Abstract
Addiction is promoted by pathological motivation for addictive substances, and, despite extensive efforts, alcohol use disorders (AUDs) continue to extract a very high social, physical, and economic toll. Compulsive drinking of alcohol, where consumption persists even when alcohol is paired with negative consequences, is considered a particular obstacle for treating AUDs. Aversion-resistant alcohol intake in rodents, e.g. where rodents drink even when alcohol is paired with the bitter tastant quinine, has been considered to model some compulsive aspects of human alcohol consumption. However, the critical mechanisms that drive compulsive-like drinking are only beginning to be identified. The neuropeptide orexin has been linked to high motivation for cocaine, preferred foods, and alcohol. Thus, we investigated the role of orexin receptors in compulsive-like alcohol drinking, where C57BL/6 mice had 2-hr daily access to 15% alcohol with or without quinine (100 µM). We found that systemic administration of the widely used selective orexin-1 receptor (OX1R) blocker, SB-334867 (SB), significantly reduced compulsive-like consumption at doses lower than those reported to reduce quinine-free alcohol intake. The dose of 3-mg/kg SB, in particular, suppressed only compulsive-like drinking. Furthermore, SB did not reduce concurrent water intake during the alcohol drinking sessions, and did not alter saccharin+quinine consumption. In addition, the OX2R antagonist TCS-OX2-29 (3 or 10 mg/kg) did not alter intake of alcohol with or without quinine. Together, our results suggest that OX1R signaling is particularly important for promoting compulsive-like alcohol drinking, and that OX1Rs might represent a novel therapy to counteract compulsive aspects of human AUDs.
Keywords: alcohol, compulsive, orexin, SB-334867
1. INTRODUCTION
Addiction to alcohol and other abused substances is typified by enhanced, and often pathological, motivation for the substance (Koob and Volkow, 2010; Larimer et al., 1999; Sinha, 2009). Despite decades of research, alcohol use disorders (AUDs) remain a major problem with a very high social, personal, and economic toll (Blincoe et al., 2002; Bouchery et al., 2011; CDC, 2014; Harwood et al., 1998; Hingson et al., 2005; Mokdad et al., 2004; Sacks et al., 2013; SAMHSA, 2014), especially due to a lack of effective pharmacotherapies (Spanagel, 2009; World Health Organization, 2014).
Compulsive alcohol intake, where drinking persists despite the knowledge of associated negative consequences, is considered a major obstacle when treating AUDs (Anton, 2000; Anton et al., 1996; Hopf and Lesscher, 2014; Koob and Volkow, 2010; Larimer et al., 1999; Modell et al., 1992; Naqvi et al., 2014; Sinha, 2009; Tiffany and Conklin, 2000). Therefore, to effectively reduce the impact of AUDs, it is critical to understand the mechanisms that drive this consequence-resistant, compulsive-like alcohol consumption. In this regard, voluntary drinking paradigms, where animals will drink despite pairing the alcohol with an aversive consequence (such as bitter-tasting quinine or foot-shock), have been utilized to model some compulsive-like aspects of AUDS in humans (Hopf et al., 2010; Hopf and Lesscher, 2014; Lesscher et al., 2010; Loi et al., 2010; Marchant et al., 2013; Spanagel and Holter, 1999; Spoelder et al., 2015; Vengeliene et al., 2009). In addition, we previously demonstrated that a similar corticoaccumbens circuit promotes both shock-resistant and quinine-resistant alcohol drinking (Seif et al., 2013). Since shock-resistant intake is considered to have face and predictive validity for compulsive intake in humans, e.g. for cocaine (Everitt and Robbins, 2005; Hopf and Lesscher, 2014; Lesscher and Vanderschuren, 2012), the observation of a similar circuit for shock- and quinine-resistant alcohol drinking (Seif et al., 2013) validates the use of alcohol-quinine drinking to model compulsive-like drives for alcohol (Hopf et al., 2010; Hopf and Lesscher, 2014; Lesscher et al., 2010; Loi et al., 2010; Marchant et al., 2013; Spanagel and Holter, 1999; Spoelder et al., 2015; Vengeliene et al., 2009). For the present studies, we utilized a recently developed compulsive-like drinking model in mice, modified from Lesscher et al. (2010) where aversion-resistant alcohol drinking is apparent after only a single protracted session of alcohol-only consumption, and with binge-like blood alcohol levels (Lei et al., in press). This is in contrast to studies in outbred rats, where months of alcohol drinking are required to develop compulsive-like alcohol drinking patterns (Hopf et al., 2010; Spanagel and Holter, 1999), and allows more rapid investigation into the mechanisms contributing to compulsive-like alcohol drinking.
Unfortunately, the central signaling mechanisms and circuits that promote compulsive-like alcohol consumption have been understudied until very recently (Barbier et al., 2015; Lesscher et al., 2012; Seif et al., 2013; Seif et al., 2015; Vendruscolo et al., 2012; Warnault et al., 2016). Here, we focus in particular on the importance of orexin (OX) receptors (OXRs) in promoting compulsive-like alcohol consumption. Over the years, OX – a neuropeptide synthesized in a subgroup of lateral hypothalamic cells which have projections throughout the brain – has been identified to play a role in a number of homeostatic and regulatory behaviors, including feeding, sleep-wake cycle, as well as emotional and neuroendocrine regulation (Brown et al., 2015a; Li et al., 2016; Mahler et al., 2014). OXRs have also been addressed in many studies of drugs of abuse, including cocaine, opioids, nicotine and alcohol (Barson and Leibowitz, 2016; Boutrel et al., 2013; Mahler et al., 2014; Mahler et al., 2012). There are two subtypes of OX receptors, OX1R and OX2R (Mahler et al., 2012). Both OX1Rs and OX2Rs can contribute to promoting alcohol drinking (Mahler et al., 2012), although extant studies suggest a stronger overall role for OX1R signaling during addictive behaviors relative to OX2Rs (Baimel et al., 2014; Barson et al., 2014; Brown et al., 2015b; Mahler et al., 2014; Moorman and Aston-Jones, 2009; but see Anderson et al., 2014; Brown et al., 2013). OX1Rs in particular appear to play a greater role in behaviors directed towards highly salient reinforcers, such as cocaine and high fat diet (Baimel et al., 2014; Borgland et al., 2009; Cason et al., 2010; Mahler et al., 2014), higher alcohol preference and intake (Moorman and Aston-Jones, 2009), and increased alcohol drinking in dependent mice (Lopez et al., 2016).
Since OX1Rs play a preferential role in pursuit of highly motivating substances, and compulsive-like intake represents a state of high motivation (willingness to drink alcohol despite the strong negative consequences) (Hopf et al., 2010; Hopf and Lesscher, 2014; Naqvi et al., 2014; Seif et al., 2013; Seif et al., 2015; Tiffany and Conklin, 2000), we hypothesized that mice engaging in compulsive-like intake would be more sensitive to the effects of OX1Rs inhibition than mice drinking regular, quinine-free alcohol. OX and OX1R signaling could represent an important and interesting mechanism that promotes compulsive-like alcohol drinking, with possible utility for clinical and therapeutic settings (Khoo and Brown, 2014; Li et al., 2016).
2. METHODS
2.1 Animals
Male C57BL/6 mice, 7–8-wks of age (Jackson Laboratories) were single-housed on a 12:12 light:dark cycle with the lights off at 10:00 am. Food and water were available ad libitum. All procedures followed the Guide for Care and Use of Laboratory Animals provided by the National Institutes of Health, and approval of the Institutional Animal Care and Use Committee of UCSF.
2.2 Limited daily access (LDA) two-bottle choice to alcohol or quinine adulterated alcohol
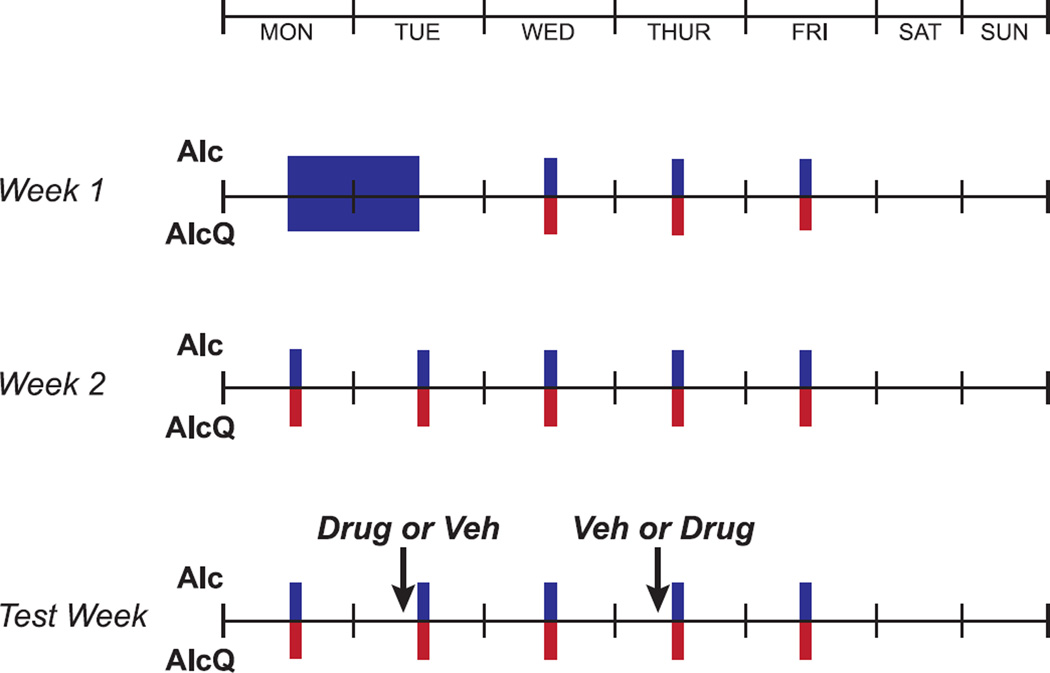
Our drinking model was modified from Lesscher et al. (2010), where mice consistently engaged in alcohol drinking despite pairing with the aversive consequence of bitter-tasting quinine. Mice were acclimated to housing conditions for approximately 2-wks. Afterwards, mice were first given one 24-hr session of drinking of alcohol-only under two-bottle choice access (one bottle with 15% alcohol in water, one bottle with water), and then a 24-hr withdrawal period. Thereafter, mice drank under a Limited Daily Access (LDA) paradigm, with 2-hr/day, 5-d/wk of two-bottle choice (Fig. 1). Each day, access to alcohol began approximately 3-hr into the dark cycle. To test for compulsive-like drinking, mice were then divided into two groups: (1) those drinking alcohol without quinine (Alc) and (2) those drinking alcohol adulterated with quinine (100 µM; AlcQ). To test whether the effects of the selective OX1R antagonist, SB-334867, were specific to alcohol-quinine, a separate group of mice were given the same drinking schedule, but instead of alcohol, in each drinking session they were presented with a 0.05% saccharin solution adulterated with 100 µM quinine (SacQ), a taste which alcohol has been reported to reflect (Goodwin and Amit, 1998). In order to account for spillage, bottles containing water, alcohol or saccharin were placed onto an empty cage on the same animal racks as the experimental subjects. Furthermore, in order to control for side preference, the bottle placements of the solutions were alternated between each drinking session.
Figure 1. Timeline of mouse drinking paradigm.
See Methods for details. Briefly, mice initially had 24-hr two-bottle choice access to alcohol-only (15% v/v) in one bottle and water in the other bottle, followed by subsequent limited daily access (LDA) two-bottle drinking of either alcohol-only (blue) or alcohol-quinine (red) for 2-hr/day, 5-d/wk.
2.3 Drugs
After 3-wk of LDA, mice began to be handled for habituation and systemic injections. The selective OX1R antagonist, SB-334867 (SB, Tocris), was dissolved in 2% DMSO in 25% (2-Hydroxypropyl)-β-cyclodextrin (Sigma) and 0.9% saline; the OX2R antagonist TCS-OX2-29 (TCS, Tocris) (Huang et al., 2010; Plaza-Zabala et al., 2013) was dissolved in 0.9% saline. Animals were injected, intraperitoneally (i.p.), 30-min prior to the drinking sessions. Subjects received either 0, 0.3, 1, 3 or 10-mg/kg-body-weight of SB, or 3 or 10-mg/kg-body-weight of TCS at a volume of 10-ml/kg (similar to previously used volumes, e.g. Olney et al., 2015). The selected SB doses were lower than what was previously used because we hypothesized mice drinking alcohol-quinine would be more sensitive to the effects of OX1R inhibition. Each given dose was injected twice (on different test days) and averaged per mouse; injections were counter-balanced with their vehicle across test days and mice using a Latin Squares design. There was at least one drinking session day between drug treatments to limit the impact of carryover effects after drug treatment, and drinking levels returned to baseline on these untreated days (not shown). Also, no more than two injections were performed in a given week (Fig. 1). The SacQ group was treated with either 0- or 3-mg/kg-body-weight of SB.
2.4 Data analysis
After each drinking session, we measured the weight of liquid consumed to determine the intake level of alcohol (g/kg-body-weight) or saccharin (mL/kg-body-weight), as well as the preference ratio (volume of intake/total volume of fluid intake). Spillage was taken into account by subtracting the fluid lost from bottles placed on the empty cages. All data were statistically analyzed by SPSS (IBM). For drug treatments, the average alcohol or saccharin intake and preference ratio were calculated for each dose and expressed as a percentage of the vehicle group average. Alcohol intake level and preference ratio were compared across the different doses of SB by a two-way ANOVA (group × dose) followed by a Tukey’s posthoc test. For the impact of TCS on alcohol drinking, a two-way ANOVA was used (cohort × dose). A paired t-test was used to analyze saccharin intake and preference between vehicle and drug groups.
3. RESULTS
3.1 OX1Rs play a greater role in regulating compulsive-like alcohol drinking versus more regular alcohol intake
To examine whether OX1Rs play a particular role in driving compulsive-like drinking, we administered different i.p. doses of the OX1R-selective antagonist, SB-334867 (SB) prior to drinking sessions in a counter-balanced manner (see Methods). Alcohol intake (Supplemental Table 1) and preference (Supplemental Table 2) and concurrent water intake (Supplemental Table 3) for each dose were compared to their own vehicle, within-mouse, and between Alc and AlcQ mice, using a two-way ANOVA (drug × group). Because multiple cohorts of animals were used over time, we also then normalized the alcohol intake for each dose to the group average of the corresponding vehicle sessions. Specifically, the intake for the vehicle sessions for each dose was averaged, so that the vehicle for each dose was on average 100%. The intake values for each animal for a given dose of SB or TCS were then expressed as a percentage of this vehicle group mean. This allowed us to directly compare consumption levels across different SB doses in the two groups (Alc and AlcQ) using a two-way ANOVA (dose × group).
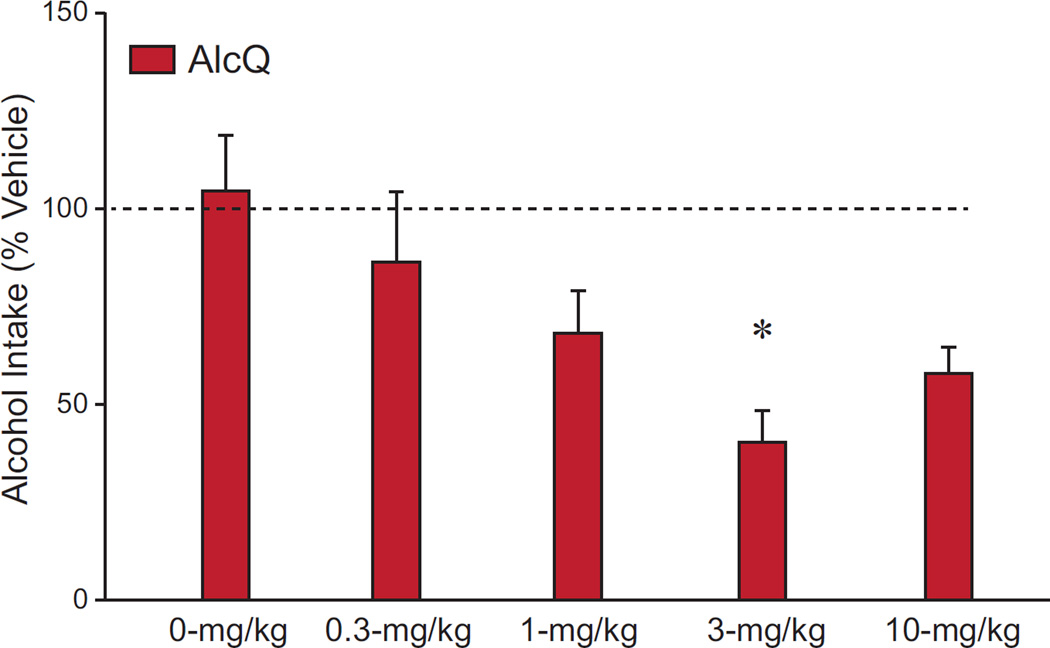
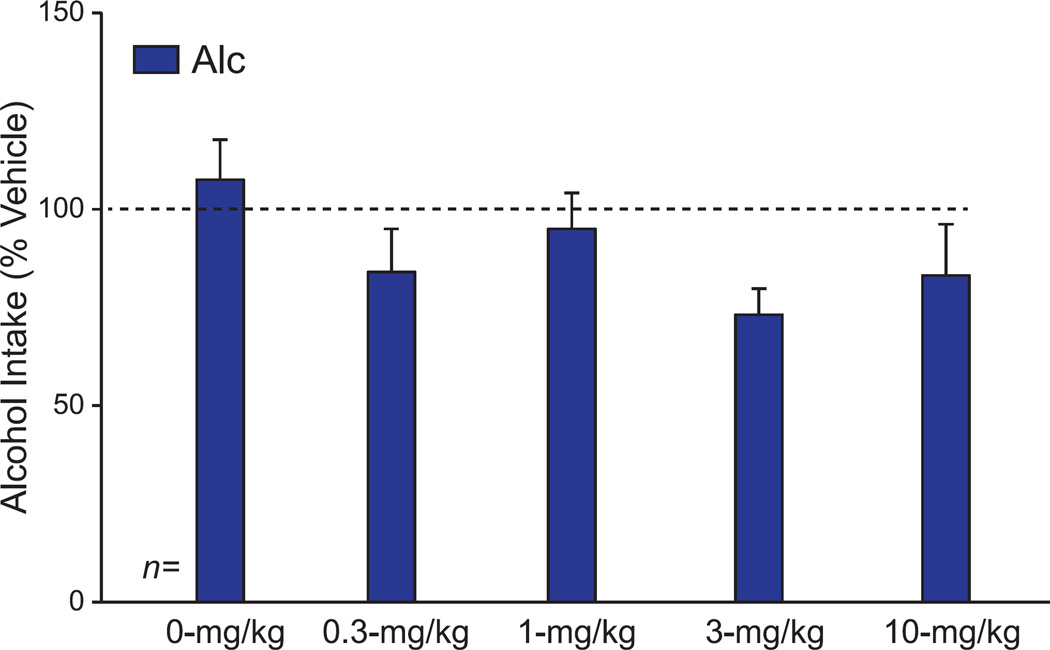
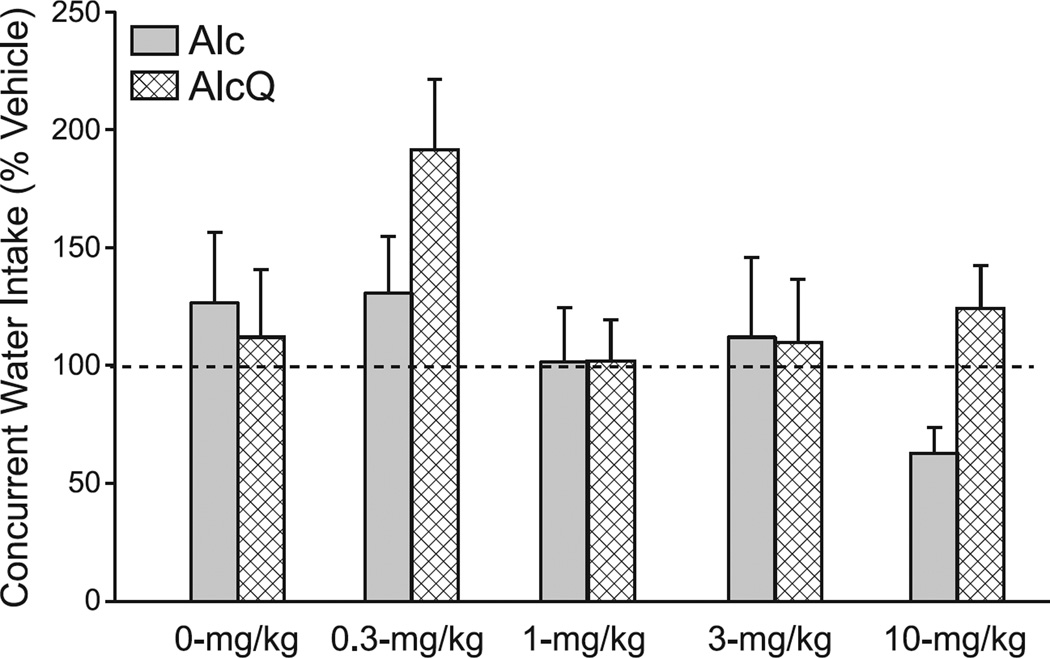
Our results overall suggest that OX1R inhibition with SB reduced AlcQ intake at a lower dose was effective against Alc consumption. Although we found no dose-group interaction (F(4,132)=0.94, p=0.44), there were main effects of both dose (F(4,132)=4.62, p=0.002) and group (F(1,132)=5.39, p=0.02) (Supplemental Table 1). Posthoc tests revealed that mice that received 3- or 10-mg/kg SB drank significantly less than those that received 0-mg/kg SB (p < 0.05). Interestingly, when the Alc and AlcQ groups were analyzed separately with one-way ANOVAs, there was a significant effect of SB in the AlcQ mice (Fig. 2; F(4,62)=3.45, p=0.013), while no drug effects were found among Alc mice (Fig. 3; F(4,70)=1.54, p=0.20). Furthermore, a posthoc test showed that AlcQ mice receiving 3-mg/kg SB drank significantly less than those that received 0-mg/kg SB (Fig. 2). Interestingly, 3-mg/kg SB is a lower dose than required in many studies to reduce different forms of alcohol drinking (see Discussion). Furthermore, at the doses we examined, SB had no effect on concurrent water consumption during the 2-hr drinking sessions (Fig. 4; interaction: F(4,132)=1.03, p=0.39; group: F(1,132)=1.69, p=0.20; drug: F(4,132)=2.30, p=0.06). Thus, low doses of SB reduced compulsive-like alcohol consumption with no impact on alcohol-only or water drinking, strongly suggesting that OX1Rs played a particular role in promoting compulsive-like alcohol drinking in AlcQ mice, with much less of a role for the regular LDA alcohol drinking (i.e., in Alc mice) or general consumption.
Figure 2. Systemic OX1R blockade significantly reduced compulsive-like alcohol drinking at relatively low doses.
For each animal, data for a given dose were normalized to vehicle exposure in that animal. Overall, 3-mg/kg SB-334867 significantly reduced compulsive-like consumption compared to the 0-mg/kg dose. A one-way ANOVA showed a significant effect across doses on alcohol-quinine consumption (F(4,62)=3.45, p=0.013). Data are shown as mean±SEM. *: p<0.05 compared to 0-mg/kg.
Figure 3. Systemic OX1R blockade had no overall impact on quinine-free alcohol intake.
For each animal, data for a given dose were normalized to vehicle exposure in that animal. Groups were compared using a one-way ANOVA. The selected doses of SB-334867 had no effect on alcohol-only consumption (F(4,70)=1.54, p=0.20). Data are shown as mean±SEM.
Figure 4. Systemic OX1R blockade had no overall impact on concurrent water drinking during intake of alcohol-only or alcohol+quinine.
For each animal, data for a given dose were normalized to vehicle exposure in that animal. Groups were compared using a two-way ANOVA (group × drug). The selected doses of SB-334867 did not alter concurrent water drinking in either Alc or AlcQ mice (interaction: F(4,132)=1.03, p=0.39; group: F(1,132)=1.69, p=0.20; drug: F(4,132)=2.30, p=0.06). Data are shown as mean±SEM.
3.2 Effects of OXR signaling on drinking are stimulus- and receptor-specific
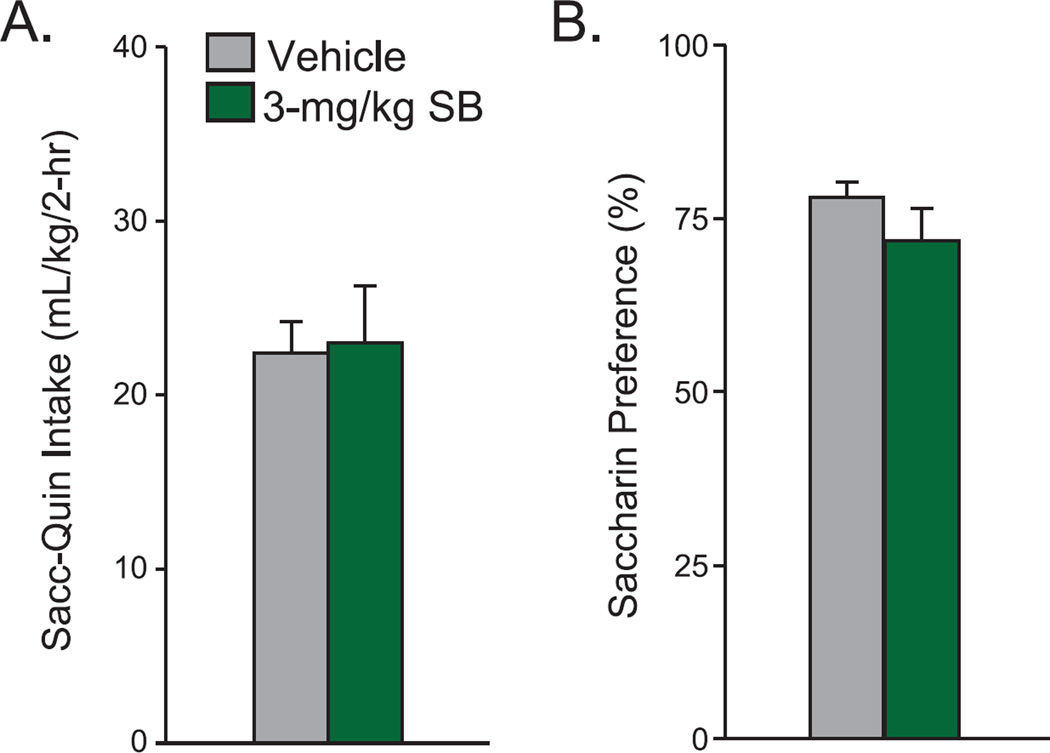
Our results suggest that OX1Rs selectively promote compulsive-like alcohol drinking, as indicated by a selective effect of low dose SB on compulsive-like alcohol consumption with no change in intake of alcohol-only and concurrent water. In addition, to further understand whether the impact of SB on compulsive-like alcohol drinking might reflect effects on intake of mixed bitter-sweet substances, we tested whether SB reduced drinking of saccharin+quinine (see Methods). However, 3-mg/kg SB had no effect on intake or preference of quinine-adulterated saccharin in AlcQ mice (n=12) (Fig. 5; intake: t(1,11)=0.25, p=0.81; preference: t(1,17)=1.23, p=0.24). Together with no effect of SB on alcohol-only drinking (Fig. 3) or concurrent water intake (Fig. 4), these results strongly suggest that OX1Rs played a selective role in compulsive-like alcohol consumption.
Figure 5. Systemic OX1R blockade did not alter saccharin-quinine consumption.
Paired t-tests showed that 3-mg/kg SB-334867 did not change saccharin-quinine (A) intake (t(1,11)=0.25, p=0.81) and (B) preference (t(1,17)=1.23, p=0.24) compared to vehicle. Data are shown as mean±SEM.
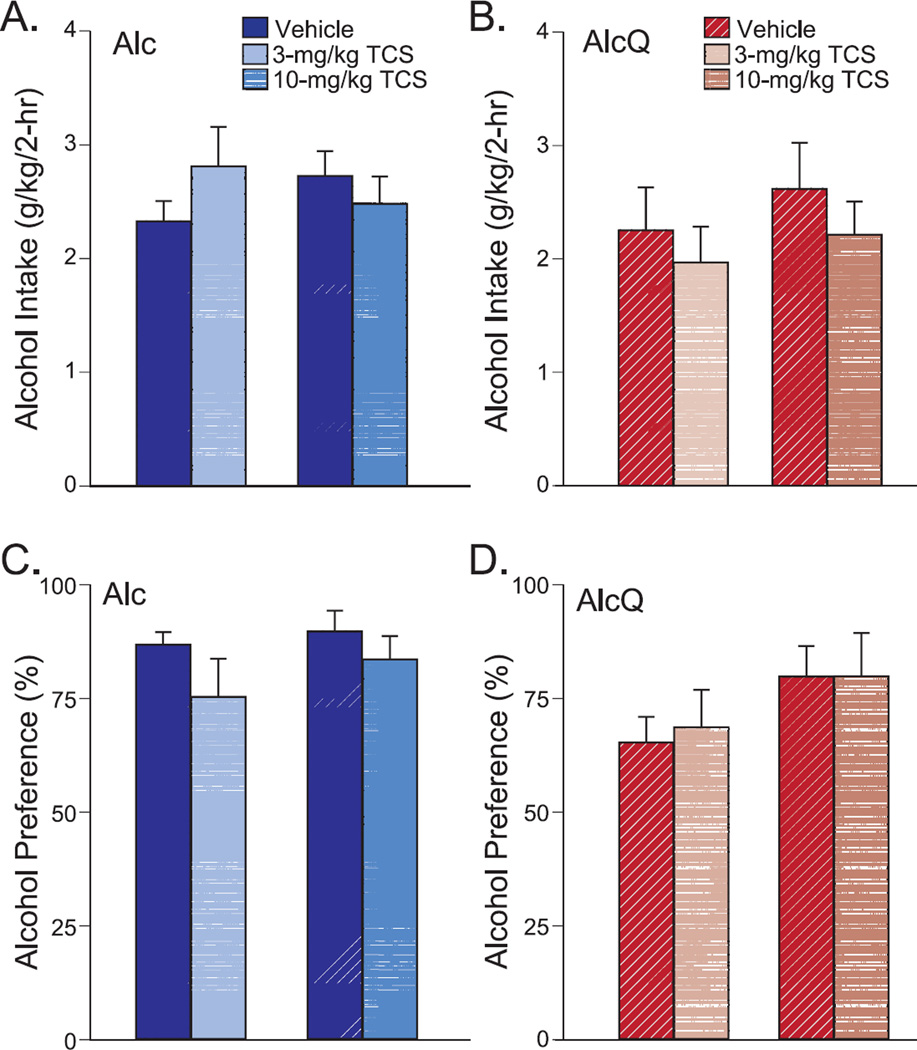
In addition, in order to examine whether the effects of OXR blockade on AlcQ drinking were receptor-specific, we examined whether the OX2R specific antagonist TCS (3 or 10-mg/kg) (Huang et al., 2010; Plaza-Zabala et al., 2013) could alter alcohol drinking in Alc (3 mg/kg: n=14; 10 mg/kg: n=11) or AlQ (3 mg/kg: n=14; 10 mg/kg: n=11) mice. Because the mice used to test the 3-mg/kg and 10-mg/kg groups were separate cohorts, a two-way ANOVA with repeated measures (dose × cohort) was used to compare TCS with vehicle between the different cohorts separately for the Alc and AlcQ groups. We found that neither the 3 nor the 10-mg/kg TCS dose affected consumption levels of alcohol-only (Fig. 6A; interaction: F(1,23)=2.62, p=0.12; drug: F(1,23)=0.28, p=0.6; cohort: F(1,23)=0.01, p=0.91) or alcohol-quinine (Fig. 6B; interaction: F(1,23)=0.03, p=0.86; drug: F(1,23)=1.08, p=0.31; cohort: F(1,23)=0.65, p=0.43). TCS also did not alter alcohol preference for alcohol-only (Fig. 6C; interaction: F(1,23)=0.34, p=0.57; drug: F(1,23)=3.71, p=0.07; cohort: F(1,23)=0.7, p=0.41) or alcohol-quinine (Fig. 6D; interaction: F(1,23)=0.06, p=0.81; drug: F(1,23)=0.06, p=0.81; cohort: F(1,23)=2.37, p=0.14). Thus, our results implicate OX1Rs but not OX2Rs in promoting compulsive-like alcohol drinking, with much less of an OX1R role during regular alcohol drinking or general consumption of water or SacQ.
Figure 6. Systemic OX2R blockade had no effect on alcohol drinking.
The 3 or 10-mg/kg TCS-OX2-29 were compared to their respective vehicle groups by a two-way ANONA with repeated measures which showed that TCS did not change (A) alcohol-only (interaction: F(1,23)=2.62, p=0.12; drug: F(1,23)=0.28, p=0.6; cohort: F(1,23)=0.01, p=0.91) or (B) alcohol-quinine (interaction: F(1,23)=0.03, p=0.86; drug: F(1,23)=1.08, p=0.31; cohort: F(1,23)=0.65, p=0.43) intake. Nor did TCS altered preference of (C) alcohol-only (interaction: F(1,23)=0.34, p=0.57; drug: F(1,23)=3.71, p=0.07; cohort: F(1,23)=0.7, p=0.41) or (D) alcohol-quinine (interaction: F(1,23)=0.06, p=0.81; drug: F(1,23)=0.06, p=0.81; cohort: F(1,23)=2.37, p=0.14). Data are shown as mean±SEM.
4. DISCUSSION
Understanding the mechanisms that regulate compulsive drinking is important for developing effective clinical treatments for AUDs. In this regard, the role of the OX system is understudied; while OXRs have been implicated in alcohol drinking, their role in driving compulsive-like alcohol consumption remains unknown. Here, we used a recently developed mouse model for compulsive-like alcohol drinking, where mice drink alcohol despite addition of the aversive-tasting quinine; these mice drink binge-like alcohol drinking levels, as evidenced by blood alcohol concentrations >80 mg% (Lei et al., in press). Interestingly, compulsive-like alcohol drinking and preference were more sensitive to inhibition by an OX1R antagonist and were significantly reduced by low doses of the OX1R antagonist; these doses were ineffective at reducing alcohol-only consumption (see below). Furthermore, the effects of OX1R inhibition were specific to alcohol, since SB did not reduce concurrent water intake during the alcohol consumption sessions, or decrease consumption of or preference for saccharin+quinine, which suggest that the effects of SB were not simply through changes in general consumption of liquids or taste palatability. In addition, alcohol-quinine drinking was not reduced by an OX2R antagonist, suggesting that OX regulated compulsive-like drinking in a receptor-specific manner. Together, our results indicate that compulsive-like drinking, compared to alcohol-only intake, was selectively and more sensitively regulated by OX1Rs, and that OX1Rs may represent a potentially effective and immediately available therapy (Khoo and Brown, 2014; Li et al., 2016) to address compulsive aspects of human AUDs.
In recent years, a number of studies have examined the importance of OXR signaling during animal models of alcohol and drug addiction (see Introduction). OX1Rs have been more extensively studied, in part because of the widely used and selective OX1R inhibitor, SB. Of the two OXRs, OX1R have been repeatedly shown to promote addictive behaviors, with a particularly important role for driving highly motivated intake. For example, OX1R blockers only reduce drinking in outbred rats with higher alcohol intake and preference, with no effect in rats with lower drinking and preference (Moorman and Aston-Jones, 2009). Similarly, OX1R blockers reduce alcohol drinking in dependent but not non-dependent mice, and with no reduction in sucrose intake (Lopez et al., 2016). These studies concur with findings that OX1Rs promote intake of highly motivating substances such as cocaine and high-fat food, with little impact of consumption of regular lab chow (Baimel et al., 2014; Cason et al., 2010; Mahler et al., 2014), and also promote cue-induced reinstatement for several drugs of abuse (Brown et al., 2015b; Cason and Aston-Jones, 2013; Smith and Aston-Jones, 2012). In addition, OX1R signaling regulates alcohol drinking in several lines of genetically alcohol-preferring rats (Anderson et al., 2014; Brown et al., 2015b; Dhaher et al., 2010; Jupp et al., 2011). However, OX1R blockers do not reduce alcohol conditioned place preference in mice (Voorhees and Cunningham, 2011), suggesting that OX1Rs may not directly regulate reward per se. OX2Rs have also been implicated in regulating alcohol intake (Anderson et al., 2014; Brown et al., 2013; Mahler et al., 2012), although our results indicate that OX2Rs were not necessary for promoting compulsive-like consumption. Importantly, compulsive-like drinking is a state of elevated motivation, where individuals are willing to consume alcohol even though drinking is paired with negative consequences (Hopf et al., 2010; Hopf and Lesscher, 2014; Naqvi et al., 2014; Seif et al., 2013; Seif et al., 2015; Tiffany and Conklin, 2000). Our findings provide novel evidence that OX1Rs play a more selective and sensitive role in promoting compulsive-like drinking. In agreement, OXRs are also proposed to play a central role in compulsive-like food binging (Merlo Pich and Melotto, 2014). Thus, OX1Rs play a critical role in the context of high motivation for intake, and we propose that the particularly pronounced role of OX1Rs in promoting compulsive-like consumption patterns may reflect the excessive motivation required to promote intake in the face of challenge.
One notable finding from our studies is that compulsive-like drinking was inhibited by low doses of SB. This was in contrast to drinking of alcohol-only, which overall was not impacted by SB. Previous studies have found that higher SB concentrations were required to decrease alcohol-only intake. For example, 30- but not 10-mg/kg SB reduces binge-like alcohol drinking in mice in one study (Anderson et al., 2014); another study found that 5- and 10-mg/kg did reduce binge-like intake in mice during the first hour of intake, but only 10-mg/kg had an overall effect across two hours of access to alcohol (Olney et al., 2015). In addition, 10- and 30-but not 3-mg/kg SB decrease drinking in the alcohol-preferring P-rat (Anderson et al., 2014), and 10-mg/kg reduces intake of cocaine and high-fat but not regular food (Borgland et al., 2009). We note that the 10- and 30-mg/kg SB dose also can reduce intake of sweet substance (Anderson et al., 2014; Olney et al., 2015; but see Jupp et al., 2011), perhaps calling into question the specificity of the impact of these concentrations. However, 30-mg/kg SB specifically decreases intake in outbred rats with higher but not lower preference for alcohol (Moorman and Aston-Jones, 2009), perhaps suggesting that, even at 30-mg/kg, OX1Rs play a more preferential role in intake under conditions of greater motivation. Although we did not see a similar effect of 10-mg/kg SB in our alcohol-only mice compared to the study from Olney et al. (2015), this could be due to variations in the drinking paradigms between the study from Olney and colleagues and our present study (e.g. 20% vs 15% alcohol, absence or presence of a second bottle containing water-only, results from 1 session test vs average of 2 sessions) or statistical power (3 vs 5 doses tested). However, the lack of effect of the 10-mg/kg SB dose under our drinking paradigm further highlights the sensitivity of the alcohol-quinine mice to OX1R inhibition. It is also possible that higher motivation for a particular substance is associated with certain neuroadaptive changes, particularly those related to OX signaling, which would explain the greater sensitivity to OX1R inhibition in the alcohol-quinine mice. Taken together, the extensive literature addressing the role of OX1Rs during addictive behaviors supports our hypothesis that compulsive-like alcohol consumption is more sensitive to OX1R inhibition relative to many other forms of motivated, addiction-related behaviors.
One limitation of our studies is that we have not addressed the exact brain regions in which OX1Rs act to promote compulsive-like alcohol consumption. OXRs in several brain regions, including the medial prefrontal cortex, lateral hypothalamus, ventral tegmental area, and paraventricular thalamus have been implicated in promoting different addictive behaviors (Baimel et al., 2014; Brown et al., 2013; Brown et al., 2015b; Mahler et al., 2014; Mahler et al., 2012; Matzeu et al., 2014; Schneider et al., 2007). In addition, alcohol intake has been associated with changes in OX production in the LH (e.g Olney et al., 2015), which could also contribute to greater sensitivity of compulsive-like drinking to OX1R blockers. Future experiments can address the brain regions through which OX1Rs regulate compulsive-like drinking and whether there are adaptations in OX1Rs or OX which are responsible for the greater sensitivity of compulsive-like alcohol drinking to lower doses of the OX1R inhibitor. Importantly, the present study used systemic administration of OXR inhibitors to be more directly applicable to translational applications. In this regard, Suvorexant is a dual-OX1R/OX2R antagonist that is FDA-approved and used to treat sleep disorders (Sheridan 2014; Kishi et al., 2015), and this compound may reflect a readily accessible intervention to treat compulsive aspects of humans AUDs.
Since our aversion-resistant drinking paradigm involves consumption across several weeks, it is possible that the greater OX1R sensitivity of the aversion-resistance could be a consequence of the repeated quinine-alcohol intake. While we cannot completely rule out this possibility, we note that we have recently demonstrated that aversion-resistant alcohol drinking in C57BL/6 mice develops very rapidly, after a single 24-hr session of alcohol-only intake (Lei et al., in press). This is in strong contrast to outbred rats, which require months to develop aversion-resistant consumption patterns (Hopf et al., 2010; Seif et al., 2013). Thus, it is likely that whatever changes might occur to promote aversion-resistant drinking would develop during the initial alcohol drinking session, rather than as a consequence of repeated alcohol-quinine intake. Also, it is likely that mice that drink alcohol-only and quinine-alcohol would both exhibit aversion-resistant consumption if tested, but aversion-resistance was not assessed in alcohol-only drinkers. This would be similar to what we previously reported in rats, where protracted alcohol drinking leads to development of neuro-adaptations that promote aversion-resistant alcohol intake (Seif et al., 2013; 2015). However, a critical difference between aversion-resistant alcohol consumption and “regular” alcohol drinking, in absence of overtly paired challenge, is that aversion-resistant intake recruits cortical-subcortical circuits that process conflict, which are then needed to promote alcohol consumption in the face of aversive challenge. In contrast, “regular” alcohol drinking does not recruit these cortical-related circuits, and thus the alcohol-related neuro-adaptations in the cortical-subcortical circuits play no role in promoting the regular alcohol consumption. Here, we have not explicitly identified whether there are alcohol-related adaptations in OX1R signaling, but we speculate that only aversion-resistant alcohol intake recruits cortical-subcortical circuits which are more sensitive to OX1R inhibition, and this might or might not require adaptations in OX1R signaling. Considerable future experiments would be required to identify any such adaptations.
Compulsive-like intake of alcohol, where drinking persists despite negative consequences, is a major obstacle when treating alcohol abuse in humans (Anton, 2000; Anton et al., 1996; Hopf and Lesscher, 2014; Koob and Volkow, 2010; Larimer et al., 1999; Modell et al., 1992; Naqvi et al., 2014; Sinha, 2009; Tiffany and Conklin, 2000). Here, we demonstrate that compulsive-like alcohol drinking in a rodent model was suppressed by low doses of an OX1R inhibitor that did not alter regular alcohol intake. Thus, OX1Rs may represent a novel and particularly potent pharmacotherapeutic target for suppressing compulsive-like drives for alcohol, especially since lower concentrations would minimize the impact on other reward-related behaviors.
Supplementary Material
Compulsive drives for alcohol represent a major global social and economic problem and also a major obstacle to treatment
Orexin-1 receptors are implicated in responding for highly motivating substances
Compulsive-like alcohol drinking is inhibited by lower concentrations of Orexin-1 receptor antagonists, relative to binge alcohol intake.
Compulsive-like alcohol consumption is not inhibited by Orexin-2 receptor antagonists.
Orexin-1 receptor blockers may represent a novel therapeutic intervention for compulsive aspects of alcohol addiction.
Acknowledgments
We thank Dr. Dorit Ron for her critical review of this manuscript. Supported by NIAAA P50 AA017072.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anderson RI, Becker HC, Adams BL, Jesudason CD, Rorick-Kehn LM. Orexin-1 and orexin-2 receptor antagonists reduce ethanol self-administration in high-drinking rodent models. Front Neurosci. 2014;8:33. doi: 10.3389/fnins.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF. Obsessive-compulsive aspects of craving: development of the Obsessive Compulsive Drinking Scale. Addiction. 2000;95(Suppl 2):S211–S217. doi: 10.1080/09652140050111771. [DOI] [PubMed] [Google Scholar]
- Anton RF, Moak DH, Latham PK. The obsessive compulsive drinking scale: A new method of assessing outcome in alcoholism treatment studies. Arch Gen Psychiatry. 1996;53:225–231. doi: 10.1001/archpsyc.1996.01830030047008. [DOI] [PubMed] [Google Scholar]
- Baimel C, Bartlett SE, Chiou LC, Lawrence AJ, Muschamp JW, Patkar O, Tung LW, Borgland SL. Orexin/hypocretin role in reward: implications for opioid and other addictions. Br J Pharmacol. 2014;172:334–348. doi: 10.1111/bph.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier E, Tapocik JD, Juergens N, Pitcairn C, Borich A, Schank JR, Sun H, Schuebel K, Zhou Z, Yuan Q, Vendruscolo LF, Goldman D, Heilig M. DNA methylation in the medial prefrontal cortex regulates alcohol-induced behavior and plasticity. J Neurosci. 2015;35:6153–6164. doi: 10.1523/JNEUROSCI.4571-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Ho HT, Leibowitz SF. Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: role of orexin receptor 2. Addict Biol. 2014;20:469–481. doi: 10.1111/adb.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Leibowitz SF. Hypothalamic neuropeptide signaling in alcohol addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:321–329. doi: 10.1016/j.pnpbp.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blincoe L, Seay A, Zaloshnja E, Miller T, Romano E, Luchter S, Spicer R. Washington D.C.: Department of Transportation; 2002. The Economic Impact of Motor Vehicle Crashes, 2000 U.S; pp. 1–82. [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. American journal of preventive medicine. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Steiner N, Halfon O. The hypocretins and the reward function: what have we learned so far? Front Behav Neurosci. 2013;7:59. doi: 10.3389/fnbeh.2013.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JA, Woodworth HL, Leinninger GM. To ingest or rest? Specialized roles of lateral hypothalamic area neurons in coordinating energy balance. Front Syst Neurosci. 2015a;9:9. doi: 10.3389/fnsys.2015.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Khoo SY, Lawrence AJ. Central orexin (hypocretin) 2 receptor antagonism reduces ethanol self-administration, but not cue-conditioned ethanol-seeking, in ethanol-preferring rats. Int J Neuropsychopharmacol. 2013;16:2067–2079. doi: 10.1017/S1461145713000333. [DOI] [PubMed] [Google Scholar]
- Brown RM, Kim AK, Khoo SY, Kim JH, Jupp B, Lawrence AJ. Orexin-1 receptor signalling in the prelimbic cortex and ventral tegmental area regulates cue-induced reinstatement of ethanol-seeking in iP rats. Addict Biol. 2015b doi: 10.1111/adb.12251. [DOI] [PubMed] [Google Scholar]
- Cason AM, Aston-Jones G. Role of orexin/hypocretin in conditioned sucrose-seeking in rats. Psychopharmacology (Berl) 2013;226:155–165. doi: 10.1007/s00213-012-2902-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Smith RJ, Tahsili-Fahadan P, Moorman DE, Sartor GC, Aston-Jones G. Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiol Behav. 2010;100:419–428. doi: 10.1016/j.physbeh.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Atlanta, GA: Center for Disease Control; 2014. Excessive Drinking Costs U.S. $223.5 Billion. [Google Scholar]
- Dhaher R, Hauser SR, Getachew B, Bell RL, McBride WJ, McKinzie DL, Rodd ZA. The Orexin-1 Receptor Antagonist SB-334867 Reduces Alcohol Relapse Drinking, but not Alcohol-Seeking, in Alcohol-Preferring (P) Rats. J Addict Med. 2010;4:153–159. doi: 10.1097/ADM.0b013e3181bd893f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Goodwin FL, Amit Z. Do taste factors contribute to the mediation of ethanol intake? Ethanol and saccharin-quinine intake in three rat strains. Alcohol Clin Exp Res. 1998;22:837–844. [PubMed] [Google Scholar]
- Harwood HJ, Fountain D, Livermore G. Economic costs of alcohol abuse and alcoholism. Recent Dev Alcohol. 1998;14:307–330. doi: 10.1007/0-306-47148-5_14. [DOI] [PubMed] [Google Scholar]
- Hingson R, Heeren T, Winter M, Wechsler H. Magnitude of alcohol-related mortality and morbidity among U.S. college students ages 18–24: changes from 1998 to 2001. Annual review of public health. 2005;26:259–279. doi: 10.1146/annurev.publhealth.26.021304.144652. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Chang SJ, Sparta DR, Bowers MS, Bonci A. Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self-administration. Alcohol Clin Exp Res. 2010;34:1565–1573. doi: 10.1111/j.1530-0277.2010.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Lesscher HM. Rodent models for compulsive alcohol intake. Alcohol. 2014;48:253–264. doi: 10.1016/j.alcohol.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SC, Dai YW, Lee YH, Chiou LC, Hwang LL. Orexins depolarize rostral ventrolateral medulla neurons and increase arterial pressure and heart rate in rats mainly via orexin 2 receptors. J Pharmacol Exp Ther. 2010;334:522–529. doi: 10.1124/jpet.110.167791. [DOI] [PubMed] [Google Scholar]
- Jupp B, Krivdic B, Krstew E, Lawrence AJ. The orexin(1) receptor antagonist SB-334867 dissociates the motivational properties of alcohol and sucrose in rats. Brain Res. 2011;1391:54–59. doi: 10.1016/j.brainres.2011.03.045. [DOI] [PubMed] [Google Scholar]
- Khoo SY, Brown RM. Orexin/hypocretin based pharmacotherapies for the treatment of addiction: DORA or SORA? CNS Drugs. 2014;28:713–730. doi: 10.1007/s40263-014-0179-x. [DOI] [PubMed] [Google Scholar]
- Kishi T, Matsunaga S, Iwata N. Suvorexant for Primary Insomnia: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials. PLoS One. 2015;28:e0136910. doi: 10.1371/journal.pone.0136910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of Addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larimer ME, Palmer RS, Marlatt GA. Relapse prevention. An overview of Marlatt's cognitive-behavioral model. Alcohol Res Health. 1999;23:151–160. [PMC free article] [PubMed] [Google Scholar]
- Lei K, Wegner SA, Yu J-H, Simms JA, Hopf FW. A single alcohol drinking session is sufficient to enable subsequent aversion-resistant consumption in mice. Alcohol. doi: 10.1016/j.alcohol.2016.07.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesscher HM, Houthuijzen JM, Groot Koerkamp MJ, Holstege FC, Vanderschuren LJ. Amygdala 14-3-3zeta as a novel modulator of escalating alcohol intake in mice. PLoS One. 2012;7:e37999. doi: 10.1371/journal.pone.0037999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesscher HM, van Kerkhof LW, Vanderschuren LJ. Inflexible and indifferent alcohol drinking in male mice. Alcohol Clin Exp Res. 2010;34:1219–1225. doi: 10.1111/j.1530-0277.2010.01199.x. [DOI] [PubMed] [Google Scholar]
- Lesscher HM, Vanderschuren LJ. Compulsive drug use and its neural substrates. Rev Neurosci. 2012;23:731–745. doi: 10.1515/revneuro-2012-0066. [DOI] [PubMed] [Google Scholar]
- Li SB, Jones JR, de Lecea L. Hypocretins, Neural Systems, Physiology, and Psychiatric Disorders. Curr Psychiatry Rep. 2016;18:7. doi: 10.1007/s11920-015-0639-0. [DOI] [PubMed] [Google Scholar]
- Loi B, Lobina C, Maccioni P, Fantini N, Carai MA, Gessa GL, Colombo G. Increase in alcohol intake, reduced flexibility of alcohol drinking, and evidence of signs of alcohol intoxication in Sardinian alcohol-preferring rats exposed to intermittent access to 20% alcohol. Alcohol Clin Exp Res. 2010;34:2147–2154. doi: 10.1111/j.1530-0277.2010.01311.x. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Moorman DE, Aston-Jones G, Becker HC. The highly selective orexin/hypocretin 1 receptor antagonist GSK1059865 potently reduces ethanol drinking in ethanol dependent mice. Brain Res. 2016 doi: 10.1016/j.brainres.2016.01.049. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci. 2014;17:1298–1303. doi: 10.1038/nn.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G. Multiple roles for orexin/hypocretin in addiction. Prog Brain Res. 2012;198:79–121. doi: 10.1016/B978-0-444-59489-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Li X, Shaham Y. Recent developments in animal models of drug relapse. Curr Opin Neurobiol. 2013;23:675–678. doi: 10.1016/j.conb.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzeu A, Zamora-Martinez ER, Martin-Fardon R. The paraventricular nucleus of the thalamus is recruited by both natural rewards and drugs of abuse: recent evidence of a pivotal role for orexin/hypocretin signaling in this thalamic nucleus in drug-seeking behavior. Front Behav Neurosci. 2014;8:117. doi: 10.3389/fnbeh.2014.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo Pich E, Melotto S. Orexin 1 receptor antagonists in compulsive behavior and anxiety: possible therapeutic use. Front Neurosci. 2014;8:26. doi: 10.3389/fnins.2014.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modell JG, Glaser FB, Mountz JM, Schmaltz S, Cyr L. Obsessive and compulsive characteristics of alcohol abuse and dependence: quantification by a newly developed questionnaire. Alcohol Clin Exp Res. 1992;16:266–271. doi: 10.1111/j.1530-0277.1992.tb01374.x. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. Jama. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol--preferring Sprague--Dawley rats. Alcohol. 2009;43:379–386. doi: 10.1016/j.alcohol.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Gaznick N, Tranel D, Bechara A. The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann N Y Acad Sci. 2014;1316:53–70. doi: 10.1111/nyas.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JJ, Navarro M, Thiele TE. Binge-like consumption of ethanol and other salient reinforcers is blocked by orexin-1 receptor inhibition and leads to a reduction of hypothalamic orexin immunoreactivity. Alcohol Clin Exp Res. 2015;39:21–29. doi: 10.1111/acer.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza-Zabala A, Flores A, Martin-Garcia E, Saravia R, Maldonado R, Berrendero F. A role for hypocretin/orexin receptor-1 in cue-induced reinstatement of nicotine-seeking behavior. Neuropsychopharmacology. 2013;38:1724–1736. doi: 10.1038/npp.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks JJ, Roeber J, Bouchery EE, Gonzales K, Chaloupka FJ, Brewer RD. State costs of excessive alcohol consumption, 2006. Amer J Prevent Med. 2013;45:474–485. doi: 10.1016/j.amepre.2013.06.004. [DOI] [PubMed] [Google Scholar]
- SAMHSA. USA: Substance Abuse and Mental Health Services Administration; 2014. Risk and Protective Factors and Initiation of Substance Use: Results from the 2014 National Survey on Drug Use and Health. [Google Scholar]
- Schneider ER, Rada P, Darby RD, Leibowitz SF, Hoebel BG. Orexigenic peptides and alcohol intake: differential effects of orexin, galanin, and ghrelin. Alcohol Clin Exp Res. 2007;31:1858–1865. doi: 10.1111/j.1530-0277.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A, Hopf FW. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci. 2013;16:1094–1100. doi: 10.1038/nn.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif T, Simms JA, Lei K, Wegner S, Bonci A, Messing RO, Hopf FW. D-Serine and D-Cycloserine Reduce Compulsive Alcohol Intake in Rats. Neuropsychopharmacology. 2015;40:2357–2367. doi: 10.1038/npp.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan C. Insomniacs get new mechanism sleep drug Belsomra. Nat Biotechnol. 2014;32:968. doi: 10.1038/nbt1014-968. [DOI] [PubMed] [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addict Biol. 2009;14:84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Orexin / hypocretin 1 receptor antagonist reduces heroin self-administration and cue-induced heroin seeking. Eur J Neurosci. 2012;35:798–804. doi: 10.1111/j.1460-9568.2012.08013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Holter SM. Long-term alcohol self-administration with repeated alcohol deprivation phases: an animal model of alcoholism? Alcohol Alcohol. 1999;34:231–243. doi: 10.1093/alcalc/34.2.231. [DOI] [PubMed] [Google Scholar]
- Spoelder M, Hesseling P, Baars AM, Lozeman-van 't Klooster JG, Rotte MD, Vanderschuren LJ, Lesscher HM. Individual Variation in Alcohol Intake Predicts Reinforcement, Motivation, and Compulsive Alcohol Use in Rats. Alcohol Clin Exp Res. 2015;39:2427–2437. doi: 10.1111/acer.12891. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Conklin CA. A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction. 2000;95(Suppl 2):S145–S153. doi: 10.1080/09652140050111717. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr, Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Celerier E, Chaskiel L, Penzo F, Spanagel R. Compulsive alcohol drinking in rodents. Addict Biol. 2009;14:384–396. doi: 10.1111/j.1369-1600.2009.00177.x. [DOI] [PubMed] [Google Scholar]
- Voorhees CM, Cunningham CL. Involvement of the orexin/hypocretin system in ethanol conditioned place preference. Psychopharmacology (Berl) 2011;214:805–818. doi: 10.1007/s00213-010-2082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnault V, Darcq E, Morisot N, Phamluong K, Wilbrecht L, Massa SM, Longo FM, Ron D. The BDNF Valine 68 to Methionine Polymorphism Increases Compulsive Alcohol Drinking in Mice that Is Reversed by Tropomyosin Receptor Kinase B Activation. Biol Psychiatry. 2016;79:427–429. doi: 10.1016/j.biopsych.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. World Health Organization; 2014. Global status report on alcohol and health-2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.