Abstract
Purpose
This study aimed to establish a large-scale database of patients with gastric cancer to facilitate the development of a national-cancer management system and a comprehensive cancer control policy.
Materials and Methods
An observational prospective cohort study on gastric cancer was initiated in 2010. A total of 14 cancer centers throughout the country and 152 researchers were involved in this study. Patient enrollment began in January 2011, and data regarding clinicopathological characteristics, life style-related factors, quality of life, as well as diet diaries were collected.
Results
In total, 4,963 patients were enrolled until December 2014, and approximately 5% of all Korean patients with gastric cancer annually were included. The mean age was 58.2±11.5 years, and 68.2% were men. The number of patients in each stage was as follows: 3,394 patients (68.4%) were in stage IA/B; 514 patients (10.4%), in stage IIA/B; 469 patients (9.5%), in stage IIIA/B/C; and 127 patients (2.6%), in stage IV. Surgical treatment was performed in 3,958 patients (79.8%), endoscopic resection was performed in 700 patients (14.1%), and 167 patients (3.4%) received palliative chemotherapy. The response rate for the questionnaire on the quality of life was 95%; however, diet diaries were only collected for 27% of patients.
Conclusions
To provide comprehensive information on gastric cancer for patients, physicians, and government officials, a large-scale database of Korean patients with gastric cancer was established. Based on the findings of this cohort study, an effective cancer management system and national cancer control policy could be developed.
Keywords: Stomach neoplasms, Cohort studies, Korea, Quality of life
Introduction
In Korea, both the incidence of cancer and the number of cancer survivors have been continuously increasing. The age-adjusted cancer incidence increased to 290.5/100,000 cases in 2013 from 210.5/100,000 cases in 1999.1 The overall survival rates for all types of cancer cases have also increased, from 41.2% in 1993 to 1995 to 69.4% in 2008 to 2013.1
The high incidence of cancer and high survival rates among cancer patients have led to an increase in cancer survivors. In Korea, the number of patients diagnosed with any cancer from 1999 to 2013 who were alive in January 2014 totaled 1,370,049 patients.1 These cancer patients and survivors face considerable physical, psychological, social, spiritual, and economic challenges after being diagnosed with cancer, and these problems present a significant socioeconomic burden beyond the problems experienced by the individual.2,3 As a result, the demand for systematic management of cancer patients and survivors has grown, and a long-term cancer policy is required.
To develop an effective cancer management system, comprehensive information is essential. Management systems should include not only clinical information such as diagnoses, treatments, and outcomes, but also information regarding the quality of life (QOL) and socioeconomic status of the patients and the family caregivers. An appropriate study population that is representative of all cancer patients is also required. Many large-scale cohort studies on different types of cancer have been initiated for this purpose, and many studies have been performing using data from the developed databases.4,5,6
Gastric cancer is the most common cancer in men and has the second highest prevalence rate in Korea.1 The 5-year survival rates of patients with gastric cancer rapidly increased from 42.8% in 1993 to 1995 to 73.1% in 2008 to 2013, and with respect to the number of cancer survivors, gastric cancer ranked second after thyroid cancer in 2013.1 Therefore, effective management systems and policies, especially for gastric cancer, are needed; the Korean government has also proposed the development of a nationwide database of patients with gastric cancer. As Korea has the highest incidence of gastric cancer in the world, large-scale studies should be performed and an effective management system for patients with gastric cancer should be developed.7
This study aimed to establish a large-scale database of Korean patients with gastric cancer and to develop a database network and management system. This cohort will provide comprehensive information on patients with gastric cancer and their family caregivers, and this information will enable the development of cancer prevention policies and management strategies for patients with gastric cancer.
Materials and Methods
1. Study design and gastric cancer cohort
The Ministry of Health and Welfare of the Republic of Korea launched a project to build a nationwide database of patients with gastric cancer in 2010. This cohort study was a large-scale multicenter observational prospective study focused on the clinical outcomes and QOL of patients with gastric cancer in Korea. The National Cancer Center, 9 Regional Cancer Centers, and 4 high-volume cancer centers were included: Gangwon, Gyeongnam, Daegu-Gyeongbuk, Daejeon, Busan, Jeonnam, Jeonbuk, Jeju, and Chungbuk Regional Cancer Centers, as well as Seoul National University Hospital, Asan Medical Center, Severance Hospital, and Samsung Medical Center (Table 1). Patients who were newly diagnosed with primary gastric cancer in the 14 centers and who met the following inclusion criteria were recruited: (1) age ≥18 years; (2) histologically confirmed primary gastric adenocarcinoma; and (3) agreement to participate in the study by provision of written informed consent. To obtain reliable survival data, patients who met the following conditions were excluded: (1) previous use of any therapy for gastric cancer, including surgery, endoscopic therapy, chemotherapy, and radiotherapy and (2) a secondary primary malignancy (except in situ carcinoma of the cervix, adequately treated basal cell carcinoma of the skin, and prior malignancy treatment received more than 5 years prior without recurrence).
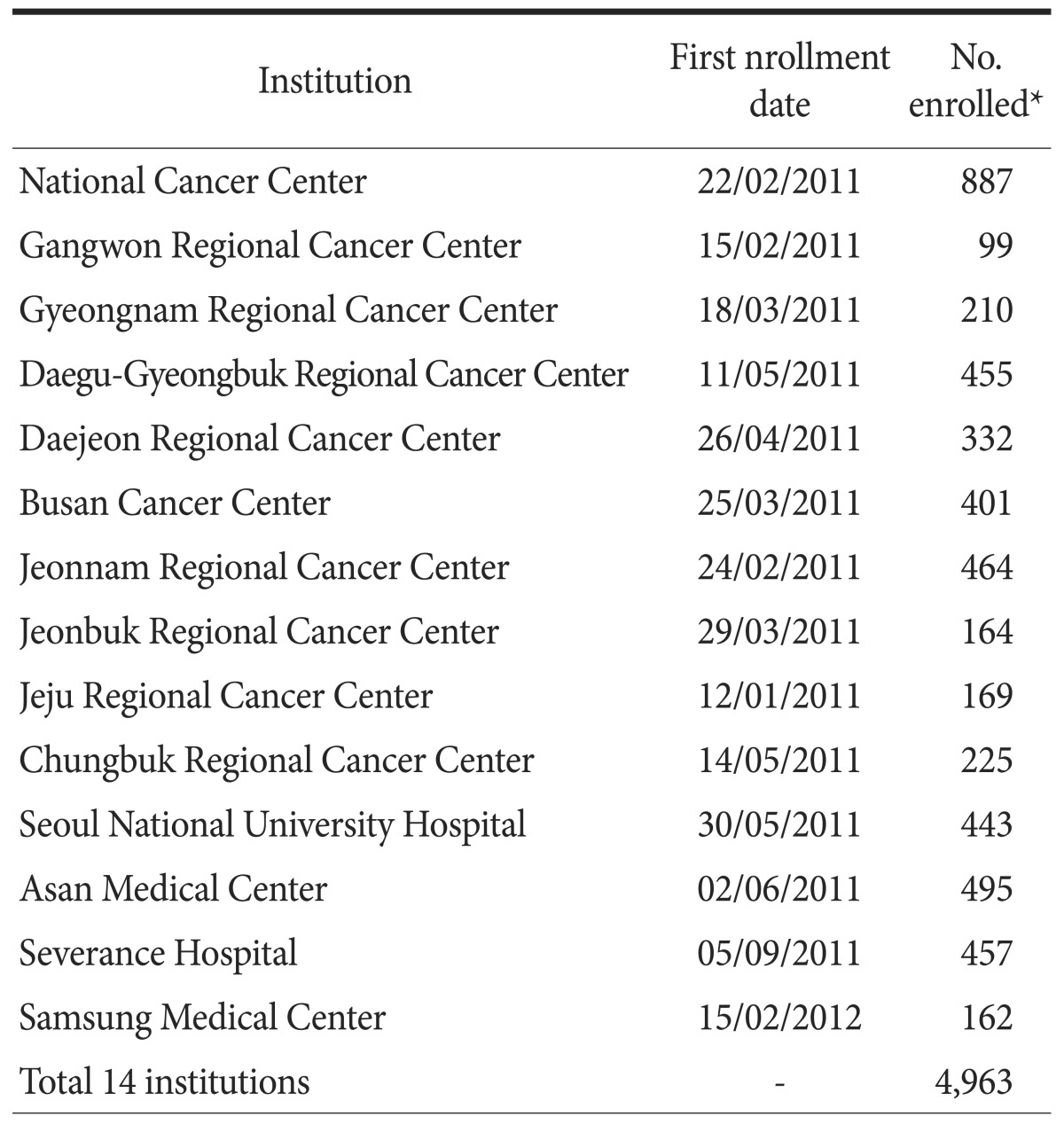
Table 1. Study enrollment for each institution.
*Number of patients enrolled before December 9, 2014.
This study was open not only to patients with gastric cancer but also to family caregivers, and the following inclusion criteria for family caregivers were considered: (1) being identified byth e enrolled patients as the unpaid person who was most involved with their actual care; (2) providing care to enrolled patients for at least 3 hours per day more than 3 days per week; (3) being aged ≥18 years; and (4) providing written informed consent before study participation. Family caregivers who were not able to communicate with the researchers were excluded.
All 14 institutional review boards approved this study protocol (National Cancer Center No. NCCNCS 10399, Chungnam National University Hospital No. 1101-01, Chonnam National University Hospital No. 2010-094, Gyeongsang National University Hospital No. GNUHIRB-2010-077, Seoul National University Hospital No. H-1302-033-463, Kyungpook National University Medical Center No. KNUMC_11-1001, Asan Medica l Center No. 2011-1006, Pusan National University Hospital No. H-1012-001-001, Chungbuk National University Hospital No. 2010-12-075, Jeju National University Hospital No. JEJUNUH 2011-11-007-012, Severance Hospital No. 4-2011-0387, Kangwon National University Hospital No. KNUH-2010-12-001-009, Chonbuk National University Hospital No. CUH 2010-12-207-016, and Samsung Medical Center No. SMC 2012-03-002-023), and patients have been continuously enrolling in the cohort since January 2011. This study was registered at the International Clinical Trial Registry Platform of the World Health Organization (KCT 0000073).
2. Data collection
1) Clinical and health related information
Clinical information such as age, sex, diagnostic test results, therapeutic procedures, and pathological results were collected from patients' medical records. Diagnostic tests included laboratory test values, such as complete blood count, liver panel data, tumor marker levels, and endoscopy and abdominopelvic computed tomography findings. Clinical or pathological stages were assigned according to the American Joint Committee on Cancer staging system, 7th edition.
Demographic data (e.g., marital status, education, and religion) and baseline socioeconomic data, including occupation, regularity of business hours, night shifts, family income, and medical insurance was determined. Health-related information such as past medical history, family history of cancer, previous health screening history, reproductive history, smoking habit, alcohol consumption, physical activity levels, dietary habits, and gastric cancer-related symptoms were obtained by a trained interviewer. Reproductive history included initial and last menstrual period as well as information regarding contraception use. Gastric cancer-related symptoms consisted of a total of 13 symptoms, including nausea, vomiting, and general weakness.
After being treated for gastric cancer, patients were followed at each hospital according to the institution's follow-up schedule. Clinical information, such as patient weight, laboratory findings, additional treatments, and disease progression/recurrence status, was collected every 6 months for 3 years. Thereafter, a trained coordinator interviewed and collected data annually until the patient's death.
2) Assessment of quality of life
QOL data were obtained using 3 self-administered questionnaires: the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ)-C30, the EORTC QLQ-STO22, and the Caregiver Quality of Life Index-Cancer (CQOLC).8,9,10 The EORTC QLQ-C30 questionnaire consists of 30 health-related QOL questions: specifically, 5 function scales (physical, role, cognitive, emotional, and social), 3 symptom scales (fatigue, pain, and nausea and vomiting), a global health and QOL scale, single items for other symptoms commonly experienced by cancer patients (e.g., dyspnea, appetite loss, sleep disturbance, constipation, and diarrhea), and perceived financial impact of the disease and treatment. The EORTC QLQ-STO22 is a 22-item gastric cancer-specific questionnaire that includes 5 hypothesized scales (dysphasia, eating restrictions, pain, reflux, and anxiety) and 4 single items (dry mouth, body image, taste, and hair loss) covering disease and treatment-related symptoms and specific emotional consequences of gastric cancer. The CQOLC evaluates the QOL of the cancer patient's family caregiver and consists of a 10-item scale on burden (physical, emotional, family, and social functioning), 7-item scale on disruptiveness, 7-item scale on positive adaptation, 3-item scale on financial concerns, and 8 single items on overall QOL.11 These 35 items are measured on a five-point Likert-type scale (ranging from 0" not at all" to 4 "very much").
3) Assessment of dietary intake
Dietary intake was evaluated using self-reported 3-day diet diaries. When a patient was enrolled in the study, a trained coordinator instructed the patient on how to complete the diet diary. Patients were asked to record any food or drink they consumed for 3 days, and the amount was reported with reference to visual measurements available in a source book. A dietitian collected the diet diaries and entered the data into a computer software program (CAN-Pro 4.0; The Korean Nutrition Society, Seoul, Korea) that converted food intake into energy, macronutrient intake, and specific micronutrient intake and provided averages.
Diet diaries were completed before treatment, 3 months after treatment, and annually during the follow-up period.
4) Data management
All information collected after enrollment was entered into a n electronic case report form (eCRF) by trained coordinators using the internet-based eVelos system (http://kcpc.ncc.re.kr; Velos Inc., Fremont, CA, USA). Data were entered into the eCRF was performed within 2 weeks of the patient's visit, and the completed eCRFs were reviewed and signed by the investigator or sub-investigator. A data management group monitors all eCRFs and regularly submits "queries" when data are not entered or are inappropriately entered.
3. Statistical analysis
All continuous values are presented as the mean (standard deviation), and categorical variables are shown as proportions. Data analyses were performed using SAS ver. 9 (SAS Institute Inc., Cary, NC, USA), R software (ver. 2.12.1), and Stata ver. 12 (STATA, College Station, TX, USA).
Results
1. Patient characteristics
From January 2011 to December 2014, 4,963 patients were enrolled in this study. The numbers of enrolled patients at each institution ranged from 99 to 888 patients (Table 1). Approximately 1,500 patients with gastric cancer were registered annually since 2012, which corresponds to approximately 5% of all new patients with gastric cancer in Korea.
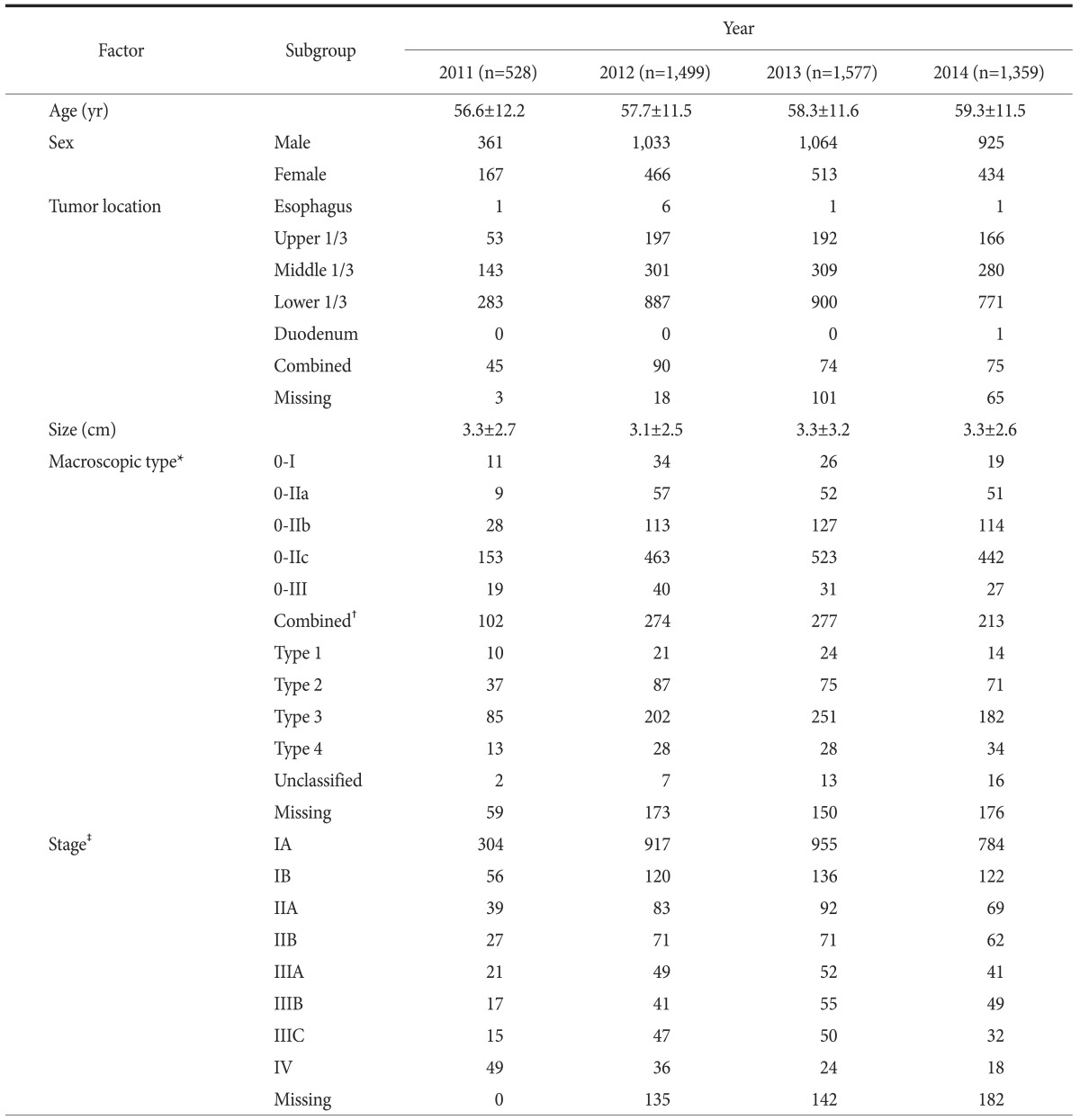
Table 2 shows the patients' clinicopathological characteristics. The mean age was 58.2±11.5 years, and 68.2% of the patients were men. Approximately 64.6% (3,205/4,963) of the patients had type 0 gastric cancer, a superficial macroscopic type, representing early gastric cancer in endoscopic findings. The most common stage was IA/B (3,394 patients, 68.4%), followed by IIA/B (514 patients, 10.4%), IIIA/B/C (469 patients, 9.5%), and IV (127 patients, 2.6%). Staging data were not available for 459 patients.
Table 2. Baseline clinicopathological characteristics.
Values are presented as mean±standard deviation or number only. *Macroscopic type was categorized based on the Japanese classification of gastric carcinoma. †Combined superficial type, such as type IIc+IIa. ‡Stage was categorized based on the American Joint Committee on Cancer staging system, 7th edition.
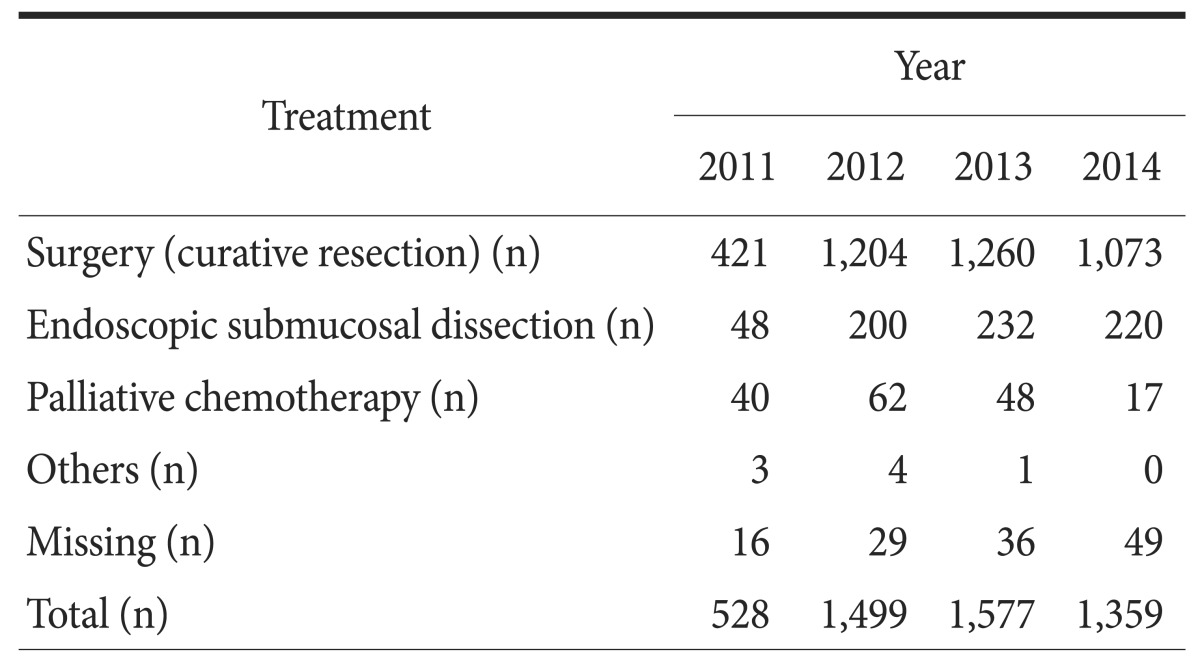
2. Treatment status
Of the 4,963 patients, 3,958 patients (79.8%) underwent curative resection, 700 patients (14.1%) were treated with endoscopic submucosal dissection, and 167 patients (3.4%) received palliaivte chemotherapy (Table 3). Four patients did not receive further treatment after undergoing explorative laparotomy owing to distant metastasis, and 3 patients underwent palliative procedures, such as gastrojejunostomy (1 patient), endoscopic stent insertion (1 patient), and endoscopic coagulation for tumor bleeding (1 patient), without further treatment. Of the 130 patients classified as missing, 44 patients refused any treatment, 27 patients were transferred to other hospitals, and data were not available for 56 patients. Of the remaining 3 patients, 1 patient withdrew from the study, 1 patient was lost to follow-up, and 1 patient had pending diagnostic results.
Table 3. Treatment for gastric cancer.
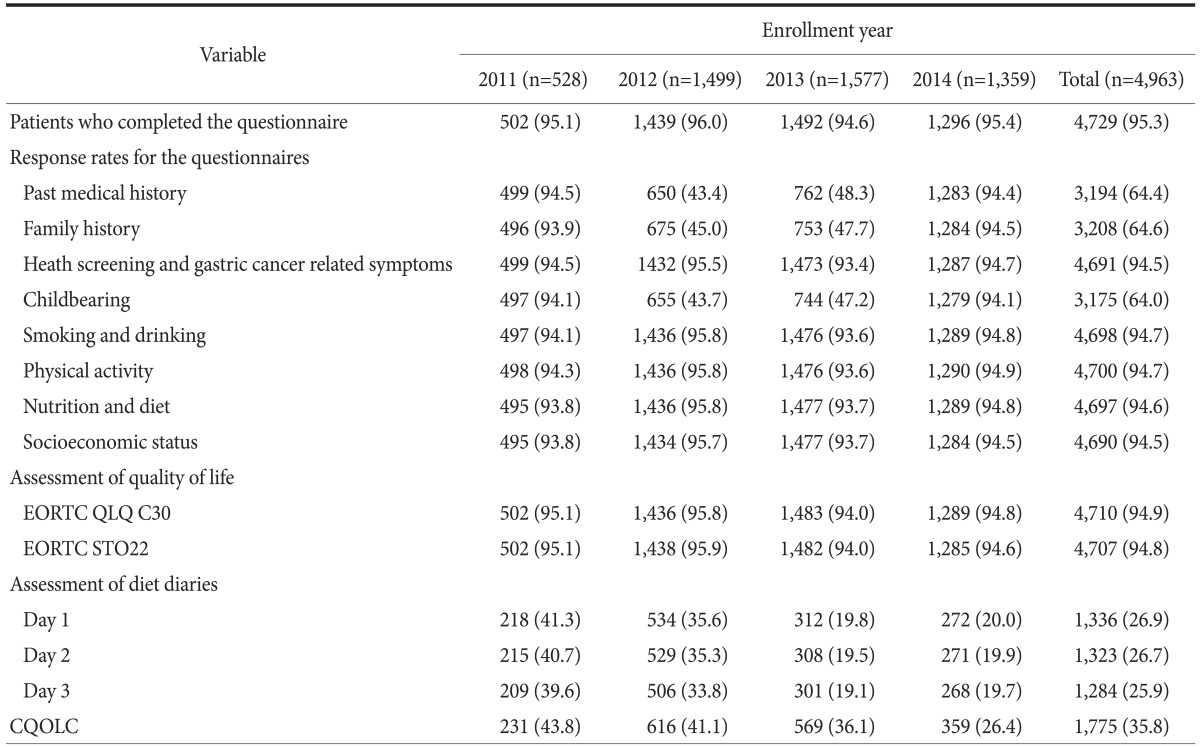
3. Baseline questionnaire collection status
Table 4 shows the response rates for the questionnaires on health-related information and QOL. Responses regarding health-related information and QLQs were collected from approximately 93.7% to 100% of participants annually, with the exception of 2012 and 2013. However, diet diaries were collected from only 19.1% to 41.3% of patients, and a low response rate was observed for the CQOLC 2(6.4%~43.8%).
Table 4. Baseline questionnaire collection.
Values are presented as number (%). EORTC QLQ = European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; CQOLC = Caregiver Quality of Life Index-Cancer.
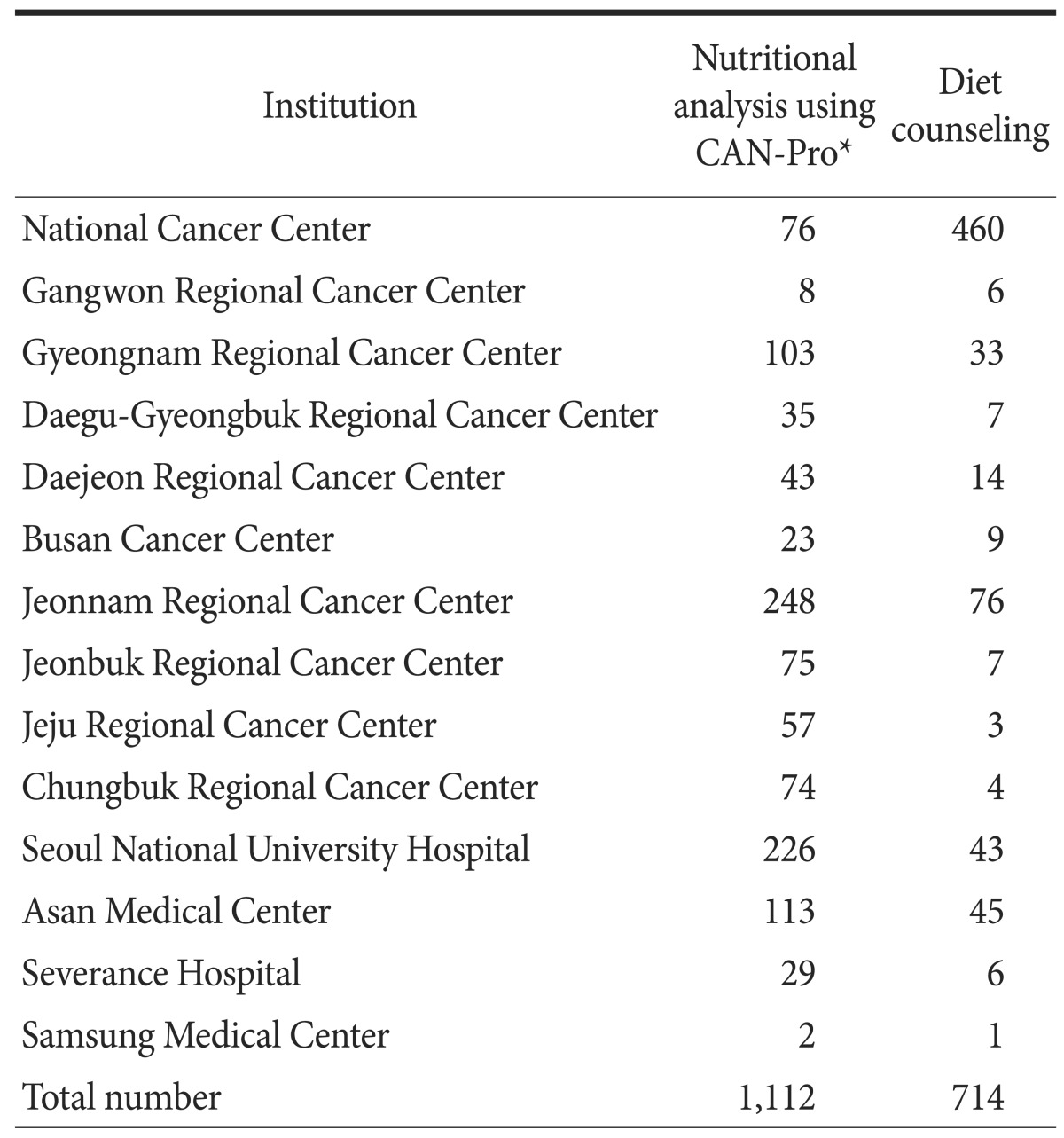
Nutritional analysis was performed in 1,112 patients and diet counseling was provided to 714 patients (Table 5).
Table 5. Nutritional analysis and diet counseling between 2011 and 2014.
*A computer software program that converted food intake into nutrients.
Discussion
In this prospective cohort study, a large-scale database of patients with gastric cancer was established. Fourteen hospitals, including 9 Regional Cancer Centers located in each province, were involved, and 4,963 patients were recruited as of December 2014. Based on this cohort study findings, an effective cancer management system and comprehensive cancer control policies could be developed.
Several cancer registries have previously been established in Korea. The Korean Breast Cancer Society has conducted a multi-center survey for patients newly diagnosed with primary breast cancer since 1996, in which 41 university and 61 surgical training hospitals were involved.6 The Online Korean Breast Cancer Registration Program was launched in 2001, and the corresponding data have been collected through a web-based database system. That program includes more than 80% of Korean patients with breast cancer, which is highly representative. However, the recorded items are limited to several clinical factors; the essential items include age, sex, surgical method used, cancer stage, and 5 optimal clinical items.
The Korean Cancer Prevention Study (KCPS) is a large-scale prospective cohort study of 1,309,275 Koreans who received biennial medical evaluations from the National Health Insurance Service from 1992 to 1995.12,13 The objective of that study was to assess risk factors for all types of cancer, and the participants were asked to complete questionnaires for risk factors such as smoking, alcohol consumption, and other health-related habits. An advantage of the KCPS is that it was a population-based nationwide study; however, the obtained data were limited to cancer risk factors.
In Japan, the Japanese Gastric Cancer Association began conducting a nationwide gastric cancer registration study using electronic data collection systems in 1998, and it started a new registration program in 2008.14,15 The new database consists data regarding clinical factors, such as surgical procedures, pathological diagnoses, and survival outcomes, that are collected from approximately 200 participating hospitals; additionally, annual reports based on data from the database have been presented. This Japanese gastric cancer cohort enrolled a large number of patients corresponding to approximately 13% of all patients with gastric cancer in Japan. However, it was limited to patients who underwent surgical treatment, and the structure of the items was simple because the objective of the new registry was to calculate the stage-specific, 5-year survival rates of patients who underwent gastrectomy. Low follow-up rates and a lax system for checking errors in data entry also constitute limitations of the Japanese cohort.
Before this project, there were no large-scale gastric cancer-specific cohort studies in Korea. The Korean Gastric Cancer Association (KGCA) has conducted a nationwide survey targeting surgically treated patients with gastric cancer every 5 years since 1999.16,17,18 In this survey, all data are retrieved from each member institution of the KGCA via e-mail. A total of 59 institutions participated, and data from 14,658 patients who had undergone surgery for gastric cancer in 2009 were analyzed in the most recent report.18 Because this survey was not a cohort study, there are no follow-up data. The KGCA survey only included demographic and clinicopathological characteristics and surgery-related information and contained no information on QOL or nutrition. Moreover, this survey was limited to surgical cases, as in the Japanese cohort.
The present cohort study of patients with gastric cancer focused on the QOL of patients with gastric cancer and that of their family caregivers, and comprehensive data were collected. The database items included not only data regarding clinical factors related to gastric cancer treatment but also detailed information regarding lifestyle and ongoing changes in QOL. Changes in the QOL of patients with gastric cancer and their family caregivers can provide important evidence of the need for institutional support. Moreover, many patients undergoing gastrectomy experience dumping syndrome and nutritional deficiency-related symptoms, such as anemia or osteoporosis.19,20,21 Therefore, diet diaries are useful tools for evaluating the changes in eating habits and nutritional status of patients with gastric cancer, and they provide important evidence regarding the long-term QOL of patients with gastric cancer.
Rigorous data processing and management procedures are advantages of this study. Well-trained coordinators entered data into the web-based system, and an independent data management group monitored all entered data. This management group sent "queries" requiring errors to be reviewed, and the data entry coordinators corrected any errors. Additionally, the data management group regularly visited each participating institution to review the data entry processes. An annual workshop for participating coordinators was held to standardize the data management practices and to share details regarding updated items. Therefore, high-quality data and reliable results are expected.
This study does have certain limitations. First, data regarding baseline clinical characteristics were missing for a considerable number of patients. Probably, lots of collected data has not yet entered to eVelos system, and the missing data would be filled afterward. Moreover, the differences in the description of endoscopic or pathological findings in each hospital likely affected these missing data. The data management group should intensify the monitoring and education program for coordinators and strive to reduce missing values in the future. Second, although the questionnaire response rates were high for most items, they were low for some items, in particular in 2012 and 2013. In May 2012, the questionnaire format changed, and information on past medical history, family history, and reproductive information was obtained from medical records instead of from interviews.
After 1 year, the previous format of the questionnaire was used, and these items were again obtained from interviews. Therefore, the response rates for the above items during this period were low. Third, the overall response rates to the diet diaries and the CQOLC questionnaires were also low. To complete the diet diaries, patients were required to record everything that they ate for 3 days, which is time consuming and requires a considerable amount of attention from both the patient and the family caregiver. Patients likely experienced difficulty recording details regarding all food items they consumed and many patients may not have completed the diet diaries. The CQOLC is a QLQ that is completed by the family caregiver of a patient with gastric cancer, and one family member should have completed the questionnaire at every hospital visit. However, the patient's companion may have changed at each visit, and collecting the questionnaire from the appointed family caregiver was difficult. Researchers and coordinators have discussed how to increase the response rates for diet diaries, but the yield remains low. More specific solutions and greater effort will be necessary in the future. Fourth, the majority of enrolled patients underwent surgery, and the proportion of patients who received endoscopic therapy or palliative chemotherapy was low. Currently, as the incidence of early gastric cancer is increasing, the number of patients who undergo endoscopic resection for early gastric cancer will also increase.18,22 Therefore, more patients who receive endoscopic therapy or palliative chemotherapy should be enrolled in the future, and hence, further efforts are needed to encourage gastroenterologists and oncologists to participate in this study.
In conclusion, a large-scale database of Korean patients with gastric cancer was established, and this database can provide comprehensive information on gastric cancer for patients, physicians, and government officials. Each physician or institution will be able to compare their management or management systems to our results, and government ministries will be able to develop cancer management strategies and policies with multidirectional approaches to address medical, psychological, economical, and social issues. Various secondary studies also can be derived using data from this cohort study.
Acknowledgments
This study was supported by a grant from the National Health Promotion Fund of the Ministry of Health and Welfare (N10399).
The data for this study were obtained from the database of the Korean Gastric Cancer Cohort Study. A total of 14 institutions participated in this cohort study, and the investigators are as follows: JSK and HSC from the National Cancer Center; HYJ, SYK, JYS, JS, HSM, and SHK from the Chungnam National University Hospital; YKP, SSK, YEJ, and IJC from the Chonnam National University Hospital; YJL, SHJ, and JHP from the Gyeongsang National University Hospital; HKY, HJL, SHK, and YSS from the Seoul National University Hospital; WY, OKK, SKK, SWJ, JGK, and BWK from the Kyungpook National University Medical Center; JHY, MHL, HYC, and YKK from the University of Ulsan College of Medicine; GAS, GHK, DHK, DHK, YMS, BEL, TYJ, and CIC from the Pusan National University Hospital, SJY, YJS, HK, HYY, KSP, SMP, SMY, JHH, WDK, HSH, HCL, and DHK from the Chungbuk National University Hospital; HEK, IHJ, JMK, and HJS from the Jeju National University School of Medicine; SHN, SYR, WJH, JHC, JYA, HIK, SKL, YCL, JCP, and SKS, from the Yonsei University Severance Hospital; SBP, SSK, KHL, SKH, HYL, YHK, and SJN from the Gangwon National University Hospital; DHY, STL, EKS, and JSK from the Jeonbuk National University Hospital; and SK, WKK, KMK, ERK, JJK, JHN, BHM, SHP, YSP, JOP, CKP, HCP, JMB, TSS, MWL, WJL, JHL, JHL, JYL, PLL, DHL, HYL, KTJ, DIC, and MGC from the Samsung Medical Center.
Footnotes
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Oh CM, Won YJ, Jung KW, Kong HJ, Cho H, Lee JK, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2013. Cancer Res Treat. 2016;48:436–450. doi: 10.4143/crt.2016.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee KS, Chang HS, Lee SM, Park EC. Economic burden of cancer in Korea during 2000-2010. Cancer Res Treat. 2015;47:387–398. doi: 10.4143/crt.2014.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim D, Yoon SJ, Gong YH, Kim YA, Seo HY, Yoon J, et al. The economic burden of cancers attributable to metabolic syndrome in Korea. J Prev Med Public Health. 2015;48:180–187. doi: 10.3961/jpmph.15.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng C, Wen W, Morgans AK, Pao W, Shu XO, Zheng W. Disparities by race, age, and sex in the improvement of survival for major cancers: results from the national cancer institute surveillance, epidemiology, and end results (SEER) program in the United States, 1990 to 2010. JAMA Oncol. 2015;1:88–96. doi: 10.1001/jamaoncol.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mertens AC, Yong J, Dietz AC, Kreiter E, Yasui Y, Bleyer A, et al. Conditional survival in pediatric malignancies: analysis of data from the Childhood Cancer Survivor Study and the Surveillance, Epidemiology, and End Results Program. Cancer. 2015;121:1108–1117. doi: 10.1002/cncr.29170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahn SH, Yoo KY. Chronological changes of clinical characteristics in 31,115 new breast cancer patients among Koreans during 1996-2004. Breast Cancer Res Treat. 2006;99:209–214. doi: 10.1007/s10549-006-9188-x. [DOI] [PubMed] [Google Scholar]
- 7.Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, et al. The global burden of cancer 2013. JAMA Oncol. 2015;1:505–527. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weitzner MA, Jacobsen PB, Wagner H, Jr, Friedland J, Cox C. The Caregiver Quality of Life Index-Cancer (CQOLC) scale: development and validation of an instrument to measure quality of life of the family caregiver of patients with cancer. Qual Life Res. 1999;8:55–63. doi: 10.1023/a:1026407010614. [DOI] [PubMed] [Google Scholar]
- 9.Fayers P, Bottomley A. Quality of life research within the EORTC-the EORTC QLQ-C30. European organisation for research and treatment of cancer. Eur J Cancer. 2002;38(Suppl 4):S125–S133. doi: 10.1016/s0959-8049(01)00448-8. [DOI] [PubMed] [Google Scholar]
- 10.Vickery CW, Blazeby JM, Conroy T, Arraras J, Sezer O, Koller M, et al. Development of an EORTC disease-specific quality of life module for use in patients with gastric cancer. Eur J Cancer. 2001;37:966–971. doi: 10.1016/s0959-8049(00)00417-2. [DOI] [PubMed] [Google Scholar]
- 11.Lafaye A, De Chalvron S, Houédé N, Eghbali H, Cousson-Gélie F. The Caregivers Quality of Life Cancer index scale (CQoLC): an exploratory factor analysis for validation in French cancer patients' spouses. Qual Life Res. 2013;22:119–122. doi: 10.1007/s11136-012-0113-y. [DOI] [PubMed] [Google Scholar]
- 12.Jee SH, Samet JM, Ohrr H, Kim JH, Kim IS. Smoking and cancer risk in Korean men and women. Cancer Causes Control. 2004;15:341–348. doi: 10.1023/B:CACO.0000027481.48153.97. [DOI] [PubMed] [Google Scholar]
- 13.Yun JE, Jo I, Park J, Kim MT, Ryu HG, Odongua N, et al. Cigarette smoking, elevated fasting serum glucose, and risk of pancreatic cancer in Korean men. Int J Cancer. 2006;119:208–212. doi: 10.1002/ijc.21816. [DOI] [PubMed] [Google Scholar]
- 14.Isobe Y, Nashimoto A, Akazawa K, Oda I, Hayashi K, Miyashiro I, et al. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer. 2011;14:301–316. doi: 10.1007/s10120-011-0085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nashimoto A, Akazawa K, Isobe Y, Miyashiro I, Katai H, Kodera Y, et al. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013;16:1–27. doi: 10.1007/s10120-012-0163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korea Gastric Cancer Association. Nationwide gastric cancer report in Korea. J Korean Gastric Cancer Assoc. 2002;2:105–114. [Google Scholar]
- 17.The Information Committee of the Korean Gastric Cancer Association. 2004 nationwide gastric cancer report in Korea. J Korean Gastric Cancer Assoc. 2007;7:47–54. [Google Scholar]
- 18.Jeong O, Park YK. Clinicopathological features and surgical treatment of gastric cancer in South Korea: the results of 2009 nationwide survey on surgically treated gastric cancer patients. J Gastric Cancer. 2011;11:69–77. doi: 10.5230/jgc.2011.11.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JH, Hyung WJ, Kim HI, Kim YM, Son T, Okumura N, et al. Method of reconstruction governs iron metabolism after gastrectomy for patients with gastric cancer. Ann Surg. 2013;258:964–969. doi: 10.1097/SLA.0b013e31827eebc1. [DOI] [PubMed] [Google Scholar]
- 20.Heiskanen JT, Kröger H, Pääkkönen M, Parviainen MT, Lamberg-Allardt C, Alhava E. Bone mineral metabolism after total gastrectomy. Bone. 2001;28:123–127. doi: 10.1016/s8756-3282(00)00404-x. [DOI] [PubMed] [Google Scholar]
- 21.Mine S, Sano T, Tsutsumi K, Murakami Y, Ehara K, Saka M, et al. Large-scale investigation into dumping syndrome after gastrectomy for gastric cancer. J Am Coll Surg. 2010;211:628–636. doi: 10.1016/j.jamcollsurg.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Lee JH, Kim JG, Jung HK, Kim JH, Jeong WK, Jeon TJ, et al. Clinical practice guidelines for gastric cancer in Korea: an evidence-based approach. J Gastric Cancer. 2014;14:87–104. doi: 10.5230/jgc.2014.14.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]