Abstract
Certain Salmonella enterica serovars belonging to subspecies I carry low-copy-number virulence plasmids of variable size (50–90 kb). All of these plasmids share the spv operon, which is important for systemic infection. Virulence plasmids are present at low copy numbers. Few copies reduce metabolic burden but suppose a risk of plasmid loss during bacterial division. This drawback is counterbalanced by maintenance modules that ensure plasmid stability, including partition systems and toxin-antitoxin (TA) loci. The low-copy number virulence pSLT plasmid of Salmonella enterica serovar Typhimurium encodes three auxiliary maintenance systems: one partition system (parAB) and two TA systems (ccdABST and vapBC2ST). The TA module ccdABST has previously been shown to contribute to pSLT plasmid stability and vapBC2ST to bacterial virulence. Here we describe a novel assay to measure plasmid stability based on the selection of plasmid-free cells following elimination of plasmid-containing cells by ParE toxin, a DNA gyrase inhibitor. Using this new maintenance assay we confirmed a crucial role of parAB in pSLT maintenance. We also showed that vapBC2ST, in addition to contribute to bacterial virulence, is important for plasmid stability. We have previously shown that ccdABST encodes an inactive CcdBST toxin. Using our new stability assay we monitored the contribution to plasmid stability of a ccdABST variant containing a single mutation (R99W) that restores the toxicity of CcdBST. The “activation” of CcdBST (R99W) did not increase pSLT stability by ccdABST. In contrast, ccdABST behaves as a canonical type II TA system in terms of transcriptional regulation. Of interest, ccdABST was shown to control the expression of a polycistronic operon in the pSLT plasmid. Collectively, these results show that the contribution of the CcdBST toxin to pSLT plasmid stability may depend on its role as a co-repressor in coordination with CcdAST antitoxin more than on its toxic activity.
Keywords: virulence plasmid, toxin-antitoxin, plasmid stability, transcriptional regulation, Salmonella Typhimurium
Introduction
During evolution bacterial pathogens acquire new genes dedicated to manipulate host processes. Many of these pathogen functions are encoded by chromosomal genes. Others, however, can be encoded by genes present in mobile genetic elements such as virulence plasmids. Horizontal transfer of these mobile genetic components has shaped the host adaptation strategies in several bacterial pathogens (Jackson et al., 2011). The presence of a virulence gene in a mobile element also facilitates its rapid acquisition or loss under distinct selective pressures. Enteric bacteria such as Escherichia coli, Shigella spp. and Salmonella enterica, frequently carry virulence genes in large transmissible low-copy-number plasmids (Sasakawa et al., 1986; Makino et al., 1988; Gulig et al., 1993). The S. enterica species are facultative intracellular bacteria that cause disease ranging from self-limiting gastroenteritis to more severe systemic infections (Rivera-Chávez and Bäumler, 2015). S. enterica subdivides into seven subspecies (I, II, IIIa, IIIb, IV, VI, and VII) (Tindall et al., 2005; Grimont and Weill, 2007) and subspecies I includes more than 2500 serovars (Grimont and Weill, 2007). Most of these serovars have adapted to infect warm-blooded hosts. One of the most extensively studied serovars of subspecies I is Typhimurium, which infects both humans and livestock. Serovar Typhimurium, together with a few other serovars of subspecies I, possesses a virulence plasmid (Jones et al., 1982). These plasmids have a variable size of 50–90 kb and share common features such as low copy number (1-2 plasmids per chromosome), a similar repC replicon (similar to the repFIB family) and a conserved set of virulence genes encoding toxins and fimbrial proteins (including spv and pef operons) (Bäumler et al., 1998; Rotger and Casadesús, 1999). The low copy number of the S. Typhimurium virulence plasmid (also called pSLT) could theoretically compromise its heritability to daughter cells during cell division. Despite this, pSLT is extremely stable in the host with ~10−7 segregants per cell generation, in a similar rate to that observed for other low-copy-number plasmids such as F and P1 (Austin and Abeles, 1983; Kline, 1985; Tinge and Curtiss, 1990a). Low-copy-number plasmids carry maintenance modules such as partition systems and toxin-antitoxin (TA) systems that ensure their proper segregation to nascent cells (Million-Weaver and Camps, 2014). Partition systems significantly increase the stability of plasmids by ensuring segregation of one copy of the plasmid to each sibling cell (Ebersbach and Gerdes, 2005). On the other hand, TA modules are typically bicistronic operons that encode an unstable antitoxin and a stable toxin (Chan et al., 2016; Lobato-Márquez et al., 2016). As a consequence of their different stabilities, antitoxin must be continuously produced to efficiently neutralize its cognate toxin (Gerdes et al., 1986). However, if the TA-encoding plasmid is lost, the antitoxin cannot be replenished and the free toxin eliminates or reduces the growth of daughter cells thus diluting plasmid-free cells in the population (Yamaguchi and Inouye, 2011). This phenomenon is called post-segregational killing (Gerdes et al., 1986).
Classically, plasmid stability has been measured using antibiotic-resistance plasmid derivates. Cells harboring the studied plasmid are positively selected in the presence of the selection antibiotic and those that have lost the plasmid are killed (Gerdes et al., 1985; del Solar et al., 1987). The main drawback of this technique is its sensitivity. Highly stable plasmids such as S. Typhimurium pSLT are below the sensitivity range of these assays. To solve this problem other methods relying in the direct selection of plasmid-free cells have been developed; for instance, the one based on the tetAR-chlortetracycline system (Bochner et al., 1980; Maloy and Nunn, 1981). The tetA gene encodes a protein which resides in the cytoplasmic membrane and prevents cellular accumulation of tetracycline, thereby conferring resistance (Reyrat et al., 1998). However, TetA location in the bacterial membrane also causes the cell to become hypersensitive to lipophilic chelators such as fusaric or quinalic acids (Bochner et al., 1980). Therefore, it is possible to select those cells that have lost the tetAR cassette. Inserted in a plasmid, the tetAR cassette can be used to select plasmid-free cells in special agar plates (Bochner-Maloy) containing fusaric acid (García-Quintanilla et al., 2006). Limitations of this method include poor reproducibility and the frequent occurrence of false positives (Li et al., 2013). Here, we have developed a novel, highly sensitive stability assay based on the negative selection of plasmid-containing cells. This assay is based on a cassette containing the ParE toxin-encoding gene of the parDE TA system and a kanamycin resistance gene (aph). ParE toxin targets DNA gyrase, blocks DNA replication and induces DNA breaks leading to cell death (Jiang et al., 2002). In our system ParE synthesis is controlled by a rhamnose-inducible promoter (PparE) (Maisonneuve et al., 2011). Once the aph-parE cassette has been inserted in the plasmid of interest and upon induction of PparE, only plasmid-free cells survive. Using this new tool we studied the contribution of the three main maintenance modules of the pSLT virulence plasmid of S. Typhimurium: the parAB partition system (Tinge and Curtiss, 1990b) and the ccdABST and vapBC2ST TA loci (Lobato-Márquez et al., 2015). We show that vapBC2ST TA module, which we recently demonstrated to be important to S. Typhimurium survival during non-phagocytic cells infection (Lobato-Márquez et al., 2015, 2016), also stabilizes pSLT plasmid. We show that the ccdABST TA system, known to impact pSLT heritability and encoding an inactive toxin (García-Quintanilla et al., 2006; Lobato-Márquez et al., 2015), conserves its TA transcriptional regulatory activity. Of interest, the ccdABST operon extends beyond the toxin gene including four additional open reading frames. Moreover, CcdABST TA complexes influence expression of downstream genes. We also demonstrate that stability of pSLT plasmid is not affected by a mutation (R99W) that restores CcdBST toxicity. We propose that the contribution of ccdABST to pSLT stability could be related to the regulatory activity of CcdAST-CcdBST complexes rather than to a post-segregational killing effect mediated only by CcdBST toxicity.
Results
Development of a new assay to measure plasmid stability
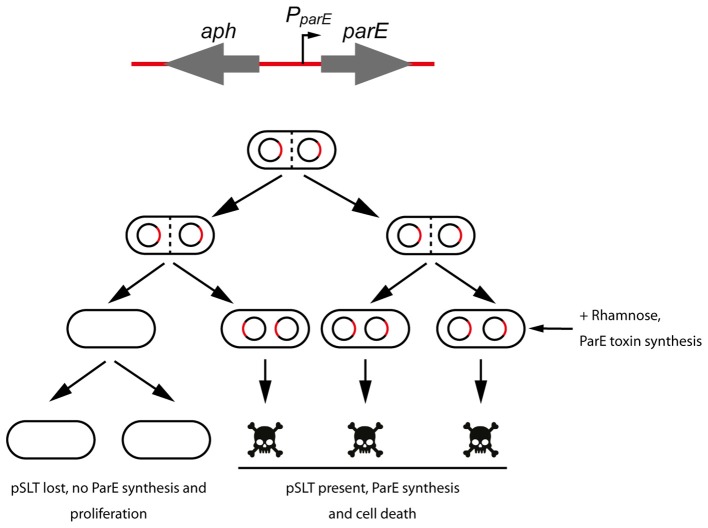
Due to the recognized problems of the tetAR-chlortetracycline method to measure plasmid stability, we decided to develop a novel negative selection method to measure the contribution to stability of the different maintenance modules encoded in pSLT plasmid (Figure 1). We took advantage of an aph-parE cassette of the pKD267 plasmid (Maisonneuve et al., 2011). This cassette carries a kanamycin resistance gene (aph) and the parE gene, which encodes the toxin of the parDE TA system. ParE toxin interacts with and blocks the DNA gyrase, causing inhibition of DNA synthesis, induction of breaks and nicks in the DNA and finally cell death (Jiang et al., 2002). In the aph-parE cassette, previously used for chromosomal scarless deletions (Maisonneuve et al., 2011; Lobato-Márquez et al., 2015), the toxin-encoding parE gene is controlled by a rhamnose-inducible promoter. Thus, when rhamnose is present as the only carbon source in the medium, ParE is synthesized and the cell is killed (Figure 1). Using aph-parE cassette to disrupt the maintenance modules of pSLT plasmid we could select plasmid-free bacteria. To distinguish plasmid curing from other events causing rhamnose resistance (e.g., mutations in PparE promoter or parE gene), we took advantage of the kanamycin resistance gene also present in the aph-parE cassette. The resulting pSLT plasmid derivates were thus tagged with two different markers.
Figure 1.
Scheme of the method used to measure plasmid stability of S. Typhimurium pSLT derivates. A cassette containing a kanamycin resistance gene (aph) and the DNA gyrase inhibitor ParE-encoding gene was used to disrupt genes of interest (top). Grown cultures were plated in rhamnose-containing agar plates and synthesis of ParE toxin was induced. Cells that kept pSLT plasmid and therefore aph-parE cassette, were selectively killed (bottom).
parAB partition system and vapBC2ST promote stability of S. Typhimurium pSLT plasmid
Previous studies proposed two important regions involved in S. Typhimurium pSLT plasmid stability: the parAB partition system and the TA module ccdABST (Tinge and Curtiss, 1990b; García-Quintanilla et al., 2006). Additionally, we identified another TA system, called vapBC2ST, encoded within the trbH gene (Lobato-Márquez et al., 2015) and homologous to the mvpAT locus encoded in the virulence plasmid of Shigella flexneri (Sayeed et al., 2000). We reported that vapBC2ST promotes Salmonella survival inside infected host cells. We now evaluated if similarly to ccdABST, and to other plasmidic TA loci, vapBC2ST may play a role in pSLT stability. Additionally, to test the sensitivity and the reproducibility of our method, we reevaluated the contribution of parAB and ccdABST using the new stability assay. We compared pSLT plasmids derivates lacking parAB, ccdABST or vapBC2ST with an isogenic strain in which aph-parE cassette was inserted in the gene spvA, which was previously shown to be innocuous for the stability of pSLT (García-Quintanilla et al., 2006). Stability assays demonstrated that disruption of vapBC2ST TA system resulted in a 5.5 ± 0.1 fold increase in the fraction of segregants after ~10 generations of growth without selection pressure (Figure 2). This increase was more important than in the case of the pSLT derivate lacking ccdABST (4 ± 0.2) under the same growth conditions (Figure 2). In accordance to previous studies, disruption of parAB or ccdABST decreased pSLT stability (Tinge and Curtiss, 1990a; García-Quintanilla et al., 2006). The parAB partition system stabilizes pSLT plasmid 119 ± 3 and 163 ± 9 fold more efficiently than the vapBC2ST or ccdABST TA systems, respectively (Figure 2). Moreover, the pSLT wild type plasmid was 650.1 ± 190.2 fold more stable than pSLT lacking parAB (Figure 2). These data strongly suggested that parAB is the main contributor to pSLT heritability. However, ccdABST and vapBCST TA systems showed a moderate contribution to pSLT stability. Together, these results demonstrated the potential of this new stability assay to determine accurately plasmid lost rates, being able to detect ~1 segregant in 2·106 bacteria. Moreover, we demonstrated that vapBC2ST, apart from its contribution to S. Typhimurium virulence, also mediates pSLT heritability.
Figure 2.
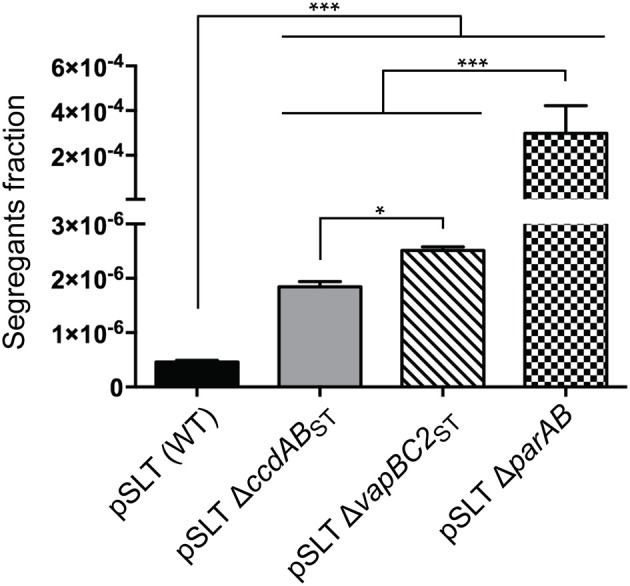
Plasmid stability assays of S. Typhimurium pSLT derivates. Segregants fraction measurement of pSLT plasmid comparing bacteria bearing the following plasmids: (i) wild type pSLT, (ii) pSLT lacking ccdABST TA module, (iii) pSLT lacking vapBC2ST TA system, and (iv) pSLT lacking parAB partition system. Disruption of all maintenance modules significantly decreased pSLT stability. The fraction of segregants was determined dividing the number of colony forming units grown in M9-rhamnose-agar plates (plasmid-free bacteria) by total bacteria grown in LB-agar plates. Data represent the means and standard deviations from five independent experiments. *P < 0.05; ***P < 0.001 by one-way ANOVA and Tukey's multiple comparison post-test.
CcdBST toxicity is not required for ccdABST-mediated stability of pSLT
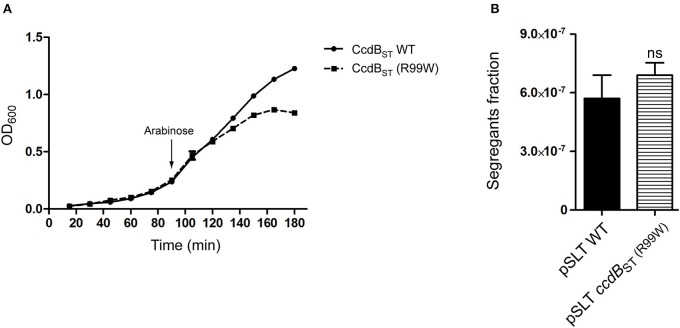
Our stability assays agreed with a previous study reporting contribution of ccdABST to pSLT plasmid stability (García-Quintanilla et al., 2006). We have recently demonstrated that CcdBST toxin of S. Typhimurium is not functional due to an amino acid substitution in the position 99 (W99R) (Lobato-Márquez et al., 2015). This residue is essential for the binding of CcdB to the subunit A of DNA Gyrase (GyrA) (Bahassi et al., 1995; Dao-Thi et al., 2005). The lack of toxic activity in CcdBST was further confirmed in liquid cultures of S. Typhimurium expressing either wild type (inactive) or active (R99W) versions of CcdBST (Figure 3A).
Figure 3.
The toxic activity of CcdBST is dispensable for the CcdABST-mediated pSLT stability. (A) Growth curves of S. Typhimurium strains expressing wild type non-active or CcdBST (R99W) toxic proteins. Bacteria were grown at 37°C with shaking in LB medium. The expression of the ccdBST (R99W) toxin-encoding gene ceased bacterial growth. The arrow indicates the time point (90 min) at which CcdBST synthesis was induced by arabinose addition. (B) Segregants fraction measurement of pSLT plasmid comparing pSLT wild type and a pSLT variant harboring the toxic version ccdBST (R99W). Data represent the means and standard deviations from five independent experiments. Data were compared using Student's T-test. ns, non significant.
In the F plasmid, the ortholog TA module ccdAB contributes to plasmid stability through a mechanism called “post-segregational killing” (Gerdes et al., 1986). Cells that do not inherit a copy of the TA-encoding plasmid cannot synthesize new antitoxin, leading the toxin free to kill or reduce the growth of plasmid-free cells (Van Melderen et al., 1994). We asked whether in S. Typhimurium the toxicity of CcdBST could be important for pSLT stability. To test this hypothesis, we carried out stability assays using a pSLT plasmid in which de non-functional ccdBST was substituted by an activated ccdBST (R99W) variant. Stability assays showed no differences between the pSLT plasmid derivates containing wild type ccdBST or the toxic version ccdBST (R99W), suggesting that CcdBST toxicity is dispensable for ccdABST-dependent stability (Figure 3B). Due to the ability of CcdABST TA system to stabilize pSLT plasmid independently of CcdBST toxicity, we characterized the ccdABST operon in more detail.
The non-functional TA system ccdABST of S. Typhimurium conserves transcriptional regulatory activity
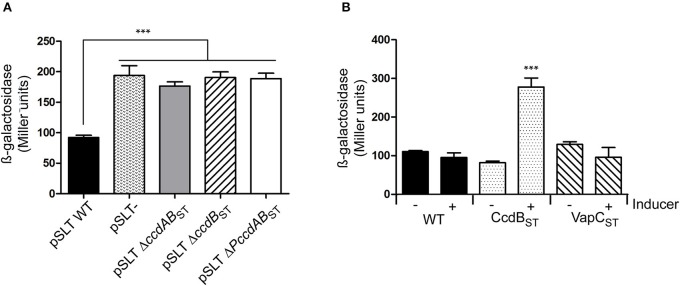
We tested if the type II TA module ccdABST of S. Typhimurium behaves as a bona fide TA system in terms of transcriptional regulation. In the F plasmid the antitoxin CcdA of the ccdAB ortholog acts as a transcriptional repressor and the toxin enhances the repressor activity when TA complexes are formed in a proper stoichiometry (Tam and Kline, 1989; Salmon et al., 1994). Mutations in the last three amino acids of the CcdB toxin in the F plasmid eliminate its toxicity while maintain its regulatory activity (Bahassi et al., 1995). To test the transcriptional activity of S. Typhimurium ccdABST, we fused the promoter of the TA system (PccdABST) to a promoter-less lacZ reporter gene. We measured β-galactosidase activity in the following genetic backgrounds: (i) pSLT wild type, (ii) pSLT plasmid cured, (iii) pSLT deficient for ccdBST gene, (iv) pSLT deficient for ccdABST operon, and (v) pSLT only lacking promoter PccdABST. β-galactosidase assays demonstrated that ccdABST TA module behaves as a classical type II TA system. When the whole system is present (wild type background), transcription of the operon is repressed. However, this repression is lost in the absence of CcdABST repressor complexes due to the lost of either ccdBST or ccdABST (Figure 4A). Interestingly, we did not observe differences in β-galactosidase activity when the system lacked only the toxin ccdBST or the whole operon arguing for an important role of CcdBST in transcriptional regulation.
Figure 4.
Transcriptional regulation of the ccdABST TA system. The 300 bp region upstream to ccdAST was cloned as a transcriptional fusion with a promoter-less lacZ reporter gene in plasmid pMP220. (A) β-galactosidase was measured in different genetic backgrounds of S. Typhimurium SV5015 transformed with pPccdAB-lacZ: (i) pSLT wild type, (ii) a strain cured of pSLT, (iii) a pSLT plasmid deficient for ccdABST TA system (including the promoter of the system), (iv) a pSLT lacking the toxin ccdBST gene, and (v) a pSLT in which the PccdABST promoter was eliminated. Absence of TA complexes releases the CcdABST transcriptional repression, resulting in increasing β-galactosidase activity (B) Wild type S. Typhimurium SV5015 was transformed with pPccdAB-lacZ and either a plasmid expressing the non-functional copies of CcdBST or VapCST to further measure β-galactosidase activity. Cultures were grown to OD600 of 0.3 and then expression of ccdBST or vapCST genes was induced by adding 0.3% arabinose during 1 h. Excess of CcdBST specifically shows conditional cooperativity effect as its overexpression derepresses transcription at PccdABST promoter of PSLT plasmid. Data represent the means and standard deviations from four independent experiments. ***P < 0.001 by one-way ANOVA.
In many type II TA modules, transcriptional regulation relies on the toxin:antitoxin ratio. Thus, an excess of antitoxin results in TA complexes that are efficient repressors; however, when the number of toxin molecules increases, the stoichiometry of the complex changes and repression is relieved. This regulation feature is termed “conditional cooperativity” (Overgaard et al., 2008). Taking advantage of the inactive CcdBST toxin, we analyzed the conditional cooperativity phenomenon in the ccdABST TA module of pSLT plasmid by supplying in trans an extra dose of the inactive CcdBST toxin. We employed a plasmid that contains inactive ccdBST gene controlled by an arabinose-inducible promoter. To discard unspecific effects derived from protein over-production, the same experiment was carried out with the unrelated non-toxic VapCST toxin encoded in the S. Typhimurium chromosome (Lobato-Márquez et al., 2015). Upon arabinose addition, we specifically observed an increased transcriptional activity of the PccdABST promoter following CcdBST but not VapCST production (Figure 4B). These data demonstrate that the ccdABST TA system responds to conditional cooperativity.
ccdABST of S. Typhimurium pSLT plasmid conforms a six-gene polycistronic operon
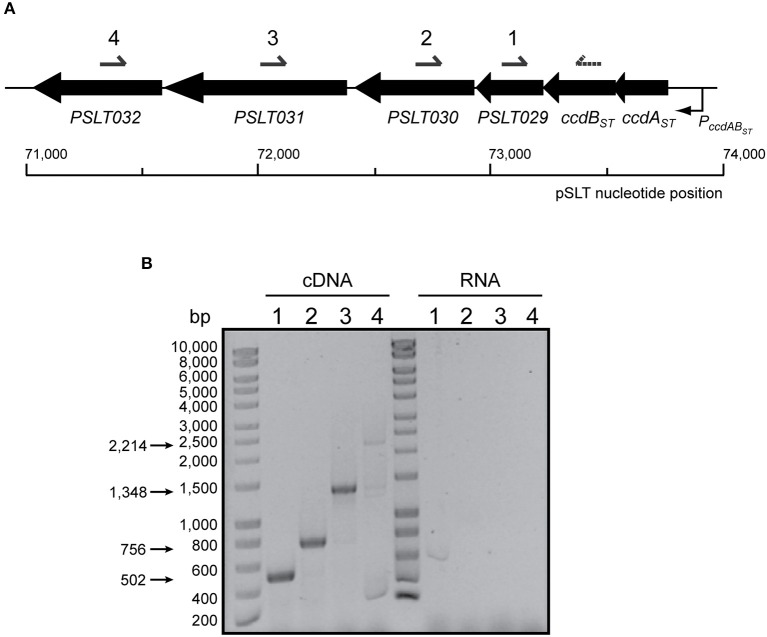
In the E. coli F plasmid, ccdAB maps upstream of the resolvase-encoding gene resD. However, analysis of the regions flanking ccdABST TA system of pSLT showed that this locus could be genetically linked to four other downstream genes (Figure 5A). ccdBST gene is separated by only one single nucleotide from the downstream gene SL1344_P1_0078 (PSLT029), which itself overlaps 4 bp with SL1344_P1_0077 (PSLT030). The next downstream gene is SL1344_P1_0076 (PSLT031 or rsdB). PSLT031 maps 33 bp downstream from the 3′-end of SL1344_P1_0077 (PSLT030) and 8 bp upstream from the 5′-end of SL1344_P1_0075 (PSLT032) (Figure 5A). These short intergenic regions led us hypothesize that the TA system ccdABST of pSLT plasmid could be encoded within a six-gene polycistronic operon. RT-PCR assays confirmed a polycistronic operon encompassing from ccdABST to PSLT032 (Figure 5B).
Figure 5.
Genetic organization and transcriptional analysis of the ccdABST locus present in S. Typhimurium pSLT plasmid. (A) Genetic organization of ccdABST in S. Typhimurium pSLT. Black arrows represent the location and orientation of the genes (scaled). Under the scheme it is marked pSLT DNA sequence coordinates. Primers used for RT-PCR (number 4) and cDNA amplification (numbers 1–4) are denoted above. (B) Co-transcription analysis of ccdABST and downstream genes. cDNA was synthesized using a primer annealing with PSLT032 (named as 4). Primers named as 1, 2, 3, and 4 were used in combination to a primer annealing in 3′-end of ccdBST (dashed arrow) to PCR-amplify each region, and PCR products were resolved in a 0.8% agarose gel. DNA marker is shown on the left. The expected sizes of PCR-amplified DNA fragments are indicated with arrows on the left. All genes are co-transcribed with those encoding the ccdABST TA system.
ccdABST transcriptional regulation is important to control the polycistronic operon
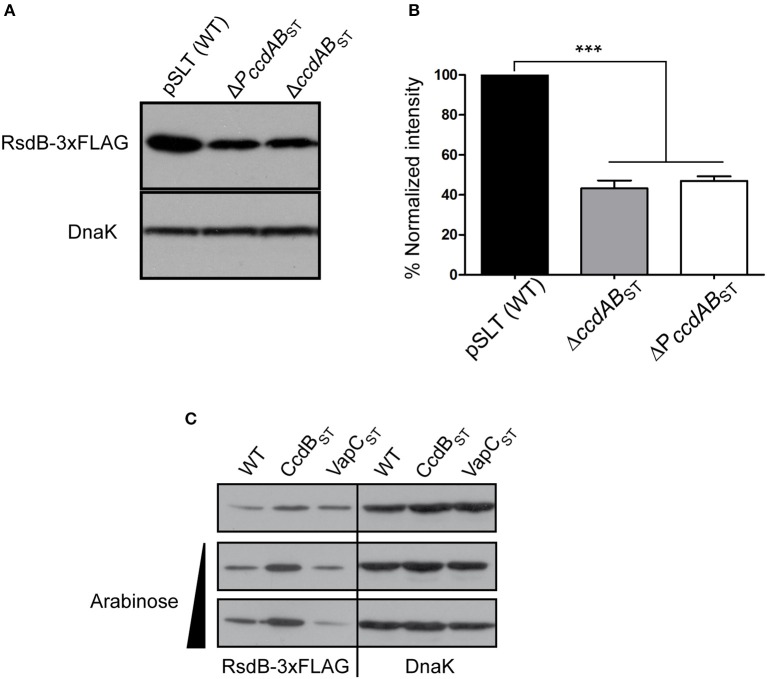
To further analyze the role of ccdABST in the polycistronic operon transcriptional control we asked if placed at the beginning of the operon, CcdAST-CcdBST TA complexes could modulate transcriptional expression of the operon in a TA system “classic” manner. PSLT031 or rsdB, placed at the penultimate position of the polycistronic operon, is annotated as a putative resolvase that could be important in multimer resolution during pSLT plasmid replication (Krause and Guiney, 1991). Thus, we used rsdB as a reporter to monitor the operon transcriptional regulation exerted by ccdABST. We tagged the rsdB gene with a 3xFLAG epitope at the 3′-end, and measured its protein levels in strains carrying: (i) wild type pSLT, (ii) engineered pSLT lacking the whole ccdABST, and (iii) pSLT lacking the 300 bp containing the ccdABST promoter. RsdB levels significantly decreased when the ccdABST TA system was altered, thus indicating that ccdABST acts as transcriptional repressor for the polycistronic operon (Figures 6A,B). As described above for the ccdABST TA system, we tested if the polycistronic operon could also respond to conditional cooperativity. We expressed the non-toxic CcdBST variant and measured RsdB levels. Complementary, we used as a negative control the production of the unrelated toxin VapCST. When CcdBST was provided in trans (Supplementary Figure 1), RsdB levels increased accordingly to conditional cooperativity (Figure 6C). Altogether, these data demonstrate that CcdABST TA complexes influence the transcription of the polycistronic operon.
Figure 6.
ccdABST TA system influences the transcription of the polycistronic operon. rsdB of pSLT plasmid was tagged with a 3xFLAG epitope in its 3′-end. (A,B) RsdB-3xFLAG levels were analyzed in pSLT plasmid lacking the PccdABST promoter or the entire ccdABST locus (including toxin, antitoxin and the promoter of the system). Bands intensity in (A) were quantified taking into account the number of cells loaded in the acrylamide gels and the band intensity of the loading control DnaK (B) Deletion of either the ccdABST operon or the promoter of the system, PccdABST, reduced RsdB 2.3 and 2.1 fold, respectively. (C) Conditional cooperativity phenomenon was analyzed in a wild type pSLT carrying the tagged 3xFLAG rsdB. CcdBST or VapCST (negative control) proteins were supplement in trans from pCcdB and pVapC plasmids. Due to the conditional cooperativity phenomenom, overexpression of CcdBST results in derepression of PccdABST and increased amount of RsdB. Quantification was carried out as described for A and B. Protein expression of CcdBST and VapCST was confirmed in SDS-acrylamide gels stained with Coomassie blue (Supplementary Figure 1). Histograms represent the quantification of at least four independent experiments. ***p < 0.001 by one-way ANOVA.
Discussion
In this report we describe a novel method to measure plasmid stability in bacteria. This procedure is based on the use of an aph-parE cassette in which a rhamnose-inducible promoter controls synthesis of ParE toxin. When the aph-parE cassette is inserted in the plasmid of interest and rhamnose is present in the medium as the only carbon source, ParE is synthesized and plasmid-containing cells are selectively eliminated. This methodology allows direct selection of plasmid-free segregants in a reproducible and highly sensitive manner. As it has been described previously for many low-copy-number plasmids, the pSLT virulence plasmid of S. Typhimurium possesses at least three main mechanisms to ensure its stable maintenance in the cell:(i) a copy number control of replication mediated by repB and repC replicons; (ii) the parAB partition system; and (iii) the TA systems ccdABST and vapBC2ST. In our study, we did not considered the influence of the conjugation machinery because although S. Typhimurium SV5015 pSLT is mobilizable, it is not self-transmissible (Ahmer et al., 1999). Using our novel stability assay, we reevaluated the contribution of ParAB and CcdABST to pSLT plasmid stability as a proof of concept for the reliability of our methodology. In accordance with the literature, we show that the ParAB partition system stabilizes the pSLT plasmid very efficiently. Moreover, as described for other plasmids, the partition system appeared more important for pSLT stability than the vapBC2ST or ccdABST TA systems (Sia et al., 1995; Sengupta and Austin, 2011; Hernández-Arriaga et al., 2014). Several studies have demonstrated a moderately stabilizing effect of TA systems. Two examples are the ccdAB TA module of the fertility factor F (Ogura and Hiraga, 1983) and the kis-kid (also called parD) TA locus of the R1 plasmid (Bravo et al., 1987). These systems increase the stability of their host plasmids around 10-fold compared to mini-derivate plasmids (Hernández-Arriaga et al., 2014). However, there are exceptions to this rule. For instance, the parDE module of RK2 has a more important role in the stabilization of this plasmid than other TA systems (Roberts et al., 1994; Easter et al., 1997). Interestingly, the mvpTA TA system of the virulence plasmid pWR100 in S. flexneri is the principal contributor to plasmid stability, more than the partition system (Sayeed et al., 2005). This differs from the stability contribution of its ortholog in S. Typhimurium, vapBC2ST. Of note, MvpAT and VapBC2ST show more than 96% amino acid sequence identity. However, it has also been described that diverse experimental variables, including temperature, growth media or the strain analyzed in the assay can alter plasmid stability (Easter et al., 1997; Sayeed et al., 2005). The toxin MvpT is a specific endonuclease that cleaves the initiator tRNA (Winther and Gerdes, 2011), and the mvpTA TA system has been shown to stabilize the virulence plasmid of S. flexneri by post-segregational killing (Sayeed et al., 2000). On the other hand, the plasmidic toxin VapC2ST and its chromosomal paralog VapCST of S. Typhimurium conserve 82% amino acid sequence identity (Lobato-Márquez et al., 2015). Moreover, similar to MvpT toxin, the chromosomal VapCST toxin possesses tRNA endonuclease activity (Winther and Gerdes, 2011). These evidences imply that VapBC2ST may mediate pSLT plasmid stability by post-segregational killing.
The other TA system of pSLT is ccdABST. In this work we demonstrate that this TA module shows classic characteristics of type II TA loci, such as autorepression and conditional cooperativity. Moreover, ccdABST is highly conserved to its ortholog present in the F plasmid: 90 and 83% amino acid identity to CcdA and CcdB, respectively. One important amino acid substitution is the tryptophan 99 to arginine in CcdBST of pSLT, an indispensable residue for the toxic activity of CcdB (Bahassi et al., 1995). Using a pSLT plasmid derivate encoding a CcdBST (R99W) variant we demonstrate that CcdBST toxicity is not necessary for the contribution of this TA module to plasmid stability. Intriguingly, ccdABST forms part of a polycistronic operon with four other downstream genes. Moreover, CcdAST-CcdBST TA complexes contribute to the regulation of the expression of this operon. This result is surprising given that few exceptions escape the general rule of TA operons organization. These exceptions include TA modules with a third gene acting as the transcriptional repressor of the system (Zielenkiewicz and Ceglowski, 2005; Hallez et al., 2010) and a single case in which a chaperone, co-transcribed with a TA operon, facilitates the folding of the antitoxin and, therefore, its activity (Bordes et al., 2011). Although, RsdB levels decreased upon deletion of either the promoter of ccdABST TA module or the whole TA locus, we still detected RsdB by western blot. These results indicate that PccdABST does control the transcription of the operon but it may exist at least another additional promoter regulating the operon.
Future work should address how the unprecedented TA genomic organization of this novel polycistronic operon including ccdABST and its transcriptional regulation influence pSLT stability. pSLT is evolutionary related to F plasmid, yet in F ccdAB does not constitute such a polycistronic operon. The study of this particular TA system could shed light on the evolution and adaptation of TA modules to its bacterial host.
Materials and methods
Bacterial strains, plasmids and growth conditions
S. enterica serovar Typhimurium SV5015 (a SL1344 His+ derivate strain Mariscotti and García-del Portillo, 2009) was used as parental strain (S. Typhimurium SL1344 accession number: NC_016810.1). All strains and plasmids used in this study are listed in Supplementary Table 1. Bacteria were grown at 37°C with shaking at 150 rpm in Luria-Bertani (LB) medium. When necessary antibiotics were added at the following concentrations: kanamycin, 50 μg/ml; ampicillin, 50 μg/ml; cloramphenicol, 20 μg/ml.
A transcriptional fusion PccdABST-lacZ was designed to measure the transcriptional activity of PccdABST promoter. A 300 bp DNA sequence upstream of ccdAST containing the promoter of ccdABST (Tam and Kline, 1989; Madl et al., 2006) was PCR-amplified, digested with EcoRI-KpnI and ligated with the large EcoRI-KpnI fragment of plasmid pMP220 (Spaink et al., 1987). The resulting plasmid was confirmed by DNA sequencing.
Construction of S. Typhimurium mutants
Oligonucleotide primers used in these procedures are listed in Supplementary Table 2. For disruption of pSLT plasmid maintenance modules, the deletion method described by Maisonneuve et al., was used (Maisonneuve et al., 2011). The strain used as control on stability assays was design inserting an aph-parE cassette in the spvA gene of pSLT. Disruption of this gene does not alter pSLT stability (Ahmer et al., 1999; García-Quintanilla et al., 2006).
A similar protocol to that involving generation of deletion mutants was used to introduce the amino acid substitution R99W in CcdBST. Briefly, the aph-parE module was first introduced in ccdBST gene. Then the cassette was cleaned up with a PCR-amplified DNA fragment bearing the nucleotide change C–T in the position 73,232 of pSLT plasmid corresponding to the first nucleotide of the arginine 99 (R99) codon.
Construction of S. Typhimurium recombinant strain expressing tagged RsdB-3xFLAG was carried out as previously described (Uzzau et al., 2001). 3x-FLAG tagging was performed at the 3′-end of the PSLT031 gene.
All mutants were verified and confirmed by PCR.
Plasmid stability assays
Before starting stability assays, bacteria were grown in LB containing 50 μg/ml kanamycin. For plasmid stability assays all bacterial strains were grown in 10 ml LB medium (10:1 flask:medium volume ratio) without selection pressure for 16 h (~10 generations) at 37°C and 150 rpm. We did not observe alterations in the growth rate of the pSLT plasmid derivates lacking parAB, ccdABST or vapBC2ST compared to pSLT wild type plasmid. Aliquots of 1 ml of the culture were collected into 1.5 ml eppendorf tubes and bacteria were pelleted in a MiniSpin® Eppendorf centrifuge 1 min at 12,000 rpm at room temperature. Supernatants were discarded and bacterial pellets were washed twice with phosphate buffered saline (PBS) pH 7.4. This ensures proper elimination of LB medium traces that otherwise could interfere with the growth in M9-rhamnose plates. Serial dilutions were done in PBS pH 7.4 and 100 μl of the appropriate aliquots plated onto LB- or M9-rhamnose-agar plates. Typically a 1:107 dilution was used to quantify total bacterial population in LB-agar plates, and dilutions in the range 1:1–103 were used to determine the number of segregants in M9-rhamnose-agar plates. Plates were incubated for 24 h (LB-agar) or 48–72 h (M9-rhamnose-agar) at 37°C before counting of the colony forming units. Colony forming units grown in M9-rhamnose-agar were tested for their kanamycin resistance on antibiotic-containing LB plates. This is a sensitive assay that effectively eliminates plasmid-containing cells, thus allowing a direct selection of plasmid-free segregants.
β-galactosidase activity measurements
Bacteria containing the plasmid with transcriptional fusion PccdAB-lacZ were grown to an optical density (OD)600 of 0.6 at 37°C and 150 rpm in LB. Then, β-galactosidase activity was measured as previously described (Miller, 1972).
For the conditional cooperativity experiments, bacteria containing pCcdB or pVapC plasmids (Lobato-Márquez et al., 2015) were grown in LB to an OD600 of 0.3 at 37°C and 150 rpm in the presence of 50 μg/ml kanamycin. Inactive CcdBST or VapCST toxins were synthesized upon induction with 0.3 % (w/v) L-arabinose. β-galactosidase activity was assessed as in the rest of strains after 1 h of induction. The chromosomally-encoded S. Typhimurium VapCST was used as a control to discard unspecific effects of protein expression in β-galactosidase measurements.
Reverse transcriptase PCR (RT-PCR)
To determine the presence of a polycistronic operon controlled by ccdABST total RNA was extracted from wild type S. Typhimurium SV5015 (Mariscotti and García-del Portillo, 2009) grown in LB at 37°C until OD600 ~ 0.3. Volume corresponding to 1 absorbance unit at OD600 was lysed in 100 μl lysis buffer (lysozime 50 mg/ml, 0.3% SDS). Cells extracts were processed using RNeasy minit kit (#74104, Quiagen). cDNA was constructed employing ThermoScript RT-PCR system (#11146-016, Invitrogen), using 600 ng of total RNA as template, a tm of 60°C and 0.6 μM of a oligonucleotide annealing with the 3′-end of PSLT032 (Supplementary Table 2). cDNA was amplified by PCR (Pfu DNA polymerase, #M774B, Promega) using 0.5 μM of primers annealing with ccdBST, SL1344_P1_0078 (PSLT029), SL1344_P1_0077 (PSLT030), rsdB, and SL1344_P1_0075 (PSLT032) (Supplementary Table 2). PCR amplification was carried out in duplicate using cDNA and RNA as a negative control. PCR products were visualized in 0.8% (w/v) agarose gels stained with ethidium bromide.
Detection of RsdB levels by western blotting and protein levels quantification
Bacterial cultures were grown 16 h at 37°C and 150 rpm. Same amount of bacterial cells were collected (volumes were adjusted based on OD600), centrifuged (1 min at 12,000 rpm) and re-suspend in Laemmli buffer (Laemmli, 1970). Bacterial protein extracts were resolved in SDS-PAGE using 15% polyacrylamide gels and processed for Western blot assays. Levels of the S. Typhimurium DnaK protein were used as loading control. RsdB or DnaK detection were performed using anti-FLAG antibody (#F3165, Sigma-Aldrich) 1:2000 (2 h) or anti-DNAK 1:10,000 (1 h), respectively, disolved in TBS-Tween buffer (137 mM NaCl, 0.1% m/v Tween 20 and 20 mM Tris-HCl pH 7.5) containing 3% non-fat milk. RsdB expression levels were calculated by western blotting experiments using extracts prepared from at least four independent experiments and pSLT plasmid variants expressing 3xFLAG-tagged RsdB. Mean data were taken as the relative expression levels of the proteins. Band densitometry was determined using Quantity One v.4.6.3 software (Bio-Rad, Berkeley, CA) as previously described (Molina-García and Giraldo, 2014; López-Villarejo et al., 2015).
Statistical analyses
Statistical significance was analyzed with GraphPad Prism v7 software (GraphPad Inc., La Jolla, CA) using one-way analysis of variance (ANOVA) with Dunnett's multiple comparison post-test for Figures 2, 4, 6B. In the comparison test used for Figure 3B a Student's T-test analysis was used. A P ≤ 0.05 was considered significant. Data are presented as mean ± standard deviation of the mean (SEM).
Author contributions
DL and RD: Conceived and designed the experiments; DL, LM, and IM: Performed the experiments; DL, LM, IM, FG, and RD: Analyzed the data; DL: Wrote the paper.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer GDS declared a shared affiliation, though no other collaboration, with several of the authors IM, RD to the handling Editor, who ensured that the process nevertheless met the standards of a fair and objective review.
Acknowledgments
We are grateful to Josep Casadesus Pursals for his critical comments about the manuscript and for providing the strain SV3081 of S. Typhimurium. We thank RD and FG lab members for their comments and help, and Dr. Serge Mostowy and Alexandra Willis for their critical review of the manuscript. The work in RD and FG's laboratories is supported by grants BFU2011-25939 (RD), CSD2008-00013 (RD and FG), and BIO2013-46281-P/BIO2015-69085-REDC (FG) from the Spanish Ministry of Economy and Competitiveness.
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmolb.2016.00066
Control assays showing proper protein synthesis of CcdBST and VapCST in the experiments involving conditional cooperativity regulation of rsdB in Figure 6C (main text). Equal amounts of total protein extracts were loaded in each lane. Bacteria were grown in LB medium to OD600 of 0.3, time at which CcdBST or VapCST expression was induced with 0.3% arabinose.
Bacterial strains and plasmids used in this study.
Oligonucleotides used in this study.
References
- Ahmer B. M., Tran M., Heffron F. (1999). The virulence plasmid of Salmonella Typhimurium is self-transmissible. J. Bacteriol. 181, 1364–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin S., Abeles A. (1983). Partition of unit-copy miniplasmids to daughter cells. I. P1 and F miniplasmids contain discrete, interchangeable sequences sufficient to promote equipartition. J. Mol. Biol. 169, 353–372. 10.1016/S0022-2836(83)80055-2 [DOI] [PubMed] [Google Scholar]
- Bahassi E. M., Salmon M. A., Van Melderen L., Bernard P., Couturier M. (1995). F plasmid CcdB killer protein: ccdB gene mutants coding for non-cytotoxic proteins which retain their regulatory functions. Mol. Microbiol. 15, 1031–1037. 10.1111/j.1365-2958.1995.tb02278.x [DOI] [PubMed] [Google Scholar]
- Bäumler A. J., Tsolis R. M., Ficht T. A., Adams L. G. (1998). Evolution of host adaptation in Salmonella enterica. Infect. Immun. 66, 4579–4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N. (1980). Positive selection for loss of tetracycline resistance. J. Bacteriol. 143, 926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordes P., Cirinesi A. M., Ummels R., Sala A., Sakr S., Bitter W., et al. (2011). SecB-like chaperone controls a toxin-antitoxin stress-responsive system in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U.S.A. 108, 8438–8443. 10.1073/pnas.1101189108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo A., de Torrontegui G., Díaz R. (1987). Identification of components of a new stability system of plasmid R1, ParD, that is close to the origin of replication of this plasmid. Mol. Gen. Genet. 210, 101–110. 10.1007/BF00337764 [DOI] [PubMed] [Google Scholar]
- Chan W. T., Espinosa M., Yeo C. C. (2016). Keeping the wolves at Bay: antitoxins of prokaryotic type ii toxin-antitoxin systems. Front. Mol. Biosci. 3:9. 10.3389/fmolb.2016.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao-Thi M. H., Van Melderen L., De Genst E., Afif H., Buts L., Wyns L., et al. (2005). Molecular basis of gyrase poisoning by the addiction toxin CcdB. J. Mol. Biol. 348, 1091–1102. 10.1016/j.jmb.2005.03.049 [DOI] [PubMed] [Google Scholar]
- del Solar G. H., Puyet A., Espinosa M. (1987). Initiation signals for the conversion of single stranded to double stranded DNA forms in the streptococcal plasmid pLS1. Nucleic Acids Res. 15, 5561–5580. 10.1093/nar/15.14.5561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easter C. L., Sobecky P. A., Helinski D. R. (1997). Contribution of different segments of the par region to stable maintenance of the broad-host-range plasmid RK2. J. Bacteriol. 179, 6472–6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersbach G., Gerdes K. (2005). Plasmid segregation mechanisms. Annu. Rev. Genet. 39, 453–479. 10.1146/annurev.genet.38.072902.091252 [DOI] [PubMed] [Google Scholar]
- García-Quintanilla M., Prieto A. I., Barnes L., Ramos-Morales F., Casadesús J. (2006). Bile-induced curing of the virulence plasmid in Salmonella enterica serovar Typhimurium. J. Bacteriol. 188, 7963–7965. 10.1128/JB.00995-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes K., Larsen J. E., Molin S. (1985). Stable inheritance of plasmid R1 requires two different loci. J. Bacteriol. 161, 292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes K., Rasmussen P. B., Molin S. (1986). Unique type of plasmid maintenance function: postsegregational killing of plasmid-free cells. Proc. Natl. Acad. Sci. U.S.A. 83, 3116–3120. 10.1073/pnas.83.10.3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimont P., Weill F. (2007). Antigenic Formulae of the Salmonella Serovars, 9th Edn. Paris: World Health Organization Collaborating Center for Reference and Research on Salmonella. Pasteur Institute. [Google Scholar]
- Gulig P. A., Danbara H., Guiney D. G., Lax A. J., Norel F., Rhen M. (1993). Molecular analysis of spv virulence genes of the Salmonella virulence plasmids. Mol. Microbiol. 7, 825–830. 10.1111/j.1365-2958.1993.tb01172.x [DOI] [PubMed] [Google Scholar]
- Hallez R., Geeraerts D., Sterckx Y., Mine N., Loris R., Van Melderen L. (2010). New toxins homologous to ParE belonging to three-component toxin-antitoxin systems in Escherichia coli O157:H7. Mol. Microbiol. 76, 719–732. 10.1111/j.1365-2958.2010.07129.x [DOI] [PubMed] [Google Scholar]
- Hernández-Arriaga A. M., Chan W. T., Espinosa M., Díaz-Orejas R. (2014). Conditional activation of toxin-antitoxin systems: postsegregational killing and beyond. Microbiol. Spectr. 2:PLAS-0009-2013. 10.1128/microbiolspec.PLAS-0009-2013 [DOI] [PubMed] [Google Scholar]
- Jackson R. W., Vinatzer B., Arnold D. L., Dorus S., Murillo J. (2011). The influence of the accessory genome on bacterial pathogen evolution. Mob. Genet. Elem. 1, 55–65. 10.4161/mge.1.1.16432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Pogliano J., Helinski D. R., Konieczny I. (2002). ParE toxin encoded by the broad-host-range plasmid RK2 is an inhibitor of Escherichia coli gyrase. Mol. Microbiol. 44, 971–979. 10.1046/j.1365-2958.2002.02921.x [DOI] [PubMed] [Google Scholar]
- Jones G. W., Rabert D. K., Svinarich D. M., Whitfield H. J. (1982). Association of adhesive, invasive, and virulent phenotypes of Salmonella Typhimurium with autonomous 60-megadalton plasmids. Infect. Immun. 38, 476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline B. C. (1985). A review of mini-F plasmid maintenance. Plasmid 14, 1–16. 10.1016/0147-619X(85)90027-7 [DOI] [PubMed] [Google Scholar]
- Krause M., Guiney D. G. (1991). Identification of a multimer resolution system involved in stabilization of the Salmonella Dublin virulence plasmid pSDL2. J. Bacteriol. 173, 5754–5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. 10.1038/227680a0 [DOI] [PubMed] [Google Scholar]
- Li X. T., Thomason L. C., Sawitzke J. A., Costantino N., Court D. L. (2013). Positive and negative selection using the tetA-sacB cassette: recombineering and P1 transduction in Escherichia coli. Nucleic Acids Res. 41, e204. 10.1093/nar/gkt1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobato-Márquez D., Díaz-Orejas R., García-del Portillo F. (2016). Toxin-antitoxins and bacterial virulence. FEMS Microbiol. Rev. 40, 592–609. 10.1093/femsre/fuw022 [DOI] [PubMed] [Google Scholar]
- Lobato-Márquez D., Moreno-Córdoba I., Figueroa V., Díaz-Orejas R., García-del Portillo F. (2015). Distinct type I and type II toxin-antitoxin modules control Salmonella lifestyle inside eukaryotic cells. Sci. Rep. 5:9374. 10.1038/srep09374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Villarejo J., Lobato-Márquez D., Díaz-Orejas R. (2015). Coupling between the basic replicon and the Kis-Kid maintenance system of plasmid R1: modulation by Kis antitoxin levels and involvement in control of plasmid replication. Toxins (Basel). 7, 478–492. 10.3390/toxins7020478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madl T., Van Melderen L., Mine N., Respondek M., Oberer M., Keller W., et al. (2006). Structural basis for nucleic acid and toxin recognition of the bacterial antitoxin CcdA. J. Mol. Biol. 364, 170–185. 10.1016/j.jmb.2006.08.082 [DOI] [PubMed] [Google Scholar]
- Maisonneuve E., Shakespeare L. J., Jørgensen M. G., Gerdes K. (2011). Bacterial persistence by RNA endonucleases. Proc. Natl. Acad. Sci. U.S.A. 108, 13206–13211. 10.1073/pnas.1100186108 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Makino S., Sasakawa C., Yoshikawa M. (1988). Genetic relatedness of the basic replicon of the virulence plasmid in shigellae and enteroinvasive Escherichia coli. Microb. Pathog. 5, 267–274. 10.1016/0882-4010(88)90099-X [DOI] [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. (1981). Selection for loss of tetracycline resistance by Escherichia coli. J. Bacteriol. 145, 1110–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariscotti J. F., García-del Portillo F. (2009). Genome expression analyses revealing the modulation of the Salmonella Rcs regulon by the attenuator IgaA. J. Bacteriol. 191, 1855–1867. 10.1128/JB.01604-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. H. (1972). Experiments in Molecular Genetics. New York, NY: Cold Spring Harbor Laboratory. [Google Scholar]
- Million-Weaver S., Camps M. (2014). Mechanisms of plasmid segregation: have multicopy plasmids been overlooked? Plasmid 75, 27–36. 10.1016/j.plasmid.2014.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-García L., Giraldo R. (2014). Aggregation interplay between variants of the RepA-WH1 prionoid in Escherichia coli. J. Bacteriol. 196, 2536–2542. 10.1128/JB.01527-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura T., Hiraga S. (1983). Mini-F plasmid genes that couple host cell division to plasmid proliferation. Proc. Natl. Acad. Sci. U.S.A. 80, 4784–4788. 10.1073/pnas.80.15.4784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard M., Borch J., Jørgensen M. G., Gerdes K. (2008). Messenger RNA interferase RelE controls relBE transcription by conditional cooperativity. Mol. Microbiol. 69, 841–857. 10.1111/j.1365-2958.2008.06313.x [DOI] [PubMed] [Google Scholar]
- Reyrat J. M., Pelicic V., Gicquel B., Rappuoli R. (1998). Counterselectable markers: untapped tools for bacterial genetics and pathogenesis. Infect. Immun. 66, 4011–4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Chávez F., Bäumler A. J. (2015). The pyromaniac inside you: Salmonella metabolism in the host gut. Annu. Rev. Microbiol. 69, 31–48. 10.1146/annurev-micro-091014-104108 [DOI] [PubMed] [Google Scholar]
- Roberts R. C., Ström A. R., Helinski D. R. (1994). The parDE operon of the broad-host-range plasmid RK2 specifies growth inhibition associated with plasmid loss. J. Mol. Biol. 237, 35–51. 10.1006/jmbi.1994.1207 [DOI] [PubMed] [Google Scholar]
- Rotger R., Casadesús J. (1999). The virulence plasmids of Salmonella. Int. Microbiol. 2, 177–184. [PubMed] [Google Scholar]
- Salmon M. A., Van Melderen L., Bernard P., Couturier M. (1994). The antidote and autoregulatory functions of the F plasmid CcdA protein: a genetic and biochemical survey. Mol. Gen. Genet. 244, 530–538. 10.1007/BF00583904 [DOI] [PubMed] [Google Scholar]
- Sasakawa C., Kamata K., Sakai T., Murayama S. Y., Makino S., Yoshikawa M. (1986). Molecular alteration of the 140-megadalton plasmid associated with loss of virulence and Congo red binding activity in Shigella flexneri. Infect. Immun. 51, 470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayeed S., Brendler T., Davis M., Reaves L., Austin S. (2005). Surprising dependence on postsegregational killing of host cells for maintenance of the large virulence plasmid of Shigella flexneri. J. Bacteriol. 187, 2768–2773. 10.1128/JB.187.8.2768-2773.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayeed S., Reaves L., Radnedge L., Austin S. (2000). The stability region of the large virulence plasmid of Shigella flexneri encodes an efficient postsegregational killing system. J. Bacteriol. 182, 2416–2421. 10.1128/JB.182.9.2416-2421.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta M., Austin S. (2011). Prevalence and significance of plasmid maintenance functions in the virulence plasmids of pathogenic bacteria. Infect. Immun. 79, 2502–2509. 10.1128/IAI.00127-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia E. A., Roberts R. C., Easter C., Helinski D. R., Figurski D. H. (1995). Different relative importances of the par operons and the effect of conjugal transfer on the maintenance of intact promiscuous plasmid RK2. J. Bacteriol. 177, 2789–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaink H. P., Okker R. J., Wijffelman C. A., Pees E., Lugtenberg B. J. (1987). Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL1JI. Plant Mol. Biol. 9, 27–39. 10.1007/BF00017984 [DOI] [PubMed] [Google Scholar]
- Tam J. E., Kline B. C. (1989). Control of the ccd operon in plasmid F. J. Bacteriol. 171, 2353–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindall B. J., Grimont P. A., Garrity G. M., Euzeby J. P. (2005). Nomenclature and taxonomy of the genus Salmonella. Int. J. Syst. Evol. Microbiol. 55(Pt 1), 521–524. 10.1099/ijs.0.63580-0 [DOI] [PubMed] [Google Scholar]
- Tinge S. A., Curtiss R., III. (1990a). Conservation of Salmonella Typhimurium virulence plasmid maintenance regions among Salmonella serovars as a basis for plasmid curing. Infect. Immun. 58, 3084–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinge S. A., Curtiss R., III. (1990b). Isolation of the replication and partitioning regions of the Salmonella Typhimurium virulence plasmid and stabilization of heterologous replicons. J. Bacteriol. 172, 5266–5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzzau S., Figueroa-Bossi N., Rubino S., Bossi L. (2001). Epitope tagging of chromosomal genes in Salmonella. Proc. Natl. Acad. Sci. U.S.A. 98, 15264–15269. 10.1073/pnas.261348198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Melderen L., Bernard P., Couturier M. (1994). Lon-dependent proteolysis of CcdA is the key control for activation of CcdB in plasmid-free segregant bacteria. Mol. Microbiol. 11, 1151–1157. 10.1111/j.1365-2958.1994.tb00391.x [DOI] [PubMed] [Google Scholar]
- Winther K. S., Gerdes K. (2011). Enteric virulence associated protein VapC inhibits translation by cleavage of initiator tRNA. Proc. Natl. Acad. Sci. U.S.A. 108, 7403–7407. 10.1073/pnas.1019587108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y., Inouye M. (2011). Regulation of growth and death in Escherichia coli by toxin-antitoxin systems. Nat. Rev. Microbiol. 9, 779–790. 10.1038/nrmicro2651 [DOI] [PubMed] [Google Scholar]
- Zielenkiewicz U., Ceglowski P. (2005). The toxin-antitoxin system of the streptococcal plasmid pSM19035. J. Bacteriol. 187, 6094–6105. 10.1128/JB.187.17.6094-6105.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Control assays showing proper protein synthesis of CcdBST and VapCST in the experiments involving conditional cooperativity regulation of rsdB in Figure 6C (main text). Equal amounts of total protein extracts were loaded in each lane. Bacteria were grown in LB medium to OD600 of 0.3, time at which CcdBST or VapCST expression was induced with 0.3% arabinose.
Bacterial strains and plasmids used in this study.
Oligonucleotides used in this study.