Abstract
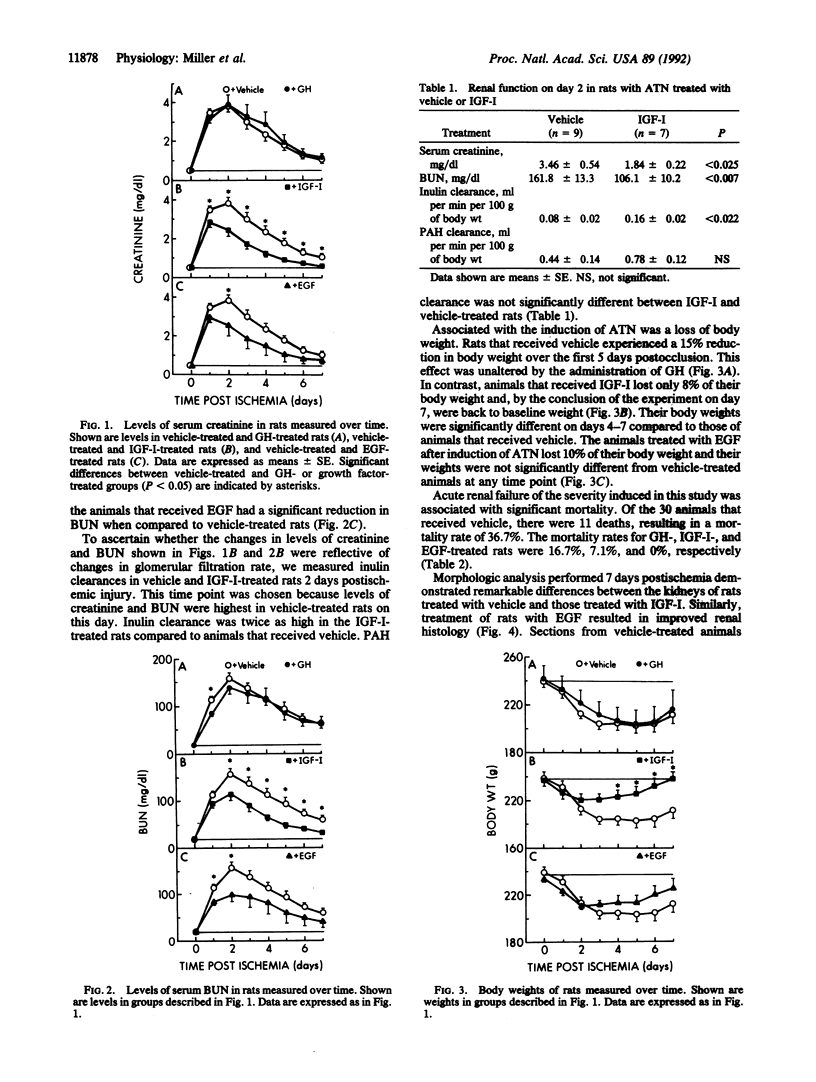
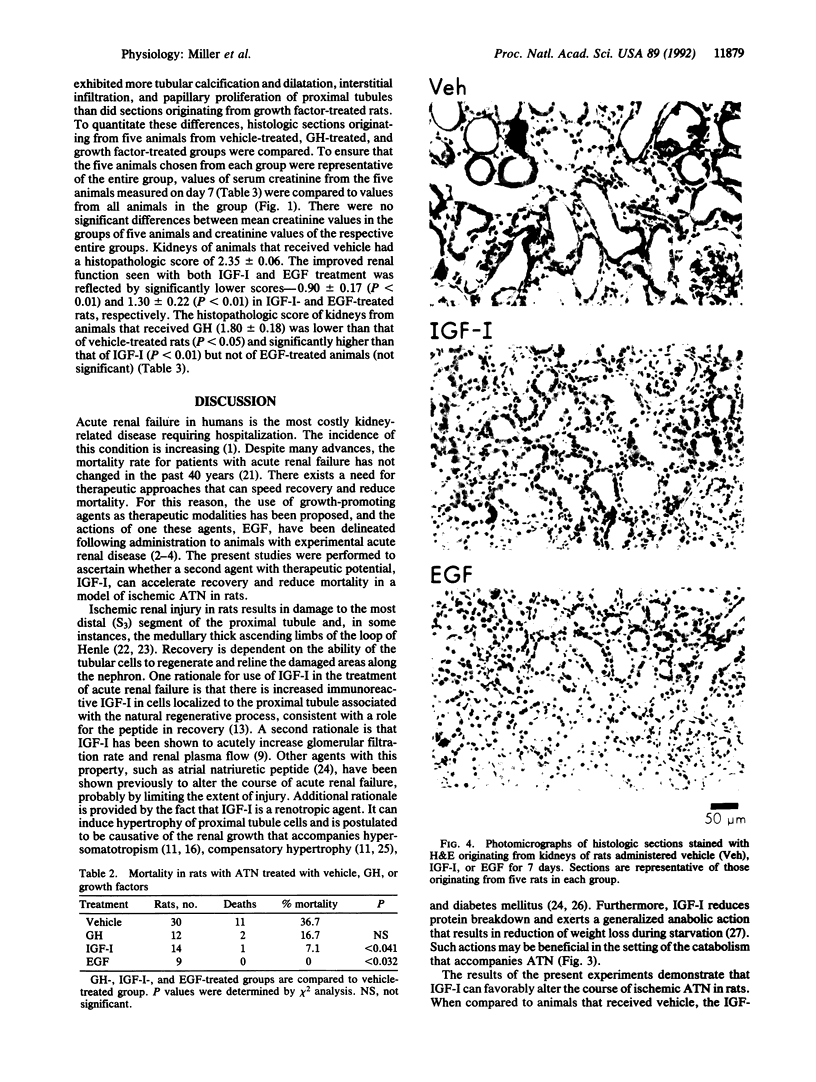
The effects of administering insulin-like growth factor I (IGF-I) were examined in a model of ischemic acute tubular necrosis in rats. Injury was induced by 75 min of bilateral renal artery occlusion. Compared to rats administered vehicle, rats administered IGF-I (100 micrograms/day via continuous subcutaneous infusion) had significantly lower serum creatinine and blood urea nitrogen levels over the course of 7 days postocclusion. Glomerular filtration rate as determined by inulin clearance was examined on day 2 postocclusion and was significantly increased in IGF-I-treated animals (0.16 +/- 0.02 ml per min per 100 g of body weight) compared to vehicle-treated controls (0.08 +/- 0.02 ml per min per 100 g of body weight). The weight loss that occurred during the course of acute tubular necrosis was ameliorated by IGF-I. Mortality was reduced from 36.7% in vehicle-treated rats to 7.1% in rats administered IGF-I. Histologically, there was much less renal injury evident at day 7 postocclusion in the IGF-I-treated rats compared to vehicle-treated controls. In contrast, growth hormone (200 micrograms administered subcutaneously for 4 days) did not affect recovery of renal function or reduce mortality postreperfusion. This report demonstrates a beneficial effect of IGF-I administration in the setting of acute tubular necrosis. Several properties of IGF-I render it a pharmacological agent with excellent potential for treatment of this condition in humans.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson G., Jennische E. IGF-I immunoreactivity is expressed by regenerating renal tubular cells after ischaemic injury in the rat. Acta Physiol Scand. 1988 Apr;132(4):453–457. doi: 10.1111/j.1748-1716.1988.tb08352.x. [DOI] [PubMed] [Google Scholar]
- Barreca A., Voci A., Minuto F., de Marchis M., Cecchelli E., Fugassa E., Giordano G., Gallo G. Effect of epidermal growth factor on insulin-like growth factor-I (IGF-I) and IGF-binding protein synthesis by adult rat hepatocytes. Mol Cell Endocrinol. 1992 Mar;84(1-2):119–126. doi: 10.1016/0303-7207(92)90078-k. [DOI] [PubMed] [Google Scholar]
- Behringer R. R., Lewin T. M., Quaife C. J., Palmiter R. D., Brinster R. L., D'Ercole A. J. Expression of insulin-like growth factor I stimulates normal somatic growth in growth hormone-deficient transgenic mice. Endocrinology. 1990 Sep;127(3):1033–1040. doi: 10.1210/endo-127-3-1033. [DOI] [PubMed] [Google Scholar]
- Bier D. M. Growth hormone and insulin-like growth factor I: nutritional pathophysiology and therapeutic potential. Acta Paediatr Scand Suppl. 1991;374:119–128. doi: 10.1111/j.1651-2227.1991.tb12014.x. [DOI] [PubMed] [Google Scholar]
- Brezis M., Rosen S., Silva P., Epstein F. H. Renal ischemia: a new perspective. Kidney Int. 1984 Oct;26(4):375–383. doi: 10.1038/ki.1984.185. [DOI] [PubMed] [Google Scholar]
- Butkus D. E. Persistent high mortality in acute renal failure. Are we asking the right questions? Arch Intern Med. 1983 Feb;143(2):209–212. [PubMed] [Google Scholar]
- Clemmons D. R. Multiple hormones stimulate the production of somatomedin by cultured human fibroblasts. J Clin Endocrinol Metab. 1984 May;58(5):850–856. doi: 10.1210/jcem-58-5-850. [DOI] [PubMed] [Google Scholar]
- Coimbra T. M., Cieslinski D. A., Humes H. D. Epidermal growth factor accelerates renal repair in mercuric chloride nephrotoxicity. Am J Physiol. 1990 Sep;259(3 Pt 2):F438–F443. doi: 10.1152/ajprenal.1990.259.3.F438. [DOI] [PubMed] [Google Scholar]
- FUHR J., KACZMARCZYK J., KRUTTGEN C. D. Eine einfache colorimetrische Methode zur Inulinbestimmung für Nieren-Clearance-Untersuchungen bei Stoffwechselgesunden und Diabetikern. Klin Wochenschr. 1955 Aug 1;33(29-30):729–730. doi: 10.1007/BF01473295. [DOI] [PubMed] [Google Scholar]
- Flyvbjerg A., Bornfeldt K. E., Marshall S. M., Arnqvist H. J., Orskov H. Kidney IGF-I mRNA in initial renal hypertrophy in experimental diabetes in rats. Diabetologia. 1990 Jun;33(6):334–338. doi: 10.1007/BF00404636. [DOI] [PubMed] [Google Scholar]
- Flyvbjerg A., Thorlacius-Ussing O., Naeraa R., Ingerslev J., Orskov H. Kidney tissue somatomedin C and initial renal growth in diabetic and uninephrectomized rats. Diabetologia. 1988 May;31(5):310–314. doi: 10.1007/BF00277413. [DOI] [PubMed] [Google Scholar]
- Guler H. P., Schmid C., Zapf J., Froesch E. R. Effects of recombinant insulin-like growth factor I on insulin secretion and renal function in normal human subjects. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2868–2872. doi: 10.1073/pnas.86.8.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler H. P., Zapf J., Scheiwiller E., Froesch E. R. Recombinant human insulin-like growth factor I stimulates growth and has distinct effects on organ size in hypophysectomized rats. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4889–4893. doi: 10.1073/pnas.85.13.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerman M. R. The growth hormone-insulin-like growth factor axis in kidney. Am J Physiol. 1989 Oct;257(4 Pt 2):F503–F514. doi: 10.1152/ajprenal.1989.257.4.F503. [DOI] [PubMed] [Google Scholar]
- Hirschberg R., Kopple J. D., Blantz R. C., Tucker B. J. Effects of recombinant human insulin-like growth factor I on glomerular dynamics in the rat. J Clin Invest. 1991 Apr;87(4):1200–1206. doi: 10.1172/JCI115119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hizuka N., Takano K., Shizume K., Asakawa K., Miyakawa M., Tanaka I., Horikawa R. Insulin-like growth factor I stimulates growth in normal growing rats. Eur J Pharmacol. 1986 Jun 5;125(1):143–146. doi: 10.1016/0014-2999(86)90093-2. [DOI] [PubMed] [Google Scholar]
- Humes H. D., Cieslinski D. A., Coimbra T. M., Messana J. M., Galvao C. Epidermal growth factor enhances renal tubule cell regeneration and repair and accelerates the recovery of renal function in postischemic acute renal failure. J Clin Invest. 1989 Dec;84(6):1757–1761. doi: 10.1172/JCI114359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R., Barrett E., Plewe G., Fagin K. D., Sherwin R. S. Acute effects of insulin-like growth factor I on glucose and amino acid metabolism in the awake fasted rat. Comparison with insulin. J Clin Invest. 1989 May;83(5):1717–1723. doi: 10.1172/JCI114072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher S. P., Robinette J. B., Miller F., Conger J. D. Effect of hemorrhagic reduction in blood pressure on recovery from acute renal failure. Kidney Int. 1987 Mar;31(3):725–730. doi: 10.1038/ki.1987.58. [DOI] [PubMed] [Google Scholar]
- Miller S. B., Hansen V. A., Hammerman M. R. Effects of growth hormone and IGF-I on renal function in rats with normal and reduced renal mass. Am J Physiol. 1990 Nov;259(5 Pt 2):F747–F751. doi: 10.1152/ajprenal.1990.259.5.F747. [DOI] [PubMed] [Google Scholar]
- Miller S. B., Rotwein P., Bortz J. D., Bechtel P. J., Hansen V. A., Rogers S. A., Hammerman M. R. Renal expression of IGF I in hypersomatotropic states. Am J Physiol. 1990 Aug;259(2 Pt 2):F251–F257. doi: 10.1152/ajprenal.1990.259.2.F251. [DOI] [PubMed] [Google Scholar]
- Norman J., Tsau Y. K., Bacay A., Fine L. G. Epidermal growth factor accelerates functional recovery from ischaemic acute tubular necrosis in the rat: role of the epidermal growth factor receptor. Clin Sci (Lond) 1990 May;78(5):445–450. doi: 10.1042/cs0780445. [DOI] [PubMed] [Google Scholar]
- O'Sullivan U., Gluckman P. D., Breier B. H., Woodall S., Siddiqui R. A., McCutcheon S. N. Insulin-like growth factor-1 (IGF-1) in mice reduces weight loss during starvation. Endocrinology. 1989 Nov;125(5):2793–2794. doi: 10.1210/endo-125-5-2793. [DOI] [PubMed] [Google Scholar]
- Ogata K. Clinicopathological study of kidneys from patients on chronic dialysis. Kidney Int. 1990 May;37(5):1333–1340. doi: 10.1038/ki.1990.119. [DOI] [PubMed] [Google Scholar]
- Rogers S. A., Miller S. B., Hammerman M. R. Insulin-like growth factor I gene expression in isolated rat renal collecting duct is stimulated by epidermal growth factor. J Clin Invest. 1991 Jan;87(1):347–351. doi: 10.1172/JCI114992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safirstein R., Zelent A. Z., Price P. M. Reduced renal prepro-epidermal growth factor mRNA and decreased EGF excretion in ARF. Kidney Int. 1989 Nov;36(5):810–815. doi: 10.1038/ki.1989.266. [DOI] [PubMed] [Google Scholar]
- Shaw S. G., Weidmann P., Hodler J., Zimmermann A., Paternostro A. Atrial natriuretic peptide protects against acute ischemic renal failure in the rat. J Clin Invest. 1987 Nov;80(5):1232–1237. doi: 10.1172/JCI113197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W., Finkelstein N., Aliminosa L., Crawford B., Graber M. THE RENAL CLEARANCES OF SUBSTITUTED HIPPURIC ACID DERIVATIVES AND OTHER AROMATIC ACIDS IN DOG AND MAN. J Clin Invest. 1945 May;24(3):388–404. doi: 10.1172/JCI101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
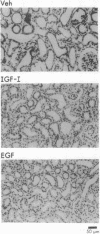
- Toback F. G. Regeneration after acute tubular necrosis. Kidney Int. 1992 Jan;41(1):226–246. doi: 10.1038/ki.1992.32. [DOI] [PubMed] [Google Scholar]
- Venkatachalam M. A., Bernard D. B., Donohoe J. F., Levinsky N. G. Ischemic damage and repair in the rat proximal tubule: differences among the S1, S2, and S3 segments. Kidney Int. 1978 Jul;14(1):31–49. doi: 10.1038/ki.1978.87. [DOI] [PubMed] [Google Scholar]