotin compounds in the rat, with particular reference to a gastric lesion. J Appl Toxicol. 1981;1(5):247–55. doi: 10.1002/jat.2550010503. [DOI] [PubMed] [Google Scholar]
- 21.Schilling G, Coonfield ML, Ross CA, Borchelt DR. Coenzyme Q10 and remacemide hydrochloride ameliorate motor deficits in a Huntington's disease transgenic mouse model. Neurosci Lett. 2001;315(3):149–53. doi: 10.1016/s0304-3940(01)02326-6. [DOI] [PubMed] [Google Scholar]
- 22.Ates O, Cayli S, Altinoz E, Gurses I, Yucel N, Sener M, et al. Neuroprotection by resveratrol against traumatic brain injury in rats. Mol Cell Biochem. 2007;294(1-2):137–44. doi: 10.1007/s11010-006-9253-0. [DOI] [PubMed] [Google Scholar]
- 23.McDonald SR, Sohal RS, Forster MJ. Concurrent administration of coenzyme Q10 and alpha-tocopherol improves learning in aged mice. Free Radic Biol Med. 2005;38(6):729–36. doi: 10.1016/j.freeradbiomed.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Papucci L, Schiavone N, Witort E, Donnini M, Lapucci A, Tempestini A, et al. Coenzyme q10 prevents apoptosis by inhibiting mitochondrial depolarization independently of its free radical scavenging property. J Biol Chem. 2003;278(30):28220–8. doi: 10.1074/jbc.M302297200. [DOI] [PubMed] [Google Scholar]
- 25.Earley B, Burke M, Leonard BE. Behavioural, biochemical and histological effects of trimethyltin (TMT) induced brain damage in the rat. Neurochem Int. 1992;21(3):351–66. doi: 10.1016/0197-0186(92)90186-u. [DOI] [PubMed] [Google Scholar]
- 26.Mignini F, Nasuti C, Artico M, Giovannetti F, Fabrizi C, Fumagalli L, et al. Effects of trimethyltin on hippocampal dopaminergic markers and cognitive behaviour. Int J Immunopathol Pharmacol. 2012;25(4):1107–19. doi: 10.1177/039463201202500428. [DOI] [PubMed] [Google Scholar]
- 27.Ishikawa K, Kubo T, Shibanoki S, Matsumoto A, Hata H, Asai S. Hippocampal degeneration inducing impairment of learning in rats: model of dementia? Behav Brain Res. 1997;83(1-2):39–44. doi: 10.1016/s0166-4328(97)86043-3. [DOI] [PubMed] [Google Scholar]
- 28.Florea AM, Dopp E, Busselberg D. Elevated Ca2+(i) transients induced by trimethyltin chloride in HeLa cells: types and levels of response. Cell Calcium. 2005;37(3):251–8. doi: 10.1016/j.ceca.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Geloso MC, Vercelli A, Corvino V, Repici M, Boca M, Haglid K, et al. Cyclooxygenase-2 and caspase 3 expression in trimethyltin-induced apoptosis in the mouse hippocampus. Exp Neurol. 2002;175(1):152–60. doi: 10.1006/exnr.2002.7866. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Li L, Prabhakaran K, Borowitz JL, Isom GE. Trimethyltin-induced apoptosis is associated with upregulation of inducible nitric oxide synthase and Bax in a hippocampal cell line. Toxicol Appl Pharmacol. 2006;216(1):34–43. doi: 10.1016/j.taap.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Fiedorowicz A, Figiel I, Kaminska B, Zaremba M, Wilk S, Oderfeld-Nowak B. Dentate granule neuron apoptosis and glia activation in murine hippocampus induced by trimethyltin exposure. Brain Res. 2001;912(2):116–27. doi: 10.1016/s0006-8993(01)02675-0. [DOI] [PubMed] [Google Scholar]
- 32.Tawfik MK. Coenzyme Q10 enhances the anticonvulsant effect of phenytoin in pilocarpine-induced seizures in rats and ameliorates phenytoin-induced cognitive impairment and oxidative stress. Epilepsy Behav. 2011;22(4):671–7. doi: 10.1016/j.yebeh.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 33.Li H, Chen G, Ma W, Li PA. Water-soluble coenzyme q10 inhibits nuclear translocation of apoptosis inducing factor and cell death caused by mitochondrial complex I inhibition. Int J Mol Sci. 2014;15(8):13388–400. doi: 10.3390/ijms150813388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hwang JY, Min SW, Jeon YT, Hwang JW, Park SH, Kim JH, et al. Effect of coenzyme Q(1)(0) on spinal cord ischemia-reperfusion injury. J Neurosurg Spine. 2015;22(4):432–8. doi: 10.3171/2014.12.SPINE14487. [DOI] [PubMed] [Google Scholar]
- 35.Aboul-Fotouh S. Coenzyme Q10 displays antidepressant-like activity with reduction of hippocampal oxidative/nitrosative DNA damage in chronically stressed rats. Pharmacol Biochem Behav. 2013;104:105–12. doi: 10.1016/j.pbb.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 36.Choi H, Park HH, Koh SH, Choi NY, Yu HJ, Park J, et al. Coenzyme Q10 protects against amyloid beta-induced neuronal cell death by inhibiting oxidative stress and activating the P13K pathway. Neurotoxicology. 2012;33(1):85–90. doi: 10.1016/j.neuro.2011.12.005. [DOI] [PubMed] [Google Scholar]