Abstract
Background:
Substantial numbers of children with HIV present to health care services in older childhood and adolescence, previously undiagnosed. These “slow-progressors” may experience considerable chronic ill health, which is not well characterized. We investigated the prevalence of chronic morbidity among children aged 6–15 years at diagnosis of HIV infection.
Methods:
A cross-sectional study was performed at 7 primary care clinics in Harare, Zimbabwe. Children aged 6–15 years who tested HIV positive following provider-initiated HIV testing and counseling were recruited. A detailed clinical history and standardized clinical examination was undertaken. The association between chronic disease and CD4 count was investigated using multivariate logistic regression.
Results:
Of the 385 participants recruited [52% female, median age 11 years (interquartile range 8–13)], 95% were perinatally HIV infected. The median CD4 count was 375 (interquartile range 215–599) cells per cubic millimeter. Although 78% had previous contact with health care services, HIV testing had not been performed. There was a high burden of chronic morbidity: 23% were stunted, 21% had pubertal delay, 25% had chronic skin disease, 54% had a chronic cough of more than 1 month-duration, 28% had abnormal lung function, and 12% reported hearing impairment. There was no association between CD4 count of <500 cells per cubic millimeter or <350 cells per cubic millimeter with WHO stage or these chronic conditions.
Conclusions:
In children with slow-progressing HIV, there is a substantial burden of chronic morbidity even when CD4 count is relatively preserved. Timely HIV testing and prompt antiretroviral therapy initiation are urgently needed to prevent development of chronic complications.
Key Words: HIV, Africa, adolescence, slow-progressors, chronic lung disease
BACKGROUND
Untreated vertically acquired HIV infection is associated with high mortality rates with a 50% probability of dying by 2 years of age among African cohorts in the pre–antiretroviral therapy (ART) era.1 However, as HIV epidemics have matured, survival estimates have been successively revised upward, and a substantial number of perinatally infected children are presenting with HIV infection in later childhood or adolescence.2–4 It is estimated that a third of HIV-infected infants have slow-progressing disease with a median survival of at least 16 years.5 Unlike children who have rapid disease progression and present with AIDS-defining illness in infancy, children with slow-progressing HIV are either paucisymptomatic or typically have a history of multiple, nonspecific complaints, including recurrent upper respiratory tract or skin infections that are also common among their HIV-uninfected peers.6,7 Recognition of HIV is consequently often delayed until presentation with advanced disease.6
There is strong evidence that immediate initiation of ART reduces mortality in infants, in particular those under 1 year of age.1 The WHO 2013 HIV treatment guidelines recommend immediate ART initiation on diagnosis regardless of clinical or immune stage in all children aged under 5 years.8 Such a benefit has not been demonstrated for older children.9 For those over 5 years, WHO 2013 guidelines (which were standard of care when this study was being conducted) recommended that, as for adults, ART is deferred until the clinical stage 3 or 4 HIV disease is apparent or if the CD4 count drops below 500 cells per millimeter.8 In 2015, the WHO further revised HIV treatment guidelines recommending that ART be commenced in all age groups regardless of clinical or immune status, although evidence for immediate ART commencement is lacking in older children and adolescents.10
While the risk of AIDS-defining infections is low above a CD4 threshold of 500 cells per cubic millimeter, long-standing infection is associated with development of chronic complications, which may not fulfill the criteria for ART initiation.11 For example, we have previously demonstrated a high prevalence of chronic respiratory symptoms among adolescents with vertically acquired HIV infection. However, objective measures of lung function were not performed, and the study included both ART-naive and ART-treated individuals.12
We investigated the prevalence of chronic symptoms, including a standardized assessment of growth and lung function and whether these were associated with immunological status among children aged 6–15 years at HIV diagnosis of HIV infection.
METHODS
Study Setting
A cross-sectional study was performed in 7 public sector primary health care clinics (PHCs) in 7 high population density suburbs in southwest Harare, Zimbabwe between January 2013 and December 2014. Each suburb in Harare is served by one main PHC, termed polyclinic, which provides acute primary care, antenatal and postnatal care, mother and child health services, HIV testing and care. Primary health care provision is nurse led, supported by weekly consultation visits by a general medical practitioner. Children are managed using WHO Integrated Management of Childhood Illness protocols with referral to the local hospital where required.13
ART and cotrimoxazole prophylaxis are provided free of charge, although each HIV consultation visit incurs a cost of US $1. Provider-initiated HIV testing and counseling (PITC) ie, offering HIV testing to all individuals attending health facilities regardless of the reason for presentation has been part of WHO and Zimbabwean national guidelines since 2007 and testing is free of charge.14,15 Nurse-led ART initiation for children was introduced at the 7 PHCs with supervision from a physician (clinics having previously only provided ART for those having been initiated on treatment in secondary health care facilities). Nurses were trained on HIV counseling and management, and ART was provided according to national guidelines. Until February 2014, participants were initiated on ART if their CD4 was below 350 cells per cubic millimeter or had WHO stage 3 or 4 infection. From March 2014, Zimbabwe adopted the WHO 2013 consolidated guidelines, with the threshold for ART initiation revised to 500 cells per millimeter.8 All children were commenced on cotrimoxazole prophylaxis at diagnosis of HIV infection.
Children aged between 6 and 15 years who tested HIV positive at the study PHCs were offered enrollment into the study. Children were excluded from the study if they resided outside Harare, as follow-up may have been difficult due to distance to travel to attend clinical review.
Clinical Assessment
Clinical history was recorded using an interviewer-administered questionnaire. Contact with health services, history of past illness, exposure to ART including maternal ART for prevention of mother-to-child HIV transmission (PMTCT), drug history, and acute and chronic symptoms were recorded, including respiratory and gastrointestinal symptoms, and problems with hearing, vision, speech, and gross motor function. HIV infection was staged using the WHO Staging System and a CD4 count.16
A standardized physical examination was performed, including inspection of the skin for herpes zoster scarring, papular pruritic eruptions, planar warts, verrucous warts, molluscum contagiosum, fungal infection, and Kaposi sarcoma. The oral cavity was examined for gingivitis, periodontitis, candidiasis, and Kaposi sarcoma. Anthropometric examination included measurement of height and weight. The head circumference was determined by measuring the greatest occipitofrontal circumference, with the larger of 2 readings recorded. Tanner pubertal staging was performed in children aged >9 years.17 In females, breast and pubic hair development and age at menarche were recorded. In males, testicular volume, measured using an orchidometer, pubic hair development, and penile length were assessed.
Cardiorespiratory function was assessed by the Medical Research Council Dyspnea Scale Score and modified incremental shuttle walk test (ISWT) with pretest and posttest recording of respiratory rate, heart rate, and peripheral oxygen saturations.18,19 ISWT was not performed if baseline oxygen saturation was less than 88%, or if resting heart rate or respiratory rate exceeded 120 and 30 per minute, respectively. Spirometry was performed by trained nurses using an EasyOne World spirometer (NDD Medical Technologies Inc., Andover, MA). After demonstration of the procedure, the participant performed forced exhalation maneuvers while seated until quality criteria had been reached or 8 trials had been completed. The trial with the highest forced expiratory volume at 1 second (FEV1) and forced vital capacity (FVC) was selected for interpretation. Reversibility testing by repeat spirometry was performed 15 minutes after administration of 2.5 mg of nebulized salbutamol, if any abnormality ie, either obstruction as defined by FEV1:FVC of less than 1.64 SD below the mean or restriction as defined by FVC <1.64 SD below mean with normal FEV1:FVC ratio, was present.20
Laboratory Investigations
CD4 count was measured using an Alere PIMA CD4 (Waltham, MA) machine. Full blood count, renal, and liver function tests are not a prerequisite for starting ART in national guidelines, and these were only performed if clinically indicated. Participants who screened positive on the WHO TB screen were asked for a sputum sample.21 Sputum was examined onsite by Ziehl-Nielsen smear microscopy and Xpert TB (Cepheid, Sunnyvale, CA).
Data Management and Analysis
Data were collected from paper forms by optical mark recognition (Cardiff TELEFORM Intelligent Character, version 10.7) and analyzed using STATA, version 12.1 (STATA Corporation, College Station, TX)). Z-scores for height-for-age, weight-for-age, and head circumference–for-age were calculated using the 1990 British growth reference curves.22 Spirometric indices were expressed as z-scores using GLI2012 reference ranges.23 The association between a priori defined variables and CD4 count was determined using logistic regression, adjusting for age and sex. Missing variables were excluded in logistic regression analysis.
Clinical Definitions
A z-score of <−2 for height-for-age and weight-for-age was considered to represent stunting and wasting, respectively. Pubertal delay was defined as girls not having reached Tanner 2 breast development by age 12 years and boys not having reached Tanner 2 testicular volume by age 14 years.24 An obstructive lung defect was defined as FEV1 less than 1.64 SDs below the mean and FEV1:FVC of less than 1.64 SD below the mean. A reduced FVC (which would be suggestive of restrictive defect) was defined as an FVC below 1.64 SD below the mean with a normal or increased FEV1:FVC ratio.23 Reversibility of either obstructive or restrictive defect was defined as greater than or equal to 12% improvement in FEV1 after repeat spirometric testing postadministration of salbutamol.20
Ethical Considerations
Written informed consent was obtained from all caregivers and written assent obtained from participants. Ethical approval for the study was obtained from the Medical Research Council of Zimbabwe, the Harare City Health Department Ethics Committee, the Biomedical Research and Training Institute Institutional Review Board, and the London School of Hygiene and Tropical Medicine Ethics Committee.
RESULTS
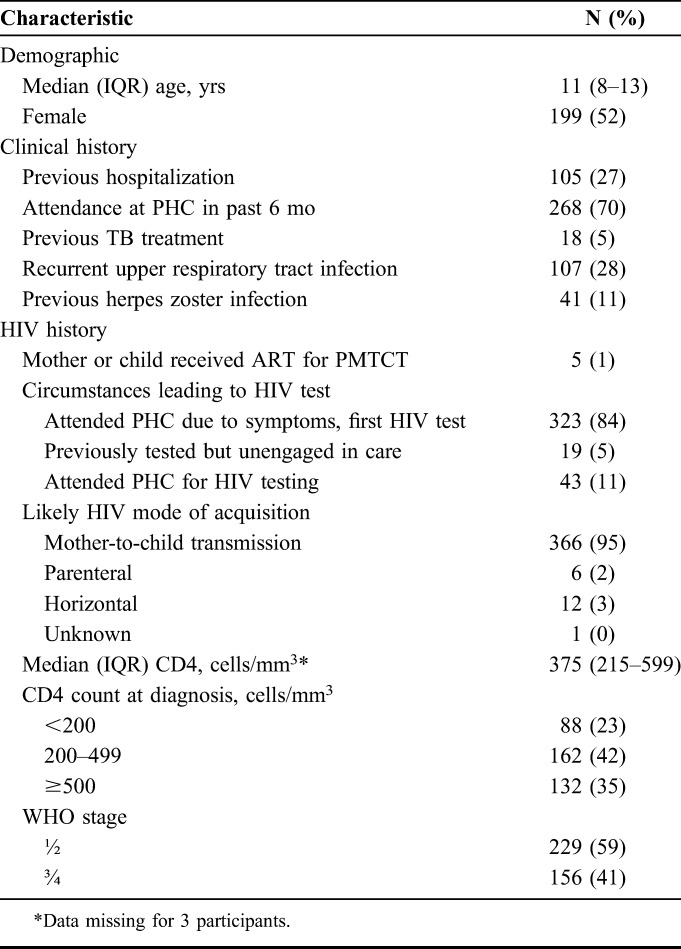
During the study period, 9655 children were tested for HIV of whom 449 (4.6%) tested HIV positive. An additional 21 participants were identified who had been diagnosed before study commencement but had not engaged in care and/or had not commenced ART. In total, 385 (86%) participants were recruited into the study. The remainder were not enrolled because of residence outside the study area (n = 24, 5%), declined consent (n = 25, 5.5%), care sought elsewhere (n = 34, 7.5%), and wrong age (n = 2, 0.4%). The median age at diagnosis was 11 years (interquartile range (IQR) 8–13), and 52% were female. Most participants were infected through mother-to-child transmission based on a history of maternal or natural sibling HIV or death, and self-report of no sexual debut, blood transfusions or surgery, and less than 2% of mothers of participants had received ART for PMTCT. Although 78% of participants had attended a PHC in the past 6 months and/or been previously hospitalized, HIV testing had not been performed (Table 1).
TABLE 1.
Baseline Characteristics of Participants at HIV Diagnosis (N = 385)
More than half of participants rated their health in the past 3 months as poor (Table 2). Chronic respiratory symptom was frequent with 54% reporting cough of more than 1 month-duration and 16% reporting dyspnea. At rest, 12% had oxygen saturations <88% and a further 10% dropped their oxygen saturations below 88% after exercise. Of the 238 participants who underwent spirometry and were able to produce quality traces, 23 (10%) had obstructive lung function defect with only 3 demonstrating reversibility with salbutamol, and 43 (18%) had a reduced FVC. Notably, despite 155 (40%) screening positive for TB on the WHO screen, only 1 participant was found to have TB on GeneXpert.
TABLE 2.
Chronic Morbidity in Study Participants
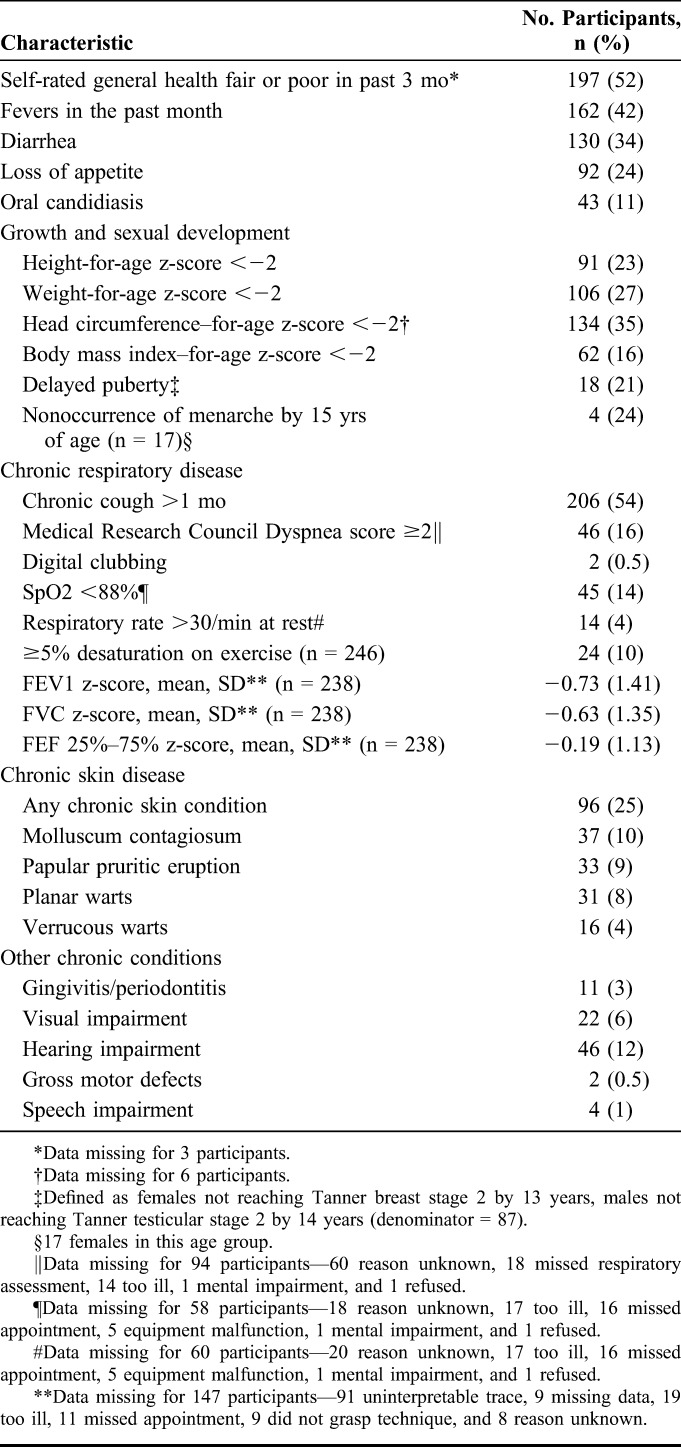
The median height-for-age, weight-for-age, and head circumference–for-age z-scores were −1.2 (IQR −1.9 to −0.41), −1.1 (IQR −2.1 to −0.45), and −1.4 (IQR −2.4 to −0.66), respectively. In addition, 27% of girls and 13% of boys were considered to have pubertal delay, and 24% of girls aged 15 years had not experienced menarche. Of the 80 participants with WHO stage 4 disease, 66% were classified as such based on stunting alone. Other chronic impairments included hearing difficulties self-reported by 12%, visual impairment by 6%, and speech impairment or gross motor defects by 2%. Chronic skin disease was found in 25% of participants on examination.
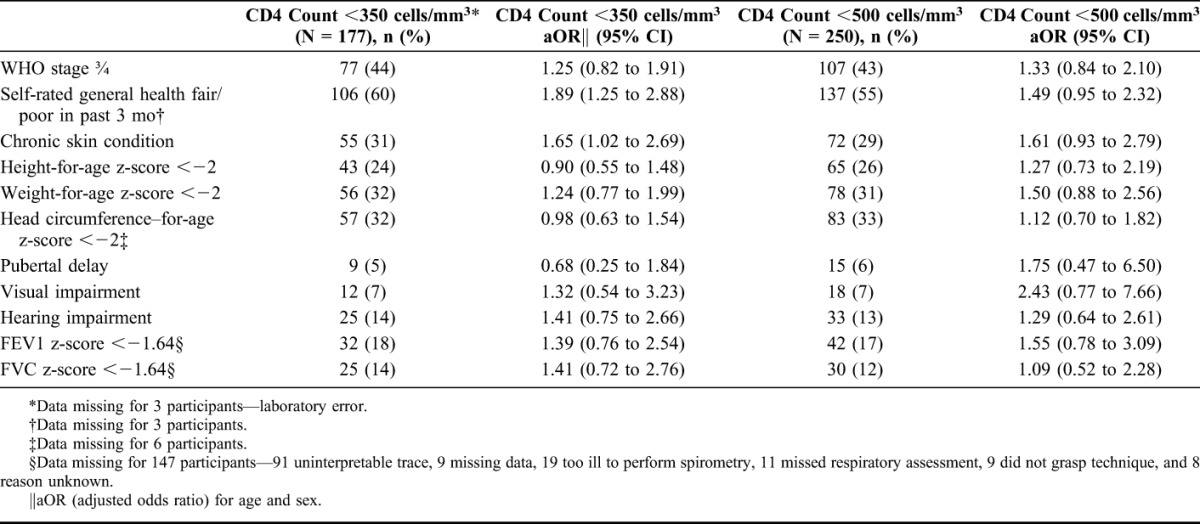
The median CD4 count was 375 cells per cubic millimeter (IQR 215–599) and 40% had WHO stage 3 or 4 HIV infection. Using a CD4 count threshold of 350 cells per cubic millimeter and/or WHO stage 3/4 disease, 67% children were eligible for ART, this figure increasing to 78% if the threshold was changed to 500 cells per cubic millimeter. Using logistic regression analysis adjusted for age and sex, participant self-report of ill health in the past 3 months was associated with a CD4 count <350 cells per cubic millimeter (adjusted odds ratio 1.89, 95% CI: 1.25 to 2.88) as was having a chronic skin condition (adjusted odds ratio 1.65, 95% CI: 1.02 to 2.69). However, a CD4 count <350 cells per cubic millimeter or <500 cells per cubic millimeter was not associated with WHO stage, reduced FEV1 or FVC z-score or growth parameters such as height-for-age, weight-for-age, or head circumference z-scores (Table 3).
TABLE 3.
Association of Clinical Conditions With CD4 Count at Study Enrollment
DISCUSSION
The main finding of this study was the heavy burden of chronic morbidity among older children and adolescents at time of HIV diagnosis. Consistent with other studies, a quarter of participants had stunting and 23% had pubertal delay.25–27 Although catch-up growth can be achieved after initiation of ART, children who begin treatment in later childhood are typically unable to regain their height potential.28,29 In addition, more than a third of participants had a head circumference–for-age z-score less than −2, which could partly be explained by suboptimal brain growth in early childhood. Unlike the progressive encephalopathy, manifest as failure to attain or loss of developmental milestones or intellectual capacity and motor defects, that is typical in HIV-infected infants and young children, gross motor defects and speech impairment were rare among our participants with slow-progressive disease. However, studies have reported defects in seemingly asymptomatic HIV-infected children in fine motor function, memory, perceptual performance, quantitative abilities, and mental processing and language abilities.30,31 These deficits are subtle and are not easily identified on routine questionnaire, and therefore, without formative testing may have been an underrepresentation of degree of morbidity in relation to higher functioning capacity in our study. These findings have to be interpreted with caution; however, as Western reference ranges for head circumference were used because of a lack of normative data from African children, and head circumference does vary by ethnic group.22
Chronic respiratory disease was common with cough, hypoxia, reduced exercise tolerance, and obstructive lung defects being the predominant features. Reversibility was rare, making asthma unlikely. Other studies have also shown a high prevalence of obstructive lung defects, with little or no reversibility.32,33 A study which performed chest computed tomography in adolescents with long-standing, vertically acquired HIV infection showed that constrictive obliterative bronchiolitis was the predominant cause of chronic lung disease, with lymphoid interstitial pneumonitis (the most commonly recognized cause of lung disease in HIV-infected children) being a rare finding.12,34,35 Obliterative bronchiolitis is a progressive, life-threatening condition and is well recognized as sequelae of respiratory tract infections, which were commonly reported by participants.36
A quarter of all participants had chronic dermatological conditions. Although not life-threatening, these are commonly recognized as stigmata of HIV infection, may take long periods to resolve after ART and for some conditions particularly planar warts, effective treatments are not available.37,38
Importantly, we observed no association between CD4 count and WHO HIV disease stage, a finding also noted previously.39 There was also no association between CD4 count and chronic conditions, including stunting and poor lung function. Taken together, this implies that CD4 count may not be an appropriate criterion for starting ART in older children. This also has potential implications with respect to timing of ART initiation. Based on the WHO 2013 HIV treatment guidelines, 12% of participants would not have been eligible for ART. Given the lack of evidence of the mortality benefit of immediate ART in older children and the concern about drug toxicity and adherence, guidelines until recently have recommended deferring ART in older children.8 Recent trials in adults have demonstrated that early initiation of ART reduces the risk of AIDS and non-AIDS events,40,41 but these trials excluded older children and adolescents. Our findings demonstrate that children with slow-progressing disease may have preserved CD4 counts but do develop chronic complications such as poor growth and chronic lung disease. It is, thus, possible that immediate ART may also prevent development of chronic complications in children. A randomized controlled trial would definitively establish whether immediate ART would prevent development or progression of chronic complications, but this is not going to be possible given the recent change in WHO guidelines recommending immediate ART in all adults and children.
The scale-up of programs to PMTCT has resulted in a substantial decline in the numbers of children infected with HIV, but coverage remains suboptimal in many high burden countries.42 The limited availability of HIV testing services for children and the low rates of early infant diagnosis mean that for many infected infants, HIV diagnosis occurs in late childhood or adolescence after many years of ill health.43 In this study, 78% of participants had previous contact with health facilities, including 27% having been hospitalized, but had not had HIV testing. Despite recommendations that PITC should be offered as standard in health facilities in HIV high prevalence settings, in practice, HIV testing is often prompted only after presentation with typical HIV-related symptoms.44 Children with slow-progressing disease may take years before they develop the well-recognized HIV indicator conditions that prompt an offer of HIV testing, by which time they develop chronic complications and organ damage which may not be reversible with ART once established.
To our knowledge, this is the first study to report the prevalence of morbidity at HIV diagnosis in children and adolescents with long-standing HIV infection. Most children were vertically infected, implying living with HIV infection for up to 15 years without previous treatment. Participants were diagnosed after optimized PITC of attendees regardless of cause of presentation, with 80% of all attendees with previously unknown HIV status undergoing HIV testing (data not shown). The study was based in primary care facilities and therefore was not biased toward sicker children. We acknowledge several limitations. There was no HIV-uninfected or an HIV-treated comparison group in our study. Vision, hearing, neurocognitive, and musculoskeletal function were not formally assessed but relied on self-report. Lung function assessment was not performed in 5% of participants. This was partly due to logistic reasons as respiratory assessment could not be performed at the initial assessment necessitating a second appointment within 2 weeks which some participants did not attend. Spirometric traces could not be interpreted in 100 (26%) participants. In addition, 19 (5%) participants were too ill to perform both spirometry and/or ISWT, which may have led to an underestimation of degree of lung disease present among the participants. Local reference ranges for lung function, puberty, and head circumference are not available. British reference ranges were used for height and weight z-score calculations because of a lack of WHO weight-for-age reference ranges for children over 9 years of age, which may not be appropriate for an African population.
Our study shows a substantial burden of chronic morbidity among HIV-infected children diagnosed in later childhood, even among those with preserved CD4 counts. Recognition of this burden is needed to stimulate earlier diagnosis and improve access to HIV care for this age group. There is a pressing need to strengthen PITC and potentially provide other more effective services for HIV testing in this age group, and for timely institution of ART. Current recommendations of deferred ART in children may put them at risk of developing chronic complications. The recent WHO guidelines recommending treatment of all HIV-infected individuals regardless of age and disease stage may reduce the risk of development of chronic complications. Studies investigating the impact of immediate ART on AIDS and non-AIDS events in children and the pathogenesis of chronic complications will inform development of optimum care provision for HIV-infected children.
Footnotes
Supported by the Wellcome Trust through an Intermediate Fellowship awarded to RAF (095878/Z/11/Z).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Newell M-L, Coovadia H, Cortina-Borja M, et al. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–1243. [DOI] [PubMed] [Google Scholar]
- 2.UNAIDS. AIDS by the Numbers 2015: UNAIDS; 2015. Available at: http://www.unaids.org/en/resources/documents/2015/AIDS_by_the_numbers_2015. Accessed January 27, 2016. [Google Scholar]
- 3.Marston M, Becquet R, Zaba B, et al. Net survival of perinatally and postnatally HIV-infected children: a pooled analysis of individual data from sub-Saharan Africa. Int J Epidemiol. 2011;40:385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stover J, Walker N, Grassly NC, et al. Projecting the demographic impact of AIDS and the number of people in need of treatment: updates to the Spectrum projection package. Sex Transm Infect. 2006;82(Suppl 3):iii45–iii50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrand RA, Corbett EL, Wood R, et al. AIDS among older children and adolescents in Southern Africa: projecting the time course and magnitude of the epidemic. AIDS. 2009;23:2039–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrand RA, Munaiwa L, Matsekete J, et al. Undiagnosed HIV infection among adolescents seeking primary health care in Zimbabwe. Clin Infect Dis. 2010;51:844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mokgatle MM, Madiba S. The burden of disease on HIV-infected orphaned and non-orphaned children accessing primary health facilities in a rural district with poor resources in South Africa: a cross-sectional survey of primary caregivers of HIV-infected children aged 5-18 years. Infect Dis Poverty. 2015;4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. WHO, Geneva, Switzerland; 2013. Available at: http://www.ncbi.nlm.nih.gov/pubmed/?term=24716260. Accessed September 24, 2015. [PubMed] [Google Scholar]
- 9.Siegfried N, Davies M-A, Penazzato M, et al. Optimal time for initiating antiretroviral therapy (ART) in HIV-infected, treatment-naive children aged 2 to 5 years old. Cochrane Database Syst Rev. 2013;10:CD010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Guideline on When to Start Antiretroviral Therapy and on Pre-Exposure Prophylaxis for HIV. WHO, Geneva, Switzerland; 2015. Available at: http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf?ua=1. Accessed January 18, 2016. [PubMed] [Google Scholar]
- 11.Devendra A, Makawa A, Kazembe PN, et al. HIV and childhood disability: a case-controlled study at a paediatric antiretroviral therapy centre in Lilongwe, Malawi. PLoS One. 2013;8:e84024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrand RA, Desai SR, Hopkins C, et al. Chronic lung disease in adolescents with delayed diagnosis of vertically acquired HIV infection. Clin Infect Dis. 2012;55:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Integrated Management of Childhood Illness for High HIV Settings. WHO, Geneva, Switzerland; 2008. Available at: http://www.ncbi.nlm.nih.gov/pubmed/?term=23805440. Accessed September 24, 2015. [PubMed] [Google Scholar]
- 14.World Health Organization. Guidance on Provider-Initiated HIV Testing and Counselling in Health Facilities. Geneva, Switzerland: WHO; 2007. Available at: http://www.who.int/hiv/pub/vct/pitc/en/. Accessed March 29, 2016. [Google Scholar]
- 15.Welfare M of H and C. Zimbabwe Guidelines for Implementation of Provider-Initiated HIV Testing and Counselling; Harare, Zimbabwe: Ministry of Health and Child Care, Zimbabwe, 2007. [Google Scholar]
- 16.World Health Organization. Interim WHO Clinical Staging of HIV/AIDS and HIV/AIDS Case Defintions for Surveillance. WHO, Geneva, Switzerland; 2005. Available at: http://www.who.int/hiv/pub/guidelines/clinicalstaging.pdf. Accessed June 12, 2015. [Google Scholar]
- 17.Tanner JM. Normal growth and techniques of growth assessment. Clin Endocrinol Metab. 1986;15:411–451. [DOI] [PubMed] [Google Scholar]
- 18.Bradley J, Howard J, Wallace E, et al. Validity of a modified shuttle test in adult cystic fibrosis. Thorax. 1999;54:437–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stenton C. The MRC breathlessness scale. Occup Med (Lond). 2008;58:226–227. [DOI] [PubMed] [Google Scholar]
- 20.Crapo RO, Casaburi R, Coates AL, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161:309–329. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. Systematic Screening for Active Tuberculosis: Principles and Recommendations. WHO, Geneva, Switzerland; 2013. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25996015. Accessed September 28, 2015. [PubMed] [Google Scholar]
- 22.Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med. 1998;17:407–429. [PubMed] [Google Scholar]
- 23.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun SS, Schubert CM, Chumlea WC, et al. National estimates of the timing of sexual maturation and racial differences among US children. Pediatrics. 2002;110:911–919. [DOI] [PubMed] [Google Scholar]
- 25.Lepage P, Msellati P, Hitimana DG, et al. Growth of human immunodeficiency type 1-infected and uninfected children: a prospective cohort study in Kigali, Rwanda, 1988 to 1993. Pediatr Infect Dis J. 1996;15:479–485. [DOI] [PubMed] [Google Scholar]
- 26.Buchacz K, Rogol AD, Lindsey JC, et al. Delayed onset of pubertal development in children and adolescents with perinatally acquired HIV infection. J Acquir Immune Defic Syndr. 2003;33:56–65. [DOI] [PubMed] [Google Scholar]
- 27.de Martino M, Tovo PA, Galli L, et al. Puberty in perinatal HIV-1 infection: a multicentre longitudinal study of 212 children. AIDS. 2001;15:1527–1534. [DOI] [PubMed] [Google Scholar]
- 28.Gsponer T, Weigel R, Davies M-A, et al. Variability of growth in children starting antiretroviral treatment in Southern Africa. Pediatrics. 2012;130:e966–e977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jesson J, Koumakpaï S, Diagne NR, et al. Effect of age at antiretroviral therapy initiation on catch-up growth within the first 24 months among HIV-infected children in the IeDEA West African pediatric cohort. Pediatr Infect Dis J. 2015;34:e159–e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laughton B, Cornell M, Boivin M, et al. Neurodevelopment in perinatally HIV-infected children: a concern for adolescence. J Int AIDS Soc. 2013;16:18603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puthanakit T, Ananworanich J, Vonthanak S, et al. Cognitive function and neurodevelopmental outcomes in HIV-infected children older than 1 year of age randomized to early versus deferred antiretroviral therapy: the predict neurodevelopmental study. Pediatr Infect Dis J. 2013;32:501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mwalukomo T, Rylance SJ, Webb EL, et al. Clinical characteristics and lung function in older children vertically infected with human immunodeficiency virus in Malawi. J Pediatr Infect Dis Soc. 2015;5:161–169. 10.1093/jpids/piv045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drummond MB, Kirk GD. HIV-associated obstructive lung diseases: insights and implications for the clinician. Lancet Respir Med. 2014;2:583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeena PM, Coovadia HM, Thula SA, et al. Persistent and chronic lung disease in HIV-1 infected and uninfected African children. AIDS. 1998;12:1185–1193. [DOI] [PubMed] [Google Scholar]
- 35.Sharland M, Gibb DM, Holland F. Respiratory morbidity from lymphocytic interstitial pneumonitis (LIP) in vertically acquired HIV infection. Arch Dis Child. 1997;76:334–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lynch JP, Weigt SS, DerHovanessian A, et al. Obliterative (constrictive) bronchiolitis. Semin Respir Crit Care Med. 2012;33:509–532. [DOI] [PubMed] [Google Scholar]
- 37.Lowe S, Ferrand RA, Morris-Jones R, et al. Skin disease among human immunodeficiency virus-infected adolescents in Zimbabwe: a strong indicator of underlying HIV infection. Pediatr Infect Dis J. 2010;29:346–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee KC, Risser J, Bercovitch L. What is the evidence for effective treatments of acquired epidermodysplasia verruciformis in HIV-infected patients? Arch Dermatol. 2010;146:903–905. [DOI] [PubMed] [Google Scholar]
- 39.Shroufi A, Gunguwo H, Dixon M, et al. HIV-infected adolescents in Southern Africa can achieve good treatment outcomes: results from a retrospective cohort study. AIDS. 2013;27:1971–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Danel C, Moh R, Gabillard D, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373:808–822. [DOI] [PubMed] [Google Scholar]
- 41.Lundgren JD, Babiker AG, Gordin F, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sibanda EL, Weller IVD, Hakim JG, et al. The magnitude of loss to follow-up of HIV-exposed infants along the prevention of mother-to-child HIV transmission continuum of care: a systematic review and meta-analysis. AIDS. 2013;27:2787–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Govindasamy D, Ferrand RA, Wilmore SMS, et al. Uptake and yield of HIV testing and counselling among children and adolescents in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2015;18:20182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kranzer K, Meghji J, Bandason T, et al. Barriers to provider-initiated testing and counselling for children in a high HIV prevalence setting: a mixed methods study. PLos Med. 2014;11:e1001649. [DOI] [PMC free article] [PubMed] [Google Scholar]


