Abstract
Elevated NaCl concentrations of the cerebrospinal fluid (CSF) increase sympathetic nerve activity (SNA) in salt-sensitive hypertension. Neurons of the rostral ventrolateral medulla (RVLM) play a pivotal role in the regulation of SNA and receive mono- or poly-synaptic inputs from several hypothalamic structures responsive to hypernatremia. Therefore, the present study investigated the contribution of RVLM neurons to the SNA and pressor response to CSF hypernatremia. Lateral ventricle infusion of 0.15M, 0.6M, and 1.0M NaCl (5µL/10 min) produced concentration-dependent increases in lumbar SNA, adrenal SNA, and arterial blood pressure (ABP) despite no change in splanchnic SNA and a decrease in renal SNA. Ganglionic blockade with chlorisondamine or acute lesion of the lamina terminalis blocked or significantly attenuated these responses, respectively. RVLM microinjection of the GABAA agonist muscimol abolished the sympathoexcitatory response to ICV infusion of 1M NaCl. Furthermore, blockade of ionotropic glutamate, but not angiotensin II type 1, receptors significantly attenuated the increase in lumbar SNA, adrenal SNA, and ABP. Finally, single-unit recordings of spinally-projecting RVLM neurons revealed three distinct populations based on discharge responses to ICV infusion of 1M NaCl: Type I excited (46%, 11/24), Type II inhibited (37%, 9/24), and Type III no change (17%, 4/24). All neurons with slow conduction velocities were Type I cells. Collectively, these findings suggest that acute increases in CSF NaCl concentrations selectively activate a discrete population of RVLM neurons through glutamate receptor activation to increase SNA and ABP.
Keywords: sodium, blood pressure, salt-sensitive hypertension, cerebrospinal fluid, medulla, hypothalamus
INTRODUCTION
Salt-sensitive hypertension is mediated, in part, by an increase in cerebrospinal fluid (CSF) [NaCl] and elevated sympathetic nerve activity (SNA) 1, 2. Experimental models of salt-sensitive hypertension such as the Dahl-salt-sensitive and Spontaneously Hypertensive (SHR) rat are associated with significant increases in CSF [Na+] during high salt diet 3-5. The increase in CSF [Na+] parallels or even precedes the increase in ABP. To our knowledge, only one study is available in salt-sensitive humans and has reported that a chronic high salt diet increased CSF [Na+] and ABP6. Importantly, these changes in CSF [Na+] do not occur in salt-resistant counterparts 3, 4, 6. However, it is not known whether CSF [Na+] of salt-sensitive subjects fluctuate from meal to meal or vary across the circadian cycle. Consistent with the above notion, acute or chronic intracerebroventricular (ICV) infusion of hypertonic NaCl increases ABP in rodents 7-11. Such responses are enhanced by a high salt diet 12 or exaggerated in salt-sensitive strains such as the Dahl-salt-sensitive rat 7, 8. Lastly, lesion or interruption of neurotransmission in various hypothalamic structures including the lamina terminalis and hypothalamic paraventricular nucleus (PVH) attenuate the increase in ABP produced by acute or chronic central infusions of hypertonic NaCl 12-14 and also antagonize the development of salt-sensitive hypertension in several experimental models 15-19. These hypothalamic circuits raise SNA and ABP through a pathway that involves the epithelial sodium channel and ouabain signaling 1, 2.
Despite evidence for a central NaCl-driven increase in SNA and ABP initiated by the forebrain hypothalamus, the downstream circuitry and signaling mechanisms are unknown. Antunes and colleagues 20 reported that acute increases in circulating NaCl concentrations activate a spinal vasopressinergic pathway originating in the PVH. In contrast, salt-sensitive hypertension including the Dahl-salt-sensitive rat depends on neurotransmission in the PVH and rostral ventrolateral medulla (RVLM) 19, 21. RVLM neurons play a pivotal role in the regulation of SNA and ABP during various physiological and pathophysiological conditions 22. These neurons are barosensitive, tonically-active and support basal SNA through direct projections to preganglionic neurons of the intermediolateral cell column. Moreover, RVLM neurons receive direct mono- or polysynaptic inputs from several hypothalamic structures activated by hypernatremia 1, 2, 22-27. Therefore, these observations prompted us to perform a series of experiments to address the extent by which RVLM neurons mediate changes in ABP and SNA to various end organs during acute increases in CSF NaCl concentration.
METHODS
All of the experimental procedures conform to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the Pennsylvania State College of Medicine. All experiments were performed in male Sprague-Dawley rats (250-400 g, Charles River Laboratories), anesthetized with Inactin (120mg/kg, IV), and prepared for simultaneous recordings of ABP and SNA (lumbar, renal, splanchnic, and adrenal) as described previously 12, 28, 29. A brain cannula was implanted into the lateral ventricle 30, 31 for ICV infusion of aCSF, 0.6M NaCl, or 1.0M NaCl (5µL/10 min). The vasopressin antagonist Manning compound (10µg/kg, IV) was also administered prior to ICV infusions to eliminate the contribution of vasopressin to the NaCl-induced responses. In preliminary experiments, vasopressin receptor blockade attenuated the pressor response by 2±1 mmHg but did not alter SNA responses to ICV infusion of 1M NaCl. A detailed methods section is available in the online supplement (www.hyper.ahajournals.org).
RESULTS
There were no differences in baseline mean ABP or heart rate for the various experimental groups (see Table S1 at www.hyper.ahajournals.org).
Central Components of the NaCl-Induced Pressor Response
Experiment 1
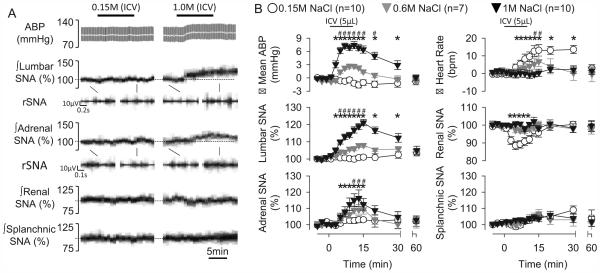
Initial experiments were performed to determine how changes in CSF NaCl concentration altered SNA to various end-organs. ICV infusion of 0.15M, 0.6M, and 1.0M NaCl produced concentration-dependent increases in lumbar SNA, adrenal SNA, heart rate, and mean ABP (Figure 1). However, the increase in adrenal SNA and heart rate was delayed and increased ~8-10 min after start of the ICV infusion. ICV infusion of 1.0M NaCl decreased renal SNA but did not alter splanchnic SNA. All variables returned to baseline values within 60 min (Figure 1).
Figure 1.
(A) Arterial blood pressure (ABP), mean ABP (grey line), and lumbar, adrenal, renal, and splanchnic SNA during ICV infusion of aCSF or 1M NaCl (5µL per 10 min). (B) Summary data presented as mean±SEM. *P<0.05 1M NaCl vs aCSF, #P<0.05, 0.6M vs 1.0M NaCl
Experiment 2
In a separate group of animals, CSF was collected from the 4th ventricle to measure changes in Na+ and Cl- during infusion of aCSF or 1.0M NaCl. Infusion of 1.0M increased 4th ventricular CSF [Na+] (baseline: 155.3±0.3mM vs 15 min: 159.0±1.6mM; n=6, P<0.05) and [Cl−] (baseline: 116.4±0.5mM vs 15 min: 118.6±0.7mM; n=6, P<0.05). Infusion of 0.15M CSF did not alter CSF [Na+] (baseline: 155.7±0.3mM vs 15 min: 155.0±0.6mM; n=6, P<0.05) and [Cl−] (baseline: 116.7±0.3mM vs 15 min: 117.0±0.2mM; n=6, P<0.05).
Experiment 3
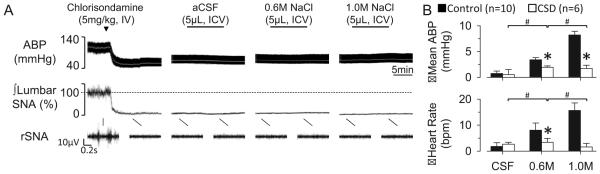
To assess the contribution of elevated SNA to the NaCl-induced pressor responses, ICV infusions were performed after ganglionic blockade. IV injection of chlorisondamine promptly decreased baseline SNA and mean ABP and abolished the SNA, tachycardic, and pressor response to 0.6M and 1.0M NaCl (Figure 2).
Figure 2.
(A) ABP, mean ABP (grey line), and lumbar SNA during ICV infusion of aCSF, 0.6M, and 1.0M NaCl after treatment with the ganglionic blocker chlorisondamine. (B) Mean±SEM peak changes in mean ABP and heart rate. *P<0.05 vs control group, #P<0.05 within treatment group
Experiment 4
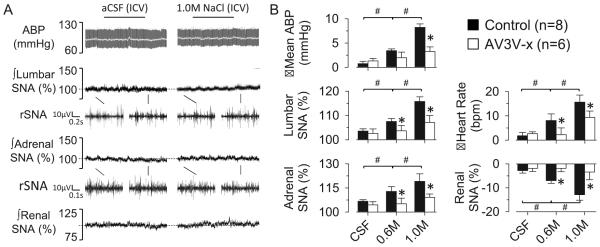
To demonstrate these SNA responses depend on the lamina terminalis, ICV infusions were performed in a fourth set of animals with acute electrolytic lesion of the AV3V. AV3V lesions significantly attenuated the increase in lumbar SNA, adrenal SNA, mean ABP, and heart rate to infusion of 0.6M and 1.0M NaCl (Figure 3, see Figure S2 for histology). The lesion also significantly attenuated the renal sympathoinhibitory response to 0.6M and 1.0M NaCl.
Figure 3.
(A) ABP, mean ABP (grey line), and lumbar, adrenal, and renal SNA during ICV infusion of aCSF or 1.0M NaCl in AV3V-lesioned rat. (B) Mean±SEM peak changes of control and AV3V-lesioned animals during ICV infusion of aCSF, 0.6M NaCl, and 1.0M NaCl. *P<0.05 vs control, #P<0.05 between NaCl concentrations (aCSF vs 0.6M vs 1.0M)
RVLM Neurons Mediate Sympathoexcitatory and Pressor Responses to ICV NaCl
Experiment 5
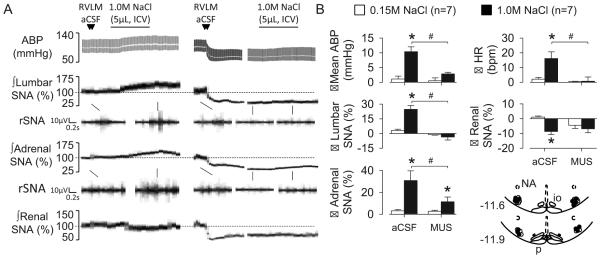
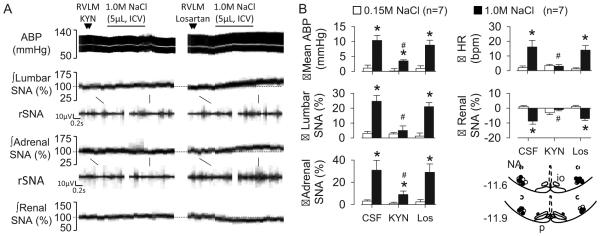
To test whether RVLM neurons mediate the sympathoexcitatory response to CSF NaCl, ICV infusions were performed after inhibition of the RVLM. Bilateral injection of the GABAA agonist muscimol promptly reduced lumbar SNA (−76±6%, P<0.01), renal SNA (−53±10%, P<0.01), adrenal SNA (−62±6%, P<0.01), splanchnic SNA (−35±9%, P<0.01), mean ABP (88±6 to 57±7 mmHg, P<0.01), and heart rate (405±12 to 365±19 bpm, P<0.01). Importantly, bilateral injection of muscimol abolished the lumbar sympathoexcitatory and pressor response to ICV infusion of 1.0M NaCl (Figure 4). The adrenal sympathoexcitatory response was partially attenuated. Bilateral injection of aCSF into the RVLM did not significantly alter any baseline variable (data not shown) or alter the responses to ICV infusion of NaCl (Figure 4).
Figure 4.
(A) ABP, mean ABP, and lumbar, adrenal, and renal SNA during ICV infusion of 1.0M NaCl after bilateral RVLM injection of aCSF or the GABAA agonist muscimol. (B) Mean±SEM peak changes and schematic illustration of RVLM injection sites. *P<0.05 vs 0.15M NaCl, #P<0.05, aCSF vs muscimol.
Experiment 6
To identify the neurotransmitter receptor in the RVLM that mediates the above responses, ICV infusions were performed after blockade of ionotropic glutamate or AT1 receptors in the RVLM. Bilateral injection of KYN into the RVLM produced a small reduction in lumbar SNA (−6±1%, P<0.05) but did not alter renal SNA (−4±5%, P>0.01), adrenal SNA (−4±7%, P>0.05), splanchnic SNA (−1±1%, P>0.05), mean ABP (85±5 to 84±4 mmHg, P>0.05), and heart rate (350±18 to 346±13 bpm, P>0.05). Blockade of RVLM glutamate receptors with KYN significantly attenuated the increase in lumbar SNA, adrenal SNA, heart rate, and mean ABP to ICV infusion of 1.0M NaCl (Figure 5). In addition, KYN also reduced the renal sympathoinhibitory response. In contrast, bilateral injection of the AT1 receptor antagonist losartan did not alter the sympathetic, tachycardic, and pressor responses to ICV infusion of 1.0M NaCl (Figure 5). RVLM injection of losartan did not alter baseline lumbar SNA (10±7%, P>0.05), renal SNA (2±3%, P>0.05), adrenal SNA (−1±3%, P>0.05), splanchnic SNA (3±2%, P>0.05), mean ABP (90±4 to 92±5 mmHg, P>0.05), and heart rate (371±15 to 368±14 bpm, P>0.05).
Figure 5.
(A) ABP, mean ABP, and lumbar, adrenal, and renal SNA during ICV infusion of 1.0M NaCl after bilateral RVLM injection of the iontropic glutamate receptor agonist kynurenic acid (KYN) or the AT1R antagonist losartan. (B) Mean±SEM peak changes and schematic illustration of RVLM injection sites. *P<0.05 vs 0.15M NaCl, #P<0.05, aCSF vs muscimol.
CSF Hypernatremia Differentially Affects the Discharge of RVLM Neurons
Experiment 7
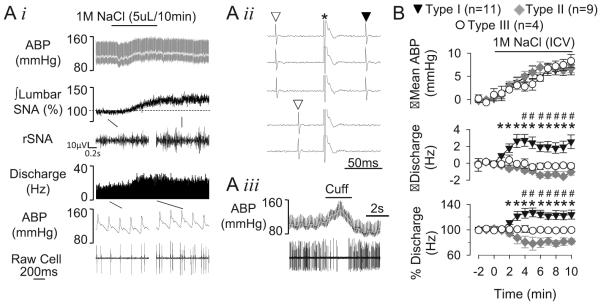
A final set of experiments was performed to establish that CSF hypernatremia alter the activity of barosensitive, spinally-projecting RVLM neurons. Neurons were divided into 3 types based on the discharge responses to ICV infusion of 1M NaCl. Type I neurons (11/24, 46%) had a baseline discharge of 11±2 Hz and conduction velocity of 2.1±0.40 m/s. ICV infusion of 1.0M NaCl increased cell discharge within 2 min after onset of the infusion, and the activity remained elevated throughout the infusion despite an increased mean ABP (Figure 6). Type II neurons (9/24, 37%) had a baseline discharge and conduction velocity of 11±3 Hz and 2.6±0.3 m/s, respectively. However, infusion of 1M NaCl decreased the discharge rate of these neurons at 4 min after the onset of the ICV infusion (Figure 6, Figure S3, www.hyper.ahajournals.org). Type III neurons (4/24, 17%) displayed a baseline discharge and conduction velocity of 17±8 Hz and 2.3±0.5 m/s, respectively. Infusion of 1M NaCl did not alter discharge rate in these neurons (Figure 6, Figure S4, www.hyper.ahajournals.org). A previous study suggested that RVLM neurons may be distinguished between C1 and non-C1 cells based on the conduction velocity as slowly conducting neurons (<1 m/s) were consistently identified as C1 neurons 32. Therefore, we performed a retrospective analysis of RVLM neurons and the respective conduction velocities using a criteria of <1 m/s to identify putative C1 neurons. This analysis identified 4 RVLM neurons with a conduction velocity of 0.8±0.1 m/s and baseline discharge rate of 7.5±3.0 Hz. Interestingly, all 4 neurons and displayed an increase discharge response to ICV infusion of 1 M NaCl (Figure 6).
Figure 6.
(A, i) Example of ABP, lumbar SNA, and cell discharge of a Type I RVLM neuron during ICV infusion of 1M NaCl. Type I neurons were antidromically activated from the spinal cord (ii) and barosensitive (iii). ∇, spontaneous spike; ▼, antidromic spike; *stimulus artifact. (B) Summary data presented as mean±SEM for Type I, II, and III RVLM neurons. *P<0.05 vs baseline values (Type I), #P<0.05 vs baseline values (Type II). See online supplement for examples of Type II and III neurons
DISCUSSION
Previous studies have documented that increased CSF [Na+] elevates ABP 1, 2, but the neural pathways and contribution of RVLM neurons have not been determined previously. The present findings provide several novel observations: 1) acute ICV infusion of NaCl differentially increases lumbar and adrenal SNA, decreases renal SNA, and does not change splanchnic SNA, 2) acute AV3V lesion prevents these changes, 3) inhibition of RVLM neurons with the GABAA agonist muscimol or blockade of RVLM ionotropic glutamate receptors significantly attenuates the sympathetic and ABP responses to ICV infusion of hypertonic NaCl, and 4) acute ICV infusion of hypertonic NaCl differentially affected the discharge frequency of spinally-projecting RVLM neurons. Collectively, these findings suggest that the sympathoexcitatory response to CSF hypernatremia depends on AV3V neurons to increase glutamatergic drive onto a selective population of RVLM neurons.
Plasma or CSF hypernatremia increases SNA and ABP in both rodents and humans1, 33. However, the sympathoexcitatory response is likely end-organ dependent. Indeed, our current findings document, for the first time, that acute ICV infusion of hypertonic NaCl produced a differential activation of lumbar and adrenal SNA but inhibition of renal SNA and no change in splanchnic SNA. In agreement, several studies have acutely raised NaCl concentrations in different species and through different routes to produce qualitatively similar responses and include: 1) IV infusion in rodents produce a similar differential SNA response34, 2) intracarotid infusion of NaCl to produce physiological changes decreases renal SNA35, and 3) IV infusion in humans increases muscle SNA 36, 37. This SNA pattern may promote increased sodium excretion through a pressure-natriuresis mechanism and concurrent inhibition of renal SNA through a direct Na+-sympathoinhibitory pathway or a baroreceptor-mediated inhibition of RVLM discharge. The latter may be particularly evident under Inactin-anesthesia; however, unpublished findings in our laboratory indicate a similar pattern of SNA to ICV infusion of hypertonic NaCl across a number of anesthetics (urethane, chloralose, isoflurane). The elevated SNA is significant as the pressor response was prevented by the ganglionic blocker chlorisondamine. In contrast, Leenen and colleagues7, 8, 38 have reported that ICV infusion of hypertonic NaCl increases renal SNA. Although our studies were performed in anesthetized animals, the above-referenced renal SNA recordings were performed within a few hours of surgery and implantation of the renal nerve electrodes 7, 8, 38. Second, the infusion volumes into the lateral ventricle were much larger in those studies (30-40 µL over 8-10 min) versus the current experiments (5µL over 10 min). Although the ICV infusion of 1M NaCl increased CSF [Na+] and [Cl−] in the 4th ventricle by 2-4mM, the changes are likely greater in the hypothalamus. Clearly, future studies are needed in which chronic SNA recordings across different end-organs during ICV infusion of hypertonic NaCl or in salt-sensitive models associated with elevated CSF NaCl concentration.
The forebrain lamina terminalis is a pivotal site for the interaction between NaCl and SNA1, 33, 39. AV3V lesions blunt osmotically-dependent responses including thirst, vasopressin secretion, and natriuresis40. These same lesions attenuate or reverse several experimental models of salt-sensitive hypertension15, 16. Our findings extend these observations and demonstrate that both sympathoexcitatory (lumbar and adrenal) and sympathoinhbitory (renal) responses to increased CSF NaCl are prevented by AV3V lesions. The manner by which AV3V neurons contribute to these responses likely reflect a Na+-specific versus osmosensitive mechanism as ICV or intracarotid infusions of NaCl versus other solutes produce greater increases in SNA and/or ABP 10, 35, 41. In this regard, Leenen and colleagues have postulated that NaCl activates an epithelial sodium channel-ouabain pathway in the hypothalamus to increase SNA and ABP 2 ; however, future studies are needed to directly link this signaling pathway to excitatory responses of Na+-sensing neurons to hypertonic NaCl.
A major goal of these experiments was to identify whether RVLM neurons contribute to the sympathetic and pressor effects of central hypernatremia. Indeed, our findings demonstrate that inhibition of RVLM neurons or blockade of ionotropic glutamate receptors on RVLM neurons prevents or attenuates, respectively, the sympathetic and pressor responses to ICV NaCl. Interestingly, blockade of glutamate receptors in the RVLM lowers ABP in Dahl-Salt-Sensitive rats 21. Although we did not identify the origin of glutamatergic drive to the RVLM, it likely involves a polysynaptic pathway from the AV3V region as injection of retrograde tracers into the RVLM does not produce robust labeling in these nuclei 22, 42. Although all sources of glutamatergic drive to the RVLM have not been identified, the majority of PVH neurons projecting to the RVLM express vesicular glutamate transporter-2 mRNA – a marker of glutamatergic neurons 27. Furthermore, the PVH contributes to the sympathetic and pressor responses to acute ICV NaCl infusions 14 and elevated ABP in Dahl-Salt-Sensitive rats 17, 18, 21. Altogether, these findings suggest that central hypernatremia activates hypothalamic pathways to increase glutamatergic drive onto RVLM neurons to increase SNA and ABP in salt-sensitive hypertension.
The single-unit recordings of RVLM neurons demonstrate three populations distinguished by the discharge response to ICV infusion of NaCl. Approximately one-half of these neurons increased discharge to central NaCl infusion – a response consistent with the increase in lumbar and adrenal SNA. The other populations (Type II and III) may reflect neurons that regulate renal and splanchnic SNA, respectively. It is unclear whether the discharge responses reflect the integration of glutamatergic, GABAergic, and other synaptic inputs at the level of RVLM versus a selective increase in glutamatergic drive onto RVLM neurons regulating lumbar and adrenal SNA. Finally, RVLM neurons have been neurochemically distinguished by the presence or absence of PNMT and deemed C1 versus non-C1 neurons, respectively 43. A previous electrophysiological study reported that all neurons with a conduction velocity <1m/s were C1 neurons 32. Here, a retrospective analysis identified 4 neurons with a conduction velocity <1 m/s, and all 4 neurons displayed an increase in discharge during ICV infusion of hypertonic NaCl. Although the immunocytochemical processing for tyrosine hydroxylase in our study was unsuccessful, these findings raise the interesting possibility that increased CSF NaCl concentrations may increase SNA and ABP through a selective activation of C1 neurons in the RVLM.
The current study used an acute ICV infusion of hypertonic NaCl to gain insight into the pathways and mechanisms that may be activated in salt-sensitive hypertension. A clinical study 6 and various experimental animal models 3, 4 have reported that a high salt diet increases CSF Na+ concentrations in salt-sensitive subjects or animals but not salt-resistant counterparts. Evidence suggests that a tonic activation of forebrain Na+ sensitive mechanisms persist in salt-sensitive hypertension as acute intracarotid infusion of hypotonic fluid lowers SNA and ABP in DOCA-salt hypertension rats 44, 45. However, there are likely additional neuroplastic changes that occur during chronic increases in CSF Na+ concentrations or dietary salt intake to further elevate SNA and ABP 2. For example, a high salt diet exaggerates SNA and ABP responses of Sprague-Dawley, Dahl-Sal-Resistant, and Dahl-Salt-Sensitive rats to various stimuli including subsequent ICV infusion of hypertonic NaCl 8, 12, 46, 47 thereby suggesting salt sensitizes central sympathetic networks. Independent of salt intake, salt-sensitive individuals and animal strains display exaggerated SNA and ABP responses to various stressors 8, 46, 48, 49 and/or activation of RVLM neurons21. Future studies are needed to investigate whether these differences reflect altered neuronal function within discrete populations of RVLM neurons that are affected by acute or chronic changes in CSF [Na+] or dietary salt.
Perspectives
Salt-sensitive hypertension is mediated in part by elevated SNA driven by a central hypernatremia 1, 2. The present findings highlight the accumulating evidence for the ability of the central nervous system to differentially control SNA and end-organ function. Such control mechanisms have important implications for nerve denervation studies. For example, acute ICV infusion of hypertonic NaCl decreased renal SNA. Moreover, renal denervation has little effect on the development of hypertension in salt-sensitive models associated with a central hypernatremia (ie, Dahl-salt-sensitive rat) 50, 51. In addition, the findings also highlight the presence of distinct population of neurons in the RVLM that provide the cellular basis for the differential control of SNA. The identity and cellular phenotype of these cells may represent a future therapeutic target for the treatment of salt-sensitive hypertension.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
1) What is new?
An acute increase in CSF NaCl concentration elevates lumbar and adrenal SNA, decreases renal SNA, and does not affect splanchnic SNA.
The sympathoexcitatory response depends on glutamatergic receptor activation in the RVLM.
Acute ICV infusion of hypertonic NaCl differentially affects the activity of bulbospinal RVLM neurons including an increased discharge frequency.
Every slowly-conducting RVLM neuron (<1m/s) displayed an increased firing rate to ICV infusion of hypertonic NaCl.
2) What is relevant?
Increased CSF NaCl concentrations differentially alters sympathetic outflow to increase ABP.
The sympathetic and pressor responses depend on glutamatergic inputs onto a select population of RVLM neurons.
3) Summary
The findings suggest that acute increases in CSF NaCl concentrations differentially elevates SNA and ABP through increased glutamatergic inputs onto bulbospinal RVLM neurons that originates from the AV3V region.
ACKNOWLEDGEMENTS
The authors acknowledge Jennifer Lay for technical assistance.
SOURCES OF FUNDING
This work was supported by National Heart, Lung, and Blood Institute Grant HL-113270 (S.D.S.), an American Heart Association Established Investigator Grant (S.D.S.), and the Pennsylvania Department of Health using Tobacco CURE Funds (S.D.S.). The Department specifically disclaims responsibility for any analyses, interpretations, or conclusions. SML was supported by an American Heart Association Summer Undergraduate Research Fellowship (10UFEL3900000).
Footnotes
CONFLICTS OF INTEREST/DISCLOSURE
None.
REFERENCES
- 1.Stocker SD, Monahan KD, Browning KN. Neurogenic and Sympathoexcitatory Actions of NaCl in Hypertension. Curr Hypertens Rep. 2013;15:538–546. doi: 10.1007/s11906-013-0385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaustein MP, Leenen FH, Chen L, Golovina VA, Hamlyn JM, Pallone TL, Van Huysse JW, Zhang J, Wier WG. How NaCl raises blood pressure: a new paradigm for the pathogenesis of salt-dependent hypertension. Am J Physiol Heart Circ Physiol. 2012;302:H1031–1049. doi: 10.1152/ajpheart.00899.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang BS, Van Vliet BN, Leenen FH. Increases in CSF [Na+] precede the increases in blood pressure in Dahl S rats and SHR on a high-salt diet. Am J Physiol Heart Circ Physiol. 2004;287:H1160–1166. doi: 10.1152/ajpheart.00126.2004. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura K, Cowley AW., Jr. Sequential changes of cerebrospinal fluid sodium during the development of hypertension in Dahl rats. Hypertension. 1989;13:243–249. doi: 10.1161/01.hyp.13.3.243. [DOI] [PubMed] [Google Scholar]
- 5.Haywood JR, Buggy J, Fink GD, DiBona GF, Johnson AK, Brody MJ. Alterations in cerebrospinal fluid sodium and osmolality in rats during one-kidney, one-wrap renal hypertension. Clin Exp Pharmacol Physiol. 1984;11:545–549. doi: 10.1111/j.1440-1681.1984.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 6.Kawano Y, Yoshida K, Kawamura M, Yoshimi H, Ashida T, Abe H, Imanishi M, Kimura G, Kojima S, Kuramochi M, et al. Sodium and noradrenaline in cerebrospinal fluid and blood in salt-sensitive and non-salt-sensitive essential hypertension. Clin Exp Pharmacol Physiol. 1992;19:235–241. doi: 10.1111/j.1440-1681.1992.tb00444.x. [DOI] [PubMed] [Google Scholar]
- 7.Huang BS, Ahmad M, Deng AY, Leenen FH. Neuronal responsiveness to central Na+ in 2 congenic strains of Dahl salt-sensitive rats. Hypertension. 2007;49:1315–1320. doi: 10.1161/HYPERTENSIONAHA.106.086363. [DOI] [PubMed] [Google Scholar]
- 8.Huang BS, Wang H, Leenen FH. Enhanced sympathoexcitatory and pressor responses to central Na+ in Dahl salt-sensitive vs. -resistant rats. Am J Physiol Heart Circ Physiol. 2001;281:H1881–1889. doi: 10.1152/ajpheart.2001.281.5.H1881. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura M, Ohtsuka K, Nanbu A, Takahashi H, Yoshimura M. Benzamil blockade of brain Na+ channels averts Na(+)-induced hypertension in rats. Am J Physiol. 1998;274:R635–644. doi: 10.1152/ajpregu.1998.274.3.R635. [DOI] [PubMed] [Google Scholar]
- 10.Bunag RD, Miyajima E. Sympathetic hyperactivity elevates blood pressure during acute cerebroventricular infusions of hypertonic salt in rats. J Cardiovasc Pharmacol. 1984;6:844–851. doi: 10.1097/00005344-198409000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Miyajima E, Bunag RD. Chronic cerebroventricular infusion of hypertonic sodium chloride in rats reduces hypothalamic sympatho-inhibition and elevates blood pressure. Circ Res. 1984;54:566–575. doi: 10.1161/01.res.54.5.566. [DOI] [PubMed] [Google Scholar]
- 12.Simmonds SS, Lay J, Stocker SD. Dietary salt intake exaggerates sympathetic reflexes and increases blood pressure variability in normotensive rats. Hypertension. 2014;64:583–589. doi: 10.1161/HYPERTENSIONAHA.114.03250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veerasingham SJ, Leenen FH. Excitotoxic lesions of the ventral anteroventral third ventricle and pressor responses to central sodium, ouabain and angiotensin II. Brain Res. 1997;749:157–160. doi: 10.1016/s0006-8993(96)01381-9. [DOI] [PubMed] [Google Scholar]
- 14.Gabor A, Leenen FH. Mechanisms in the PVN mediating local and central sodium-induced hypertension in Wistar rats. Am J Physiol Regul Integr Comp Physiol. 2009;296:R618–630. doi: 10.1152/ajpregu.90417.2008. [DOI] [PubMed] [Google Scholar]
- 15.Berecek KH, Barron KW, Webb RL, Brody MJ. Vasopressin-central nervous system interactions in the development of DOCA hypertension. Hypertension. 1982;4:131–137. [PubMed] [Google Scholar]
- 16.Goto A, Ganguli M, Tobian L, Johnson MA, Iwai J. Effect of an anteroventral third ventricle lesion on NaCl hypertension in Dahl salt-sensitive rats. Am J Physiol. 1982;243:H614–618. doi: 10.1152/ajpheart.1982.243.4.H614. [DOI] [PubMed] [Google Scholar]
- 17.Gabor A, Leenen FH. Cardiovascular effects of angiotensin II and glutamate in the PVN of Dahl salt-sensitive rats. Brain Res. 2012;1447:28–37. doi: 10.1016/j.brainres.2012.01.060. [DOI] [PubMed] [Google Scholar]
- 18.Gabor A, Leenen FH. Mechanisms mediating sodium-induced pressor responses in the PVN of Dahl rats. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1338–1349. doi: 10.1152/ajpregu.00246.2011. [DOI] [PubMed] [Google Scholar]
- 19.Ito S, Hiratsuka M, Komatsu K, Tsukamoto K, Kanmatsuse K, Sved AF. Ventrolateral medulla AT1 receptors support arterial pressure in Dahl salt-sensitive rats. Hypertension. 2003;41:744–750. doi: 10.1161/01.HYP.0000052944.54349.7B. [DOI] [PubMed] [Google Scholar]
- 20.Antunes VR, Yao ST, Pickering AE, Murphy D, Paton JF. A spinal vasopressinergic mechanism mediates hyperosmolality-induced sympathoexcitation. J Physiol. 2006;576:569–583. doi: 10.1113/jphysiol.2006.115766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito S, Komatsu K, Tsukamoto K, Sved AF. Tonic excitatory input to the rostral ventrolateral medulla in Dahl salt-sensitive rats. Hypertension. 2001;37:687–691. [PubMed] [Google Scholar]
- 22.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 23.Budzikowski AS, Vahid-Ansari F, Leenen FH. Chronic activation of brain areas by high-sodium diet in Dahl salt-sensitive rats. Am J Physiol. 1998;274:H2046–2052. doi: 10.1152/ajpheart.1998.274.6.H2046. [DOI] [PubMed] [Google Scholar]
- 24.Budzikowski AS, Vahid-Ansari F, Robertson GS, Leenen FH. Patterns of neuronal activation during development of sodium sensitive hypertension in SHR. Hypertension. 1997;30:1572–1577. doi: 10.1161/01.hyp.30.6.1572. [DOI] [PubMed] [Google Scholar]
- 25.Larsen PJ, Mikkelsen JD. Functional identification of central afferent projections conveying information of acute "stress" to the hypothalamic paraventricular nucleus. J Neurosci. 1995;15:2609–2627. doi: 10.1523/JNEUROSCI.15-04-02609.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oldfield BJ, Badoer E, Hards DK, McKinley MJ. Fos production in retrogradely labelled neurons of the lamina terminalis following intravenous infusion of either hypertonic saline or angiotensin II. Neuroscience. 1994;60:255–262. doi: 10.1016/0306-4522(94)90219-4. [DOI] [PubMed] [Google Scholar]
- 27.Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J Comp Neurol. 2006;494:673–685. doi: 10.1002/cne.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward KR, Bardgett JF, Wolfgang L, Stocker SD. Sympathetic response to insulin is mediated by melanocortin 3/4 receptors in the hypothalamic paraventricular nucleus. Hypertension. 2011;57:435–441. doi: 10.1161/HYPERTENSIONAHA.110.160671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steiner JL, Bardgett ME, Wolfgang L, Lang CH, Stocker SD. Glucocorticoids attenuate the central sympathoexcitatory actions of insulin. J Neurophysiol. 2014;112:2597–2604. doi: 10.1152/jn.00514.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bardgett ME, McCarthy JJ, Stocker SD. Glutamatergic receptor activation in the rostral ventrolateral medulla mediates the sympathoexcitatory response to hyperinsulinemia. Hypertension. 2010;55:284–290. doi: 10.1161/HYPERTENSIONAHA.109.146605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stocker SD, Smith CA, Kimbrough CM, Stricker EM, Sved AF. Elevated dietary salt suppresses renin secretion but not thirst evoked by arterial hypotension in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1521–1528. doi: 10.1152/ajpregu.00658.2002. [DOI] [PubMed] [Google Scholar]
- 32.Schreihofer AM, Guyenet PG. Identification of C1 presympathetic neurons in rat rostral ventrolateral medulla by juxtacellular labeling in vivo. J Comp Neurol. 1997;387:524–536. doi: 10.1002/(sici)1096-9861(19971103)387:4<524::aid-cne4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 33.Stocker SD, Osborn JL, Carmichael SP. Forebrain osmotic regulation of the sympathetic nervous system. Clin Exp Pharmacol Physiol. 2008;35:695–700. doi: 10.1111/j.1440-1681.2007.04835.x. [DOI] [PubMed] [Google Scholar]
- 34.Weiss ML, Claassen DE, Hirai T, Kenney MJ. Nonuniform sympathetic nerve responses to intravenous hypertonic saline infusion. J Auton Nerv Syst. 1996;57:109–115. doi: 10.1016/0165-1838(95)00108-5. [DOI] [PubMed] [Google Scholar]
- 35.Frithiof R, Xing T, McKinley MJ, May CN, Ramchandra R. Intracarotid hypertonic sodium chloride differentially modulates sympathetic nerve activity to the heart and kidney. Am J Physiol Regul Integr Comp Physiol. 2014;306:R567–575. doi: 10.1152/ajpregu.00460.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farquhar WB, Wenner MM, Delaney EP, Prettyman AV, Stillabower ME. Sympathetic neural responses to increased osmolality in humans. Am J Physiol Heart Circ Physiol. 2006;291:H2181–2186. doi: 10.1152/ajpheart.00191.2006. [DOI] [PubMed] [Google Scholar]
- 37.Wenner MM, Rose WC, Delaney EP, Stillabower ME, Farquhar WB. Influence of plasma osmolality on baroreflex control of sympathetic activity. Am J Physiol Heart Circ Physiol. 2007;293:H2313–2319. doi: 10.1152/ajpheart.01383.2006. [DOI] [PubMed] [Google Scholar]
- 38.Huang BS, Leenen FH. Sympathoexcitatory and pressor responses to increased brain sodium and ouabain are mediated via brain ANG II. Am J Physiol. 1996;270:H275–280. doi: 10.1152/ajpheart.1996.270.1.H275. [DOI] [PubMed] [Google Scholar]
- 39.Toney GM, Stocker SD. Hyperosmotic activation of CNS sympathetic drive: implications for cardiovascular disease. J Physiol. 2010;588:3375–3384. doi: 10.1113/jphysiol.2010.191940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci. 2008;9:519–531. doi: 10.1038/nrn2400. [DOI] [PubMed] [Google Scholar]
- 41.Chen QH, Toney GM. AT(1)-receptor blockade in the hypothalamic PVN reduces central hyperosmolality-induced renal sympathoexcitation. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1844–1853. doi: 10.1152/ajpregu.2001.281.6.R1844. [DOI] [PubMed] [Google Scholar]
- 42.Sved AF, Ito S, Madden CJ, Stocker SD, Yajima Y. Excitatory inputs to the RVLM in the context of the baroreceptor reflex. Ann N Y Acad Sci. 2001;940:247–258. doi: 10.1111/j.1749-6632.2001.tb03681.x. [DOI] [PubMed] [Google Scholar]
- 43.Guyenet PG, Stornetta RL, Bochorishvili G, Depuy SD, Burke PG, Abbott SB. C1 neurons: the body's EMTs. Am J Physiol Regul Integr Comp Physiol. 2013;305:R187–204. doi: 10.1152/ajpregu.00054.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Donaughy TL, Brooks VL. Deoxycorticosterone acetate-salt rats: hypertension and sympathoexcitation driven by increased NaCl levels. Hypertension. 2006;47:680–685. doi: 10.1161/01.HYP.0000214362.18612.6e. [DOI] [PubMed] [Google Scholar]
- 45.O'Donaughy TL, Qi Y, Brooks VL. Central action of increased osmolality to support blood pressure in deoxycorticosterone acetate-salt rats. Hypertension. 2006;48:658–663. doi: 10.1161/01.HYP.0000238140.06251.7a. [DOI] [PubMed] [Google Scholar]
- 46.Mark AL. Sympathetic neural contribution to salt-induced hypertension in Dahl rats. Hypertension. 1991;17:I86–90. doi: 10.1161/01.hyp.17.1_suppl.i86. [DOI] [PubMed] [Google Scholar]
- 47.Adams JM, Madden CJ, Sved AF, Stocker SD. Increased dietary salt enhances sympathoexcitatory and sympathoinhibitory responses from the rostral ventrolateral medulla. Hypertension. 2007;50:354–359. doi: 10.1161/HYPERTENSIONAHA.107.091843. [DOI] [PubMed] [Google Scholar]
- 48.Deter HC, Buchholz K, Schorr U, Mathiak K, Sharma AM. Salt-sensitivity and other predictors of stress-related cardiovascular reactivity in healthy young males. Clin Exp Hypertens. 2001;23:213–225. doi: 10.1081/ceh-100102661. [DOI] [PubMed] [Google Scholar]
- 49.Deter HC, Buchholz K, Schorr U, Schachinger H, Turan S, Sharma AM. Psychophysiological reactivity of salt-sensitive normotensive subjects. J Hypertens. 1997;15:839–844. doi: 10.1097/00004872-199715080-00006. [DOI] [PubMed] [Google Scholar]
- 50.Kandlikar SS, Fink GD. Mild DOCA-salt hypertension: sympathetic system and role of renal nerves. Am J Physiol Heart Circ Physiol. 2011;300:H1781–1787. doi: 10.1152/ajpheart.00972.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wyss JM, Sripairojthikoon W, Oparil S. Failure of renal denervation to attenuate hypertension in Dahl NaCl-sensitive rats. Can J Physiol Pharmacol. 1987;65:2428–2432. doi: 10.1139/y87-385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.