Abstract
Dysregulation of mRNA translation leads to aberrant activation of cellular pathways that promote expansion and survival of leukemic clones. A key element of the initiation translation complex is eIF4E (eukaryotic translation initiation factor 4E). The mitogen-activated protein kinase (MAPK) and mammalian target of rapamycin (mTOR) pathways play important roles in the regulation of eIF4E expression and downstream functional outcomes. Mitogen-activated protein kinase interacting protein kinases (Mnks) control translation by phosphorylation of eIF4E, whereas the mTOR kinase phosphorylates/de-activates the eIF4E inhibitor, 4E-BP1, to release translational repression. Both pathways are often abnormally activated in leukemia cells and promote cell survival events by controlling expression of oncogenic proteins. Targeting these pathways may provide approaches to avoid aberrant proliferation and neoplastic transformation.
Keywords: translation initiation, eIF4E, MNK, mTOR, acute myeloid leukemia
1. Introduction
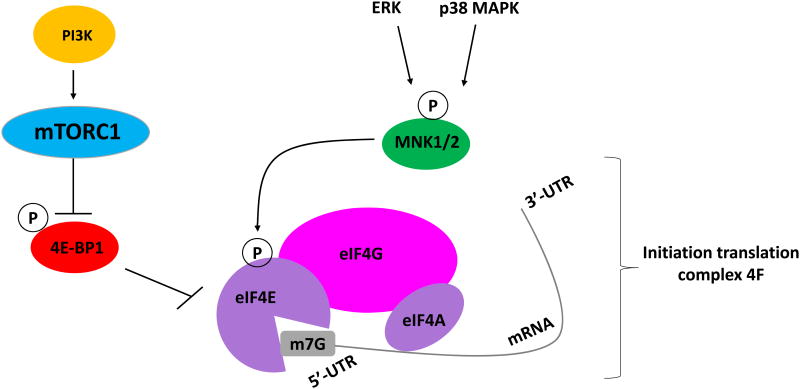
Acute myeloid leukemia (AML) is a genetically heterogeneous leukemia characterized by subtypes with different molecular abnormalities and by the activation of multiple signalling pathways that promote cell survival and proliferation. Despite the currently available therapies, most subtypes of AML remain difficult to treat [1]. The control of mRNA translation plays a pivotal role in the regulation of expression of genes that are responsible for many cellular processes such as cell proliferation, differentiation and apoptosis. Translation processes are tightly regulated. The critical step for initiating translation of mRNAs is the availability of eIF4E (eukaryotic translation initiation factor 4E) to participate in the eukaryotic initiation complex 4F, along with RNA helicase eIF4A and the scaffolding protein eIF4G [2, 3] (Figure 1). eIF4E is a key component of this complex because it recognizes and directly binds the 5′-cap of the mRNA structure, which includes a 7-methylguanosine (m7G) moiety [4, 5]. The eIF4G scaffolding protein also binds to mRNA by interaction with eIF4E and the m7G cap structure. This complex also includes the eIF4B protein that helps in the RNA-helicase function of eIF4A, thus regulating the translation of mRNAs that contain 5′-UTRs (untranslated regions) [6, 7]. The study of eIF4E has become a major focus in cancer research due to its key role in controlling translation of mRNAs that lead to the expression of tumor-associated proteins, such as c-Myc, cyclins D1 and D3, and Mcl-1 (Myeloid cell leukemia 1). The activity of these proteins has been linked to proliferation of leukemic cells and other type of malignant cells [8, 9, 10]. In contrast, the activation of eIF4E plays a minor role in the expression of mRNAs for housekeeping genes, such as GAPDH (glyceraldehyde-3-phosphate dehydrogenase) and β-actin [11]. In addition to its role in translation, eIF4E also seems to facilitate the nucleocytoplasmic transport of certain mRNAs, which is enhanced by eIF4E phosphorylation. This process enables the production of proteins that are involved in cell cycle progression and cell survival [12]. This could represent an independent mechanism required for expression of oncogenic proteins and potentially provide a unique cellular target for therapeutic approaches.
Figure 1.
Schematic illustration depicting the cellular pathways that lead to eIF4E activation.
The function of eIF4E is strictly regulated in cells under normal physiological conditions and is controlled by its repressor proteins, 4E-BPs (eIF4E-binding proteins), whose function does not allow formation of eIF4F complex [8]. eIF4E activity can be regulated by two major signalling pathways which play critical roles in leukemogenesis, the MAPK (Mitogen-activated protein kinases) and mTOR (mammalian target of rapamycin) pathways [13, 14]. The selective targeting of these pathways, alone or in combination with other therapies, could conceptually increase the anti-leukemic activity of the currently available and generally insufficient treatments for patients with AML and has been the major focus of study by different groups in the field.
2. The oncogenic activity of eIF4E and its phosphorylation by MNKs
The oncogenic activity of eIF4E can be modified by its phosphorylation at Serine209 (Ser209) by the MAPK-interacting kinases MNK1 and MNK2 (Figure 1). MNK1 and MNK2 belong to a family of serine/threonine protein kinases that are activated downstream of either ERK or p38 MAPK in response to extracellular factors (growth factors and stress) [15, 9, 16]. In human cells, two Mnk genes have been identified as MNK1 and MNK2. Each one of these genes, after alternative splicing events, translate two protein isoforms: MNK1a, MNK1b, MNK2a, and MNK2b, respectively, which share a similar N-terminal region (involved in binding to eIF4G), but vary in their C-terminal domains [17, 18]. The C-terminal regions of the longer MNK1a and MNK2a isoforms have a MAPK-biding site that allows their interaction/phosphorylation by ERK and p38 MAPK [19]. However, unlike MNK1a, which has a high affinity for both kinases, MNK2a has greater affinity for ERK. Moreover, the activation of ERK or p38 MAPK increases the low basal activity of MNK1a, but does not have a significant impact on the constitutive high basal activity of MNK2a [20, 17]. On the other hand, the shorter b-isoforms of Mnks lack the MAP-kinase biding site in their C-terminal region. Initial studies have shown that basal activity of MNK1b is higher than that of MNK2b [17, 21].
Recent studies have shown that phosphorylation and activation of eIF4E at Ser209 by MNK1/MNK2 is critical for eIF4E to promote oncogenic activity [22], but not essential for normal development [15, 16]. As a proof of concept of this perspective, a study using Mnk1/2 double knockout PTEN-/- mice (T-cell-specific PTEN conditional knockout mice) showed resistance to lymphogenesis in these mice, when compared to the parental PTEN-/- mice [23]. Phosphorylation of eIF4E is also involved in development and progression of other type of cancer [11]. Notably, phosphorylation of eIF4E was shown to promote invasion, metastasis and epithelial to mesenchymal transition [24]. eIF4E is overexpressed in many types of cancer and in most cases is connected with poor prognosis (increased cancer recurrence and decreased patient survival) [11]. There has been accumulating evidence implicating Mnks in the pathophysiology of leukemogenesis. AML is often characterized by a collection of several mutations that support proliferation and survival of leukemic clones [25, 26]. It has been shown that MNK1 activity is induced by several AML fusion genes and has an important role in hematopoietic proliferation [27]. A recent study on chronic myeloid leukemia (CML) provides evidence that targeting the MNK-eiF4E axis can inhibit the function of blast crisis leukemia stem cells (BC LSCs) by affecting production of β-catenin, without affecting normal hematopoietic stem cell functions [28]. Thus, Mnks can play an important role in leukemia progression and, therefore, future development of new small molecule inhibitors could be important for the treatment of leukemia.
3. Regulation of eIF4E by the mTOR pathway
The mTOR signaling cascade controls initiation of mRNA translation and plays significant roles in cellular processes such as protein synthesis, lipid production, cell growth and proliferation, and ribosome biogenesis [29, 30]. This kinase is present in two unique, separate complexes: mTORC1 and mTORC2 [31]. In those complexes there are common and distinct subunits and effectors [32, 33]. mTORC1 contains the common subunits mLST8 (mammalian lethal with sec13 protein 8) and Deptor (DEP domain-containing mTOR-interacting protein), and the unique components PRAS 40 (proline rich Akt substrate of 40 kDA) and Raptor (regulatory-associated protein of mTOR) [34, 29, 32]. The mTORC2 includes the unique subunits Rictor (rapamycin-insensitive companion of mTOR), mSin1 (mammalian stress activated protein-kinase interacting protein), and Protor, in addition to the common proteins mLST8 and Deptor [30]. A defining function of mTORC2 is the control of phosphorylation of AKT at Ser473, a site which is essential for activation of AKT and anti-apoptotic downstream effectors [31, 32, 35]. Additionally, mTORC2 regulates cytoskeletal organization and glucose and lipid metabolism [36]. It has also been shown that in addition to AKT, mTORC2 also phosphorylates SGK (glucocorticoid-regulated kinase) and PKCα (protein kinase C-α) [37, 38, 39].
mTORC1 is activated upstream by engagement of the PI3K/AKT cascade. PI3K (phosphoinositide 3-kinase) is a membrane-associated lipid kinase that when activated promotes conversion of phosphatidyl inositol 3,4 (PIP2) to phosphatidyl inositol 3,4,5 (PIP3) [29, 40]. Once active, PI3K and PIP3 bind PDK1 which phosphorylates AKT at Threonine 308 [41, 42]. In its active form, AKT plays an important role in regulation of mTORC1 activity by phosphorylation of PRAS40 and tuberous sclerosis complex 2 (TSC2) [43, 44, 45].
mTORC1 signaling is induced by multiple growth factors, hormones, cytokines, as well as, stress [46, 40] and regulates pathways involved in initiation of mRNA translation of oncogenic proteins, cellular growth, cell cycle progression and autophagy [30, 47]. Two key substrates of mTORC1 are S6K and translation repressor 4E-BP1 [48, 49, 50]. After its phosphorylation by mTOR, 4E-BP1 is inactivated, leading to its dissociation from eIF4E. Consequently, eIF4E can become phosphorylated on Ser209 by MNK1 and MNK2, triggering initiation of mRNA translation of-mitogenic proteins [51] (Figure 1). S6K regulates two downstream substrates, the S6 ribosomal protein (rpS6) and eukaryotic initiation factor 4B (eIF4B). eIF4B is recruited, along with eIF3, to contribute in the formation of the translation initiation complex [52].
Dysregulation of mTOR pathways plays an important role in tumorigenesis as shown by promotion of proliferation and survival of various types of malignant cells [31, 53]. In fact, PI3K/AKT/mTOR pathways were shown to promote leukemic cell proliferation and survival and have been implicated in resistance to antileukemic drugs [54, 55]. It has been reported that in 50-80% of AML cases there is constitutive activation of the PI3K and mTOR kinases [56]. Therefore, targeting the PI3K/AKT/mTOR pathway could improve pro-apoptotic an antiproliferative effects on hematological malignancies.
4. Strategies for targeting eIF4E in AML
4.1 Direct eIF4E inhibitors
The use of eIF4E anti-sense oligonucleotides was one of the first approaches used to specifically and directly target eIF4E, which inhibited the expression of eIF4E–regulated proteins, such as cyclin D1, c-Myc and Bcl-2, and induced apoptosis [57]. However, this approach does not appear to be clinically meaningful [58]. A more effective approach seems to be the use of the antiviral drug ribavirin. There is evidence that ribavirin mimics the m7G cap, binding eIF4E, and thus blocking its activity. A clinical trial with ribavirin has shown to reduce eIF4E activity and to induce partial remission in some patients with M4/M5 subtypes of AML [59, 60]. This approach appears to be promising, but future studies are warranted to confirm its value in a larger cohort of patients.
4.2 MNK inhibitors
Another strategy to reduce the activity of eIF4E is by developing compounds that target MNK and inhibit eIF4E phosphorylation on Ser209. Until now, only a few small-molecule inhibitors have been described. CGP57380 is a Mnk inhibitor which exhibits cytotoxic effects against cancer cells at low micromolar concentration, yet was also shown to inhibit other kinases [10, 61]. CGP57380 blocks eIF4E phosphorylation on Ser209, but also was shown to decrease rpS6 phosphorylation in CML cell lines [62]. Another Mnk inhibitor, cercosporamide, is a natural product isolated from Cercosporidium henningsii and was initially identified as a broad spectrum antifungal agent [63]. However, cercosporamide also targets other kinases, including Jak3 [64]. We have previously reported that cercosporamide suppresses tumor growth in an AML xenograft model [65]. Additionally, cercosporamide blocks MNK1/2-induced phosphorylation of eIF4E in human AML cells and such inhibitory effects correlate with decreased cell viability [65]. Most recently, Diab et al. [66] identified a series of 5-(2-(phenyloamino)pyrymidin-4-yl)thiazole-2(3H)-one derivatives as Mnk inhibitors, most of which showed potent MNK2 inhibition and resulted in reduced levels of the anti-apoptotic protein Mcl-1 and promoted apoptosis in MV4-11 AML cells.
4.3 Allosteric mTOR inhibitors
It has been known that deregulation of the mTOR pathway plays an important role in AML pathogenesis. Notably, constitutive activation of PI3K is essential for survival of AML blasts [67, 68]. This has provided the rationale for investigating the potential use of mTOR inhibitors in the treatment of AML. Initial studies investigated the antileukemic effects of the first generation of mTOR inhibitors, rapamycin and several analogs (everolimus, temsirolimus, ridaforolimus), which target mTORC1 [69]. It has been shown that RAD001 (everolimus) induced apoptosis and enhanced tumor necrosis factor-related apoptosis-inducing ligand (TRIAL) in Jurkat T leukemia cells [70]. In addition, CCI-779 (temsirolimus) has been shown to co-operate with ABT-737 (Bcl-2 family proteins inhibitor) to induce apoptotic cell death in acute lymphoblastic leukemia (ALL), which was accompanied by downregulation of Mcl-1 expression [71]. Unfortunately, the efficacy of rapamycin analogs in leukemia treatment has been clinically lower than expected [72, 73, 11]. Directly blocking mTORC1 activity results in only some cytostatic effects on AML cells in vitro [67]. In addition, rapamycin incompletely blocks 4E-BP1 phosphorylation, while inhibiting the negative feedback loops between mTORC1-S6K and AKT, thus resulting in activation of AKT that can promote cell survival [74, 75]. For all these reasons, the future clinical use of first generation mTOR inhibitors is unclear in leukemia.
4.4 Catalytic mTOR inhibitors
Because of the limited success of rapalogs, most recent therapeutic studies have been focused on developing mTOR catalytic inhibitors which can target both mTORC1 and mTORC2. PP242 and OSI-027 have both showed potent suppressive effects on cell viability in BCR-ABL transformed cell lines when compared with rapamycin treatment [39, 70]. This dual inhibition of the mTOR complexes has also demonstrated tumor growth suppressive effects in a human AML xenograft mouse model [69]. Moreover, PP242 treatment reduced colony formation of leukemic progenitor cells derived from CML patients [76] Additionally, in AML models, OSI-027 has shown anti-leukemic effects in cell lines, as well as, in primary leukemic precursors from patients with AML. Importantly, when compared to rapamycin treatment, OSI-027 was able to completely block phosphorylation of 4E-BP1 in AML cells [40]. Other recent studies have shown that Torin-2, a second-generation ATP-competitive inhibitor, induces apoptosis and autophagy in B-precursor acute lymphoblastic leukemia (B-pre ALL) cell lines [77].
4.5 Dual PI3K/mTOR inhibitors
Another potential approach to avoid negative feedback mechanisms is the development of inhibitors that can target both mTOR and PI3K, which is the main AKT-activating kinase [78]. PI-103, a dual PI3K/mTOR inhibitor, was found to strongly induce antiproliferative effects compared to rapamycin in various leukemia cell lines [79, 80, 81]. NVP-BEZ-235 is a new orally available inhibitor, which is currently under clinical investigation, including in acute leukemia (European Clinical Trials Database number EUDRACT2011-005050-61) [82]. These dual inhibitors were shown to fully inhibit the rapamycin-resistant phosphorylation of 4E-BP1, resulting in suppression of cell proliferation and induction of apoptosis in AML cells [83] and chronic lymphocytic leukemia (CLL) cells [84]. NVP-BEZ-235 induces cytostatic effects in resistant lymphoid malignant models, by reducing Mcl-1 expression and activating BAX (BCL2-Associated × Protein), which results in cellular death [85]. Further studies have also demonstrated that NVP-BEZ-235 induces apoptosis in primary AML blast cells, and inhibits growth/viability of AML cell lines [54]. Additionally, this PI3K/mTOR inhibitor delayed tumor growth and prolonged survival in an AML xenograft mouse model [56]. NVP-BGT226, a novel dual PI3K/mTOR inhibitor, has shown greater anti-leukemic and pro-apoptotic effects against a broad range of leukemic cells [86].
4.6 Combinatorial targeting of MNK-eIF4E-mTOR and other pathways in leukemia treatment
Based on most recent data there is strong evidence indicating that combinatorial inhibition of mTOR-eIF4E-MNK pathways may provide an important strategic advantage in cancer treatment [65, 87, 88]. Inhibition of mTORC1 pathways leads to activation of pro-survival pathways/negative feedback loops, which results in upregulation of eIF4E activity. Thus, combination of MNK inhibitors with rapalogs may provide anti-leukemic synergistic effects, by blocking eIF4E phosphorylation, which may result in greater anti-tumorigenic effects. In fact, cercosporamide and rapamycin treatment resulted in greater inhibition of colony formation of leukemic progenitor cells derived from AML cell lines and patients with AML, when compared to each drug used alone [65]. Additionally, Teo et al. [87] established that sensitivity of different leukemia cell lines to Mnk inhibitors correlates with the levels of phosphorylated 4E-BP1 at Thr70. Co-treatment of leukemic cells with Mnk inhibitors and rapamycin showed superior anti-leukemic effects compared to each drug alone [87], further suggesting that combined MNK and mTORC1 inhibition can provide an advantage in inducing antileukemic responses.
Bruton's tyrosine kinase (BTK) plays a key role in malignant cell proliferation and survival in several B-cell malignancies [89]. Recently, Wu et al. [90] suggested that dual inhibition of BTK and Mnks may provide an effective approach for the treatment of B-cell malignancies. The authors successfully developed a first selective dual BTK/MNK inhibitor, QL-X-138, which induced antiproliferative effects in leukemia cell lines in vitro, as well as, in CLL/AML-patient derived primary leukemia cells [90]. Another recent study showed that treatment of MLL-rearranged AML cells, which can lead to activation of both Ras/Raf/MEK/ERK and PI3K/AKT/mTOR pathways, with the dual PI3K/mTOR inhibitor NVP-BEZ-235 and selumetinib MEK-inhibitor strongly induces apoptosis in patient-derived cells [56]. Additionally, co-treatment with selumetinib and the catalytic mTOR inhibitor AZD8055 resulted in further induction of apoptosis in AML cell lines and primary AML samples, when compared to each drug alone [91].
In another study, co-treatment of BCR-ABL-positive leukemia cells with the dual PI3K/mTOR inhibitor NVP-BEZ-235 and nilotinib, a BCR-ABL kinase inhibitor, effectively blocked phosphorylation of 4E-BP1, AKT and S6K, induced cellular apoptosis, and suppressed colony formation and in vivo tumor growth [92]. Additionally, in primary blast cells from AML patients, targeting of AKT and mTOR by allosteric inhibitors was shown to induce AKT phosphorylation and activation of compensatory mechanisms, such as activation of receptor tyrosine kinase (RTK) pathways [93, 94]. Therefore, treatment of AML with RTK inhibitors (linstinib, sunitinib, guizartinib) in combination with dual PI3K/mTOR inhibitors could provide a better therapeutic strategy in the treatment of this disease [94].
5. Conclusions
Significant progress has been made to understand the role of MAPK and mTOR in oncogenic transformation and development. Recent evidence suggests a dysregulation of both of these pathways, in addition to either upstream or downstream effectors, in hematologic malignancies, which has led to development of new targeted therapeutics drugs. Nevertheless, there are still some limitations due to activation of multiple feedback loops and due to crosslinking between different pathways that hinder the effective targeted treatment of cancer. One approach that could improve potency of future anti-leukemic therapy is the use of multimodal treatment strategies. The combination of mTORC1/mTORC2 pathways inhibitors with MAPK signalling inhibitors warrant further development and clinical evaluation.
Acknowledgments
D.S. was supported by NIH/NCI training grant T32 CA080621. The research of LCP was supported by grants CA77816, CA155566, and CA121192 from the National Institutes of Health and by grant I01CX000916 from the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dash A, Gilliland DG. Molecular genetics of acute myeloid leukaemia. Best Pract Res Clin Haematol. 2001;14:49–64. doi: 10.1053/beha.2000.0115. [DOI] [PubMed] [Google Scholar]
- 2.Jackson RJ, Hellen CU, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. N Rev Mol Cell Biol. 2010;11:113–127. doi: 10.1038/nrm2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sonenberg N, Morgan MA, Merrick, Shatkin AJ. A polypeptide in eukaryotic initiation factors that crosslinks specifically to the 5′-terminal cap in mRNA. Proc Natl Acad Sci U S A. 1978;75:4843–7. doi: 10.1073/pnas.75.10.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Benedetti A, Graff JR. eIF4E expression and its role in malignancies and metastases. Oncogene. 2004;23:3189–3199. doi: 10.1038/sj.onc.1207545. [DOI] [PubMed] [Google Scholar]
- 5.Mamane Y, Petroulakis E, Rong L, Yoshida K, Ler LW, Sonenberg N. eIF4E — from translation to transformation. Oncogene. 2004;23:3172–3179. doi: 10.1038/sj.onc.1207549. [DOI] [PubMed] [Google Scholar]
- 6.Hinnebusch AG, Asano K, Olsen DS, Phan L, Nielsen KH, Valasek L. Study of translational control of eukaryotic gene expression using yeast. Ann N Y Acad Sci. 2004;1038:60–74. doi: 10.1196/annals.1315.012. [DOI] [PubMed] [Google Scholar]
- 7.Tarun SZ, Jr, Sachs AB. Association of the yeast poly(A) tail binding protein with translation initiation factor eIF-4G. EMBO J. 1996;15:7168–7177. [PMC free article] [PubMed] [Google Scholar]
- 8.Culjkovic B, Topisirovic I, Borden KL. Controlling gene expression through RNA regulons: The role of the eukaryotic translation initiation factor eIF4E. Cell Cycl. 2007;6:65–69. doi: 10.4161/cc.6.1.3688. [DOI] [PubMed] [Google Scholar]
- 9.Joshi S, Platanias LC. Mnk kinases in cytokine signaling and regulation of cytokine responses. Biomol Concepts. 2015;6:85. doi: 10.1515/bmc-2011-2000. [DOI] [PubMed] [Google Scholar]
- 10.Diab S, Kumarasiri M, Yu M, Teo T, Proud C, Milne R, Wang S. MAP Kinase-Interacting Kinases—Emerging Targets against Cancer. Chem Biol. 2014;21:441–52. doi: 10.1016/j.chembiol.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Bhat M, Robichaud N, Hulea L, Sonenberg N, Pelletier J, Topisirovic I. Targeting the translation machinery in cancer. Nat Rev Drug Discov. 2015 Apr;14(4):261–78. doi: 10.1038/nrd4505. [DOI] [PubMed] [Google Scholar]
- 12.Topisirovic I, Ruiz-Gutierrez M, Borden KL. Phosphorylation of the eukaryotic translation initiation factor eIF4E contributes to its transformation and mRNA transport activities. Cancer Res. 2004 Dec 1;64(23):8639–42. doi: 10.1158/0008-5472.CAN-04-2677. [DOI] [PubMed] [Google Scholar]
- 13.Gilliland DG, Jordan CT, Felix CA. The molecular basis of leukemia. Hematology Am Soc Hematol Educ Program. 2004:80–97. doi: 10.1182/asheducation-2004.1.80. [DOI] [PubMed] [Google Scholar]
- 14.Martelli AM, Evangelisti C, Chiarini F, Grimaldi C, Manzoli L, McCubrey JA. Targeting the PI3K/AKT/mTOR signaling network in acute myelogenous leukemia. Expert Opin Investig Drugs. 2009;18(9):1333–1349. doi: 10.1517/14728220903136775. [DOI] [PubMed] [Google Scholar]
- 15.Waskiewicz AJ, Flynn A, Proud CG, Cooper JA. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 1997;16:1909–1920. doi: 10.1093/emboj/16.8.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furic L, Rong L, Larsson O, Koumakpay IH, Yoshida K, Brueschke A, et al. eIF4E phosphorylation promotes tumorigenesis and is associated with prostate cancer progression. Proc Natl Acad Sci U S A. 2010;107:14134–9. doi: 10.1073/pnas.1005320107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Loghlen A, González VM, Piñeiro D, Pérez-Morgado MI, Salinas M, Martín ME. Identification and molecular characterization of Mnk1b, a splice variant of human MAP kinase-interacting kinase Mnk1. Exp Cell Res. 2004 Oct 1;299(2):343–55. doi: 10.1016/j.yexcr.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Scheper GC, Parra JL, Wilson M, Van Kollenburg B, Vertegaal AC, Han ZG, Proud CG. The N and C termini of the splice variants of the human mitogen-activated proteinkinase-interacting kinase Mnk2 determine activity and localization. Mol Cell Biol. 2003 Aug;23(16):5692–705. doi: 10.1128/MCB.23.16.5692-5705.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parra JL, Buxade M, Proud CG. Features of the catalytic domains and C termini of the MAPK signal-integrating kinases Mnk1 and Mnk2 determine their differing activities and regulatory properties. J Biol Chem. 2005;280:37623–37633. doi: 10.1074/jbc.M508356200. [DOI] [PubMed] [Google Scholar]
- 20.Scheper GC, van Wijk R, Thomas AM. Regulation of the activity of eukaryotic initiation factors in stressed cells. Prog Mol Subcell Biol. 2001;27:40–56. [PubMed] [Google Scholar]
- 21.O'Loghlen A, González VM, Jurado T, Salinas M, Martín ME. Characterization of the activity of human MAP kinase-interacting kinase Mnk1b. Biochim Biophys Acta. 2007 Sep;1773(9):1416–27. doi: 10.1016/j.bbamcr.2007.05.009. Epub 2007 May 29. [DOI] [PubMed] [Google Scholar]
- 22.Wendel HG, Silva RL, Malina A, Mills JR, Zhu H, Ueda T, et al. Dissecting eIF4E action in tumorgenesis. Genes Dev. 2007;21:3232–7. doi: 10.1101/gad.1604407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ueda T, Sasaki M, Elia AJ, Chio IIC, Hamada K, Fukunaga R, Mak TW. Combined deficiency for MAP kinase-interacting kinase 1 and 2 (Mnk1 and Mnk2) delays tumor development. Proc Natl Acad Sci USA. 2010;107:13984–13990. doi: 10.1073/pnas.1008136107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robichaud N, del Rincon SV, Huor B, Alain T, Petruccelli LA, Hearnden J. Phosphorylation of eIF4E promotes EMT and metastasis via translational control of SNAIL and MMP-3. Oncogene. 2015;34:2032–2042. doi: 10.1038/onc.2014.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shlush LI, Zandi S, Mitchell A, Chen WC, Brandwein JM, Gupta V, et al. Identification of pre-leukaemia haematopoietic stem cells in acute leukaemia. Nature. 2014 Feb 20;506(7488):328–33. doi: 10.1038/nature13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welch JS, Ley TJ, Link DC, Miller CA, Larson DE, et al. The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012;150(2):264–278. doi: 10.1016/j.cell.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Worch J, Tickenbrock L, Schwäble J, Steffen B, Cauvet T, Mlody B, et al. The serinethreonine kinase MNK1 is post-translationally stabilized by PML-RARalpha and regulates differentiation of hematopoietic cells. Oncogene. 2004 Dec 9;23(57):9162–72. doi: 10.1038/sj.onc.1208164. [DOI] [PubMed] [Google Scholar]
- 28.Lim S, Saw TY, Zhang M, Janes MR, Nacro K, Hill J, Lim AQ, et al. Targeting of the MNK-eIF4E axis in blast crisis chronic myeloid leukemia inhibits leukemia stem cell function. Proc Natl Acad Sci USA. 2013;110:E2298–E2307. doi: 10.1073/pnas.1301838110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–45. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 30.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012 Apr 13;149(2):274–93. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 32.Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 33.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 34.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Khokhar NZ, Altman JK, Platanias LC. Emerging roles for mammalian target of rapamycin inhibitors in the treatment of solid tumors and hematological malignancies. Curr Opin Oncol. 2011;23:578–86. doi: 10.1097/CCO.0b013e32834b892d. [DOI] [PubMed] [Google Scholar]
- 36.Oh WJ, Jacinto E. mTOR complex 2 signaling and functions. Cell Cycle. 2011 Jul 15;10(14):2305–16. doi: 10.4161/cc.10.14.16586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan QW, Cheng C, Knight ZA, Haas-Kogan D, Stokoe D, James CD, McCormick F, Shokat KM, Weiss WA. EGFR signals to mTOR through PKC and independently of Akt in glioma. Sci Signal. 2009;2:ra4. doi: 10.1126/scisignal.2000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Facchinetti V, Ouyang W, Wei H, Soto N, Lazorchak A, Gould C, Lowry C, et al. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J. 2008;27:1932–43. doi: 10.1038/emboj.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan L, Mieulet V, Lamb RF. mTORC2 is the hydrophobic motif kinase for SGK1. Biochem J. 2008;416:e19–21. doi: 10.1042/BJ20082202. [DOI] [PubMed] [Google Scholar]
- 40.Altman JK, Sassano A, Platanias LC. Targeting mTOR for the treatment of AML. New agents and new directions. Oncotarget. 2011;2:510–17. doi: 10.18632/oncotarget.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001 Nov;26(11):657–64. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- 42.Scheid MP, Woodgett JR. Phosphatidylinositol 3′ kinase signaling in mammary tumorigenesis. J Mammary Gland Biol Neoplasia. 2001 Jan;6(1):83–99. doi: 10.1023/a:1009520616247. [DOI] [PubMed] [Google Scholar]
- 43.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 44.Dibble CC, Elis W, Menon S, Qin W, Klekota J, Asara JM, et al. TBC1D7 is a third subunit of the TSC1-TSC2 complex upstream of mTORC1. Mol Cell. 2012 Aug 24;47(4):535–46. doi: 10.1016/j.molcel.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saleiro D, Platanias LC. Intersection of mTOR and STAT signaling in immunity. Trends Immunol. 2015 Jan;36(1):21–9. doi: 10.1016/j.it.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dazert E, Hall MN. mTOR signaling in disease. Curr Opin Cell Biol. 2011;23:744–55. doi: 10.1016/j.ceb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 47.Beauchamp EM, Platanias LC. The evolution of the TOR pathway and its role in cancer. Oncogene. 2013 Aug 22;32(34):3923–32. doi: 10.1038/onc.2012.567. [DOI] [PubMed] [Google Scholar]
- 48.Ma XM, Yoon SO, Richardson CJ, Julich K, Blenis J. SKAR links pre-mRNA splicing to mTOR/S6K1-mediated enhanced translation efficiency of spliced mRNAs. Cell. 2008;133:303–313. doi: 10.1016/j.cell.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 49.Raught B, Peiretti F, Gingras AC, Livingstone M, Shahbazian D, Mayeur GL, et al. Phosphorylation of eucaryotic translation initiation factor 4B Ser422 is modulated by S6 kinases. EMBO J. 2004;23:1761–1769. doi: 10.1038/sj.emboj.7600193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang X, Li W, Williams M, Terada N, Alessi DR, Proud CG. Regulation of elongation factor 2 kinase by p90(RSK1) and p70 S6 kinase. EMBO J. 2001;20:4370–4379. doi: 10.1093/emboj/20.16.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gingras AC, Gygi SP, Raught B, et al. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev. 1999;13:1422–37. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holz MK, Ballif BA, Gygi SP, et al. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2005 Nov 18;123(4):569–80. doi: 10.1016/j.cell.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 53.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 54.Bertacchini J, Guida M, Accordi B, Mediani L, Martelli AM, Barozzi P. Feedbacks and adaptive capabilities of the PI3K/Akt/mTOR axis in acute myeloid leukemia revealed by pathway selective inhibition and phosphoproteome analysis. Leukemia. 2014 Nov;28(11):2197–205. doi: 10.1038/leu.2014.123. [DOI] [PubMed] [Google Scholar]
- 55.Fransecky L, Mochmann LH, Baldus CD. Outlook on PI3K/AKT/mTOR inhibition in acute leukemia. Mol Cell Ther. 2015 Mar 20;3:2. doi: 10.1186/s40591-015-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sandhöfer N, Metzeler KH, Rothenberg M, Herold T, Tiedt S, Groiß V. Dual PI3K/mTOR inhibition shows antileukemic activity in MLL-rearranged acute myeloid leukemia. Leukaemia. 2015 Apr;29(4):828–38. doi: 10.1038/leu.2014.305. [DOI] [PubMed] [Google Scholar]
- 57.Graff JR, Konicek BW, Vincent TM, Lynch RL, Monteith D, Weir SN, et al. Therapeutic suppression of translation initiation factor eIF4E expression reduces tumor growth without toxicity. J Clin Invest. 2007;117:2638–48. doi: 10.1172/JCI32044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hong DS, Kurzrock R, Oh Y, Wheler J, Naing A, Brail L, et al. A phase one dose selection, pharmacokinetic, and pharmacodynamics evaluation of eIF-4E antisense oligonucleotide LY2275796 in patients with advanced cancer. Clin Cancer Res. 2011;17:6582–91. doi: 10.1158/1078-0432.CCR-11-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Assouline S, Culjkovic B, Cocolakis E, Rousseau C, Beslu N, Amri A, et al. Molecular targeting of the oncogene eIF4E in acute myeloid leukemia (AML): a proof-of-principle clinical trial with ribavirin. Blood. 2009;114:257–60. doi: 10.1182/blood-2009-02-205153. [DOI] [PubMed] [Google Scholar]
- 60.Borden KL. Targeting the oncogene eIF4E in cancer: from the bench to clinical trials. Clin Invest Med. 2011;34:E315. doi: 10.25011/cim.v34i6.15889. [DOI] [PubMed] [Google Scholar]
- 61.Yu M, Li P, C Basnet SK, Kumarasiri M, Diab S, Teo T, Albrecht H, Wang S. Discovery of 4-(dihydropyridinon-3-yl)amino-5-methylthieno[2,3-d] pyrimidine derivatives as potent Mnk inhibitors: synthesis, structure, structure-activity relationship analysis and biological evaluation. Eur J Med Chem. 2015 May 5;95:116–26. doi: 10.1016/j.ejmech.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 62.Zhang M, Fu W, Prabhu S, Moore JC, Ko J, Kim JW, Druker BJ, et al. Inhibition of polysome assembly enhances imatinib activity against chronic myelogenous leukemia and overcomes imatinib resistance. Mol Cell Biol. 2008;28:6496–6509. doi: 10.1128/MCB.00477-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sussman A, Huss K, Chio LC, Heidler S, Shaw M, Ma D, Zhu G, Campbell RM, Park TS, Kulanthaivel P, Scott JE, Carpenter JW, Strege MA, Belvo MD, Swartling JR, Fischl A, et al. Discovery of cercosporamide, a known antifungal natural product, as a selective Pkc1 kinase inhibitor through high-throughput screening. Eukaryotic cell. 2004;3(4):932–943. doi: 10.1128/EC.3.4.932-943.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Konicek BW, Stephens JR, McNulty AM, Robichaud N, Peery RB, Dumstorf CA, et al. Therapeutic inhibition of MAP kinase interacting kinase blocks eukaryotic initiation factor 4E phosphorylation and suppresses outgrowth of experimental lung metastases. Cancer Res. 2011;71:1849–57. doi: 10.1158/0008-5472.CAN-10-3298. [DOI] [PubMed] [Google Scholar]
- 65.Altman JK, Szilard A, Konicek BW, Iversen PW, Kroczynska B, Glaser H, et al. Inhibition of Mnk kinase activity by cercosporamide and suppressive effects on acute myeloid leukemia precursors. Blood. 2013;121:3675–81. doi: 10.1182/blood-2013-01-477216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Diab S, Teo T, Kumarasiri M, Li P, Yu M, Lam F, Basnet SK, Sykes MJ, Albrecht H, Milne R, et al. Discovery of 5-(2-(phenylamino)pyrimidin-4-yl)thiazol-2 (3H)-one derivatives as potent Mnk2 inhibitors: synthesis, SAR analysis and biological evaluation. Chem Med Chem. 2014 May;9(5):962–72. doi: 10.1002/cmdc.201300552. [DOI] [PubMed] [Google Scholar]
- 67.Recher C, Beyne-Rauzy O, Demur C, et al. Antileukemic activity of rapamycin in acute myeloid leukemia. Blood. 2005;105:2527–34. doi: 10.1182/blood-2004-06-2494. [DOI] [PubMed] [Google Scholar]
- 68.Xu Q, Simpson SE, Scialla TJ, et al. Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood. 2003;102:972–80. doi: 10.1182/blood-2002-11-3429. [DOI] [PubMed] [Google Scholar]
- 69.Janes MR, Limon JJ, So L, Chen J, Lim RJ, Chavez MA, et al. Effective and selective targeting of leukemia cells using a TORC1/2 kinase inhibitor. Nat Med. 2010;16:205–213. doi: 10.1038/nm.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee MW, Kim DS, Eom JE, Ko YJ, Sung KW, Koo HH, Yoo KH. RAD001 (everolimus) enhances TRAIL cytotoxicity in human leukemic Jurkat T cells by upregulating DR5. Biochem Biophys Res Commun. 2015 Aug 7;463(4):894–9. doi: 10.1016/j.bbrc.2015.05.133. [DOI] [PubMed] [Google Scholar]
- 71.Iacovelli S, Ricciardi MR, Allegretti M, Mirabilii S, Licchetta R, Bergamo P. Co- targeting of Bcl-2 and mTOR pathway triggers synergistic apoptosis in BH3 mimetics resistant acute lymphoblastic leukemia. Oncotarget. 2015 Oct 13;6(31):32089–10. doi: 10.18632/oncotarget.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Callera F, Lopes CO, Rosa ES, et al. Lack of antileukemic activity of rapamycin in elderly patients with acute myeloid leukemia evolving from a myelodysplastic syndrome. Leuk Res. 2008;32:1633–4. doi: 10.1016/j.leukres.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 73.Menon S, Manning BD. Common corruption of the mTOR signalling network in human tumors. Oncogene. 2009;27(Suppl 2):S43–51. doi: 10.1038/onc.2009.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choo AY, Blenis J. Not all substrates are treated equally: implications for mTOR, rapamycin-resistance and cancer therapy. Cell Cycle. 2009 Feb 15;8(4):567–72. doi: 10.4161/cc.8.4.7659. [DOI] [PubMed] [Google Scholar]
- 75.Hsu PP, Kang SA, Rameseder J, Zhang Y, Ottina KA, Lim D, et al. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science. 2011 Jun 10;332(6035):1317–22. doi: 10.1126/science.1199498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsai BP, Jimenez J, Lim S, Fitzgerald KD, Zhang M, Chuah CT. A novel Bcr-Abl-mTOR-eIF4A axis regulates IRES-mediated translation of LEF-1. Open Biol. 2014 Nov;4(11):140180. doi: 10.1098/rsob.140180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simioni C, Cani A, Martelli AM, Zauli G, Tabellini G, McCubrey J. Activity of the novel mTOR inhibitor Torin-2 in B-precursor acute lymphoblastic leukemia and its therapeutic potential to prevent Akt reactivation. Oncotarget. 2014 Oct 30;5(20):10034–47. doi: 10.18632/oncotarget.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chiarini F, Fala F, Tazzari PL, Ricci F, Astolfi A, Pession A, et al. Dual inhibition of class IA phosphatidylinositol 3-kinase and mammalian target of rapamycin as a new therapeutic option for T-cell acute lymphoblastic leukemia. Cancer Res. 2009;69:3520–3528. doi: 10.1158/0008-5472.CAN-08-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kharas MG, Janes MR, Scarfone VM, et al. Ablation of PI3K blocks BCR-ABL leukemogenesis in mice, and a dual PI3K/mTOR inhibitor prevents expansion of human BCR-ABL+ leukemia cells. J Clin Invest. 2008;118:3038–50. doi: 10.1172/JCI33337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kojima K, Shimanuki M, Shikami M, et al. The dual PI3 kinase/mTOR inhibitor PI-103 prevents p53 induction by Mdm2 inhibition but enhances p53-mediated mitochondrial apoptosis in p53 wild-type AML. Leukemia. 2008;22:1728–36. doi: 10.1038/leu.2008.158. [DOI] [PubMed] [Google Scholar]
- 82.Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, Brachmann S, Chene P, De Pover A, Schoemaker K. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008 Jul;7(7):1851–63. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 83.Chapuis N, Tamburini J, Green AS, Vignon C, Bardet V, et al. Dual inhibition of PI3K and mTORC1/2 signaling by NVPBEZ235 as a new therapeutic strategy for acute myeloid leukemia. Clin Cancer Res. 2010 Nov 15;16(22):5424–35. doi: 10.1158/1078-0432.CCR-10-1102. [DOI] [PubMed] [Google Scholar]
- 84.Shull AY, Noonepalle SK, Awan FT, Liu J, Pei L, Bollag RJ. RPPA based protein profiling reveals eIF4G overexpression and 4EBP1 serine 65 phosphorylation a molecular events that correspond with a pro-survival phenotype in chronic lymphocytic leukemia. Oncotarget. 2015 Jun 10;6(16):14632–45. doi: 10.18632/oncotarget.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Choudhary GS, Al-Harbi S, Mazumder S, Hill BT, Smith MR, Bodo J, Hsi ED, Almasan A. MCL-1 and BCL-xL-dependent resistance to the BCL-2 inhibitor ABT-199 can be overcome by preventingPI3K/AKT/mTOR activation in lymphoid malignancies. Cell Death Dis. 2015 Jan 15;6:e1593. doi: 10.1038/cddis.2014.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kampa-Schittenhelm KM, Heinrich MC, Akmut F, et al. Cell cycle-dependent activity of the novel dual PI3KMTORC1/2 inhibitor NVP-BGT226 in acute leukemia. Mol Cancer. 2013;12:46. doi: 10.1186/1476-4598-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Teo T, Yu M, Yang Y, Gillam T, Lam F, Sykes MJ, Wang S. Pharmacologic co inhibition of Mnks and mTORC1 synergistically suppresses proliferation and perturbs cell cycle progression in blast crisis-chronic myeloid leukemia cells. Cancer Lett. 2015 Feb 28;357(2):612–23. doi: 10.1016/j.canlet.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 88.Eckerdt F, Beauchamp E, Bell J, Iqbal A, Su B, Fukunaga R, Platanias LC. Regulatory effects of a Mnk2-eIF4E feedback loop during mTORC1 targeting of human medulloblastoma cells. Oncotarget. 2014 Sep 30;5(18):8442–51. doi: 10.18632/oncotarget.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hendriks RW, Yuvaraj S, Kil LP. Targeting Bruton's tyrosine kinase in B cell malignancies. Nat Rev Cancer. 2014 Apr;14(4):219–32. doi: 10.1038/nrc3702. [DOI] [PubMed] [Google Scholar]
- 90.Wu H, Hu C, Wang A, Weisberg EL, Chen Y, Yun CH, Wang W. Discovery of a BTK/MNK dual inhibitor for lymphoma and leukemia. Leukemia. 2016 Jan;30(1):173–81. doi: 10.1038/leu.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang W, Ruvolo VR, Gao C, Zhou L, Bornmann W, Tsao T, Schober WD, Smith P. Evaluation of apoptosis induction by concomitant inhibition of MEK, mTOR, and Bcl-2 in human acutemyelogenous leukemia cells. Mol Cancer Ther. 2014 Jul;13(7):1848–59. doi: 10.1158/1535-7163.MCT-13-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Okabe S, Tauchi T, Tanaka Y, Kitahara T, Kimura S, Maekawa T, Ohyashiki K. Efficacy of the dual PI3K and mTOR inhibitor NVP-BEZ235 in combination with nilotinib against BCR-ABL-positive leukemia cells involves the ABL kinase domain mutation. Cancer Biol Ther. 2014 Feb;15(2):207–15. doi: 10.4161/cbt.26725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bertacchini J, Guida M, Accordi B, Mediani L, Martelli AM, Barozzi P. Feedbacks and adaptive capabilities of the PI3K/Akt/mTOR axis in acute myeloid leukemia revealed by pathway selective inhibition and phosphoproteome analysis. Leukemia. 2014;28(11):2197–2205. doi: 10.1038/leu.2014.123. [DOI] [PubMed] [Google Scholar]
- 94.Bertacchini J, Heidari N, Mediani L, Capitani S, Shahjahani M, Ahmadzadeh A, Saki N. Targeting PI3K/AKT/mTOR network for treatment of leukemia. Cell Mol Life Sci. 2015 Jun;72(12):2337–47. doi: 10.1007/s00018-015-1867-5. [DOI] [PMC free article] [PubMed] [Google Scholar]