Abstract
Background
Growing evidence points to a key role for somatostatin (SST) in schizophrenia (SZ) and bipolar disorder (BD). In the amygdala, neurons expressing SST play an important role in the regulation of anxiety, often comorbid in these disorders. We tested the hypothesis that SST-immunoreactive (IR) neurons are decreased in the amygdala of subjects with SZ and BD. Evidence for circadian SST expression in the amygdala and disrupted circadian rhythms and rhythmic peaks of anxiety in BD suggest a disruption of rhythmic expression of SST in this disorder.
Methods
Amygdala sections from 12 SZ, 15 BD, and 15 control subjects were processed for immunocytochemistry for SST and neuropeptide Y (NPY), a neuropeptide partially co-expressed in SST-IR neurons. Total numbers (Nt) of IR neurons were measured. Time of death (TOD) was used to test associations with circadian rhythms.
Results
SST-IR neurons were decreased in the lateral amygdala nucleus in BD (Nt, p= 0.003) and SZ (Nt, p=0.02). In normal controls, Nt of SST-IR neurons varied according to TOD. This pattern was altered in BD, characterized by decreases of SST-IR neurons selectively in subjects with TOD corresponding to the day (06:00–17:59). Numbers of NPY-IR neurons were not affected.
Conclusions
Decreased SST-IR neurons in the amygdala of SZ and BD, interpreted here as decreased SST expression, may disrupt responses to fear and anxiety regulation in these subjects. In BD, our findings raise the possibility that morning peaks of anxiety depend on a disruption of circadian regulation of SST expression in the amygdala.
Keywords: anxiety, stress, mood disorder, circadian, interneuron, GABA
Introduction
Growing evidence indicates expression of somatostatin (SST) and neuropeptide-Y (NPY) in amygdala neurons plays key roles in fear and stress responses, and in modulation of anxiety (1–6). Intraventricular, and intra-amygdala infusions of SST in rodents result in anxiolytic and antidepressant effects (1, 7). Mice lacking SST display increased anxiety-like behaviors (2), and neuroendocrine and molecular abnormalities reported in subjects with anxiety and depression (8). NPY infusion counteracts the effects of corticotropin releasing factor (CRF) (4, 5, 9), a molecule essential for stress response (10, 11). In the amygdala, NPY levels decrease following restraint stress (12). Together, these observations point to marked anxiolytic effects of SST and NPY, with prominent involvement of amygdalar circuitry.
Severe anxiety is often comorbid in schizophrenia (SZ) and bipolar disorder (BD) (13–15). Approximately 38% of subjects with SZ and 50% with BD meet criteria for anxiety (16, 17). In both disorders, anxiety is associated with more severe symptoms and/or poorer treatment responses (16, 17). Findings from postmortem and genetic studies suggest abnormal SST and NPY signaling in SZ and BD (18–23). A somatostatin receptor SSTR5 genetic polymorphism has been associated with BD (24). Decreased SST and NPY mRNA was reported in the prefrontal cortex in SZ (18–20). In two of these studies, SST showed the largest decrease with respect to all other interneuron markers examined in SZ (19, 20). Furthermore, decreased SST mRNA was observed in the orbitofrontal cortex (21), and decreased SST mRNA and SST-IR neurons were observed in the hippocampus in both SZ and BD (22, 23). In a quantitative meta-analysis of gene expression studies in BD, SST was identified as one of the most consistently decreased genes (25). Surprisingly, the hypothesis that amygdala neurons expressing SST and NPY are impacted in SZ and BD has not been tested thus far.
Although anxiety represents a shared phenotype in SZ and BD, some differences are notable. For instance, SZ is commonly associated with social phobias, followed by post-traumatic stress disorder and obsessive compulsive disorder (16), while panic disorders and generalized anxiety disorder are common in BD (17). Furthermore, anxiety in BD may be linked more distinctly to disease states, such as depression and, notably, circadian rhythm dysfunction (26–33). In BD, the most severe anxiety and depression symptoms commonly occur in the morning (34–37) (“morning-worse“), with a less common peak in the evening (“evening-worse”) (34–36), suggesting a circadian component to severity. Consistent with these observations, mounting evidence supports a role for circadian rhythm abnormalities in BD (27–32). Sleep and biological rhythms are implicated in this disorder, and genetic polymorphisms for clock-associated genes are associated with BD and lithium responsiveness (32, 38–44). Notably, the most effective treatments, lithium and valproic acid, lengthen circadian period and modulate the expression of clock genes (45–49). A link between SST and circadian rhythms in BD is suggested by evidence that SST is decreased in cerebrospinal fluid (CSF) sampled in the morning, but not in samples taken in the evening, from the same subjects (50).
Together, these considerations support the hypothesis that SST and NPY expression is decreased in the amygdala of SZ and BD subjects. In BD, altered amygdalar SST expression may be associated with circadian dysfunction (34–37). The observation that SST and NPY expression in the rodent amygdala varies in a circadian manner (2) supports this possibility. The present studies tested the hypothesis that SST- and NPY- immunoreactive (IR) neurons are reduced in the amygdala of subjects with SZ or BD, and that reductions in BD are pronounced in the morning, coinciding with reported increased severity of anxiety and depression at this time (34–37).
Methods and Materials
Human Subjects
Tissue blocks containing the whole amygdala from 12 SZ, 15 BD and 15 normal control donors were obtained from the Harvard Brain Tissue Resource Center, McLean Hospital, Belmont, MA (Tables 1 and S3). Diagnoses were made by two psychiatrists on the basis of retrospective review of medical records and extensive questionnaires concerning social and medical history provided by family members. A neuropathologist examined several regions from each brain for a neuropathology report. The cohort for this study did not include subjects with evidence for gross and/or macroscopic brain changes, or clinical history consistent with cerebrovascular accident or other neurological disorders. Subjects with Braak & Braak stages III or higher were not included. Subjects had no significant history of substance dependence, other than nicotine and alcohol, within 10 years from death.
Table 1.
Disease-Related Descriptive Characteristics
| case | AGE OF ONSET | Duration of illness (years) | CPZ (g) | CPZ last 6 (g) | LI (g) | LI last 6 months (g) | nicotine | ethanol | ECT | SSRI | VPA (g) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SCHIZOPHRENIA | |||||||||||
| 4375 | 19 | 12 | 1654 | 171 | 0 | 0 | 0 | 0 | 0 | no | 0 |
| 4496 | 19 | 30 | 801.5 | 36 | 0 | 0 | 1 | 2 | 2 | no | 913.1 |
| 4544 | 16 | 16 | 671 | 30 | 0 | 0 | 3 | 2 | 0 | no | 0 |
| 4548 | - | - | 0 | 0 | 0 | 0 | - | - | 0 | no | 0 |
| 4702 | 35 | 20 | 793 | 27 | 0 | 0 | 1 | 2 | 2 | yes | 0 |
| 4707 | 38 | 22 | 365.2 | 0 | 0 | 0 | 1 | NA | 0 | yes | 0 |
| 4907 | 22 | 51 | 498 | 24 | 0 | 0 | 3 | 0 | 4 | yes | 0 |
| 4942 | 20 | 41 | 3550 | 36.18 | 0 | 0 | 2 | 2 | 3 | yes | 0 |
| 5100 | 24 | 48 | 420 | 31.5 | 0 | 0 | 1 | 0 | 0 | yes | 0 |
| 5656 | 19 | 54 | 37 | 0 | 36.8 | 0 | 2 | 0 | 0 | no | 0 |
| 5785 | 31 | 31 | 1169 | 162 | 0 | 0 | 0 | 3 | 1 | yes | 1370 |
| 5920 | 20 | 38 | 2430 | 81 | 648 | 108 | 3 | - | 0 | no | 730 |
| case | AGE OF ONSET | Duration of illness (years) | CP Z (g) | CPZ last 6 (g) | LI (g) | LI last 6 months (g) | nicotine | ethanol | ECT | SSRI | VPA (g) |
| BIPOLAR DISORDER | |||||||||||
| 4403 | 67 | 9 | 77.9 | 2.7 | 1321.0 | 27.0 | 4 | 4 | 0 | no | 0 |
| 4464 | 52 | 22 | 102.5 | 29.25 | 3945 | 0 | 1 | 2 | 0 | no | 547.9 |
| 4518 | - | - | 0 | 0 | 0 | 0 | - | - | 0 | - | - |
| 4545 | 30 | 43 | 10.8 | 3.6 | 0 | 0 | 1 | 0 | 0 | no | 0 |
| 4661 | 15 | 10 | 328.7 | 90 | 3945 | 216 | 3 | 2 | 0 | yes | 0 |
| 4665 | 35 | 31 | 731.3 | 1.0 | 1096.0 | 54.0 | 4 | 0 | 0 | no | 0 |
| 4914 | 20 | 53 | 202.7 | 13.95 | 4273.0 | 0 | 1 | 0 | 0 | no | 0 |
| 4928 | 27 | 48 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | no | 0 |
| 5044 | 35 | 38 | 82.2 | 20.3 | 2191.5 | 0 | 0 | 0 | 0 | no | 182.6 |
| 5265 | 43 | 40 | 0 | 0 | 2190.0 | 0 | - | 0 | 0 | yes | 803.6 |
| 5357 | 50 | 12 | 0 | 0 | 216.0 | 0 | 1 | 0 | 0 | yes | 292.2 |
| 5391 | 23 | 44 | 237.5 | 54 | 4131 | 0 | 4 | 2 | 3 | no | 1825 |
| 5431 | 22 | 18 | 1116 | 55.8 | 4320 | 0 | 2 | 4 | 0 | - | - |
| 5802 | 33 | 18 | 1242.0 | 81.0 | 0 | 0 | 1 | 4 | yes | 912 | |
| 5888 | 27 | 43 | 0 | 0 | 3240 | 0 | 4 | 4 | 3 | no | 456.25 |
Tissue Processing (see Supplemental Materials)
Immunocytochemistry
Free-floating tissue sections were carried through antigen retrieval in citric acid buffer (0.1 M citric acid, 0.2 M Na2HPO4) heated to 80 degrees °C for 30 minutes, and incubated in primary antibody monoclonal rat anti-SST (1:500, Millipore, MAB354, lot# NG1934075) raised against synthetic SST peptide corresponding to amino acids 1–14; rabbit anti-NPY (1:1000, Chemicon, AB1915, lot#0604027825), raised against synthetic porcine neuropeptide tyrosine) and subsequently in biotinylated secondary antibody (SST, goat anti-rat IgG; 1:500; Vector Labs, Inc. Burlingame, CA; NPY, goat anti-rabbit IgG (1:500; Vector Labs, Inc. Burlingame, CA), followed by streptavidin conjugated with horse-radish peroxidase for two hours (1:5000 μl, Zymed, San Francisco, CA), and, finally, in nickel-enhanced diaminobenzidine/ peroxidase reaction (0.02% diaminobenzidine, Sigma-Aldrich, 0.08% nickel-sulphate, 0.006% hydrogen peroxide in PB). All solutions were made in PBS with 0.5% Triton X (PBS-Tx) unless otherwise specified.
Immunostained sections were mounted on gelatin-coated glass slides, coverslipped and coded for blinded quantitative analysis. All sections included in the study were processed simultaneously within the same session to avoid procedural differences. Each six-well staining dish contained sections from SZ, BD and control subjects and was carried through each step for the same duration of time. Omission of the primary or secondary antibodies did not result in detectable signal. The primary antibody for SST has been shown to have no cross-reactivity with enkephalins, endorphins, or substance P (Spec sheet, Millipore Corporation, Temecula, CA). Specificity of these antibodies was confirmed by our group (Supplemental Materials) and others (51).
Data Collection
SST- and NPY-IR neurons were counted in the lateral (LN), basal (BN), accessory basal (AB) and cortical (CO) nuclei of the amygdala using a Zeiss Axioskop-2 Plus interfaced with Stereo Investigator 6.0 (Microbrightfield Inc., Willinston, VT). Intra-rater (J.W.) reliability of at least 95% was established before formal data collection and reassessed regularly. The borders of amygdala nuclei (Fig. 1) were traced and confirmed in adjacent Nissl stained sections according to cytoarchitectonic criteria described by Amaral et al, 1992 and Sims and Williams, 1990 (52, 53). The nomenclature adopted was used by Sorvari et 1995 (54). The central, medial and anterior nuclei could not be quantified because their dorso-medial portion was damaged in several samples. Each traced nucleus was systematically scanned through the full x, y, and z-axes to count each SST- and NPY-IR neuron over complete sets of serial sections (6–10 sections) representing the whole extent of the amygdala from each subject (section interval 1040 μm).
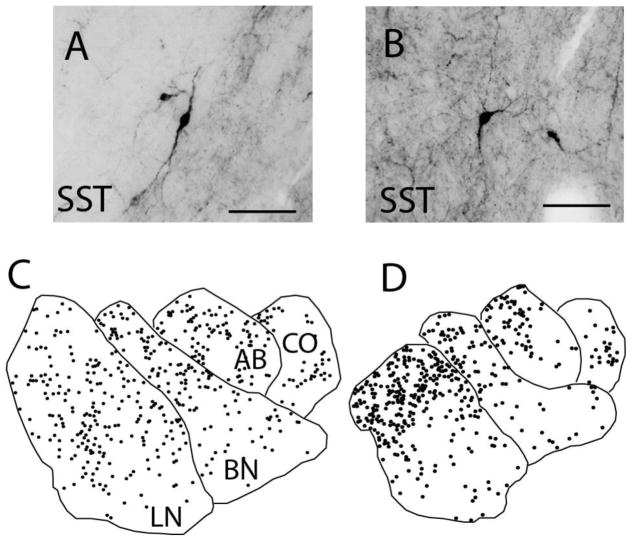
Figure 1. Somatostatin Immunoreactive Neurons in the Human Amygdala.
Somatostatin immunoreactive (SST-IR) neurons display various morphological subtypes in the human amygdala, including fusiform (A) and multipolar (B) neurons. SST-IR neurons are widely distributed across all of the amygdala nuclei examined, as shown by representative plots from a control subject depicting the distribution of SST-IR neurons in the rostral (C) and caudal (D) amygdala. Scale bars = 50 μm. LN: lateral nucleus, BN: basal nucleus, AB: accessory basal nucleus, CO: cortical nucleus.
Statistical Analysis
Differences between groups relative to the main outcome measures were assessed for statistical significance using stepwise linear regression (ANCOVA). Effect sizes were calculated according Hedges’ g. Logarithmic transformation was uniformly applied to all values because data were not normally distributed. Statistical analyses were performed using JMP v5.0.1a (SAS Institute Inc., Cary, NC). BD and SZ were compared separately to normal controls. Potential confounds (Supplemental Materials) were tested systematically for their effects on main outcome measures, and included in the model if they significantly improved goodness-of-fit. Time of death (TOD) was obtained from the death certificate for each subject and tested for potential effects on outcome measures. TOD was also used to divide subjects into subjective day (s-Day TOD, 06:00–17:59 hours) and subjective night (s-Night, 18:00–05.59 hours) groups on the basis of previous literature indicating circadian fluctuations in SST levels (50, 55, 56). Effects of TOD on outcome measures were analyzed using three steps: 1) Effect of TOD was tested in stepwise linear regression analyses. 2) Subjects were divided into s-Day vs. s-Night groups for comparisons using stepwise linear regression analysis 3) We used quartic regression analysis on plots of Nt of SST-IR neurons by TOD for each group according to methods used to detect similar relationships in postmortem studies (57–59). Quartic regression models were chosen on the basis of expression patterns reported in the mouse amygdala consisting of two peaks and two troughs in SST expression levels (2). Quartic regression, commonly used to fit plots consisting of four real roots, or x-intercepts of a graph, represented by two expected peaks, has been used to fit dual-peak circadian rhythms in several studies (60–63). The t-ratios and p-values for all main outcome comparisons are reported in Supplementary Table S2. Covariates found to significantly affect outcome measures are also reported.
Numerical Densities and Total Numbers Estimates
Total number (Nt) of IR neurons was calculated as Nt = i • Σn where Σn = sum of the cells counted in each subject, and i is the section interval (i.e. number of serial sections between each section and the next within each compartment=26) as described previously (64). Numerical densities were calculated as Nd= ΣN / ΣV where V is the volume of each amygdala nucleus, calculated as V= z • ssf • Σ a where z is the thickness of the section (40 μm), ssf is the section sampling fraction (1/26; i.e. number of serial sections between each section within a compartment) and a is the area of the region of interest.
Results
Amygdala Volumes
Volumetric results confirm previous findings (64). In subjects with SZ, no volume changes were detected (Supplementary Fig. 1). A significant effect of hemisphere was observed on the volume of the overall amygdala. In subjects with BD, significant volume decreases were detected in the LN (p<0.005, g=−1.22; adjusted for a significant effect of lifetime exposure to CPZ), and CO (p<0.007, g=−1.09) (Supplementary Fig. 1).
SST-IR Neurons
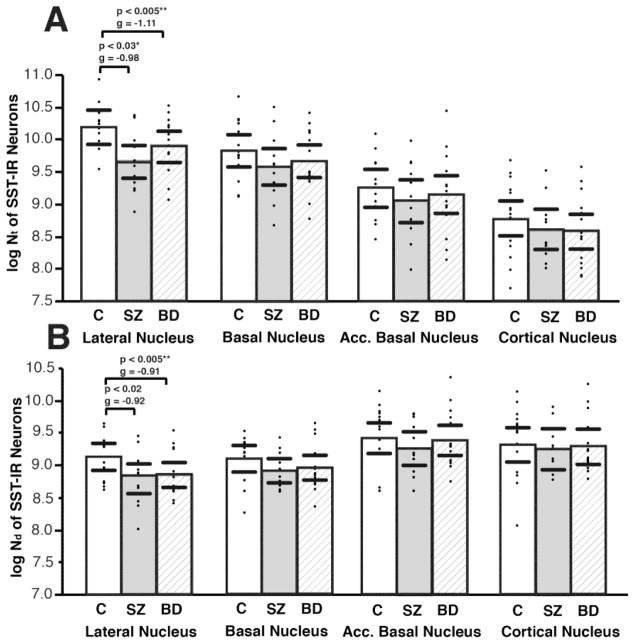
In the healthy human amygdala, SST-IR neurons were detected in all amygdala nuclei examined, with the highest numbers in the LN and the highest densities in the AB and CO (Figure 1, Supplementary Table S1), consistent with observations in primates (3). In subjects with SZ, Nt and Nd of SST-IR neurons were decreased selectively in the LN (Nt, p= 0.03, g=−0.98; adjusted for hemisphere p=0.03; Nd, p = 0.02, g=−0.92; Figure 2, Supplementary Table S2). Similarly, in subjects with BD, SST-IR neurons were decreased in the LN (Nt, p = 0.005, g=−1.11; Nd, p = 0.005, g=−0.91; Figure 2, Supplementary Table S2, with a significant effect of TOD, p<0.005). In both disease groups, decreases of SST-IR neurons did not correlate with years of illness, and were not affected by exposure to antipsychotics, lithium, SSRIs, alcohol or nicotine (Supplemental Materials). No changes were observed in any of the other amygdala nuclei examined (Figure 2, Supplementary Table S2).
Figure 2. Decreased Total Numbers and Numerical Densities of Somatostatin Neurons in the Lateral Nucleus of SZ and BD Subjects.
Scatterplots depicting total numbers (Nt) (A), and numerical densities (Nd) of SST-IR neurons in control, SZ, and BD subjects. Significant decreases of Nt (A) and Nd (B) were detected in the lateral nucleus of SZ and BD subjects. Significance values are derived from stepwise linear regression models. Scatterplots show the mean (histogram) and 95% confidence intervals (black lines). *Adjusted for significant effect of hemisphere. ** Adjusted for significant effect of time of death.
NPY-IR Neurons
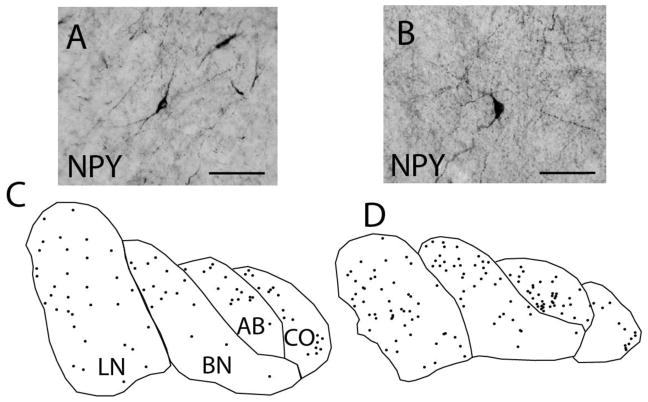
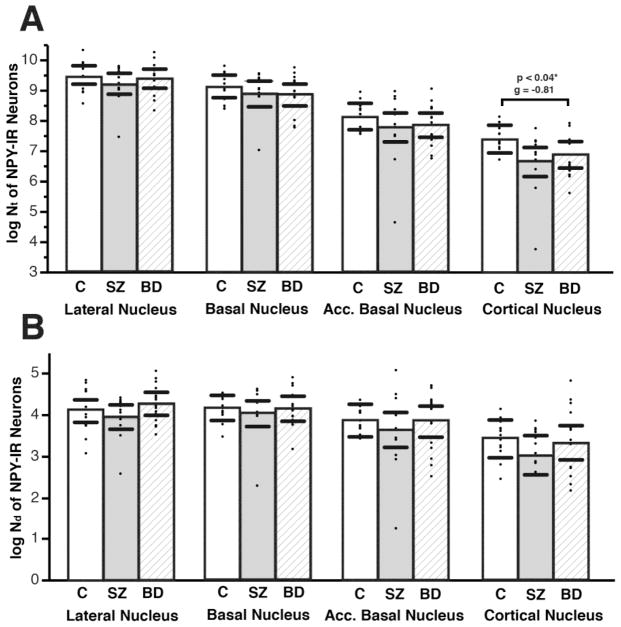
NPY-IR neurons are less numerous than SST-IR neurons, and evenly distributed across amygdala nuclei (Figure 3, Supplementary Table S1). In subjects with SZ, no changes were detected in any of the nuclei examined. In subjects with BD, a marginally significant decrease of Nt, but not Nd, of NPY-IR neurons was detected in the CO (Nt, p<0.04, g= −0.81, adjusted for PMI) (Figure 4, Supplementary Table S2).
Figure 3. Neuropeptide-Y Immunoreactive Neurons in the Human Amygdala.
Neuropeptide-Y immunoreactive (NPY-IR) neurons morphological subtypes in the human amygdala, including fusiform (A) and multipolar (B) neurons. NPY-IR neurons are widely distributed across all of the amygdala nuclei examined as shown by representative plots from a control subject depicting the distribution of NPY-IR neurons in the rostral (C) and caudal (D) amygdala. Scale bars = 50 μm. LN: lateral nucleus, BN: basal nucleus, AB: accessory basal nucleus, CO: cortical nucleus.
Figure 4. Total Numbers and Numerical Densities of Neuropeptide-Y Immunoreactive Neurons Are Not Altered in the Amygdala of SZ or BD Subjects.
Scatterplots depicting total numbers (Nt) (A), and numerical densities (Nd) of NPY-IR neurons in control, SZ, and BD subjects. A marginally significant decrease of Nt, but not Nd, of NPY-IR neurons was detected only in the CO nucleus (Nt, p<0.04, g= −0.81, adjusted for effect of PMI). No other significant changes were observed in Nt or Nd of NPY-IR neurons when SZ or BD subjects were compared to normal control subjects. Significance values are derived from stepwise linear regression models. Scatterplots show the mean (histogram) and 95% confidence intervals (black lines).
Effects of Time of Death
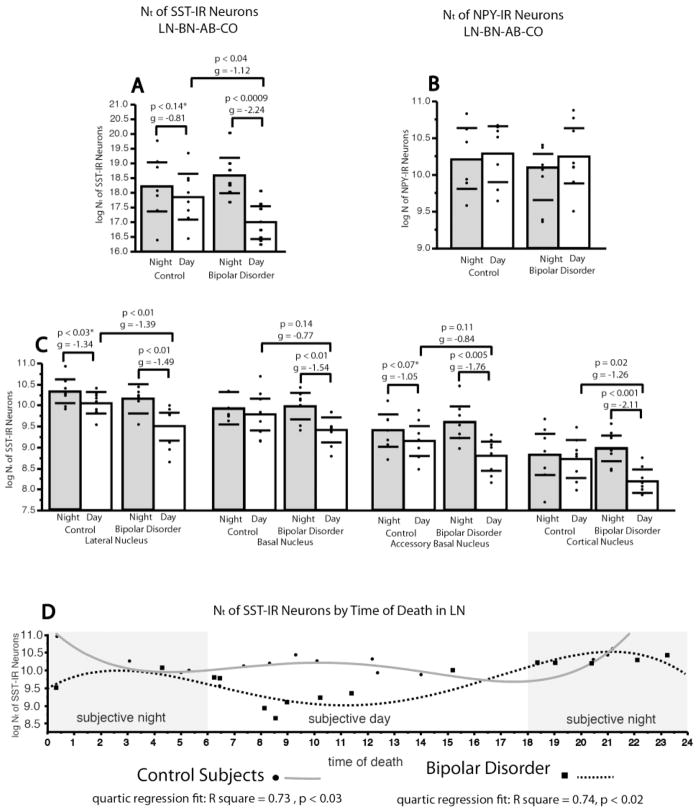
In control subjects, Nt of SST-IR neurons in the LN, as well as in the combined LN-BN-AB-CO, were significantly higher in subjects with s-Night TOD, as compared to subjects with a s-Day TOD (LN, Nt, p<0.03, g= −1.34; with a significant effect of sex (p<0.01), and cause of death (p<0.02); Nd, p<0.03, g= −1.24; LN-BN-AB-CO, Nt, p= 0.14, g= −0.81; Nd, p<0.04, g= −1.15). To further test this relationship, Nt of SST-IR neurons in the LN were plotted by TOD and fit to quartic regression analysis. A rhythmic-like relationship was observed, with a significant quartic regression fit (F=6.9, p<0.006; Fig. 5), displaying two peaks of SST-IR neurons in diurnal humans, identical to amygdala SST rhythms reported in nocturnal mice (2). A first peak in Nt of SST-IR neurons at approximately 12 AM was followed by a trough at approximately 6 AM, and by a second, smaller, peak at approximately 12 PM, followed by a second trough at approximately 6 PM.
Figure 5. SST-IR Neurons are Decreased Selectively in BD Subjects with Subjective Day Time of Death.
(A) Scatterplot depicts total numbers (Nt) of SST-IR neurons in the combined LN-BNAB-CO of subjects with a time of death (TOD) in the subjective day (06:00–17:59) in comparison to subjects with a time of death in the subjective night (18:00–05:59). Within the normal control group, there is a trend toward higher numbers of SST-IR neurons in the subjective night TOD group with respect to subjective day TOD group. Within the BD group, this comparison is significant, with subjective night TOD group showing higher numbers of SST-IR neurons with respect to the subjective day TOD group (p< 0.009). Between group comparisons of subjective day and subjective night respectively show a significant effect of diagnosis selectively for the subjective day TOD group (p< 0.04).. (B) No relationship was observed between TOD and total number of NPY-IR neurons in the LN in either diagnosis group. (C) Scatterplots of Nt of SST-IR neurons in the subjective day TOD vs. subjective night TOD across each amygdala nucleus. SST-IR neuron numbers were found to be lower in the subjective day TOD in both control and BD subjects in most amygdala nuclei. In subjects with BD, decreases of total number of SST-IR neurons were present in the subjective day TOD group in the LN, and CO, with statistical trends for decreases in the BN and AB. Furthermore, comparisons of subjective day vs. subjective night groups in subjects with BD revealed a significant decrease of Nt of SST-IR neurons in the subjective day across all amygdala nuclei examined in this disorder (C). (D) Plots display quartic regression analysis of total numbers of SST-IR neurons in the LN by TOD for each diagnosis group. A rhythmic relationship is evident for both normal control and BD groups. In control subjects, total numbers of SST-IR neurons display a peak at approximately 12 AM, followed by a trough at approximately 6 AM, and a second peak at approximately 12 PM followed by a second trough at approximately 6 PM (Black circles, gray solid line). Subjects with BD show a reverse rhythmic-like relationship, with a trough at approximately 12 AM, a peak at approximately 6 AM, a second trough at approximately 12 PM and a second peak at approximately 6 PM (black squares, black dashed line). Scatterplots show the mean (histogram) and 95% confidence intervals (black lines). *Adjusted for significant effect of sex and cause of death.
Subjects with BD showed an enhanced day/night difference with respect to controls. Nt of SST-IR neurons in the LN were significantly higher in BD subjects with a s-Night TOD (Nt, p<0.01, g= −1.49; Nd, p<0.002, g= −2.04; Fig. 5) and in the combined LN-BN-AB-CO (Nt, p<0.0009, g= −2.24; Fig. 5) with respect to BD subjects with a s-Day TOD. Notably, healthy control versus BD between-group comparison showed that Nt of SST-IR neurons were significantly lower in BD in the s-Day groups (LN-BN-AB-CO: p= 0.04, g= −1.12; LN: p= 0.01, g= −1.39) but not in the s-Night groups (Fig. 5). Quartic regression analysis showed Nt of SST-IR neurons in subjects with BD display an altered rhythmic-like relationship with respect to control subjects (quartic regression fit: F= 5.08, p<0.02, Fig. 5). The appearance of a ‘reversed rhythm’ with a trough at approximately 12 AM, a peak at approximately 6 AM, a second trough at approximately 12 PM and a second peak at approximately 6 PM, is driven by the sharp reduction of SST-IR neurons between 6AM–12PM in the BD group.
TOD analyses could not be performed in subjects with SZ because of insufficient number of subjects with SZ with TOD between 4 PM and 12 AM. Nt of NPY-IR neurons in the LN did not vary between the s-Day and s-Night groups in controls or in subjects with BD (Fig. 5), consistent with lack of effect of TOD in stepwise linear regression models.
Discussion
The present studies resulted in three main, previously unreported, findings: i) SST-IR neurons are decreased in the amygdala in SZ and BD, ii) the expression of SST in the healthy human amygdala displays a circadian-like rhythm, and iii) this circadian-like SST expression is altered in BD. These findings add to growing evidence for involvement of SST in SZ and BD (18–20, 22, 23), and suggest that amygdalar SST decreases represent a common denominator, contributing to elevated anxiety in both disorders. In BD, we observed enhanced rhythmic-like SST expression in the LN defined by a marked decrease of SST-IR neurons in the morning, when numbers of SST-IR neurons increase in control subjects. The lowest portion of that cycle corresponds to the ‘morning worse’ time period typical of BD. Consistent with these findings, comparisons between control and BD subjects show a significant effect of TOD on SST-IR neuron numbers. These results contribute to emerging evidence for circadian rhythm disruption in BD, and suggest that dysregulation of circadian SST expression in the amygdala may contribute to morning peaks of anxiety and depression (34–37). In subjects with SZ, relationships with circadian rhythms were not investigated for technical reasons.
Technical Considerations
TOD Analysis as a Proxy for Circadian Time
In these postmortem studies, TOD for each subject was used to monitor expression changes as they may relate to circadian rhythms. TOD represents a single measure per subject at a specific time point, rather than repeated measures across time. Therefore, we refer to SST-IR neuron numbers plotted by TOD as “circadian-like” and “rhythmiclike”. This approach has been successfully used in several postmortem brain studies (57–59, 65, 66). In the present study, the validity of this method is supported by the observation that “rhythmic-like’ changes of SST expression observed in healthy human amygdala (Fig. 5) are consistent with those reported in the rodent amygdala (2). In addition, SST rhythmic-like abnormalities detected in BD parallel similar abnormalities reported in cerebrospinal fluid of live subjects with mood disorders (50).
Treatment of Antipsychotics and Lithium
We did not detect significant effects of exposure to antipsychotics, lithium, or valproic acid on SST-IR and NPY-IR neurons. Although effects of these confounding factors cannot be ruled out with certainty, our results indicate that pharmacological treatment did not contribute to decreases of SST-IR neurons. In support, chronic treatment with antipsychotics or lithium in rodents increases SST and NPY expression (67–71). For additional considerations, see ‘Supplemental Material’.
Decreases of SST-IR neurons
Decreased numbers of SST-IR neurons in the amygdala may reflect neuronal loss or decreased expression. Several considerations point to decreased expression as the most likely interpretation. First, in both SZ and BD, numbers of NPY-IR neurons, co-expressed in a large percentage of SST neurons, were not altered. This discrepancy suggests that either changes in SST expression occur in NPY-negative neurons, or differential factors may regulate SST and NPY in the same neurons. This latter possibility is supported by studies on SST mutant mice and chronic stress reporting altered SST expression but normal levels of NPY (72). Second, previous studies in a largely overlapping cohort showed that amygdalar Nt and Nd of Nissl-stained neurons and volumes were unchanged in SZ (64), a finding inconsistent with neuronal loss. Third, in BD, significant decreases of Nt and Nd of SST-IR neurons in the LN do not parallel normal Nd of Nissl-stained neurons in a largely overlapping subject cohort, even in the presence of volume decreases (64). Fourth, fluctuations of SST in the amygdala of rodents (2) and SST-IR neurons in healthy humans (this study), and s-Day decreases of SST-IR neurons in BD across all amygdala nuclei (i.e. including those with normal total neuron number and volume (64)) are not consistent with neuronal loss. The likelihood that we detect SST-IR cells above a certain threshold of protein expression rather than absolute measures of protein, together with the short circulating half-life of SST (<3 minutes) (73, 74), further suggest that SST-IR neuron numbers across TOD represent fluctuating levels of SST within neurons. Together, these observations provide strong support for decreased SST expression in the amygdala of subjects with SZ and BD.
Decreased SST expression in SZ and BD: Implications for Amygdala Activity and Comorbidity with Anxiety and Stress Vulnerability
Amygdala SST-IR neurons are primarily GABAergic (75), and form inhibitory synapses onto distal dendrites of local pyramidal neurons (76). The proximity of these synapses to excitatory inputs suggests that SST synapses on pyramidal neurons affect synaptic plasticity related to emotional learning (76). SST exerts an inhibitory effect on amygdala pyramidal neurons and, in several brain regions, modulates GABAergic inhibition (77–82). These considerations suggest that decreased SST may contribute to reduced inhibitory regulation and disruption of amygdala intrinsic circuits. Consistent with this possibility, increased amygdala activity has been reported in SZ during the processing of emotional stimuli (83, 84), and in subjects with BD during mania (85). Predominant SST-IR neuron decreases in the LN, known to mediate plasticity and rapid behavioral responses to fearful stimuli (86, 87), point to abnormal processing of sensory stimuli and fear response. In addition, it is possible that small populations of LN SST-IR neurons projecting to the entorhinal cortex (88), and BN and CO SST-IR neurons to the basal forebrain (89) may be involved, potentially contributing to disrupted sensory gating in SZ (90–94), and dysregulation of sleep-wake patterns in BD (95–97) through the basal forebrain (98, 99), respectively.
Growing evidence suggest that SST in the amygdala powerfully reduces anxiety (1, 2, 4–6), indicating that SST represents a potential pharmacological target against depression and anxiety (100, 101). Animal models show anxiolytic, and possibly antidepressant, effects of SST in the amygdala, and suggest a role in fear learning and expression (102, 103). Increased SST receptors in the amygdala in response to threatening stimuli (104) suggest that SST activation in this region may counteract anxiety-related responses, perhaps contributing to maintain a balance between adaptive fear responses and maladaptive, or generalized, anxiety. Thus, it is reasonable to speculate that decreased SST expression in the amygdala of subjects with SZ and BD may result in increased anxiety and heightened vulnerability to stress. Consistent with this hypothesis, SST−/− mice display high cortisol levels and increased anxiety and depressive-like behaviors (8). Furthermore, decreased SST levels in subjects with depression correlate with increased cortisol levels (50, 105–107), and are associated with a greater plasma cortisol response to dexamethasone (50), suggesting that decreased SST contributes to disinhibited stress response. Finally, SST expression in subjects with SZ and BD was reported to correlate with levels of inflammatory cytokines (108), in turn associated with altered expression of stress-related markers (109). Alternatively, amygdala SST expression may decrease as a consequence to chronic stress experienced by patients with SZ or BD. Lack of correlation of SST-IR neuron numbers with years of illness suggests otherwise.
SST-IR Neurons in the Amygdala of Subjects with BD: Potential Link to Circadian Rhythm Dysregulation
In subjects with BD, SST-IR neuron decreases across amygdala nuclei were selective for the s-Day group, suggesting a link with circadian rhythm dysregulation (34, 35, 37, 50, 110). These findings are consistent with results from another group, showing decreased levels of SST in the CSF of subjects with affective disorders selectively in the morning (50). Notably, the significant difference in rhythmic-like SST expression in the LN of subjects with BD (Fig. 5) points to an abnormal circadian phase in the amygdala. The enhanced rhythmic-like distribution observed, with its appearance of a reversed rhythm in the regression plot driven by the sharp decrease of SST-IR neurons in BD subjects with a morning TOD, represents an enhanced day/night difference with respect to the moderate s-Day decrease of SST-IR neurons in control subjects. In BD, the sharp decrease between 6AM–12 PM may contribute to the morning-worse phase of anxiety and depression (34–37).
Circadian rhythms are controlled by clock genes in the suprachiasmatic nucleus (SCN), which coordinates rhythms throughout the brain and body (111, 112). However, clock genes exist in many neural and non-neural tissues (113–115), and can impact mood in a variety of manners (116). Animal studies have shown that clock gene rhythms in distinct brain regions can change phase irrespective of the SCN rhythm (117, 118). Thus, altered circadian rhythm of SST expression in the amygdala of subjects with BD may result from at least two, non-mutually exclusive, mechanisms, i.e. i) core circadian dysfunction in the SCN, and ii) dysregulation of intrinsic amygdala circadian rhythms. Support for reduced synchrony of rhythms by the SCN comes from studies reporting reduced circadian amplitude of activity rhythms in subjects with BD (119–121), and from observations on the effects of bright-light therapy (122), which may resynchronize the circadian clock through established effects of light input to the SCN (123–127). Support for intrinsic amygdala dysregulation is based on evidence that molecular rhythms within this region change phase in response to fearful stimuli (118). Thus, altered amygdala SST rhythms may result from heightened responses to negative environmental factors, as observed in subjects with BD (128–130). Notably, glucocorticoids are regulated by the circadian clock and affect amygdalar circadian rhythms (131–136). It is plausible that altered SST rhythms in this region may be induced by interactions between heighted responses to stress and circadian rhythm dysregulation. In addition, the basolateral amygdala displays a circadian rhythm anti-phase to the central amygdala (137), the later of which is regulated by glucocorticoids (135). Glucocorticoid receptors (GR) are present throughout the amygdala (138, 139), with highest concentrations in the central nucleus (140, 141). Furthermore, glucocorticoids also directly regulate CLOCK genes through glucocorticoid response elements located in the promoter region of Per1 and Per2 genes (142, 143). A complex interaction of stress and CLOCK genes may contribute to enhanced SST amygdala rhythms in BD.
Evidence that neurons in the hypothalamus switch between dopamine and SST expression under altered light-dark cycles (144), suggest a complex relationship of circadian rhythms with SST and dopamine. CLOCK has been shown to regulate dopamine expression (145–147). Altered CLOCK function resulting in enhanced dopamine transmission to the amygdala, together with disrupted SST rhythms, may contribute to an imbalance of mood regulation.
In summary, reductions of amygdalar SST-IR neurons add to growing evidence of the involvement of this neuropeptide in SZ and BD. Reduced SST expression in the amygdala may contribute to anxiety and stress vulnerability frequently comorbid in these disorders. Our findings also suggest SST expression in the normal human amygdala varies according to circadian rhythms. In BD, a disruption of this rhythm, with decreased SST expression in the subjective day, may contribute to peaks of anxiety and depression in the morning.
Supplementary Material
Acknowledgments
Disclosures and acknowledgments: The authors thank the Harvard Brain Tissue Resource Center, brain donors and their families for the tissue samples used in these studies and NIH and the Phyllis and Jerome Lyle Rappaport Foundation for funding this work. The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yeung M, Engin E, Treit D. Anxiolytic-like effects of somatostatin isoforms SST 14 and SST 28 in two animal models (Rattus norvegicus) after intra-amygdalar and intra-septal microinfusions. Psychopharmacology (Berl) 2011;216:557–567. doi: 10.1007/s00213-011-2248-x. [DOI] [PubMed] [Google Scholar]
- 2.Albrecht A, Thiere M, Bergado-Acosta JR, Poranzke J, Muller B, Stork O. Circadian modulation of anxiety: a role for somatostatin in the amygdala. PLoS One. 2013;8:e84668. doi: 10.1371/journal.pone.0084668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonald AJ, Mascagni F, Augustine JR. Neuropeptide Y and somatostatin-like immunoreactivity in neurons of the monkey amygdala. Neuroscience. 1995;66:959–982. doi: 10.1016/0306-4522(94)00629-j. [DOI] [PubMed] [Google Scholar]
- 4.Sajdyk TJ, Shekhar A, Gehlert DR. Interactions between NPY and CRF in the amygdala to regulate emotionality. Neuropeptides. 2004;38:225–234. doi: 10.1016/j.npep.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Sajdyk TJ, Fitz SD, Shekhar A. The role of neuropeptide Y in the amygdala on corticotropin-releasing factor receptor-mediated behavioral stress responses in the rat. Stress. 2006;9:21–28. doi: 10.1080/10253890600557315. [DOI] [PubMed] [Google Scholar]
- 6.Sajdyk TJ, Johnson PL, Leitermann RJ, Fitz SD, Dietrich A, Morin M, et al. Neuropeptide Y in the amygdala induces long-term resilience to stress-induced reductions in social responses but not hypothalamic-adrenal-pituitary axis activity or hyperthermia. J Neurosci. 2008;28:893–903. doi: 10.1523/JNEUROSCI.0659-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engin E, Stellbrink J, Treit D, Dickson CT. Anxiolytic and antidepressant effects of intracerebroventricularly administered somatostatin: behavioral and neurophysiological evidence. Neuroscience. 2008;157:666–676. doi: 10.1016/j.neuroscience.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 8.Lin LC, Sibille E. Somatostatin, neuronal vulnerability and behavioral emotionality. Mol Psychiatry. 2015 doi: 10.1038/mp.2014.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheriff S, Dautzenberg FM, Mulchahey JJ, Pisarska M, Hauger RL, Chance WT, et al. Interaction of neuropeptide Y and corticotropin-releasing factor signaling pathways in AR-5 amygdalar cells. Peptides. 2001;22:2083–2089. doi: 10.1016/s0196-9781(01)00549-6. [DOI] [PubMed] [Google Scholar]
- 10.Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JM, Stalla GK, et al. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- 11.Sherrin T, Todorovic C, Zeyda T, Tan CH, Wong PT, Zhu YZ, et al. Chronic stimulation of corticotropin-releasing factor receptor 1 enhances the anxiogenic response of the cholecystokinin system. Mol Psychiatry. 2009;14:291–307. doi: 10.1038/sj.mp.4002121. [DOI] [PubMed] [Google Scholar]
- 12.Thorsell A, Svensson P, Wiklund L, Sommer W, Ekman R, Heilig M. Suppressed neuropeptide Y (NPY) mRNA in rat amygdala following restraint stress. Regul Pept. 1998;75–76:247–254. doi: 10.1016/s0167-0115(98)00075-5. [DOI] [PubMed] [Google Scholar]
- 13.Bosanac P, Mancuso S, Castle D. Anxiety symptoms in psychotic disorders. Clinical schizophrenia & related psychoses. 2013:1–22. doi: 10.3371/CSRP.BOMA.091313. [DOI] [PubMed] [Google Scholar]
- 14.Cosoff SJ, Hafner RJ. The prevalence of comorbid anxiety in schizophrenia, schizoaffective disorder and bipolar disorder. Aust N Z J Psychiatry. 1998;32:67–72. doi: 10.3109/00048679809062708. [DOI] [PubMed] [Google Scholar]
- 15.Cassano GB, Pini S, Saettoni M, Rucci P, Dell’Osso L. Occurrence and clinical correlates of psychiatric comorbidity in patients with psychotic disorders. J Clin Psychiatry. 1998;59:60–68. doi: 10.4088/jcp.v59n0204. [DOI] [PubMed] [Google Scholar]
- 16.Braga RJ, Reynolds GP, Siris SG. Anxiety comorbidity in schizophrenia. Psychiatry Res. 2013;210:1–7. doi: 10.1016/j.psychres.2013.07.030. [DOI] [PubMed] [Google Scholar]
- 17.Vazquez GH, Baldessarini RJ, Tondo L. Co-occurrence of anxiety and bipolar disorders: clinical and therapeutic overview. Depress Anxiety. 2014;31:196–206. doi: 10.1002/da.22248. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167:1479–1488. doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- 21.Fung SJ, Fillman SG, Webster MJ, Shannon Weickert C. Schizophrenia and bipolar disorder show both common and distinct changes in cortical interneuron markers. Schizophr Res. 2014;155:26–30. doi: 10.1016/j.schres.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 22.Konradi C, Yang CK, EZ, Lohman KM, Gresch P, Pantazopoulos H, et al. Hippocampal Interneurons in Schizophrenia. Schizophrenia Research. 2011 doi: 10.1016/j.schres.2011.06.007. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konradi C, Zimmerman EI, Yang CK, Lohmann KM, Gresch P, Pantazopoulos H, et al. Hippocampal interneurons in bipolar disorder. Archives of general psychiatry. 2011;68:340–350. doi: 10.1001/archgenpsychiatry.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nyegaard M, Borglum AD, Bruun TG, Collier DA, Russ C, Mors O, et al. Novel polymorphisms in the somatostatin receptor 5 (SSTR5) gene associated with bipolar affective disorder. Mol Psychiatry. 2002;7:745–754. doi: 10.1038/sj.mp.4001049. [DOI] [PubMed] [Google Scholar]
- 25.Seifuddin F, Pirooznia M, Judy JT, Goes FS, Potash JB, Zandi PP. Systematic review of genome-wide gene expression studies of bipolar disorder. BMC Psychiatry. 2013;13:213. doi: 10.1186/1471-244X-13-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aydin A, Selvi Y, Besiroglu L, Boysan M, Atli A, Ozdemir O, et al. Mood and metabolic consequences of sleep deprivation as a potential endophenotype’ in bipolar disorder. Journal of affective disorders. 2013 doi: 10.1016/j.jad.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 27.McClung CA. Circadian rhythms and mood regulation: insights from pre-clinical models. Eur Neuropsychopharmacol. 2011;21(Suppl 4):S683–693. doi: 10.1016/j.euroneuro.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165:830–843. doi: 10.1176/appi.ajp.2008.08010077. [DOI] [PubMed] [Google Scholar]
- 29.Kripke DF, Nievergelt CM, Joo E, Shekhtman T, Kelsoe JR. Circadian polymorphisms associated with affective disorders. J Circadian Rhythms. 2009;7:2. doi: 10.1186/1740-3391-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benedetti F, Dallaspezia S, Colombo C, Pirovano A, Marino E, Smeraldi E. A length polymorphism in the circadian clock gene Per3 influences age at onset of bipolar disorder. Neurosci Lett. 2008;445:184–187. doi: 10.1016/j.neulet.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Nievergelt CM, Kripke DF, Barrett TB, Burg E, Remick RA, Sadovnick AD, et al. Suggestive evidence for association of the circadian genes PERIOD3 and ARNTL with bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:234–241. doi: 10.1002/ajmg.b.30252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soria V, Martinez-Amoros E, Escaramis G, Valero J, Perez-Egea R, Garcia C, et al. Differential association of circadian genes with mood disorders: CRY1 and NPAS2 are associated with unipolar major depression and CLOCK and VIP with bipolar disorder. Neuropsychopharmacology. 2010;35:1279–1289. doi: 10.1038/npp.2009.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Etain B, Milhiet V, Bellivier F, Leboyer M. Genetics of circadian rhythms and mood spectrum disorders. Eur Neuropsychopharmacol. 2011;21(Suppl 4):S676–682. doi: 10.1016/j.euroneuro.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Wirz-Justice A. Diurnal variation of depressive symptoms. Dialogues in clinical neuroscience. 2008;10:337–343. doi: 10.31887/DCNS.2008.10.3/awjustice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray G. Diurnal mood variation in depression: a signal of disturbed circadian function? Journal of affective disorders. 2007;102:47–53. doi: 10.1016/j.jad.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Murray G. Major depressive disorder: afternoon and evening diurnal mood variation is common. Evidence-based mental health. 2008;11:59. doi: 10.1136/ebmh.11.2.59. [DOI] [PubMed] [Google Scholar]
- 37.Murray G, Allen NB, Trinder J. Mood and the circadian system: investigation of a circadian component in positive affect. Chronobiol Int. 2002;19:1151–1169. doi: 10.1081/cbi-120015956. [DOI] [PubMed] [Google Scholar]
- 38.Rybakowski JK. Factors associated with lithium efficacy in bipolar disorder. Harv Rev Psychiatry. 2014;22:353–357. doi: 10.1097/HRP.0000000000000006. [DOI] [PubMed] [Google Scholar]
- 39.McCarthy MJ, Welsh DK. Cellular circadian clocks in mood disorders. J Biol Rhythms. 2012;27:339–352. doi: 10.1177/0748730412456367. [DOI] [PubMed] [Google Scholar]
- 40.Pandey A, Davis NA, White BC, Pajewski NM, Savitz J, Drevets WC, et al. Epistasis network centrality analysis yields pathway replication across two GWAS cohorts for bipolar disorder. Transl Psychiatry. 2012;2:e154. doi: 10.1038/tp.2012.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCarthy MJ, Nievergelt CM, Kelsoe JR, Welsh DK. A survey of genomic studies supports association of circadian clock genes with bipolar disorder spectrum illnesses and lithium response. PLoS One. 2012;7:e32091. doi: 10.1371/journal.pone.0032091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mansour HA, Talkowski ME, Wood J, Chowdari KV, McClain L, Prasad K, et al. Association study of 21 circadian genes with bipolar I disorder, schizoaffective disorder, and schizophrenia. Bipolar Disord. 2009;11:701–710. doi: 10.1111/j.1399-5618.2009.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wulff K, Gatti S, Wettstein JG, Foster RG. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci. 2010;11:589–599. doi: 10.1038/nrn2868. [DOI] [PubMed] [Google Scholar]
- 44.Leibenluft E, Suppes T. Treating bipolar illness: focus on treatment algorithms and management of the sleep-wake cycle. Am J Psychiatry. 1999;156:1976–1981. doi: 10.1176/ajp.156.12.1976. [DOI] [PubMed] [Google Scholar]
- 45.Klemfuss H. Rhythms and the pharmacology of lithium. Pharmacol Ther. 1992;56:53–78. doi: 10.1016/0163-7258(92)90037-z. [DOI] [PubMed] [Google Scholar]
- 46.Abe M, Herzog ED, Block GD. Lithium lengthens the circadian period of individual suprachiasmatic nucleus neurons. Neuroreport. 2000;11:3261–3264. doi: 10.1097/00001756-200009280-00042. [DOI] [PubMed] [Google Scholar]
- 47.Yin L, Wang J, Klein PS, Lazar MA. Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock. Science. 2006;311:1002–1005. doi: 10.1126/science.1121613. [DOI] [PubMed] [Google Scholar]
- 48.Li J, Lu WQ, Beesley S, Loudon AS, Meng QJ. Lithium impacts on the amplitude and period of the molecular circadian clockwork. PLoS One. 2012;7:e33292. doi: 10.1371/journal.pone.0033292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johansson AS, Brask J, Owe-Larsson B, Hetta J, Lundkvist GB. Valproic acid phase shifts the rhythmic expression of Period2::Luciferase. J Biol Rhythms. 2011;26:541–551. doi: 10.1177/0748730411419775. [DOI] [PubMed] [Google Scholar]
- 50.Rubinow DR. Cerebrospinal fluid somatostatin and psychiatric illness. Biol Psychiatry. 1986;21:341–365. doi: 10.1016/0006-3223(86)90163-0. [DOI] [PubMed] [Google Scholar]
- 51.Xu X, Roby KD, Callaway EM. Mouse cortical inhibitory neuron type that coexpresses somatostatin and calretinin. J Comp Neurol. 2006;499:144–160. doi: 10.1002/cne.21101. [DOI] [PubMed] [Google Scholar]
- 52.Sims KS, Williams RS. The human amygdaloid complex: a cytologic and histochemical atlas using Nissl, myelin, acetylcholinesterase and nicotinamide adenine dinucleotide phosphate diaphorase staining. Neuroscience. 1990;36:449–472. doi: 10.1016/0306-4522(90)90440-f. [DOI] [PubMed] [Google Scholar]
- 53.Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. New York: Wiley-Liss; 1992. [Google Scholar]
- 54.Sorvari H, Soininen H, Paljarvi L, Karkola K, Pitkanen A. Distribution of parvalbumin-immunoreactive cells and fibers in the human amygdaloid complex. J Comp Neurol. 1995;360:185–212. doi: 10.1002/cne.903600202. [DOI] [PubMed] [Google Scholar]
- 55.Berelowitz M, Perlow MJ, Hoffman HJ, Frohman LA. The diurnal variation of immunoreactive thyrotropin-releasing hormone and somatostatin in the cerebrospinal fluid of the rhesus monkey. Endocrinology. 1981;109:2102–2109. doi: 10.1210/endo-109-6-2102. [DOI] [PubMed] [Google Scholar]
- 56.Arnold MA, Reppert SM, Rorstad OP, Sagar SM, Keutmann HT, Perlow MJ, et al. Temporal patterns of somatostatin immunoreactivity in the cerebrospinal fluid of the rhesus monkey: effect of environmental lighting. J Neurosci. 1982;2:674–680. doi: 10.1523/JNEUROSCI.02-06-00674.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hofman MA. Circadian oscillations of neuropeptide expression in the human biological clock. Journal of comparative physiology A, Neuroethology, sensory, neural, and behavioral physiology. 2003;189:823–831. doi: 10.1007/s00359-003-0458-3. [DOI] [PubMed] [Google Scholar]
- 58.Li JZ, Bunney BG, Meng F, Hagenauer MH, Walsh DM, Vawter MP, et al. Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proc Natl Acad Sci U S A. 2013;110:9950–9955. doi: 10.1073/pnas.1305814110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou JN, Riemersma RF, Unmehopa UA, Hoogendijk WJ, van Heerikhuize JJ, Hofman MA, et al. Alterations in arginine vasopressin neurons in the suprachiasmatic nucleus in depression. Arch Gen Psychiatry. 2001;58:655–662. doi: 10.1001/archpsyc.58.7.655. [DOI] [PubMed] [Google Scholar]
- 60.Iwata O, Okamura H, Saitsu H, Saikusa M, Kanda H, Eshima N, et al. Diurnal cortisol changes in newborn infants suggesting entrainment of peripheral circadian clock in utero and at birth. J Clin Endocrinol Metab. 2013;98:E25–32. doi: 10.1210/jc.2012-2750. [DOI] [PubMed] [Google Scholar]
- 61.Dumont M, Macchi MM, Carrier J, Lafrance C, Hebert M. Time course of narrow frequency bands in the waking EEG during sleep deprivation. Neuroreport. 1999;10:403–407. doi: 10.1097/00001756-199902050-00035. [DOI] [PubMed] [Google Scholar]
- 62.Schmal C, Reimann P, Staiger D. A circadian clock-regulated toggle switch explains AtGRP7 and AtGRP8 oscillations in Arabidopsis thaliana. PLoS Comput Biol. 2013;9:e1002986. doi: 10.1371/journal.pcbi.1002986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Monk TH, Buysse DJ, Reynolds CF, 3rd, Berga SL, Jarrett DB, Begley AE, et al. Circadian rhythms in human performance and mood under constant conditions. Journal of sleep research. 1997;6:9–18. doi: 10.1046/j.1365-2869.1997.00023.x. [DOI] [PubMed] [Google Scholar]
- 64.Berretta S, Pantazopoulos H, Lange N. Neuron numbers and volume of the amygdala in subjects diagnosed with bipolar disorder or schizophrenia. Biol Psychiatry. 2007;62:884–893. doi: 10.1016/j.biopsych.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 65.Bunney BG, Li JZ, Walsh DM, Stein R, Vawter MP, Cartagena P, et al. Circadian dysregulation of clock genes: clues to rapid treatments in major depressive disorder. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Wamelen DJ, Aziz NA, Anink JJ, van Steenhoven R, Angeloni D, Fraschini F, et al. Suprachiasmatic nucleus neuropeptide expression in patients with Huntington’s Disease. Sleep. 2013;36:117–125. doi: 10.5665/sleep.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sakai K, Maeda K, Chihara K, Kaneda H. Increases in cortical neuropeptide Y and somatostatin concentrations following haloperidol-depot treatment in rats. Neuropeptides. 1995;29:157–161. doi: 10.1016/0143-4179(95)90018-7. [DOI] [PubMed] [Google Scholar]
- 68.Marin C, Engber TM, Bonastre M, Chase TN, Tolosa E. Effect of long-term haloperidol treatment on striatal neuropeptides: relation to stereotyped behavior. Brain Res. 1996;731:57–62. doi: 10.1016/0006-8993(96)00461-1. [DOI] [PubMed] [Google Scholar]
- 69.Zachrisson O, Mathe AA, Stenfors C, Lindefors N. Region-specific effects of chronic lithium administration on neuropeptide Y and somatostatin mRNA expression in the rat brain. Neurosci Lett. 1995;194:89–92. doi: 10.1016/0304-3940(95)11735-f. [DOI] [PubMed] [Google Scholar]
- 70.Husum H, Mathe AA. Early life stress changes concentrations of neuropeptide Y and corticotropin-releasing hormone in adult rat brain. Lithium treatment modifies these changes. Neuropsychopharmacology. 2002;27:756–764. doi: 10.1016/S0893-133X(02)00363-9. [DOI] [PubMed] [Google Scholar]
- 71.Arif M, Ahmed MM, Kumabe Y, Hoshino H, Chikuma T, Kato T. Clozapine but not haloperidol suppresses the changes in the levels of neuropeptides in MK-801-treated rat brain regions. Neurochem Int. 2006;49:304–311. doi: 10.1016/j.neuint.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 72.Lin LC, Sibille E. Somatostatin, neuronal vulnerability and behavioral emotionality. Mol Psychiatry. 2015;20:377–387. doi: 10.1038/mp.2014.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, et al. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- 74.Harris AG. Somatostatin and somatostatin analogues: pharmacokinetics and pharmacodynamic effects. Gut. 1994;35:S1–4. doi: 10.1136/gut.35.3_suppl.s1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McDonald AJ, Pearson JC. Coexistence of GABA and peptide immunoreactivity in non-pyramidal neurons of the basolateral amygdala. Neurosci Lett. 1989;100:53–58. doi: 10.1016/0304-3940(89)90659-9. [DOI] [PubMed] [Google Scholar]
- 76.Muller JF, Mascagni F, McDonald AJ. Postsynaptic targets of somatostatin-containing interneurons in the rat basolateral amygdala. J Comp Neurol. 2007;500:513–529. doi: 10.1002/cne.21185. [DOI] [PubMed] [Google Scholar]
- 77.Vincens M, Mauvais-Jarvis F, Behar S. A novel recognition site for somatostatin-14 on the GABA(A) receptor complex. Eur J Pharmacol. 1998;344:R1–2. doi: 10.1016/s0014-2999(97)01610-5. [DOI] [PubMed] [Google Scholar]
- 78.Arancibia S, Estupina C, Pesco J, Belmar J, Tapia-Arancibia L. Responsiveness to depolarization of hypothalamic neurons secreting somatostatin under stress and estrous cycle conditions: involvement of GABAergic and steroidal interactions. J Neurosci Res. 1997;50:575–584. doi: 10.1002/(SICI)1097-4547(19971115)50:4<575::AID-JNR8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 79.Chan-Palay V, Ito M, Tongroach P, Sakurai M, Palay S. Inhibitory effects of motilin, somatostatin, [Leu]enkephalin, [Met]enkephalin, and taurine on neurons of the lateral vestibular nucleus: interactions with gamma-aminobutyric acid. Proc Natl Acad Sci U S A. 1982;79:3355–3359. doi: 10.1073/pnas.79.10.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chigr F, Ba M’hamed S, Najimi M, Vincens M. Modulation of central GABAA receptor complex by somatostatin: a pharmacological study. Therapie. 1999;54:579–584. [PubMed] [Google Scholar]
- 81.Chigr F, M’Hamed SB, Najimi M. Modulation Of [35S]-tert-butylbicyclophosphorothionate binding by somatostatin in rat hypothalamus. Clinical and experimental pharmacology & physiology. 2002;29:291–298. doi: 10.1046/j.1440-1681.2002.03645.x. [DOI] [PubMed] [Google Scholar]
- 82.Lopez-Huerta VG, Tecuapetla F, Guzman JN, Bargas J, Galarraga E. Presynaptic modulation by somatostatin in the neostriatum. Neurochem Res. 2008;33:1452–1458. doi: 10.1007/s11064-007-9579-3. [DOI] [PubMed] [Google Scholar]
- 83.Suslow T, Lindner C, Dannlowski U, Walhofer K, Rodiger M, Maisch B, et al. Automatic amygdala response to facial expression in schizophrenia: initial hyperresponsivity followed by hyporesponsivity. BMC Neurosci. 2013;14:140. doi: 10.1186/1471-2202-14-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rauch AV, Reker M, Ohrmann P, Pedersen A, Bauer J, Dannlowski U, et al. Increased amygdala activation during automatic processing of facial emotion in schizophrenia. Psychiatry Res. 2010;182:200–206. doi: 10.1016/j.pscychresns.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 85.Altshuler L, Bookheimer S, Proenza MA, Townsend J, Sabb F, Firestine A, et al. Increased amygdala activation during mania: a functional magnetic resonance imaging study. Am J Psychiatry. 2005;162:1211–1213. doi: 10.1176/appi.ajp.162.6.1211. [DOI] [PubMed] [Google Scholar]
- 86.Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- 87.LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McDonald AJ, Zaric V. GABAergic somatostatin-immunoreactive neurons in the amygdala project to the entorhinal cortex. Neuroscience. 2015;290:227–242. doi: 10.1016/j.neuroscience.2015.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McDonald AJ, Mascagni F, Zaric V. Subpopulations of somatostatin-immunoreactive non-pyramidal neurons in the amygdala and adjacent external capsule project to the basal forebrain: evidence for the existence of GABAergic projection neurons in the cortical nuclei and basolateral nuclear complex. Frontiers in neural circuits. 2012;6:46. doi: 10.3389/fncir.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Basu J, Zaremba JD, Cheung SK, Hitti FL, Zemelman BV, Losonczy A, et al. Gating of hippocampal activity, plasticity, and memory by entorhinal cortex long-range inhibition. Science. 2016;351:aaa5694. doi: 10.1126/science.aaa5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hajos M, Hoffmann WE, Kocsis B. Activation of cannabinoid-1 receptors disrupts sensory gating and neuronal oscillation: relevance to schizophrenia. Biol Psychiatry. 2008;63:1075–1083. doi: 10.1016/j.biopsych.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 92.Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry. 1982;17:639–654. [PubMed] [Google Scholar]
- 93.Freedman R, Adler LE, Gerhardt GA, Waldo M, Baker N, Rose GM, et al. Neurobiological studies of sensory gating in schizophrenia. Schizophr Bull. 1987;13:669–678. doi: 10.1093/schbul/13.4.669. [DOI] [PubMed] [Google Scholar]
- 94.Siegel C, Waldo M, Mizner G, Adler LE, Freedman R. Deficits in sensory gating in schizophrenic patients and their relatives. Evidence obtained with auditory evoked responses. Arch Gen Psychiatry. 1984;41:607–612. doi: 10.1001/archpsyc.1984.01790170081009. [DOI] [PubMed] [Google Scholar]
- 95.Harvey AG, Schmidt DA, Scarna A, Semler CN, Goodwin GM. Sleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problems. Am J Psychiatry. 2005;162:50–57. doi: 10.1176/appi.ajp.162.1.50. [DOI] [PubMed] [Google Scholar]
- 96.Harvey AG, Talbot LS, Gershon A. Sleep Disturbance in Bipolar Disorder Across the Lifespan. Clin Psychol (New York) 2009;16:256–277. doi: 10.1111/j.1468-2850.2009.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ng TH, Chung KF, Ho FY, Yeung WF, Yung KP, Lam TH. Sleep-wake disturbance in interepisode bipolar disorder and high-risk individuals: a systematic review and meta-analysis. Sleep medicine reviews. 2015;20:46–58. doi: 10.1016/j.smrv.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 98.Xu M, Chung S, Zhang S, Zhong P, Ma C, Chang WC, et al. Basal forebrain circuit for sleep-wake control. Nat Neurosci. 2015;18:1641–1647. doi: 10.1038/nn.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hoyer D, Bartfai T. Neuropeptides and neuropeptide receptors: drug targets, and peptide and non-peptide ligands: a tribute to Prof. Dieter Seebach. Chemistry & biodiversity. 2012;9:2367–2387. doi: 10.1002/cbdv.201200288. [DOI] [PubMed] [Google Scholar]
- 101.Chaki S, Okubo T, Sekiguchi Y. Non-monoamine-based approach for the treatment of depression and anxiety disorders. Recent patents on CNS drug discovery. 2006;1:1–27. doi: 10.2174/157488906775245318. [DOI] [PubMed] [Google Scholar]
- 102.Kahl E, Fendt M. Injections of the somatostatin receptor type 2 agonist L-054,264 into the amygdala block expression but not acquisition of conditioned fear in rats. Behav Brain Res. 2014;265:49–52. doi: 10.1016/j.bbr.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 103.Kluge C, Stoppel C, Szinyei C, Stork O, Pape HC. Role of the somatostatin system in contextual fear memory and hippocampal synaptic plasticity. Learn Mem. 2008;15:252–260. doi: 10.1101/lm.793008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nanda SA, Qi C, Roseboom PH, Kalin NH. Predator stress induces behavioral inhibition and amygdala somatostatin receptor 2 gene expression. Genes Brain Behav. 2008;7:639–648. doi: 10.1111/j.1601-183X.2008.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Molchan SE, Hill JL, Martinez RA, Lawlor BA, Mellow AM, Rubinow DR, et al. CSF somatostatin in Alzheimer’s disease and major depression: relationship to hypothalamic-pituitary-adrenal axis and clinical measures. Psychoneuroendocrinology. 1993;18:509–519. doi: 10.1016/0306-4530(93)90044-l. [DOI] [PubMed] [Google Scholar]
- 106.Molchan SE, Lawlor BA, Hill JL, Martinez RA, Davis CL, Mellow AM, et al. CSF monoamine metabolites and somatostatin in Alzheimer’s disease and major depression. Biol Psychiatry. 1991;29:1110–1118. doi: 10.1016/0006-3223(91)90253-i. [DOI] [PubMed] [Google Scholar]
- 107.Kling MA, Rubinow DR, Doran AR, Roy A, Davis CL, Calabrese JR, et al. Cerebrospinal fluid immunoreactive somatostatin concentrations in patients with Cushing’s disease and major depression: relationship to indices of corticotropin-releasing hormone and cortisol secretion. Neuroendocrinology. 1993;57:79–88. doi: 10.1159/000126345. [DOI] [PubMed] [Google Scholar]
- 108.Fillman SG, Cloonan N, Miller LC, Weickert CS. Markers of inflammation in the prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18:133. doi: 10.1038/mp.2012.199. [DOI] [PubMed] [Google Scholar]
- 109.Fillman SG, Sinclair D, Fung SJ, Webster MJ, Weickert CS. Markers of Inlfammation and Stress Distinguish Subsets of Individuals with Schizophrenia and Bipolar Disorder. Translational Psychiatry. 2014 doi: 10.1038/tp.2014.8. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Murray G, Allen NB, Trinder J, Burgess H. Is weakened circadian rhythmicity a characteristic of neuroticism? Journal of affective disorders. 2002;72:281–289. doi: 10.1016/s0165-0327(01)00465-7. [DOI] [PubMed] [Google Scholar]
- 111.Moore RY. Circadian rhythms: basic neurobiology and clinical applications. Annual review of medicine. 1997;48:253–266. doi: 10.1146/annurev.med.48.1.253. [DOI] [PubMed] [Google Scholar]
- 112.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 113.Schibler U, Ripperger J, Brown SA. Peripheral circadian oscillators in mammals: time and food. J Biol Rhythms. 2003;18:250–260. doi: 10.1177/0748730403018003007. [DOI] [PubMed] [Google Scholar]
- 114.Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, et al. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- 115.Weigl Y, Harbour VL, Robinson B, Dufresne L, Amir S. Peripheral circadian clocks--a conserved phenotype? Chronobiol Int. 2013;30:559–576. doi: 10.3109/07420528.2012.754451. [DOI] [PubMed] [Google Scholar]
- 116.McClung CA. How might circadian rhythms control mood? Let me count the ways. Biol Psychiatry. 2013;74:242–249. doi: 10.1016/j.biopsych.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Verwey M, Lam GY, Amir S. Circadian rhythms of PERIOD1 expression in the dorsomedial hypothalamic nucleus in the absence of entrained food-anticipatory activity rhythms in rats. Eur J Neurosci. 2009;29:2217–2222. doi: 10.1111/j.1460-9568.2009.06766.x. [DOI] [PubMed] [Google Scholar]
- 118.Pantazopoulos H, Dolatshad H, Davis FC. A fear-inducing odor alters PER2 and c-Fos expression in brain regions involved in fear memory. PLoS One. 2011;6:e20658. doi: 10.1371/journal.pone.0020658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rock P, Goodwin G, Harmer C, Wulff K. Daily rest-activity patterns in the bipolar phenotype: A controlled actigraphy study. Chronobiol Int. 2014;31:290–296. doi: 10.3109/07420528.2013.843542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McKenna BS, Drummond SP, Eyler LT. Associations between circadian activity rhythms and functional brain abnormalities among euthymic bipolar patients: a preliminary study. Journal of affective disorders. 2014;164:101–106. doi: 10.1016/j.jad.2014.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gonzalez R. The relationship between bipolar disorder and biological rhythms. J Clin Psychiatry. 2014;75:e323–331. doi: 10.4088/JCP.13r08507. [DOI] [PubMed] [Google Scholar]
- 122.Sit D, Wisner KL, Hanusa BH, Stull S, Terman M. Light therapy for bipolar disorder: a case series in women. Bipolar Disord. 2007;9:918–927. doi: 10.1111/j.1399-5618.2007.00451.x. [DOI] [PubMed] [Google Scholar]
- 123.Colwell CS, Foster RG. Photic regulation of Fos-like immunoreactivity in the suprachiasmatic nucleus of the mouse. J Comp Neurol. 1992;324:135–142. doi: 10.1002/cne.903240202. [DOI] [PubMed] [Google Scholar]
- 124.Donaldson JA, Stephan FK. Entrainment of circadian rhythms: retinofugal pathways and unilateral suprachiasmatic nucleus lesions. Physiol Behav. 1982;29:1161–1169. doi: 10.1016/0031-9384(82)90314-6. [DOI] [PubMed] [Google Scholar]
- 125.Fuchs JL, Moore RY. Development of circadian rhythmicity and light responsiveness in the rat suprachiasmatic nucleus: a study using the 2-deoxy[1-14C]glucose method. Proc Natl Acad Sci U S A. 1980;77:1204–1208. doi: 10.1073/pnas.77.2.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Meijer JH. Physiological basis for photic entrainment. Eur J Morphol. 1990;28:308–316. [PubMed] [Google Scholar]
- 127.Stetson MH, Watson-Whitmyre M. Nucleus suprachiasmaticus: the biological clock in the hamster? Science. 1976;191:197–199. doi: 10.1126/science.942799. [DOI] [PubMed] [Google Scholar]
- 128.Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 129.Passarotti AM, Sweeney JA, Pavuluri MN. Fronto-limbic dysfunction in mania pre-treatment and persistent amygdala over-activity post-treatment in pediatric bipolar disorder. Psychopharmacology (Berl) 2011;216:485–499. doi: 10.1007/s00213-011-2243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pavuluri MN, O’Connor MM, Harral E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry. 2007;62:158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 131.Oster H, Damerow S, Kiessling S, Jakubcakova V, Abraham D, Tian J, et al. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 2006;4:163–173. doi: 10.1016/j.cmet.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 132.Son GH, Chung S, Choe HK, Kim HD, Baik SM, Lee H, et al. Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proc Natl Acad Sci U S A. 2008;105:20970–20975. doi: 10.1073/pnas.0806962106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Morimoto M, Morita N, Ozawa H, Yokoyama K, Kawata M. Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: an immunohistochemical and in situ hybridization study. Neurosci Res. 1996;26:235–269. doi: 10.1016/s0168-0102(96)01105-4. [DOI] [PubMed] [Google Scholar]
- 134.Al-Safadi S, Branchaud M, Rutherford S, Amir S. Glucocorticoids and Stress-Induced Changes in the Expression of PERIOD1 in the Rat Forebrain. PLoS One. 2015;10:e0130085. doi: 10.1371/journal.pone.0130085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Segall LA, Milet A, Tronche F, Amir S. Brain glucocorticoid receptors are necessary for the rhythmic expression of the clock protein, PERIOD2, in the central extended amygdala in mice. Neurosci Lett. 2009;457:58–60. doi: 10.1016/j.neulet.2009.03.083. [DOI] [PubMed] [Google Scholar]
- 136.Segall LA, Perrin JS, Walker CD, Stewart J, Amir S. Glucocorticoid rhythms control the rhythm of expression of the clock protein, Period2, in oval nucleus of the bed nucleus of the stria terminalis and central nucleus of the amygdala in rats. Neuroscience. 2006;140:753–757. doi: 10.1016/j.neuroscience.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 137.Lamont EW, Robinson B, Stewart J, Amir S. The central and basolateral nuclei of the amygdala exhibit opposite diurnal rhythms of expression of the clock protein Period2. Proc Natl Acad Sci U S A. 2005;102:4180–4184. doi: 10.1073/pnas.0500901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sarrieau A, Dussaillant M, Agid F, Philibert D, Agid Y, Rostene W. Autoradiographic localization of glucocorticosteroid and progesterone binding sites in the human post-mortem brain. J Steroid Biochem. 1986;25:717–721. doi: 10.1016/0022-4731(86)90300-6. [DOI] [PubMed] [Google Scholar]
- 139.Perlman WR, Webster MJ, Kleinman JE, Weickert CS. Reduced glucocorticoid and estrogen receptor alpha messenger ribonucleic acid levels in the amygdala of patients with major mental illness. Biol Psychiatry. 2004;56:844–852. doi: 10.1016/j.biopsych.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 140.Sanchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of corticosteroid receptors in the rhesus brain: relative absence of glucocorticoid receptors in the hippocampal formation. J Neurosci. 2000;20:4657–4668. doi: 10.1523/JNEUROSCI.20-12-04657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Honkaniemi J, Pelto-Huikko M, Rechardt L, Isola J, Lammi A, Fuxe K, et al. Colocalization of peptide and glucocorticoid receptor immunoreactivities in rat central amygdaloid nucleus. Neuroendocrinology. 1992;55:451–459. doi: 10.1159/000126156. [DOI] [PubMed] [Google Scholar]
- 142.So AY, Bernal TU, Pillsbury ML, Yamamoto KR, Feldman BJ. Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proc Natl Acad Sci U S A. 2009;106:17582–17587. doi: 10.1073/pnas.0909733106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yamamoto T, Nakahata Y, Tanaka M, Yoshida M, Soma H, Shinohara K, et al. Acute physical stress elevates mouse period1 mRNA expression in mouse peripheral tissues via a glucocorticoid-responsive element. J Biol Chem. 2005;280:42036–42043. doi: 10.1074/jbc.M509600200. [DOI] [PubMed] [Google Scholar]
- 144.Dulcis D, Jamshidi P, Leutgeb S, Spitzer NC. Neurotransmitter switching in the adult brain regulates behavior. Science. 2013;340:449–453. doi: 10.1126/science.1234152. [DOI] [PubMed] [Google Scholar]
- 145.Mukherjee S, Coque L, Cao JL, Kumar J, Chakravarty S, Asaithamby A, et al. Knockdown of Clock in the ventral tegmental area through RNA interference results in a mixed state of mania and depression-like behavior. Biol Psychiatry. 2010;68:503–511. doi: 10.1016/j.biopsych.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Spencer S, Torres-Altoro MI, Falcon E, Arey R, Marvin M, Goldberg M, et al. A mutation in CLOCK leads to altered dopamine receptor function. J Neurochem. 2012;123:124–134. doi: 10.1111/j.1471-4159.2012.07857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.